Abstract
Microporous organic polymers (MOPs) are promising materials for gas sorption because of their intrinsic and permanent porosity, designable framework, and low density. The introduction of nitrogen-rich building block in MOPs will greatly enhance the gas sorption capacity. Here, we report the synthesis of MOPs from the 2,4,6-tris(4-ethynylphenyl)-1,3,5-triazine unit and aromatic azides linkers by click polymerization reaction. Fourier transform infrared (FTIR) and solid-state 13C CP-MAS (Cross Polarization-Magic Angle Spinning) NMR confirm the formation of the polymers. CMOP-1 and CMOP-2 exhibit microporous networks with a BET (Brunauer–Emmett–Teller) surface area of 431 m2·g−1 and 406 m2·g−1 and a narrow pore size distribution under 1.2 nm. Gas sorption isotherms including CO2 and H2 were measured. CMOP-1 stores a superior CO2 level of 1.85 mmol·g−1 at 273 K/1.0 bar, and an H2 uptake of up to 2.94 mmol·g−1 at 77 K/1.0 bar, while CMOP-2, with its smaller surface area, shows a lower CO2 adsorption capacity of 1.64 mmol·g−1 and an H2 uptake of 2.48 mmol·g−1. In addition, I2 vapor adsorption was tested at 353 K. CMOP-1 shows a higher gravimetric load of 160 wt%. Despite the moderate surface area, the CMOPs display excellent sorption ability for CO2 and I2 due to the nitrogen-rich content in the polymers.
Keywords: microporous organic polymers, nitrogen-rich, CO2 adsorption, H2 adsorption, I2 vapor sorption
1. Introduction
During the last few decades, microporous organic polymers (MOPs) have been intensively investigated and applied in various fields such as heterogeneous catalysis [1], gas adsorption and separation [2,3], the adsorption of radioactive iodine [4], drug delivery [5,6] and photovoltaics [7,8,9] duo to the low density, large surface area, high thermal/chemical stability, functional surface, and diverse building blocks. Until now, numerous MOPs including crystalline covalent organic frameworks (COFs) [10], covalent triazine frameworks (CTFs) [11], benzimidazole-linked polymers (BILPs) [12], porous polymer networks (PPNs) [13], hyper-crosslinked polymers (HCPs) [14], conjugated microporous polymers (CMPs) [15], polymers of intrinsic microporosity (PIMs) [16], and porous aromatic frameworks (PAFs) [17] have been synthesized via different polymerization reactions and versatile synthons. Amorphous polymers have received more and more attention owing to their scale-up preparation, high stability, and controllable synthesis compared with crystalline networks.
The excessive emission of carbon dioxide is believed to be the main contribution to the global warming problem resulting from the burning of fossil fuels. Great effort has been devoted to exploring the technology for CO2 capture and storage. Recently, MOPs have generated enormous interest in the area of gas sorption for carbon dioxide, hydrogen, iodine, and methane because of their low weight and remarkable microporous framework [18,19,20]. To achieve higher gas uptake by MOPs, a large surface area, suitable pore size, and strong interaction are crucial and desired. The introduction of heteroatoms, especially nitrogen or oxygen, has been demonstrated to be feasible and effective to enhance adsorption load by means of stronger interaction between gas molecules and adsorbent [21,22,23]. Triazine-based building blocks that have inherent nitrogen atoms can not only combine metal ions for catalysis [24], but also provide polarizable sites for gas uptake. Pan et al. reported imide functionalized 1,3,5-triazine frameworks named TPIs@IC. The polymers had ultramicropores at 5.4–6.8 Å, which can adsorb 3.2 mmol·g−1 CO2 (273 K/1 bar) and 1.47 wt% H2 (77 K/1 bar) [25]. Bu et al. synthesized aminal-linked porous organic polymers (APOPs) with a moderate to high surface area (SBET = 724–1402 m2·g−1), but significant gas adsorption (CO2: 4.54 mmol·g−1 at 273 K/1 bar; H2: 8.95 mmol·g−1 at 77 K/1 bar), especially high CO2 adsorption, and good selectivity over N2 at low pressure (0.15 bar) [26]. In 2015, Lotsch et al. reported amorphous CTFs synthesized at different temperatures. For these polymers, excellent CO2 (5.58 mmol·g−1) and H2 (2.12 wt %) adsorption capacities were observed [27]. In addition, many CIFs with microporous frameworks for gas storage and separation have been reported, such as the cCTF (SBET = 1247 m2·g−1; CO2: 13.3 wt%), fl-CTFs (SBET = 15–2862 m2·g−1; CO2: 1.27–4.28 mmol·g−1), CTF-TB-3 (SBET = 612 m2·g−1; CO2: 16.84 wt%), and CTF-BIs (CO2: 21.68 wt%) [28,29,30,31]. Zhang et al. synthesized polymers with N-heterocyclic groups (NAPOPs). NAPOP-1 and NAPOP-3 exhibit high adsorption toward iodine (>240 wt%) and moderate CO2 adsorption [32]. Geng et al. constructed novel triazine-based TCMPs by Friedel–Crafts polymerization reaction [33]. Among them, TTPPA displays excellent reversible iodine vapor uptake of 4.90 g·g−1. Furthermore, the TCMPs show sensitive sensing ability toward o-nitrophenol. Zhang et al. synthesized a triptycene-based polymer (NTP) by modified Yamamoto-type Ullmann cross-coupling reaction. The NTP displays good gas uptake (CO2: 15.2 wt%; H2: 1.58 wt%) and high iodine sorption capacity (180 wt%) [34]. Liao et al. constructed NCMPs with high N content and ultramicroporosity, which showed 215 wt% iodine vapor uptake at ambient pressure [35]. Zhao et al. reported a new nitrogen-rich COF with two kinds of micropores that exhibited extremely high iodine uptake (481 wt%) [36].
