Abstract
Appreciation of mechanisms that affect steroidogenesis is critical to identifying compromising signals that may decrease reproductive efficiency. Follicle maturation and steroidogenesis requires coordinated actions from the pituitary gonadotropins and local ovarian signaling molecules. β-Catenin (CTNNB1), the lynchpin molecule of canonical wingless-type mouse mammary tumor virus integration site (WNT) signaling, is required for maximal gonadotropin stimulation of steroid production from granulosa (GC) and luteal cells. WNTs are locally secreted glycoproteins involved in ovarian development and folliculogenesis. In cultured bovine GC, WNT2 and AKT mRNAs and CTNNB1 protein increase after FSH stimulation. Likewise, CTNNB1 protein is greater in large antral follicles with high intrafollicular estradiol concentrations, suggesting the hormonal milieu responsible for increased estradiol content modulates CTNNB1 accumulation. In addition, concurrent treatment of FSH and WNT3A in GC results in reduced steroidogenic enzymes and ovarian differentiation factors. It is likely that FSH regulation of WNT signaling establishes a negative feedback loop to ensure CTNNB1 remains controlled. To explore the mechanism resulting in this inhibitory effect, AKT pathway modulators were utilized and unveiled a requirement for AKT activity in FSH-mediated CTNNB1 accumulation. Cells treated with FSH, IGF-1, and IGF-1 + FSH had increased CTNNB1 protein accumulation compared with controls. Similarly, estradiol medium concentrations increased in treated cells compared with non-treated controls, while co-treatment of FSH and IGF-1 with the AKT inhibitor LY294002 reduced CTNNB1 and estradiol production. Subsequent studies evaluated whether FSH regulation of CTNNB1 occurs through a specific phosphorylation event. In bovine GC, phosphorylation of CTNNB1 at Ser-552 was demonstrated in FSH-treated cells, whereas IGF-1 treatment did not phosphorylate CTNNB1 Ser-552. Data indicate that in cattle phosphorylation on CTNNB1 Ser-552 is a protein kinase A (PKA) dependent, protein kinase B (AKT) independent event. Data suggest that CTNNB1 regulated by AKT is a fundamental component of FSH-induced estrogen production. However, AKT’s role in estradiol synthesis does not appear to be through phosphorylation of CTNNB1 Ser-552. The complex interplay between FSH and ovarian WNT/CTNNB1 signaling is key to regulation of follicle maturation and steroidogenesis.
Keywords: β-catenin, bovine, estradiol, ovary, WNT
INTRODUCTION
Estrogen is a steroidogenic hormone involved in regulating a broad range of physiological and pathological processes. Although estrogens have been associated with the development and progression of various cancers including those of the breast, endometrium, and ovary (Shang, 2007), endogenous estrogen also influence bone density, lipid metabolism, and key aspects of reproduction (Simpson et al., 2005). In the female, estrogens play an essential role in folliculogenesis (Drummond and Findlay, 1999), sexual behavior, mammary duct development, and preparation of the uterus for arrival of the conceptus (Couse and Korach, 1999). The production of estrogen from the ovarian follicle relies on coordinated actions of the pituitary gonadotropin, FSH, and intraovarian factors that influence steroidogenic enzymes including aromatase. A more recently identified intraovarian regulator of ovarian function is the wingless-type mouse mammary tumor virus integration site (WNT) family of signaling molecules. Components of the WNT signaling pathway exhibit regulated expression at specific stages of follicular development in the adult ovary (Hsieh et al., 2002; Ricken et al., 2002). In addition, β-catenin (CTNNB1), a transcriptional cofactor of the WNT signaling pathway, is required for maximal FSH stimulation of aromatase (Parakh et al., 2006) and subsequent estrogen production (Hernandez Gifford et al., 2009) in primary cultures of rat and mice granulosa cells. Although these studies have begun to define a clear role for WNT/CTNNB1 in adult ovarian function, the vast majority of studies have been conducted in rodent models. As such, a paucity of information on the physiological significance of this pathway in livestock species remains. This review will provide an overview of our studies on the contribution of the WNT/CTNNB1 pathway on estradiol production and follicle maturation in bovine granulosa cells.
WNT SIGNALING
The WNT family of signaling molecules are highly conserved secreted glycoproteins. These short-range signaling molecules regulate numerous cellular processes including proliferation, differentiation, cell fate determination, embryonic induction, and axis specification (Cadigan and Nusse, 1997; Logan and Nusse, 2004; Komiya and Habas, 2008). There are 19 structurally related Wnt genes in mammals that can signal via 3 distinct pathways, known as the canonical (WNT/CTNNB1), WNT/Ca2+, and planar cell polarity pathways. The most intensely studied WNT pathway is the canonical WNT signaling cascade that signals through the linchpin molecule CTNNB1 to regulate gene expression. β-Catenin is a downstream coactivator that restores transcription of TCF/LEF genes normally repressed by Groucho-related proteins (Roose et al., 1998; Chen et al., 1999; Brantjes et al., 2001). As a transcriptional cofactor of WNT signaling, CTNNB1 regulates numerous genes involved in development and a variety of pathologies including cancers of breast, colon, skin (Logan and Nusse, 2004), and granulosa cell tumors (Boerboom et al., 2006).
The role of WNTs in the female gonad was first demonstrated by characterization of Wnt4-deficient mice. Female mice null for Wnt4 exhibit partial female to male sex reversal and a loss of oocyte reserve at birth (Vainio et al., 1999), elucidating a requirement for WNT in ovarian development. Growing evidence has since indicated that WNT proteins play a critical in regulation of postnatal ovarian function. Expressions of Wnt family member transcripts Wnt4, Fz4, and Fz1 are differentially expressed throughout specific stages of follicular development (Hsieh et al., 2002). In immature rat ovaries, Wnt2, Wnt2b, and Fz4 are localized to follicular granulosa cells (Wnt2 and Fz4) and ovarian surface epithelium (Wnt2b; Ricken et al., 2002). In addition, Wang et al. (2010) detected the greatest expression of Wnt-2 mRNA in granulosa cells of healthy antral follicles. Furthermore, hormonal regulation of the Wnt family of genes has been demonstrated in adult ovaries of mice and rats (Hsieh et al., 2002; Owens et al., 2002).
