Abstract
Heat stress is one of the limiting factors negatively affecting pig production, health, and fertility. Characterizing genomic regions responsible for variation in HS tolerance would be useful in identifying important genetic factor(s) regulating physiological responses to HS. In the present study, we performed genome-wide association analyses for respiration rate (RR), rectal temperature (TR), and skin temperature (TS) during HS in 214 crossbred gilts genotyped for 68,549 single nucleotide polymorphisms (SNP) using the Porcine SNP 70K BeadChip. Considering the top 0.1% smoothed phenotypic variances explained by SNP windows, we detected 26, 26, 21, and 14 genes that reside within SNPs explaining the largest proportion of variance (top 25 SNP windows) and associated with change in RR (ΔRR) from thermoneutral (TN) conditions to HS environment, as well as the change in prepubertal TR (ΔTR), change in postpubertal ΔTR, and change in TS (ΔTS), respectively. The region between 28.85 Mb and 29.10 Mb on chromosome 16 explained about 0.05% of the observed variation for ΔRR. The growth hormone receptor (GHR) gene resides in this region and is associated with the HS response. The other important candidate genes associated with ΔRR (PAIP1, NNT, and TEAD4), ΔTR (LIMS2, TTR, and TEAD4), and ΔTS (ERBB4, FKBP1B, NFATC2, and ATP9A) have reported roles in the cellular stress response. The SNP explaining the largest proportion of variance and located within and in the vicinity of genes were related to apoptosis or cellular stress and are potential candidates that underlie the physiological response to HS in pigs.
Keywords: genome-wide association, gilt, heat stress, pig
INTRODUCTION
Heat stress (HS) is a hurdle to efficient animal agriculture productivity (Renaudeau et al., 2012; Baumgard and Rhoads, 2013) and the global changes in temperature are expected to become increasingly erratic (IPCC, 2007). In pigs, HS is an annual limiting factor affecting production, health, and fertility and results in significant economic losses (St-Pierre et al., 2003; Ross et al., 2017). From a traditional production parameter standpoint, HS increases mortality (D’Allaire et al., 1996), reduces milk production (Renaudeau and Noblet, 2001) and litter survival (Wettemann and Bazer, 1985; Renaudeau et al., 2003; St-Pierre et al., 2003), markedly decreases growth rate and feed intake (FI) (Collin et al., 2001; Campos et al., 2014), and substantially increases the variability in market weight (Baumgard and Rhoads, 2013). Pigs are particularly sensitive to HS due to their inability to sweat and the presence of a thick layer of subcutaneous adipose tissue that prevents heat dissipation (Renaudeau et al., 2006; Fernandez et al., 2015). Commercial pig breeds have been intensely selected for economically important phenotypes, such as increased growth rate and leaner body composition, and this has inadvertently resulted in increased HS susceptibility (Renaudeau et al., 2012) since synthesizing and maintaining lean tissue increases basal heat production.
Genetic variation exists in thermal tolerance among species, between breeds, and within breed (Blackshaw and Blackshaw, 1994; Hoffmann, 2010; Renaudeau et al., 2012), and thus, may provide opportunity to improve thermal tolerance through using genetic tools to identify genomic regions of importance in the response to HS. For instance, recent genome-wide association studies (GWAS) in dairy cattle have identified genomic regions associated with TR during HS (Dikmen et al., 2013). The development of a high-density Porcine SNP BeadChip has aided the implementation of efficient genomic evaluation and selection in the commercial pig industry (Fernández et al., 2012). Despite the economic and animal welfare effects of HS on pork production and pig health, identifying genomic regions responsible for variation in HS tolerance has not yet been thoroughly explored. In the pig, single nucleotide polymorphism (SNP) markers have been chiefly used for association analysis of growth, meat, and carcass quality traits. The objectives of this study were to conduct GWAS to identify genomic regions associated with thermotolerance traits in crossbred gilts.
MATERIALS AND METHODS
Animals and Experimental Design
The Iowa State University Institutional Animal Care and Use Committee approved all procedures involving animals. Detailed description of experimental designs and how the body temperature variables were calculated during prepubertal and postpubertal development have been described in two other studies that established the HS phenotypes. (Graves et al., 2018; Seibert et al., 2018). Seibert et al. (2018) established the production phenotypes in response to HS while Graves et al. (2018) utilized a subset of the same group of gilts and established the repeatability of the phenotypes later in life and the relationship between the HS response and reproductive success. Collectively, crossbred gilts (n = 235; PIC maternal × Duroc terminal sire) from the same cohort were received on the 24th day of age and arrived immediately after weaning. Due to logistical constraints of the facilities, the experiment was conducted in five replications (n = 44 to 48/replicate). The initial BW from replications 1 to 5 were 59 ± 1.0, 64 ± 1.2, 77 ± 1.2, 88 ± 1.1, and 103 ± 1.6 kg, respectively (Seibert et al., 2018). During the experiment, water and feed were provided ad libitum during the entire experiment. All pigs were fed a standard diet consisting mainly of corn and soybean meal formulated to meet or exceed nutrient requirements (NRC, 2012). The study was divided into three experimental periods (P) for each replicate: P0, P1, and P2. Period 0 (72 h) served as an acclimation period in which all pigs were housed individually in thermoneutral (TN) conditions (21.9 ± 0.5 °C, 62 ± 13% relative humidity [RH]). After P0, pigs remained in TN conditions for 24 h (period 1; P1) and then exposed to HS (29.7 ± 1.3 °C, 49 ± 8% RH) conditions for 24 h (period 2; P2). Pigs were exposed to a 12:12 h light:dark cycle during P0, but continuous light during P1 and P2 to allow for accurate data collection.
TR (°C) was measured with a lubricated, calibrated digital thermometer (Welch Allyn SureTemp Plus 690, Skaneateles Falls, NY). TS (°C) was measured using a calibrated infrared thermometer (ST 380A Infrared Thermometer, HDE, Allentown, PA), and RR (breaths per minute) was determined by counting the number of flank movements in 15 s and multiplying by four. During the initial study, FI was measured daily and body temperature indices were monitored during both the 24 h TN (21.9 ± 0.5 °C, 62 ± 13% RH) and HS (29.7 ± 1.3 °C, 49 ± 8% RH) phases. BW were collected at the beginning of the acclimation and TN periods and at the end of the HS period. The difference (Δ) for physiological traits (e.g. TR, TS, and RR) was determined by subtracting the TN from the HS value.
Following boar exposure and heat detection, the second study (Graves et al., 2018) utilized 100 cyclic (postpubertal) animals from the initial 235 gilts. Selecting these postpubertal 100 gilts was based on their ability or inability to maintain a minimal TR during the 24 h HS challenge. During this study, TR, RR, and TS were collected at 0800, 1400, 1500, 1600, 1900, 2000, and 2100 h during TN (20 °C) conditions and condensed into a single average to represent each individual’s TN thermoregulatory set point. All body temperature indices measured at the same time points during 9 d of HS were condensed into a single average value, representing HS thermortolerance parameters. The difference for each physiological trait (ΔTR, ΔTS, and ΔRR) was calculated by subtracting TN from HS values for each trait.
Marker Data/Genotyping and Quality Control
All animals (235) were genotyped using the GGP-Porcine HD BeadChip (GeneSeek, Lincoln, NE), which contains 68,249 SNP that uniformly span the porcine genome according to Illumina’s standard protocols (http://www.illumina.com). Autosomal and X chromosome markers were filtered for the call rate ≥95%; Hardy–Weinberg equilibrium (HWE) <0.0001 and minor allele frequency (MAF) ≥0.05. Additionally, of the total animals genotyped, 21 individual samples failed to have at least a call rate of 95% and were excluded. After applying the above quality control criteria, a total of 52,528 SNP for 214 animals remained for the subsequent GWAS analysis. Quality control measures were performed using SNP and Variation Suit v8.3.1 (Golden Helix, Inc., Bozeman, MT, www.goldenhelix.com).
