Abstract
Vacuoles are essential pleomorphic organelles that undergo dynamic changes during cell growth and differentiation in plants. How developmental signals are linked to vacuole biogenesis and development is poorly understood. In this report, we show that a Rop GTPase is localized to developing vacuoles in pea (Pisum sativum cv Extra Early Alaska). Rop belongs to the RHO family of Ras-related small GTP-binding proteins that are key molecular switches in a wide variety of eukaryotic signal transduction pathways. Using indirect immunofluorescence and an anti-Rop antibody, we showed that Rop proteins accumulate to high levels in rapidly growing tapetal cells of pea anthers. In these cells, Rop is localized to an endomembrane system that exists as dynamic pleomorphic networks: a perinuclear fine network decorated with punctate dots, a network composed of small spheres and tubules, and interconnected chambers. Colocalization with a tonoplast annexin VCaB42 shows that these dynamic networks represent the tonoplast. Our results suggest that the dynamic Rop-containing tonoplast networks represent a unique stage of vacuole development. The specific localization of Rop to developing vacuoles supports a role for Rop in signal transduction that mediates vacuole development in plants.
Plant vacuoles are multifunctional post-Golgi organelles critical for cell growth, ion homeostasis, signal transduction, and storage of nutrients, defense compounds, and other metabolites. The development and dynamic morphogenesis of vacuoles are usually associated with rapid cell growth and differentiation in plants. Vacuole biogenesis is thought to occur through one of or a combination of the following pathways: fusion of post-Golgi vesicles to prevacuoles, enlargement of endoplasmic reticulum (ER) tubules, fusion of young vacuoles (for review, see Okita and Rogers, 1996; Marty, 1999). These pathways most likely account for the occurrence of different types of vacuoles within a given cell and of specialized vacuoles in specific tissues and cell types. As a consequence, the molecular machinery for vacuole biogenesis must be coupled to the mechanism that controls plant cell growth and development. Many plant homologs of the yeast molecules that modulate membrane trafficking to vacuoles, e.g. the phosphotidylinositol 3-kinase VPS34p, the syntaxin AtPEP12p, the SNARE AtVTI1, AtVPS45p, have recently been identified and implicated in vacuole biogenesis in plants (Welters et al., 1994; da Silva Conceicao et al., 1997; Bassham and Raikhel, 1998; Sanderfoot et al., 1998; Sanderfoot and Raikhel, 1999; Zheng et al., 1999). However, the molecular machinery for vacuole biogenesis remain poorly characterized, and it is unknown how the machinery is linked to developmental signals that control vacuole biogenesis and development.
Studies in mammalian cells suggest that the biogenesis and dynamics of endosomes and lysosomes, which share some functional and biogenetic similarities to plant vacuoles, involves RHO GTPase-dependent signaling pathways (Adamson et al., 1992; Lamaze et al., 1996; Murphy et al., 1996). The RHO-family GTPases belong to the RAS superfamily of small GTP-binding proteins (Chardin, 1993; Yang, 1996). RHO GTPases have emerged as one of the most important and versatile groups of signaling proteins (Ridley, 1996; Mackay and Hall, 1998). In animals and yeast, RHO signaling controls a large variety of key cellular processes, including actin cytoskeletal organization, membrane trafficking and organization (e.g. exocytosis and endocytosis), cell cycle progression, the activation of MAP kinase cascades, the formation of focal adhesion, the establishment of cell polarity, and the activation of glucan synthase and NADPH oxidase (Heyworth et al., 1994; Nobes and Hall, 1994; Vojtek and Cooper, 1995; Arellano et al., 1996; Larochelle et al., 1996; Nagata and Hall, 1996; Qadota et al., 1996; Tapon and Hall, 1997; Hall, 1998; Mackay and Hall, 1998). The RHO-family GTPases from fungi and animals can be categorized into at least three major subfamilies: Cdc42, Rac, and Rho, according to sequence similarities (Chardin, 1993). Each subfamily has distinct multiple cellular functions, e.g. mammalian CDC42 is known to mediate cellular polarization and cell cycle progression, whereas Rac regulates the activation of NADPH oxidase and cell movement (Ridley, 1996; Mackay and Hall, 1998).
Plants possess a unique subfamily of RHO GTPases, termed Rop, that is specific to plants (Yang et al., 1993; Delmer et al., 1995; Winge et al., 1997; Li et al., 1998; Zheng and Yang, 2000b). Rop is also emerging as an important signaling switch in plants (Zheng and Yang, 2000b). Several studies indicate that Rop GTPases are localized to the apical region of the pollen tube plasma membrane and play a pivotal role in the control of polar growth in pea (Pisum sativum cv Extra Early Alaska) and Arabidopsis pollen tubes by regulating the tip-localized Ca2+ signaling (Lin et al., 1996; Lin and Yang, 1997; Yang, 1998; Kost et al., 1999; Li et al., 1999; Zheng and Yang, 2000a). Rop is also involved in the activation of active oxygen production, leading to cell death in rice leaves (Kawasaki et al., 1999) and possibly the initiation of cellulose synthesis in cotton fibers (Potikha et al., 1999). Evidence suggests that Rops are involved in the regulation of many other processes, e.g. actin organization and cell morphogenesis (Li and Yang, 2000a; Zheng and Yang, 2000b).
In this study, we demonstrate that one or more Rop proteins are localized to the tonoplast of developing vacuoles. The tonoplast localization and accumulation of Rop undergo dynamic changes that are correlated with coalescence of early pleomorphic vacuoles to become large central vacuoles during rapid cell expansion in the pea tapetum. This novel localization suggests that Rop GTPases may participate in a signaling pathway that regulates the development of vacuoles or the function of young vacuoles.
