In countries that lack prenatal screening and treatment of pregnant women for chlamydia, neonatal chlamydial conjunctivitis is still a common infection. Ours is the first systematic review and meta-analysis of treatment for neonatal chlamydial conjunctivitis.
Keywords: azithromycin, Chlamydia trachomatis, conjunctivitis, erythromycin, neonate
Abstract
Background
With the continued high prevalence of chlamydia worldwide and high risk of transfer from mothers to their infant during delivery, a need for safe and effective therapies for infants who acquire a chlamydial infection remains. We conducted a systematic review and meta-analysis of antibiotic treatments, including oral erythromycin, azithromycin, and trimethoprim, for neonatal chlamydial conjunctivitis.
Methods
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception to July 14, 2017. We included randomized and nonrandomized studies that evaluated the effects of erythromycin, azithromycin, or trimethoprim in neonates with chlamydial conjunctivitis. A meta-analysis using a random-effects generic inverse-variance method was performed, and the certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.
Results
We found 12 studies (n = 292 neonates) and were able to meta-analyze 7 studies that used erythromycin at a dose of 50 mg/kg body weight per day for 14 days. The clinical and microbiological cure were 96% (95% confidence interval [CI], 94%–100%) and 97% (95% CI, 95%–99%), respectively, and adverse gastrointestinal effects occurred in 14% (95% CI, 1%–28%) of the neonates. The microbiological cure in the study that assessed azithromycin at 20 mg/kg per day were 60% (95% CI, 27%–93%) when it was given in a single dose and 86% (95% CI, 61%–100%) when given in a 3-day course. Two studies reported compliance with treatments, and 1 study reported no pyloric stenosis events. Because of the risk of bias and the few neonates included across the studies, the certainty of evidence is low to very low. No studies assessed trimethoprim.
Conclusions
Although evidence suggests that erythromycin at 50 mg/kg per day for 14 days results in higher numbers of cure than does azithromycin, compliance and risk of pyloric stenosis related to their use for other infections in neonates will factor into treatment recommendations. More data are needed to compare these treatments directly.
Chlamydia is the most common bacterial sexually transmitted infection worldwide [1]. The estimated global incidence of chlamydial infection is approximately 131 million new cases annually [2]. Although chlamydial infection typically affects sexually active adults, it can be passed on to newborns by their infected mother during delivery. Approximately 30% to 50% of infants born to a mother with active chlamydial infection will develop neonatal conjunctivitis, also known as ophthalmia neonatorum (ON) [3]. Infection in neonates commonly affects the conjunctivae, but it can occur also in the nasopharynx, lungs, vagina, urethra, and rectum [4].
Neonatal conjunctivitis caused by Chlamydia trachomatis is an acute infection of the conjunctiva that is characterized by erythema and edema of the eyelids, palpebral conjunctivae, and purulent eye discharge. It typically occurs between 5 and 14 days after delivery, although it can present earlier [5]. Neonatal conjunctivitis caused by C trachomatis is now significantly more prevalent than gonococcal conjunctivitis and has been reported to be the most common infectious cause of neonatal conjunctivitis worldwide [5–7]. Although it is generally a mild illness, complications can occur if it is left untreated; cases of untreated infection resulting in complications, such as scarring of the cornea or conjunctiva, have been reported [8, 9]. In addition, up to 20% of neonates exposed to chlamydial infection during birth can develop pneumonia, and evidence of previous conjunctivitis is found in approximately 50% of them [4, 10].
Newborn ocular prophylaxis has been found to be ineffective in protecting against chlamydial conjunctivitis [11, 12]. Several high-income countries have abandoned ocular prophylaxis of newborns and replaced it with routine prenatal screening and treatment of mothers with a sexually transmitted infection [12]. Indeed, the incidence of neonatal chlamydial and gonorrheal infection has decreased dramatically since the widespread initiation of routine prenatal screening and treatment of pregnant women in North America [3, 13]. However, in countries where prenatal screening is not a common practice and maternal chlamydial infections are prevalent, chlamydial infection remains a common cause of ON [3, 14]. In Hong Kong, where routine prenatal screening and treatment are not performed, an incidence of 4 cases of ON per 1000 live births between 2004 and 2005 was reported [15]. In contrast, in the United States, where routine prenatal screening and treatment are standard of care, the overall ON rate in 2002 was 8.5 per 100000 live births [16]. In 2015 in Canada, the rate of reported cases of chlamydial infection in children aged less than 1 year was approximately 10 per 100000 [17]. A 2017 survey of approximately 300 pediatric ophthalmologists, primarily in North America, estimated the number of neonatal conjunctivitis cases, most commonly caused by C trachomatis, to be up to 5 per practitioner per year [7]. Because of the continued high prevalence of chlamydia worldwide and the lack of prenatal screening in certain countries, a need for safe and effective therapies for infants who acquire a chlamydial infection remains. Therefore, we conducted a systematic review and meta-analysis of studies that tested antibiotic treatments, including oral erythromycin, azithromycin, or trimethoprim, for treating neonatal chlamydial conjunctivitis.
METHODS
A World Health Organization (WHO) guideline panel of clinicians, neonatologists, pathologists, researchers, and program managers with expertise in sexually transmitted infections [18] informed our inclusion and exclusion criteria for this review. We followed the Cochrane Handbook for Systematic Reviews of Interventions [19] and completed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [20].
