Abstract
Non-coding small RNA molecules, the microRNAs (miRNAs), contribute decisively to the epigenetic regulation processes in cancer cells. Problematic pathogenic properties of cancer cells and the response of cancers towards anticancer drugs are highly influenced by miRNAs. Both increased drug activity and formation of tumor resistance are regulated by miRNAs. Further to this, the survival and proliferation of cancer cells and the formation of metastases is based on the modulated expression of certain miRNAs. In particular, drug-resistant cancer stem-like cells (CSCs) depend on the presence and absence of specific miRNAs. Fortunately, several small molecule natural compounds were discovered that target miRNAs involved in the modulation of tumor aggressiveness and drug resistance. This review gives an overview of the effects of a selection of naturally occurring small molecules (alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins) on miRNAs that are closely tangled with cancer diseases.
Keywords: MiRNA, Alkaloids, Organosulfur compounds, Aliphatic carboxylic acids, Water-soluble vitamins, Anticancer drugs
Abbreviations: AM, allyl mercaptan; AOM, azoxymethane; CPT, camptothecin; DADS, diallyl disulfide; DHA, docosahexaenoic acid; DIM, 3,3′-diindolylmethane; EPA, eicosapentaenoic acid; FA, folic acid; GTC, green tea catechins; I3C, indole-3-carbinol; NaB, sodium butyrate; PEITC, phenethylisothiocyanate; PUFA, polyunsaturated fatty acid; SAMC, S-allylmercaptocysteine; SFN, sulforaphane; TSA, trichostatin A
1. Introduction
MicroRNAs (miRNAs) feature short and highly conserved non-coding RNAs of 22–23 nucleotides and there are more than two thousand miRNAs that regulate about one-third of all human genes [1]. In fact, the regulation of important cellular processes such as proliferation, apoptosis, and differentiation of cells involves miRNAs [2]. MiRNAs also feature valuable diagnostic and prognostic factors because characteristic miRNA expression patterns were identified in tumor tissues [3], [4], [5], [6], [7]. The survival of drug-resistant cancer stem-like cells (CSCs) is regulated by special miRNAs as well [8], [9]. MiRNAs also play an essential part concerning the formation of metastases due to regulation of epithelial-to-mesenchymal transition (EMT) of cancer cells [10], [11].
The translation of target messenger RNAs (mRNAs) is blocked by mature miRNAs via binding to the 3′-untranslated region (3′UTR) of the mRNAs [12]. Single miRNAs can target various genes while dysregulation of tumor suppressor or oncogenic miRNAs was observed in many human cancer types [13], [14]. Thus, miRNAs with effects on carcinogenesis, cell proliferation and differentiation, apoptosis, and on the formation of metastasis and drug resistance feature valuable targets for the design of potent anticancer drugs [15], [16], [17]. Next generation anticancer drugs should either inhibit the activity of oncogenic miRNAs or promote the activity of tumor suppressor miRNAs. A recent review published in this journal before dealt with miRNA-modulating natural phenols and terpenoids and their effects on various tumors [18]. This review provides an overview of further anticancer active natural products (alkaloids, organosulfur and aliphatic carboxylic acid derivatives, water-soluble vitamins) and their manifold and versatile interactions with miRNAs involved in cancer diseases.
2. Dietary compounds and natural products (alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins) with effects on miRNA expression and activity
Nature provides us with a plethora of drug candidates we only need to explore. In order to develop potent agents against deadly diseases such as cancer, it is necessary to investigate the modes of action of these dietary and natural products thoroughly. There are very promising derivatives from the natural compound classes of alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins. Their multiple modes of action and their manifold interactions with miRNAs represent the key issues of this review manuscript.
2.1. Alkaloids
Alkaloids feature a rich compound class (ca. 12.000 alkaloids were investigated as bioactive compounds) comprising small molecule natural amines and N-heterocyclic natural products [19]. Several alkaloids have shown potent anticancer activity and some of them (e.g., Vinca alkaloids, camptothecins) have already been approved for chemotherapy of certain cancer and leukemia diseases, while others (e.g., DIM, berberine, vitamin C) seem to be valuable additives for chemotherapeutic regimens. The interactions of anticancer active alkaloids subdivided into indole alkaloids on the one hand, and quinoline, isoquinoline and quinolizidine alkaloids on the other hand, are presented in this chapter.
2.1.1. Indole alkaloids
Indole-3-carbinol was detected in various Brassica vegetables (e.g., broccoli, cabbage, cauliflower, sprouts) and its condensation product 3,3′-diindolylmethane (DIM) is generated after consumption in the stomach under acidic conditions (Fig. 1) [20]. The more stable compound DIM revealed distinct anticancer activities via suppression of the PI3K-Akt-mTOR signaling pathway and via inhibition of epigenetic factors such as DNA-methyl transferases/DNMTs (hypomethylation) and histone deacetylases (HDACs) [21], [22]. Several clinical trials of I3C and DIM have revealed that both I3Ca and DIM were well tolerable and further trials with DIM in prostate cancer patients, in patients with cervical dysplasia and as preventive agent in healthy non-smokers are ongoing [23]. DIM (more precisely, formulated DIM called BR-DIM with improved bioavailability) increased the expression of tumor suppressing let-7 miRNAs associated with suppression of the let-7-target EZH2 (a histone-lysine N-methyltransferase) and inhibition of prostate tumor growth (LNCaP, C4-2B, PC-3) [24]. In pancreatic cancer cells (Colo357, Panc-1), DIM induced miR-146a expression leading to suppression of EGFR (epidermal growth factor receptor), IRAK-1 (interleukin 1 receptor-associated kinase 1), NF-κB (nuclear factor κB) and MTA2 (metastasis-associated protein 2) and to inhibition of cancer cell invasion [25]. DIM also upregulated let-7b/c/d/e and miR-200b/c in gemcitabine-resistant pancreatic cancer cells (MiaPaCa-2) causing a reversal of the EMT (epithelial-to-mesenchymal transition) via suppression of ZEB1 (zinc finger E-box binding homeobox 1), slug and vimentin and upregulation of E-cadherin [26]. In combination with Herceptin, DIM increased miR-200 expression accompanied by FoxM1 suppression and induction of apoptosis in HER-2 positive breast cancer cells (SKBR3, MDA-MB-468) [27]. DIM also induced miR-34 expression in castrate-resistant prostate cancer cells associated with inhibition of Notch-1 and androgen receptor (AR) signaling and lowered the self-renewal potential of prostate cancer cells [28]. In breast cancer models (T47D, MDA-MB-231), DIM increased the expression of the miRNA cluster miR-212/132 via activation of the aryl hydrocarbon receptor (Ahr) leading to the suppression of the pro-metastatic protein Sox4 (SRY-related HMG-box 4) both in vitro and in vivo, to reduced tumor growth and to inhibition of metastasis formation [29].
Fig. 1.

Structures of the indole alkaloids I3C and DIM.
Concerning oncogenic miRNAs (oncomiRs), I3C suppressed miR-21 expression in pancreatic cancer cells (Panc-1) leading to upregulation of the miR-21 target PDCD4 (programmed cell death protein 4) and enhanced gemcitabine-sensitivity of the I3C-treated cancer cells [30]. In vinyl carbamate-induced lung cancer, I3C was able to suppress the upregulated miRNAs miR-21, miR-31, miR-130a, miR-146b, and miR-377 [31]. In addition, DIM downregulated miR-221 in pancreatic cancer cells (MiaPaCa-2, Pan377 c-1) associated with upregulation of the miR-221 targets PTEN (phosphatase and tensin homolog deleted on chromosome 10), PUMA (p53 upregulated modulator of apoptosis), and the CDK(cyclin-dependent kinase)-inhibitors p27 and p57 [32]. Interestingly, DIM revealed inhibitory effects on bone metastasis formation by prostate cancers via suppression of miR-92 [33]. Surprisingly, DIM enhanced the expression of miR-21 in breast cancer cells leading to degradation of Cdc25A and cancer cell growth inhibition [34]. Thus, in this special case miR-21 can also function as a tumor suppressor. A list of miRNAs regulated by DIM and I3C is shown in Table 1.
Table 1.
Regulation of microRNAs by DIM and I3C.
Tubulin-binding Vinca alkaloids (e.g., vinblastine, vincristine) were first isolated from the Madagascar periwinkle (Catharanthus roseus) in the 1960's and feature complex indole alkaloids which represent valuable and approved anticancer drugs for the treatment of advanced cancer diseases (Fig. 2) [35]. The semi-synthetic Vinca alkaloid vinorelbine (Fig. 2) revealed an improved toxicity profile compared with vinblastine and vincristine. Due to clinical drawbacks such as emerging multidrug-resistance upon treatment with Vinca alkaloids, the role of miRNAs for the induction of resistance to Vinca alkaloids was investigated in various cancer models. In breast cancer cells, miR-27a and miR-451 induced expression of the ABC-transporter MDR1/Pgp, and suppression of miR-27a and miR-451 was accompanied by increased accumulation of vinblastine in cancer cells [36]. Similar results were observed from MCF-7 breast cancer cells, where vinblastine suppressed the expression of miR-27a, miR-27b, miR-324-3p, miR-328, miR-148a and miR-451, while these miRNAs were not affected by vinblastine in colon cancer cells (Caco-2) [37]. In particular, the downregulation of miR-27b, miR-148a, and miR-451 by vinblastine was associated with increased expression of ABC-transporters such as ABCB1 and ABCC1 leading to enhanced drug efflux from the cancer cell [38]. MiR-34a expression enhanced the antiproliferative activity of vincristine in retinoblastoma cells by suppression of HMGB1 (high mobility group box 1) [38]. Vincristine-resistant hepatocellular carcinoma (HCC) cells exhibited upregulated miRNAs such as miR-27b, miR-181a, miR-146b-5p, miR-181d and miR-146a [39]. In addition, vincristine-resistant laryngeal cancer cells (Hep-2/v) exhibited increased expression of miR-210 and miR-923, while five miRNAs were suppressed (miR-93, miR-93*, miR-424*, miR-25*, miR-494) [40]. Overexpression of the oncogenic miR-125b in Ewing sarcoma cells decreased the anticancer activity of vincristine [41]. R-CHOP (rituximab, cyclophosphamide, adriamycin, vincristine, prednisone) is clinically approved for the treatment of diffuse large B-cell lymphoma (DLBCL) and DLBCL-patients with high miR-224 levels (associated with low CD59 expression) responded better to R-CHOP treatment (longer overall survival and progression free survival) than patients with low miR-224 expression [42]. Key components of R-CHOP such as vincristine showed increased anticancer activity in DLBCL cells overexpressing miR-199a or miR-497, and expression of miR-199a and miR-497 led to prolonged overall survival of DLBCL patients [43]. Inhibition of miR-133a/b and miR-361-3p induced cell cycle arrest (S phase arrest) and apoptosis (activation of caspase-3/7) and enhanced the anti-proliferative effects of vinorelbine and of another tubulin targeting drug (paclitaxel) in H1993 non-small cell lung cancer cells (=NSCLC cells) significantly [44]. In vinorelbine-resistant MDA-MB-231/Nvb breast cancer cells, four miRNAs (miR-138-5p, miR-140-3p, miR-210-3p and miR-3613-5p) were upregulated both in the cancer cells and in the exosomes [45]. It is assumed that drug resistance can be transmitted to other cancer cells via exosomes loaded with oncogenic miRNAs. MAPK, mTOR, Wnt and TGF-β signaling pathways contributed to vinorelbine-resistance in resistant MDA-MB-231/Nvb breast cancer cells via dysregulated miRNA expression [46]. Eleven miRNAs (miR-138-5p, miR-182-5p, miR-18a-5p, miR-193b-3p, miR-199a-5p, miR-210-3p, miR-21-5p, miR-378a-3p, miR-4262, miR-4725-5p, and miR-92b-3p) were upregulated while 6 miRNAs were downregulated (let-7a-5p, miR-130a-3p, miR-146a-5p, miR-221-3p, miR-23b-3p, and miR-4319) in vinorelbine-resistant breast cancer cells [46]. Patients suffering from NSCLC treated with a combination of vinorelbine and cisplatin were examined for prognostic miRNA markers and the microRNAs miR-149 and miR-375 were found predictive for response to the combination treatment and progression free survival [47]. In addition, four other miRNAs (miR-29c, miR-124, miR-200c, and miR-424) were applied as a signature for overall survival of NSCLC patients treated with cisplatin-vinorelbine [47].