RESULTS
Rop GTPases Are Localized to a Dynamic Endomembrane System in Tapetal Cells
We previously showed that Rop GTPases are most abundant in the anther among various pea tissues (Lin et al., 1996). To begin assessing potential roles for Rop in the anther, we determined its cellular and subcellular localization using anti-Rop1Ps antibody and immunocytochemical techniques. We have shown that this antibody reacts with all Rop isoforms from Arabidopsis (Li et al., unpublished data). Thus, pea proteins detected by this antibody can be any members of the pea Rop family. Using frozen thin sections stained with the primary antibody and alkaline phosphatase-conjugated second antibodies, we found that Rop is preferentially localized to tapetal cells, microsporogenic cells, and microspores in the anther (see below). To analyze the subcellular localization of Rop GTPases, squashed anthers were stained with the affinity-purified primary antibody and fluorescein-conjugated second antibodies. Two types of cells were readily released from squashed young anthers: tapetal cells and microspore mother cells. They are easily distinguished by cellular and chromatin morphology. Microspore mother cells are oval or spindle-shaped and contain condensed chromatin, whereas tapetal cells are irregular-shaped and contain interphase chromatin (Fig. 1).
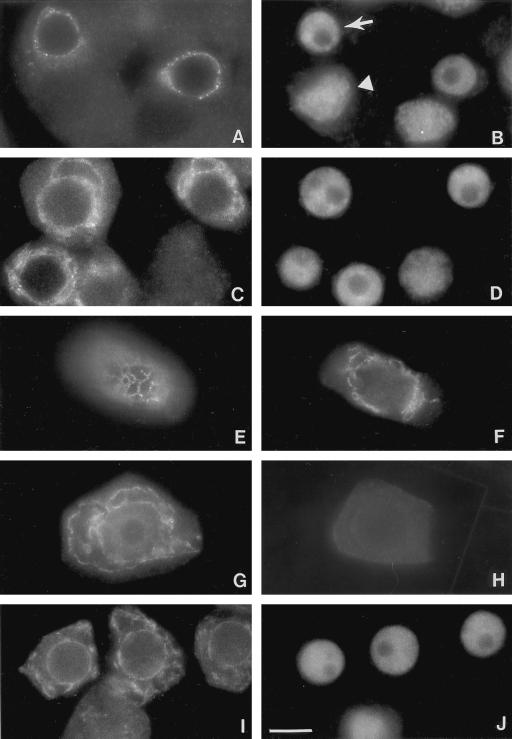
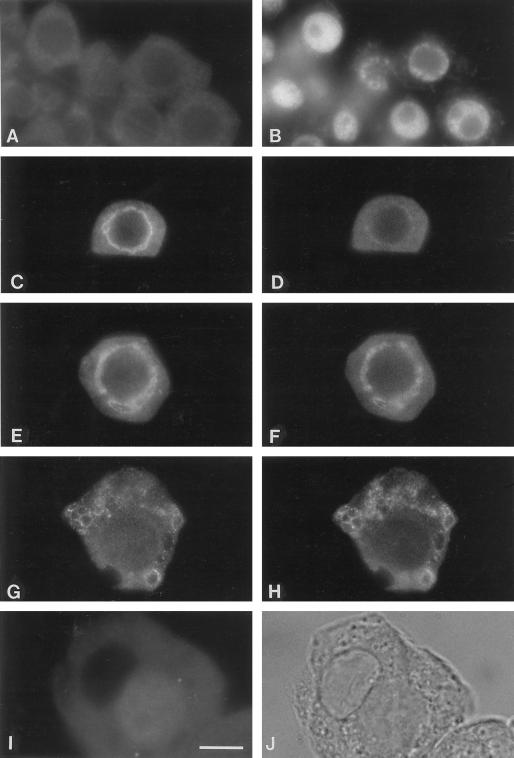
Figure 1.
Localization of Rop GTPase to an endomembrane system in pea tapetal cells. Pea anthers prior to anthesis were collected and squashed as described in text. Squashed anthers were stained with affinity-purified anti-Rop1Ps antibodies (A, C, E, F, and G) or anti-BiP antibodies (I) and an anti-rabbit secondary antibody conjugated with fluorescein. Cells shown in A, C, and I were counterstained with DAPI to reveal the localization of the nucleus (B, D, and J), respectively. Arrow in B indicates the location of the nucleus in tapetal cells, whereas arrowhead indicates the nucleus of microspore mother cells. Negative control is shown in H, in which the primary anti-Rop1Ps antibody was replaced with a pre-immune serum. Bar = 25 μm.
In tapetal cells, Rop is localized to an endomembrane system composed of several types of dynamic structures (Fig. 1). First, fluorescence forms a circle aligning just outside of the nucleus. Under an epifluorescence microscope, the circle consists of dots that seem to be linked by thin threads (Fig. 1A). Dotted fluorescence is much stronger than thin lines. Upon careful examination under confocal microscope, the network appears to be composed of spheres and small tubules that are connected to one another (Fig. 2). Second, tubules and spheres enlarge but remain interconnected and can be easily visualized under epifluorescence microscope (Fig. 1, C through F). Third, each tapetal cell contains several chamber-like structures that are stained with Rop antibodies, some chambers are bigger than the others (Fig. 1G). Certain chambers remain connected to neighboring chambers. The fluorescence is not evenly distributed throughout the endomembrane system. Certain regions are much brighter than others. In most cases, small spheres or tubules have strong fluorescence, whereas larger chambers contain weaker fluorescence. Fluorescence on chambers is frequently discontinuous and punctated. The punctates line up to form interconnected chambers. A small number of punctates are particularly bright. Pre-immune control or anti-Rop1Ps premixed with GST-Rop1Ps fusion proteins did not stain these cells, demonstrating that the staining pattern was specific to Rop GTPases (Fig. 1H).
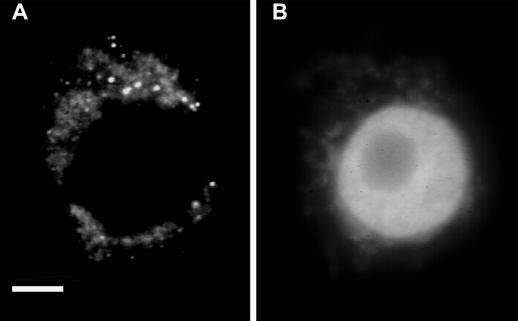
Figure 2.