Search Strategy and Study Selection
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception date to July 14, 2017. The search strategy included keywords and text words for chlamydia, ON, conjunctivitis, and the drug names. We did not impose language or study type restrictions. We also reviewed the reference lists of relevant study, guideline, and systematic review reports (see “Search Strategy” in Supplementary Material). Two investigators (A. Z. and N. S.) independently screened citations according to title and abstract and screened the full text of the relevant articles. Disagreements were resolved by consensus or by consulting with a third investigator.
We included primary studies on the effects of oral erythromycin, azithromycin, or trimethoprim in treating neonatal chlamydial conjunctivitis. Although other oral and topical antimicrobial agents are available, these 3 antimicrobials were chosen by the WHO guideline panel as a high priority for review, because they are used more widely in lower- and middle-income countries, and there is an outstanding question about which of them should be used in practice. We included any dosing regimen but were particularly interested in the effects of doses commonly used in lower- and middle-income countries, that is, erythromycin in 4 daily divided doses orally for 14 days at 20, 30, or 50 mg/kg body weight per day, azithromycin at 20 mg/kg per day orally (1 dose or for 3 days), and trimethoprim at 40 mg plus sulfa at 200 mg orally, twice daily for 14 days. We allowed randomized controlled trials and nonrandomized studies, including before–after studies, that followed 1 or 2 groups of neonates who underwent an eligible treatment. We included studies if more than 90% of the neonates were younger than 2 months at disease onset and diagnosed with chlamydial conjunctivitis on the basis of clinical symptoms and microbiological confirmation of C trachomatis using culture, a direct fluorescent antibody (DFA) test, or nucleic acid amplification testing (NAAT). The WHO guideline panel of experts identified critically important outcomes to include in the review by using methods for prioritizing outcomes [21]. Outcomes included clinical cure, microbiological cure, adverse effects (including pyloric stenosis), complications, antimicrobial resistance, and compliance. We also recorded data regarding treatment relapse, nasopharyngeal infection, and pneumonia. Clinical cure was defined as the resolution of signs and symptoms of neonatal chlamydial conjunctivitis, including erythema and edema of the eyelids or conjunctiva and ocular discharge. Microbiological cure was recorded when the results of chlamydial follow-up tests determined by culture, a DFA, or NAAT were negative.
Data Extraction and Risk of Bias Assessment
We created and piloted a data-extraction form. One investigator extracted the data, and a second investigator verified the data. We extracted data to determine the characteristics of the study, the effects of treatments, and the risk of bias, and the risk of bias. We used the Newcastle-Ottawa Scale for nonrandomized studies, because the articles we found reported the effects of only 1 treatment with no comparison [22]. For before–after studies of 1 treatment, we did not answer the question of comparability of the groups. We resolved disagreements by discussion between the 2 investigators
Analysis and Assessment of the Evidence
We conducted a meta-analysis of dichotomous outcomes by using a random-effects generic inverse-variance method to calculate pooled proportions and 95% confidence intervals (CIs) in Review Manager 5.1 (Nordic Cochrane Center and the Cochrane Collaboration, Copenhagen, Denmark). Two investigators evaluated the certainty of evidence for each outcome by using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach and resolved any discrepancies [23]. We assessed all GRADE domains, that is, risk of bias, inconsistency, imprecision, indirectness, publication bias, large effect, presence of a dose response, and plausible opposing confounders. According to the GRADE, we present the certainty of evidence as high, moderate, low, or very low [24].
RESULTS
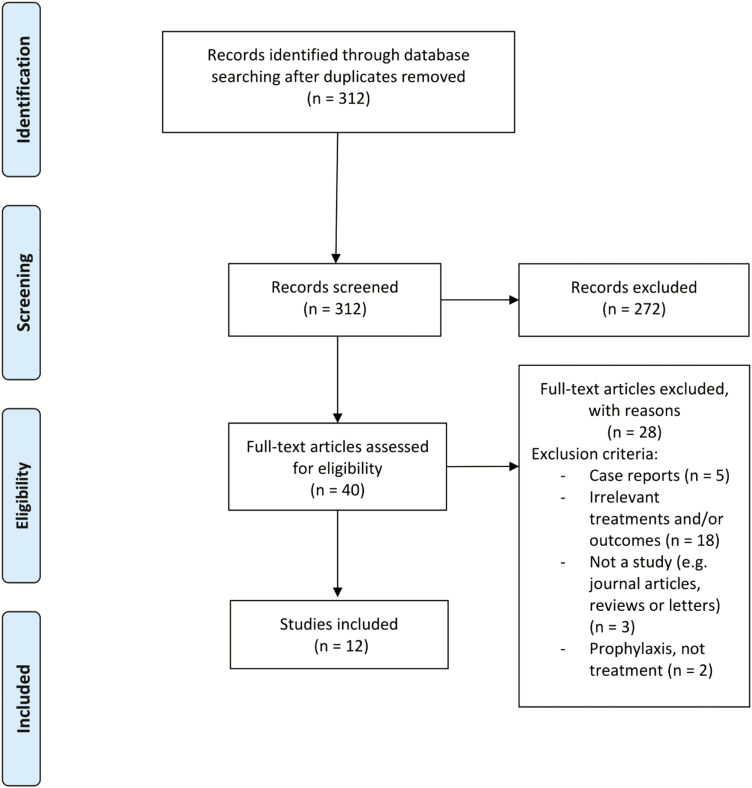
We found 312 nonduplicate records from our search of electronic databases and included 12 studies [4, 15, 25–34] presented as a narrative synthesis or meta-analysis (see Figure 1 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the study-selection process.
