Abstract
In people without lower extremity peripheral artery disease (PAD), mitochondrial DNA copy number declines with aging, and this decline is associated with declines in mitochondrial activity and functional performance. However, whether lower extremity ischemia is associated with lower mitochondrial DNA copy number and whether mitochondrial DNA copy number is associated with the degree of functional impairment in people with PAD is unknown. In people with and without PAD, age 65 years and older, we studied associations of the ankle–brachial index (ABI) with mitochondrial DNA copy number and associations of mitochondrial DNA copy number with functional impairment. Calf muscle biopsies were obtained from 34 participants with PAD (mean age: 73.5 years (SD 6.4), mean ABI: 0.67 (SD 0.15), mean 6-minute walk distance: 1191 feet (SD 223)) and 10 controls without PAD (mean age: 73.1 years (SD 4.7), mean ABI: 1.14 (SD 0.07), mean 6-minute walk distance: 1387 feet (SD 488)). Adjusting for age and sex, lower ABI values were associated with higher mitochondrial DNA copy number, measured in relative copy number (ABI<0.60: 914, ABI 0.60–0.90: 731, ABI 0.90–1.50: 593; p trend=0.016). The association of mitochondrial DNA copy number with the 6-minute walk distance and 4-meter walking velocity differed significantly between participants with versus without PAD (p-value for interaction=0.001 and p=0.015, respectively). The correlation coefficient between mitochondrial DNA copy number and the 6-minute walk distance was 0.653 (p=0.056) among people without PAD and −0.254 (p=0.154) among people with PAD and ABI < 0.90. In conclusion, lower ABI values are associated with increased mitochondrial DNA copy number. Associations of mitochondrial DNA copy number with the 6-minute walk distance and 4-meter walking velocity significantly differed between people with versus without PAD, with stronger positive associations observed in people without PAD than in people with PAD. The cross-sectional and exploratory nature of the analyses precludes conclusions regarding causal inferences.
ClinicalTrials.gov Identifier: NCT02246660
Keywords: functional performance, mitochondria, peripheral artery disease (PAD), vascular medicine
Introduction
Growing evidence suggests that lower extremity peripheral artery disease (PAD) is associated with calf skeletal muscle abnormalities, and these calf muscle abnormalities are associated with functional impairment and decline in PAD.1–7 The association of PAD with calf muscle mitochondrial activity is conflicting, with some studies reporting increased activity and others reporting lower activity of mitochondrial markers in people with PAD compared to those without PAD.5–13 Further information about the association of lower extremity ischemia with measures of mitochondrial function is needed.
In healthy men without PAD, mitochondrial DNA copy number correlates positively with mitochondrial volume and abundance of mitochondrial enzyme mRNA in response to exercise training.14 In people without PAD, older age is associated with a lower abundance of mitochondrial DNA copy number and poorer activity of mitochondrial enzymes.15–17 Among older people, lower mitochondrial DNA copy number is associated with poorer health and poorer lower extremity functioning.15–17 If lower extremity ischemia accelerates the decline in mitochondrial DNA copy number that occurs with aging, people with PAD may have a lower abundance of mitochondrial DNA copy number and lower oxidative capacity than people without PAD. Alternatively, lower extremity ischemia could increase mitochondrial DNA copy number, if ischemia damages mitochondria and promotes a compensatory mitochondrial biogenesis or if ischemia impairs mitophagy and prevents removal of defective mitochondria.18,19 If lower extremity ischemia damages mitochondrial DNA and also impairs mitophagy,20–23 mitochondrial DNA copy number could increase without improving mitochondrial activity.
This study delineated the association of lower extremity ischemia with abundance of mitochondrial DNA and the association of mitochondrial DNA copy number with mitochondrial activity and other meaningful outcomes in the setting of PAD. This study had the following aims. First, to assess the association of lower extremity ischemia severity, measured by the ankle–brachial index (ABI), with calf muscle mitochondrial DNA copy number in people with and without PAD. Second, to study the association of calf muscle mitochondrial DNA copy number with walking performance, measured by the 6-minute walk and 4-meter walking velocity, in people with PAD. Third, to determine whether associations of mitochondrial DNA copy number with 6-minute walk distance and 4-meter walking velocity differ in people with versus without PAD. Fourth, to determine whether greater mitochondrial DNA copy number is associated with an increased abundance of peroxisome proliferative activated receptor-γ co-activator 1α (PGC-1α), a marker of mitochondrial biogenesis, in people with PAD. Fifth, in people with PAD, to study the association of mitochondrial measures with brachial artery flow-mediated dilation (FMD). For this fifth aim, we hypothesized that poorer mitochondrial health and oxidative capacity would be associated with poorer brachial artery FMD. We proposed this hypothesis because poorer mitochondrial health and oxidative capacity are associated with increased oxidative stress and reduced nitric oxide availability, which is associated with poorer FMD.2
Methods
PAD participants in this study were in the RESTORE trial, a pilot randomized trial of resveratrol in people with PAD, age 65 years and older.22 Participants without PAD were identified for comparison. Analyses reported here are exploratory and represent cross-sectional data obtained from PAD participants in the RESTORE trial at baseline, prior to randomization, and participants without PAD identified from among those evaluated for eligibility in RESTORE and other randomized trials of PAD participants or observational studies of participants with and without PAD.22–25 The Institutional Review Board of Northwestern University approved the protocol. Participants provided written informed consent.