Statistical Analyses
Genome-wide association tests were performed using single-locus mixed linear model Efficient Mixed-Model Association eXpedited (EMMAX), which includes a kinship matrix as random effect and implemented by SNP and Variation Suite Version 8.3.1 software (Golden Helix, Inc.). In GWAS, lack of accounting for population structure may lead to spurious association results (Kang et al., 2010). It has been demonstrated that the EMMAX approach can correct for population stratification and relatedness between samples (Kang et al., 2010). To correct for confounding effects due to population structure and relatedness between individuals; an identity-by-state (IBS) between samples was computed from the genotype data and included as a random effect in the model. The EMMA approach and algorithm have been well described in SNP and Variation Suite Version 8.3.1 documentation (Golden Helix, Inc.).The model used can be expressed as:
where y is an n × 1 the vector of observed phenotypic values, X is an n × f matrix of fixed SNP effects, β is a q × 1 vector representing coefficients of the fixed effect, Z is an n × t relating the instances of the random effects, u the vector of random effect, and e the residual effect.
Initial BW, replication, and room were included in the analyses as covariates for all of the traits. For each trait, pseudo-heritability, the fraction of phenotypic variance explained by the empirically estimated relationship matrix (Kang et al., 2010; Segura et al., 2012) was estimated with the SNP and Variation Suite (Golden Helix, Inc.).
As for several genome-wide analysis using small sample size (Dockery et al., 2017), we did not detect any SNP that passed Bonferroni adjusted P value threshold; therefore, we considered the top SNP explaining the largest proportion of variance. To reduce the specious noise from single SNP based analyses, the observed phenotypic variance accounted by an individual SNP was smoothed over five SNP sliding windows. This approach has been applied to GWAS studies in cattle and poultry (Dikmen et al., 2013; Fragomeni et al., 2014). As previously demonstrated, SNP windows explaining the largest SNP variance were considered to represent candidate gene regions associated with variation in phenotypes (Dikmen et al., 2013; Fragomeni et al., 2014). In those studies, SNP window thresholds were arbitrarily selected. For instance, Fragomeni et al. (2014) considered the top 10 windows (~200 SNPs) explaining the largest genetic variance using windows of 20 SNP, whereas Dikmen et al. (2013) considered the top 20 loci explaining the largest proportion of variance using three- and five-SNP sliding windows. Therefore, we considered the top 0.1% (25 windows) smoothed variance explained by SNP windows. The candidate genes associated with the top 0.1% SNPs were searched for from the NCBI database (http://www.ncbi.nlm.nih.gov/).
RESULTS AND DISCUSSION
Heritability estimates for prepubetal ΔTR, ΔRR, postpubetal ΔTR, and ΔTS were 0.49, 0.39, 0.83, and 0.00, respectively. There are only limited studies on the heritabilities of thermotolerance traits in pigs to compare with our results. To the best of our knowledge, no prior study reported estimates of heritability for thermotolerance traits in pigs from genome-wide SNP data. Very recently, Gourdine et al. (2017) reported heritability estimates of 0.35 and 0.39 for TR and RR, respectively, in lactating sows reared in a tropical climate. Generally, the value observed for TR in the present study is higher than the range of values reported in cattle (0.11 to 0.44) (Da Silva, 1973; Morris et al., 1989) and poultry (0.36) (Taouis et al. 2002). The higher heritability in this study could be partly attributed to small sample size. Concurrent with this assumption, Baco et al. (1997) showed that the average heritability decreased as the sample size increase from 100 to 400. The moderate and high heritabilities observed in this study imply that there is genetic variation in thermotolerance in pigs that can be exploited to improve heat tolerance.
In the present study, we performed GWAS for ΔRR, prepubertal or postpubertal ΔTR, and ΔTS, to identify genomic regions associated with thermoregulatory and production responses to HS in pigs using the Porcine SNP 70 BeadChip technology. Significant SNP were declared when the P value was less than the genome-wide type I error rate, adjusted with Bonferroni correction by using α/K, where α = 0.05 and K = number of SNPs. We did not detect any SNP displaying the set significant threshold (0.05/52528 = 9.5187 × 10−7) but this was not unexpected given the limited number of observations (214 prepubertal animals and 91 postpubertal animals).
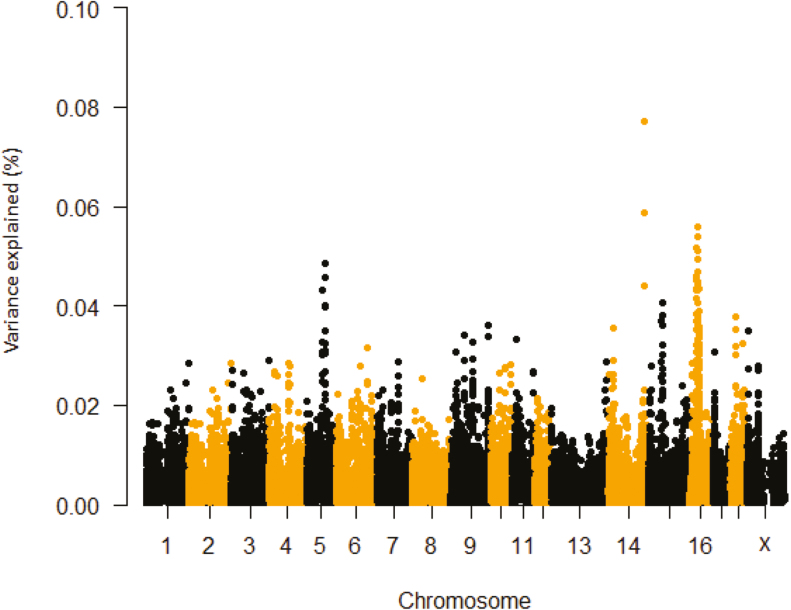
We therefore considered the top 0.1% of the smoothed phenotypic variance explained by five SNP windows. The total number of genes associated with these SNPs were 26, 26, 21, and 14 for ΔRR, prepubertal ΔTR, postpubertal ΔTR, and ΔTS, respectively. The region between 28.85 Mb and 29.10 Mb on chromosome 16 (five SNPs) explained about 0.05% of the observed variation for ΔRR and includes the growth hormone receptor genomic locus (GHR; Table 1 and Figure 1). This is not surprising as growth hormone (GH) variables are influenced by HS. For example, HS decreases GHR mRNA abundance in hepatic tissue of lactating Holstein dairy cows (Deane and Woo, 2005; Rhoads et al., 2010) and avian species (Gasparino et al., 2014; Del Vesco et al., 2015), and is independent of the heat-induced feed intake reduction (Collier et al., 2008). Additionally, although not always observed (Rhoads et al., 2009), circulating GH levels decline in HS compared to TN cattle (Farooq et al., 2010); this decrease in circulating GH is attributed to reduced GH secretion at the pituitary gland. Furthermore, primiparous cattle treated with growth hormone-releasing hormone (GHRH) during HS had increased BW gain, milk yield, pregnancy rates, and circulating prolactin (PRL), and reduced mortality (Brown et al., 2008). Polymorphisms within GHR have known to significantly affect growth traits including in pigs and goats (An et al., 2011; Tian et al., 2014). Considering the critical physiological and metabolic role of GHR, SNPs within this gene are likely potential selection candidates for HS tolerance.
Table 1.
