Abstract
Background
Human papillomavirus (HPV) infection has contributed to an increased incidence of squamous cell carcinoma of the head and neck (SCCHN). Fatigue is a major side effect of SCCHN and its treatment. However, the association between HPV and fatigue has not been examined, nor is it known whether HPV influences biological mechanisms of fatigue including inflammation.
Methods
SCCHN patients without distant metastasis were assessed at baseline (pre-radiotherapy) and one- and three-month post-radiotherapy. Fatigue was measured by the Multidimensional Fatigue Inventory. Peripheral inflammation was assessed by plasma C-reactive protein (CRP), interleukin-1 receptor antagonist (IL-1ra), soluble tumor necrosis factor receptor2 (sTNFR2), and IL-6. Mixed effect models were used to examine associations.
Results
Ninety-four newly diagnosed patients were enrolled; 53% had HPV-related tumors. Patients with HPV-unrelated tumors had higher fatigue and higher CRP, sTNFR2, and IL-6 over time, especially at baseline and 3 months post-IMRT compared to those with HPV-related tumors (all p<0.05). However, fatigue and sTNFR2 increased more significantly from baseline to one-month post-radiotherapy in the HPV-related group than in the HPV-unrelated group (both p<0.01). Controlling for significant covariates, HPV status and inflammation were independent predictors of fatigue over time.
Conclusion
HPV status is an important marker of vulnerability to behavioral and immune consequences of SCCHN and its treatment, providing support for different symptom management strategies. Special emphasis should be placed on addressing marked persisting fatigue in patients with HPV-unrelated tumors, while attention should be paid to the large increases in fatigue during treatment for patients with HPV-related tumors.
Keywords: HPV, fatigue, inflammation, head and neck cancer
INTRODUCTION
Head and neck cancer (HNC) is the 6th leading cancer by incidence worldwide;1 the estimate of new cases in the US is approximately 61,760,2 the majority of which are squamous cell carcinoma (SCC). The incidence of SCCHN has been consistently increasing in the US, due mainly to the increased incidence of human papillomavirus (HPV) infection.2 Patients with HPV-related SCCHN have better responses to treatment and better survival than patients with HPV-unrelated SCCHN.3 However, studies have not explored the association of HPV status with cancer-related fatigue and whether HPV status plays a role in biological mechanisms related to fatigue including inflammation. Fatigue is the most common side effect of cancer and its treatment.4 SCCHN patients receiving intensity-modulated radiation therapy (IMRT), a commonly used radiotherapy, experience even greater fatigue compared to conventional radiotherapy.5 Our studies on SCCHN showed that fatigue is consistently ranked among the top three severe side effects.6, 7 Fatigue negatively affects patients’ quality of life, functional status,8 and adherence to medical treatment.9 Pre- or post-treatment fatigue is prognostic of poor treatment response10 and reduced survival for cancer patients including SCCHN.11 However, the management of cancer-related fatigue is still challenging, and understanding the biological mechanisms of fatigue will help reveal potential targets for intervention.
Inflammation has been proposed as one biological mechanism that contributes to cancer-related fatigue.12 Our published findings have supported the positive association between inflammation and fatigue from pre- to one-month post-treatment in SCCHN patients.7 Long term follow-up with a larger sample size is necessary to verify and extend our understanding of this association. Moreover, the effect of HPV status on inflammation and fatigue has not been examined. Given recent published evidence of an association between HPV and improved quality of life,13 we hypothesized that fatigue might differ as a function of HPV status, and that HPV may influence inflammatory profiles and their association with fatigue. Thus, the purpose of this study was to assess the relationship among HPV status, inflammation and fatigue in SCCHN patients from pre-IMRT to one and three-month post-IMRT.
METHODS
Participants and Procedures
This was a longitudinal, prospective study on SCCHN patients receiving IMRT at Radiation Oncology Clinics at Emory University between 2012 to 2015. Patients were studied before IMRT and one and three months post-IMRT. All cancer treatments were completed upon completion of IMRT, and 1 month and 3 months post IMRT are typical time points for clinical follow-up. The study was approved by the Emory Institutional Review Board, and all subjects provided informed consent.