Recruitment
Participants with PAD were identified through postcards mailed to community dwelling older men and women living in the Chicago area. People with PAD who had previously participated in PAD-related research at Northwestern University and expressed interest in future research participation were contacted. Non-PAD participants were identified from among potential participants for RESTORE and other studies of participants with and without PAD22–25 who were found to have a normal ABI at their baseline visit and provided informed consent for muscle biopsy. Participants without PAD underwent the 6-minute walk test and measures of usual and fast paced 4-meter walking velocity, but did not have treadmill testing, measurement of brachial artery FMD, or mitochondrial measures other than mitochondrial DNA copy number.
Inclusion and exclusion criteria
Age ≥65 years was an inclusion criterion. PAD was defined as an ABI < 0.90 in either leg.22 Individuals with PAD who had a resting ABI ≥ 0.90 were potentially eligible if their medical record documented lower extremity revascularization or evidence of PAD from a non-invasive vascular laboratory test performed at a medical center. Participants without PAD had an ABI between 0.90 and 1.40 at their baseline study visit.
Exclusion criteria have been reported22 and are summarized briefly. For those with PAD, potential participants with a below-knee or above-knee amputation and those whose walking was primarily limited by a reason other than PAD were excluded. Participants with PAD and planned lower extremity revascularization in the next 6 months were excluded. Potential participants who were treated for cancer in the past 2 years were excluded unless their prognosis was excellent. Potential participants participating in another clinical trial were excluded. Potential participants with a Mini-Mental Status Examination score < 23 at baseline were excluded.22
ABI measurement
A hand-held Doppler probe (Pocket Dop II; Nicolet Biomedical Inc., Golden, CO, USA) was used to obtain systolic pressures twice in the right and left brachial, dorsalis pedis, and posterior tibial arteries using established methods.22–26 The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the four brachial pressures.26 Mean pressures in the arm with the higher pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the two brachial pressures differed by ≥ 10 mmHg in one measurement set.22–26
Medical history
Medical history, race, and demographics were obtained through patient report by a trained and certified health interviewer using a questionnaire.
Six-minute walk test
Following a standardized protocol,22,23,27–29 participants walked up and down a 100-foot hallway for 6 minutes after instructions to cover as much distance as possible. The distance completed after 6 minutes was recorded.
Treadmill walking performance
Treadmill walking performance was measured among participants with PAD using the Gardner–Skinner protocol.22,23,30,31
Brachial artery flow-mediated dilation
Among participants with PAD, brachial artery FMD was measured after a 12-hour fast by trained Registered Diagnostic Cardiac Sonographers, at baseline and 6-month follow-up, using standard procedures.22,23,31 The proximal brachial artery was imaged (B-mode and Doppler) using a linear array vascular ultrasound transducer (frequency 8 MHz, range 5–8 MHz) (Sequoia Model #256; Siemens Medical Solutions, Erlangen, Germany).
Four-meter walking velocity
Walking velocity was measured with a 4-meter walk performed at ‘usual’ and ‘fastest’ pace. For the ‘usual’ pace walk, participants were instructed to walk at their usual pace, ‘as if going down the street to the store’. Each walk was performed twice. The faster walk in each pair was used in analyses.27,28
Calf skeletal muscle biopsy procedure
An open muscle biopsy was performed in the medial head of the gastrocnemius muscle of the leg with the lower ABI in participants with and without PAD. Anesthesia was achieved with subcutaneous lidocaine. Subcutaneous and adipose tissue were dissected until muscle was identified. Muscle tissue was removed and immediately prepared for freezing at −70°C.
Mitochondrial DNA copy number and measures of mitochondrial activity
Total DNA was isolated from human muscle using the Wizard Genomic DNA Purification Kit according to the manufacturer’s instructions (Promega, Madison, WI, USA). After purification, DNA was evaluated spectrophotometrically by NanoDrop 1000 (Thermo Scientific, Rockford, IL, USA). Quantification of the relative number of copies of mitochondrial DNA copy number (using nuclear DNA as a standard) was determined with the Human Mitochondrial DNA Monitoring Primer Kit (#7246; Takara Bio USA, Mountain View, CA, USA) using real-time polymerase chain reaction (RT-PCR). RT-PCR amplification reactions were performed on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA). Primers were used to amplify genes corresponding to mitochondrial NADH dehydrogenase subunits 1 and 5 (ND1, ND5) and the nuclear genes corresponding to solute carrier organic anion transporter family member 2b1 (SLCO2B1) and serpin family A member 1 (SERPINA1).32 The reaction mixture consisted of 12.5 μL Terra qPCR Direct SYBR Premix (2X), 0.4 μM forward and reverse primers, and genomic DNA template (10 ng). Each sample was analyzed in triplicate. The quantification of the relative mitochondrial DNA content was performed according to the Pfaffl mathematical model.33 The difference in threshold cycle values for the ND1/SLCO2B1 pair (ΔCt1 = Ct for SLCO2B1 − Ct for ND1) and the ND5/SERPINA1 pair (ΔCt2 = Ct for SERPINA1 − Ct for ND5) were calculated and the average of 2ΔCt1 and 2ΔCt2 was used as the measure of mitochondrial DNA copy number expressed as arbitrary units.