AtPEP12 is localized to organelles distinct from Rop-containing organelles. Squashed anthers were stained with anti-AtPEP12 antiserum (A) and counterstained with DAPI (B) as described in Figure 1. Bar = 10 μm.
The endomembrane localization of Rop GTPases appears to be developmentally regulated. In microspore mother cells and microspores, only diffuse cytoplasmic staining was found. In anther primordia in which tapetal cells have not been differentiated or are being differentiated, Rop is also diffuse throughout the cytoplasm. The perinuclear dense network-like staining becomes obvious as soon as tapetal cells are distinguishable from microsporogenic cells. However, we cannot exclude the possibility that lack of network-like staining in other cell types was due to the inability of the primary antibodies to penetrate these cells as these cells contain thicker cell walls compared with tapetal cells.
Rop Is Not Localized to ER, Golgi Complex, or Prevacuoles
To determine whether Rop is localized to the ER, squashed anthers were stained with antibodies against BiP, an ER lumen protein. As shown in Figure 1I, anti-BiP staining gave a completely different pattern from Rop localization. In contrast to the dense network that is outside of the nucleus, anti-Bip stained nuclear envelopes and network-like structures typical of ER networks that are scattered throughout the cytoplasm. Costaining with the fluorescent ceramide analog BODIPY FL C5-ceramide, which presumably stains both ER and Golgi (Kawazu et al., 1995), show that Rop is not localized to the Golgi complex either (data not shown). In addition, Rop is not colocalized with AtPEP12p, an Arabidopsis syntaxin; AtPEP12p is localized to a novel post-Golgi structure called prevacuolar compartment (da Silva Conceicao et al., 1997). Indirect immunofluorescence studies show that in pea tapetal cells AtPEP12p is localized to various sizes of spherical or patchy structures, presumably representing the prevacuolar compartment (Fig. 2). Unlike Rop-containing organelles, AtPEP12p-containing organelles do not form networks and are not restricted to the perinuclear region. Instead, they are distributed throughout the cytoplasm, although more concentrated in the perinuclear region. Pre-immune serum did not stain these organelles, indicating that AtPEP12 staining is specific (data not shown).
Rop Is Colocalized with a Vacuolar Annexin
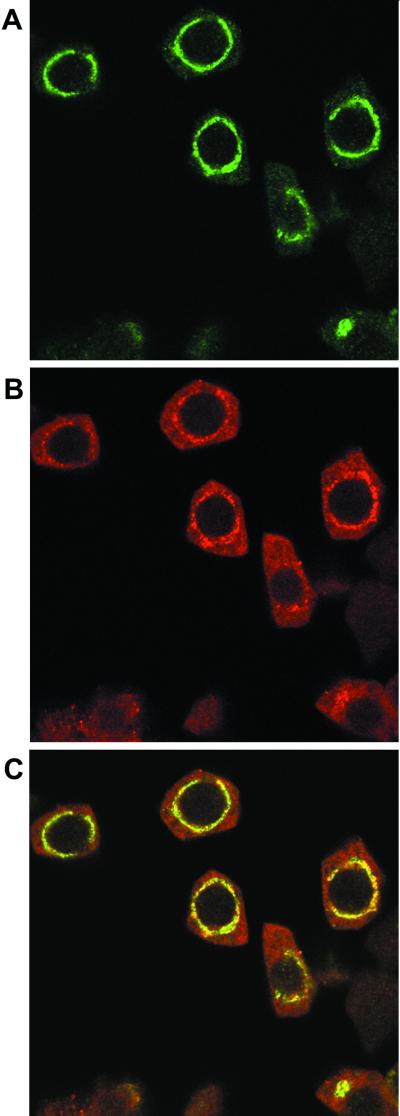
The above results led us to suspect that Rop is localized to vacoules. To test this notion, pea tapetal cells were costained with anti-Rop1Ps and a mouse polyclonal antibody raised against the celery VCaB42. VCaB42 is an annexin that displays Ca2+-dependent localization to vacuolar membranes (Seals et al., 1994; Seals and Randall, 1997). As shown in Figure 3, Rop and VCaB42 stained identical patterns. Similar patterns were obtained when tapetal cells were separately stained with Rop and VcaB42 antibodies. These results suggest that Rop GTPases and VCab42 are precisely colocalized. Because VCaB42 has been localized to vacuolar membranes in several tissues and cells from different plant species (Seals et al., 1994; Seals and Randall, 1997), our results clearly indicate that Rop is localized to the tonoplast in tapetal cells.
Figure 3.
Colocalization of Rop with the vacuolar annexin VCaB42. Squashed anthers were co-incubated with the rabbit anti-Rop1Ps antibody and mouse anti-VCaB42 antibody. The reaction was first treated with FITC-conjugated anti-rabbit IgG secondary antibodies and then with the Texas Red-conjugated anti-mouse IgG sheep F(ab)2 fragment. After washes, the samples were observed under a laser scanning confocal microscope as described in text. A, Localization of Rop as revealed by FITC fluorescence. B, Localization of VCaB42 as revealed by Texas Red fluorescence. C, Overlay between A and B.
