Summary
Neutrophils are essential in the fight against invading pathogens. They utilize antimicrobial effector mechanisms, such as phagocytosis, release of proteases and other antimicrobial products, robust oxidative bursts and neutrophil extracellular traps to combat infections. Neutrophils also modulate immune responses through the production of eicosanoids, cytokines and chemokines, as well as via direct communication with other immune cells. This system of high‐intensity offense against pathogens is exquisitely balanced through regulation to limit damage to host tissue. Unfortunately, the control of neutrophils is not failproof. In cases of sterile injury, autoimmunity and even during an infection, neutrophils can cause tissue destruction and become detrimental to the host. For that reason, there is a need to find means to regulate the aberrant activation of these cells. We found that alphaB‐crystallin (α BC), a heat‐shock protein known to have anti‐inflammatory abilities, affects certain properties of mouse neutrophils that subsequently influence the pro‐inflammatory state of antigen‐presenting cells (APCs). More specifically, α BC mediated small but significant increases in the levels of IL‐10 and matrix metalloproteinase 8, and altered hydrogen peroxide secretion by stimulated neutrophils. Further, the heat‐shock protein influenced the communication between neutrophils and dendritic cells by decreasing the production of pro‐inflammatory cytokines, specifically IL‐12p40, by the APCs. α BC could thus contribute to dampening neutrophil inflammatory responses by impacting the effect of neutrophils on other immune cells.
Keywords: alphaB‐crystallin, dendritic cells, interleukin‐10, interleukin‐12, matrix metalloproteinases
Abbreviations
- APC
antigen‐presenting cell
- CD
cluster of differentiation
- ELISA
enzyme‐linked immunosorbent assay
- GM‐CSF
granulocyte macrophage‐colony‐stimulating factor
- H2O2
hydrogen peroxide
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- JNK
c‐Jun N‐terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MCP‐1
macrophage chemotactic protein‐1
- MIP‐1α
macrophage inflammatory protein‐1α
- MMP
matrix metalloproteinase
- NET
neutrophil extracellular trap
- p38
class of mitogen‐activated protein kinases
- PI3K
phosphoinositol‐3‐kinase
- ROS
reactive oxygen species
- Th
helper T lymphocyte
- TLR
toll‐like receptor
- αBC
alphaB‐crystallin
Introduction
Following infection with bacteria, viruses, fungi and parasites, or after tissue injury, neutrophils are mobilized rapidly from blood and bone marrow niches to areas of damage or contagion via inflammatory agents such as N‐formylated peptides, chemokines and danger signals.1, 2, 3 With respect to infection, a variety of effector mechanisms, including release of reactive oxygen species (ROS), anti‐microbial peptides and proteases are employed by the granulocytes to efficiently kill invading microbes.1, 4 Neutrophils are thus considered essential beneficial cells, and their importance became evident when their removal from the inflammatory equation, such as in the case of neutropaenia or following the use of immunosuppressive agents, resulted in a substantial increase in the susceptibility to infection.5, 6 However, exuberant infiltration and activation of neutrophils during sterile injury, autoimmunity or even during infection can lead to tissue damage. For example, matrix metalloproteinases (MMPs) secreted by neutrophils are thought to digest collagen, tendon and bone, which severely damages joints in rheumatoid arthritis (RA).7 It is thus important to decipher how the physiological properties of neutrophils can be regulated in order to control over‐zealous neutrophil responses.
In addition to direct effects during injury and infection, neutrophils can also modulate the responses of other immune cells, such as dendritic cells (DCs), and thus further influence inflammatory responses. Through direct cell‐to‐cell contact or indirectly through secreted factors, neutrophils can interact with DCs to alter their antigen‐presenting abilities, cytokine production and/or polarization of T‐cells.8, 9, 10 For instance, DCs can engulf neutrophils and present the antigen(s) that was being processed by the neutrophils. In other situations, the granulocyte can directly transfer antigen(s) to DCs.11, 12, 13 In addition, neutrophils are able to induce DCs to produce the pro‐inflammatory cytokine interleukin (IL)‐12p40, and potentiate the ability of these antigen‐presenting cells (APCs) to differentiate T lymphocytes into a Th1 or Th17 phenotype.10, 14 Because of these cell‐to‐cell interactions, over‐exuberant neutrophil activation could augment and extend the pro‐inflammatory properties of APCs such as DCs. Thus, the ability to control neutrophil function would indirectly regulate the actions of other potentially harmful immune cells.
The small heat‐shock protein, alphaB‐crystallin (αBC), has many protective functions, including chaperoning, anti‐neurotoxic and immunosuppressive properties.15 We, and colleagues, have shown that αBC treatment is beneficial in autoimmune diseases and after peripheral nerve injury due to its chaperoning, remyelinating and immune‐dampening effects.16, 17, 18, 19, 20 Most of these studies focused on the anti‐inflammatory effect of αBC on T‐cells17, 18 and macrophages17, 21 because of the involvement of these cell types in the models used. It is suggested that exogenous αBC exerts its cellular effects by binding to and crossing the plasma membrane,22 or signalling through the TLR2/CD14 receptor.21 Whether αBC also impacts the biology of neutrophils remains unclear. van Noort et al.21 have shown that microparticle‐encapsulated αBC‐treated macrophages reduced neutrophil activation and migration following lung injury. Additionally, Dieterich et al.23 found that αBC regulated the release and expansion of Gr‐1+ immunosuppressive immature immune cells from the bone marrow during tumour progression. Whether αBC directly influences neutrophil function to subsequently impact the activation of other immune cells has not, however, been elucidated. We therefore sought to establish whether the small heat‐shock protein affects how neutrophils respond to inflammatory stimulation by assessing for cytokine secretion, activation state and effector mechanisms, such as ROS and MMP secretion. We also examined whether αBC‐treated neutrophils could influence the response of other immune cells, specifically DCs. Altogether, we found that the crystallin mediated a small increase in IL‐10 and MMP8 production by neutrophils, and that αBC‐treated neutrophils reduced the production of IL‐12p40 by DCs in both a contact‐ and non‐contact‐dependent manner.
Materials and methods
Mice
129S6 mice from Taconic (129SVETac, Germantown, NY) were housed and bred in a specific pathogen‐free conventional unit at the University of Calgary where they had access to food and water ad libitum. Eight–12‐week‐old female mice were used for all experiments. All procedures were carried out in accordance with guidelines of the Canadian Council of Animal Care, and received approval by the University of Calgary Animal Resources and Ethics Committee.
Immune cell isolation from bone marrow
Hind limbs were removed from naïve 129S6 mice, and bones carefully extracted from the surrounding tissue. Bone marrow was then flushed from the femurs and tibias using cold sterile phosphate‐buffered saline (PBS). Cells were centrifuged at 295 × g (Beckman Coulter Allegra X‐12R) at 4° for 10 min.
Neutrophil isolation and stimulation
Neutrophils were isolated from the bone marrow of naïve 129S6 animals using a neutrophil isolation kit (MACS Miltenyi, 130‐097‐658, Auburn, CA, USA). Briefly, cells were incubated with biotin‐labelled non‐neutrophil antibodies for 10 min at 4°, washed, incubated with anti‐biotin magnetic beads for 15 min at 4° and then passed through magnetized ferromagnetic matrix columns. Eluted cells were collected and plated at 1 million cells/ml of media containing RPMI 1640, 5% heat‐inactivated fetal bovine serum (FBS), 100 U/ml penicillin/streptomycin and 2 mm l‐glutamine. For neutrophil‐only experiments, cells were stimulated with 50 ng/ml recombinant mouse (rm)‐granulocyte macrophage‐colony‐stimulating factor (GM‐CSF; Invitrogen, PMC2015, Waltham, MA, USA) plus 1 μg/ml lipopolysaccharide (LPS; Sigma, L2654, St. Louis, MO, USA), and then treated with 2 μg/ml αBC73–92 peptide (Stanford Pan Facility) for 20 hr at 37° and 5% CO2. We, and colleagues, have previously shown that the αBC73–92 peptide as well as full‐length αBC17 reduces central nervous system inflammation24 and activation of CD4+ T‐cells.25
DC isolation and co‐culture
Dendritic cells were derived from neutrophil‐depleted bone marrow. After neutrophils were eluted from the bone marrow cell suspension using the neutrophil isolation kit, columns were removed from the magnet and the remaining cells flushed from the columns. The neutrophil‐depleted cells were grown in RPMI 1640 media containing 10% heat‐inactivated FBS, 100 U/ml penicillin/streptomycin, 2 mm l‐glutamine and 20 ng/ml rm‐GM‐CSF for 4 days at 37° and 5% CO2. Wells were replenished with fresh rm‐GM‐CSF‐containing media and grown for an additional 3 days. DCs were harvested using 0·25% trypsin and gentle scraping, and plated at 20 000, 40 000 and 100 000 cells/well in 96‐well plates, 50 000 cells/well in 24‐well plates, or 100 000 cells/well in 96‐well transwell plates (Corning, 3391, Corning, NY, USA). Cells were starved from rm‐GM‐CSF for 48 hr. Pre‐stimulated and αBC‐treated neutrophils were added on top of the DCs at 100 000 DCs/well in 96‐well plates or 250 000 DCs/well in 24‐well plates. For the transwell system, 200 000 neutrophils/well were added on top of the 0·4‐μm pore polycarbonate membrane. For these co‐cultures, neutrophils were prior stimulated with 5 ng/ml rm‐GM‐CSF plus 100 ng/ml LPS, and treated with 200 ng/ml αBC73–92 peptide at 37° and 5% CO2 for 20 hr.