The N-heterocycle derived from the efficient click reaction can further increase the nitrogen content for abundant sorption sites. Keeping this consideration in mind, we synthesized nitrogen-rich polymers named CMOPs from 2,4,6-tris(4-ethynylphenyl)-1,3,5-triazine (TEPT) and azides by click polymerization reaction (Scheme 1). The polymers were characterized by FTIR, solid state 13C CP-MAS, NMR, and elemental analysis (EA). Thermogravimetric analysis (TGA) showed the high thermal stability. Powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM) confirmed the amorphous but uniform phase. Nitrogen adsorption–desorption isotherms at 77 K were investigated to explore the surface area, porosity, and pore size. In addition, adsorption measurements of CO2, H2, and I2 were researched to evaluate the gas sorption capacity.
Scheme 1.
The synthetic route to themicroporous organic polymers.
2. Results
2.1. Synthesis and Character
The CMOPs were synthesized by click reaction in DMF solvent from TEPT and aromatic azides, according to the procedure in Scheme 1. The resulting brown powders were insoluble in common organic solvent such as tetrahydrofuran, dichloromethane, methanol, chloroform, acetone, and N,N-dimethylformamide.
In the Fourier transform infrared (FTIR) spectra of CMOPs (Figure S1), characteristic peaks for TEPT at 1505 cm−1 and 3252 cm−1 attenuated dramatically, while peaks at 2106 cm−1 and 2095 cm−1 for azide-1 and azide-2 disappeared, and new peaks at 1617, 2921 cm−1 for CMOP-1 and 1612, 2927 cm−1 for CMOP-2 could be observed, confirming the occurring of click reaction and the formation of a triazole ring. The solid state 13C CP-MAS NMR measurements gave similar spectra for the two CMOPs (Figure S2). The peak at ~169 ppm could be attributed to the carbon atom in triazine ring, while the peak at ~ 146 ppm was ascribed to phenyl carbon, which was directly linked with the triazine ring. The main peaks at ~141 ppm and ~134 ppm originated from the two carbon atoms of the triazole ring. The broad signals at about ~ 128–110 ppm corresponded to the phenyl rings.
Thermogravimetric analysis in the N2 atmosphere showed that the polymers could keep thermal stability under 240 °C (CMOP-1) and 210 °C (CMOP-2). The first 5% weight loss was mainly due to the volatilization of solvent adsorbed in the polymers (Figure S3). Powder X-ray diffraction (PXRD) analysis in the angle range of 5° to 35° suggested the amorphous nature of the CMOPs (Figure S4). We considered that the relative strong peaks may originate the light copper complex due to the interaction between Cu2+ and nitrogen atoms. Scanning electron microscopy (SEM) images revealed the uniform morphology (Figure S5).
