Abstract
Objective
To measure the effects of iron supplementation and anthelmintic treatment on iron status, anaemia, growth, morbidity, and development of children aged 6-59 months.
Design
Double blind, placebo controlled randomised factorial trial of iron supplementation and anthelmintic treatment.
Setting
Community in Pemba Island, Zanzibar.
Participants
614 preschool children aged 6-59 months.
Main outcome measures
Development of language and motor skills assessed by parental interview before and after treatment in age appropriate subgroups.
Results
Before intervention, anaemia was prevalent and severe, and geohelminth infections were prevalent and light—Plasmodium falciparum infection was nearly universal. Iron supplementation significantly improved iron status, but not haemoglobin status. Iron supplementation improved language development by 0.8 (95% confidence interval 0.2 to 1.4) points on the 20 point scale. Iron supplementation also improved motor development, but this effect was modified by baseline haemoglobin concentrations (P=0.015 for interaction term) and was apparent only in children with baseline haemoglobin concentrations <90 g/l. In children with a baseline haemoglobin concentration of 68 g/l (one standard deviation below the mean value), iron treatment increased scores by 1.1 (0.1 to 2.1) points on the 18 point motor scale. Mebendazole significantly reduced the number and severity of infections caused by Ascaris lumbricoides and Trichuris trichiura, but not by hookworms. Mebendazole increased development scores by 0.4 (−0.3 to 1.1) points on the motor scale and 0.3 (−0.3 to 0.9) points on the language scale.
Conclusions
Iron supplementation improved motor and language development of preschool children in rural Africa. The effects of iron on motor development were limited to children with more severe anaemia (baseline haemoglobin concentration <90 g/l). Mebendazole had a positive effect on motor and language development, but this was not statistically significant.
What is already known on this topic
Iron is needed for development and functioning of the human brain
Anaemic children show developmental delays, but it is not yet clear whether iron deficiency causes these deficits or whether iron supplementation can reverse them
Helminth infections in schoolchildren are associated with cognitive deficits, but few studies have been made of helminth infection and early child development
What this study adds
Low doses of oral iron supplementation given daily improved language development in children aged 1-4 years in Zanzibar
Iron supplementation improved motor development, but only in children with initial haemoglobin concentrations below 90 g/l
The effects of routine anthelmintic treatment on motor and language milestones were positive, but non-significant, with our sample size
Introduction
Iron deficiency anaemia is associated with comparatively poor performance in tests of mental and motor development in infants and toddlers and of intelligence and cognitive function in preschool and school children.1–4 In young children aged 12-18 months, one randomised trial found that development improved in children treated for iron deficiency anaemia5; however, most quasi-experimental studies in children of a similar age have shown no such benefit.6 Prospective trials have also produced discrepant findings.7,8
A causal link between iron deficiency anaemia and delays in child development may be mediated by a variety of direct or indirect pathways; the most obvious are associated decreases in haemoglobin concentration and oxygen delivery to tissues. Alternative theories relate to reductions in cerebral iron concentrations, including hypomyelination and impaired dopaminergic function.9–11
Kvalsvig et al found that geohelminth infections impair children's cognition and learning,12 but the focus of their work was in schoolchildren. Associations between geohelminth infection and mental performance in schoolchildren have been reported,12–16 but results from randomised trials have been inconclusive.17 We are not aware of published investigations of the relation between geohelminth infections and development in preschool children.
We report measures of child development from a subsample of children of an appropriate age enrolled in a double blind placebo controlled randomised factorial trial of iron supplementation and anthelmintic treatment. The trial was designed to measure the effects of iron supplementation and anthelmintic treatment on iron status, anaemia, growth, morbidity, and development of children aged 6-59 months at the start of the trial. In studies of the relation between iron concentrations and child development, our trial is unique because it included a considerable number of children with moderate to severe anaemia, and it was carried out in a population exposed to numerous health risks, including year round of Plasmodium falciparum malaria and geohelminths, and widespread malnutrition.
Participants and methods
Location
The study was conducted in Kengeja village on the island of Pemba north of Zanzibar. The environment is rural, with fishing and farming as the main occupations. P falciparum is holoendemic and transmitted throughout the year, and P malariae is also present. A number of helminths are highly endemic in this population, including two hookworm species (Ancylostoma duodenale and Necator americanus), Ascaris lumbricoides, Trichuris trichiura, and Schistoma haematobium.
Study sample and randomisation
Before the study, we estimated the sample size that was needed to be recruited to show a 5 g/l difference in mean haemoglobin response in two age subgroups, with α=0.05 and β=0.10 as 640 children. Prior power calculations were not made for developmental outcomes, as the rating scales were developed specifically to be filled out by parents in this study.
During June and July 1996, a census was conducted in Kengeja and a database of all children whose age was reported by their parents as 3-56 months was created (the trial was planned to begin three months later). The database contained 684 children from 451 households, all of whose parents gave informed consent to participate in the census and the trial; these parents were invited to enter their children in the trial.