WNT/β-CATENIN IN GRANULOSA CELL FUNCTION
Reviews have been published on the emerging field in which WNT functions in the adult ovary (Boyer et al., 2010; Hernandez Gifford, 2015); however, to date, limited focus has been given to involvement of the WNT signaling pathways in bovine ovarian function. To appreciate the role WNT/CTNNB1 plays in bovine estrogen production, it is important to recognize a few foundational studies. The study that puts CTNNB1 at the forefront of estrogen production and follicle maturation was conducted by Parakh et al. (2006) utilizing a human tumor granulosa cell line (KGN) and overexpression assays. These studies demonstrated a requirement of CTNNB1 in FSH-mediated aromatase expression that was funneled at least in part through an interaction with steroidogenic factor-1 (NR5A1). Follow-up studies employed conditional deletion of CTNNB1 in a primary mouse granulosa cell model that resulted in a marked decrease in the ability of FSH to stimulate aromatase gene expression and subsequent estradiol production (Hernandez Gifford et al., 2009). Furthermore, transgenic mice expressing stabilized CTNNB1 results in an increased number of growing small antral follicles, aromatase mRNA, and reduced follicle apoptosis (Fan et al., 2010) further implicating this pathway in ovarian follicle steroid production.
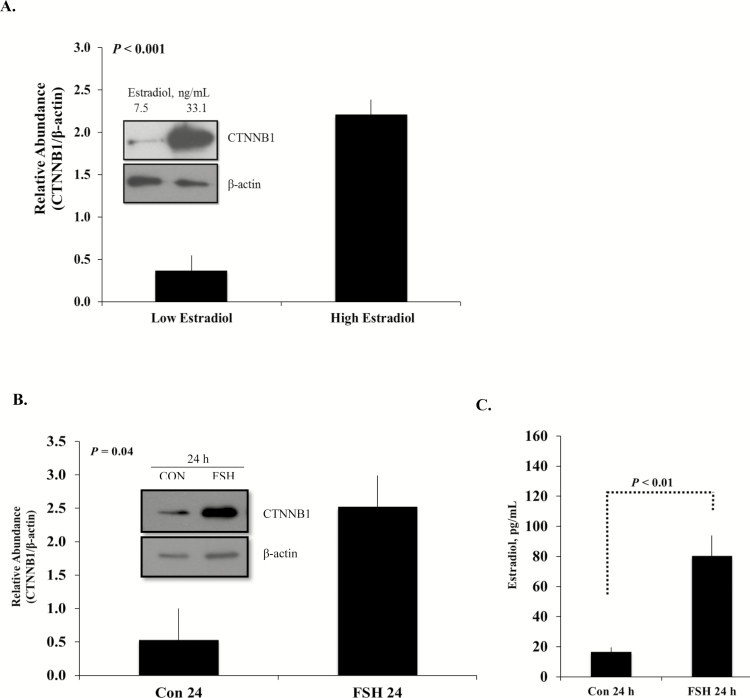
Based on these observations in rodents, our lab became interested in the role WNT signaling that might play in bovine ovarian function. To ascertain specific contributions of WNT to bovine folliculogenesis, an initial study was set up in which granulosa cells were collected from large antral bovine follicles of nonpregnant females containing a stage-III corpus luteum (Castañon et al., 2012). The follicles were retrospectively designated as high estradiol (≥25 ng/mL) or low estradiol (≤14 ng/mL) producing follicles based on intrafollicular estradiol concentrations. That data clearly demonstrate that CTNNB1 protein abundance increases significantly in high estradiol-producing follicles compared with low-estradiol follicles (Fig. 1A). Because the increased intrafollicular concentrations of estradiol suggest that the follicles were exposed to an elevated FSH milieu, a subsequent study was designed to evaluate specific effects of FSH to the modulated CTNNB1 abundances. To recapitulate the developmental environment of the high estradiol-producing follicles, primary bovine granulosa cells were cultured in the presence or absence of FSH for 24 h. This in vitro experiment resulted in a similar increase in CTNNB1 protein in granulosa cells treated with FSH compared with the vehicle-treated controls. In addition, changes in CTNNB1 paralleled the demonstrated increase in the estradiol concentrations found in the media of FSH-treated cells (Fig. 1B and C).
Figure 1.
(A) Representative Western blot (inset) and quantitative analysis of CTNNB1 protein abundance from granulosa cells with low (7.5 ng/mL) or high (33.1 ng/mL) intrafollicular estradiol concentrations quantified by RIA (n = 3 per group). Total protein was collected from freshly isolated granulosa cells from each of the largest follicles in a pair of ovaries. (B) Representative Western blot (inset) and quantitative analysis of replicate experiments (n = 3) demonstrating effect of FSH treatment on primary cultures of bovine granulosa cells on β-catenin (CTNNB1) protein accumulation. Small follicle granulosa cells were isolated from randomly staged bovine ovaries and treated with FSH (100 ng/mL) for 24 h. β-Actin served as the loading control. (C) Subsequent estradiol production measured in the medium of the cultured cells demonstrated a corresponding rise in estradiol concentrations. Least square means ± SEM are presented (n = 6). Con = nontreated controls. Data are from Castañon et al. (2012).