Characteristics of the Studies
Study Design and Country
Characteristics of the included studies are summarized in Table 1. Of the 12 studies, 9 [4, 15, 27–30, 32–34] were nonrandomized. Three studies [25, 26, 31] were randomized controlled trials; however, only 1 group in each study assessed an eligible treatment. Of the 9 nonrandomized studies, 8 collected data prospectively [4, 15, 27–30, 32, 33] and 1 collected data retrospectively [34]. Two of the studies used the same cohort of neonates but reported different outcomes [28, 29]. Four studies were conducted in the United States [4, 25, 26, 33], 4 in Sweden [28–31], 1 in Kenya [27], 1 in Mexico [32], and 2 in Hong Kong [15, 34].
Table 1.
Characteristics of Included Studies
| Study (year) | Study Design | Country | No. of Neonates With Confirmed Chlamydial Conjunctivitis | Primary Outcome(s) Measured |
Follow-Up Time(s) | No. Lost to Follow-Up | Intervention(s) |
|---|---|---|---|---|---|---|---|
| Hammerschlag et al [4] (1982) | NRS | USA | 12 | Clinical cure, microbiological cure, complications, compliance | 2 and 6 wk, 3 and 6 mo, and 1 year | 1 | Erythromycin 50 mg/kg per day × 14 days |
| Patamasucon et al [25] (1982) | RCTa | USA | 41 | Clinical cure, microbiological cure, adverse effects, compliance | 1, 3, 7, 10, 14, 21, and 28 days | 12 | Erythromycin 20, 30, or 40 mg/kg × 21 days |
| Heggie et al [26] (1985) | RCTa | USA | 37 | Clinical cure, microbiological cure, adverse effects | 14 days and 4–6 wk | 8 | Erythromycin 50 mg/kg per day × 14 days |
| Fransen et al [27] (1986) | NRS | Kenya | 22 | Clinical cure, microbiological cure, adverse effects | 7–10 and 30 days | 5 | Erythromycin 50 mg/kg per day × 10 days |
| Sandström [28] (1987) | NRS | Sweden | 19 | Adverse effects | 0, 3, 4, 10, 18, and 32 days | NR | Erythromycin 50 mg/kg per day × 14 days |
| Sandström et al [29] (1988) | NRS | Sweden | 33 | Clinical cure, microbiological cure, complications, compliance | 1, 3, 4, 10, 14, 18, and 32 days and 2, 3, 4, 6, and 12 mo | 9 | Erythromycin 50 mg/kg per day × 14 days |
| Stenberg et al [30] (1990) | NRS | Sweden | 45 | Clinical cure, microbiological cure, complications, adverse effects | 1 mo and 1 year | 16 | Erythromycin 40–50 mg/kg per day × 14 days |
| Stenberg et al [31] (1991) | RCTa | Sweden | 14 | Clinical cure, microbiological cure, complications, adverse effects | 1 mo | NR | Erythromycin 200 mg/day × 10 days |
| Yescas-Buendía et al [32] (1993) | NRS | Mexico | 32 | Clinical cure, complications | 5 and 6 days and 3 and 6 mo | 0 | Erythromycin 30–40 mg/kg per day × 14 days (intravenously or orally)b |
| Hammerschlag [33] (1998) | NRS | USA | 13 | Clinical cure, microbiological cure, adverse effects | 1–6 wk | 1 | Azithromycin 20 mg/kg single dose or × 3 days; erythromycin 50 mg/kg per day × 14 days |
| Chang et al [34] (2006) | NRS | Hong Kong | 19 | Clinical cure, complications, adverse effects | 2 wk and 6 mo | NR | Erythromycin 50 mg/kg per day × 14 days |
| Yip et al [15] (2007) | NRS | Hong Kong | 24 | Microbiological cure, complications | 3 wk and 3 mo | 4 | Erythromycin 50 mg/kg per day × 14 days |
Abbreviations: NR, not reported; RCT, randomized controlled trial; NRS, nonrandomized study.
aOnly 1 treatment arm in which neonates received erythromycin was used in the analysis. The other treatment arm provided neonates with a treatment not covered in this review.
bIntravenous erythromycin was administered to 16 of 32 neonates with concomitant chlamydial pneumonia.
Participants
In total, 292 neonates with a confirmed microbiological diagnosis of neonatal chlamydial conjunctivitis were included. Sample sizes ranged from 12 to 45. Two studies [25, 31] included older infants, but they comprised less than 10% of the sample size; all other studies included neonates with disease onset in the first month of life. When reported, the distribution of male to female neonates seemed equal within the individual studies. Seven studies reported that neonates had received a previous prophylactic intervention such as topical silver nitrate solution [4], erythromycin ointment [4], tetracycline ointment [25], chloramphenicol drops [30, 31], or fusidic acid eye ointment [30]. One study reported the use of intramuscular injections of 50000 U of penicillin G potassium [25]. The remaining study reports did not specify the type of prophylaxis given or did not report any prophylaxis use [15, 26–29, 32–34]. However, in all cases, any previous antibiotics were stopped before starting the treatment of interest.
Interventions
Table 1 provides detailed information about dosage regimens; erythromycin was the primary antibiotic used [4, 15, 25–32, 34]. The most common treatment regimen was the established standard of care [35], erythromycin at 50 mg/kg per day orally in 4 divided doses daily for 14 days. One study [33] analyzed the efficacy of 2 different durations of azithromycin treatment (single dose vs once daily for 3 days). None of the studies assessed the effects of trimethoprim.