Households, rather than children, were randomly allocated to receive iron or placebo, so that mothers of siblings would have to manage only one bottle of supplement. Households were grouped into three strata—those with children <36 months, those with children ⩾36 months, and those with one or more child in each age subgroups. Within these strata, households were randomly allocated to receive iron or placebo in blocks of four. Random allocation to mebendazole or mebendazole placebo was carried out by child, stratified by iron allocation and household, in blocks of four.
Of the 684 children identified in the census and randomised to treatment, 614 attended the baseline clinic at the local primary healthcare centre during September 1996. In total, 538 children completed the follow up period of 12 months, including the final clinical assessment during September 1997.
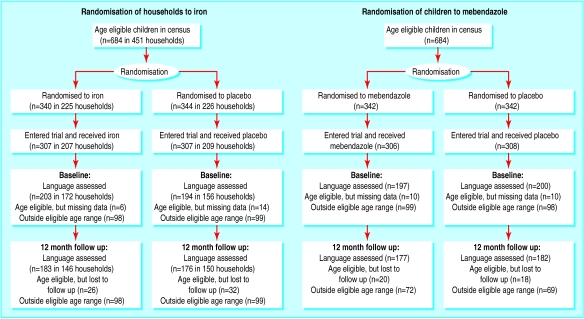
Developmental data were collected in August 1996 and August 1997, one month before each clinical assessment. The language development scale was appropriate for children from 12 to 48 months, and 417 children between these ages entered the trial. Of these, 397 children were assessed in August 1996, and 359 children—the language scale cohort—were assessed again in August 1997. The motor development scale was appropriate only for children between 12 and 36 months, and 293 children between these ages entered the trial. Of these, 267 were assessed in August 1996, and 255 children—the motor scale cohort—were assessed again in August 1997. The motor scale cohort is a younger subset of the language scale cohort. The randomisation and retention of children in the trial is shown in figure 1 .
Figure 1.
Randomisation of preschool children in Zanzibar to iron and mebendazole or placebo, and retention in trial
The 58 children eligible by age for inclusion in the language scale cohort who were not followed up were similar to the 359 who completed the study in sex, age, and other characteristics. The children who were not followed up had slightly better baseline haemoglobin values than those who were (88.6 (SD 14.7) g/l v 85.6 (14.7) g/l, P=0.15).
Interventions
Iron treatment
Iron treatment consisted of a ginger flavoured liquid supplement containing 20 mg/ml ferrous sulphate, or an identical placebo (both supplied by Alpharma USPD, Baltimore). The supplement was packaged in 50 ml bottles, with childproof 1 ml dropper caps. Each bottle label included one of six batch numbers, three of which corresponded to iron and three to placebo; these treatment codes were assigned by Alpharma USPD and were kept in sealed envelopes in Baltimore and Zanzibar. At the baseline clinic, each mother was trained on how to give a 0.5 ml dose (equivalent to 10 mg iron) to her children, and she was instructed to give this dose daily for the next year. During the 12 month trial, study staff visited each mother weekly to ask how many days in the past week she had given the supplement to her child and to deal with any compliance problems, using a problem solving algorithm. The study staff member also replenished the supplement as needed. The potency of the supplement was monitored by the manufacturer, and it was found to retain 80% of its potency after being stored for 12 months at room temperature.
Despite the childproof packaging, two children each consumed a large portion of a bottle of iron supplement. These children were monitored closely for toxic sequelae (none of which were found), and they were included in the analyses. After these events, the amount of iron supplement in each bottle was reduced to 25 ml so that the total amount of elemental iron in each bottle was reduced from 1000 mg to 500 mg.
Anthelmintic treatment
Anthelmintic treatment consisted of 500 mg mebendazole in an orange flavoured chewable tablet, or an identical placebo tablet (Pharmamed, Zejtun). The pills were packaged in six bottles with unique treatment codes, three corresponding to mebendazole and three to placebo. The treatment codes were assigned by one study investigator, and they were kept in sealed envelopes in Baltimore and Zanzibar. After the baseline clinic, study staff made home visits to all children every three months to give the anthelmintic treatment.
Oral iron
Children with severe anaemia (haemoglobin concentrations <70 g/l) at the baseline clinic were treated with oral iron 60 mg/day for 30 days, but they were also given their randomly allocated iron. The total iron dosage for these severely anaemic children for the first month of the study, therefore, was 60 mg/day or 70 mg/day. These children were also given mebendazole (500 mg) in place of their randomly allocated anthelmintic treatment, but they subsequently received their randomly allocated treatment at the baseline clinic. Parents of these children were informed that their child was severely anaemic and the treatments given were explained. These children were included in the subsequent analyses on an intention to treat basis.
Clinical assessments
Parents were given a container in which to take a small sample of their child's faeces to the baseline clinic. Faecal samples were stained on the same day and examined within one hour by the Kato-Katz method.18 The numbers of helminth eggs in faecal samples were counted for 591/614 (96%) of study children.
