Abstract
INTRODUCTION
We tested the hypothesis that poor sense of smell is associated with lower cognitive function and higher MCI prevalence.
METHODS
Olfaction, measured by the Sniffin’ Sticks test, was categorized as OI (score ≤6) or no OI (score >6). MCI was adjudicated based on review of a neuropsychological examination. Linear regression estimated the mean difference in cognitive factor scores, and log-binomial regression quantified MCI prevalence among participants with vs. without OI.
RESULTS
Participants with OI had lower mean factor scores [memory: -0.27 standard deviation (SD), 95% CI: -0.35,-0.19; language: -0.24 SD, 95% CI: -0.30,-0.17; executive function/processing speed: -0.09 SD, 95% CI: -0.12,-0.06; general cognitive performance: -0.25 SD, 95% CI: -0.30,-0.20. OI was also associated with MCI (n=204; Prevalence Ratio=1.56, 95% CI: 1.37,1.78).
DISCUSSION
An impaired sense of smell may serve as a readily accessible early marker of neurodegeneration and improve upon the prevailing delayed diagnoses and under-ascertainment of MCI/dementia.
Keywords: cognition, mild cognitive impairment, olfaction
1. Background
The prevalence of olfactory impairment (OI) is almost 25% in individuals aged 53 years or older, rising to 60% at ages 80-97.1 OI can lead to reduced quality of life (i.e. loss of pleasure in food2) and increased health hazards (i.e. inability to detect spoiled food and gas leaks3), and it is a strong and independent risk factor for mortality.4,5 The most salient predictor of olfactory impairment among healthy adults is age.6 Other predictors include race, sex, inflammation of the nasal passages, upper respiratory infections, viral infections, exposure to toxins, and head trauma.1
Impaired olfaction is an early symptom of neurodegenerative pathogeneses due to Alzheimer’s disease (AD), as well as Parkinson’s disease.7 Autopsy data show that a greater loss in the sense of smell is associated with plaques and tangles in the central olfactory region of the brain8 that connects to the hippocampal region. More specifically, damage due to Braak neurofibrillary tangle (NFT) stage I of AD occurs preferentially in areas including the olfactory cortex.9-11 Recent data suggest OI is associated with reduced cognitive performance, incident MCI, and rate of progression from MCI to dementia due to AD.12 Additional population-based studies are needed both for confirmation and to examine cognitive decline and individual cognitive domains.
Differences by race and sex also warrant investigation. Preliminary data suggest that African Americans and Hispanics have worse olfactory function than individuals of European ancestry,13 with greater differences than by sex.14 Few studies have examined differences by race in the associations between olfaction and neurocognitive outcomes. Non-demented community-dwelling older adults from the Health Aging, and Body Composition (Health ABC) study with poorer odor identification had a three-fold greater risk of dementia compared to those with good odor identification; this association was stronger in whites compared to Blacks.15 In a multiethnic cohort of 1,037 participants from Northern Manhattan, those in the lowest quartile of a smell test score had a higher risk of transitioning to Alzheimer’s dementia compared to participants in the highest quartile.16
Our objective was to examine the associations of olfactory function with cognitive function in the absence of dementia. Here we add to the literature by testing the hypothesis that poor sense of smell is associated with (1) lower domain-specific cognitive function, specifically in the domain of memory, (2) greater prior cognitive decline, and (3) increased prevalence of mild cognitive impairment (MCI).