Phenotypic variance explained by SNP windows for delta respiration rate prior to puberty (prepubertal ΔRR)
| SSCa | Position start (bp)b | Position end (bp)c | Variance explained (%)d | Candidate gene(s)e |
|---|---|---|---|---|
| 14 | 139721921 | 139813511 | 0.077 | — |
| 14 | 139607069 | 139757205 | 0.059 | RAB11FIP2 |
| 16 | 29375218 | 29645155 | 0.056 | LOC100524404, CCL28, PAIP1, LOC100524913 |
| 16 | 29513888 | 29742940 | 0.054 | LOC100524404, PAIP1, LOC100524913, NNT |
| 16 | 26931779 | 27129171 | 0.052 | HEATR7B2, MROH2B |
| 16 | 28409425 | 28629545 | 0.051 | — |
| 16 | 27848815 | 28627099 | 0.049 | OXCT1, FBXO4,LOC102165724 |
| 5 | 69383487 | 69487000 | 0.049 | TSPAN9, TEAD4, TULP3/TUBl3 |
| 16 | 28850217 | 29102419 | 0.047 | GHR |
| 16 | 26415650 | 26619363 | 0.046 | — |
| 5 | 69487000 | 69597659 | 0.046 | TULP3/TUBl3, LOC100524913, LOC102162709, ITFG2, LOC102164154 |
| 16 | 26861794 | 27039793 | 0.045 | LOC100737708, HEATR7B2 |
| 16 | 29200306 | 29513888 | 0.045 | CCL28, LOC100524404 |
| 16 | 29645155 | 29881595 | 0.045 | PAIP1,LOC100524913, PAIP1, NNT |
| 16 | 29102419 | 29375218 | 0.044 | LOC106506477, CCL28 |
| 16 | 29742940 | 30016395 | 0.044 | NNT |
| 14 | 139757205 | 139906120 | 0.044 | C14H10orf84 |
| 16 | 35074836 | 35176313 | 0.043 | ARL15 |
| 16 | 26552965 | 26755662 | 0.043 | — |
| 16 | 27039793 | 27242934 | 0.043 | MROH2B, LOC106505864, C6 |
| 16 | 28627099 | 28800253 | 0.043 | GHR,LOC102158502, GHR |
| 5 | 60978291 | 61121151 | 0.043 | ARHGDIB, ART4 |
| 16 | 26755662 | 26931779 | 0.042 | LOC100737708 |
| 16 | 32429434 | 32520142 | 0.041 | — |
Gene abbreviations: RAB11FIP2 = RAB11 family interacting protein 2; CCL28 = C-C motif chemokine ligand 28; PAIP1 = poly(A) binding protein interacting protein 1; NNT = nicotinamide nucleotide transhydrogenase; HEATR7B2 = maestro heat-like repeat-containing protein family member 2B; MROH2B = maestro heat-like repeat family member 2B; OXCT1 = 3-oxoacid CoA-transferase 1; FBXO4 = F-box protein 4; TSPAN9 = tetraspanin 9; TULP3 = tubby like protein 3; TEAD4 = TEA domain transcription factor 4; GHR = growth hormone receptor; ITFG2 = integrin alpha FG-GAP repeat containing 2; ARL15 = ADP ribosylation factor like GTPase 15; ARHGDIB = Rho GDP dissociation inhibitor beta; ART4 = ADP-ribosyltransferase 4.
aChromosome number of the pig genome for which the SNP window location is mapped.
bSNP window positions start location on the chromosome.
cSNP window position end location on the chromosome.
dPercentage of variance explained by five SNP windows.
eCandidate genes located within the SNP window.
Figure 1.
Manhattan plot of delta respiration rate during first HS challenge prior to puberty (prepubertal ΔRR) percentage of variance explained by SNP windows in crossbred gilts. The variance accounted by an individual SNP was smoothed over five SNP sliding windows.
Another important candidate gene with close proximity to GHR is poly(A) binding protein interacting protein 1 (PAIP1) which falls within a five SNP window that explained about 0.06% the variance on SSC16 at 29.37 to 29.64 Mb. Based on an in vitro experiment using HeLa cells, the abundance of PAIP1 protein decreases in response to HS (Datu and Bag, 2013). In mammals, HS increases free radical formation (reactive oxygen species; ROS) and induces oxidative stress (Lord-Fontaine and Averill-Bates, 2002). HS also induces oxidative damage in pigs (Montilla et al., 2014) and fish (Heise et al., 2006) and oxidative stress is involved in heat-induced cell death (Davidson et al., 1996). Interestingly, we detected SNP on chromosome 16 that explain 0.05% of the variance for ΔRR and contained the nicotinamide nucleotide transhydrogenase (NNT) gene (Table 1 and Figure 1). The NNT gene product is necessary to prevent ROS accretion (Arkblad et al., 2005; Nickel et al., 2015) and loss of its activity has been implicated in increased mitochondrial oxidative damage, ultimately resulting in overall increased sensitivity to oxidative stress (Arkblad et al., 2005; Navarro et al., 2012). Moreover, Nnt knockdown in mice leads to increased ROS production and a stronger inflammatory response in macrophages (Ripoll et al., 2012). Interestingly, it has been reported that a mutated Nnt gene in mice results in loss of B-cell lymphoma 2 (BCL-2 ) (Navarro et al., 2012), a major antiapoptotic protein implicated in the prevention of heat-induced cell death (Setroikromo et al., 2007). In vitro heat shock downregulates Bcl-2 expression (Khar et al., 2006), which may inhibit its activity to prevent permeability of the outer mitochondrial membrane and ultimate release of apoptogenic factors (Beere, 2004). The effect of HS-induced autophagy signaling in the pig ovary demonstrated that BECN1 abundance correlates with an increase in phosphorylation of BCL2 (Hale et al., 2017). Thus, NNT could be involved in variation of HS-induced oxidative stress and autophagy in pigs.
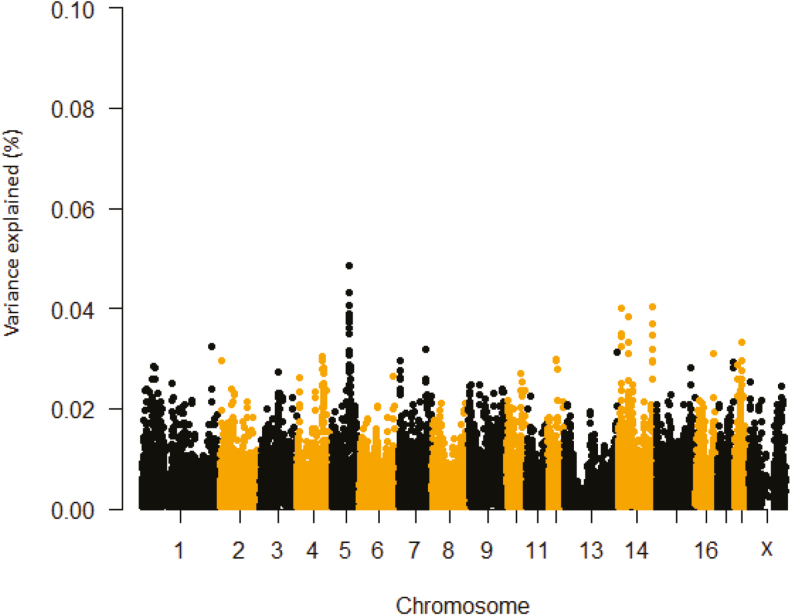
For ΔRR and prepubertal ΔTR, the SSC 5: 69.38 to 69.48 Mb region accounted for 0.05% the observed variance and contained TEA domain transcription factor 4 (TEAD4) or related transcription enhancer factor-1 (RTEF-1) (Tables 1 and 2; Figures 1 and 2). However, this region was not detected for postpubertal ΔTR. The lack of detecting a common significant region for prepubertal ΔTR and postpubertal ΔTR could be ascribed to differences in either animal age or sample size or both. TEAD4 protein prevents oxidative stress in blastocoels (Kaneko and DePamphilis, 2013). Also, hypoxic inducible factor 1 alpha (HIF-1α) gene expression was decreased when RTEF-1 was knocked down in endothelial cells (Jin et al., 2011). HIF-1α can interact with HSP90, which mediates heat-induced stabilization of HIF-1α (Katschinski et al., 2002). The region extending from 136.70 Mb to 139.10 Mb (10 loci) on SSC 14 accounted for about 0.05% of the observed variance for the prepubertal ΔTR and encompasses the attractin-like 1 (ATRNL1) gene locus. Previous studies suggest selecting certain alleles in this gene may improve high-altitude adaptation (Simonson et al., 2010). Thus, TEAD4 and ATRNL1 represent gene candidates that could be explored as targets to improve heat tolerance in pigs.