Ultrastructural Evidence for Perinuclear Vacuolar Network and Dynamic Vacuole Biogenesis in Pea Tapetal Cells
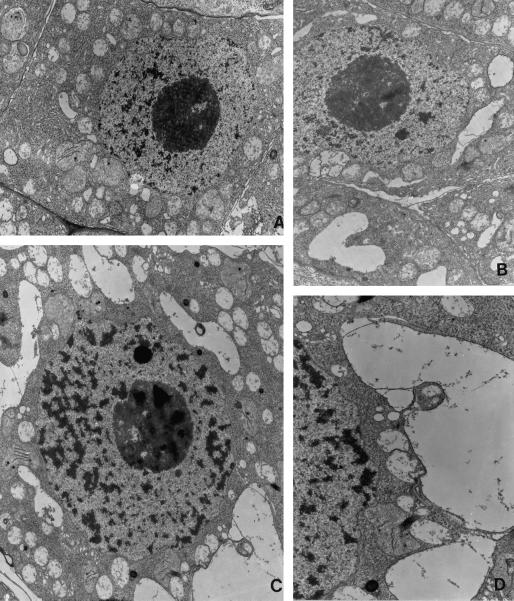
To demonstrate whether or not pea tapetal cells contain a vacuolar network as suggested by the Rop immunofluorescence staining, we investigated the ultrastructure of these cells using transmission electron microscopy (TEM). As shown in Figure 4, TEM analyses revealed a highly dynamic vacuolar system in young tapetal cells. Small vacuoles apparently line up outside of the nucleus. They seem to fuse with each other as well as with larger vacuoles. In addition to coalescence of vacuolar membranes, the development of vacuoles also appears to involve endosomal activity-like events as revealed by the presence of multivesicular structures and apparent engulfment of smaller vacuoles by larger vacuoles. These ultrastructural results conform to the dynamic changes in the localization of Rop shown by indirect immunofluorescence. Rop antigenicity seems to be extremely labile because treatment of tissues with buffer or fixatives used for TEM sections resulted in the loss of antigenicity. For this reason, we failed to detect any Rop labeling by EM immunocytochemistry.
Figure 4.
Ultrastructural analyses show a dynamic vacuolar network surrounding the nucleus in pea tapetal cells. Ultra-thin sections of chemically fixed pea anthers were prepared and examined under a transmission microscope as described in text. A, Tapetum initial cells shows no sign of vacuole development, consistent with lack of Rop staining in these cells. B, A few small vacuole-like structures start to fuse with each other. C, Developing tapetal cells show an extensive vacuolar network and numerous small vacuole-like compartments. D, An enlarged section of C, showing small vacuole-like structures fuse with each other and with vacuoles or are being engulfed by vacuoles (D). Magnification, ×8,000 (A, B, and C), ×14,000 (D).
The Relationship of Subcellular Rop Localization with Vacuole Development
Our TEM analyses also suggest that active vacuole development in tapetal cells occurs during the meiotic stage of microsporogenesis. We suspected that various patterns of Rop subcellular localization described above represent different stages of vacuole development in pea tapetal cells. To confirm this, anthers at various stages of microsporogenesis were stained (Fig. 5). Stages of microsporogenesis were determined by costaining cells with 4′,6-diamino-phenylindole (DAPI) for the visualization of chromosome morphology. Prior to meiosis (at the stage of microsporogenic cells), Rop exhibits diffuse cytoplasmic localization in tapetum initial cells. As soon as meiosis (of microspore mother cells) begins, Rop becomes concentrated on the perinuclear network. Interconnecting tubules and spheres appear during anaphase I. Large chamber-like staining was observed near the end of meiosis. At tetrad stage, endomembrane Rop staining completely disappears. It is interesting that VCaB42 showed identical localization patterns except that its appearance on the early perinuclear network is later than Rop (Fig. 5) and that it remains on the mature vacuoles (data not shown) as seen in other tissues and cell types (Seals et al., 1994; Seals and Randall, 1997).
Figure 5.
The dynamic localization of Rop to the tonoplast is correlated with vacuole development in pea tapetal cells. Pea anthers from different developmental stages were squashed and co-stained with anti-Rop1Ps (A, C, E, G, and I) and anti-VCaB42 (D, F, and H) antibodies as described in Figure 3. Stained cells were examined under an epifluorescence microscope. At the stage of microsporogenic cells (giving rise to microspore mother cells) and parietal cells (giving rise to the tapetum), only diffuse cytoplasmic staining (A) for Rop is found. At this stage, it is difficult to distinguish the two cell types either by cell shape (A) or nuclear morphology (B). Localization to the tonoplast of tapetal cells (C) appears at the stage of microspore mother cells, whereas little tonoplast localization is observed for VCaB42 (D) at this stage. The tonoplast localization of Rop persists through the early and late meiosis I (E and G, respectively), during which VCaB42 is colocalized with Rop (F and H). By early tetrad stage, Rop staining (I) completely disappears from the tonoplast of the large mature vacuole (see differential interference contrast image in J), whereas VCaB42 staining in the tonoplast remains (data not shown).
Dynamic Localization of Rop Proteins to the Tonoplast Is Correlated with Their Accumulation in Tapetal Cells
To assess whether the changes in subcellular localization patterns are the result of developmental regulation of Rop accumulation, we analyzed the kinetics of Rop accumulation in the tapetum during microsporogenesis. Cross-sections of pea flowers at various stages were stained with the anti-Rop1Ps antibodies. As shown in Figure 6, Rop proteins are primarily localized to a microsporogenic cells, microspores of various developmental stages, the tapetum, and vascular tissues. The accumulation of Rop in the tapetum showed dynamic changes. In the tapetum initial cells, moderate levels of Rop proteins are found (data not shown). Rop proteins accumulate to the highest level in the tapetal cells at the stages of micorspore mother cells and their meiosis (Fig. 6, A and C). At the tetrad stage, the tapetal accumulation of Rop proteins declined to a low level (Fig. 6D). By the time when young microspores are released from the tetrad, Rop completely disappears from the tapetum (data not shown). Sections stained with a pre-immune serum did not show any signals (Fig. 5B). Therefore, the abundant accumulation of Rop proteins in the tapetum coincides with the localization to developing vacuoles, demonstrating a tight link between vacuole biogenesis and Rop accumulation in the tapetum.
