Abstract
The human-mediated introduction of marine non-indigenous species is a centuries- if not millennia-old phenomenon, but was only recently acknowledged as a potent driver of change in the sea. We provide a synopsis of key historical milestones for marine bioinvasions, including timelines of (a) discovery and understanding of the invasion process, focusing on transfer mechanisms and outcomes, (b) methodologies used for detection and monitoring, (c) approaches to ecological impacts research, and (d) management and policy responses. Early (until the mid-1900s) marine bioinvasions were given little attention, and in a number of cases actively and routinely facilitated. Beginning in the second half of the 20th century, several conspicuous non-indigenous species outbreaks with strong environmental, economic, and public health impacts raised widespread concerns and initiated shifts in public and scientific perceptions. These high-profile invasions led to policy documents and strategies to reduce the introduction and spread of non-indigenous species, although with significant time lags and limited success and focused on only a subset of transfer mechanisms. Integrated, multi-vector management within an ecosystem-based marine management context is urgently needed to address the complex interactions of natural and human pressures that drive invasions in marine ecosystems.
Introduction
Marine ecosystems are affected by several well-known human-induced global pressures, such as exploitation of living resources, land-based pollution, eutrophication, physical destruction and climate change (e.g., [1,2]). Many studies have documented human-mediated introductions of non-indigenous species (NIS), yet only relatively recently NIS have been recognized as a major threat that may cause significant changes in the structure and function of marine ecosystems [3].
Multiple human-induced pressures, which vary across Earth’s oceans, interact in complex and often non-linear ways [4]. Evaluation of the cumulative effects is essential to successful ecosystem-based management (e.g., [5,6]). Although our ability to comprehend interactions between human pressures and evaluate their cumulative effects is improving, managerial response still mostly relies on sectoral approaches. Whereas alleviation of specific pressures (e.g., pollution, fisheries) have resulted in some instances in the improvement of local marine environments and their living resources [7], there is no such evidence available for bioinvasion management, where many historically well-documented regions with sound biodiversity baselines exhibit clear temporal increases in detection rates of new NIS introductions (e.g., [8,9]). We consider that ‘NIS remain NIS,’ regardless of the time passed since their first detected presence.
Herein we address the “shifting baseline” syndrome in marine bioinvasions. This syndrome was first recognized in fisheries science wherein the state of the fishery was assessed based on a contemporary stock size and species composition, overlooking the prior history of the fishery, leading to underestimation of the magnitude of change and the degree of overexploitation [10,11]. The extent of marine bioinvasions may be similarly occluded. Carlton [12] presented an overview of the taxonomic, historical, and shifting baseline impediments to understanding of marine bioinvasions. Over the past 30 years, invaluable historical overviews on marine bioinvasions have confirmed their ancient origins (e.g., [13–16]). The advancement and application of new molecular and genomic methods will continue broadening our view of past invasions (e.g., [17–19]). However, a lack of quantitative, high -resolution analyses and detection methods aimed at marine bioinvaders and their histories further deepens the “shifting baseline” syndrome effect, and prevents a more complete understanding and acknowledgment of the full extent of the problem.
This paper provides a synopsis of the essential aspects related to the history of marine bioinvasions globally, through collating and synthesizing information on i) early evidence of species introductions by different vectors, ii) dynamics of introduction vectors and human perceptions over time, and iii) evolution of methodologies used for detection, identification and surveillance. We frame the assembled historical information into policy and management perspectives through i) outlining milestones in relevant policy and management acts and ii) making broad comparisons among the vector dynamics in the recent past and the content and efficacy of legislative management acts. In doing so we identify key messages crucial to the effective management of NIS, as well as redress some of the historical legacies.
A history of vectors dynamics and associated introductions
Vessels
Early shipping
Throughout history, the maritime shipping has played a fundamental role as means of transportation of goods and people [20–22]. However, we know little of the relationship between the early sea voyages and the dispersal of species on (as fouling communities), in (as boring communities) and inside (as ballast communities) ancient wooden sailing ships. We do know that there were extensive biofouling communities on these vessels, that shipworms were known to the ancients, and that solid ballast was loaded into ships since the Bronze age. It is highly likely that the dispersal and introduction of marine animals and plants by sea-going ships, in hull fouling and in damp rock-, shingle-, and sand- ballasted holds, commenced long ago, millennia before marine biologists began documenting the biogeography of organisms [12]. Persuasive insights and a strong signal into the probable scale of early invasions comes from the archaeo-entomologists who have traced the expansion of the European insect fauna via Roman and Viking ships around Europe and across the Atlantic Ocean ([23,24] and references therein). The same ships transporting terrestrial life would, of course, have transported marine life as well. A compelling example of an ancient invasion is the North American clam Mya arenaria. No fossil record is known in Europe, where it likely appeared by the 1200s ([25,26], see also Table 1).
Table 1. Examples of evidence of early introductions of selected marine non-indigenous species.
| Taxon | Species | First detected presence | Origin to recipient region | Likely vector | Reference |
|---|---|---|---|---|---|
| Mollusca: Bivalvia | Mya arenaria (soft-shelled clam) | 1200s | North America to Europe | Hull fouling, rock ballast | [25,27] |
| Mytilus spp. (mussels) | 1500s | Northern hemisphere to South America | Hull fouling | [14] | |
| Crassostrea angulata (oyster) | 1500s | Western North Pacific to Southern Europe | Hull fouling, ballast | [12] | |
| Mollusca: Gastropoda | Littorina saxatilis (rock periwinkle) | 1792 | Western Europe to Adriatic Sea | Rock ballast | [28] |
| Littorina littorea (shore periwinkle) | 1840s | Europe to North America | Rock ballast | [29] | |
| Crustacea: Brachyura | Carcinus maenas (green crab) | 1817 | Europe to North America | Hull fouling, rock ballast | [30,31] |
| Crustacea: Isopoda | Sphaeroma terebrans (pill bug) | 1860s | Indian Ocean to Brazil | Ship hull fouling or boring | [32] |
| Plantae: Chlorophyta | Halimeda opuntia (green alga) | 1699 | Indo-West Pacific to the Caribbean | Hull fouling | [33] |
| Plantae: Tracheophyta | Spartina alterniflora (cordgrass) | 1803 | North America to France | Shore ballast | [34] |
Shipping expanded dramatically in the late 1500s [26]. However, as with antiquity, we have limited insight into marine bioinvasions of this era. Both vessel hull fouling and ballast likely played significant roles. Lindroth [35] notes that solid ballast discharge regulations were already in place by 1611 in the New World, which suggests the early awareness of the sheer volume of ballast being transported. Carlton and Hodder [36] undertook the first experimental studies on the fouling communities on a vessel in transit, focusing on a replica of a 16th century sailing ship, and thus providing insights into what may have been transported by vessels in the 1500s. The vessel sequentially accumulated species along the voyage route, such that it arrived in one port with species accumulated from previous ports (harbors) of call. This vessel also sank into mud at low tide in one port, acquiring benthic species not normally thought to have been transported by ships. In addition, Carlton [37] reconstructed the potential assemblage of marine animals and plants that may have been transported by a wooden sailing ship of 1750, suggesting that two dozen or more species (certainly an underestimate) could have been transported in ballast alone. However, we have no early records of the fauna transported by ballast, and only limited records of the flora, thanks to 19th century sampling of the latter, known as “ballast waifs”, on ballast dumping grounds [38].
Records of ballast-mediated introductions begin to appear by the late 1700s and early 1800s. The type specimen of one of the world's best-known salt marsh plants, the North American Spartina alterniflora, was collected in France in 1803 [34], and thus likely introduced to the region in the 1700s in ships' ballast. It was transported to South America by 1817 either from North America or Europe. As an ecosystem engineer, it caused profound changes on the west coast of South America: marshes now occupy vast areas where mudflats used to exist, with concomitant changes in bird, fish, and invertebrate diversity and trophic relationships [34].
Rock ballast was the probable vector for the arrival and spread of the European periwinkle Littorina littorea in North America. This well-known snail is one of the most meticulously documented invasions of the early 1800s [29,39,40]. The large-shelled, intertidal marine molluscan fauna of Eastern North America (present day Canada and the United States) was already reasonably well known to European scientists by the mid- to late-1700s, such that the discovery of this western European snail L. littorea in Nova Scotia circa 1840s was greeted with a good deal of surprise by British scientists. Its southward spread over the following decades to the mid-Atlantic coast has been well documented. Through detailed investigation of shipping and ballast history commencing in the 1770s, Brawley et al. [40] linked the introduction of both L. littorea and the European seaweed Fucus serratus (in the 1860s) to the discharge of solid ballast from Western Europe. Carlton [41] noted that the invasion of this small snail effectively re-organized the structure and function of rocky, soft bottom, and salt marsh intertidal shores of the Northwest Atlantic Ocean. Even before L. littorea appeared in North America, Littorina saxatilis was carried by rock ballast to the Adriatic Sea, where it was found to be established by 1792 [28]. The same era saw the ship-mediated arrival in North America of the European green crab Carcinus maenas [42], which became one of the major shoreline predators of the Atlantic seaboard.
Modern shipping
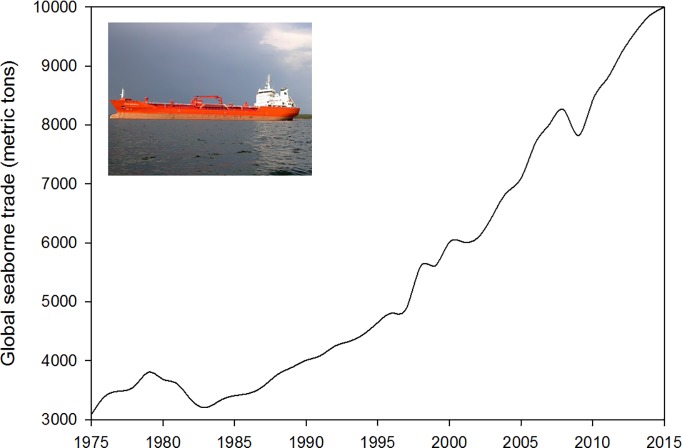
The 19th and 20th centuries saw key innovations to ship design and manufacturing (e.g., engine powered steel-hulled vessels) which resulted in major changes in ship operations and behavior [43]. As markets became increasingly globalized, shipping volumes soared. The massive increase in shipping since the 1950s, boosted by the development of container-shipping in the 1960s [44], underpins the growth in world trade. According to data from the United Nations Conference on Trade and Development [45], global seaborne trade has increased by 3.8 times from 1970 to 2015, exceeding 9 billion tonnes loaded worldwide in 2015 (Fig 1) with developing countries increasingly contributing to the total volumes of international seaborne trade [45].
Fig 1. Global seaborne trade, volume in metric tons, 1975–2015 (data from [45]).
Photo credit: Maiju Lehtiniemi.
Global shipping routes have evolved since the end of the 20th century, shifting from one based on direct port to port services along the major East–West routes, which linked the three poles of the global economy (Europe, the United States and East Asia), to a ‘hub and spoke’ network, linking the major East–West maritime motorway with the secondary North–South services [46]. This shift in trade routes has functionally increased direct and indirect connectivity among global ports and harbors [47], whereby a decade ago approximately ten billion tonnes of ballast water were transported around the world by ships annually [48]. As vessel size, speed and number increased, so too have the likely number of organisms transported alive across oceans in hull fouling and ballast. For example, an early study of ships’ ballast water entering the North American Great Lakes revealed an average of 17 live species with densities varying from 10,000 to 8 billion individuals per vessel [49]. Additional studies have further demonstrated the magnitude and diversity of marine organisms delivered in ballast throughout the world [50]. In addition to ballast, slow speed transits of recreational vessels, drilling rigs, barges and floating docks have been documented to further contribute to the dispersal of a wide diversity of fouling organisms [51–54].
Despite the realization of such broad scale species transfers, it took a confluence of economically disastrous events to gain management response (see Global policy and legislation). Some of the high-impact examples include dinoflagellates, comb jellies and mussels. The ballast-water introduction of the carnivorous comb jelly Mnemiopsis leidyi into the Black Sea in the 1980s was associated with major ecosystem and severe adverse economic effects [55]. In the 1980s vessels entering the North American Great Lakes dumped ballast water from freshwater ports in Europe with propagules of the now notorious zebra mussel Dreissena polymorpha and quagga mussel Dreissena bugensis–quite likely the most economically and biologically disruptive NIS in North America [56]. Evidence from historic plankton samples, cyst surveys in sediment cores and genetic studies implicated ballast water as the source of introduction of the photosynthetic dinoflagellate Gymnodinium catenatum and the likely source of neurotoxic poisoning, leading to the closure of 15 shellfish farms for periods up to six months in Tasmania in the 1980s [57].
Recreational boating
The use of recreational craft is increasingly considered a high-risk vector for primary introductions and secondary spread of marine NIS, owing to their numbers, spatial distribution, travel patterns, and connectivity between high risk NIS hubs [58–62]. Mass marine recreational boating is a relatively recent phenomenon, initiated in the 1920s-30s and greatly expanded since the 1960s [63]. The number of coastal marinas grew from 5 in 1960 to 54 in 2000 in Queensland, Australia, and from 403 in 1985 to 716 in 2002 in Italy. In Florida and California, USA, in 2010, 914,535 and 810,008 boats, respectively, were registered [64]. In Ireland 29 marinas operated in the early 2000s, whereas none existed in mid-1970s [65]. Based on satellite images from 2007, the number of recreational boats in the Mediterranean Sea was approximately 1.5 million at the time [66].
Recreational craft are often moored for long periods and may accumulate organisms from the local fouling communities, transporting them to the next marina or mooring place, or to even distant ports. Largely overlooked, water entrained in bilge spaces during the transit also may contribute to spread of marine organisms [67]. In regions favoured by boaters (Caribbean Sea, Mediterranean Sea, and generally subtropical and temperate seas near affluent population centres), leisure craft provides high connectivity between high and low NIS locales (‘hub and spoke’), enhancing invasion risk by increasing potential propagule pressure [68]. But even in cold-temperate areas risks are high: in British Columbia, Canada, over a quarter of boats surveyed (25.7%) were fouled with one or more NIS [60,69].
Despite the growing number and geographical distribution of marinas and seaworthy leisure craft, investigations of introduction and translocation of NIS mediated by recreational boating only began in the 1990s (e.g., [70] and references therein), and to date the data remain geographically restricted, thus often underestimating the problem [71].
Trade in live organisms
Culture
Farming of marine and partly marine (anadromous, catadromous) organisms (including fish, invertebrates and plants) for food and other products has a long history. Some target species are bred and raised in enclosed systems, whereas others are cultured to a certain life stage and placed in the sea in enclosures (cages, rafts), or released to roam freely. Farming is increasing to address the demand for marine food and to replace or restore declining coastal fisheries [72,73].
In the first century AD the Romans constructed Ostriaria for rearing of oysters [74], and transported them regionally within the Mediterranean Sea (e.g. from Brindisi in the southern Adriatic Sea to be reared in the Gulf of Baia in the Tyrrhenian Sea), in effect an early form of sea ranching [75]. Stock enhancement has been long practiced too: in the 11th century, “…King Knud the Great brought oysters home from England and introduced them to the Wadden Sea” [76].
The intentional transplantation of alien edible marine species in the late 19th century occurred partly in response to increased demand for seafood and to native stock failures. In 1860, the east Asian oyster Crassostrea angulata was imported to France from Portugal to compensate for shortage of seed of the native oyster Ostrea edulis [77], as well as the northern quahog Mercenaria mercenaria [78]. A century later, mass mortality of C. angulata triggered introduction of the Japanese cupped oyster Crassostrea gigas to France (Table 2; [79]). Of the current global production of C. gigas, about 15% originate from Europe and 7% from America [80]. Vast numbers of the North American Atlantic oyster Crassostrea virginica were transplanted in the 19th century to the American Pacific coast (see ‘Live seafood trade’), as well as released into European waters before marketing [81,82].
Table 2. Examples of records of four widely introduced non-indigenous cultured marine species.
| First record | Country/region of origin | Country/region of introduction | Reference |
|---|---|---|---|
| Crassostrea gigas (Japanese cupped oyster) | |||
| 1902 | Japan | USA: Washington State | [83] |
| 1919 | Japan | USA: Washington State | [84] |
| 1925 | Japan | Canada: British Columbia | [85] |
| 1947 | Japan | Australia: Tasmania | [86] |
| 1966 | Japan | France | [79] |
| 1972 | USA | French Polynesia | [87] |
| 1973 | France | South Africa: Cape Province | [88] |
| 1975 | Taiwan | USA: Guam | [89] |
| 1982 | Chile | Argentina | [90] |
| Oncorhynchus mykiss (Rainbow trout) | |||
| 1882 | USA: Pacific coast | Germany | [91] |
| 1884 | USA: Atlantic coast | Belgium | [91] |
| 1890 | Russia | Lithuania | [91] |
| 1898 | Germany | Finland | [91] |
| 1902 | Northeast Pacific | Norway | [91] |
| 1983 | Northeast Pacific | Iceland | [91] |
| Penaeus vannamei (Whiteleg shrimp) | |||
| 1972 | Mexico, Panama | New Caledonia | [92] |
| 1978–1985 | USA | USA: Hawaii | [93] |
| 1985 | Panama: Pacific coast | USA: South Carolina | [92] |
| 1985 | Panama: Pacific coast | Cuba | [94] |
| 1988 | USA: Texas, Hawaii | China | [92,95] |
| 1997 | Taiwan | Philippines | [96] |
| 1998 | Taiwan | Thailand | [96] |
| 2000 | China | Vietnam | [96] |
| 2001 | Taiwan | India | [96] |
| Ruditapes philippinarum (Manila clam) | |||
| 1930s | Japan | USA/Canada: Pacific coast | [97] |
| 1972 | USA: Pacific coast | France | [98] |
| 1980 | USA: Pacific coast | England | [99] |
| 1983 | England | Italy: Adriatic Sea | [100] |
| 1984 | Spain | Portugal | [101] |
Attempts to augment marine finfish production by releasing hatched larvae started in the 1870s, mainly using cod and plaice [102]. In the beginning of the 1880s marine hatcheries were built in Europe and North America, mainly rearing anadromous fish [103]. In the 20th century, the Soviet Union pursued extensive marine fisheries enhancement (MFE) programs, introducing the king crab Paralithodes camtschaticus and pink salmon Oncorhynchus gorbuscha to the Barents Sea, and sturgeons (Acipenser gueldenstaedtii, Huso huso) and salmonids (Coregonus baerii, O. gorbuscha, O. keta) to the Baltic Sea, together with several mysids introduced to increase the diversity of fish diet [104–107]. Farming of non-indigenous salmonids continues to be widespread phenomenon—a sizable share of the global production of Atlantic salmon is now located in Chile and Tasmania, Australia [80].
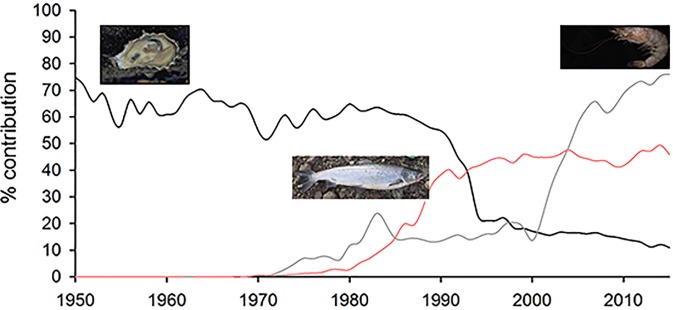
The number of species involved and the geographic spread of transplantations appears to have increased in the late 20th century: between 1984 and 1997, 64 countries reported the stocking of 180 species that spend at least part of their life in marine and coastal areas (46 confined to marine environments), although the authors admit these numbers are only a fraction of the global activity [108]. The whiteleg shrimp Penaeus vannamei, native to the Pacific coast of Latin America, was introduced widely in the 1970s [92], and constitutes 76% of the world production of cultured penaeids (Fig 2), mainly due to rising production in China and Southeast Asia [71]. In the last decades China has promoted MFE programs [109]. By 2008, over 100 species of finfish, crustaceans, shellfish and jellyfish have been stocked, and almost 20 billion juveniles were released annually [110].
Fig 2. Temporal trends in global aquaculture: % of whiteleg shrimp Penaeus vannamei of shrimp and prawn culture in marine and brackish environment, % of Japanese cupped oyster Crassostrea gigas of oyster culture in marine environment, and % of Atlantic salmon Salmo salar of fish culture in marine environment.
Decrease in relative contribution of C. gigas is related to increase in oyster culture in China, where C. plicatula and C. rivularis are cultured on a large commercial scale (Data from [111]). Photo credits: IFREMER (France), Ralf Mae and Nicholas Yap.
A few species, unintentionally introduced with cultured target species, have been farmed as well. For instance, the seaweed Undaria pinnatifida (wakame) was accidentally introduced with C. gigas into the Mediterranean Sea in 1971 [112,113]. In 1983 it was intentionally transplanted to Brittany, France, for farming, with the risk of its dispersal from the farming sites considered minimal by the French authorities [114]. However, by 1987 reproducing individuals were found on mussel lines next to one farm, and the alga has subsequently spread along the coast from Portugal to the Netherlands [115]. It fouls oyster and mussel lines, aquaculture equipment and boats; massive development may impair aquaculture harvests [116].
Experimental evidence shows that shells of shipped oysters, even if visibly clean, can host a wide range of macroalgal species, including the Japanese seaweed Sargassum muticum [117,118], which was introduced to Western Europe in the 1970s with oyster imports [119]. The introduction and rapid expansion of S. muticum caused one of the most dramatic changes in the vegetation of the upper sublittoral zone, inducing sedimentation, changes in community composition, replacement of native species, and interference with coastal fisheries and recreational activities [120].
The Manila clam Ruditapes philippinarum, unintentionally introduced to the North American Pacific coast in the 1930s with Japanese oysters, has become the basis of major mariculture production in the Pacific Northwest [97]. Intentionally introduced in 1983 into the Italian Adriatic to supplement the declining fishery of the indigenous carpet clam Ruditapes decussatus, R. philippinarum ended up supplanting it [98]. Similarly, introduced to the south coast of England for mariculture, R. philippinarum has spread into the wild providing fishermen with a new crop [99]. Commercial fishing of R. philippinarum is also very important along the French Atlantic coastline, reaching thousands of tons annually [100].
Concerns about the impact of hatchery fry on wild populations of the same species have been raised since the late 1980s [121]. Of 70 studies which compared hatchery reared and wild stocks, 23 studies showed significant negative effects of hatchery rearing on the fitness of stocked fish, and 28 studies showed reduced genetic variation in hatchery populations [122]. The main concerns are impacts on wild populations such as changes in genetic composition and structure, breakdown of genetic adaptations and loss of genetic diversity [123–125].
Disease agents detrimental to the cultured stocks, associated with the target species, have been of particular concern to the stakeholders for a long time. Some of the early examples include the loss of income following large-scale disease epidemics and mass mortalities of commercially important molluscs infected by introduced “protozoans” (e.g., Haplosporidium nelsoni [= Minchinia nelsoni]) depressing the mollusc production in Chesapeake and Delaware Bays since the late 1950s [126], and Bonamia ostreae affecting Ostrea edulis in European waters [127]. These occurrences prompted policymakers and stakeholders to start establishing regulations to limit disease spread and prevent pathogen introductions (see Global policy and legislation).
Extreme weather events may be expected to escalate in intensity and frequency with climate change. Such events play a role in release of NIS from marine as well as land-based mariculture farms and holding pens and causing possible impacts on wild populations [128–130].
Live seafood and bait
Humans have moved living species for food and other purposes for a long time (e.g., the 10,000 years timeline from pre-domestication cultivation has been well studied [131]. However, little is known about the historical movement of live edible marine species (see above).