Inclusion criteria were histological proof of SCCHN; ≥21 years of age; no evidence of uncontrolled major organ disease or immunologic conditions that might confound the relationship between fatigue and inflammation; and patients who were to receive IMRT with or without concurrent chemotherapy. Exclusion criteria included evidence of distant metastases; simultaneous primaries; pregnancy; and patients diagnosed with major psychiatric disorders or who could not understand English. Over-the-counter anti-inflammatory medications and antidepressants were allowed.
Patients’ eligibility was determined by reviewing the electronic medical record. After giving consent, eligible patients were enrolled before the start of IMRT. Demographic and clinical variables were collected at baseline and/or follow-up as appropriate. Behavioral measures were completed pre-IMRT, and then at one and three-month post-IMRT. Blood samples for inflammatory markers were collected on the same day as the behavioral questionnaires.
Measurements
Demographic and clinical variables were collected through patient-reported questionnaires or chart review. 6 Demographic variables included age, sex, race (white vs. other), marital status (married vs. other), tobacco use (current/history vs. not), and alcohol use (current/history vs. not). Clinical variables included HPV status (HPV-related vs. -unrelated), body mass index (BMI), antidepressant use (yes vs. no), comorbidities (Charlson Comorbidity Index), primary cancer site (oropharynx vs. oral cavity vs. larynx vs. other), cancer stage (TNM: I-III vs. IV), radiation dose, chemotherapy (yes vs. no), chemotherapy regimen (Cisplatin vs. Carboplatin/Paclitaxel vs. others) and surgery (yes vs. no). Patients’ p16 or HPV status were determined based on pathology reports of the tumor tissue before treatment and were obtained through chart review. According to current practice, p16 or HPV positive were counted as HPV-related; otherwise they were counted as HPV-unrelated.
Fatigue, the primary outcome, was measured by the Multidimensional Fatigue Inventory (MFI). The MFI is a 20-item self-report instrument covering five dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity.14 Each dimension includes four items. The total score, ranging from 20 to 100 (higher score indicates more fatigue), is calculated as the sum of the five dimensions. The MFI has well-established validity and reliability (α=0.84) in use with cancer,14 including SCCHN.7
Other symptoms as covariates included depressive symptoms, sleep problems, cognitive dysfunction, pain, dry mouth, difficulty swallowing, skin burn from radiation, mouth or throat sores, taste change, nausea, and vomiting. These were chosen based on literature reviews and our studies.6, 7, 12 All symptoms were measured by the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) initiated by NCI.15
In order to reduce the number of variables in data analysis, 8 of above-described symptoms were grouped into two symptom clusters as supported by our published studies.6 These two symptom clusters were the HNC cluster (including pain, dry mouth, difficulty swallowing, skin burn from radiation, mouth or throat sores, and taste change) and the gastrointestinal (GI) cluster (including nausea and vomiting).
Whole blood was collected into chilled EDTA tubes for plasma isolation. The time of collection was not standardized based on subject availability. Plasma was separated by centrifugation at 1000xg for 10 minutes at 4ºC, and then aliquoted into siliconized polypropylene tubes and stored at −80ºC until batched assay. Plasma concentrations of IL-1ra, sTNFR2 and IL-6 were determined in duplicate using Magnetic Luminex Screening Assay (R&D Systems, Minneapolis, MN).16, 17 CRP was measured using a standard turbidimetric assay.7 Assay sensitivity was 39.01 pg/ml for IL-1ra, 5.72 pg/ml for sTNFR2, 0.17 pg/ml for IL-6, and 0.1 mg/l for CRP. Mean intra- and inter-assay CVs were reliably less than 10%. All assay results were in the detectable range. These inflammatory markers were selected because of their association with cancer-related fatigue in our previous studies of SCCHN and other cancers.7
Statistical Analysis
Descriptive statistics were performed for sample characteristics. T-tests or chi-square tests were conducted to examine differences between HPV-related and unrelated groups. Mixed effect models were used to examine whether HPV status was a predictor for inflammation or fatigue over time. Similar mixed effect models were also used to examine whether HPV and inflammation were predictors for fatigue. Covariates (demographic and clinical variables, and other symptoms) with significant bivariate associations with inflammation or fatigue over time at p<0.1 were adjusted for as fixed effects in the mixed effect models. Grand mean centering was used for inflammatory markers. A random subject intercept was included to account for correlations within subjects over time. Given the potential influence of diurnal patterns on inflammatory markers,18 the blood collection time was also entered into the models. If a significant interaction effect with time was noticed for the covariates, their interaction effects were added into the models as well. A two-sided significance level of 0.05 was used. SAS 9.4 was used for data analysis.
