Abstract
The absolute requirement for reproduction implies that hypothalamo-pituitary-gonadal axis, controlling fertility, is an evolutionary robust mechanism. The gonadotropin-releasing hormone (GnRH) neurons of the hypothalamus represent the key cell type within the body dictating fertility. However, the level of functional redundancy within the GnRH neuron populations is unknown. As a result of a fortuitous transgene insertion event, GNR23 mice exhibit a marked allele-dependent reduction in GnRH neuron number within their brain. Wild-type mice have ~600 GnRH neurons compared with ~200 (34%) and ~70 (12%) in GNR23+/- and GNR23-/- mice, respectively. Using these mice we have examined the minimal GnRH neuron requirements for fertility. Male GNR23-/- mice exhibited normal fertility. In contrast, female GNR23-/- mice were markedly sub-fertile, failing to produce normal litters, estrous cycles or ovulate. The failure of ovulation resulted from an inability of the few existing GnRH neurons to generate the luteinizing hormone surge. This was not the case, however, for the first cycle at puberty that appeared normal. Together, these observations demonstrate that 12% of the GnRH neuron population is sufficient for pulsatile gonadotropin secretion and puberty onset, whereas between 12 and 34% are required for cyclical control in adult female mice. This indicates that substantial redundancy exists within the GnRH neuronal population and suggests that the great majority of GnRH neurons must be dysfunctional before fertility is affected.
Keywords: GnRH, LHRH, puberty, transgenic, redundancy, ovulation
It is well established that gonadotropin-releasing hormone (GnRH) neurons are the critical output cells of the neuronal network controlling fertility in all mammalian species (1, 2). These cells have the unique property of migrating from the nose into the brain during embryonic development (3, 4) and exist postnatally as a scattered population within the hypothalamus and septum. The GnRH neurons extend axons to the median eminence from where they release GnRH into the pituitary portal system to regulate secretion of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary. Humans suspected of having dysfunctional or absent GnRH neurons are infertile (5), as are mutant mice with defective GnRH biosynthesis (6).
Given the critical importance of GnRH neurons to the survival of all mammalian species, a degree of functional redundancy within this cell population might be expected. Indeed the seminal grafting studies by Gibson and colleagues using the mutant hypogonadal (hpg) mouse, that exhibits defective post-transcriptional processing of the GnRH transcript (7, 8), have suggested that only a few GnRH neurons are necessary to support pituitary gonadotroph secretion (6, 9, 10). Grafts of normal GnRH neurons into hpg mice can restore pulsatile LH release (11, 12) but do not permit estrous cycles to occur in females (6, 13, 14). Hence, as yet, the GnRH neuron requirements for cyclical activity, spontaneous ovulation and puberty onset remain unknown.
We recently generated a mutant mouse line (GNR23) in which the migration of GnRH neurons into the brain during embryogenesis is defective (15). In these mice, the integration of a transgene into chromosome 5 resulted in a 26kb deletion approximately 67kb from the gene epha5. The deletion of these distant regulatory elements resulted in an up-regulation of EphA5 expression in the specific regions of the developing brain, including the migrating GnRH neurons. This was associated with an allele-dependent inability of most GnRH neurons to migrate out of the nose during embryogenesis. As such, adult male homozygous (hmz) GNR23 mice have only approximately 80 GnRH neurons within their brain while hemizygous GNR23 mice have around 200, compared with the normal complement of >500 in adult wild-type mice (15). All other neuroendocrine populations examined to date in these mice appear normal (15). Thus, adult hemizygous and hmz GNR23 mice exhibit step-wise, allele-dependent reductions in their complement of GnRH neurons. This provided an opportunity to examine the numbers of GnRH neurons required for different facets of reproductive function in mice. Our goals were two-fold; first to verify that only small numbers of GnRH neurons are required to reproductive function in male mice and second, to examine how many GnRH neurons are required for puberty and subsequent cyclical activity to occur in the female.
Materials & Methods
Animals
All experimentation was approved by the University of Otago Animal Welfare and Ethics Committee under application 66/02. Transgenic GNR23 mice (C57BL6/J x CBA/Ca) were maintained under 12:12 lighting conditions (lights on 08:00h) with food and water available ad libitum. Genotyping was undertaken as detailed previously (15). Where indicated, estrous cyclicity in 2-3 month females was evaluated using vaginal smears for a period of 2 weeks. Pre-pubertal female mice were examined every day for vaginal opening and once this had occurred, vaginal smears were taken each day until the first estrous smear was encountered. Hemizygous and hmz GNR23 mice exhibit normal growth rates and have body weights the same as wild-type littermates (Herbison, unpublished observations).
Immunocytochemistry
Free-floating immunocytochemical analysis of GnRH expression was undertaken as described previously using the polyclonal rabbit LR1 (1:20,000; gift of R. Benoit, Montreal) antiserum with secondary biotinylated anti-rabbit immunoglobulins (1:200; Vector Laboratories, Burlingham, CA), Vector Elite avidin-peroxidase (1:100; Vector Labs) and nickel-DAB substrate (15). Experiments were undertaken on six adult female mice of each genotype. The total number of GnRH-immunoreactive neurons found in a 1-in-3 set of coronal sections taken from the medial septum (MS) through to the level of the anterior hypothalamus (AHA) was determined in each mouse and multiplied by 3 to give an estimate of total GnRH neuron number in this area. The small number of GnRH neurons that exist more rostrally in the regions of the septohippocampal and olfactory nuclei were not counted. To establish the topographical distribution of GnRH neurons in the three genotypes, two 30μm-thick coronal brain sections at each of the three different levels (MS, rostral preoptic area (rPOA), AHA; see Fig. 1) were counted. Statistical analysis was undertaken with ANOVA followed by post-hoc Tukey-Kramer tests.
