Abstract
Introduction –
Intraventricular hemorrhage (IVH) affects both premature infants and adults. In both demographics, it has high mortality and morbidity. There is no FDA approved therapy that improves neurological outcome in either population highlighting the need for additional focus on therapeutic targets and treatments emerging from preclinical studies.
Areas Covered –
IVH induces both initial injury linked to the physical effects of the blood (mass effect) and secondary injury linked to the brain response to the hemorrhage. Preclinical studies have identified multiple secondary injury mechanisms following IVH, and particularly the role of blood components (e.g. hemoglobin, iron, thrombin). This review, with an emphasis on pre-clinical IVH research, highlights therapeutic targets and treatments that may be of use in prevention, acute care, or repair of damage.
Expert Opinion –
An IVH is a potentially devastating event. Progress has been made in elucidating injury mechanisms, but this has still to translate to the clinic. Some pathways involved in injury also have beneficial effects (coagulation cascade/inflammation). A greater understanding of the downstream pathways involved in those pathways may allow therapeutic development. Iron chelation (deferoxamine) is in clinical trial for intracerebral hemorrhage and preclinical data suggest it may be a potential treatment for IVH.
Keywords: Intraventricular hemorrhage, Post-hemorrhagic hydrocephalus, Treatments, Preclinical Models, Prevention, Therapeutic Targets
1. INTRODUCTION
Intraventricular hemorrhage (IVH) occurs when blood from a cerebral hemorrhage expands into the brain ventricular system. This can happen via a variety of mechanisms with both the elderly and preterm neonates being high-risk demographics. In adults, IVH occurs as an extension of intracerebral hemorrhage (ICH) in ~50% patients and it is an independent predictor of worse outcome1. In preterm neonates, IVH typically occurs as a result of germinal matrix hemorrhage (GMH), a brain region abutting the lateral ventricles. This combined phenomenon is referred to as GMH-IVH and affects over a third of extreme pre-term birth infants with mortality rates of over 50% in that population2. Those that survive often develop significant neurological sequelae such as cerebral palsy, developmental delay, deafness, and blindness. In particular, post-hemorrhagic hydrocephalus (PHH) is a common co-morbidity with IVH in neonatal populations and an independent predictor of poor prognosis3.
Currently, no treatment has demonstrated improved clinical outcome in either adult or neonatal IVH. Current treatments focus on methods of CSF drainage to limit PHH4, 5. While there is continued interest in the use of intraventricular thrombolytics to remove IVH, the recent large CLEAR III trial in adult IVH, failed to show a significant benefit on neurological outcome with tissue plasminogen activator (tPA), although mortality was reduced6. As yet, no neuroprotectant or any approach to improve brain recovery after IVH has shown clinical benefit.
Preclinical studies on IVH have, however, identified several potential therapeutic targets for reducing brain injury. This review examines the current state of research on IVH in both adults and neonates. Exploration of those findings may give important information for improving our ability to care for those with IVH.
2. IVH MODELING
2.1. Adult IVH models
A variety of models have been utilized to investigate adult IVH. Pang et al. injected pre-clotted autologous blood directly into the lateral ventricles of dogs7. This model demonstrated ventricular enlargement simulating post-hemorrhagic hydrocephalus. Another porcine model was created to study post-hemorrhagic ventriculomegaly8. This model also employed direct injection of autologous blood into the ventricles, but co-injected thrombin to accelerate coagulation. Despite the relative successes of these two models at developing enlarged ventricles, the majority of current IVH animal models use small rodents such as rats or mice.
In rodents, the primary method of simulating hemorrhage is via autologous blood injection. Some models utilize direct intracerebroventricular (ICV) injection9 while others attempt to simulate combined ICH and IVH by injecting into peri-ventricular brain regions10. Ventricular injection provides a greater degree of control over the analysis by solely investigating the effects of intraventricular blood, but a parenchymal injection more fully replicates human IVH and induces greater damage10. In other studies, ICV injections of iron, lysed blood cells, hemoglobin, or thrombin have been used to study the effects of particular blood components11–15. One recent study utilized ICV injection of human cerebrospinal fluid (CSF) taken from patients with subarachnoid hemorrhage16. Such models each demonstrated enlarged ventricles and periventricular cell death and have subsequently identified distinct pathways for IVH-induced damage, which will be covered in later sections.
A common model used in ICH studies is collagenase injection to induce blood vessel disruption. However, this model has not seen much use in the investigation of adult IVH, although it has been used in neonatal models, as discussed below.
For adult animals, there are few models of spontaneous ICH. These include the spontaneously hypertensive rat-stroke prone (SHRSP) and some hypertensive models in mice, as well as some murine models of cerebral amyloid angiopathy17, 18. Because of its primarily lobar location, it is not a major cause of IVH in patients19. In the hypertensive models, ICH occurrence is generally inconsistent as is the location of those hemorrhages. This greatly limits the utility of such models for ICH/IVH research. A lack of models of spontaneous ICH/IVH is particularly a major limitation for studies of IVH prevention.
2.2. Neonatal IVH models
As in adults, direct ICV injection of blood and blood components such as hemoglobin and iron into neonatal rodents has been used to model IVH14, 20. These models have allowed investigation of the role of blood components in the formation of PHH and in neuronal degeneration. Neonatal GMH-IVH has also been studied using periventricular injections of collagenase in postnatal day six rats21. Ventricular enlargement following blood extension into the ventricles was observed. Similarly, another study utilized collagenase injection directly into the germinal matrix22. That study did not investigate for morphological changes, but rather studied cognitive and sensorimotor function.
For neonatal IVH there are also models of genetic and stress-induced IVH. One spontaneous GMH-IVH model is a transgenic mouse embryo model wherein vascular endothelial growth factor (VEGF) overexpression is induced specifically in the germinal matrix via the tetracycline regulatory system23. VEGF is involved in angiogenesis and its overexpression leads to an outgrowth of weak vasculature that is prone to rupture. This model reported 90% incidence of intracranial hemorrhage that extended into the ventricles, but also had low efficiency with 80% of embryos dying before birth. Despite this, such a model is encouraging and further efforts should be made into creating models of spontaneous IVH.
There are other genetic mutations that cause vascular disruption and neonatal or fetal ICH. Gould et al. reported a semi-dominant mutation in procollagen type IV alpha 1 that causes ICH and death within one day of birth24. Similarly, McCarty et al. found that mice null for αv integrin develop ICH in utero and die soon after birth25. Whether it is possible to induce IVH by manipulating such genes specifically in the periventricular zone merits investigation.
There are also models of stress-induced GMH-IVH. Ballabh and colleagues have developed a model where rabbit pups are delivered prematurely and then treated with glycerol to induce hyper-osmolality26. The majority of pups (~80%) develop IVH, and they also have periventricular cell death, axonal damage, neuroinflammation and behavioral deficits, and approximately half the animals develop PHH26. In newborn beagle puppies GMH-IVH can be induced by a number of stressors, hypercarbia, hypertension and hypotension with volume re-expansion27, 28. These GMH-IVH models have important clinical relevance, but there can be difficulties determining whether the cause of particular injuries is the GMH or the IVH (or the combination), which has therapeutic consequences.
2.3. In vitro models
There are no in vitro models that fully replicate an IVH. However, standard cell culture techniques have been used to examine the impact of clot-derived factors (e.g. hemoglobin, iron, thrombin) on multiple types of brain cells (e.g. neurons, astrocytes, microglia, endothelial cells). Developments in inducible pluripotent stem cells (iPSCs) will now allow such experiments in patient-derived cells31. Similarly, the use of those cells to produce brain organoids may allow dissection of the effects of clot-derived factors on cell:cell interactions. Such organoids produce ventricle-like structures32.
3. PREVENTION
There are multiple potential strategies to reduce IVH occurrence or size. The first is to reduce the risk factors for IVH. In adults, the major risk factor for ICH (the underlying cause of IVH) is hypertension and there is evidence that increased access to anti-hypertensive medication may decrease ICH incidence1. As occurs in other vascular beds, hypertension causes cerebrovascular remodeling and altered function. This includes misaligned smooth muscle cells, reduced autoregulatory ability and blood-brain barrier leakage that may precede hemorrhage33. Targeting those changes33, as well as lowering blood pressure, may be a way of reducing adult ICH/IVH. A second, and growing, cause of ICH is anti-coagulant use. Warfarin use is associated with IVH risk, hematoma volume, and poor prognosis in adults suffering from ICH34. However, for anti-coagulant use there is a trade-off between increased hemorrhagic stroke risk and reduced ischemic stroke risk. Reduced platelet activity is also associated with more IVH in adult ICH patients35. A third major cause of ICH is cerebral amyloid angiopathy, but because of the generally lobar location, it is not a major cause of IVH19.