RESUTLS
Ninety-four patients were consented and enrolled; 35 of them were reported in our previous publication of pre-IMRT to one-month post.7 Similarly, the sample was primarily white (88%), male (75%) and middle-aged (58±10). Most of the participants were cancer stage III (31%) or IV (57%), and 51% were diagnosed with oropharyngeal cancer. Seventy-seven percent received concurrent chemotherapy, among which 65% had cisplatin. Patients with HPV-related tumors (53%) were significantly more likely to be male, have no history of tobacco use, have higher BMI, be diagnosed with oropharyngeal cancer, receive concurrent chemoradiotherapy, and receive higher dose of radiotherapy (Table 1).
Table 1.
Baseline demographic and clinical characteristics of the participants (N=94)
| Variables | HPV-related Mean ± SD or N (%) N=50 |
HPV-unrelated Mean ± SD or N (%) N=44 |
p | |
|---|---|---|---|---|
| Age (years) | 57.82 ± 8.96 | 58.68 ± 10.85 | 0.673 | |
|
| ||||
| Gender | Male | 42 (84) | 28 (64) | |
|
| ||||
| Female | 8 (16) | 16 (36) | 0.033 | |
|
| ||||
| Race | White | 46 (92) | 37 (84) | |
| Non-White | 4 (8) | 7 (16) | 0.337 | |
|
| ||||
| Marital statusa | Married | 38 (76) | 28 (64) | |
| Unmarried | 12 (24) | 16 (36) | 0.259 | |
|
| ||||
| History of tobacco use | No | 25 (50) | 8 (18) | |
| Yes | 25 (50) | 36 (82) | 0.002 | |
|
| ||||
| History of alcohol use | No | 21 (42) | 21 (48) | |
| Yes | 29 (58) | 23 (52) | 0.678 | |
|
| ||||
| BMI | 28.29 ± 4.62 | 26.06 ± 5.03 | 0.028 | |
|
| ||||
| Comorbiditiesb | 0 | 38 (76) | 31 (70) | |
| 1 | 8 (16) | 11 (25) | ||
| 2 | 4 (8) | 2 (5) | 0.495 | |
|
| ||||
| Antidepressants | No | 45 (54) | 38 (46) | |
| Yes | 5 (46) | 6 (54) | 0.584 | |
|
| ||||
| Cancer site | Oropharynx | 43 (86) | 5 (12) | |
| Oral cavity | 3 (6) | 15 (34) | ||
| larynx | 2 (4) | 12 (27) | ||
| Other | 2(4) | 12 (27) | <0.0001 | |
|
| ||||
| Stage | ≤ III | 24 (48) | 16 (36) | |
| IV | 26 (52) | 28 (64) | 0.299 | |
|
| ||||
| Treatment Type | IMRT | 1 (2) | 4 (9) | |
| IMRT + Surgery | 1 (2) | 12 (27) | ||
| IMRT + Chemo | 44 (88) | 21 (48) | ||
| IMRT + Chemo + Surgery | 4 (8) | 7 (16) | <0.0001 | |
|
| ||||
| Chemotherapy | Cisplatin | 32 (70) | 16 (57) | |
| Carboplatin/Paclitaxel | 10 (22) | 8 (29) | ||
| Other | 4 (6) | 4 (14) | 0.137 | |
|
| ||||
| Radiation dose | 69.20 ± 2.55 | 66.53 ± 4.16 | <0.0001 | |
|
| ||||
| Feeding tubes | No | 22 (44) | 16 (36) | |
| Yes | 28 (56) | 28 (64) | 0.530 | |
|
| ||||
| Fatigue | 40.60 ± 14.45 | 50.93 ± 15.36 | 0.001 | |
Note. T-test, Fisher’s Exact Test or Pearson Chi-Square used for the comparison. BMI = Body Mass Index, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy, SD = Standard deviation.