The children's weights were measured to the nearest 0.1 kg using a digital scale (Seca, Columbia, NY). Length was measured in triplicate to the nearest 0.1 cm using a wooden length board (Shorr Productions, Olney). Age was ascertained from the birth certificate or, in one case where the birth certificate was missing, by the parent's report. Anthropometric z scores were computed with Anthro version 3.0 (CDC, Atlanta). Stunting was defined as height for age z score <−2.0, wasting as weight for height z score <−2.0, and underweight as weight for age z score <−2.0. Mothers were asked to recall all foods and drinks, including breast milk, given to the child in the previous 24 hours.
Blood samples (3 ml) were collected by venipuncture into a Vacutainer tube (BD, Franklin Lakes). Drops of whole blood were dispensed immediately to make a blood film that was used to determine haemoglobin concentration (HemoCue haemoglobinometer; HemoCue, Angelhom) and erythrocyte protoporphyrin concentration (haematofluorometer; Aviv Biomedical, Lakewood, NJ). The remaining blood was centrifuged at 1000 × g for 20 minutes at room temperature and the serum was collected. Thin blood films were fixed with ethanol and stained with Giemsa, and the numbers of malaria parasites were counted against leukocytes using standard methods.19 Serum samples were stored in Pemba at −10°C for up to three months, and they were then transported in liquid nitrogen to Baltimore, where they were stored at −70°C until they were analysed. Ferritin was assayed using a fluorescence-linked immunoassay (DELFIA system; Wallac, Gaithersburg). The average coefficient of variation for this assay was 3% (range 0.2%-7.0%).
Developmental assessments
Motor and language development were assessed by the parents reporting gross motor and language milestones—a method known to have considerable accuracy and sensitivity for identifying developmental delays.20–23 Mothers were asked if their child could do each of the tasks listed in table 1 ; one point was scored for each item that the mother reported the child could do. When the scales were being constructed, a wide ranging collection of items was taken from a variety of sources, including the Griffiths and McCarthy scales of motor and mental development.24,25 To ensure reliability of the scales, the number of items was reduced through a series of piloting procedures, initially in Zulu in a rural African community (Umbumbulu, KwaZulu-Natal, South Africa) and then in Kiswahili in a peri-urban community in Pemba (Mkoroshoni).
Table 1.
Mean item scores on language and motor scales for preschool children in Zanzibar
| Scale | Mean |
|---|---|
| Language* | |
| The child can say one word | 0.9176 |
| When the child's holding something and I ask them to give it to me they do | 0.8764 |
| The child points and makes some sounds when they want something | 0.8379 |
| The child can say three words | 0.7843 |
| If I ask the child, they can point to a dog | 0.7775 |
| If I ask the child, they can point to a person that is walking | 0.6484 |
| The child can say six words | 0.6429 |
| The child uses the words I, me, and you | 0.6264 |
| The child is constantly asking the names of objects | 0.5467 |
| The child asks a lot of questions beginning “What,” “Where,” and “Who” | 0.5220 |
| The child can say many words (20 or more) | 0.4918 |
| The child uses plurals when they speak | 0.4657 |
| The child can tell people their full name | 0.4437 |
| The child can tell me what a knife is for | 0.4368 |
| The child asks the meanings of words | 0.3544 |
| The child can talk about things that happened in the past | 0.3516 |
| The child knows a few rhymes‡ | 0.3063 |
| The child can tell me the opposite of the word “big” | 0.1360 |
| Motor development† | |
| The child can crawl | 0.9835 |
| The child can walk when both hands are held | 0.9325 |
| The child can walk with only one hand held | 0.9008 |
| The child can stand for a moment on their own | 0.8452 |
| The child can stand on their own for a long time | 0.8056 |
| The child walks and then collapses down | 0.7460 |
| The child can run | 0.7063 |
| The child can bend down and straighten up again without falling | 0.6984 |
| The child can walk up steps | 0.6905 |
| The child can throw a ball overhand | 0.6865 |
| The child can walk up and down steps | 0.6845 |
| The child can kick a ball forward | 0.6310 |
| The child can walk forward along a straight line (10 paces)‡ | 0.5635 |
| The child jumps with both feet | 0.5020 |
| The child can stand on one foot for several seconds‡ | 0.4444 |
| The child can walk backward along a straight line (10 paces)‡ | 0.4187 |
| The child can walk on tiptoe | 0.3968 |
| The child can sit up without using their hands‡ | 0.2639 |
| The child can skip using alternate legs‡ | 0.2321 |
| The child can hop 20 times on one leg‡ | 0.1885 |
Number of items=18, Kohnbach's α=0.9482, age 12-48 months.
Number of items=20, Kohnbach's α=0.9492, age 12-36 months.
Action was demonstrated to observer.
