Abstract
Rapid eye movement sleep behavior disorder (RBD) is diagnosed by a clinical history of dream enactment accompanied by polysomnographic rapid eye movement sleep atonia loss (rapid eye movement sleep without atonia). Rapid eye movement sleep behavior disorder is strongly associated with neurodegenerative disease, especially synucleinopathies such as Parkinson disease, dementia with Lewy bodies, and multiple system atrophy. A history of RBD may begin several years to decades before onset of any clear daytime symptoms of motor, cognitive, or autonomic impairments, suggesting that RBD is the presenting manifestation of a neurodegenerative process. Evidence that RBD is a synlucleinopathy includes the frequent presence of subtle prodromal neurodegenerative abnormalities including hyposmia, constipation, and orthostatic hypotension, as well as abnormalities on various neuroimaging, neurophysiological, and autonomic tests. Up to 90.9% of patients with idiopathic RBD ultimately develop a defined neurodegenerative disease over longitudinal follow-up, although the prognosis for younger patients and antidepressant-associated RBD is less clear. Patients with RBD should be treated with either melatonin 3 to 12 mg or clonazepam 0.5 to 2.0 mg to reduce injury potential. Prospective outcome and treatment studies of RBD are necessary to enable accurate prognosis and better evidence for symptomatic therapy and future neuroprotective strategies.
Rapid eye movement sleep behavior disorder (RBD) is diagnosed when dream enactment and complex motor behaviors occur during rapid eye movement (REM) sleep, accompanied by supportive evidence from loss of normal REM sleep muscle atonia known as REM sleep without atonia (RSWA) during polysomnography.1 The prevalence of RBD has been estimated to be in the range of 0.5% to 2%,2–4 yet larger population-based studies of probable dream enactment symptoms suggest that RBD is likely considerably more frequent and present in between 5% and 13% of older community-dwelling adults aged 60 to 99 years.5–8 Rapid eye movement sleep behavior disorder appears to be more common in men than women in older adults,9–13 yet below the age of 50 years it is equally frequent in women and men.14–17 Rapid eye movement sleep behavior disorder is 5-fold more likely to develop in patients receiving antidepressants and 10-fold more likely to develop in those with a psychiatric diagnosis.15 Rapid eye movement sleep behavior disorder usually onsets in the fifth or sixth decade, although it may be seen in younger patients with antidepressant use, narcolepsy, autoimmunity, or developmental disorders.15,18–21 Risk factors for RBD are similar to Parkinson disease (PD), including lower educational level, previous head injury, occupational pesticide exposure, and farming, yet some distinct risk factors have also been reported, including smoking, ischemic heart disease, and inhaled corticosteroids,22,23 whereas caffeine use and smoking are not protective in RBD.
Rapid eye movement sleep behavior disorder is idiopathic when unassociated with neurological disorders or symptomatic when underlying causes such as autoimmune or inflammatory disorders, brain lesions, or provoking antidepressant medications are present.10,12,15,18,21,23–32 In both idiopathic and symptomatic categories, RBD is strongly associated with neurodegenerative diseases, especially synucleinopathies including PD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and pure autonomic failure.1,10,12,24–28,31,33–41 Rapid eye movement sleep behavior disorder may manifest initially as an idiopathic prodromal state that occurs years to decades before the evolution of overt motor, cognitive, or autonomic impairments as the presenting manifestation of synucleinopathy. Dream enactment symptoms and idiopathic RBD diagnosis may be accompanied by other subtle prodromal features such as subjective cognitive symptoms without evidence of impairment on neuropsychological testing, asymptomatic cognitive or motor deficits, hyposmia, constipation, and orthostatic hypotension; many of these features are associated with a higher risk of pheno-conversion to a defined neurodegenerative disorder.20,22,24,30,31,37,42–46
We will review evidence of the strong association between RBD and synucleinopathies, especially PD, mild cognitive impairment (MCI), DLB, and MSA. We will also review diagnosis and differential diagnosis of RBD as well as its pathophysiology and treatment. The article begins with an illustrative case typical of an initially idiopathic RBD diagnosis, that progresses to PD.
ILLUSTRATIVE CASE
Mr F was a 52-year-old man with a history of depression and anxiety. Because of loud disruptive snoring and a 10-year history of excessive daytime sleepiness (Epworth Sleepiness Scale score, 14), he underwent polysomnography, which exhibited an apnea-hypopnea index of 82 per hour and an oxyhemoglobin saturation nadir of 79%, consistent with severe obstructive sleep apnea. Rapid eye movement sleep atonia loss was also noted (Figure 1), prompting further collateral history from his wife that revealed that the patient had also developed violent sleep behaviors of arm flailing movements beginning at the age of 30 years, escalating to nightly shouting or arm flailing movements over the past 5 years for which he had not previously sought medical attention. He had inadvertently struck his spouse on occasion. He received fluoxetine 20 mg daily, which had been initiated 1 month before his polysomnogram. His family history was pertinent for parkinsonism and dementia in his mother, who had developed rest tremor in her 70s and died after the development of severe dementia in her 80s. Symptoms of hyposmia or constipation were absent.
FIGURE 1.
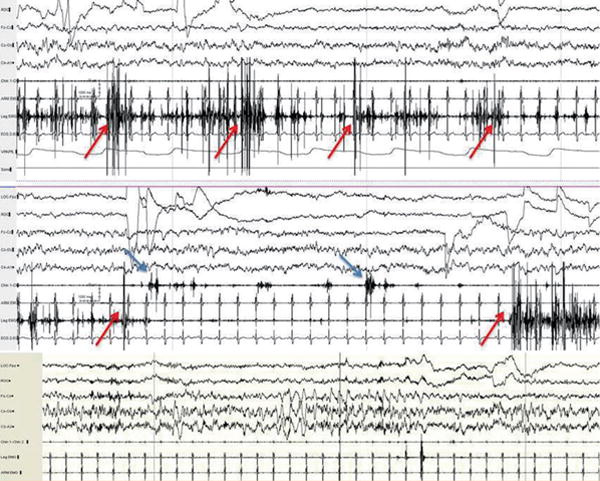
Rapid eye movement (REM) sleep atonia loss, also known as REM sleep without atonia, in a 52-year-old man with REM sleep behavior disorder. Note that the predominant abnormality in the top epoch is excessive phasic/transient muscle activity confined to the anterior tibialis muscle (seventh channel, red arrows) and the middle epoch shows additional activations of abnormal phasic bursting in the submentalis muscle (blue arrows, sixth channel). By contrast, the bottom polysomnogram epoch shows normal REM atonia levels in the chin, leg, and arm muscles (in channels 6-8).
Mr F’s initial neurological examination results at the age of 52 years were normal, without any signs of parkinsonism or cognitive decline. He was treated with nasal continuous positive airway pressure for severe obstructive sleep apnea, and with faithful adherence to treatment he noted some improvement in sleep quality, but no further improvement in parasomnia behaviors or daytime sleepiness; so modafinil 200 mg twice daily was added with incomplete benefit for his tendency to doze off, and he still needed a daily 15- to 20-minute nap. Melatonin was then prescribed, and after raising the dose to 6 mg, dream-enactment behaviors ceased entirely.
He was seen annually without further change until the age of 56 years, when repeat neurological examination demonstrated an intermittent right upper extremity postural and rest tremor, increased tone, and reduction of right arm swing during gait assessment. Because of concern about whether the tremor was more consistent with essential or parkinsonian tremor, a subsequent dopamine transporter uptake scan revealed asymmetrical reduced uptake in the tail of the left putamen. A diagnosis of PD was made, and carbidopa-levodopa 25/100 mg thrice daily was prescribed, with improvement in his tremor. Dream enactment frequency had decreased substantially within the year and he had discontinued melatonin.