Married includes patients married or living as married; Unmarried includes patients single, separated, divorced, or widowed.
Comorbidities was assessed using the Charlson Comorbidity Index excluding tumor.
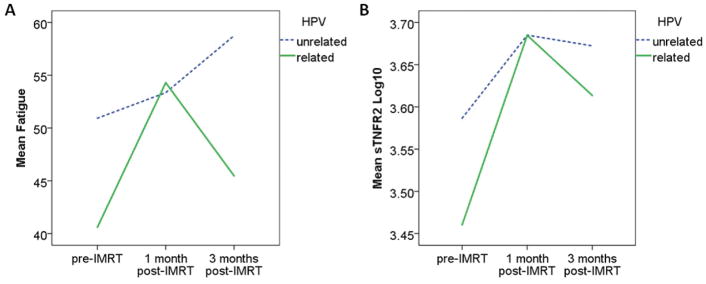
Fatigue increased significantly from pre- to one-month post-IMRT and remained increased at three-month post-IMRT (45.44±15.68 vs. 53.86±15.49 vs. 51.80±17.84, p<0.0001) for all patients. Patients with HPV-unrelated tumors experienced significantly higher levels of fatigue over the course of the study (p=0.0097, Table 2), especially at pre-IMRT (p=0.001) and three-month post-IMRT (p=0.002), compared to those with HPV-related tumors (Figure 1a). These findings were unchanged after controlling for significant covariates (Table 3). Of note, treatment type, chemotherapy or surgery were not significant individual predictors of fatigue. Similar patterns were observed for inflammation as represented by CRP, sTNFR2, and IL-6. Patients with HPV-unrelated tumors had significantly higher levels of CRP (p<0.0001), sTNFR2 (p=0.0369), and IL-6 (p=0.0064) over time (Table 2), especially at pre-IMRT (CRP, p<0.0001; sTNFR2, p<0.0001; IL-6, p=0.021) and three-month post-IMRT (CRP, P=0.005; IL-6, p=0.030), compared to those with HPV-related tumors (Figure 1b). CRP and sTNFR2 remained significantly associated with HPV over time after controlling for significant covariates of feeding tube and HNC cluster for CRP (p=0.0003) and medical comorbidities, BMI, chemotherapy, and GI cluster for sTNFR2 (p=0.0101).
Table 2.
Mean fatigue and inflammatory markers in patients with HPV-related and -unrelated SCCHN, assessed pre-, one-month, and three-month post-IMRT
| Pre-IMRT | 1-month Post Mean ± SD |
3-month Post | p for Time | p for HPV | ||||
|---|---|---|---|---|---|---|---|---|
| HPV-related N=50 |
HPV-unrelated N=44 |
HPV-related N=47 |
HPV-unrelated N=40 |
HPV-related N=38 |
HPV-unrelated N=30 |
|||
| Fatigue | 40.60 ± 14.45 | 50.93 ± 15.36 | 54.29 ± 15.42* | 53.35 ± 15.74 | 45.47 ± 15.38* | 59.88 ± 17.72* | <0.0001 | 0.0097 |
| CRP (mg/L) | 4.14 ± 7.74 | 10.79 ± 13.00 | 4.36 ± 6.51 | 7.82 ± 9.10 | 2.56 ± 4.23 | 7.67 ± 12.09 | 0.0287 | <0.0001 |
| IL-1ra (pg/mL) | 782.19 ± 614.43 | 1154.71 ± 1767.34* | 911.43 ± 1426.32 | 663.01 ± 521.60* | 466.06 ± 271.98 | 741.33 ± 942.20* | <0.0001 | 0.4774 |
| sTNFR2 (pg/mL) | 3175.74 ± 1230.40 | 4210.56 ± 1380.48* | 5450.49 ± 2458.59 | 5259.93 ± 2238.37 | 4544.14 ± 1917.29* | 5368.30 ± 2759.06 | <0.0001 | 0.0369 |
| IL-6 (pg/ml) | 1.28 ± 1.51 | 2.78 ± 3.87* | 2.06 ± 3.07 | 3.19 ± 5.71* | 1.08 ± 0.89 | 3.67 ± 6.07* | 0.0696 | 0.0064 |
Note. Mean and SD are raw scores. Mixed effect modeling was used to assess the effects of Time and HPV status on fatigue and the inflammatory markers. CRP = C-reactive protein, HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy, IL-1ra = interleukin 1 receptor antagonist, SCCHN = squamous cell carcinoma of the head and neck, SD = Standard deviation, sTNFR2 = soluble tumor necrosis factor receptor 2.
