Abstract
Oral squamous cell carcinoma (OSCC) is a major malignant cancer of the head and neck. Long non-coding RNAs (lncRNAs) have emerged as critical regulators during the development and progression of cancers. This study aimed to identify a lncRNA-related signature with prognostic value for evaluating survival outcomes and to explore the underlying molecular mechanisms of OSCC. Associations between overall survival (OS), disease-free survival (DFS) and candidate lncRNAs were evaluated by Kaplan–Meier survival analysis and univariate and multivariate Cox proportional hazards regression analyses. The robustness of the prognostic significance was shown via the Gene Expression Omnibus (GEO) database. A total of 2,493 lncRNAs were differentially expressed between OSCC and control samples (fold change >2, p < 0.05). We used Kaplan–Meier survival analysis to identify 21 lncRNAs for which the expression levels were associated with OS and DFS of OSCC patients (p < 0.05) and found that down-expression of lncRNA AC012456.4 especially contributed to poor DFS (p = 0.00828) and OS (p = 0.00987). Furthermore, decreased expression of AC012456.4 was identified as an independent prognostic risk factor through multivariate Cox proportional hazards regression analyses (DFS: p = 0.004, hazard ratio (HR) = 0.600, 95% confidence interval(CI) [0.423–0.851]; OS: p = 0.002, HR = 0.672, 95% CI [0.523–0.863). Gene Set Enrichment Analysis (GSEA) indicated that lncRNA AC012456.4 were significantly enriched in critical biological functions and pathways and was correlated with tumorigenesis, such as regulation of cell activation, and the JAK-STAT and MAPK signal pathway. Overall, these findings were the first to evidence that AC012456.4 may be an important novel molecular target with great clinical value as a diagnostic, therapeutic and prognostic biomarker for OSCC patients.
Keywords: Long non-coding RNAs (lncRNAs), Oral squamous cell carcinoma (OSCC), AC012456.4, Prognostic biomarkers
Introduction
The five-year survival rate is approximately 50% for oral squamous cell carcinoma (OSCC), which is one of the most common malignancies of the head and neck region (Bozec et al., 2009; Ferlay et al., 2015; Kamangar, Dores & Anderson, 2006; Kim et al., 2017; Verusingam et al., 2017). The predisposition of OSCC to distant metastases and metastases in the lymph nodes, its highly invasive nature, and its tendency towards local recurrence are important factors that contribute to the poor prognosis of OSCC patients (Massano et al., 2006; Singh & Schenberg, 2013). Hence, more effective novel tumor diagnostic and prognostic biomarkers (Mehrotra & Gupta, 2011), which can improve the survival rate and can be used to assess treatment outcomes, are urgently needed.
The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov) database, which is primarily used to collate specimens from cancer patients and adjacent normal tissue specimens, contains large data sets collected with high-throughput methods at multiple genomic and proteomic levels (Chin, Andersen & Futreal, 2011; Wang, Gerstein & Snyder, 2009). The Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) is the largest and most comprehensive public gene expression repository for high-throughput data at NCBI (Barrett & Edgar, 2006; Clough & Barrett, 2016). Both the GEO and TCGA collect macroscopic clinical information, such as stage and grade of tumor, survival time, age, sex, and race. Therefore, the TCGA and GEO databases can be analyzed systematically and comprehensively to explore important potential value and information.
In this study, we first sought to use the existing GEO microarrays and TCGA RNA-seq data to identify differential expression of lncRNAs between OSCC and control tissue samples. Then, the differentially expressed lncRNAs were evaluated by Kaplan–Meier survival analysis and univariate, multivariate Cox proportional hazards regression analyses and Gene Set Enrichment Analysis (GSEA). Ultimately, through systematic and objective analysis, we first discovered that lncRNA AC012456.4 is significantly associated with survival outcomes of OSCC patients based on TCGA data. Then, AC012456.4 was further successfully confirmed as a potential prognostic biomarker for the prediction of overall survival (OS) in the GEO database. We hope that the lncRNA AC012456.4 revealed in our study may serve as a novel biomarker and potential target for the diagnosis, treatment, and prognosis of OSCC.
Materials and Methods
Data source
The RNA-seq data and corresponding patient information data of head and neck cell carcinoma (HNSC) were downloaded from the TCGA database. Clinical samples from the oral cavity (buccal mucosa, tongue, lip, hard palate, alveolar ridge, floor of the mouth and oral cavity) were chosen, while some samples from other parts (hypopharynx, larynx, oropharynx and tonsil, for example) were excluded. The original microarray data between OSCC and adjacent normal tissue samples were downloaded from the NCBI GEO databases. The accession numbers were GSE36820 and GSE41613, respectively. The microarray data of GSE36820 and GSE41613 were based on GPL570 (Affymetrix Human Genome U133 Plus 2.0 Array).
Data pre-processing and differential expression analysis
The edgeR package was downloaded from the Stanford University website. The original microarray data from the GEO were converted into expression measures using the affy R package. Then, the differentially expressed lncRNAs were identified by the Limma R package (Ritchie et al., 2015; Teufel et al., 2016). The differentially expressed lncRNAs that were screened from the TCGA were analyzed by the edgeR package (Robinson, McCarthy & Smyth, 2010). To improve screen accuracy and simplify the screening process, the cut-off criteria, which was in accordance with the procedure of Benjamini & Hochberg (BH), was as follows: 1. the false discovery rate was controlled at 0.01; 2. the fold change should be more than 2. The differentially expressed lncRNAs among GSE36820, GSE41613 and the TCGA were identified by the intersect function in the R package. Tumor and normal tissue data were recorded and were statistically analyzed.
Identification of lncRNAs with prognostic value in OSCC
The differences between expressed lncRNAs (fold change >2, p < 0.05) are involved in the prognostic value for OSCC. The OSCC patients were divided into two parts, depending on the average expression level of candidate lncRNAs: a high expression group and a low expression group. Survival differences and p-values were compared between the two groups and were evaluated using a Kaplan–Meier survival analysis and a log-rank test. After this, a univariate Cox proportional hazards regression analysis (Bair & Tibshirani, 2004) was conducted to assess the correlation between candidate lncRNAs and patient overall survival (OS) and disease-free survival (DFS) (p < 0.05). Statistically significant lncRNAs and clinical candidate predictors were further evaluated by multivariate Cox proportional hazards regression analyses to identify independent prognostic lncRNAs. Candidate predictors included age, gender, grade, and stage. We then performed subgroup analyses. The hazard ratio (HR) and 95% confidence interval (CI) were also assessed.
Gene set enrichment analysis (GSEA)
GSEA 2-2.2.3 (JAVA version) was downloaded from the Gene Set Enrichment Analysis website (http://software.broadinstitute.org/gsea/index.jsp). Then, the downloaded dataset was imported using the GSEA software. Gene sets identified as related to biological signal conduction on the MSigDB (Molecular Signatures Database) (http://software.broadinstitute.org/gsea/msigdb), which may be found on the GSEA website, served as reference gene sets. This process was repeated 1,000 times for each analysis according to the default weighted enrichment statistical method. Gene sets with a false discovery rate (FDR) <0.25 and a family-wise error rate (FWR) <0.05. The GSEA analysis includes four key statistics: Enrichment Score (ES), Normalized Enrichment Score (NES), False Discovery Rate (FDR) and P-value.
Statistical analysis
In this study, all analyses, including the t-test, heat map, and survival analyses, were performed with the R, GraphPad and SPSS software packages. p values less than 0.05 were considered significant. All statistical tests were two-sided.