In neonates, the major risk factor for GMH-IVH is prematurity as the germinal matrix almost completely involutes at about gestational week 33 in humans. Preventing premature birth is a complicated area of research that has been reviewed elsewhere36, but the number of premature infants, and especially extremely premature infants, that are surviving after birth is increasing37. Cerebral blood flow fluctuations in neonates can add stress to the germinal matrix vasculature and are seen as a potential factor underlying neonatal IVH. Elimination of such fluctuation via intravenous pancuronium infusion has long been shown to reduce IVH incidence38. Current use of synchronized ventilator modes in neonatal care also reduces the amount of cerebral blood flow fluctuation39.
Another potential strategy to reduce IVH occurrence is to strengthen potentially fragile vessels40. Prenatal administration of glucocorticoids reduces the severity and frequency of IVH, as these can stabilize the germinal matrix vasculature41. However, glucocorticoids can have side effects by affecting brain development42. There is a growing understanding of the developmental regulation of brain angiogenesis and barriergenesis43. There has been a dissection of the roles of pericytes and astrocytes, and signaling pathways including platelet derived growth factor B, sonic hedgehog and angiopoietin in strengthening the links between brain endothelial cells43. Further studies are needed to determine whether modulating such pathways might be a way of reducing IVH without affecting brain development.
A third potential approach is to limit the amount of bleeding from already ruptured vessels. In adult ICH, ~40% of patients undergo hematoma expansion within the first 24 hours44 and there has been considerable effort to try and reduce that expansion by using hemostatic agents or by acutely reducing blood pressure. None of these trials have shown a significant improvement in outcome although there is evidence of reduced hematoma expansion. The Factor VIIa in Acute Intracerebral Hemorrhage (FAST) trial showed reduced hematoma expansion over the first 24 hours45 and a secondary analysis of the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trials (INTERACT 1 and 2) found that blood pressure reduction reduced ICH plus IVH hematoma expansion46.
A fourth potential strategy is to identify whether there are specific genetic vulnerabilities for IVH that might be amenable to therapeutic intervention. The majority of such efforts have focused on coagulation or fibrinolytic genes, with special focus on the Factor V Leiden (Arg506Gln) and prothrombin (G20210A) mutations47. These mutations have been associated with increased risk of GMH-IVH in preterm birth infants48–50. Additionally, certain haplotypes of the gene encoding vascular endothelial growth factor A are correlated to increased incidence of IVH in preterm infants51. However, as of yet, few if any pre-clinical studies have been reported that manipulate these genes, so no causal relationship has been demonstrated.
For adult IVH, in particular, preclinical work on prevention with any of these strategies is hampered by the relative paucity of spontaneous ICH models. There has been work on examining the impact of different anticoagulants on ICH after collagenase injection52. Experiments examining the impact of such anticoagulants on combined ICH/IVH models are warranted although there are concerns that the collagenase models may differ from human hemorrhage (bacterial protein and underlying mechanism of vessel disruption;53).
4. IVH-INDUCED BRAIN INJURY
After ICH/IVH there is both a primary injury, caused by the physical presence of the blood within the brain, and secondary injury caused by the effects of neurotoxic compounds released from the hematoma and the brain response to blood (e.g. inflammation). The time windows for these injury processes differ and most preclinical research has focused in secondary injury.
4.1. Physical effect of IVH
Three physical effects of an intraventricular hematoma are the displacement of neural tissue (mass effect), increased intracranial pressure (ICP) and blocking of the CSF flow pathway. The immediate physical impact of an IVH will involve stretching of the wall of the ventricles and periventricular structures. The importance of such stretch in IVH-induced brain injury has not been well studied preclinically. It is known that there can be extensive loss of the ependymal cells that line the ventricle walls after IVH54, but the relative importance of physical stretch versus clot-derived factors is still unclear. Thus, for example, it is known that ICV injection of iron can cause ependymal damage12 without having a mass effect.
In adults, the volume of the intracranial space is fixed, so the presence of blood increases ICP unless there is a compensatory fluid movement (e.g. CSF displacement). The CLEAR III trial reported intracranial pressures of >30 mmHg in 30% of patients with ICH/IVH who didn’t require a CSF shunt and ~44% of patients who did55. High intracranial pressure may impact cerebral blood flow and cause brain herniation. In neonates, there is some skull flexibility that may limit the increases in ICP caused by increased intracranial volume. The role of transient global ischemia on brain injury after IVH has received little attention in preclinical models.
Another effect of an IVH is that it can block the CSF drainage pathway, at least transiently9, 56, either within the ventricular system or at the CSF outflow sites. This may lead to, or contribute to, PHH. It should be noted that as well as a physical block by the blood clots, there may be other alterations to the CSF pathways contributing to PHH (e.g. outflow site fibrosis). Very recent evidence also indicates that CSF secretion rate is increased after experimental IVH via Toll-Like receptor (TLR)-4 activation. That causes activation of Ste20-type stress kinase (SPAK) which phosphorylates and activates Na/K/Cl cotransporter (NKCC)-1 at the apical surface of the choroid plexus epithelium57. CSF hypersecretion may help clear potentially harmful clot-derived factors from the brain, but it may also contribute to PHH particularly if CSF absorption is impaired. Thus, targeting the TLR4, SPAK, NKCC1 pathway may be a method of reduced PHH57.
4.2. Mass effect
Reducing the mass effect after IVH requires clearing the hematoma from the ventricles. Identifying methods of clearing IVH has been a goal in many preclinical and clinical studies5, 6, 58. This has generally been performed by ICV injection of a thrombolytic such as tPA or urokinase to lyse the hematoma. Whether fibrinolytics improve outcome is still uncertain. A meta-analysis of intraventricular fibrinolytic therapy in adults found that tissue plasminogen activator (tPA) administration reduced ventricular dilation and mortality, and improved functional outcomes in adults59. One recent meta-analysis observed that using fibrinolytics in conjunction with external ventricular drainage provided the best improvement in mortality rates60. However, another meta-analysis reported that while fibrinolytic treatment did reduce mortality, the data failed to reach significance58. The very recent CLEAR III trial compared outcomes ICV tPA versus placebo in patients with an EVD. It found that tPA significantly reduced IVH volume and mortality but it did not improve the number of patients with good functional outcome (as assessed by modified Rankin score_ , the primary endpoint of the tiral6.
In premature infants, there have been multiple studies of ICV fibrinolytics (tPA, urokinase, streptokinase) to reduce the need of shunt placement after PHH5. While there have been some studies that have suggested a benefit, others have not and have shown increased secondary IVH. Thus, currently, the clinical recommendation is not to use fibrinolytics in children with PHH5.
In relation to IVH clearance studies, it is important to note two points. First, that this approach may have effects on both primary (mass effect) and secondary injury (removing the clot as a source of neurotoxic factors). Secondly, there is the question of timing. Giving a thrombolytic in a patient with an intracranial bleed might result in new bleeding. Thus, the CLEAR III trial delayed tPA administration until the clot was stable on CT scan to limit this possibility. This meant that the average time from ictus to randomization between tPA and saline groups was 52 hours6 which may reduce the effect on the initial primary injury.
There have been few preclinical studies examining the effects of fibrinolytics on IVH8, 61. Such studies may be useful for direct comparison of tPA and urokinase. It has been proposed that urokinase lacks some of the pro-inflammatory properties of tPA making it a potentially preferable choice58. In addition, preclinical models may be used to study combination therapies (e.g. a fibrinolytic and a neuroprotectant).
4.3. Imbalance in CSF secretion and absorption
The choroid plexuses are responsible for the majority of CSF secretion62, 63 and reducing that secretion is a target for IVH therapy and particularly for ameliorating PHH. The choroid plexus is a secretory epithelium where the epithelial cells are linked by tight junctions and contain a wide array of ion transporters as well as water channels (primarily aquaporin 1, AQP1). The epithelium is involved in vectoral ion transport that can drive water transport62. Clinically, a combination of acetazolamide (a carbonic anhydrase inhibitor) and furosemide (a Na/Cl cotransport inhibitor) has been evaluated as a method of reducing CSF secretion but it doesn’t reduce the need for shunt placement in preterm infants with PHH and actually increased neurological morbidity5. Currently, therefore, CSF is regulated in PHH patients via one of several CSF drainage methods5.
Our greater understanding of ion and water transport across the choroid plexus is, however, suggesting some new therapeutic targets including the tight junction protein claudin-2 and the water channel AQP1. Unlike the claudin-5 present in the tight junctions of the blood-brain barrier, the claudin-2 present in choroid plexus epithelial cells is ion and water permeable62 and may represent a paracellular route for fluid movement. AQP1 is important in choroid plexus fluid secretion64 and aquaporin inhibitors are beginning to be developed65.
It should be noted that surprisingly little is known about how the choroid plexuses are impacted by IVH66. Such changes may impact fluid secretion (e.g. aquaporin expression67) and alter therapeutic targets.