Enzyme‐linked immunosorbent assay (ELISA)
Supernatants were collected from cells at 20 hr after stimulation, and 10–50 μl assayed for the following cytokines and proteins according to the manufacturer's instructions: tumour necrosis factor (TNF)‐α (R&D Systems, DY410, Minneapolis, MN, USA), IL‐1β (BD Biosciences, 559603, San Jose, CA, USA), IL‐4 (BD Biosciences, 555232), IL‐6 (BD Biosciences, 555240), IL‐10 (BD Biosciences, 555252), IL‐12p40 (BD Biosciences, 555165), MMP8 (Abcam, ab206982, Cambridge, MA, USA), MMP9 (R&D Systems, MMPT90), macrophage inflammatory protein‐1α (MIP‐1α; R&D Systems, DY450) and macrophage chemotactic protein‐1 (MCP‐1; eBiosciences, 88‐7391‐88, Waltham, MA, USA).
Extracellular hydrogen peroxide (H2O2)
As an indicator of ROS generation, extracellular secretion of H2O2 was assessed using Amplex UltraRed reagent, which reacts 1 : 1 with H2O2 to produce fluorescent resorufin. Neutrophils were primed with 50 ng/ml rm‐GM‐CSF plus 1 μg/ml LPS, and treated with 2 μg/ml αBC73–92 peptide for 2, 4 or 20 hr. Plates were centrifuged at 1200 rpm for 5 min at 24°, and supernatants collected and stored at 4° for 1 hr to assess for H2O2 secreted by cells prior to zymosan stimulation. Cells were then stimulated with 1 mg/ml serum‐opsonized zymosan for 1 hr, their supernatant removed and diluted 20 × in PBS. A fresh substrate solution consisting of 20 μg/ml Amplex UltraRed Reagent (ThermoFisher, A36006, Waltham, MA, USA) and 20 U/ml horseradish peroxidase was prepared in PBS. Fifty microlitres of the substrate solution was added to 50 μl of diluted supernatant or a prepared standard curve of H2O2 used for quantification of reactive oxygen. The plate was incubated in the dark for 15 min at room temperature, and fluorescence emission measured at 571 nm/585 nm (excitation λ/emission λ). Five measurements were collected per well.
RNA isolation and quantitative polymerase chain reaction (PCR)
Following neutrophil stimulation for 20 hr in a six‐well plate, cells were lysed in 1 ml of Trizol reagent (Life Technologies, 15596018, Waltham, MA, USA) and RNA isolated from the aqueous phase of a Trizol chloroform mixture using a Qiagen RNeasy mini kit (74106, Germantown, MD, USA) according to manufacturer's instructions. RNA was eluted with 30 μl of RNase‐free water and quantified using a Nanodrop spectrophotometer. RNA was then converted to cDNA by reverse transcription with a QuantiTect RT kit (Qiagen 205311) according to the company's directions. Contaminating genomic DNA was eliminated by an incubation with gDNA Wipeout Buffer at 42° for 2 min. QuantiTect Reverse Transcriptase and RT primer mix were added to the RNA (70–150 pg of RNA was used in each reaction) followed by an incubation at 42° for 15 min. Reverse transcriptase was inactivated by incubating for 3 min at 95°. Gene expression was subsequently analysed by real‐time PCR. Briefly, cDNA samples were incubated with Quantifast Sybr Green master mix (Qiagen Quantifast Sybr Green PCR kit, 204054) along with inducible nitric oxide synthase (iNOS; Mm_LOC673161_1_SG QuantiTect Primer Assay) and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH; Mm_Gapdh_3_SG QuantiTect Primer Assay) primers. Samples were run in triplicate with a MyiQ2 qPCR machine (BioRad, Hercules, CA, USA). The real time cycle consisted of a 5‐min incubation at 95° followed by 45 cycles of 15 seconds at 95° and 1 min at 60°. All experiments were concluded with a melt curve of 55°–95° in 81 steps to ensure correct gene product was replicated. biorad iq5 software was used to set up the experiments and to analyse the data, including calculation of the threshold cycle. Fold change was calculated over gene expression level in the GM‐CSF + LPS‐treated cells using the following equations: (i) threshold cycle of gene of interest − threshold cycle of housekeeping gene (GAPDH) = Delta; (ii) Delta − average Delta of GM‐CSF‐stimulated samples = Delta‐Delta; (iii) 2−(Delta‐Delta) = fold change.
Flow cytometry
Cells were suspended in PBS containing 3% heat‐inactivated FBS and their Fc receptors blocked for 5 min at room temperature using unlabelled anti‐CD16/CD32 antibodies (BD Biosciences 553142). Cells were then stained for 30 min at 4° with the following BD Biosciences fluorescently‐conjugated antibodies: FITC‐conjugated CD45 (553080), PerCP‐conjugated CD45 (557235), APC‐conjugated CD45 (559864), APC‐Cy7‐conjugated CD45 (557659), FITC‐conjugated Ly6G (551460), PerCP‐conjugated Ly6G (560602), FITC‐conjugated CD11b (553310), PE‐conjugated CD11c (557401), APC‐conjugated CD11c (550261), PE‐conjugated CD40 (561846), PerCP‐conjugated CD80 (560526), PE‐conjugated CD86 (560582) and FITC‐conjugated I‐A/I‐E (MHC class II, 562009). An Attune acoustic focusing cytometer (Applied Biosystems, Waltham, MA, USA) was used with its blue and red lasers configured to measure the size, granularity and fluorescence intensity of cells bound with antibodies.
DC : neutrophil interaction assay
Neutrophils were stimulated with 5 ng/ml rm‐GM‐CSF plus 100 ng/ml LPS, and treated with 200 ng/ml αBC73–92 peptide for 20 hr, washed and co‐cultured with DCs for 24 hr. Cells were then fixed and images captured on an inverted microscope using phase contrast. metamorph Advanced software version 7·7·8·0 was used to capture images in five locations in each well. Under phase contrast, neutrophils and DCs were distinguished by size and appearance (Fig. S5e both panels: DC – black arrowhead; neutrophils – black arrow) and interactions were counted when the two cell types were touching (Fig. S5e right panel: white arrowheads). Each DC was counted along with the number of interacting neutrophils.
Kinase inhibition assay
The following kinase inhibitors were purchased from Millipore (Burlington, MA, USA): LY294002 (Cat# 440202), a phosphoinositol‐3‐kinase (PI3K) inhibitor that blocks the phosphorylation of Akt; SB203580 (Cat# 559389), a p38 mitogen‐activated protein kinase (MAPK) inhibitor that prevents the activation of MAPKAPK‐2 by p38 MAPK; U0126 (Cat# 662005) that inhibits the kinase activity of MEK1/2 and downstream activation of MAPK p42/44; and c‐Jun N‐terminal kinase (JNK) inhibitor II (Cat# 420119), a small molecule inhibitor that blocks the kinase activity of JNK by competitive inhibition with respect to adenosine triphosphate. LY294002, SB203580, U0126 or JNK II (1 μm) was administered to neutrophils simultaneously with 50 ng/ml rm‐GM‐CSF + 1 μg/ml LPS stimulation and 2 μg/ml αBC73–92 peptide, and incubated for 20 hr at 37° and 5% CO2. Supernatants were collected, and the amount of IL‐10 secretion assessed by ELISA.
Viability assay
Neutrophils were stimulated with 50 ng/ml rm‐GM‐CSF + 1 μg/ml LPS, and treated with 2 μg/ml αBC73–92 peptide in the presence or absence of 1 μm LY294002, SB203580, U0126 or JNK inhibitor II inhibitors for 20 hr. The Fc receptors on cells were blocked for 5 min at room temperature with unlabelled anti‐CD16/CD32 antibodies. Cells were subsequently stored in 300 μl of PBS containing 3% heat‐inactivated FBS on ice, resuspended, and 5 μl of propidium iodide added to the tube 30 seconds before processing by flow cytometry. Alternatively, neutrophils were stimulated with 5 ng/ml rm‐GM‐CSF + 100 ng/ml LPS, and treated with 200 ng/ml αBC73–92 peptide for 20 hr and incubated with 5 μl of propidium iodide and 5 μl of Annexin V in binding buffer for 10 min before processing by flow cytometry. Cells that stained positive for propidium iodide and/or annexinV were considered non‐viable.
Statistics
Results are expressed as the mean of each experiment in individual scatter plot data sets. Comparisons between groups were made by repeated‐measures two‐way anova with Šídák post hoc tests, or repeated‐measures one‐way anova with Dunnett's multiple comparison test. All statistical measures were completed using graphpad prism 6 software (GraphPad, La Jolla, CA). A value of P < 0·05 was considered statistically significant.
Results
αBC induced a small increase in the secretion of IL‐10 but did not alter the production of other cytokines in neutrophils
Neutrophils secrete a plethora of cytokines including TNF‐α, IL‐12p40, IL‐6, IL‐1β, IL‐17 and IL‐10 upon activation by various stimuli.26, 27, 28 To assess if αBC affected cytokine production by these cells, bone‐marrow‐derived CD45+ CD11b+ Ly6G+ neutrophils (Fig. 1a) were stimulated with rm‐GM‐CSF + LPS with or without αBC addition. Although TNF‐α and IL‐6 production increased with stimulation, αBC did not alter the secretion of these cytokines from either stimulated or unstimulated neutrophils (Fig. 1b,c). For other pro‐inflammatory cytokines, such as IL‐1β and IL‐12p40, production of these signalling factors was not altered following stimulation or upon αBC treatment relative to media levels (Fig. 1d,e). With respect to immunosuppressive cytokines, very low levels of IL‐4 could be detected and remained unchanged among the various groups (Fig. 1f). For IL‐10, however, a significant increase was evident following GM‐CSF + LPS stimulation, which was modestly, but consistently significantly enhanced when αBC was present (Fig. 1g).