This case reports several typical and remarkable features about the clinical course of idiopathic RBD as it evolves to a defined neurodegenerative disease. In this case, RBD was detected and brought to clinical attention only because of recognition of marked REM sleep atonia loss (RSWA) during polysomnography that had been performed for another indication for sleep apnea suspicion. Such cases of RBD diagnosed incidentally are not uncommon at the time of polysomnography in sleep medicine practice, and isolated REM sleep atonia loss without dream enactment is relatively common in the general population.47 A recent large idiopathic RBD case series from Barcelona has also similarly suggested that up to 44% of idiopathic RBD cases may be unaware of their sleep behaviors, suggesting that the diagnosis may be detected secondarily when patients present with other sleep problems.13 In this case, prompt diagnosis and treatment with melatonin helped prevent injury and enabled surveillance and early detection and treatment of symptomatic parkinsonism that may improve function and quality of life, and hopefully one day, serial follow-up may enable application of neuroprotective therapy.
PATHOPHYSIOLOGY OF RBD
The mechanisms for RSWA and RBD currently remain poorly understood. Most insights into REM sleep atonia control and its abnormal loss have been drawn from animal lesion studies and functional models, which have shown that REM sleep regulation predominantly involves the key pontine centers including the predominantly glutamatergic subcoeruleus/sublateral dorsal nucleus, the noradrenergic locus coeruleus, the cholinergic pedunculopontine and laterodorsal tegmental nuclei, as well as the medullary magnocellular reticular formation, with additional modulation by the hypothalamus, thalamus, substantia nigra, basal forebrain, limbic system, and frontal cortex.48–50 The possibility of RBD was foreseen by the eminent neurophysiologist Michel Jouvet, who initially described that dorsal pontine lesions near the locus coeruleus caused REM sleep atonia loss and RSWA, anticipating the eventual recognition of RBD in humans by Dr Carlos Schenck and Dr Mark Mahowald and their team at Hennepin County Medical Center in Minnesota.48–50 In rats, REM onset and offset is mediated by a complex reciprocal flip-flop circuit between the “REM-active” sublateral dorsal nucleus, pontine “REM-inactive” nuclei, and the inhibitory gamma-aminobutyric acid/galaninergic non-REM sleep “active” center in the basal forebrain ventrolateral preoptic nucleus, and it appears this mechanism for REM sleep duration and latency remains largely unaffected in REM sleep behavior disorder.48,49,51–55 During REM sleep, excitatory sublateral dorsal/subcoeruleus nucleus glutamatergic neurons also activate spinal cord inhibitory interneurons to hyperpolarize and thereby inhibit the spinal motoneuron pool, causing REM sleep atonia, so sublateral dorsal/subcoeruleus nucleus lesions resulting from brain lesions in the dorsal pons cause REM sleep atonia loss and may cause clinical REM sleep behavior disorder (Figure 2).29,56,57 Posterior hypothalamic hypocretin may also further stabilize the REM-active and REM-inactive centers and networks, and in the context of hypocretin deficiency as in narcolepsy type 1, RBD may also occur.58
FIGURE 2.
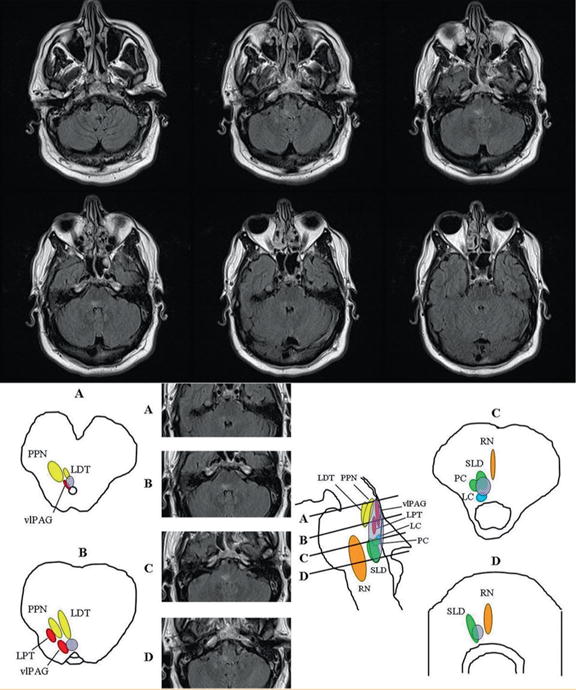
Rapid eye movement (REM) sleep behavior disorder results from a lesion in the neuronal network regulating REM atonia in the dorsal medial pons. A 47-year-old man evolved mononeuritis multiplex with biopsy-proven vasculitis, followed by multiple cranial neuropathies involving the III, IV, VI, VII, IX, and X cranial nerves, and within weeks, he also began exhibiting complex motor behavior during sleep paralleling dream mentation of defense against attack during which he would punch, kick, flail his arms, or stand up in bed. Magnetic resonance imaging of the brain exhibited a hyperintense FLAIR signal abnormality in the dorsal pontomedullary region, neighboring the sublateral dorsal nucleus, which is the “REM-on” center governing REM sleep atonia. A lesion in this area leads to REM sleep atonia loss (REM sleep without atonia), a permissive state for dream enactment and REM sleep behavior disorder. Coronal fluid-attenuated inversion recovery (FLAIR) intensity MRI sections at the level of the medulla and pons show a discrete longitudinally extensive hyperintense lesion at the level of the dorsomedial pons extending rostrally to the right superior pons ventral to the superior cerebellar peduncle. The brainstem nuclei thought to be involved in REM sleep atonia regulation are shown on human brainstem templates. Letters for each template and corresponding MRI FLAIR sections selected from our case represent cross-sectional views through the brainstem as shown in the midsagittal figure, with sections representing (A) the pontomesencephalic junction, (B) the upper/mid pons, (C) the lower/mid pons, and (D) the pontomedullary junction. The approximate location of the lesion is shown in the superimposed pink oval. LC = locus ceruleus; LDT = laterodorsal tegmental nucleus; LPT = lateral pontine tegmentum; PPN = pedunculopontine nucleus; RN = raphe nucleus; SLD = sublateral dorsal nucleus; vlPAG = ventrolateral part of the periaqueductal gray matter. Reproduced from Neurology,29 with permission from Wolters Kluwer.
The hypothesis of Braak, based on pathologic staging of Lewy body progression in PD, suggests that pathology begins in the medulla and pons, which could be associated with initial RSWA and RBD development in idiopathic RBD. Then as Lewy disease ascends to the substantia nigra, the motor expression of PD evolves, and eventually as Lewy disease progresses to the cortex, PD dementia unfolds.56,59 In those who develop DLB, RBD typically begins many years before the onset of cognitive decline, with parkinsonism and other core features of DLB usually evolving sometime later. The onset of cognitive decline prior to parkinsonism in the DLB phenotype may be explained by the temporal sequence of evolution from RBD to DLB, such that the nigrostriatal system may be impacted in a less severe and/or later fashion than in the evolution of RBD to PD/PD dementia. Limbic and/or neocortical structures are impacted more severely and/or earlier in the DLB phenotype, thereby explaining the onset of cognitive decline prior to parkinsonism. However, not all RBD, PD, and DLB patients follow this typical ascending Braak model of progression, since RBD may also follow cognitive, motor, or autonomic symptoms in some patients. Rapid eye movement sleep behavior disorder is not universally seen in patients with all of the synucleinopathies, suggesting that topographic onset and progression varies considerably across individual patients. Finally, not all patients with RSWA are aware of symptoms of sleep disturbance, implying that either the neurophysiologic property of RSWA may dissociate from clinical dream enactment, or that patients’ dream enactment behaviors may be subtle and remain subclinical and covert.
Lastly, genetic studies of RBD remain limited, but RBD has been associated with glucocerebrosidase sequence variation and PD-related genetic loci including the microtubule associated protein tau (MAPT) gene in genome-wide association studies,41,60,61 and the leucine rich repeat kinase (LRRK2) mutation carriers with Parkinson disease.41,62,63 It has been proposed that the variability in RBD expression in some genetic Parkinsonian disorders may reflect heterogeneous neuropathological substrate with less marked involvement of the brainstem REM atonia control structures in LRRK2 mutation carriers than in idiopathic PD.