Table 2.
Phenotypic variance explained by SNP windows for the change in TR during heat stress prior to puberty (prepubertal ΔTR)
| SSCa | Position start (bp)b | Position end (bp)c | Variance explained (%)d | Candidate gene(s)e |
|---|---|---|---|---|
| 5 | 69383487 | 69487000 | 0.049 | TSPAN9, TEAD4 |
| 5 | 72245513 | 72424166 | 0.043 | MICAL3, LOC102162673 |
| 5 | 69487000 | 69597659 | 0.041 | TULP3, LOC102162709, ITFG2, LOC102164154 |
| 14 | 136702381 | 136891524 | 0.040 | ATRNL1, LOC102161079 |
| 14 | 13443000 | 13564731 | 0.040 | FZD3 |
| 5 | 69437477 | 69555670 | 0.039 | TEAD4, TULP3, LOC102162709 |
| 14 | 39275817 | 39773984 | 0.038 | — |
| 5 | 69333042 | 69437477 | 0.038 | TSPAN9, TEAD4 |
| 5 | 72352991 | 72500090 | 0.037 | LOC102162673 |
| 14 | 139721921 | 139813511 | 0.037 | — |
| 5 | 72141748 | 72352991 | 0.036 | BID, MICAL3 |
| 5 | 69555670 | 69691307 | 0.035 | LOC102162709, ITFG2, LOC10216415, LOC102164154, LOC106510369, LOC100512907 |
| 14 | 13564731 | 13666604 | 0.035 | LOC102157783, EXTL3 |
| 14 | 136824061 | 136939847 | 0.035 | ATRNL1 |
| 14 | 13356571 | 13497286 | 0.034 | FBXO16, FZD3 |
| 18 | 27560376 | 27761494 | 0.033 | ING3, TSPAN12 |
| 14 | 39616077 | 39904195 | 0.033 | LOC102157597 |
| 14 | 13497286 | 13621432 | 0.033 | FZD3 |
| 1 | 283482216 | 283609728 | 0.032 | SUSD1 |
| 7 | 111019262 | 111170076 | 0.032 | |
| 14 | 136939847 | 137099653 | 0.032 | ATRNL1 |
| 5 | 70488420 | 70775116 | 0.032 | ERC1, RAD52 |
| 13 | 215584218 | 215697149 | 0.031 | C2CD2, LOC102161849 |
| 5 | 69597659 | 69759629 | 0.031 | LOC102164154, LOC106510369, LOC100512907, IQSEC3 |
Gene abbreviations: TSPAN9 = tetraspanin 9; TEAD4 = TEA domain transcription factor 4; MICAL3 = microtubule associated monooxygenase, calponin and LIM domain containing 3; ITFG2 = integrin alpha FG-GAP repeat containing 2; EXTL3 = exostosin like glycosyltransferase 3; ATRNL1 = attractin-like 1; FBXO16 = F-box protein 16; FZD3 = frizzled class receptor 3; ING3 = inhibitor of growth family member 3; TSPAN12 = tetraspanin 12; SUSD1 = sushi domain containing 1; ERC1 = ELKS/RAB6-interacting/CAST family member 1; RAD52 = RAD52 homolog, DNA repair protein; C2CD2 = C2 calcium dependent domain containing 2; IQSEC3 = IQ motif and Sec7 domain 3.
aChromosome number of the pig genome for which the SNP window location is mapped.
bSNP window positions start location on the chromosome.
cSNP window position end location on the chromosome.
dPercentage of variance explained by five SNP windows.
eGenes located within the SNP window.
Figure 2.
Manhattan plot of delta TR first HS challenge (prepubertal ΔTR) percentage of variance explained by SNP windows in crossbred gilts. The variance accounted by an individual SNP was smoothed over five SNP sliding windows.
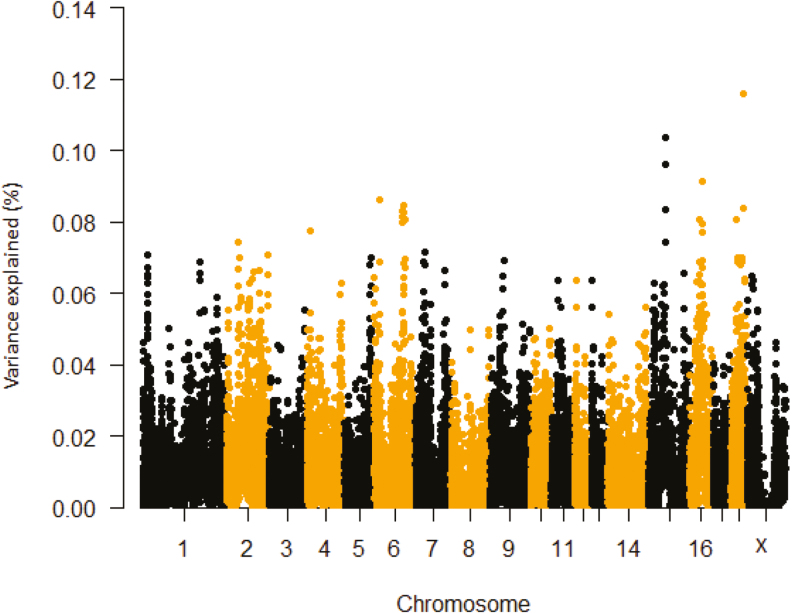
Phenotypic variances explained by SNP windows postpubertal ΔTR are shown in Table 3 and Figure 3. The region between 65.86 and 66.79 Mb encompassed the LIM and senescent cell antigen-like domains 2 (LIMS2) gene. Hepatic LIMS2 is differentially expressed in response to high ambient temperature (Coble et al., 2014). Another candidate region on SSC15 extending from 65.58 to 66.44 Mb contained the transthyretin (TTR) gene. Studies have revealed that the expression patterns of Ttr was altered by chronic stress in different rat strains (Andrus et al., 2012). In addition, various stress stimuli upregulate Ttr and calcium binding-related genes in the prefrontal cortex of the cerebrum in mice. Our single marker based analyses also detected the death-domain association protein (DAXX) candidate gene for postpubertal ΔTR (Supplementary Table S1 and Supplementary Figure S1). This gene product plays a key role as a mediator of heat shock factor 1 (HSF1) activation (Nefkens et al., 2003; Boellmann et al., 2004). Other studies have reported that heat shock protein (HSP) expression is modulated by DAXX (Boellmann et al., 2004). Thus, taking into account the known direct and indirect association of these genes (LIMS2, TTR, and DAXX) with stress, they represent potential candidates for HS tolerance in pigs.
Table 3.
