Abstract
Purpose
Daratumumab and its use in combination with other agents is becoming a new standard of care for treatment of multiple myeloma (MM). We mechanistically studied how daratumumab acts on NK cells.
Experimental design
Quantities of NK cells in peripheral blood (PB) and/or bone marrow (BM) of MM patients or healthy donors were examined by flow cytometry. NK cell apoptosis and the associated mechanism were assessed by flow cytometry and immunoblotting. Patients' NK cells were expanded in vitro using feeder cells. Combination treatment of daratumumab and expanded NK cells was performed using an MM.1S xenograft animal model.
Results
CD38−/low NK cells survived, while CD38+ NK cells were almost completely eliminated, in PB and BM of daratumumab-treated MM patients. NK cell depletion occurred due to daratumumab-induced NK cell fratricide via antibody-dependent cellular cytotoxicity (ADCC). Consequently, CD38−/low NK cells were more effective for eradicating MM cells than were CD38+ NK cells in the presence of daratumumab. Blockade of CD38 with the F(ab)2 fragments of daratumumab inhibited the antibody-mediated NK cell fratricide. CD38−/low NK cells displayed a significantly better potential for expansion than CD38+ NK cells, and the expanded NK cells derived from the former population were more cytotoxic than those derived from the latter against MM cells. Therefore, infusion of ex vivo-expanded autologous NK cells from daratumumab-treated patients may improve the antibody therapy.
Conclusion
We unravel a fratricide mechanism for daratumumab-mediated NK cell depletion and provide a potential therapeutic strategy to overcome this side effect in daratumumab-treated MM patients.
Introduction
Multiple myeloma (MM) is one of most frequently diagnosed hematological cancers occurring in developed countries, accounting for approximately 2% of all cancer deaths and 10-15% of all hematologic malignancies in the United States (1). The recent development and FDA approval of therapeutic monoclonal antibodies (mAbs), including daratumumab (a mAb against CD38) and elotuzumab (a mAb against CS1), is changing the treatment algorithm for MM. However, MM still relapses and remains incurable, with especially short progression-free survival periods (less than 21 months) (2-5).
As a single agent, elotuzumab is safe but has low efficacy, while daratumumab has a response rate of over 30% (6,7). As with many other cancers, combination therapy has always been more successful compared to single agents in treating MM, as each patient has multiple MM clones at the time of diagnosis and more clones develop after relapse; daratumumab combined with lenalidomide and/or dexamethasone is becoming a new standard of care treatment for MM (2). Recent clinical trials of bortezomib and dexamethasone with daratumumab showed a significant improvement in the rate of progression-free survival (2,8). It was also shown that this combination treatment lacked dose-limiting toxic effects and had a greater than 80%overall rate of response(6,7).
Daratumumab binds the CD38 molecule and mediates tumor cell killing via various mechanisms of action, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), and direct induction of tumor cell apoptosis (9). ADCC, including that induced by daratumumab, is mediated by natural killer (NK) cells. Moreover, accumulating evidence implicates NK cells as an indispensable component of immune surveillance preventing tumor occurrence as well asrelapse (10,11).
In the current study, we demonstrate that peripheral blood (PB)as well as bone marrow (BM) NK cells were depleted in MM patients who have undergone daratumumab therapy. This NK cell depletion occurs as a result of daratumumab-induced fratricide among NK cells, due to high levels of CD38 surface expression on these cells. We observed that the remaining NK cells in these MM patients are CD38−/low and ex vivo-expanded NK (eNK) cells from daratumumab-treated MM patients are highly proliferative and have the potential to rescue this daratumumab-induced NK cell depletion in MM patents. We believe that our study is clinically significant as daratumumab-treated MM patients frequently relapse, an observation which may be at least partially explained by daratumumab-induced NK cell depletion.
Materials and Methods
Mice
NOD. Cg-prkdcscid IL2rgtm1Wjl/szJ (NSG) mice (6- to 8-week-old) were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). All experiments were approved by The Ohio State University Animal Care and Use Committee. Mice were checked once a day for signs of discomfort, weight loss, ataxia, and paralysis to measure MM progression.
Cell lines
MM cell lines (MM.1S) were obtained from the American Type Culture Collection and were maintained in Roswell Park Memorial Institute 1640 (RPMI-1640) medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma, St. Louis, MO). The NK-92 cell line and K562 feeder cells were received from Dr. Michael A. Caligiuri's lab and cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma, St. Louis, MO). Interleukin (IL)-2 (100 U/ml) was also included in the culture of the NK-92 cell line. These cell lines have not been authenticated since receipt but were routinely tested to ensure they are negative for mycoplasma using MycoAlert™ PLUS Mycoplasma Detection Kit from Lonza (Walkersville, MD).
Patient and healthy donor samples
PB and BM samples were collected from MM patients who had undergone treatment with or without daratumumab at the James Cancer Hospital. All patient samples were obtained with an IRB approval. PB mononuclear cells (PBMCs) of healthy donors were derived from leukopaks obtained from the American Red Cross.
NK cell expansion
Primary NK cells were purified using a negative selection method, as detailed in the Supplemental Information. NK cells were expanded using PBMCs or purified NK cells cultured in the presence of IL-2 (100 U/mL) and K562 feeder cells expressing membrane-bound IL-21, as previously described (12). In brief, NK cells or PBMCs and irradiated feeder cells (1:1 ratio for NK:feeder cells and 1:2 for PBMCs:feeder cells) were co-cultured in RPMI 1640 supplemented with 20% heat-inactivated FBS, L-Glutamine, and IL-2 (100 U/mL) at 37 °C in a 5% CO2 incubator. Media were changed based on cell density, and an equal number of irradiated feeder cells were added every 7 days. Cells preserved for future use were stored at -80 °C in a solution of FBS containing 10% DMSO at a maximum density of 2.5 ×107 cells per vial.
MM mouse model and bioluminescence imaging
Firefly luciferase-expressing MM.1S cells were established using a Pinco-pGL3-luc/GFP virus as previously described (13), and GFP positive cells were purified by FACS. An orthotopic xenograft MM model was then established using NSG mice injected i.v. with 8 × 106 cells in 200 μL of saline on day 0 (13). A pilot study was then performed to measure the anti-MM activity of ex vivo-expanded NK cells. In particular, on days 7 and 14 post tumor inoculation, mice were injected with 5×106 NK cells expanded from the PBMC of daratumumab-treated MM patients. To determine whether NK cells expanded ex vivo from the PBMCs of daratumumab-treated MM patients were able to improve the outcome of daratumumab therapy, on days 14, 21, and 28 post tumor inoculation, mice were also injected i.v. with daratumumab at a dose of 8 mg/kg, as previously described (14), followed by i.v. injection with 5×106 expanded NK cells on the following days (i.e., on days 15, 22, and 29). To monitor tumor growth, mice were infused i.p. with D-luciferin (150 mg/kg; Gold Biotechnology, St. Louis, MO) (13) for in vivo bioluminescence imaging by In Vivo Imaging System (IVIS-100) with Living Image software (PerkinElmer, Waltham, Massachusetts) (13).
