Abstract
Recent RNA sequencing studies have revealed that most of the human genome is transcribed, but very little of the total transcriptomes has the ability to encode proteins. Long non-coding RNAs (lncRNAs) are non-coding transcripts longer than 200 nucleotides. Members of the non-coding genome include microRNA (miRNA), small regulatory RNAs and other short RNAs. Most of long non-coding RNA (lncRNAs) are poorly annotated. Recent recognition about lncRNAs highlights their effects in many biological and pathological processes. LncRNAs are dysfunctional in a variety of human diseases varying from cancerous to non-cancerous diseases. Characterization of these lncRNA genes and their modes of action may allow their use for diagnosis, monitoring of progression and targeted therapies in various diseases. In this review, we summarize the functional perspectives as well as the mechanism of action of lncRNAs.
Keywords: LncRNA, X-chromosome inactivation, Genome imprinting, Transcription regulation, Cancer, Immunity
1. Introduction
The overwhelming use of technologies and new discoveries has led to the understanding of functional properties of what was once considered as junk DNA. The International Human Genome Sequencing Consortium 2004 surprised everyone after revealing that there are only 20,000 protein-coding genes in the human genome, that is just about >2% of the total genomic sequences. Through the extensive annotation efforts, some dedicated consortiums such as the ENCODE project, have broadened our knowledge of what lies in the dark recesses of the genome [1]. These findings in relation to previous studies have underscored the pervasiveness of genome transcription [2], [3], and it became clear that nonprotein-coding regions too have various functions and regulatory roles. A mere number of protein-coding genes probably can't explain the organizational complexity of organisms, as there are other less complex eukaryotes with a more or less similar number of protein-coding genes (e.g., Caenorhabditis elegans). This increased complexity may be explained by pre-mRNA splicing of protein-coding transcripts and post-translational modification of proteins, which increase the diversity and functionality of the proteome. The human genome sequencing has provided an opportunity for the systematic survey of genomic regions for their biological activity. As a first logical proxy for activity, many groups began their quest by mapping observed transcriptional events in the genome [4], [5]. A project called FANTOM3 identified approximately 35,000 non-coding transcripts from approximately 10,000 distinct loci bearing many signatures of mRNAs, including capping, splicing, and poly-adenylation, but has little or no open reading frames (ORF) [6]. Thousands of long non-coding RNA (lncRNA) transcripts were identified that did not appear to be coding for proteins due to these early efforts [7], [8].
LncRNAs are up to ∼100 kilobases (kb) long, more specifically their nucleotide span range from 200 nt to 100 kilobases (kb). These are mRNA-like transcripts, but lack stable open reading frames. Like other protein coding genes, lncRNAs are probably transcribed by RNA polymerase II (RNA pol II) and are polyadenylated [9], [10]. Generally, the extent or levels of expression of lncRNAs appear to be lower than protein-coding genes [11], [12], [13], [14], and specific to the tissues [15]. Although the function of most of the lncRNAs is unknown, the number of characterized lncRNAs is increasing. Studies suggest their role in regulation of gene expression at transcriptional as well as at post-transcriptional level in development, differentiation, and human diseases, that could be negative or positive [16], [17], [18]. It has been reported that many lncRNAs functions as key regulators of translational output (also transcriptional) and therefore affect cell identity and function [19], [20], [21], [22]. In a short period, lncRNAs have emerged as legitimate, major new class of genes, as more and more functions are being attributed to them. In the near future, more functions associated with them will emerge.
2. Mechanism of action
2.1. LncRNAs as chromatin regulators
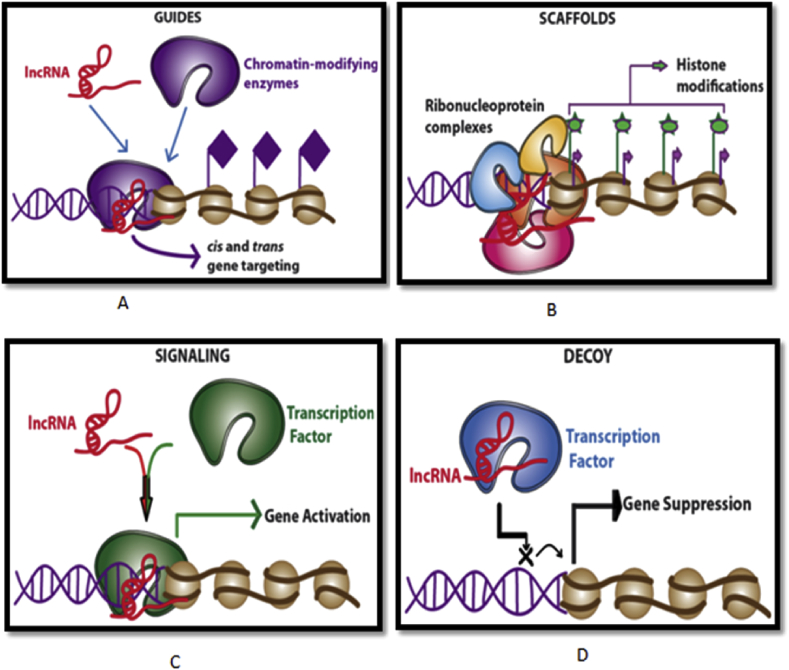
The number of lncRNAs with described functions is steadily increasing, and most of the reports revolve around their regulatory capacity. LncRNAs often function as important cis and trans-acting modulators for the expression of protein-coding genes [23], [24]. LncRNAs can mediate epigenetic modification by recruiting chromatin-remodelling complex to a specific chromatin locus (Fig. 1A). At least 38% of the lncRNAs present in several tissues bind to the polycomb repressive complex 2 or the chromatin modifying proteins, CoREST and SMCX [24]. Others bind to trithorax chromatin-activating complexes and/or activated chromatin [25]. The well-characterized lncRNAs, ANRIL, XIST, HOTAIR and KCNQ1OT1 are able to recruit epigenetic modifiers to specific loci for reprogramming the chromatin state. For example, KCNQ1OT1 binds to PRC2 and the methyl-transferase G9A (also known as EHMT2), whereas ANRIL binds to PRC1 and PRC2 [26], [27]. HOTAIR and other lncRNAs function as scaffolds (Fig. 1B) coordinate the targeting of specific repressive histone modifying complexes to target loci [26]. However, within this framework, the detailed mechanism of how specific DNA regions are targeted by lncRNAs remains unclear. An example of such principle approaches together is the XIST locus that controls the X chromosome dosage compensation. XIST, in many ways can be considered as a model example of lncRNA biology, and the dissection of its mechanism of action can serve as a guide for deeper and thorough future lncRNA studies. Principally, X chromosome inactivation is due to ablation of the DNA and promoter of XIST [27], [28], yet such models can't undermine the effect of the local act of transcription.