Results
Characteristics of OSCC patients according to the TCGA
In this study, the datasets of 350 OSCC patient and 44 controls were acquired and downloaded from the TCGA (http://cancergenome.nih.gov) database; these datasets contained expression data and clinical information related to 14,448 lncRNAs. The clinicopathological features of all patients are shown in Table 1. The mean ± standard deviation (STDEV) for all patient ages is 61.590 ± 12.886.
Table 1. The clinicopathological characteristics of patients from the TCGA database.
| Characteristics | Number of case | No. of patients (%) |
|---|---|---|
| Age (years) | 346 | |
| ≦60 | 152(41.33%) | |
| ≧60 | 194(58.67%) | |
| Median (range) | 61.590(19–90) | |
| Gender | 347 | |
| Male | 236(68.01%) | |
| Female | 111(31.99%) | |
| Alcohol history | 339 | |
| No | 111(32.74%) | |
| Yes | 228(67.26%) | |
| Perineural invasion present | 263 | |
| No | 123(46.77%) | |
| Yes | 140(53.23%) | |
| Margin status | 324 | |
| Close | 39(12.04%) | |
| Negative | 244(75.31%) | |
| Positive | 41(12.65%) | |
| Lymphovascular invasion present | 250 | |
| Yes | 76(30.40%) | |
| No | 174(69.60%) | |
| Tumor stage | 314 | |
| Stage I | 21(6.69%) | |
| Stage II | 56(17.83%) | |
| Stage III | 64(20.38%) | |
| Stage IV | 173(55.10%) | |
| T stage | 335 | |
| T1 | 34(10.15%) | |
| T2 | 103(%) | |
| T3 | 70(%) | |
| T4 | 128(%) | |
| N stage | 334 | |
| NO | 126(37.72%) | |
| N1 | 52(15.57%) | |
| N2 | 110(32.93%) | |
| N3 | 46(13.77%) | |
| M stage | 170 | |
| M0 | 125(73.53%) | |
| M1 | 45(26.47%) | |
| Histologic grade | 344 | |
| G1 | 53(15.41%) | |
| G2 | 210(61.05%) | |
| G3 | 71(20.64%) | |
| G4 | 10(2.91%) | |
| Vital status | 347 | |
| Alive | 227(65.42%) | |
| Dead | 120(34.58%) |
Significant differentially expressed lncRNAs in OSCC
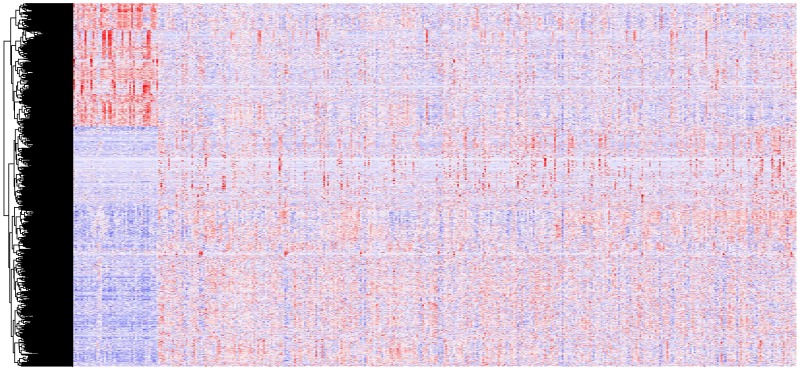
In all, 2,493 differentially expressed lncRNAs were identified through analysis of 14,448 lncRNAs using the edgeR packages (fold change >2, p < 0.05) (Fig. 1). Moreover, 855 lncRNAs were down-regulated and 1,638 lncRNAs were up-regulated in the OSCC samples compared to normal tissue. Down-regulated and up-regulated lncRNAs account for 34.2% and 65.6% of the differentially expressed lncRNAs, respectively.
Figure 1. A heat map drawn to show differential lncRNA expression in OSCC and normal tissue samples from the TCGA datasets, which were analyzed with R software.
Representative genes of each cluster were selected and represented as a heat map. Genes shown in red are upregulated and genes in blue are downregulated. The magnitude of the regulation is illustrated by the intensity of the color.
Identification of survival differences lncRNAs in OSCC
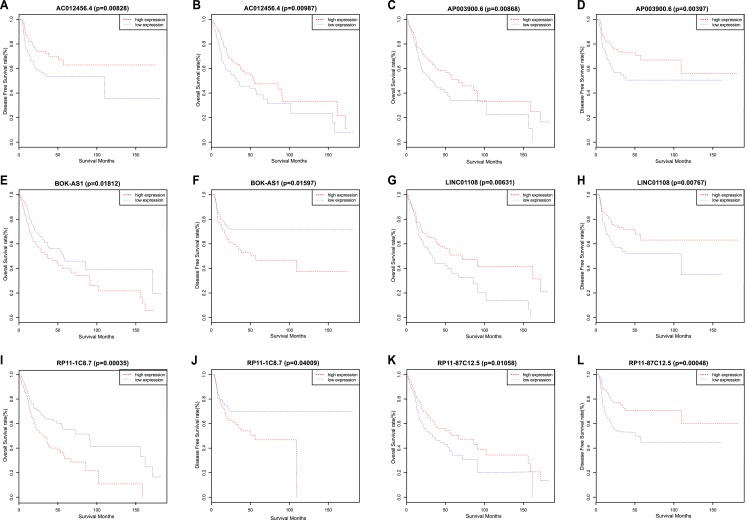
We used a Kaplan–Meier survival analysis with the log-rank test to identify relationships between the above 2,493 lncRNA signatures and the survival of OSCC patients. Then, we determined the levels of 21 lncRNA signatures that were significantly related to OS and DFS. Among these 21 lncRNAs, a significant positive correlation was observed between the signatures of 13 lncRNAs (TTC39A-AS1, RP11-93B14.9, AC012456.4, RP11-87C12.5, RP11-464F9.21, LINC01549, RP11-897M7.1, AP003900.6, LINC01343, RP11-181E10.3, CTD-2545H1.2, RP11-796E2.4 and LINC01108) and OS/DFS. In contrast, the signatures of the remaining 8 lncRNAs (AC007879.2, BOK-AS1, CTB-161M19.4, CTD-2033A16.3, FAM95B1, RP11-1C8.7, RP11-285G1.14 and RP11-286E11.1) were significantly negatively correlated with OS and DFS. That is, low expression of the 13 lncRNAs described above correlated with a poor prognosis of OSCC patients, while the up-regulation of the latter 8 lncRNAs correlated with a shorter survival time (Fig. 2) (Table 2).
Figure 2. Kaplan–Meier survival analyses and log-rank tests for OS and DFS in OSCC.
(A) OS and (B) DFS rates of all patients according to AC012456.4 expression. (C) OS and (D) DFS rates of all patients according to AP003900.6 expression. (E) OS and (F) DFS rates of all patients according to BOK-AS1 expression. (G) OS and (H) DFS rates of all patients according to LINC01108 expression. (I) OS and (J) DFS rates of all patients according to RP11-1C8.7 expression. (K) OS and (L) DFS rates of all patients according to RP11-87C12.5 expression.