As indicated previously, one of the roles of CTNNB1 is to serve as a transcriptional regulator of the WNT signaling pathway. Specific WNT ligands have been recognized for their role in follicle formation, growth, and ovulation/luteinization (Hsieh et al., 2002; Ricken et al., 2002). The distinct expression of WNTs is both stage specific and hormonally regulated. Therefore, to determine whether changes in CTNNB1 abundance after FSH treatment was modulated by WNT ligands, gene expressions of several WNT transcripts and downstream signaling molecules were evaluated. The only WNT mRNA demonstrating a change after FSH treatment was WNT2, which was increased 6-fold over vehicle-treated controls (Castañon et al., 2012). This is consistent with data detecting Wnt2 in granulosa cells of the postnatal rat ovary (Ricken et al., 2002). Furthermore, overexpression of Wnt2 in a rat granulosa cell line promotes cytosolic and nuclear accumulation of CTNNB1 (Finnson et al., 2012) and regulates DNA synthesis in mouse granulosa cells through CTNNB1 (Wang et al., 2010).
INDUCTION OF β-CATENIN BY FSH IS A DIRECT EFFECT OF AKT ACTIVITY
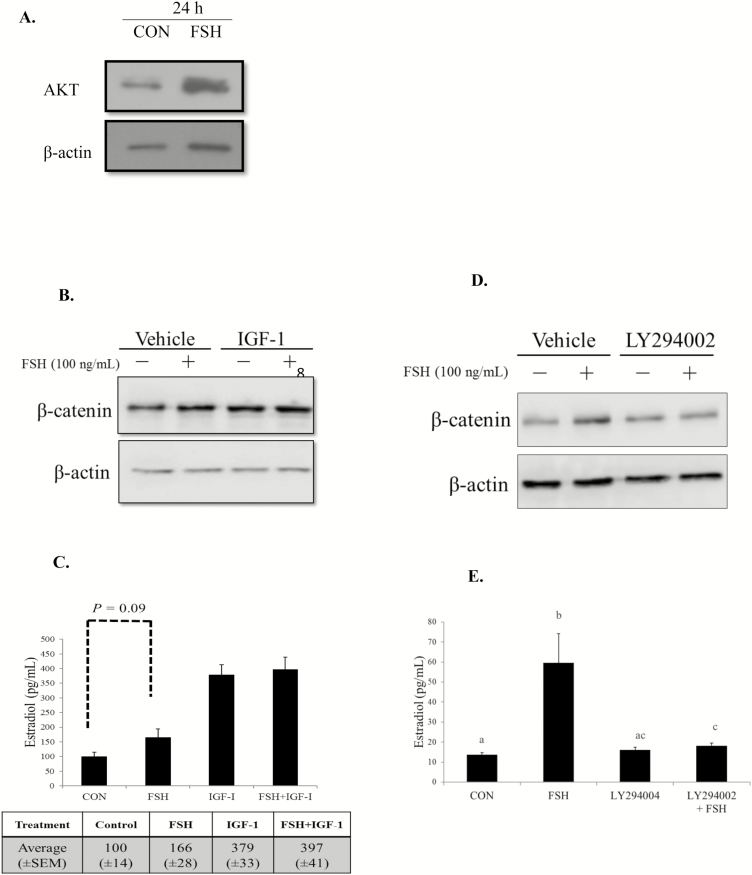
The dynamic changes occurring during follicle maturation require coordinated regulation by the hypothalamic–pituitary–gonadal axis. Pituitary-derived FSH initiates cAMP/protein kinase A (PKA) and phosphoinositide 3-kinase (PI3K) to regulate granulosa cell target genes involved in proliferation, maturation, and estradiol production (Hunzicker-Dunn and Maizels, 2006). Phosphoinositide 3-kinase is an essential component of FSH signaling that phosphorylates and activates intraovarian factors such as protein kinase B (PKB/AKT; Richards et al., 2002; Gloaguen et al., 2011). Previous studies have demonstrated that AKT is essential for granulosa cell function, as granulosa cells treated with a negative regulator of AKT fail to induce aromatase mRNA and subsequent estradiol in media (Zeleznik et al., 2003). These studies also reveal that AKT is an obligatory component in FSH stimulation of granulosa cell differentiation, but not proliferation (Zeleznik et al., 2003). In addition, IGF-1 signals via PI3K/AKT and synergizes with FSH to stimulate gonadal steroid production (Zhou et al., 2013). In cattle, IGF-1 contributes to estradiol production and dominant follicles contain greater concentrations of IGF-1 and estradiol than their subordinate cohorts (Beg et al., 2001). Therefore, we evaluated AKT protein in cultures of primary bovine granulosa cells following stimulation with FSH. Consistent with the data provided by rodent models, AKT protein abundance was increased in cultured bovine granulosa cells evaluated 24 h after FSH treatment compared with nontreated controls (Fig. 2A; Castañon et al., 2012). To evaluate if AKT induces CTNNB1 accumulation in granulosa cells of cattle, known pathway stimulators including FSH and IGF-1 were utilized in subsequent experiments. Insulin-like growth factor-1 and FSH are both important regulators of ovarian function, and disruption to either gene or their receptors has deleterious effects on fertility (Baker et al., 1996; Abel et al., 2000). Furthermore, FSH and IGF-1 signal at least in part through the PI3K/AKT pathway to regulate steroid output (Zhou et al., 2013). Therefore, granulosa cells collected from “all natural” (not exposed to growth-promoting implants, ionophores, or antibiotics) nonpregnant cows and heifers were treated with the AKT stimulators alone or in combination. After 24 h of treatment, cells demonstrated an increase in CTNNB1 protein with similar abundance for all treatment groups compared with nontreated controls (Fig. 2B; Gomez et al., 2015). Likewise, media concentrations of estradiol increased from 100 (±14 pg/mL) in nontreated controls with 166 (±28 pg/mL) following FSH treatment; a similar albeit greater response in estradiol media concentrations was demonstrated in granulosa cells stimulated with IGF-1 or coincubated with IGF-1 and FSH (Fig. 2C). The actions of FSH and IGF-1 in inducing estradiol are reported in bovine granulosa cells (Mani et al., 2010; Castañon et al., 2012), and mounting evidence also suggests that circulating FSH and ovarian-produced IGF-1 increase estradiol by enhancing AKT activity.
Figure 2.