DIAGNOSIS OF RBD
Diagnosis of RBD requires either a clinical history of sleep-related complex motor behaviors or REM sleep complex vocal or motor behaviors recorded during polysomnography, accompanied by RSWA.1 Rapid eye movement sleep behavior disorder diagnosis also requires that the sleep disturbance is not better explained by another disorder, such as obstructive sleep apnea or an alternative non-rapid eye movement (NREM) sleep parasomnia. Idiopathic RBD is diagnosed when there is clinical sleep-related complex motor dream enactment behavior, without a clearly associated underlying pathology, such as PD or related synucleinopathies.1 Even when idiopathic, RBD has a very strong association with PD and other synucleinopathies. Rapid eye movement sleep behavior disorder is considered symptomatic RBD when it occurs in direct association with previously diagnosed PD, DLB, or MSA, or when there is another known underlying pathology such as a brain lesion (Figure 2).29,57,64
Confirmatory collateral history from a bed partner is necessary, especially when the patient has cognitive impairment.24,65 Dream content in RBD usually involves aggressive themes, like being chased or defense against attack by animals or people.24,66–69 Screaming or shouting, arm flailing, punching, kicking, or running movements paralleling action-filled dream content is common, complicated by minor or serious injuries such as bruising, lacerations, fractures, and subdural hematomas.12,70,71 Vivid dream recall and falls from bed have been associated with injury.70
Loss of REM sleep atonia, known as RSWA, is required for diagnosis, although probable RBD may be diagnosed on clinical grounds when a clear history of dream enactment—type behaviors is present. There are several well-validated RBD screening measures for the diagnosis of probable RBD when polysomnography is unavailable or when REM sleep is not captured during polysomnogram recording.65,72–77 These various instruments include the REM Sleep Behavior Disorder Screening Questionnaire,75 the Innsbruck REM Sleep Behavior Disorder Inventory,76 the RBD-HK,74 and the Mayo Sleep Questionnaire (MSQ).65,72,77 The MSQ is an especially well-validated diagnostic tool for RBD screening in older patients with cognitive impairment and/or parkinsonism, administered to either the bed partner (informant version) or the patient (patient version) when a bed partner is not available. The REM Sleep Behavior Disorder Single-Question Screen is a similar patient-administered tool containing essentially the same core question regarding dream enactment.73 The REM Sleep Behavior Disorder Single-Question Screen and MSQ have each shown reasonable sensitivity and specificity in comparison to polysomnography when it is unavailable or impractical, yet polysomnography remains essential to support a clinical diagnosis of RBD.
Rapid eye movement sleep without atonia may be identified either qualitatively in clinical practice or quantitatively defined by visual or automated methods for research purposes.47,78–84 Rapid eye movement sleep without atonia is of 3 types: phasic/transient (short muscle activity bursts), tonic (sustained increase in the muscle activity background voltage), or “any” (either phasic or tonic). Several quantitative methods are available as references for RBD diagnostic cutoffs for use in research or for difficult or questionable cases in clinical settings. Currently there is no consensus on the best standard for RSWA, although widely validated visual quantitative techniques include the Sleep Innsbruck Barcelona, Montreal, American Academy of Sleep Medicine, and Mayo visual scoring methods, and the automated REM atonia index.76,78,79,83–85 The Sleep Innsbruck Barcelona submentalis (chin) combined with flexor digitorum superficialis phasic muscle activity is the most specific method as recommended by the American Academy of Sleep Medicine, although it is not yet widely used in most sleep laboratories.1,47
Rapid eye movement sleep without atonia may also be an incidental or isolated finding during polysomnography without clinical accompaniment. The significance and natural history of isolated RSWA has not been defined. In a recent study of motor events, phasic RSWA exceeded defined cutoffs for RBD in 25% of a community sample of people without symptoms or signs of dream enactment.86 Isolated RSWA is most frequent in older men and is a common finding in patients receiving antidepressant medications.21,87 Isolated RSWA has been associated with positive neurodegenerative biomarkers such as loss of smell or color vision88 and has been associated with gait freezing and cognitive impairment in PD.89,90 Rapid eye movement sleep without atonia amounts have also been shown to progress over time in patients with idiopathic RBD91 and have been associated with a higher risk of phenoconversion to PD in idiopathic RBD.92 Isolated RSWA may also phenoconvert to idiopathic RBD, as shown in a recent study, with 7% to 14% of patients developing clinical RBD during longitudinal follow-up.88 Further research of isolated RSWA is necessary to clarify whether it could be a biomarker for an underlying synucleinopathy.
CLINICAL IMPLICATIONS OF RBD
Rapid eye movement sleep behavior disorder is strongly associated with synucleinopathy neurodegeneration. There are several lines of converging evidence substantiating that idiopathic RBD is a prodromal form of synucleinopathy, including longitudinal cohort outcome studies, neurodegenerative biomarker studies, and pathological evidence from both autopsy series and demonstration of extranigral α-synuclein pathology in living patients with RBD. One very recent large cross sectional study of 171 RBD patients found that 74% (95% CI 66, 80%) met Movement Disorders Society criteria for a diagnosis of prodromal Parkinson’s disease.41 Longitudinal cohort studies of patients with idiopathic RBD have shown consistent evidence for a strong association with eventual phenoconversion to a defined neurodegenerative disease, predominantly the synucleinopathy phenotypes of PD, nonamnestic MCI, DLB, and MSA (Figure 3).10,11,28,31,93–97 Phenoconversion risk over 2 to 5 years is approximately 15% to 35%, and longitudinal follow-up between 12 and 25 years increases to 41% to 90.9%, although the risk of phenoconversion has substantial interindividual variability, sometimes occurring over 50 years after initial symptom onset.10,11,20,28,31,84,95,97 In a general community sample of elderly individuals older than 70 years, probable RBD symptoms endorsement on the MSQ was associated with a ratio for phenoconversion to PD or MCI over three years of 2.2 (95% CI, 1.3-3.9).5 Given the high lifetime risk for phenoconversion to overt synucleinopathy in patients with idiopathic RBD, when and how best to counsel patients about this risk remains a current controversy and uncertain point in practice.98
FIGURE 3.
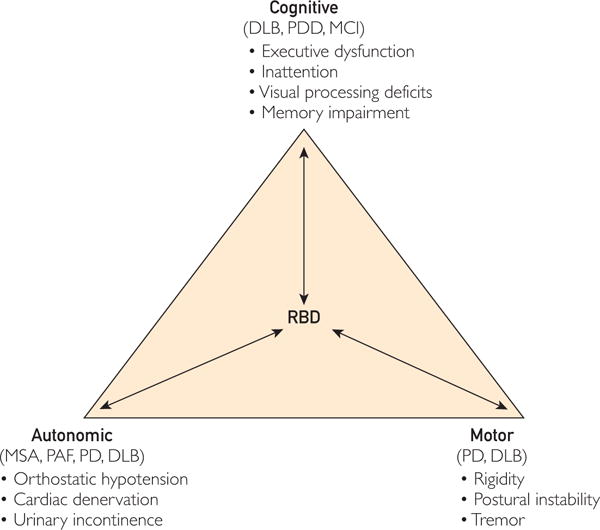
Theoretical model of rapid eye movement sleep behavior disorder (RBD) and its relationship with different clinical manifestations of synucleinopathies. Idiopathic RBD may remain as an isolated syndrome with or without additional cognitive, autonomic, or motor “soft signs” that may or may not evolve toward more definitive, clinically overt, “full-blown” synucleinopathy subtypes of dementia with Lewy bodies (DLB), multiple system atrophy (MSA), Parkinson disease (PD), or PD with dementia (PDD). Patients with parkinsonism and dementia are considered to have PDD if cognitive decline occurs longer than 1 year after the emergence of parkinsonism and DLB if patients present with cognitive decline less than 1 year after the emergence of parkinsonism. Patients with PD and patients without RBD may represent different clinical phenotypes, given different and more severe motor signs, cognitive impairments, and autonomic signs in those with PD compared with those without PD. MCI = mild cognitive impairment; PAF = pure automatic failure. Reproduced from Sleep Med,93 with permission from Elsevier, Inc.