The development of fast, reliable refrigerated transportation for valuable perishable cargo brought about the expansion of a retail market for live seafood around the globe. This has resulted in large amounts of live fish, shellfish and algae being transported and occasionally dumped or released, accidentally or intentionally. Still, live marine seafood trade has received limited attention as a vector of introduction [132]. Information concerning intentional transportation of live marine organisms for consumption is rare until the 19th century, when fast transport, refrigeration and growing affluence provided the means for a global marketplace in live seafood. The American oyster C. virginica, native to the North Atlantic, was likely the first commercial success of the long-distance live marine seafood trade. As the supply of European indigenous oysters had greatly fallen off due to overharvesting, oysters were shipped from New York to Europe, where they were evidently greatly appreciated: 5000 barrels a week of live oysters packed in flour were shipped in 1882 from New York alone [133,134]. The oysters were shipped live in North America “as far as railroads and careful packing could get oysters without spoilage” throughout the 19th and early 20th century [135]. The completion of the transcontinental Central Pacific Railroad in 1869 and the expansion of the ice industry in the late 1800s made possible shipping fresh oysters from the USA East coast to California and eventually as far north as British Columbia [81,136]. The eastern oyster trade is thought to be responsible for a significant percentage of Western Atlantic invaders in San Francisco Bay [137]. Many species of estuarine mollusks, polychaetes, bryozoans, and crustaceans, for example, were inadvertently but successfully introduced with live oyster shipments from the Western Atlantic to the Eastern Pacific [138]. Long after these introductions, other Northwest Atlantic species arrived with a vector that did not exist in the 19th century: live marine worm bait wrapped in seaweed dunnage, the latter hosting many associated species. By this means both the European green crab C. maenas and the rock periwinkle L. saxatilis were added to the North American Pacific coast fauna [139,140]. More broadly, the live marine bait trait represents another live trade vector that can transport diverse species to potentially many global regions [141].
Evidence is scant of marine species that have been transported live for the seafood and bait trade and eventually established in the wild. Indeed, only a small number of live imported seafood organisms end up in an environment suitable for their survival. American lobsters, Homarus americanus, some with their claws still bound with rubber bands, have been reported from the wild in a number of European countries. Their presence raised concerns about disease transfer, ecological interactions and hybridization with the European lobster, H. gammarus [142,143]. However, and despite the request, H. americanus, was not included into the list of invasive alien species of European Union (EU) concern [144]. If numbers of released/discarded organisms are large enough, or if an asexually reproducing organism is released frequently enough, the risk of establishment can increase [145]. Cecere et al. [146] highlight the disregard for regulations concerning storage and handling of imported live seafood and the risk from live seafood organisms held in water in holding facilities and quayside jettisoned discards. While few regulations exist for live bait trade, various studies have explored both the potential importance and possible management strategies [147].
Income and population growth are shifting the live seafood trade from developed to developing countries (China, Southeast Asia), while improvements in chilled cargo shipping and air cargo sustain the emergent long distance live seafood trade patterns [148]. High volumes of lightly regulated transshipment, storage and handling of live organisms pose a clear bioinvasion risk.
Ornamental
The horseshoe crab Limulus polyphemus is the earliest (1866) marine species considered to have been transported from the United States to Europe as a consequence of the ornamental trade (Table 3; [149]). The aquarium trade vector gained notoriety following the highly-publicized introduction of the seaweed Caulerpa taxifolia into the Mediterranean Sea in 1984 [150]. DNA fingerprinting linked the introduction of this invasive alga to public aquaria in Europe [151]. It was established successfully in the Mediterranean Sea and has proven highly disruptive [152], but was eradicated in California, USA [153], and failed to establish in Japan [154,155]. The report of accidental release of lionfish due to a breakage of a large aquarium by Hurricane Andrew is probably erroneous [156], but their subsequent spread across the Atlantic seaboard created a media storm and increased the scrutiny of the ornamental trade as a marine vector [157–159].
Table 3. Examples of first records of non-indigenous marine species attributed to the ornamental trade vector.
First record is the date of reported collection. “Status” indicates whether species has established self-sustaining populations. “Certainty” refers to confidence of vector assignment; “possible” indicates ornamental as one of several possible vectors, “probable” indicates most likely or sole vector ascribed in reference(s), “certain” indicates a verified aquarium release.
| First record | Species | Marine realm | Country | Status, vector certainty | Native realm | Reference |
|---|---|---|---|---|---|---|
| 1866 | Limulus polyphemus (Horseshoe crab) | Temperate Northern Atlantic | Germany | failed; probable | west Temperate Northern Atlantic | [149,160] |
| 1969 | Poecilia latipinna (Sailfin molly) | Central Indo-Pacific | Australia | established; possible | east North America | [161] |
| 1984 | Caulerpa taxifolia (Australian green algae) | Mediterranean Sea | Monaco | established; certain | circumtropical to temperate Australasia | [151] |
| 1985 | Pterois volitans (Red lionfish) | Tropical Atlantic | USA, Florida | established; probable | Indo-Pacific | [158] |
| 1994 | Cromileptes altivelis (Humpback grouper) | Tropical Atlantic | USA, Florida | established; probable | Western/Central Indo-Pacific | [162] |
| 1995 | Etroplus suratensis (Pearlspot) | Central Indo-Pacific | Singapore | established; probable | Western Indo-Pacific | [163] |
| 2007 | Scatophagus argus (Spotted scat) | Mediterranean Sea | Malta | established; probable | Indo West Pacific | [164] |
| 2011 | Acanthurus coeruleus (Blue tang surgeonfish) | Mediterranean Sea | Cyprus | failed; probable | Tropical Atlantic | [165] |
| 2015 | Zebrasoma xanthurum (Yellowtail tang) | Mediterranean Sea | Italy | failed, probable | Western Indian Ocean | [166] |
Records of marine NIS attributed probably or possibly to the ornamental vector have proliferated in recent decades, although this is likely an underestimate given the lack of marine vector information, let alone ornamental, from many regions [167,168]. However, few of those have established free-living populations (e.g., [157,169,170]).
The marine aquaria trade supplying home and public aquaria has grown into a global industry since the 2000s. The United States and the European Union constitute the largest markets, although trade in Japan, China and Southeast Asia is increasing. The number of marine fish species traded in the US has increased from 1000 in 2001 and 1471 in 2005, to about 2300 in 2011, in addition to 725 invertebrate species [171,172]. Despite the high numbers of species and individuals traded [173], due to its late emergence, the largely tropical origin of the species, and rare instances of release into the sea, few introductions have been attributed to the ornamental trade vector. Increasing trade volumes and global climate change may increase establishment rates for ornamental species introduced to coastal regions of importing countries.
Maritime canals
The first navigable canal was constructed in the 6th century BCE to join the Mediterranean Sea with the Red Sea by way of the Nile ([174]; Table 4). In the 19th century the same purpose was achieved by excavation of a canal through the Isthmus of Suez. This was followed by another monumental interoceanic canal excavated through the Isthmus of Panama. By breaching natural barriers to the dispersal of marine organisms and altering shipping routes, the interoceanic canals have provided marine biota with new opportunities for dispersal by natural means as well as by shipping.
Table 4. Examples of canals connecting different seas (data from [174–177]).
| Canal name | Opened | Comments |
|---|---|---|
| Mediterranean and Red Seas | ||
| 6th century BCE | by way of the Nile | |
| Suez Canal | 1869 | Cross section area increased from initial 300 m2 to 5200 m2 |
| Ponto-Caspian and Baltic Seas | ||
| Oginskij Canal | 1768 | This and below: riverine canals |
| Bug-Pripet Canal | 1775 | |
| Mariinskij Waterway | 1810 | |
| Severo-Dvinskiy Waterway | 1829 | |
| Volga-Don Canal | 1952 | |
| Baltic and North Seas | ||
| Kiel Canal | 1895 | First inland waterway in the region in 1398 |
| Pacific and Atlantic Oceans | ||
| Panama Canal | 1914 | Proposed in 1534 |
| Nicaragua Canal | Under consideration | |
Prior to the opening of the Suez Canal the French malacologist Vaillant [178] had already argued that cutting through the Isthmus of Suez offered an opportunity to examine the immigration of species and the mix of faunas. Within a decade of its opening, two Red Sea bivalves, the Gulf pearl oyster, Pinctada imbricata radiata and the mussel Brachidontes pharaonis were collected in the Port of Alexandria and Port Said respectively (as Malaegrina sp., and Mytilus variabilis); the former was already abundant in the port by 1874 and sold in the market [179]. Erythraean biota may traverse the canal by “natural” dispersal, by autonomous active or passive larval or adult movements, but the fouling habits of both bivalve species and their early finding in ports incline us to assume they were vessel-transported. Indeed, Fox [180] observed that fouled tugs and barges employed in the Canal could transport biota from one end to the other. Bivalves are uniquely suited to withstand temperature, salinity and desiccation stress, therefore it was to be expected they would successfully traverse the hypersaline Bitter lakes that served as a salinity barrier in the first decades of the Suez Canal's existence [181]. Successive enlargements of the canal (from 1962 to 2014 its depth increased from 15.5 to 24 m, and its cross-sectional area from 1800 to 5200 m2; [182]), combined with the decline of a hypersaline barrier (through dilution), permitted passage to ever larger number of propagules, resulting in the establishment of over 400 Erythraean species in the Mediterranean Sea [183].
The Panama Canal serves as a “bridge of water” between the Caribbean and the Pacific side of the isthmus. The earliest and best-known species reported to have traversed the canal and established a population on the opposite coast is the Atlantic tarpon, Megalops atlanticus. This fish, known from the eastern and western Atlantic Ocean, was reported from Lake Gatun and Miraflores lakes in 1935 [184], and later from the sea level end of the canal below Miraflores locks [185]. Recently the species was recorded from Pejeperro Lagoon, on the Pacific coast of Costa Rica [186]. Most of the Atlantic biota that has been recorded from the canal reached Miraflores Third Lock lagoon next to the Pacific entrance of the canal, but failed to establish along the Pacific coast [187]. The freshwater Lake Gatún has formed an efficient barrier to the movement of all but the most euryhaline marine species (except, of course, for any species travelling inside vessels in ballast water). Yet, a large number of organisms have undoubtedly been transported by vessels traversing the canal to be introduced elsewhere ([187] and references therein).
The new, 300-kilometre long Nicaragua Canal joining the Pacific and Atlantic oceans intends to compete for interoceanic traffic by servicing ships too big to pass through Panama’s recently expanded canal. At present, financial problems, along with ongoing environmental and engineering reviews, have delayed the project [175].
Development of methodologies for detection, identification and surveillance
Field surveys
Major research focus on marine invasions is relatively recent, emerging initially in the 1960s and 1970s in a few regions, such as the Panama Canal, Suez Canal, and the Pacific coast of North America [137,181,188,189], long after these canals and vectors have been in operation. As a result, NIS data varies considerably among geographic regions and taxonomic groups, resulting in significant imbalance among marine taxa in inventories [190,191]. The data in the available syntheses and checklists (see World Register of Introduced Marine Species, WRIMS [192]), is therefore a product of taxonomic studies, museum collections, field surveys and inventories, rather than standardized surveys designed to detect NIS. While these records are invaluable, providing insights into invasion dynamics and vectors, they are “bycatch” data, collected by different methods for diverse goals. The data quality is uneven across geographic regions, time, and taxonomic groups, making it challenging-to-impossible to interpret patterns of invasion with confidence [8,193]. The historical data generally fail to: (a) estimate the full extent (richness) of marine habitats or taxonomic groups, even at one location, or (b) provide comparable estimates of NIS present across locations or time periods [194,195].
Since the 1970s, survey methodologies have been designed and implemented explicitly to detect marine NIS richness and composition (Table 5; [196]). Most of these have focused on bays and estuaries, especially surrounding ports and marinas [197], as well as canals and offshore structures [198–201]). Most surveys were single events, providing a snapshot documentation of particular area/habitat/taxon. The identities and richness of detected NIS depends upon the methodologies (tools, replication, spatial and temporal scales) employed, season, duration and taxonomic expertise (but see [196]). Often smaller organisms (e.g., meiofauna) and plankton are not included.
Table 5. Examples of field surveys designed and implemented to detect non-indigenous marine species.
| Survey type | Target group | First applied | Examples of later applications |
|---|---|---|---|
| Rapid assessment surveys | Visual scans for target species and qualitative sampling and analysis, to detect NIS in benthic and pelagic habitats [195,196]. | Pacific coast of North America, 1976 [137] | US Atlantic and Pacific coasts, England, Scotland, Ireland, and Panama [202–206]. |
| Quantitative port surveys | Sampling benthic, epifaunal, and plankton communities | Australia, 1996 [207] | Many ports in Australia, New Zealand, and other countries, including adoption by the GloBallast Programme of the International Maritime Organization [196] |
| Quantitative fouling panel surveys | Sampling hard substrate communities | US Pacific and Atlantic coasts, 1999 [195] | At 36 different bays in the continental US, Hawaiian Islands, and Puerto Rico, with additional bays in Australia, Belize, Ecuador, Panama and other countries [208,209], Canada [210] and Portugal [211]. |
At the present time, baseline data collected by several survey types exist across multiple global regions. Unfortunately, there is no single global standard survey methodology that has been adopted to allow inter-comparisons among regions. However, several survey types have been replicated spatially, providing some opportunities for regional comparisons. While the value of repeated measures and surveillance is widely recognized, both for evaluating management and rapid response to new incursions [193,212], NIS detection programs comprising repeated community-level surveys appear to still be rare [196] and largely in the formative stages [194,213].
Application of molecular tools
Molecular tools are increasingly argued as instrumental in overcoming the difficulties associated with conventional taxonomic identification approaches—morphological complexities, cryptic life stages, globally declining taxonomic expertise [214–216]—and addressing the urgent need for efficient and timely detection of new incursions and robust identification of suspected NIS.
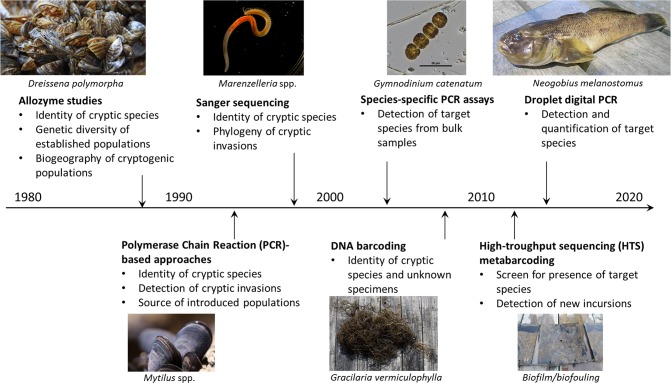
The earliest applications of molecular techniques to bioinvasions date to 1980s (Fig 3), when allozyme studies addressed the identity and genetic diversity of Dreissena spp. and Mytilus spp. [217–219]. Subsequently, DNA-based genetic analyses (e.g., fingerprinting, multilocus genotyping, Sanger sequencing) have been increasingly applied to detect cryptic invasions [220–224].
Fig 3. Timeline of molecular methods applications to marine bioinvasions research and surveillance, with images visualising examples of species or biological matrices to which the method was applied in the context of bioinvasions (data from [217,220,221,222,223,225,226,236,238,250]).
Photo credits: APRAE SOD (Italy), Jan-Erik Bruun, Vivian Husa, Pixabay, Heli Spilev and Anastasija Zaiko.
Molecular techniques facilitate targeted surveillance as species-specific Polymerase Chain Reaction (PCR) and quantitative or real-time (qPCR) assays are cost-efficient tools for biosecurity surveillance, whereby specificity, sensitivity and applicability to environmental DNA (eDNA) enhance scalability of NIS surveillance. In recent decades, an increasing number of species-specific PCR assays have been designed for marine NIS and applied for pre-border [225–227] and post-border [228–231] detection and monitoring.
In early 2000s, a molecular approach to taxonomic diagnosis involving sequencing of short species-specific DNA fragments (DNA barcodes) was introduced to biological research [232]. DNA barcoding has evolved into metabarcoding, allowing potential taxonomic assignment of specimens across entire biotic assemblages [233] from eDNA samples. The uptake of metabarcoding was fostered by the recent development of the High-Throughput Sequencing (HTS) techniques [234,235]. Despite the remaining gaps in understanding the detection limits and quantification capacities of HTS metabarcoding, it is generally recognized as a game-changing approach to environmental surveillance [236–238], including early detection of new incursions, pathway screening, propagule pressure assessment and monitoring of established NIS populations [239–244]).
To date, molecular techniques have been recognized as an important complementary tool for invasion biologists and ecosystem managers [216,245–247]. These methods can provide fast, specific, standardized, high quality and ecosystem-wide information on biodiversity (from microorganisms to macrozoobenthos) and all life stages (including juveniles or larvae–the common spreadable stages of many NIS). The ongoing technological developments and introduction of yet new methods, like shotgun sequencing, digital droplet PCR, gene enrichment techniques and single-molecule sequencers [248–251] make molecular surveillance approaches even more appealing for routine biosecurity applications. Certain caveats remain relative to the specificity of non-target molecular methods (such as metabarcoding), given that reference sequence databases are far from complete and error-free, and truly universal marker genes do not exist yet [58,97,200]. Another shortcoming is the current lack of quantitative capacity, especially when applied to multicellular organisms. As yet robust biodiversity or abundance information required for impact assessments, management and enforcement is unattainable [252]. Taxonomic expertise remains a critical requirement for NIS assessment and management, and the advantages of integrated taxonomic approaches using both molecular and morphology-based methods are repeatedly emphasized by researchers.
Citizen science
Historically, members of the public have played a key role in detection and surveillance, advancing our understanding of changes in species distributions and abundances through time and across diverse ecosystems and taxonomic groups [253,254]. The valuable contribution of such observations, and their potential as an information resource, have gained increasing recognition over the past decades. This has led to a surge in development of citizen science programs with a diverse range of applications, including the detection and study on NIS in marine systems [254,255].
The discovery of new marine NIS in a region has often been through chance encounter by fishermen, divers, and the public at large–who report novel and conspicuous organisms–providing an informal and diffuse detection network. For example, a fisherman in Chesapeake Bay provided the initial report of the Chinese mitten crab Eriocheir sinensis for the Atlantic coast of the United States [256].
The opportunity for “crowdsourcing” NIS detection and surveillance has been greatly enhanced by broad accessibility of new technologies, including the ability to instantly collect and share georeferenced data and photographs through mobile phone and web-based platforms, and also by increased focus and tools for optimizing the structure of citizen science efforts [257–260]. This has led to increasingly organized and formally structured campaigns–from bioblitz activities to sustained detection and monitoring for conspicuous NIS–including those in the marine realm.
The contribution of citizen science programs for NIS detection and surveillance is expected to expand over time, helping to address the limited funding and spatial/temporal coverage available with current programs [261]. Current research is demonstrating the high-quality data possible for particular types of measures and marine taxa [254,255]. There are some constraints that need to be considered in program design and expectations, including selecting large-bodied, conspicuous taxa with easy-to-recognize diagnostic characteristics. In the future, genetic tools may be adopted by citizen science programs to enhance the potential taxonomic scope and validation.
Post-invasion management
Post-introduction management efforts date back to the mid-20th century if not earlier [262]. Management attempts may be directed at, (1) the eradication of small, spatially restricted populations of newly introduced NIS, (2) reducing the local abundance of already established NIS, or (3) preventing their spread. Williams and Grosholz [263] have summarized nearly 20 examples of successful, unsuccessful, and ongoing eradication programs for introduced estuarine and coastal species from 1951 to 2006. Very few programs result in the permanent removal of NIS.
Efforts that seek ways and methods to control the abundance and spread of abundant pest species continue. Examples include the Asian seaweed Sargassum horneri in southern California [264], the grape algae Caulerpa racemosa in the Mediterranean Sea [265,266], the Asian ascidian Didemnum vexillum in the North Atlantic Ocean [267], and the Indo-Pacific lionfish Pterois spp. in the Caribbean Sea [268,269]. We emphasize that prevention through the restriction and reduction of introduction pathways and vectors is the overwhelmingly preferred option, given that management of already established NIS is increasingly viewed as unfeasible and unsustainable (e.g., [194,270]).
Impacts quantification
One of the earliest quantitative evaluations of ecological impact of NIS dates back to the 1920s, when the Atlantic mussel Geukensia demissa endangered the California clapper rail Rallus obsoletus in San Francisco Bay. It was estimated that at least 75% of the adult rail and 25% of the chicks were negatively affected [271]. However, only in the late 1970s, with documentation of the increasing domination of non-indigenous biota and associated changes in native biota (e.g., the Baltic Sea [272] and San Francisco Bay [273]), did quantitative evaluation become firmly established (Table 6). The last two decades have substantially increased our knowledge base through experimental and quantitative studies on the impacts of NIS worldwide (Europe, North and South America, South Africa, and Australasia), although the number of studies remains relatively small compared to the number of marine introductions.
Table 6. Examples of the ecological and environmental impacts of non-indigenous marine species.