(A) Representative Western blot of AKT abundance in bovine granulosa cells cultured with and without FSH (100 ng/mL) for 24 h indicates specific FSH responsiveness of the granulosa cells in culture. Data from Castañon et al. (2012). (B) Representative Western blot of bovine granulosa cells and cultured with AKT pathway stimulator IGF-1 (50 ng/mL) in the presence or absence of FSH (100 ng/mL) for 24 h. (C) Subsequent estradiol concentrations measured in the medium of cultured granulosa cells were analyzed by RIA. Quantitative analysis of 4 biological experiments evaluated in duplicate is presented as least squares means ± SEM. A tendency (P = 0.09) exist between CON and FSH. (D, E) Bovine granulosa cells were cultured and treated with AKT inhibitor LY294002 (30 µM) or vehicle for 30 min, prior to incubation in the presence or absence of FSH (100 ng/mL) for 24 h. Total protein was collected from 3 biological experiments. (D) Representative Western blot of β-catenin (E) and accompanying estradiol concentrations are presented. Bars without a common superscript differ (P < 0.05). Least squares means ± SEM are presented. Reprinted from Gomez et al. (2015), with permission from Elsevier.
Follicle-stimulating hormone activation of the AKT pathway also promotes phosphorylation of glycogen synthase kinase 3 β (GSK3β), a component of the CTNNB1 degradation complex (Fan et al., 2010). Appreciation of this pathway interaction suggests a mechanism for regulating CTNNB1 protein abundance. To further examine the effect of AKT activity on CTNNB1 abundance in bovine granulosa cells, the PI3K-family inhibitor LY294002 was used to mute AKT activity. As demonstrated in earlier experiments, treatment with FSH consistently increased CTNNB1 protein. However, abrogation of AKT signaling by the pathway inhibitor reduced the ability of FSH to increase CTNNB1 abundance (Fig. 2D). The decrease in CTNNB1 protein was associated with a similar decrease in medium estradiol concentrations of granulosa cells incubated with the LY294002 inhibitor (Fig. 2E). Likewise, granulosa cells of cattle coexposed to the AKT inhibitor and IGF-1 demonstrated a reduction in CTNNB1 protein abundance and estradiol concentrations compared with IGF-1 alone (Gomez et al., 2015). Constraint of AKT signaling and consequent reduction in CTNNB1 indicates AKT activity is an essential component of FSH and IGF-1 directed CTNNB1 protein abundance. Collectively, these data demonstrate the importance of FSH in regulation of CTNNB1 protein in granulosa cells of cattle. The ability of AKT to be mediated by both FSH and IGF-1 indicates signaling downstream of the FSH and IGF-1 receptors. Furthermore, these data reveal that the mechanism for CTNNB1 accumulation in bovine granulosa cells relies on important contributions of the PI3K/AKT/GSK3β signaling pathway in FSH and IGF-1-mediated estradiol biosynthesis.
We suggest a model whereby activation of protein kinase B (AKT) by FSH and IGF-1 phosphorylates and thus blocks GSK3β-mediated degradation of CTNNB1 thereby allowing activation of FSH target genes. A multiprotein complex with Axin, adenomatous polyposis coli, and GSK3β regulates cytoplasmic concentrations of CTNNB1 (Kishida et al., 1998). Glycogen synthase kinase 3β associated with this degradation complex will phosphorylate CTNNB1 at Thr41, Ser-37, and Ser-33, targeting CTNNB1 for ubiquitination and subsequent degradation by the proteasome (Aberle et al., 1997). Stimulation by FSH and IGF-1 activates PI3K/AKT, resulting in inhibition of GSK3β thereby disrupting the CTNNB1 degradation complex. This process allows CTNNB1 to accumulate in cytoplasm and translocate to the nucleus where it associates with transcription factors such as T-cell factor (TCF) and steroidogenic factor-1 to initiate transcription of FSH target genes regulating key steroidogenic enzymes (Parakh et al., 2006) and ovarian estradiol production (Hernandez Gifford et al., 2009). Although canonical WNT signaling is characterized by CTNNB1/TCF binding to activate target genes, Parakh et al. (2006) demonstrated that FSH-induced aromatase expression occurs at least in part through a functional interaction of CTNNB1 with steroidogenic factor-1.
CONTRIBUTIONS OF WNT TO OVARIAN STEROIDOGENESIS
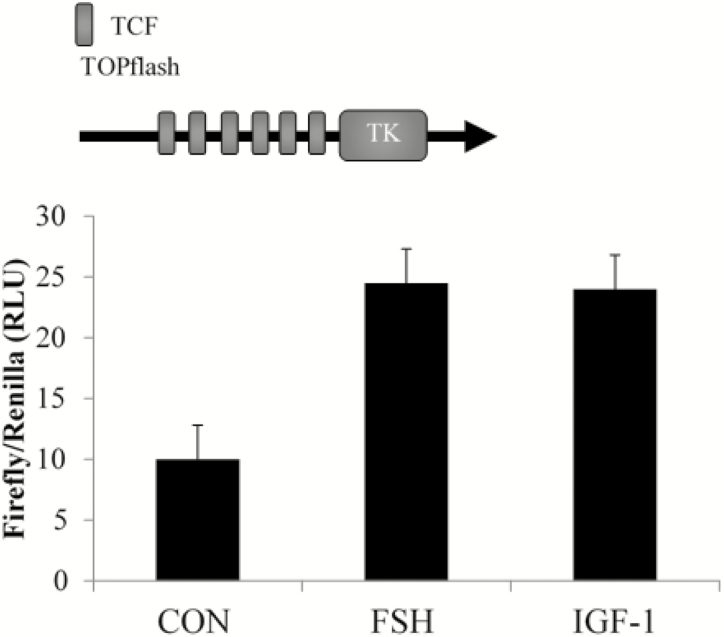
The paracrine or autocrine quality of WNT activity is dependent on the cellular context and specific combination in which the cognate frizzled receptors and coreceptors are expressed. The 10 frizzled membrane-bound receptors are thought to bind promiscuously to the 19 structurally related WNT genes (Slusarski et al., 1997; Liu et al., 2001). Importantly, differences in affinity of individual WNT proteins with frizzled receptors may determine which of the 3 WNT signaling pathways are activated (Van Amerongen and Nusse, 2009). Previous studies from our lab demonstrated that low concentrations of WNT (1 or 5 ng/mL) were unable to stimulate WNT signaling; however, WNT3A at higher concentrations (50 or 500 ng/mL) induced canonical WNT signaling pathway in primary rat granulosa cells (Stapp et al., 2014). In addition, studies in bovine granulosa cells suggested canonical WNT signaling, specifically WNT2 may be participating in CTNNB1 accumulation, supporting a role for the canonical WNT signaling pathway in ovarian function. Therefore, we next assessed the ability of CTNNB1 to associate with TCF transcriptional factors of the canonical WNT signaling pathway after FSH and IGF-1 stimulation. A TOPflash TCF luciferase reporter assay was conducted in primary bovine granulosa cells. The ability of FSH and IGF-1 to increase CTNNB1 abundance subsequently resulted in nuclear translocation and transcription activity of CTNNB1/TCF. Bovine granulosa cells incubated for 24 h with FSH or IGF-1-potentiated TOPflash activity by approximately 2.5-fold compared with vehicle-treated controls (Fig. 3; unpublished data). Together these data demonstrate that CTNNB1 is an important component of the AKT pathway and probably contributes to IGF-1 and FSH-mediated estradiol biosynthesis through an interaction with TCF transcription factors. Thus, the pituitary gonadotropin FSH along with the intraovarian molecules IGF-1 and WNT appear to work together to coordinate steroid production from the granulosa cell.