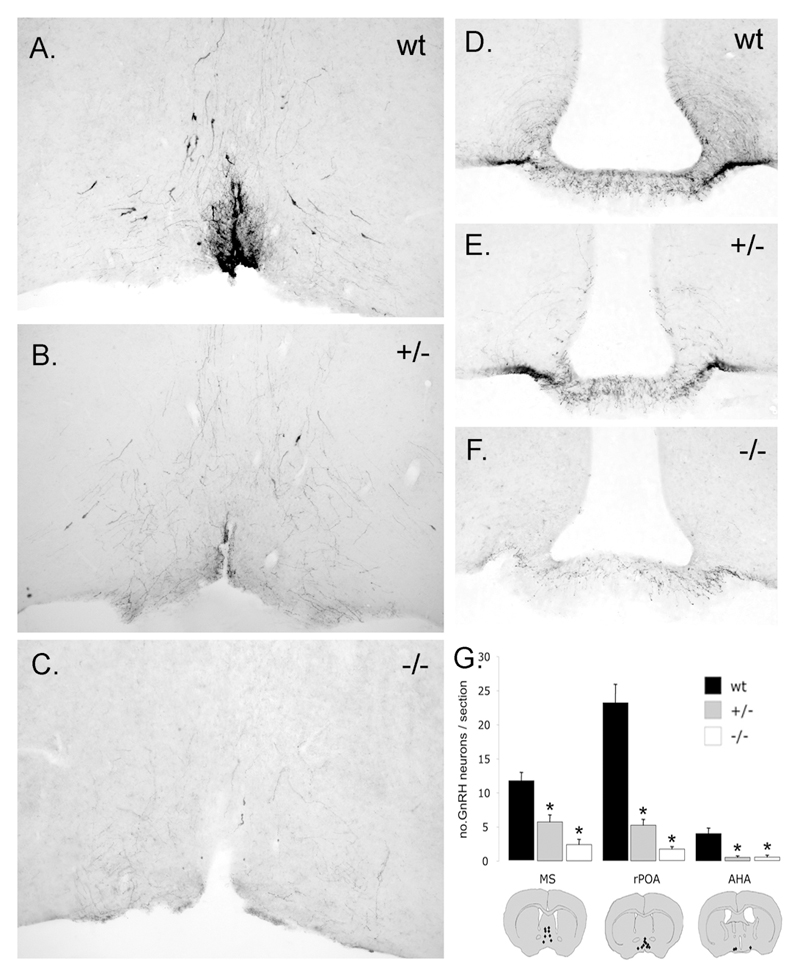
Figure 1.
Allele-dependent reduction in GnRH neuron numbers in the brains of GNR23 homozygous (-/-) and hemizygous (-/-) female mice. A-F, coronal brain sections showing GnRH immunoreactivity in the rostral preoptic area (rPOA) (A-C) and median eminence (D-F). Note that the numbers of GnRH cell bodies and their projections to the median eminence are diminished in a step-wise manner according to genotype. G, mean + SEM numbers of GnRH neurons per 30μm-thick brain section in the medial septum, rPOA and anterior hypothalamic area (AHA). The distribution of GnRH neurons (black dots) at these three levels is given in schematic coronal brain sections at the bottom. * p<0.01 (ANOVA with post-hoc Tukeys) compared with wild-type.
Dual label c-Fos/GnRH immunocytochemistry was undertaken as described previously (16) by cutting the rostral forebrain into three sets of 30μm-thick coronal sections and immunostaining one set for c-Fos using a polyclonal rabbit anti-cFos antibody (1:10,000, SC52, Santa Cruz Biotechnology Inc., Santa Cruz, CA) followed by biotinylated anti-rabbit immunoglobulins (1:200; Vector, Burlingame, CA) and Vector Elite avidin-peroxidase (1:100), and revealed using nickel-diaminobenzedine hydrochloride. A second sequential staining using the rabbit anti-GnRH antibody (1:40,000; LR1, gift of R. Benoit, Montreal) and peroxidase-labelled anti-rabbit immunoglobulins (1:400; Vector Labs) revealed by diaminobenzidine hydrochloride alone was used to visualize the GnRH neurons. The removal of either primary antibodies resulted in a complete absence of the respective immunoreactivity. Analysis was undertaken by counting the numbers of single-labelled (brown cytoplasm only) and dual-labelled (brown cytoplasm and black nucleus) GnRH neurons in two coronal brain sections at each of the levels of the MS, rPOA and AHA (Fig.1).
Ovarian histology
Reproductive tracts including both ovaries were dissected intact and weighed from perfusion-fixed animals for each of the 3 genotypes (n=4 each group, 3 months old). Mean weights were compared by ANOVA followed by Tukey’s test. Ovaries were fixed for a further hour in freshly prepared 4% paraformaldehyde in phosphate buffered saline and processed for wax embedding in a Shandon Hypercenter XP processor. A systematic sample of 5 sections were prepared at 5µm that included profiles through the central portions of the specimens, and were stained with haematoxylin and eosin. Sections were viewed and photographed in an Olympus BX Provis microscope and follicular stages of development and the presence or absence of luteal tissues recorded. Atresia was recorded if pycnotic nuclei of granulosa and also oocytes was evident, as well as the presence of uneven mural granulosa and cell fragments within the antral spaces.
Fertility testing
The fertility of hemizygous and hmz GNR23 mice was examined by pairing adult (2-3 months of age at mating) female mice (n=5-6) with wild-type stud males for a period of 4 months. Wild-type littermate females of GNR23 mice were paired with wild-type mice as controls. Male fertility was evaluated in the same manner by placing wild-type littermates and hmz GNR23 mice with wild-type females for three months. The day of birth and number of pups was recorded for each pair. Mean numbers of litters and pup numbers over this period were determined for each genotype and analysed using ANOVA followed by post-hoc Tukey-Kramer tests for females and Mann-Whitney U-tests for males.
Radioimmunoassay for LH and FSH
To establish baseline hormone levels, adult male wild-type littermates and hmz GNR23 mice were killed by cervical dislocation and trunk blood collected for hormone analysis (n=5/group). The same process was performed for females (n=5/group), with wild-type and hemizygous GNR23 being killed at diestrus and hmz GNR23 mice killed during (persistent) estrus. Plasma LH and FSH concentrations were determined by radioimmunoassay using the anti-rLH-S-11 antiserum and mLH-RP reference provided by A.F. Parlow (NHPP), and an FSH Biotrak Assay kit (RPA 550; Amersham Biosciences, UK). For LH, the intra-and inter-assay coefficients of variation were 8.3% and 11.2%, respectively. The inter-assay coefficient of variation was 11.1% for the FSH.
OVX-E-P GnRH/LH surge protocol
Adult (2-3 months of age) female mice of all three genotypes were anesthetized with Avertin (0.1 ml/10g body weight), ovariectomized (OVX) and an estradiol-filled Silastic capsule implanted subcutaneously. Estradiol capsules were made according to the protocol of Bronson (17), and involved filling Silastic tubing (1.0 mm internal, 2.1mm external diameter; Dow Corning, Michigan) with Silastic medical-grade adhesive (Dow Corning) containing 0.1mg 17-β-estradiol (Sigma, Missouri)/ml adhesive. Each mouse was given an ~ 1cm length of Silastic tubing (1μg estradiol/20g body weight). Six days after ovariectomy, mice received a subcutaneous injection of estradiol benzoate (1μg/20g body weight; Intervet, Castle Hill, Australia) at 11:00h. On the following day, animals were given a single subcutaneous injection of progesterone (500mg/20g body weight; Sigma) at 11:00h. Later that day, groups of 4-5 wild-type and hemizygous GNR23 were killed every hour from 16:00h to 24:00h by cervical dislocation and trunk blood collected after decapitation for LH radioimmunoassay. As this showed that 18:00h represented the time of peak LH secretion during the surge, a second identical experiment was undertaken using a group of wild-type littermates and hmz GNR23 mice (n=5/group) with all mice killed at 18:00h.