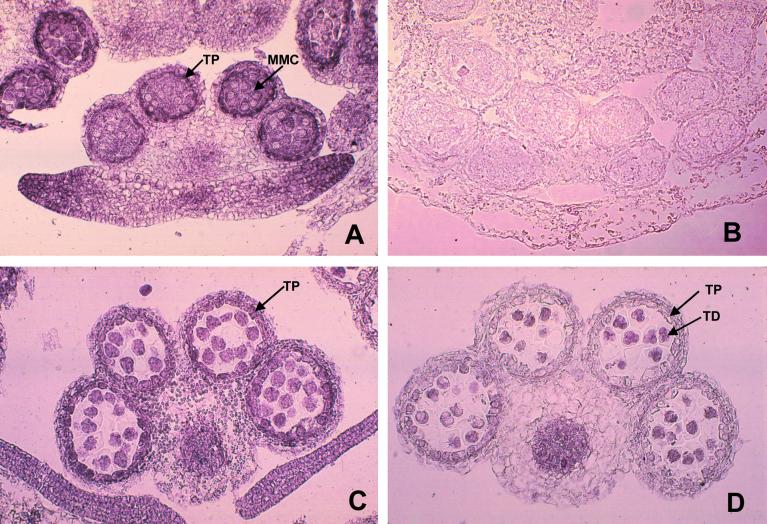
Figure 6.
Accumulation of Rop protein in tapetal cells is associated with their development. Cross-sections of pea anthers at different developmental stages were stained with anti-Rop1Ps antibody as described in text. Immediately before meiosis (A) high levels of Rop accumulate, correlated with the appearance of Rop localization to the tonoplast (Fig. 5C). Rop accumulation in the tapetum persists throughout meiosis (C), during which tapetal cells rapidly expand (Fig. 5, E and G), prior to its disappearance at the tetrad stage (D). Staining with pre-immune serum did not detect any signals (B). TP, Tapetum; MMC, microspore mother cells; TD, tetrads.
DISCUSSION
The current data provide convincing evidence for novel subcellular localization of a Rho-type GTPase (Rop) to the tonoplast in pea tapetal cells. To our knowledge, this is the only signaling GTPase known to be localized to plant vacuoles. Importantly, Rop is specifically found on the membrane of developing vacuoles but not that of mature vacuoles. This finding may be especially significant in the light of the coupling of vacuole development to the control of plant cell growth and differentiation. Hence, the tonoplast-localized Rop GTPase may provide an important marker to investigate how growth and developmental signals regulate the development of vacuoles in plants.
Vacuole development in plants is a highly regulated process that most likely involves multiple morphological pathways, complex molecular machinery, and signaling events (Marty, 1999). Ultrastructural and biochemical analyses suggest the existence of both ER-origin and Golgi-origin biogenetic pathways (Palevitz and O'Kane, 1981; Palevitz et al., 1981; Herman et al., 1994; Okita and Rogers, 1996; Marty, 1999). Recent work also reveals a novel subcellular compartment, i.e. prevacuole, that may function as an intermediate between post-Golgi vesicles and vacuoles. Furthermore, the development of mature central vacuoles involves coalescence of dynamic pleomorphic vacuolar networks, as found in several cell types in different plant species (for review, see Okita and Rogers, 1996; Marty, 1999). Our TEM analyses also reveal the presence of various shapes of interconnected vacuolar compartments in pea tapetal cells, which evidently coalescence to become central vacuoles.
We have demonstrated that Rop proteins are localized to a pleomorphic dynamic endomembrane network in pea tapetal cells by using a unique indirect immunofluorescence method. The unique feature of this method is that no cell wall degrading enzymes was necessary to allow the penetration of antibodies into the tapetal cells, because these cells only contain a thin and incomplete cell wall. This was an important distinction from the conventional immunofluorescence techniques involving cell wall digestion, which frequently leads to the distortion of cellular structures and the loss of antigenicity. We found that anti-Rop1Ps antibodies gave a staining pattern in tapetal cells that resembles the vacuolar network observed in living stomatal cells (Palevitz and O'Kane, 1981; Palevitz et al., 1981). The Rop-associated network is clearly not ER, Golgi apparatus, as markers for these organelles do not colocalize with Rop. However, the appearance of the Rop-associated network and its morphological dynamics during tapetal cell development parallel those of the vacuolar network revealed by TEM. The tonoplastic nature of this network was further confirmed by the precise colocalization of Rop with the vacuolar annexin VCab42 (Seals et al., 1994; Seals and Randall, 1997) revealed by both epifluorescent and laser confocal microscopy.
RHO-family GTPases are believed to be synthesized on cytosolic ribosomes and are targeted to membranes as a result of attachment of an isoprenyl group to the C terminus-a post-translational process called protein isoprenylation. Targeting to specific membrane systems requires additional internal amino acid sequences, especially the variable region proximal to the isoprenylation site. In mammalian cells, a polybasic domain in this region targets Rho-type GTPases to the plasma membrane. It is interesting that different subgroups of Rop GTPases have distinct sequences in this region and show differential subcellular localization (Li et al., 1998; Bischoff et al., 2000; Y. Lin, H. Li, Z. Yang, unpublished data; J. Fowler, personal communication). Arabidopsis Rop1At and Rop6At and maize Rop1 and Rop6 are localized to the plasma membrane in tobacco suspension cultures and maize leaves, respectively; whereas Rop2At and Rop4At are preferentially localized to a perinuclear region in suspension-cultured tobacco cells. It is unknown whether the perinuclear Rop proteins are localized to small vacuoles similar to those in pea tapetal cells. Nonetheless, it is important to determine which Rop GTPases are localized to the tonoplast. Identification of such Rop GTPases will provide necessary tools for the elucidation of the mechanism for the tonoplast localization and the function of these Rop GTPases.