Phenotypic variance explained by SNP windows for the change in TR during the heat stress challenge following puberty (postpubertal ΔTR)
| SSCa | Position start (bp)b | Position end (bp)c | Variance explained (%)d | Candidate gene(s)e |
|---|---|---|---|---|
| 18 | 45115484 | 45291167 | 0.116 | DST |
| 15 | 65866223 | 66790298 | 0.104 | FMNL2, GPR17, LIMS2 |
| 15 | 65585973 | 66442766 | 0.096 | UGGT1, GPR17, LIMS2 |
| 16 | 45509140 | 45700636 | 0.091 | — |
| 6 | 20211611 | 20449928 | 0.086 | LOC102165723 |
| 6 | 110474964 | 110566591 | 0.085 | — |
| 18 | 45058469 | 45173869 | 0.084 | — |
| 15 | 66442766 | 66907405 | 0.083 | GPR17, LIMS2, FMNL2 |
| 6 | 108129231 | 108545750 | 0.083 | TTR, LOC106510687, RNF125 |
| 6 | 109474291 | 109870440 | 0.083 | ASXL3 |
| 6 | 108138639 | 108646700 | 0.081 | LOC106510687, RNF125 |
| 18 | 20063484 | 20338092 | 0.081 | LOC100516838, LOC100737195, STRIP2, AHCYL2 |
| 6 | 115147146 | 115788549 | 0.081 | — |
| 16 | 37793490 | 38004706 | 0.081 | — |
| 6 | 107975607 | 108138639 | 0.080 | — |
| 16 | 48647762 | 48792525 | 0.079 | LOC106504115, MAST4 |
| 4 | 13581654 | 13825367 | 0.077 | LOC102164882 |
| 16 | 47786015 | 47968058 | 0.077 | ERBB2IP, LOC102167060 |
| 15 | 65327301 | 65866223 | 0.074 | UGGT1 |
| 2 | 46429654 | 46587388 | 0.074 | — |
| 7 | 34708292 | 34803564 | 0.072 | — |
| 2 | 159763907 | 159898148 | 0.071 | — |
| 1 | 16981336 | 17160649 | 0.071 | CCDC170 |
| 2 | 51076948 | 51244889 | 0.070 | — |
Gene abbreviations: DST = dystonin; FMNL2 = formin like 2; GPR17 = G protein-coupled receptor 17; LIMS2 = LIM zinc finger domain containing 2; TTR = transthyretin; ASXL3 = additional sex combs like 3; STRIP2 = striatin interacting protein 2; AHCYL2 = adenosylhomocysteinase like 2; MAST4 = microtubule associated serine/threonine kinase family member 4; ERBB2IP = erbb2 interacting protein; UGGT1 = UDP-glucose glycoprotein glucosyltransferase 1; CCDC170 = coiled-coil domain containing 170.
aChromosome number of the pig genome for which the SNP window location is mapped.
bSNP window positions start location on the chromosome.
cSNP window position end location on the chromosome.
dPercentage of variance explained by five SNP windows.
eGenes located within the SNP window.
Figure 3.
Manhattan plot of delta TR during the second HS challenge (postpubertal ΔTR) percentage of variance explained by SNP windows in crossbred gilts. The variance accounted by an individual SNP was smoothed over five SNP sliding windows.
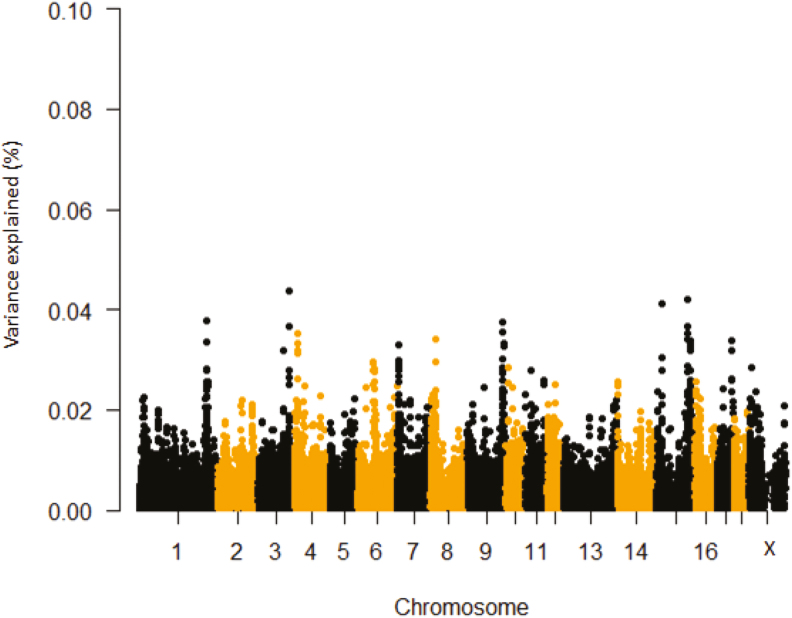
In Table 4 and Figure 4, phenotypic variance explained by SNP windows for ΔTS is presented. The region of interest is flagged on SSC 15 (125.96 to 126.47 Mb) comprising the erb-b2 receptor tyrosine kinase 4 (ERBB4) genomic locus, which is a member of the tyrosine kinase family and is involved in the DNA damage response (Gilmore-Hebert et al., 2010). Expression of this gene can be induced in response to various cellular stresses and it plays a key role in preventing apoptosis (Hua et al., 2012). Furthermore, this gene induces HSPs in a HSF1-dependent manner (Khaleque et al., 2005) and is associated with maximum lifespan in rodents (Edrey et al., 2012). Another potential candidate region associated with ΔTs is the SSC 17: 59.36 to 59.44 Mb, which includes the ATPase phospholipid transporting 9A (ATP9A) gene. ATPases move ions across cellular membranes (Altshuler et al., 2012) and are involved in maintaining ion homeostasis during heat stroke or stress (Kourtis et al., 2012). For instance, HSP-16.1 functions with the Ca2+- and Mn2+-transporting ATPase calcium-transporting (PMR-1) to maintain Ca2+ homeostasis under heat stroke (Kourtis et al., 2012). Moreover, it has been shown that mutant protein lacking ATPase domain resulted in loss of key activities of HSP72 (Volloch et al., 1999). The SNPs on SSC15 at 127.76 Mb to 127.88 Mb accounted for 0.04% of the observed SNP variance and contained the IKAROS family zinc finger 2 (IKZF2) gene. This is a stress-related gene and expressed in various lymphomas and leukemia (Antica et al., 2008) and is also associated with QTL regions for T lymphocyte subpopulations in swine (Lu et al., 2012). On SSC 3, the highest proportion of phenotypic variance explained (0.04%) by SNP windows was observed at 121.85 to 122.02 Mb and encompassed the FK506 binding protein 1B (FKBP1B) locus, which is differentially expressed in response to HS in catfish (Liu et al., 2013). In addition, members of the FKBP protein family are involved in modulating thermotolerance by interacting with HSP90.1 and are essential for survival at high temperatures (Meiri and Breiman, 2009). Another potential candidate gene detected on SSC17 (59.31 to 59.40 Mb) is nuclear factor of activated T-cells 2 (NFATC2). The NFAT gene family mediated transcription is induced in epidermal cells in response to UV light (Horsley and Pavlath, 2002). NFATC2 is a novel HSF1 target that strongly inhibits polyglutamine aggregation (polyQ) and is required for HSF1-mediated suppression of ployQ aggregation (Hayashida et al., 2010). Single marker based analyses for ΔTS identified a SNP on SSC 6 explaining 0.05% of the observed variance and located within the U6 snRNA gene (Supplementary Table S1 and Supplementary Figure S1). U6 snRNA is essential for mRNA splicing and interestingly enough, this gene has been associated with TR under HS in Holstein cattle (Dikmen et al., 2013).
Table 4.