Experiments examining c-Fos expression in GnRH neurons were undertaken using the same OVX-E-P protocol. At 18:00h on the seventh day, mice were anesthetized with pentobarbitone and perfusion-fixed through the left ventricle with 15ml 4% paraformaldehyde fixative solution. Brains were then removed and post-fixed for 90 min at room temperature in 4% paraformaldehyde before being processed for dual labelling c-Fos/GnRH.
Pituitary GnRH stimulation test
Six adult wild-type and 6 hmz GNR23 female mice (3 months of age) were anesthetized with Avertin (0.1 ml/10g body weight) and a 50 μl tail blood sample obtained. Following the protocol of Xu and colleagues (18), mice were then administered GnRH (200ng/kg in 100μl saline, s.c.; Sigma) and a 100 μl blood sample obtained from the right atrium 10 min later. Blood sera were stored at –70°C until assayed for LH by radioimmunoassay.
Results
GnRH neuron number is reduced in an allele-dependent manner in female GNR23 mice
As our previous study reported GnRH neuron number in detail only in male GNR23 mice (15), we first examined GnRH neuron number and distribution in adult female GNR23 animals. Immunocytochemistry revealed the typical “inverted Y” distribution of GnRH cell bodies located within the MS, rPOA and AHA of adult female wild-type mice (Fig.1A). Cell counts in coronal sections extending from the medial septum through to the anterior hypothalamus estimated that the total GnRH neuron population in this region was 569±27 in wild-type females (n=6), 193±16 (34% of wild-type) in hemizygous GNR23 mice (n=6) and 66±15 (12% of wild-type) in hmz GNR23 mice (n=6). When examined on a topographical basis, hemizygous female GNR23 mice exhibited significant reductions to (p<0.01) 50%, 22% and 12% of normal GnRH neuron cell numbers in the MS, rPOA (Fig.1B) and AHA, respectively, while the equivalent values for hmz female GNR23 mice (p<0.01) were 20%, 7% (Fig.1C) and 12% (Fig.1G). GnRH neuron cell counts per 30μm-thick coronal section at the three levels are given in Fig.1G. Immunostaining of the median eminence, the terminal field for neuroendocrine GnRH neurons, showed a similar, marked loss of GnRH fibers, with hmz more depleted than hemizygous GNR23 mice (Fig.1D,E,F). Fibers immunoreactive for GnRH were also detected in the lateral septum, organum vasculosum of the lamina terminalis, subfornical organ, bed nucleus of the stria terminalis, paraventricular nucleus of the thalamus, medial habenula and periaqueductal grey in all three genotypes but, as in the median eminence, were less abundant in hemizygous mice and even further reduced in hmz animals. Thus, as previously reported for male GNR23 mice (15), female GNR23 hemizygous and hmz mice also possess only approximately 34% and 12%, respectively, of the normal complement of GnRH neurons.
To confirm the GnRH neuron-specificity of the GNR23 mutant, we also undertook a quantitative assessment of arcuate nucleus dopaminergic neurons, periventricular somatostatin neurons and medial septal acetylcholine neurons using immnocytochemistry. In each case an entirely normal distribution and number of neurons was found in GNR23 hmz mice (data not shown).
Puberty in GNR23 female mice
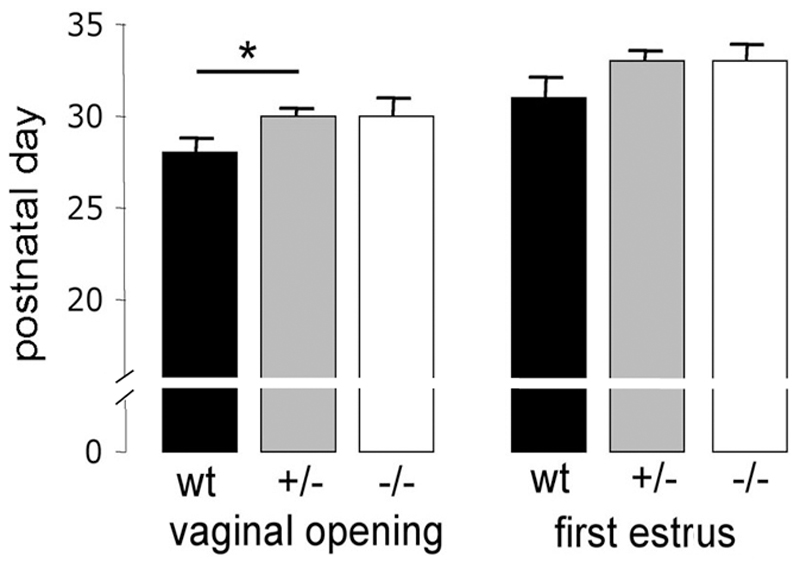
The activation of GnRH neurons is responsible for the initiation of puberty in humans and all other mammals (19–21). We examined whether mice with only ~ 70 or ~ 200 GnRH cells could support the normal onset of puberty. Wild-type, hemizygous and hmz female GNR23 mice were examined for the onset of vaginal opening and the day of first estrous by vaginal smear. Vaginal opening was delayed by ~2 days in hemizygous GNR23 mice (postnatal day 30±0.3, n=23) compared with wild-type littermates (p<0.05; postnatal day 28±0.7, n=11), but was not significantly different between hmz GNR23 (postnatal day 30±0.9, n=6) and controls (Fig.2). However, no differences were detected between genotypes for the first day of estrus, occurring ~3 days following vaginal opening (wild-type postnatal day 31±1; hemizygous 33±0.5; hmz 33±0.8; Fig.2).
Figure 2.
Puberty onset in GNR23 female mice. The mean (+SEM) day of vaginal opening and first estrus is given for each of the three genotypes. Wild-type (wt, n=11); hemizygous (+/-, n=23); homozygous (-/-, n=6), * p<0.05 (ANOVA with post-hoc Tukeys).