Statistical analysis
Student's t-test or paired t-test was used to compare two independent or paired groups. Linear or linear mixed models were used to compare multiple groups and account for the covariance structure due to repeated measures. Kaplan-Meier method was used to estimate survival functions and log-rank test was used to compare any two survival curves. P values were corrected for multiple comparisons. A P value less than 0.05 was considered statistically significant.
See Supplementary Materials and Methods for additional details.
Results
Daratumumab-induced NK cell activation
Both daratumumab and NK cells have been shown to play roles in eradicating MM cells. For this reason, we set out to determine whether daratumumab activates NK cells, and to characterize potential mechanisms by which these effects may occur. We found that daratumumab indeed stimulates NK cells, as evidenced by an increase in expression of IFNG mRNA and protein (Fig. S1A and S1B). To assess whether daratumumab can also promote NK-mediated ADCC against MM.1S target cells, which robustly express CD38 (Supplementary Fig. S2), we performed standard 51Cr release assays using primary NK cells from healthy donors as effectors and the MM.1S MM tumor cell line as targets. Results suggested that daratumumab can indeed significantly enhance NK cell-mediated cytotoxicity against MM.1S targets (9) (Supplementary Fig. S3A). In particular, this enhanced cytotoxicity seemed to be occurring via ADCC, as the addition of an anti-CD16 blocking Ab greatly diminished the effects of daratumumab (Supplementary Fig. S3A). These daratumumab-mediated effects on NK cell activation occurred concomitantly with induction of STAT1 phosphorylation and activation of NF-κB p65 (Supplementary Fig. S3B). Notably, even a low dose of daratumumab (1 μg/mL) was sufficient to trigger phosphorylation of STAT1 and activation of NF-κB (Supplementary Fig. S3B). Thus, the aforementioned finding lends further support to the data depicted in Supplementary Fig. S1, which shows that an increase in NK cell FNG mRNA expression occurs in response to treatment with the same doses of daratumumab. NF-κB and STAT1 activation occurs downstream of factors containing immunoreceptor tyrosine-based activation motifs (ITAMs) (15,16), which are recruited by CD16 in NK cells (17). Accordingly, we found that daratumumab was able to induce IFNG expression in NK-92 cells that were CD16 (158V/F) positive, but not in those that were CD16 negative. Because both of the aforementioned populations expressed similar levels of CD38 (Supplementary Fig. S4A and S4B), our findings together implicate CD16 as a factor necessary for daratumumab-triggered activation of NK cells.
CD38+ but not CD38−/low NK cells are depleted in daratumumab-treated MM patients
The above data together demonstrate that daratumumab is indeed capable of activating NK cells in vitro. Therefore, we set out to next determine how daratumumab affects NK cells in daratumumab-treated MM patients. To this end, we assessed serial samples taken from MM patients undergoing treatment at the James Cancer Hospital. Daratumumab treatment was administered once per week (16 mg/kg) for 8 weeks, then once every other week (8 mg/kg) for 16 weeks, and then once per month (16 mg/kg). Of note, it was reported that after the first 16 mg/kg dose was administered, the mean serum concentration of daratumumab was >250 μg/mL (18). We found that daratumumab treatment significantly reduced the relative abundance of NK cells in the PB of MM patients, whose NK cells comprised approximately 2% of PB lymphocytes;in contrast, NK cells represented approximately 10% of PB lymphocytesin healthy donors or MM patients that had not undergone daratumumab treatment (Fig. 1A and B). NK cell presence was also diminished within the BM of daratumumab-treated compared to non-daratumumab-treated MM patients (Supplementary Fig. S5A and S5B). In contrast, we did not observe any significant differences in the relative abundance of PB T or B lymphocytes among healthy donors, non-daratumumab-treated MM patients, and daratumumab-treated MM patients (Fig. 1C and D). NK cells typically express high levels of CD38 in healthy donors (19) and in non-daratumumab-treated MM patients (Fig. 1E); however, the PB NK cell population in daratumumab-treated MM patients was comprised almost entirely of CD38−/low NK cells (Figs 1E and F and Supplementary Fig. S5). We found that daratumumab binds to CD38 in a manner that prevents detection with many of the individual anti-CD38 antibodies that are commercially available, while a multi-epitope polyclonal anti-CD38 antibody can be used to detect CD38 expression in daratumumab-treated patients. Accordingly, we made use of this multi-epitope polyclonal anti-CD38 antibody to stain PB and BM NK cells from daratumumab-treated MM patients as described in Fig.1E and F and Supplementary Fig. S5C. The above data suggest that CD38+ NK cells were susceptible to daratumumab-mediated depletion, while CD38−/low NK cells were resistant.
Figure 1.
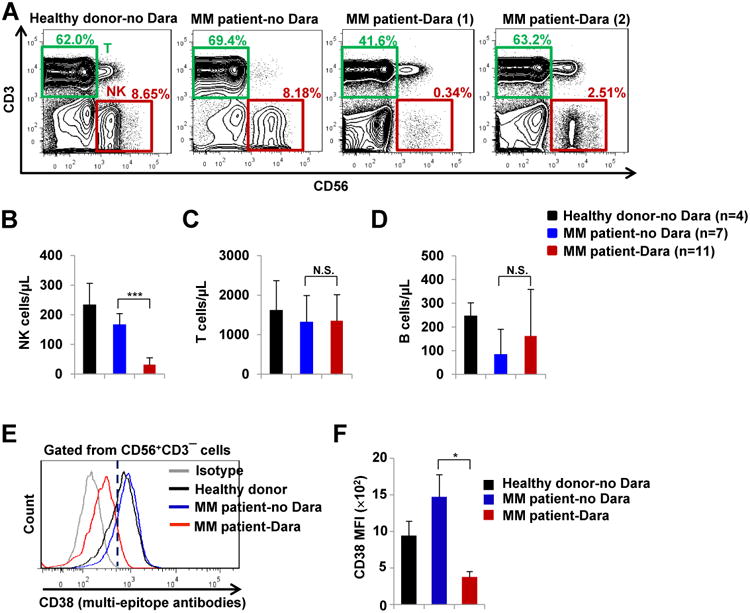
Dara depletes CD38+ NK cells in the peripheral blood of patients with multiple myeloma (MM). A, Flow cytometric analysis of NK cells (CD56+CD3−) and T cells (CD56−CD3+) in PBMCs from healthydonors (n=4), MM patients without Dara treatment (MM patient-no Dara, n=7), and MM patients treated with Dara (MM patient-Dara, n=11). The representative MM patient-Dara (1) sample was collected after the patient was treated with Dara once per week for 3 weeks; The representative MM patient-Dara (2) sample was collected after the patient was treated with Dara once per week for 8 weeks followed by every other week for 3 weeks. B-D, Quantitative assessmentsof NK cells (CD56+CD3−), T cells (CD56−CD3+), and B cells (CD3−CD19+) in the peripheral blood of healthy donors-no Dara, MM patients-no Dara, and MM patients-Dara were analyzed by flow cytometry. E and F, Flow cytometric analysis of CD38 surface expression, as determined by flow cytometric analysisin samples stained with a multi-epitope anti-CD38 antibody, in NK cells from healthy donors-no Dara, MM patients-no Dara, and MM patients-Dara (n=3 for each group). Dara, daratumumab; MFI, mean fluorescence intensity; Error bars, S.D.; N.S., not significant; *, P< 0.05; ***, P< 0.001.