Phenotypic variance explained by SNP windows for delta TS prior to puberty (prepubertal ΔTS)
| SSCa | Position start (bp)b | Position end (bp)c | Variance explained (%)d | Candidate gene(s)e |
|---|---|---|---|---|
| 3 | 121853700 | 122019392 | 0.044 | LOC100521960, FKBP1B, ATAD2B |
| 15 | 126029433 | 126285452 | 0.042 | ERBB4 |
| 15 | 25747414 | 25910541 | 0.041 | — |
| 1 | 271950519 | 272028559 | 0.038 | — |
| 9 | 140242929 | 140385683 | 0.038 | — |
| 15 | 125958213 | 126098646 | 0.037 | ERBB4 |
| 3 | 121766459 | 121896763 | 0.037 | ITSN2, LOC100521960, FKBP1B |
| 9 | 142991886 | 143124394 | 0.036 | — |
| 4 | 12197136 | 12282429 | 0.035 | — |
| 15 | 127761392 | 127886086 | 0.035 | IKZF2, LOC100737978 |
| 15 | 126098646 | 126467297 | 0.034 | ERBB4 |
| 8 | 19747348 | 19780058 | 0.034 | — |
| 15 | 136273347 | 136352463 | 0.034 | — |
| 17 | 59360910 | 59447126 | 0.034 | ATP9A |
| 1 | 271984966 | 272080412 | 0.034 | LOC100153054 |
| 9 | 143029683 | 143164935 | 0.033 | — |
| 4 | 12025385 | 12095880 | 0.033 | — |
| 15 | 136764149 | 136868267 | 0.033 | EPHA4, LOC102159610 |
| 7 | 9126277 | 9208606 | 0.033 | — |
| 9 | 143124394 | 143242124 | 0.033 | RPS6KC1 |
| 15 | 136981095 | 137108151 | 0.033 | LOC106506372 |
| 17 | 59314509 | 59400449 | 0.032 | NFATC2, ATP9A |
| 4 | 12237013 | 12315209 | 0.032 | — |
| 15 | 136181273 | 136319027 | 0.032 | — |
Gene abbreviations: FKBP1B = FK506 binding protein 1B; ATAD2B = ATPase family, AAA domain containing 2B; ERBB4 = erb-b2 receptor tyrosine kinase 4; ATP9A = ATPase phospholipid transporting 9A (putative); EPHA4 = EPH receptor A4; RPS6KC1 = ribosomal protein S6 kinase C1; NFATC2 = nuclear factor of activated T-cells 2; ATP9A = ATPase phospholipids’ transporting 9A.
aChromosome number of the pig genome for which the SNP window location is mapped.
bSNP window positions start location on the chromosome.
cSNP window position end location on the chromosome.
dPercentage of variance explained by five SNP windows.
eCandidate genes located within the SNP window.
Figure 4.
Manhattan plot of delta TS first HS challenge (prepubertal ΔTS) percentage of variance explained by SNP windows in crossbred gilts. The variance accounted by an individual SNP was smoothed over five SNP sliding windows.
LIMITATIONS AND CONCLUSIONS
To identify loci associated with thermotolerance traits in pigs, we employed a classical GWAS approach. GWAS using a large number of markers require thousands of samples to attain an adequate statistical power (Spencer et al., 2009; Hong and Park, 2012). As indicated in several studies, GWAS undertaken using smaller sample size, have little power to identify loci with small polygenic effects and only loci with very large effects are expected to reach the genome-wide significant threshold (Davenport et al., 2015). As expected, with our small sample size, no SNPs reached the set genome-wide significance threshold. Therefore, we conclude that the results of the present study are suggestive and warrant further replication and follow-up study using reasonable sample sizes.
Despite the above-indicated limitation of this study, we have identified some genes that are known to be involved in physiological adaptation to general stressors. The SNPs explaining the largest proportion of variance and associated with or located within (GHR, PAIP1, TEAD, NNT, ERBB4, FKBP1B, and NFATC2) and related to apoptosis and cellular stress and may prove to be potential candidates for further validation studies.
Conflict of interest statement. Any opinion, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Pork Board. No conflicts of interest, financial, or otherwise are declared by the author(s).
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
Supplementary Material
This work was supported by the National Pork Board, the Iowa Pork Producers Association, the Iowa Pork Industry Center, The Ensminger program, and Hatch and State of Iowa Funds.
LITERATURE CITED
- Altshuler I., Vaillant J. J., Xu S., and Cristescu M. E.. 2012. The evolutionary history of sarco(endo)plasmic calcium ATPase (SERCA). Plos One 7:e52617. doi:10.1371/journal.pone.0052617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X., Wang L., Hou J., Li G., Song Y., Wang J., Yang M., Cui Y., and Cao B.. 2011. Novel polymorphisms of goat growth hormone and growth hormone receptor genes and their effects on growth traits. Mol. Biol. Rep. 38:4037–4043. doi:10.1007/s11033-010-0522-3 [DOI] [PubMed] [Google Scholar]
- Andrus B. M., Blizinsky K., Vedell P. T., Dennis K., Shukla P. K., Schaffer D. J., Radulovic J., Churchill G. A., and Redei E. E.. 2012. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol. Psychiatry 17:49–61. doi:10.1038/mp.2010.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antica M., Cicin-Sain L., Kapitanovic S., Matulic M., Dzebro S., and Dominis M.. 2008. Aberrant Ikaros, Aiolos, and Helios expression in Hodgkin and non-Hodgkin lymphoma. Blood 111:3296–3297. doi:10.1182/blood-2007-12-125682 [DOI] [PubMed] [Google Scholar]
- Arkblad E. L., Tuck S., Pestov N. B., Dmitriev R. I., Kostina M. B., Stenvall J., Tranberg M., and Rydström J.. 2005. A Caenorhabditis elegans mutant lacking functional nicotinamide nucleotide transhydrogenase displays increased sensitivity to oxidative stress. Free Radic. Biol. Med. 38:1518–1525. doi:10.1016/j.freeradbiomed.2005.02.012 [DOI] [PubMed] [Google Scholar]
- Baco S., Inoue S., Sudo S., Hisadome H., Harada H., and Fukuhara R.. 1997. Effects of sample size on heritability estimates for field carcass characters in Wagyu population. The West Japan J. Anim. Sci. 40:33–39. doi:10.11461/jwaras1968.40.33 [Google Scholar]
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi:10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Beere H. M. 2004. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. j. Cell Sci. 117:2641–2651. doi:10.1242/jcs.01284 [DOI] [PubMed] [Google Scholar]
- Blackshaw J. K., and Blackshaw A.. 1994. Heat stress in cattle and the effect of shade on production and behaviour: a review. Anim. Prod. Sci. 34:285–295. doi:10.1071/EA9940285 [Google Scholar]
- Boellmann F., Guettouche T., Guo Y., Fenna M., Mnayer L., and Voellmy R.. 2004. DAXX interacts with heat shock factor 1 during stress activation and enhances its transcriptional activity. Proc. Natl. Acad. Sci. USA 101:4100–4105. doi:10.1073/pnas.0304768101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. A., Bodles-Brakhop A. M., and Draghia-Akli R.. 2008. Effects of plasmid growth hormone-releasing hormone treatment during heat stress. DNA Cell Biol. 27:629–635. doi:10.1089/dna.2008.0758 [DOI] [PubMed] [Google Scholar]
- Campos P. H., Labussière E., Hernández-García J., Dubois S., Renaudeau D., and Noblet J.. 2014. Effects of ambient temperature on energy and nitrogen utilization in lipopolysaccharide-challenged growing pigs. J. Anim. Sci. 92:4909–4920. doi:10.2527/jas.2014-8108 [DOI] [PubMed] [Google Scholar]
- Coble D. J., Fleming D., Persia M. E., Ashwell C. M., Rothschild M. F., Schmidt C. J., and Lamont S. J.. 2014. RNA-seq analysis of broiler liver transcriptome reveals novel responses to high ambient temperature. BMC Genomics 15:1084. doi:10.1186/1471-2164-15-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Collier J. L., Rhoads R. P., and Baumgard L. H.. 2008. Invited review: genes involved in the bovine heat stress response. J. Dairy Sci. 91:445–454. doi:10.3168/jds.2007-0540 [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. 2001. Effect of high temperature and feeding level on energy utilization in piglets. J. Anim. Sci. 79:1849–1857. doi:10.2527/2001.7971849x [DOI] [PubMed] [Google Scholar]