Adult GNR23 male mice exhibit normal fertility
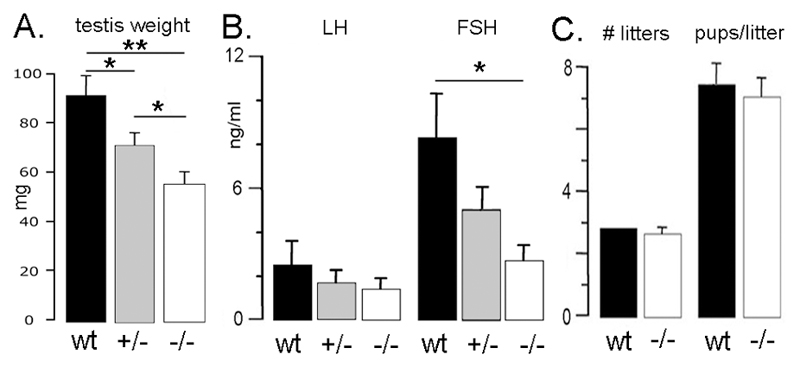
Gross examination of the testis showed a significant step-wise reduction in testicular weight for hmz (56±3mg) and hemizygous (72±5mg) GNR23 mice compared with wild-type males (92±8mg; ANOVA with post-hoc Tukey’s test; n=6-9 for each group). Histological examination did not, however, reveal any differences between the testes of the three genotypes (not shown). Plasma LH levels were not significantly different between the genotypes (Fig. 3B), whereas FSH concentrations were significantly reduced in hmz GNR23 males compared with wild-type mice (Fig.3B).
Figure 3.
Reproductive phenotype of GNR23 male mice. Histograms show mean (+SEM) (A) testis weight, (B) gonadotropin secretion and (C) numbers of litters born to GNR23 male mice/3 months and numbers of pups/litter for wild-type (wt), hemizygous (+/-) and homozygous (-/-) GNR23 mice. * p<0.05, ** p<0.01 (ANOVA with post-hoc Tukeys).
To evaluate fertility in GNR23 male mice, adult wild-type (n=5) and hmz GNR23 (n=5) male mice were placed with adult wild-type females for a period of 3 months and litter number and size recorded. No differences were detected between the two groups. Time to first litter (22.0±0.6 and 21.6±0.2 days), numbers of litters in the 3 month period (3.0± 0.0 and 2.8± 0.2) and numbers of pups per litter (7.7±0.7 and 7.3±0.6) were identical between wild-type and hmz GNR23 mice (Fig. 3C). These observations indicate that, despite reduced FSH levels and testicular size, a population of ~ 70 GnRH neurons is sufficient to maintain reproductive competency in males.
Hmz GNR23 female mice exhibit abnormal fertility
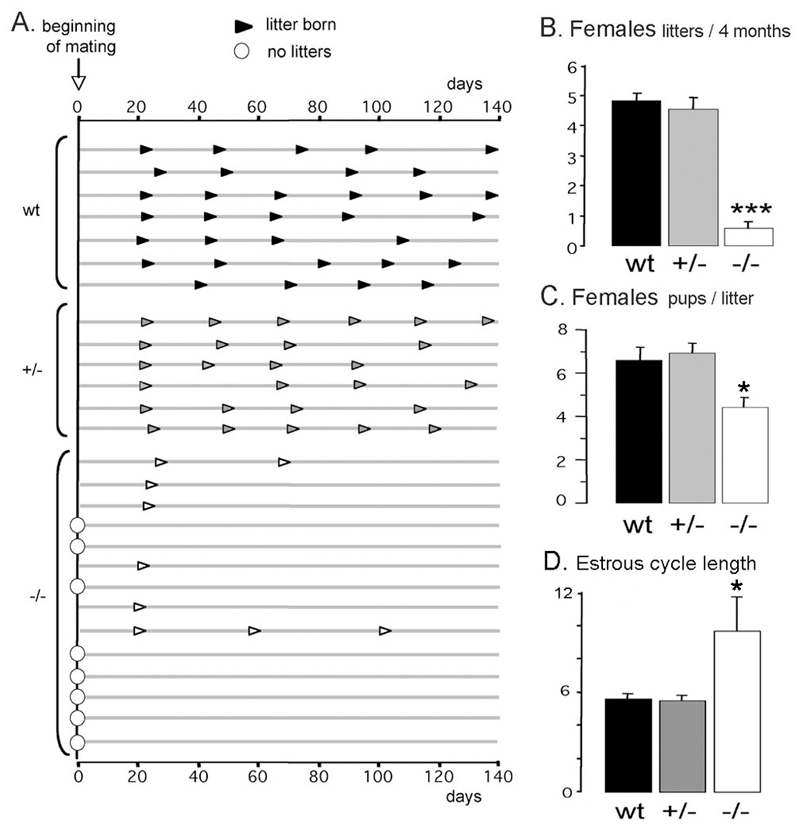
The fertility of GNR23 female mice was assessed by pairing with wild-type males for a period of 4 months and observing the frequency and size of litters. Wild-type litter-mates (n=7) gave birth to their first litter 23.2±0.8 days after pairing and subsequently had litters on a regular ~22 day interval for the rest of the assessment period (Fig.4A). Hemizygous GNR23 females (n=6) exhibited an identical profile (Fig.4A); the first litters were born on day 22.2±0.3 after pairing and, overall, produced 4.5±0.3 litters in the 4 month period compared with 4.9±0.3 for wild-type mice (Fig.4B). In contrast, hmz GNR23 mice were either infertile or sub-fertile. Of the 14 hmz mice paired, eight (58%) failed to get pregnant, while the remaining six mice generated only 9 litters amongst them over the 4 month period (Fig.4A). Interestingly, all these mice produced their first litter at the same time as that of wild-type and hemizygous GNR23 mice (day 22.5±1.1 after pairing) but then either failed to have a second litter, or had a greatly prolonged interval between litters (Fig.4A). The number of pups born to hmz GNR23 mice was significantly reduced (p<0.05; 4.7±0.5) compared with wild-type (6.6±0.4) or hemizygous (7.0±0.6) GNR23 mice (Fig.4C). These data indicate that 34% of the normal GnRH neuron population in females is compatible with normal fertility, but that a further reduction to only ~%12 is insufficient.
Figure 4.
Fertility of GNR23 mice. A, Schematic representation of females’ reproductive history following introduction of a wild-type male mouse (beginning of mating, arrow = day 0). Each line represents time across 140 days for an individual female mouse with arrowheads indicating the day each litter was born. Data for 7 wild-type, 6 hemizygous (+/-) and 14 homozygous (-/-) females are given. B, Mean (+SEM) number of litters for each genotype. C, Mean (+SEM) number of pups born/litter for the three genotypes. D, Mean (+SEM) number of days of the estrous cycle for wild-type (wt), and GNR23 female mice. Note that -/- data only include those GNR23 female mice that showed evidence of a cycle; most were in persistent estrus. *** p<0.01, * p<0.05 (ANOVA with post-hoc Tukeys).