The data collection team consisted of Pemban men and women whose first language was Kiswahili. To forestall any tendency by parents to over-rate or underrate their child's ability, care was taken to secure parental cooperation in providing accurate responses and the child was asked to demonstrate the ability to perform some of the items. The procedures used to check the motor items were similar to those used in the McCarthy scales.25
A preliminary analysis established that the scales were appropriate to the age range and were sensitive to changes over time within the study environment. At the 12 month retest, the mean motor score for the children in the group who were now aged 36-48 months was nearing asymptote, and children older than 48 months at retest were excluded from further analysis on this measure. The language score neared asymptote at a later stage, and children who were 60 months and over at retest were excluded from further language development analysis. The scales were tested for internal consistency using Cronbach's α (table 1).26
Analysis of treatment effects
We compared the characteristics of children in the motor and language cohorts by treatment group (table 2). Several characteristics (sex, breast feeding, stunting and developmental scores), were unbalanced between the groups.
Table 2.
Characteristics of children in Zanzibar aged 12-48 months at baseline with complete developmental assessments at baseline and after treatment. Values are numbers (percentages) unless otherwise specified
| Iron
|
Mebendazole
|
||||
|---|---|---|---|---|---|
| Placebo | Treatment | Placebo | Treatment | ||
| General characteristics: | |||||
| Female sex | 85/176 (48) | 82/183 (45) | 86/182 (47) | 81/177 (46) | |
| Age (mean (SD) months) | 29.4 (11.1) | 29.6 (10) | 29.4 (10.8) | 29.7 (11) | |
| Breast fed in the past 24 hours | 56/176 (32) | 45/183 (25) | 53/182 (29) | 48/177 (27) | |
| Stunted | 58/171 (34) | 76/179 (43) | 66/179 (37) | 68/171 (40) | |
| Wasted | 10/171 (6) | 9/180 (5) | 10/179 (6) | 9/172 (5) | |
| Underweight | 58/171 (34) | 63/180 (35) | 64/179 (36) | 57/172 (33) | |
| Malaria positive blood film | 149/176 (85) | 153/183 (84) | 154/182 (85) | 148/177 (84) | |
| Anaemia and iron deficiency: | |||||
| Mean (SD) haemoglobin (g/l) | 86 (15) | 86 (15) | 86 (15) | 85 (15) | |
| Mean erythrocyte protoporphyrin (μmol/mol)* | 153 | 162 | 155 | 160 | |
| Mean serum ferritin (μg/l) | 29.2 | 32.5 | 30.9 | 30.8 | |
| Helminth infections: | |||||
| Hookworm | 74/170 (44) | 88/181 (49) | 86/180 (48) | 76/171 (44) | |
| Trichuris trichiura | 115/170 (68) | 125/181 (69) | 125/180 (69) | 115/171 (67) | |
| Ascaris lumbricoides | 73/170 (43) | 74/181 (41) | 79/180 (44) | 68/171 (40) | |
| Developmental scores: | |||||
| Language scale (mean (SD) score) | 9.6 (5.8) | 10.4 (5.8) | 10.0 (5.8) | 10.0 (5.8) | |
| Motor scale (mean (SD) score)† | 11.5 (6.2) (n=123) | 12.0 (6.2) (n=132) | 11.7 (6.3) (n=128) | 11.8 (6.0) (n=127) | |
Differences in denominators result from missing data on anthropometry and helminth egg counts.
Geometric mean.
Children 12-36 months old at baseline.
Other general characteristics, baseline iron status, baseline developmental score, and baseline helminth infections were tested for significance using P<0.10 as the criterion for inclusion in the comparison—only age, sex, and baseline developmental score met this criterion. When covariates were included, the results changed only slightly; however, we present the adjusted results because they provide the most accurate estimates of treatment effects. An iron with mebendazole (iron-mebendazole) treatment interaction term was tested in all models, but it did not approach statistical significance in any model. To look at variables that might modify the effects of treatment on developmental outcomes, we tested the interaction term of treatment (iron or mebendazole) with each variable. Finally, we repeated the final models for motor and language scores, excluding those children who were severely anaemic (and treated therapeutically) at baseline, to determine whether inclusion of these children attenuated the randomised treatment effects. In final regressions, we used generalised estimation equations to account for the intrahousehold clustering induced by randomising the iron treatment by household.27,28 Statistical analyses were conducted in SYSTAT 7.0 for Windows (SPSS, Chicago).
The study was approved by the ethical review boards of Johns Hopkins University School of Hygiene and Public Health, the World Health Organization, and the Ministry of Health of Zanzibar.
Results
The characteristics of children in the language scale cohort are shown in table 2. In total, 101/358 (28%) of participants aged 12-48 months were breast fed at the time of the study. Breast feeding was common in children aged 12-23 months (96/121, 79%) and rare in children ⩾2 years (5/237, 2%). Stunted growth was common, while wasting was relatively uncommon. P falciparum malarial infection was nearly universal. Most characteristics were similar among the treatment groups, but more children who received iron than those who received the iron placebo had stunted growth at baseline (76/179, 43% v 58/171, 34%).