2. Methods
2.1. Study population and design
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based prospective cohort study that recruited 15,792 participants aged 45 to 64 years between 1987-1989 from 4 U.S. communities (Washington County, Maryland; Forsyth County, North Carolina; Minneapolis, Minnesota; and Jackson, Mississippi). ARIC was designed to investigate the etiology of atherosclerosis and its clinical sequelae. Detailed information about the ARIC Study has been described.17 The current study is based on the fifth cohort examination (2011-2013, n=6,538), the ARIC Neurocognitive Study (NCS). The primary aim of ARIC-NCS was to evaluate the contribution of mid-life vascular risk factors to cognitive decline and risk of MCI and dementia. ARIC-NCS participants completed a smell identification test and comprehensive neuropsychological battery. Neurologists and neuropsychologists adjudicated MCI and dementia.18 Due to small numbers, we excluded non-Black/non-White participants (n=18); Blacks from Minneapolis, Minnesota and Washington County, Maryland (n=25); and participants who were missing the smell test data (n=440). Participants without smell test data (eTable 1 in the Supplement) were older, more often Black, had lower educational attainment and a higher prevalence of hypertension and stroke. Given our interest in examining associations of olfaction with early impairments, and to avoid the artifact of poor smell test performance in persons with dementia, participants with diagnosed dementia (n=247) were excluded from the analysis. We excluded an additional 787 participants due to incomplete covariate/outcome data. Our analytic sample included 5,021 older adults. Institutional review boards at each study site approved the study.
2.2. Exposure: olfactory function
The sense of smell identification was measured by the 12-item Sniffin’ Sticks screening test at ARIC visit 5.19 Participants were asked to smell 12 common odorants in a felt-tip pen (orange, leather, cinnamon, peppermint, banana, lemon, licorice, coffee, cloves, pineapple, rose, and fish), one at a time, and asked to identify each using a multiple-choice format of 4 possible answer choices. One point was assigned to each correctly identified odorant, yielding a total possible score of 12. The smell test score was dichotomized according to a conventional cut point for OI (score ≤ 6).20 We also examined associations of interest across the continuous values of the smell test score (0-12, higher score=better olfactory function). The Sniffin’ Sticks test is comparable to other brief olfactory screening tests, including the Brief Smell Identification Test (B-SIT), and has been widely used in both clinical and epidemiologic studies.20,21
2.3. Outcomes: cognitive function and MCI
Cognitive function was assessed with a comprehensive neuropsychological battery administered at ARIC visit 5. The following domains and cognitive tests were examined: memory [delayed word recall, logical memory, and incidental learning], executive functioning/processing speed [Trail Making Tests, Parts A and B; Digit Symbol Substitution Test], and language [semantic and phonemic fluency, Boston Naming Test]. Using data from these tests in a factor analysis, factor scores for general cognitive performance, executive functioning/processing speed, memory and language were derived.22 Briefly, factor analysis is a structured approach for identifying common covariation between specific indicators, in this case the cognitive tests, to reduce measurement error when combining data across multiple cognitive tests. The interpretations of factor scores are similar to that for z scores because they were scaled to have a mean of 0 and variance of 1 at ARIC visit 2 when the participant’s cognitive function was first tested.
Algorithms based on the National Institute of Aging-Alzheimer’s Association (NIA-AA) workgroups23,24 and Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)25 were used to determine a diagnosis of MCI. An MCI diagnosis was assigned to participants if they met the following criteria: (1) performing worse than -1.5 standard deviations on at least one cognitive domain; (2) scored >0.5 and ≤ 3 on the Clinical Dementia Rating Score (CDR) sum of boxes; (3) scored ≤ 5 on the Functional Assessment Questionnaire (FAQ); and (4) declined below the 10th percentile on one test or below the 20th percentile on two tests in a serial ARIC cognitive battery first administered at visit 2. A classification committee reviewed participants with algorithm-diagnosed MCI, and the diagnosis was confirmed by two reviewers (one neurologist and one neuropsychologist) and adjudicated when necessary by a third reviewer. The prevalence of MCI among ARIC visit 5 participants was 21%, which is comparable to the prevalence of MCI in our analytic sample (n=1,092, 21.7%).18 For participants with cerebral imaging data, a primary and secondary MCI etiology were determined by review,18 categorizing etiologic diagnoses as MCI with Alzheimer’s disease (AD, with or without a secondary diagnosis, n=768). Details of these etiologic diagnoses has been previously described.18
2.4. Covariates
All covariates included in the regression models were assessed at ARIC visit 5, except race-field center (Minnesota Whites; Maryland Whites; North Carolina Whites; North Carolina Blacks; Mississippi Blacks), sex, and education (less than high school, high school or equivalent, and greater than high school), which were assessed at visit 1. Additional covariates included: age, cigarette smoking status (never vs. ever); diabetes mellitus (defined as fasting glucose ≥126 mg/dL, self-reported history of diabetes mellitus diagnosis, or use of diabetes mellitus medication); hypertension (defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of blood pressure-lowering medication); and apolipoprotein (APOE) ε4 genotype (0 or ≥ 1 allele).