Daratumumab induces NK cell fratricide via NK-to-NK ADCC
To explore the mechanism by which daratumumab induces NK cell depletion as shown in Fig. 1, we first tested the effects of daratumumab on NK cell death. We showed that daratumumab triggers NK cell apoptotic activity in a dose-dependent manner, which results in a significant reduction in the absolute quantity of primary NK cells (Fig. 2A and B and Supplementary Fig. S6). In addition, we observed that treatment with daratumumab also significantly increased the rate of NK cell degranulation (Fig. 2C). Interestingly, this daratumumab-induced degranulation did not require the presence of target cells (i.e., tumor cells), as it could be observed when NK cells were treated in the absence of target cells. Based on this observation, we predicted that daratumumab may be inducing NK cell apoptosis through a mechanism involving NK cell fratricide (NK-mediated cytotoxicity against neighboring NK cells) occurring via ADCC, as a majority of NK cells highly express CD38 (19,20). To this end, we performed a 51Cr release assay and a flow cytometry-based cytotoxicity assay, both of which indicated that NK cells were indeed able to lyse one another in the presence of daratumumab (Fig. 2D and E). The low percentages of killing in both the daratumumab-treated and untreated groups in the 51Cr release assay could be due to the fact that the 51Cr uptake by primary NK cells tends to be very low [e.g., 1,054 counts per minutes (cpm) for primary NK vs. 10,998 cpm for MM.1S tumor cells in our experiment]. In contrast, we did not observe NK cell fratricide by instead trying to target CS1 on NK cells (21) with elotuzumab (an anti-CS1 monoclonal antibody) (Fig. 2E). Moreover, we found that when daratumumab was digested into Fc and F(ab)2 fragments using an IgG-specific protease (IdeZ) (Supplementary Fig. S7), the fragments failed to increase NK cell apoptosis (Fig. 2F and G), indicating that daratumumab-augmented NK cell apoptosis requires the integrity of daratumumab, as depicted in Fig 2H. NK-92 cells, an NK cell line that is naturally CD16-deficient but robustly expresses CD38 (Supplementary Fig. S4A), were resistant to daratumumab-induced apoptosis; however, this effect was reversed in NK-92 cells engineered to overexpress native CD16 or a high-affinity version of CD16 (158V/F) (Supplementary Fig. S4C). These results indicate that in addition to being required for NK cell activation (Supplementary Fig. S4B) and being essential for NK-mediated ADCC (22), the presence of functional CD16, also called FcγRIIIA, may also be required for daratumumab-induced NK cell apoptosis. Furthermore, although the digested F(ab)2 and Fc fractions of daratumumab were still individually able to recognize CD38 and CD16 molecules, respectively, the enzyme-digested version of daratumumab was unable to form a molecular bridge between the CD38 and CD16 receptors on neighboring NK cells, thus lacking a necessary condition for ADCC, and then were incapable of inducing apoptosis of primary NK cells (Fig. 2F and G). These results demonstrate that daratumumab-mediated NK cell apoptosis occurs through a mechanism of NK cell fratricide.
Figure 2.
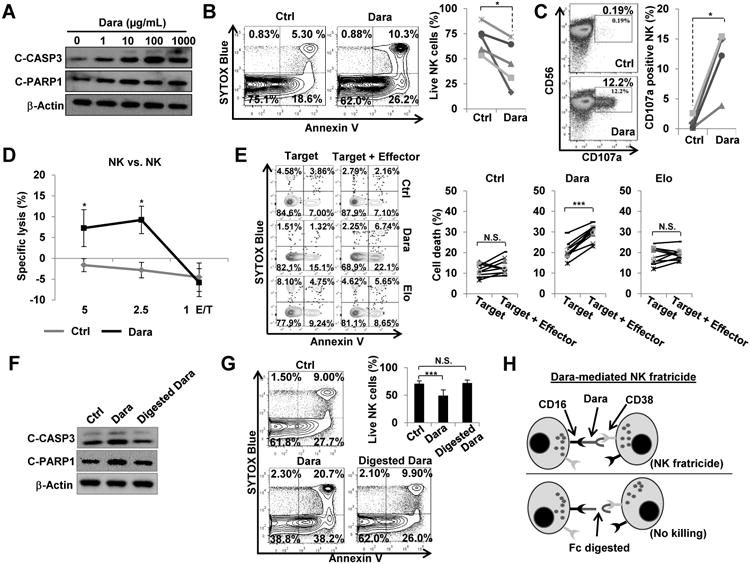
Dara induces primary NK cell fratricide, which occurs via non-traditional ADCC between NK cells in the absence of tumor targets. A, The expression of cleaved (C)-CASP3 and C-PARP1 were detected by immunoblotting after NK cells were treated for 16 h with various doses of Dara as indicated. B, NK cells were treated for 16 h with 10 μg/mL of Dara, then stained with an anti-Annexin V antibody and SYTOX Blue viability dye. NK cell apoptosis was analyzed by flow cytometry (n=5). Each line in the panel on the right indicates survival of Dara-treated vs. untreated NK cells from the same individual donors. C, NK cells were treated for 4 h with 10 μg/mL of Dara, then expression of CD107a, an NK degranulation marker, was analyzed by flow cytometry (n=4). Each line in the panel on the right indicates Dara-treated vs. untreated NK cells from the same individual donors. D, Dara-mediated NK cell fratricide (both effector cells and target cells are NK cells) was performed using a standard 4-h 51Cr-release assay (n=3). E, A 4-h flow cytometry-based cytotoxicity assay was performed (Effector/Target is 1:1; n=9). NK cells serving as target cells were labeled with CFSE and pre-treated with or without Dara or Elo, an anti-CS1 monoclonal antibody, for 30 min. Effector NK cells (effector) were pre-treated with F(ab)2 fragments of Dara or Elo for 30 min to block binding of intact Dara or Elo to CD38 or CS1, respectively, thus preventing fratricide among the effector cells. Target cells were gated from CFSE+ events, and cell death was detected by flow cytometric analysis via staining with an anti-Annexin V antibody and SYTOX Blue viability dye. Each line in the panel on the right compares target alone vs. target+effector group from the same individual donors. F, Expression of cleaved(C)-CASP3 and C-PARP-1 were detected by immunoblotting after 16 h treatment with 10 μg/mL of intact Dara or Dara enzyme-digested by an IgG-specific protease (IdeZ). G, NK cells were treated for 16 h with 10 μg/mL of Dara or enzyme-digested Dara, then apoptosis was analyzed (n=9) as described in (B). H, Schematic detailing mechanism for Dara-induced NK cell fratricide. CASP3, Caspase 3; Dara, daratumumab; Elo, elotuzumab; Error bars, S.D; N.S., not significant; *, P< 0.05; ***, P< 0.001.