Having missing data:
HPV-related: One month post-IMRT: fatigue (1); Three months post-IMRT: fatigue (1), sTNFR2 (1).
HPV-unrelated: Pre-IMRT: IL-1ra (1), sTNFR2 (1), IL-6 (1); One month post-IMRT: IL-1ra (1), IL-6 (1); Three months post-IMRT: fatigue (1), IL-1ra (1), IL-6 (1).
Fig 1.
Fatigue (A), and (B) sTNFR2 in HPV-related and -unrelated groups over time.
Note. HPV = Human papillomavirus, IMRT = Intensity-Modulated Radiation Therapy, sTNFR2 = soluble tumor necrosis factor receptor 2.
Table 3.
Effect of inflammation and HPV status on fatigue over time
| Model 1 (CRP#) | Model 2 (IL-1ra#) | Model 3 (sTNFR2#) | Model 4 (IL-6#) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| Timea: | 0.1790 | 0.2021 | 0.8774 | 0.3733 | ||||
| baseline | −1.7006 | −2.0430 | 0.5703 | −1.1856 | ||||
| 1-month | 4.6762 | 4.4139 | 4.1642 | 4.7143 | ||||
|
| ||||||||
| HPV statusb | 5.6632 | 0.1441 | 6.79447 | 0.0304 | 5.5684 | 0.1646 | 6.0354 | 0.0914 |
|
| ||||||||
| Time*HPV statusc | 0.0084 | 0.0082 | 0.0278 | 0.0076 | ||||
|
| ||||||||
| Inflammatory marker | 3.2670 | 0.0103 | 5.8329 | 0.0123 | 12.0137 | 0.0063 | 2.7873 | 0.0956 |
|
| ||||||||
| Depressive symptoms | 2.9688 | 0.0005 | 2.8992 | 0.0014 | 3.2077 | 0.0002 | 3.1014 | 0.0004 |
|
| ||||||||
| Cognitive dysfunction | 7.3810 | <.0001 | 7.2671 | <.0001 | 7.2530 | <.0001 | 7.2459 | <.0001 |
|
| ||||||||
| HNC cluster | 0.4392 | 0.0389 | 0.5086 | 0.0165 | 0.5437 | 0.0109 | 0.4800 | 0.0254 |
|
| ||||||||
| GI cluster | 1.0574 | 0.0107 | 1.0107 | 0.0189 | 0.8207 | 0.0515 | 1.0060 | 0.0162 |
|
| ||||||||
| Chemotherapyd | 0.4096 | 0.8734 | −0.3948 | 0.8760 | 1.6907 | 0.5177 | 0.6375 | 0.8071 |
Note. Multivariate mixed effect modeling was used to assess the effect of time, HPV status, and their interaction on fatigue as a function of each inflammatory marker individually. CRP = C-reactive protein, GI = Gastrointestinal, HNC = Head and neck cancer, HPV = Human papillomavirus, IL-1ra = interleukin 1 receptor antagonist, sTNFR2 = soluble tumor necrosis factor receptor 2.
Data were log10 transformed and centered by the grand mean.