Table 2. Twenty-one lncRNA levels significantly correlated to OS and DFS.
| LncRNA | Gene ID | Chromosome | OS (P value ) | DFS (P value ) |
|---|---|---|---|---|
| AC012456.4 | ENSG00000230790 | chr2 | 0.00987 | 0.00828 |
| AP003900.6 | ENSG00000271308 | chr21 | 0.00868 | 0.00397 |
| BOK-AS1 | ENSG00000234235 | chr2 | 0.01812 | 0.01597 |
| LINC01108 | ENSG00000226673 | chr6 | 0.00631 | 0.00767 |
| RP11-1C8.7 | ENSG00000271830 | chr8 | 0.00035 | 0.04009 |
| RP11-87C12.5 | ENSG00000255856 | chr12 | 0.01058 | 0.00048 |
| TTC39A-AS1 | ENSG00000261664 | chr1 | 0.04276 | 0.00371 |
| RP11-93B14.9 | ENSG00000277496 | chr20 | 0.01279 | 0.00352 |
| AC007879.2 | ENSG00000234902 | chr2 | 0.00811 | 0.03607 |
| RP11-464F9.21 | ENSG00000234606 | chr10 | 0.01486 | 0.03221 |
| LINC01549 | LINC01549 | chr21 | 0.00021 | 0.0165 |
| CTB-161M19.4 | ENSG00000249494 | chr5 | 0.04807 | 0.01152 |
| RP11-286E11.1 | ENSG00000245293 | chr4 | 0.03618 | 0.0041 |
| RP11-897M7.1 | ENSG00000256209 | chr12 | 0.03129 | 0.02265 |
| LINC01343 | ENSG00000237290 | chr1 | 0.01115 | 0.03191 |
| FAM95B1 | ENSG00000223839 | chr9 | 0.04778 | 0.01648 |
| RP11-181E10.3 | ENSG00000271590 | chr2 | 0.00597 | 0.00934 |
| CTD-2545H1.2 | ENSG00000262445 | chr17 | 0.02892 | 0.02929 |
| RP11-796E2.4 | ENSG00000245904 | chr12 | 0.04276 | 0.00371 |
| CTD-2033A16.3 | ENSG00000262136 | chr16 | 0.04586 | 0.02714 |
| RP11-285G1.14 | ENSG00000273363 | chr10 | 0.01276 | 0.00503 |
Through the above Kaplan–Meier survival analysis, the variables of age, gender, grade, tumor stage, and TNM stage were identified as statistically significant factors that are related to the above 21 lncRNAs and patient prognosis. We also applied univariate and multivariate Cox regression analyses to evaluate the ability of 21 candidate lncRNA signatures to serve as independent prognostic variables. The univariate analysis indicated that decreased AC012456.4 expression (HR = 0.706, 95% CI [0.551–0.903], p = 0.006), age, tumor stage, and TNM stage were all significantly related to worse OS in OSCC patients (Table 3). Decreased AC012456.4 expression (HR = 0.601, 95% CI [0.423–0.853], p = 0.004) was the only variable that could predict poorer DFS for OSCC. Finally, multivariate Cox regression analysis revealed that low expression of AC012456.4 was the only independent prognostic variable for both OS (HR = 0.672, 95% CI [0.523–0.863], p = 0.002) and DFS (HR = 0.600, 95% CI [0.423–0.851], p = 0.004) in OSCC patients (Table 4). In addition, age and N stage were highly significantly correlated with shorter OS or DFS.
Table 3. Univariate and multivariate Cox regression analysis for OS in patients with OSCC.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Age (years) | 0.003 | 1.021 | 1.007, 1.036 | 0.001 | 1.026 | 1.011, 1.041 |
| Gender | 0.459 | 1.150 | 0.794, 1.665 | 0.481 | 1.145 | 0.786, 1.666 |
| Grade | 0.127 | 1.215 | 0.946, 1.560 | 0.062 | 1.276 | 0.988, 1.648 |
| Stage | ||||||
| (age ≦ 60) | 0.034 | 1.425 | 1.026, 1.978 | 0.210 | 0.765 | 0.503, 1.163 |
| (age > 60) | 0.523 | 1.080 | 0.853, 1.367 | |||
| N | 0.015 | 1.263 | 1.046, 1.524 | 0.011 | 1.279 | 1.059, 1.546 |
| T (age ≦ 60) | 0.003 | 1.551 | 1.160, 2.075 | 0.293 | 1.101 | 0.921, 1.316 |
| (age > 60) | 0.873 | 0.982 | 0.783, 1.230 | |||
| AC012456.4 | 0.006 | 0.706 | 0.551, 0.903 | 0.002 | 0.672 | 0.523, 0.863 |
Notes.
- N
- Regional Lymph Nodes
- T
- Primary Tumor
Table 4. Univariate and multivariate Cox regression analysis for DFS in patients with OSCC.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Age (years) | 0.093 | 1.017 | 0.997, 1.036 | 0.071 | 1.018 | 0.999, 1.037 |
| Gender | 0.627 | 1.132 | 0.687, 1.867 | 0.678 | 1.113 | 0.672, 1.841 |
| Grade | 0.817 | 1.043 | 0.732, 1.485 | 0.533 | 1.125 | 0.777, 1.627 |
| Stage | 0.625 | 1.064 | 0.830, 1.363 | 0.482 | 0.852 | 0.545, 1.332 |
| N | 0.539 | 1.085 | 0.7837, 1.407 | 0.167 | 1.286 | 0.900, 1.836 |
| T | 0.191 | 1.167 | 0.926, 1.470 | 0.295 | 1.134 | 0.896, 1.434 |
| AC012456.4 | 0.004 | 0.601 | 0.423, 0.853 | 0.004 | 0.600 | 0.423, 0.851 |
lncRNA AC012456.4 was low expressed in OSCC tissues and associated with clinicopathological parameters
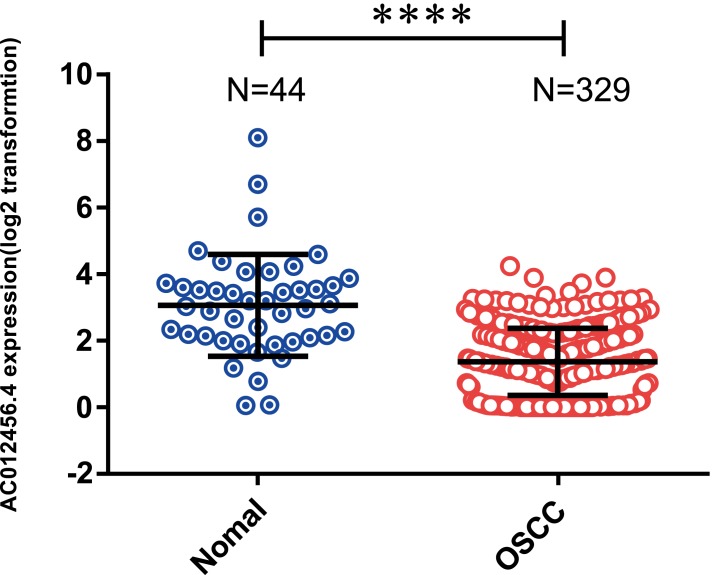
OSCC patients were further classified into high or low expression groups based on the median value of the relative lncRNA expression. The expression of lncRNA AC012456.4 was significantly weaker in OSCC tissue samples (1.360 ± 0.05569) relative to normal tissue samples (3.062 ± 0.2304) in the TCGA (p < 0.0001) (Fig. 3). The correlation between lncRNA AC012456.4 expression and clinicopathologic parameters of OSCC patients was also further analyzed. As shown in Table 5, lncRNA AC012456.4 expression was significantly correlated with alcohol history consumption (p = 0.033). Additionally, decreased expression of lncRNA AC012456.4 expression was nearly significantly associated with T stage (p = 0.075). However, no significant association was found between other clinicopathological factors and lncRNA AC012456.4 expression.