4.4. Blood-Components and Brain Injury
A significant volume of recent research on IVH has indicated that secondary damage caused by blood components is responsible for much of the observed injury. After a hemorrhage, erythrocytes can lyse and release their potentially toxic contents into the ventricular system. Moreover, other blood components such as elements of the coagulation cascade and immune cells can independently induce brain damage. There are likely similarities in injury mechanisms between adult and neonatal IVH. However, it should be noted that for ischemic stroke, there are age-dependent differences in the impact of microglia and neuroinflammation68. Given the amount of pre-clinical research that has aimed at elucidating blood-component-based mechanisms of injury, several therapeutic targets have been identified. The following section will describe the mechanisms of blood -derived toxicity.
4.4.1. Erythrocyte Lysis, Hemoglobin and its Degradation Products
Early after IVH, erythrocytes can start to lyse. ICV injection of lysed erythrocytes causes brain injury indicating that there are neurotoxic compound(s) within the red blood cell12. This also suggests that erythrocyte lysis may be a therapeutic target in IVH. The mechanism of such lysis after IVH has not been well established. It may reflect gradual energy depletion in the erythrocyte, but it may also result from complement activation and insertion of membrane attack complex in the cell membrane. The extent to which erythrocytes lyse before they can undergo phagocytosis by microglia/macrophages may have an important impact on brain injury.
One erythrocyte component released into the ventricular system is hemoglobin. Free hemoglobin in extracellular spaces has been shown to exhibit cytotoxic effects and increase the inflammatory response69; consequently, the role of hemoglobin in post-hemorrhagic damage has been extensively investigated (Fig. 1). ICV injection of hemoglobin results in inflammatory responses characterized by increased levels of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) in the CSF, as well as by periventricular brain damage70, 71. The ependymal cells that line the cerebral ventricles are injured by IVH and iron resulting in areas of denudation12, 54. This may facilitate the movement of hemoglobin deeper into the periventricular brain. The periventricular region includes the sub-ventricular zone, a site of neurogenesis in neonates and mature adults. The presence of ventricular hemoglobin can also affect more distal brain region. There is significant c-Jun N-terminal kinase (JNK)-linked neurodegeneration in the hippocampus within three days of an ICV injection of hemoglobin20. The mechanism of distal damage has not been fully elucidated. Possibilities include diffusion of hemoglobin to the hippocampus, inflammatory injury, or damage of nearby axons that belong to distant soma.
Figure 1.
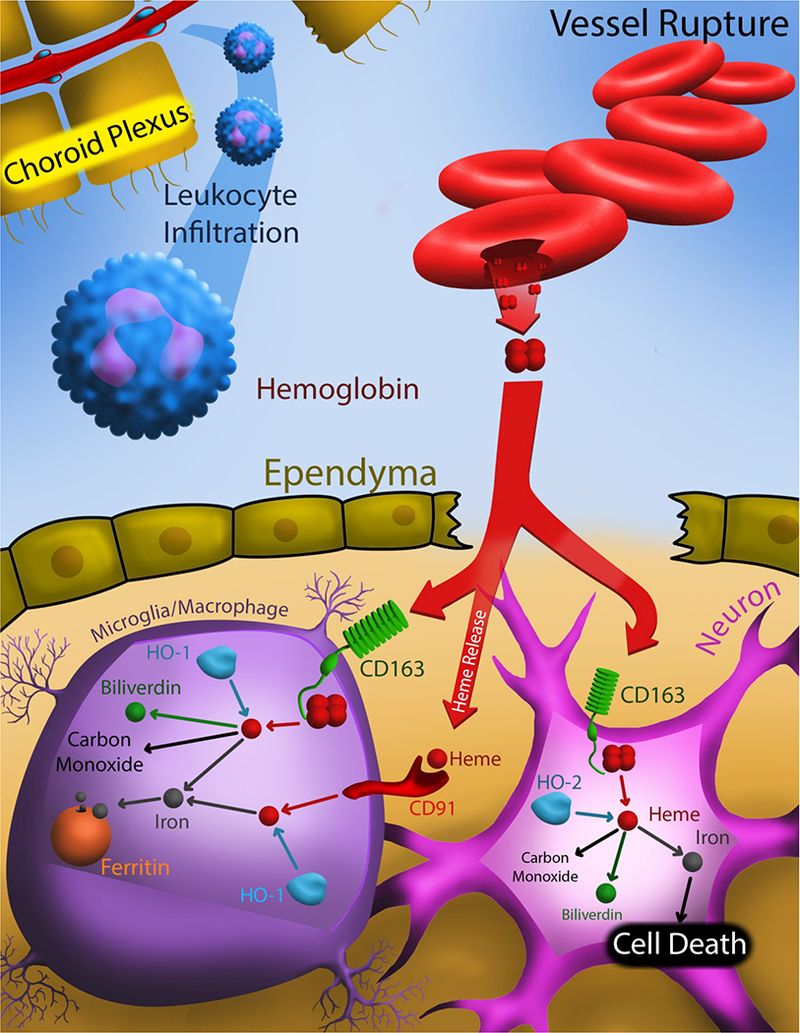
Schematic showing effects of IVH related to erythrocyte lysis and hemoglobin release within the ventricular system. IVH causes ependymal damage and this may facilitate the penetration of hemoglobin from CSF into brain parenchyma. Hemoglobin may then be taken up into microglia/ macrophages via the CD163 receptor, particularly when hemoglobin is complexed to haptoglobin. Alternately, heme released from hemoglobin may be taken up by microglial cells via the CD91 receptor. Inside microglia, hemoglobin/heme is degraded by heme oxygenase-1 (HO-1) to iron, carbon monoxide and biliverdin. HO-1 is inducible and it is markedly upregulated after cerebral hemorrhage. In microglia, iron produced by HO-1 can be chelated by ferritin reducing iron-mediated damage. Recent evidence indicates that neurons can also express CD163 and hemoglobin uptake into those cells may be more toxic due to a lack of ferritin. Neurons express a constitutive form of heme oxygenase, HO-2. Hemoglobin in CSF can also cause damage to the choroid plexus and this may impact CSF homeostasis, result in infiltration of leukocytes and potentially contribute to hydrocephalus, an understudied area.
Hemoglobin is capable of inducing PHH as well as cellular degeneration. In neonatal rat models of IVH, ICV injections of hemoglobin result in significant ventriculomegaly compared to injections with artificial CSF14. Again, the mechanism of this injury is not fully known. It is possible, however, that the pathway includes the choroid plexus, which is not only the primary site of CSF production, but which is also a crucial component of the blood-CSF barrier70. Karimy et al. found the IVH induced CSF hypersecretion via activation of TLR4 on the choroid plexus epithelium and stimulation of NKCC1 activity57. Methemoglobin and heme, a hemoglobin degradation product, can activate TLR4 72. In addition, ICV injection of hemoglobin can induce significant structural damage to the choroid plexus, paired with increased levels of cellular activation, oxidative stress, and inflammatory responses73. Moreover, ventricular hemoglobin can alter aquaporin expression in the choroid plexus67. Aquaporins are proteins that facilitate the passage of water through cell membranes and they are important in CSF production and circulation64. AQP alterations have been linked to the induction of hydrocephalus74. In a preterm rabbit pup model, IVH resulted in choroid plexus AQP1 and AQP5 protein levels67. In unrelated studies, it has been shown that AQP5 overexpression can result in increased RAS-related cell proliferation75. If such cell proliferation should occur in the choroid plexus, it may result in increased CSF production and consequential buildup of intracranial pressure. Thus, there is a need for greater understanding of the role of choroid plexus AQPs in PHH.
The mechanism underlying hemoglobin-induced damage is likely tied to its degradation. Within the ventricular system, hemoglobin naturally dissociates into αβ dimers which may be bound by haptoglobin molecules derived from the hematoma. Haptoglobin is a plasma protein with very affinity for hemoglobin76 that plays a key role in clearing intravascular hemoglobin. While the brain may start to produce haptoglobin after a cerebral hemorrhage77, normal adult CSF levels of haptoglobin are very low, meaning that there may be insufficient haptoglobin, at least initially, to fully scavenge free hemoglobin after hemolysis78. Moreover, plasma haptoglobin levels in neonates are also extremely low; this results in an inability to quickly bind free hemoglobin, resulting in the higher morbidity rates of neonatal hemorrhage79.
Once securely bound, the haptoglobin-hemoglobin complexes undergo receptor-mediated endocytosis via CD163, a protein expressed in monocytes and macrophages, and recently identified in neurons20, 80. After endocytosis, haptoglobin and hemoglobin dissociate, and heme is released, which is subsequently degraded by the heme oxygenase (HO) to form biliverdin, carbon monoxide, and ferrous iron81. In certain cell types in the brain, this iron is quickly sequestered by ferritin. However, in cell types lacking ferritin, such as neurons, this Fe2+ can participate in oxidative Fenton reactions. In that reaction, ferrous iron reacts with hydrogen peroxide to produce dangerous radical oxygen species:
The resulting ferric iron is free to be reduced back to Fe2+ by cellular reducing agents. Once in the ferrous form, the reaction can begin again with a new equivalent of hydrogen peroxide, thus continuously generating reactive oxygen species.