Several clinical investigational studies have clearly shown that patients with idiopathic RBD have frequent symptoms or signs, indicating likely underlying synucleinopathy pathology, including hyposmia and constipation, orthostatic hypotension, and gait abnormalities, which have been associated with a higher risk of phenoconversion.23,30,31,41,42,45,89,99–101 In addition, neuropsychological deficits of dysexecutive, attentional, visuoperceptual, and short-term memory impairments appear to progress over time,38,44,96,102–107 and neurophysiological markers such as electroencephalographic slowing,108–111 and neuroimaging studies have also demonstrated findings indicating likely underlying synucleinopathy pathology.
Neuroimaging has shown altered neuromelanin signal intensity in the locus coeruleus/subcoeruleus nucleus region,29,57,112–115 and functional imaging studies, including dopamine transporter uptake, have found decreased nigrostriatal putamenal dopaminergic uptake in patients with idiopathic RBD95,116–120 as well as decreased cortical thickness and diffuse resting state metabolic network dysfunction with similar patterns to PD.121–123 Lastly, autopsy series of patients with RBD have found underlying synucleinopathy in 94% of patients,25,97,124,125 and tissues from living patients with idiopathic RBD have exhibited abnormal α-synuclein immunoreactivity in peripheral tissues such as the submandibular gland126 and colonic submucosal nerve fibers or ganglia.127 Other neurodegenerative pathologies have been reported, sometimes intermingling with typical synucleinopathy pathology of Lewy bodies and neurites with neuronal loss, including Alzheimer disease pathology of amyloid beta and tau proteins, progressive supranuclear palsy, neuronal brain iron accumulation type 1, other neurodegenerative pathologies, or brain lesions. However, the implication from converging evidence from cohort outcome studies, clinical neurodegenerative biomarker studies, and pathological series is that in most cases, particularly in older adults with symptom onset after the age of 50 years, RBD is associated with underlying synucleinopathy pathology.
In younger patients with RBD symptom onset before the age of 50 years, prodromal synucleinopathy is still possible, but alternative nondegenerative causes should also be considered, such as narcolepsy, autoimmunity, and antidepressant-associated RBD. In narcolepsy type 1 (previously known as narcolepsy with cataplexy), REM sleep dream imagery (hypnogogic hallucinations) and atonia (cataplexy, sleep paralysis) intrude into wakefulness, and conversely, waking motor tone (RSWA) and complex motor behavior giving rise to RBD may also be seen.128–130 Rapid eye movement sleep without atonia may occur with or without RBD, which may occur in 36% to 50% of those with narcolepsy.129,131 Another cause of RBD in younger and some older patients is paraneoplastic or autoimmune disorders, such as the anti-voltage-gated potassium channel antibody complex syndrome (including the CASPR-2 and LGI-1 epitopes), IgLON5 disease, and brainstem lesions caused by inflammatory, neoplastic, or cerebrovascular disorders.29,57,64,132–139 In addition, both RBD and RSWA have been strongly associated with antidepressant use, especially selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and tricyclic antidepressants, and it remains unclear whether this association is mediated by reversible pharmacological effects or whether antidepressants are causing earlier expression of RSWA and RBD in predisposed individuals with covert synucleinopathy15,16,21,30 in a manner analogous to drug-induced parkinsonism.
RAPID EYE MOVEMENT SLEEP BEHAVIOR DISORDER TREATMENT
All patients with RBD should be counseled about bedroom safety principles to prevent injury or serious consequences, including lowering the mattress to the floor or safe-guarding against falls by placing mattresses or foam cushions on the floor, padding any sharp bedside furniture surfaces, and removing firearms from the bedroom environment. In some cases, advising separate bedrooms to prevent bed partner injury may be necessary. A bed alarm system that could reassure and alert the patient during RBD behaviors may be useful in some patients, especially if they also have sleep walking behaviors.140 Comorbid obstructive sleep apnea treatment with nasal continuous positive airway pressure may also improve the frequency and severity of RBD behaviors.
The 2 main pharmacological treatments of RBD are melatonin and clonazepam.141–147 Both have been shown to prevent injury and reduce the frequency and severity of RBD behaviors, with melatonin having fewer adverse effects and better tolerability than clonazepam.142 Most adverse effects are dose related, such as the carryover of sedation to the next morning, headache, or daytime sleepiness, and these can often be improved by lowering the dose used. Melatonin is particularly desirable in symptomatic RBD treatment for patients with comorbid sleep apnea or memory problems, and the recommended starting dose is 3 mg, increased gradually to the range of 6 to 12 mg at bedtime, with the average effective dose being 6 mg. Melatonin has been shown to increase REM sleep atonia levels, thereby diminishing RSWA.53,144,148 Clonazepam 0.25 to 2.0 mg at bedtime is also a useful treatment for RBD. However, clonazepam does not appear to reduce RSWA and may instead modulate dreaming or complex motor behaviors. Clonazepam may exacerbate comorbid obstructive sleep apnea and cognitive impairment, so it should be used with caution in elderly patients, especially those with PD, DLB, or MSA.145,146 Adverse effects include sedation, sexual dysfunction, and imbalance. Other RBD treatments with reported benefit for RBD in small uncontrolled case series include pramipexole, donepezil, ramelteon, Yi-Gan San, and cannabinoids,141,149,150 and further evidence basis for the use of all symptomatic treatment of RBD is greatly needed. Some medications such as the antidepressants mirtazapine, b-blockers, or tramadol may worsen the frequency and severity of RBD, and when possible should be either discontinued or reduced.149 Patients should also be counseled to avoid alcohol abuse and withdrawal, which have been reported to precipitate RBD.
FUTURE DIRECTIONS
Considering the compelling evidence of RBD usually reflecting an underlying synucleinopathy, there is considerable interest among clinicians and investigators to plan for future therapeutic trials in the hope of modifying the course, delaying the onset, or preventing the development of the disabling manifestations of PD, DLB, and MSA. A schematic framework for such an effort is shown in Figure 4.
FIGURE 4.