Figure 1.
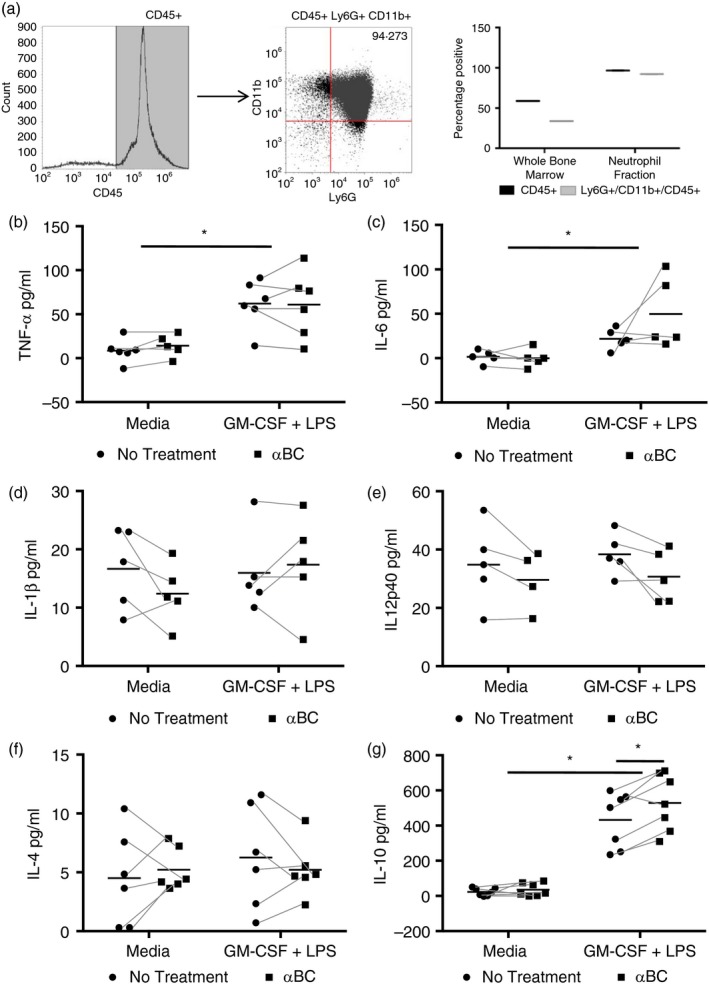
Stimulated neutrophils secrete more interleukin (IL)‐10 after treatment with alphaB‐crystallin (α BC). (a) Gating strategy and purity of bone marrow‐derived CD45+ Ly6G+ CD11b+ neutrophils in the whole bone marrow and in the neutrophil fraction after isolation with a neutrophil isolation kit. Graph represents one of three independent experiments, with each data point representing pooled cells isolated from four mice. (b–g) Tumour necrosis factor (TNF)‐α (b), IL‐6 (c), IL‐1β (d), IL‐12p40 (e), IL‐4 (f) and IL‐10 (g) production by granulocyte macrophage‐colony‐stimulating factor (GM‐CSF) + lipopolysaccharide (LPS)‐stimulated bone marrow‐derived neutrophils following treatment with (squares) or without (circles) α BC for 20 hr. The results represent the mean and spread of six (TNF‐α), five (IL‐6), five (IL‐1β), five (IL‐12p40), six (IL‐4) and seven (IL‐10) independent experiments. Statistical analyses were performed by repeated‐measures two‐way anova with Šídák post hoc test, *P < 0·05 of main effect (stimulation on TNF‐α, IL‐6 and IL‐10) and post hoc test (treatment on IL‐10).
H2O2 and MMP secretion by neutrophils were not altered by αBC
Neutrophils exhibit an array of effector mechanisms that are vital in the fight against pathogens, but which are not always beneficial to the host if they are not controlled. For example, proteases and ROS released by neutrophils can cause significant tissue damage and pathology if not abated.29 We therefore evaluated whether the secretion of ROS and two prominent MMPs in the neutrophil repertoire (neutrophil collagenase, MMP8, gelatinase B, MMP9) were impacted by αBC. MMPs play an important role in neutrophil migration and tissue damage in diseases such as RA.30, 31 As well, both immune‐suppressing and immune‐activating functions of MMP8 have been reported. Both MMP8 and MMP9 secretion were enhanced when neutrophils were stimulated with rm‐GM‐CSF + LPS (Fig. 2). However, while MMP9 levels remained unchanged in the presence of the heat‐shock protein (Fig. 2a), MMP8 production displayed a small significant increase upon application of αBC to stimulated cells (Fig. 2b). We then proceeded to assess if the crystallin impacted the secretion of H2O2, an indicator of the ROS effector program in neutrophils. Using an AmplexRed assay, we found that neutrophils stimulated with GM‐CSF + LPS alone did not release measurable amounts of H2O2 after stimulation (Fig. S1). The protocol was therefore modified whereby cells were first activated with GM‐CSF + LPS with and without αBC, and then activated with serum‐opsinized zymosan. Neutrophils primed for 2 hr displayed no increase in H2O2 secretion (Fig. 3a). However, an enhancement was evident at 4 hr (Fig. 3a). At this time point, a small but significant decrease in H2O2 synthesis towards control levels was observed in the presence of αBC (Fig. 3b). Intriguingly, but not unprecedented,32 a reduction in ROS production was seen at 20 hr post‐stimulation relative to media controls (Fig. 3c). Here again, a small but significant increase in H2O2 levels again towards control levels was seen when αBC was applied to cells (Fig. 3c).
Figure 2.
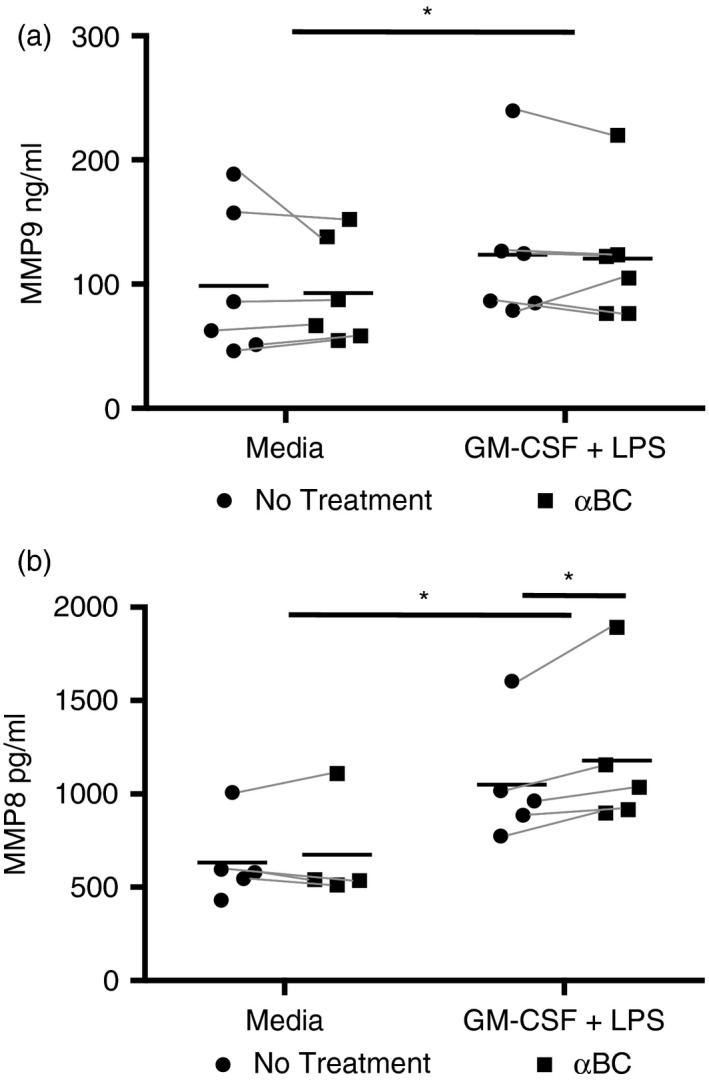
AlphaB‐crystallin (α BC) treatment enhanced secretion of matrix metalloproteinase (MMP)8 by stimulated neutrophils. MMP9 (a) and MMP8 (b) secretion by granulocyte macrophage‐colony‐stimulating factor (GM‐CSF) + lipopolysaccharide (LPS)‐activated neutrophils grown in the presence (squares) or absence (circles) of α BC. Data represent the mean and spread of six (MMP9) and five (MMP8) independent experiments. Statistical analyses were done by repeated‐measures two‐way anova with Šídák post hoc test, *P < 0·05 of main effect (stimulation on MMP8 and MMP9) and post hoc test (treatment on MMP8).
Figure 3.