Figure 3.
Transcriptional regulation of CTNNB1/TCF is demonstrated in bovine granulosa cells treated with FSH and IGF-1. Primary bovine granulosa cells were transfected with a TOPflash luciferase reporter containing TCF-response elements and treated with FSH (100 ng/mL) or IGF-1 (50 ng/mL) for 24 h. Cell lysate was collected for luciferase assays in 2 highly repeatable experiments.
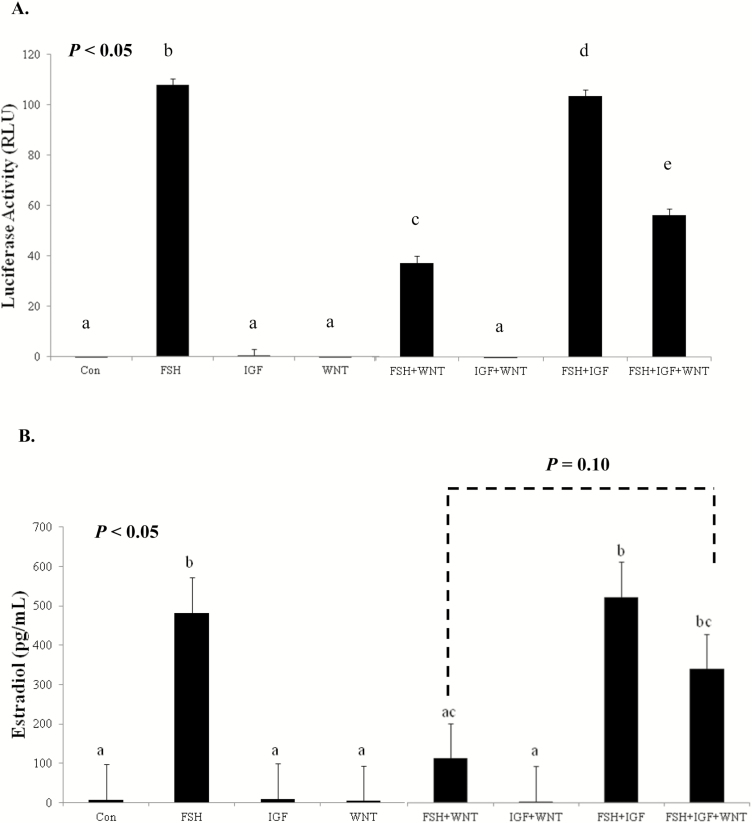
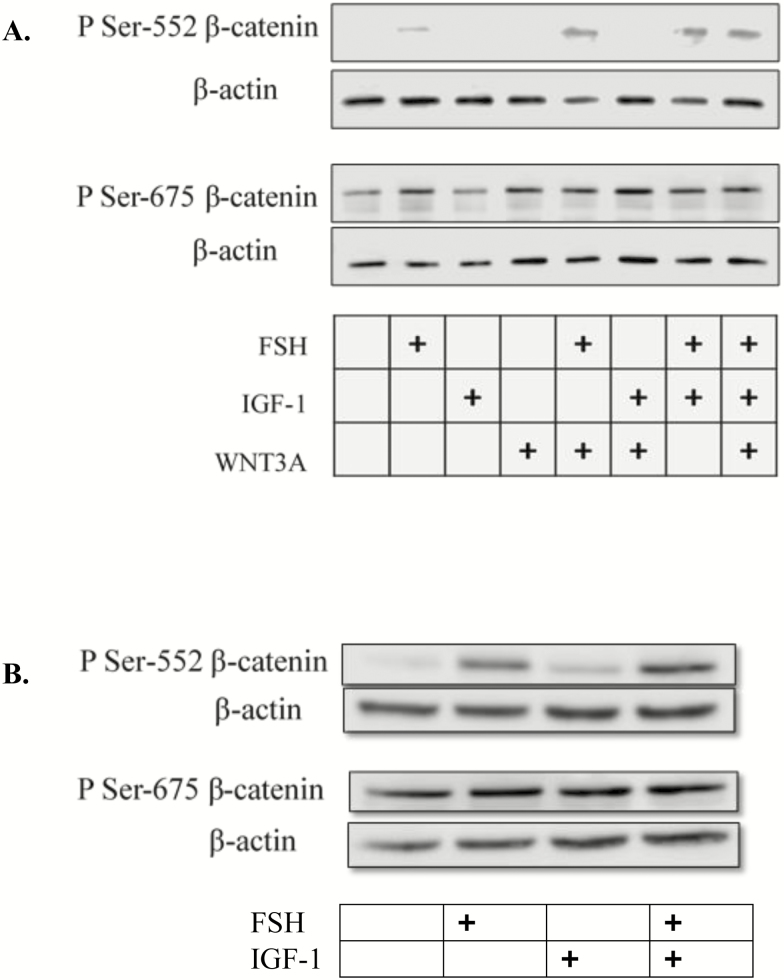
With the growing complexity of players in ovarian steroid production and the differential temporal expression of IGF-1 and FSH, we next wanted to determine if WNT signaling via CTNNB1 was contributing directly to estradiol production in bovine granulosa cells. In the adult bovine ovary, locally secreted WNTs and pathway members are expressed at different stages of follicle maturation (Gupta et al., 2014). In addition, hormonal regulation of the WNT family of genes has been detected in bovine granulosa cell. Expression of WNT2 mRNA was elevated in primary cultures of bovine granulosa cells treated with FSH stimulation (Castañon et al., 2012). In addition, mRNA expression of noncanonical WNT5A is detected and downregulated by FSH in long-term cultures of bovine granulosa cells (Abedini et al., 2015). In bovine corpora lutea, CTNNB1 regulates StAR mRNA expression and contributes to increased progesterone production in response to LH (Roy et al., 2009). These studies suggest that normal bovine ovarian function requires regulated contributions from WNT and CTNNB1. Moreover, early studies from our lab utilizing a primary rat granulosa cell culture model demonstrated an unexpected inhibition of WNT3A on FSH signaling. Simultaneous activation of canonical WNT and FSH signaling pathways reduced the ability of FSH to stimulate key steroidogenic enzymes and subsequent estradiol and progesterone production (Stapp et al., 2014). Recognizing that IGF-1 and FSH mediate AKT and subsequent CTNNB1 accumulation suggest signaling overlap as well as the possibility that AKT could be a potential mediator of WNT signaling. Therefore, a study was designed to investigate whether or not IGF-1 could overcome the inhibitory effects of WNT3A on FSH-mediated steroidogenesis. Once again employing a rat granulosa cell model to recapitulate the earlier inhibition of WNT3A on FSH target genes, primary cultures of rat granulosa cells were exposed to FSH (100 ng/mL) and WNT3A (50 ng/mL) in the presence or absence of IGF-1 (50 ng/mL) for 24 h. Follicle-stimulating hormone increased activation of an aromatase type II promoter (PII)-luciferase reporter with or without the addition of IGF-1 (Fig. 4A) and confirming our earlier studies, WNT3A muted FSH-mediated aromatase-PII activity (Stapp et al., 