CD38−/low NK cells are resistant to daratumumab-induced NK cell fratricide, and thus are better at killing MM cells via daratumumab-mediated ADCC
Because daratumumab-induced NK cell fratricide (Fig. 2) depletes only the CD38+ subset of NK cells in MM patients (Fig. 1), we next tested whether CD38−/low NK cells are resistant to daratumumab-induced apoptosis. Approximately 15% of NK cells in human PB are CD38−/low (Fig. 3A) and, remarkably, daratumumab-induced apoptosis was frequently observed in CD38+, but rarely detected in CD38−/low NK cells (Fig. 3B and C). We also observed that in the absence of daratumumab, the survival rate of CD38−/low NK cell was significantly higher than seen in CD38+ NK cells (Supplementary Fig. 8). Interestingly, though eradication of MM.1S targets was enhanced when CD38−/low and CD38+ NK cells were combined with daratumumab, this effect was stronger within the CD38−/low NK subset (Fig. 3D and E). Presumably, this difference may be attributable, at least in part, to the daratumumab-mediated induction of NK cell apoptosis as we reported above, which would be happening concurrently in the CD38+ population but not in the CD38−/low population. In accordance with this presumption, the rate of apoptosis was indeed higher within the CD38+ NK cell subset than in the CD38−/low NK cell subset during daratumumab-mediated ADCC against MM.1S targets (Fig. 3F). Together, these results indicate that compared to CD38+ NK cells, CD38−/low NK cells are superiorat acting cooperatively with daratumumab to eradicate MM cells via ADCC.
Figure 3.
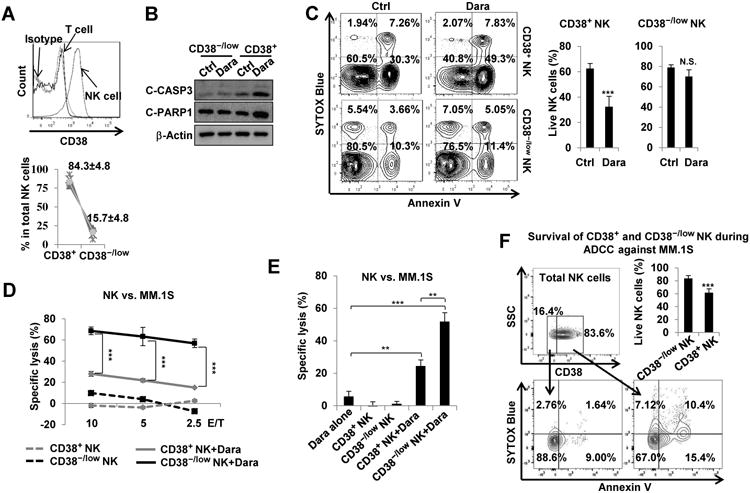
CD38−/lowprimary NK cells are resistant to Dara-induced apoptosis and, compared to CD38+ NK cells, show better efficacy at Dara-mediated ADCC against MM cells. A, Expression of CD38 was detected by flow cytometry using healthy donor PBMCs. Percentage of CD38−/low and CD38+ populations among total NK cells were analyzed (n=6). Each line in the lower panel indicates proportion of CD38+ and CD38−/low NK cells within the same individual donors. B, Immunoblotting analysis was performed after CD38−/low or CD38+ NK cells were cultured for 16 h in the presence or absence of 10 μg/mL of Dara. C, CD38−/low or CD38+ NK cells were treated for 16 h with 10 μg/mL of Dara, then stained with an anti-Annexin V and SYTOX Blue viability dye (n=9). D, Dara-mediated ADCC against MM.1S target cells, assessed by a 4-h standard 51Cr-release assay. Effectors were CD38−/low or CD38+ NK cells FACS-sorted from healthy donors (n=3). E,. Comparison of synergistic effect on tumor eradication, as assessed by a 4-h standard 51Cr-release assay, between Dara and CD38−/low or CD38+ NK cells FACS-sorted from healthy donors (Effector/Target ratio is 5:1, n=3). F, Purified NK cells were co-cultured with MM.1S cells in the presence of Dara for 16 h. Cells were stained with anti-Annexin V and SYTOX Blue dye (n=9). ADCC, antibody-dependent cellular cytotoxicity; Dara, daratumumab; Error bars, S.D; N.S., not significant; **,P< 0.01; ***,P< 0.001.
CD38−/low NK cells from healthy donors are more proliferative than their CD38+ counterparts, and expanded cells from the former population are more cytotoxic than those from the latter
The NK cells that remain in MM patients following daratumumab treatment are primarily CD38−/low and resistant to daratumumab-induced fratricide, and we show above that compared to CD38+ NK cells, CD38−/low NK cells have the better potential to cooperate with daratumumab to eradicate MM tumor cells. However, CD38−/low NK cells represent a relatively minor population in healthy donors, accounting for approximately 15% of their PB NK cells (Fig. 3A). We therefore investigated the potential to expand these cells in vitro, and then we characterized the expanded cells. For this purpose, PB NK cells from healthy donors were FACS-purified into two separate populations:CD38−/low or CD38+. Both of these NK cell subsets were then expanded on a K562 feeder cell line modified to express membrane-bound IL-21, as previously reported (23). We observed a significant increase in proliferation of NK cells that were derived from CD38−/low progenitors (CD38−/low exp. cells) compared to those derived from CD38+ NK progenitors(CD38+ exp. cells) (Fig. 4A and Supplementary Fig. S9A). Both CD38−/low exp. and CD38+ exp. cells had some capacity to kill MM target cells; however, when compared to CD38+ exp. cells, CD38−/low exp. cells were more cytotoxic against the MM.1S cell line (Supplementary Fig. S9B, left panel) in the absence of daratumumab. Interestingly, the previous observation occurred despite the fact that each of the two expanded subsets expressed similar levels of GZMB protein (Supplementary Fig. S9C). Moreover, though treatment with daratumumab was able to further increase cytotoxicity against the MM.1S cell line in both CD38−/low exp. and CD38+ exp. NK cell subsets, daratumumab-mediated killing of the cell line remained higher in the CD38−/low exp. cells relative to that seen in CD38+ exp. cells (Supplementary Fig. S9B, right panel). Likewise, when challenged with primary MM cells as targets, in the presence of daratumumab, CD38−/low exp. cells displayed higher levels of cytotoxicity than that of CD38+ exp. cells (Fig. 4B). We speculated that this may be occurring due to differential expression of CD38 on the progeny of these two expanded NK cell subsets, as we found that CD38 expression could lead to NK-cell fratricide in the presence of daratumumab. Indeed, we observed that the expanded NK cells derived from CD38−/low NK cells expressed lower levels of CD38 than those derived from CD38+ progenitors at all tested time points (Fig. 4C). Although CD38−/low exp. cells have higher levels of daratumumab-mediated ADCC against MM cells than CD38+ exp. cells (Fig. 4B), the former subset also acquired lower levels of CD38 expression on the cell surface than the latter subset. We then tested whether daratumumab F(ab)2 fragments can block surface CD38 and this blockade may prevent fratricide and improve daratumumab-mediated ADCC in expanded NK cells. For this purpose, we first tested the blockade effect using CD38+ exp. NK cells through pre-incubation with varying concentrations of daratumumab F(ab)2 fragments. Pre-incubation with F(ab)2daratumumabfragments was indeed sufficient for preventing the increased apoptosis mediated by daratumumab in CD38+ exp. NK cells, which occurred in a dose-dependent manner (Fig. 4D). Furthermore, this blockade of CD38 indeed significantly enhanced the capacity of CD38+ exp. NK cells for daratumumab-mediated ADCC against MM.1S targets (Fig. 4E).