The association of Rop with vacuoles presents a tantalizing model by which Rop may function in the early signaling events of vacuole development. This is supported by the changes in the accumulation of Rop proteins and their localization to the tonoplast during the development of tapetal cells in pea. Kinetic analyses reveal that the subcellular localization of both Rop and VCab42 to the tonoplast is remarkably dynamic during the early stage of rapid expansion of tapetal cells. Rop is first seen on a network-like vacuoles composed of dense globular cisternae and small tubules and then remains on interconnected chamber-like vacuoles. It is interesting that these dynamic Rop localization patterns are consistent with the various developmental states of dynamic vacuoles in differentiating stomatal cells, root tip cells, and trichome cells (Palevitz and O'Kane, 1981; Palevitz et al., 1981; for reviews, see Okita and Rogers, 1996; Marty, 1999). However, Rop is absent from both central vacuoles with which VCab42 remains to be associated, and the AtPEP12p-localized prevacuolar compartment (da Silva Conceicao et al., 1997; Sanderfoot et al., 1998). Thus, we conclude that the Rop-associated vacuolar networks appear to represent a biochemically and structurally distinct developmental stage of vacuoles that is different from prevacuoles and mature central vacuoles.
The exact role of Rop during these early stages of development is currently unknown, but its unique association with vacuoles is consistent with a role as a signal transducer in vacuole biogenesis. Rop might modulate coalescence of young vacuoles, the dynamic spatial organization of these vacuoles, or endocytosis-associated vacuole development. The development of vacuoles in tapetal cells may involve endocytic events, as suggested by the presence of multivesicular structures associated with the vacuoles of the pea tapetum. In mammalian cells, Rho GTPases have been localized to endosomes and are implicated in the control of endocytosis (Adamson et al., 1992; Lamaze et al., 1996). By analogy, the putative Rop-dependent pathway might be involved in the vacuole development by regulating late endocytic events. Rop alternatively could regulate the spatial organization of developing vacuoles in the perinuclear region. Such a spatial distribution of young vacuoles may facilitate their rapid fusion. It is interesting that a novel Rho member RhoD has been shown to modulate the organization of endosomes in mammalian cells (Murphy et al., 1996). Evidence suggests that the dynamics of vacuoles in Allium stomatal cells requires the actin cytoskeleton (Palevitz and O'Kane, 1981). Because Rho GTPases are conserved regulators of the actin cytoskeleton, Rop could be a signaling molecule that controls the vacuole-associated actin cytoskeleton.
The most attractive model for the role of Rop is its participation in a signaling pathway that controls the fusion of the early pleiomorphic vacuoles to form the mature central vacuoles. Our finding that Rop is colocalized with the vacuole annexin VCaB42 and the appearance of Rop on the tonoplast in the tapetum precedes that of VCaB42 provides some support for this hypothesis. Annexins belong to a large family of Ca2+-dependent phospholipid-binding proteins that potentially play a key role in the process of membrane fusion during endocytosis and exocytosis in animal cells (Moss, 1997). In plant cells, there is also evidence that annexins are involved in Ca2+-dependent membrane fusion and annexins are proposed to control both vacuole biogenesis and exocytosis (Blackbourn and Battey, 1993; Battey et al., 1996; Seals and Randall, 1997). We have previously shown Rop regulates tip growth of pollen tubes (i.e. fusion of post-Golgi to the apical plasma membrane) through the tip-localized Ca2+ activities (Lin and Yang, 1997; Li et al., 1999). Thus, it is reasonable to speculate that an early Rop-dependent pathway regulates late-step, Ca2+- and VCaB42-dependent, and membrane fusion during the formation of large vacuoles. Analyses of transgenic dominant mutants and knockout mutants for the tonoplast-localized Rop should shed light on the function of Rop in the regulation of vacuole development.
MATERIALS AND METHODS
Plant Materials
Pea flower buds were harvested from pea (Pisum sativum cv Extra Early Alaska) plants grown in a growth chamber at 22°C with a 16-h-light and 8-h-dark regime.
Antibodies
Production and purification of anti-Rop1Ps polyclonal antibodies were described previously (Lin et al., 1996; Lin and Yang, 1997). Mouse VCaB42 antisera were prepared as described (Seals et al., 1994; Seals and Randall, 1997). Anti-AtPEP12p and anti-BiP polyclonal antibodies were provided by Drs. Natasha Raikhel and Maarten Chrispeels, respectively.
Indirect Immunofluorescence Microscopy
For subcellular immunolocalization, pea anthers at the stage of meiosis were dissected from young flower buds and fixed in 4% (v/v) paraformaldehyde, 50 mm PIPES (1,4-piperazinediethanesulfonic acid) buffer, pH 6.9, 2 mm MgSO4 at room temperature for 2 h. After washing with PBS (10 mm phosphate-buffered saline, pH 7.4, 138 mm NaCl, 2.7 mm KCl), fixed anthers were placed on a microscope slide coated with poly-Lys and sandwiched with a second slide. The anthers were then gently squashed with a thumb to release meiocytes and tapetal cells. The slides were separated and immediately immersed in PBS for 2 to 5 min. The squashed anther cells were then blocked with 3% (v/v) non-fat dry milk in PBS at room temperature for 1 h. The cells were then reacted with purified anti-Rop1Ps antibodies (1:40 dilution), anti-BiP sera (1:200 dilution), or anti-VCaB42 sera (1:40 dilution) at 30°C for 1 to 2 h. The primary antibodies were diluted in a PBS buffer containing 1% (v/v) non-fat dry milk. After three 10-min washes in PBST (PBS containing 0.05% [v/v] Triton X-100), the slides were incubated with a secondary antibody (fluorescein isothiocyanate [FITC]-conjugated affinity-purified anti-rabbit IgG or Texas-Red-conjugated sheep F(ab)2 fragment against mouse IgG, Chappel Organon Teknika) at 30°C for 1 h. The secondary antibodies were diluted 1:100 in PBS containing 1% (v/v) non-fat milk. The slides were then washed as described above.