2.5. Statistical analysis
Descriptive analysis used chi-square and ANOVA tests to examine differences in baseline demographic and disease characteristics among ARIC-NCS participants with and without OI. We first examined the adjusted mean cognitive factor scores and MCI prevalence across smell test scores (0-12). Multivariable linear regression was used to estimate the cross-sectional associations of olfactory function with domain-specific cognitive factor scores. Log-binomial regression was used to estimate the prevalence of MCI (and MCI due to AD) comparing participants with and without OI. Models were adjusted for age, education, race-ARIC field center interaction, smoking status, and APOE ε4. Additional analyses examined prevalent hypertension and diabetes as confounders.
We further examined the association between olfactory function and prior change in domain-specific cognitive function from this analytic cohort’s visit 2 (1990-1992) to visit 5 (2011-2013) test scores. Three cognitive tests and domains (memory: Delayed Word Recall Test, language: verbal fluency, executive function/processing speed: Digit Symbol Substitution Test) that were administered at the visit 5 examination were also collected at visits 2 (1990-1992) and 4 (1996-1998). Using time on study, we performed a longitudinal analysis using mixed effects models with random intercepts and slopes. A linear spline was included at 6 years (visit 4, 1996-1998) to estimate the change in cognition from (a) 0-6 years and (b) 6 years- end of study. A random slope for spline 1 and a random slope for spline 2 were included in the models. We specified an independent covariance matrix for the random effects. An interaction term between OI and each time spline was incorporated to estimate the change separately for years 0-6 and 6 years-end of study, which were then combined to provide 20-year change estimates. All analyses were performed using STATA version 14.0 (StataCorp LLC, College Station, Texas).
3. Results
Demographic and clinical characteristics of the study population (n=5,021) are provided in Table 1 by OI at ARIC visit 5. The prevalence of OI was 11% (n=574). Participants with OI were older, more often male, and had lower education levels, slightly higher diabetes and stroke prevalence, and lower domain-specific and general cognitive performance scores.
Table 1.
Population Characteristics by Olfactory Impairment, ARIC Visit 5 (2011-2013, n=5,021)
| Characteristics | No Olfactory Impairment Smell Score > 6 n=4447 | Olfactory Impairment Smell Score ≤ 6 n=574 |
|---|---|---|
| Age, mean (SD), years | 75.2 (5.0) | 76.9 (5.4) |
| Female sex, n (%) | 2680 (60.3) | 268 (46.7) |
| Black Race, n (%) | 843 (19.0) | 204 (35.5) |
| < High School Education, n (%) | 521 (11.7) | 108 (18.8) |
| Diabetes, n (%) | 1193 (27.3) | 178 (31.8) |
| Hypertension, n (%) | 3268 (74.0) | 429 (75.5) |
| Prevalent stroke, n (%) | 137 (3.1) | 29 (5.1) |
| Ever smoker, n (%) | 2581 (58.0) | 338 (58.9) |
| ApoE4 allele ≥ 1, n (%) | 1195 (26.9) | 194 (33.8) |
| Memory factor score, mean (SD) | -1.82 (1.00) | -2.34 (1.00) |
| Language factor score, mean (SD) | 0.07 (0.83) | -0.39 (0.89) |
| Executive functioning/processing speed factor score, mean (SD) | -0.26 (0.39) | -0.50 (0.39) |
| General cognitive function factor score, mean (SD) | -0.49 (0.80) | -1.04 (0.92) |
SD=standard deviation
3.1. Olfactory function and cognitive performance
Adjusted mean cognitive factor scores were higher across smell test scores in an almost linear fashion (P trend<0.05, Figure 1 and eFigures 1-3 in the Supplement). The adjusted mean difference in domain-specific cognitive factor scores between participants with vs. those without OI showed that participants with OI had statistically significantly lower scores across all cognitive domains [Table 2, memory: Beta= -0.27 (95% confidence interval [CI]: -0.35, -0.20); language: Beta= -0.24 (95% CI: (-0.30, -0.17); executive function/processing speed: Beta= -0.09 (95% CI: -0.12, -0.06); general cognition: Beta= -0.25 (95% CI: -0.31, -0.20). Neither race, sex nor APOE ε4 modified these associations. Results were robust to further adjustment for prevalent diabetes and hypertension.