| Species and Origin | Introduced location | Impact | Reference(s) |
|---|---|---|---|
| Chlorophyta (green algae) | |||
| Caulerpa taxifolia (Australian green alga) | Mediterranean Sea | Reduces productivity of two native macrophytes (Cystoseira barbata f. aurantia and Gracilaria bursa-pastoris). Decrease in mean species richness, mean density and mean biomass of fish | [274,275] |
| Codium fragile fragile (Japanese green alga) | USA: New England | Reduces diversity of other seaweeds; impacts shellfish populations; transports large numbers of native slipper limpets (Crepidula fornicata) onto shore | [276] |
| Canada: Nova Scotia | Competitive advantage over native seaweeds (kelps and other algae) through opportunistic exploitation of disturbed patches in kelp beds; once established as dense meadows, prevents kelp recolonization and persists as the dominant canopy-forming seaweed | [277] | |
| Tracheophyta (flowering plants) | |||
| Zostera japonica (Japanese eelgrass) | Canada and USA: British Columbia to Oregon | Converted vast areas from open soft-sediment habitat to rooted vegetation, a profound habitat alteration influencing sediment patterns (mean sediment grain size and sediment volatile organics) and resident fauna richness and densities, which alters interactions between pre-existing species | [32,278] |
| Spartina alterniflora (Northwest Atlantic saltmarsh cordgrass) | Argentina and Atlantic coast of South America | Changed previous soft-bottom habitat to coastal marshes, with vast unrecorded and thus overlooked shifts in bird, fish, and invertebrate biodiversity and immense shifts in algal vs. detritus production, with the concomitant trophic cascades | [279] |
| USA: California and Washington | Changed sediment dynamics, decrease algal production through shading, loss of shorebird feeding habitat, reduction of shrimp and oyster habitat, altering fish and wildlife habitat | [32] | |
| Ctenophora (comb jellyfish) | |||
| Mnemiopsis leidyi (West Atlantic comb jelly) | Black Sea | Predation on fish eggs and larvae and their food (zooplankton) in addition to increased nutrients and high fishing pressure caused a collapse of small planktivorous fish | [280,281] |
| Beroe ovate (West Atlantic comb jelly) | Black Sea | Predation combined with seawater warming and decreased fishing pressure, caused a marked decrease in the density of M. leidyi with concomitant increase in the abundance of zooplankton (about 5-fold) and ichthyoplankton (about 20-fold) | [280,282,283] |
| Annelida: Polychaeta (worms) | |||
| Ficopomatus enigmaticus (Australian tubeworm) | Argentina | Reef-building species providing habitat for native species, such as the crab Cyrtograpsus angulatus, which dramatically increases and then negatively impacts the abundance of native worms and a major effect on habitat integrity; reefs alter bedload transport and water flow | [284,285] |
| Sabella spallanzani (Mediterranean fan worm) | New Zealand | Dense aggregations significantly alter communities, outcompeting native species for space and food. Form ‘canopies’, affecting the recruitment, survival and growth of other biofouling organisms, by overgrowing and dislodging native taxa | [286–289] |
| Marenzelleria spp. (North American spionid worms) | Baltic Sea | Alters benthic community and nutrient regulation, including enhancing phosphorus flux from sediment to water on a basin-wide scale, potentially countering eutrophication mitigation. Re-oxygenates oxygen depleted deep sediments. | [290,291] |
| Mollusca: Gastropoda (snails) | |||
| Littorina littorea (European periwinkle) | USA: New England | Regulate much of intertidal diversity directly or indirectly, including reducing algal diversity and abundance through direct consumption; controls species composition and diversity in tidepools; may impact salt-marsh dynamics by consuming Spartina rhizomes; displaces native mudsnail Tritia obsoleta, setting upper and lower limits of native's distribution; increased abundance in some regions of native hermit crabs | [32,276,292,293] |
| Batillaria attramentaria (Japanese mudsnail) | USA: California | Competitive displacement of the native mudsnail Cerithideopsis californica | [294] |
| USA: Washington | Leads to increase abundance of NIS on Batillaria shells, of non-indigenous eelgrass, and of native hermit crabs | [295] | |
| Zeacumantus subcarinatus (New Zealand rock pool snail) | Australia: Sydney | Competitive displacement of the native rock pool snail Bembicium nanum | [296] |
| Tritia obsoleta (= Ilyanassa obsoleta) (Atlantic mud snail) | USA California: San Francisco Bay | Competitive displacement of the native Pacific mud snail Cerithideopsis californica | [297] |
| Rapana venosa (Japanese rapa whelk) | Black Sea | Significant impact on the native bivalves Ostrea edulis, Pecten ponticus, and Mytilus galloprovincialis due to predation | [298] |
| Uruguay: Rio de la Plata estuary | Predominate top-down effect on abundance of most native bivalves | [299] | |
| Crepidula fornicata (West Atlantic slipper limpet) | France Atlantic coast: Bay of Saint-Brieuc | Conversion of former soft substrate to hard, shelled substrate, resulting in decreased abundance of certain suprabenthic species (such as mysids) | [300] |
| Germany: Wadden Sea | Reduces survival and growth of native mussel Mytilus edulis | [301] | |
| France Atlantic coast: Arcachon Bay | Homogenizes benthic community (decreasing beta-diversity) but increases local diversity (alpha-diversity), which may alter interactions between species | [302] | |
| Mollusca: Bivalvia (mussels, clams) | |||
| Corbula amurensis (= Potamocorbula amurensis) (Asian corbula) | USA California: San Francisco Bay | Seasonal loss of water column productivity, with cascading trophic impacts |
[303–305] |
| Chronic depression of estuarine copepods that are food of several fish species that are also in decline | [306] | ||
| Arcuatula senhousia (= Musculista senhousia), Ruditapes philippinarum (= Venerupis philippinarum), Mya arenaria, Gemma gemma (Japanese mussel, Manila clam, Atlantic softshell clam, and Atlantic gem clam, respectively) | USA California: San Francisco Bay | Control of water column productivity through grazing (filtering) | [307,308] |
| Arcuatula senhousia (= Musculista senhousia) (Japanese mussel) | USA California: Mission and San Diego Bays | Intertidal reef-like mussel mats dominate shores, depressing native clam and seagrass populations | [309–311] |
| New Zealand: Auckland region | Decline in infaunal bivalves | [312] | |
| Mytilus galloprovincialis (Mediterranean mussel) and Semimytilus algosus(Pacific mussel) | South Africa | Now, alien mussels and barnacles (e.g. Balanus glandula) dominate on some wave-swept shores, but see [314] for changes to habitat complexity and abundance of both native and introduced species following sequential invasions of rocky shores on Marcus Island on west coast | [313,314] |
| Mytilus galloprovincialis (Mediterranean mussel) | South Africa | Competitive exclusion of indigenous mussel Aulacomya ater and large limpets; enhancement of recruitment of juvenile limpets and increased habitat availability for mussel infauna | [315] |
| New Zealand | Competitive domination in subtidal benthic community | [316] | |
| California | Replaced native mussel Mytilus trossulus | [317] | |
| Arthropoda: Crustacea: Isopoda (pill bugs) | |||
| Sphaeroma quoianum (New Zealand burrowing isopod) | USA California: San Francisco Bay | Severely erodes marsh and peat-bank edges | [32] |
| USA: Oregon to California | Major intertidal bioeroder, damaging and destabilizing marsh banks, friable rock, and polystyrene marine floats (for the latter, leading to production of fine plastic dust, exacerbating plastic pollution in the ocean) | [318] | |
| Sphaeroma terebrans (Indian Ocean boring isopod) | USA: Florida mangroves | Bores into and destroys mangrove (Rhizophora mangle) prop roots | [32] |
| Arthropoda: Crustacea: Amphipoda (amphipods) | |||
| Corophium volutator (European amphipod) | Atlantic North America: Bay of Fundy | Significant ecosystem engineer and often major prey of migratory birds; long overlooked as an invasion | [319] |
| Arthropoda: Crustacea: Decapoda (crabs) | |||
| Carcinus maenas (European green crab) | USA: New England | Alters diversity and abundance of many native prey species; alters abundance and morphology (phenotypes) of native intertidal snails; precipitous declines in native soft-shell clam Mya arenaria | [276] |
| Canada Atlantic coast | Significantly alters mud-bottom community structure through habitat disruption | [320] | |
| Hemigrapsus sanguineus (Asian shore crab) | USA: New England | The most abundant crab on many intertidal shores, leading to significant declines in abundance of other crabs, snails, mussels, barnacles, and many other species | [321,322] |
| Arthropoda: Crustacea: Stomatopoda (mantis shrimps) | |||
| Gonodactylaceus falcatus (Indo-Pacific mantis shrimp) | USA: Hawaiian Islands | Competitive displacement of native mantis shrimp Pseudosquilla ciliata | [323] |
| Bryozoa (moss animals) | |||
| Membranipora membranacea (European bryozoan) | Canada: Nova Scotia | Significant loss of native seaweeds due to epibiotic colonization of blades | [277,324] |
| Tricellaria inopinata (Pacific Bryozoan) | Italy: Northern Adriatic Sea | Significant loss of a highly diverse native bryozoan community | [325] |
| Echinodermata (sea stars) | |||
| Asterias amurensis (Japanese sea star) | Australia | A major predator and a keystone species exerting top-down control of its prey, especially native bivalve populations; caused local extinctions of several species; long-term decline of certain demersal fish due to competition with Asterias | [326–328] |
| Chordata: Ascidiacea (sea squirts) | |||
| Didemnum vexillum (Japanese compound sea squirt) | USA: New England, Georges Bank | The key driver of biodiversity decline in the epibenthos, restructuring invertebrate community | [329] |
| Clavelina oblonga (Caribbean sea squirt) | USA: North Carolina | Dominates fouling community with significant declines in biodiversity | [330] |
| Ciona robusta (Japanese Ciona sea squirt) | USA California: San Francisco Bay | Significantly depresses biofouling community species richness | [331] |
| Teleostei (fish) | |||
| Siganus rivulatus and Siganus luridus (Red Sea rabbitfish) | Mediterranean Sea: Levant, Aegean Sea | Replaces canopy-forming algae with ‘barrens,’ causing reduction in biogenic habitat complexity, biodiversity and biomass | [332,333] |
| Pterois volitans and Pterois miles (Indo-Pacific lionfish) | Caribbean Sea | Predation caused a 95% decrease in abundance of small reef fish at some invaded sites and a 65% decline in native fish biomass on heavily invaded reefs; concomitant cascading effects on reef food webs and benthic community structure, including altering balance of competition between native coral reef fish | [334–339] |
The first attempts to actually define, evaluate and compare measures of impact in a comprehensive manner started in the late 1990s, when recommendations were made on how the field of invasion biology might proceed in order to build a general framework for understanding and predicting impacts [340]. The first comprehensive ecological impact assessment study was conducted by Ruiz et al. [341], who analysed the reported ecological impacts of 196 species in the Chesapeake Bay through incorporation of various types of information (such as the impact type, information type and the effect of magnitude into the analysis). The more integrated impact evaluation framework–BINPAS (Biological Invasion Impact / Biopollution Assessment System)–to translate the existing data on invasive alien species impacts into uniform biopollution measurement units was developed in the 2000s [342]. Perhaps the most comprehensive and inclusive, but very data-hungry NIS introduction consequence (impacts) matrix has been developed by Hewitt et al. [343], where impacts are assessed against eleven value sets (habitat and habitat forming species, biodiversity, trophic interactions, nationally important and ecologically valuable species, assets (places) of environmental significance, economic values, social values, cultural values, national image (iconic places or species), aesthetic values and human health at a 5-grade level (from negligible to very low to extreme). During the last decade, a few additional impact evaluation frameworks, with inclusion of both qualitative and quantitative data, and ecological and socioeconomic information, were proposed (e.g., [344–349]). However, and despite pilot evaluations, none of them have proven so far robust enough to be able to reach the status of wide cross-regional applications in the marine realm.
Known/unknown/unknowable–some long-standing dilemmas
Implications of overlooked invasions
If between 1500 and 1800 only three marine species a year were successfully introduced but undetected as such around the world, “then nearly 1,000 coastal species of marine organisms that are now regarded as naturally cosmopolitan are in fact simply early introductions” [350]. These were referred to as the “Missing 1000” [351]. The estimate may be far too low, given that international shipping had commenced within ocean basins more than 2000 years ago and that more than 200 years have passed since 1800. Overlooked invasions may have profoundly altered the structure and function of pre-existing marine communities, which have long been studied as if they resulted from long term evolutionary processes. This phenomenon was referred to as “ecological mirages: illusions that have seriously hampered our ability to recognize the nature of pre-existing native ecosystems” [34]. Some examples include the wood-boring isopod Sphaeroma terebrans, and the stoloniferous fouling bryozoan Amathia verticillata. The isopod S. terebrans was transported by ships prior to the 1860s from the Indian Ocean to the Western Atlantic, where it altered the mangrove forest communities over a vast area, and yet their remarkable ecological consequences have been rarely noted [352]. The bryozoan Amathia verticillata (“zoobotryon”) occurs worldwide in tropical and warm-temperate waters, mostly in ports and marinas, or anthropogenically altered areas such as shellfish farming bays and lagoons [353]. Although long considered native to the Mediterranean Sea, it may be native to the Caribbean Sea and introduced elsewhere [354].
Non-indigenous vs. cryptogenic species
Species that we are unable to determine as to whether they are native or non-indigenous are termed cryptogenic [93]. The failure in classification may be due to their early introduction/establishment, misinterpretation due to systematics (pseudoindigenous species, imperfect or low-resolution taxonomy); complex biogeographic and community histories (widespread intraoceanic and interoceanic corridor species, neritic species with presumptive oceanic dispersal); or sampling (unexplored or little known habitats or communities, small population sizes) [12]. Even widely distributed, seemingly well-known species are prone to these issues. For example, the mussel Mytilus galloprovincialis, native to the Mediterranean Sea, was mistakenly re-described as a native species following introduction (e.g., re-described as M. diegensis in California, and M. planulatus in Australia [14]). Similarly, the "endangered" European seaslug Corambe batava was eventually recognized as the common American seaslug C. obscura, but only 125 years after it had been described [12]. Resolution of cryptogenic status [355,356] relies greatly on data availability and molecular tools, and is therefore a subject for continuous improvement and change. Similarly, the sea squirt Ciona intestinalis was recently recognized as comprising two species, both introduced elsewhere, one widely [357–359].
Certainty in introduction pathways
Vectors of introduction are known with high certainty only for a selected group of NIS (i.e., documented deliberate introductions, or where linkage between donor/regional regions, life history, and historical records point to a sole possible vector). Establishing the vector of introduction for the majority of NIS is still largely a matter of inference rather than evidence. Vectors are deduced from biological and ecological traits of the species, the habitats they occupy in the native and introduced range, the timing of first record, e.g. before or after the advent of ballast water use (see Modern shipping), relative to regional trade patterns and vector activity, e.g. mariculture or shipping [9,356,360]. Nevertheless, many NIS display traits and habitat preferences that may give a good reason to expect association with multiple vectors, e.g. NIS commonly found in harbours may have been introduced by ships in fouling or in ballast [361]. The compilation of regional inventories of marine NIS in the 1990s supplied the impetus for discussion of vectors. Carlton and Ruiz [362] provided terminology (polyvectic, cryptovectic) and a conceptual framework for marine bioinvasion vectors that distinguished cause, route, and vector for an invasion, as well as a vector's tempo, biota and strength.
Despite a burgeoning interest in invasion science in the last 25 years, a surprising number of gaps exists in our knowledge and understanding of how vectors operate. It is widely accepted that “the detailed invasion history of most species, which may include multiple introductions via multiple pathways, will never be known with absolute certainty” [360]. Over the past 20 years, designation of vector probability has been discussed (see Table 7 for a classification and examples). Most authors prefer the multiple vectors scheme, which allows for a range of possible introduction scenarios, and can be weighted depending on probability/certainty, or simply accorded equal value (as in most literature). No consensus has been reached on the optimal strategy to deal with the vexing issue of vector uncertainty.
Table 7. Schemes describing vector uncertainty in marine bioinvasions.
| Scheme | Region | Reference |
|---|---|---|
| Single vector: each species assigned only to its most-likely vector | Britain | [363] |
| Baltic Sea | [364] | |
| Mediterranean Sea | [346] | |
| Multiple vectors (A): each species assigned to one or more distinct vectors, all equally probable | USA: California | [361,365] |
| South Africa | [366] | |
| Great Britain | [367] | |
| Malta | [368] | |
| Portugal | [369] | |
| Multiple vectors (B): each species assigned to one or more distinct vectors, each vector scored in accordance with its probability | Australia: Port Phillip Bay | [356] |
| Multiple vectors (C): each species assigned to one or more distinct vectors, each vector assigned certainty value | USA: Puget Sound | [202] |
| Baltic Sea | [370] | |
| Multiple vectors (D): each species assigned to one vector category; some categories are polyvectic (e.g. “Culture + Vessels”) | USA, Canada: northern California to British Columbia | [360] |
| Europe | [9] | |
| Mediterranean Sea | [183] |
Perceptions of marine bioinvasions
In interpreting historical processes, one is aware of the influence of the societal drivers underpinning human perceptions and actions and how these change over time. Introduction of marine NIS was not widely considered a potential threat until the early 1980s. Since then, increasing evidence of the impacts of marine NIS (see Impacts quantification) has helped raise public awareness and altered community perception of marine introductions, followed by a growing realization that the “shifting baselines” syndrome [10] applies to introductions as it does to fisheries. Although invasion scientists provide ever more evidences of socio-economic and ecological impacts of marine bioinvasions (which are context dependent, i.e., not all NIS manifest the same level of environmental, economic, societal and other impacts, and these may vary over time and space; further, some NIS with known ecological impacts may also be considered to impart ecological or socioeconomic advantage [371–373]), the discipline has elicited criticism and has been intensely disputed [374,375].
Public awareness and perceptions, driven by environmental, economic and social consequences of invasive NIS, can determine the level of support for policy and management actions used to control/manage (potentially) harmful NIS. Unlike terrestrial and inland aquatic bioinvasions, quantitative data or assessments for impacts for most marine NIS are scarce. This is a “catch-22” situation–the impacts for the vast majority of marine NIS remain unknown for want of funding, which depends on public support, which in turn is decided according to public concerns and priorities. “Unless impacts are conspicuous, induce direct economic cost, or impinge on human welfare, they fail to arouse public awareness” [270]. Indeed, media recently scanned for coverage of NIS introductions to the Mediterranean Sea, highlighted species considered human health hazards rather than those of high ecological risk [376].
Despite evidence of major irreversible ecological impacts by many NIS and some shift in societal perceptions, NIS are not yet at the forefront in marine management. An online survey of more than 10,000 respondents from 10 European nations examined “the public’s informedness and concern regarding marine impacts …and priorities for policy and funding” revealed that respondents were the least informed on NIS issues and prioritized marine invasive species at the bottom of research funding needs [377]. The same attitude is apparent even amongst marine conservationists. A recent literature review found that biological invasions are being widely disregarded when planning for conservation in the marine environment; of 119 articles on marine spatial plans in the Mediterranean Sea, only three (2.5%) explicitly took NIS and marine bioinvasions into account [378], even in the NIS-beset Levantine Basin [379].
Policy and legislation: Honored in the breach
Global policy and legislation
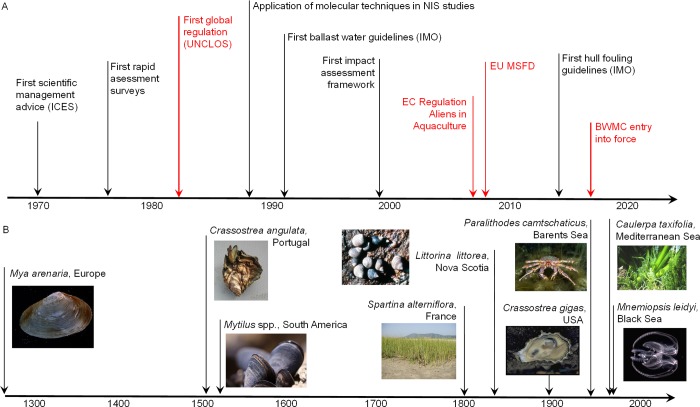
As NIS are often introduced or spread by global transport and trade and just as often have transboundary impacts, their prevention and management is an international issue requiring global policy. To date, only two global instruments are strictly legally binding.
The United Nations Convention on the Law of the Sea (UNCLOS) is the first global legally binding legislation to deliver a clear message: “States shall take all measures necessary to prevent, reduce and control … the intentional or accidental introduction of species, alien or new, to a particular part of the marine environment, which may cause significant and harmful changes thereto.” [380]. Considering the negative environmental effects of intentional and unintentional introductions into the marine environment, the uncertainty as to which of the present and continually introduced NIS will have an impact and at what scale, the unfeasibility of eradication and restoration and vectors’ build-up (e.g., commercial and recreational maritime transport, mariculture, canals), one would expect decision makers to follow UNCLOS and adopt a preventive and precautionary, if not environmentally-focused approach. Disappointingly, examination of policy and legislation actions reveals reactive, piecemeal development, often following disastrous and costly NIS outbreaks.
Article 8(h) of the Convention on Biological Diversity (CBD) requires Parties, as possible and as appropriate “to prevent the introduction of, control or eradicate those alien species which threaten ecosystems, habitats or species” [381]. A decade after the adoption of the CBD, noting “… that there are certain gaps and inconsistencies in the international regulatory framework from the perspective of the threats of invasive alien species to biological diversity”, the Conference of the Parties adopted the ‘Guiding Principles for the Prevention, Introduction, and Mitigation of Impacts of Alien Species That Threaten Ecosystems, Habitats, or Species’ and urged the development of national and regional invasive species strategies and action plans [382]. The revised Strategic Plan for 2011–2020 adopted by the CBD in 2010, supported by 20 “Aichi Biodiversity Targets”, states “By 2020, invasive alien species and pathways are identified and prioritized, priority species are controlled or eradicated, and measures are in place to manage pathways to prevent their introduction and establishment.” [383]. 2020 will now pass without these targets achieved, and they remain a major challenge.
After establishing the Working Group on the Introduction and Transfers of Marine Organisms (WGITMO), the International Council for the Exploration of the Sea (ICES) adopted the first version of what was to become an internationally recognized Code of Practice on the movement and translocation of non-native species for fisheries enhancement and mariculture purposes. The Code contained two recommended procedures: i) for all species prior to reaching a decision regarding new introductions, and ii) for introductions or transfers which are part of current commercial practice [384]. The Code of Conduct for Responsible Fisheries, promulgated by the Food and Agriculture Organization (FAO) of the United Nations, based on ICES’ Code of Practice, includes recommendations concerning non-indigenous aquaculture species [385]. Article 9.3.1 urges “… efforts should be undertaken to minimize the harmful effects of introducing non-native species … especially where there is a significant potential for the spread of such non-native species … into waters under the jurisdiction of other States as well as waters under the jurisdiction of the State of origin. States should, whenever possible, promote steps to minimize adverse … effects of escaped farmed fish on wild stocks”. Although widely endorsed, few people report applying its principles [386]. Further recommendations as to management and disease surveillance and notification have developed into a comprehensive Aquatic Animal Health Code [387,388]. However, the legislation is primarily focused on the economic issues, by stating: “The principal aim of the International Aquatic Animal Health Code…. is to facilitate international trade in aquatic animals and aquatic animal products. The International Aquatic Animal Health Code… attempts to achieve this aim by providing detailed definitions of minimum health guarantees to be required of trading partners in order to avoid the risk of spreading aquatic animal diseases.” [387]. The industry’s precautionary principle does not extend to feral introduced shellfish and fish, nor the many non-pathogenic organisms introduced with the target species. In 2006, considerations and suggestions to be taken into account by decision makers and managers when using–or deciding on the use of–NIS for aquaculture purposes were developed under the IUCN umbrella [389].
In response to national concerns, the US Congress passed the “Non-indigenous Aquatic Nuisance Prevention and Control Act” in 1990, and the Commonwealth Government of Australia, Australian Quarantine and Inspection Service, introduced voluntary ballast water guidelines for ships entering Australian ports from overseas. The guidelines developed under both the US and Australian initiatives were adopted the next year by IMO’s Marine Environment Protection Committee (MEPC) as the “International Guidelines for preventing the introduction of unwanted aquatic organisms and pathogens from ships' ballast water and sediment discharges” [390], and adopted by the IMO Assembly in 1993 [391]. A few years later, “Guidelines for Control and Management of Ships’ Ballast Water to Minimize the Transfer of Harmful Aquatic Organisms and Pathogens” were published [392]. The second legally binding instrument is the “International Convention for the Control and Management of Ships' Ballast Water and Sediments” (BWMC), which is directed at managing the discharge of ballast water and sediments through ballast water exchange and treatment [393]. The BWMC sets a global standard for the minimum amount and size of organisms permissible in ballast water discharged by ships. The BWMC formally entered into force in September 2017. However, on July 2017, the MEPC accepted an amended implementation scheme for ships to comply with the D-2 biological standard and set new schedules for ship owners to meet the requirements for ballast water treatment, in some cases delaying by two years the deadlines for installing those systems on ships already in operation. The deadline for mandatory installation of an approved BWMC system is now, in some cases, as late as 2024, twenty years after adoption of BWMC [394].
Vessel biofouling, a major vector in the translocation of NIS (see Modern shipping), was for many decades, and still is, held in partial check by the application of toxic paints. In 2001 IMO adopted the “International Convention on the Control of Harmful Anti-fouling Systems on Ships” [395], which entered into force in 2008, following studies that attributed the failure of some oyster culture operations and severe pathological conditions in some marine organisms to leachate from antifouling paints, particularly tributyltins (TBT). Concerns over the surge of vessel biofouling moved IMO to adopt voluntary “Guidelines for the control and management of ships' biofouling to minimize the transfer of invasive aquatic species” [396], followed by approving “Guidelines for the control and management of ships’ biofouling to minimize the transfer of invasive aquatic species” [397].
European Union policy and legislation
The European Union (EU) has a substantial body of environmental laws. Its biodiversity legislation, most notably the Habitats Directive, forms the cornerstone of Europe's nature conservation policy [398]. Article 22(b) states that in implementing the provisions of this Directive, Member States shall “ensure that the deliberate introduction into the wild of any species which is not native to their territory is regulated so as not to prejudice natural habitats within their natural range or the wild native fauna and flora” [398].
The “Convention on the Conservation of European Wildlife and Natural Habitats” (Bern Convention) requires Contracting Parties “to strictly control the introduction of non-native species” [399]. In 1984 the Committee of Ministers concerning the introduction of non-native species recommended that “the governments of the member states prohibit the introduction of non-native species into the natural environment” (with exceptions following risk assessment), “take the necessary steps to prevent as far as possible the accidental introduction of non-native species, and inform governments of neighboring countries concerned of introduction schemes or accidental introductions” [400]. However, with the single exception of controlling proliferation of C. taxifolia in the Mediterranean Sea, these recommendations concern terrestrial and inland waters [401].
Some preventive measures to curb introductions of NIS with cultured organisms were initiated in Europe (France) as early as the 1930s, with a state decree limiting oyster transfer due to the concomitant occurrence of the American slipper limpet Crepidula fornicata [402]. Also, brood stock of C. gigas imported to France from Canada and Japan underwent in the 1970s at the customs clearance “histological analysis, presence of predators and commensal species… spat were immersed in freshwater to destroy fouling organisms and predators” [403]; despite this, the authors acknowledge that a long list of “concomitant exotic species” were still introduced. Building on the ICES Code of Practice (see 7.1), the European Community (EC) adopted in 2007 a regulation concerning use of alien and locally absent species in aquaculture, reasoning that as “Aquaculture is a fast-growing sector… it is important for the aquaculture industry to diversify the species reared” [404]. The policy objective, developed to control new intentional introductions “… is to optimise benefits associated with introductions and translocations while at the same time avoiding alterations to ecosystems, preventing negative biological interaction, including genetic change, with indigenous populations and restricting the spread of non-target species and detrimental impacts on natural habitats.” [404]. Yet records of culture-transported NIS established in the wild–including macrophytes, molluscs, crustaceans–continue unabated [405–410].
The EU Marine Strategy Framework Directive (MSFD) aims to protect the marine environment by achieving “Good Environmental Status” (GES) in European Seas by 2020 [5,411], a target again no longer feasible. It comprises an explicit regulatory objective “Descriptor 2: Non-indigenous species introduced by human activities are at levels that do not adversely alter the ecosystems.” A recent report assessing Member States’ monitoring programmes found low adequacy and compliance for Descriptor 2, as only 5% of the monitoring activities were linked with NIS, and warned that “Monitoring programmes for NIS will require a clear acceleration to ensure proper coverage given the MSFD deadlines for the update of marine strategies by 2018, and achieving GES by 2020” [412]. A later, even less sanguine document “…highlighted that more efforts were urgently needed if Member States are to reach good environmental status by 2020. The results showed the necessity to significantly improve the quality and coherence of the determination of good environmental status by the Member States. In addition, the assessment recognised that regional cooperation must be at the very heart of the implementation of Directive 2008/56/EC. It also emphasised the need for Member States to more systematically build upon standards stemming from Union legislation or, where they do not exist, upon standards set by Regional Sea Conventions or other international agreements” [412].