For colocalization of Rop and VCaB42, slides containing squashed anthers were reacted with 200 μL of a PBS buffer containing 1% (v/v) non-fat milk and 5 μL of purified anti-Rop1Ps antibodies and 1 μL of Vcab42 antisera. The slides were covered with a parafilm and incubated at 30°C for 1 to 2 h. After washing as described above, the slides were first incubated with the FITC-conjugated anti-rabbit IgG secondary antibodies and then with the Texas Red-conjugated anti-mouse IgG sheep F(ab)2 fragment at 30°C for 1 h each. Following each incubation with the secondary antibodies, the slides were washed with PBST as described above. The slides were mounted in a PBS solution containing 0.1% (v/v) p-phenylenediamine and 50% (v/v) glycerol. The samples were observed under an Axiovert 100 microscope (Zeiss, Jena, Germany) equipped with epifluorescence optics or a confocal microscope (MRC-1024, Bio-Rad Laboratories, Hercules, CA). For confocal observation, 2-μm sections of laser scanning were taken. As control, pre-immune sera were used instead of the primary antibodies.
Cryosection and Cellular Immunolocalization
For cellular immunolocalization, pea flower buds were fixed in 50 mm PIPES buffer (pH 6.9) containing 4% (v/v) paraformaldehyde and 2 mm MgSO4 at room temperature for 4 h. After washing with PBS (10 mm phosphate-buffered saline, pH 7.4, 138 mm NaCl, 2.7 mm KCl), fixed flower buds were embedded in Tissue-Tek OCT compound (Miles, Elkhart, IN) and frozen on a dry ice block. Embedded frozen tissues were sectioned to approximately 10-μm thickness using a cryostat microtome (Reichert HistoStat). The sections were collected on the poly-Lys-coated microscope slides, immersed in PBS for 10 min to remove OCT, and then blocked with 3% (v/v) non-fat dry milk in PBS at room temperature for 1 h. The slides were incubated with the purified anti-Rop1Ps polyclonal antibodies (1:40 dilution with 1% [v/v] non-fat dry milk in PBS) at room temperature for 1 to 2 h. After three 10-min washes in PBST (0.05% [v/v] Tween 20 in PBS) and 10 min in TBS (50 mm Tris, 138 mm NaCl, 2.7 mm KCl, pH 7.4), the slides were incubated with a secondary antibody (alkaline phosphatase-conjugated, affinity-purified goat anti-rabbit IgG; Boehringer Mannheim) at room temperature for 1 to 2 h. The slides were washed three times (10 min each) in TBST and incubated in 120 μL of alkaline phosphatase reaction mixture (0.66 mg/mL nitro blue tetrazolium and 0.12 mg/mL bromochloroindoly phosphate in a buffer containing 100 mm Tris, pH 9.5, 100 mm NaCl, and 5 mm MgCl2) at room temperature for 30 min. The slides were washed with TBS for 5 min and mounted with 50% (v/v) glycerol in PBS. Tissue sections were examined under a Zeiss Axiophot microscope and photographed using a 35-mm camera.
TEM
Pea anthers were dissected from fresh flower buds in a Petri dish containing a fixative (2% [v/v] glutaraldehyde in 0.1 m phosphate buffer, pH 7.2). The samples were transferred into 1.5-mL tube with pipette and fixed in a fresh fixative at room temperature for 4 h. Following three washes (15 min each) with 0.1 m phosphate buffer (pH 7.2), the samples were post-fixed in 1% (v/v) OsO4 (Electron Microscopy Sciences, Fort Washington, PA) at room temperature for 2 h. Fixed tissues were washed with double-distilled water three times (5 min each) and dehydrated in a water/ethanol series (30%, 50%, 70%, 90%, 95%, and 100% [v/v] ethanol twice for 10–15 min each step). The dehydrated samples were infiltrated and embedded with LR White resin (London Resin Company) in BEEM capsules and curved in a vacuum oven at 55°C for 48 h. Ultra-thin sections were prepared with an ultramicrotome (Reichert Ultracut E). The sections were mounted on 100 mesh nickel grids coated with formvar film and stained with a 1% (v/v) solution of aqueous uranyl acetate for 5 to 10 min in darkness. After a wash in double-distilled water, the sections were then stained with 0.3% (v/v) lead citrate (Electron Microscope Sciences) for 5 min and washed in double-distilled water. Observations and photography were conducted using Philips 301 transmission electron microscope.
ACKNOWLEDGMENTS
We thank Maarten Chrispeels for his generous gift of anti-BiP antibodies and Natasha Raikhel for anti-AtPEP12p antibodies.
Footnotes
This work was supported by the U.S. Department of Agriculture (grant no. 96–35304–3861), the National Science Foundation (grant no. MCB–9724047 to Z.Y.), and a Purdue Research Foundation grant (to S.R.).