Figure 1.
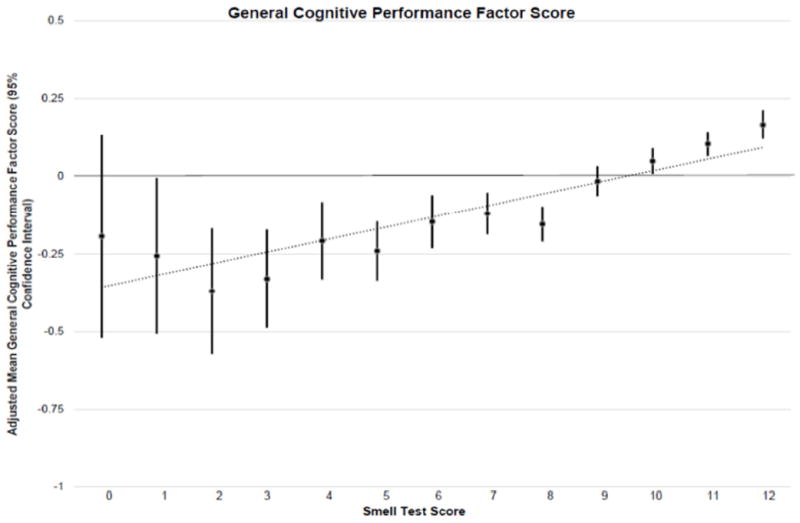
Table 2.
Adjusted mean standard deviation difference in domain-specific cognitive function comparing participants with vs. those without olfactory impairment, ARIC Visit 5 (2011-2013, n=5,021)
| Factor Domain | Standard Deviation Difference | 95% Confidence Interval | P |
|---|---|---|---|
| Memory | -0.27 | -0.35, -0.19 | <0.001 |
| Language | -0.24 | -0.30, -0.17 | <0.001 |
| Executive function/processing speed | -0.09 | -0.12, -0.06 | <0.001 |
| General cognitive function | -0.25 | -0.30, -0.20 | <0.001 |
Adjusted for age, sex, education, race-field center, APOE ε4, ever smoker status
3.2. Olfactory function and mild cognitive impairment
The prevalence of MCI in the analytic sample was 21.7% (n=1,092, eTable 2 in the Supplement). Its association with smell test scores was also nearly linear (P trend<0.05, eFigure 4 in the Supplement). OI was associated with an MCI Prevalence Ratio [PR] of 1.56 (95% CI: 1.37, 1.78) (Table 3). The PR for MCI due to AD (n=768) was 1.46 (95% CI: 1.23, 1.73). Race, sex and APOE ε4 did not modify the OI-MCI associations. Although results were slightly attenuated, the overall inferences were not affected by additional adjustment for diabetes and hypertension.
Table 3.