Recognizing that “…the ecological, economic and social consequences of IS [invasive species] in the EU are significant and require a coordinated response” the EC made in 2008 a formal commitment to develop an EU Strategy on Invasive Alien Species [413]. The EU biodiversity strategy, initially conceived as being achieved by 2020 comprises six targets, one of which relates to invasive alien species (IAS), undertaken to “… fill policy gaps in combating IAS by developing a dedicated legislative instrument by 2012” [414]. The legally binding instrument, EU Regulation on the prevention and management of the introduction and spread of invasive alien species, was adopted in 2014 [415]. The regulation imposes restrictions on a list of invasive alien species known as “species of Union concern”. However, the criteria for their selection pose a conundrum: the species shall only be included on the Union list if “they are … likely to have a significant adverse impact on biodiversity or the related ecosystem services, and may also have an adverse impact on human health or the economy”, and if risk assessment described their “…adverse impact on biodiversity and related ecosystem services, including on native species, protected sites, endangered habitats, as well as on human health, safety, and the economy including an assessment of the potential future impact having regard to available scientific knowledge” [415]. Yet, it is compulsory “…that the inclusion on the Union list will effectively prevent, minimise or mitigate their adverse impact” [415]. Since existing data on marine NIS impacts are scarce, by the time the requisite information is assembled, a given species may have spread and colonized a larger area and thus successful removal, control or containment will likely prove futile [270]. Indeed, only a single estuarine/marine species, the crab Eriocheir sinensis, is included in the ‘List of Alien Invasive Species of Union concern’ [144].
Other regions
In other international, regional-level responses (besides the EU; see Table 8), NIS were considered only marginally without clear demand for actions or appropriate follow-up mechanisms, resulting in a lack of efficacious actions [416–421]. There are several national-level responses, including those in the Canada and US, and Australia and New Zealand [213,422–425], which are largely consistent with those of the IMO (as above) and carry separate enforcement and some cross-border coordination.
Table 8. Selected management responses to non-indigenous marine species, by international organizations, in chronological order of response.
| Organisation | Established | Management response | Action, reference |
|---|---|---|---|
| International Council for the Exploration of the Sea (ICES) | 1902 | 1969 (Working Group of Non-indigenous Marine Organisms established) | Code of Practice to reduce the risks of adverse effects arising from introduction of non-indigenous marine species [384] |
| The Convention on Conservation of Nature in the South Pacific | 1976 (entry into force 1990) | 1976 | Apia Convention [416] |
| United Nations | 1982 | 1982 | Convention on the Law of the Sea (UNCLOS) [380] |
| Barcelona Convention | 1976 | 1982 | Protocol Concerning Mediterranean Specially Protected Areas and Biological Diversity in the Mediterranean [417] |
| Nairobi Convention | 1985 (entry into force 1996) | 1985 | Protocol Concerning Protected Areas and Wild Fauna and Flora in the Eastern African Region [418] |
| Caribbean Regional Coordinating Unit | 1981 | 1990 (entry into force 2000) | Protocol Concerning Protected Areas and Wildlife in the wider Caribbean Region [419] |
| The Antarctic Treaty | 1959 (entry into force 1961) | 1991 (entry into force 1998) | Protocol on Environmental Protection to the Antarctic Treaty, [420] |
| International Maritime Organisation (IMO) | 1948 | 1991 | Guidelines for preventing the introduction of unwanted aquatic organisms and pathogens from ships' ballast water and sediment discharges [390] |
| Convention on Biological Diversity (CBD) | 1992 | 1992 | [381] |
| IMO | 1948 | 2004 (entry into force 2017, but see text) | Convention for the Control and Management of Ships’ Ballast Water and Sediments [393] |
| United Nations Environmental Programme (UNEP) | 1972 | 2005 | Action Plan concerning species introductions and invasive species in the Mediterranean Sea [426] |
| International Union for Conservation of Nature (IUCN) | 1948 | 2006 | Alien Species in Aquaculture: Considerations for responsible use [389] |
| Baltic Sea Environmental Protection Commission (HELCOM) | 1974 | 2007 | Baltic Sea Action Plan [427] |
| European Commission (EC) | 1992 | 2007 | Regulation concerning use of alien and locally absent species in aquaculture [403] |
| EC | 1992 | 2008 | Marine Strategy Framework Directive [5] |
| Oslo and Paris Commission (OSPAR) | 1972 | 2008 | The General Guidance on the Voluntary Interim application of the D1 Ballast Water Exchange Standard [428] |
| UNEP | 1972 | 2008 | New strategic direction for the Coordinating Body on the Seas of East Asia COBSEA (2008–2012) [420] |
| IUCN | 1948 | 2009 (Invasive Species Specialist Group established 1993) | Marine Menace—Alien invasive species in the marine environment [429] |
| IMO | 1948 | 2011 | Guidelines for the control and management of ships' biofouling to minimize the transfer of invasive aquatic species [395] |
| EU | 1992 | 2014 | Regulation on invasive species [414] |
| Arctic Council | 1996 | 2017 | Arctic invasive alien species strategy and action plan [430] |
The future is now
Human perception of marine NIS introductions changed in the mid-20th century, following several conspicuous outbreaks that resulted in negative environmental, economic, and public health outcomes. Since then, the role of NIS in biodiversity, habitat and ecological community erosion and the loss of ecosystem services, together with increasing management costs, has been widely recognized. Historical milestones reveal that: i) some marine bioinvasions are millennia-old, ii) the drivers of marine introductions have greatly intensified, diversified and accelerated in recent decades, iii) NIS community baselines vary by region, taxa and time scale, iv) regulatory policies and instruments have been reactive and slow to evolve, attempting to address only a subset of vectors and factors that drive invasions, and v) most major introduction pathways lack legally binding, timely implemented, and strictly monitored instruments (see also Fig 4). Not surprisingly, therefore, the milestones of "2020" noted above for robust NIS action and management cannot now be realized.
Fig 4.
Milestones of management responses to marine bioinvasions, in red–legally binding instruments (panel A); key non-indigenous species introductions since 1200s (panel B). For details, see Global policy and legislation and Table 8. Photo credits: Jim Carlton, IFREMER (France), IMR (Norway), Lauri Laitila, Maiju Lehtiniemi and Pixabay.
It is our sincere conviction that protecting marine ecosystems from further disturbance and preventing socio-economic damages requires urgent changes and advances in the response to NIS, from global to local scales, to minimize invasion impacts. We call for the following key actions:
Recognize and acknowledge that effective marine ecosystem management must address both NIS introductions and their interactions with other human stressors (e.g., pollution, fisheries, physical degradation), given that the latter affect invasion dynamics and impacts.
Adopt management strategies at multiple spatial scales (as below) that consider the shifting global landscape of invasion risks, due to changing climate and human responses (e.g., changing trade routes/volumes and coastal infrastructure), affecting patterns of propagule delivery, likelihood of invasions, and consequences.
Create integrative and comprehensive legal instruments that control the transfer of species by the diverse range of existing and possible future vectors, in order to move beyond the current single vector approach that ignores the multi-vectic nature of both primary and secondary introductions.
Provide a robust legal base to enforce controls on species transfers by vectors at both international and regional or national levels. We suggest that the regional sea / large marine ecosystem (or similar) management bodies would be especially instrumental in the implementation of international obligations/legislative acts and the coordination/harmonisation of countries’ responsibilities.
Assess the performance of existing and new NIS legal instruments by documenting the rate of new introductions, secondary spread of established NIS populations, and the implementation (management and enforcement). Such performance measures should be a required component of legal instruments, to evaluate efficacy and whether modification (i.e., adaptive management) is needed to meet management objectives.
Without these critical steps to address conspicuous and existing gaps, invasions will remain a major force of change in coastal marine ecosystems, impacting many dimensions of ecosystem function and human society.
Acknowledgments
This work is dedicated to the memory of our late colleague and co-author Professor Susan L. Williams, who was tragically lost in a traffic accident on April 24, 2018. The authors thank the Working Group on Introductions and Transfers of Marine Organisms (WGITMO) of the International Council for the Exploration of the Sea (ICES) for facilitating this research.
Data Availability
All relevant data are within the paper with all data sources being listed in the References section.
Funding Statement
This article is based upon work from COST Action Oceans Past Platform (supported by COST project number IS1403; European Cooperation in Science and Technology). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lotze HK, Reise K, Worm B, van Beusekom J, Busch M, Ehlers A, et al. Human transformations of the Wadden Sea ecosystem through time: a synthesis. Helgol Mar Res. 2005;59(1): 84–95. 10.1007/s10152-004-0209-z [DOI] [Google Scholar]
- 2.Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D'Agrosa C, et al. A global map of human impact on marine ecosystems. Science. 2008;319(5865): 948–952. 10.1126/science.1149345 [DOI] [PubMed] [Google Scholar]
- 3.Costello MJ, Coll M, Danovaro R, Halpin P, Ojaveer H, Miloslavich P. A census of marine biodiversity knowledge, resources and future challenges. PLoS ONE. 2010;5(8): e12110 10.1371/journal.pone.0012110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Occhipinti Ambrogi A. Global change and marine communities: alien species and climate change. Mar Poll Bull. 2007;55(7–9): 342–352. 10.1016/j.marpolbul.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Directive 2008/56/EC of the European Parliament and the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). European Commission. Official Journal of the European Union. 2008;L164/19 (Jun 25, 2008).
- 6.Stelzenmüller V, Coll M, Mazaris AAD, Giakoumi S, Katsanevakis S, Portman ME, et al. A risk-based approach to cumulative effect assessments for marine management. Sci Total Environ. 2018;612: 1132–1140. 10.1016/j.scitotenv.2017.08.289 [DOI] [PubMed] [Google Scholar]
- 7.State of the Baltic Sea [internet]. First version of the ‘State of the Baltic Sea’ report–June 2017 –to be updated in 2018 [about 197 pages]. HELCOM [cited 2018 Mar 09]. Available from: http://stateofthebalticsea.helcom.fi
- 8.Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH. Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst. 2000;31: 481–531. 10.1146/annurev.ecolsys.31.1.481 [DOI] [Google Scholar]
- 9.Galil BS, Marchini A, Occhipinti-Ambrogi A, Minchin D, Narščius A, Ojaveer H, et al. International arrivals: widespread bioinvasions in European Seas. Ethol Ecol Evol. 2014;26(2–3): 152–171. 10.1080/03949370.2014.897651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol. 1995;10: 430 10.1016/S0169-5347(00)89171-5 [DOI] [PubMed] [Google Scholar]
- 11.Klein ES, Thurstan RH. Acknowledging long-term ecological change: The problem of shifting baselines In: Schwerdtner Máñez K, Poulsen B, editors. Perspectives on oceans past. Dordrecht: Springer; 2016. pp. 11–29. [Google Scholar]
- 12.Carlton JT. Deep invasion ecology and the assembly of communities in historical time In: Rilov G, Crooks JA, editors. Biological invasions in marine ecosystems. Berlin: Springer-Verlag; 2009. pp. 13–56. [Google Scholar]
- 13.Carlton JT. Patterns of transoceanic marine biological invasions in the Pacific Ocean. Bull Mar Sci. 1987;41: 452–465. [Google Scholar]
- 14.Carlton JT. Molluscan invasions in marine and estuarine communities. Malacologia. 1999;41(2): 439–454. [Google Scholar]
- 15.Carlton JT. Community assembly and historical biogeography in the North Atlantic Ocean: the potential role of human-mediated dispersal vectors. Hydrobiologia. 2003;503(1–3): 1–8. 10.1023/B:HYDR.0000008479.90581.e1 [DOI] [Google Scholar]
- 16.Carlton JT. The inviolate sea? Charles Elton and biological invasions in the world's oceans In: Richardson DM, editor. Fifty years of invasion ecology. The legacy of Charles Elton. Oxford (England): Wiley-Blackwell; 2011. pp. 25–33. [Google Scholar]
- 17.Roman J. Diluting the founder effect: cryptic invasions expand a marine invader’s range. Proc R Soc Lond B Biol Sci. 2006;273: 2453–2459. 10.1098/rspb.2006.3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blakeslee AMH, Kamakukra Y, Onufrey J, Makino W, Urabe J, Park S, et al. Reconstructing the invasion history of the Asian shorecrab, Hemigrapsus sanguineus (De Haan 1835) in the western Atlantic. Mar Biol. 2017;164: 47 10.1007/s00227-017-3069-1 [DOI] [Google Scholar]
- 19.Carlton JT, Chapman JW, Geller JB, Miller JA, Carlton DA, McCuller MI, et al. Tsunami-driven rafting: Transoceanic species dispersal and implications for marine biogeography. Science. 2017;357(6358): 1402–1406. 10.1126/science.aao1498 [DOI] [PubMed] [Google Scholar]
- 20.Casson L. Ships and seamanship in the ancient world 1st ed. Baltimore: Johns Hopkins University Press; 1971. [Google Scholar]
- 21.Woodman R. The history of the ship: The comprehensive story of seafaring from the earliest times to the present day 1st ed. London: Conway Maritime Press; 1997. [Google Scholar]
- 22.Barnett R, Yamaguchi N, Shapiro B, Sabin R. Ancient DNA analysis indicates the first English lions originated from North Africa. Contrib Zool. 2008;77: 7–16. [Google Scholar]
- 23.Sadler J. Beetles, boats and biogeography: insect invaders of the North Atlantic. Acta Archaeol. 1990;61: 199–211. [Google Scholar]
- 24.Buckland PC, Panagiotakopulu E, Sveinbjarnardottir G. A failed invader in the North Atlantic, the case of Aglenus brunneus Gyll. (Col., Colydiidae), a blind flightless beetle from Iceland. Biol Invasions. 2009;11(6): 1239–1245. 10.1007/s10530-008-9339-6 [DOI] [Google Scholar]
- 25.Petersen KS, Rasmussen KL, Heinemeler J, Rud N. Clams before Columbus? Nature. 1992;359(6397): 679 10.1038/359679a0 [DOI] [Google Scholar]
- 26.Natkiel R, Preston A 1986. Atlas of maritime history 1st ed. New York: Facts on File Inc.; 1986. [Google Scholar]
- 27.Cross ME, Bradley CR, Cross TF, Culloty S, Lynch S, McGinnity P, et al. Genetic evidence supports recolonisation by Mya arenaria of Western Europe from North America. Mar Ecol Prog Ser. 2016;549: 99–112. 10.3354/meps11672 [DOI] [Google Scholar]
- 28.Panova M, Blakeslee AMH, Miller AW, Makinen T, Ruiz GM, Johannesson K, et al. Glacial history of the North Atlantic marine snail, Littorina saxatilis, inferred from distribution of mitochondrial DNA lineages. PLoS ONE. 2011;6(3): e17511 10.1371/journal.pone.0017511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman JW, Blakeslee AMH, Carlton JT, Bellinger MR. Parsimony dictates a human introduction: on the use of genetic and other data to distinguish between the natural and human-mediated invasion of the European snail Littorina littorea in North America. Biol Invasions. 2008;10(2): 131–133. 10.1007/s10530-007-9115-z [DOI] [Google Scholar]
- 30.Audet D, Miron G, Moriyasu M. Biological characteristics of a newly established green crab (Carcinus maenas) population in the southern Gulf of St. Lawrence, Canada. J Shellfish Res. 2008;27: 427–441. 10.2983/0730-8000(2008)27[427:BCOANE]2.0.CO;2 [DOI] [Google Scholar]
- 31.Edgell T, Hollander J 2011. The evolutionary ecology of European green crab, Carcinus maenas, in North America In: Galil BS, Clark PF, Carlton JT, editors. In the wrong place: alien marine crustaceans—distribution, biology and impacts. Dordrecht: Springer; 2011. pp. 641–659. [Google Scholar]
- 32.Carlton JT, Ruiz GM. The magnitude and consequences of bioinvasions in marine ecosystems: implications for conservation biology In: Norse EA, Crowder LB, editors. Marine conservation biology: The science of maintaining the sea's biodiversity. Washington DC: Island Press; 2005. pp. 123–148. [Google Scholar]
- 33.Kooistra WHCF, Verbruggen H. Genetic patterns in the calcified tropical seaweeds Halimeda opuntia, H. distorta, H. hederacea, and H. minima (Bryopsidales, Chlorophyta) provide insights in species boundaries and interoceanic dispersal. J Phycol. 2005;41: 177–187. 10.1111/j.1529-8817.2005.04095.x [DOI] [Google Scholar]
- 34.Bortolus A, Carlton JT, Schwindt E. Reimagining South American coasts: unveiling the hidden invasion history of an iconic ecological engineer. Divers Distrib. 2015;21(11): 1267–1283. 10.1111/ddi.12377 [DOI] [Google Scholar]
- 35.Lindroth CH. The faunal connections between Europe and North America 1st ed. New York: John Wiley and Sons Inc.; 1957. [Google Scholar]
- 36.Carlton JT, Hodder J. Biogeography and dispersal of coastal marine organisms: experimental studies on a replica of a 16th-century sailing vessel. Mar Biol. 1995;121: 721–730. 10.1007/BF00349308 [DOI] [Google Scholar]
- 37.Carlton JT. The scale and ecological consequences of biological invasions in the world’s oceans In: Sandlund OT, Schei PJ, Viken Å, editors. Invasive species and biodiversity management. Dordrecht: Kluwer Academic Publishers; 1999. pp. 195–212. [Google Scholar]
- 38.Mack RN. Global plant dispersal, naturalization, and invasion: pathways, modes, and circumstances In: Ruiz GM, Carlton JT, editors. Invasive species. Vectors and management strategies. Washington: Island Press; 2003. pp. 3–30. [Google Scholar]
- 39.Blakeslee AMH, Byers JE, Lesser MP. Solving cryptogenic histories using host and parasite molecular genetics: the resolution of Littorina littorea's North American origin. Mol Ecol. 2008;17: 3684–3696. 10.1111/j.1365-294X.2008.03865.x [DOI] [PubMed] [Google Scholar]
- 40.Brawley SH, Coyer JA, Blakeslee AMH, Hoarau G, Johnson LE, Byers JE, et al. Historical invasions of the intertidal zone of Atlantic North America associated with distinctive patterns of trade and emigration. Proc Natl Acad Sci U S A. 2009;106: 8239–8244. 10.1073/pnas.0812300106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlton JT. Invertebrates, Marine In: Simberloff D, Rejmanek M, editors. Encyclopedia of biological invasions. Berkeley: University of California Press, 2011. pp. 385–390. [Google Scholar]
- 42.Carlton JT, Cohen AN. Episodic global dispersal in shallow water marine organisms: the case history of the European shore crabs Carcinus maenas and Carcinus aestuarii. J Biogeogr. 2003;30: 1809–1820. 10.1111/j.1365-2699.2003.00962.x [DOI] [Google Scholar]
- 43.Encyclopedia Britannica [internet]. History of ships [about 22 pages]. [cited 2018 Mar 09] Available from: https://www.britannica.com/technology/ship/History-of-ships
- 44.Talley WK. Ocean container shipping: impacts of a technological improvement. J Econ Issues. 2000;34, 933–948. 10.1080/00213624.2000.11506322 [DOI] [Google Scholar]
- 45.Barki D, Deleze-Black L. Review of maritime transport New York and Geneva: United Nations Publication; 2016. UNCTAD/RMT/2016, ISBN 978-92-1-112904-5. [Google Scholar]
- 46.Fremont A. Global maritime networks: The case of Maersk. J Transp Geogr. 2007;15(6): 431–442. 10.1016/j.jtrangeo.2007.01.005 [DOI] [Google Scholar]
- 47.Seebens H, Schwartz N, Schupp PJ, Blasius B. Predicting the spread of marine species introduced by global shipping. Proc Natl Acad Sci U S A. 2016;113(20): 5646–5651. 10.1073/pnas.1524427113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamelander J, Riddering L, Haag F, Matheickal J. Guidelines for development of a national ballast water management strategy GloBallast Monograph Series No. 18 1st Ed. London and Gland: GloBallast Partnerships & IUCN; 2010. [Google Scholar]
- 49.Howarth RS. The presence and implication of foreign organisms in ship ballast waters discharged into the Great Lakes Georgetown: Bio-Environmental Services Ltd; 1981. [Google Scholar]
- 50.Hewitt CL, Gollasch S, Minchin D. The vessel as a vector—biofouling, ballast water and sediments In: Rilov G, Crooks JA, editors. Biological invasions in marine ecosystems: ecological, management, and geographic perspectives. Berlin Heidelberg: Springer-Verlag; 2009. pp. 117–131. [Google Scholar]
- 51.Coutts ADM. Slow-moving barge introduces biosecurity risk to the Marlborough Sounds, New Zealand. In: Godwin LS, editor. Hull fouling as a mechanism for marine invasive species introductions. Proceedings of a workshop on current issues and potential management strategies; 2003 Feb 12–13; Honolulu, Hawaii. Honolulu: Hawaii Department of Agriculture; 2005. pp. 29–36.
- 52.Coutts ADM, Piola RF, Taylor MD, Hewitt CL, Gardner JPA. The effect of vessel speed on the survivorship of biofouling organisms at different hull locations. Biofouling 2010;26(5): 539–553. 10.1080/08927014.2010.492469 [DOI] [PubMed] [Google Scholar]
- 53.Simard N, Pelletier-Rousseau M, Clarke Murray C, McKindsey CW, Therriault TW, Lacoursière-Roussel A, et al. National risk assessment of recreational boating as a vector for marine nonindigenous species Research Document 2017/006. Ottawa: Canadian Science Advisory Secretariate; 2017. Available from: http://waves-vagues.dfo-mpo.gc.ca/Library/40601699.pdf. [Google Scholar]
- 54.Koplovitz G, Shmue Y, Shenkar N. Floating docks in tropical environments—a reservoir for the opportunistic ascidian Herdmania momus. Manag Biol Invasion. 2016;7(1): 43–50. 10.3391/mbi.2016.7.1.06 [DOI] [Google Scholar]
- 55.Oguz T, Fach B, Salihoglu B. Invasion dynamics of the alien ctenophore Mnemiopsis leidyi and its impact on anchovy collapse in the Black Sea. J Plankton Res. 2008; 30(12): 1385–1397. 10.1093/plankt/fbn094 [DOI] [Google Scholar]
- 56.Nalepa TF, Schloesser DW. Quagga and zebra mussels: biology, impacts, and control 2nd ed. Boca Raton: CRC Press; 2013. [Google Scholar]
- 57.Hallegraeff GM. Harmful algal blooms in the Australian region. Mar Pollut Bull. 1992;25(5–8): 186–190. 10.1016/0025-326X(92)90223-S [DOI] [Google Scholar]
- 58.Floerl O, Inglis GJ. Starting the invasion pathway: the interaction between source populations and human transport vectors. Biol Invasions. 2015;7(4): 589–606. 10.1007/s10530-004-0952-8 [DOI] [Google Scholar]
- 59.Davidson IC, Zabin CJ, Chang AL, Brown CW, Sytsma MD, Ruiz GM. Recreational boats as potential vectors of marine organisms at an invasion hotspot. Aquat Biol. 2010;11: 179–191. 10.3354/ab00302 [DOI] [Google Scholar]
- 60.Clarke Murray C, Pakhomov EA, Therriault TW. Recreational boating: a large unregulated vector transporting marine invasive species. Divers Distrib. 12011;7(6): 1161–1172. 10.1111/j.1472-4642.2011.00798.x [DOI] [Google Scholar]
- 61.Ashton G, Davidson I, Ruiz G. Transient small boats as a long-distance coastal vector for dispersal of biofouling organisms. Estuaries Coast. 2014;37(6): 1572–1581. 10.1007/s12237-014-9782-9 [DOI] [Google Scholar]
- 62.Zabin C, Ashton GV, Brown CW, Davidson IC, Sytsma MD, Ruiz GM. Small boats provide connectivity for nonindigenous marine species between a highly invaded international port and nearby coastal harbors. Manag Biol Invasion. 2014;5: 97–112. 10.3391/mbi.2014.5.2.03 [DOI] [Google Scholar]
- 63.Boating Industry [internet]. 85 years of boating history [about 7 screens]. [cited 2018 Mar 09]. Available from: http://boatingindustry.com/top-stories/2014/06/12/85-years-of-boating-history/
- 64.National Marine Manufacturers Association [internet]. NMMA releases 2010 U.S. recreational boat registration statistics report [about 2 screens]. [cited 2017 Jul 15]. Available from: http://www.nmma.org/press/article/18028
- 65.Minchin D, Floerl O, Savini D, Occhipinti-Ambrogi A. Small craft and the spread of exotic species In: Davenport L L., Davenport J, editors. The ecology of transportation: managing mobility for the environment. Berlin: Springer-Verlag; 2006. pp. 99–118. [Google Scholar]
- 66.Cappato A. Cruises and recreational boating in the Mediterranean Sophia Antipolis: Plan Bleu/UNEP MAP Regional Activity Centre; 2011. [Google Scholar]
- 67.Fletcher LM, Zaiko A, Atalah J, Richter I, Dufour CM, Pochon X, et al. Bilge water as a vector for the spread of marine pests: a morphological, metabarcoding and experimental assessment. Biol Invasions. 2017;19(10): 2851–2867. 10.1007/s10530-017-1489-y [DOI] [Google Scholar]
- 68.Ferrario J, Caronni S, Occhipinti-Ambrogi A, Marchini A. Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species. Biofouling. 2017;33(8): 651–660. 10.1080/08927014.2017.1351958 [DOI] [PubMed] [Google Scholar]
- 69.Clarke Murray C, Gartner H, Gregr EJ, Chan K, Pakhomov E, Therriault TW. Spatial distribution of marine invasive species: environmental, demographic and vector drivers. Divers Distrib. 2014;20(7): 824–836. 10.1111/ddi.12215 [DOI] [Google Scholar]
- 70.Bax N, Hewitt C, Campbell M, Thresher R. Man-made marinas as sheltered islands for alien marine organisms: Establishment and eradication of an alien invasive marine species In: Veitch CR, Clout MN, editors. Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group. Gland Switzerland and Cambridge, UK: IUCN; 2002. pp. 26–39. [Google Scholar]
- 71.Ashton G, Zabin C, Davidson I, Ruiz G. Aquatic invasive species vector risk assessments: Recreational vessels as vectors for non-native marine species in California. Final Report. Portland: The Aquatic Bioinvasion Research & Policy Institute; 2012. [Google Scholar]
- 72.Bell JD, Bartley DM, Lorenzen K, Loneragan NR. Restocking and stock enhancement of coastal fisheries: Potential, problems and progress. Fish Res. 2006;80(1): 1–8. 10.1016/j.fishres.2006.03.008 [DOI] [Google Scholar]
- 73.Lorenzen K. Understanding and managing enhancement fisheries systems. Rev Fish Sci. 2008;16: 10–23. 10.1080/10641260701790291 [DOI] [Google Scholar]
- 74.Günther R. The oyster culture of the ancient Romans. J Mar Biol Assoc UK. 1897;4(4): 360–365. 10.1017/S0025315400005488 [DOI] [Google Scholar]
- 75.Pliny, IX, [cited 2018 Mar 09] Available from: https://www.loebclassics.com/view/pliny_elder-natural_history/1938/pb_LCL353.279.xml
- 76.Kristensen PS. Oyster and mussel fisheries in Denmark. In: MacKenzie CLJr, Burrell VGJr, Rosenfield A, Hobart WL, editors. The history, present conditions, and future of the molluscan fisheries of north and central America and Europe: Volume 3, Europe. Seattle: NOAA Technical Report NMFS 129; 1997. pp. 25–47.