Hmz GNR23 female mice have disrupted estrous cycles
Wild-type litter-mates (n=8), hemizygous (n=24) and hmz (n=12) GNR23 adult female mice were examined for estrous cyclicity by daily vaginal smear. Wild-type mice exhibited a mean cycle length of 5.5±0.3 days with 36±3% of the time spent in estrus. Hemizygous GNR23 mice had a very similar profile with mean cycle length of 5.4±0.3 days and 30±4% of time in estrus (Fig.4D). In contrast, 7 of the 12 GNR23 mice did not show any cyclical activity remaining in persistent estrus. The remaining 5 hmz GNR23 mice exhibited a mean cycle length of 9.5±2 days with 64±10% of the time spent in estrus (p<0.05 compared with wild-type and hemizygous GNR23; Fig.4D).
Single-point measurements of plasma LH and FSH levels in diestrous wild-type (n=4) and in hmz GNR23 (n=4) females did not reveal any differences (FSH; wild-type, 1.2±0.2 ng/ml; hmz, 1.4±0.3 ng/ml; LH; wild-type, 1.3±0.4 ng/ml; hmz, 1.4±0.4 ng.ml).
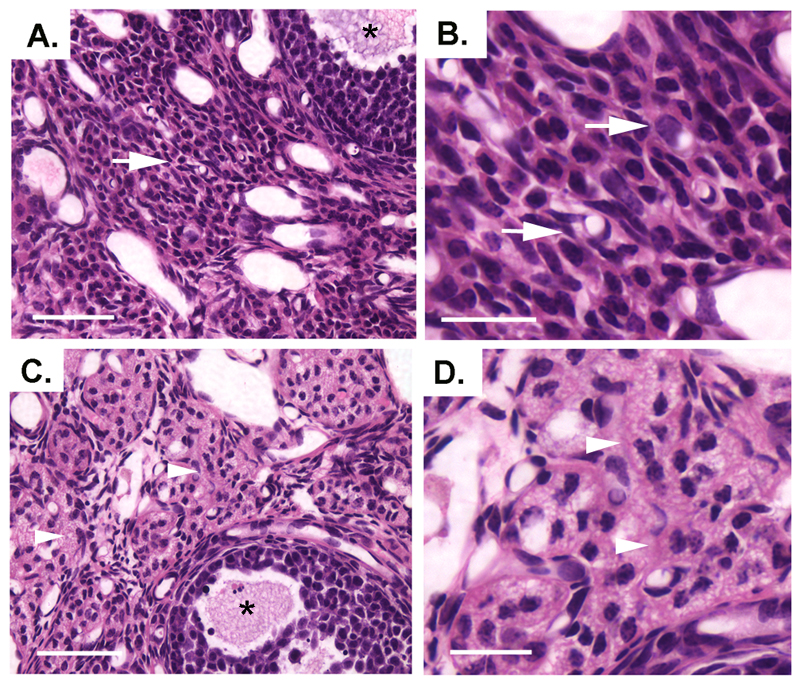
Reproductive tract weights of wild-type (0.069±0.007g) and hemizygous (0.071±0.013) mice were the same, but each was significantly heavier than that of hmz females (0.043±0.006, p<0.05). Ovarian histology showed the presence of primordial, primary and antral staged follicles in all three genotypes (Fig.5). The vast majority of primordial and primary staged small follicles had normal features of granulosa and oocyte morphology. Both healthy and atretic antral follicles were evident in all samples with more atretic antral follicles noted in the hmz ovaries (Fig.5). The main difference between genotypes was seen in luteal tissue. All wild-type, and most of the hemizygous, ovaries had areas of normal luteal tissue indicative of recent ovulation. However, no evidence of normal luteal tissue was evident in hmz GNR23 females (Fig.5C,D). Instead, there were areas of stromal tissue with aggregates of dispersed cells containing marked flocculent cytoplasm (Fig.5C,D) indicative of attempted granulosa cell transformation into luteal cells that had become moribund and disorganised.
Figure 5.
Histological sections of wt (A, B) and hmz (C, D) ovaries. Normal stromal tissue with vascular elements is present throughout the wt samples (arrows). Extensive areas of flocculent cellular material is seen in the stroma of the hmz specimen (arrowheads). Antral follicles are indicated in A and C (asterisks). Scale bars represent 45μm in A and C and 15μm in B and D.
GNR23 mice are unable to generate a normal LH surge
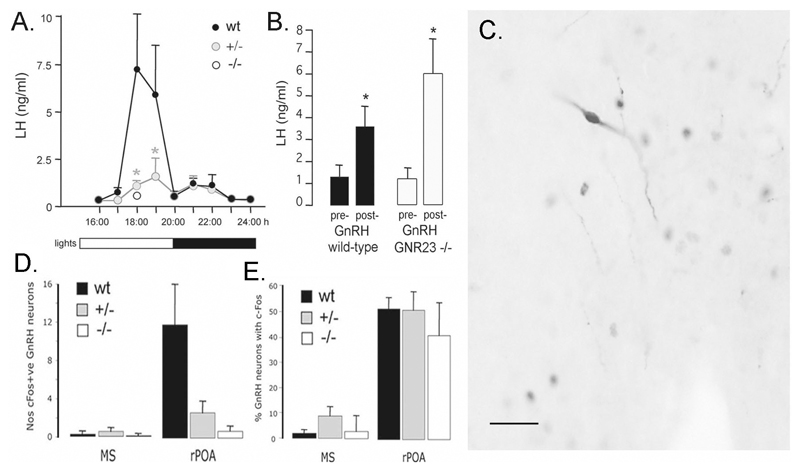
One explanation for the abnormal estrous cyclicity and fertility of hmz GNR23 female mice may be that these mice have insufficient numbers of GnRH neurons to generate the GnRH surge that, in turn, evokes the LH surge and ovulation. To assess this possibility, we used an ovariectomized-estrogen-progesterone (OVX-E-P) replacement model to examine the LH surge in these mice. In the first experiment, we examined wild-type and hemizygous GNR23 mice at hourly intervals from 16:00h to 24:00h to examine the profile of the LH surge in our OVX-E-P protocol. Wild-type mice exhibited an LH surge beginning at 17:00h and finishing 3h later at 20:00h (Fig.6A). Of the 8 mice examined at 18:00 and 19:00h, seven exhibited an LH surge ([LH] > mean + 2 standard deviations of 16:00h [LH]). Surprisingly, considering the normal fertility of hemizygous GNR23 mice, the LH surge in hemizygous GNR23 mice was markedly flattened in shape compared with wild-type mice (Fig.6A). Five of eight hemizygous GNR23 mice examined at 18:00 and 19:00h exhibited an LH surge. Peak LH levels were 7.4±3.0 ng/ml in wild-type mice compared with 1.6±0.9 ng/ml in hemizygous GNR23 mice (p<0.05). To evaluate the LH surge levels in hmz GNR23 females, we examined a group of OVX-E-P wild-type mice alongside OVX-E-P hmz GNR23 animals at 18:00h, the time of peak LH secretion. Whereas LH levels in wild-type mice were 8.5±4.1 ng/ml, LH levels in GNR23 hmz females were 0.3±0.1 ng/ml (Fig.6A) with no mice showing evidence of an LH surge.