Risk of Bias of Included Studies
Details of our risk-of-bias assessment are presented in Supplementary Material. Most of the studies objectively and accurately ascertained exposure of neonates to erythromycin either through secure hospital records or at clinic or trial visits and assessed clinical and microbiological cure outcomes according to the results of clinical examination and microbiological tests of cure. However, other outcomes, such as adverse effects, complications, and compliance, were likely not measured objectively or independently. Several studies were also at risk of bias as a result of their high percentage of loss to follow-up or lack of a statement regarding follow-up [25–27, 29, 31]. Overall, the risk of bias in the included studies was very serious because of their high loss to follow-up and lack of objective assessment of most outcomes.
Effects of Interventions
Table 2 provides a summary of the findings and a detailed assessment of the certainty of evidence for all treatments and critical outcomes in our meta-analysis. For most of the outcomes, the certainty of evidence is very low across the doses of erythromycin and azithromycin. We found serious risk of bias in all the studies and serious to very serious imprecision as a result of the small numbers of neonates in the trials. Considering the greater numbers of neonates in the studies that evaluated erythromycin at 50 mg/kg per day for 14 days and the large effects, the evidence for cure was of low certainty.
Table 2.
Summary of Findings for Outcomes of Neonatal Chlamydial Conjunctivitis Treatments (Presented as Pooled Proportions)
| Findings | Overall Certainty of Evidencea | Erythromycin % (95% CI); n/N | Azithromycin % (95% CI); n/N | |||||
|---|---|---|---|---|---|---|---|---|
| 50 mg/kg per day × 14 days [4, 15, 26, 29, 30, 33, 34] |
200 mg per day × 10 days [31] |
50 mg/kg per day × 10 days [27] |
30–40 mg/kg per day for 14 days [32] | 20, 30, or 40 mg/kg per day × 21 days [25] |
20 mg/kg, single dose [33] | 20 mg/kg per day × 3 days [33] | ||
| Outcome | ||||||||
| Clinical cure | Lowb,c ⊕⊕OO |
96 (92 to 101); 121/128 |
93 (78 to 108); 13/14 |
78 (60 to 96); 14/18 |
100 (95 to 105); 32/32 |
100 (93 to 107); 22/22 |
60 (27 to 93); 3/5 |
100 (82 to 118); 7/7 |
| Microbiological cure | Lowb,c ⊕⊕OO |
97 (94 to 100); 124/132 |
71 (50 to 93); 10/14 |
78 (60 to 96); 14/18 |
NM | 100 (93 to 107); 22/22 |
60 (27 to 93); 3/5 |
86 (61 to 110); 6/7 |
| Treatment relapsed | NA | 2 (−3 to 7); 4/69 |
NM | 6 (−7 to 18); 1/18 |
NM | 14 (−1 to 28); 3/22 |
NM | NM |
| Adverse effects | ||||||||
| Gastrointestinale | Very lowb,f ⊕OOO |
14 (1 to 28); 10/71 |
43 (20 to 66); 6/14 |
7 (1 to 13); 6/85 |
NM | 18 (3 to 34); 4/22 |
0 (−22 to 22); 0/5 |
0 (−18 to 18); 0/7 |
| Pyloric stenosis | NA | 0 (−8 to 8); 0/19 |
NM | NM | NM | NM | NM | NM |
| Complication | ||||||||
| Conjunctival scarring | Very lowb,f ⊕OOO |
6 (−1 to 12); 4/70 |
NM | NM | NM | NM | NM | NM |
| Antibiotic resistance | NM | |||||||
Abbreviations: n, number of events; N, total number of patients followed up; NA, not assessed; NM, not measured.
bNonrandomized studies were at serious risk of bias because most outcomes were not measured objectively and there was high loss to follow-up; evidence was rated down by 2 levels.
cImprecision resulted from small sample sizes and few events; however, the effects were large compared to those of no treatment and, therefore, the evidence was not rated down.
dClinical and/or microbiological relapse.
eIncludes diarrhea, watery stools, vomiting, and apparent abdominal pain.
fImprecision resulted from small sample sizes and few events, and the evidence was rated down 1 level.
aCertainty of the evidence: high (++++), we are very confident that the true effect lies close to that of the estimate of the effect; moderate (+++O), we are moderately confident in the effect estimate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different); low (++OO), our confidence in the effect estimate is limited (the true effect may be substantially different from the estimate of the effect); very low (+OOO), we have very little confidence in the effect estimate (the true effect is likely to be substantially different from the estimate of effect).