Due to its role in the endocytosis of hemoglobin into cells like neurons that lack the appropriate iron sequestration systems, CD163 has been suggested to be a target for reducing neuronal injury after IVH82. However, microglial and macrophage CD163 has been shown to be beneficial for clearing hemoglobin from the extracellular space and thereby decreasing injury after cerebral hemorrhage83. This creates a therapeutic dilemma: any treatment that attempts to address neuronal expression of CD163 must be careful to avoid decreasing CD163 levels on microglia/macrophages.
The ability of iron from hemoglobin to participate in radical reactions has made iron a focus of pre-clinical studies investigating hemoglobin during IVH. ICV injection of lysed red blood cells induces both heme oxygenase-1 expression in brain, an enzyme that degrades heme and releases iron, and ferritin, the primary iron sequestration protein in brain12. Convincing evidence of the role of intra-hemoglobin iron came from Strahle et al. who demonstrated that injection of protoporphyrin IX (essentially an iron-less heme compound) does not induce hydrocephalus while iron and hemoglobin both did14, implicating iron as the key component of the heme moiety in the mechanism behind PHH. Moreover, it is possible that this iron may be released into the CSF via iron export proteins such as ferroportin. In a clinical study of infants with PHH, 75% had free iron present in their CSF, while no control subjects had such iron84. Significant evidence supports the notion that the accumulation of iron following IVH results in ventricular dilation and brain damage10. Moreover, it has been shown to correlate with brain edema, neuronal cell death in the basal ganglia, and motor function deficits85. It has also been suggested that iron induces hydrocephalus by activating the Wnt signaling pathway after IVH, which is closely implicated in subarachnoid fibrosis in chronic hydrocephalus86. This finding warrants further investigation.
Deferoxamine (DFX) is a ferric ion chelator that is used clinically for systemic iron overload and that shows promise as a treatment for cerebral hemorrhage87, 88. In intracranial hemorrhage, DFX has beneficial effects on iron-induced edema89, neuronal death90, hippocampal degeneration20, and inflammation89, 91, 92. A phase II clinical trial is ongoing for ICH (Intracerebral Hemorrhage Deferoxamine Trial - iDEF Trial; NCT02175225). In models specific to IVH, DFX has been shown to reduce the amount of cell death and neuronal degeneration in peri-ventricular areas10, 11, 20.
One effect of DFX is to ameliorate PHH10, 93. DFX co-injection reduces ventricular enlargement following ICV injection of lysed erythrocytes12, hemoglobin, and iron injection in both adults and neonates14. While it is most likely DFX acts via iron chelation, it is possible that it can also affect important signaling pathways94. The Wnt signaling pathway plays a role in the coagulation cascade, and may be involved in the formation of obstructive non-communicating hydrocephalus following IVH. DFX has been shown to counteract Wnt activity following IVH86.
Minocycline is another commonly-studied pre-clinical treatment for intracranial hemorrhage due to its ability to chelate iron and inhibit microglia. Like DFX, minocycline has been repeatedly demonstrated to diminish damage in a variety of intracranial hemorrhage models95, 96. In cell cultures, it has been shown to reduce iron-induced cortical neuron degeneration with greater efficiency than DFX97. Guo et al. that demonstrated the efficacy of minocycline at diminishing iron accumulation in an experimental GMH-IVH model, resulting in decreased brain edema, hydrocephalus, and brain damage85. The mechanism of its action was likely iron-based in nature, as it additionally reduced ferritin upregulation following the hemorrhage, but they may also be beneficial effects on microglia activation. Minocycline should be more rigorously evaluated as a potential treatment for iron-induced injury following IVH.
4.4.2. Coagulation Components
Plasma, as well as erythrocyte, components play a role in IVH-induced brain injury. Significant among these are the elements of the coagulation cascade including prothrombin/thrombin (Factor IIa). Thrombin is produced following the cleavage of prothrombin, a process upregulated during hemorrhage. Thrombin functions primarily by cleaving fibrinogen into insoluble fibrin to prevent bleeding. However, following ICH and IVH, other thrombin functions can lead to detrimental outcomes98 (Fig. 2). For example, recently, Klebe et al.99 found that a thrombin antagonist, dabigatran, reduced hydrocephalus and behavioral deficits in a rat GMH model.
Figure 2.
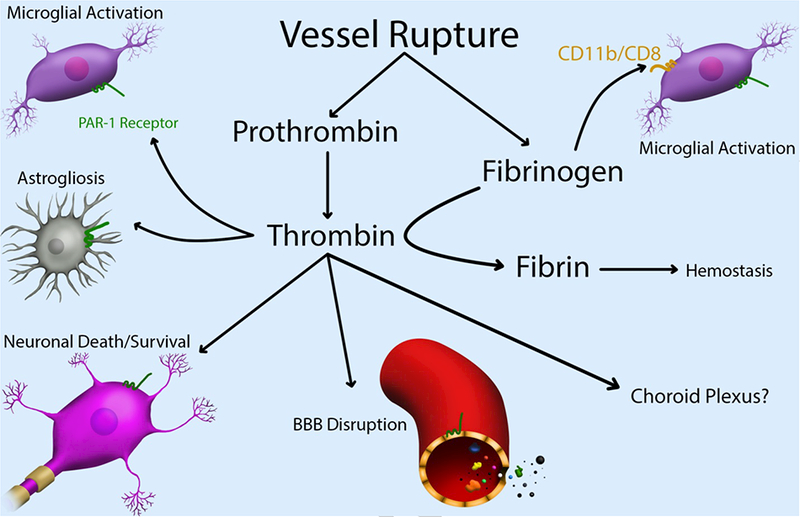
After vessel rupture, prothrombin and fibrinogen enter the brain and there is activation of the coagulation cascade and the final cleavage of fibrinogen to fibrin by thrombin. The main role of the coagulation cascade is to stop bleeding (hemostasis). However, thrombin and fibrinogen have other actions within brain, some of which may contribute to injury after IVH. Thrombin can activate microglia, induce astrogliosis and cause BBB disruption. In addition, thrombin has neuronal effects. These can be beneficial at low concentrations but harmful at high concentrations. Some of the effects of thrombin are via protease activated receptor-1 (PAR-1). Fibrinogen also causes microglial activation, via the CD11b/CD8 (Mac-1) receptor. While there is mounting evidence of the role of the coagulation cascade in hemorrhagic brain injury, targeting that cascade therapeutically is complicated by the essential role of that cascade in hemostasis. Targeting downstream mediators of injury may be an approach.
Some of the functions of thrombin involve protease-activated receptors (PARs). PAR-1, a G-protein-coupled receptor, is directly activated by the serine protease activity of thrombin. Following its activation, PAR-1 can trigger multiple biological cascades98. Increased thrombin production during IVH results in PAR-1 activation which can damage the ependymal wall and lead to the induction of hydrocephalus13. The Src family of kinases are activated by the thrombin-PAR-1 system and are responsible for the phosphorylation (activation) of metalloproteinases, such as matrix metalloproteinase-9, which are known to be a significant cause of damage in other types of intracranial hemorrhage, although their specific roles in IVH and clot clearance are yet to be investigated100.
Some treatments identified by studies investigating thrombin and PAR-1 following IVH include SCH79797, a PAR-1 antagonist101. This antagonist has been shown to significantly reduce ependymal wall damage following experimental IVH13. Inhibition of Src family kinases (and potential subsequent metalloproteinase activation) with PP2 blocks brain edema and blood brain barrier disruption100. In subarachnoid hemorrhage animal models, thrombin-induced inflammation has been linked to transforming growth factor β (TGF β)102. Normally associated with platelets in the blood, TGF β can be released into CSF following platelet extravasation during IVH; it has suggested that TGF β could play some role in the creation of obstructive hydrocephalus103. Recent studies of kaolin-induced hydrocephalus indicate that decorin, an antagonist of TGF β, can reverse ventriculomegaly and white matter injury104, 105. However, further research into this topic will be necessary in order to fully elucidate the mechanism by which TGF β is involved in hydrocephalus following IVH.
Another part of the coagulation cascade that may play a part in IVH-induced brain injury is fibrinogen (Factor I). While the conversion of fibrinogen to fibrin by thrombin is essential for hemostasis, those clots can obstruct the passage of CSF. In addition, even un-cleaved fibrinogen may have negative effects once released into the ventricles. Evidence indicates that extravascular fibrinogen can induce powerful inflammatory response, activating microglial via the CD11b/CD18 receptor106, and it may also play a role in subsequent brain injury.