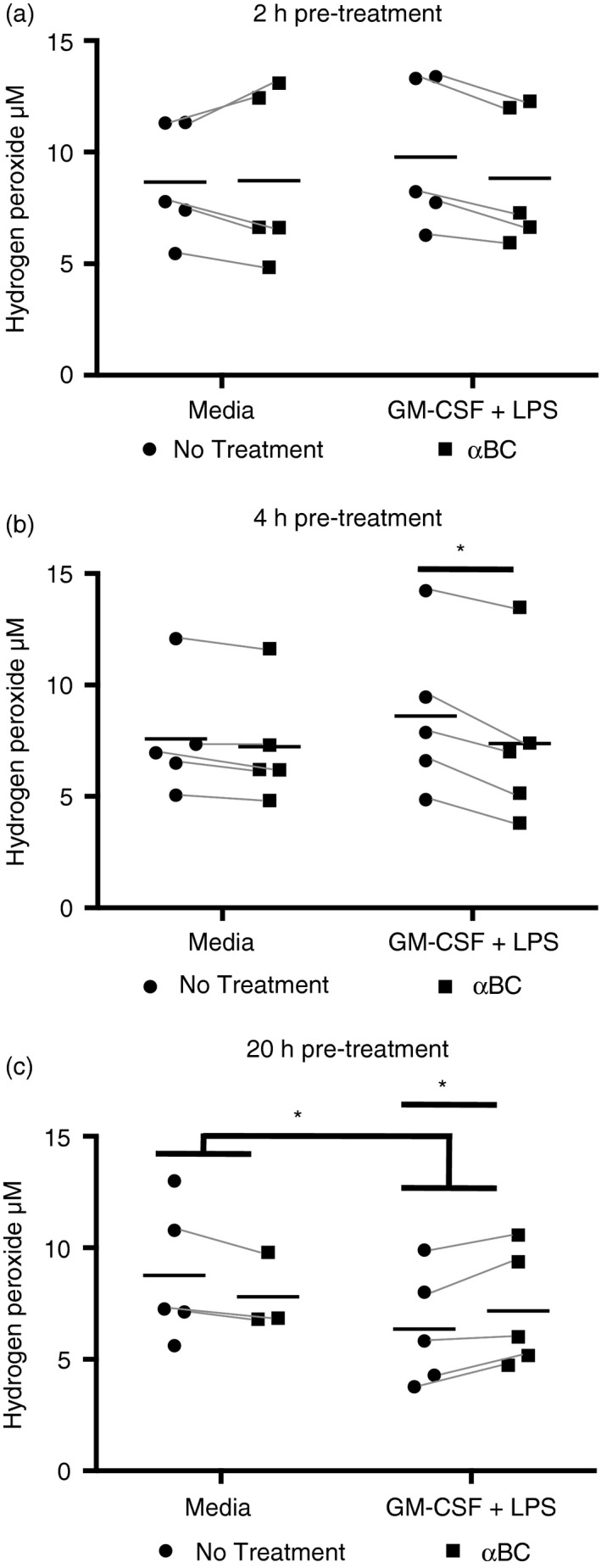
Hydrogen peroxide (H2O2) secretion is altered by alphaB‐crystallin (α BC) treatment of stimulated neutrophils. Hydrogen peroxide, as an indicator of reactive oxygen species (ROS), secreted by zymosan‐stimulated neutrophils following (a) 2 hr, (b) 4 hr and (c) 20 hr pre‐stimulation with granulocyte macrophage‐colony‐stimulating factor (GM‐CSF) + lipopolysaccharide (LPS) with (squares) or without (circles) addition of α BC. The results represent the combined mean and spread of five (2 hr), five (4 hr) and five (20 hr) independent experiments. Statistical analyses were performed by repeated‐measures two‐way anova with Šídák post hoc test (2 and 4 hr), and repeated‐measures one‐way anova with Dunnett's multiple comparison test for 20 hr, *P < 0·05 of main effect (2 hr), interaction (4 hr stimulation) and post hoc/multiple comparison tests (4 and 20 hr treatment).
αBC did not affect the expression of CD11b or iNOS
Upon activation, neutrophils usually upregulate iNOS production and membrane expression of CD11b.33, 34, 35 During the degranulation process, granules containing CD11b in their membranes fuse with the cell membrane to increase the presence of this protein on the cell surface. Neutrophils then use this CD11b for migration into tissue and to communicate directly with other immune cells, such as DCs and T‐cells.9, 36 We therefore assessed whether CD11b expression was altered by αBC at 20 hr post‐GM‐CSF + LPS stimulation. Although stimulated neutrophils showed an increase in CD11b mean fluorescence index as compared with media control, αBC treatment did not alter cell surface expression of CD11b in stimulated cells (Fig. S2a). We then considered the effect of αBC treatment on iNOS synthesis following GM‐CSF + LPS stimulation in the presence or absence of αBC. Neutrophils stimulated for 2 or 6 hr but not 20 hr displayed increased levels of iNOS mRNA as compared with media control (Fig. S2b). Treatment of activated cells with αBC did not, however, alter the level of iNOS mRNA at any of the time points (Fig. S2b).
DCs co‐cultured with αBC‐treated neutrophils produce less IL‐12p40
Because neutrophils are known to communicate with DCs to modulate the activation of both DCs and lymphocytes,8, 10 and that αBC was found to have a small effect on some of the effector properties of neutrophils (augmentation of IL‐10 and MMP8 secretion, altered H2O2 release), we evaluated if αBC‐treated neutrophils could change the activation of DCs. Neutrophils were stimulated with GM‐CSF + LPS with and without αBC, and seeded on top of CD45+ CD11c+ DCs (Fig. 4a) in a 1 : 5, 1 : 2·5 and 1 : 1 ratio. We then measured for the levels of IL‐1β and IL‐12p40, which are produced by DCs, but not our neutrophils grown in isolation (Fig. 1d,e). DCs co‐cultured with GM‐CSF + LPS‐stimulated neutrophils produced significant amounts of IL‐1β and IL‐12p40 (Fig. 4b–g). Following αBC treatment of GM‐CSF + LPS‐stimulated neutrophils, secretion of IL‐1β by DCs remained unchanged relative to stimulation alone (Fig. 4b–d). For IL‐12p40, however, a reduction was evident in the group whose neutrophils had been previously treated with the crystallin as compared with those DCs cultured with untreated stimulated neutrophils (Fig. 4e–g). Moreover, the reduction in IL‐12p40 by DCs occurred in a cell ratio‐dependent manner. Specifically, DCs cultured in a 1 : 5 ratio with αBC‐treated neutrophils displayed the largest reduction in IL‐12p40 secretion (Fig. 4e), followed by the cells grown in a 1 : 2.5 ratio (Fig. 4f). Cells cultured at a 1 : 1 density did not have a significant reduction in IL‐12p40 secretion (Fig. 4g). It is also important to note that DCs that were grown with the washed supernatant from neutrophil‐free wells containing only stimulation reagents did not exhibit any secretion of IL‐1β and IL‐12p40 (Fig. S3), indicating that the changes in IL‐1β and IL‐12p40 were specifically related to the neutrophils and not to stimulation contamination. In addition, αBC treatment did not affect the viability of these cells as assessed by propidium iodide and Annexin V staining (Fig. S4a,b), suggesting that in this case the viability of the neutrophils did not influence the communication between neutrophils and DCs. This effect of αBC‐treated neutrophils on DCs appears to be specific to pro‐inflammatory cytokine production, particularly IL‐12p40, and not the antigen‐presenting machinery or interactive ability between the two cell types because the expression of antigen‐presenting molecules (CD40, CD80, CD86, MHC II) on DCs (Fig. S5a–d) and number of contacts (Fig. S5e,f) between DCs and neutrophils were unchanged by the presence of the heat‐shock protein. The latter observation is likely related to the finding that the secretion of two chemokines that are produced by neutrophils and known to attract DCs to allow for direct cell‐to‐cell engagement, MIP‐1α (CCL3) and MCP‐1 (CCL2),14, 28, 37, 38, 39 was also not altered by αBC in unactivated or stimulated neutrophils (Fig. S5g,h).
Figure 4.
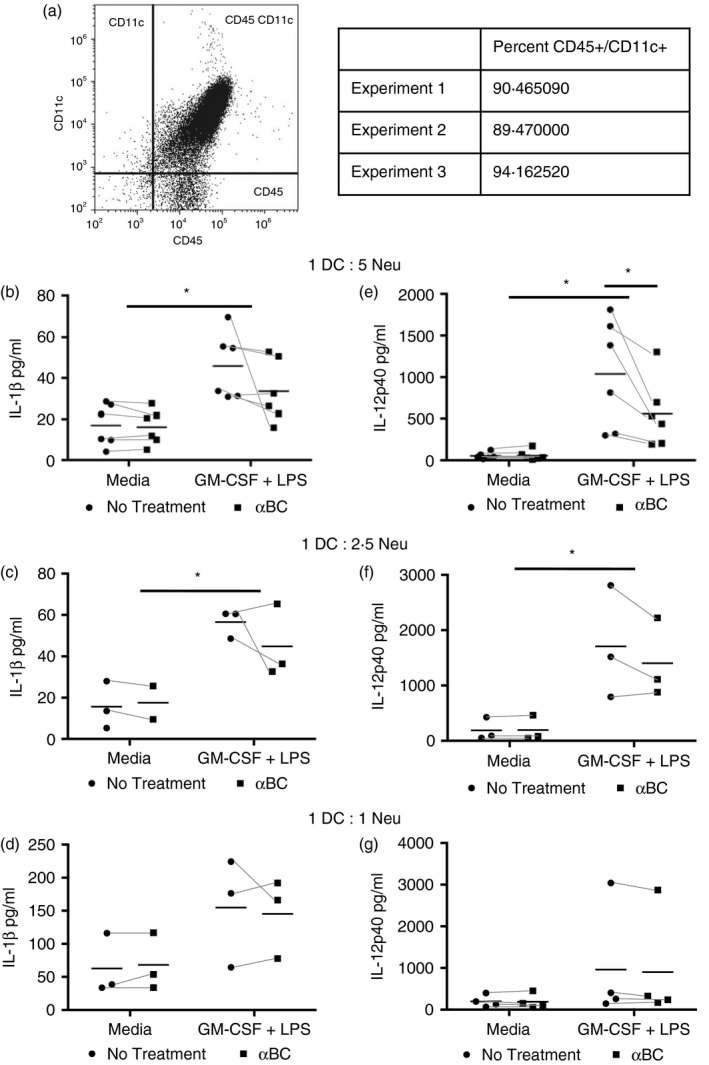
Stimulated neutrophils previously treated with alphaB‐crystallin (α BC) induced less interleukin (IL)‐12p40 production by DCs. (a) Flow cytometric gating strategy to determine purity of bone marrow‐derived DCs after culturing for 1 week in granulocyte macrophage‐colony‐stimulating factor (GM‐CSF)‐containing media, and 48 hr in GM‐CSF‐free media. The purity of DCs from three separate experiments is shown. (b–g) The presence of IL‐1β (b–d) and IL‐12p40 (e–g) in the supernatants of DCs co‐cultured with neutrophils previously stimulated with GM‐CSF + lipopolysaccharide (LPS) with (squares) or without (circles) of α BC in a 1 : 5 (b, e), 1 : 2·5 (c, f) or 1 : 1 (d, g) DC : neutrophil ratio. The data represent the mean and spread of data from (b) six, (c) three, (d) three, (e) six, (f) three and (g) four individual experiments. Statistical analyses were performed by two‐way anova with Šídák post hoc test, *P < 0·05 of main effect (stimulation on IL‐1β and IL‐12p40) and post hoc test (treatment on IL‐12p40).