2014). Interestingly, the addition of IGF-1 partially rescued the inhibition of WNT3A on FSH’s ability to regulate the aromatase-PII promoter. Media concentrations of estradiol in granulosa cells cotreated with FSH and WNT3A were lower than cells treated with FSH alone, whereas the addition of IGF-1 in the presence of WNT3A and FSH tended to increase estradiol production (Fig. 4B). To identify a possible mechanism by which IGF-1 ameliorates WNT3A inhibition on FSH activity, CTNNB1 phosphorylation status was next evaluated. Site-specific phosphorylation of CTNNB1 on the N-terminal region is an indicator of transcriptional activity (Taurin et al., 2006). In primary rat granulosa cells, PKA phosphorylation of CTNNB1 at Ser-552 and Ser-675 promotes TCF-dependent transcriptional activity (Law et al., 2013). Therefore, phosphorylation status on residues Ser-552 or Ser-675 were evaluated in response to FSH, IGF (rat and bovine granulosa cells), and WNT3A (rat granulosa cells). Treatment with FSH promoted CTNNB1 phosphorylation at Ser-552 and Ser-675 irrespective of cotreatments when compared with controls (P < 0.05; Fig. 5A). Cotreatment of FSH with WNT3A or IGF-1 did not modulate CTNNB1 phosphorylation status. In a subsequent study, primary bovine granulosa cells treated with FSH and IGF-1 demonstrated a similar PKA-dependent pattern and AKT-independent pattern of CTNNB1 phosphorylation as previously seen in rat granulosa cells (Fig. 5B). Specifically, treatment with FSH-induced phosphorylation of CTNNB1 at Ser-552, whereas IGF-1 treatment had no effect of phosphorylation status compared with vehicle-treated controls. Phosphorylation of CTNNB1 at Ser-675 does not appear to be impacted by FSH or IGF-1 treatment in the bovine granulosa cells. These data indicate that IGF-1 plays an important role in FSH and WNT signaling by increasing CTNNB1 protein abundance, but not by regulating CTNNB1 phosphorylation status. The interplay between FSH, IGF-1, and WNT/CTNNB1 is essential for normal ovarian function and granulosa cell synthesis of estradiol in the bovine ovary.
Figure 4.
(A) Cyp19a1 promoter activity and (B) estradiol concentrations. Cyp19a1 PII activity and estradiol production in rat granulosa cells treated with FSH, WNT3A, and IGF-1. Primary rat granulosa cells were transfected with a Cyp19a1 PII luciferase reporter plasmid and treated for 24 h with 1) vehicle control, 2) FSH (100 ng/mL), 3) IGF-1 (50 ng/mL), 4) WNT3A (50 ng/mL), 5) FSH + WNT3A, 6) IGF-1 + WNT3A, 7) FSH + IGF-1, or 8) FSH + WNT3A + IGF-1. Cell lysate was collected for luciferase assay and treatment culture medium for quantification of estradiol concentrations. Least squares means ± SEM are presented, and bars without a common superscript differ (P < 0.05; n = 3).
Figure 5.