Figure 3. Expression of AC012456.4 in normal tissues and OSCC tissues.
AC012456.4 expression is significantly down-regulated in OSCC samples (1.360 ± 0.05569) in comparison to adjacent non-cancerous tissues (3.062 ± 0.2304) in the TCGA dataset.
Table 5. AC012456.4 expression and clinicopathological characteristics of patients with OSCC.
| Characteristics | Number of case | AC012456.4 expression | P value | |
|---|---|---|---|---|
| Decreased number (%) | Non-decreased number (%) | |||
| Age (years) | 0.082 | |||
| ≥60 | 186 | 96(51.61%) | 90(48.39%) | |
| <60 | 143 | 60(41.96%) | 83(58.08%) | |
| Gender | 0.745 | |||
| Female | 102 | 47(46.08%) | 55(59.92%) | |
| Male | 227 | 109(48.02%) | 118(51.98%) | |
| Alcohol history | 0.033 | |||
| Yes | 213 | 109(51.17%) | 104(48.83%) | |
| No | 104 | 40(38.46%) | 64(61.54%) | |
| M stage | 0,511 | |||
| M0 | 119 | 56(47.06%) | 63(52.94%) | |
| M1 | 39 | 16(41.03%) | 23(58.97%) | |
| T stage | 0.075 | |||
| T1 + T2 | 128 | 54(42.19%) | 74(57.81%) | |
| T3 + T4 | 189 | 99(52.38%) | 90(47.62%) | |
| N stage | 0.163 | |||
| N0 + N1 | 168 | 87(51.79%) | 81(48.21%) | |
| N2 + N3 | 148 | 65(43.92%) | 83(56.08%) | |
Notes.
- M0
- No distant metastasis (no pathologic M0; use clinical M to complete stage group)
- M1
- Distant metastasis
- N0
- No regional lymph node metastasis
- N1
- Metastasis in a single ipsilateral lymph node, 3 cm or less in greatest dimension
- N2
- Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension; or in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension; or in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension
- N3
- Metastasis in a lymph node more than 6 cm in greatest dimension
- T1
- Tumor 2 cm or less in greatest dimension
- T2
- Tumor more than 2 cm but not more than 4 cm in greatest dimension
- T3
- Tumor more than 4 cm in greatest dimension
- T4a
- Moderately advanced local disease
- T4b
- T4b Very advanced local disease
Tumor invades masticator space, pterygoid plates, or skull base and/or encases internal carotid artery.
Evaluation of the prognostic value of lncRNA AC012456.4 via the GEO
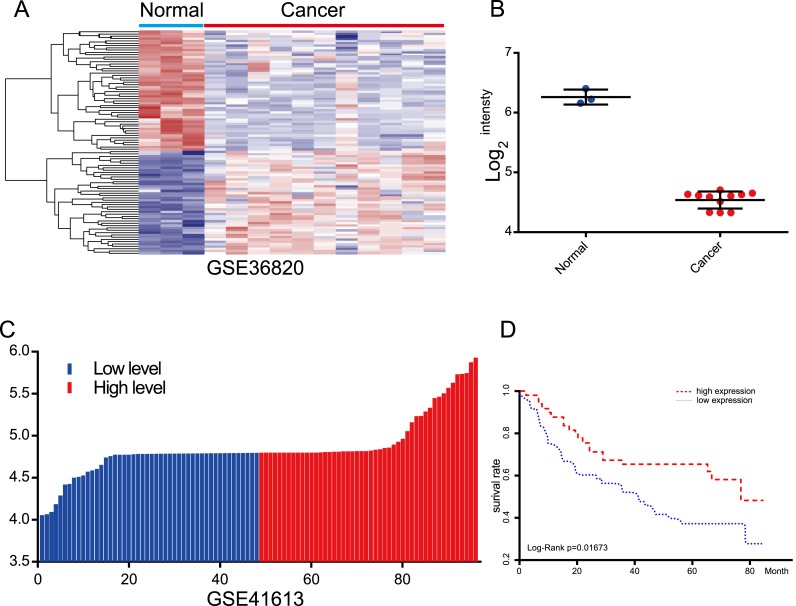
For the purpose of evaluating the robustness of lncRNA AC012456.4 expression in the prediction of OS of OSCC patients, we acquired other independent datasets from the GEO with accession numbers of GSE36820 and GSE41613, which contained OSCC samples, but samples with incomplete clinical information were excluded. The prognostic signatures and the Kaplan–Meier analysis were calculated and performed for each OSCC sample. In agreement with the result of the TCGA datasets, low expression levels of lncRNA AC012456.4 were associated with lower OS (Fig. 4). The lncRNA AC012456.4 was also expressed at low levels in OSCC tissues (p < 0.0001).
Figure 4. Evaluation of the prognostic value of lncRNA AC012456.4 via the GEO.
(A) Heatmap of lncRNA AC012456.4 expression in GEO. (B) lncRNA AC012456.4 expression was significantly low in OSCC. (C) OSCC patients were divided into the high expression group and the low expression group according to the median lncRNA AC012456.4 expression. (D) The low expression of lncRNA AC012456.4 was significantly associated with poor prognosis in patients with OSCC (p < 0.0001).
Relationship between lncRNA AC012456.4 and biological pathways and functions
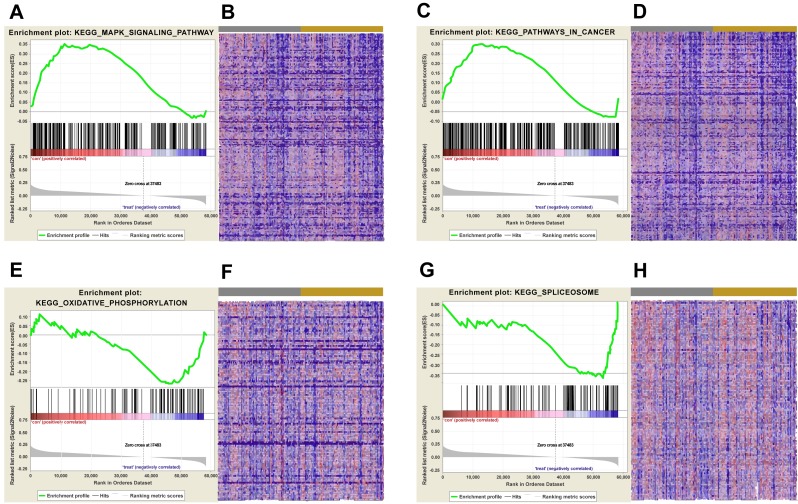
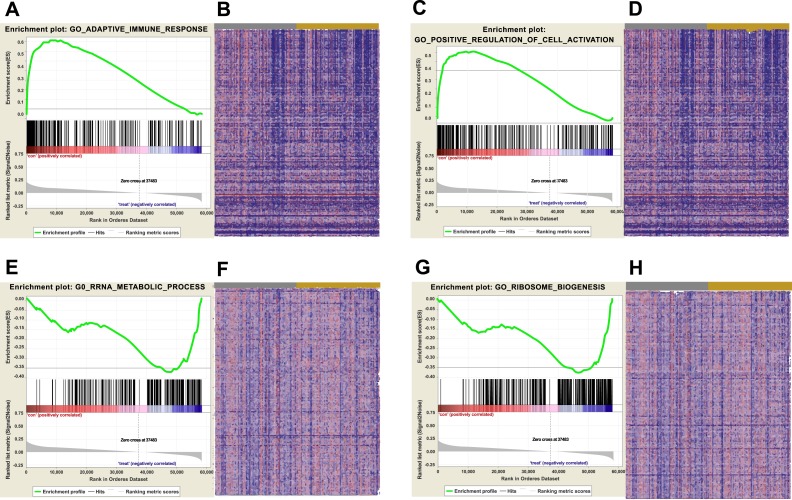
Biological pathways and functions of lncRNA AC012456.4 were identified by GSEA. This analysis revealed that lncRNA AC012456.4 was involved in many critical pathways and correlated with tumorigenesis. A total of 150 pathways listed in the high-risk group were enriched, including KEGG MAPK SIGNALING PATHWAY, KEGG JAK-STAT SIGNALING PATHWAY, KEGG CALCIUM SIGNALING PATHWAY and KEGG PATHWAYS IN CANCER. Twenty-seven pathways in the low-risk group were also identified, including the KEGG OXIDATIVE PHOSPHORYLATION, KEGG PROTEASOME and KEGG SPLICEOSOME (Fig. 5). Similarly, 3073 GO annotations in the high-risk group and 516 GO annotations in the low-risk group were enriched (Fig. 6). Relevant partial results for KEGG pathways and GO analysis are listed in Table 6 and Table 7.