COX and citrate synthase were measured to assess mitochondrial enzyme activity. PGC-1α was measured because it is a major regulator of mitochondrial biogenesis. COX (nmol/min/mg total protein) and citrate synthase (μmol/min/g total protein) activities were measured in duplicate spectrophotometrically in whole muscle homogenates33 and normalized to a standard cross sample included within each batch. For immunoblotting of PGC-1α, whole muscle tissue homogenates were prepared as described previously.22,34 Proteins were separated on 20% polyacrylamide gels, transferred to PVDF membranes and blocked for 1 hour in 5% milk in Tris-buffered saline Tween. Blots were probed with the appropriate primary antibodies (1:500; Millipore #516557, PGC-1α) and anti-rabbit secondary antibody conjugated with horseradish peroxidase (1:5000; #7074; Cell Signaling Technology, (Danvers, MA)). Proteins were visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) using a ChemiDoc XRS imager from Bio-Rad. Bands were quantified using Image Lab Software from Bio-Rad and normalized to a standard cross sample included within each gel and to Ponceau stain for loading control.
Other measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight (kg)/[height (meters)]2.
Statistical analyses
Baseline characteristics of participants with versus without PAD were summarized as means and standard deviations for continuous variables and as frequencies and percentages for categorical variables. T-tests were used to compare continuous characteristics and chi-squared tests and Fisher’s exact test were used to compare categorical characteristics of participants with versus without PAD, when appropriate. Linear regression analyses were used to test for trend across mitochondrial DNA copy number tertiles, including an independent variable with values of ‘1, 2, and 3’ to represent 1st, 2nd, 3rd tertiles, respectively. Pearson correlation coefficients were used to measure and compare the association of the ABI with mitochondrial DNA copy number among participants with and without PAD and separately in PAD participants. Pearson correlation coefficients were used to relate mitochondrial DNA copy number to 6-minute walk performance, COX enzyme activity, citrate synthase activity, PGC-1α, and brachial artery FMD. Based on scatterplot data, in which associations appeared to vary by severity of lower extremity ischemia, analyses were repeated according to PAD severity. For the 6-minute walk, analyses were repeated among participants with normal ABI, participants with an ABI of 0.60–0.90, and participants with an ABI < 0.60, respectively. Associations of mitochondrial DNA copy number with remaining mitochondrial measures and the brachial artery FMD were estimated separately among participants with an ABI of 0.60–0.90 and among participants with an ABI < 0.60. The interaction terms for the indicator ‘PAD’ in the association between mitochondrial DNA copy number and functional measures were tested using regression models. One PAD participant with an ABI > 0.90 was excluded from analyses of interaction testing. Analyses were exploratory and were not adjusted for multiple comparisons. Analyses were performed using SAS version 9.4 (Cary, NC, USA).
Results
Of 66 participants in the RESTORE trial, 23 refused muscle biopsy, three did not receive permission from their physician to stop antiplatelet or anticoagulation therapy for muscle biopsy, one withdrew from the study, and the remaining 39 had a muscle biopsy. Of the 39, two did not yield sufficient muscle for analyses, and three did not have sufficient muscle tissue after analysis for the RESTORE trial to analyze mitochondrial DNA copy number. One participant with PAD had a history of revascularization and an ABI > 0.90. Ten participants without PAD, age 65 years and older, had muscle available for mitochondrial DNA analyses. Characteristics of participants are in Table 1. Participants with PAD included a higher proportion of men than those without PAD. Lower ABI values and higher COX enzyme activity were associated with significantly higher mitochondrial DNA copy number among participants with PAD (Table 2).
Table 1.