Clinical and Microbiological Cure
Erythromycin
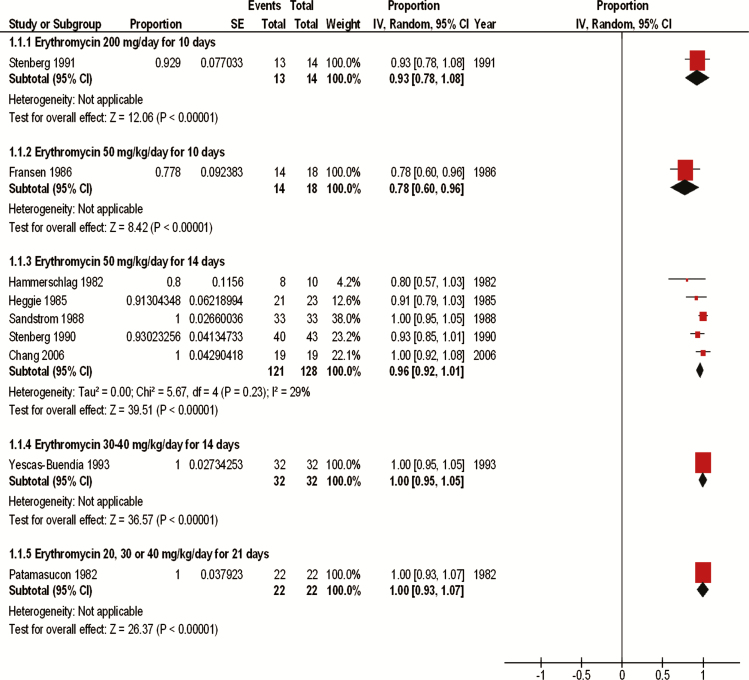
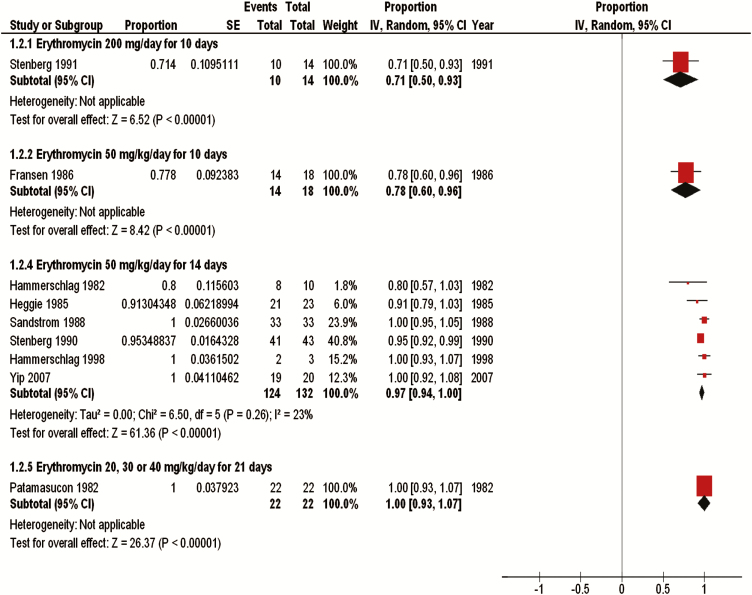
We pooled results from 7 studies that evaluated erythromycin at 50 mg/kg per day for 14 days (161 neonates) [4, 15, 26, 29, 30, 33, 34]. This dose resulted in a clinical cure of 96% (95% CI, 92%–101%) and a microbiological cure of 97% (95% CI, 94%–100%) (Figures 2 and 3). Four of 69 infants (2% [95% CI, −3% to 7%]) experienced a microbiological relapse after being treated with erythromycin at 50 mg/kg per day for 14 days, but none of the infants had a clinical relapse at 1 year [4, 29, 30]. Yescas-Buendía et al [32] treated infants with a lower dose of erythromycin (30–40 mg/kg per day for 14 days), and 100% (95% CI, 95%–105%) of the infants were clinically cured. Patamasucon et al [25] also tested erythromycin at lower doses, including 20, 30, and 40 mg/kg per day for 21 days, and reported clinical and microbiological cure of 100% (95% CI, 93%–107%) after 7 days of treatment. However, 3 (14%) of these neonates either experienced a relapse or were reinfected after completing the 21-day treatment regimen [25]. Two of these relapses were both clinical and microbiological, and 1 was only a microbiological relapse. The study report did not specify which neonates received which dose specifically. One study [27] evaluated erythromycin at 50 mg/kg per day for a shorter duration of 10 days and reported a clinical and microbiological cure for 78% (95% CI, 60%–96%) of the neonates. Of 18 infants, 1 (6% [95% CI, −7% to 18%]) treated with erythromycin at 50 mg/kg per day for 10 days experienced a clinical and microbiological relapse [27]. Another study [31] used a fixed dosage of erythromycin (200 mg/day) for 10 days and reported a clinical cure of 93% (95% CI, 78%–108%) and a microbiological cure of 71.4% (95% CI, 50%–93%), the lowest microbiological cure found among all the interventions. This study did not weight-adjust the dose; however, 12 of the 14 neonates received a dosage that ranged from 49 to 54 mg/kg per day. When reported, neonates whose treatment failed across all studies were re-treated and cured with a second or, rarely, third course of erythromycin, unless they were lost to follow-up.
Figure 2.
Clinical cure data for erythromycin treatment. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse-variance method; SE, standard error.
Figure 3.
Microbiological cure data for erythromycin treatment. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse-variance method; SE, standard error.
Azithromycin
Five infants received 1 dose of azithromycin at 20 mg/kg, and 3 (60% [95% CI, 27%–93%]) of these 5 infants were both clinically and microbiologically cured after follow-up [33]. The 2 infants who experienced treatment failure underwent re-treatment with erythromycin at 50 mg/kg per day for 14 days. However, 1 of these infants required 2 courses of erythromycin to recover fully [33]. Of 7 infants, 6 (86% [95% CI, 61%–110%]) treated with azithromycin at 20 mg/kg per day for 3 days were microbiologically cured after treatment, and all of them were clinically asymptomatic. The remaining infant with culture-positive (both eye and nasopharyngeal swab) chlamydial conjunctivitis was re-treated with erythromycin and achieved both clinical and microbiological cure after the 14-day follow-up [33]. The authors reported that the 3 infants whose culture results remained positive did not receive the entire dose of azithromycin successfully.