Fig. 2.
Structures of the Vinca alkaloids and staurosporine.
The indole alkaloid staurosporine features a natural pan-kinase inhibitor and the prototype for the design of more selective protein kinase inhibitors approved for clinical application as anticancer agents (Fig. 2) [48]. Staurosporine induced apoptosis in proliferating human cells and overexpression of miR-31 augmented the apoptosis induction by staurosporine via downregulation of PKCε and Bcl-2 in MCF10A breast epithelial cells [49]. In Wi38 fibroblast cells, staurosporine-induced apoptosis was mediated by functional miR-34 in a p53-dependent way and suppression of miR-34 reduced apoptosis induction by staurosporine due to upregulation of Bcl-2 [50]. In addition, apoptosis induction by staurosporine was mediated by expression of miR-145 leading to inhibition of DFF45 (DNA fragmentation factor-45) [51]. In contrast to that, miR-125b expression suppressed apoptosis induction by staurosporine via downregulation of pro-apoptotic Bmf and KLF13 in MPP (multipotent progenitor) cells and Jurkat T-cell leukemia cells [52]. Suppression of miR-24 potentiated the apoptosis induction by staurosporine in prostate cancer cells via upregulation of pro-apoptotic FAF1 (Fas associated factor 1) [53]. Table 2 shows a list of miRNAs involved in sensitivity or resistance to indole alkaloids such as Vinca alkaloids and staurosporine.
Table 2.
Influence of microRNAs on cellular sensitivity or resistance to Vinca alkaloids and staurosporine.
| Compounds | Sensitivity | Resistance |
|---|---|---|
| Vinblastine | − | miR-27a [36], [37], miR-27b [37], miR-148a [37], miR-324-3p [37], miR-328 [37], miR-451 [36], [37] |
| Vincristine | miR-25* [40], miR-34a [38], miR-93 [40], miR-93* [40], miR-199a [43], miR-224 [42], miR-424* [40], miR-494 [40], miR-497 [43] | miR-27b [39], miR-125b [41], miR-146a [39], miR-146b-5p [39], miR-181a [39], miR-181d [39], miR-210 [40], miR-923 [40] |
| Vinorelbine | let-7a-5p [46], miR-23b-3p [46], miR-130a-3p [46], miR-146a-5p [46], miR-149 [47], miR-221-3p [46], miR-375 [47], miR-4319 [46] | miR-18a-5p [46], miR-21-5p, miR-92b-3p [46], miR-133a/b [44], miR-138-5p [45], [46], miR-140-3p [45], miR-182-5p [46], miR-193b-3p [46], miR-199a-5p [46], miR-210-3p [45], [46], miR-361-3p [44], miR-378a-3p [46], miR-3613-5p [45], miR-4262 [46], miR-4725-5p [46] |
| Staurosporine | miR-31 [49], miR-34 [50], miR-145 [51] | miR-24 [53], miR-125b [52] |
Anticancer agents that bind covalently to nucleotides likely modulate the expression and activity of oligonucleotides such as non-coding RNAs and miRNAs as well. The indole alkaloid and approved anticancer drug mitomycin C was initially isolated from Streptomyces caspitosus and causes DNA damage via mono- and bifunctional alkylations (formation of cross-links) (Fig. 3) [54], [55]. Alkylation of nucleobases (guanine-N2) by mitomycin C requires the activation of mitomycin C via enzymatic reduction (e.g., by DT-diaphorase) of its quinone ring system [56]. In urothelial bladder cancer (UBC) cells, miR-31 served as a tumor suppressor and sensitized UBC cells to mitomycin C via direct suppression of ITGA5 (integrin α5) and inhibition of Akt and ERK signaling [57]. MiR-31 also increased the tumor growth inhibitory activity of mitomycin C in vivo in UBC xenografts (T24) [57]. However, the nucleotide binding properties of alkylating agents such as mitomycin C harbor the danger of long-term genotoxicity and of inheritable aberration of miRNA expression leading to new cancer diseases as side-effects [58]. Indeed, treatment of HeLa cells with mitomycin C revealed increased inherited expression of five oncogenic miRNAs (miR-19b-3p, miR-21-3p, miR-30a-3p, miR-30e-3p, miR-182-5p) and inherited downregulation of nine tumor suppressor miRNAs (miR-23b-3p, miR-29b-3p, miR-99a-5p, miR-99b-5p, miR-100-5p, miR-148a-3p, miR-193a-3p, miR-340-5p, miR-365a-3p) [58].
Fig. 3.

Chemical structure of mitomycin C.
2.1.2. Quinoline, isoquinoline and quinolizidine alkaloids
The anticancer active quinoline alkaloid camptothecin (CPT) was isolated from the Chinese tree Camptotheca acuminata [59]. CPT and its water-soluble derivatives topotecan and irinotecan represent important approved anticancer drugs for the treatment of various hematological malignancies and solid tumor diseases (Fig. 4) [60]. CPT and its derivatives induce apoptosis by inhibition of topoisomerase 1 (Top1) and stabilization of the Top1-DNA cleavage complex leading to toxic DNA damage [61]. The connection between CPT activity and miRNA expression was investigated. For instance, downregulation of oncogenic miR-125b was observed in apoptotic cancer cells upon treatment with CPT [62]. MiR-125b-mediated mitochondrial apoptotic pathways were induced by CPT associated with increased expression of the pro-apoptotic factors Bak1, Mcl1, and p53 [62]. In line with these findings, the ectopic expression of miR-125b blocked apoptosis induction by CPT [62]. In contrast to that, overexpression of the tumor suppressing miRNAs miR-15a and miR-16 sensitized HeLa cervical carcinoma cells to CPT treatment and enhanced autophagy in HeLa cells via suppression of Rictor, a component of the mTORC2 complex, followed by the inhibition of phosphorylation of mTORC1 and p70S6K [63]. CPT derivatives modulated the response of glioma cells to hypoxia and the connection between HIF-1 (hypoxia-inducible factor 1) and microRNAs upon treatment with CPT was investigated [64], [65]. Indeed, CPT upregulated the expression of miR-155 and miR-17-5p leading to inhibitory effects on HIF-1α expression and activity in hypoxic HeLa cancer cells [65]. In colorectal cancer stem cells, the upregulation of miR-451 reduced resistance to the water-soluble CPT derivative irinotecan associated with suppression of Wnt-signaling and COX-2 (cyclooxygenase 2) expression as well as downregulation of the ABC-transporter ABCB1 (which eliminates intracellular irinotecan molecules) [66]. The activity of irinotecan was increased by suppressed miR-21 expression in gastric cancer and low miR-21 expression resulted into irinotecan-sensitivity [67]. The expression of the multidrug resistance mediating ABC transporter ABCG2 is regulated by miR-519c and irinotecan-resistant colorectal cancer showed low levels of miR-519c but high levels of ABCG2 [68]. In HCC cells, overexpression of miR-23a induced irinotecan-resistance via downregulation of topoisomerase 1 expression [69]. In patients suffering from metastatic colorectal cancer, the appearance of single-nucleotide polymorphisms (SNPs) in the miR-26a-1 gene and in the 5′UTR of pre-miR-100 led to prolonged overall survival and progression-free time when treated with a combination of 5-FU (5-fluorouracil) and irinotecan [70]. In addition, the efficacy of anti-EGFR therapy (cetuximab) in combination with irinotecan was distinctly enhanced in a group of KRAS-mutated, chemotherapy-refractory colorectal cancer patients with high let-7a levels accompanied by improved survival which paves the way for new therapy options in these cases [71]. In addition, high levels of miR-345 were correlated with resistance to irinotecan plus cetuximab 3rd line treatment in colorectal cancer patients and in non-KRAS mutant patient sub-groups [72]. Across the panel of the NCI-60 cell lines, topotecan and irinotecan suppressed miR-24 expression, while upregulation of miR-24 decreased Top1 sensitivity to Top1 poisons [73]. In breast cancer cells (MCF-7), oncogenic miR-21 was involved in topotecan-resistance and downregulation of miR-21 re-sensitized breast cancer cells to topotecan [74]. In addition, the modulation of miRNAs associated with drug resistance in 16 ovarian cancer cell lines upon treatment with topotecan was investigated [75]. Three microRNAs (miR-34b, miR-431, miR-518c-AS) were upregulated by topotecan treatment, while miR-142-5p was downregulated by topotecan in drug resistant ovarian cancer cells [75].
Fig. 4.
Chemical structures of camptothecin derivatives and trabectedin.
The natural compound trabectedin (Ecteinascidin 743, Yondelis®) represents an alkylating tetrahydroisoquinoline alkaloid initially isolated from the tunicate Ecteinascidia turbinata of the Caribbean Sea, which is the host of the trabectedin-forming bacterial symbiont Endoecteinascidia frumentensis (Fig. 4) [76]. Trabectedin exhibited strong cytotoxic activity against various tumors and was approved for the therapy of soft tissue sarcoma and ovarian cancer [76]. Trabectedin damages DNA by a unique mechanism, the trabectedin molecule binds to nitrogen-N2 of guanine bases of the DNA minor groove which causes a bended DNA molecule followed by interaction with DNA binding proteins of the TC-NER (transcription-coupled nucleotide excision repair) DNA-repair system leading to cell death in the end via formation of double strand breaks in particular in HR(homologous recombination)-deficient cells [76]. In addition, trabectedin inhibited the transcription activity of FUS-CHOP in sensitive myxoid liposarcoma (MLS) [77]. Facing the influence of trabectedin on transcription, D'Incalci and coworkers investigated the effects of trabectedin on miRNA expression in MLS cells (402-91 sensitive and 402-91/ET trabectedin-resistant MLS cells) [78]. In the trabectedin-resistant cells, the tumor suppressor let-7e was suppressed (three-fold) and oncogenic miR-21 was upregulated (two-fold) when compared with the sensitive MLS cells which was consequently accompanied by the upregulation of let-7e targets (CCDN1, SEMA4C, E2F5) and suppression of miR-21 targets (PDCD4) in the resistant cells [78]. In addition, the tumor suppressors miR-192, miR-130a and miR-98 were downregulated in 402-91/ET cells, while oncogenic miR-7 was induced. The genes of the miRNAs miR-7, miR-21 and miR-130a harbor CHOP-binding motifs in their promoters and, thus, are probably modulated by trabectedin in a FUS-CHOP dependent way [78]. A list of miRNAs involved in drug resistance or sensitivity to camptothecins and trabectedin is shown in Table 3.
Table 3.