Therapeutically, the targeting elements of the coagulation cascade in IVH poses significant problems. While they may contribute to brain injury, they also play a vital role in hemostasis. It may be possible to ameliorate this difficulty by targeting downstream mediators; e.g. targeting thrombin-mediated PAR-1 receptor activation while not affecting fibrinogen cleavage, or targeting fibrinogen-mediated microglial activation.
4.4.3. Other blood components
Most attention has focused on the role of hemoglobin/iron, prothrombin/thrombin and fibrinogen/fibrin as clot-derived factors that may cause brain injury. However, it should be noted that other erythrocyte, plasma and platelet derived factors may impact surrounding brain tissue and this merits further investigation. Thus, for example, carbonic anhydrase from erythrocytes has been implicated in inducing brain injury after ICH107. Similarly, recent evidence has shown that lysophosphatidic acid, present in serum, can induce hydrocephalus and impair ependymal integrity108. Lysophosphatidic acid is produced by activated platelets109 and the role of platelets in IVH-induced brain injury has received very little attention.
4.5. Neuroinflammation
Many of the clot-derived factors described above have pro-inflammatory properties (e.g. thrombin, fibrinogen, hemoglobin). After IVH there is an upregulation of pro-inflammatory cytokines, microglial activation and leukocyte infiltration into brain110, 111. Microglia are the resident brain immune cells that upon activation can have a range of phenotypes. Broadly, these include polarization into a “M1” phenotype that is generally pro-inflammatory, or “M2” phenotype that is associated with the resolution of inflammation112. Following IVH, microglia with both M1 and M2 polarization accumulate in the periventricular regions beginning ~48 hours post-hemorrhage111. Excessive microglial activation after injury to the immature brain has been linked to impairment and even development of cerebral palsy113, 114. The inflammatory response after IVH also involves the infiltration of circulating leukocytes into brain111. Following IVH, very-low-birth-weight infants exhibit increased total leukocyte counts115. Inhibiting microglia activation/polarization and blocking leukocyte infiltration into brain are potential therapeutic targets in IVH. It should be noted, however, that neuroinflammation has an important role in brain repair as well as inducing brain injury and that the effects of inflammatory cells in brain injury may vary with age68
5. PRE-CLINICAL FINDINGS ON DAMAGE REPAIR
While the majority of pre-clinical studies have focused on blocking or reducing IVH-induced brain damage, some studies have investigated the possibility of enhancing brain repair. These include examining enhancing neurogenesis, stem cell therapy and reversal of hyaluronan buildup.
In neonates, IVH is generally caused by hemorrhage in the germinal matrix, a region that is a source of new neurons and glial cells in the developing brain. Attempts to repair this region, and thereby reduce developmental impairment, have included using recombinant erythropoietin (rEPO), which not only stimulates red blood cell production but also displays neuroprotective capabilities116. rEPO administration can enhance neuro- and oligodendrogenesis in neonates with white matter injury117. The efficacy of rEPO at improving the long-term cognitive outcomes of infants suffering from IVH is currently under study118. Unfortunately, an earlier large prospective clinical trial studying rEPO treatment in pre-term birth infants demonstrated that while rEPO is safe to use, it does not decrease IVH incidence or mortality rates119. Nevertheless, further investigation into rEPO as a treatment for IVH-induced damage is warranted.
An alternative approach is to use stem cell therapy. Currently, the main goal of such therapy is to modulate the immune response following IVH. The production of new neurons that can survive and integrate is a long-term goal. Mesenchymal stem cells (MSC) have powerful immune-modulating abilities after stroke120, 121. Intraventricular transplantation of MSCs derived from the umbilical cord has been shown to prevent the development of brain injury and hydrocephalus following IVH122. The mechanism of this prevention and repair is likely due to the treatments ability to impact inflammatory cytokine production. However, additional studies have highlighted the neurotropic factors secreted by MSCs which promote astrogliosis and myelination123. The optimal route of delivery of MSCs after IVH appears to be intravenously rather than directly into the ventricles of the brain124; despite the greater efficiency of ICV delivery, there was no improvement in treatment efficacy over intravenous delivery. MSC use after IVH has been thoroughly reviewed elsewhere125.
Following IVH, a buildup of hyaluronan in white matter lesions has been linked to inhibition of oligodendrocyte precursor cell maturation and myelination. Hyaluronan is a glycosaminoglycan polymer that inhibits remyelination126. Vinukonda et al. identified the possibility of treating this buildup of hyaluronan with hyaluronidase127. They found that hyaluronidase treatment in a rabbit IVH model reduced inflammation, increased oligodendrocyte precursor cell maturation, and restored myelination in the white matter lesions. While no other studies have focused on the possibility of repairing IVH-induced damage via hyaluronidase, it is a promising new lead that warrants further investigation.
6. CONCLUSION
Intraventricular blood can be a devastating complication of cerebral hemorrhage in preterm infants to adults. As yet, there is no therapy that reduces IVH-induced neurological deficits. However, preclinical data has identified multiple potential mechanisms for reducing such damage and enhancing brain repair. It is essential that more research be devoted to addressing whether those therapeutic avenues merit being pursued into clinical trial.
7. EXPERT OPINION
Intraventricular blood can have devastating effects in both preterm infants and in adults. As in all stroke, prevention would be the best therapeutic option. While there are preclinical GMH/IVH models that can be used to study prevention in neonates, a paucity of adult spontaneous ICH/IVH models limits research and such models are sorely needed. One approach to limit GMH/IVH is to enhance vessel stability. There have been great strides made in understanding barriergenesis in the CNS and this may provide new therapeutic targets. A potential concern with this approach is that those pathways may have side effects on brain development either via the vasculature (e.g. angiogenesis) or on other types of brain cell. Detailed dissection of the signaling pathways may provide more focused therapies.
IVH induces both primary (physical, mass effect) and secondary injury. Much of the primary injury may occur acutely after the IVH making it more difficult to treat. Fibrinolytic trials are, in part, designed to reduce the hematoma size and reduce the mass effect. Because of safety concerns, the initiation of fibrinolysis is delayed and while this may reduce further mass effects (e.g. on CSF drainage) it may be too late to prevent initial damage. Reducing the time for initiating fibrinolysis, by combining systemic hemostatic therapy with ICV fibrinolysis, may be of benefit.
There is growing data that clot-derived factors have an important role in IVH-induced secondary injury. Such factors include hemoglobin, iron, thrombin and fibrinogen/fibrin, although there should be greater examination of the role other potential factors. Some of these clot-derived factors have multiple actions in IVH that might hamper them being targeted therapeutically (e.g. the role of thrombin in hemostasis as well as injury). There needs to be a dissection of downstream pathways activated by clot-derived factors to help identify better therapeutic targets.
Targeting hemoglobin- and iron-induced toxicity is attractive. There is a current phase II clinical trial for deferoxamine in ICH (iDEF Trial; NCT02175225). If the clinical experience with deferoxamine in ICH trial is successful, the experience with that treatment may be extended to other forms of cerebral hemorrhage including IVH. It should be noted that expanding deferoxamine to preterm IVH will require especial attention to potential side effects. For example, iron deficiency is associated with a variety of neurological problems and iron is required for oligodendrocyte maturation128. It is possible that limiting the duration of deferoxamine exposure and/or using different routes of administration (e.g. intracerebroventricular or intranasal) may limit some side effects.
Multiple of the clot-derived factors have a role in inflammation and inflammation itself may be a therapeutic target. As with thrombin, however, inflammation may have beneficial (e.g. in repair) as well as detrimental actions, effects which vary with time after hemorrhage. This makes it a difficult target. There is an enormous amount of preclinical information on the role of inflammation in brain injury after cerebral ischemia that has led to multiple clinical trials without success. While there are multiple potential reasons, the complex role of inflammation in brain repair as well as brain injury may be a contributing factor.
A new unexpected role of inflammation in IVH is in regulating CSF secretion57. Activation of choroid plexus epithelial TLR4, probably by hemoglobin or heme, activates a signal transduction pathway that stimulates NKCC1 activity and CSF secretion, contributing to PHH. That pathway needs to be examined in patients, but it may be inhibitable at several different steps57.
As well as targeting the actions of clot-derived factors, there is also the possibility of reducing their release from the hematoma. The resolution of the hematoma after IVH isn’t well studied, but altering the fate of the hematoma, phagocytosis versus erythrolysis, may have important consequences for brain injury by determining whether hemoglobin/iron is released extracellularly or within microglia/macrophages. In ICH, there is evidence that phagocytosis can be enhanced by peroxisome proliferator-activated receptor (PPAR)-γ agonists 129 or manipulating eat-me/don’t eat me signals on erythrocytes 130. Alternatively, it may be possible to slow erythrolysis by targeting the complement cascade. Thus, the hematoma as well as neural tissue may be a therapeutic target, an understudied area.