Figure 6.
LH surge in GNR23 mice. A, Profile of LH surge in OVX mice of all three genotypes treated with estrogen and progesterone. Note that the LH surge in hemizygous (+/-) mice (* p<0.05) is markedly flattened in profile whereas evaluation of LH levels at the time of peak LH secretion (18:00h) reveals an absence of the LH surge in homozygous (-/-) GNR23 females. Bar at bottom indicates light schedule. B, Pituitary response to exogenous GnRH. Mean (+SEM) LH levels before (pre-) and 10 min after (post-) 200ng/kg GnRH s.c. to wild-type (black bars) and homozygous GNR23 (open bars) female mice. * p<0.05. C, Dual-label immunocytochemistry showing a GnRH neuron expressing c-Fos (black nuclear staining) in the rostral preoptic area (rPOA) of a hmz GNR23 mouse. D, Mean (+SEM) number of medial septal (MS) and rPOA GnRH neurons/coronal section expressing c-Fos at 18:00h in the three genotypes. E, Mean (+SEM) percentage of rPOA GnRH neurons expressing c-Fos at 18:00h. Scale bar in C represents 50μm.
The failure of GNR23 mice to exhibit a surge may result from dysregulation at the level of the pituitary gonadotroph. To address this possibility, we examined the sensitivity of the pituitary to exogenous GnRH. Basal LH levels in adult wild-type and female hmz GNR23 mice (n=5 each group) were the same, and exogenous GnRH significantly elevated LH levels in both genotypes (p<0.05, Mann-Whitney U-test; Fig.6B). Although there was a trend for a greater response in hmz GNR23 mice, this was not statistically different. This result suggests that pituitary sensitivity to GnRH is intact in hmz GNR23 mice.
To understand better the relative and absolute failure of the LH surge in hemizygous and hmz GNR23 mice, we examined the expression of c-Fos in GnRH neurons following the OVX-E-P surge protocol. The GnRH neurons activated by estrogen to evoke the GnRH/LH surge express c-Fos coincident with the beginning of the GnRH surge (22). Wild-type, hemizygous and hmz GNR23 adult female mice (n=5-6/group) underwent the OVX-E-P protocol. As noted previously in wild-type mice (23), GnRH neurons expressing c-Fos were located almost exclusively within the rPOA (Fig.6C,D) with only a few dual labelled cells detected at the levels of the MS (Fig.6D) and AHA (not shown). The dual-labelling immunocytochemical staining procedure did not reduce our ability to detect GnRH neurons, as the numbers of GnRH neurons detected in dual-labeled sections were the same as those found in the single-label immuncytochemical experiments (e.g. wild-type mice had 23±3 and 23±4 GnRH neurons/rPOA section in dual- and single-labelling experiments, respectively). Although there were fewer GnRH neurons in hemizygous and hmz GNR23 mice, we nevertheless found evidence for c-Fos expression in a sub-population of rPOA GnRH neurons in both genotypes (Fig.6C,D). A mean of 0.7±0.5, 2.6±1.2 and 12.0±4.2 c-Fos-positive GnRH neurons/brain section were detected in hmz, hemizygous and wild-type mice, respectively (Fig.6D). When the numbers of c-Fos-positive GnRH neurons were examined as a percentage of the total rPOA GnRH population, we found that 40-50% of GnRH neurons showed evidence of activation in each genotype (wild-type = 51±5%; hemizygous = 50±7%, hmz = 41±12%; Fig.6E). Together, these observations suggest that the estrogen positive feedback mechanism is present in all genotypes, but that it only activates a very small number of GnRH neurons in hmz GNR23 mice.
Discussion
Using a unique mouse model, we provide here evidence for substantial functional redundancy within the GnRH neuronal population. Male mice with only approximately 80 GnRH neurons (15) exhibit normal fertility. In contrast, whereas females with approximately 200 GnRH neurons (34%) are fertile, reducing GnRH neuron number to 70 (~12%) results in severe reproductive deficits. Analyses at multiple levels indicate the sub-fertility of female GNR23 mice arises from a failure of ovulation due to an inability of hmz GNR23 mice to generate an LH surge. Remarkably, the process of puberty, including the first pubertal ovulation, occurs with only ~70 GnRH neurons. These findings indicate that features of the reproductive axis that require relatively simple pulsatile GnRH secretion are likely to be sustained by only a very few GnRH neurons. In contrast, more complex cyclical elements require between 12-34% of the normal GnRH neuron population.
We show here with immunocytochemistry that hemizygous and hmz GNR23 mice have approximately 34% and 12%, respectively, of the normal number of GnRH neurons in their forebrain. We believe that this provides a good index of the actual number of GnRH neurons present in the brain as (i) our previous work evaluated GNR23 mice with in situ hybridization and found an identical reduction in GnRH mRNA-expressing cell number in hemizygous and hmz GNR23 male mice (15), and (ii) during development, the GnRH neurons absent in the brain can be accounted for by those GnRH neurons identified to remain within the nose (15). Hence, the reduction in GnRH-immunoreactive neuron numbers observed in GNR23 mice represents a real loss of neurons rather than their biosynthetic capacity.
GnRH neurons in GNR23 mice fail to migrate through the nose during embryogenesis due to an up-regulation of epha5 gene expression, and remain trapped in the nose where they stop producing GnRH (15). Why a few GnRH neurons are able to migrate from the nose in an apparently normal manner while others remain trapped is unknown. One possibility is that the temporal pattern of epha5 up-regulation in GNR23 mice favours the migration out of the brain of the very earliest or very latest differentiating GnRH neurons. In this light, it is interesting to note that, topographically, the depletion in GnRH neuron numbers is progressively more severe in those brain regions requiring the longest migratory route. However the relationship between the initiation of migration and eventual position within the brain for individual GnRH neurons is unknown at present. Intriguingly, the few GnRH neurons that end up in the brain of GNR23 mice appear to innervate all of the hypothalamic, as well as extra-hypothalamic, brain regions normally targeted by the full GnRH neuron population.