αBC pre‐treated neutrophils reduce IL‐12p40 secretion by DCs in a non‐contact‐dependent manner
Thus far, we discovered that prior αBC‐treated neutrophils were capable of reducing pro‐inflammatory cytokine (IL‐12p40) production by DCs when co‐cultured together. Neutrophils can indeed alter the response of other immune cells through direct cell‐to‐cell contact, which could be the case in our situation. However, because no observed difference was seen in the interactions between neutrophils and DCs, we assessed whether αBC‐treated neutrophils could also impact DC function in a non‐contact‐dependent manner, that is, via secretory factors. To test this idea, a transwell system was implemented. Similar to that seen in the contact‐dependent assay (Fig. 4), IL‐1β secretion by DCs was not altered when co‐cultured with stimulated and αBC‐treated neutrophils in the transwell scenario (Fig. 5a). For IL‐12p40, however, DCs secreted significantly less of this cytokine when grown with αBC‐treated stimulated neutrophils as compared with untreated neutrophils (Fig. 5b). Our data therefore suggest that αBC treatment of neutrophils results in repressed IL‐12p40 production by DCs via a secreted factor(s).
Figure 5.
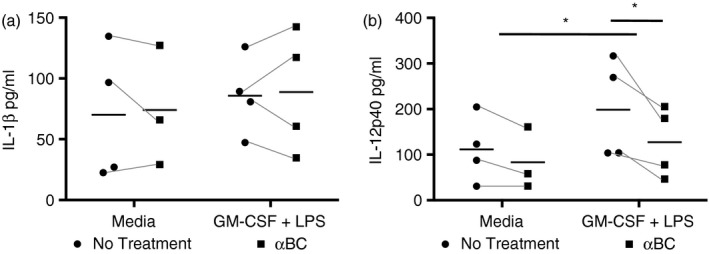
Secretory components from stimulated neutrophils treated with alphaB‐crystallin (α BC) induced less interleukin (IL)‐12p40 production from dendritic cells (DCs). (a) IL‐1β and (b) IL‐12p40 cytokine production in the supernatants of DCs cultured in a transwell system together with neutrophils that had been previously activated with granulocyte macrophage‐colony‐stimulating factor (GM‐CSF) + lipopolysaccharide (LPS) with (squares) or without (circles) the addition of α BC. The results represent the combined mean and spread of three individual experiments. Statistical analyses were completed by repeated‐measures two‐way anova with Šídák post hoc test, *P < 0·05 of main effect (stimulation on IL‐12p40) and post hoc test (treatment on IL‐12p40).
αBC‐mediated increase in IL‐10 production may involve PI3K and MEK/ERK
Finally, in an effort to decipher the molecular mechanism(s) underlying the small effect of αBC on IL‐10, MMP and ROS production by neutrophils, we evaluated the importance of the MAPK and PI3K pathways as they are known to be involved in the secretion of IL‐10 and degranulation by neutrophils.40, 41 Also, αBC treatment has been shown to affect various MAPK pathways as well as PI3K.42, 43 We therefore investigated if these signalling pathways were involved in the enhanced secretion of IL‐10 using inhibitors against p38 (SB203580), PI3K (LY294002), MEK/ERK (U0126) and JNK (JNKII).40 Inhibition of the JNK pathway did not alter the secretion of IL‐10 by either unstimulated or activated neutrophils (Fig. 6a), whereas SB203580 reduced the production of IL‐10 in both non‐αBC‐stimulated and αBC‐treated neutrophils (Fig. 6b). Of interest, PI3K and MEK/ERK inhibition resulted in a reduction of IL‐10 in stimulated neutrophils, but the decrease was only significant in cells that had been incubated with αBC (Fig. 6c,d). To ensure that the PI3K and MEK/ERK‐induced reduction in IL‐10 secretion was not due to cell death, the viability of the cells was evaluated by propidium iodide staining for non‐viable cells. None of the kinase inhibitors altered the viability of non‐stimulated, GM‐CSF + LPS‐stimulated or αBC‐treated cells (Fig. S4c), suggesting that the reduction in IL‐10 production with the inhibitors was not related to cell death.
Figure 6.
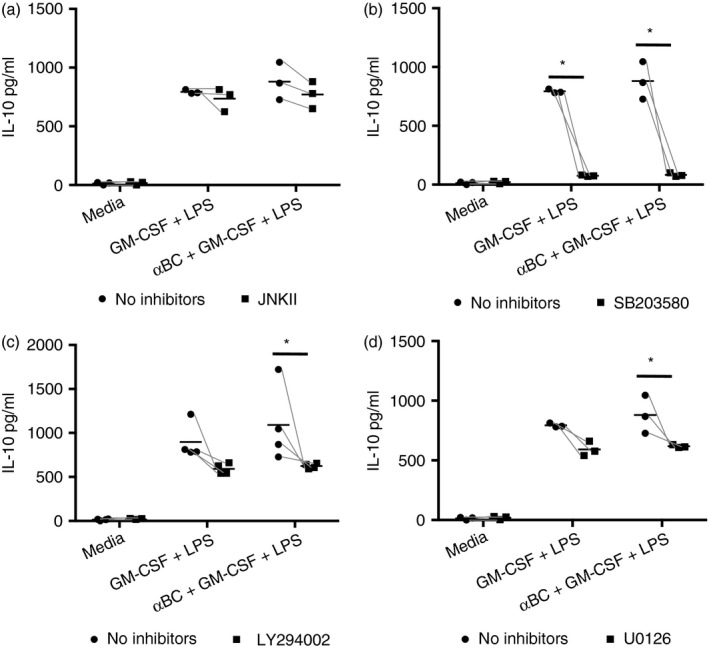
Phosphoinositol‐3‐kinase (PI3K) and MEK/ERK kinase inhibitors reduce interleukin (IL)‐10 secretion by alphaB‐crystallin (α BC)‐treated stimulated neutrophils. Production of IL‐10 by granulocyte macrophage‐colony‐stimulating factor (GM‐CSF) + lipopolysaccharide (LPS)‐activated neutrophils treated with or without α BC in the presence or absence of (a) c‐Jun N‐terminal kinase (JNK), (b) p38, (c) PI3K or (d) MEK/ERK kinase inhibitors. The results represent the combined mean and spread of (a) three, (b) three, (c) four and (d) three independent experiments. Statistical analyses were completed by repeated‐measures two‐way anova with Šídák post hoc test, *P < 0·05 of post hoc test.
Discussion
Neutrophils are imperative for protecting the host against invading microbes. Unfortunately, over‐exuberant activation of these granulocytes in autoimmune diseases, sterile injury and infection can result in direct damage to host tissue and/or prolongation of the inflammatory reaction due to interactions between neutrophils and other immune cells. It is thus important to understand if suppressors of neutrophil activation could be identified so as to reduce aberrant neutrophil responses. Neutrophils can dampen inflammation by acting directly on T lymphocytes and APCs, or through their own engulfment by APCs such as DCs.44, 45, 46 Because αBC has been shown to suppress the activation and function of T‐cells and macrophages, we investigated if the small heat‐shock protein could also reduce neutrophil activation. We found that the heat‐shock protein had a small but significant effect on selective properties of neutrophils, specifically IL‐10, MMP8 and H2O2 secretion, and that αBC‐treated neutrophils were capable of suppressing pro‐inflammatory cytokine production, specifically IL‐12p40, by DCs.