2.2. Porosity
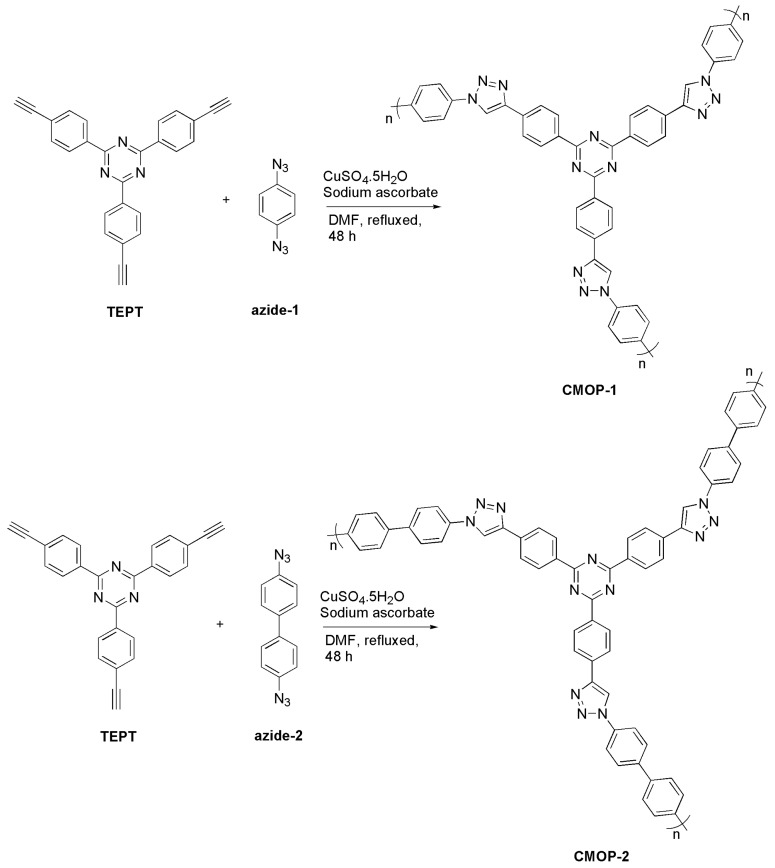
The surface area and porosity of CMOPs was investigated by N2 adsorption–desorption measurements at 77 K. The results were illustrated in Figure 1. CMOP-1 and CMOP-2 exhibited type-I isotherms that showed a sharp adsorption at relative low pressure (P/Po < 0.05), indicating a permanent microporous network. The Brunauer–Emmett–Teller (BET) surface areas were calculated to be 431 m2·g−1 and 406 m2·g−1, while the microporous areas were 209 m2·g−1 and 188 m2·g−1. The total pore volumes of CMOP-1 and CMOP-2 were 0.458 cm3·g−1 and 0.387 cm3·g−1, with 0.09 cm3·g−1 and 0.08 cm3·g−1 microporous volume. The pore size distribution was estimated on the basis of the density functional theory (DFT) model. It revealed that the CMOPs exhibited two main distribution at 0.6 nm, 1.2 nm, and 0.7, 1.2 nm for CMOP-1 and CMOP-2, indicating the existence of ultramicroporosity (<1 nm), which was consistent with the microporous N2 sorption isotherm (Figure S6). Although the azide-2 molecule is longer than that of azide-1, the pore sizes were almost the same due to the disordered overlap in the formation of the porous network.
Figure 1.
N2 adsorption–desorption isotherm at 77 K (filled: adsorption; empty: desorption).
2.3. Gas Sorption Capacity
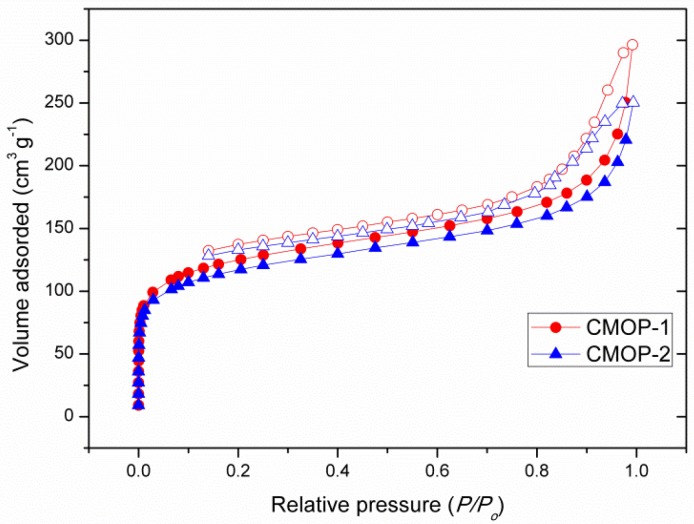
The microporous framework with a narrow pore size distribution and nitrogen-rich character inspired us to explore CO2 adsorption capacity. As shown in Figure 2, CMOP-1 exhibited a higher CO2 adsorption ability of 1.85 mmol·g−1 (8.2 wt%) at 273 K and 0.80 mmol·g−1 (3.5 wt%) at 298 K, while CMOP-2, with its smaller surface area, showed a slightly lower uptake to 1.64 mmol·g−1 (7.3 wt%) and 0.57 mmol·g−1 (2.5 wt%) at 1.0 bar, which was superior to many heteroatom-rich polymers such as FPOP-1 (SBET: 538 m2·g−1, 6.3 wt%), NOP-55 (SBET: 526 m2·g−1, 8.6 wt%), CPOP-22 (SBET: 440 m2·g−1, 6.9 wt%), and CuPor-BPDC (SBET: 442 m2·g−1, 5.5 wt%) [37,38,39,40], but lower than other triazine-based polymers [26,27,28,29,30,31].