Influence of microRNAs on cellular sensitivity or resistance to camptothecin derivatives and to trabectedin.
| Compounds | Sensitivity | Resistance |
|---|---|---|
| Camptothecin | miR-15a [63], miR-16 [63] | miR-125b [62] |
| Irinotecan | let-7a [71], miR-451 [66], miR-519c [68] | miR-21 [67], miR-23a [69], miR-24 [73], miR-345 [72] |
| Topotecan | miR-124-5p [75] | miR-21 [74], miR-24 [73], miR-34b [75], miR-431 [75], miR-518c-AS [75] |
| Trabectedin | let-7e [78], miR-98 [78], miR-130a [78], miR-192 [78] | miR-7 [78], miR-21 [78] |
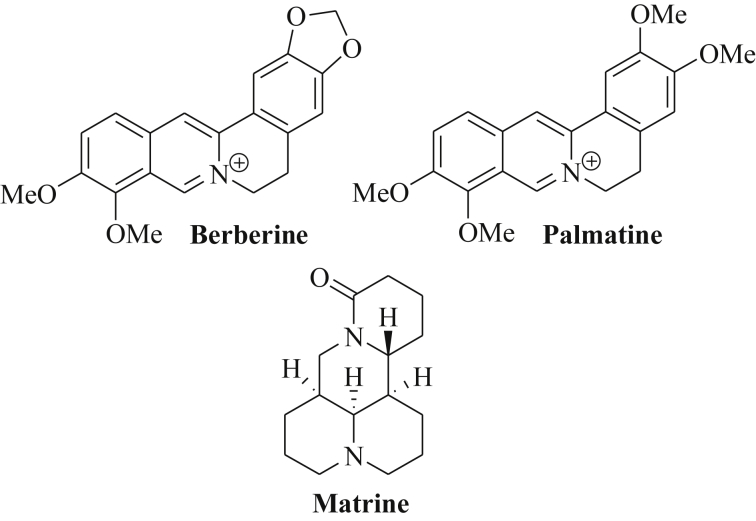
The isoquinoline alkaloid berberine features a quaternary cationic quinolizinium derivative found in various medicinal plants such as Berberis aristata, Coptis chinensis and C. japonica, Phellondendron amurense and P. chinense (Fig. 5) [79]. Berberine induces cytotoxic effects in cancer cells by interaction with DNA leading to double-strand breaks, telomerase inhibition by triplex and G-quadruplex formation and stabilization of the topoisomerase-DNA “cleavable complex” [79]. In multiple myeloma cells (U266), berberine induced apoptosis via suppression of oncogenic miR-21 and anti-apoptotic Bcl-2, and via inhibition of NF-κB translocation [80]. Berberine-mediated suppression of miR-21 in multiple myeloma cells was associated with upregulation of PDCD4 expression [81]. It is assumed that berberine inhibits miR-21 expression via suppression of IL6 (interleukin 6) and STAT3 (signal transducer and activator of transcription) [81]. In addition, berberine sensitized ovarian cancer cells (SKOV3) to cisplatin treatment via suppression of miR-21 and increased expression of the tumor suppressor PDCD4 [82].
Fig. 5.
Structures of berberine, palmatine and matrine.
During the processing of the precursor miRNA hairpin a miRNA duplex is formed that consists of a guide strand (termed miRNA) that forms the miRISC complex upon binding to Argonaute (AGO), and a passenger strand (termed miRNA*) that was initially believed to be non-functional in most cases and to be degraded soon. However, the passenger strand leading to miR-21* (=miR-21-3p) plays an important role for the regulation of cell growth as well. In hepatocellular carcinoma cells (HepG2), berberine increased the levels of miR-21-3p associated with tumor growth inhibition and apoptosis induction [83]. It was shown that miR-21-3p acted as a tumor suppressor in the hepatoma cells and directly inhibited the expression of the methionine adenosyltransferases MAT2A and MAT2B accompanied by increased SAM (S-adenosyl-methionine) levels as a mechanism of the anti-hepatoma activity of miR-21-3p and berberine [83]. Berberine also significantly suppressed the miRNA clusters miR-99a∼125b, miR-17–92 and miR-106–25 in multiple myeloma cells [84]. In particular, downregulation of the oncogenic miR-99a∼125b cluster by berberine (mediated by modulation of p53, Erb and MAPK signaling pathways) induced apoptosis and cell cycle arrest in the G2-phase in multiple myeloma cells [84].
Palmatine features another isoquinoline alkaloid of the roots of Coptis japonica whose chemical structure is closely related to berberine (Fig. 5) [85]. Palmatine revealed anticancer activity against prostate cancer cells by inhibition of NF-κB [86]. In breast cancer cells (MCF-7), the expression of the tumor suppressor miR-200c was increased after treatment with palmatine chloride leading to ZEB1 inhibition and E-cadherin upregulation [87]. In addition, palmatine chloride induced the expression of the tumor suppressors miR-34a and miR-141 in MCF-7 breast cancer cells [87].
The quinolizidine alkaloid matrine was isolated from Sophora flavescens and exhibited strong anticancer activity (Fig. 5) [88]. In breast cancer cells, matrine induced cell cycle arrest and apoptosis via suppression of oncogenic miR-21 and inhibition of Akt signaling as well as upregulation of PTEN (phosphatase and tensin homolog) [88]. Matrine also modulated miRNA levels in gastric cancer cells and suppressed the expression of 20 oncomirs overexpressed in gastric cancer cells including let-7b-5p, miR-10a-5p, miR-15b-5p, miR18a-5p, miR-19a-3p, miR-19b-3p, miR-20b-5p, mir-21-5p, miR-23a-3p, miR-26b-5p, miR-27a-3p, miR-27b-3p, miR-32-5p, miR-34a-5p, miR-98, miR-106b-5p, miR-181a-5p, miR-183-5p and miR-338-3p [89]. A list of miRNAs regulated by isoquinoline and quinolizidine alkaloids is shown in Table 4.
Table 4.
Regulation of microRNAs by isoquinoline and quinolizidine alkaloids.
| Compounds | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Berberine | miR-21-3p [83] | miR-21 [80], miR-99a∼125b cluster [84], miR-17∼92 cluster [84], miR-106∼25 cluster [84] |
| Palmatine chloride | miR-34a [87], miR-141 [87], miR-200c [87] | – |
| Matrine | – | let-7b-5p [89], miR-10a-5p [89], miR-15b-5p [89], miR-18a-5p [89], miR-19a-3p [89], miR-19b-3p [89], miR-20b-5p [89], miR-21 [88], mir-21-5p [89], miR-23a-3p [89], miR-26b-5p [89], miR-27a-3p [89], miR-27b-3p [89], miR-32-5p [89], miR-34a-5p [89], miR-98 [89], miR-106b-5p [89], miR-181a-5p [89], miR-183-5p [89], miR-338-3p [89] |
2.2. Organosulfur-based natural products
Organosulfur-based natural products include sulfides and isothiocyanates that occur in Allium species (garlic) and in cruciferous vegetables (e.g., broccoli, watercress) and revealed distinct epigenetic effects in cancer cells [90]. The natural garlic disulfide diallyl disulfide (DADS, Fig. 6) is usually metabolized to S-allylmercaptocysteine (SAMC) and to allyl mercaptan (AM) and exhibited tumor cell growth inhibition, apoptosis induction as well as inhibition of metastasis formation and angiogenesis [91], [92]. DADS and to a greater extend its metabolite AM strongly inhibited histone deacetylases (HDACs) [93], [94]. In gastric cancer cells (MGC-803), DADS inhibited cell growth and induced apoptosis via induction of miR-200b and miR-22 expression leading to inhibition of Wnt-1 signaling [95]. In addition, the expression of the tumor suppressors miR-200b and miR-22 enhanced the growth inhibitory activity and the anticancer effects of DADS both in vitro and in vivo [95]. DADS also inhibited breast cancer proliferation and metastasis formation by upregulation of the tumor suppressor miR-34a associated with blocked Src/Ras/ERK signaling (with the Src gene as a direct target of miR-34a) [96]. In gastric cancer cells (SGC-7901), DADS-mediated induction of miR-34a suppressed the PI3K/Akt signaling pathway and successfully blocked cancer cell invasion while apoptosis was strongly induced in DADS-treated cells [97].
Fig. 6.
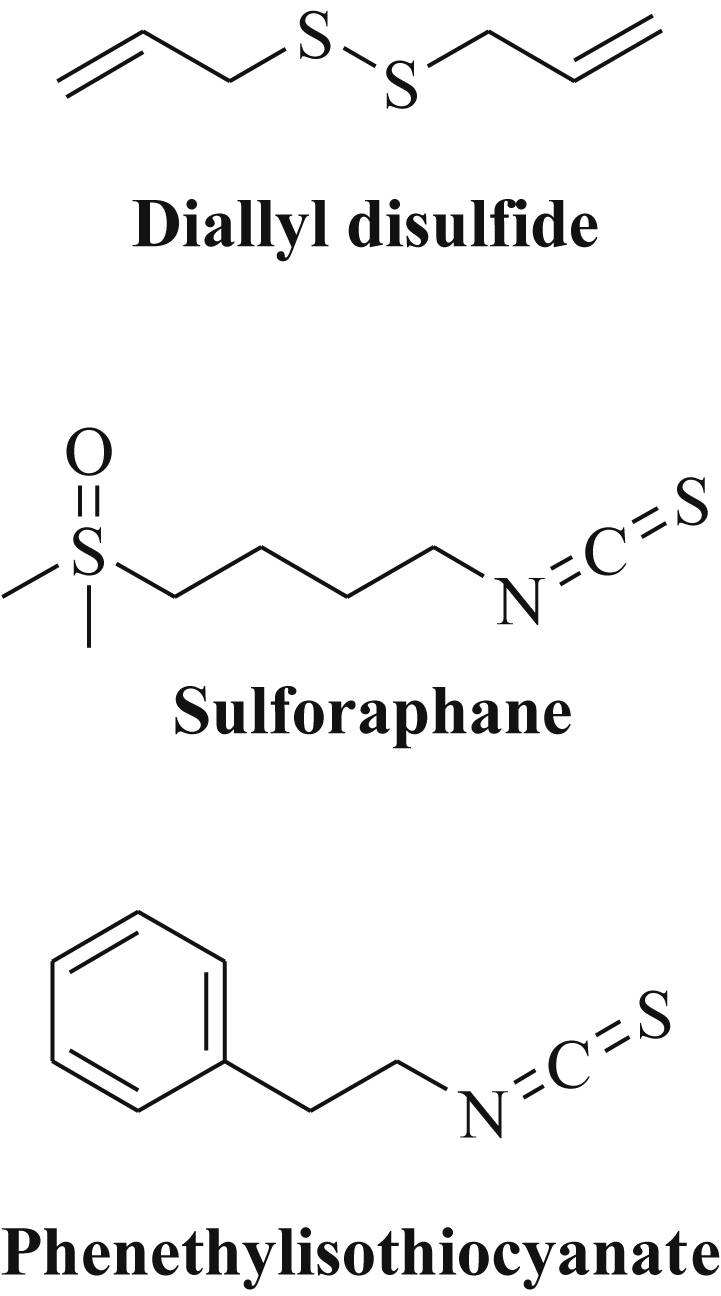
Structures of natural organosulfur compounds.
Sulforaphane (SFN) features an isothiocyanate derivative of cruciferous vegetables, which inhibits carcinogenesis as well as tumor growth in vivo (Fig. 6) [98]. In the plants, isothiocyanates with strong effects on various epigenetic mechanisms occur as more stable glucosinolate prodrugs that release the reactive isothiocyanate agent upon consumption catalyzed by the enzyme myrosinase [99]. SFN itself induced cell cycle arrest via p21 activation and inhibited HDAC enzymes such as HDAC6 [90]. In bladder cancer cells (T24), SFN induced the expression of the tumor suppressor miR-200c associated with blocked EMT and inhibition of metastasis formation [100]. Similar effects (miR-200c upregulation, suppression of cancer stemness) were observed from oral squamous cell carcinomas after treatment with SFN [101]. In addition, miR-140 was upregulated by SFN in breast cancer cells (MCF10DCIS, and MDA-MB-231) leading to the suppression of SOX9 and aldehyde dehydrogenase 1 (ALDH1) [102]. The combination of SFN with green tea catechins (GTCs) was much more anticancer active against advanced pancreatic cancer than GTCs alone because of the SFN-mediated increased expression of the tumor suppressor let-7a leading to K-Ras inhibition [103]. In non-malignant colon epithelial cells (NCM460, NCM356), SFN suppressed three miRNAs (miR-633, miR-155, miR-106a*) while 15 miRNAs (miR-372, miR-342-3p, miR-486-5p, miR-9, miR-9*, miR-145, miR-146a, miR-629, miR-505, miR-758, miR-30*, miR-27b*, miR-135*, miR-27b, miR-23b) were upregulated by SFN which was associated with a reduced risk of colon cancer development [104].