(A) Representative Western blot of phosphorylation of β-catenin at Ser-552 and Ser-675 primary rat granulosa cells (n = 3) treated for 24 h with 1) vehicle control, 2) 100 ng/mL highly purified human FSH, 3) IGF-1 (50 ng/mL), 4) WNT3A (50 ng/mL), 5) FSH + WNT3A, 6) IGF-1 + WNT3A, 7) FSH + IGF-1, or 8) FSH + WNT3A + IGF-1. β-Actin was used as a loading control. (B) Representative Western blot of β-catenin at Ser-552 and Ser-675 primary bovine granulosa cells (n = 3) treated for 24 h with 1) vehicle control, 2) FSH (100 ng/mL), 3) IGF-1 (50 ng/mL), or 4) FSH + IGF-1.
SUMMARY AND CONCLUSIONS
There is a growing body of evidence that WNT/CTNNB1 signaling plays a critical role in normal ovarian function. Until recently, the possibility that these locally secreted glycoproteins contribute to bovine ovarian follicle maturation and steroidogenesis has been largely overlooked. The data herein indicate that WNT signaling components not only participate in granulosa cell steroid production in cattle but also work in coordination with the pituitary gonadotropin, FSH, and other local ovarian molecules such as IGF-1. Given the temporal expression of FSH, IGF-1, and WNT signaling molecules in the ovary, our data suggest that IGF-1 is capable of overriding a negative feedback system set up by wingless-type mammary tumor virus integration site signaling on FSH target genes to ensure that follicle maturation and estrogen production do not go unregulated, which would result in negative consequences on fertility.
Footnotes
Based on a presentation titled “WNTs Role in Folliculogenesis,” presented at the ASAS-SSR Triennial Reproductive Symposium, July 13, 2017, Washington, DC.
The original research reported in this review was supported, in part, by the Oklahoma Agricultural Experiment Station, Stillwater, OK (OKL02789).
LITERATURE CITED
- Abedini A., Zamberlam G., Boerboom D., and Price C. A.. 2015. Non-canonical WNT5A is a potential regulator of granulosa cell function in cattle. Mol. Cell. Endocrinol. 403:39–45. doi:10.1016/j.mce.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Abel M. H., Wootton A. N., Wilkins V., Huhtaniemi I., Knight P. G., and Charlton H. M.. 2000. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141:1795–1803. doi:10.1210/endo.141.5.7456 [DOI] [PubMed] [Google Scholar]
- Aberle H., Bauer A., Stappert J., Kispert A., and Kemler R.. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797–3804. doi:10.1093/emboj/16.13.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J., Hardy M. P., Zhou J., Bondy C., Lupu F., Bellvé A. R., and Efstratiadis A.. 1996. Effects of an igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 10:903–918. doi:10.1210/mend.10.7.8813730 [DOI] [PubMed] [Google Scholar]
- Beg M. A., Bergfelt D. R., Kot K., Wiltbank M. C., and Ginther O. J.. 2001. Follicular-fluid factors and granulosa-cell gene expression associated with follicle deviation in cattle. Biol. Reprod. 64:432–441. [DOI] [PubMed] [Google Scholar]
- Boerboom D., White L. D., Dalle S., Courty J., and Richards J. S.. 2006. Dominant-stable beta-catenin expression causes cell fate alterations and wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Res. 66:1964–1973. doi:10.1158/0008-5472.CAN-05-3493 [DOI] [PubMed] [Google Scholar]
- Boyer A., Goff A. K., and Boerboom D.. 2010. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol. Metab. 21:25–32. doi:10.1016/j.tem.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Brantjes H., Roose J., van De Wetering M., and Clevers H.. 2001. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 29:1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan K. M. and Nusse R.. 1997. Wnt signaling: A common theme in animal development. Genes Dev. 11:3286–3305. [DOI] [PubMed] [Google Scholar]
- Castañon B. I., Stapp A. D., Gifford C. A., Spicer L. J., Hallford D. M., and Hernandez Gifford J. A.. 2012. Follicle-stimulating hormone regulation of estradiol production: Possible involvement of WNT2 and β-catenin in bovine granulosa cells. J. Anim. Sci. 90:3789–3797. doi:10.2527/jas.2011-4696 [DOI] [PubMed] [Google Scholar]
- Chen G., Fernandez J., Mische S., and Courey A. J.. 1999. A functional interaction between the histone deacetylase rpd3 and the corepressor Groucho in Drosophila development. Genes Dev. 13:2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse J. F., and Korach K. S.. 1999. Estrogen receptor null mice: What have we learned and where will they lead us?Endocr. Rev. 20:358–417. doi:10.1210/edrv.20.3.0370 [DOI] [PubMed] [Google Scholar]
- Drummond A. E., and Findlay J. K.. 1999. The role of estrogen in folliculogenesis. Mol. Cell. Endocrinol. 151:57–64. [DOI] [PubMed] [Google Scholar]
- Fan H. Y., O’Connor A., Shitanaka M., Shimada M., Liu Z., and Richards J. S.. 2010. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol. Endocrinol. 24:1529–1542. doi:10.1210/me.2010-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnson K. W., Kontogiannea M., Li X., and Farookhi R.. 2012. Characterization of wnt2 overexpression in a rat granulosa cell line (DC3): Effects on CTNNB1 activation. Biol. Reprod. 87:12, 1–12, 8. doi:10.1095/biolreprod.111.096396 [DOI] [PubMed] [Google Scholar]