Figure 2.
CO2 adsorption isotherm at 273/298 K.
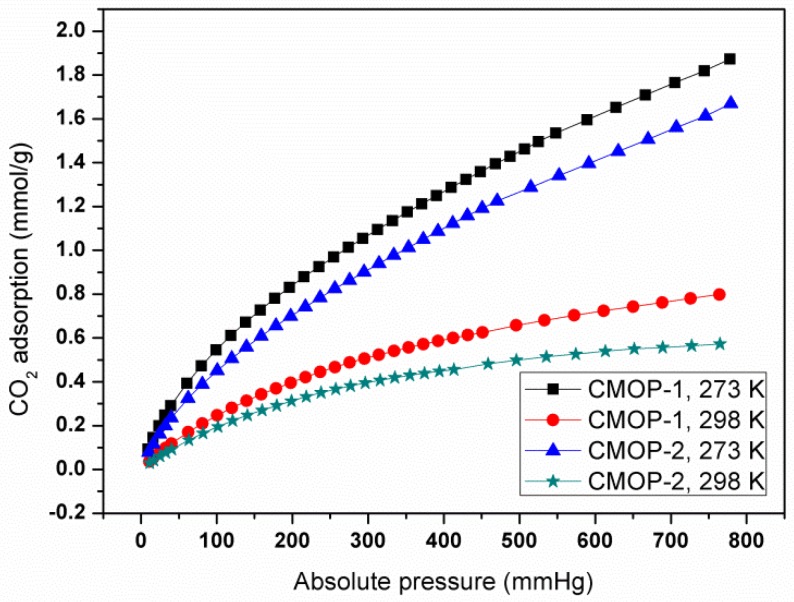
In addition, H2 uptake was also investigated at 77 K (Figure 3). The adsorption capacity was increasing along with the pressure, and then increased up to saturation. The maximum uptakes were 2.94 mmol·g−1 (0.6 wt%) and 2.48 mmol·g−1 (0.5 wt%) for CMOP-1 and CMOP-2. The moderate capacity originated from the low surface area.
Figure 3.
H2 adsorption isotherm at 77 K.
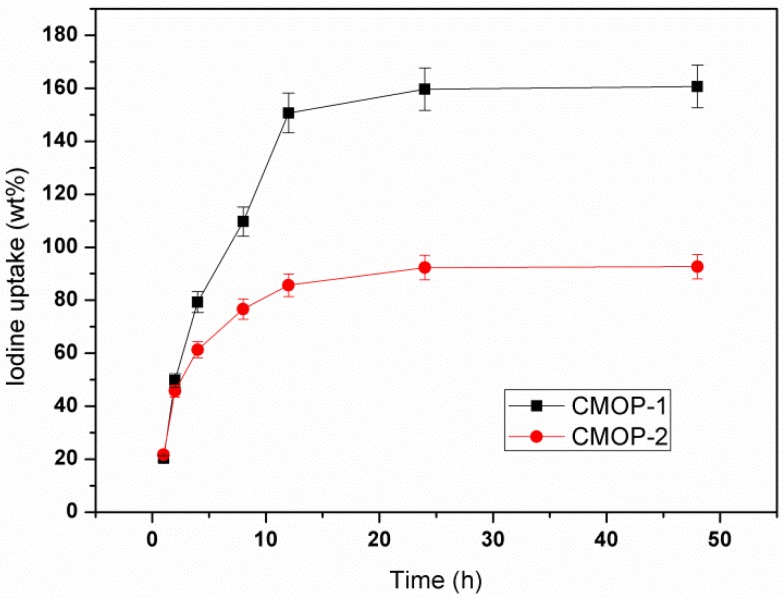
It was found that nitrogen-rich polymer favored the sorption toward iodine, owing to the stronger interaction between nitrogen atom and electron-deficient I2 [4,32,33]. The images in Figure S7 showed the color change of I2-cyclohexane (1 mg·mL−1) solution after adding 25 mg of CMOP-1 and CMOP-2. As time passed, the pink color of I2-cyclohexane solution faded clearly, indicating the good removal efficiency. To evaluate the I2 uptake ability of CMOPs, the sorption of I2 vapor was investigated at 353 K. The solid powder was placed in an open bottle that was exposed to vapor of I2 in a sealed container. As shown in Figure 4, the gravimetric uptake was gradually increasing, and then reached a plateau after about 24 h. The sorption ability was 160 wt% and 93 wt% for CMOP-1 and CMOP-2. The larger capacity of CMOP-1 was mainly attributed to the larger surface area and greater amount of nitrogen atoms in the surface.