The closely related isothiocyanate phenethylisothiocyanate (PEITC) from watercress exhibited enhanced histone acetylation accompanied by p21 induction in prostate cancer cells (Fig. 6) [105]. Indeed, PEITC suppressed the oncogenic high-risk factor miR-141 in prostate cancer cells (LNCaP) leading to increased expression of the miR-141-target Shp (small heterodimer partner protein, a small orphan receptor and corepressor) and suppressed androgen receptor (AR) signaling [106]. In order to examine the chemopreventive effects of PEITC, rats exposed to cigarette smoke were treated with PEITC and a strong reversal of smoke-induced miRNA suppression was observed in the lungs of the PEITC-treated rats. These PEITC-induced miRNAs include miR-125b (stress response), miR-26a (TGF-β expression), miR-146-prec (NF-κB activation), let-7a and let-7c (Ras activation, cell proliferation and angiogenesis), miR-192 (Ras activation), miR-222-prec and miR-123-prec (cell proliferation and angiogenesis) [107]. It is assumed that PEITC is able to modulate carcinogen metabolism leading to enhanced inactivation and excretion of cigarette/tobacco smoke carcinogens [107]. A list of miRNAs regulated by dietary organosulfur compounds is given in Table 5.
Table 5.
Regulation of microRNAs by natural organosulfur derivatives.
| Compounds | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Diallyl disulfide | miR-22 [95], miR-34a [96], [97], miR-200b [95] | – |
| Sulforaphane | let-7a [103], miR-9 [104], miR-9* [104], miR-23b [104], miR-27b [104], miR-27b* [104], miR-30* [104], miR-135* [104], miR-140 [102], miR-145 [104], miR-146a [104], miR-200c [100], [101], miR-342-3p [104], miR-372 [104], miR-486-5p [104], miR-505 [104], miR-629 [104], miR-758 [104] | miR-106a* [104], miR-155 [104], miR-633 [104] |
| PEITC | let-7a [107], let-7c [107], miR-26a [107], miR-123-prec [107], miR-125b [107], miR-146-prec [107], miR-192 [107], miR-222-prec [107] | miR-141 [106] |
2.3. Natural aliphatic carboxylates
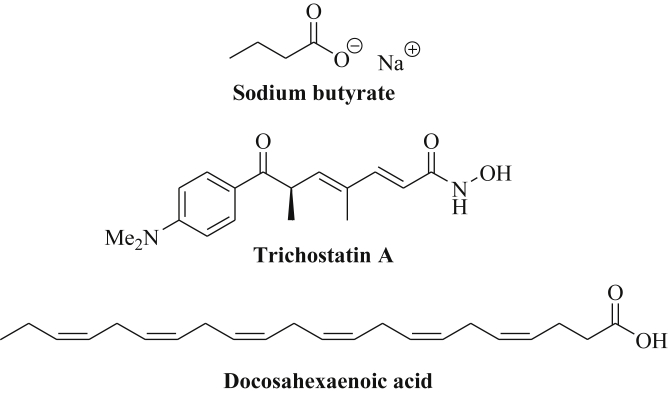
The simple aliphatic short-chain carboxylic acid butyric acid, which was initially isolated from glycerol esters of butter, and its sodium salt, sodium butyrate (NaB), were identified as non-competitive pan-HDAC inhibitors (inhibition of class I and II HDAC enzymes) with modulatory effects on microRNA expression (Fig. 7) [108], [109]. In colorectal cancer cells (HCT-116), high doses of NaB (1–2 mM) downregulated the oncogenic clusters miR-17-92, miR-106a-363 and miR-106b-25 as well as miR-34a, miR-222-5p, miR-7, miR-221, miR-886, let-7b-3p, and miR-196b leading to p21 induction [110]. Several miRNAs were upregulated in colorectal cancer cells upon treatment with NaB (miR-96, miR-183, miR-487b, miR-381, miR-300, miR-215, miR-194, miR-874, miR-602, miR-95, miR-320b, miR-492, miR-583, miR-184, miR-202, miR-1908, miR-637, miR-424) [110]. NaB also downregulated the oncogenes miR-135a and miR-106b in colon adenoma cells accompanied by upregulation of p21 [111]. The tumor suppressors miR-15a and miR-16-1 were upregulated by NaB in lung cancer cells (A549, H1299) [112]. In addition, the expression of miR-101, miR-143 and miR-145 was induced by NaB in Burkitt's lymphoma cells (Raji) associated with suppression of PI3K/Akt signaling and inhibition of tumor cell growth [113]. In combination with VP-16, NaB suppressed methylation of the p16INK4a promoter in Burkitt's lymphoma cells [113]. NaB also induced miR-125a-5p expression in breast cancer stem cells leading to increased apoptosis of breast cancer cells [114]. In breast cancer cells (MDA-MB-231, MCF-7), NaB induced cellular senescence via increased expression of miR-31 leading to suppression of BMI1 [115]. Because of the low bioavailability of NaB, NaB prodrugs such as AN-9 (Pivanex, pivaloyloxymethyl butyrate) and tributyrin (tributyryl glyceride) were developed which showed improved biological activities and pharmacokinetics [116], [117].
Fig. 7.
Structures of sodium butyrate, trichostatin A and docosahexaenoic acid.
The natural hydroxamic acid trichostatin A (TSA) represents an antifungal antibiotic able to inhibit HDACs of the classes I and II (Fig. 7) [118]. The hydroxamate moiety of TSA coordinates to the catalytic zinc ion of the active site of class I and class II HDAC enzymes leading to HDAC inhibition and hyperacetylation of histones and of other HDAC targets in the TSA-treated cells [119]. TSA reduced the expression of the oncogenic miR-106b-93-25 cluster via MYC downregulation in endometrial cancer cells [120]. On the other hand, TSA induced the expression of the tumor suppressor miR-7 leading to EGFR suppression in triple-negative breast cancer cells [121]. TSA-mediated upregulation of miR-125a-5p led to enhanced apoptosis induction in breast cancer cells [122]. The miRNA profile of apoptosis-resistant breast cancer cells was modified by TSA, i.e., the oncomirs miR-500 and miR-645 were downregulated by TSA, and the tumor suppressors miR-1, miR-22, miR-139, miR-143, miR-144, miR-153, miR-191*, miR-194, miR-202*, miR-215, miR-335, miR-486 and miR-559 were upregulated by TSA [123]. Oncogenic miR-155 was also upregulated by TSA-treated breast cancer cells and combination of TSA with miR-155 targeting agents might improve the outcome of TSA-based therapies [123]. In lung cancer cells, the levels of the tumor suppressors miR-15a, miR-16-1, and miR-373 were increased after treatment with TSA [124], [125]. MiR-15a and miR-16-1 were also induced by TSA in mantle cell lymphoma cells [126]. In liver cancer cells, miR-129, miR-182-3p and miR-449 were upregulated by TSA, as well as miR-129-5p in thyroid cancer cells and miR-375 in tongue cancer cells [127], [128], [129]. In addition, the overexpression of the microRNAs miR-30d, miR-181a and miR-199a-5p enhanced the anticancer activity of TSA via suppression of the ER-chaperone GRP78 (glucose-regulated protein) [130]. Colon cancer cells treated with TSA showed increased levels of the tumor suppressor miR-449a/b leading to inhibition of Fra-1 and of tumor cell growth and migration [131]. In combination with the antimetabolite 5-aza-CdR, TSA increased the expression of miR-29b, miR-30b and miR-31 [132]. In gastric cancer cells, TSA plus 5-aza-2′-deoxycytidine increased the expression of the tumor suppressor miR-10b leading to tumor growth inhibition, reduced migration and invasion, and enhanced apoptosis induction [133]. Similarly, the tumor suppressor miR-219-2-3p was induced by TSA and 5-aza-2′-deoxycytidine in gastric cancer cells associated with suppression of p-ERK1/2 [134]. In HCC cells, TSA plus 5-aza-2′-deoxycytidine increased the expression of miR-362-3p leading to suppression of the cell cycle regulator Tob2 and to reduced tumor cell proliferation and suppressed anchorage-independent growth [135]. In addition, combination of TSA with 5-azaC enhanced tumor suppressor miR-1-1 expression while combination of TSA with the DNMT inhibitor zebularine induced miR-26b and let-7a expression [136], [137], [138].
Polyunsaturated fatty acids (PUFAs), in particular, n-3 PUFAs such as docosahexaenoic acid (DHA, Fig. 7) and eicosapentaenoic acid (EPA) feature significant constituents of fish oil and revealed distinct tumor suppressive effects, for instance, enhanced protection against colon tumorigenesis [139], [140], [141], [142]. Rats fed with fish oil rich in n-3 PUFAs revealed reduced carcinogenic effects of azoxymethane (AOM) in rat colons by upregulation of let-7d, miR-15b, miR-107, miR-191, and miR-324-5p [143]. PUFAs such as DHA and γ-linolenic acid induced apoptosis in glioma cells by specific downregulation of miR-143 while miR-20b was upregulated [144]. DHA distinctly increased the levels of tumor suppressing (let-7a) and anti-angiogenic miRNAs (miR-21, miR-23b, miR-27b, miR-320b) in the exosomes of breast cancer cells (MCF-7, MDA-MB-231) while non-malignant breast cells exhibited no differences [145]. Increased expression of miR-23b and miR-320b downregulated the expression of pro-angiogenic targets (PLAU, AMOTL1, NRP1, ETS2) and contributed to the anti-angiogenic activity of DHA [145]. Another study reported the suppression of the oncomir miR-21 by DHA in breast cancer cells (MCF-7, MDA-MB-231) leading to increased expression of PTEN and reduction of CSF-1 [146]. Mice injected with HT-29 colon cancer showed reduced tumor growth when fed with a walnut diet leading to increased levels of n-3 PUFAs such as DHA and EPA in the mice [147]. The anticancer effects of the walnut diet were mediated by suppression of miR-467c, miR-1903, and miR-3068, and overexpression of miR-297* [147]. A list of miRNAs regulated by aliphatic carboxylic acids is given in Table 6.
Table 6.
Regulation of microRNAs by natural aliphatic carboxylic acids.
| Compounds | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| NaB | miR-15a [112], miR-16-1 [112], miR-31 [115], miR-95 [110], miR-96 [110], miR-101 [113], miR-125a-5p [114], miR-143 [113], miR-145 [113], miR-183 [110], miR-184 [110], miR-202 [110], miR-487b [110], miR-194 [110], miR-215 [110], miR-300 [110], miR-320b [110], miR-381 [110], miR-424 [110], miR-492 [110], miR-583 [110], miR-602 [110], miR-637 [110,], miR-874 [110], miR-1908 [110] | miR-17-92 cluster [110], miR-106a-363 cluster [110], miR-106b-25 cluster [110], let-7b-3p [110], miR-7 [110], miR-34a [110], miR-106b [111], miR-135a [110], miR-196b [110], miR-221 [110], miR-222-5p [110], miR-886 [110] |
| TSA | miR-1 [123], miR-7 [121], miR-15a [124], [125], [126], miR-16-1 [124], [125], [126], miR-22 [123], miR-125a-5p [122], miR-129 [127], [128], miR-139 [123], miR-143 [123], miR-144 [123], miR-153 [123], miR-182-3p [127], miR-191* [123], miR-194 [123], miR-202* [123], miR-215 [123], miR-335 [123], miR-373 [124], [125], miR-375 [129], miR-449a/b [127], [131], miR-486 [123], miR-559 [123] | miR-106b-93-25 cluster [120], miR-500 [123], miR-645 [123] |
| TSA + antimetabolites | miR-1-1 [136], [137], miR-10b [133], miR-29b [132], miR-30b [132], miR-31 [132], miR-219-2-3p [134], miR-362-3p [135] | – |
| TSA + zebularine | let-7a [138], miR-26b [138] | – |
| n-3 PUFAs | let-7a [145], let-7d [143], miR-15b [143], miR-20b [144], miR-21 (anti-angiogenic) [145], miR-23b [145], miR-27b [145], miR-107 [143], miR-191 [143], miR-297* [147], miR-324-5p [143], miR-320b [145] | miR-21 [146], miR-143 [144], miR-467c [147], miR-1903 [147], miR-3068 [147] |
2.4. Water-soluble vitamins
Folic acid (FA, vitamin B9) is a natural vitamin occurring in grains, fruits and vegetables and plays an important role concerning DNA biosynthesis, DNA repair and DNA methylation (Fig. 8) [148]. FA also modulated miRNAs involved in the development of different cancer types. Rats fed with a folate-deficient diet produced HCC after one year associated with upregulation of let-7a, miR-21, miR-23, miR-130, miR-190, and miR-17-92 as well as suppression of miR-122 in the developed hepatomas [149]. FA consumption was accompanied by upregulation of miR-122 leading to efficient blocking of liver tumorigenesis [149], [150]. In addition, increased expression of miR-222 was observed from lymphoblastoid cells devoid of FA supplement [151]. FA was also applied for certain “theranostic” purposes involving miRNAs since FA receptors are often overexpressed on the cell surface of cancer cells. A nanoprobe consisting of a gold nanoparticle modified with a fluorescein isothiocyanate (FITC)-labelled molecular beacon (MB that binds to miR-21) and PEGylated FA was prepared which docked to cancer cells via the FA receptor [152]. Inside the cell, miR-21 bound to the MBs of the nanoprobes induced a fluorescence signal which determined the miR-21 level within the cancer cells. Subsequent irradiation with NIR (near infrared) light caused apoptosis via photothermal effects of the gold nanoparticle which enhanced the fluorescence signal since remaining unbound MBs were unwinded at higher temperatures [152]. Similarly, gold nanocages modified with PEGylated FA were able to bind and to deliver anti-miR-181b molecules into HCC cells and the combined effects of the nanoprobes comprising photothermal therapy and miR-181b suppression distinctly reduced tumor growth both in vitro and in vivo after NIR irradiation [153].