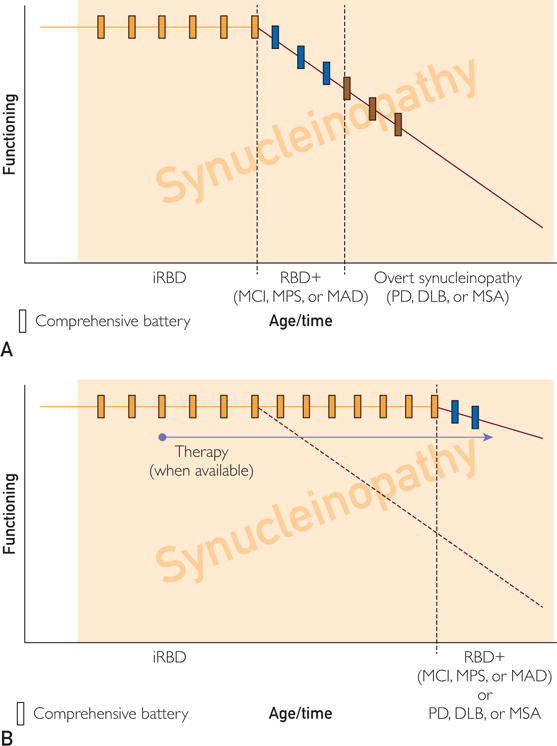
Schematic framework for viewing natural history studies (A) and showing efficacy of disease-modifying therapies (B) in patients with RBD and/or other neurological symptoms and findings. See text for details. DLB = dementia with Lewy bodies; iRBD = idiopathic RBD (RBD without other neurological symptoms or signs); MAD = mild autonomic dysfunction (phase preceding overt MSA); MCI = mild cognitive impairment (phase preceding overt DLB); MPS = mild parkinsonian signs (phase preceding overt PD); MSA = multiple system atrophy; PD = Parkinson disease; RBD = rapid eye movement sleep behavior disorder
The basic tenet of this framework (Figure 4, A) is that most patients with idiopathic RBD will develop other features reflecting accumulating synucleinopathy pathology in the central and peripheral nervous system such that phenoconversion to a definable transitional state, and then to an overt synucleinopathy phenotype, will evolve over time. One would predict that most of those who are destined to develop a parkinsonism-predominant syndrome will develop mild parkinsonian signs (as well as other degrees of neurological/neuropsychiatric dysfunction) before meeting clinical criteria for PD; most of those destined to develop a dementia-predominant syndrome will develop MCI (as well as other degrees of neurological/neuropsychiatric dysfunction) before meeting clinical criteria for DLB; and most of those destined to develop an autonomic dysfunction-predominant syndrome will develop mild autonomic dysfunction (as well as other degrees of neurological/neuropsychiatric dysfunction) before meeting clinical criteria for MSA or a Lewy body disorder such as PD or DLB.150–152 Additionally, some individuals do not develop either initial predominant phenotypic manifestations, or evolve along a clearly delineated motor, cognitive, or autonomic phenotypic pathway, and instead develop parkinsonism, dementia, and dysautonomia simultaneously in tandem. Among those with idiopathic RBD, predicting when phenoconversion will occur and which phenotype will evolve is not yet possible on the basis of available data. A comprehensive battery of clinical, neuropsychological, biofluid, neuroimaging, and electrophysiological measures performed at regular intervals (eg, every 1-3 years) involving a large number of patients with idiopathic RBD, as well as the application of outcome criteria developed by consensus panels and analytical models, will be required to develop clinical trial methodology to plan for future disease-modifying trials. Many envision the future to appear as shown in Figure 4, B, and liken the goals to be similar to previous and ongoing therapeutic trials in those with hypertension or hyperlipidemia in delaying the onset or preventing cardiovascular and cerebrovascular morbidity/mortality. With adequate research funding and infrastructure development for these efforts and the development of putative therapies for synucleinopathy pathophysiology, the future appears brighter to potentially affect the burden of synuclein-associated neurodegenerative disease.
CONCLUSION
Rapid eye movement sleep behavior disorder is diagnosed when patients present with a clinical history of complex motor dream enactment behaviors during REM sleep and exhibit REM sleep atonia loss (RSWA) on polysomnography. Both idiopathic RBD and symptomatic RBD are strongly associated with synucleinopathy neurodegenerative diseases, including PD, nonamnestic MCI, DLB, and MSA. Evidence suggests that RBD is likely to represent prodromal synucleinopathy, preceding overt motor, cognitive, or autonomic impairments by years to decades. Melatonin 3 to 12 mg or clonazepam 0.5 to 2.0 mg are the treatments of choice for RBD to reduce the potential for injury. Further evidence regarding the clinical course of RBD and its treatment are needed to clarify its rate of phenoconversion to overt synucleinopathy and to determine a defined time point at which neuroprotective therapies could be offered to prevent or delay more devastating motor, cognitive, and autonomic sequelae of synucleinopathy.
CME Activity.
Target Audience
The target audience for Mayo Clinic Proceedings is primarily internal medicine physicians and other clinicians who wish to advance their current knowledge of clinical medicine and who wish to stay abreast of advances in medical research.
Statement of Need
General internists and primary care physicians must maintain an extensive knowledge base on a wide variety of topics covering all body systems as well as common and uncommon disorders. Mayo Clinic Proceedings aims to leverage the expertise of its authors to help physicians understand best practices in diagnosis and management of conditions encountered in the clinical setting.
Accreditation
In support of improving patient care, Mayo Clinic College of Medicine and Science is accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the health care team.
Credit Statement
Mayo Clinic College of Medicine designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s).™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Credit Statement
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1 MOC point in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Learning Objectives
On completion of this article, you should be able to (1) recognize rapid eye movement sleep behavior disorder, a potentially injurious parasomnia strongly associated with synucleinopathy neurodegeneration; (2) delineate idiopathic and symptomatic forms of rapid eye movement sleep behavior disorder; and (3) choose efficacious and tolerable treatments for rapid eye movement sleep behavior disorder.
Disclosures
As a provider accredited by ACCME, Mayo Clinic College of Medicine and Science (Mayo School of Continuous Professional Development) must ensure balance, independence, objectivity, and scientific rigor in its educational activities. Course Director(s), Planning Committee members, Faculty, and all others who are in a position to control the content of this educational activity are required to disclose all relevant financial relationships with any commercial interest related to the subject matter of the educational activity. Safeguards against commercial bias have been put in place. Faculty also will disclose any off-label and/or investigational use of pharmaceuticals or instruments discussed in their presentation. Disclosure of this information will be published in course materials so that those participants in the activity may formulate their own judgments regarding the presentation.
In their editorial and administrative roles, Karl A. Nath, MBChB, Terry L. Jopke, Kimberly D. Sankey, and Nicki M. Smith, MPA, have control of the content of this program but have no relevant financial relationship(s) with industry.
Dr St. Louis receives research support from Mayo Clinic CCaTS, NIH/NHLBI, and Sunovion and book royalties from Wiley-Blackwell for Epilepsy and the Interictal State: Co-morbidities and Quality of Life. Dr Boeve reports that he is an investigator in clinical trials sponsored by Cephalon, Allon Pharmaceuticals, and GE Healthcare. He receives royalties from the publication of a book titled The Behavioral Neurology of Dementia (Cambridge Medicine) (2009). He has received honoraria from the American Academy of Neurology. He receives research support from the National Institute on Aging (grant nos. P50 AG16574 [coinvestigator], U01 AG06786 [coinvestigator], RO1 AG32306 [coinvestigator]) and the Mangurian Foundation.
Method of Participation
In order to claim credit, participants must complete the following:
Read the activity.
Complete the online CME Test and Evaluation. Participants must achieve a score of 80% on the CME Test. One retake is allowed.
Visit www.mayoclinicproceedings.org, select CME, and then select CME articles to locate this article online to access the online process. On successful completion of the online test and evaluation, you can instantly download and print your certificate of credit.
Estimated Time
The estimated time to complete each article is approximately 1 hour.
Hardware/Software
PC or MAC with Internet access.
Date of Release
11/1/2017
Expiration Date
10/31/2019 (Credit can no longer be offered after it has passed the expiration date.)
Privacy Policy
Questions?
Contact dletcsupport@mayo.edu.
Acknowledgments
Grant Support: The work was supported by Clinical and Translational Science Awards grant UL1 TR000135 from the National Center for Advancing Translational Science (NCATS). Dr Boeve’s support relevant to this work includes grants P50 AG016574, UO1 AG006786, and RO1 AG015866, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, and the Little Family Foundation.
Abbreviations and Acronyms
- DLB
dementia with Lewy bodies
- MCI
mild cognitive impairment
- MSA
multiple system atrophy
- MSQ
Mayo Sleep Questionnaire
- PD
Parkinson disease
- RBD
rapid eye movement sleep behavior disorder
- REM
rapid eye movement
- RSWA
rapid eye movement sleep without atonia
Footnotes
Potential Competing Interests: Dr St. Louis receives research support from Mayo Clinic CCaTS, NIH/NHLBI, and Sunovion and book royalties from Wiley-Blackwell for Epilepsy and the Interictal State: Co-morbidities and Quality of Life. Dr Boeve reports that he is an investigator in clinical trials sponsored by Cephalon, Allon Pharmaceuticals, and GE Healthcare. He receives royalties from the publication of a book titled The Behavioral Neurology of Dementia (Cambridge Medicine) (2009). He has received honoraria from the American Academy of Neurology. He receives research support from the National Institute on Aging (grant nos. P50 AG16574 [coinvestigator], U01 AG06786 [coinvestigator], RO1 AG32306 [coinvestigator]) and the Mangurian Foundation.