Fig. 1.
LncRNA-mediated transcriptional regulation. Long non-coding RNAs (lncRNAs; red solid lines) regulate gene transcription through three main mechanisms: (A) Interaction with and recruitment of chromatin-modifying enzymes (e.g., histone methylases, acetylases, and deacetylases) to the target gene locus. Modulation of the chromatin state by these enzymes leads to activation or repression of local genes. (B) Interaction with other RNA-binding factors such as hnRNPs to form RNA–protein complexes (RNPs). RNPs can either promote transcription by recruiting key proteins to the target gene promoters or repress gene transcription by binding to existing gene repressors. (C) LncRNAs also have enhancer functions and help to change the chromatin architecture and recruit transcriptional machinery proteins to adjacent target gene locus to promote its transcription. (D) LncRNAs are also involved in the repression of some pro-apoptotic genes, such as FAS and BIK, by acting as a decoy for the transcription factor (NF-YA).
2.2. Transcriptional regulation
Long non-coding RNAs act as co-factors to modify the activity of transcriptional factor. For example, the ncRNA Evf2 is transcribed from conserved distal enhancer and recruits the transcription factor (Fig. 1C) DlX2 to the same enhancer to induce expression of adjacent protein-coding genes [29]. Various developmental genes are regulated in a similar fashion by transcribing the enhancers in the cells in which they are active [30]. LncRNAs can modify RNA polymerase (RNAP) II activity by interplaying with the initiation complex to steer promoter choice e.g., in the humans transcription of a ncRNA from an upstream region of the dihydrofolate reductase (DHFR) locus to form a triplex in the major promoter of DHFR inhibits the binding of the transcriptional co-factor TFIID31. This could be a general mechanism for controlling the usage of the promoter, as thousands of these triplex structures exist in eukaryotic chromosomes [31]. Long lncRNAs may also affect global changes by interacting with some basic components of the RNAP II-dependent transcription machinery. LncRNAs interacting with RNAP II machinery are primarily transcribed by RNAP III, thereby delinking their expression from the RNAP II-dependent transcription reaction they regulate. For example, transcription of Alu elements in response to heat shock, they bind tightly to RNAP II to rule out the formation of active pre-initiation complexes [32]. Alu elements can independently mediate polymerase binding and repression by their respective domain interaction. Due to their abundance and distribution in the mammalian genome, these functional domains might have been co-operated into other ncRNAs during evolution [33].
2.3. Post-transcriptional regulation
The ability of ncRNAs to identify complementary sequences allows some specific interactions capable of regulating post-transcriptional processing of mRNAs like capping, splicing, editing, transport, translation, degradation, and stability at various control sites. For example, MALAT1 affects alternate splicing by interacting with splicing factors. Another lncRNA, Gomafu/MIAT, which is localized to a nuclear domain and has a neuron-specific expression, may block spliceosome formation and affect the mRNA splicing by sequestering splicing factor 1 (SF1) [34], [35]. The NAT lncRNAs also plays an essential role in regulating mRNA dynamics (Fig. 1D). About 61–72% of all transcribed regions possess lncRNAs in antisense orientation (NATs) [36]. Unlike classical NAT lncRNAs to the imprinting genes (Tsix, Air, HOTAIR, and Evf-2) which are responsible for recruiting repressor complexes like PRC2 to the target site, certain NATs result in RNA duplexes to inhibit cis-regulatory elements, leading to an alternate splicing pattern of the paired gene. For example, the Zeb2/Sip1 NAT complementary binds to the 5′ splice site of an intron in the 5′-UTR of the zinc finger Hox mRNA Zeb2, which plays a crucial role in epithelial-mesenchymal transition (EMT). Expression of the Zeb2 NAT upon EMT masks the splice site, hence preventing spliceosome action. Subsequently the translation machinery can then recognize and bind to an internal ribosome entry site (IRES), resulting in more efficient Zeb2 translation.
3. LncRNAs: a functional perspective
Though they are different from mRNAs exported to the cytoplasm for translation, few lncRNAs are known to be restrained in certain sub-nuclear compartments [37], [38], [39], which suggest potential functions in these compartments.
3.1. X chromosome inactivation
Epigenetic regulation of allelic expression (process of dosage compensation and genomic imprinting) is the best-studied function of lncRNAs. XCI (X chromosome inactivation) compensates the imbalance between XX and XY with respect to X-linked genes by inactivation of one of the X chromosomes in females through hetero-chromatinization [40]. Two different types of XCIs occur in placental mammals, the first one is the imprinted XCI, in the embryo, where the paternal X chromosome is always inactivated and another type is the random XCI, which occurs in the inner cell mass where epigenetic markers have been silenced after embryo implantation [41]. In random XCI, either the paternal or the maternal X chromosome is randomly inactivated, producing a mosaic female. XCI in placental mammals is regulated by a cluster of lncRNA loci which is known as the X-inactivation centre (Xic) [42]. The Xi-specific transcript (Xist) is highly expressed from Xi (X inactive) during the onset of XCI, but not from Xa (Xactive) chromosome. Xist RNA recruits silencing factors like Polycomb repressive complex 2 (PRC2) [43] by forming a “Xist cloud” around the X chromosome [44]. As it turns out, Xist is regulated by other lncRNAs. Tsix, which is transcribed in antisense orientation from a promoter downstream of Xist, is expressed at a higher level before initiating XCI and then goes silent from the future Xi chromosome, but persists on the presumptive Xa chromosome, thus exhibiting the reverse pattern as Xist expression. Tsix has a role in coordinating X chromosome pairing to bring epigenetic asymmetry within the Xist locus and to downregulate Xist by different mechanisms [45].