At baseline, anaemia was prevalent and severe. In total, 347 of 359 (97%) children were anaemic by international standards (haemoglobin <110 g/l) and 54/305 (18%) were severely anaemic (haemoglobin <70 g/l). Haemoglobin concentration was strongly and positively associated with age at baseline.29 An overall increase in haemoglobin was seen in all treatment groups; this was mostly attributable to the increased age of the children at follow up. Erythrocyte protoporphyrin concentrations were high, and they were strongly associated with haemoglobin concentrations,29 suggesting that iron deficiency was prevalent. Serum ferritin values were higher than expected given the prevalence of anaemia and the raised erythrocyte protoporphyrin concentrations; this probably reflects the prevalence of malaria and other subclinical infections.29 Iron supplementation significantly increased serum ferritin and erythrocyte protoporphyrin concentrations, but it had little impact on haemoglobin concentrations (table 3). The effect of iron treatment on haemoglobin concentration was greater (but still not statistically significant) in children who were more anaemic at the start: 0.2 (−4.8 to 5.2) g/l in children with baseline haemoglobin concentrations ⩾80 g/l compared with 5.4 (−2.7 to 13.1) g/l in those with initial haemoglobin <80 g/l. Mebendazole had no impact on indicators of iron status, except to decrease the average concentrations of serum ferritin (perhaps by reducing the gut inflammatory response secondary to helminth infection).
Table 3.
Anaemia and iron status before and after treatment in preschool children. Values are numbers (percentages) unless otherwise specified
| Iron
|
Mebendazole
|
||||
|---|---|---|---|---|---|
| Placebo (n=176) | Treatment (n=183) | Placebo (n=182) | Treatment (n=177) | ||
| Mean (SD) haemoglobin (g/l): | |||||
| Baseline | 86 (15) | 86 (15) | 86 (15) | 85 (15) | |
| 12 months | 97 (16) | 99 (15) | 99 (15) | 98 (16) | |
| Haemoglobin <110 g/l: | |||||
| Baseline | 169 (96) | 178 (97) | 174 (96) | 173 (98) | |
| 12 months | 142 (81) | 144 (79) | 147 (81) | 139 (79) | |
| Haemoglobin <70 g/l: | |||||
| Baseline | 26 (15) | 28 (15) | 21 (12) | 33 (19) | |
| 12 months | 8 (5) | 5 (3) | 6 (3) | 7 (4) | |
| Mean erythrocyte protoporphyrin (μmol/mol): | |||||
| Baseline* | 153 | 162 | 155 | 160 | |
| 12 months | 86 | 72† | 76 | 80 | |
| Mean serum ferritin (μg/l): | |||||
| Baseline* | 29.2 | 32.5 | 30.9 | 30.8 | |
| 12 months | 45.8 | 60.8‡ | 58.1 | 47.9§ | |
Geometric mean.
Iron v placebo, P=0.032.
Iron v placebo, P=0.002.
Mebendazole v placebo, P=0.037.
Helminth infections were prevalent (table 2), but of light intensity (number of eggs/g faeces; table 4). Regular treatment with mebendazole was highly efficient in reducing the prevalence and intensity of A lumbricoides infection (table 4). It was less efficacious against T trichiura, although the intensity of this infection was still greatly reduced in the participants who received mebendazole. The effect of mebendazole on hookworm prevalence and intensity three months after treatment was not statistically significant.
Table 4.
Helminth infections before and after treatment in preschool children. Values are numbers (percentages) unless otherwise specified
| Infection | Iron
|
Mebendazole
|
|||
|---|---|---|---|---|---|
| Placebo | Treatment | Placebo | Treatment | ||
| Children* | |||||
| Baseline | 170 | 181 | 180 | 177 | |
| 12 months | 159 | 168 | 166 | 161 | |
| Tested positive for hookworms | |||||
| Baseline | 74 (44) | 88 (49) | 86 (48) | 76 (44) | |
| 12 months | 103 (65) | 103 (61) | 109 (66) | 97 (60) | |
| Hookworms (eggs/g faeces)† | |||||
| Baseline | 11.4 | 16.4 | 14.7 | 12.8 | |
| 12 months | 181.6 | 125.1 | 187.9 | 118.9 | |
| Tested positive for Trichuris trichiura | |||||
| Baseline | 115 (68) | 125 (69) | 125 (69) | 115 (67.3) | |
| 12 months | 109 (69) | 108 (64) | 124 (75) | 93 (57.8)‡ | |
| T trichiura (eggs/g faeces)† | |||||
| Baseline | 53.2 | 60.9 | 52.4 | 61.8 | |
| 12 months | 260.9 | 179.3 | 511.3 | 88.1‡ | |
| Tested positive for Ascaris lumbricoides | |||||
| Baseline | 73 (43) | 74 (41) | 79 (44) | 68 (39.8) | |
| 12 months | 62 (39) | 62 (37) | 82 (49.4) | 42 (27.1)‡ | |
| A lumbricoides (eggs/g faeces)† | |||||
| Baseline | 29.3 | 23.5 | 29.5 | 23.1 | |
| 12 months | 47.6 | 31.8 | 126.3 | 11.8† | |
Differences between numbers at baseline and 12 months due to missing faecal samples.