LITERATURE CITED
- Adamson P, Paterson HF, Hall A. Intracellular localization of the p21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SU, Bar-Peled M, Raikhel NV. Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 1997;114:325–336. doi: 10.1104/pp.114.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano M, Duran A, Perez P. Rho1 GTPase activates the (1–3) β-d-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Raikhel NV. An Arabidopsis VPS45p homolog implicated in protein transport to the vacuole. Plant Physiol. 1998;117:407–415. doi: 10.1104/pp.117.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey NH, James NC, Greenhand AJ. cDNA isolation and gene expression of maize annexin p33 and p35. Plant Physiol. 1996;112:1391–1396. doi: 10.1104/pp.112.3.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbourn HD, Battey NH. Annexin-mediated secretory aggregation in plants. Physiol Plant. 1993;89:27–32. [Google Scholar]
- Chardin P. ras homologs: a comparison of primary structures. In: Lacal JC, McCormick F, editors. The Ras Superfamily of GTases. Boca Raton, FL: CRC Press; 1993. pp. 203–229. [Google Scholar]
- Coughlan SJ, Hastings C, Winfrey RJ. Molecular characterization of plant endoplasmic reticulum: identification of protein disulfide-isomerase as the major reticuloplasmin. Eur J Biochem. 1996;235:215–224. doi: 10.1111/j.1432-1033.1996.00215.x. [DOI] [PubMed] [Google Scholar]
- da Silva Conceicao A, Marty-Mazars D, Bassham DC, Sanderfoot AA, Marty F, Raikhel NV. The syntaxin homolog AtAtPEP12p resides on a late post-Golgi compartment in plants. Plant Cell. 1997;9:571–582. [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Pear JR, Andrawis A, Stalker DM. Genes for small GTP-binding proteins analogous to mammalian Rac are preferentially expressed in developing cotton fibers. Mol Gen Genet. 1995;248:43–51. doi: 10.1007/BF02456612. [DOI] [PubMed] [Google Scholar]
- Griesbach RJ, Sink KC. Evacuolation of mesophyll protoplasts. Plant Sci Lett. 1983;30:297–301. [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Herman EM, Li SH, Su RT, Larsen P, Hsu HT, Sze H. Vacuolar-type H+-ATPases are associated with the endoplasmic reticulum and provacuoles of root tip cells. Plant Physiol. 1994;106:1313–1324. doi: 10.1104/pp.106.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth PG, Bohl BP, Bokoch GM, Curnutte JT. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. J Biol Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K. The small GTP-binding protein rac is a regulator of cell death in plants. Proc Natl Acad Sci USA. 1999;96:10922–10926. doi: 10.1073/pnas.96.19.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu T, Kawano S, Kuroiwa T. Distribution of the Golgi apparatus in the mitosis of cultured tobacco cells as revealed by DioC6 fluorescence microscopy. Protoplasma. 1995;186:183–192. [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua N-H. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Chuang T-H, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Larochelle DA, Vithalani KP, De Lozanne A. A novel member of the rho family of small GTP-binding proteins is specifically required for cytokinesis. J Cell Biol. 1996;133:1321–1329. doi: 10.1083/jcb.133.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to the tip-localized calcium influx. Plant Cell. 1999;11:1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu G, Ware D, Davis KR, Yang Z. Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 1998;118:407–417. doi: 10.1104/pp.118.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yang Z. Rho GTPase and the actin cytoskeleton. In: Staiger CJ, Baluska F, Volkmann D, Barlow P, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 301–321. [Google Scholar]
- Lin Y, Wang Y, Zhu J, Yang Z. Localization of a rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Yang Z. Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell. 1997;9:1647–1659. doi: 10.1105/tpc.9.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DJ, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SE. Annexins. Trends Cell Biol. 1997;7:87–89. doi: 10.1016/S0962-8924(96)10049-0. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;115:753–761. [Google Scholar]
- Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lütcke A, Parton RG, Zerial M. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Nagata K-I, Hall A. The Rho GTPase regulates protein kinase activity. BioEssays. 1996;7:529–531. [Google Scholar]
- Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Okita TW, Rogers JC. Compartmentation of proteins in the endomembrane system of plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:327–350. doi: 10.1146/annurev.arplant.47.1.327. [DOI] [PubMed] [Google Scholar]
- Palevitz BA, O'Kane DJ. Epifluorescence and video analysis of vacuole motility and development in stomatal cells of Allium. Science. 1981;214:443–445. doi: 10.1126/science.214.4519.443. [DOI] [PubMed] [Google Scholar]
- Palevitz BA, O'Kane DJ, Kobres RE, Raikhel NV. The vacuole system in stomatal cells of Allium: vacuole movements and changes in morphology in differentiating cells as revealed by epifluorescence, video and electron microscopy. Protoplasma. 1981;109:23–55. [Google Scholar]
- Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, Python CP, Inoue SB, Arisawa M, Anraku Y, Zheng Y, Watanabe T, Levin DE, Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- Sanderfoot AA, Ahmed SU, Marty-Mazars D, Rapoport I, Kirchhausen T, Marty F, Raikhel NV. A putative vacuolar cargo receptor partially colocalizes with AtAtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc Natl Acad Sci USA. 1998;95:9920–9925. doi: 10.1073/pnas.95.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Raikhel NV. The specificity of vesicle trafficking: coat proteins and SNAREs. Plant Cell. 1999;11:629–642. doi: 10.1105/tpc.11.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Parrish ML, Randall SK. A 42-kilodalton annexin-like protein is associated with plant vacuoles. Plant Physiol. 1994;106:1403–1412. doi: 10.1104/pp.106.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Randall SK. Vacuole-associated annexin protein, VCaB42, correlates with the expansion of tobacco cells. Plant Physiol. 1997;115:753–761. doi: 10.1104/pp.115.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Cooper JA. Rho family members: activators of MAP kinase cascade. Cell. 1995;82:527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Welters P, Takegawa K, Eer S, Chrispeels M. Atvps34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc Natl Acad Sci USA. 1994;91:11398–11402. doi: 10.1073/pnas.91.24.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge P, Brembu T, Bones AM. Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol Biol. 1997;35:483–495. doi: 10.1023/a:1005804508902. [DOI] [PubMed] [Google Scholar]
- Yang W, Burkhard W, Cavallius J, Merrick WC, Boss WF. Purification and characterization of a phosphatidylinositol 4-kinase activator in carrot cells. J Biol Chem. 1993;268:392–398. [PubMed] [Google Scholar]
- Yang Z. Signal transducing proteins in plants: an overview. In: Verma DPS, editor. Signal Transduction in Plant Growth and Development. New York: Springer Wien; 1996. pp. 1–37. [Google Scholar]
- Yang Z. Signaling tip growth in plants. Curr Opin Plant Biol. 1998;1:525–530. doi: 10.1016/s1369-5266(98)80046-0. [DOI] [PubMed] [Google Scholar]
- Zheng H, von Mollard GF, Kovaleva V, Stevens TH, Raikhel NV. The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol Biol Cell. 1999;10:2251–2264. doi: 10.1091/mbc.10.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z-L, Yang Z. The Rop GTPase switch turns on polar growth in pollen. Trends Plant Sci. 2000a;7:298–303. doi: 10.1016/s1360-1385(00)01654-x. [DOI] [PubMed] [Google Scholar]
- Zheng Z-L, Yang Z (2000b) The Rop GTPase: an emerging signaling switch in plants. Plant Mol Biol (in press) [DOI] [PubMed]