Adjusted prevalence of MCI comparing participants with vs. without olfactory impairment, ARIC Visit 5 (2011-2013, n=5,021)
| Olfactory Impairment n=574 | No Olfactory Impairment n=4447 | P | |
|---|---|---|---|
| # MCI cases | 204 | 888 | |
|
| |||
| Prevalence Ratio | 1.56 | Referent | <0.001 |
| 95% CI | 1.37, 1.78 | ||
|
| |||
| # MCI cases due to AD | 137 | 631 | |
|
| |||
| Prevalence Ratio | 1.46 | Referent | <0.001 |
| 95% CI | 1.23, 1.73 | ||
Adjusted for age, sex, education, race-field center, APOE ε4, ever smoker status
3.3. Olfactory function and domain-specific cognitive decline between ARIC visit 2 and visit 5
Compared with ARIC participants without OI at visit 5, those with OI had had faster 20-year rates of decline in memory (Beta=-0.40 (95% CI: -0.48, -0.32)), language (Beta=-0.28 (95% CI: -0.34, -0.22)), and global cognitive performance (Beta=-0.20 (95% CI: -0.25, -0.15)). No significant differences in 20-year declines in executive function/processing speed between visits 2 and 5 were observed (P >0.05, Table 4).
Table 4.
20-year change in domain-specific cognitive factor scores comparing participants with vs. without olfactory impairment (n=4,948)
| EXPOSURE GROUP / CONTRAST | Standard deviation | 95% Confidence Interval |
|---|---|---|
|
GENERAL COGNITIVE PERFORMANCE
| ||
| Olfactory impairment | -0.99 | -1.03, -0.94 |
| No Olfactory impairment | -0.79 | -0.80, -0.77 |
| Difference | -0.20 | -0.25, -0.15 |
|
| ||
|
MEMORY
| ||
| Olfactory impairment | -2.23 | -2.31, -2.15 |
| No Olfactory impairment | -1.83 | -1.86, -1.80 |
| Difference | -0.40 | -0.48, -0.32 |
|
| ||
|
LANGUAGE
| ||
| Olfactory impairment | -0.36 | -0.41, -0.30 |
| No Olfactory impairment | -0.08 | -0.10, -0.06 |
| Difference | -0.28 | -0.34, -0.22 |
|
| ||
|
EXECUTIVE FUNCTION/PROCESSING SPEED
| ||
| Olfactory impairment | -0.48 | -0.51, -0.46 |
| No Olfactory impairment | -0.46 | -0.47, -0.45 |
| Difference | -0.03 | -0.05, 0.003 |
Bolded estimates indicate P <0.05 for difference;
Adjusted for age, sex, education, race-field center, APOE ε4, ever smoker status
4. Discussion
Consistent with our a priori hypothesis, among individuals without dementia, poor olfactory function was associated with lower general cognitive performance and across specific cognitive domains, particularly memory and language, and was associated with a higher prevalence of MCI. We also observed poor olfactory function in association with greater prior 20-year cognitive decline. These results consistently support the notion that OI may be an important and readily accessible marker of cerebral neuropathologic changes.16,26 The average decline in global cognitive function is estimated at 0.04-0.05 standard deviation units per year in most older adults.22,27-29 Therefore, the 0.25 SD lower performance in general cognition observed among those with OI in our study is of a magnitude comparable to ~5 years of cognitive aging, relative to those without OI. The more notable deficit in memory than in executive function/processing speed is consistent with pathologic changes in the hippocampus and olfactory cortex, where neuropathologic changes identified as Braak 1 stage AD are first observed. Therefore, our findings may be more reflective of changes due to AD rather than vascular-pathology.
Also in support of prior studies, 16,30,31 we observed differences in the 20-year rates of cognitive decline among participants with compared to those without OI. Our results add to the knowledge base by overcoming several of the limitations of prior reports, including, small sample sizes (50-1500 participants) and homogenous study populations (primarily White and educated). The association we find of OI with prior cognitive decline provides new assurance because of its reduced potential for confounding by stable characteristics of individuals (e.g. education, other elements of cognitive reserve), which might otherwise greatly affect the cognitive scores.
Previous studies reported that OI was also associated with MCI in middle-aged and older community-dwelling participants.32 For example, participants enrolled in the Rush Memory and Aging Project who made four errors on the Brief Smell Identification Test (BSIT, range: 0-12) were 50% more likely to develop MCI as compared to those participants with only one error on the test.33 We observed a similar effect size estimate for ARIC participants who had 6 or more errors (range: 0-12) on the ‘Sniffin’ Sticks’ Test.