Geometric mean of all children; values of 0 set to 1 before taking logarithm.
Mebendazole treatment effect, P<0.001.
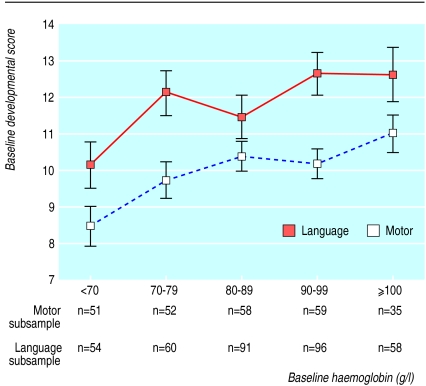
Baseline scores on the motor and language scales were strongly associated with age (motor r=0.691, P<0.001; language r=0.741, P<0.001) and with each other. After age was controlled for, the partial correlation of baseline motor score with language score was 0.563 (P<0.001). There was also a strong within-child correlation of baseline score to the score at the end of the trial (motor r=0.578, P<0.001; language r=0.642, P<0.001). After age was adjusted for, haemoglobin was positively associated with motor scores (partial correlation=0.175, P=0.005) and language scores (partial correlation=0.158, P=0.003) (fig 2).
Figure 2.
Relation of pretreatment language and motor scores to haemoglobin concentration. Points are age adjusted least squares means with standard errors
When adjusted for age, the association of erythrocyte protoporphyrin concentration with motor score was weaker than that of haemoglobin concentration (partial correlation=−0.113, P=0.071), and it became non-significant when haemoglobin concentration was also included in the model. In contrast, when we adjusted for age, the association of protoporphyrin concentration with language score was nearly as strong as that of haemoglobin concentration (partial correlation=−0.134, P=0.01), and it remained marginally significant (P=0.065) when haemoglobin concentration was also included in the model. Each heme increment in protoporphyrin of 100 μmol/mol was associated with a decrease in language score of 0.4 (95% confidence interval 0 to 0.8) points. Serum ferritin concentration was not associated with motor or language scores. After we adjusted for age, the presence of helminth infections at baseline (prevalence of each worm, intensity of each worm, or the number of worm species present) was not associated with baseline motor or language scores.
The adjusted main effects of iron and mebendazole treatments on motor scores were positive, but they were not statistically significant. However, there was a significant interaction between baseline haemoglobin concentration and iron treatment (P=0.015). Iron treatment was associated with higher post-treatment motor scores only in children with low baseline haemoglobin. There was a benefit from iron treatment in children with baseline haemoglobin concentrations <90 g/l, and this was statistically significant at concentrations <80 g/l. In children with baseline haemoglobin of 68 g/l (one standard deviation below the mean value), the iron treatment effect was 1.1 (0.1 to 2.1) points on the 18 point motor scale. The adjusted effect of mebendazole treatment on motor scores in this final model was 0.44 (−0.22 to 1.10); this was not significantly modified by the concentration of haemoglobin at baseline or by iron treatment.
Because baseline haemoglobin was strongly related to age and erythrocyte protoporphyrin, we compared multivariate models that included iron with haemoglobin (iron-haemoglobin), iron-protoporphyrin, and iron-age interaction terms. Baseline protoporphyrin did not modify the iron treatment effect. The iron-age interaction term was statistically significant if the iron-haemoglobin interaction was not included in the model; younger children benefited most from iron supplementation. The iron-haemoglobin interaction was statistically stronger and produced a higher model R2 value than the iron-age interaction.
The adjusted main effects of iron and mebendazole treatments on language scores were positive, and the effect of iron treatment was significant (P=0.011) (table 5). Although children who received iron and mebendazole treatments had the highest final language scores, the iron-mebendazole interaction term did not approach significance (P=0.48). There was no significant interaction between either treatment and any characteristics of the children included in table 2. The effect of iron treatment was similar for all children within the wide range of haemoglobin concentrations and other measured characteristics in the study sample.
Table 5.
Language scores in four treatment groups with differences in scores showing effects of treatment
| Treatment | Final language score* | Difference in score† |
|---|---|---|
| Placebo iron and placebo mebendazole (n=85) | 13.1 (12.7 to 13.9) | |
| Mebendazole (n=85) | 13.5 (12.6 to 14.4) | |
| Iron (n=94) | 14.0 (13.2 to 14.8) | |
| Mebendazole and iron (n=85) | 14.2 (13.4 to 15.0) | |
| Effect of iron v no iron | 0.8 (0.2 to 1.4) | |
| Effect of mebendazole v no mebendazole | 0.3 (−0.3 to 0.9) |
Adjusted for age, haemoglobin, sex, baseline language score, weight for height z score, height for age z score, and presence of ⩾5000 malaria parasites/μl blood.