- 77.Hinard G, Lambert L. Tableau de l'ostréiculture française (deuxième partie). Rev Trav Off Pêches Marit. 1928;1(4): 61–127. French. [Google Scholar]
- 78.Lambert L. Note complémentaire sur le clam Venus mercenaria. Rev Trav Off Pêches Marit. 1947–1949;15(1–4): 118–122. French. [Google Scholar]
- 79.Le Borgne M, Gras MP, Comps M, Carruesco G, Razet D. Observations sur la reproduction des huîtres dans la Seudre (Bassin de Marennes-Oléron) en 1972. ICES. 1973;C.M.1973/K:16: 1–5. French. Available from: http://archimer.ifremer.fr/doc/00009/12006/8698.pdf
- 80.Global Aquaculture Production, FAO. [cited 2017 Jul 17]. Available from: http://www.fao.org/fishery/statistics/global-aquaculture-production/en
- 81.MacKenzie CL Jr, Burrell VG Jr, Rosenfield A, Hobart WL. The history, present condition and future of the molluscan fisheries of North, and Central America and Europe. Volume 2, Pacific coast and supplemental topics. Seattle: NOAA Technical Report NMMFS, 128; 1997.
- 82.Goulletquer P, Heral M. Marine molluscan production trends in France: from fisheries to aquaculture. In: MacKenzie CLJr, Burrell VGJr, Rosenfield A, Hobart WL, editors. The history, present conditions, and future of the molluscan fisheries of north and central America and Europe: Volume 3, Europe. Seattle: NOAA Technical Report NMFS 129; 1997. pp. 137–164.
- 83.Chew KK. Global bivalve shellfish introductions. World Aquacult. 1990;21: 9–21. [Google Scholar]
- 84.Lindsay C, Simons D. The Fisheries for Olympia oysters, Ostreola canchaphila; Pacific oysters, Crassostrea gigas; and Pacific razor clams, Siliqua patula, in the State of Washington. In: MacKenzie CL Jr, Burrell VG Jr, Rosenfield A, Hobart WL, editors. The history, present condition and future of the molluscan fisheries of North, and Central America and Europe. Volume 2, Pacific coast and supplemental topics. Seattle: NOAA Technical Report NMMFS, 128; 1997. pp. 89–113.
- 85.Quayle DB. Distribution of introduced marine mollusca in British Columbia Waters. J Fish Res Board Can. 1964;21: 1155–1181. 10.1139/f64-102 [DOI] [Google Scholar]
- 86.Ayres P. Introduction of Pacific oysters in Australia In: Leffler M, Greer J, editors. The ecology of Crassostrea gigas in Australia, New Zealand, France and Washington State. College Park: Maryland Sea Grant College; 1991. pp. 3–8. [Google Scholar]
- 87.FAO [internet]. Database on Introduced Aquatic Species (DIAS). [cited 2017 Jul 10]. Available from: http://www.fao.org/fishery/topic/14786/en
- 88.Haupt TM, Griffiths CL, Robinson TB, Tonin AFG, De Bruin PA. The history and status of oyster exploitation and culture in South Africa. J Shellfish Res. 2010;29(1): 151–159. 10.2983/035.029.0109 [DOI] [Google Scholar]
- 89.Fitzgerald WJ. Aquaculture development plan for the territory of Guam Guam: Government of Guam, Department of Commerce; 1982. [Google Scholar]
- 90.Orensanz JM, Schwindt E, Pastorino G, Bortolus A, Casas G, Darrigran G, et al. No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biol Invasions. 2002;4: 115–143. 10.1023/A:1020596916153 [DOI] [Google Scholar]
- 91.AquaNIS [internet]. Information system on aquatic non-indigenous and cryptogenic species. Version 2.36+. [cited 2017 Oct 7]. Available from: www.corpi.ku.lt/databases/aquanis
- 92.Briggs M, Funge-Smith S, Subasinghe R, Phillips M. Introductions and movement of Penaeus vannamei and Penaeus stylirostris in Asia and the Pacific. Bangkok: FAO; 2004. RAP Publication 2004/10. [Google Scholar]
- 93.Carlton JT. Marine bioinvasions: the alteration of marine ecosystems by nonindigenous species. Oceanography. 1996;9(1): 36–43. [Google Scholar]
- 94.Food and Agriculture Organization of the United Nations [internet]. Fisheries and Aquaculture Department. National aquaculture sector overview–Cuba. [about 14 screens]. [cited 2018 Mar 09]. Available from: http://www.fao.org/fishery/countrysector/naso_cuba/en
- 95.Weimin M. Status of aquaculture of Penaeus vannamei in China In: Sulit VT, Aldon MET, Tendencia IT, Ortiz AMJ, Alayon SB, Ledesma AS, editors. Regional technical consultation on the aquaculture of P. vannamei and other exotic shrimps in southeast Asia, Manila, Philippines. Manila: SEAFDEC Aquaculture Department; 2005. pp. 84–91. [Google Scholar]
- 96.Funge-Smith S, Briggs M. 2005 The introduction of Penaeus vannamei and P stylirostris into the Asia-Pacific region. In: Bartley DM, Bhujel RC, Funge-Smith S, Olin PG, Phillips MJ, editors. International mechanisms for the control and responsible use of alien species in aquatic ecosystems. Report of an Ad Hoc Expert Consultation; 2003 Aug 27–30; Xishuangbanna, People's Republic of China. Rome: FAO; 2005. pp. 149–168. Available from: http://www.fao.org/docrep/009/a0113e/a0113e00.htm [Google Scholar]
- 97.Shean R. 2011. Venerupis philippinarum, Japanese littleneck clam. [internet]. FISH 423: Aquatic invasion ecology. [about 14 pages]. [cited 2018 Mar 09]. Available from: http://depts.washington.edu/oldenlab/wordpress/wp-content/uploads/2013/03/Venerupis-philippinarum_Shean.pdf
- 98.Breber P. Introduction and acclimatisation of the Pacific carpet clam, Tapes philippinarum, to Italian waters In: Leppäkoski E, Gollasch S, Olenin S, editors. Invasive aquatic species of Europe. Distribution, impacts and management. Dordrecht: Kluwer; 2002. pp. 120–126. [Google Scholar]
- 99.Humphreys J, Caldow RWG, McGrorty S, West AD, Jensen AC. Population dynamics of naturalised Manila clams Ruditapes philippinarum in British coastal waters. Mar Biol. 2007;151: 2255–2270. 10.1007/s00227-007-0660-x [DOI] [Google Scholar]
- 100.De Montaudouin X, Arzul I, Caill-Milly N, Khayati A, Labrousse JM, Lafitte C, et al. Asari clam (Ruditapes philippinarum) in France: history of an exotic species 1972–2015. Bulletin of Japan Fisheries Research and Education Agency. 2016;42: 35–42. Available from: http://archimer.ifremer.fr/doc/00366/47767/. [Google Scholar]
- 101.Ruano F, Sobral DV. Marine non-indigenous species–current situation in Portugal. In: Rodrigues L, Reino L, Godinho LO, Freitas H, editors. Proceedings of the 1st Symposium on Nonindigenous Species: Introduction, Causes and Consequences. Lisboa: Liga para a Protecção da Natureza; 2000. pp. 58–63.
- 102.Blaxter JHS. The enhancement of marine fish stocks. Adv Mar Biol. 2000;38: 1–54. 10.1016/S0065-2881(00)38002-6 [DOI] [Google Scholar]
- 103.Kirk R. A history of marine fish culture in Europe and North America Farham: Fishing News Books; 1987. [Google Scholar]
- 104.Orlov YuI Ivanov BG. On the introduction of the Kamchatka king crab Paralithodes camtschatica (Decapoda: Anomura: Lithodidae) into the Barents Sea. Mar Biol. 1978;48(4): 373–375. 10.1007/BF00391642. [DOI] [Google Scholar]
- 105.Baltz DM. Introduced fishes in marine systems and inland seas. Biol Conserv. 1991;56: 151–177. 10.1016/0006-3207(91)90015-2 [DOI] [Google Scholar]
- 106.Arbaciauskas K. Ponto-Caspian amphipods and mysids in the inland waters of Lithuania: history of introduction, current distribution and relations with native malacostracans In: Leppäkoski E, Gollasch S, Olenin S, editors. Invasive aquatic species of Europe: Distribution, impacts and management. Dordrecht: Kluwer Academic Publishers; 2002. pp. 104–115. [Google Scholar]
- 107.Berezina NA, Petryashev VV, Razinkovas A, Lesutienė J. Alien Malacostracan crustaceans in the eastern Baltic Sea: Pathways and consequences. In: Galil BS, Clark PF, Carlton JT, editors. In the wrong place—alien marine crustaceans: Distribution, biology and impacts. Invading Nature—Springer Series in Invasion Ecology; 2011. pp. 301–322.
- 108.Born AF, Immink AJ, Bartley DM. Marine and coastal stocking: global status and information needs In: Bartley DM, Leber KM, editors. Marine ranching. FAO Fisheries Technical Paper 429. Rome: FAO; 2004. pp. 1–18. [Google Scholar]
- 109.Wang Q, Zhuang Z, Deng J, Ye Y. Stock enhancement and translocation of the shrimp Penaeus chinensis in China. Fish Res. 2006;80(1): 67–79. 10.1016/j.fishres.2006.03.015 [DOI] [Google Scholar]
- 110.Cao L, Chen Y, Dong S, Hanson A, Huang B, Leadbitter D, et al. Opportunity for marine fisheries reform in China. Proc Natl Acad Sci U S A. 2017;114(3): 435–442. 10.1073/pnas.1616583114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.FAO Global Aquaculture Production, 1950–2015. Available from: http://www.fao.org/figis/servlet/TabSelector
- 112.Activité de l'Institut des Pêches en 1979. Principales actions en matière de cultures marines. Science et Pêche. Bull Inst Pêche Marit. 1980;306: 1–39. French. [Google Scholar]
- 113.Boudouresque CF, Gerbal M, Knoepffler-Peguy M. L'algue japonnaise Undaria pinnatifida (Phaeophyceae, Laminariales) en Méditerranée. Phycologia. 1985;24(3): 364–266. French. 10.2216/i0031-8884-24-3-364.1 [DOI] [Google Scholar]
- 114.Floc'h JY, Pajot R, Wallentinus I. The Japanese brown alga Undaria pinnatifida on the coast of France and its possible establishment in European waters. ICES J Mar Sci. 1991;47(3): 379–390. 10.1093/icesjms/47.3.379 [DOI] [Google Scholar]
- 115.Floc’h JY, Pajot R, Mouret V. Undaria pinnatifida (Laminariales, Phaeophyta) 12 years after its introduction into the Atlantic Ocean. In: Lindstrom SC, Chapman DJ, editors. Fifteenth International Seaweed Symposium; 1995 Jan; Valdivia, Chile. Developments in Hydrobiology. Dordrecht: Springer. 1996;116(326/327): 217–222. 10.1007/978-94-009-1659-3_30 [DOI]
- 116.Delivering alien invasive species inventories for Europe. [internet]. Undaria pinnatifida [about 4 pages]. [cited 2018 Mar 09]. Available from: http://www.europe-aliens.org/pdf/Undaria_pinnatifida.pdf
- 117.Mineur F, Belsher T, Johnson MP, Maggs CA, Verlaque M. Experimental assessment of oyster transfers as a vector for macroalgal introductions. Biol Conserv. 2007;137(2): 237–247. 10.1016/j.biocon.2007.02.001 [DOI] [Google Scholar]
- 118.Barille L, Le Bris A, Meleder V, Launeau P, Roobin M, Louvrou I, et al. Photosynthetic epibionts and endobionts of Pacific oyster shells from oyster reefs in rocky versus mudflat shores. PLoS ONE. 2017;12(9): e0185187 10.1371/journal.pone.0185187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engelen AH, Serebryakova A, Ang P, Britton-Simmons K, Mineur F, Pedersen MF, et al. Circumglobal invasion by the brown seaweed Sargassum muticum. Oceanogr Mar Biol. 2015;53: 81–126. [Google Scholar]
- 120.Josefsson M, Jansson K. 2011. NOBANIS–Invasive Alien Species Fact Sheet Sargassum muticum [internet]. [10 pages]. [cited 2018 Mar 3]. Available from: https://www.nobanis.org/globalassets/speciesinfo/s/sargassum-muticum/sargassum_muticum.pdf
- 121.Salini J, Shaklee JB. Genetic structure of barramundi (Lates calcarifer) stocks from northern Australia. Mar Freshw Res. 1988;39(3): 317–329. 10.1071/MF9880317 [DOI] [Google Scholar]
- 122.Araki H, Schmid C. Is hatchery stocking a help or harm?: Evidence, limitations and future directions in ecological and genetic surveys. Aquaculture. 2010;308: S2–S11. 10.1016/j.aquaculture.2010.05.036 [DOI] [Google Scholar]
- 123.Blanco Gonzalez E, Nagasawa K, Umino T. Stock enhancement program for black sea bream (Acanthopagrus schlegelii) in Hiroshima Bay: monitoring the genetic effects. Aquaculture. 2008;276: 36–43. 10.1016/j.aquaculture.2008.02.004 [DOI] [Google Scholar]
- 124.Hansen MM, Fraser DJ, Meier K, Mensberg K-LD. Sixty years of anthropogenic pressure: a spatio-temporal genetic analysis of brown trout populations subject to stocking and population declines. Mol Ecol. 2009;18(12): 2549–2562. 10.1111/j.1365-294X.2009.04198.x [DOI] [PubMed] [Google Scholar]
- 125.Laikre L, Schwartz MK, Waples RS, Ryman N. Compromising genetic diversity in the wild: unmonitored large-scale release of plants and animals. Trends Ecol Evol. 2010;25: 520–529. 10.1016/j.tree.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 126.Andrews JD. MSX disease of oysters caused by Haplosporidium nelsoni Revised and updated by Ford Susan E. ICES Identification leaflets for diseases and parasites of fish and shellfish. Leaflet No. 38. Copenhagen: ICES; 2010. Available from: http://www.ices.dk/sites/pub/Publication%20Reports/Disease%20Leaflets/Sheet%20no%2038.pdf [Google Scholar]
- 127.Pichot Y, Comps M, Tigé F, Grizel H, Rabouin MA. Recherches sur Bonamia ostreae gen. n., sp. n., parasite nouveau d l'huître plate Ostrea edulis L. Rev Trav Inst Pêches Marit. 1980;43: 131–140. French. [Google Scholar]
- 128.Naylor R, Hindar K, Fleming IA, Goldburg R, Williams S, Volpe J, et al. Fugitive salmon: assessing risks of escaped fish from aquaculture. BioScience. 2005;55: 427–437. 10.1641/0006-3568(2005)055[0427:FSATRO]2.0.CO;2 [DOI] [Google Scholar]
- 129.Handisyde NT, Ross LG, Badjeck M-C, Allison EH. The effects of climate change on world aquaculture: A global perspective. Final technical report Stirling: DFID Aquaculture and Fish Genetics Research Programme, Stirling Institute of Aquaculture; 2006. Available from: http://www.ecasa.org.uk/Documents/Handisydeetal_000.pdf [Google Scholar]
- 130.Jackson D, Drumm A, McEvoy S, Jensen O, Mendiola D, Gabina G, et al. A pan-European valuation of the extent, causes and cost of escape events from sea cage fish farming. Aquaculture. 2015;436: 21–26. 10.1016/j.aquaculture.2014.10.040 [DOI] [Google Scholar]
- 131.Silva F, Stevens CJ, Weisskopf A, Castillo C, Qin L, Bevan A, et al. Modelling the geographical origin of rice cultivation in Asia using the Rice Archaeological Database. PLoS ONE. 2015;10(9): e0137024 10.1371/journal.pone.0137024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weigle SM, Smith LD, Carlton JT, Pederson J. Assessing the risk of introducing exotic species via the live marine species trade. Conserv Biol. 2005;19: 213–223. 10.1111/j.1523-1739.2005.00412.x [DOI] [Google Scholar]
- 133.Carlton JR, Mann R. 1996. Transfers and world-wide introductions In: Kennedy VS, Newell RIE, Eble AF, editors. The eastern oyster: Crassostrea virginica. College Park: University of Maryland Sea Grant Press; 1996. pp. 691–706. [Google Scholar]
- 134.Kurlansky M. The big oyster: History on the half shell 1st ed. New York: Ballantine Books; 2006. [Google Scholar]
- 135.Furnas JC. The Americans, a social history of the United States 1587–1914 1st ed. New York: G. P. Putnams's Sons; 1969. [Google Scholar]
- 136.Calisphere University of California [internet]. The eastern oyster industry in California, 1869–1910 [about 13 pages]. [cited 2018 Mar 09]. Available from: http://content.cdlib.org/view?docId=kt629004n3;NAAN=13030&doc.view=frames&chunk.id=d0e540&toc.id=0&brand=calisphere
- 137.Carlton JT. 1979. Introduced invertebrates of San Francisco Bay. In: Conomos TJ, Leviton AE, Berson M, editors. San Francisco Bay: The urbanized estuary. Investigations into the natural history of San Francisco Bay and Delta with reference to the influence of man. Proceedings of the 58th annual meeting of the Pacific division of American association for the advancement of science; 1977 Jun 12–16; San Francisco, USA. Lawrence: Allen Press; 1979. pp. 427–444.
- 138.National Exotic Marine and Estuarine Species Information System [internet]. Smithsonian Environmental Research Center. [cited 2018 Mar 09] Available from: http://invasions.si.edu/nemesis/
- 139.Miller AW, Ruiz GM, Minton MS, Ambrose RF. Differentiating successful from failed molluscan invaders in estuarine ecosystems. Mar Ecol Progr Ser. 2007;332: 41–51. doi: 3354/meps332041 [Google Scholar]
- 140.Fowler AE, Blakeslee AMH, Canning-Clode J, Repetto MF, Phillip AM, Carlton JT, et al. Opening Pandora’s bait box: a potent vector for biological invasions of live marine species. Divers Distrib. 2015;22: 30–42. 10.1111/ddi.12376 [DOI] [Google Scholar]
- 141.Font T, Gil J, Lloret J. The commercialization and use of exotic baits in recreational fisheries in the north‐western Mediterranean: Environmental and management implications. Aquatic Conserv. 2018;28(3): 651–661. 10.1002/aqc.2873 [DOI] [Google Scholar]
- 142.van der Meeren GI, Ekeli KO, Jørstad, KE, Tveite S. Americans on the wrong side—the lobster Homarus americanus in Norwegian waters. ICES. 2000;C.M. 2000/U:20: 1–15. Available from: http://www.ices.dk/sites/pub/CM%20Doccuments/2000/U/U2000.pdf.
- 143.Stebbing P, Johnson P, Delahunty A, Clark PF, McCollin T, Hale C, et al. Reports of American lobsters, Homarus americanus (H. Milne Edwards, 1837), in British waters. BioInvasions Rec. 2012;1(1): 17–23. 10.3391/bir.2012.1.1.04 [DOI] [Google Scholar]
- 144.European Commission Environment [internet]. List of invasive alien species of Union concern [about 3 screens]. [cited 2017 Dec 28]. Available from: http://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm;
- 145.Cohen AN. Aquatic invasive species vector risk assessments: Live marine seafood and the introduction of non-native species into California. Final Report. Submitted to the California Ocean Science Trust. Richmond, CA: Center for Research on Aquatic Bioinvasions (CRAB); 2012. Available from: http://www.opc.ca.gov/webmaster/ftp/project_pages/AIS/AIS_LiveSeafood.pdf [Google Scholar]
- 146.Cecere E, Petrocelli A, Belmonte M, Portacci G, Rubino F. Activities and vectors responsible for the biological pollution in the Taranto Seas (Mediterranean Sea, southern Italy): a review. Environ Sci Pollut Res. 2016;23(13): 12797–810. 10.1007/s11356-015-5056-8 [DOI] [PubMed] [Google Scholar]
- 147.Blakeslee A, Fowler A, Couture J, Grosholz E, Ruiz G, Miller A. Effective approaches for reducing diversity of marine organisms transferred with live bait. Manag Biol Invasion. 2016;7: 389–398. 10.3391/mbi.2016.7.4.08 [DOI] [Google Scholar]
- 148.ITEFood&Drink [internet]. Shanghai opens first port dedicated to live seafood [about 3 screens]. [cited 2018 Mar 09]. Available from: https://www.food-exhibitions.com/Market-Insights/China/Shanghai-opens-first-port-dedicated-live-seafood
- 149.Wolff T. The horseshoe crab (Limulus polyphemus) in north European waters. Vidensk Meddr Dansk Naturh Foren. 1977;140: 39–52. [Google Scholar]
- 150.Meinesz A. Killer algae 1st ed. Chicago: University of Chicago Press; 1999. [Google Scholar]
- 151.Jousson O, Pawlowski J, Zaninetti I, Zechman FW, Dini F, Di Giuseppe G, et al. Invasive alga reaches California. Nature. 2000;408: 157–158. 10.1038/35041623 [DOI] [PubMed] [Google Scholar]
- 152.Piazzi L, Ceccherelli G, Cinelli F. Expansion de Caulerpa taxifolia et de Caulerpa racemosa le long des côtes Toscanes (Italie), situation en 1998. In: Gravez V, Ruitton S, Boudouresque CF, Le Direach L, Meinesz A, Scabbia G, Verlaque M, editors. Proceedings of the Fourth International Workshop on Caulerpa taxifolia, 1999 Feb 1–2; Lerici, Italy. Marseille: GIS Posidonie Publisher; 2001. pp. 71–77. French.