Fig. 8.
Structures of folic acid and vitamin C.
Vitamin C (ascorbic acid) features a natural tetronic acid with potent antioxidant properties and occurs in many easily available fruits and vegetables (Fig. 8). Already Cameron and Campbell had discovered the potent anticancer properties of vitamin C and an initial clinical trial with terminal cancer patients who received a combination of vitamin C infusions (10 g/day, i.v. for 10 days) and oral vitamin C (10 g/day, 10 days) exhibited distinctly longer medium survival in the vitamin C group (210 days) when compared with untreated patients (50 days) [154], [155]. A clinical study of the Mayo Clinic used only oral vitamin C and, thus, could not reproduce the promising results of Cameron and coworkers which underlines the high importance of intravenous application of vitamin C in order to reach sufficient blood plasm concentrations of vitamin C (up to 5.5 mmol/L after 10 g vitamin C, i.v.; up to 13.5 mmol/L after 50 g vitamin C, i.v.) [156], [157], [158]. Several newer cancer case studies and trials that applied high-dose vitamin C infusions reported of significant tumor remission and reduced side-effects when combined with chemotherapeutic agents (e.g., paclitaxel) [159], [160], [161], [162], [163], [164]. A phase I clinical trial of the combination of vitamin C and gemcitabine in metastatic pancreatic cancer patients (PACMAN study) revealed a mean survival time of 13 months, which was more than twice of the mean survival time of patients treated only with gemcitabine (5.65 months) [164], [165]. The mode of action of vitamin C comprises the production of toxic H2O2 molecules that kill the cancer cells in a selective way [158], [166]. Resistance to vitamin C was mediated by hypoxic conditions and HIF-1α expression [167]. The effects of ascorbic acid on the expression of microRNAs were investigated as well. In breast tissues, vitamin C inhibited carcinogenesis via suppression of oncogenic miR-93 associated with increased NRF2 (nuclear factor erythroid 2-related factor 2) expression [168]. Similarly, in a more recent study vitamin C inhibited breast carcinogenesis by suppression of oncogenic miR-153 leading likewise to upregulation of NRF2 [169]. In melanoma cells, vitamin C increased the expression of 14 tumor suppressing and EMT reversing miRNAs (miR-596, miR-630, miR-422a, miR-490-5p, miR-375, miR-708, miR-345, miR-125b-2, miR-516a-3p, miR-135a, miR-1228, miR-1915, miR-134, miR-663) [170]. The expression of miR-596, miR-630, miR-490, miR-375 and miR-708 prolonged the overall survival of cancer patients when compared with patient groups exhibiting only low expression of these miRNAs [170]. A list of miRNAs regulated by water-soluble vitamins is given in Table 7.
Table 7.
Regulation of microRNAs by water-soluble vitamins.
| Compounds | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Folic acid | miR-122 [149], [150] | let-7a [149], miR-21 [149], miR-23 [149], miR-130 [149], miR-190 [149], miR-17-92 [149], miR-222 [151] |
| Vitamin C | miR-125b-2 [170], miR-134 [170], miR-135a [170], miR-345 [170], miR-375 [170], miR-422a [170], miR-490-5p [170], miR-516a-3p [170], miR-596 [170], miR-630 [170], miR-663 [170], miR-708 [170], miR-1228 [170], miR-1915 [170] | miR-93 [168], miR-153 [169] |
3. Conclusions
The expression of microRNAs involved in cancer-related processes such as cell death, cell differentiation and proliferation, angiogenesis and metastasis formation was modulated by several natural and dietary compounds of known anticancer activity. The effects of natural drugs selected from the classes of alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins on microRNAs contribute to their sound anticancer activities. Low toxicity and reduced side-effects were already observed from animal studies and clinical trials for several of these natural compounds (e.g., DIM, vitamin C, etc.) that add significantly to conventional cancer therapies in consequence. In addition, the activity of approved alkaloid anticancer agents such as Vinca alkaloids and camptothecin and its derivatives was regulated and strongly influenced by various tumor suppressor and oncogenic miRNAs. In order to improve currently applied anticancer therapies, a thorough knowledge of the regulation of relevant miRNAs by small molecule natural products and dietary factors is crucial. The therapeutic modulation of miRNAs harbors outstanding prospects for anticancer patients such as better prognosis, improved quality of life, and prevention of drug resistance.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Srivastava S.K., Arora S., Averett C., Singh S., Singh A. Modulation of microRNAs by phytochemicals in cancer: underlying mechanisms and translational significance. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/848710. Article ID 848710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarnieri D.J., DiLeone R.J. MicroRNAs: a new class of gene regulators. Ann. Med. 2008;40:197–208. doi: 10.1080/07853890701771823. [DOI] [PubMed] [Google Scholar]
- 3.Jeong H.C., Kim E.K., Lee J.H., Yoo H.N., Kim J.K. Aberrant expression of let-7a miRNA in the blood of non-small cell lung cancer patients. Mol. Med. Rep. 2011;4:383–387. doi: 10.3892/mmr.2011.430. [DOI] [PubMed] [Google Scholar]
- 4.Mallick R., Patnaik S.K., Yendamuri S. Micro RNAs and lung cancer: biology and applications in diagnosis and prognosis. J. Carcinog. 2010;9:8. doi: 10.4103/1477-3163.67074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patnaik S.K., Mallick R., Yendamuri S. Detection of microRNAs in dried serum blots. Anal. Biochem. 2010;407:147–149. doi: 10.1016/j.ab.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothe F., Ignatiadis M., Chaboteaux C., Haibe-Kains B., Kheddoumi N., Majjaj S., Badran B., Fayyad-Kazan H., Desmedt C., Harris A.L., Piccart M., Sotiriou C. Global microRNA expression profiling identifies miR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enerly E., Steinfeld I., Kleivi K., Leivonen S.-K., Aure M.R., Russnes H.G., Ronneberg J.A., Johnsen H., Navon R., Rodland E., Mäkelä R., Naume B., Perälä M., Kallioniemi O., Kristensen V.N., Yakhini Z., Borresen-Dale A.-L. MiRNA-mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS One. 2011;6:e16915. doi: 10.1371/journal.pone.0016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang-Verslues W.W., Chang P.-H., Wei P.-C., Yang C.-Y., Huang C.-K., Kuo W.-H., Shew J.-Y., Chang K.-J., Lee E.Y.-H.P., Lee W.-H. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via down-regulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 9.Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., Dirbas F.M., Somlo G., Pera R.A., Lao K., Clarke M.F. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory P.A., Bracken C.P., Bert A.G., Godall G.J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 11.Yu F., Jiao Y., Zhu Y., Wang Y., Zhu J., Cui X., Liu Y., He Y., Park E.-Y., Zhang H., Lv X., Ma K., Su F., Park J.H., Song E. MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J. Biol. Chem. 2012;287:465–473. doi: 10.1074/jbc.M111.280768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Wu S., Huang S., Ding J., Zhao Y., Liang L., Liu T., Zhan R., He X. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene. 2010;29:2302–2308. doi: 10.1038/onc.2010.34. [DOI] [PubMed] [Google Scholar]
- 14.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.di Leva G., Garofolo M., Croce C.M. MicroRNAs in cancer. Ann. Rev. Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouyssou J.M., Manier S., Huynh D., Issa S., Roccaro A.M., Ghobrial I.M. Regulation of microRNAs in cancer metastasis. Biochim. Biophys. Acta. 2014;22:255–265. doi: 10.1016/j.bbcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biersack B. Current state of phenolic and terpenoidal dietary factors and natural products as non-coding RNA/microRNA modulators for improved cancer therapy and prevention. Non-coding RNA Res. 2016;1(1):12–34. doi: 10.1016/j.ncrna.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler J., Facchini P.J. Alkaloid biosynthesis: metabolism and trafficking. Ann. Rev. Plant Biol. 2008;59:735–769. doi: 10.1146/annurev.arplant.59.032607.092730. [DOI] [PubMed] [Google Scholar]
- 20.Anderton M.J., Manson M.M., Verschoyle R.D., Gescher A., Lamb J.H., Farmer P.B., Steward W.P., Williams M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad A., Li Y., Sarkar F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food Res. 2016 doi: 10.1002/mnfr.201500867. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad A., Biersack B., Li Y., Kong D., Bao B., Schobert R., Padhye S.B., Sarkar F.H. Targeted regulation of PI3K/Akt/mTOR/NF-kB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anti-Cancer Agents Med. Chem. 2013;13:1002–1013. doi: 10.2174/18715206113139990078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar F.H., Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat. Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong D., Heath E., Chen W., Cher M.L., Powell I., Heilbrun L., Li Y., Ali S., Sethi S., Hassan O., Hwang C., Gupta N., Chitale D., Sakr W.A., Menon M., Sarkar F.H. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One. 2012;7:e33729. doi: 10.1371/journal.pone.0033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Vandenboom T.G., II, Wang Z., Kong D., Ali S., Philip P.A., Sarkar F.H. MiR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Vandenbloom T.G., II, Kong D., Wang Z., Ali S., Philip P.A., Sarkar F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad A., Ali S., Ahmed A., Ali A.S., Raz A., Sakr W.A., Rahman K.M. 3,3′-Diindolylmethane enhances the effectiveness of Herceptin against HER-2/neu-expressing breast cancer cells. PLoS One. 2013;8:e54657. doi: 10.1371/journal.pone.0054657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashat M., Azzouz L., Sarkar S.H., Kong D., Li Y., Sarkar F.H. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am. J. Transl. Res. 2012;4:432–442. [PMC free article] [PubMed] [Google Scholar]
- 29.Hanieh H. Aryl hydrocarbon receptor-micrRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol. Cancer. 2015;14:172. doi: 10.1186/s12943-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paik W.H., Kim H.R., Park J.K., Song B.J., Lee S.H., Hwang J. Chemosensitivity induced by down-regulation of microRNA-21 in gemcitabine-resistant pancreatic cancer cells by indole-3-carbinol. Anticancer Res. 2013;33:1473–1482. [PubMed] [Google Scholar]
- 31.Melkamu T., Zhang X., Tan J., Zeng Y., Kassie F. Alteration of microRNA expression in vinyl carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2010;31:252–258. doi: 10.1093/carcin/bgp208. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S., Dubayo H., Ali S., Goncalves P., Kollepara S.L., Sethi S., Philip P.A., Li Y. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27(kip1), p57(kip2), and PUMA. Am. J. Cancer Res. 2013;3:465–477. [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Kong D., Ahmad A., Bao B., Sarkar F.H. Targeting bone remodeling by isoflavone and 3,3′-diindolylmethane in the context of prostate cancer bone metastasis. PLoS One. 2012;7:e33011. doi: 10.1371/journal.pone.0033011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y. 3,3′-Diindolylmethane inhibits breast cancer cell growth via miR-21-mediated CDC25A degradation. Mol. Cell. Biochem. 2011;358:345–354. doi: 10.1007/s11010-011-0985-0. [DOI] [PubMed] [Google Scholar]