The Symposium on Neurosciences will continue in an upcoming issue.
References
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. Third. Chicago: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Ohayon MM, Schenck CH. Violent behavior during sleep: prevalence, comorbidity and consequences. Sleep Med. 2010;11(9):941–946. doi: 10.1016/j.sleep.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frauscher B, Gschliesser V, Brandauer E, et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11(2):167–171. doi: 10.1016/j.sleep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147–1152. doi: 10.5665/sleep.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71(1):49–56. doi: 10.1002/ana.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417–1421. doi: 10.1002/mds.26350. [DOI] [PubMed] [Google Scholar]
- 7.Adler CH, Hentz JG, Shill HA, et al. Probable RBD is increased in Parkinson’s disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord. 2011;17(6):456–458. doi: 10.1016/j.parkreldis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WK, Hermann DM, Chen YK, et al. Brainstem infarcts predict REM sleep behavior disorder in acute ischemic stroke. BMC Neurol. 2014;14:88. doi: 10.1186/1471-2377-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wing YK, Lam SP, Li SX, et al. REM sleep behaviour disorder in Hong Kong Chinese: clinical outcome and gender comparison. J Neurol Neurosurg Psychiatry. 2008;79(12):1415–1416. doi: 10.1136/jnnp.2008.155374. [DOI] [PubMed] [Google Scholar]
- 10.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9(2):e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(pt 2):331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Arcos A, Iranzo A, Serradell M, Gaig C, Santamaria J. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep. 2016;39(1):121–132. doi: 10.5665/sleep.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonakis A, Howard RS, Ebrahim IO, Merritt S, Williams A. REM sleep behaviour disorder (RBD) and its associations in young patients. Sleep Med. 2009;10(6):641–645. doi: 10.1016/j.sleep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Teman PT, Tippmann-Peikert M, Silber MH, Slocumb NL, Auger RR. Idiopathic rapid-eye-movement sleep disorder: associations with antidepressants, psychiatric diagnoses, and other factors, in relation to age of onset. Sleep Med. 2009;10(1):60–65. doi: 10.1016/j.sleep.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Ju YE, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12(3):278–283. doi: 10.1016/j.sleep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Bodkin CL, Schenck CH. Rapid eye movement sleep behavior disorder in women: relevance to general and specialty medical practice. J Womens Health (Larchmt) 2009;18(12):1955–1963. doi: 10.1089/jwh.2008.1348. [DOI] [PubMed] [Google Scholar]
- 18.Hancock KL, St Louis EK, McCarter SJ, et al. Quantitative analyses of REM sleep without atonia in children and adolescents with REM sleep behavior disorder. Minn Med. 2014;97(5):43. [PubMed] [Google Scholar]
- 19.Lloyd R, Tippmann-Peikert M, Slocumb N, Kotagal S. Characteristics of REM sleep behavior disorder in childhood. J Clin Sleep Med. 2012;8(2):127–131. doi: 10.5664/jcsm.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75(6):494–499. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarter SJ, St Louis EK, Sandness DJ, et al. Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep. 2015;38(6):907–917. doi: 10.5665/sleep.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postuma RB, Iranzo A, Hogl B, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol. 2015;77(5):830–839. doi: 10.1002/ana.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frauscher B, Jennum P, Ju YE, et al. Comorbidity and medication in REM sleep behavior disorder: a multicenter case-control study. Neurology. 2014;82(12):1076–1079. doi: 10.1212/WNL.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeve BF, Silber MH, Ferman TJ, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14(8):754–762. doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61(1):40–45. doi: 10.1212/01.wnl.0000073619.94467.b0. [DOI] [PubMed] [Google Scholar]
- 27.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 28.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 29.St Louis EK, McCarter SJ, Boeve BF, et al. Lesional REM sleep behavior disorder localizes to the dorsomedial pons. Neurology. 2014;83(20):1871–1873. doi: 10.1212/WNL.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postuma RB, Gagnon JF, Tuineaig M, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 2013;36(11):1579–1585. doi: 10.5665/sleep.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain. 2009;132(pt 12):3298–3307. doi: 10.1093/brain/awp244. [DOI] [PubMed] [Google Scholar]
- 32.Wing YK, Li SX, Mok V, et al. Prospective outcome of rapid eye movement sleep behaviour disorder: psychiatric disorders as a potential early marker of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83(4):470–472. doi: 10.1136/jnnp-2011-301232. [DOI] [PubMed] [Google Scholar]
- 33.Boeve BF, Silber MH, Ferman TJ. REM sleep behavior disorder in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2004;17(3):146–157. doi: 10.1177/0891988704267465. [DOI] [PubMed] [Google Scholar]
- 34.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77(9):875–882. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferman TJ, Boeve BF, Smith GE, et al. Dementia with Lewy bodies may present as dementia and REM sleep behavior disorder without parkinsonism or hallucinations. J Int Neuropsychol Soc. 2002;8(7):907–914. doi: 10.1017/s1355617702870047. [DOI] [PubMed] [Google Scholar]
- 36.McCarter SJ, St Louis EK, Boeve BF. Mild cognitive impairment in rapid eye movement sleep behavior disorder: a predictor of dementia? Sleep Med. 2013;14(11):1041–1042. doi: 10.1016/j.sleep.2013.08.780. [DOI] [PubMed] [Google Scholar]
- 37.McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease [published correction appears in Curr Neurol Neurosci Rep. 2012;12(2):226] Curr Neurol Neurosci Rep. 2012;12(2):182–192. doi: 10.1007/s11910-012-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133(pt 2):540–556. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray ME, Ferman TJ, Boeve BF, et al. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology. 2013;81(19):1681–1689. doi: 10.1212/01.wnl.0000435299.57153.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St Louis EK, Boeve AR, Boeve BF. REM sleep behavior disorder in Parkinson’s disease and other synucleinopathies. Mov Disord. 2017;32(5):645–658. doi: 10.1002/mds.27018. [DOI] [PubMed] [Google Scholar]
- 41.Barber TR, Lawton M, Rolinski M, et al. Prodromal Parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017;40(8) doi: 10.1093/sleep/zsx071. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fantini ML, Postuma RB, Montplaisir J, Ferini-Strambi L. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Res Bull. 2006;70(4–6):386–390. doi: 10.1016/j.brainresbull.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Massicotte-Marquez J, Décary A, Gagnon JF, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70(15):1250–1257. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 44.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann Neurol. 2009;66(1):39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 45.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011;69(5):811–818. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- 46.Vendette M, Gagnon JF, Soucy JP, et al. Brain perfusion and markers of neurodegeneration in rapid eye movement sleep behavior disorder. Mov Disord. 2011;26(9):1717–1724. doi: 10.1002/mds.23721. [DOI] [PubMed] [Google Scholar]
- 47.Frauscher B, Iranzo A, Gaig C, et al. SINBAR (Sleep Innsbruck Barcelona) Group Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835–847. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967;47(2):117–177. doi: 10.1152/physrev.1967.47.2.117. [DOI] [PubMed] [Google Scholar]
- 49.Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. CR Soc Biol. 1965;159:895–899. [Google Scholar]
- 50.Sakai K, Sastre JP, Salvert D, Touret M, Tohyama M, Jouvet M. Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: an HRP study. Brain Res. 1979;176(2):233–254. doi: 10.1016/0006-8993(79)90981-8. [DOI] [PubMed] [Google Scholar]
- 51.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 52.Luppi PH, Clément O, Sapin E, et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15(3):153–163. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32(29):9785–9795. doi: 10.1523/JNEUROSCI.0482-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peever J, Luppi PH, Montplaisir J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37(5):279–288. doi: 10.1016/j.tins.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Fraigne JJ, Torontali ZA, Snow MB, Peever JH. REM sleep at its coredcircuits, neurotransmitters, and pathophysiology. Front Neurol. 2015;6:123. doi: 10.3389/fneur.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(pt 11):2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 57.McCarter SJ, Tippmann-Peikert M, Sandness DJ, et al. Neuroimaging-evident lesional pathology associated with REM sleep behavior disorder. Sleep Med. 2015;16(12):1502–1510. doi: 10.1016/j.sleep.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133(pt 2):568–579. doi: 10.1093/brain/awp320. [DOI] [PubMed] [Google Scholar]