- 153.Anderson LWJ. California’s reaction to Caulerpa taxifolia: a model for invasive species rapid response. Biol Invasions. 2005;7: 1003–1016. 10.1007/s10530-004-3123-z [DOI] [Google Scholar]
- 154.Komatsu T, Ishikawa T, Yamaguchi N, Hori Y, Ohba H. But next time?: Unsuccessful establishment of the Mediterranean strain of the green seaweed Caulerpa taxifolia in the Sea of Japan. Biol Invasions. 2003;5: 275–278. 10.1023/A:1026164902868 [DOI] [Google Scholar]
- 155.Fujikura K, Lindsay D, Kitazato H, Nishida S, Shirayama. 2010. Marine biodiversity in Japanese waters. PLoS ONE. 2010;5(8): e11836 10.1371/journal.pone.0011836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.U.S. Geological Survey Nonindigenous Aquatic Species Database [internet]. Pterois volitans/miles [about 5 screens]. [cited 2018 Mar 09]. Available from: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=963
- 157.Semmens BX, Buhle ER, Salomon AK, Pattengill-Semmens CV. A hotspot of non-native marine fishes: evidence for the aquarium trade as an invasion pathway. Mar Ecol Prog Ser. 2004;266: 239–244. 10.3354/meps266239 [DOI] [Google Scholar]
- 158.Schofield PJ. Geographic extent and chronology of the invasion of non-native lionfish (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea. Aquat Invasions. 2009;4: 473–479. 10.3391/ai.2009.4.3.5 [DOI] [Google Scholar]
- 159.Carballo-Cárdenas E. Controversies and consensus on the lionfish invasion in the western Atlantic Ocean. Ecol Society. 2015;20: 24 10.5751/ES-07726-200324 [DOI] [Google Scholar]
- 160.Lloyd WA. On the occurrence of Limulus polyphemus off the coast of Holland, and on the transmission of aquarium animals. Zoologist. 1874;9(2): 3845–3855. [Google Scholar]
- 161.Corfield J, Diggles B, Jubb C, McDowall RM, Moore A., Richards A, et al. Review of the impacts of introduced ornamental fish species that have established wild populations in Australia Prepared for the Australian Government Department of the Environment, Water, Heritage and the Arts. NIWA Australia; 2008. Available from: http://www.environment.gov.au/system/files/resources/fb1584f5-1d57-4b3c-9a0f-b1d5beff76a4/files/ornamental-fish.pdf [Google Scholar]
- 162.Johnston MW, Purkis SJ. Correction: modeling the potential spread of the recently identified non-native Panther grouper (Chromileptes altivelis) in the Atlantic using a cellular automaton approach. PLoS ONE. 2013;8(9): 10.1371/annotation/54b58291-1a3f-4a38-b48a-fe4466b7f61b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ng TH, Tan HH. The introduction, origin and life-history attributes of the non-native cichlid Etroplus suratensis in the coastal waters of Singapore. J Fish Biol. 2010;76(9): 2238–2260. 10.1111/j.1095-8649.2010.02642.x [DOI] [PubMed] [Google Scholar]
- 164.Zammit E, Schembri P. An overlooked and unexpected introduction? Occurrence of the spotted scat Scatophagus argus (Linnaeus, 1766) (Osteichthyes: Scatophagidae) in the Maltese Islands. Aquat Invasions. 2011;6(Suppl 1): S79–S83. 10.3391/ai2011.6.S1.018 [DOI] [Google Scholar]
- 165.Langeneck J, MarcellI M, Simak HC. Unexpected alien species in Cyprus waters: Acanthurus coeruleus (Actinopterygii: Acanthuridae). Mar Biodivers Rec. 2012;5: e116 10.1017/S1755267212001042 [DOI] [Google Scholar]
- 166.Guidetti P, Magnali L, Navone A. First record of the acanthurid fish Zebrasoma xanthurum (Blyth, 1852) in the Mediterranean Sea, with some considerations on the risk associated with aquarium trade. Mediterr Mar Sci. 2016;17(1): 147–151. 10.12681/mms.1470 [DOI] [Google Scholar]
- 167.Shine C, Reaser JK, Gutierrez AT. Invasive alien species in the Austral-Pacific region: national reports & directory of resources Cape Town: Global Invasive Species Programme; 2003. Available from: http://www.issg.org/pdf/publications/GISP/Resources/AP-1.pdf [Google Scholar]
- 168.HEAR Hawaiian Ecosystems at Risk Program [internet]. Galapagos invasive species: invasive species in the sea. [about 1 screen]. [cited 2017 Jan 26]. Available from: http://www.hear.org/galapagos/invasives/topics/management/marine/index.html
- 169.Randall JE. New records of fishes from the Hawaiian Islands. Pac Sci. 1980;34(3): 211–232. [Google Scholar]
- 170.Lelong P. Capture d’un macabit, Epinephelus merra Bloch, 1793 (Poisson, Serranidae), en Méditerranée nord-occidentale. Mar Life. 2005;15(1–2): 63–66. French. Available from: http://www.marinelife-revue.fr/IMG/pdf/Lelong-2005-MarLife.pdf. [Google Scholar]
- 171.Rhyne AL, Tlusty MF, Schofield PJ, Kaufman L, Morris JA Jr. Revealing the appetite of the marine aquarium fish trade: the volume and biodiversity of fish imported into the United States. PLoS ONE. 2012;7(5): e35808 10.1371/journal.pone.0035808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Biondo MV. Quantifying the trade in marine ornamental fishes into Switzerland and an estimation of imports from the European Union. Glob Ecol Conserv. 2017;11: 95–105. 10.1016/j.gecco.2017.05.006 [DOI] [Google Scholar]
- 173.Marine aquarium biodiversity and trade flow [internet]. [cited 2018 Mar 09]. Available from: https://www.aquariumtradedata.org/
- 174.Herodotus. The Histories Translated by Waterfield R. Oxford world’s classics. Unknown edition Oxford: Oxford University Press; 2008. [Google Scholar]
- 175.Fortune [internet]. Why China and Nicaragua’s canal project is floundering [about 4 screens]. [cited 2017 Feb 23]. Available from: http://fortune.com/2016/02/29/china-nicaragua-canal/
- 176.Olenin S. Black Sea–Baltic Sea invasion corridors. In: Briand F, editor. Alien marine organisms introduced by ships in the Mediterranean and Black Seas. CIESM Workshops Monograph 20. Comission Internationale pour l’Exploration Scientifique de la mer Mediterranee; 2002. pp. 29–33.
- 177.Gollasch S, Galil BS, Cohen AN. Bridging divides: Maritime canals as invasion corridors Monographiae Biologicae 83 Dordrecht: Springer; 2006. [Google Scholar]
- 178.Vaillant L. Recherches sur la faune malacologique de la baie de Suez. J Conchyliol. 1865;13: 97–127. French. [Google Scholar]
- 179.Monterosato TA. Enumerazione e sinonimia delle conchiglie mediterranee. G Sci Nat Econ Palermo. 1878;13: 61–115. Italian. [Google Scholar]
- 180.Fox HM. General part. Zoological results of the Cambridge expedition to the Suez Canal, 1924. 1. Trans Zool Soc London. 1926;22: 1–64. [Google Scholar]
- 181.Galil BS. The marine caravan–The Suez Canal and the Erythrean invasion In: Gollasch S, Galil BS, Cohen AN, editors. Bridging Divides. Dordrecht: Springer; 2006. pp. 207–300. [Google Scholar]
- 182.Suez Canal authority [internet]. Canal characteristics [about 3 screens]. [cited 2017 Nov 20]. Available from: http://www.suezcanal.gov.eg/English/About/SuezCanal/Pages/CanalCharacteristics.aspx
- 183.Galil BS, Marchini A, Occhipinti-Ambrogi A. East is east and west is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar Coast Shelf Sci. 2018;201: 7–16. 10.1016/j.ecss.2015.12.021 [DOI] [Google Scholar]
- 184.Hildebrand SF. The tarpon in the Panama Canal. Sci Mon. 1937;44: 239–248. [Google Scholar]
- 185.Hildebrand SF. The Panama Canal as a passageway for fishes, with lists and remarks on the fishes and invertebrates observed. Zoologica (N.Y.). 1939;24(3): 15–45. Available from: http://www.bio-nica.info/biblioteca/Hildebrand1939PanamaCanal.pdf. [Google Scholar]
- 186.The Tico Times [internet]. Tarpon on the Pacific coast? You betcha [about 4 screens]. [cited 2018 Mar 09]. Available from: http://www.ticotimes.net/2011/07/06/tarpon-on-the-pacific-coast-you-betcha
- 187.Cohen AN. Species introduction in the Panama Canal In: Gollasch S, Galil BS, Cohen AN, editors. Bridging Divides. Dordrecht: Springer; 2006. pp. 127–206. [Google Scholar]
- 188.Rubinoff RW, Rubinoff I. Interoceanic colonization of a marine goby through the Panama Canal. Nature. 1968;217: 476–478. 10.1038/217476a0 [DOI] [Google Scholar]
- 189.Por FD. One hundred years of Suez canal–a century of Lessepsian migration: retrospect and viewpoints. Syst Zool. 1971;20(2): 138–159. 10.2307/2412054 [DOI] [Google Scholar]
- 190.Hewitt CL. distribution and diversity of tropical Australian marine bio-invasions. Pac Sci. 2002;56: 213–222. 10.1353/psc.2002.0016 [DOI] [Google Scholar]
- 191.Ruiz GM, Torchin ME, Grant K. Using the Panama Canal to test predictions about tropical marine invasions In: Lang MA, Macintyre IG, Rutzler K, editors. Proceedings of the Smithsonian Marine Science Symposium. Smith Contributions to the Marine Sciences, vol 38 Washington D.C.: Smithsonian Institution Scholarly Press; 2009. pp. 291–299. [Google Scholar]
- 192.World register of introduced marine species [internet]. Ahyong S, Costello MJ, Dolan J, Galil BS, Gollasch S, Hutchings P, et al. [cited 2018 Mar 09]. Available from: http://www.marinespecies.org/introduced/
- 193.Ruiz GM, Carlton JT. Invasion vectors: a conceptual framework for management In: Ruiz GM, Carlton JT, editors. Invasive species: Vectors and management strategies. Washington: Island Press; 2003. pp. 459–504. [Google Scholar]
- 194.Lehtiniemi M, Ojaveer H, David M, Galil BS, Gollasch S, McKenzie S, et al. Dose of truth—Monitoring marine non-indigenous species to serve legislative requirements. Mar Policy. 2015;54: 26–35. 10.1016/j.marpol.2014.12.015 [DOI] [Google Scholar]
- 195.Ruiz GM, Hewitt CL. Toward understanding patters of coastal marine invasions: A prospectus In: Leppäkoski E, Olenin S, Gollasch S, editors. Invasive aquatic species of Europe. Dordrecht: Kluwer Academic Publishers, 2002. pp. 529–547. [Google Scholar]
- 196.Campbell ML, Gould B, Hewitt CL. Survey evaluations to assess marine bioinvasions. Mar Pollut Bull. 2007;55: 360–378. 10.1016/j.marpolbul.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 197.Ruiz GM, Freestone AL, Fofonoff PW, Simkanin C. Habitat distribution and heterogeneity in marine invasion dynamics: The importance of hard substrate and artificial structure In: Wahl M, editor. Marine hard bottom communities. Berlin: Springer-Verlag; 2009. pp. 321–332. [Google Scholar]
- 198.Page HM, Dugan JE, Culver CS, Hoesterey JC. Exotic invertebrate species on offshore oil platforms. Mar Ecol Prog Ser. 2006; 325: 101–107. 10.3354/meps325101 [DOI] [Google Scholar]
- 199.Bulleri F, Chapman MG. The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol. 2010;47: 26–35. 10.1111/j.1365-2664.2009.01751.x [DOI] [Google Scholar]
- 200.Carman MR, Bullard SG, Rocha RM, Lambert G, Dijkstra JA, Roper JJ, et al. Ascidians at the Pacific and Atlantic entrances to the Panama Canal. Aquat Invasions. 2011;6: 371–380. 10.3391/ai.2011.6.4.02 [DOI] [Google Scholar]
- 201.Adams TP, Miller RG, Alleyne D, Burrows MT. Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J Appl Ecol. 2014;51: 330–338. 10.1111/1365-2664.12207 [DOI] [Google Scholar]
- 202.Cohen AN, Mills C, Berry H, Wonham M, Bingham B, Bookheim B, et al. A rapid assessment survey of non-indigenous species in the shallow waters of Puget Sound: Report of the Puget Sound expedition, September 8–16, 1998. Nearshore Habitat Program, Aquatic Resources Division. Olympia: Washington State Department of Natural Resource; 1998.
- 203.Pederson J, Bullock R, Carlton J, Dijkstra J, Dobroski N, Dyrynda P, et al. 2005. Rapid assessment survey of non-native and native marine species of floating dock communities, August 2003 Cambridge: MIT Sea Grant College Program; 2005. [Google Scholar]
- 204.Arenas F, Bishop JDD, Carlton JT, Dyrynda PJ, Farnham WF, Gonzalez DJ, et al. Alien species and other notable records from a rapid assessment survey of marinas on the south coast of England. J Mar Biol Assoc UK. 2006;86(6): 1329–1337. 10.1017/S0025315406014354 [DOI] [Google Scholar]
- 205.Ashton GV, Boos K, Shucksmith R, Cook EJ. Risk assessment of hull fouling as a vector for marine non-natives in Scotland. Aquat Invasions. 2006;1(4): 214–218. [Google Scholar]
- 206.Minchin D. Rapid coastal survey for targeted alien species associated with floating pontoons in Ireland. Aquat Invasions. 2007;2(1): 63–70. 10.3391/ai2007.2.1.8 [DOI] [Google Scholar]
- 207.Hewitt CL, Martin RB. Revised protocols for baseline port surveys for introduced marine species: survey design, sampling protocols and specimen handling. CRIMP Technical Report Number 22. Hobart: CSIRO Marine Research; 2001.
- 208.Ruiz GM, Huber T, Larson K, McCann L, Steves B, Fofonoff P, et al. Biological invasions in Alaska’s coastal marine ecosystems: establishing a baseline. Final Report submitted to Prince William Sound Regional Cizens’ Advsiory council & U. S. fish and Wildlife Service. 2006. [cited 2018 Feb 17]. Available from: http://www.uaf.edu/files/ces/aiswg/resources/BioInvasionsAKCoastal.pdf
- 209.Smithsonian environmental research centre [internet]. Research project ‘Large-scale surveys of fouling, zooplantkon and soft sediment benthic habitats. [about 3 screens]. [cited 2018 Mar 09]. Available from: https://serc.si.edu/research/projects/large-scale-surveys-fouling-zooplankton-and-soft-sediment-benthic-habitats
- 210.Gartner HN, Murray CC, Frey MA, Nelson J, Larson K, Ruiz GM, Therriault TW. Nonindigenous invertebrate species in the marine fouling communities of British Columbia, Canada. BioInvasion Records 2016;5(4): 205–212. 10.3391/bir.2016.5.4.03 [DOI] [Google Scholar]
- 211.Canning-Clode J, Fofonoff P, McCann L, Carlton JT, Ruiz GM. Marine invasions on a subtropical island: fouling studies and new records in a recent marina on Madeira Island (Eastern Atlantic Ocean). Aquatic Invasions 2013;8(3): 261–270. 10.3391/ai.2013.8.3.02 [DOI] [Google Scholar]
- 212.Wittenberg R, Cock MJW. Best practices for the prevention and management of invasive alien species In: Mooney HA, Mack RN, McNeely JA, Neville LE, Schei PJ, Waage JK, editors. Invasive alien species: A new synthesis. Washington, D.C.: Island Press; 2005. pp. 209–232. [Google Scholar]
- 213.National Invasive Species Council. Management Plan: 2016–2018. Washington D.C.: National Invasive Species Council; 2016. [cited 2018 Feb 12]. [Google Scholar]
- 214.Hopkins GW, Freckleton RP. Declines in the numbers of amateur and professional taxonomists: implications for conservation. Anim Conserv. 2002;5: 245–249. 10.1017/S1367943002002299 [DOI] [Google Scholar]
- 215.Kelly RP, Port JA, Yamahara KM, Martone RG, Lowell N, Thomsen PF, et al. Harnessing DNA to improve environmental management. Science. 2014;344(6191): 1455–1456. 10.1126/science.1251156 [DOI] [PubMed] [Google Scholar]
- 216.Darling JA, Galil BS, Carvalho GR, Rius M, Viard F, Piraino S. Recommendations for developing and applying molecular genetic tools to assess and manage biological invasions in marine ecosystems. Mar Policy. 2017;85: 54–64. 10.1016/j.marpol.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McDonald JH, Koehn RK. The mussels Mytilus galloprovincialis and Mytilus trossulus on the Pacific coast of North America. Mar Biol. 1988;99: 111–18. 10.1007/BF00644984 [DOI] [Google Scholar]
- 218.McDonald JH, Seed R, Koehn RK. Allozymes and morphometric characters of three species of Mytilus in the northern and southern hemispheres. Mar Biol. 1991;111: 323–33. 10.1007/BF01319403 [DOI] [Google Scholar]
- 219.Spidle AP, Marsden JE, May B. Identification of the Great Lakes quagga mussel as Dreissena bugensis from the Dnieper River, Ukraine, on the basis of allozyme variation. Can J Fish Aquat Sci. 1994;51: 1485–1489. 10.1139/f94-148 [DOI] [Google Scholar]
- 220.Geller JB, Carlton JT, Powers DA. PCR-based detection of mtDNA haplotypes of native and invading mussels on the northeastern Pacific coast: latitudinal pattern of invasion. Mar Biol. 1994;119: 243–49. 10.1007/BF00349563 [DOI] [Google Scholar]
- 221.Geller JB, Walton ED, Grosholz ED, Ruiz GM. Cryptic invasions of the crab Carcinus detected by molecular phylogeography. Mol Ecol. 1997;6: 901–906. 10.1046/j.1365-294X.1997.00256.x. [DOI] [PubMed] [Google Scholar]
- 222.Heath DD, Rawson PD, Hilbish TJ. PCR-based nuclear markers identify alien blue mussel (Mytilus spp.) genotypes on the west coast of Canada. Can J Fish Aquat Sci. 1995;52(12): 2621–2627. 10.1139/f95-851 [DOI] [Google Scholar]
- 223.Bastrop R, Jurss K, Sturmbauer C. Cryptic species in a marine polychaete and their independent introduction from North America to Europe. Mol Biol Evol. 1998;15: 97–103. 10.1093/oxfordjournals.molbev.a025919 [DOI] [PubMed] [Google Scholar]
- 224.Davies N, Villablanca FX, Roderick GK. Determining the source of individuals: multilocus genotyping in nonequilibrium population genetics. Trends Ecol Evol. 1999;14: 17–21. 10.1016/S0169-5347(98)01530-4 [DOI] [PubMed] [Google Scholar]
- 225.Aridgides LJ, Doblin MA, Berke T, Dobbs FC, Matson DO, Drake LA. Multiplex PCR allows simultaneous detection of pathogens in ships' ballast water. Mar Pollut Bull. 2004;48, 1096–1101. 10.1016/j.marpolbul.2003.12.017 [DOI] [PubMed] [Google Scholar]
- 226.Patil JG, Gunasekera RM, Deagle BE, Bax NJ, Blackburn SI. Development and evaluation of a PCR based assay for detection of the toxic dinoflagellate, Gymnodinium catenatum (Graham) in ballast water and environmental samples. Biol Invasions. 2005;7: 983–994. 10.1007/s10530-004-3119-8 [DOI] [Google Scholar]
- 227.Doblin MA, Coyne KJ, Rinta-Kanto JM, Wilhelm SW, Dobbs FC. Dynamics and short-term survival of toxic cyanobacteria species in ballast water from NOBOB vessels transiting the Great Lakes—implications for HAB invasions. Harmful Algae. 2007;6: 519–530. 10.1016/j.hal.2006.05.007 [DOI] [Google Scholar]
- 228.Smith KF, Wood SA, Mountfort D, Cary SC. Development of a real-time PCR assay for the detection of the invasive clam, Corbula amurensis, in environmental samples. J Exp Mar Bio Ecol. 2012;412: 52–57. 10.1016/j.jembe.2011.10.021 [DOI] [Google Scholar]
- 229.Gillum JE, Jimenez L, White DJ, Goldstien SJ, Gemmell NJ. Development and application of a quantitative real-time PCR assay for the globally invasive tunicate Styela clava. Manag Biol Invasion. 2014;5: 133–142. 10.3391/mbi.2014.5.2.06 [DOI] [Google Scholar]
- 230.Ardura A, Zaiko A, Martinez JL, Samuiloviene A, Semenova A, Garcia-Vazquez E. eDNA and specific primers for early detection of invasive species- a case study on the bivalve Rangia cuneata, currently spreading in Europe. Mar Environ Res. 2015;112(B): 48–55. 10.1016/j.marenvres.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 231.Wood SA, Zaiko A, Richter I, Inglis G, Pochon X. Development of a real-time polymerase chain reaction assay for the detection of the invasive Mediterranean fanworm, Sabella spallanzanii, in environmental samples. Environ Sci Pollut Res. 2017;24(21): 17373–17382. 10.1007/s11356-017-9357-y [DOI] [PubMed] [Google Scholar]
- 232.Hebert P.D.N., Cywinska A., Ball S.L., de Waard J.R. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003;270: 313–321. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Taberlet P, Coissac E, Pompanon F, Brochmann C, Willerslev E. next-generation biodiversity assessment using DNA metabarcoding. Mol Ecol. 2012;21(8): 2045–50. 10.1111/j.1365-294X.2012.05470.x [DOI] [PubMed] [Google Scholar]
- 234.Ansorge WJ. Next-generation DNA sequencing techniques. N Biotechnol. 2009;25(4): 195–203. 10.1016/j.nbt.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 235.Hajibabaei M, Shokralla S, Zhou X, Singer GA, Baird DJ. Environmental barcoding: a next-generation sequencing approach for biomonitoring applications using river benthos. PloS ONE. 2011;6(4): e17497 10.1371/journal.pone.0017497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pochon X, Bott NJ, Smith KF, Wood SA. Evaluating detection limits of next-generation sequencing for the surveillance and monitoring of international marine pests. PloS ONE. 2013;8(9): e73935 10.1371/journal.pone.0073935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ji Y, Ashton L, Pedley SM, Edwards DP, Tang Y, Nakamura A, et al. Reliable, verifiable and efficient monitoring of biodiversity via metabarcoding. Ecol Lett. 2013;16(10): 1245–1257. 10.1111/ele.12162 [DOI] [PubMed] [Google Scholar]
- 238.Wood SA, Smith KF, Banks JC, Tremblay LA, Rhodes L, Mountfort D, et al. Molecular genetic tools for environmental monitoring of New Zealand's aquatic habitats, past, present and the future. N Z J Mar Freshwater Res. 2013;47: 90–119. 10.1080/00288330.2012.745885 [DOI] [Google Scholar]
- 239.Collins RA, Armstrong KF, Holyoake AJ, Keeling S. Something in the water: biosecurity monitoring of ornamental fish imports using environmental DNA. Biol Invasions. 2013;15: 1209–1215. 10.1007/s10530-012-0376-9 [DOI] [Google Scholar]
- 240.Comtet T, Sandionigi A, Viard F, Casiraghi M. DNA (meta)barcoding of biological invasions: a powerful tool to elucidate invasion processes and help managing aliens. Biol Invasions. 2015;17: 905–922. 10.1007/s10530-015-0854-y [DOI] [Google Scholar]
- 241.Pochon X, Zaiko A, Hopkins GA, Banks JC, Wood SA. Early detection of eukaryotic communities from marine biofilm using high-throughput sequencing: an assessment of different sampling devices. Biofouling. 2015;31(3): 241–251. 10.1080/08927014.2015.1028923 [DOI] [PubMed] [Google Scholar]
- 242.Zaiko A, Martinez JL, Schmidt-Petersen J, Ribicic D, Samuloviene A, Garcia-Vazquez E. Metabarcoding approach for the ballast water surveillance—an advantageous solution or an awkward challenge? Mar Pollut Bull. 2015;92: 25–34. 10.1016/j.marpolbul.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 243.Zaiko A, Samulioviene A, Ardura A, Garcia-Vazquez E. Metabarcoding approach for non-indigenous species surveillance in marine coastal waters. Mar Pollut Bull. 2015;100: 53–59. 10.1016/j.marpolbul.2015.09.030 [DOI] [PubMed] [Google Scholar]
- 244.Zaiko A, Schimanski K, Pochon X, Hopkins GA, Goldstien S, Floerl O, et al. Metabarcoding improves detection of eukaryotes from early biofouling communities: implications for pest monitoring and pathway management. Biofouling 2016;32(6): 671–684. 10.1080/08927014.2016.1186165 [DOI] [PubMed] [Google Scholar]
- 245.Bott NJ, Ophel-Keller KM, Sierp MT, Rowling KP, McKay AC, Loo MG, et al. Toward routine, DNA-based detection methods for marine pests. Biotechnol Adv. 2010;28(6): 706–714. 10.1016/j.biotechadv.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 246.Mountfort D, Smith KF, Kirs M, Kuhajek JM, Adamson JE, Wood SA. Development of single and multispecies detection methods for the surveillance and monitoring of marine pests in New Zealand. Aquat Invasions. 2012;7(1): 125–128. 10.3391/ai.2012.7.1.013 [DOI] [Google Scholar]
- 247.Viard F, David P, Darling J. Marine invasions enter the genomic era: Three lessons from the past, and the way forward. Curr Zool. 2016;62: 629–642. 10.1093/cz/zow053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou X, Li Y, Liu S, Yang Q, Su X, Zhou L, et al. Ultra-deep sequencing enables high-fidelity recovery of biodiversity for bulk arthropod samples without PCR amplification. GigaScience. 2013;2: 4 10.1186/2047-217X-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bohmann K, Evans A, Gilbert MTP, Carvalho GR, Creer S, Knapp M, et al. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol Evol. 2014;29(6): 358–367. 10.1016/j.tree.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 250.Nathan LM, Simmons M, Wegleitner BJ, Jerde CL, Mahon AR. Quantifying environmental DNA signals for aquatic invasive species across multiple detection platforms. Environ Sci Techol Lett. 2014;48: 12800–12806. 10.1021/es5034052 [DOI] [PubMed] [Google Scholar]