Neutrophils, IL‐10 and MMP8
IL‐10 production is upregulated during the height of inflammatory reactions where it contributes to refraction of the reaction.47 For example, IL‐10 plays a role in suppressing Th1 CD4+ T‐cell and CD8+ T‐cell responses by downregulating MHC II and co‐stimulatory molecule expression on APCs, thereby reducing antigen presentation by APCs and thus T‐cell activation.48 With respect to MMP8, this metalloproteinase has been reported to possess both pro‐ and anti‐inflammatory functions49, 50, 51 in addition to its expected proteinase activity – it is involved in the breakdown of type I, II and III collagens.52 Further, MMP8 secretion is differentially altered depending on the activator.53 We speculate that the small effect of αBC in augmenting the secretion of IL‐10 and MMP8 in neutrophils is by mediating the release of certain granules within neutrophils that contain these factors. Neutrophils contain granules that are formed sequentially during development in the bone marrow and contain particular molecules based on what is being synthesized at the time of formation.54 Secondary (or specific) granules contain MMP8, and are speculated to be formed during myelocyte and metamyelocyte phases of neutrophil maturation. The IL‐10 receptor is also known to reside within secondary granules55 and, although not well characterized, IL‐10 secretion may also be associated with release of these granules.56 Further, IL‐10 transcription can be regulated by C/EBPα, which is the dominant transcription factor active during secondary granule formation.54, 57 It is possible that αBC treatment is enhancing the release of secondary granules to result in the dual secretion of IL‐10 and MMP8. A feedback loop may also be involved whereby MMP8 mediates IL‐10 production as MMP8 is thought to have a role in processing IL‐10.58
Neutrophils and PI3K/MAPK signalling
The PI3K and MAPK pathways have a demonstrated involvement in not only primary and secondary granule release59, 60 by neutrophils, but also the secretion of IL‐10 by these cells.40 For instance, the release of lactoferrin, one of the defining molecules present within secondary granules, is regulated by PI3K, p38 and ERK61 in response to the complement protein, c5a. We found that the small αBC‐augmented IL‐10 secretion was significantly reduced by PI3K and MEK/ERK inhibitors, while others have shown that MMP8 production is augmented by c‐MET through the PI3K and MEK/ERK pathways.62 Because the crystallin has been found to act through the PI3K, MEK/ERK and p38 signalling in relation to its anti‐apoptotic and immunosuppressive functions,63, 64 it is possible that αBC is acting through these kinase pathways to enhance the release of secondary granules and/or IL‐10 and MMP8 production directly.
Neutrophils, ROS and αBC
We observed that neutrophils that were primed with GM‐CSF + LPS for short periods of time and further stimulated with zymosan showed a small increase in H2O2 secretion, whereas those cells primed for longer periods displayed decreased H2O2 secretion. This divergence in H2O2 (indicative of ROS) production over time has been observed by others. Saturnino et al.32 found that mast cells also experience this effect when primed for long periods with endotoxin. Further, ‘immunosuppressive’ neutrophils have been associated with both low and high levels of ROS generation that was related to the length of stimulation of the cells.65 Regarding αBC and ROS, the crystallin has been shown to inhibit the generation of ROS in response to Cu2+ stimulation of lens epithelial cells.66 In this situation, αBC sequestered the Cu2+ to reduce the reason for ROS production, but this is unlikely in our case as αBC was removed before stimulating these cells with zymosan. Also, because treatment of neutrophils with the heat‐shock protein during priming appeared to return H2O2 production by neutrophils to an unprimed level of secretion, it suggested that αBC could affect the priming of these cells. However, because the effects of αBC on IL‐10, H2O2 and MMP8 are small, the crystallin is likely not a major modulator of neutrophil priming and effector abilities.
Neutrophils and IL‐12p40 production in DCs
Stimulated neutrophils are known to upregulate the production of IL‐12p40 and TNF‐α by DCs.67 DCs utilize IL‐12p40 to mediate differentiation of naïve T lymphocytes into a Th168 or Th1769, 70 phenotype. IL‐10 opposes this process, and the ratio of IL‐12 : IL‐10 is often used to assess inflammatory status.71, 72 Interestingly, even though the effects of αBC on IL‐10, H2O2 and MMP8 were small, treatment of neutrophils with the crystallin was capable of reducing secretion of IL‐12p40 by co‐cultured DCs in a cell‐ratio‐dependent manner. It is possible then that αBC‐treated neutrophils could contribute to reducing the overall pro‐inflammatory milieu by suppressing DC pro‐inflammatory state, while at the same time suppressing the ability of DCs to polarize T‐cells towards a Th1 or Th17 phenotype and thus tipping the inflammatory reaction towards a dampening phenotype.
An interesting observation was the ability of αBC‐treated neutrophils to suppress IL‐12p40 secretion by DCs in both a contact‐dependent and non‐dependent manner. Because the interactions between neutrophils and DCs were not altered in the presence of αBC, the transwell experiment suggests that stimulated neutrophils treated with αBC secreted less of an unknown factor(s) that normally drives the secretion of IL‐12p40 by DCs. One mechanism that neutrophils employ to induce IL‐12p40 secretion by DCs is through secretion of TNF‐α.14 We do not think that TNF‐α is the unknown secretory factor because production of the cytokine was not changed in αBC‐treated neutrophils (Fig. 1b). An alternate possibility is that αBC‐treated neutrophils secreted a molecule(s) that suppressed the production of IL‐12p40 by DCs. Many factors are known to regulate the expression of IL‐12p40 by DCs including IL‐10.73 Since we found that IL‐10 was elevated in αBC‐treated neutrophils, it is conceivable that the immunosuppressive cytokine could contribute to suppressing DC IL‐12p40 production. Another possibility is that neutrophil elastase (NE), which was found to be inhibited by αBC,74 may contribute to the reduced secretion of IL‐12p40 by DCs. NE has been shown to reduce IL‐12 production by macrophages,75 and it is possible that a similar process may occur in mature DCs.
In summary, treatment of neutrophils with αBC peptide led to small increases in IL‐10 and MMP8 secretion as well as suppressed secretion of the pro‐inflammatory cytokine, IL‐12p40, by DCs. These data suggest that the small heat‐shock protein is likely not a major player in neutrophil priming and effector abilities, but its limited effects on neutrophil physiology may contribute to impacting overall inflammatory responses via dampening of DC IL‐12p40 production.
Author contributions
SSO and TMF conceived the study and experiments, and wrote the manuscript. TMF and ALP performed experiments and analyses. The authors thank Melissa Flancia for isolating the RNA, Keir Pittman from Dr Paul Kubes's lab for demonstrating neutrophil isolation, and Dr Robin Yates's lab for advice regarding the ROS‐AmplexRed assay.
Disclosures
The authors have no conflicting financial interests.
Supporting information
Figure S1. Hydrogen peroxide secreted by neutrophils stimulated with GM‐CSF + LPS and treated with αBC for two (black bars), four (dark grey bars), or 20 hr (light grey bars) with no further stimulation. Combined data from 3 individual experiments. Error bars represent spread of data from minimum to maximum. Statistical analyses were completed by repeated measures two‐way ANOVA with Šídák post hoc test.
Figure S2. αBC treatment does not modulate CD11b and iNOS expression by GM‐CSF + LPS‐stimulated neutrophils. (a) Expression of CD11b by untreated (circles) or αBC‐treated (squares) neutrophils in the combined mean and spread of 6 individual experiments. Statistical analyses were completed by repeated measured two‐way ANOVA with Šídák post hoc test, *P < 0·05 of main effect. (b) Relative fold change of iNOS mRNA in neutrophils treated with or without αBC for 2, 6, or 20 h compared to GM‐CSF + LPS stimulation and normalized to amplified GAPDH. Data represents 5 combined individual experiments. Error bars represent spread of minimum to maximum. Statistical analyses were performed by repeated measures one‐way ANOVA with Dunnett's post hoc test, *P < 0·05 of post hoc test.
Figure S3. IL‐1β (a–c) and IL‐12p40 (d–f) secretion in the supernatants from 20 000 (a, d), 40 000 (b, e) or 100 000 (c, f) DCs grown in the washed supernatant from neutrophil‐free wells containing GM‐CSF + LPS stimulants with or without the addition of αBC. One representative experiment of 3 individual experiments. Error bars represent spread of data from minimum to maximum.
Figure S4. (a, b) Flow cytometric analysis of the percentage (a) and number (b) of Annexin V and/or PI stained bone marrow‐derived neutrophils that were stimulated with GM‐CSF + LPS for 20 hr while in the presence or not of αBC, n = 4 animals per group. (c) Percentage of PI positive GM‐CSF + LPS stimulated neutrophils treated with αBC in the presence or absence of 1 μm of kinase inhibitors. Results show the combined mean and minimum to maximum spread of data from 3 individual experiments. Statistical analyses were performed by repeated measures two‐way ANOVA with Šídák post hoc test, *P < 0·05 of main effect.
Figure S5. Dendritic cell antigen presenting marker expression, chemokine secretion and interactive ability were not altered when co‐cultured with αBC pre‐treated neutrophils. (a‐d) Mean fluorescence index relative to control of CD40 (a), CD80 (b), CD86 © and MHCII (d) surface expression on DCs grown in a 1:5 ratio with GM‐CSF + LPS‐activated neutrophils that had been grown in the presence (squares) or absence (circles) of αBC. Results represent combined mean and spread of 4 independent experiments. Statistical analyses were performed by repeated measures two‐way ANOVA with Šídák post hoc test. (e) Sample images of DCs (black arrowheads) with no interactions (left panel) and multiple interactions (white arrowheads in right panel) with co‐cultured neutrophils (black arrows). (f) Combined mean cell interactions of 3 individual experiments showing the mean number of DCs with zero (white bars), one (light grey bars), and two or more neutrophil interactions (dark grey bars). Error bars represent standard deviation. Statistical analyses were performed by repeated measures two‐way ANOVA with Šídák post hoc test. (g, h) MIP‐1α (g) and MCP‐1 (h) secretion in supernatants from neutrophils that had been treated with (squares) or without (circles) αBC. Data show the combined mean and spread of 9 (MIP‐1α) (g) and 6 (MCP‐1) (h) independent experiments. Statistical analyzes were completed by repeated measures two‐way ANOVA with Dunnett's multiple comparison test (MCP‐1), *P < 0·05 of main effect of stimulation on MIP‐1.