Figure 4.
The uptake of I2 vapor at 353 K.
3. Materials and Methods
3.1. General Information
TEPT and azides were synthesized according to the methods in the literature [41,42,43]. All of the other reagents were purchased from J&K (J&K, ShangHai, China) and used without further purification.
Before all of the measurements, polymers were dried under vacuum at 120 °C overnight. FTIR spectra were recorded on a Thermo-Nicolet iS10 spectrometer using KBr discs (Thermo Scientific Co., Ltd., Madison, WI, USA). Solid state 13C CP-MAS NMR spectra data were collected on a BRUKER AVANCE III HD 400MHz NMR spectrometer (Bruker, Leipzig, Germany). Elemental analysis for C, H, and N were carried out with Elementar VarioMICRO Cube analyzer (Elementar, Frankfurt, Germany). Thermogravimetric analysis was performed on SDT Q600 V20.9 Build 20 (TA Instrument, New Castle, DE, USA) with a temperature ramping rate of 10 °C·min−1 from 30 °C to 850 °C. Scanning electron microscopy (SEM) was performed on ZEISS Evo 18 scanning electron microscope at 20.0 KV (Zeiss, Oberkochen, Germany). Powder X-ray diffraction (PXRD) data were recorded on Bruker D8 Advance (Bruker, Leipzig, Germany), from 5° up to 35° with 4°/min increment. Nitrogen adsorption–desorption isotherms were carried out with a Demo 2020 instrument at 77 K (Micromeritics Instrument Corp., Norcross, GA, USA). Carbon dioxide adsorption isotherms were measured at 273 K and 298 K on 3Flex Version 4.02 (Micromeritics Instrument Corp., Norcross, GA, USA). Hydrogen adsorption isotherms were conducted at 77 K on Quadrasorb SI (Quantachrome, FL, USA).
3.2. Synthesis
CMOP-1: Under nitrogen atmosphere, TEPT (80 mg, 0.209 mmol), azide-1 (51 mg, 0.318 mmol), CuSO4·5H2O (15 mg, 0.06 mmol), sodium ascorbate (13 mg, 0.05 mmol), and dry DMF (7 mL) were placed in a flask, and then heated to reflux for 48 h. The resulting brown solid was filtrated and washed with water, methanol, and DCM. The residue was stirred in distilled water overnight, and then filtrated and dried in vacuum (101 mg, 77%). Anal. calculated for (C12H7N4)n: C 69.54, H 3.41, N 27.04; found (C12H7N4·0.05SO42−)n: C 57.37, H 4.17, N 18.75, S 0.733
CMOP-2: Under nitrogen atmosphere, TEPT (68 mg, 0.178 mmol), azide-2 (64 mg, 0.271 mmol), CuSO4·5H2O (14 mg, 0.05 mmol), sodium ascorbate (11 mg, 0.045 mmol), and dry DMF (7 mL) were placed in a flask, and then heated to reflux for 48 h. The resulting brown solid was filtrated and washed with water, methanol, and DCM. The residue was stirred in distilled water overnight, and then filtrated and dried in vacuum (106 mg, 80%). Anal. calculated for (C15H9N4)n: C 73.44, H 3.70, N 22.84; found (C15H9N4·0.01SO42−)n : C 63.20, H 4.27, N 15.49, S 0.433.
3.3. The Gravimetric Sorption of I2 Vapor
10 mg of polymer was placed in an open bottle (10 mL) and the total weight was weighed (W0, mg). The open bottle was then putted into a sealed vessel (50 mL) that contained 200 mg of iodine at 353 K in the oil bath. The open bottle was taken out and weighed after 1 h, 4 h, 8 h, 12 h, 24 h, and 48 h (Wi, mg), respectively. The gravimetric sorption (wt%) was calculated using the following formula:
| wt% = (Wi − W0)/10 × 100% |
4. Conclusions
In conclusion, two nitrogen-rich triazine-based microporous organic polymers were designed and synthesized by the condensation of a 2,4,6-tris(4-ethynylphenyl)-1,3,5-triazine building block and aromatic azides linkers for the first time. The resulting polymers possessed an ultramicroporosity network, good thermal stability, and high nitrogen content (CMOP-1: 18.17 wt%; CMOP-2: 15.49 wt%, from EA). Despite the moderate surface areas of 431 m2·g−1 and 406 m2·g−1 based on nitrogen adsorption, CMOPs exhibited relatively high CO2 sorption and moderate H2 sorption capacity that was comparable to or superior than many POPs with similar surface area. We ascribed the excellent CO2 sorption capacity to nitrogen-rich content, which improved the dipole–quadruple interaction between CO2 and polymers. Furthermore, the CMOPs displayed significant gravimetric uptake ability toward I2 vapor up to 160 wt%. The successfully constructed networks confirmed the advantage of nitrogen atoms for gas capture, and inspired our great interest to explore more functional MOPs.