3.2. Regulation of allelic expression: genomic imprinting
LncRNAs also play their role in genomic imprinting by expression of a gene monoallelically according to its line of descent [46], [47]. Like XCI, imprinting is also controlled by some specific genomic loci, known as imprinting control regions. Depending on parental origins, differentially methylated regions, unmethylated DNA imprinting control regions (ICRs), result in specific expression of nearby lncRNAs genes and suppression of neighbouring genes in cis [48]. Most of the imprinted clusters are associated with protein coding genes and some specific lncRNAs which are inversely expressed, e.g., Air and Kcnq1ot1/LIT1 (Kcnq1 opposite transcript 1, or long QT intronic transcript 1) cause suppression of paternally inherited genes. Precisely, Kcnq1ot1/LIT1 is involved in repression of several protein-coding genes in cis by interacting with repressive chromatin-modifying complexes [49], [50]. Kcnq1ot1/LIT1 is an imprinted region with at least eight genes expressed exclusively or preferentially from the maternal allele [51]. Kcnq1ot1/LIT1 acts as an organizer on a tissue/lineage-specific nuclear domain, involving in epigenetic silencing of the Kcnq1 imprinting control region [51], [52], [53], [54] other examples being Igf2r/Air [26], Dlk1/Gtl2 [27], Nesp/Nespas/Gnas [55], and the Beckwith–Wiedemann syndrome (BWS) associated Kcnq1/Kcnq1ot1 [56]. These lncRNAs regulate the expression of imprinted genes by recruiting epigenetic factors, such as PRC2 and G9a [57]. H19 was one of the first lncRNAs to be identified and is reported to be the most highly expressed transcripts within the embryo [58]. It is inversely imprinted with Igf2. However, it has been observed that H19 does not seem to function as a lncRNAs [59], but acts as miRNA precursor [60].
4. LncRNAs in cancer
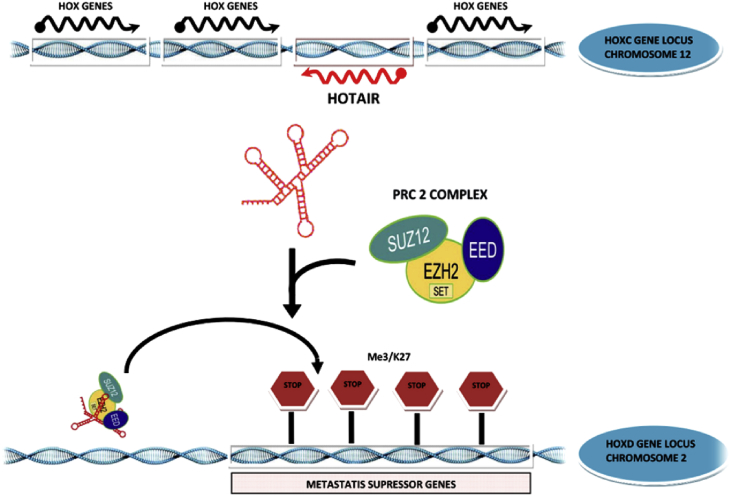
Cancer is a condition where gene expression is aberrant. The study of genetic background of cancer has revealed that the majority of the cancers are attributed to non-coding regions of the genome. Recent developments indicate that several cancer loci are transcribed into lncRNAs and that these transcripts play key roles in tumorigenesis. LncRNAs contribute to cancer development through diverse mechanisms. LncRNAs are reported to be implicated in serial steps of cancer development [61]. These lncRNAs interact with nucleic material and protein molecules and/or their combinations and act as an essential regulator of chromatin organization, transcriptional and posttranscriptional regulation. Their misexpression confers the cancer cell potential to initiate tumour growth, and metastasis. A review demonstrating the roles of lncRNAs in cancer diagnosis and therapy, reported different expression profiles for numerous lncRNAs in urothelial cancer [62]. Various evidences suggest that most of the lncRNAs result in epigenetic changes by recruiting various chromatin modifying agents [63], [64], like polycomb repressive complex 2 or the chromatin modifying proteins CoREST and SMCX [65]. The best studied lncRNAs (Table 1); ANRIL, MALAT1, HOTAIR and KCNQ1OT1, Taurine-upregulated gene 1 (TUG1), LINC00152, RP11-385J1.2 and TUBA4B which recruit epigenetic modifiers to their respective specific loci to regulate the chromatin state and their misexpression are linked to diverse cancers such as ANRIL: prostate cancer, HOTAIR: breast cancer KCNQ1OT1: colorectal cancer, TUG1: esophageal cancer, LINC00152: gastric cancer, RP11-385J1.2 and TUBA4B: non-small cell lung cancer [66], [67], [68], [69], [70]. One of the first lncRNAs described to have a functional role in the development of cancer is the metastasis-associated HOX Antisense Intergenic RNA (HOTAIR). HOTAIR lncRNAs promotes cancer metastasis by inducing epigenetic variations in the chromatin state of cancer cells [71]. The mechanism of action is well demonstrated in Fig. 2. It was observed to be highly up-regulated in metastatic and primary breast tumours, about 2000 times over the normal breast tissue. The upregulation of HOTAIR could be associated with poor prognosis in breast, liver, colorectal, GIT, and pancreatic cancers. Meanwhile, it may probably contribute to promote the tumour invasiveness and metastasis [72], [73], [74], [75], [76], [77]. A substantial growth of primary tumours was observed in mouse mammary fat pads by the grafting cells expressing HOTAIR [78]. Interestingly, certain reports indicate various lncRNAs emanating from the HOX locus suggesting its global regulatory role [79]. Taurine-upregulated gene 1 (TUG1) has shown significant over expression in esophageal squamous cell carcinoma (ESCC) tissues, and in vitro silencing of TUG1 inhibits the proliferation and migration of ESCC cells [80]. POU3F3 (Linc-POU class 3 homeobox 3) acts as a regulator in ESCC, which promotes the methylation of POU3F3 by interacting with EZH2 [67], [68]. Another significant lncRNA (LINC00152) has been associated with the development of gastric cancer (GC) and can be used as biomarker for GC diagnosis [69]. Recent comprehensive studies have shown that wide range of lncRNAs are significantly associated with non-small cell lung cancer (NSCLC). Important among these, RP11-385J1.2 and TUBA4B, and are aberrantly over expressed lncRNAs, thus exhibit an important developmental role in NSCLC [70]. The MALAT1 gene, or the metastasis-associated lung adenocarcinoma transcript 1, is associated with metastatic potential and is widely expressed in various human tissues [81], [82] and was also found to be up-regulated in various types of cancers like breast, prostate, colon, liver and uterine cancers [83]. Some lncRNAs are constituents of macromolecular complexes involved in RNA processing. MALAT1 is proposed to be acting at a post-transcriptional level by controlling the alternative splicing of pre-mRNA molecules. It modulates the activity of serine/arginine (SR) splicing factors [84]. MALAT1 is up-regulated in various cancer types and is over-expressed in metastatic cells of lung and colorectal cancers [85].