Figure 4.
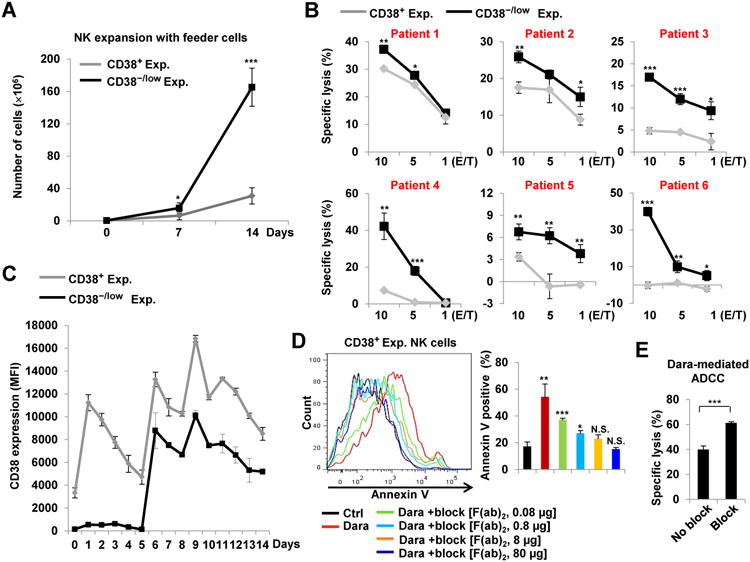
Compared to their CD38+ counterparts, CD38−/lowprimary NK cells are more proliferative and more cytotoxic against MM target cells. A, 0.5×106 NK cells were expanded on K562 feeder cells. At days 7 and 14, cells were counted and analyzed to compare CD38−/low exp. and CD38+ exp. cells (n=5). B, A standard 4-h 51Cr-release assay was used to measure Dara-mediated ADCC capacity of CD38−/low exp. and CD38+ exp. NK cells against primary MM cells, isolated from BM of patients with MM (n=6). C, Expression of CD38 on CD38−/low exp. and CD38+ exp. NK cells was detected by flow cytometry (n=3). D, 0.5×106 CD38+ expanded NK cells were pre-treated for 1 h with the indicated doses of F(ab)2 Dara fragments, followed by 4 h treatment with 10 μg/mL of intact Dara. An anti-Annexin V antibody was then used to analyze apoptosis by flow cytometry (n=3). E, CD38+ expanded NK cells were pre-treated for 1 h with F(ab)2 fragments of Dara. A standard 4-h 51Cr-release assay was then used to assess Dara-mediated ADCC against MM.1S target cells (Effector/Target ratio is 5:1, n=3). MFI, median fluorescence intensity; ADCC, antibody-dependent cellular cytotoxicity; Dara, daratumumab; Error bars, S.D; *, P< 0.05; **,P< 0.01; ***,P< 0.001.
Alternatively, it's possible that, in the absence of CD38 blockade, CD38+ exp. NK cells compete with MM cells for the pool of available daratumumab, and co-culture withCD38+ exp. NK cells would lead to lower levels of daratumumab bound to CD38 on the surface of MM cells; whereas the higher levels of daratumumab available to MM cells could help explain the increase in ADCC observed when MM cells were instead co-cultured with CD38−/low exp. NK cells. However, we found that this problem of NK cell competition with MM cells for daratumumab binding could be eliminated through administering daratumumab at 10 and 100 μg/mL doses in our cytotoxicity experiments because all tumor cells were bound with daratumumab at these concentrations in the presence of the potential competitor, CD38+ exp. NK cells (Supplementary Fig. S10). This also strengthens our hypothesis that NK-cell fratricide occurs in vitro and in patients because the 10 μg/mL concentration used in our culture system and the >250 μg/mL serum concentration achieved in patients treated with daratumumab at a dose16 mg/kg(18)are both within the range of daratumumab concentrations (i.e., 10 to 100 μg/mL) where there is no antibody binding competition between NK cells and MM cells.
Because the data above suggest that CD38−/low NK cells and CD38+ NK cells appear to be two functionally different subsets, we used freshly isolated bulk NK cells to further characterize each of these subsets. We showed that levels of CD16 and NKp46 expression were lower, while CXCR4, KLRG1, CD69, and CD96 expression were higher in CD38−/low NK cells than in CD38+ NK cells (Supplementary Fig. S11). The expression of NKG2D, TIGIT, CD94 and CD226 was not significantly different between the two subsets of NK cells (Supplementary Fig. S11). Furthermore, we found that freshly isolated CD56dim and CD56bright NK cells both contained CD38+ and CD38−/low subsets (Supplementary Fig. S12A). Consistent with previous studies (24,25), while GZMB expression was not detected in CD56bright NK cells, CD56dim NK cells expressed GZMB in abundance (Supplementary Fig. S12C). Among CD56dim NK cells, the CD56dimCD38−/low NK cell subset expressed GZMB at levels significantly lower than those found in the CD56dimCD38+ NK subset (Supplementary Fig. S12B and S12C). Previous reports have indicated that high levels of GZMB expression occur following NK cell terminal maturation (26) which would suggest that CD38+ NK cells may be more mature than CD38−/low NK cells; however, it has also been reported that CD38−/lowand CD38+ NK cell subsets may be generated from distinct populations of hematopoietic stem cells (HSCs) that are CD38− and CD38+, respectively (27). Thus, further in-depth studies characterizing the nature of the developmental relationship the between CD38−/low and CD38+ NK cell subsets are warranted.
NK cells from daratumumab-treated MM patients are more proliferative than those from non-daratumumab-treated MM patients or healthy donors
Because only a small number of CD38−/low NK cells can be found in the PB of daratumumab-treated MM patients, we set out to determine whether NK cells, when freshly isolated or from samples that had been previously frozen, can be expanded from MM patient PBMCs ex vivo on K562 feeder cells. For this reason, MM patient PBMCs (stored at -80°C) were thawed and expanded with the aforementioned feeder cells plus IL-2. We observed that both non-daratumumab-treated and daratumumab-treated MM patients' NK cells had rapidly expanded by day 7 (Supplementary Fig. S13). The purity of these expanded NK cells reached approximately 90% at day 7, and further increased to more than 95% by day 19 (Supplementary Fig. S13 and Fig. 5A). Moreover, we observed that NK cells from the PBMC of daratumumab-treated MM patients expanded at a significantly higher rate than NK cells from the PBMC of non-daratumumab-treated MM patients (P <0.05) or healthy donors (P <0.01) (an average of 60,000-fold increase vs. 10,000-fold and 3,100-fold at day 19, respectively) (Fig. 5B). This finding was consistent with experiments performed using NK cells from healthy donors, in which we compared expansion capacity of CD38−/low to CD38+ and total NK cells (Supplementary Fig. S9A), because the majority of NK cells from daratumumab-treated MM patients are CD38−/low, while NK cells from non-daratumumab-treated mimic total NK cells of healthy donors in terms of NK cell subsets defined by CD38 surface expression (Fig. 1E). Both non-daratumumab-treated and daratumumab-treated MM patients'eNK cells had remarkable cytotoxic activity against MM target cells, killing at much higher rates than NK cells from healthy counterparts (Fig. 5C). Together, these data demonstrate that daratumumab-treated MM patients' NK cells, which are largely CD38−/low, have an outstanding capacity for proliferation and the eNK cells derived from PBMCs of these patients are potent killers of MM cells.