Previous hpg-grafting studies by Gibson, Silverman and colleagues indicated that only a few GnRH neurons were necessary to enable pulsatile LH secretion (9). Our present findings are in agreement with those observations as hmz GNR23 male mice exhibit normal levels of fertility. This suggests that pulsatile LH secretion is sufficient in male mice with only ~70 GnRH neurons. Interestingly, however, we uncovered a differential effect of reduced GnRH neuron numbers on plasma gonadotrophin levels; plasma LH levels were normal whereas FSH concentrations were reduced by ~60% in the male. The low concentrations of FSH are very likely to be responsible for the reduced overall size of the otherwise relatively normal testis in these GNR23 mice. Indeed, the testicular findings and reproductive phenotype are very similar to that of mice with activin receptor type-II and FSHβ subunit mutations that exhibit low or no circulating FSH levels, respectively (24, 25).
Female mice with approximately 200 GnRH neurons, or 34% of their normal number, exhibited normal levels of fertility. This was despite the observation that the amplitude of the LH surge in hemizygous GNR23 was only ~20% of its normal size. Early studies indicated that as little as 10-20% of the LH surge was required for normal levels of ovulation in the rat (26, 27). Our present results suggest a similar scenario in the mouse and confirm the substantial redundancy in LH signalling at the level of the ovary in terms of fertility.
The hmz GNR23 female mice with 12% of the GnRH neuron population exhibited severe deficits in their fertility. Just over half of mice failed to become pregnant while others showed much reduced litter numbers and sizes. Only 2 of 14 hmz GNR23 mice were able to generate more than one litter over a period of 4 months. Curiously, those hmz GNR23 mice that were able to generate a litter, did so at the normal time approximately 20 days after first pairing with a male, but then failed from then on. This could be explained by GNR23 mice exhibiting a relatively normal male-induced, reflex LH surge upon introduction to the male, followed by absent, or markedly defective, spontaneous ovulations in the following months of co-habitation. An evaluation of estrus cyclicity in hmz GNR23 mice showed that these mice were either in persistent estrus or exhibited prolonged 9-day estrous cycles with 63% of the time spent in estrous. The ovaries of hmz GNR23 mice were found to be significantly smaller in size compared with controls and histological examination revealed an increase in the number of atretic follicles and an absence of luteal tissue. Together, these observations clearly indicate that hmz GNR23 mice fail to exhibit normal cyclical ovulatory behavior.
One reason for the anovulatory phenotype of GNR23 mice would be that the reduced numbers of GnRH neurons in these mice are insufficient to generate a GnRH surge. Indeed, we found that hmz GNR23 mice were unable to exhibit an LH surge. As pituitary sensitivity to GnRH is not altered in GNR23 mice, the absence of the LH surge is unlikely to have resulted from a defect at the level of the gonadotroph. To examine further the reason for the failure of the LH surge, we questioned whether the GnRH neurons were being activated. Previous studies in the mouse have shown that 40-50% of GnRH neurons located in the rPOA express c-Fos at the time of the LH/GnRH surge and that they are activated by an indirect estrogen positive feedback mechanism (16, 22). Remarkably, we found that 40-50% of GnRH neurons exhibited c-Fos at the time of the surge in hmz, as well as hemizygous, GNR23 mice. This suggests that despite the low numbers of GnRH neurons in GNR23 mice, they remain innervated in a relatively normal heterogeneous manner. When translated into actual numbers of GnRH neurons (total number of dual-labelled cells with sample size correction), however, no more than 10-15 individual GnRH neurons are activated by positive feedback in each hmz GNR23 mouse. Although it is clear that redundancy exists in terms of the amount of GnRH required to evoke an LH surge (28), it seems that 10-15 activated GnRH neurons in hmz GNR23 mice do not generate a sufficient amount, or profile, of GnRH in portal blood to evoke an LH surge.
Given the severe fertility defects in female hmz GNR23 mice, it was surprising to find that puberty onset was relatively normal in these mice. Although hemizygous GNR23 mice exhibited a slight delay in vaginal opening, first estrus occurred at the normal time. Vaginal opening occurs in response to increasing levels of circulating estrogen levels that are driven by the emergence of pulsatile gonadotropin secretion at puberty (21). As it is likely that even very low numbers of GnRH neurons are sufficient to drive pulsatile LH secretion (see above), it was not unexpected to find that vaginal opening occurred at the normal time in GNR23 mice. However, given the severe ovulatory defects of adult mice, it was surprising that hmz GNR23 mice were able to exhibit their first pubertal ovulation at the correct time. A similar disassociation in dysfunction between first and latter ovulations has been observed in glutamic acid decarboxylase-over expressing GnRH neurons in the rat (29). Equally, the first pubertal ovulation of rats has been observed to be more robust than subsequent ovulations in the face of pharmacological manipulations (30). This may result from a particularly heightened release of GnRH, and/or the relative absence of a robust estrogen negative feedback mechanism, at the time of puberty (31). These results raise the likelihood that failure of puberty in mammals, including humans, only occurs when the great majority of GnRH neurons become dysfunctional. Certainly, it is suspected that anosmic hypogonadal hypogonadotropic individuals with Kalmann Syndrome have no GnRH neurons in their brain (32, 33).
Finally, it is important to consider these results with respect to the potential caveats of studies undertaken in transgenic mouse models. It is clear that GNR23 mice have had depleted GnRH neuron numbers all of their postnatal life and, as such, some manner of compensation may have occurred to enhance their efficacy. To date, we have examined GnRH transcript levels in these cells, and found that they are the same in GNR23 mice and wild-type littermates (Herbison, unpublished observations), and also shown that pituitary sensitivity to GnRH is normal (Fig.6). It is also possible that the epha5 dysregulation has had effects upon other components of the hypothalamo-pituitary-gonadal axis in GNR23 mice. Although possible, we note that GNR23 mice appear normal in all other respects and immunocytochemical analyses of several other neuroendocrine and neural populations in these mice have all been normal.
In summary, we report here on a mouse model that has enabled the GnRH neuron requirements for puberty onset, ovulation and fertility to be assessed. Overall, the data show that a remarkable degree of redundancy exists within the GnRH neuron population in terms of successful fertility. Whereas males exhibit normal fertility with only 12% of the normal GnRH neurons, females require between 12-34% of the population. Taken together with data from hpg-grafted mice (9), these observations suggest that pulsatile GnRH secretion can be achieved by only a small number (<70) of GnRH neurons. In contrast, the cyclical nature of female fertility requires between 12-34% of the GnRH neuron population to ensure the generation of an effective GnRH/LH surge. These findings are relevant to the various transgenic strategies currently being employed to modulate GnRH neuron function in vivo, as at least 70-90% of GnRH neurons will need to be deleted or made dysfunctional before a loss-of-neuron reproductive phenotype appears. An unsuspected finding of this study has been that puberty can occur with only ~70 GnRH neurons. From the perspective of the clinic, these observations on minimal GnRH neuron requirements, suggest that the majority of GnRH neurons must be dysfunctional before patients will present with impuberty or infertility due to hypogonadal hypogonadism.