Table 1.
LncRNAs in cancer.
| S.No. | lncRNA | Function | Reference |
|---|---|---|---|
| 1 | ANRIL | Transcription control by chromatin modifications | [52] |
| 2 | MALAT1 | Promotes cancer by controlling the alternative splicing of pre-mRNA molecules | [67], [68], [69] |
| 3 | HOTAIR | Promotes cancer metastasis and progression through epigenetic variations in the chromatin state | [61] |
| 4 | KNCQ10T1 | Promoting cancer progression | |
| 5 | TUG1 | Promoting proliferation, migration, cell cycle | [53], [54], [66] |
| 6 | LINC00152 | Promoting cancer progression | [55] |
| 7 | RP11-385J1.2 | Suppressing proliferation and invasion | [56] |
| 8 | TUBA4B | Promoting proliferation, migration, invasion | [56] |
Fig. 2.
Model of long noncoding RNA (lncRNA) HOX transcript antisense RNA (HOTAIR) regulating expression of HOX genes in trans. LncRNA HOTAIR transcribed from the HOXC cluster of genes (chromosome no.2) binds PRC2 complex of polycomb-group of proteins and targets it to the HOXD cluster (chromosome no.12) leading to H3K27 methylation and silencing of neighboring HOXD genes.
Many tumor suppressor genes are known to carry antisense transcripts [86]. For example, p15, a cyclin-dependent kinase inhibitor implicated in leukemia, possesses an antisense transcript, which silences its transcription in cis and trans by inducing heterochromatin formation without changing the DNA methylation in a Dicer-independent manner [87], [88], [89], [90]. It is possible that these antisense transcripts directly bind and recruit chromatin-modifying complexes to their associated sense transcripts [91].
In summary, lncRNAs can act through different mechanisms to regulate cancer state. Although the molecular mechanisms of various lncRNAs functions are well studied, but their modes of action mostly remain unclear. A comprehensive study of the lncRNA mechanism and their functions will aid in better understanding of their role in cancer and will open up new therapeutic possibilities to modulate their function.
4.1. Role of LncRNAs in immunity
The role of non-coding RNAs in the regulation of gene expression in immune cells is still poorly understood [92]. Reports suggest their role in T cell development, differentiation, and activation of adaptive and innate immunity. LncRNAs are reported to be present in various immune cells like monocytes, macrophages, dendritic cells, neutrophils, T cells and B cells. The role of lnc-DC in the differentiation of monocytes to dendritic cells has been well established [93], [94]. Many lncRNAs like Lethe [95] and lnc-IL7R [96] have been reported to possess immune system associated functions. Moreover, recent reports reveal that the regulatory functions of many of these lncRNAs are in RNA-protein binding [97], [98], chromatin remodeling [99], [100] and in interactions with transcriptional factors and signaling molecules [101] as described in Fig. 1D. Although the functions of most of the lncRNAs in infections are still unknown, recent reports suggest that many lncRNAs may be playing a pivotal role in gene regulation at transcriptional and posttranscriptional level during innate immune responses. Their role in gene expression regulation in macrophages and other innate immune cells has also been reported. Such discoveries suggest that lncRNAs may contribute to gene regulatory networks governing host–pathogen interactions. Similarly, inflammatory mediation or cytokine regulation by lncRNAs is also poorly understood. The innate immunity is the frontline defense response of a cell. The Innate immune response is important to the host to defend itself from the invasion of various pathogenic organisms like bacteria or viruses. As cells encounter pathogens, an innate immune response is triggered by the production of inflammatory mediators or cytokines through transcription factors, such as API, NF-kB, and miRNAs [102], [103]. The innate immune response also triggers adaptive immune responses by recognizing pathogens by certain specific receptors known as TLRs (Toll-like receptors) resulting in the elimination of the pathogen. It has been found that TLRs are a major receptor type for pathogen recognition [104]. Following this ligand recognition by these receptors, lncRNAs like Nest/tmvpg1 [105], [106], IL1β-eRNA [107] and IL1β-RBT46 [108] recruit transcription activators through RNA–protein complex formation or remove repressors of transcription, leading to rapid expression of cytokines. This also involves transcriptional and post-transcriptional gene regulation via transcription factors (API and NF-kB, and miRNAs) [109]. Phagocytes such as macrophages and polymorphonuclear neutrophilic granulocytes (PMNs) represent the first line of defense against invading pathogens. Macrophages are key components of host defense mechanisms against invading pathogens. Many lncRNAs were also found to be up-regulated in macrophages exposed to Toll-like receptor 2 (TLR2) ligands [110]. It has been found that a large number of lncRNAs are involved in regulation of interferon stimulated genes (ISG) like PACER[111], NRON[112], [113], antisense transcript of IL1β and a long intergenic non-coding RNA (lincRNA) Cox2 [114], [115] (Table 2). LincRNA Cox2 was found to have a role in TLR-induced expression of interleukin-6 (Il6) [116]. TNF and HNRNPL-related immunoregulatory lincRNA (THRIL) were reported to regulate expression of tumor necrosis factor (TNF) in human monocytes through interactions with HNRNPL [118], [117]. In addition to the phagocytosis and secretion of pro-inflammatory mediators by macrophages, autophagy is the most recent mechanism of the immune response to bacterial infection. Although, the role of lncRNAs in autophagy response to the intracellular bacteria is still unknown [119], it has been reported that IFN-γ induced autophagy in infected macrophages resulted in sustained lncRNA MEG3 down-regulation and lack of IFN-γ allowed for counter-regulation of lncRNA MEG3 by viable M. bovis BCG. Knockdown of lncRNA MEG3 in macrophages resulted in autophagy and enhanced removal of intracellular M. bovis BCG [120], [121]. It has also been reviewed that lncRNAs may have a function in a variety of human polygenic diseases from cancers of different organs to non-cancerous diseases, such as Alzheimer's disease [122], [125], [124], [123]. Further research may enable us to better understand their infectious and inflammatory pathologies and may provide a new insight for effective therapeutics.