- 59.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 60.Gan-Or Z, Mirelman A, Postuma RB, et al. GBA mutations are associated with Rapid Eye Movement Sleep Behavior Disorder. Ann Clin Transl Neurol. 2015;2(9):941–945. doi: 10.1002/acn3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gan-Or Z, Girard SL, Noreau A, et al. Parkinson’s disease genetic loci in rapid eye movement sleep behavior disorder. J Mol Neurosci. 2015;56(3):617–622. doi: 10.1007/s12031-015-0569-7. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez-Santiago R, Iranzo A, Gaig C, et al. MAPT association with REM sleep behavior disorder. Neurol Genet. 2017;3(1):e131. doi: 10.1212/NXG.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pont-Sunyer C, Iranzo A, Gaig C, et al. Sleep disorders in Parkinsonian and nonparkinsonian LRRK2 mutation carriers. PLoS One. 2015;10(7):e0132368. doi: 10.1371/journal.pone.0132368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tippmann-Peikert M, Boeve BF, Keegan REM sleep behavior disorder initiated by acute brainstem multiple sclerosis. Neurolo BMgy. 2006;66(8):1277–1279. doi: 10.1212/01.wnl.0000208518.72660.ff. [DOI] [PubMed] [Google Scholar]
- 65.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12(5):445–453. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valli K, Frauscher B, Peltomaa T, Gschliesser V, Revonsuo A, Högl B. Dreaming furiously? A sleep laboratory study on the dream content of people with Parkinson’s disease and with or without rapid eye movement sleep behavior disorder. Sleep Med. 2015;16(3):419–427. doi: 10.1016/j.sleep.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Uguccioni G, Golmard JL, de Fontréaux AN, Leu-Semenescu S, Brion A, Arnulf I. Fight or flight? Dream content during sleepwalking/sleep terrors vs. rapid eye movement sleep behavior disorder. Sleep Med. 2013;14(5):391–398. doi: 10.1016/j.sleep.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 68.D’Agostino A, Manni R, Limosani I, Terzaghi M, Cavallotti S, Scarone S. Challenging the myth of REM sleep behavior disorder: no evidence of heightened aggressiveness in dreams. Sleep Med. 2012;13(6):714–719. doi: 10.1016/j.sleep.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Fantini ML, Corona A, Clerici S, Ferini-Strambi L. Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology. 2005;65(7):1010–1015. doi: 10.1212/01.wnl.0000179346.39655.e0. [DOI] [PubMed] [Google Scholar]
- 70.McCarter SJ, St Louis EK, Boswell CL, et al. Factors associated with injury in REM sleep behavior disorder. Sleep Med. 2014;15(11):1332–1338. doi: 10.1016/j.sleep.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry. 1989;146(9):1166–1173. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 72.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. 2013;9(5):475–480. doi: 10.5664/jcsm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Med. 2010;11(1):43–48. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaireda new diagnostic instrument. Mov Disord. 2007;22(16):2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 76.Frauscher B, Ehrmann L, Zamarian L, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012;27(13):1673–1678. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- 77.McCord SV, McCarter SJ, St Louis EK, Silber MH, Boeve BF. Validation of the Mayo sleep questionnaire patient version in a clinical sleep medicine practice. Sleep. 2015;38(Suppl):A254. [Google Scholar]
- 78.Ferri R, Manconi M, Plazzi G, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res. 2008;17(1):89–100. doi: 10.1111/j.1365-2869.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 79.Ferri R, Rundo F, Manconi M, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med. 2010;11(9):947–949. doi: 10.1016/j.sleep.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Frauscher B, Iranzo A, Högl B, et al. SINBAR (Sleep Innsbruck Barcelona Group) Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31(5):724–731. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarter SJ, St Louis EK, Sandness DJ, et al. Diagnostic REM sleep muscle activity in idiopathic REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep Med. 2017;33:23–29. doi: 10.1016/j.sleep.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42(7):1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 83.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25(13):2044–2051. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 84.McCarter SJ, St Louis EK, Sandness DJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med. 2017;33:23–29. doi: 10.1016/j.sleep.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 86.Frauscher B, Gabelia D, Mitterling T, et al. Motor events during healthy sleep: a quantitative polysomnographic study. Sleep. 2014;37(4):763–773. 773A–773B. doi: 10.5665/sleep.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCarter SJ, St Louis EK, Boeve BF, Sandness DJ, Silber MH. Greatest rapid eye movement sleep atonia loss in men and older age. Ann Clin Transl Neurol. 2014;1(9):733–738. doi: 10.1002/acn3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stefani A, Gabelia D, Högl B, et al. Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a pilot study. J Clin Sleep Med. 2015;11(11):1273–1279. doi: 10.5664/jcsm.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alibiglou L, Videnovic A, Planetta PJ, Vaillancourt DE, MacKinnon CD. Subliminal gait initiation deficits in rapid eye movement sleep behavior disorder: a harbinger of freezing of gait? Mov Disord. 2016;31(11):1711–1719. doi: 10.1002/mds.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Videnovic A, Marlin C, Alibiglou L, Planetta PJ, Vaillancourt DE, Mackinnon CD. Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology. 2013;81(12):1030–1035. doi: 10.1212/WNL.0b013e3182a4a408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iranzo A, Ratti PL, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J. Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep. 2009;32(9):1149–1153. doi: 10.1093/sleep/32.9.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Postuma RB, Gagnon JF, Rompré S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74(3):239–244. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCarter SJ, St Louis EK, Boeve BF. Is rapid eye movement sleep behavior disorder in Parkinson disease a specific disease subtype? Sleep Med. 2013;14(10):931–933. doi: 10.1016/j.sleep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder [published correction appears in Neurology. 1996;46(6):1787] Neurology. 1996;46(2):388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 95.Iranzo A, Lomeña F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] [published correction appears in Lancet Neurol. 2010;9(11):1045] Lancet Neurol. 2010;9(11):1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 96.Iranzo A, Molinuevo JL, Santamaría J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5(7):572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 97.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 98.Arnaldi D, Antelmi E, St Louis EK, et al. Idiopathic REM sleep behavior disorder and neurodegenerative risk: To tell or not to tell to the patient? How to minimize the risk? Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.11.002. [Epub ahead of print] [DOI] [PubMed]
- 99.Frauscher B, Nomura T, Duerr S, et al. Investigation of autonomic function in idiopathic REM sleep behavior disorder. J Neurol. 2012;259(6):1056–1061. doi: 10.1007/s00415-011-6298-0. [DOI] [PubMed] [Google Scholar]
- 100.Mahlknecht P, Iranzo A, Högl B, et al. Sleep Innsbruck Barcelona Group Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015;84(7):654–658. doi: 10.1212/WNL.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 101.Arnulf I, Neutel D, Herlin B, et al. Sleepiness in idiopathic REM sleep behavior disorder and parkinson disease. Sleep. 2015;38(10):1529–1535. doi: 10.5665/sleep.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferini-Strambi L, Di Gioia MR, Castronovo V, Oldani A, Zucconi M, Cappa SF. Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): does the idiopathic form of RBD really exist? Neurology. 2004;62(1):41–45. doi: 10.1212/01.wnl.0000101726.69701.fa. [DOI] [PubMed] [Google Scholar]
- 103.Marques A, Dujardin K, Boucart M, et al. REM sleep behaviour disorder and visuoperceptive dysfunction: a disorder of the ventral visual stream? J Neurol. 2010;257(3):383–391. doi: 10.1007/s00415-009-5328-7. [DOI] [PubMed] [Google Scholar]