- 35.Johnson I.S., Armstrong J.G., Gorman M., Burnett J.P. The vinca alkaloids: a new class of oncolytic agents. Cancer Res. 1963;23:1390–1427. [PubMed] [Google Scholar]
- 36.Zhu H., Wu H., Liu X., Evans B.R., Medina D.J., Liu C.G., Yang J.M. Role of microRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem. Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues A.C., Li X., Radecki L., Pan Y.-Z., Winter J.C., Huang M., Yu A.-M. MicroRNA expression is differentially altered by xenobiotic drugs in different human cell lines. Biopharm. Drug Dispos. 2011;32:355–367. doi: 10.1002/bdd.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K., Huang J., Xie M., Yu Y., Zhu S., Kang R., Cao L., Tang D., Duan X. MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy. 2014;10:442–452. doi: 10.4161/auto.27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuo L., Liu J., Wang B., Gao M., Huang A. Differential miRNA expression profiles in hepatocellular carcinoma cells and drug-resistant sublines. Oncol. Rep. 2013;29:555–562. doi: 10.3892/or.2012.2155. [DOI] [PubMed] [Google Scholar]
- 40.Yin W., Wang P., Wang X., Song W., Cui X., Yu H., Zhu W. Identification of microRNAs and mRNAs associated with multidrug resistance of human laryngeal cancer Hep-2 cells. Braz. J. Med. Biol. Res. 2013;46:546–554. doi: 10.1590/1414-431X20131662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iida K., Fukushi J., Matsumoto Y., Oda Y., Takahashi Y., Fujiwara T., Fujiwara-Okada Y., Hatano M., Nabashima A., Kamura S., Iwamoto Y. MiR-125b develops chemoresistance in Ewing sarcoma/primitive neuroectodermal tumor. Cancer Cell Int. 2013;13:21. doi: 10.1186/1475-2867-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song G., Song G., Ni H., Gu L., Liu H., Chen B., He B., Pan Y., Wang S., Cho W.C. Deregulated expression of miR-224 and its target gene: CD59 predicts outcome of diffuse large B-cell lymphoma patients treated with R-CHOP. Curr. Cancer Drug Targets. 2014;14:659–670. doi: 10.2174/1568009614666140818211103. [DOI] [PubMed] [Google Scholar]
- 43.Troppan K., Wenzel K., Pichler M., Pursche B., Schwarzenbacher D., Feichtinger J., Thallinger G.G., Beham-Schmid C., Neumeister P., Deutsch A. MiR-199a and miR-497 are associated with better overall survival due to increased chemosensitivity in diffuse large B-cell lymphoma patients. Int. J. Mol. Sci. 2015;16:18077–18095. doi: 10.3390/ijms160818077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du L., Borkowski R., Zhao Z., Ma X., Yu X., Xie X.-J., Pertsemlidis A. A high-throughput screen identifies miRNA inhibitors regulating lung cancer cell survival and response to paclitaxel. RNA Biol. 2013;10:1700–1713. doi: 10.4161/rna.26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong S., Chen X., Wang D., Zhang X., Shen H., Yang S., Lv M., Tang J., Zhao J. MicroRNA expression profiles of drug-resistance breast cancer cells and their exosomes. Oncotarget. 2016;7:19601–19609. doi: 10.18632/oncotarget.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong S., Ma T., Zhang X., Lv M., Chen L., Tang J., Zhao J. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in vinorelbine-resistant breast cancer cells. Gene. 2015;556:113–118. doi: 10.1016/j.gene.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 47.Berghmans T., Ameye L., Willems L., Paesmans M., Mascaux C., Lafitte J.J., Meert A.P., Scherpereel A., Cortot A.B., CsToth I., Dernies T., Toussaint L., Leclerq N., Sculier J.P. Identification of microRNA-based signatures for response and survival for non-small cell lung cancer treated with cisplatin vinorelbine A ELCWP prospective study. Lung Cancer. 2013;82:340–345. doi: 10.1016/j.lungcan.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Jänne P.A., Gray N., Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat. Rev. Drug Discov. 2009;8:709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 49.Körner C., Keklikoglu I., Bender C., Wörner A., Münstermann E., Wiemann S. MicroRNA-31 sensitizes human breast cells to apoptosis by direct targeting of protein kinase C ε (PKCε) J. Biol. Chem. 2013;288:8750–8761. doi: 10.1074/jbc.M112.414128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bommer G.T., Gerin I., Feng Y., Kaczorowski A.J., Kuick R., Love R.E., Zhai Y., Giordano T.J., Qin Z.S., Moore B.B., MacDougald O.A., Cho K.R., Fearon E.R. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr. Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J., Guo H., Qian G., Ge S., Ji H., Hu X., Chen W. MiR-145, a new regulator of the DNA fragmentation factor-45 (DFF45)-mediated apoptotic network. Mol. Cancer. 2010;9:211. doi: 10.1186/1476-4598-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ooi A.G.L., Sahoo D., Adorno M., Wang Y., Weissman I.L., Park C.Y. MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21505–21510. doi: 10.1073/pnas.1016218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin W., Shi Y., Zhao B., Yao C., Jin L., Ma J., Jin Y. MiR-24 regulates apoptosis by targeting the open reading frame (ORF) region of FAF1 in cancer cells. PLoS One. 2010;5:e9429. doi: 10.1371/journal.pone.0009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakaki S., Marumo H., Tomioka K., Shimizu G., Kato E., Kamada H., Kudo S., Fujimoto Y. Isolation of new fractions of antitumor mitomycins. Antibiot. Chemother. 1958;8:228–240. [PubMed] [Google Scholar]
- 55.Iyer V., Szybalski W. A molecular mechanism of mitomycin action: linking of complementary DNA strands. Proc. Natl. Acad. Sci. U. S. A. 1963;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomasz M., Palom Y. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 1997;76:73–87. doi: 10.1016/s0163-7258(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 57.Xu T., Qin L., Zhu Z., Wang X., Liu Y., Fan Y., Zhong S., Wang X., Zhang X., Xia L., Zhang X., Xu C., Shen Z. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin α5. Oncotarget. 2016 doi: 10.18632/oncotarget.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarasov V.A., Matishov D.G., Shin E.F., Boyko N.V., Timoshkina N.N., Makhotkin M.A., Lomonosov A.M., Kirpiy A.A. Inheritable changes in miRNAs expression in HeLa cells after x-ray and mitomycin C treatment. Russ. J. Gen. 2014;50:798–806. [PubMed] [Google Scholar]
- 59.Wall M.E., Wani M.C. Camptothecin and taxol: discovery to clinic – thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753–760. [PubMed] [Google Scholar]
- 60.Zunino F., Pratesi G. Camptothecins in clinical development. Expert. Opin. Investig. Drugs. 2004;13:269–284. doi: 10.1517/13543784.13.3.269. [DOI] [PubMed] [Google Scholar]
- 61.Staker B.L., Hjerrild K., Feese M.D., Behnke C.A., Burgin A.B., Jr., Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng C.-W., Zhang X.-J., Lin K.-Y., Ye H., Feng S.-Y., Zhang H., Chen Y.-Q. Camptothecin induces apoptosis in cancer cells via micro-RNA-125b-mediated mitochondrial pathways. Mol. Pharmacol. 2012;81:578–586. doi: 10.1124/mol.111.076794. [DOI] [PubMed] [Google Scholar]
- 63.Huang N., Wu J., Qiu W., Lyu Q., Xie W., Xu N., Zhang Y. MiR-15a and miR-16 induce autophagy and enhance chemosensitivity of camptothecin. Cancer Biol. Ther. 2015;16:941–948. doi: 10.1080/15384047.2015.1040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapisarda A., Uranchimeg B., Scudiero D.A., Selby M., Sausville E.A., Shoemaker R.H., Melillo G. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- 65.Bertozzi D., Marinello J., Manzo S.G., Fornari F., Gramantieri L., Capranico G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1α activity by changing miR expression patterns in human cancer cells. Mol. Cancer Ther. 2014;13:239–248. doi: 10.1158/1535-7163.MCT-13-0729. [DOI] [PubMed] [Google Scholar]
- 66.Bitarte N., Bandres E., Boni V., Zarate R., Rodriguez J., Gonzalez-Huarriz M., Lopez I., Sola J.J., Alonso M.M., Fortes P., Garcia-Foncillas J. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 67.Xu D., Liu B., Shen J. Circulating and tumor miR-21 could predict irinotecan sensitivity in gastric cancer. Zhongguo Aizheng Zazhi. 2013;23:744–750. [Google Scholar]
- 68.To K.K.W., Leung W.W., Ng S.S.M. Exploiting a novel miR-519c-HuR-ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer. Exp. Cell Res. 2015;338:222–231. doi: 10.1016/j.yexcr.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Wang N., Zhu M., Tsao S.-W., Man K., Zhang Z., Feng Y. MiR-23a-mediated inhibition of topoisomerase 1 expression potentiates cell response to etoposide in human hepatocellular carcinoma. Mol. Cancer. 2013;12:119. doi: 10.1186/1476-4598-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boni V., Zarate R., Villa J.C., Bandrés E., Gomez M.A., Maiello E., Garcia-Foncillas J., Aranda E. Role of primary miRNA polymorphic variants in metastatic colon cancer patients treated with 5-fluorouracil and irinotecan. Pharmacogen. J. 2011;11:429–436. doi: 10.1038/tpj.2010.58. [DOI] [PubMed] [Google Scholar]
- 71.Ruzzo A., Graziano F., Vincenzi B., Canestrari E., Perrone G., Galluccio N., Catalano V., Loupakis F., Rabitti C., Santini D., Tonini G., Fiorentini G., Rossi D., Falcone A., Magnani M. High let-7a microRNA levels in KRAS-mutated colorectal carcinomas may rescue anti-EGFR therapy effects in patients with chemotherapy-refractory metastatic disease. Oncologist. 2012;17:823–829. doi: 10.1634/theoncologist.2012-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schou J.V., Rossi S., Jensen B.V., Nielsen D.L., Pfeiffer P., Hogdall E., Yilmaz M., Tejpar S., Delorenzo M., Kruhoffer M., Johansen J.S. MiR-345 in metastatic colorectal cancer: a non-invasive biomarker for clinical outcome in non-KRAS mutant patients treated with 3rd line cetuximab and irinotecan. PLoS One. 2014;9:e99886. doi: 10.1371/journal.pone.0099886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gmeiner W.H., Reinhold W.C., Pommier Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol. Cancer Ther. 2010;9:3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corcoran C., Friel A.M., Duffy M.J., Crown J., O'Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin. Chem. 2011;57:18–32. doi: 10.1373/clinchem.2010.150730. [DOI] [PubMed] [Google Scholar]
- 75.Boren T., Xiong Y., Hakam A., Wenham R., Apte S., Chan G., Kamath S.G., Chen D.-T., Dressman H., Lancaster J.M. MicroRNAs and their target messenger RNAs associated with ovarian cancer response to chemotherapy. Gynecol. Oncol. 2009;113:249–255. doi: 10.1016/j.ygyno.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Le V.H., Inai M., Williams R.M., Kan T. Ecteinascidins. A review of the chemistry, biology and clinical utility of potent tetrahydroisoquinoline antitumor antibiotics. Nat. Prod. Rep. 2015;32:328–347. doi: 10.1039/c4np00051j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forni C., Minuzzo M., Virdis E., Tamborini E., Simone M., Tavecchio M., Erba E., Grosso F., Gronchi A., Aman P., Casali P., D'Incalci M., Pilotti S., Mantovani R. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol. Cancer Ther. 2009;8:449–457. doi: 10.1158/1535-7163.MCT-08-0848. [DOI] [PubMed] [Google Scholar]