Table 2.
LncRNA in immunity.
| S.N.o. | lncRNA | Function | Reference |
|---|---|---|---|
| 1 | LincRNA-COX2 | Role in TLR-induced expression of interleukin-6 | [114], [115] |
| 2 | Lnc-DC | Required for the differentiation of monocytes to dendritic cells | [93] |
| 3 | NRON | Transcription regulator for immune regulation | [112], [113] |
| 4 | Lnc-IL7R | Epigenetically regulates inflammation | [96] |
| 5 | NeST/Tmevpg1 | Epigenetically regulates the adaptive immunity through IFN-gamma | [105], [106] |
| 6 | IL1b-eRNA | Overexpressed in LPS induced inflammation | [107] |
| 7 | PACER | Involved in multiple processs related to regulation of immunogene expression | [111] |
| 8 | THRIL | regulate expression of tumour necrosis factor (TNF) in human monocytes | [117] |
| 9 | IL1β-RBT46 | Regulates the homeostasis of IL-1β in monocytes | [108] |
| 10 | lethe | Upregulated during inflammation | [95] |
5. Conclusion
Despite the fast increase in understanding the functional role of lncRNA, this field is still in its infancy and many questions and challenges are debatable. Their role in human diseases is a mystery. Undoubtedly, in this review article we tried to explain how lncRNAs play a diverse role in biological processes and contribute in physiological processes of the cell. A better understanding of chromosomal architecture and affiliation between the organization of RNA structure and protein recruitment as well as gene regulation shall help in prognosis and design novel therapeutics to target gene expression for the treatment of many dreadful human diseases. Many of the lncRNAs are reported to have diverse functions, identified through genomic and transcriptomic approaches. The Much deeper study is needed to discover many more lncRNAs that have their functions annotated. Another challenge in lncRNA research is to explore their coding prospective. The possibility that code for short peptides has not been ruled out as of now.
References
- 1.The ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carninci P. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 3.Koch F., Jourquin F., Ferrier P., Andrau J.C. Genome-wide RNA polymerase II: not genes only. Trends Biochem. Sci. 2008;33:265–273. doi: 10.1016/j.tibs.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Maeda N., Kasukawa T., Oyama R., Gough J., Frith M., Engström P.G., Lenhard B., Aturaliya R.N., Batalov S., Beisel K.W. Transcript annotation in FANTOM3:mouse gene catalog based on physical cDNAs. PLoS Genet. 2006;2(4):62. doi: 10.1371/journal.pgen.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordström K.J.V., Mirza M.A.I., Almén M.S., Gloriam D.E., Fredriksson R., Schiöth H.B. Critical evaluation of the FANTOM3 non-coding RNA transcripts. Genomics. 2009;94:169–176. doi: 10.1016/j.ygeno.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki Y., Furuno M., Kasukawa T., Adachi J., Bono H., Kondo S., Nikaido I., Osato N., Saito R., Suzuki H. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 7.Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J., Kapranov P., Drenkow J., Dike S., Brubaker S., Patel S., Long J., Stern D., Tammana H., Helt G. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science. 2005;308:1149–1154. doi: 10.1126/science.1108625. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q., Kim Y.C., Lu J., Xuan Z., Chen J., Zheng Y., Zhou T., Zhang M.Q., Wu C.I., Wang S.M. Poly A- transcripts expressed in HeLa cells. PLoS One. 2008;3(7):e2803. doi: 10.1371/journal.pone.0002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramskold D., Wang E.T., Burge C.B., Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoSComput. Biol. 2009;5(12):1000598. doi: 10.1371/journal.pcbi.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttman M., Garber M., Levin J.Z., Donaghey J., Robinson J., Adiconis X., Fan L., Koziol M.J., Gnirke A., Nusbaum C. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 2010;28:503–510. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babak T., Blencowe B.J., Hughes T.R. A systematic search for new mammalian noncoding RNAs indicates little conserved intergenic transcription. BMC Genomics. 2005;6:104. doi: 10.1186/1471-2164-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bono H., Yagi K., Kasukawa T., Nikaido I., Tominaga N., Miki R., Mizuno Y., Tomaru Y., Goto H., Nitanda H. Systematic expression profiling of the mouse transcriptome using RIKEN cDNA microarrays. Genome Res. 2003;13(6B):1318–1323. doi: 10.1101/gr.1075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer T.R., Dinger M.E., Sunkin S.M., Mehler M.F., Mattick J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. U. S. A. 2008;105(2):716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer T.R., Dinger M.E., Sunkin S.M., Mehler M.F., Mattick J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. U. S. A. 2008;105(2):716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taft R.J., Pang K.C., Mercer T.R., Dinger M., Mattick J.S. Non-coding RNAs: regulators of disease. J. Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 18.Yap K.L., Li S., Munoz-Cabello A.M., Raguz S., Zeng L., Mujtaba S., Gil J., Walsh M.J., Zhou M.M. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer T.R., Qureshi I.A., Gokhan S., Dinger M.E., Li G., Mattick J.S., Mehler M.F. Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11:14. doi: 10.1186/1471-2202-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L.L., Carmichael G.G. Decoding the function of nuclear long non-coding RNAs. Curr. Opin. Cell Biol. 2010;22:357–364. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinger M.E., Amaral P.P., Mercer T.R., Pang K.C., Bruce S.J., Gardiner B.B., Askarian-Amiri M.E., Ru K., Soldà G., Simons C., Sunkin S.M., Crowe M.L., Grimmond S.M., Perkins A.C., Mattick J.S. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–1445. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loewer S., Cabili M.N., Guttman M., Loh Y.H., Thomas K., Park I.H., Garber M., Curran M., Onder T., Agarwal S., Manos P.D., Datta S., Lander E.S., Schlaeger T.M., Daley G.Q., Rinn J.L. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Y.S., Sunwoo H., Zhang B., Spector D.L. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat. Cell Biol. 2011;13:95–101. doi: 10.1038/ncb2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fejes-Toth K., Sotirova V., Sachidanandam R., Assaf G., Hannon G.J., Kapranov P., Foissac S., Willingham A.T., Duttagupta R., Dumais E., Gingeras T.R. Post-transcriptional processing generates a diversity of 5′-modified long and short RNAs. Nature. 2009;457:1028–1032. doi: 10.1038/nature07759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapranov P., St Laurent G., Raz T., Ozsolak F., Reynolds C.P., Sorensen P.H., Reaman G., Milos P., Arceci R.J., Thompson J.F., Triche T.J. The majority of total nuclear-encoded non-ribosomal RNA in a human cell is ‘dark matter’ un-annotated RNA. BMC Biol. 2010;8:149. doi: 10.1186/1741-7007-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J.T. Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control. Nat. Rev. Mol. Cell Biol. 2011;12:815–826. doi: 10.1038/nrm3231. [DOI] [PubMed] [Google Scholar]