The reference group was 3-month.
The reference group was the HPV-related group.
Fatigue was significantly higher in the HPV-unrelated group at baseline and 3-month post-IMRT but not at 1-month post-IMRT for all models including different inflammatory markers.
The reference group was chemotherapy group.
Of note, compared to patients with HPV-unrelated tumors, patients with HPV-related tumors exhibited larger increases in fatigue and inflammation from pre- to one-month post-IMRT, and larger decreases in fatigue and inflammation from one-month to three-month post IMRT (Figure 1). Thus, we conducted post hoc t-tests for changes from pre- to one-month post-IMRT and from one-month to three-month post-IMRT. The results showed that increases in fatigue from pre- to one-month post-IMRT were significantly higher in patients with HPV-related tumors than patients with HPV-unrelated tumors (p=0.001). Inflammation as represented by sTNFR2 demonstrated a similar pattern (p=0.003). Patients with HPV-related tumors also had a larger drop in fatigue from one-month to three-month post-IMRT than those with HPV-unrelated tumors, who remained high in fatigue (p=0.002) at 3 months post-treatment. Although inflammatory markers showed a similar pattern during this time frame, they did not differ significantly between patients with HPV-related and -unrelated tumors.
Mixed effects modeling was used to examine the impact of inflammation (considering each inflammatory marker independently), HPV status, time and their interaction (HPV status x time) on fatigue. The results indicated that inflammatory markers and HPV status x time were independent predictors of fatigue over time after adjusting for significant and relevant covariates including chemotherapy (Table 3). Patients with higher levels of inflammation as represented by CRP (Estimate=3.2670; p=0.0103), IL-1ra (Estimate=5.8329; p=0.0123), and sTNFR2 (Estimate=12.0137; p=0.0063) were more likely to have higher fatigue during the course of the study. Additionally, there were significant interaction effects between HPV and time on fatigue (Table 3). Patients with HPV-unrelated tumors were more likely to experience higher fatigue at pre- and three-month post-IMRT than those with HPV-related tumors, while fatigue was similar between the two groups at one-month post-IMRT.
DISCUSSION
Our findings indicate that HPV status is associated with levels of fatigue and inflammation before and after IMRT. Fatigue in patients with HPV-unrelated tumors was persistently high both pre- and post IMRT, whereas patients with HPV-related tumors experienced lower levels of fatigue before and 3 month post IMRT, but experienced a larger increase in fatigue from pre- to one-month post-IMRT. These data support the notion that HPV status may play an important role in the vulnerability to the effects of SCCHN and its treatment on both fatigue and inflammation.
Our findings of higher level of fatigue and inflammation in patients with HPV-unrelated tumors are consistent with our published studies on quality of life where HPV-unrelated SCCHN patients, compared to HPV-related SCCHN patients, experienced worse quality of life before and after radiotherapy.13, 19, 20 Of note, the higher levels of fatigue and inflammation in HPV-unrelated SCCHN patients at baseline and three-month post-treatment in the current study were apparent even after considering surgery (typically one month prior to IMRT) and other clinical variables that differed between groups including tumor site and stage as well as treatment regimen in the analyses. Indeed, patients with HPV-related tumors were more likely to receive concurrent chemotherapy and radiation, both of which have been associated with more fatigue and potentially more inflammation in previous studies.21 It is unclear why fatigue and inflammation is high at baseline and remains high three-month post-treatment in the HPV-unrelated group; however, our finding suggests that patients in the HPV-unrelated group need continuous support for their symptom burden.