- 78.Uboldi S., Calura E., Beltrame L., Nerini I.F., Marchini S., Cavalieri D., Erba E., Chiorino G., Ostano P., D'Angelo D., D'Incalci M., Romualdi C. A systems biology approach to characterize the regulatory networks leading to trabectedin resistance in an in vitro model of myxoid liposarcoma. PLoS One. 2012;7:e35423. doi: 10.1371/journal.pone.0035423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortiz L.M.G., Lombardi P., Tillhon M., Scovassi A.I. Berberine, an epiphany against cancer. Molecules. 2014;19:12349–12367. doi: 10.3390/molecules190812349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu H., Li K., Wang X., Liu Y., Lu Z., Dong R., Guo H., Zhang M. Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 2013;34:157–166. doi: 10.1038/aps.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo X., Gu J., Zhu R., Feng M., Zhu X., Li Y., Fei J. Integrative analysis of differential miRNA and functional study of miR-21 by seed-targeting inhibition in multiple myeloma cells in response to berberine. BMC Syst. Biol. 2014;8:82. doi: 10.1186/1752-0509-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S., Fang Y., Shen H., Xu W., Li H. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim. Biophys. Sin. 2013;45:756–762. doi: 10.1093/abbs/gmt075. [DOI] [PubMed] [Google Scholar]
- 83.Lo T.-F., Tsai W.-C., Chen S.-T. MicroRNA-21–3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8:e75628. doi: 10.1371/journal.pone.0075628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng M., Luo X., Gu C., Li Y., Zhu X., Fei J. Systematic analysis of berberine-induced signaling pathway between miRNA clusters and mRNAs and identification of mir-99a∼125b cluster function by seed-targeting inhibitors in multiple myeloma cells. RNA Biol. 2015;12:82–91. doi: 10.1080/15476286.2015.1017219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee H.-S. Rat lens aldose reductase inhibitory activities of Coptis japonica root-derived isoquinoline alkaloids. J. Agric. Food Chem. 2002;50:7013–7016. doi: 10.1021/jf020674o. [DOI] [PubMed] [Google Scholar]
- 86.Hambright H.G., Batth I.S., Xie J., Ghosh R., Kumar A.P. Palmatine inhibits growth and invasion in prostate cancer cell: potential role for rpS6/NFkB/FLIP. Mol. Carcinog. 2014;54:1227–1234. doi: 10.1002/mc.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hagiwara K., Gailhouste L., Yasukawa K., Kosaka N., Ochiya T. A robust screening method for dietary agents that activate tumour-suppressor microRNAs. Sci. Rep. 2015;5:14697. doi: 10.1038/srep14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li L.Q., Li X.L., Wang L., Du W.J., Guo R., Liang H.H., Liu X., Liang D.S., Lu Y.J., Shan H.L., Jiang H.C. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell. Physiol. Biochem. 2012;30:631–641. doi: 10.1159/000341444. [DOI] [PubMed] [Google Scholar]
- 89.Li H., Xie S., Liu X., Wu H., Lin X., Gu J., Wang H., Duan Y. Matrine alters microRNA expression profiles in SGC-7901 human gastric cancer cells. Oncol. Rep. 2014;32:2118–2126. doi: 10.3892/or.2014.3447. [DOI] [PubMed] [Google Scholar]
- 90.Huang J., Plass C., Gerhäuser C. Cancer chemoprevention by targeting the epigenome. Curr. Drug Targets. 2011;12:1925–1956. doi: 10.2174/138945011798184155. [DOI] [PubMed] [Google Scholar]
- 91.Nian H., Delage B., Ho E., Dashwood R.H. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulphoraphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 2009;50:213–221. doi: 10.1002/em.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Powolny A.A., Singh S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lea M.A., Rasheed M., Randolph V.M., Khan F., Shareef A., des Bordes C. Induction of histone acetylation and inhibition of growth of mouse erythroleukemia cells by S-allylmercaptocysteine. Nutr. Cancer. 2002;43:90–102. doi: 10.1207/S15327914NC431_11. [DOI] [PubMed] [Google Scholar]
- 94.Nian H., Delage B., Pinto J.T., Dashwood R.H. Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. Carcinogenesis. 2008;29:1816–1824. doi: 10.1093/carcin/bgn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang H., Kong Y., Guo J., Tang Y., Xie X., Yang L., Su Q., Xie X. Diallyl disulfide suppresses proliferation and induces apoptosis in human gastric cancer through Wnt-1 signaling pathway by up-regulation of miR-200b and miR-22. Cancer Lett. 2013;340:72–81. doi: 10.1016/j.canlet.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 96.Xiao X., Chen B., Liu X., Liu P., Zheng G., Ye F., Tang H., Xie X. Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by up-regulating miR-34a. PLoS ONE. 2014;9:e112720. doi: 10.1371/journal.pone.0112720. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Wang G., Liu G., Ye Y., Fu Y., Zhang X. Upregulation of miR-34a by diallyl disulfide suppresses invasion and induces apoptosis in SGC-7901 cells through inhibition of the PI3K/Akt signaling pathway. Oncol. Lett. 2016;11:2661–2667. doi: 10.3892/ol.2016.4266. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Zhang Y., Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 99.Gerhauser C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:405–410. doi: 10.1097/MCO.0b013e328362014e. [DOI] [PubMed] [Google Scholar]
- 100.Shan Y., Zhang L., Bao Y., Li B., He C., Gao M., Feng X., Xu W., Zhang X., Wang S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013;24:1062–1069. doi: 10.1016/j.jnutbio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Liu C.-M., Peng C.-Y., Liao Y.-W., Lu M.-Y., Tsai M.-L., Yeh J.-C., Yu C.-H., Yu C.-C. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J. Formos. Med. Assoc. 2016 doi: 10.1016/j.jfma.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Li Q., Yao Y., Eades G., Liu Z., Zhang Y., Zhou Q. Down-regulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589–2600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Appari M., Babu K.R., Kaczorowski A., Gross W., Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int. J. Oncol. 2014;45:1391–1400. doi: 10.3892/ijo.2014.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Slaby O., Sachlova M., Brezkova V., Hezova R., Kovarikova A., Bischofová S., Sevcikova S., Bienertova-Vasku J., Vasku A., Svoboda M., Vyzula R. Identification of microRNAs regulated by isothiocyanates and association of polymorphisms inside their target sites with risk of sporadic colorectal cancer. Nutr. Cancer. 2013;65:247–254. doi: 10.1080/01635581.2013.756530. [DOI] [PubMed] [Google Scholar]
- 105.Wang L.G., Liu X.M., Fang Y., Dai W., Chiao F.B., Puccio G.M., Feng J., Liu D., Chiao J.W. De-repression of the p21 promoter in prostate cancer cells by an isothiocyanate via inhibition of HDACs and c-Myc. Int. J. Oncol. 2008;33:375–380. [PubMed] [Google Scholar]
- 106.Xiao J., Gong A.Y., Eischeid A.N., Chen D., Deng C., Young C.Y., Chen X.M. miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate. 2012;72:1514–1522. doi: 10.1002/pros.22501. [DOI] [PubMed] [Google Scholar]
- 107.Izzotti A., Calin G.A., Steele V.E., Cartiglia C., Longobardi M., Croce C.M., de Flora S. Chemoprevention of cigarette smoke-induced alterations of microRNA expression in rat lungs. Cancer Prev. Res. 2010;3:62–72. doi: 10.1158/1940-6207.CAPR-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ali S.R., Humphreys K.J., McKinnon R.A., Michael M.Z. Impact of histone deacetylase inhibitors on microRNA expression and cancer therapy: a review. Drug Dev. Res. 2015;76:296–317. doi: 10.1002/ddr.21268. [DOI] [PubMed] [Google Scholar]
- 109.Cousens L.S., Gallwitz D., Alberts B.M. Different accessibilities in chromatin to histone acetylase. J. Biol. Chem. 1979;254:1716–1723. [PubMed] [Google Scholar]
- 110.Hu S., Dong T.S., Dalal S.R., Wu F., Bissonnette M., Kwon J.H., Chang E.B. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS ONE. 2011;6:e16221. doi: 10.1371/journal.pone.0016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schlörmann W., Naumann S., Renner C., Glei M. Influence of miRNA-106b and miRNA-135a on butyrate-regulated expression of p21 and cyclin D2 in human colon adenoma cells. Genes Nutr. 2015;10:50. doi: 10.1007/s12263-015-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen C.Q., Chen C.S., Chen J.J., Zhou L.P., Xu H.L., Jin W.W., Wu J.B., Gao S.M. Histone deacetylase inhibitor trichostatin A increases the expression of Dleu2/miR-15a/16-1 via HDAC3 in non-small cell lung cancer. Mol. Cell. Biochem. 2013;383:137–148. doi: 10.1007/s11010-013-1762-z. [DOI] [PubMed] [Google Scholar]
- 113.Ferreira A.C., Robaina M.C., Rezende L.M., Severino P., Klumb C.E. Histone deacetylase inhibitor prevents cell growth in Burkitt's lymphoma by regulating PI3K/Akt pathways and leads to upregulation of miR-143, miR-145, and miR-101. Ann. Hematol. 2014;93:983–993. doi: 10.1007/s00277-014-2021-4. [DOI] [PubMed] [Google Scholar]
- 114.Hsieh T.H., Hsu C.Y., Tsai C.F., Long C.Y., Wu C.H., Wu D.C., Lee J.N., Chang W.C., Tsai E.M. HDAC inhibitors target HDAC5, upregulate microRNA-125-5p, and induce apoptosis in breast cancer cells. Mol. Ther. 2015;23:656–666. doi: 10.1038/mt.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cho J.-H., Dimri M., Dimri G.P. MicroRNA-31 is a transcriptional target of histone deacetylase inhibitors and a regulator of cellular senescence. J. Biol. Chem. 2015;290:10555–10567. doi: 10.1074/jbc.M114.624361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patnaik A., Rowinsky E.K., Villalona M.A., Hammond L.A., Britten C.D., Siu L.L., Goetz A., Felton S.A., Burton S., Valone F.H., Eckhardt S.G. A phase I study of pivaloyloxymethyl butyrate, a prodrug of the differentiating agent butyric acid, in patients with advanced solid malignancies. Clin. Cancer Res. 2002;8:2142–2148. [PubMed] [Google Scholar]
- 117.Edelman M.J., Bauer K., Khanwani S., Tait N., Trepel J., Karp J., Nemieboka N., Chung E.-J., Echo D.V. Clinical and pharmacologic study of tributyrin: an oral butyrate prodrug. Cancer Chemother. Pharmacol. 2003;51:439–444. doi: 10.1007/s00280-003-0580-5. [DOI] [PubMed] [Google Scholar]
- 118.Seidel C., Florean C., Schnekenburger M., Dicato M., Diederich M. Chromatin-modifying agents in anti-cancer therapy. Biochimie. 2012;94:2264–2279. doi: 10.1016/j.biochi.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 119.Finnin M.S., Donigian J.R., Cohen A., Richon V.M., Rifkind R.A., Marks P.A., Breslow R., Pavletich N.P. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Z.N., Bai J.X., Zhou Q., Yan B., Qin W.W., Jia L.T., Meng Y.L., Jin B.Q., Yao L.B., Wang T., Yang A.G. TSA suppresses miR-106b-93-25 cluster expression through downregulation of MYC and inhibits proliferation and induces apoptosis in human EMC. PLoS ONE. 2012;7:e45133. doi: 10.1371/journal.pone.0045133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tu C.Y., Chen C.H., Hsia T.C., Hsu M.H., Wei Y.L., Yu M.C., Chen W.S., Hsu K.W., Yeh M.H., Liu L.C., Chen Y.J., Huang W.C. Trichostatin A suppresses EGFR expression through induction of microRNA-7 in an HDAC-independent manner in lapatinib-treated cells. Biomed. Res. Int. 2014;2014:168949. doi: 10.1155/2014/168949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsieh T.H., Hsu C.Y., Tsai C.F., Long C.Y., Wu C.H., Wu D.C., Lee J.N., Chang W.C., Tsai E.M. HDAC inhibitors target HDAC5, upregulate microRNA-125a-5p, and induce apoptosis in breast cancer cells. Mol. Ther. 2015;23:656–666. doi: 10.1038/mt.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rhodes L.V., Nitschke A.M., Segar H.C., Martin E.C., Driver J.L., Elliott S., Nam S.Y., Li M., Nephew K.P., Burow M.E., Collins-Burow B.M. The histone deacetylase inhibitor trichostatin A alters microRNA expression profiles in apoptosis-resistant breast cancer cells. Oncol. Rep. 2012;27:10–16. doi: 10.3892/or.2011.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen C.Q., Chen C.S., Chen J.J., Zhou L.P., Xu H.L., Jin W.W., Wu J.B., Gao S.M. Histone deacetylases inhibitor trichostatin A increases the expression of Dleu2/miR-15a/16-1 via HDAC 3 in non-small cell lung cancer. Mol. Cell. Biochem. 2013;383:137–148. doi: 10.1007/s11010-013-1762-z. [DOI] [PubMed] [Google Scholar]