Acknowledgements
The authors wish to thank Dr. Stephen Assinder and Ryan Davis for their help with the testicular analysis, Dr. A.F. Parlow, NIDDK National Hormone & Peptide Program, CA, USA for radioimmunoassay reagents, and Dr. R. Benoit for LR1 antiserum. Work was supported by The Wellcome Trust and the Health Research Council of New Zealand.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biology of Reproduction. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 3.Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316. doi: 10.1016/s0091-3022(02)00001-8. [DOI] [PubMed] [Google Scholar]
- 4.Tobet SA, Bless EP, Schwarting GA. Developmental aspect of the gonadotropin-releasing hormone system. Mol Cell Endocrinol. 2001;185:173–184. doi: 10.1016/s0303-7207(01)00616-5. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Martinez D, Hu Y, Bouloux PM. Ontogeny of GnRH and olfactory neuronal systems in man: novel insights from the investigation of inherited forms of Kallmann's syndrome. Front Neuroendocrinol. 2004;25:108–130. doi: 10.1016/j.yfrne.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Charlton H. Neural transplantation in hypogonadal (hpg) mice - physiology and neurobiology. Reproduction. 2004;127:3–12. doi: 10.1530/rep.1.00066. [DOI] [PubMed] [Google Scholar]
- 7.Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- 8.Seong JY, Kim BW, Park S, Son GH, Kim K. First intron excision of GnRH pre-mRNA during postnatal development of normal mice and adult hypogonadal mice. Endocrinology. 2001;142:4454–4461. doi: 10.1210/endo.142.10.8449. [DOI] [PubMed] [Google Scholar]
- 9.Gibson MJ, Wu TJ, Miller GM, Silverman A. What nature's knockout teaches us about GnRH activity: hypogonadal mice and neuronal grafts. Hormones and Behavior. 1997;31:212–220. doi: 10.1006/hbeh.1997.1387. [DOI] [PubMed] [Google Scholar]
- 10.Silverman AJ, Zimmerman EA, Gibson MJ, Perlow MJ, Charlton HM, Kokoris GJ, Krieger DT. Implantation of normal fetal preoptic area into hypogonadal mutant mice: temporal relationships of the growth of gonadotropin-releasing hormone neurons and the development of the pituitary/testicular axis. Neuroscience. 1985;16:69–84. doi: 10.1016/0306-4522(85)90048-x. [DOI] [PubMed] [Google Scholar]
- 11.Gibson MJ, Miller GM, Silverman AJ. Pulsatile luteinizing hormone secretion in normal female mice and in hypogonadal female mice with preoptic area implants. Endocrinology. 1991;128:965–971. doi: 10.1210/endo-128-2-965. [DOI] [PubMed] [Google Scholar]
- 12.Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology. 1988;48:45–52. doi: 10.1159/000124988. [DOI] [PubMed] [Google Scholar]
- 13.Gibson MJ, Kokoris GJ, Silverman AJ. Positive feedback in hypogonadal female mice with preoptic area brain transplants. Neuroendocrinology. 1988;48:112–119. doi: 10.1159/000124998. [DOI] [PubMed] [Google Scholar]
- 14.Gibson MJ, Silverman AJ. Effects of gonadectomy and treatment with gonadal steroids and luteinizing hormone secretion in hypogonadal male and female mice with preoptic area implants. Endocrinology. 1989;125:1525–1532. doi: 10.1210/endo-125-3-1525. [DOI] [PubMed] [Google Scholar]
- 15.Gamble JA, Karunadasa DK, Pape JR, Skynner MJ, Todman MG, Bicknell RJ, Allen JP, Herbison AE. Disruption of ephrin signaling associates with disordered axophilic migration of the gonadotropin-releasing hormone neurons. J Neurosci. 2005;25:3142–3150. doi: 10.1523/JNEUROSCI.4759-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. doi: 10.1210/endo-108-2-506. [DOI] [PubMed] [Google Scholar]
- 18.Xu M, Hill JW, Levine JE. Attenuation of luteinizing hormone surges in neuropeptide Y knockout mice. Neuroendocrinology. 2000;72:263–271. doi: 10.1159/000054595. [DOI] [PubMed] [Google Scholar]
- 19.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57(Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 20.Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 2177–2230. [Google Scholar]
- 21.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 2061–2126. [Google Scholar]
- 22.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 23.Wintermantle T, Campbell CE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons and fertility. Neuron. 2006 doi: 10.1016/j.neuron.2006.07.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 25.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 26.Greig F, Weisz J. Preovulatory levels of luteinizing hormone, the critical period and ovulation in rats. J Endocrinol. 1973;57:235–245. doi: 10.1677/joe.0.0570235. [DOI] [PubMed] [Google Scholar]
- 27.Gosden RG, Everett JW, Tyrey L. Luteinizing hormone requirements for ovulation in the pentobarbital-treated proestrous rat. Endocrinology. 1976;99:1046–1053. doi: 10.1210/endo-99-4-1046. [DOI] [PubMed] [Google Scholar]
- 28.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56:303–309. doi: 10.1095/biolreprod56.2.303. [DOI] [PubMed] [Google Scholar]
- 29.Heger S, Seney M, Bless E, Schwarting GA, Bilger M, Mungenast A, Ojeda SR, Tobet SA. Overexpression of glutamic acid decarboxylase-67 (GAD-67) in gonadotropin-releasing hormone neurons disrupts migratory fate and female reproductive function in mice. Endocrinology. 2003;144:2566–2579. doi: 10.1210/en.2002-221107. [DOI] [PubMed] [Google Scholar]
- 30.Field E, Tyrey L. Blockade of first ovulation in pubertal rats by delta-9-tetrahydrocannabinol: requirement for advanced treatment due to early initiation of the critical period. Biol Reprod. 1986;34:512–517. doi: 10.1095/biolreprod34.3.512. [DOI] [PubMed] [Google Scholar]
- 31.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 32.Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6:311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 33.Hardelin JP. Kallmann syndrome: towards molecular pathogenesis. Mol Cell Endocrinol. 2001;179:75–81. doi: 10.1016/s0303-7207(01)00462-2. [DOI] [PubMed] [Google Scholar]