Our findings suggest that an impaired sense of smell may serve as a readily accessible early marker of neurodegeneration and therefore improve upon the prevailing delayed diagnoses and under-ascertainment of MCI and dementia. Almost all patients with dementia due to AD have olfactory dysfunction,34 and tests of olfaction have shown high sensitivity and specificity for distinguishing persons with dementia due to AD from controls.35 Individuals at high risk for AD (e.g., carriers of ≥ 1 APOE ε4 allele, those with familial history of dementia or MCI) have also been identified as performing worse on olfactory tests than controls.36-39 Furthermore, recent data suggests faster progression from MCI to dementia among those with poor olfactory function.12 Autopsy studies have shown that sense of smell is associated with plaques and tangles in the central olfactory region of the brain,8 that connects to the hippocampal region of the brain where neurofibrillary tangles caused by AD are first sited. Similar findings are evident amongst individuals without clinically manifest dementia due to AD or MCI and after accounting for behavioral and genetics confounding factors,33 suggesting that olfactory dysfunction may precede overt manifestations of MCI or dementia due to AD.
Some limitations of our study should be noted. First, detailed information related to participants’ pertinent olfaction history (i.e. nasal diseases, head trauma) was not available. However, participants were asked to report on reasons that their sense of smell may have decreased. The most commonly reported reasons were smoking, sinus problems and allergies. Due to study size, we lack power to examine the associations with cognitive function within these subgroups. The cross-sectional nature of our study limits the interpretation of our findings and introduces the potential for reverse causality; however, we excluded those participants with dementia since it might affect odor identification.
Several strengths should be mentioned. First, the well-characterized ARIC-NCS cohort provides over 20 years of collected data, allowing us to examine the rate of cognitive change over 3 domains. The longitudinal analysis results provide useful corroboration for those observed cross-sectionally by reducing the potential effects of unmeasured confounders. Second, the in-person assessments, brain imaging at visit 5, and expert review over a clinically derived algorithm enabled good definition of MCI and its etiology.18 An additional strength of our study is in the use of calibrated general and domain-specific cognitive factor scores, validated as previously reported22 by comparing the associations of diabetes with cognitive outcomes derived as factor scores versus averaged standardized tests. The associations were stronger when using factor scores in comparison to averages of standardized tests, which were attributed to the reduced level of measurement error in measuring cognitive traits as factor scores when derived from latent variable methods in comparison to the traditional methods of averaging standardized cognitive test scores.
Discriminating the causes of early cognitive declines has depended on costly or invasive testing such as cerebrospinal fluid assays and PET scans. The ‘Sniffin’ Sticks’ test is easily administered, noninvasive, inexpensive, and has a conventional threshold for impaired olfaction to enable interpretable results. The results reported suggest it may help discriminate the causes of early impairments either alone or together with the more costly or invasive methods now in use.
Supplementary Material
Research in Context.
Systematic Review
We reviewed the relevant literature using PubMed. Although recent data suggests olfactory impairment (OI) is associated with reduced cognitive performance and incident MCI, additional population-based studies are needed both for confirmation and to examine cognitive decline and individual cognitive domains. Furthermore, an examination of differences by race and sex are needed.
Interpretation
OI was associated with lower general cognitive performance and across specific cognitive domains, particularly memory and language, and was associated with a higher prevalence of MCI. We also observed poor olfactory function in late-life to be associated with greater prior 20-year cognitive decline. Neither race nor sex modified these associations.