Adverse Effects
The most commonly reported adverse effects were related to the gastrointestinal tract [25, 27, 28, 30, 31], including diarrhea, watery stools, vomiting, and apparent abdominal pain; the authors did not report whether the effects were severe or whether treatment was stopped as a result of the adverse effects. In the treatment group given erythromycin at 50 mg/kg per day for 14 days, 14% (95% CI, 1%–28%) of the neonates developed adverse gastrointestinal effects [28, 30]. Adverse gastrointestinal effects occurred in 43% (95% CI, 20%–66%) of the neonates treated with erythromycin at 200 mg/day for 10 days and 18% (95% CI, 3%–34%) of those treated with erythromycin at 20, 30, or 40 mg/kg per day for 21 days [25, 31]. The authors of the study that treated neonates with erythromycin at 50 mg/kg per day for 10 days reported that 7% (95% CI, 1%–13%) experienced adverse gastrointestinal effects [27]. No adverse effects, including gastrointestinal adverse effects, were observed in the 12 infants treated with azithromycin [33]. None of the 19 neonates treated with erythromycin at 50 mg/kg per day for 14 days experienced pyloric stenosis after 6 months of follow-up [34]. No other studies reported or measured for pyloric stenosis.
Complications
Four studies assessed the development of pneumonia after treatment with antibiotics [4, 25, 29, 30], and 3 of these studies [4, 29, 30] had a follow-up duration of 1 year (see Table 1), which is long enough to detect the occurrence of pneumonia (the typical age of onset is between 1 and 3 months) [35]. Of 76 neonates, 1 (1% [95% CI, −3% to 5%]) treated with erythromycin at 50 mg/kg per day for 14 days developed pneumonia [4, 29, 30] at one month after treatment and were treated with another 2-week course of erythromycin [30]. None of the 22 neonates treated with erythromycin at 20, 30, or 40 mg/kg per day for 21 days developed pneumonia [25]. Three studies assessed rates of conjunctival scarring [28–30]. By pooling data from 2 studies, we found that 4 (6% [95% CI, −3% to 17%]) of 70 neonates treated with erythromycin at 50 mg/kg per day for 14 days developed conjunctival scarring [29, 30]. Ten studies reported nasopharyngeal infection [4, 25–31, 33], which occurred in 118 (50.2%) of 235 neonates. Of 90 neonates treated with erythromycin at 50 mg/kg per day for 14 days, 4 (3% [95% CI, −1% to 8%]) had a nasopharynx specimen that they were culture positive for Chlamydia trachomatis [4, 15, 26, 29, 30, 33]. Of 5 neonates given erythromycin at 50 mg/kg per day for 10 days, 3 (60% [95% CI, 27%–93%]) had persistent nasopharyngeal infection [27]; 0 of 6 given erythromycin at 20, 30, or 40 mg/kg per day for 21 days had persistent nasopharyngeal infection [25]; and 3 (21% [95% CI, 1%–41%]) of 14 given erythromycin at 200 mg/day for 10 days [31] had persistent nasopharyngeal infection. Only 1 neonate treated with azithromycin at 20 mg/kg per day given in a single dose and 1 (50% [95% CI, 9%–91%]) of 2 neonates treated with azithromycin at 20 mg/kg per day for 3 days had persistent nasopharyngeal infection [33].
Antibiotic Resistance
We did not find any studies that measured antibiotic resistance.
Compliance
Only 2 studies reported compliance issues [4, 25]. Patamasucon et al [25] measured the erythromycin content of serum and tears as an indicator of compliance. In that study, the authors reported that of the 22 neonates who received oral erythromycin, all of them had a detectable erythromycin level. However, the authors also reported that of all 53 neonates initially included in the study, 5 were excluded because of noncompliance, but they did not indicate who received topical or oral therapy. Hammerschlag et al [4] reported 1 incident of noncompliance in which an infant received the prescribed therapy for only 5 days instead of 14 days.
DISCUSSION
Ours is the first systematic review and meta-analysis of treatments for neonatal chlamydial conjunctivitis. We included 12 studies that assessed the effects of erythromycin or azithromycin, but we found no randomized studies that directly compared different drugs and no studies that evaluated trimethoprim. The certainty of evidence is low to very low because of the high risk of bias and the imprecision of the results across the studies. Overall, we found very large effects for cure for some treatments. We found high proportions of clinical (96%) and microbiological (97%) cure when erythromycin at 50 mg/kg per day was given in 4 divided doses for 14 days. These effects are large when compared to those with topical therapy alone [4, 26]. Several studies assessed topical therapy alone [4, 25, 26, 28], and their microbiological cure ranged from 0% with chloramphenicol ointment [28] to 78% with 1% erythromycin ophthalmic ointment. We found some data from small individual studies that suggest that lower doses or shorter courses of erythromycin might reduce the proportion of neonates cured, but the evidence is uncertain because of the very low-quality evidence. Adverse gastrointestinal effects, including diarrhea, watery stools, vomiting, and abdominal pain, can occur in 14% of neonates receiving erythromycin and might be higher when the dose of erythromycin is not adjusted to the infant’s weight. The only complication noted across the studies was scarring of the conjunctiva, which occurred in 6% (95% CI, −3% to 17%) of the neonates treated with erythromycin at 50 mg/kg per day for 14 days. We found 1 study that examined the efficacy of azithromycin in 12 neonates [33]. Although it seems that clinical and microbiological cure rates might be lower with azithromycin than with the standard dose of erythromycin, we are very uncertain in this result. Also, very few data regarding adverse effects and complications of azithromycin have been reported. Evidence on compliance was sparse; however, given the less-frequent dosing of azithromycin, it is possible that its use could improve compliance [36]. No studies in this review measured antibiotic resistance, but recent evidence suggests that antibiotic resistance is not currently an issue with C trachomatis infection [37, 38].