Figure 5. KEGG pathway enrichment analysis of lncRNA AC012456.4.
(A) Enrichment of genes in the KEGG MAPK SIGNALING PATHWAY by GSEA. (B) Heat map of core enrichment genes in the gene set KEGG MAPK SIGNALING PATHWAY. (C) Enrichment of genes in KEGG PATHWAYS IN CANCER by GSEA. (D) Heat map of core enrichment genes from the gene set KEGG PATHWAYS IN CANCER. (E) Enrichment of genes in KEGG OXIDATIVE PHOSPHORYLATION by GSEA. (F) Heat map of core enrichment genes from the gene set KEGG OXIDATIVE PHOSPHORYLATION. (G) Enrichment of genes in KEGG SPLICEOSOME by GSEA. (H) Heat map of core enrichment genes from the gene set KEGG SPLICEOSOME. The GSEA software was used to calculate enrichment levels.
Figure 6. GSEA were carried out to identify upregulated or downregulated GO.
(A) Enrichment of genes in GO ADAPTIVE IMMUNE RESPONSE by GSEA. (B) Heat map of core enrichment genes in the gene set GO ADAPTIVE IMMUNE RESPONSE. (C) Enrichment of genes in GO POSITIVE REGULATION OF CELL ACTIVATION by GSEA. (D) Heat map of core enrichment genes in the gene set GO POSITIVE REGULATION OF CELL ACTIVATION. (E) Enrichment of genes in GO RRNA METABOLIC PROCESS by GSEA. (F) Heat map of core enrichment genes in the gene set GO RRNA METABOLIC PROCESS. (G) Enrichment of genes in GO RIBOSOME BIOGENESIS by GSEA. (H) Heat map of core enrichment genes in the gene set GO RIBOSOME BIOGENESIS. The GSEA software was used to calculate the enrichment levels.
Table 6. KEGG Pathways enriched in high-risk and low-risk groups by using GSEA.
| NAME | SIZE | ES | NES | NOM p-val | FDR q-val | FWER p-val | Rank at max | Leading edge |
|---|---|---|---|---|---|---|---|---|
| KEGG_PRIMARY_IMMUNODEFICIENCY | 35 | 0.783950 | 2.003367 | 0.002036 | 0.080199 | 0.032 | 5022 | tags=63%, list=9%, signal=69% |
| KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | 258 | 0.503302 | 1.751613 | 0.016227 | 0.351688 | 0.258 | 13393 | tags=46%, list=23%, signal=60% |
| KEGG_JAK_STAT_SIGNALING_PATHWAY | 151 | 0.462485 | 1.585162 | 0.051020 | 0.356088 | 0.496 | 11252 | tags=35%, list=19%, signal=43% |
| KEGG_PATHWAYS_IN_CANCER | 324 | 0.296756 | 1.015304 | 0.442386 | 0.570524 | 0.968 | 12772 | tags=28%, list=22%, signal=36% |
| KEGG_MAPK_SIGNALING_PATHWAY | 265 | 0.353983 | 1.239951 | 0.226804 | 0.527153 | 0.881 | 11268 | tags=29%, list=19%, signal=35% |
| KEGG_PROTEASOME | 46 | −0.542264 | −1.310828 | 0.249049 | 1 | 0.849 | 11204 | tags=54%, list=19%, signal=67% |
| KEGG_CYTOSOLIC_DNA_SENSING_PATHWAY | 55 | −0.342477 | −1.059409 | 0.361581 | 1 | 0.958 | 6866 | tags=33%, list=12%, signal=37% |
| KEGG_SNARE_INTERACTIONS_IN_VESICULAR_TRANSPORT | 38 | −0.365953 | −0.983674 | 0.481132 | 1 | 0.969 | 6863 | tags=32%, list=12%, signal=36% |
| KEGG_OXIDATIVE_PHOSPHORYLATION | 118 | −0.269338 | −0.724989 | 0.681050 | 1 | 0.992 | 11643 | tags=38%, list=20%, signal=48% |
| KEGG_SPLICEOSOME | 123 | −0.362891 | −0.936620 | 0.566473 | 1 | 0.978 | 5025 | tags=24%, list=9%, signal=26% |
Table 7. GO annotation enriched in high-risk and low-risk groups by using GSEA.
| Name | Size | ES | NES | NOM p-val | FDR q-val | FWER p-val | Rank at max | Leading edge |
|---|---|---|---|---|---|---|---|---|
| GO_B_CELL_RECEPTOR_SIGNALING_PATHWAY | 54 | 0.749803 | 1.963207 | 0.003838 | 0.974954 | 0.161 | 6389 | tags=67%, list=11%, signal=75% |
| GO_ADAPTIVE_IMMUNE_RESPONSE | 279 | 0.614785 | 1.932954 | 0.007648 | 0.761521 | 0.202 | 7793 | tags=46%, list=13%, signal=53% |
| GO_NEGATIVE_REGULATION_OF_INTERLEUKIN _6_PRODUCTION | 33 | 0.711452 | 1.897834 | 0 | 0.660863 | 0.28 | 10264 | tags=67%, list=18%, signal=81% |
| GO_REGULATION_OF_B_CELL_ACTIVATION | 121 | 0.626420 | 1.886616 | 0.003883 | 0.617897 | 0.294 | 9579 | tags=55%, list=16%, signal=65% |
| GO_POSITIVE_REGULATION_OF_CELL_ACTIVATION | 305 | 0.540650 | 1.725436 | 0.031496 | 0.464684 | 0.631 | 11768 | tags=47%, list=20%, signal=58% |
| GO_CELLULAR_RESPONSE_TO_ZINC_ION | 16 | −0.60868 | −1.550511 | 0.056310 | 1 | 0.883 | 4440 | tags=56%, list=8%, signal=61% |
| GO_RIBOSOMAL_LARGE_SUBUNIT_BIOGENESIS | 48 | −0.60318 | −1.496404 | 0.109343 | 1 | 0.925 | 5330 | tags=42%, list=9%, signal=46% |
| GO_POSITIVE_REGULATION_OF_PEPTIDYL_SERINE _PHOSPHORYLATION_OF_STAT_PROTEIN | 21 | −0.52630 | −1.392874 | 0.115079 | 1 | 0.962 | 6411 | tags=48%, list=11%, signal=53% |
| GO_RRNA_METABOLIC_PROCESS | 249 | −0.38387 | −1.055244 | 0.457925 | 1 | 0.998 | 10606 | tags=36%, list=18%, signal=44% |
| GO_RIBOSOME_BIOGENESIS | 300 | −0.38284 | −1.050548 | 0.456692 | 1 | 0.998 | 11706 | tags=38%, list=20%, signal=47% |
Discussion
OSCC is a common, highly invasive type of oral cancer prone to early recurrence and metastasis (Massano et al., 2006; Singh & Schenberg, 2013). Therefore, early diagnosis and treatment of OSCC is essential (Bozec et al., 2009). While cytology- and pathology-based methods have been applied to the clinical differential diagnosis of OSCC, limitations in the detection methods and poor prognoses have limited the five-year survival rate (Omar, 2013). Hence, more reliable, accurate and sensitive prognosis biomarkers and tools for early diagnosis are urgently needed (Mehrotra & Gupta, 2011). In recent years, many studies have revealed a close association between aberrant expression of lncRNAs and tumorigenesis (Alessandro & Irene, 2014; Batista & Chang, 2013; Espinosa, 2017; Rinn & Chang, 2012; Slaby, Laga & Sedlacek, 2017), which may aid in cancer diagnosis and prognosis.