Acknowledgments
The authors thank Junmin Liu for gas sorption measurement.
Supplementary Materials
Supplementary materials are available online. Figure S1. FTIR spectra of TEPT, azides and CMOPs. Figure S2. 13C CP-MAS NMR spectra of CMOP-1 and CMOP-2. Figure S3. Thermogravimetric analysis (TGA) curves of CMOP-1 and CMOP-2. Figure S4. Powder X-ray diffraction (PXRD) spectra of CMOP-1 and CMOP-2. Figure S5. SEM images (a) CMOP-1, (b) CMOP-2. Figure S6. Brunauer–Emmett–Teller (BET) plot and pore size distribution (a)/(c) CMOP-1, (b)/(d) CMOP-2. Figure S7. Color change of iodine–cyclohexane solution (a) CMOP-1, (b) CMOP-2.
Author Contributions
J.-R.S. conceptualized and designed the experiment route, performed all of the experiment work, participated in the discussion of result and wrote the paper. W.-G.D. and D.-P.L. supervised the work and discussed the result.
Funding
This research was funded by Fundamental Research Fund of Guangxi Institute of Botany [No. 16002] and the Director of Capital Projects of Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization [No. ZRJJ2016-2].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds CMOP-1 and CMOP-2 are available from the authors.
References
- 1.Wang C.-A., Wang W. Advances in Porous Organic Catalysis. Acta Chim. Sin. 2015;73:498–529. doi: 10.6023/A15010019. [DOI] [Google Scholar]
- 2.Wang W., Zhou M., Yuan D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A. 2017;5:1334–1347. doi: 10.1039/C6TA09234A. [DOI] [Google Scholar]
- 3.Kato R., Nishide H. Polymers for carrying and storing hydrogen. Polym. J. 2018;50:77–82. doi: 10.1038/pj.2017.70. [DOI] [Google Scholar]
- 4.Lin Y., Zhu Y., Kuang G., Yu G., Jin R. Application of porous organic polymers in the radioactive iodine adsorption. Prog. Chem. 2017;29:766–775. [Google Scholar]
- 5.Bai L., Phua S.Z.F., Lim W.Q., Jana A., Luo Z., Tham H.P., Zhao L., Gao Q., Zhao Y. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 2016;52:4128–4131. doi: 10.1039/C6CC00853D. [DOI] [PubMed] [Google Scholar]
- 6.Fang Q., Wang J., Gu S., Kaspar R.B., Zhuang Z., Zheng J., Guo H., Qiu S., Yan Y. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 2015;137:8352–8355. doi: 10.1021/jacs.5b04147. [DOI] [PubMed] [Google Scholar]
- 7.Bai L., Gao Q., Zhao Y. Two fully conjugated covalent organic frameworks as anode materials for lithium ion batteries. J. Mater. Chem. A. 2016;4:14106–14110. doi: 10.1039/C6TA06449C. [DOI] [Google Scholar]
- 8.He Q., Zhang C., Li X., Wang X., Pan M., Jiang J. Pyrene-based conjugated microporous polymer as high performance electrode for lithium-ion batteries. Acta Chim. Sin. 2018;76:202–208. doi: 10.6023/A17110477. [DOI] [Google Scholar]
- 9.Liu X., Xu Y., Jiang D. Conjugated microporous polymers as molecular sensing devices: microporous architecture enables rapid response and enhances sensitivity in fluorescence-on and fluorescence-off sensing. J. Am. Chem. Soc. 2012;134:8738–8741. doi: 10.1021/ja303448r. [DOI] [PubMed] [Google Scholar]
- 10.Ding S.-Y., Wang W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013;42:548–568. doi: 10.1039/C2CS35072F. [DOI] [PubMed] [Google Scholar]
- 11.Sakaushi K., Antonietti M. Carbon- and nitrogen-based porous solids: A recently emerging class of materials. Bull. Chem. Soc. Jpn. 2015;88:386–398. doi: 10.1246/bcsj.20140317. [DOI] [Google Scholar]
- 12.Rabbani M.G., El-Kaderi H.M. Synthesis and characterization of porous benzimidazole-linked polymers and their performance in small gas storage and selective uptake. Chem. Mater. 2012;24:1511–1517. doi: 10.1021/cm300407h. [DOI] [Google Scholar]