There is the possibility of a combination therapy for reducing IVH-induced brain injury. There is evidence in adults that fibrinolytic therapy reduces IVH-induced mortality although it didn’t improve overall neurological outcome 6. Fibrinolytic therapy does not fully remove the hematoma and perhaps the combination of that therapy with one targeting clot-mediated brain injury or one targeting brain repair might have greater efficacy.
Progress is being made in identifying therapeutic targets for IVH. As with other forms of stroke, the big hurdle is translating that knowledge to the clinic.
HIGHLIGHTS.
Intraventricular hemorrhage can be a devastating consequence of intracerebral hemorrhage, in adults, and germinal matrix hemorrhage, in premature infants.
There are a number of potential strategies to try and limit the occurrence and size of IVH including targeting cerebrovascular remodeling and stability.
Intraventricular hemorrhage results in a physical disruption that is a target of hematoma evacuation and efforts to accelerate hematoma resolution pharmacologically.
Intraventricular hemorrhage also causes secondary injury via the release of factors from the hematoma that induce damage, neuroinflammation and cause CSF hypersecretion.
Such factors include hemoglobin, iron and thrombin. These factors (or their downstream mediators) may be therapeutic targets for reducing IVH-induced brain injury.
Improving brain recovery after IVH is also a therapeutic target, e.g. via stem cells.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
This work was supported by the National Institutes of Health grants NS073595, NS079157, NS090925, NS091545, NS093399 and NS096917.
Abbreviations:
- AQP
aquaporin
- CLEAR III
clot lysis evaluation of accelerated resolution of intraventricular hemorrhage III
- CSF
cerebrospinal fluid
- DFX
deferoxamine
- GMH
germinal matrix hemorrhage
- ICH
intracerebral hemorrhage
- ICV
intracerebroventricular
- INTERACT
intensive blood pressure reduction in acute cerebral haemorrhage trials
- IVH
intraventricular hemorrhage
- MSC
mesenchymal stem cell
- PAR-1
protease-activated receptor 1
- PHH
post-hemorrhagic hydrocephalus
- rEPO
recombinant erythropoietin
- NKCC1
sodium potassium chloride cotransporter 1
- SPAK
Ste20-type stress kinase
- TGF β3
transforming growth factor β1
- TLR4
Toll-like receptor 4
- tPA
tissue plasminogen activator
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet (London, England) 2009. May 09;373(9675):1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 2014. January;133(1):55–62. [DOI] [PubMed] [Google Scholar]
- 3.Robinson S Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr 2012. March;9(3):242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar N, Al-Faiadh W, Tailor J, Mallucci C, Chandler C, Bassi S, et al. Neonatal post-haemorrhagic hydrocephalus in the UK: a survey of current practice. British journal of neurosurgery 2016. September 30:1–5. [DOI] [PubMed] [Google Scholar]
- 5.Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr., Rogido M, Mitchell L, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. Journal of Neurosurgery Pediatrics 2014;14 Suppl 1:8–23. [DOI] [PubMed] [Google Scholar]
- 6. ••.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet (London, England) 2017. February 11;389(10069):603–11. A recent clinical trial of thrombolytic therapy for adult IVH showing no improvement in functional outcome but reduced mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1. Canine intraventricular blood cast model. Neurosurgery 1986. October;19(4):540–6. [DOI] [PubMed] [Google Scholar]
- 8.Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Kuker W, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke 1997. January;28(1):141–8. [DOI] [PubMed] [Google Scholar]
- 9.Lodhia KR, Shakui P, Keep RF. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta Neurochir Suppl 2006;96:207–11. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Tang J, Tan L, Guo J, Tao Y, Li L, et al. Intracerebral Hematoma Contributes to Hydrocephalus After Intraventricular Hemorrhage via Aggravating Iron Accumulation. Stroke 2015. October;46(10):2902–8. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke 2011. February;42(2):465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 2014. June;34(6):1070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ••.Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 2014. March;34(3):489–94. Prclinical evidence that hematoma-derived factors including iron contribute to post hemorrhagic hydrocephalus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strahle JM, Garton T, Bazzi AA, Kilaru H, Garton HJ, Maher CO, et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery 2014. December;75(6):696–705; discussion 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni W, Gao F, Zheng M, Koch LG, Britton SL, Keep RF, et al. Effects of Aerobic Capacity on Thrombin-Induced Hydrocephalus and White Matter Injury. Acta Neurochir Suppl 2016;121:379–84. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Chaudhary N, Gemmete JJ, Thompson BG, Hua Y, Xi G, et al. Intraventricular Injection of Noncellular Cerebrospinal Fluid from Subarachnoid Hemorrhage Patient into Rat Ventricles Leads to Ventricular Enlargement and Periventricular Injury. Acta Neurochir Suppl 2016;121:331–4. [DOI] [PubMed] [Google Scholar]
- 17.Alharbi BM, Tso MK, Macdonald RL. Animal models of spontaneous intracerebral hemorrhage. Neurol Res 2016. May;38(5):448–55. [DOI] [PubMed] [Google Scholar]
- 18.Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke 1976. Jan-Feb;7(1):46–53. [DOI] [PubMed] [Google Scholar]
- 19.Mehndiratta P, Manjila S, Ostergard T, Eisele S, Cohen ML, Sila C, et al. Cerebral amyloid angiopathy-associated intracerebral hemorrhage: pathology and management. Neurosurg Focus 2012. April;32(4):E7. [DOI] [PubMed] [Google Scholar]
- 20.Garton TP, He Y, Garton HJ, Keep RF, Xi G, Strahle JM. Hemoglobin-induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage. Brain Res 2016. March 15;1635:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alles YC, Greggio S, Alles RM, Azevedo PN, Xavier LL, DaCosta JC. A novel preclinical rodent model of collagenase-induced germinal matrix/intraventricular hemorrhage. Brain Res 2010. October 14;1356:130–8. [DOI] [PubMed] [Google Scholar]
- 22.Lekic T, Manaenko A, Rolland W, Tang J, Zhang JH. A novel preclinical model of germinal matrix hemorrhage using neonatal rats. Acta Neurochir Suppl 2011;111:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D, Baumann JM, Sun YY, Tang M, Dunn RS, Akeson AL, et al. Overexpression of vascular endothelial growth factor in the germinal matrix induces neurovascular proteases and intraventricular hemorrhage. Sci Transl Med 2013. July 10;5(193):193–90. [DOI] [PubMed] [Google Scholar]
- 24.Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science (New York, NY) 2005. May 20;308(5725):1167–71. [DOI] [PubMed] [Google Scholar]
- 25.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Molecular and cellular biology 2002. November;22(21):7667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. •.Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, et al. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke 2009. October;40(10):3369–77. This paper describes an important model of intraventricular hemorrhage in premature rabbit pups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goddard J, Lewis RM, Alcala H, Zeller RS. Intraventricular hemorrhage--an animal model. Biol Neonate 1980;37(1–2):39–52. [DOI] [PubMed] [Google Scholar]
- 28.Ment LR, Stewart WB, Duncan CC, Lambrecht R. Beagle puppy model of intraventricular hemorrhage. J Neurosurg 1982. August;57(2):219–23. [DOI] [PubMed] [Google Scholar]
- 29.Del Bigio MR, Di Curzio DL. Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS 2016. February 05;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAllister JP, 2nd. Pathophysiology of congenital and neonatal hydrocephalus. Seminars in fetal & neonatal medicine 2012. October;17(5):285–94. [DOI] [PubMed] [Google Scholar]
- 31.Canfield SG, Stebbins MJ, Morales BS, Asai SW, Vatine GD, Svendsen CN, et al. An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. Journal of neurochemistry 2017. March;140(6):874–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013. September 19;501(7467):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. American journal of physiology Heart and circulatory physiology 2013. June 15;304(12):H1598–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biffi A, Battey TW, Ayres AM, Cortellini L, Schwab K, Gilson AJ, et al. Warfarin-related intraventricular hemorrhage: imaging and outcome. Neurology 2011. November 15;77(20):1840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naidech AM, Bendok BR, Garg RK, Bernstein RA, Alberts MJ, Bleck TP, et al. Reduced platelet activity is associated with more intraventricular hemorrhage. Neurosurgery 2009. October;65(4):684–8; discussion 88. [DOI] [PubMed] [Google Scholar]
- 36.Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med 2014. November 12;6(262):262sr5. [DOI] [PubMed] [Google Scholar]