- 251.Dowle E, Pochon X, Banks J, Shearer K, Wood SA. Targeted gene enrichment and high throughput sequencing for environmental biomonitoring: a case study using freshwater macroinvertebrates. Mol Ecol Resour. 2016;16: 1240–54. 10.1111/1755-0998.12488 [DOI] [PubMed] [Google Scholar]
- 252.Yu DW, Ji YQ, Emerson BC, Wang XY, Ye CX, Yang CY, et al. 2012. Biodiversity soup: metabarcoding of arthropods for rapid biodiversity assessment and biomonitoring. Methods Ecol Evol. 2012;3(4): 613–623. 10.1111/j.2041-210X.2012.00198.x [DOI] [Google Scholar]
- 253.Silvertown J. A new dawn for citizen science. Trends Ecol Evol. 2009;24: 467–471. 10.1016/j.tree.2009.03.017 [DOI] [PubMed] [Google Scholar]
- 254.Thiel M, Penna-Díaz MA, Luna-Jorquera G, Sala S, Sellanes J, Stotz W. Citizen scientists and marine research: Volunteer participants, their contributions and projection for the future. Oceanogr Mar Biol. 2014;52: 257–314. [Google Scholar]
- 255.Delaney DG, Sperling CD, Adams CS, Leung B. Marine invasive species: validation of citizen science and implications for national monitoring networks. Biol Invasions. 2008;10: 117–128. 10.1007/s10530-007-9114-0 [DOI] [Google Scholar]
- 256.Ruiz GM, Fegley L, Fofonoff P, Cheng X, Lemaitre R. First records of Eriocheir sinensis H. Milne Edwards, 1853 (Crustacean: Brachyura: Varunidae) for Chesapeake Bay and the mid-Atlantic coast of North America. Aquat Invasions. 2006;1(3): 137–142. [Google Scholar]
- 257.Crall AW, Newman GJ, Jarnevich C, Stohlgren TJ, Waller DM, Graham, J. Improving and integrating data on invasive species collected by citizen scientists. Biol Invasions. 2010; 12(10): 3419–3428. 10.1007/s10530-010-9740-9 [DOI] [Google Scholar]
- 258.Dickinson JL, Shirk J, Bonter D, Bonney R, Crain RL, Martin J, et al. The current state of citizen science as a tool for ecological research and public engagement. Front Ecol Environ. 2012;10(6): 291–297. 10.1890/110236 [DOI] [Google Scholar]
- 259.Newman G, Wiggins A, Crall A, Graham E, Newman S, Crowston K. The future of citizen science: emerging technologies and shifting paradigms. Front Ecol Environ. 2012;10(6): 298–304. 10.1890/110294 [DOI] [Google Scholar]
- 260.Cigliano JA, Meyer R, Ballard HL, Freitag A, Phillips TB, Wasser A. Making marine and coastal citizen science matter. Ocean Coast Manag. 2015;115: 77–87. 10.1016/j.ocecoaman.2015.06.012 [DOI] [Google Scholar]
- 261.Ricciardi A, Blackburn TM, Carlton JT, Dick JTA, Hulme PE, Iacarella JC, et al. Invasion science: A horizon scan of emerging challenges and opportunities. Trends Ecol Evol. 2017;32(6): 464–474. 10.1016/j.tree.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 262.Carlton JT. Introduced species in U.S. coastal waters: environmental impacts and management priorities Arlington: Pew Oceans Commission; 2001. [Google Scholar]
- 263.Williams SL, Grosholz ED. The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coast. 2008;31(1): 3–20. 10.1007/s12237-007-9031-6 [DOI] [Google Scholar]
- 264.Marks LM, Reed DC, Obaza AK. Assessment of control methods for the invasive seaweed Sargassum horneri in California, USA. Manag Biol Invasion. 2017;8(2): 205–213. 10.3391/mbi.2017.8.2.08 [DOI] [Google Scholar]
- 265.Ceccherelli G, Piazzi L. Exploring the success of manual eradication of Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta): the effect of habitat. Cryptogam Algol. 2005;26(3): 319–328. [Google Scholar]
- 266.Klein JC, Verlaque M. Experimental removal of the invasive Caulerpa racemosa triggers partial assemblage recovery. J Mar Biol Assoc U.K. 2017; 91(1):117–125. 10.1017/S0025315410000792 [DOI] [Google Scholar]
- 267.McKenzie CH,Reid V, Lambert G, Matheson K, Minchin D,Pederson J, et al. Alien species alert: Didemnum vexillum Kott, 2002: Invasion, impact, and control. ICES Cooperative Research Report 335. Copenhagen: International Council for the Exploration of the Sea; 2017. 10.17895/ices.pub.2138 [DOI]
- 268.Huth WL, McEvoy DM, Morgan OA. Controlling an invasive species through consumption: the case of lionfish as an impure public good. Ecol Econ 2018;149: 74–79. 10.1016/j.ecolecon.2018.02.019 [DOI] [Google Scholar]
- 269.Malpica-Cruz L, Chaves LCT, Cote IM. Managing marine invasive species through public participation: Lionfish derbies as a case study. Mar Policy. 2016;74: 158–164. 10.1016/j.marpol.2016.09.027 [DOI] [Google Scholar]
- 270.Ojaveer H, Galil B, Campbell ML, Carlton JT, Canning-Clode J, Cook EJ, et al. Classification of non-indigenous species based on their impacts: considerations for application in marine management. PLoS Biol. 2015;13(4): e1002130 10.1371/journal.pbio.1002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.De Groot DS. The California clapper rail: Its nesting habits, enemies, and habitat. Condor. 1927;29(6): 259–270. 10.2307/1362957 [DOI] [Google Scholar]
- 272.Leppäkoski E. Introduced species in the Baltic Sea and its coastal ecosystems. Ophelia. 1984;Suppl. 3: 123–135. [Google Scholar]
- 273.Nichols FH, Thompson JK. Persistence of an introduced mudflat community in South San Francisco Bay, California. Mar Ecol Prog Ser. 1985;24: 83–97. [Google Scholar]
- 274.Ferrer E, Garreta AG, Ribera MA. Effect of Caulerpa taxifolia on the productivity of two Mediterranean macrophytes. Mar Ecol Progr Ser. 1997;149: 279–287. 10.3354/meps149279 [DOI] [Google Scholar]
- 275.Harmelin-Vivien M, Francour P, Harmelin J-G. Impact of Caulerpa taxifolia on Mediterranean fish assemblages: a six years study. Proceedings of the Workshop on Invasive Caulerpa Species in the Mediterranean, 1998 Mar 18–20; Heraklion, Crete, Greece. UNEP MAP Technical Report Series 125. Athens: UNEP; 1999. pp. 127–138. Available from: https://wedocs.unep.org/bitstream/handle/20.500.11822/566/mts125.pdf?sequence=2&isAllowed=y.
- 276.Steneck R, Carlton JT. Human alterations of marine communities: students beware! In: Bertness MD, Gaines SD, Hay ME, editors. Marine Community Ecology. Sunderland: Sinauer Associates Inc.; 2001. pp. 445–468.
- 277.Scheibling RE, Gagnon P. Competitive interactions between the invasive green alga Codium fragile ssp. tomentosoides and native canopy-forming seaweeds in Nova Scotia (Canada). Mar Ecol Prog Ser. 2006;325: 1–14. 10.3354/meps325001 [DOI] [Google Scholar]
- 278.Posey M. Community changes associated with the spread of an introduced seagrass, Zostera japonica. Ecology. 1988;69: 974–983. 10.2307/1941252 [DOI] [Google Scholar]
- 279.Bortolus A, Carlton JT, Schwindt E. Reimagining South American coasts: unveiling the hidden invasion history of an iconic ecological engineer. Divers Distrib. 2015;21(11): 1267–1283. 10.1111/ddi.12377 [DOI] [Google Scholar]
- 280.Daskalov GM, Grishin AN, Rodionov S, Mihneva V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. PNAS. 2007;104(25): 10518–10523. 10.1073/pnas.0701100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Oguz T, Fach B, Salihoglu B. Invasion dynamics of the alien ctenophore Mnemiopsis leidyi and its impact on anchovy collapse in the Black Sea. J Plankton Res. 2008; 30(12): 1385–1397. 10.1093/plankt/fbn094 [DOI] [Google Scholar]
- 282.Finenko GA, Anninsky BE, Datsyk NA. Mnemiopsis leidyi A. Agassiz, 1865 (Ctenophora: Lobata) in the inshore areas of the Black Sea: 25 years after its outbreak. Russ J Biol Invasions. 2018;9(1): 86–93. 10.1134/S2075111718010071 [DOI] [Google Scholar]
- 283.Shiganova TA, Dumont HJ, Mikaelyan A, Glazov DM, Bulgakova YV, Musaeva EI, et al. Interactions between the invading ctenophores Mnemiopsis leidyi (A. Agassiz) and Beroe ovata Mayer 1912, and their influence on the pelagic ecosystem of the Northeastern Black Sea In: Dumont H, Shiganova TA, Niermann U, editors. Aquatic Invasions in the Black, Caspian, and Mediterranean Seas. Nato Science Series: IV: Earth and Environmental Sciences (IV: Earth and Environmental Science), vol 35 Dordrecht: Springer; 2004. pp. 33–70. 10.1007/1-4020-2152-6_2 [DOI] [Google Scholar]
- 284.Schwindt E, Bortolus A, Iribarne OO. Invasion of a reef-builder polychaete: direct and indirect impacts on the native benthic community structure. Biol Invasions. 2001;3(2): 137–149. 10.1023/A:1014571916818 [DOI] [Google Scholar]
- 285.Schwindt E, Iribarne OO, Isla FI. Physical effects of an invading reef-building polychaete on an Argentinean estuarine environment. Estuar Coast Shelf Sci. 2004;59(1): 109–120. 10.1016/j.ecss.2003.06.004 [DOI] [Google Scholar]
- 286.Holloway MG, Keough MJ. An introduced polychaete affects recruitment and larval abundance of sessile invertebrates. Ecol Appl. 2002;12(6): 1803–1823. 10.1890/1051-0761(2002)012[1803:AIPARA]2.0.CO;2 [DOI] [Google Scholar]
- 287.O'Brien AL, Ross DJ, Keough MJ. Effects of Sabella spallanzanii physical structure on soft sediment macrofaunal assemblages. Mar Freshw Res. 2006;57(4): 363–371. 10.1071/MF05141 [DOI] [Google Scholar]
- 288.Ross DJ, Longmore AR, Keough MJ. Spatially variable effects of a marine pest on ecosystem function. Oecologia. 2013;172(2): 525–538. 10.1007/s00442-012-2497-3 [DOI] [PubMed] [Google Scholar]
- 289.Ahyong ST, Kupriyanova E, Burghardt I, Sun Y, Hutchings PA, Capa M, et al. Phylogeography of the invasive Mediterranean fan worm, Sabella spallanzanii (Gmelin, 1791), in Australia and New Zealand. J Mar Biol Assoc U.K. 2017;97(5): 985–991. 10.1017/S0025315417000261 [DOI] [Google Scholar]
- 290.Norkko J, Reed DC, Timmermann K, Norkko A, Gustafsson BG, Bonsdorff E, et al. A welcome can of worms? Hypoxia mitigation by an invasive species. Glob Chang Biol. 2012;18(2): 422–434. 10.1111/j.1365-2486.2011.02513.x [DOI] [Google Scholar]
- 291.Sandman AN, Naslund J, Gren I-M, Norling K. Effects of an invasive polychaete on benthic phosphorus cycling at sea basin scale: An ecosystem disservice. Ambio. 2018; 10.1007/S13280-018-1050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lubchenko J. 1978. Plant species diversity in a marine intertidal community: Importance of herbivore food preference and algal competitive abilities. Am Natur. 1978;112(983): 23–39. 10.1086/283250 [DOI] [Google Scholar]
- 293.Brenchley GA, Carlton JT. Competitive displacement of native mud snails by introduced periwinckles in the New England intertidal zone. Biol Bull. 1983;165: 543–558. 10.2307/1541464 [DOI] [PubMed] [Google Scholar]
- 294.Byers JE. Exposing the mechanism and timing of impact of nonindigenous species on a native species. Ecology. 2001;82(5): 1330–1343. 10.1890/0012-9658(2001)082[1330:ETMATO]2.0.CO;2 [DOI] [Google Scholar]
- 295.Wonham MJ, O'Connor M, Harley CDG. Positive effects of a dominant invader on introduced and native mudflat species. Mar Ecol Prog Ser. 2005;289: 109–116. 10.3354/meps289109 [DOI] [Google Scholar]
- 296.Hendrickx JP, Creese RG, Gribben PE. Impacts of a non-native gastropod with a limited distribution; less conspicuous invaders matter too. Mar Ecol Prog Ser 2015;537: 151–162. 10.3354/meps11469 [DOI] [Google Scholar]
- 297.Race MS. Competitive displacement and predation between introduced and native mud snails. Oecologia. 1982;54: 337–347. 10.1007/BF00380002 [DOI] [PubMed] [Google Scholar]
- 298.Ciuhcin VD. Ecology of the gastropod molluscs of the Black Sea Academy of Sciences of the USSR. Kiev: Naukova Dumka; 1984. Russian. [Google Scholar]
- 299.Lercari D, Bergamino L. Impacts of two invasive mollusks, Rapana venosa (Gastropoda) and Corbicula fluminea (Bivalvia), on the food web structure of the Río de la Plata estuary and nearshore oceanic ecosystem. Biol Invasions. 2011;13(9): 2053–2061. 10.1007/s10530-011-0023-x [DOI] [Google Scholar]
- 300.Vallet C, Dauvin J-C, Hamon D, Dupuy C. Effect of the introduced common slipper shell on the suprabenthic biodiversity of the subtidal communities in the Bay of Saint-Brieuc. Conserv Biol. 2001;15(6): 1686–1690. 10.1046/j.1523-1739.2001.99295.x [DOI] [Google Scholar]
- 301.Thieltges DW. Impact of an invader: epizootic American slipper limpet Crepidula fornicata reduces survival and growth in European mussels. Mar Ecol Prog Ser. 2005;286: 13–19. 10.3354/meps286013 [DOI] [Google Scholar]
- 302.de Montaudouin X, Blanchet H, Hippert B. Relationship between the invasive slipper limpet Crepidula fornicata and benthic megafauna structure and diversity, in Arcachon Bay. J Mar Biol Assoc U.K. 2017; 10.1017/S0025315417001655 [DOI] [Google Scholar]
- 303.Nichols FH, Thompson JK, Schemel LE. Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam Potamocorbula amurensis. II. Displacement of a former community. Mar Ecol Prog Ser. 1990;66: 95–101. [Google Scholar]
- 304.Thompson JK. One estuary, one invasion, two responses: phytoplankton and benthic community dynamics determine the effect of an estuarine invasive suspension-feeder In: Dame RF, Olenin S, editors. The Comparative Roles of Suspension-Feeders in Ecosystems. Springer. NATO Science Series IV: Earth and Environmental Series, vol 47 Dordrecht, The Netherlands:Springer; 2005. pp. 291–316. 10.1007/1-4020-3030-4_17 [DOI] [Google Scholar]
- 305.Dugdale RC, Wilkerson FP, Parker AE. The effect of clam grazing on phytoplankton spring blooms in the low-salinity zone of the San Francisco Estuary: a modelling approach. Ecol Modell. 2016;340(C): 1–16. 10.1016/j.ecolmodel.2016.08.018 [DOI] [Google Scholar]
- 306.Kimmerer WJ, Lougee L. Bivalve grazing causes substantial mortality to an estuarine copepod population. J Exp Mar Bio Ecol. 2015;473: 53–63. 10.1016/j.jembe.2015.08.005 [DOI] [Google Scholar]
- 307.Cloern JE. Does the benthos control phytoplankton biomass in south San Francisco Bay? Mar Ecol Progr Ser. 1982;9: 191–202. [Google Scholar]
- 308.Officer CB, Smayda TJ, Mann R. Benthic filter feeding: A natural eutrophication control. Mar Ecol Progr Ser. 1982;9: 203–210. [Google Scholar]
- 309.Crooks JA. Habitat alteration and community-level effects of an exotic mussel, Musculista senhousia. Mar Ecol Progr Ser. 1998;162: 137–152. 10.3354/meps162137 [DOI] [Google Scholar]
- 310.Crooks JA. Assessing invader roles within changing ecosystems: Historical and experimental perspectives on an exotic mussel in an urbanized lagoon. Biol Invasions. 2001;3(1): 23–36. 10.1023/A:1011404914338 [DOI] [Google Scholar]
- 311.Reusch TBH, Williams SL. Variable responses of native eelgrass Zostera marina to a non- indigenous bivalve Musculista senhousia. Oecologia. 1998;113(3): 428–441. 10.1007/s004420050395 [DOI] [PubMed] [Google Scholar]
- 312.Creese R, Hooker S, DeLuca S, Wharton W. Ecology and environmental impact of Musculista senhousia (Mollusca: Bivalvia: Mytilidae) in Tamaki Estuary, Auckland, New Zealand. N Z J Mar Freshwater Res. 1997;31(2): 225–236. 10.1080/00288330.1997.9516760 [DOI] [Google Scholar]
- 313.de Greef K, Griffiths CL, Zeeman Z. Deja vu? A second mytilid mussel, Semimytilus algosus, invades South Africa's west coast. Afr J Mar Sci. 2013;35(3): 307–313. 10.2989/1814232X.2013.829789 [DOI] [Google Scholar]
- 314.Sadchatheeswaran S, Branch GM, Robinson TB. Changes in habitat complexity resulting from sequential invasions of a rocky shore: implications for community structure. Biol Invasions. 2015;17(6): 1799–1816. 10.1007/s10530-014-0837-4 [DOI] [Google Scholar]
- 315.Griffiths CL, Hockey PAR, Van Erkom Schurink C, Le Roux PJ. Marine invasive aliens on South African shores: implications for community structure and tropillc functioning. S Afr J Marine Sci. 1992;12(1): 713–722. 10.2989/02577619209504736 [DOI] [Google Scholar]
- 316.Witman JD, Grange GR. Links between rain, salinity, and predation in a rocky subtidal community. Ecology. 1998;79(7): 2429–2447. 10.1890/0012-9658(1998)079[2429:LBRSAP]2.0.CO;2 [DOI] [Google Scholar]
- 317.Geller JB. Decline of a native mussel masked by sibling species invasion. Conserv Biol. 1999;13(3): 661–664. 10.1046/j.1523-1739.1999.97470.x [DOI] [Google Scholar]
- 318.Davidson TM, de Rivera CE, Carlton JT. Small increases in temperature exacerbate the erosive effects of a non-native burrowing crustacean. J Exp Mar Bio Ecol. 2013;446: 115–121. 10.1016/j.jembe.2013.05.008 [DOI] [Google Scholar]
- 319.Einfeldt AL, Addison JA. Anthropocene invasion of an ecosystem engineer: resolving the history of Corophium volutator (Amphipoda: Corophiidae) in the North Atlantic. Biol J Linn Soc Lond. 2015;115(2): 288–304. 10.1111/bij.12507 [DOI] [Google Scholar]
- 320.Lutz-Collins V, Cox R, Quijón PA. Habitat disruption by a coastal invader: local community change in Atlantic Canada sedimentary habitats. Mar Biol. 2016;163: 177 10.1007/s00227-016-2947-2 [DOI] [Google Scholar]
- 321.Blakeslee AMH, Kamakura Y, Onufrey J, Makino W, Urabe J, Park S, et al. Reconstructing the invasion history of the Asian shorecrab, Hemigrapsus sanguineus (De Haan 1835) in the Western Atlantic. Mar Biol. 2017;164: 47 10.1007/s00227-017-3069-1 [DOI] [Google Scholar]
- 322.O’Connor NJ. Invasion dynamics on a temperate rocky shore: from early invasion to establishment of a marine invader. Biol Invasions. 2014;16(1): 73–87. 10.1007/s10530-013-0504-1 [DOI] [Google Scholar]
- 323.Kinzie RA III. The ecology of the replacment of Pseudosquilla ciliata (Fabricius) by Gonodactylus falcatus (Forskal) (Crustacea: Stomatopoda) recently introduced into the Hawaiian Islands. Pac Sci. 1968;22: 465–475. [Google Scholar]
- 324.O'Brien JM, Krumhansl KA, Scheibling RE. Invasive bryozoan alters interaction between a native grazer and its algal food. J Mar Biol Assoc U.K. 2013;93(5): 1393–1400. 10.1017/S0025315412001683 [DOI] [Google Scholar]
- 325.Occhipinti Ambrogi A. Biotic invasions in a Mediterranean Lagoon. Biol Invasions. 2000;2(2): 165–176. 10.1023/A:101000492 [DOI] [Google Scholar]
- 326.Ross DJ, Johnson CR, Hewitt CL. Impact of introduced seastars Asterias amurensis on survivorship of juvenile commercial bivalves Fulvia tenuicostata. Mar Ecol Prog Ser. 2002;241: 99–112. 10.3354/meps241099 [DOI] [Google Scholar]
- 327.Parry GD, Hirst AJ. Decadal decline in demersal fish biomass coincident with a prolonged drought and the introduction of an exotic starfish. Mar Ecol Prog Ser. 2016;544: 37–52. 10.3354/meps11577 [DOI] [Google Scholar]
- 328.Aguera A, Byrne M. A dynamic energy budget model to describe the reproduction and growth of invasive starfish Asterias amurensis in southeast Australia. Biol Invasions. 2018; 20(8): 2015–2031. 10.1007/s10530-018-1676-5 [DOI] [Google Scholar]
- 329.Kaplan KA, Hart DR, Hopkins K, Gallager S, York A, Taylor R, et al. Invasive tunicate restructures invertebrate community on fishing grounds and a large protected area on Georges Bank. Biol Invasions. 2018;20(1): 87–103. 10.1007/s10530-017-1517-y [DOI] [Google Scholar]
- 330.Theuerkauf KW, Eggleston DB, Theuerkauf SJ. An exotic species alters patterns of marine community development. Ecol Monogr. 2018;88(1): 92–108. 10.1002/ecm.1277 [DOI] [Google Scholar]
- 331.Blum JC, Chang AL, Liljesthrom M, Schenk ME, Steinberg MK, Ruiz GM. The non-native solitary ascidian Ciona intestinalis (L.) depresses species richness. J Exp Mar Biol Ecol. 2007;342(1): 5–14. 10.1016/j.jembe.2006.10.010 [DOI] [Google Scholar]
- 332.Sala E, Kizilkaya Z, Yildirim D, Ballesteros E. Alien marine fishes deplete algal biomass in the eastern Mediterranean. PLoS ONE. 2011;6(2): e17356 10.1371/journal.pone.0017356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Vergés A, Tomas F, Cebrian E, Ballesteros E, Kizilkaya Z, Dendrinos P, et al. Tropical rabbitfish and the deforestation of a warming temperate sea. J Ecol. 2014;102(6): 1518–1527. 10.1111/1365-2745.12324 [DOI] [Google Scholar]
- 334.Green SJ, Akins JL, Maljkovic A, Cote IM. Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE. 2012;7(3): e32596 10.1371/journal.pone.0032596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hackerott S, Valdivia A, Cox CE, Silbiger NJ, Bruno JF. Invasive lionfish had no measurable effect on prey fish community structure across the Belizean Barrier Reef. PeerJ 2017;5: e3270 10.7717/peerj.3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Albins MA. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol Invasions. 2013;15(1): 29–43. 10.1007/s10530-012-0266-1 [DOI] [Google Scholar]
- 337.Lesser MP, Slattery M. Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biol Invasions. 2011;13(8): 1855–1868. 10.1007/s10530-011-0005-z [DOI] [Google Scholar]
- 338.Peake J, Bogdanoff AK, Layman CA, Castillo B, Reale-Munroe K, Chapman J, et al. Feeding ecology of invasive lionfish (Pterois volitans and Pterois miles) in the temperate and tropical western Atlantic. Biol Invasions. 2018; 10.1007/s10530-017-1634-7 [DOI] [Google Scholar]
- 339.Cote IM, Smith NS. The lionfish Pterois sp. invasion: has the worst-case scenario come to pass? J Fish Biol. 2018;92(3): 660–689. 10.1111/jfb.13544 [DOI] [PubMed] [Google Scholar]
- 340.Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, et al. Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions. 1999;1: 3–19. 10.1023/A:1010034312781 [DOI] [Google Scholar]
- 341.Ruiz G, Fofonoff P, Hines AH. Non-indigenous species as stressors in estuarine and marine communities: assessing invasion impacts and interactions. Limnol Oceanogr. 1999;44: 950–972. 10.4319/lo.1999.44.3_part_2.0950 [DOI] [Google Scholar]
- 342.Olenin S, Minchin D, Daunys D. Assessment of biopollution in aquatic ecosystems. Mar Pollut Bull. 2007;55: 379–394. 10.1016/j.marpolbul.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 343.Hewitt CL, Campbell ML, Coutts ADM, Dahlstrom A, Shields D, Valentine J. (2011) Species biofouling risk assessment Commissioned by the Department of Agriculture, Fisheries and Forestry (DAFF); 2011. Available from: http://www.agriculture.gov.au/SiteCollectionDocuments/animal-plant/pests-diseases/marine-pests/biofouling-consult/species-biofouling-risk-assessment.pdf [Google Scholar]
- 344.Lovell S, Stone S, Fernandez L. The economic impacts of aquatic invasive species: A review of the literature. Agric Resour Econ Rev. 2006;35(1): 195–208. 10.1017/S1068280500010157 [DOI] [Google Scholar]
- 345.Simberloff D. How common are invasion-induced ecosystem impacts? Biol Invasions. 2011;13: 1255–68. 10.1007/s10530-011-9956-3 [DOI] [Google Scholar]
- 346.Katsanevakis S, Coll M, Piroddi C, Steenbeek J, Ben Rais Lasram F, Zenetos A, et al. Invading the Mediterranean Sea: biodiversity patterns shaped by human activities. Front Mar Sci. 2014;1: 32 10.3389/fmars.2014.00032 [DOI] [Google Scholar]
- 347.Kumschick S, Gaertner M, Vilà M, Essl F, Jeschke JM, Pyšek P, et al. Ecological impacts of alien species: quantification, scope, caveats, and recommendations. BioScience. 2015;65(1): 55–63. 10.1093/biosci/biu193 [DOI] [Google Scholar]
- 348.Thomsen MS, Byers JE, Schiel DR, Bruno JF, Olden JD, Wernberg T, et al. Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Mar Ecol Prog Ser. 2014;495: 39–47. 10.3354/meps10566 [DOI] [Google Scholar]
- 349.Blackburn TM, Essl F, Evans T, Hulme PE, Jeschke JM, Kühn I, et al. A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol. 2014;12(5): e1001850 10.1371/journal.pbio.1001850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Carlton JT. Bioinvasion ecology: Assessing invasion impact and scale In: E. Leppäkoski E, Gollasch S, Olenin S, editors. Invasive aquatic species of Europe. Distribution, impacts, and management, Dordrecht: Kluwer Academic Publishers; 2002. pp. 7–19. [Google Scholar]
- 351.Carlton JT. Quo vadimus exotica oceanica? Marine bioinvasion ecology in the twenty-first century. In: Pederson J, editor. Marine bioinvasions: Proceedings of the first national conference. Cambridge: MIT Sea Grant College Program; 2000. pp. 6–23.