Figure 5.
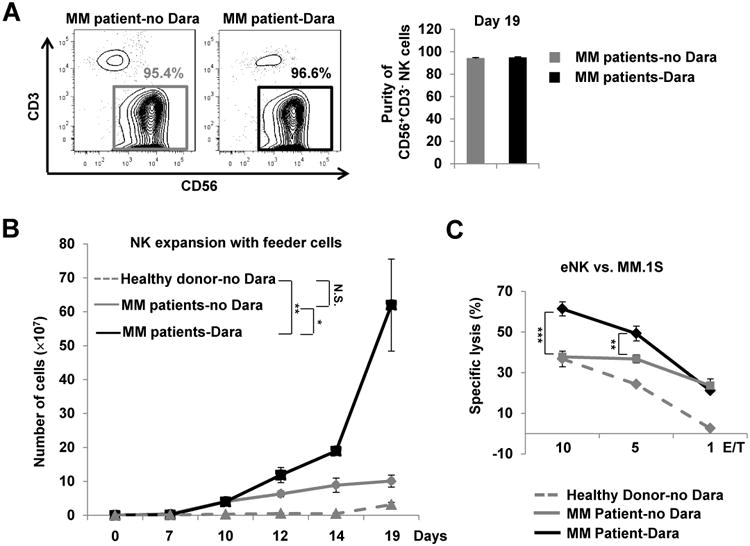
Expansion of primary NK cells from MM patients' PBMCs. A, Purity of NK cells expanded (eNK) from non-Dara-treated or Dara-treated MM patients assessed by flow cytometry at day 19 and presented as the percentage of CD56+CD3− lymphocytes among total lymphocytes (n=3). B, Expansion of NK cells derived in vitro from the PBMCs of healthy donors and MM patients treated with or without Dara (Dara and no Dara, respectively), as assessed on days 7, 10, 12, 14 and 19 (n=3). C, A 4-h standard 51Cr-release assay was performed using NK effector cells expanded from the PBMCs of healthy donors or MM patients treated with or without Dara (n=3). MM.1S served as targets. Dara, daratumumab; Error bars, S.D; N.S., not significant; *, P< 0.05;**,P< 0.01; ***, P< 0.001.
NK cells expanded from daratumumab-treated MM patient PBMCs display better efficacy following combination treatment with daratumumab than with single agents in vivo
We first tested whether expanded NK (eNK) cells from MM patients possess the capacity to kill MM. For this and the remaining in vivo experiments, we used the MM.1S xenograft model that we previously described (28). Indeed, we observed that eNK cells from daratumumab-treated MM patients' cells have significant anti-tumor activity in MM tumor-bearing mice (Supplementary Fig. S14). We applied a combination treatment consisting of daratumumab with eNK derived from daratumumab-treated MM patients' PBMCs in order to test whether eNK cells from PB of daratumumab-treated MM patients synergize with daratumumab to kill MM and provide a survival advantage. Tumor growth was monitored by bioluminescence imaging twice a week, starting at day 14 after tumor inoculation. A diagram illustrating the scheme for treatment is shown in Fig. 6A. We found that, compared to the control group and groups treated with only a single agent, combination treatment significantly improved tumor growth suppression (Fig. 6B and C and Supplementary Fig. S15A and S15B). Likewise, survival of MM tumor-bearing mice was improved to a greater degree in the group receiving combined treatment than in any other treatment groups (Fig. 6D and Supplementary Fig. S15C). Thus, combination treatment with daratumumab and eNK cells from daratumumab-treated MM patients displayed more effective and potent anti-tumor activity compared to treatment with daratumumab alone. However, since the aforementioned effects of combination treatment on prolonging survival may not be durable, subsequent injections with eNK cells should be performed frequently following daratumumab treatment.
Figure 6.
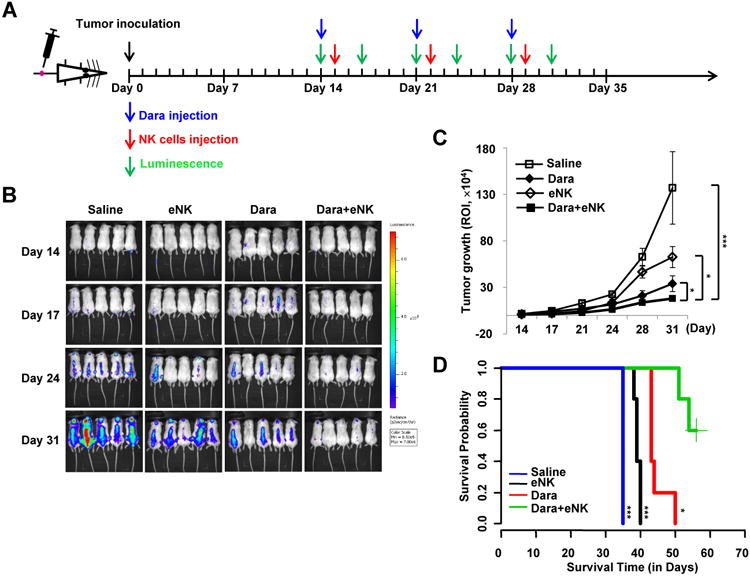
Treatment of MM-bearing mice with or without Dara, expanded NK cells (eNK), or their combination. A, Treatment and imaging schedules. B, Images of mice in each treatment group at 14, 17, 24, and 31 days after MM.1S cell inoculation. C, Bioluminescent quantification of tumor growth (n=5; **, P< 0.01; ***,P< 0.001, combination vs. saline, Dara, or eNK), indexed by region-of-interest (ROI). D, Survival analysis (n=5 for each group);Dara, daratumumab; *, P< 0.05; ***,P< 0.001, combination vs. saline, Dara, or eNK.
Discussion
Daratumumab's efficacy has been proven in a series of clinical trials, both as a single agent to target the CD38 molecule, and as part of combination treatments for MM (2,6,8,29). Daratumumab works to eliminate tumor cells via several mechanisms, including ADCC and direct induction of tumor cell apoptosis (9,30). ADCC is mediated by NK cells (31,32); however, as we demonstrate in this study, NK cell depletion in PB and BM can simultaneously occur in MM patients who are undergoing daratumumab therapy, consistent with previous studies (33,34). Though the mechanism by which daratumumab depletes NK cells was previously unclear, in this study, we revealed that daratumumab can enhance NK cell apoptosis through NK-to-NK ADCC, wherein fratricide occurs among NK cells without the involvement of tumor cells. Furthermore, the CD38−/low NK cells remaining in daratumumab-treated MM patients are highly proliferative ex vivo, and following expansion, these NK cells can acquire potent in vivo anti-MM activity. Therefore, we propose combined treatment with daratumumab and eNK cells as a potential therapeutic strategy for treatment of patients with MM. To the best of our knowledge, this combination renders our study novel compared to previous studies (33,34).