From multivariate model described above using general estimating equations.
We repeated the final multivariate regression models for motor and language scores, excluding those children with baseline haemoglobin concentrations <70 g/l who were treated therapeutically for the first month of the trial. In the motor cohort (204 children without severe anaemia), the iron-haemoglobin interaction term was marginally significant (P=0.067), although its magnitude was similar to that of the full cohort. The weakening in statistical significance can be attributed to the smaller sample size and the fact that we censored the cohort in the range of haemoglobin concentrations where the iron treatment effect was greatest.
In the language cohort (298 children without severe anaemia), exclusion of severely anaemic children resulted in an effect of iron treatment that was larger and more statistically significant despite the smaller sample size (0.9 (0.2 to 1.5) scale units). This is consistent with the lack of haemoglobin-iron interaction in this model and suggests that including children who were therapeutically treated with iron at the start of the trial in the placebo group diluted our measurement of the effect of iron supplementation at low doses on a long term basis.
Discussion
Several distinct features characterise this study of the effects of iron and anthelmintic treatments on child development. Anaemia in the study children was prevalent and more severe than that reported in any published study with similar objectives of which we are aware. P falciparum malaria was endemic and contributed considerably to the degree of anaemia observed.29 The randomised, placebo controlled, community based intervention design provides a strong basis for causal inference.
Effects of iron and anaemia on motor and language development
Our results shed partial light on the contributions of anaemia and iron deficiency as causes of developmental delays. At baseline, motor scores were more strongly related to haemoglobin concentrations than to erythrocyte protoporphyrin concentrations (an indicator quite specific to iron deficiency in this population).29 Iron supplementation improved motor development only in children with very low baseline haemoglobin concentrations. A similar plateau in the association of haemoglobin with motor development was recently found in children in the United Kingdom.30 It is possible that the aetiology of anaemia changes with its severity, with more severe anaemia being more strongly related to iron deficiency. There is some evidence for this in our data, as the effect of iron treatment was lower in children with baseline haemoglobin concentrations ⩾80 g/l than <80 g/l, suggesting that the effect of iron on motor development was mediated through improved haemoglobin concentrations. The younger age of children with more severe anaemia may have been an important determinant of their increased response to iron.
In contrast, language scores at baseline were strongly related to haemoglobin and erythrocyte protoporphyrin concentrations. Iron supplementation improved language development across a wide range of baseline haemoglobin concentrations, despite the small effect of iron treatment on haemoglobin in all but the most severely anaemic children. This suggests that the effect of iron on language development was mediated through mechanisms that are independent of haemoglobin concentration.
Effects of anthelmintic treatment on motor and language development
We are not aware of previous reports on the effect of anthelmintic treatment on motor and language development of children in this young age range. In this study, children who received mebendazole had slightly better developmental scores, but the size of the effect was small and did not approach statistical significance. The power of our study to detect a statistically significant difference was <80% for effect sizes smaller than 1 unit on our scales. During the 12 month study period, the most rapid increases in motor and language development scores were in the children aged 12-24 months. This group had low prevalences of the target parasite species, making it less likely that we would detect the effects of mebendazole treatment. At baseline, only 6% of the children aged 12-24 months were infected with all three helminth species compared with 39% of the children aged 37-48 months.
The lack of effect of mebendazole on motor and language development might be explained by the drug's relatively low cure rates for two of the species—different species having different effects on development—or by a causal relation too small to be detected with our methods or not present at all. The potential public health benefits of treating young children for worms include immunological and nutritional outcomes as well as motor and language development, and additional research is needed.
Summary
Our results highlight that in African communities in which malaria is endemic there are severely anaemic children who are not detected by the current healthcare system and who seem to be at considerable risk of poor development. Identifying the optimal treatment and follow up regimen for severely anaemic children is a high priority and, although the best dose and duration needs to be clarified, long term treatment with oral iron may be an important component.
Footnotes
Funding: Thrasher Research Fund, Cooperative Agreement #HRN-A-00-97-00015-00 between the Johns Hopkins University and the United States Agency for International Development, and Alpharma USPD, Baltimore, MD.
Competing interests: None declared.