Characteristics of participants with versus without PAD.
| With PAD (n=34) | Without PAD (n=l0)a | p-value | |
|---|---|---|---|
| Age, years, mean (SD) | 73.54 (6.40) | 73.07 (4.68) | 0.83 |
| Male sex, n (%) | 24 (70.59) | 3 (30.00) | 0.030 |
| African American race, n (%) | l9 (55.88) | 5 (50.00) | l.00 |
| Current smoker, n (%) | 9 (26.47) | 0 (0) | 0.l7 |
| Former smoker, n (%) | l9 (55.88) | 6 (66.67) | 0.7l |
| Ankle-brachial index, mean (SD) | 0.67 (0.15) | l.l4 (0.07) | <0.000l |
| Body mass index, kg/m2, mean (SD) | 29.58 (4.01) | 27.59 (5.06) | 0.22 |
| Diabetes, n (%) | 15 (44.12) | 1 (11.11) | 0.l2 |
| Myocardial infarction, n (%) | l (2.94) | 0 (0.00) | l.00 |
| Angina, n (%) | 4 (11.76) | 1 (ll.ll) | l.00 |
| Pulmonary disease, n (%) | 6 (17.65) | 1 (ll.ll) | l.00 |
| Cancer, n (%) | ll (32.35) | 2 (22.22) | 0.70 |
| History of revascularization, n (%) | ll (32.35) | 0 (0) | 0.046 |
| Six-minute walk distance, feet, mean (SD) | 1190.6 (223.4) | l387.3 (488.3) | 0.08 |
| Usual paced 4-meter walking velocity, m/s, mean (SD) | 0.81 (0.14) | 0.92 (0.25) | 0.08 |
| Fast paced 4-meter walking velocity, m/s, mean (SD) | l.l2 (0.22) | l.l6 (0.26) | 0.68 |
| MtDNA copy number, arbitrary units, mean (SD)b | 766 (312) | 626 (269) | 0.2l |
BMI, body mass index; MtDNA, mitochondrial DNA; PAD, peripheral artery disease
One person without PAD had missing data for smoking, BMI, comorbidities, and functional performance measurements.
Mitochondrial DNA copy number is measured relative to nuclear DNA and is expressed as arbitrary units.
Table 2.
Characteristics associated with higher mitochondrial DNA copy number among people with PAD (n=34).a
| Tertile 1 MtDNA 249–559 arbitrary units (n=ll) |
Tertile 2 MtDNA 606–874 arbitrary units (n=l2) |
Tertile 3 MtDNA 908-1540 arbitrary units (n=ll) |
Trend p-value | |
|---|---|---|---|---|
| Age, years | 75.5 (6.9) | 73.9 (7.8) | 7l.20 (3.l) | 0.ll |
| Male | 9 (8l.8) | 8 (66.7) | 7 (63.6) | 0.35 |
| African American race | 7 (63.6) | 6 (50.0) | 6 (54.6) | 0.67 |
| Current smoker | 1 (9.l) | 5 (4l.7) | 3 (27.3) | 0.33 |
| Former smoker | 7 (63.6) | 5 (4l.7) | 7 (63.6) | l.00 |
| Ankle-brachial index | 0.72 (0.l5) | 0.72 (0.l3) | 0.56 (0.l4) | 0.0ll |
| Body mass index, kg/m2 | 28.48 (3.30) | 30.50 (2.36) | 29.67 (5.8l) | 0.50 |
| Diabetes | 6 (54.6) | 5 (4l.7) | 4 (36.4) | 0.39 |
| Myocardial infarction | 0 (0.00) | 0 (0.00) | l (9.09) | 0.2l |
| Angina | 1 (9.l) | 1 (8.3) | 2 (l8.2) | 0.5l |
| Pulmonary disease | 2 (l8.2) | 2 (l6.7) | 2 (l8.2) | l.00 |
| Cancer | 3 (27.3) | 2 (l6.7) | 6 (54.6) | 0.l7 |
| Six-minute walk, feet | l282.2 (l96.5) | ll69.7 (220.0) | ll2l.8 (240.6) | 0.09 |
| Usual paced 4-meter walking velocity, m/s | 0.83 (0.l5) | 0.82 (0.l3) | 0.79 (0.l6) | 0.5l |
| Fast paced 4-meter walking velocity, m/s | l.ll (0.24) | l.l5 (0.24) | l.l0 (0.20) | 0.90 |
| Relative COX enzyme activityb | 59.ll (28.8l) | l07.40 (59.02) | ll8.56 (58.02) | 0.0l8 |
| PGC-1 quantity, arbitrary units | 3.ll (8.8l) | 0.46 (0.87) | l.09 (l.47) | 0.38 |
| Relative citrate synthase enzymenb | l07.59 (l6.47) | l04.40 (8.82) | 99.66 (l9.59) | 0.24 |
MtDNA, mitochondrial DNA; PAD, peripheral artery disease.
Data shown for continuous variables are means and standard deviations. Categorical variables are presented as n (%). The test for trend was assessed using linear regression and by entering the tertiles as ‘1, 2, 3’ in a linear regression model.
MtDNA copy number is measured relative to nuclear DNA and is expressed as arbitrary units.
COX and citrate synthase enzyme activity were measured in batches and results are reported relative to a standard sample run within each batch to allow comparison of results from different batches.
The ABI was associated positively with distance achieved in the 6-minute walk test among participants with PAD (Pearson correlation coefficient = 0.417, p=0.014), but there was no association of ABI with the 6-minute walk distance among participants without PAD (Figure 1).
Figure 1.