Another important observation was that patients with HPV-related tumors had a larger increase in fatigue one-month post-treatment compared to those without HPV. Inflammatory markers showed a similar pattern. This change in fatigue over time also echoes our published study on quality of life, in which patients with HPV-related tumors experienced a larger decrease in quality of life at the end of radiotherapy than patients without HPV.13 The reason behind this is unclear; however, it might be related to the lower baseline levels of fatigue and inflammation in these individuals. Given the similar intensive treatment regimens received by both groups at the time of this study, this larger increase in fatigue and inflammation indicates that HPV-related patients are more likely to be affected by treatment intensity, further supporting the rationale for current dose reduction trials in HPV-related SCCHN patients.22
Given the significant association between HPV and oropharyngeal cancer, we also examined whether oropharyngeal cancer might be the major driver of the different fatigue and inflammation profiles seen as a function of HPV. Mixed effect models showed that cancer site (oropharyngeal vs non-oropharyngeal) explained significant differences in CRP, but not in fatigue, IL1-ra, sTNFR2, and IL-6. Thus, our data suggested that HPV status, not the cancer site, plays the major role in differences in fatigue and inflammation in these patients.
Fatigue, in general, was significantly associated with inflammation in our study subjects. This positive association is consistent with previous evidence,12, 23 and extends our published finding of positive associations between inflammation and fatigue to three months post-IMRT in SCCHN patients.7 Longer follow-up studies are warranted to explore whether this association persists long after the completion of cancer treatment and whether HPV plays a role in the long-term association. Additionally, treatment type was not significantly associated with fatigue, although treatment-associated side effects, such as HNC and GI clusters were significant. HNC and GI clusters were significantly higher in patients receiving carboplatin/paclitaxel than those receiving cisplatin. These data suggest that treatment types can play a role in some side effects, but not necessarily in fatigue, at least in this study. More data are needed to further evaluate these associations.
The study’s major strengths include a relatively large sample size and data collected prospectively and longitudinally. Nevertheless, with the longitudinal design, we had some missing data at the three-month post treatment, which might have biased the results. However we did sensitivity analyses, and with the exception of race and gender, there were no significant differences in baseline measures, including fatigue and inflammatory markers, between subjects having missing data at three months and those without missing data at that time point. Also, different fatigue and inflammation profiles in HPV-related and unrelated groups should be interpreted with caution as other confounders might explain the differences, even though they were not significant in this study. Although the timing of blood collection and behavioral surveys was included in the data analyses as a control variable (and was not significant), circadian variations in inflammatory markers (and potentially behavioral responses) may have also influenced the relationship among inflammation, fatigue and HPV status. Additionally, the heterogeneous nature of head and neck cancer sites, as noted in other published studies, represents a limitation.24 However, cancer site did not significantly predict the relationship between inflammation and fatigue in this study.
CONCLUSION
This is the first study to report an association between HPV and behavioral and inflammatory responses to cancer and its treatment. Patients with HPV-unrelated tumors experienced persistently high levels of fatigue even at three-month post-IMRT, while patients with HPV-related tumors exhibited lower fatigue and inflammation at baseline and three-month post-treatment. However, patients with HPV-related tumors experienced a larger increase in fatigue and inflammation from baseline to one-month post-IMRT. These different symptom profiles suggest that clinicians may want to utilize different symptom management strategies for patients with or without HPV-related tumors. More specifically, HPV-unrelated patients need constant support before and after treatment, whereas one-month post-IMRT is when HPV-related patients need the most attention. Studies with longer follow-up and larger sample sizes are needed to verify our findings, especially among patients with oropharyngeal cancer only. It also would be interesting to explore why HPV-unrelated SCCHN patients have higher fatigue and inflammation than HPV-related SCCHN patients.
Acknowledgments
The authors appreciate the support from Emory University School of Nursing, School of Medicine, and Winship Cancer Institute.
Funding:
The study was supported by NIH/NINR K99/R00NR014587, NIH/NINR R01NR015783, NIH/NCI P30CA138292 and Oncology Nursing Society Foundation.
Footnotes
Conflicts of Interest: None
Author Contributions:
Canhua Xiao: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, writing - original draft, and writing - review and editing. Jonathan J. Beitler: conceptualization, investigation, resources, and writing - review and editing. Kristin A. Higgins: investigation, resources, and writing - review and editing. Toby Glazer: investigation, project administration, and writing - review and editing. Linh Kha Huynh: investigation, project administration, and writing - review and editing. Sudeshna Paul: data curation, formal analysis, methodology, resources, validation, and writing - review and editing. Jennifer C. Felger: methodology, resources, and writing - review and editing. Evanthia C Wommack: methodology, resources, and writing - review and editing. Nabil F. Saba: investigation, resources, and writing - review and editing. Dong M. Shin: investigation, resources, and writing - review and editing. Deborah W. Bruner: conceptualization, funding acquisition, supervision, and writing - review and editing. Andrew H. Miller, conceptualization, funding acquisition, investigation, methodology, supervision, and writing - review and editing.