- 27.Mak W., Nesterova T.B., de Napoles M., Appanah R.S., Yamanaka Reactivation of the paternal X chromosome in early mouse embryos. Science. 2004;303:666–669. doi: 10.1126/science.1092674. [DOI] [PubMed] [Google Scholar]
- 28.Brown C.J., Lafrenière R.G., Powers V.E., Sebastio G., Ballabio A. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature. 1999;349:82–84. doi: 10.1038/349082a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J., Sun B.K., Erwin J.A., Song J.J., Lee J.T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemson C.M., McNeil J.A., Willard H.F., Lawrence J.B. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 1996;132:259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sado T., Hoki Y., Sasaki H. Tsix silences Xist through modification of chromatin structure. Dev. Cell. 2005;9:159–165. doi: 10.1016/j.devcel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Wan L.B., Bartolomei M.S. Regulation of imprinting in clusters: noncoding RNAs vs. insulators. Adv. Genet. 2008;61:207–223. doi: 10.1016/S0065-2660(07)00007-7. [DOI] [PubMed] [Google Scholar]
- 33.Edwards C.A., Ferguson-Smith A.C. Mechanisms regulating imprinted genes in clusters. CurrOpin Cell Biol. 2007;19:281–289. doi: 10.1016/j.ceb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 34.He Y., Meng X.M., Huang C., Wu B.M., Zhang L., Lv X.W., Li J. Long noncoding RNAs: novel insights into hepatocelluar carcinoma. Cancer. 2014;344:20–27. doi: 10.1016/j.canlet.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Pandey R.R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D., Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Kanduri C., Thakur N., Pandey R.R. The length of the transcript encoded from the Kcnq1ot1 antisense promoter determines the degree of silencing. EMBO J. 2006;25:2096–2106. doi: 10.1038/sj.emboj.7601090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanduri C. Kcnq1ot1: a chromatin regulatory RNA. Semin. Cell Dev. Biol. 2011;22:343–350. doi: 10.1016/j.semcdb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P., Dinger M.E., Mattick J.S. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones M.J., Bogutz A.B., Lefebvre L. An extended domain of Kcnq1ot1 silencing revealed by an imprinted fluorescent reporter. Mol. Cell Biol. 2011;31:2827–2837. doi: 10.1128/MCB.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammad F., Mondal T., Guseva N., Pandey G.K., Kanduri C. Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development. 2010;137:2493–2499. doi: 10.1242/dev.048181. [DOI] [PubMed] [Google Scholar]
- 41.Lyle R., Watanabe D., teVruchte D., Lerchner W., Smrzka O.W. The imprinted antisense RNA at the Igf2r locus overlaps but does not imprint Mas1. Nat. Genet. 2000;25:19–21. doi: 10.1038/75546. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt J.V., Matteson P.G., Jones B.K., Guan X.J., Tilghman S.M. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 2000;14:1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson C.M., Turner M.D., Ball S.T., Nottingham W.T., Glenister P. Identification of an imprinting control region affecting the expression of all transcripts in the Gnascluster. Nat. Genet. 2006;38:350–355. doi: 10.1038/ng1731. [DOI] [PubMed] [Google Scholar]
- 44.Kanduri C., Thakur N., Pandey R.R. The length of the transcript encoded from the Kcnq1ot1 antisense promoter determines the degree of silencing. EMBO J. 2006;25:2096–2106. doi: 10.1038/sj.emboj.7601090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J., Ohsumi T.K., Kung J.T., Ogawa Y., Grau D.J. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brannan C.I., Dees E.C., Ingram R.S., Tilghman S.M. The product of the H19 gene may function as an RNA. Mol. Cell Biol. 1990;20:28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu D., Xu B., Chen S., Yang Y., Zhang X., Liu J., Lu K., Zhang L., Liu C., Zhao Y., Jiang H., Liu N., Chen M. Long non-coding RNAs and prostate cancer. J. NanosciNanotechnol. 2013;13:3186–3194. doi: 10.1166/jnn.2013.6870. [DOI] [PubMed] [Google Scholar]
- 48.Gibb E.A., Brown C.J., Lam W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer. 2011;10:38–55. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabory A., Jammes H., Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays. 2010;32:473–480. doi: 10.1002/bies.200900170. [DOI] [PubMed] [Google Scholar]
- 50.Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012;14:859–865. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattick J.S., Gagen M.J. The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol. Biol. Evol. 2001;18:1611–1630. doi: 10.1093/oxfordjournals.molbev.a003951. [DOI] [PubMed] [Google Scholar]
- 52.Mattick J.S., Amaral P.P., Dinger M.E., Mercer T.R., Mehler M.F. RNA regulation of epigenetic processes. Bioessays. 2009;31:51–59. doi: 10.1002/bies.080099. [DOI] [PubMed] [Google Scholar]
- 53.Guo H., Wu L., Yang Q., Ye M., Zhu X. Functional linc-POU3F3 is overexpressed and contributes to tumorigenesis in glioma. Gene. 2015;554:114–119. doi: 10.1016/j.gene.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 54.Li W., Zheng J., Deng J., You Y., Wu H., Li N., Lu J., Zhou Y. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology. 2014;146:1714–1726. doi: 10.1053/j.gastro.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Li Q., Shao Y., Zhang X., Miao M., Qin L., Wang B., Ye G., Xiao B., Guo J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36:2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Xu G., Chen W., Pan Q., Huang K., Pan J., Zhang W., Chen J. Detection of long-chain non-encoding RNA differential expression in non-small cell lung cancer by microarray analysis and preliminary verification. Mol. Med. Rep. 2015;11:1925–1932. doi: 10.3892/mmr.2014.2944. [DOI] [PubMed] [Google Scholar]