- 37.Battin MR, Knight DB, Kuschel CA, Howie RN. Improvement in mortality of very low birthweight infants and the changing pattern of neonatal mortality: the 50-year experience of one perinatal centre. Journal of paediatrics and child health 2012. July;48(7):596–9. [DOI] [PubMed] [Google Scholar]
- 38.Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med 1985. May 23;312(21):1353–7. [DOI] [PubMed] [Google Scholar]
- 39.Rennie JM, South M, Morley CJ. Cerebral blood flow velocity variability in infants receiving assisted ventilation. Arch Dis Child 1987. December;62(12):1247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathogenesis Ballabh P. and prevention of intraventricular hemorrhage. Clin Perinatol 2014. March;41(1):47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006. July 19(3):CD004454. [DOI] [PubMed] [Google Scholar]
- 42.Carson R, Monaghan-Nichols AP, DeFranco DB, Rudine AC. Effects of antenatal glucocorticoids on the developing brain. Steroids 2016. October;114:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Current opinion in neurobiology 2013. December;23(6):1057–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowlatshahi D, Brouwers HB, Demchuk AM, Hill MD, Aviv RI, Ufholz LA, et al. Predicting Intracerebral Hemorrhage Growth With the Spot Sign: The Effect of Onset-to-Scan Time. Stroke 2016. March;47(3):695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008. May 15;358(20):2127–37. [DOI] [PubMed] [Google Scholar]
- 46.Song L, Sandset EC, Arima H, Heeley E, Delcourt C, Chen G, et al. Early blood pressure lowering in patients with intracerebral haemorrhage and prior use of antithrombotic agents: pooled analysis of the INTERACT studies. J Neurol Neurosurg Psychiatry 2016. December;87(12):1330–35. [DOI] [PubMed] [Google Scholar]
- 47.Ramenghi LA, Fumagalli M, Groppo M, Consonni D, Gatti L, Bertazzi PA, et al. Germinal matrix hemorrhage: intraventricular hemorrhage in very-low-birth-weight infants: the independent role of inherited thrombophilia. Stroke 2011. July;42(7):1889–93. [DOI] [PubMed] [Google Scholar]
- 48.Petaja J, Hiltunen L, Fellman V. Increased risk of intraventricular hemorrhage in preterm infants with thrombophilia. Pediatr Res 2001. May;49(5):643–6. [DOI] [PubMed] [Google Scholar]
- 49.Melegh B, Stankovics J, Kis A, Nagy A, Storcz J, Losonczy H, et al. Increased prevalence of factor V Leiden mutation in neonatal intracranial haemorrhage. Eur J Pediatr 1998. March;157(3):261. [PubMed] [Google Scholar]
- 50.Komlosi K, Havasi V, Bene J, Storcz J, Stankovics J, Mohay G, et al. Increased prevalence of factor V Leiden mutation in premature but not in full-term infants with grade I intracranial haemorrhage. Biol Neonate 2005;87(1):56–9. [DOI] [PubMed] [Google Scholar]
- 51.Poggi C, Giusti B, Gozzini E, Sereni A, Romagnuolo I, Kura A, et al. Genetic Contributions to the Development of Complications in Preterm Newborns. PLoS One 2015;10(7):e0131741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauer A, Cianchetti FA, Van Cott EM, Schlunk F, Schulz E, Pfeilschifter W, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation 2011. October 11;124(15):1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. •.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 2012. August;11(8):720–31. Intraventricular hemorrhage is a consequence of intracerebral hemorrhage and this paper reviews mechanisms of injury and therapeutic targets in the latter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cherian SS, Love S, Silver IA, Porter HJ, Whitelaw AG, Thoresen M. Posthemorrhagic ventricular dilation in the neonate: development and characterization of a rat model. Journal of neuropathology and experimental neurology 2003. March;62(3):292–303. [DOI] [PubMed] [Google Scholar]
- 55.Murthy SB, Awad I, Harnof S, Aldrich F, Harrigan M, Jallo J, et al. Permanent CSF shunting after intraventricular hemorrhage in the CLEAR III trial. Neurology 2017. June 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aquilina K, Chakkarapani E, Thoresen M. Early deterioration of cerebrospinal fluid dynamics in a neonatal piglet model of intraventricular hemorrhage and posthemorrhagic ventricular dilation. J Neurosurg Pediatr 2012. December;10(6):529–37. [DOI] [PubMed] [Google Scholar]
- 57. ••.Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med 2017. August;23(8):997–1003. This study provides evidence that intraventricular hemorrhage stimulates CSF secretion contributing to post-hemorrhagic hydrocephalus and elucidates underlying mechanisms. [DOI] [PubMed] [Google Scholar]
- 58.Gaberel T, Montagne A, Lesept F, Gauberti M, Lemarchand E, Orset C, et al. Urokinase versus Alteplase for intraventricular hemorrhage fibrinolysis. Neuropharmacology 2014. October;85:158–65. [DOI] [PubMed] [Google Scholar]
- 59.Khan NR, Tsivgoulis G, Lee SL, Jones GM, Green CS, Katsanos AH, et al. Fibrinolysis for intraventricular hemorrhage: an updated meta-analysis and systematic review of the literature. Stroke 2014. September;45(9):2662–9. [DOI] [PubMed] [Google Scholar]
- 60.Gaberel T, Magheru C, Parienti JJ, Huttner HB, Vivien D, Emery E. Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage: a meta-analysis. Stroke 2011. October;42(10):2776–81. [DOI] [PubMed] [Google Scholar]
- 61.Wang YC, Lin CW, Shen CC, Lai SC, Kuo JS. Tissue plasminogen activator for the treatment of intraventricular hematoma: the dose-effect relationship. Journal of the Neurological Sciences 2002;202(1–2):35–41. [DOI] [PubMed] [Google Scholar]
- 62. •.Praetorius J, Damkier HH. Transport across the choroid plexus epithelium. American journal of physiology Cell physiology 2017. June 01;312(6):C673–c86. Current state of knowledge of choroid plexus ion and water transport. [DOI] [PubMed] [Google Scholar]
- 63.Spector R, Keep RF, Robert Snodgrass S, Smith QR, Johanson CE. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp Neurol 2015. May;267:78–86. [DOI] [PubMed] [Google Scholar]
- 64.Verkman AS, Tradtrantip L, Smith AJ, Yao X. Aquaporin Water Channels and Hydrocephalus. Pediatr Neurosurg 2016. December 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Migliati E, Meurice N, DuBois P, Fang JS, Somasekharan S, Beckett E, et al. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Molecular pharmacology 2009. July;76(1):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiang J, Routhe LJ, Wilkinson DA, Hua Y, Moos T, Xi G, et al. The choroid plexus as a site of damage in hemorrhagic and ischemic stroke and its role in responding to injury. Fluids Barriers CNS 2017. March 28;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sveinsdottir S, Gram M, Cinthio M, Sveinsdottir K, Morgelin M, Ley D. Altered expression of aquaporin 1 and 5 in the choroid plexus following preterm intraventricular hemorrhage. Dev Neurosci 2014;36(6):542–51. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Lopez D, Faustino J, Klibanov AL, Derugin N, Blanchard E, Simon F, et al. Microglial Cells Prevent Hemorrhage in Neonatal Focal Arterial Stroke. J Neurosci 2016. March 09;36(10):2881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong XY, Wang J, Qian ZM, Yang QW. Iron and intracerebral hemorrhage: from mechanism to translation. Transl Stroke Res 2014. August;5(4):429–41. [DOI] [PubMed] [Google Scholar]
- 70.Gram M, Sveinsdottir S, Cinthio M, Sveinsdottir K, Hansson SR, Morgelin M, et al. Extracellular hemoglobin - mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. Journal of neuroinflammation 2014;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gram M, Sveinsdottir S, Ruscher K, Hansson SR, Cinthio M, Akerstrom B, et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. Journal of neuroinflammation 2013;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwon MS, Woo SK, Kurland DB, Yoon SH, Palmer AF, Banerjee U, et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int J Mol Sci 2015. March 05;16(3):5028–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simard PF, Tosun C, Melnichenko L, Ivanova S, Gerzanich V, Simard JM. Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl Stroke Res 2011. June;2(2):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skjolding AD, Rowland IJ, Sogaard LV, Praetorius J, Penkowa M, Juhler M. Hydrocephalus induces dynamic spatiotemporal regulation of aquaporin-4 expression in the rat brain. Cerebrospinal Fluid Res 2010;7(20) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Direito I, Madeira A, Brito MA, Soveral G. Aquaporin-5: from structure to function and dysfunction in cancer. Cellular and molecular life sciences : CMLS 2016. April;73(8):1623–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, et al. Hemoglobin and heme scavenging. IUBMB life 2005. November;57(11):749–59. [DOI] [PubMed] [Google Scholar]
- 77.Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci 2009. December 16;29(50):15819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galea J, Cruickshank G, Teeling JL, Boche D, Garland P, Perry VH, et al. The intrathecal CD163-haptoglobin-hemoglobin scavenging system in subarachnoid hemorrhage. Journal of neurochemistry 2012. June;121(5):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kessel I, Leib M, Levy A, Miller-Lotan R, Waisman D, Jacobson E, et al. Does Haptoglobin Phenotype Influence Postnatal Morbidity in Preterm Neonates? American journal of perinatology 2016. January;33(2):130–5. [DOI] [PubMed] [Google Scholar]