References
- 1.WHO. Head and Neck Cancer, Union for International Cancer Control, 2014 Review of Cancer Medicines on the WHO List of Essential Medicines. 2014 Available from URL: http://www.who.int/selection_medicines/committees/expert/20/applications/HeadNeck.pdf.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006;42:846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Gulliford SL, Miah AB, Brennan S, et al. Dosimetric explanations of fatigue in head and neck radiotherapy: An analysis from the PARSPORT Phase III trial. Radiother Oncol. 2012;104:205–212. doi: 10.1016/j.radonc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol. 2013;49:360–366. doi: 10.1016/j.oraloncology.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao C, Beitler JJ, Higgins KA, et al. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav Immun. 2016;52:145–152. doi: 10.1016/j.bbi.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt ME, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. J Cancer Surviv. 2012;6:11–19. doi: 10.1007/s11764-011-0197-3. [DOI] [PubMed] [Google Scholar]
- 9.Solberg Nes L, Ehlers SL, Patten CA, Gastineau DA. Self-regulatory Fatigue in Hematologic Malignancies: Impact on Quality of Life, Coping, and Adherence to Medical Recommendations. Int J Behav Med. 2013;20:13–21. doi: 10.1007/s12529-011-9194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HC, Janjan NA, Mendoza TR, et al. Temporal patterns of fatigue predict pathologic response in patients treated with preoperative chemoradiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:775–781. doi: 10.1016/j.ijrobp.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer. 2004;100:425–432. doi: 10.1002/cncr.20010. [DOI] [PubMed] [Google Scholar]
- 12.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao C, Zhang Q, Nguyen-Tan PF, et al. Quality of Life and Performance Status From a Substudy Conducted Within a Prospective Phase 3 Randomized Trial of Concurrent Standard Radiation Versus Accelerated Radiation Plus Cisplatin for Locally Advanced Head and Neck Carcinoma: NRG Oncology RTOG 0129. Int J Radiat Oncol Biol Phys. 2017;97:667–677. doi: 10.1016/j.ijrobp.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Available from URL: http://outcomes.cancer.gov/tools/pro-ctcae.html.
- 16.Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med. 2017:1–11. doi: 10.1017/S0033291717000150. [DOI] [PubMed] [Google Scholar]
- 17.Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13:541–547. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong MT, Zhang Q, Rosenthal DI, et al. Quality of Life and Performance Status From a Substudy Conducted Within a Prospective Phase 3 Randomized Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Locally Advanced Head and Neck Carcinoma: NRG Oncology Radiation Therapy Oncology Group 0522. Int J Radiat Oncol Biol Phys. 2017;97:687–699. doi: 10.1016/j.ijrobp.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ringash J, Fisher R, Peters L, et al. Effect of p16 Status on the Quality-of-Life Experience During Chemoradiation for Locally Advanced Oropharyngeal Cancer: A Substudy of Randomized Trial Trans-Tasman Radiation Oncology Group (TROG) 02.02 (HeadSTART) Int J Radiat Oncol Biol Phys. 2017;97:678–686. doi: 10.1016/j.ijrobp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Jereczek-Fossa BA, Santoro L, Alterio D, et al. Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys. 2007;68:403–415. doi: 10.1016/j.ijrobp.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Yom SS. NRG-HN002: A Randomized Phase II Trial for Patients with p16 Positive. Non-Smoking Associated, Locoregionally Advanced Oropharyngeal Cancer, NCT02254278. Available from URL: https://www.nrgoncology.org/Clinical-Trials/NRG-HN002.
- 23.Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19:1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer. 2014;120:1975–1984. doi: 10.1002/cncr.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]