- 57.Ishibashi M., Kogo R., Shibata K., Sawada G., Takahashi Y., Kurashige J., Akiyoshi S., Sasaki S., Iwaya T., Sudo T., Sugimachi K., Mimori K., Wakabayashi G., Mori M. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol. Rep. 2013;29:946–950. doi: 10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 58.White N.M., Cabanski C.R., Silva-Fisher J.M., Dang H.X., Govindan R., Maher C.A. Transcriptome sequencing reveals altered long intergenic non-coding RNAs in lung cancer. Genome Biol. 2014;15:429. doi: 10.1186/s13059-014-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L., Wang Y., Brzoska P., Kong B., Li R., West R.B., van de R.B., Vijver M.J., Sukumar S., Chang H.Y. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Z., Zhou L., Wu L.M., Lai M.C., Xie H.Y., Zhang F., Zheng S.S. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann. SurgOncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 61.Kogo R., Shimamura T., Mimori K., Kawahara K., Imoto S., Sudo T., Tanaka F., Shibata K., Suzuki A., Komune S., Miyano S., Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 62.Niinuma T., Suzuki H., Nojima M., Nosho K., Yamamoto H., Takamaru H., Yamamoto E., Maruyama R., Nobuoka T., Miyazaki Y., Nishida T., Bamba T., Kanda T., Ajioka Y., Taguchi T., Okahara S., Takahashi H., Nishida Y., Hosokawa M., Hasegawa T., Tokino T., Hirata K., Imai K., Toyota M., Shinomura Y. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–1136. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]
- 63.Kim K., Jutooru I., Chadalapaka G., Johnson G., Frank J., Burghardt R., Kim S., Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khalil A.M., Guttman M., Huarte M., Raj Garber A., Rivea M.D., Thomas K., Presser A., Bernstein B.E., van Oudenaarden A., Lander Regev E.S., Rinn J.L. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gutschner T., Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y., Wang J., Qiu M., Xu L., Li M., Jiang F., Yin R., Xu L. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36:1643–1651. doi: 10.1007/s13277-014-2763-6. [DOI] [PubMed] [Google Scholar]
- 67.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khalil A.M., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D., Thomas K., Presser A., Bernstein B.E., van Oudenaarden A. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U. S. A. 2009;106(28):11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji P., Diederichs S., Wang W., Boing S., Metzger R., Schneider P.M., Tidow N., Brandt B., Buerger H., Bulk E. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 70.Hutchinson J.N., Ensminger A.W., Clemson C.M., Lynch C.R., Lawrence J.B., Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39. doi: 10.1186/1471-2164-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guffanti A., Iacono M., Pelucchi P., Kim N., Solda G., Crof L.J., Taft t R.J., Rizzi E., Askarian-Amiri M., Bonnal R.J. A transcriptional sketch of a primary human breast cancer by 454 deep sequencing. BMC Genomics. 2009;10:163. doi: 10.1186/1471-2164-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu W., Gius D., Onyango P., Muldoon-Jacobs K., Karp J., Feinberg A.P., Cui H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatta Y., Hirama T., Miller C.W., Yamada Y., Tomonaga M., Koeffler H.P. Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood. 1995;85:2699–2704. [PubMed] [Google Scholar]
- 74.Otsuki T., Jaffe E.S., Wellmann A., Kumar S., Condron K.S., Raffeld M. Absence of p18 mutations or deletions in lymphoid malignancies. Leukemia. 1996;10:356–360. [PubMed] [Google Scholar]
- 75.Martinez-Delgado B., Robledo M., Arranz E., Osorio A., García M.J., Echezarreta G., Rivas C., Benitez J. Hypermethylation of p15/ink4b/MTS2 gene is differentially implicated among non-Hodgkin’s lymphomas. Leukemia. 1998;12:937–941. doi: 10.1038/sj.leu.2401009. [DOI] [PubMed] [Google Scholar]
- 76.Maloney K.W., McGavran L., Odom L.F., Hunger S.P. Different patterns of homozygous p16INK4A and p15INK4B deletions in childhood acute lymphoblastic leukemias containing distinct E2A translocations. Leukemia. 1998;12:1417–1421. doi: 10.1038/sj.leu.2401124. [DOI] [PubMed] [Google Scholar]
- 77.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A., Blencowe B.J., Prasanth S.G., Prasanth K.V. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt L.H., Spieker T., Koschmieder S., Schaffers S., Humberg J., Jungen D., Bulk E., Hascher A., Wittmer D., Marra A., Hillejan L., Wiebe K., Berdel W.E., Wiewrodt R., Muller-Tidow C. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. ThoracOncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 79.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khalil A.M., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D., Thomas K., Presser A., Bernstein B.E., van Oudenaarden A., Regev A., Lander E.S., Rinn J.L. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U. S. A. 2009;106(:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dinger M.E., Amaral P.P., Mercer T.R., Pang K.C., Bruce S.J., Gardiner B.B., Askarian-Amiri M.E., Ru K., Solda G., Simons C., Sunkin S.M., Crowe M.L., Grimmond S.M., Perkins A.C., Mattick J.S. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–1445. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yap K.L. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandey R.R. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Kotake Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15INK4B tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spitale R.C., Tsai M.C., Chang H.Y. RNA templating the epigenome: long noncoding RNAs as molecular scaffolds. Epigenetics. 2011;6:539–543. doi: 10.4161/epi.6.5.15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Penny G., Kay G., Sheardown S., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 87.Feng J. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashe H.L., Monks J., Wijgerde M., Fraser P., Proudfoot N.J. Intergenic transcription and transinduction of the human β-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohno M., Fukagawa T., Lee J.S., Ikemura T. Triplex-forming DNAs in the human interphase nucleus visualized in situ by polypurine/polypyrimidine DNA probes and antitriplex antibodies. Chromosoma. 2002;111:201–213. doi: 10.1007/s00412-002-0198-0. [DOI] [PubMed] [Google Scholar]