NK cell activation can either be induced by receptor recognition between effector cells and target cells (35), or directly stimulated through treatment with antibodies directed against markers such as CD16 and/or NKG2D (36), which represent examples of cytotoxicity-related receptors on NK cells (37). Though CD38 is also expressed on the surface of NK cells, anti-CD38 F(ab)2 fails to trigger NK cell activation in freshly isolated human NK cells (20), consistent with our findings that the F(ab)2 fragment of daratumumab does not trigger NK activation or cell death. Thus, CD38 may not be a direct transducer of cell signaling that controls NK cell activation. Cytokine-mediated activation and antibody-mediated ADCC enhance NK cell activation, which can promote killing of tumor target cells or virally-infected cells (38,39); however, both of the aforementioned processes rely on the presence of target cells (38,39). Interestingly, our observations indicate that daratumumab [but not the F(ab)2 part] is able to directly trigger NK cell activation even in the absence of target cells. During this process, NK cells not only became activated, but also killed each other. Therefore, we believe the daratumumab-induced NK cell death we described occurs at least partially through NK-mediated non-classical ADCC directed against neighboring NK cells, a process termed fratricide. During this process, CD38+ NK cells serve as both “target cells” and effector cells. Interestedly, another FDA-approved antibody, elotuzumab directed against CS1, does not induce NK cell fratricide, although CS1 is expressed on NK cells (40,41). In addition, we concede that we cannot formally exclude the possibility that this phenomenon may also be attributed to activation-induced cell death (AICD), particularly because during the process of daratumumab-mediated NK cell fratricide, we indeed observed NK cells acquiring an activated phenotype. However, to our knowledge, whether ADCC in NK cells also induces AICD, a process that is more typically induced by cytokines, has not yet been explored. Thus, it would be difficult to formally test whether the daratumumab-induced NK cell death occurring through ADCC-mediated NK cell fratricide might also be accompanied by AICD-mediated NK cell suicide.
The effect of daratumumab on NK cell death requires the co-expression of CD38 and the Fc receptor, CD16, on the surface of NK cells. Findings in previous studies are consistent with our observations reported here, indicating that NK cells highly express CD38, while T cells are nearly CD38 negative (42). This detail can help explain why daratumumab treatment results in depletion of NK cells but not T cells in MM patients. Lack of daratumumab-mediated NK cell to NK cell engagement through the use of an IgG-specific protease IdeZ, which digests daratumumab into F(ab)2 and Fc fragments, completely diminishes daratumumab-induced NK cell death. Based on this finding, the F(ab)2 fragments of daratumumab may be useful for blocking the CD38 receptor on NK cells, which will prevent NK cells from succumbing to daratumumab-induced apoptosis. Indeed, blockade of CD38 with daratumumab F(ab)2 is sufficient to prevent daratumumab-induced NK cell death. Importantly, we found that this method simultaneously works to enhance daratumumab-mediated cytotoxicity of NK cells against MM cells. Thus, blocking CD38 with daratumumab F(ab)2 prevents daratumumab-induced apoptosis in CD38+ NK cells, suggesting that CD38 blockade with daratumumab F(ab)2 in NK cells isolated or expanded from PB of MM patients or allogeneic donors may represent a useful strategy for improving the outcome of daratumumab therapy in MM.
CD38 is a glycoprotein and a multifunctional enzyme that catalyzes the synthesis and hydrolysis of the reaction from NAD+ to ADP-ribose (42), a process with important functions in cell adhesion and the calcium signaling pathway (43,44). Loss of CD38 is associated with impaired immune responses (42). CD38, a receptor which lacks an intracellular domain (42), also positively regulates cytokine release and cytotoxicity in activated NK cells, likely through interacting with CD16, an event that may trigger activation of the calcium signaling pathway (45) As mentioned above, the F(ab)2 fragment of daratumumab, without an Fc proportion, failed to stimulate NK cell activation, indicating that the binding of the daratumumab-recognized epitope of CD38 did not activate downstream signaling pathways. Our data are in keeping with this concept, as we show that daratumumab fails to activate NK-92 cells that are CD16−, whereas the antibody is able to activate a variant of the NK-92 cell line that has been engineered to ectopically express CD16, an Fc receptor. Consistently, when the F(ab)2 fragment of daratumumab was used on CD16+ NK cells, activation was not observed. These data suggest that activation of CD38 signaling in NK cells may require an anti-CD38 antibody that is capable of bridging CD38 and CD16 on the same NK cell via the F(ab)2 and the Fc portion of the antibody, respectively. Indeed, this is an attractive and logical hypothesis; however, whether daratumumab bridges CD38 and CD16 on the same cell to induce NK cell suicide remains to be proven.
CD38−/low NK cells were more proliferative than CD38+ NK cells. NK cells remaining in daratumumab-treated MM patients are mainly CD38−/low and have much higher capacity for expansion than NK cells isolated from healthy donors or from MM patients who have not undergone daratumumab treatment. We also observed that freshly isolated CD38−/low NK cells expressed much lower levels of granzyme B than did CD38+ NK subsets. Alternatively, the CD38+ subset may represent NK cells that are at a more senescent stage than the CD38−/low subset. In support of this, expanded cells from CD38+ NK cells are less proliferative and less cytotoxic than those from CD38−/low NK cells. However, the relationship between these two subsets of NK cells as it pertains to differentiation and/or maturation status has not yet been characterized in the literature. It will be of great interest to determine whether PB CD38−/low NK subset represents a more immature developmental stage, or if the development of the CD38−/low and CD38+ NK cell subsets occur entirely independently from distinct subsets of hematopoietic progenitors. Indeed, if proven to be true, either of the aforementioned hypotheses might lead to the observed functional differences between the two NK cell subsets.
Interestingly, our data also showed that CD38−/low NK cells acquire a certain level of increased CD38 expression during in vitro expansion. If this occurs in vivo, it may explain why CD38−/low NK cells, with greater proliferative ability, do not expand in vivo in daratumumab-treated MM patients to reconstitute the NK cell compartment after depletion of CD38+ NK cells by daratumumab, namely because the acquisition of CD38 expression might result in daratumumab-induced apoptosis of these cells. For the same reason, in addition to the sequential treatment with daratumumab and CD38−/low exp. NK cells, a pretreatment of CD38−/low exp. NK cells with F(ab)2 fragments of daratumumab to prevent fratricide of these NK cells may represent a promising alternative approach for the use of combinational therapy with expanded NK cells and daratumumab. Although the latter approach allows for administration of daratumumab and expanded NK cells simultaneously, the potential re-binding of F(ab)2 fragments shed from NK cells onto MM cells may also result in MM cells that are resistant to daratumumab.
In conclusion, we highlight that daratumumab depletes NK cells in patients with MM through a mechanism involving NK cell fratricide. This side effect of daratumumab for MM patients may influence the efficacy of daratumumab therapy, particularly because NK-mediated ADCC against MM cells could become diminished, and may subsequently increase the risk of MM relapse. To address these issues, we propose a novel therapeutic strategy for the treatment of MM, which combines daratumumab treatment with eNK cells expanded from daratumumab-treated patients.
Supplementary Material
Translational relevance.