- 13.Lu W., Yuan D., Zhao D., Schilling C.I., Plietzsch O., Muller T., Bräse S., Guenther J., Blümel J., Krishna R., et al. Porous polymer networks: Synthesis, porosity, and applications in gas storage/separation. Chem. Mater. 2010;22:5964–5972. doi: 10.1021/cm1021068. [DOI] [Google Scholar]
- 14.Tan L., Tan B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017;46:3322–3356. doi: 10.1039/C6CS00851H. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y., Jin S., Xu H., Nagai A., Jiang D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013;42:8012–8031. doi: 10.1039/c3cs60160a. [DOI] [PubMed] [Google Scholar]
- 16.McKeown N.B., Bud P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006;35:675–683. doi: 10.1039/b600349d. [DOI] [PubMed] [Google Scholar]
- 17.Li L., Ren H., Yuan Y., Yu G., Zhu G. Construction and adsorption properties of porous aromatic frameworks via AlCl3-triggered coupling polymerization. J. Mater. Chem. A. 2014;2:11091–11098. doi: 10.1039/C4TA01252F. [DOI] [Google Scholar]
- 18.Klumpen C., Radakovitsch F., Jess A., Senker J. BILP-19-An ultramicroporous organic network with exceptional carbon dioxide uptake. Molecules. 2017;22:1343–1353. doi: 10.3390/molecules22081343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q., Luo M., Hammershøj P., Zhou D., Han Y., Laursen B.W., Yan C.-G., Han B.-H. Microporous polycarbazole with high specific surface area for gas storage and separation. J. Am. Chem. Soc. 2012;134:6084–6087. doi: 10.1021/ja300438w. [DOI] [PubMed] [Google Scholar]
- 20.Yang X., Yu M., Zhao Y., Zhang C., Wang X., Jiang J.-X. Remarkable gas adsorption by carbonized nitrogen-rich hypercrosslinked porous organic polymers. J. Mater. Chem. A. 2014;2:15139–15145. doi: 10.1039/C4TA02782E. [DOI] [Google Scholar]
- 21.Wang T., Zhao Y.-C., Zhang L.-M., Cui Y., Zhang C.-S., Han B.-H. Novel approach to hydroxy-group-containing porous organic polymers from bisphenol A. Beilstein J. Org. Chem. 2017;13:2131–2137. doi: 10.3762/bjoc.13.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S.K., Wang X., Lai Z. Facile synthesis of triazine-triphenylamine-based microporous covalent polymer adsorbent for flue gas CO2 capture. Microporous Mesoporous Mater. 2018;255:76–83. doi: 10.1016/j.micromeso.2017.07.038. [DOI] [Google Scholar]
- 23.Shao L., Li Y., Huang J., Liu Y.-N. Synthesis of triazine-based porous organic polymers derived N-enriched porous carbons for CO2 capture. Ind. Eng. Chem. Res. 2018;57:2856–2865. doi: 10.1021/acs.iecr.7b04533. [DOI] [Google Scholar]
- 24.Puthiaraj P., Lee Y.-R., Zhang S., Ahn W.-S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A. 2016;4:16288–16311. doi: 10.1039/C6TA06089G. [DOI] [Google Scholar]
- 25.Wu S., Gu S., Zhang A., Yu G., Wang Z., Jian J., Pan C. A rational construction of microporous imidebridged covalent–organic polytriazines for highenthalpy small gas absorption. J. Mater. Chem. A. 2015;3:878–885. doi: 10.1039/C4TA04734F. [DOI] [Google Scholar]
- 26.Song W.-C., Xu X.-K., Chen Q., Zhuang Z.-Z., Bu X.-H. Nitrogen-rich diaminotriazine-based porous organic polymers for small gas storage and selective uptake. Polym. Chem. 2013;4:4690–4696. doi: 10.1039/c3py00590a. [DOI] [Google Scholar]
- 27.Hug S., Stegbauer L., Oh H., Hirscher M., Lotsch B.V. Nitrogen-rich covalent triazine frameworks as high-performance platforms for selective carbon capture and storage. Chem. Mater. 2015;27:8001–8010. doi: 10.1021/acs.chemmater.5b03330. [DOI] [Google Scholar]
- 28.Buyukcaki O., Je S.H., Talapanen S.N., Kim D., Coskun A. Charged covalent triazine frameworks for CO2 capture and conversion. ACS Appl. Mater. Interfaces. 2017;9:7209–7216. doi: 10.1021/acsami.6b16769. [DOI] [PubMed] [Google Scholar]