- 90.Mariner P.D. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol. Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 91.Amaral P.P., Mattick J.S. Noncoding RNA in development. Mamm. Genome. 2008;19:454–492. doi: 10.1007/s00335-008-9136-7. [DOI] [PubMed] [Google Scholar]
- 92.Sone M., Hayashi T., Tarui H., Agata K., Takeichi M. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J. Cell Sci. 2007;12:2498–2506. doi: 10.1242/jcs.009357. [DOI] [PubMed] [Google Scholar]
- 93.Wang P., Xue Y., Han Y. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 94.Tsuiji H., Yoshimoto R., Hasegawa Y., Furuno M., Yoshida M. Competition between a noncoding exon and introns: Gomafu contains tandem UACUAAC repeats and associates with splicing factor-1. Genes cells. 2011;16:479–490. doi: 10.1111/j.1365-2443.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rapicavoli N.A., Qu K., Zhang J., Mikhail M., Laberge R.M., Chang H.Y. A mammalian pseudogene lncRNAat the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:00762. doi: 10.7554/eLife.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui H., Xie N., Tan Z. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur. J. Immunol. 2014;44:2085–2095. doi: 10.1002/eji.201344126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris K.V. Long antisense non-coding RNAs function to direct epigenetic complexes that regulate transcription in human cells. Epigenetics. 2009;4:296–301. doi: 10.4161/epi.4.5.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gang H. Tan, Xia-Di,Functional diversity of long non-coding RNAs in immune regulation. Genes Dis. 2016;3(1):72–81. doi: 10.1016/j.gendis.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atianand M.K., Fitzgerald K.A. Long non-coding RNAs and control of gene expression in the immune system. Trends Mol. Med. 2014;20:623–631. doi: 10.1016/j.molmed.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carpenter S., Aiello D., Atianand M.K. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z., Chao T.C., Chang K.Y. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1002–1007. doi: 10.1073/pnas.1313768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez J.A., Wapinski O.L., Yang Y.W. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell. 2013;152:743–754. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rossetto C.C., Tarrant-Elorza M., Verma S., Purushothaman P., Pari G.S. Regulation of viral and cellular gene expression by Kaposi's sarcoma-associated herpesvirus polyadenylated nuclear RNA. J. Virol. 2013;87:5540–5553. doi: 10.1128/JVI.03111-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turner M., Galloway A., Vigorito E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat. Immunol. 2014;15:484–491. doi: 10.1038/ni.2887. [DOI] [PubMed] [Google Scholar]
- 105.Gomez J.A., Wapinski O.L., Yang Y.W. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell. 2013;152:743–754. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vigneau S., Rohrlich P.S., Brahic M., Bureau J.F. Tmevpg1, a candidate gene for the control of Theiler's virus persistence, could be implicated in the regulation of gamma interferon. J. Virol. 2003;77:5632–5638. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.ott II N.E., Heward J.A., Roux B. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat. Commun. 2014 doi: 10.1038/ncomms4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu J., Wu X., Hong M., Tobias P., Han J. A potential suppressive effect of natural antisense IL-1beta RNA on lipopolysaccharide-induced IL-1beta expression. J. Immunol. 2013;190:6570–6578. doi: 10.4049/jimmunol.1102487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-likereceptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 110.Sonkoly E., Ståhle M., Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clin. Exp. Dermatol. 2008;33:312–315. doi: 10.1111/j.1365-2230.2008.02804.x. [DOI] [PubMed] [Google Scholar]
- 111.Krawczyk M., Emerson B.M. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-kappaB complexes. Elife. 2014;3:01776. doi: 10.7554/eLife.01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Willingham A.T., Orth A.P., Batalov S. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 113.Sharma S., Findlay G.M., Bandukwala H.S. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11381–11386. doi: 10.1073/pnas.1019711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guttman M., Amit I., Garber M. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carpenter S., Aiello D., Atianand M.K. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawai T., Akira S. Theroleofpattern-recognition receptors in innate immunity:update on toll-likereceptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 117.Li Z., Chao T.C., Chang K.Y. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1002–1007. doi: 10.1073/pnas.1313768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carpenter S., Aiello D., Atianand M.K. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y.C. Long noncoding RNAs in innate immunity. Cell MolImmunol. 2016;13(2):138–147. doi: 10.1038/cmi.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pawar K. Down regulated lncRNA MEG3 eliminates mycobacteria in macrophages via autophagy. SciRep. 2016;6:19416. doi: 10.1038/srep19416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bihl F., Brahic M., Bureau J.F. Two loci, Tmevp2 and Tmevp3, located on the telomeric region of chromosome 10, control the persistence of Theiler's virus in the central nervous system of mice. Genetics. 1999;152:385–392. doi: 10.1093/genetics/152.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vigneau S., Rohrlich P.S., Brahic M., Bureau J.F. Tmevpg1, a candidate gene for the control of Theiler's virus persistence, could be implicated in the regulation of gamma interferon. J. Virol. 2003;77:5632–5638. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karim R., Zou Li, Wang, Yun-fu The research progress of long noncoding RNAs in autoimmune diseases. J. Neurophysiol. 2016;7:2. [Google Scholar]
- 124.Li J., Xuan Z., Liu C. Long non-coding RNAs and complex human diseases. Int. J. Mol. Sci. 2013;14:18790–18808. doi: 10.3390/ijms140918790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]