- 352.Davidson TM, Ruiz GM, Torchin ME. Boring crustaceans shape the land-sea interface in brackish Caribbean mangroves. Ecosphere. 2016;7: e01430 10.1002/ecs2.1430 [DOI] [Google Scholar]
- 353.Marchini A, Ferrario J, Minchin D. Marinas may act as hubs for the spread of the pseudo-indigenous bryozoan Amathia verticillata (Delle Chiaje, 1822) and its associates. Sci Mar. 2015;79(3): 355–365. 10.3989/scimar.04238.03A [DOI] [Google Scholar]
- 354.Galil BS, Gevili R. Zoobotryon verticillatum (Delle Chiaje, 1822) (Bryozoa, Ctenostomatida, Vesiculariidae), a new occurrence in the Mediterranean coast of Israel. Mar Biodivers Rec. 2014;7: e17 10.1017/S1755267214000086 [DOI] [Google Scholar]
- 355.Eldredge L. Perspectives in aquatic exotic species management in the Pacific Islands Vol. 1 Introductions of commercially significant aquatic organisms to the Pacific Islands. south Pacific commission. Noumea: South Pacific Commission; 1994. [Google Scholar]
- 356.Hewitt CL, Campbell ML, Thresher RE, Martin RB, Boyd S, Cohen BF, et al. Introduced and cryptogenic species in Port Phillip Bay, Victoria, Australia. Mar Biol. 2004;144(1): 183–202. 10.1007/s00227-003-1173-x [DOI] [Google Scholar]
- 357.Zhan A, Macisaac HJ, Cristescu ME. Invasion genetics of the Ciona intestinalis species complex: from regional endemism to global homogeneity. Mol Ecol. 2010;19: 4678–4694. 10.1111/j.1365-294X.2010.04837.x [DOI] [PubMed] [Google Scholar]
- 358.Brunetti R, Gissi C, Pennati R, Caicci F, Gasparini F, Manni L. Morphological evidence that the molecularly determined Ciona intestinalis Type A and Type B are different species: Ciona robusta and Ciona intestinalis. J Zool Syst Evol Res. 2015;53: 186–193. 10.1111/jzs.12101 [DOI] [Google Scholar]
- 359.Bouchemousse S, Liautard-Haag C, Bierne N, Viard F. Distinguishing contemporary hybridization from past introgression with postgenomic ancestry-informative SNPs in strongly differentiated Ciona species. Mol Ecol. 2016;25: 5527–5542. 10.1111/mec.13854 [DOI] [PubMed] [Google Scholar]
- 360.Wonham MJ, Carlton JT. Trends in marine biological invasions at local and regional scales: the northeast Pacific Ocean as a model system. Biol Invasions. 2005;7(3): 369–392. 10.1007/s10530-004-2581-7 [DOI] [Google Scholar]
- 361.Ruiz GM, Fofonoff PW, Steves B, Foss SF, Shiba SN. Marine invasion history and vector analysis of California: a hotspot for western North America. Divers Distrib. 2011;17(2): 362–373. 10.1111/j.1472-4642.2011.00742.x [DOI] [Google Scholar]
- 362.Carlton JT, Ruiz GM. Vector science and integrated vector management in bioinvasion ecology: conceptual frameworks In: Mooney HA, Mack R, McNeely JA, Neville LE, Schei PJ, Waage JK, editors. Invasive alien species: a new synthesis. Covelo: Island Press; 2005. pp. 36–58. [Google Scholar]
- 363.Eno NC, Clark RA, Sanderson WG, editors. Non-native marine species in British waters: a review and directory 1st ed. Peterborough: Joint Nature Conservation Committee, 1997. [Google Scholar]
- 364.Leppäkoski E, Olenin S. Non-native species and rates of spread: lessons from the brackish Baltic Sea. Biol Invasions. 2000;2: 151–163. 10.1023/A:1010052809567 [DOI] [Google Scholar]
- 365.Cohen, AN and Carlton, JT. Non-indigenous aquatic species in a United States estuary: A case study of the biological invasions of the San Francisco Bay and delta. A report for the United States Fish and Wildlife Service and the National Sea Grant College Program Connecticut Sea Grant (NOAA Grant Number NA36RG047), 1995.
- 366.Mead A, Carlton JT, Griffiths CL, Rius M. Revealing the scale of marine bioinvasions in developing regions: a South African re-assessment. Biol Invasions. 2011;13(9): 1991–2008. 10.1007/s10530-011-0016-9 [DOI] [Google Scholar]
- 367.Minchin D, Cook EJ, Clark PF. Alien species in British brackish and marine waters. Aquat Invasions. 2013;8(1): 3–19. 10.3391/ai.2013.8.1.02 [DOI] [Google Scholar]
- 368.Evans J, Barbara J, Schembri PJ. Updated review of marine alien species and other ‘newcomers’ recorded from the Maltese Islands (Central Mediterranean). Mediterr Mar Sci. 2015;16(1): 225–244. 10.12681/mms.1064 [DOI] [Google Scholar]
- 369.Chainho P, Fernandes A, Amorim A, Avila SP, Canning-Clode J, Castro JJ, et al. Non-indigenous species in Portuguese coastal areas, coastal lagoons, estuaries and islands. Estuar Coast Shelf Sci. 2015;167(Part A): 199–211. 10.1016/j.ecss.2015.06.019 [DOI] [Google Scholar]
- 370.Ojaveer H, Olenin S, Narščius A, Florin A-B, Ezhova E, Gollasch S, et al. Dynamics of biological invasions and pathways over time: a case study of a temperate coastal sea. Biol Invasions. 2017;19: 799–813. 10.1007/s10530-016-1316-x [DOI] [Google Scholar]
- 371.Herbert RJH, Humphreys J, Davis CJ, Roberts C, Fletcher S, Crowe TP. Ecological impacts of non native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodivers Conserv. 2016;25(14): 2835–2865. 10.1007/s10531-016-1209-4 [DOI] [Google Scholar]
- 372.Mortensen S, Bodvin T, Strand Å, Holm MW, Dolmer P. Effects of a bio-invasion of the Pacific oyster, Crassostrea gigas (Thunberg, 1793) in five shallow water habitats in Scandinavia. Manag Biol Invasion. 2017;8(4): 543–552. 10.3391/mbi.2017.8.4.09 [DOI] [Google Scholar]
- 373.Zwerschke N, Hollyman PR, Wild R, Strigner R, Turner JR, King JW. Limited impact of an invasive oyster on intertidal assemblage structure and biodiversity: the importance of environmental context and functional equivalency with native species. Mar Biol. 2018;165(5): 89 10.1007/s00227-018-3338-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Richardson DM, Ricciardi A. Misleading criticisms of invasion science: A field guide. Divers Distrib. 2013;19(12): 1461–1467. 10.1111/ddi.12150 [DOI] [Google Scholar]
- 375.Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J, et al. Impacts of biological invasions—what's what and the way forward. Trends Ecol Evol. 2013;28, 58.–. 10.1016/j.tree.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 376.Marchini A, Galil BS, Occhipinti-Ambrogi A, Ojaveer H. The Suez Canal and Mediterranean marine invasions: media coverage. ICES WGITMO Report 2017 SCICOM Steering Group on Ecosystem Pressures and Impacts. ICES CM 2017/SSGEPI:09. Fort Lauderdale: ICES Annual Science Conference; 2017 10.13140/RG.2.2.24020.71046 [DOI]
- 377.Gelcich S, Buckley P, Pinnegar JK, Chilvers J, Lorenzoni I, Terry G, et al. Public awareness, concerns, and priorities about anthropogenic impacts on marine environments. Proc Natl Acad Sci USA. 2014;111(42): 15042–15047. 10.1073/pnas.1417344111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Giakoumi S, Guilhaumon F, Kark S, Terlizzi A, Claudet J, Felline S, et al. Space invaders; biological invasions in marine conservation planning. Divers Distrib. 2016;22: 1220–1231. 10.1111/ddi.12491 [DOI] [Google Scholar]
- 379.Galil B. Eyes wide shut: managing bio‐invasions in the Mediterranean marine protected areas In: Goriup PD, editor. Management of marine protected areas: A network perspective. Chichester: John Wiley & Sons Ltd; 2017. pp. 187–206. 10.1002/9781119075806.ch10 [DOI] [Google Scholar]
- 380.United Nations Convention on the Law of the Sea. United Nations; 1982.
- 381.Convention on Biological Diversity. Chapter XXVII. Environment. United Nations; 1992.
- 382.Report of the sixth meeting of the Conference of Parties to the Convention on Biological Diversity. Conference of Parties to the Convention on Biological Diversity. The Hague, 7–19 April 2002. UNEP/CBD/COP/6/20. Available from: https://www.cbd.int/doc/meetings/cop/cop-06/official/cop-06-20-en.pdf
- 383.Convention on Biological Diversity [internet]. Target 9 –Technical Rationale extended (provided in document COP/10/INF/12/Rev.1) [about 2 screens]. [cited 2018 Mar 09]. Available from: https://www.cbd.int/sp/targets/rationale/target-9/
- 384.Code of Practice to reduce the risks of adverse effects arising from introduction of marine non-indigenous species Copenhagen: ICES; 1973. [Google Scholar]
- 385.Code of Conduct for Responsible Fisheries. Rome: FAO; 1995. [Google Scholar]
- 386.Hosch G. Analysis of the implementation and impact of the FAO Code of Conduct for Responsible Fisheries since 1995 FAO Fisheries and Aquaculture Circular. No. 1038. Rome: FAO; 2009. Available from: http://www.fao.org/tempref/docrep/fao/011/i0604e/i0604e00.pdf [Google Scholar]
- 387.OIE. International aquatic animal health code, Fish, molluscs and crustaceans. Recommendations for international trade in aquatic animals and aquatic animal products 1st ed. Paris: World Organization for Animal Health; 1995. [Google Scholar]
- 388.OIE. Aquatic Animal Health Code. 20th ed. Paris: World Organization for Animal Health; 2017. Available from: http://www.oie.int/en/international-standard-setting/aquatic-code/access-online/ [Google Scholar]
- 389.Hewitt CL, Campbell ML, Gollasch S. Alien species in aquaculture Considerations for responsible use. Gland and Cambridge: IUCN; 2006. [Google Scholar]
- 390.International guidelines for preventing the introduction of unwanted aquatic organisms and pathogens from ships' ballast water and sediment discharges. International Maritime Organization. Resolution MEPC.50(31). London: IMO; 1991.
- 391.Guidelines for preventing the introduction of unwanted aquatic organisms and pathogens from ships' ballast water and sediment discharges. International Maritime Organization. Resolution A774(18). London: IMO; 1993.
- 392.Guidelines for the control and management of ships' ballast water to minimize the transfer of harmful aquatic organisms and pathogens. International Maritime Organization. Resolution A868(20). London: IMO; 1997.
- 393.International convention for the control and management of ships' ballast water and sediments International Maritime Organization. London: IMO; 2004. [Google Scholar]
- 394.International Maritime Organization [internet]. International Maritime Organization moves ahead with oceans and climate change agenda [about 5 screens]. [cited 2018 Mar 09]. Available from: http://www.imo.org/en/MediaCentre/PressBriefings/Pages/17-MEPC-71.aspx
- 395.International Convention on the Control of Harmful Anti-fouling Systems on Ships. International Maritime Organization. London: IMO; 2001. [Google Scholar]
- 396.International Maritime Organization. Guidelines for the control and management of ships' biofouling to minimize the transfer of invasive aquatic species. Resolution MEPC.207(62)). London: IMO; 2011.
- 397.International Maritime Organization. Guidance for minimizing the transfer of aquatic invasive species as biofouling (hull fouling) for recreational craft. MEPC.1/Circ.792. London: IMO; 2012
- 398.Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Communities. 1992;L206, 7–50. (May 21, 1992). Available from: http://eur-lex.europa.eu/eli/dir/1992/43/oj
- 399.Convention on the Conservation of European Wildlife and Natural Habitats. Council of Europe. European Treaty Series—No. 104. (Sep 19, 1979). Available from: https://rm.coe.int/1680078aff
- 400.Recommendation No. R(84)14 of the Committee of Ministers to Member States concerning the introduction of non-native species. Council of Europe. (Jun 21, 1984). Available from: https://rm.coe.int/16804c8381.
- 401.Convention on the Conservation of European Wildlife and Natural Habitats, standing Committee. Council of Europe. Recommendation No. 45 on controlling the proliferation of Caulerpa taxifolia in the Mediterranean (Mar 24, 1995).
- 402.Goulletquer P. Guide des organismes exotiques marins 1st ed. Paris: Belin; 2016. French. [Google Scholar]
- 403.Goulletquer P, Bachelet G, Sauriau PG, Noel P. Open Atlantic coast of Europe—A century of introduced species into French waters In: Leppäkoski E, Gollasch S, Olenin S, editors. Invasive aquatic species of Europe. Distribution, impacts and management. Dordrecht: Kluwer Academic Publishers; 2002. pp. 276–290. [Google Scholar]
- 404.Council regulation (EC) no. 708/2007 of 11 June 2007 concerning use of alien and locally absent species in aquaculture. European Commission. Official Journal of the European Communities. 2007;L168: 1–17. (Jun 11, 2007). Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32007R0708 [Google Scholar]
- 405.Petrocelli A, Cecere E, Verlaque M. Alien marine macrophytes in transitional water systems: new entries and reappearances in a Mediterranean coastal basin. BioInvasions Rec. 2013;2: 177–184. 10.3391/bir.2013.2.3.01 [DOI] [Google Scholar]
- 406.Wolf MA, Sfriso A, Moro I. Thermal pollution and settlement of new tropical alien species: the case of Grateloupia yinggehaiensis (Rhodophyta) in the Venice Lagoon. Estuar Coast Shelf Sci. 2014;147: 11–16. 10.1016/j.ecss.2014.05.020 [DOI] [Google Scholar]
- 407.Armeli Minicante S, Genovese G, Morabito M. Two new alien macroalgae identified by the DNA barcoding. Biol Mar Mediterr. 2014;21(1): 70–72. [Google Scholar]
- 408.Marchini A, Sorbe J-C, Torelli F, Lodola A, Occhipinti-Ambrogi A. The non-indigenous Paranthura japonica Richardson, 1909 in the Mediterranean Sea: travelling with shellfish? Mediterr Mar Sci. 2014;15(3): 545–553. 10.12681/mms.779 [DOI] [Google Scholar]
- 409.Cruscanti M, Innocenti G, Alvarado Bremer A, Galil BS. First report of the brown shrimp Penaeus aztecus (Crustacea, Decapoda, Penaeidae) in the Tyrrhenian Sea. Mar Biodivers Rec. 2015;8: e81 10.1017/S1755267215000664 [DOI] [Google Scholar]
- 410.Marchini A, Ferrario J, Occhipinti-Ambrogi A. Confirming predictions: the invasive isopod Ianiropsis serricaudis Gurjanova, 1936 (Crustacea: Peracarida) is abundant in the Lagoon of Venice (Italy). Acta Adriat. 2016;57(2): 331–336. [Google Scholar]
- 411.Commission Decision (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU. European Commission. Official Journal of the European Union. 2017;L125: 43–74. (May 17, 2017). Available from: http://eur-lex.europa.eu/eli/dec/2017/848/oj [Google Scholar]
- 412.Report from the Commission to the European Parliament and the Council assessing Member States’ monitoring programmes under the Marine Strategy Framework Directive (2008/56/EC). Brussels: European Commission 15; 2017. (Jan 16, 2017). Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0003&from=EN
- 413.Towards an EU Strategy on Invasive Species. Brussels: Commission of European Communities; 2008. (COM (2008) 789 final). (Dec 3, 2008). Available from: http://ec.europa.eu/environment/nature/invasivealien/docs/1_EN_ACT_part1_v6.pdf [Google Scholar]
- 414.Our life insurance, our natural capital: an EU biodiversity strategy to 2020. European Parliament resolution of 20 April 2012 on our life insurance, our natural capital: an EU biodiversity strategy to 2020 (2011/2307(INI)). Brussels: European Commission; 2012. Available from: http://ec.europa.eu/environment/nature/biodiversity/comm2006/pdf/EP_resolution_april2012.pdf [Google Scholar]
- 415.Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. European Commission. Official Journal of the European Union, 2014;L317/35 (Nov 4, 2014). Available from: http://eur-lex.europa.eu/eli/reg/2014/1143/oj [Google Scholar]
- 416.The Convention on Conservation of Nature in the South Pacific. Apia Convention 1976. (Jun 12, 1976). Available from: http://sedac.ciesin.org/entri/texts/nature.south.pacific.1976.html [cited 2017 May 5]
- 417.Protocol Concerning Mediterranean Specially Protected Areas and Biological Diversity in the Mediterranean (Apr 3, 1982). Available from: http://eur-lex.europa.eu/eli/prot/1999/800/oj [cited 2017 May 5]
- 418.Protocol concerning Protected Areas and Wild Fauna and Flora in the Eastern African Region. Nairobi Convention 1985. UNEP. (Jun 21, 1985) Available from: http://web.unep.org/nairobiconvention/protocol-concerning-protected-areas-and-wild-fauna-and-flora-eastern-african-region// [cited 2017 May 5]
- 419.Protocol concerning specially protected areas and wildlife to the convention for the protection and development of the marine environment in the wider Caribbean region. (Jan 18, 1990). Available from: http://www.cep.unep.org/content/about-cep/spaw [cited 2017 May 5]
- 420.Protocol on Environmental Protection to the Antarctic Treaty. (Oct 4, 1991). Available from http://www.ats.aq/documents/recatt/Att006_e.pdf
- 421.New Strategic Direction for COBSEA (2008–2012). Thailand: COBSEA Secretariat, United Nations Environment Programme; 2008. Available from: http://www.cobsea.org/documents/Meeting_Documents/19COBSEA/New%20Strategic%20Direction%20for%20COBSEA%202008-2012.pdf [Google Scholar]
- 422.Nonindigenous Aquatic Nuisance Prevention and Control Act of 1990. US Congress, Washington DC. (Dec 29, 2000). Available from: https://www.anstaskforce.gov/Documents/nanpca90.pdf
- 423.Canadian Action Plan to Address the Threat of Aquatic Invasive Species 2004. Canadian Council of Fisheries and Aquaculture Ministers Aquatic Invasive Species Task Group; 2014. Available from: http://waves-vagues.dfo-mpo.gc.ca/Library/365581.pdf, [cited 2018 Feb 12]. [Google Scholar]
- 424.Biosecurity Act 2015. The Biosecurity Act. Australia. No 61. (Jun 16, 2015). https://www.legislation.gov.au/Details/C2015A00061 [cited 2018 Feb 12]
- 425.Tiakina Aotearoa–Protect New Zealand: The biosecurity strategy for New Zealand. Wellington: Biosecurity Council; 2003. Available from: https://www.mpi.govt.nz/dmsdocument/7152-tiakina-aotearoa-protect-new-zealand-the-biosecurity-strategy-for-new-zealand [cited 2018 Feb 12] [Google Scholar]
- 426.Action Plan concerning species introductions and invasive species in the Mediterranean Sea Tunis: UNEP-MAP-RAC/SPA; 2005. Available from: http://www.rac-spa.org/sites/default/files/action_plans/invasive.pdf [Google Scholar]
- 427.HELCOM Baltic Sea Action Plan. Krakow: HELCOM Ministerial Meeting. Krakow; 2007. Available from: http://www.helcom.fi/Documents/Baltic%20sea%20action%20plan/BSAP_Final.pdf
- 428.General guidance on the voluntary interim application of the D1 ballast water exchange standard in the North-East Atlantic and the Baltic Sea. OSPAR Commission 2008–10. Available from: https://www.ospar.org/documents?v=32309
- 429.Marine menace: Alien invasive species in the marine environment Geneva: IUCN; 2009. Available from: https://www.iucn.org/content/marine-menace-alien-invasive-species-marine-environment [Google Scholar]
- 430.Arctic invasive Alien Species Strategy and Action Plan 2017 Conservation of Arctic Flora and Fauna and Protection of the Arctic Marine Environment Akureyri: CAFF and PAME; 2017. Iceland: Available from: https://www.caff.is/strategies-series/415-arctic-invasive-alien-species-strategy-and-action-plan [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper with all data sources being listed in the References section.