Fewer than 2% of genes in the human genome are transcribed, and up to 98% of these transcripts are non-coding RNAs (Jandura & Krause, 2017; Espinosa, 2013; Quinn & Chang, 2016). lncRNAs are a class of non-coding transcripts ≥ 200 nucleotides in length that are actively involved in many biological processes, such as epigenetic regulation, cell cycle regulation, chromatin modulation and regulation of multiple gene expression (Rinn & Chang, 2012; Wang et al., 2017). These non-coding transcripts also play key roles in the occurrence, development and progression of malignant tumors (Espinosa, 2017; Kopp & Mendell, 2018; Spizzo et al., 2012). An increasing number of studies have reported that lncRNAs can play essential roles as oncogenes or tumor suppressor genes involved in the development and progression of various cancers (Batista & Chang, 2013; Espinosa, 2017; Kopp & Mendell, 2018; Reik, 2009; Rinn & Chang, 2012; Slaby, Laga & Sedlacek, 2017; Spizzo et al., 2012), including OSCC (Fang et al., 2017; Gomes et al., 2017; Guo et al., 2017; (Li et al., 2017). For example, the down-regulation of HOTAIR is associated with cancer progression in 26 human tumor types (Bhan & Mandal, 2015).
However, most early studies focused on a single gene or the results obtained from a single cohort study of lncRNAs and OSCC. Sun et al. (2017) used qRT-PCR to analyze the expression levels of lncRNA PDIA3P in 58 OSCC and paired noncancerous tissue samples. This study found that the overexpression of lncRNA PDIA3P correlated with lower survival rates for OSCC patients. One study by Wu et al. (2015) suggested that high expression of lncRNA HOTAIR in OSCC patients would contribute to the development and progression of cancer, leading to a poor prognosis. Similarly, LINC00668 expression is increased in both 50 OSCC tissues and cells, and over-expression is significantly correlated with poorer survival for OSCC patients; Therefore, this might be a negative predictive factor for the prognosis of OSCC patients (Zhang, 2017). In the era of big data, the development of TCGA and GEO technology has allowed researchers to predict and identify new biomarkers, which has enhanced the reliability and accuracy of current research. Cui et al. (2017) used TCGA and GEO data to determine that the expression levels of several lncRNAs, including RP1-228H13.5, TMCC1-AS1, LINC00205, and RP11-307C12.11, were associated with OS and recurrence-free survival of hepatocellular carcinoma patients. Three lncRNAs (LINC01140, TGFB2-OT1, and RP11-347C12.10) were significantly correlated with prognoses of hepatocellular carcinoma patients, independent of some clinical characteristics. Using the database, three lncRNAs, which may play key roles in the development, progression, and recurrence in gastric cancer, were identified (Song et al., 2017). However, the functions, roles, and molecular mechanisms of lncRNAs associated with OSCC remain unclear.
In this study, we identified lncRNAs that are dysregulated in OSCC and evaluated the relationships between the TCGA database and the clinicopathological features of these OSCC patients. Based on the above analysis, a total of 21 lncRNAs were correlated with patient prognoses, of which 13 lncRNAs (TTC39A-AS1, RP11-93B14.9, AC012456.4, RP11-87C12.5, RP11-464F9.21, LINC01549, RP11-897M7.1, AP003900.6, LINC01343, RP11-181E10.3, CTD-2545H1.2, RP11-796E2.4 and LINC01108) were significantly positively associated with OS and DFS, while the up-regulation of the latter eight lncRNAs (AC007879.2, BOK-AS1, CTB-161M19.4, CTD-2033A16.3, FAM95B1, RP11-1C8.7, RP11-285G1.14 and RP11-286E11.1) were correlated with poorer prognoses. Lan et al. (2017) have also reported that RP11-1C8.7 predicted the progression and outcome of patients with kidney renal papillary cell carcinoma and was regarded as an independent prognostication factor for kidney renal papillary cell carcinoma. Thus far in the published literature, no report has evaluated the biological function and molecular mechanisms of other lncRNAs associated with human cancers.
To our knowledge, this study is pioneering research and identified the lncRNA AC012456.4, which exhibited significantly lower expression in OSCC tissues than in adjacent normal tissues. Additionally, a Kaplan–Meier survival analysis (Gyorffy, Lánczky & Szállási, 2012) as well as univariate and multivariate Cox regression analyses revealed that lncRNA AC012456.4 was an independent prognostic factor and was significantly correlated with shorter OS and DFS. Further validation via the GEO database was consistent with the TCGA database analysis results. Moreover, we further evaluated the relationship between AC012456.4 expression and the clinicopathological features of OSCC patients. Low levels of AC012456.4 were found to be significantly associated with the history of alcohol consumption in OSCC patients. Interestingly, according to previous studies, we found that alcohol consumption can increase the probability of G:C to A:T transitions and that alcohol drinkers exhibited a significantly higher incidence of p53 mutations in OSCC (Hsieh et al., 2001), which suggested that alcohol may play a critical role in the progression of OSCC.
Since lncRNAs perform their biological function by specifically binding to target genes, we further explored the possible biological functions and molecular pathways of AC012456.4. Through GSEA, AC012456.4 was found to be significantly involved with tumor-related signaling pathways and crucial biological functions in tumorigenesis. Key pathways and functions for tumor initiation and progression were identified, such as GO biological function annotation and KEGG pathways, including the adaptive immune response, RRNA metabolic processes, CALCIUM, MAPK, and the JAK/STAT signaling pathway. Additionally, mutation, aberrant expression and modification of these GO annotations and signaling pathways have been frequently reported in OSCC and other cancers. We found that the MAPK pathway could be activated by the low expression of the tumor suppressor QKI-5, which can promote the proliferation of OSCC cells (Fu & Feng, 2015). We also revealed the strong relationships between HOXC10 and gastric cancer cell proliferation and metastasis, which occur through the MAPK pathway (Guo et al., 2017). Other pathways and biological functions have also been reported in pancreatic ductal adenocarcinoma (Huang et al., 2017a), hepatocellular carcinoma (Huang et al., 2017b; Wonganan et al., 2017), and human papillomavirus-transformed tumors (Skeate et al., 2018).