References
- 1.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics. 1987;79:981–995. . [Published erratum appears in Pediatrics 1988;81:683.] [PubMed] [Google Scholar]
- 2.Walter T. Infancy: mental and motor development. Am J Clin Nutr. 1989;50:655–666. doi: 10.1093/ajcn/50.3.655. [DOI] [PubMed] [Google Scholar]
- 3.Oski FA, Honig AS, Helu B, Howanitz P. Effect of iron therapy on behavior performance in nonanemic, iron-deficient infants. Pediatrics. 1983;71:877–880. [PubMed] [Google Scholar]
- 4.Watkins WE, Pollitt E. Nutrition, health, and child development: Research advances and policy recommendations. Washington, DC: Pan American Health Organization, The World Bank and Tropical Medicine Research Unit; 1997. Iron deficiency and cognition among school-age children; pp. 179–197. [Google Scholar]
- 5.Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;341:1–4. doi: 10.1016/0140-6736(93)92477-b. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 7.Williams J, Wolff A, Daly A, MacDonald A, Aukett A, Booth IW. Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. BMJ. 1999;318:693–697. doi: 10.1136/bmj.318.7185.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morley R, Abbott R, Fairweather-Tait S, MacFadyen U, Stephenson T, Lucas A. Iron fortified follow on formula from 9 to 18 months improves iron status but not development or growth: a randomised trial. Arch Dis Child. 1999;81:247–252. doi: 10.1136/adc.81.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozoff B. Nutrition, health, and child development: Research advances and policy recommendations. Washington, DC: Pan American Health Organization, The World Bank and Tropical Medicine Research Unit; 1997. Explanatory mechanisms for poorer development in iron-deficient anemic infants; pp. 162–178. [Google Scholar]
- 10.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–2288. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- 12.Kvalsvig JD, Cooppan RM, Connolly KJ. The effects of parasite infections on cognitive processes in children. Ann Trop Med Parasitol. 1991;85:551–568. doi: 10.1080/00034983.1991.11812608. [DOI] [PubMed] [Google Scholar]
- 13.Sakti H, Nokes C, Hertanto WS, Hendratno S, Hall A, Bundy DA, et al. Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Trop Med Int Health. 1999;4:322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Robinson BA, Bundy DA. Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitology. 1992;104:539–547. doi: 10.1017/s0031182000063800. [DOI] [PubMed] [Google Scholar]
- 15.Simeon DT, Grantham-McGregor SM, Wong MS. Trichuris trichiura infection and cognition in children: results of a randomized clinical trial. Parasitology. 1995;110:457–464. doi: 10.1017/s0031182000064799. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson SE, Powell CA, Walker SP, Chang SM, Grantham-McGregor SM. Nutrition, anaemia, geohelminth infection and school achievement in rural Jamaican primary school children. Eur J Clin Nutr. 1997;51:729–735. doi: 10.1038/sj.ejcn.1600473. [DOI] [PubMed] [Google Scholar]
- 17.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. BMJ. 2000;320:1697–1701. doi: 10.1136/bmj.320.7251.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ash LR, Orihel TC, Savioli L. Bench aids for the diagnosis of intestinal parasites. Geneva: World Health Organization; 1994. [Google Scholar]
- 19.Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg. 1985;79:181–184. doi: 10.1016/0035-9203(85)90329-3. [DOI] [PubMed] [Google Scholar]
- 20.Glascoe FP, Sandler H. Value of parents' estimates of children's developmental ages. J Pediatr. 1995;127:831–835. doi: 10.1016/s0022-3476(95)70184-2. [DOI] [PubMed] [Google Scholar]
- 21.Ireton H, Glascoe FP. Assessing children's development using parents' reports. The Child Development Inventory. Clin Pediatr (Phila) 1995;34:248–255. doi: 10.1177/000992289503400504. [DOI] [PubMed] [Google Scholar]
- 22.Cowen EL, Work WC, Wyman PA, Jarrell DD. Relationships between retrospective parent reports of developmental milestones and school adjustment at ages 10 to 12 years. J Am Acad Child Adolesc Psychiatry. 1994;33:400–406. doi: 10.1097/00004583-199403000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Knobloch H, Stevens F, Malone A, Ellison P, Risemberg H. The validity of parental reporting of infant development. Pediatrics. 1979;63:872–878. [PubMed] [Google Scholar]
- 24.Griffiths R. The abilities of young children: a comprehensive system of mental measurement for the first eight years of life. London: Child Development Research Centre; 1970. [Google Scholar]
- 25.McCarthy D. Manual for the McCarthy scales of children's abilities. New York: Psychological Corporation; 1972. [Google Scholar]
- 26.DeVellis R. Scale development. Theory and applications. California: Sage; 1991. [Google Scholar]
- 27.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 28.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Stoltzfus RJ, Chwaya HM, Montresor A, Albonico M, Savioli L, Tielsch J. Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-y old Zanzibari children and these relationships change with age. J Nutr. 2000;130:1724–1733. doi: 10.1093/jn/130.7.1724. [DOI] [PubMed] [Google Scholar]
- 30.Sherriff A, Emond A, Bell JC, Golding J. Should infants be screened for anaemia? A prospective study investigating the relation between haemoglobin at 8, 12, and 18 months and development at 18 months. Arch Dis Child. 2001;84:480–485. doi: 10.1136/adc.84.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]