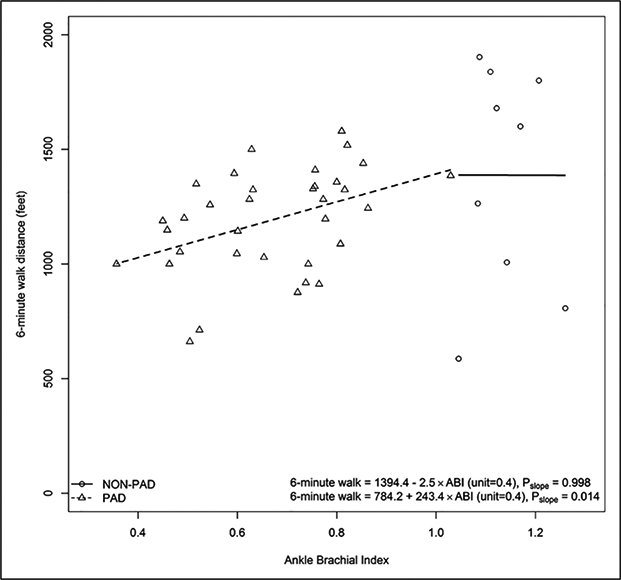
Associations of the ABI with 6-minute walk performance among participants with and without PAD.a
aOne participant with PAD and a history of lower extremity revascularization had an ABI > 0.90. One participant without PAD had a missing value for the 6-minute walk distance.
ABI, ankle–brachial index; PAD, peripheral artery disease.
There was no significant difference in mitochondrial DNA copy number between participants with versus without PAD (Table 1). Overall, the association of ABI with mitochondrial DNA copy number was Pearson correlation coefficient = −0.350, p=0.020. Among participants with PAD, lower ABI values were associated with higher mitochondrial DNA copy number (Pearson correlation coefficient = −0.355, p=0.040) (Figure 2). Among participants without PAD, the Pearson correlation coefficient for the association of ABI with mitochondrial DNA copy number was −0.299 (p=0.402) (Figure 2).
Figure 2.
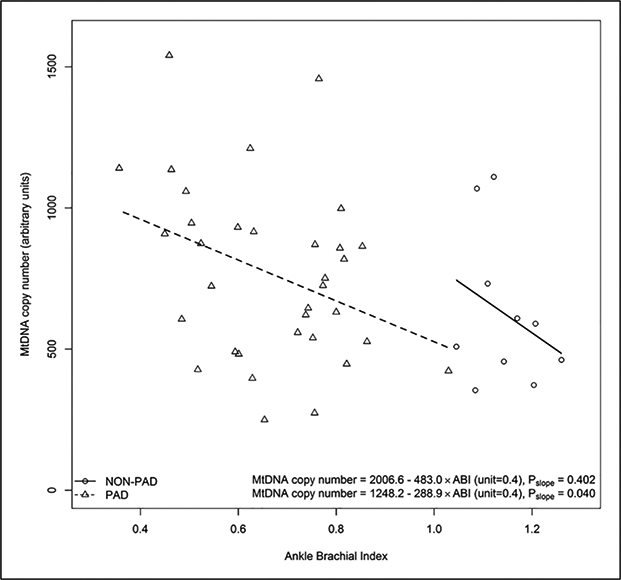
The ABI and MtDNA copy number in participants with and without PAD.a
aOne participant with PAD and a history of lower extremity revascularization had an ABI > 0.90. One participant without PAD had a missing value for the 6-minute walk distance.
ABI, ankle–brachial index; MtDNA, mitochondrial DNA; PAD, peripheral artery disease.
There was a statistically significant interaction of presence versus absence of PAD and the association of mitochondrial DNA copy number with the 6-minute walk (p for interaction = 0.001). This meant that the association of mitochondrial DNA copy number with the 6-minute walk distance was significantly different between participants with versus without PAD and this difference was not likely due to chance. The correlation coefficient between mitochondrial DNA copy number and the 6-minute walk distance was 0.653 (p=0.056) among people without PAD and −0.254 (p=0.154) among people with PAD and ABI < 0.90 (Figure 3).
Figure 3.
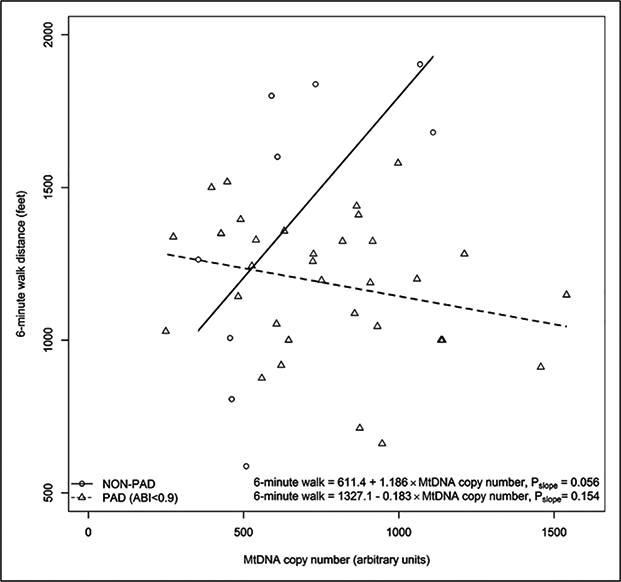
Association of MtDNA copy number and 6-minute walk distance for PAD and non-PAD participants.a
aOne PAD participant with a history of revascularization and an ABI > 0.90 was excluded from the analysis. One participant without PAD had a missing value for the 6-minute walk distance.