- D’Allaire S., Drolet R., and Brodeur D.. 1996. Sow mortality associated with high ambient temperatures. Can. Vet. J. 37:237–239. [PMC free article] [PubMed] [Google Scholar]
- Da Silva R. G. 1973. Improving tropical beef cattle by simultaneous selection for weight and heat tolerance. Heritability and correlation of the traits. J. Anim. Sci. 37:637–642. doi:10.2527/jas1973.373637x [Google Scholar]
- Datu A. K., and Bag J.. 2013. Enhanced translation of mRNAs encoding proteins involved in mRNA translation during recovery from heat shock. Plos One 8:e64171. doi:10.1371/journal.pone.0064171 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Davenport E. R., Cusanovich D. A., Michelini K., Barreiro L. B., Ober C., and Gilad Y.. 2015. Genome-wide association studies of the human gut microbiota. Plos One 10:e0140301. doi:10.1371/journal.pone.0140301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. F., Whyte B., Bissinger P. H., and Schiestl R. H.. 1996. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 93:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane E. E., and Woo N. Y.. 2005. Growth hormone increases hsc70/hsp70 expression and protects against apoptosis in whole blood preparations from silver sea bream. Ann. N. Y. Acad. Sci. 1040:288–292. doi:10.1196/annals.1327.044 [DOI] [PubMed] [Google Scholar]
- Del Vesco A. P., Gasparino E., Grieser D. O., Zancanela V., Voltolini D. M., Khatlab A. S., Guimarães S. E., Soares M. A., and Oliveira Neto A. R.. 2015. Effects of methionine supplementation on the expression of protein deposition-related genes in acute heat stress-exposed broilers. Plos One 10:e0115821. doi:10.1371/journal.pone.0115821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen S., Cole J. B., Null D. J., and Hansen P. J.. 2013. Genome-wide association mapping for identification of quantitative trait loci for rectal temperature during heat stress in Holstein cattle. Plos One 8:e69202. doi:10.1371/journal.pone.0069202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery L. E., Tritchler D., Krivak T. C., Kaufman K., Lankes H., Bae-Jump V. L., Berchuck A., Backes F. J., Rose P.G., Tewari K. S. et al. . 2017. Genome-wide association study (GWAS) of single nucleotide polymorphisms (SNPs) and the risk of platinum and taxane toxicities: an analysis of GOG 172 and 182. Gynecol. Oncol. 145:42–43. doi:10.1016/j.ygyno.2017.03.109 [Google Scholar]
- Edrey Y. H., Casper D., Huchon D., Mele J., Gelfond J. A., Kristan D. M., Nevo E., and Buffenstein R.. 2012. Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. Aging Cell 11:213–222. doi:10.1111/j.1474-9726.2011.00772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U., Samad H., Shehzad F., and Qayyum A.. 2010. Physiological responses of cattle to heat stress. World Appl. Sci. J. 8:38–43. [Google Scholar]
- Fernandez M. V. S., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S.,Elsasser T. H., Ross J. W., Isom S. C.,. et al. 2015. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi:10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández A. I., Pérez-Montarelo D., Barragán C., Ramayo-Caldas Y., Ibáñez-Escriche N., Castelló A., Noguera J. L., Silió L., Folch J. M., and Rodríguez M. C.. 2012. Genome-wide linkage analysis of QTL for growth and body composition employing the PorcineSNP60 BeadChip. BMC Genet. 13:41. doi:10.1186/1471-2156-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragomeni B. D. O., Misztal I., Lourenco D. L., Aguilar I., Okimoto R., and Muir W. M.. 2014. Changes in variance explained by top SNP windows over generations for three traits in broiler chicken. Front. Genet. 5:332. doi:10.3389/fgene.2014.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparino E., Del Vesco A. P., Voltolini D. M., Nascimento C. S., Batista E., Khatlab A. S., Grieser D. O., Zancanela V., and GuimarÃEs S. E.. 2014. The effect of heat stress on GHR, IGF-I, ANT, UCP and COXIII mRNA expression in the liver and muscle of high and low feed efficiency female quail. Br. Poult. Sci. 55:466–473. doi:10.1080/00071668.2014.925090 [DOI] [PubMed] [Google Scholar]
- Gilmore-Hebert M., Ramabhadran R., and Stern D. F.. 2010. Interactions of ErbB4 and Kap1 connect the growth factor and DNA damage response pathways. Mol. Cancer Res. 8:1388–1398. doi:10.1158/1541-7786.MCR-10-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdine J. L., Mandonnet N., Giorgi M., and Renaudeau D.. 2017. Genetic parameters for thermoregulation and production traits in lactating sows reared in tropical climate. Animal 11:365–374. doi: 10.1017/S175173111600135X [DOI] [PubMed] [Google Scholar]
- Graves K. L., Seibert J. T., Keating A. F., Baumgard L. H., and Ross J. W.. 2018. Characterization of the acute heat stress response in gilts: II. Assessing repeatability and association with fertility. j. Anim. Sci. 96:2419–2426. doi:10.1093/jas/skx037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale B. J., Hager C. L., Seibert J. T., Selsby J. T., Baumgard L. H., Keating A. F., and Ross J. W.. 2017. Heat stress induces autophagy in pig ovaries during follicular development. Biol. Reprod. 97:426–437. doi:10.1093/biolre/iox097 [DOI] [PubMed] [Google Scholar]
- Hayashida N., Fujimoto M., Tan K., Prakasam R., Shinkawa T., Li L., Ichikawa H., Takii R., and Nakai A.. 2010. Heat shock factor 1 ameliorates proteotoxicity in cooperation with the transcription factor NFAT. Embo J. 29:3459–3469. doi:10.1038/emboj.2010.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K., Puntarulo S., Nikinmaa M., Abele D., and Pörtner H. O.. 2006. Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout Zoarces viviparus L. J. Exp. Biol. 209:353–363. doi:10.1242/jeb.01977 [DOI] [PubMed] [Google Scholar]
- Hoffmann I. 2010. Climate change and the characterization, breeding and conservation of animal genetic resources. Anim. Genet. 41 (Suppl 1):32–46. doi:10.1111/j.1365- 2052.2010.02043.x [DOI] [PubMed] [Google Scholar]
- Hong E. P., and Park J. W.. 2012. Sample size and statistical power calculation in genetic association studies. Genomics Inform. 10:117–122. doi:10.5808/GI.2012.10.2.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V., and Pavlath G. K.. 2002. NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156:771–774. doi:10.1083/jcb.200111073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y., Gorshkov K., Yang Y., Wang W.,Zhang N., and Hughes D. P.. 2012. Slow down to stay alive: HER4 protects against cellular stress and confers chemoresistance in neuroblastoma. Cancer 118:5140–5154. doi:10.1002/cncr.27496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC 2007. Climate Change 2007: The Physical Science Basis. In: S. Solomon D. Qin M. Manning Z. Chen M. Marquis K. B. Averyt M. Tignor, and H. L. Miller, editors. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. [Google Scholar]
- Jin Y., Wu J., Song X., Song Q., Cully B. L., Messmer-Blust A., Xu M., Foo S. Y., Rosenzweig A., and Li J.. 2011. RTEF-1, an upstream gene of hypoxia-inducible factor-1α, accelerates recovery from ischemia. J. Biol. Chem. 286:22699–22705. doi:10.1074/jbc.M111.237024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K. J., and DePamphilis M. L.. 2013. TEAD4 establishes the energy homeostasis essential for blastocoel formation. Development 140:3680–3690. doi:10.1242/dev.093799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. M., Sul J. H., Service S. K., Zaitlen N. A., Kong S. Y., Freimer N. B., Sabatti C., and Eskin E.. 2010. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42:348–354. doi:10.1038/ng.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschinski D. M., Le L., Heinrich D., Wagner K. F., Hofer T., Schindler S. G., and Wenger R. H.. 2002. Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J. Biol. Chem. 277:9262–9267. doi:10.1074/jbc.M110377200 [DOI] [PubMed] [Google Scholar]