Dysregulated expression of lncRNA signatures has tremendous potential value, but this research has limitations. Above all, we have explored the correlation between AC012456.4 expression and OSCC prognosis based on the TCGA and GEO databases, which signifies that the exploration was performed using a bioinformatics approach. Then, further research, such as quantitative real-time PCR, as well as in vivo and in vitro experiments, will require collaborative efforts to explore the potential molecular functions and related mechanisms of these lncRNAs in OSCC.
Conclusions
In summary, this study was the first to discover that lncRNA AC012456.4 was poorly expressed in OSCC, with decreased survival rates for OSCC patients. This may be a potential novel, independent biomarker and therapeutic target for the early diagnosis, pathological classification, clinical treatment and outcome prediction for OSCC. Nevertheless, these assumptions require validation and confirmation by larger, multicenter studies.
Supplemental Information
The clinicopathological features of all OSCC patients from the TCGA database.
Univariate and multivariate Cox regression analyses were applied to evaluate the 21 candidate lncRNA signatures as independent prognostic variables.
AC012456.4 expression is significantly down-regulated in OSCC and normal tissue samples in the TCGA dataset.
The relationship between the expression of lncRNA AC012456.4 OSCC tissues with clinicopathological parameters.
Kaplan–Meier survival analysis with the log-rank test was used to identify relationships between the above 2493 lncRNA signatures and OSCC patient survival. Then, we determined the levels of 126 lncRNA signatures that were significantly related to DFS.
Kaplan–Meier survival analysis with the log-rank was used to identify relationships between the above 2493 lncRNA signatures and OSCC patient survival. Then, we determined the levels of 151 lncRNA signatures that were significantly related to OS.
Kaplan–Meierlan-Meier survival analysis with the log-rank was used to identify relationships between the above 2493 lncRNA signatures and OSCC patient survival. Then, we determined the levels of 21 lncRNA signatures that were significantly related to OS and DFS.
Biological pathways and functions of lncRNA AC012456.4 were identified by GSEA. This analysis revealed that lncRNA AC012456.4 was involved in many critical pathways and correlated with tumorigenesis.
Acknowledgments
We thank the patients and investigators who participated in TCGA Research Network (http://cancergenome.nih.gov/), which provides a Web resource for exploring, visualizing, and analyzing multidimensional cancer genomics data. In addition, Xuegang Hu wants to thank, in particular, the patience, care and support from Yao Xiong, Qun Li, Jing Lin, Qin Hu, Tingting You, and Qian Xiong in lab 302 over the past years. The authors also acknowledge the significant advice from Dr. Shan Jiang.
Abbreviations
- LncRNAs
long non-coding RNAs
- OSCC
Oral squamous cell carcinoma
- HR
hazard ratio
- CI
confidence interval
- DFS
disease-free survival
- OS
overall survival
- TCGA
The Cancer Genome Atlas
- GEO
Gene Expression Omnibus
- GSEA
Gene Set Enrichment Analysis
- KEGG
the Kyoto Encyclopedia of Genes and Genomes
- GO
Gene Ontology
- STDEV
standard deviation
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Xuegang Hu conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Zailing Qiu conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Jianchai Zeng performed the experiments, analyzed the data, prepared figures and/or tables, approved the final draft.
Tingting Xiao performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Zhihong Ke performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Hongbing Lyu conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Alessandro & Irene (2014).Alessandro F, Irene B. Long non-coding RNAs: new players in cell differentiation and development. Nature Reviews Genetics. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- Bair & Tibshirani (2004).Bair E, Tibshirani R. Semi-supervised methods to predict patient survival from gene expression data. PLOS Biology. 2004;2:E108. doi: 10.1371/journal.pbio.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett & Edgar (2006).Barrett T, Edgar R. Mining microarray data at NCBI’s Gene Expression Omnibus (GEO)*. Methods in Molecular Biology. 2006;338:175–190. doi: 10.1385/1-59745-097-9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista & Chang (2013).Batista P, Chang H. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan & Mandal (2015).Bhan A, Mandal S. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochimica et Biophysica Acta/General Subjects. 2015;1856:151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozec et al. (2009).Bozec A, Peyrade F, Fischel JL, Milano G. Emerging molecular targeted therapies in the treatment of head and neck cancer. Expert Opinion on Emerging Drugs. 2009;14:299–310. doi: 10.1517/14728210902997947. [DOI] [PubMed] [Google Scholar]
- Chin, Andersen & Futreal (2011).Chin L, Andersen JN, Futreal PA. Cancer genomics: from discovery science to personalized medicine. Nature Medicine. 2011;17:297–303. doi: 10.1038/nm.2323. [DOI] [PubMed] [Google Scholar]
- Clough & Barrett (2016).Clough E, Barrett T. The gene expression omnibus database. Methods in Molecular Biology. 2016;1418:93–110. doi: 10.1007/978-1-4939-3578-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. (2017).Cui H, Zhang Y, Zhang Q, Chen W, Zhao H, Liang J. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer Medicine. 2017;6:2932–2941. doi: 10.1002/cam4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa (2013).Espinosa JM. Epigenetic regulation by long noncoding RNAs. Chromosome Research. 2013;21:685–693. doi: 10.1126/science.1231776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa (2017).Espinosa JM. On the origin of lncRNAs: missing link found. Trends in Genetics. 2017;33:660–662. doi: 10.1016/j.tig.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang et al. (2017).Fang Z, Zhao J, Xie W, Sun Q, Wang H, Qiao B. LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Medicine. 2017;6:2897–2908. doi: 10.1002/cam4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay et al. (2015).Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fu & Feng (2015).Fu X, Feng Y. QKI-5 suppresses cyclin D1 expression and proliferation of oral squamous cell carcinoma cells via MAPK signalling pathway. International Journal of Oral and Maxillofacial Surgery. 2015;44:562–567. doi: 10.1016/j.ijom.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Gomes et al. (2017).Gomes C, De Sousa S, Calin G, Gomez R. The emerging role of long noncoding RNAs in oral cancer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2017;123:235–241. doi: 10.1016/j.oooo.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2017).Guo C, Hou J, Ao S, Deng X, Lyu G. HOXC10 up-regulation promotes gastric cancer cell proliferation and metastasis through MAPK pathway. Chinese Journal of Cancer Research. 2017;29:572–580. doi: 10.21147/j.issn.1000-9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy, Lánczky & Szállási (2012).Gyorffy B, Lánczky A, Szállási Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocrine-related Cancer. 2012;19:197–208. doi: 10.1530/ERC-11-0329. [DOI] [PubMed] [Google Scholar]