ABI, ankle–brachial index; MtDNA, mitochondrial DNA; PAD, peripheral artery disease.
There was a statistically significant interaction of presence versus absence of PAD and the association of mitochondrial DNA copy number with the usual paced 4-meter walking velocity (p for interaction = 0.015) (Table 3). This meant that the association of mitochondrial DNA copy number with the 4-meter walking velocity test was significantly different between participants with versus without PAD and this difference was not likely due to chance. The correlation coefficient between mitochondrial DNA copy number and usual paced 4-meter walking velocity was 0.579 (p=0.102) among people without PAD and −0.09 (p=0.601) among people with PAD.
Table 3.
Correlations coefficients of MtDNA copy number with functional performance measures, according to ABI value.
| ABI < 0.60 (n=l2) | ABI 0.60–0.90 (n=2l) | No PADa (n=9) | Interaction term for PAD status and association of MtDNA copy number with functional performance | |
|---|---|---|---|---|
| Six-minute walk test | ||||
| Pearson correlation coefficient with mitochondrial DNA copy number | −0.344 | −0.057 | 0.653 | <0.00l |
| p-value | 0.273 | 0.808 | 0.057 | |
| Four-meter walking velocity at usual pace | ||||
| Pearson correlation coefficient with mitochondrial DNA copy number | −0.264 | −0.003 | 0.579 | 0.0l5 |
| p-value | 0.407 | 0.990 | 0.l02 | |
| Four-meter walking velocity at fastest pace | ||||
| Pearson correlation coefficient with mitochondrial DNA copy number | −0.244 | 0.l78 | 0.624 | 0.l00 |
| p-value | 0.444 | 0.439 | 0.072 | |
| Maximal treadmill walking time | ||||
| Pearson correlation coefficient with mitochondrial DNA copy number | −0.340 | −0.l75 | No data | NA |
| p-value | 0.280 | 0.46l | NA | |
| Pain-free treadmill walking time | ||||
| Pearson correlation coefficient with mitochondrial DNA copy number | −0.044 | 0.390 | No data | NA |
| p-value | 0.892 | 0.099 | NA | |
ABI, ankle–brachial index; MtDNA, mitochondrial DNA; NA, not applicable; PAD, peripheral artery disease.
One participant without PAD did not have data for the functional performance measurements.
Among all participants with PAD, a statistically significant association of mitochondrial DNA copy number with COX enzyme activity was observed (Pearson correlation coefficient = 0.385, p=0.033). People with the most severe ischemia had no significant associations of mitochondrial DNA copy number with any mitochondrial measures or brachial artery FMD. However, among participants with mild to moderate PAD (ABI 0.60–0.90), higher mitochondrial DNA copy number was associated with greater citrate synthase activity and with greater relative brachial artery FMD, a measure of vascular function (Table 4).
Table 4.
Correlations of MtDNA copy number with markers of mitochondrial activity and mitochondrial biogenesis among participants with PAD.a
| All PAD participants (n=34)a | ABI < 0.60 (n=l2) | ABI 0.60–0.90 (n=2l) | |
|---|---|---|---|
| Relative COX activity | |||
| Pearson correlation coefficient with mitochondrial DNA copy number | 0.385 | 0.478 | 0.3l6 |
| p-value | p=0.033 | p=0.ll6 | p=0.202 |
| Relative citrate synthase activity | |||
| Pearson correlation coefficient with mitochondrial DNA copy number | 0.038 | −0.25l | 0.479 |
| p-value | p=0.835 | p=0.432 | p=0.038 |
| Log-transformed PGC-1α | |||
| Pearson correlation coefficient with mitochondrial DNA copy number | 0.253 | 0.03l | 0.453 |
| p-value | p=0.l69 | p=0.924 | p=0.059 |
| Maximum relative brachial artery FMD 60/90 (%) | |||
| Pearson correlation coefficient with mitochondrial DNA copy number | 0.255 | −0.043 | 0.465 |
| p-value | p=0.l66 | p=0.90l | p=0.039 |
ABI, ankle–brachial index; FMD, flow-mediated dilation; MtDNA, mitochondrial DNA; PAD, peripheral artery disease.
One participant with documented PAD and a history of revascularization had an ABI > 0.90.