Only 1 study reported the rate of pyloric stenosis; 0 of the 19 neonates who received erythromycin developed any signs or symptoms [34]. Infantile hypertrophic pyloric stenosis typically presents between 3 and 8 weeks of age, and the length of follow-up in several of the studies was less than this time period [25, 27, 28, 31]. In addition, most of the included studies were published before 1999, the year in which the initial association between oral erythromycin and pyloric stenosis in infants after pertussis prophylaxis was elucidated [39, 40]. More recently, a study of a large retrospective cohort with more than 1 million infants with various infections revealed an increased risk of pyloric stenosis with both azithromycin and erythromycin [41]. The authors of this study reported the highest risk in infants exposed before 2 weeks of age, and a slightly higher risk was found in infants given erythromycin than in those given azithromycin (adjusted odds ratios, 13.3 [95% CI, 6.8–15.9] and 8.3 [95% CI, 2.6–26], respectively). This result means that compared to the number of neonates between 0 and 14 days old who developed pyloric stenosis when not receiving any antimicrobial agent (approximately 2 of 1000), there might be 29 more neonates with pyloric stenosis when given erythromycin compared to 18 more when given azithromycin. Other studies also found an increased risk of pyloric stenosis after early exposure to erythromycin [42].
There are some limitations of our review. First, this systematic review was restricted to studies in which the investigators confirmed neonatal chlamydial conjunctivitis microbiologically, but in many low- and middle-income countries, microbiological diagnosis is not feasible. Nevertheless, this review measures the best possible cure rates of the drug regimens in neonates who have chlamydial conjunctivitis. Given that we found primarily nonrandomized studies that evaluated the effects of 1 drug in 1 group of neonates, we pooled proportions across studies. However, we did not attempt to compare statistically the pooled proportions of different regimens of erythromycin to each other or compare azithromycin to erythromycin because of the small numbers of neonates across studies and the degree of indirectness. Overall, the results of our review provide a synthesis of the best available evidence for the treatment of neonatal chlamydial conjunctivitis.
In summary, there is evidence of low to very low certainty for the effects of erythromycin and azithromycin and no published evidence for trimethoprim. These findings have been used to inform the development of clinical practice guidelines for the treatment of neonatal conjunctivitis by the World Health Organization [18]. In that guideline, the experts balanced the potential benefits and harms of erythromycin and azithromycin, the limited information about compliance, the costs, and the greater clinical experience with erythromycin to make a strong recommendation for the use of azithromycin rather than erythromycin to treat chlamydial conjunctivitis in neonates, primarily because of the serious risk of pyloric stenosis in neonates who are given erythromycin. However, several questions remain unanswered. Many important outcomes identified by clinical experts have not been measured in studies, such as pyloric stenosis and compliance to the different drug regimens. Research that directly compares azithromycin, erythromycin, and trimethoprim is needed to measure critical outcomes better. Indeed, routine prenatal screening and treatment of pregnant women is the best method for preventing neonatal chlamydial conjunctivitis. Nonetheless, given the significant global burden of chlamydial infection and the lack of routine prenatal screening and treatment in certain countries, this research is still necessary.
Supplementary Data
Supplementary materials are available at Journal of the Pediatric Infectious Diseases Society online.
Notes
Financial support. This work was supported as an independent review by the United Nations Development Programme (UNDP)/United Nations Population Fund (UNFPA)/United Nations Children’s Fund (UNICEF)/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), World Health Organization.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Growing Antibiotic Resistance Forces Updates to Recommended Treatment for Sexually Transmitted Infections. Geneva: World Health Organization; 2016. Available at: http://www.who.int/mediacentre/news/releases/2016/antibiotics-sexual-infections/en/. Accessed July 30, 2017. [Google Scholar]
- 2. Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis 2011; 53:99–102. [DOI] [PubMed] [Google Scholar]
- 4. Hammerschlag MR, Chandler JW, Alexander ER, et al. Longitudinal studies on chlamydial infections in the first year of life. Pediatr Infect Dis 1982; 1:395–401. [DOI] [PubMed] [Google Scholar]
- 5. Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis 2005; 16:235–44. [DOI] [PubMed] [Google Scholar]
- 6. Mallika P, Asok T, Faisal H, et al. Neonatal conjunctivitis—a review. Malays Fam Physician 2008; 3:77–81. [PMC free article] [PubMed] [Google Scholar]
- 7. Zloto O, Gharaibeh A, Mezer E, et al. Ophthalmia neonatorum treatment and prophylaxis: IPOSC global study. Graefes Arch Clin Exp Ophthalmol 2016; 254:577–82. [DOI] [PubMed] [Google Scholar]
- 8. Goscienski PJ, Sexton RR. Follow-up studies in neonatal inclusion conjunctivitis. Am J Dis Child 1972; 124:180–2. [DOI] [PubMed] [Google Scholar]
- 9. Forster RK, Dawson CR, Schachter J. Late follow-up of patients with neonatal inclusion conjunctivitis. Am J Ophthalmol 1970; 69:467–72. [DOI] [PubMed] [Google Scholar]
- 10. Tipple MA, Beem MO, Saxon EM. Clinical characteristics of the afebrile pneumonia associated with Chlamydia trachomatis infection in infants less than 6 months of age. Pediatrics 1979; 63:192–7. [PubMed] [Google Scholar]
- 11. Hammerschlag MR, Cummings C, Roblin PM, et al. Efficacy of neonatal ocular prophylaxis for the prevention of chlamydial and gonococcal conjunctivitis. N Engl J Med 1989; 320:769–72. [DOI] [PubMed] [Google Scholar]
- 12. Darling EK, McDonald H. A meta-analysis of the efficacy of ocular prophylactic agents used for the prevention of gonococcal and chlamydial ophthalmia neonatorum. J Midwifery Womens Health 2010; 55:319–27. [DOI] [PubMed] [Google Scholar]
- 13. Moore DL, MacDonald NE; Canadian Paediatric Society, Infectious Diseases and Immunization Committee Preventing ophthalmia neonatorum. Paediatr Child Health 2015; 20:93–6. [PMC free article] [PubMed] [Google Scholar]
- 14. Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis infection in pregnancy: the global challenge of preventing adverse pregnancy and infant outcomes in Sub-Saharan Africa and Asia. Biomed Res Int 2016; 2016:9315757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yip TP, Chan WH, Yip KT, et al. Incidence of neonatal chlamydial conjunctivitis and its association with nasopharyngeal colonisation in a Hong Kong hospital, assessed by polymerase chain reaction. Hong Kong Med J 2007; 13:22–6. [PubMed] [Google Scholar]
- 16. Workowski K, Levine W. Sexually transmitted diseases treatment guidelines 2002. MMWR Morb Mortal Wkly Rep 2002; 51:1–78. [Google Scholar]
- 17. Public Health Agency of Canada. Reported cases by age group in Canada, grouped by sex—notifiable diseases on-line Available at: http://diseases.canada.ca/notifiable/charts?c=abs. Accessed March 15, 2018.