We highlight that daratumumab-mediated natural killer (NK) cell depletion in multiple myeloma (MM) patients occurs via a mechanism of NK cell fratricide. This side effect of daratumumab for MM patients may disrupt NK-mediated antibody-dependent cellular cytotoxicity against MM cells, and subsequently influences the efficacy of daratumumab therapy and also increases the risk of MM relapse. We further demonstrate that ex vivo expanded autologous NK cells have the potential to overcome daratumumab-induced NK cell depletion to improve daratumumab therapy for MM.
Acknowledgments
This work was supported by grants from the NIH (AI129582, NS106170, CA185301, and CA068458), the Leukemia & Lymphoma Society Translational Research Award, the American Cancer Society Scholar Award (RSG-14-243-01-LIB), and a grant from the Gabrielle's Angel Cancer Research Foundation. The authors are grateful to Dr. Dean Lee at Nationwide Children's Hospital who helped with NK cell expansion.
Footnotes
Authors' Contribution: Conception and design: Y. Wang, J. Yu, M.A. Caligiuri, D.M. Benson
Acquisition of data (provided animals acquired and managed patients, provided facilities, etc.): Y. Wang, Y. Zhang, T. Hughes
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J. Zhang
Writing, review, and/or revision of the manuscript: Y. Wang, J. Yu, T. Hughes, M.A. Caligiuri, D.M. Benson
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): D.M. Benson, J. Zhang
Study supervision and funding acquisition: J. Yu
Conflict of interest disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 4.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 6.Blair HA. Daratumumab: A review in relapsed and/or refractory multiple myeloma. Drugs. 2017;77:2013–24. doi: 10.1007/s40265-017-0837-7. [DOI] [PubMed] [Google Scholar]
- 7.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. The New England journal of medicine. 2015;373:1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 8.Plesner T, Arkenau HT, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016 doi: 10.1182/blood-2016-07-726729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 10.Slaney CY, Rautela J, Parker BS. The emerging role of immunosurveillance in dictating metastatic spread in breast cancer. Cancer Res. 2013;73:5852–7. doi: 10.1158/0008-5472.CAN-13-1642. [DOI] [PubMed] [Google Scholar]
- 11.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Phan MT, Lee SH, Kim SK, Cho D. Expansion of NK cells using genetically engineered K562 feeder cells. Methods Mol Biol. 2016;1441:167–74. doi: 10.1007/978-1-4939-3684-7_14. [DOI] [PubMed] [Google Scholar]
- 13.Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clinical cancer research. 2014;20:3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijhof IS, Groen RW, Noort WA, van Kessel B, de Jong-Korlaar R, Bakker J, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21:2802–10. doi: 10.1158/1078-0432.CCR-14-1813. [DOI] [PubMed] [Google Scholar]
- 15.Blonska M, Lin X. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55–70. doi: 10.1038/cr.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tassiulas I, Hu X, Ho H, Kashyap Y, Paik P, Hu Y, et al. Amplification of IFN-alpha-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–9. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- 17.Lanier LL, Yu G, Phillips JH. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989;342:803–5. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- 18.Plesner T, Arkenau HT, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128:1821–28. doi: 10.1182/blood-2016-07-726729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–94. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW, et al. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–71. [PubMed] [Google Scholar]
- 21.Hagberg N, Theorell J, Schlums H, Eloranta ML, Bryceson YT, Ronnblom L. Systemic lupus erythematosus immune complexes increase the expression of SLAM family members CD319 (CRACC) and CD229 (LY-9) on plasmacytoid dendritic cells and CD319 on CD56(dim) NK cells. J Immunol. 2013;191:2989–98. doi: 10.4049/jimmunol.1301022. [DOI] [PubMed] [Google Scholar]
- 22.Lanier LL, Kipps TJ, Phillips JH. Functional properties of a unique subset of cytotoxic CD3+ T lymphocytes that express Fc receptors for IgG (CD16/Leu-11 antigen) J Exp Med. 1985;162:2089–106. doi: 10.1084/jem.162.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–81. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratke K, Kuepper M, Bade B, Virchow JC, Jr, Luttmann W. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur J Immunol. 2005;35:2608–16. doi: 10.1002/eji.200526122. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muench MO, Barcena A. Broad distribution of colony-forming cells with erythroid, myeloid, dendritic cell, and NK cell potential among CD34(++) fetal liver cells. J Immunol. 2001;167:4902–9. doi: 10.4049/jimmunol.167.9.4902. [DOI] [PubMed] [Google Scholar]
- 28.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–27. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokhorst HM, Plesner T, Gimsing P, Nahi H, Minnema M, Lassen UN, et al. Phase I/II dose-escalation study of daratumumab in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2013;31:abstr 8512. 2013 ASCO Annual Meeting. [Google Scholar]
- 30.Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197:807–13. doi: 10.4049/jimmunol.1501351. [DOI] [PubMed] [Google Scholar]
- 31.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature medicine. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. 1983;148:785–94. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- 33.Casneuf T, Xu XS, Adams HC, 3rd, Axel AE, Chiu C, Khan I, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood advances. 2017;1:2105–14. doi: 10.1182/bloodadvances.2017006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps C, Chen Y, Gopalakrishnan S, Tan D. Daratumumab and its potential in the treatment of multiple myeloma: overview of the preclinical and clinical development. Therapeutic advances in hematology. 2015;6:120–7. doi: 10.1177/2040620715572295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–12. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poggi A, Boero S, Musso A, Zocchi MR. Selective role of mevalonate pathway in regulating perforin but not FasL and TNFalpha release in human natural killer cells. PLoS One. 2013;8:e62932. doi: 10.1371/journal.pone.0062932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun PD. Structure and function of natural-killer-cell receptors. Immunol Res. 2003;27:539–48. doi: 10.1385/IR:27:2-3:539. [DOI] [PubMed] [Google Scholar]
- 38.Sun P, Morrison BJ, Beckett CG, Liang Z, Nagabhushana N, Li A, et al. NK cell degranulation as a marker for measuring antibody-dependent cytotoxicity in neutralizing and non-neutralizing human sera from dengue patients. J Immunol Methods. 2017;441:24–30. doi: 10.1016/j.jim.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Pasero C, Gravis G, Guerin M, Granjeaud S, Thomassin-Piana J, Rocchi P, et al. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016;76:2153–65. doi: 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 40.Benson DM, Jr, Byrd JC. CS1-directed monoclonal antibody therapy for multiple myeloma. Journal of clinical oncology. 2012;30:2013–5. doi: 10.1200/JCO.2011.40.4061. [DOI] [PubMed] [Google Scholar]
- 41.van de Donk NW, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia. 2012;26:199–213. doi: 10.1038/leu.2011.214. [DOI] [PubMed] [Google Scholar]
- 42.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 43.Rah SY, Kim UH. CD38-mediated Ca(2+) signaling contributes to glucagon-induced hepatic gluconeogenesis. Sci Rep. 2015;5:10741. doi: 10.1038/srep10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao YJ, Zhu WJ, Wang XW, Zhang LH, Lee HC. Determinants of the membrane orientation of a calcium signaling enzyme CD38. Biochim Biophys Acta. 2015;1853:2095–103. doi: 10.1016/j.bbamcr.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 45.Deaglio S, Zubiaur M, Gregorini A, Bottarel F, Ausiello CM, Dianzani U, et al. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99:2490–8. doi: 10.1182/blood.v99.7.2490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