- Khaleque M. A., Bharti A., Sawyer D., Gong J., Benjamin I. J., Stevenson M. A., and Calderwood S. K.. 2005. Induction of heat shock proteins by Heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene 24:6564–6573. doi:10.1038/sj.onc.1208798 [DOI] [PubMed] [Google Scholar]
- Khar A., Kumari A. L., Pardhasaradhi B. V., Varalakshmi C. h., and Rangaraj N.. 2006. Heat stress induced apoptosis in BC-8 cells derived from AK-5 tumor involves downregulation of Bcl-2 and generation of reactive oxygen species. Indian J. Exp. Biol. 44:802–808. [PubMed] [Google Scholar]
- Kourtis N., Nikoletopoulou V., and Tavernarakis N.. 2012. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 490:213–218. doi:10.1038/nature11417 [DOI] [PubMed] [Google Scholar]
- Liu S., Wang X., Sun F., Zhang J., Feng J., Liu H., Rajendran K. V., Sun L., Zhang Y., Jiang Y.,. et al. 2013. RNA-seq reveals expression signatures of genes involved in oxygen transport, protein synthesis, folding, and degradation in response to heat stress in catfish. Physiol. Genomics 45:462–476. doi:10.1152/physiolgenomics.00026.2013 [DOI] [PubMed] [Google Scholar]
- Lord-Fontaine S., and Averill-Bates D. A.. 2002. Heat shock inactivates cellular antioxidant defenses against hydrogen peroxide: protection by glucose. Free Radic. Biol. Med. 32:752–765. doi:10.1016/S0891-5849(02)00769-4 [DOI] [PubMed] [Google Scholar]
- Lu X., Fu W. X., Luo Y. R., Ding X. D., Zhou J. P., Liu Y., Liu J. F. and Zhang Q.. 2012. Genome-wide association study for T lymphocyte subpopulations in swine. BMC Genomics 13:488. doi: 10.1186/1471-2164-13-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri D., and Breiman A.. 2009. Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with hsp90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 59:387–399. doi:10.1111/j.1365-313X.2009.03878.x [DOI] [PubMed] [Google Scholar]
- Montilla S. I., Johnson T. P., Pearce S. C., Gardan-Salmon D., Gabler N. K., Ross J. W., Rhoads R. P., Baumgard L. H., Lonergan S. M., and Selsby J. T.. 2014. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature (Austin). 1:42–50. doi:10.4161/temp.28844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. A., Jones K. R., and Wilson J. A.. 1989. Heritability of rectal temperature and relationships with growth in young cattle in a temperate climate. New Zeal. J. Agr.Res. 32:375–378. doi:10.1080/00288233.1989.10421755 [Google Scholar]
- Navarro S. J., Trinh T., Lucas C. A., Ross A. J., Waymire K. G., and Macgregor G. R.. 2012. The C57BL/6J mouse strain background modifies the effect of a mutation in Bcl2l2. G3 (Bethesda). 2:99–102. doi:10.1534/g3.111.000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefkens I., Negorey D. G., Ishow A. M., Michaelson J. S., Yeh E. T., Tanguay R. M., Müller W. E., and Maul G. G.. 2003. Heat shock and Cd2+ exposure regulate PML and Daxx release from ND10 by independent mechanisms that modify the induction of heat-shock proteins 70 and 25 differently. J. Cell Sci. 116:513–524. doi: 10.1242/jcs.00253 [DOI] [PubMed] [Google Scholar]
- Nickel A. G., von Hardenberg A., Hohl M., Löffler J. R., Kohlhaas M., Becker J., Reil J. C., Kazakov A., Bonnekoh J., Stadelmaier M.,. et al. 2015. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab. 22:472–484. doi:10.1016/j.cmet.2015.07.008 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient Requirements of Swine, 11th rev. ed. Natl. Acad. Press, Washington, DC. doi: 10.17226/13298 [DOI] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi:10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Leclercq-Smekens M., and Herin M.. 2006. Differences in skin characteristics in European (Large White) and Caribbean (Creole) growing pigs with reference to thermoregulation. Anim. Res. 55:209–217. doi:10.1051/animres: 2006012 [Google Scholar]
- Renaudeau D., and Noblet J.. 2001. Effects of exposure to high ambient temperature and dietary protein level on sow milk production and performance of piglets. J. Anim. Sci. 79:1540–1548. doi:/2001.7961540x [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Noblet J., and Dourmad J. Y.. 2003. Effect of ambient temperature on mammary gland metabolism in lactating sows. J. Anim. Sci. 81:217–231.doi:/2003.811217x [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Kim J. W., Collier R. J., Crooker B. A., Boisclair Y. R., Baumgard L. H., and Rhoads R. P.. 2010. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 93:170–179. doi:10.3168/jds.2009-2469 [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi: 10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Ripoll V. M., Meadows N. A., Bangert M., Lee A. W., Kadioglu A., and Cox R. D.. 2012. Nicotinamide nucleotide transhydrogenase (NNT) acts as a novel modulator of macrophage inflammatory responses. FASEB J. 26:3550–3562. doi:10.1096/fj.11-199935 [DOI] [PubMed] [Google Scholar]
- Ross J. W., Hale B. J., Seibert J. T., Romoser M., Adur M. K., Keating A. F., and Baumgard L. H.. 2017. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 84:934–945. doi:10.1002/mrd.22859 [DOI] [PubMed] [Google Scholar]
- Segura V., Vilhjálmsson B. J., Platt A., Korte A., Seren Ü., Long Q., and Nordborg M.. 2012. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 44:825–830. doi:10.1038/ng.2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert J. T., Graves K. L., Hale B. J., Keating A. F., Baumgard L. H., and Ross J. W.. 2018. Characterizing the acute heat stress response in gilts: I. Thermoregulatory and production variables. J. Anim. Sci. 96:941–949. doi:10.1093/jas/skx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setroikromo R., Wierenga P. K., van Waarde M. A., Brunsting J. F., Vellenga E., and Kampinga H. H.. 2007. Heat shock proteins and Bcl-2 expression and function in relation to the differential hyperthermic sensitivity between leukemic and normal hematopoietic cells. Cell Stress Chaperones 12:320–330. doi:10.1379/CSC-279.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson T. S., Yang Y., Huff C. D., Yun H., Qin G., Witherspoon D. J., Bai Z., Lorenzo F. R., Xing J., Jorde L. B.,. et al. 2010. Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75. doi:10.1126/science.1189406 [DOI] [PubMed] [Google Scholar]
- Spencer C. C., Su Z., Donnelly P., and Marchini J.. 2009. Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. Plos Genet. 5:e1000477. doi:10.1371/journal.pgen.1000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre N., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi:10.3168/jds.S0022-0302 (03)74040–5 [Google Scholar]
- Taouis M., De Basilio V., Mignon-Grasteau S., Crochet S., Bouchot C., Bigot K., Collin A., and Picard M.. 2002. Early-age thermal conditioning reduces uncoupling protein messenger RNA expression in pectoral muscle of broiler chicks at seven days of age. Poult. Sci. 81:1640–1643. doi:10.1093/ps/81.11.1640 [DOI] [PubMed] [Google Scholar]
- Tian Y. G., Yue M., Gu Y., Gu W. W., and Wang Y. J.. 2014. Single-nucleotide polymorphism analysis of GH, GHR, and IGF-1 genes in minipigs. Braz. J. Med. Biol. Res. 47:753–758. doi: 10.1590/1414-431X20143945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Gabai V. L., Rits S., and Sherman M. Y.. 1999. ATpase activity of the heat shock protein hsp72 is dispensable for its effects on dephosphorylation of stress kinase JNK and on heat-induced apoptosis. FEBS Lett. 461:73–76. doi:10.1016/S0014-5793(99)01428-3 [DOI] [PubMed] [Google Scholar]
- Wettemann R. P., and Bazer F. W.. 1985. Influence of environmental temperature on prolificacy of pigs. J. Reprod. Fertil. Suppl. 33:199–208. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.