- 29.Hug S., Mesch M.B., Oh H., Popp N., Hirscher M., Senker J., Lotsch B.V. A fluorene based covalent triazine framework with high CO2 and H2 capture and storage capacities. J. Mater. Chem. A. 2014;2:5928–5936. doi: 10.1039/C3TA15417C. [DOI] [Google Scholar]
- 30.Tao L., Niu F., Liu J., Wang T., Wang Q. Troger’s base functionalized covalent triazine frameworks for CO2 capture. RSC Adv. 2016;6:94365–94372. doi: 10.1039/C6RA21196H. [DOI] [Google Scholar]
- 31.Tao L., Niu F., Wang C., Liu J., Wang T., Wang Q. Benzimidazole functionalized covalent triazine frameworks for CO2 capture. J. Mater. Chem. A. 2016;4:11812–11820. doi: 10.1039/C6TA05107C. [DOI] [Google Scholar]
- 32.Weng J.-Y., Xu Y.-L., Song W.-C., Zhang Y.-H. Tuning the adsorption and fluorescence properties of aminal-linked porous organic polymers through N-heterocyclic group decoration. J. Polym. Sci. Polym. Chem. 2016;54:1724–1730. doi: 10.1002/pola.28028. [DOI] [Google Scholar]
- 33.Geng T., Ye S., Zhu Z., Zhang W. Triazine-based conjugated microporous polymers with N,N,N′,N′-tetraphenyl-1,4-phenylenediamine, 1,3,5-tris(diphenylamino)benzene and 1,3,5-tris[(3-methylphenyl)-phenylamino]benzene as the core for high iodine capture and fluorescence sensing of o-nitrophenol. J. Mater. Chem. A. 2018;6:2808–2816. [Google Scholar]
- 34.Ma H., Chen J.-J., Tan L., Bu J.-H., Zhu Y., Tan B., Zhang C. Nitrogen-rich triptycene-based porous polymer for gas storage and iodine enrichment. ACS Macro Lett. 2016;5:1039–1043. doi: 10.1021/acsmacrolett.6b00567. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y., Cheng Z., Zuo W., Thomas A., Faul C.F.J. Nitrogen-rich conjugated microporous polymers: Facile synthesis, efficient gas storage and hetergeneous catalysis. ACS Appl. Mater. Interfaces. 2017;9:38390–38400. doi: 10.1021/acsami.7b09553. [DOI] [PubMed] [Google Scholar]
- 36.Yin Z.-J., Xu S.-Q., Zhan T.-G., Qi Q.-Y., Wu Z.-Q., Zhao X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heterpore covalent organic framework. Chem. Commun. 2017;53:7266–7269. doi: 10.1039/C7CC01045A. [DOI] [PubMed] [Google Scholar]
- 37.Sun X., Qi Y., Li J., Wang W., Ma Q., Liang J. Ferrocene-linked porous organic polymers for carbon dioxide and hydrogen sorption. J. Organomet. Chem. 2018;859:117–123. doi: 10.1016/j.jorganchem.2018.01.054. [DOI] [Google Scholar]
- 38.Chen D., Fu Y., Yu W., Yu G., Pan C. Versatile adamantane-based porous polymers with enhanced microporosity for efficient CO2 capture and iodine removal. Chem. Eng. J. 2018;334:900–906. doi: 10.1016/j.cej.2017.10.133. [DOI] [Google Scholar]
- 39.Zhang R.-R., Yin Q., Liang H.-P., Chen Q., Luo W.-H. Hypercrosslinked porous polycarbazoles from carbazolyl-bearing aldehydes or ketones. Polymer. 2018;143:87–95. doi: 10.1016/j.polymer.2018.03.062. [DOI] [Google Scholar]
- 40.Neti V.S.P.K., Wu X., Deng S., Echegoyen L. Selective CO2 capture in an imine linked porphyrin porous polymer. Polym. Chem. 2013;4:4566–4569. doi: 10.1039/c3py00798g. [DOI] [Google Scholar]
- 41.Jian Z. A nanoporous organic polymer constructed from a 1,3,5-triazine derivative via ethynyl cyclotrimerization reaction: Synthesis and carbon dioxide capture. J. Chem. Pharm. Res. 2014;6:322–326. [Google Scholar]
- 42.Li D., Wang X., Jia Y., Wang A., Wu Y. Synthesis of conjugated hyperbranched polytriazoles containing truxene units by click polymerization. Chin. J. Chem. 2012;30:861–868. doi: 10.1002/cjoc.201100339. [DOI] [Google Scholar]
- 43.Wang Y., Wang D., Xu C., Wang R., Han J., Feng S. Click polymerization: Synthesis of novel σ-π conjugated organosilicon polymers. J. Organomet. Chem. 2011;696:3000–3005. doi: 10.1016/j.jorganchem.2011.05.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.