- 125.Seol H.S., Akiyama Y., Shimada S., Lee H.J., Kim T.I., Chun S.M., Singh S.R., Jang S.J. Epigenetic silencing of microRNA-373 to epithelial-mesenchymal transition in non-small cell lung cancer through IRAK2 and LAMP1 axes. Cancer Lett. 2014;353:232–241. doi: 10.1016/j.canlet.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X., Chen X., Lin J., Lwin T., Wright G., Moscinski L.C., Dalton W.S., Seto E., Wright K., Sotomayor E. Myc represses miR-15a/miR-16-1 expression through recruitment of HDAC3 in mantle cell and other non-Hodgkin B-cell lymphomas. Oncogene. 2012;31:3002–3008. doi: 10.1038/onc.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Buurman R., Gürlevik E., Schäffer V., Eilers M., Sandbothe M., Kreipe H., Wilkens L., Schlegelberger B., Kühnel F., Skawran B. Histone deacetylases activate hepatocyte growth factor signaling by repressing microRNA-449 in hepatocellular carcinoma cells. Gastroenterology. 2012;143:811–820. doi: 10.1053/j.gastro.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 128.Brest P., Lasalle S., Hofman V., Bordone O., Tanga V.G., Bonnetaud C., Moreilhon C., Rios G., Santini J., Barbry P., Svanborg C., Mograbi B., Mari B., Hofman P. MiR-129-5p is required for histone deacetylase inhibitor-induced cell death in thyroid cancer cells. Endocr. Relat. Cancer. 2011;18:711–719. doi: 10.1530/ERC-10-0257. [DOI] [PubMed] [Google Scholar]
- 129.Jung H.M., Benarroch Y., Chan E.K. Anti-cancer drugs reactivate tumor suppressor miR-375 expression in tongue cancer cells. J. Cell. Biochem. 2015;116:836–843. doi: 10.1002/jcb.25039. [DOI] [PubMed] [Google Scholar]
- 130.Su S.-F., Wang Y.-W., Andreu-Vieyra C., Fang J.Y., Yang Z., Han B., Lee A.S., Liang G. MiR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene. 2013;32:4694–4701. doi: 10.1038/onc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu J., Fan S. MicroRNA-449a/b inhibits the expression of Fra-1 in human colon cancer cells. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao. 2013;29:161–167. [Google Scholar]
- 132.Lee K.H., Lotterman C., Karikari C., Omura N., Feldmann G., Habbe N., Goggins M.G., Mendell J.T., Maitra A. Epigenetic silencing of microRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology. 2009;9:293–301. doi: 10.1159/000186051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Z., Lei H., Luo M., Wang Y., Dong L., Ma Y., Liu C., Song W., Wang F., Zhang J., Shen J., Yu J. DNA methylation downregulated miR-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 134.Lei H., Zou D., Li Z., Luo M., Dong L., Wang B., Yin H., Ma Y., Liu C., Wang F., Zhang J., Yu J., Li Y. MicroRNA-219-2-3p functions as a tumor suppressor in gastric cancer and is regulated by DNA methylation. PLoS ONE. 2013;8:e60369. doi: 10.1371/journal.pone.0060369. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Shen H., Li W., Tian Y., Xu P., Wang H., Zhang J., Li Y. Upregulation of miR-362-3p modulates proliferation and anchorage-independent growth by directly targeting Tob2 in hepatocellular carcinoma. J. Cell. Biochem. 2015;116:1563–1573. doi: 10.1002/jcb.25110. [DOI] [PubMed] [Google Scholar]
- 136.Datta J., Kutay H., Nasser M.W., Nuovo G.J., Wang B., Majumder S., Liu C.G., Volinia S., Croce C.M., Schmittgen T.D., Ghoshal K., Jacob S.T. Methylation mediated silencing of microRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008;68:5049–5058. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Letelier P., Garcia P., Leal P., Alvarez H., Ili C., Lopez J., Castillo J., Brebi P., Roa J.C. MiR-1 and miR-145 act as tumor suppressor microRNAs in gallbladder cancer. Int. J. Clin. Exp. Pathol. 2014;7:1849–1867. [PMC free article] [PubMed] [Google Scholar]
- 138.Kitchen M.O., Yacqub-Usman K., Emes R.D., Richardson A., Clayton R.N., Farrell W.E. Epidrug mediated re-expression of miRNA targeting the HMGA transcripts in pituitary cells. Pituitary. 2015;18:674–684. doi: 10.1007/s11102-014-0630-5. [DOI] [PubMed] [Google Scholar]
- 139.Potter J.D. Colon cancer – do the nutritional epidemiology, the gut physiology and the molecular biology tell the same story. J. Nutr. 1993;123:418–423. doi: 10.1093/jn/123.suppl_2.418. [DOI] [PubMed] [Google Scholar]
- 140.Chang W.L., Chapkin R.S., Lupton J.R. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J. Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 141.Hall M.N., Chavarro J.E., Lee I.M., Willett W.C., Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol. Biomarkers Prev. 2008;17:1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.West N.J., Clark S.K., Phillips R.K., Hutchinson J.M., Leicester R.J., Belluzzi A., Hull M.A. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 143.Davidson L.A., Wang N., Shah M.S., Lupton J.R., Ivanov I., Chapkin R.S. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–2084. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Faragó N., Fehér L.Z., Kitajka K., Das U.N., Puskás L.G. MicroRNA profile of polyunsaturated fatty acid treated glioma cells reveal apoptosis-specific expression changes. Lipids Health Dis. 2011;10:173. doi: 10.1186/1476-511X-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hannafon B.N., Carpenter K.J., Berry W.L., Janknecht R., Dooley W.C., Ding W.-Q. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol. Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mandal C.C., Ghosh-Choudhury T., Dey N., Choudhury G.G., Ghosh-Choudhury N. MiR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis. 2012;33:1897–1908. doi: 10.1093/carcin/bgs198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsoukas M.A., Ko B.-J., Witte T.R., Dincer F., Hardman W.E., Mantzoros C.S. Dietary walnut suppression of colorectal cancer in mice: mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015;26:776–783. doi: 10.1016/j.jnutbio.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 148.Crider K.C., Yang T.P., Berry R.J., Bailey L.B. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv. Nutr. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pogribny I.P., Tryndyak V.P., Ross S.A., Beland F.A. Differential expression of microRNAs during hepatocarcinogenesis induced by methyl deficiency in rats. Nutr. Rev. 2008;66:S33–S35. doi: 10.1111/j.1753-4887.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 150.Kutay H., Bai S., Datta J., Motiwala T., Pogribny I., Frankel W., Jacob S.T., Goshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J. Cell. Biochem. 2006;99:671–678. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Marsit C.J., Eddy K., Kelsey K.T. MicroRNA responses to cellular stress. Cancer Res. 2006;66:10843–10848. doi: 10.1158/0008-5472.CAN-06-1894. [DOI] [PubMed] [Google Scholar]
- 152.Liu J., Zhang L., Lei J., Ju H. MicroRNA-responsive cancer cell imaging and therapy with functionalized gold nanoprobe. ACS Appl. Mater. Interfaces. 2015;7:19016–19023. doi: 10.1021/acsami.5b06206. [DOI] [PubMed] [Google Scholar]
- 153.Huang S., Duan S., Wang J., Bao S., Qiu X., Li C., Liu Y., Yan L., Zhang Z., Hu Y. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-miR-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Adv. Funct. Matter. 2016;26:2532–2544. [Google Scholar]
- 154.Cameron E., Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 1974;9:285–315. doi: 10.1016/0009-2797(74)90019-2. [DOI] [PubMed] [Google Scholar]
- 155.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U. S. A. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gröber U. Vitamin C in complementary oncology - update 2009. Med. Monatsschr. Pharm. 2009;32:263–267. [PubMed] [Google Scholar]
- 157.Moertel C.G., Fleming T.R., Creagan E.T., Rubin J., O'Connell M.J., Ames M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: a randomized double-blind comparison. N. Engl. J. Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 158.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as prodrug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Drisko J.A., Chapman J., Hunter V.J. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J. Am. Coll. Nutr. 2003;22:118–123. doi: 10.1080/07315724.2003.10719284. [DOI] [PubMed] [Google Scholar]
- 160.Riodan N.H., Riordan H.D., Meng X., Li Y., Jackson J.A. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med. Hypotheses. 1995;44:207–213. doi: 10.1016/0306-9877(95)90137-x. [DOI] [PubMed] [Google Scholar]
- 161.Padayatty S.J., Riordan H.D., Hewitt S.M., Katz A., Hoffer L.J., Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006;174:937–942. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Braschoß A. Hochdosis-Infusionstherapie mit Vitamin C in der adjuvanten Therapie des Mammakarzinoms. Onkologie. 2006;12 [Google Scholar]
- 163.Gröber U. Vitamin-C-Hochdosisinfusionstherapie bei Mammakarzinom. Dtsch. Z. Onkol. 2007;39:80–81. [Google Scholar]
- 164.Fritz H., Flower G., Weeks L., Cooley K., Callachan M., McGowan J., Skidmore B., Kirchner L., Seely D. Intravenous vitamin C and cancer: a systematic review. Integr. Cancer Ther. 2014;13:280–300. doi: 10.1177/1534735414534463. [DOI] [PubMed] [Google Scholar]
- 165.Welsh J.L., Wagner B.A., van't Erve T.J., Zehr P.S., Berg D.J., Halfdanarson T.R., Yee N.S., Bodeker K.L., Du J., Roberts L.J., II, Drisko J., Levine M., Buettner G.R., Cullen J.J. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gröber U. Mikronährstoffe: vitamin C – aktuell und kompakt. Dtsch. Apoth. Ztg. 2008;148:104–107. [Google Scholar]
- 167.Sinnberg T., Noor S., Venturelli S., Berger A., Schuler P., Garbe C., Busch C. The ROS-induced cytotoxicity of ascorbate is attenuated by hypoxia and HIF-1alpha in the NCI60 cancer cell lines. J. Cell. Mol. Med. 2014;18:530–541. doi: 10.1111/jcmm.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh B., Ronghe A.M., Chatterjee A., Bhat N.K., Bhat H.K. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34:1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang B., Teng Y., Liu Q. MicroRNA-153 regulates NRF2 expression and is associated with breast carcinogenesis. Clin. Lab. 2016;62:39–47. doi: 10.7754/clin.lab.2015.150518. [DOI] [PubMed] [Google Scholar]
- 170.Venturelli S., Sinnberg T.W., Berger A., Noor S., Levesque M.P., Böcker A., Niessner H., Lauer U.M., Bitzer M., Garbe C., Busch C. Epigenetic impacts of ascorbate on human metastatic melanoma cells. Front. Oncol. 2014;4 doi: 10.3389/fonc.2014.00227. Article 227. [DOI] [PMC free article] [PubMed] [Google Scholar]