Discussion
Results reported here showed that more severe lower extremity ischemia, measured by the ABI, was associated with significantly higher mitochondrial DNA copy number. Results also showed a statistically significant interaction for the association of mitochondrial DNA copy number with the 6-minute walk distance and 4-meter walking velocity. Specifically, results showed that the magnitude of association of mitochondrial DNA copy number with functional performance measures was statistically significantly more positive in participants without PAD, compared to those with PAD. To our knowledge, this is the first study of mitochondrial DNA copy number to include participants with and without classical symptoms of intermittent claudication in a cohort of PAD participants without critical limb ischemia.
A prior study reported no difference in mitochondrial DNA copy number between people with versus without PAD,35 consistent with results reported here. However, to our knowledge, no prior studies have reported on the association of greater lower extremity ischemia with mitochondrial DNA copy number. Our finding that more severe lower extremity ischemia was associated with higher mitochondrial DNA copy number may be due to a compensatory increase in mitochondrial DNA copy number in the setting of ischemiarelated reductions in mitochondrial activity. Our finding that mitochondrial DNA copy number was not significantly associated with any mitochondrial or functional measures or with brachial artery FMD in people with severe lower extremity ischemia suggests that a compensatory increase in mitochondrial DNA copy number was inadequate in people with severe lower extremity or that mitochondria in people with severe lower extremity ischemia are damaged. Consistent with this possibility, results reported here showed that PAD participants with mild to moderate PAD had statistically significant and positive correlations of mitochondrial DNA copy number with calf muscle citrate synthase activity and brachial artery FMD, but positive associations were not observed among PAD participants with severe PAD.
Prior research has been contradictory regarding the association of PAD with mitochondrial measures.5–13 PAD patients who successfully compensate may have higher measures of both mitochondrial activity and abundance, while those with severe ischemia may be unable to fully compensate, resulting in lower mitochondrial activity despite greater mitochondrial abundance. Increased mitochondrial damage, mitochondrial DNA deletions, and impaired mitophagy have been reported in the calf muscle of people with PAD.18–21 These phenomena may also potentially explain higher mitochondrial DNA copy number in people with more severe ischemia. For example, damaged mitochondrial DNA may accumulate in the setting of impaired mitophagy.21
To our knowledge, no prior studies have related mitochondrial DNA copy number to the 6-minute walk performance or 4-meter walking velocity in people with PAD. Our finding of a statistically significant interaction term for the presence versus absence of PAD and the association of mitochondrial measures with the 6-minute walk and 4-meter walking velocity is consistent with, but distinct from, a previous study demonstrating that calf muscle mitochondrial DNA copy number correlated with aerobic exercise capacity, measured by VO2 max, in people without PAD but not in people with PAD.35
Impaired mitochondrial activity is associated with increased oxidative stress and lower nitric oxide availability.2 For this reason, it is conceivable that impaired mitochondrial activity may be correlated with impaired brachial artery FMD, a measure of vascular function. Our finding that mitochondrial DNA copy number was correlated with brachial artery FMD in people with an ABI of 0.60–0.90 is consistent with this hypothesis. To our knowledge, this association has not been reported previously.
Limitations
This study has limitations. First, the study was cross-sectional. The biologic pathways responsible for the associations described here cannot be determined. Second, the sample size was small, limiting statistical power, particularly in the group of participants with an ABI < 0.60. Third, the analyses were performed as exploratory analyses in a trial of resveratrol for people with PAD21 and the results were not adjusted for multiple testing due to the sample size limitations. Results require confirmation. Fourth, mitochondrial DNA copy number has not been associated consistently with mitochondrial volume or abundance in young healthy males.36 Furthermore, in people with chronic disease, a compensatory increase in mitochondrial DNA copy number may occur that is not associated with improved mitochondrial activity.37 Therefore, the significance of an increase in mitochondrial DNA copy number in people with PAD remains uncertain. Fifth, calf muscle biopsy measures of COX activity, citrate synthase activity, and PGC-1α and brachial artery FMD were not measured in participants without PAD. Sixth, data on respirometry or oxidative stress were not available. Seventh, it is possible that associations of lower extremity ischemia and mitochondrial DNA copy number may differ between PAD participants with versus without a history of lower extremity revascularization. Finally, results may have been influenced by the fact that the PAD participants had more males than the non-PAD participants.