- Hsieh et al. (2001).Hsieh L, Wang P, Chen I, Liao C, Wang H, Chen M, Chang J, Cheng A. Characteristics of mutations in the p53 gene in oral squamous cell carcinoma associated with betel quid chewing and cigarette smoking in Taiwanese. Carcinogenesis. 2001;22:1497–1503. doi: 10.1093/carcin/22.9.1497. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2017b).Huang Q, Cao H, Zhan L, Sun X, Wang G, Li J, Guo X, Ren T, Wang Z, Lyu Y, Liu B, An J, Xing J. Mitochondrial fission forms a positive feedback loop with cytosolic calcium signaling pathway to promote autophagy in hepatocellular carcinoma cells. Cancer Letters. 2017b;403:108–118. doi: 10.1016/j.canlet.2017.05.034. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2017a).Huang L, Ma B, Ma J, Wang F. Fractalkine/CX3CR1 axis modulated the development of pancreatic ductal adenocarcinoma via JAK/STAT signaling pathway. Biochemical and Biophysical Research Communications. 2017a;493:1510–1517. doi: 10.1016/j.bbrc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Jandura & Krause (2017).Jandura A, Krause HM. The new RNA world: growing evidence for long noncoding RNA functionality. Trends in Genetics. 2017;33:665–676. doi: 10.1016/j.tig.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Kamangar, Dores & Anderson (2006).Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2017).Kim H, Kang Y, Byun J, Jang S, Rho G, Lee J, Park B. Midkine and NANOG have similar immunohistochemical expression patterns and contribute equally to an adverse prognosis of oral squamous cell carcinoma. International Journal of Molecular Sciences. 2017;18:E2339. doi: 10.3390/ijms18112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp & Mendell (2018).Kopp F, Mendell J. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan et al. (2017).Lan H, Zeng J, Chen G, Huang H. Survival prediction of kidney renal papillary cell carcinoma by comprehensive LncRNA characterization. Oncotarget. 2017;8:110811–110829. doi: 10.18632/oncotarget.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017).Li X, Ma C, Zhang L, Li N, Zhang X, He J, He R, Shao M, Wang J, Kang L, Han C. LncRNAAC132217.4, a KLF8-regulated long non-coding RNA, facilitates oral squamous cell carcinoma metastasis by upregulating IGF2 expression. Cancer Letters. 2017;407:45–56. doi: 10.1016/j.canlet.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Massano et al. (2006).Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontology. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Mehrotra & Gupta (2011).Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head & Neck Oncology. 2011;3:1–9. doi: 10.1186/1758-3284-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar (2013).Omar EA. The outline of prognosis and new advances in diagnosis of Oral Squamous Cell Carcinoma (OSCC): review of the literature. Journal of Oral Oncology. 2013;2013:519312. doi: 10.1155/2013/519312. [DOI] [Google Scholar]
- Quinn & Chang (2016).Quinn J, Chang H. Unique features of long non-coding RNA biogenesis and function. Nat Rev Gen. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- Reik (2009).Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Rinn & Chang (2012).Rinn J, Chang H. Genome regulation by long noncoding RNAs. Annual Review of Biochemistry. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie et al. (2015).Ritchie M, Phipson B, Wu D, Hu Y, Law C, Shi W, Smyth G. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, McCarthy & Smyth (2010).Robinson M, McCarthy D, Smyth G. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh & Schenberg (2013).Singh T, Schenberg M. Delayed diagnosis of oral squamous cell carcinoma following dental treatment. Annals of the Royal College of Surgeons of England. 2013;215:369–373. doi: 10.1308/003588413X13629960045599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeate et al. (2018).Skeate J, Da Silva D, Chavez-Juan E, Anand S, Nuccitelli R, Kast W. Nano-pulse stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLOS ONE. 2018;13:e0191311. doi: 10.1371/journal.pone.0191311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby, Laga & Sedlacek (2017).Slaby O, Laga R, Sedlacek O. Therapeutic targeting of non-coding RNAs in cancer. Biochemical Journal. 2017;474:4219–4251. doi: 10.1042/BCJ20170079. [DOI] [PubMed] [Google Scholar]
- Song et al. (2017).Song P, Jiang B, Liu Z, Ding J, Liu S, Guan W. A three-lncRNA expression signature associated with the prognosis of gastric cancer patients. Cancer Medicine. 2017;6:1154–1164. doi: 10.1002/cam4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizzo et al. (2012).Spizzo R, Almeida M, Colombatti A, Calin G. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2017).Sun CC, Zhang L, Li G, Li SJ, Chen ZL, Fu YF, Gong FY, Bai T, Zhang DY, Wu QM. The lncRNA PDIA3P interacts with miR-185-5p to modulate oral squamous cell carcinoma progression by targeting cyclin D2. Molecular Therapy Nucleic Acids. 2017;9:100–110. doi: 10.1016/j.omtn.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel et al. (2016).Teufel A, Itzel T, Erhart W, Brosch M, Wang X, Kim Y, Von Schönfels W, Herrmann A, Brückner S, Stickel F, Dufour J, Chavakis T, Hellerbr C, Spang R, Maass T, Becker T, Schreiber S, Schafmayer C, Schuppan D, Hampe J. Comparison of gene expression patterns between mouse models of nonalcoholic fatty liver disease and liver tissues from patients. Gastroenterology. 2016;151:513–525. doi: 10.1053/j.gastro.2016.05.051. [DOI] [PubMed] [Google Scholar]
- Verusingam et al. (2017).Verusingam N, Yeap S, Ky H, Paterson I, Khoo S, Cheong S, Ong A, Kamarul T. Susceptibility of human oral squamous cell carcinoma (OSCC) H103 and H376 cell lines to retroviral OSKM mediated reprogramming. PeerJ. 2017;5:e3174. doi: 10.7717/peerj.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Gerstein & Snyder (2009).Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Gen. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang C, Wang L, Ding Y, Lu X, Zhang G, Yang J, Zheng H, Wang H, Jiang Y, Xu L. LncRNA structural characteristics in epigenetic regulation. International Journal of Molecular Sciences. 2017;18:E2659. doi: 10.3390/ijms18122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonganan et al. (2017).Wonganan O, He Y, Shen X, Wongkrajang K, Suksamrarn A, Zhang G, Wang F. 6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon α/ β by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicology and Applied Pharmacology. 2017;336:31–39. doi: 10.1016/j.taap.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2015).Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren X, Wei F, Yu W, Liu T, Wang X. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. International Journal of Oncology. 2015;46:2586–2594. doi: 10.3892/ijo.2015.2976. [DOI] [PubMed] [Google Scholar]
- Zhang (2017).Zhang CZ. Long intergenic non-coding RNA 668 regulates VEGFA signaling through inhibition of miR-297 in oral squamous cell carcinoma. Biochemical and Biophysical Research Communications. 2017;489:404–412. doi: 10.1016/j.bbrc.2017.05.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The clinicopathological features of all OSCC patients from the TCGA database.
Univariate and multivariate Cox regression analyses were applied to evaluate the 21 candidate lncRNA signatures as independent prognostic variables.
AC012456.4 expression is significantly down-regulated in OSCC and normal tissue samples in the TCGA dataset.
The relationship between the expression of lncRNA AC012456.4 OSCC tissues with clinicopathological parameters.
Kaplan–Meier survival analysis with the log-rank test was used to identify relationships between the above 2493 lncRNA signatures and OSCC patient survival. Then, we determined the levels of 126 lncRNA signatures that were significantly related to DFS.
Kaplan–Meier survival analysis with the log-rank was used to identify relationships between the above 2493 lncRNA signatures and OSCC patient survival. Then, we determined the levels of 151 lncRNA signatures that were significantly related to OS.
Kaplan–Meierlan-Meier survival analysis with the log-rank was used to identify relationships between the above 2493 lncRNA signatures and OSCC patient survival. Then, we determined the levels of 21 lncRNA signatures that were significantly related to OS and DFS.
Biological pathways and functions of lncRNA AC012456.4 were identified by GSEA. This analysis revealed that lncRNA AC012456.4 was involved in many critical pathways and correlated with tumorigenesis.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.