- 80.Liu R, Cao S, Hua Y, Keep RF, Huang Y, Xi G. CD163 Expression in Neurons After Experimental Intracerebral Hemorrhage. Stroke 2017. May;48(5):1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 1988;2(10):2557–68. [PubMed] [Google Scholar]
- 82.Garton T, Keep RF, Hua Y, Xi G. CD163, a Hemoglobin/Haptoglobin Scavenger Receptor, After Intracerebral Hemorrhage: Functions in Microglia/Macrophages Versus Neurons. Transl Stroke Res 2017. April 06. [DOI] [PubMed] [Google Scholar]
- 83.Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia Activation and Polarization After Intracerebral Hemorrhage in Mice: the Role of Protease-Activated Receptor-1. Transl Stroke Res 2016. December;7(6):478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatric Research 2001;49(2):208–12. [DOI] [PubMed] [Google Scholar]
- 85.Guo J, Chen Q, Tang J, Zhang J, Tao Y, Li L, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res 2015. January 12;1594:115–24. [DOI] [PubMed] [Google Scholar]
- 86.Meng H, Li F, Hu R, Yuan Y, Gong G, Hu S, et al. Deferoxamine alleviates chronic hydrocephalus after intraventricular hemorrhage through iron chelation and Wnt1/Wnt3a inhibition. Brain Res 2015. March 30;1602:44–52. [DOI] [PubMed] [Google Scholar]
- 87.Cui HJ, He HY, Yang AL, Zhou HJ, Wang C, Luo JK, et al. Efficacy of deferoxamine in animal models of intracerebral hemorrhage: a systematic review and stratified meta-analysis. PLoS One 2015;10(5):e0127256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selim M Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke 2009. March;40(3 Suppl):S90–1. [DOI] [PubMed] [Google Scholar]
- 89.Xie Q, Gu Y, Hua Y, Liu W, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke 2014. January;45(1):290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res 2013. October;4(5):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou X, Xie Q, Xi G, Keep RF, Hua Y. Brain CD47 expression in a swine model of intracerebral hemorrhage. Brain Res 2014. July 29;1574:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab 2011. May;31(5):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao J, Chen Z, Xi G, Keep RF, Hua Y. Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl Stroke Res 2014. October;5(5):586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garton T, Keep RF, Hua Y, Xi G. Brain Iron Overload following Intracranial Haemorrhage. Stroke Vasc Neurol 2016 December 2016;1(4):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Z, Zou X, Zhu W, Mao Y, Chen L, Zhao F. Minocycline is effective in intracerebral hemorrhage by inhibition of apoptosis and autophagy. J Neurol Sci 2016. December 15;371:88–95. [DOI] [PubMed] [Google Scholar]
- 96.Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res 2009. March;31(2):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun 2009. August 21;386(2):322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? Journal of neurochemistry 2003. January;84(1):3–9. [DOI] [PubMed] [Google Scholar]
- 99.Klebe D, Flores JJ, McBride DW, Krafft PR, Rolland WB, Lekic T, et al. Dabigatran ameliorates post-haemorrhagic hydrocephalus development after germinal matrix haemorrhage in neonatal rat pups. J Cereb Blood Flow Metab 2016. January 01:271678×16684355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol 2010. April;67(4):526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garton T, Keep RF, Wilkinson DA, Strahle JM, Hua Y, Garton HJ, et al. Intraventricular Hemorrhage: the Role of Blood Components in Secondary Injury and Hydrocephalus. Transl Stroke Res 2016. June 30. [DOI] [PubMed] [Google Scholar]
- 102.Li T, Zhang P, Yuan B, Zhao D, Chen Y, Zhang X. Thrombin-induced TGF-beta1 pathway: a cause of communicating hydrocephalus post subarachnoid hemorrhage. International journal of molecular medicine 2013. March;31(3):660–6. [DOI] [PubMed] [Google Scholar]
- 103.Whitelaw A, Christie S, Pople I. Transforming growth factor-beta1: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr Res 1999. November;46(5):576–80. [DOI] [PubMed] [Google Scholar]
- 104.Aojula A, Botfield H, McAllister JP 2nd, Gonzalez AM, Abdullah O, Logan A, et al. Diffusion tensor imaging with direct cytopathological validation: characterisation of decorin treatment in experimental juvenile communicating hydrocephalus. Fluids Barriers CNS 2016;13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Botfield H, Gonzalez AM, Abdullah O, Skjolding AD, Berry M, McAllister JP 2nd, et al. Decorin prevents the development of juvenile communicating hydrocephalus. Brain : a journal of neurology 2013. September;136(Pt 9):2842–58. [DOI] [PubMed] [Google Scholar]
- 106.Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nature communications 2012;3:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo F, Hua Y, Wang J, Keep RF, Xi G. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res 2012. March;3(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park R, Moon UY, Park JY, Hughes LJ, Johnson RL, Cho SH, et al. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nature communications 2016. January 12;7:10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yao CS, Yan SG, Gao LS, Sun ZR, Liu F, Jiang B, et al. Patients with risk factors have higher plasma levels of lysophosphatidic acid: a promising surrogate marker for blood platelet activation. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis 2014. June;25(4):322–5. [DOI] [PubMed] [Google Scholar]
- 110.Dohare P, Zia MT, Ahmed E, Ahmed A, Yadala V, Schober AL, et al. AMPA-Kainate Receptor Inhibition Promotes Neurologic Recovery in Premature Rabbits with Intraventricular Hemorrhage. J Neurosci 2016. March 16;36(11):3363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, et al. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke 2008. December;39(12):3378–88. [DOI] [PubMed] [Google Scholar]
- 112.Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/Macrophage Polarization After Experimental Intracerebral Hemorrhage. Transl Stroke Res 2015. December;6(6):407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mallard C, Davidson JO, Tan S, Green CR, Bennet L, Robertson NJ, et al. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res 2014. January;75(1–2):234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Supramaniam V, Vontell R, Srinivasan L, Wyatt-Ashmead J, Hagberg H, Rutherford M. Microglia activation in the extremely preterm human brain. Pediatr Res 2013. March;73(3):301–9. [DOI] [PubMed] [Google Scholar]
- 115.Paul DA, Leef KH, Stefano JL. Increased leukocytes in infants with intraventricular hemorrhage. Pediatr Neurol 2000. March;22(3):194–9. [DOI] [PubMed] [Google Scholar]
- 116.Kaneko N, Kako E, Sawamoto K. Enhancement of ventricular-subventricular zone-derived neurogenesis and oligodendrogenesis by erythropoietin and its derivatives. Front Cell Neurosci 2013. November 27;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kako E, Kaneko N, Aoyama M, Hida H, Takebayashi H, Ikenaka K, et al. Subventricular zone-derived oligodendrogenesis in injured neonatal white matter in mice enhanced by a nonerythropoietic erythropoietin derivative. Stem Cells 2012. October;30(10):2234–47. [DOI] [PubMed] [Google Scholar]
- 118.Ruegger CM, Hagmann CF, Buhrer C, Held L, Bucher HU, Wellmann S, et al. Erythropoietin for the Repair of Cerebral Injury in Very Preterm Infants (EpoRepair). Neonatology 2015;108(3):198–204. [DOI] [PubMed] [Google Scholar]
- 119.Fauchere JC, Koller BM, Tschopp A, Dame C, Ruegger C, Bucher HU, et al. Safety of Early High-Dose Recombinant Erythropoietin for Neuroprotection in Very Preterm Infants. J Pediatr 2015. July;167(1):52–7 e1–3. [DOI] [PubMed] [Google Scholar]
- 120.Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, et al. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke 2011. July;42(7):2045–53. [DOI] [PubMed] [Google Scholar]
- 121.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 2010. March;24(3):387–93. [DOI] [PubMed] [Google Scholar]
- 122.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 2013. February;44(2):497–504. [DOI] [PubMed] [Google Scholar]
- 123.Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr 2014. June;57(6):251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Im GH, et al. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. PLoS One 2015;10(7):e0132919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Mesenchymal Stem Cells: The Magic Cure for Intraventricular Hemorrhage? Cell Transplant 2017. March 13;26(3):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med 2005. September;11(9):966–72. [DOI] [PubMed] [Google Scholar]
- 127.Vinukonda G, Dohare P, Arshad A, Zia MT, Panda S, Korumilli R, et al. Hyaluronidase and Hyaluronan Oligosaccharides Promote Neurological Recovery after Intraventricular Hemorrhage. J Neurosci 2016. January 20;36(3):872–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia 2009. April 01;57(5):467–78. [DOI] [PubMed] [Google Scholar]
- 129.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol 2007. April;61(4):352–62. [DOI] [PubMed] [Google Scholar]
- 130.Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of Erythrocyte CD47 in Intracerebral Hematoma Clearance. Stroke 2016. February;47(2):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