- Gloaguen P., Crépieux P., Heitzler D., Poupon A., and Reiter E.. 2011. Mapping the follicle-stimulating hormone-induced signaling networks. Front. Endocrinol. (Lausanne) 2:45. doi:10.3389/fendo.2011.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez B. I., Gifford C. A., Hallford D. M., and Hernandez Gifford J. A.. 2015. Protein kinase B is required for follicle-stimulating hormone mediated beta-catenin accumulation and estradiol production in granulosa cells of cattle. Anim. Reprod. Sci. 163:97–104. doi:10.1016/j.anireprosci.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Gupta P. S., Folger J. K., Rajput S. K., Lv L., Yao J., Ireland J. J., and Smith G. W.. 2014. Regulation and regulatory role of WNT signaling in potentiating FSH action during bovine dominant follicle selection. PLoS ONE 9:e100201. doi:10.1371/journal.pone.0100201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Gifford J. A. 2015. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction 150:R137–R148. doi:10.1530/REP-14-0685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Gifford J. A., Hunzicker-Dunn M. E., and Nilson J. H.. 2009. Conditional deletion of beta-catenin mediated by amhr2cre in mice causes female infertility. Biol. Reprod. 80:1282–1292. doi:10.1095/biolreprod.108.072280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M., Johnson M. A., Greenberg N. M., and Richards J. S.. 2002. Regulated expression of Wnts and frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology 143:898–908. doi:10.1210/endo.143.3.8684 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M., and Maizels E. T.. 2006. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell Signal. 18:1351–1359. doi:10.1016/j.cellsig.2006.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S., Yamamoto H., Ikeda S., Kishida M., Sakamoto I., Koyama S., and Kikuchi A.. 1998. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 273:10823–10826. [DOI] [PubMed] [Google Scholar]
- Komiya Y., and Habas R.. 2008. Wnt signal transduction pathways. Organogenesis 4:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law N. C., Weck J., Kyriss B., Nilson J. H., and Hunzicker-Dunn M.. 2013. Lhcgr expression in granulosa cells: Roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol. Endocrinol. 27:1295–1310. doi:10.1210/me.2013-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., DeCostanzo A. J., Liu X., Hy W., Hallagan S., Moon R. T., and Malbon C. C.. 2001. G protein signaling from activated rat frizzled-1 to the beta-catenin-lef-tcf pathway. Science 292:1718–1722. doi:10.1126/science.1060100 [DOI] [PubMed] [Google Scholar]
- Logan C. Y., and Nusse R.. 2004. The wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781–810. doi:10.1146/annurev.cellbio.20.010403. 113126 [DOI] [PubMed] [Google Scholar]
- Mani A. M., Fenwick M. A., Cheng Z., Sharma M. K., Singh D., and Wathes D. C.. 2010. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction 139:139–151. doi:10.260/REP-09-0050 [DOI] [PubMed] [Google Scholar]
- Owens G. E., Keri R. A., and Nilson J. H.. 2002. Ovulatory surges of human CG prevent hormone-induced granulosa cell tumor formation leading to the identification of tumor-associated changes in the transcriptome. Mol. Endocrinol. 16:1230–1242. doi:10.1210/mend.16.6.0850 [DOI] [PubMed] [Google Scholar]
- Parakh T. N., Hernandez J. A., Grammer J. C., Weck J., Hunzicker-Dunn M., Zeleznik A. J., and Nilson J. H.. 2006. Follicle-stimulating hormone/camp regulation of aromatase gene expression requires beta-catenin. Proc. Natl. Acad. Sci. U.S.A. 103:12435–12440. doi:10.1073/pnas.0603006103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. S., Russell D. L., Ochsner S., Hsieh M., Doyle K. H., Falender A. E., Lo Y. K., and Sharma S. C.. 2002. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog. Horm. Res. 57:195–220. [DOI] [PubMed] [Google Scholar]
- Ricken A., Lochhead P., Kontogiannea M., and Farookhi R.. 2002. Wnt signaling in the ovary: Identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology 143:2741–2749. doi:10.1210/endo.143.7.8908 [DOI] [PubMed] [Google Scholar]
- Roose J., Molenaar M., Peterson J., Hurenkamp J., Brantjes H., Moerer P., van de Wetering M., Destrée O., and Clevers H.. 1998. The Xenopus Wnt effector xtcf-3 interacts with Groucho-related transcriptional repressors. Nature 395:608–612. doi:10.1038/26989 [DOI] [PubMed] [Google Scholar]
- Roy L., McDonald C. A., Jiang C., Maroni D., Zeleznik A. J., Wyatt T. A., Hou X., and Davis J. S.. 2009. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology 150:5036–5045. doi:10.1210/en.2009-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y. 2007. Hormones and cancer. Cell Res. 17:277–279. doi:10.1038/cr.2007.26 [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Misso M., Hewitt K. N., Hill R. A., Boon W. C., Jones M. E., Kovacic A., Zhou J., and Clyne C. D.. 2005. Estrogen–the good, the bad, and the unexpected. Endocr. Rev. 26:322–330. doi:10.1210/er.2004-0020 [DOI] [PubMed] [Google Scholar]
- Slusarski D. C., Corces V. G., and Moon R. T.. 1997. Interaction of Wnt and a frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410–413. doi:10.1038/37138 [DOI] [PubMed] [Google Scholar]
- Stapp A. D., Gómez B. I., Gifford C. A., Hallford D. M., and Hernandez Gifford J. A.. 2014. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS ONE 9:e86432. doi:10.1371/journal.pone.0086432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S., Sandbo N., Qin Y., Browning D., and Dulin N. O.. 2006. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 281:9971–9976. doi:10.1074/jbc.M508778200 [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkilä M., Kispert A., Chin N., and McMahon A. P.. 1999. Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409. doi:10.1038/17068 [DOI] [PubMed] [Google Scholar]
- Van Amerongen R., and Nusse R.. 2009. Towards an integrated view of Wnt signaling in development. Development 136:3205–3214. doi:10.1242/dev.033910 [DOI] [PubMed] [Google Scholar]
- Wang H. X., Li T. Y., and Kidder G. M.. 2010. WNT2 regulates DNA synthesis in mouse granulosa cells through beta-catenin. Biol. Reprod. 82:865–875. doi:10.1095/biolreprod.109.080903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik A. J., Saxena D., and Little-Ihrig L.. 2003. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 144:3985–3994. doi:10.1210/en.2003-0293 [DOI] [PubMed] [Google Scholar]
- Zhou P., Baumgarten S. C., Wu Y., Bennett J., Winston N., Hirshfeld-Cytron J., and Stocco C.. 2013. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 27:511–523. doi:10.1210/me.2012-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]