Acknowledgements
This work was supported by operating grants from the Canadian Institutes of Health Research (MOP‐97884) and Alberta Innovates‐Health Solutions (AIHS; 200800409). TMF was a recipient of a Vanier Canada Graduate Scholarship, Hotchkiss Brain Institute Dr T. Chen Fong Doctoral Scholarship in Neuroscience, Multiple Sclerosis Society of Canada Doctoral Studentship and an AIHS Doctoral Studentship.
References
- 1. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11:519–31. [DOI] [PubMed] [Google Scholar]
- 2. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 3. Pittman K, Kubes P. Damage‐associated molecular patterns control neutrophil recruitment. J Innate Immun 2013; 5:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006; 6:541–50. [DOI] [PubMed] [Google Scholar]
- 5. Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. J Exp Med 2010; 207:923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welte K, Zeidler C, Dale DC. Severe congenital neutropenia. Semin Hematol 2006; 43:189–95. [DOI] [PubMed] [Google Scholar]
- 7. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci 2006; 11:529–43. [DOI] [PubMed] [Google Scholar]
- 8. Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA. Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs. J Immunol 2013; 191:4531–9. [DOI] [PubMed] [Google Scholar]
- 9. van Gisbergen KP, Ludwig IS, Geijtenbeek TB, van Kooyk Y. Interactions of DC‐SIGN with Mac‐1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett 2005; 579:6159–68. [DOI] [PubMed] [Google Scholar]
- 10. van Gisbergen KP, Sanchez‐Hernandez M, Geijtenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation‐dependent interactions between Mac‐1 and DC‐SIGN. J Exp Med 2005; 201:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Megiovanni AM, Sanchez F, Robledo‐Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol 2006; 79:977–88. [DOI] [PubMed] [Google Scholar]
- 12. Ludwig IS, Geijtenbeek TB, van Kooyk Y. Two way communication between neutrophils and dendritic cells. Curr Opin Pharmacol 2006; 6:408–13. [DOI] [PubMed] [Google Scholar]
- 13. Alfaro C, Suarez N, Onate C, Perez‐Gracia JL, Martinez‐Forero I, Hervas‐Stubbs S et al Dendritic cells take up and present antigens from viable and apoptotic polymorphonuclear leukocytes. PLoS One 2011; 6:e29300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross‐talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol 2003; 171:6052–8. [DOI] [PubMed] [Google Scholar]
- 15. Reddy VS, Reddy GB. Emerging role for alphaB‐crystallin as a therapeutic agent: pros and cons. Curr Mol Med 2015; 15:47–61. [DOI] [PubMed] [Google Scholar]
- 16. Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci 2012; 15:1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA et al Protective and therapeutic role for alphaB‐crystallin in autoimmune demyelination. Nature 2007; 448:474–9. [DOI] [PubMed] [Google Scholar]
- 18. Oyebamiji AI, Finlay TM, Hough RM, Hoghooghi V, Lim EM, Wong CH et al Characterization of migration parameters on peripheral and central nervous system T cells following treatment of experimental allergic encephalomyelitis with CRYAB. J Neuroimmunol 2013; 259:66–74. [DOI] [PubMed] [Google Scholar]
- 19. Park H, Park H, Hwang HJ, Hwang HS, Kim H, Choi BR et al Alpha B‐crystallin prevents ventricular arrhythmia by attenuating inflammation and oxidative stress in rat with autoimmune myocarditis. Int J Cardiol 2015; 182:399–402. [DOI] [PubMed] [Google Scholar]
- 20. Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC et al Therapeutic effects of systemic administration of chaperone alphaB‐crystallin associated with binding proinflammatory plasma proteins. J Biol Chem 2012; 287:9708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Noort JM, Bsibsi M, Nacken PJ, Gerritsen WH, Amor S, Holtman IR et al Activation of an immune‐regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat‐shock protein alpha B‐crystallin‐loaded PLGA microparticles. Biomaterials 2013; 34:831–40. [DOI] [PubMed] [Google Scholar]
- 22. Cobb BA, Petrash JM. Characterization of alpha‐crystallin‐plasma membrane binding. J Biol Chem 2000; 275:6664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dieterich LC, Schiller P, Huang H, Wawrousek EF, Loskog A, Wanders A et al alphaB‐Crystallin regulates expansion of CD11b(+)Gr‐1(+) immature myeloid cells during tumor progression. FASEB J 2013; 27:151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G et al Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem 2012; 287:36 423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quach QL, Metz LM, Thomas JC, Rothbard JB, Steinman L, Ousman SS. CRYAB modulates the activation of CD4+ T cells from relapsing‐remitting multiple sclerosis patients. Mult Scler 2013; 19:1867–77. [DOI] [PubMed] [Google Scholar]
- 26. Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol 2010; 10:1325–34. [DOI] [PubMed] [Google Scholar]
- 27. Tecchio C, Cassatella MA. Neutrophil‐derived cytokines involved in physiological and pathological angiogenesis. Chem Immunol Allergy 2014; 99:123–37. [DOI] [PubMed] [Google Scholar]
- 28. Tecchio C, Micheletti A, Cassatella MA. Neutrophil‐derived cytokines: facts beyond expression. Front Immunol 2014; 5:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H et al Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog 2015; 11:e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10:593–601. [DOI] [PubMed] [Google Scholar]
- 31. Lerchenberger M, Uhl B, Stark K, Zuchtriegel G, Eckart A, Miller M et al Matrix metalloproteinases modulate ameboid‐like migration of neutrophils through inflamed interstitial tissue. Blood 2013; 122:770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saturnino SF, Prado RO, Cunha‐Melo JR, Andrade MV. Endotoxin tolerance and cross‐tolerance in mast cells involves TLR4, TLR2 and FcepsilonR1 interactions and SOCS expression: perspectives on immunomodulation in infectious and allergic diseases. BMC Infect Dis 2010; 10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN et al Selective roles for Toll‐like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 2003; 170:5268–75. [DOI] [PubMed] [Google Scholar]
- 34. Tsukahara Y, Morisaki T, Kojima M, Uchiyama A, Tanaka M. iNOS expression by activated neutrophils from patients with sepsis. ANZ J Surg 2001; 71:15–20. [DOI] [PubMed] [Google Scholar]
- 35. Webb JL, Polak JM, Evans TJ. Effect of adhesion on inducible nitric oxide synthase (iNOS) production in purified human neutrophils. Clin Exp Immunol 2001; 123:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW et al A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac‐1. J Clin Invest 2012; 122:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin CL, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol 1998; 28:4114–22. [DOI] [PubMed] [Google Scholar]
- 38. Scimone ML, Lutzky VP, Zittermann SI, Maffia P, Jancic C, Buzzola F et al Migration of polymorphonuclear leucocytes is influenced by dendritic cells. Immunology 2005; 114:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C‐C chemokines bind and induce directional migration of dendritic cells in vitro . J Leukoc Biol 1996; 60:365–71. [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo‐Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 2009; 31:761–71. [DOI] [PubMed] [Google Scholar]
- 41. Simard JC, Girard D, Tessier PA. Induction of neutrophil degranulation by S100A9 via a MAPK‐dependent mechanism. J Leukoc Biol 2010; 87:905–14. [DOI] [PubMed] [Google Scholar]
- 42. Zhu Z, Li R, Stricker R, Reiser G. Extracellular alpha‐crystallin protects astrocytes from cell death through activation of MAPK, PI3K/Akt signaling pathway and blockade of ROS release from mitochondria. Brain Res 2015; 1620:17–28. [DOI] [PubMed] [Google Scholar]
- 43. Mao Y, Xiang H, Wang J, Li D. AlphaB crystallin prevents calcimycin‐induced apoptosis through repression of Erk/p38 MAP kinases and caspase‐3. Invest Ophthalmol Vis Sci 2002; 43:2990. [Google Scholar]
- 44. Vasconcelos ZF, Santos BM, Costa ES, Lima M, Tabak DG, Bouzas LF et al T‐lymphocyte function from peripheral blood stem‐cell donors is inhibited by activated granulocytes. Cytotherapy 2003; 5:336–45. [DOI] [PubMed] [Google Scholar]
- 45. Vasconcelos ZF, Dos Santos BM, Farache J, Palmeira TS, Areal RB, Cunha JM et al G‐CSF‐treated granulocytes inhibit acute graft‐versus‐host disease. Blood 2006; 107:2192–9. [DOI] [PubMed] [Google Scholar]
- 46. Ribeiro‐Gomes FL, Romano A, Lee S, Roffe E, Peters NC, Debrabant A et al Apoptotic cell clearance of Leishmania major‐infected neutrophils by dendritic cells inhibits CD8(+) T‐cell priming in vitro by Mer tyrosine kinase‐dependent signaling. Cell Death Dis 2015; 6:e2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 2012; 32:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Couper KN, Blount DG, Riley EM. IL‐10: the master regulator of immunity to infection. J Immunol 2008; 180:5771–7. [DOI] [PubMed] [Google Scholar]
- 49. Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 2014; 7:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase‐8 inactivates macrophage inflammatory protein‐1 alpha to reduce acute lung inflammation and injury in mice. J Immunol 2010; 184:1575–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee EJ, Han JE, Woo MS, Shin JA, Park EM, Kang JL et al Matrix metalloproteinase‐8 plays a pivotal role in neuroinflammation by modulating TNF‐alpha activation. J Immunol 2014; 193:2384–93. [DOI] [PubMed] [Google Scholar]