- 18. World Health Organization. WHO Guidelines for the Treatment of Chlamydia trachomatis. Geneva: World Health Organization; 2016. Available at: http://www.who.int/reproductivehealth/publications/rtis/chlamydia-treatment-guidelines/en/. Accessed November 30, 2016. [PubMed] [Google Scholar]
- 19. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62:e1–34. [DOI] [PubMed] [Google Scholar]
- 21. Santesso N, Mustafa RA, Schünemann HJ, et al. ; Guideline Support Group World Health Organization guidelines for treatment of cervical intraepithelial neoplasia 2-3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynaecol Obstet 2016; 132:252–8. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst 2013:1–4. [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schünemann HJ, Best D, Vist G, Oxman AD; GRADE Working Group Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. CMAJ 2003; 169:677–80. [PMC free article] [PubMed] [Google Scholar]
- 25. Patamasucon P, Rettig PJ, Faust KL, et al. Oral v topical erythromycin therapies for chlamydial conjunctivitis. Am J Dis Child 1982; 136:817–21. [DOI] [PubMed] [Google Scholar]
- 26. Heggie AD, Jaffe AC, Stuart LA, et al. Topical sulfacetamide vs oral erythromycin for neonatal chlamydial conjunctivitis. Am J Dis Child 1985; 139:564–6. [DOI] [PubMed] [Google Scholar]
- 27. Fransen L, Nsanze H, D’Costa L, et al. Oral erythromycin estolate in nongonococcal neonatal conjunctivitis. Eur J Sex Transm Dis 1986; 3:85–9. [Google Scholar]
- 28. Sandström I. Treatment of neonatal conjunctivitis. Arch Ophthalmol 1987; 105:925–8. [DOI] [PubMed] [Google Scholar]
- 29. Sandström I, Kallings I, Melen B. Neonatal chlamydial conjunctivitis. A long term follow-up study. Acta Paediatr Scand 1988; 77:207–13. [DOI] [PubMed] [Google Scholar]
- 30. Stenberg K, Mårdh PA. Chlamydial conjunctivitis in neonates and adults. History, clinical findings and follow-up. Acta Ophthalmol (Copenh) 1990; 68:651–7. [DOI] [PubMed] [Google Scholar]
- 31. Stenberg K, Mårdh PA. Treatment of chlamydial conjunctivitis in newborns and adults with erythromycin and roxithromycin. J Antimicrob Chemother 1991; 28:301–7. [DOI] [PubMed] [Google Scholar]
- 32. Yescas-Buendía G, Udaeta-Mora E, Arredondo-García JL, et al. Neonatal conjunctivitis caused by Chlamydia trachomatis. Bol Med Hosp Infant Mex 1993; 50:570–6. [PubMed] [Google Scholar]
- 33. Hammerschlag MR, Gelling M, Roblin PM, et al. Treatment of neonatal chlamydial conjunctivitis with azithromycin. Pediatr Infect Dis J 1998; 17:1049–50. [DOI] [PubMed] [Google Scholar]
- 34. Chang K, Cheng VY, Kwong NS. Neonatal haemorrhagic conjunctivitis: a specific sign of chlamydial infection. Hong Kong Med J 2006; 12:27–32. [PubMed] [Google Scholar]
- 35. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64. [PMC free article] [PubMed] [Google Scholar]
- 36. Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence 2013; 7:419–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohlhoff SA, Hammerschlag MR. Treatment of chlamydial infections: 2014 update. Expert Opin Pharmacother 2015; 16:205–12. [DOI] [PubMed] [Google Scholar]
- 38. West SK, Moncada J, Munoz B, et al. Is there evidence for resistance of ocular Chlamydia trachomatis to azithromycin after mass treatment for trachoma control?J Infect Dis 2014; 210:65–71. [DOI] [PubMed] [Google Scholar]
- 39. Honein MA, Paulozzi LJ, Himelright IM, et al. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study. Lancet 1999; 354:2101–5. [DOI] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention. Hypertrophic pyloric stenosis in infants following pertussis prophylaxis with erthromycin—Knoxville, Tennessee, 1999. MMWR Morb Mortal Wkly Rep 1999; 48:1117– 20. [PubMed] [Google Scholar]
- 41. Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics 2015; 135:483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cooper WO, Griffin MR, Arbogast P, et al. Very early exposure to erythromycin and infantile hypertrophic pyloric stenosis. Arch Pediatr Adolesc Med 2002; 156:647–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.