Conclusion
In conclusion, more severe lower extremity ischemia is associated with an increased mitochondrial DNA copy number. Findings reported here are consistent with the hypothesis that greater mitochondria abundance in the calf muscle of people with lower extremity ischemia may reflect a compensatory response that is inadequate, particularly in the setting of severe lower extremity ischemia. A prospective study is needed to confirm these findings and delineate the biologic pathways responsible for the associations reported here.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute on Aging (R21-AG047510), National Heart, Lung, and Blood Institute (R01-HL107510, R01HL109244, R01-HL088589, R01HL122846), and the Office of Dietary Supplements.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.McDermott MM. Lower extremity manifestations of peripheral artery disease: The pathophysiologic and functional implications of leg ischemia. Circ Res 2015; 116: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamburg NM, Creager MA. Pathophysiology of intermittentclaudication in peripheral artery disease. Circ J 2017; 81: 281–289. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Ferrucci L, Guralnik J, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation 2009; 120: 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Guralnik JM, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation 2008; 117: 2484–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AlGhatrif M, Zane A, Oberdier M, et al. Lower mitochondrial energy production of the thigh muscles in patients with low-normal ankle-brachial index. J Am Heart Assoc 2017; 6: e006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pipinos II, Sharov VG, Shepard AD, et al. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg 2003; 38: 827–832. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JD, Epstein FH, Meyer CH, et al. Multifactorial determinants of functional capacity in peripheral arterial disease: Uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol 2009; 54: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou XY, Green S, Askew CD, et al. Skeletal muscle mitochondrial ATP production rate and walking performance in peripheral arterial disease. Clin Physiol Funct Imaging 2002; 22: 226–232. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren F, Dahllof AG, Schersten T, et al. Muscle enzyme adaptation in patients with peripheral arterial insufficiency: Spontaneous adaptation, effect of different treatments and consequences on walking performance. Clin Sci (Lond) 1989; 77: 485–493. [DOI] [PubMed] [Google Scholar]
- 10.Jansson E, Johansson J, Sylven C, et al. Calf muscle adaptation in intermittent claudication. Side-differences in muscle metabolic characteristics in patients with unilateral arterial disease. Clin Physiol 1988; 8: 17–29. [DOI] [PubMed] [Google Scholar]
- 11.Bylund AC, Hammarsten J, Holm J, et al. Enzyme activities in skeletal muscles from patients with peripheral arterial insufficiency. Eur J Clin Invest 1976; 6: 425–429. [DOI] [PubMed] [Google Scholar]
- 12.Clyne CA, Mears H, Weller RO, et al. Calf muscle adaptation to peripheral vascular disease. Cardiovasc Res 1985; 19: 507–512. [DOI] [PubMed] [Google Scholar]
- 13.Brass EP, Hiatt WR, Gardner AW, et al. Decreased NADH dehydrogenase and ubiquinol-cytochrome c oxidoreductase in peripheral arterial disease. Am J Physiol Heart Circ Physiol 2001; 280: H603–609. [DOI] [PubMed] [Google Scholar]
- 14.Puntschart A, Claassen H, Jostarndt K, et al. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol 1995; 268: C619–625. [DOI] [PubMed] [Google Scholar]
- 15.Hebert SL, Rouge PM, Lanza IR, et al. Mitochondrial aging and physical decline: Insights from three generations of women. J Gerontol A Bio Sci Med Sci 2015; 70: 1409–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mengel-From J, Thinggard M, Dalgard C, et al. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet 2014; 133: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 2005; 102: 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White SH, McDermott MM, Sufit RL, et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: An observational study. J Transl Med 2016; 14: 284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat HK, Hiatt WR, Hoppel CL, et al. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 1999; 99: 807–812. [DOI] [PubMed] [Google Scholar]
- 20.Brass EP, Wang H, Hiatt WR. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vasc Med 2000; 5: 225–230. [PubMed] [Google Scholar]
- 21.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med 2000; 5: 55–59. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Leeuwenburgh C, Guralnik JM, et al. Effect of Resveratrol on walking performance in older people with peripheral artery disease: The RESTORE randomized clinical trial. JAMA Cardiol 2017; 2: 902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott MM, Ferrucci L, Tian L, et al. Effect of granulocyte-macrophage colony-stimulating factor with or without supervised exercise on walking performance in patients with peripheral artery disease: The PROPEL randomized clinical trial. JAMA 2017; 318: 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Liu K, Green D, et al. Changes in D-dimer and inflammatory biomarkers before ischemic events in patients with peripheral artery disease: The BRAVO study. Vasc Med 2016; 21: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polonsky TS, Liu K, Tian L, et al. High-risk plaque in the superficial femoral artery of people with peripheral artery disease: Prevalence and associated clinical characteristics. Atherosclerosis 2014; 237: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral artery disease. J Vasc Surg 2000; 32: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Ades PA, Dyer A, et al. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg 2008; 48: 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014; 130: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol 2008; 51: 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner AW, Skinner JS, Cantwell BW, et al. Progressive vs. single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 1991; 23: 402–408. [PubMed] [Google Scholar]
- 31.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: A randomized controlled trial. JAMA 2009; 301: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, Liu H, Ikeda Y, et al. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: Relevance to cellular therapies. Stem Cell Res 2012; 9: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondria function and biogenesis pathways in skeletal muscles of sedentary high- and low-functioning elderly individuals. Aging Cell 2012; 11: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Hiatt WR, Barstow TJ, et al. Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: Alterations with disease. Eur J Appl Physiol Occup Physiol 1999; 80: 22–27. [DOI] [PubMed] [Google Scholar]
- 36.Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 2012; 590: 3349–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu-Wai-Man P, Sitarz KS, Samuels DC, et al. OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Human Mol Genet 2010; 19: 3043–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]