- 52. Owen CA, Hu Z, Lopez‐Otin C, Shapiro SD. Membrane‐bound matrix metalloproteinase‐8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase‐resistant collagenase and serpinase. J Immunol 2004; 172:7791–803. [DOI] [PubMed] [Google Scholar]
- 53. Ramanujum R, Lin YL, Liu JK, He S. Regulatory expression of MMP‐8/MMP‐9 and inhibition of proliferation, migration and invasion in human lung cancer A549 cells in the presence of HGF variants. Kaohsiung J Med Sci 2013; 29:530–9. [DOI] [PubMed] [Google Scholar]
- 54. Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997; 89:3503–21. [PubMed] [Google Scholar]
- 55. Elbim C, Reglier H, Fay M, Delarche C, Andrieu V, El Benna J et al Intracellular pool of IL‐10 receptors in specific granules of human neutrophils: differential mobilization by proinflammatory mediators. J Immunol 2001; 166:5201–7. [DOI] [PubMed] [Google Scholar]
- 56. Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood 2011; 118:9–18. [DOI] [PubMed] [Google Scholar]
- 57. Brenner S, Prosch S, Schenke‐Layland K, Riese U, Gausmann U, Platzer C. cAMP‐induced Interleukin‐10 promoter activation depends on CCAAT/enhancer‐binding protein expression and monocytic differentiation. J Biol Chem 2003; 278:5597–604. [DOI] [PubMed] [Google Scholar]
- 58. Garcia‐Prieto E, Gonzalez‐Lopez A, Cabrera S, Astudillo A, Gutierrez‐Fernandez A, Fanjul‐Fernandez M et al Resistance to bleomycin‐induced lung fibrosis in MMP‐8 deficient mice is mediated by interleukin‐10. PLoS One 2010; 5:e13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mocsai A, Jakus Z, Vantus T, Berton G, Lowell CA, Ligeti E. Kinase pathways in chemoattractant‐induced degranulation of neutrophils: the role of p38 mitogen‐activated protein kinase activated by Src family kinases. J Immunol 2000; 164:4321–31. [DOI] [PubMed] [Google Scholar]
- 60. Fensome A, Cunningham E, Prosser S, Tan SK, Swigart P, Thomas G et al ARF and PITP restore GTP gamma S‐stimulated protein secretion from cytosol‐depleted HL60 cells by promoting PIP2 synthesis. Curr Biol 1996; 6:730–8. [DOI] [PubMed] [Google Scholar]
- 61. Hao J, Meng LQ, Xu PC, Chen M, Zhao MH. p38MAPK, ERK and PI3K signaling pathways are involved in C5a‐primed neutrophils for ANCA‐mediated activation. PLoS One 2012; 7:e38317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c‐MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer 2007; 97:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu F, Yu H, Liu J, Cheng L. alphaB‐crystallin regulates oxidative stress‐induced apoptosis in cardiac H9c2 cells via the PI3K/AKT pathway. Mol Biol Rep 2013; 40:2517–26. [DOI] [PubMed] [Google Scholar]
- 64. Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Liu L et al Human alphaA‐ and alphaB‐crystallins prevent UVA‐induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res 2004; 79:393–403. [DOI] [PubMed] [Google Scholar]
- 65. Sauce D, Dong Y, Campillo‐Gimenez L, Casulli S, Bayard C, Autran B et al Reduced oxidative burst by primed neutrophils in the elderly individuals is associated with increased levels of the CD16bright/CD62Ldim immunosuppressive subset. J Gerontol A Biol Sci Med Sci 2016; 72:163–72. [DOI] [PubMed] [Google Scholar]
- 66. Prabhu S, Srinivas V, Ramakrishna T, Raman B, Rao ChM. Inhibition of Cu2+‐mediated generation of reactive oxygen species by the small heat shock protein alphaB‐crystallin: the relative contributions of the N‐ and C‐terminal domains. Free Radic Biol Med 2011; 51:755–62. [DOI] [PubMed] [Google Scholar]
- 67. van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Close encounters of neutrophils and DCs. Trends Immunol 2005; 26:626–31. [DOI] [PubMed] [Google Scholar]
- 68. Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G et al Interleukin‐12 is produced by dendritic cells and mediates T helper 1 development as well as interferon‐gamma production by T helper 1 cells. Eur J Immunol 1996; 26:659–68. [DOI] [PubMed] [Google Scholar]
- 69. Mus AM, Cornelissen F, Asmawidjaja PS, van Hamburg JP, Boon L, Hendriks RW et al Interleukin‐23 promotes Th17 differentiation by inhibiting T‐bet and FoxP3 and is required for elevation of interleukin‐22, but not interleukin‐21, in autoimmune experimental arthritis. Arthritis Rheum 2010; 62:1043–50. [DOI] [PubMed] [Google Scholar]
- 70. Iwakura Y, Ishigame H. The IL‐23/IL‐17 axis in inflammation. J Clin Investig 2006; 116:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Watson DC, Sargianou M, Panos G. Interleukin‐12 (IL‐12)/IL‐10 ratio as a marker of disease severity in Crimean‐Congo hemorrhagic fever. Clin Vaccine Immunol 2012; 19:823–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carrieri PB, Ladogana P, Di Spigna G, de Leva MF, Petracca M, Montella S et al Interleukin‐10 and interleukin‐12 modulation in patients with relapsing‐remitting multiple sclerosis on therapy with interferon‐beta 1a: differences in responders and non responders. Immunopharmacol Immunotoxicol 2008; 30:1–9. [DOI] [PubMed] [Google Scholar]
- 73. Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL‐10 on dendritic cell functions. J Immunol 2001; 166:4312–8. [DOI] [PubMed] [Google Scholar]
- 74. Ortwerth BJ, Sharma KK, Olesen PR. A comparison of the inhibition of porcine pancreatic elastase and human neutrophil elastase by alpha‐crystallin. Curr Eye Res 1994; 13:561–7. [DOI] [PubMed] [Google Scholar]
- 75. Guimaraes‐Costa AB, Rochael NC, Oliveira F, Echevarria‐Lima J, Saraiva EM. Neutrophil extracellular Traps reprogram IL‐4/GM‐CSF‐induced monocyte differentiation to anti‐inflammatory macrophages. Front Immunol 2017; 8:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Hydrogen peroxide secreted by neutrophils stimulated with GM‐CSF + LPS and treated with αBC for two (black bars), four (dark grey bars), or 20 hr (light grey bars) with no further stimulation. Combined data from 3 individual experiments. Error bars represent spread of data from minimum to maximum. Statistical analyses were completed by repeated measures two‐way ANOVA with Šídák post hoc test.
Figure S2. αBC treatment does not modulate CD11b and iNOS expression by GM‐CSF + LPS‐stimulated neutrophils. (a) Expression of CD11b by untreated (circles) or αBC‐treated (squares) neutrophils in the combined mean and spread of 6 individual experiments. Statistical analyses were completed by repeated measured two‐way ANOVA with Šídák post hoc test, *P < 0·05 of main effect. (b) Relative fold change of iNOS mRNA in neutrophils treated with or without αBC for 2, 6, or 20 h compared to GM‐CSF + LPS stimulation and normalized to amplified GAPDH. Data represents 5 combined individual experiments. Error bars represent spread of minimum to maximum. Statistical analyses were performed by repeated measures one‐way ANOVA with Dunnett's post hoc test, *P < 0·05 of post hoc test.
Figure S3. IL‐1β (a–c) and IL‐12p40 (d–f) secretion in the supernatants from 20 000 (a, d), 40 000 (b, e) or 100 000 (c, f) DCs grown in the washed supernatant from neutrophil‐free wells containing GM‐CSF + LPS stimulants with or without the addition of αBC. One representative experiment of 3 individual experiments. Error bars represent spread of data from minimum to maximum.
Figure S4. (a, b) Flow cytometric analysis of the percentage (a) and number (b) of Annexin V and/or PI stained bone marrow‐derived neutrophils that were stimulated with GM‐CSF + LPS for 20 hr while in the presence or not of αBC, n = 4 animals per group. (c) Percentage of PI positive GM‐CSF + LPS stimulated neutrophils treated with αBC in the presence or absence of 1 μm of kinase inhibitors. Results show the combined mean and minimum to maximum spread of data from 3 individual experiments. Statistical analyses were performed by repeated measures two‐way ANOVA with Šídák post hoc test, *P < 0·05 of main effect.
Figure S5. Dendritic cell antigen presenting marker expression, chemokine secretion and interactive ability were not altered when co‐cultured with αBC pre‐treated neutrophils. (a‐d) Mean fluorescence index relative to control of CD40 (a), CD80 (b), CD86 © and MHCII (d) surface expression on DCs grown in a 1:5 ratio with GM‐CSF + LPS‐activated neutrophils that had been grown in the presence (squares) or absence (circles) of αBC. Results represent combined mean and spread of 4 independent experiments. Statistical analyses were performed by repeated measures two‐way ANOVA with Šídák post hoc test. (e) Sample images of DCs (black arrowheads) with no interactions (left panel) and multiple interactions (white arrowheads in right panel) with co‐cultured neutrophils (black arrows). (f) Combined mean cell interactions of 3 individual experiments showing the mean number of DCs with zero (white bars), one (light grey bars), and two or more neutrophil interactions (dark grey bars). Error bars represent standard deviation. Statistical analyses were performed by repeated measures two‐way ANOVA with Šídák post hoc test. (g, h) MIP‐1α (g) and MCP‐1 (h) secretion in supernatants from neutrophils that had been treated with (squares) or without (circles) αBC. Data show the combined mean and spread of 9 (MIP‐1α) (g) and 6 (MCP‐1) (h) independent experiments. Statistical analyzes were completed by repeated measures two‐way ANOVA with Dunnett's multiple comparison test (MCP‐1), *P < 0·05 of main effect of stimulation on MIP‐1.