Future Directions
These results consistently support the notion that OI may be an important and readily accessible marker of cerebral neuropathologic changes. Therefore, olfactory function may help discriminate the causes of early impairments either alone or together with the more costly/invasive methods now in use.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data was collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825. Additional support provided to HC by the Intramural Research Program of the NIH/NIEHS Z01ES101986. Dr. Palta was supported by grant K99-AG-052830 from the National Institute of Aging. Dr. Gross was supported by K01-AG050699 from the National Institute on Aging. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute of Aging; the National Institutes of Health; or the U.S. Department of Health and Human Services.3
Footnotes
Disclosures
The authors declare no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Jama. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 2.Aschenbrenner K, Hummel C, Teszmer K, et al. The influence of olfactory loss on dietary behaviors. The Laryngoscope. 2008;118(1):135–144. doi: 10.1097/MLG.0b013e318155a4b9. [DOI] [PubMed] [Google Scholar]
- 3.Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Archives of otolaryngology--head & neck surgery. 2004;130(3):317–319. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]
- 4.Devanand DP, Lee S, Manly J, et al. Olfactory identification deficits and increased mortality in the community. Annals of neurology. 2015;78(3):401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PloS one. 2014;9(10):e107541. doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cain WS, Gent JF. Olfactory sensitivity: reliability, generality, and association with aging. Journal of experimental psychology Human perception and performance. 1991;17(2):382–391. doi: 10.1037//0096-1523.17.2.382. [DOI] [PubMed] [Google Scholar]
- 7.Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Current neurology and neuroscience reports. 2006;6(5):379–386. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. Journal of neurology, neurosurgery, and psychiatry. 2007;78(1):30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.England M, Wakely J. Color Atlas of the Brain and Spinal Cord. 2. Philadelphia, PA: Mosby Elsevier; 2006. [Google Scholar]
- 11.Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport. 2001;12(2):285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RO, Christianson TJ, Kremers WK, et al. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA neurology. 2016;73(1):93–101. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(3):323–329. doi: 10.1093/gerona/glt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chemical senses. 2012;37(4):325–334. doi: 10.1093/chemse/bjr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2017;88(5):456–462. doi: 10.1212/WNL.0000000000003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015;84(2):182–189. doi: 10.1212/WNL.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 18.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimer’s & dementia. 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chemical senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 20.Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. The Annals of otology, rhinology, and laryngology. 2001;110(10):976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- 21.Dong J, Pinto JM, Guo X, et al. The Prevalence of Anosmia and Associated Factors Among U.S Black and White Older Adults . The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(8):1080–1086. doi: 10.1093/gerona/glx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross AL, Power MC, Albert MS, et al. Application of Latent Variable Methods to the Study of Cognitive Decline When Tests Change over Time. Epidemiology. 2015;26(6):878–887. doi: 10.1097/EDE.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Association AP. DSM-5: Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 26.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Odor identification and cognitive function in the Beaver Dam Offspring Study. Journal of clinical and experimental neuropsychology. 2013;35(7):669–676. doi: 10.1080/13803395.2013.809701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salthouse T. Major Issues in Cognitive Aging. New York: Oxford University Press; 2010. [Google Scholar]
- 28.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age and ageing. 2011;40(6):684–689. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: the freedom house study. Journal of the International Neuropsychological Society : JINS. 2005;11(7):899–909. doi: 10.1017/s135561770505109x. [DOI] [PubMed] [Google Scholar]
- 30.Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21(2):58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26(2):61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Archives of general psychiatry. 2007;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Annals of the New York Academy of Sciences. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou YM, Lu D, Liu LP, Zhang HH, Zhou YY. Olfactory dysfunction in Alzheimer’s disease. Neuropsychiatric disease and treatment. 2016;12:869–875. doi: 10.2147/NDT.S104886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan CD, Murphy C. Olfactory event-related potentials in Alzheimer’s disease. Journal of the International Neuropsychological Society : JINS. 2002;8(6):753–763. doi: 10.1017/s1355617702860039. [DOI] [PubMed] [Google Scholar]
- 36.Devanand DP, Michaels-Marston KS, Liu X, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease follow-up. The American journal of psychiatry. 2000;157(9):1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- 37.Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Annals of the New York Academy of Sciences. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman SS, Graham BG, Sattely-Miller EA, Zervakis J, Welsh-Bohmer K. Taste, smell and neuropsychological performance of individuals familial risk for Alzheimer’s disease. Neurobiology of aging. 2002;23(3):397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 39.Eibenstein A, Fioretti AB, Simaskou MN, et al. Olfactory screening test in mild cognitive impairment. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2005;26(3):156–160. doi: 10.1007/s10072-005-0453-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