- 104.Sasai T, Miyamoto T, Miyamoto M, et al. Impaired decision-making in idiopathic REM sleep behavior disorder. Sleep Med. 2012;13(3):301–306. doi: 10.1016/j.sleep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 105.Terzaghi M, Sinforiani E, Zucchella C, et al. Cognitive performance in REM sleep behaviour disorder: a possible early marker of neurodegenerative disease? Sleep Med. 2008;9(4):343–351. doi: 10.1016/j.sleep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 106.Rolinski M, Zokaei N, Baig F, et al. Visual short-term memory deficits in REM sleep behaviour disorder mirror those in Parkinson’s disease. Brain. 2016;139(pt 1):47–53. doi: 10.1093/brain/awv334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Youn S, Kim T, Yoon IY, et al. Progression of cognitive impairments in idiopathic REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2016;87(8):890–896. doi: 10.1136/jnnp-2015-311437. [DOI] [PubMed] [Google Scholar]
- 108.Fantini ML, Gagnon JF, Petit D, et al. Slowing of electroen-cephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003;53(6):774–780. doi: 10.1002/ana.10547. [DOI] [PubMed] [Google Scholar]
- 109.Iranzo A, Isetta V, Molinuevo JL, et al. Electroencephalographic slowing heralds mild cognitive impairment in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11(6):534–539. doi: 10.1016/j.sleep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 110.Rodrigues Brazète J, Gagnon JF, Postuma RB, Bertrand JA, Petit D, Montplaisir J. Electroencephalogram slowing predicts neurodegeneration in rapid eye movement sleep behavior disorder. Neurobiol Aging. 2016;37:74–81. doi: 10.1016/j.neurobiolaging.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 111.Rodrigues Brazète J, Montplaisir J, Petit D, et al. Electroencephalogram slowing in rapid eye movement sleep behavior disor- der is associated with mild cognitive impairment. Sleep Med. 2013;14(11):1059–1063. doi: 10.1016/j.sleep.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 112.Unger MM, Belke M, Menzler K, et al. Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions. Sleep. 2010;33(6):767–773. doi: 10.1093/sleep/33.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.García-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain. 2013;136(pt 7):2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boeve BF, St Louis EK, Kantarci K. Neuromelanin-sensitive imaging in patients with idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139(pt 4):1005–1007. doi: 10.1093/brain/aww030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ehrminger M, Latimier A, Pyatigorskaya N, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139(pt 4):1180–1188. doi: 10.1093/brain/aww006. [DOI] [PubMed] [Google Scholar]
- 116.Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797–805. doi: 10.1016/S1474-4422(11)70152-1. [DOI] [PubMed] [Google Scholar]
- 117.Miyamoto M, Miyamoto T, Iwanami M, et al. Preclinical substantia nigra dysfunction in rapid eye movement sleep behaviour disorder. Sleep Med. 2012;13(1):102–106. doi: 10.1016/j.sleep.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 118.Stiasny-Kolster K, Doerr Y, Möller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128(pt 1):126–137. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 119.Eisensehr I, Linke R, Noachtar S, Schwarz J, Gildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder: comparison with Parkinson’s disease and controls. Brain. 2000;123(pt 6):1155–1160. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 120.De Marzi R, Seppi K, Högl B, et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2016;79(6):1026–1030. doi: 10.1002/ana.24646. [DOI] [PubMed] [Google Scholar]
- 121.Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–627. doi: 10.1212/WNL.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu P, Yu H, Peng S, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2014;137(pt 12):3122–3128. doi: 10.1093/brain/awu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rahayel S, Montplaisir J, Monchi O, et al. Patterns of cortical thinning in idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2015;30(5):680–687. doi: 10.1002/mds.25820. [DOI] [PubMed] [Google Scholar]
- 124.Boeve BF, Dickson DW, Olson EJ, et al. Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med. 2007;8(1):60–64. doi: 10.1016/j.sleep.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45(4):709–712. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 126.Vilas D, Iranzo A, Tolosa E, et al. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15(7):708–718. doi: 10.1016/S1474-4422(16)00080-6. [DOI] [PubMed] [Google Scholar]
- 127.Sprenger FS, Stefanova N, Gelpi E, et al. Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85(20):1761–1768. doi: 10.1212/WNL.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schenck CH, Mahowald MW. Motor dyscontrol in narcolepsy: rapid-eye-movement (REM) sleep without atonia and REM sleep behavior disorder. Ann Neurol. 1992;32(1):3–10. doi: 10.1002/ana.410320103. [DOI] [PubMed] [Google Scholar]
- 129.Dauvilliers Y, Rompré S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30(7):844–849. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.St Louis EK. Patient management problem. Continuum (Minneap Minn) 2017;23(4):1184–1192. doi: 10.1212/CON.0000000000000511. Sleep Neurology. [DOI] [PubMed] [Google Scholar]
- 131.Ferri R, Franceschini C, Zucconi M, et al. Searching for a marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep. 2008;31(10):1409–1417. [PMC free article] [PubMed] [Google Scholar]
- 132.Compta Y, Iranzo A, Santamaría J, Casamitjana R, Graus F. REM sleep behavior disorder and narcoleptic features in anti-Ma2-associated encephalitis. Sleep. 2007;30(6):767–769. doi: 10.1093/sleep/30.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cornelius JR, Pittock SJ, McKeon A, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68(6):733–738. doi: 10.1001/archneurol.2011.106. [DOI] [PubMed] [Google Scholar]
- 134.Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59(1):178–181. doi: 10.1002/ana.20693. [DOI] [PubMed] [Google Scholar]
- 135.Josephs KA, Silber MH, Fealey RD, Nippoldt TB, Auger RG, Vernino S. Neurophysiologic studies in Morvan syndrome. J Clin Neurophysiol. 2004;21(6):440–445. doi: 10.1097/00004691-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Bonello M, Jacob A, Ellul MA, et al. IgLON5 disease responsive to immunotherapy. Neurol Neuroimmunol Neuroin flamm. 2017;4(5):e383. doi: 10.1212/NXI.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88(18):1736–1743. doi: 10.1212/WNL.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Graus F, Santamaria J. Understanding anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e393. doi: 10.1212/NXI.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Honorat JA, Komorowski L, Josephs KA, et al. IgLON5 antibody: Neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e385. doi: 10.1212/NXI.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Howell MJ, Arneson PA, Schenck CH. A novel therapy for REM sleep behavior disorder (RBD) J Clin Sleep Med. 2011;7(6):639A–644A. doi: 10.5664/jcsm.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung J, St Louis EK. Treatment options in REM sleep behavior disorder. Curr Treat Options Neurol. 2016;18(11):50. doi: 10.1007/s11940-016-0433-2. [DOI] [PubMed] [Google Scholar]
- 142.McCarter SJ, Boswell CL, St Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14(3):237–242. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281–284. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 144.Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. 2010;19(4):591–596. doi: 10.1111/j.1365-2869.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 145.Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100(3):333–337. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- 146.Li SX, Lam SP, Zhang J, et al. A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med. 2016;21:114–120. doi: 10.1016/j.sleep.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 147.Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14(3):507–511. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 148.St Louis EK, McCarter SJ, Boeve BF, et al. Ramelteon for idiopathic rem sleep behavior disorder: implications for pathophysiology and future treatment trials; commentary on esaki et al. an open-labeled trial of ramelteon in idiopathic rapid eye movement sleep behavior disorder. J Clin Sleep Med. 2016;12(5):683–693. doi: 10.5664/jcsm.5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010;6(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- 150.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, et al. Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol. 2017;81(2):287–297. doi: 10.1002/ana.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Silber MH, Boeve BF, St Louis EK. Letter on “Natural history of pure autonomic failure: A United States prospective cohort”. Ann Neurol. 2017;81(6):910. doi: 10.1002/ana.24948. [DOI] [PubMed] [Google Scholar]
- 152.Jung Y, Boot BP, Mielke MM, et al. Phenoconversion from probable rapid eye movement sleep behavior disorder to mild cognitive impairment to dementia in a population-based sample. Alzheimers Dement (Amst) 2017;8:127–130. doi: 10.1016/j.dadm.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


