Abstract
Background
Previous studies characterised the effects of calcium releasing secretagogues and substances implicated in the development of acute pancreatitis on mitochondrial Ca2+, transmembrane potential and NAD(P)H in pancreatic acinar cells. Here we aimed to characterise the effects of these substances on the ATP levels in the cytosol and the mitochondria of acinar cells.
Results
The ATP level was monitored using cytosolic or mitochondrial targeted luciferases. Inhibition of oxidative phosphorylation produced substantial decrease in cytosolic ATP which was comparable to that induced by the inhibition of glycolysis. Cholecystokinin (CCK) increased total cytosolic ATP level in spite of accelerating ATP consumption. The effects of acetylcholine (ACh) and Bombesin were similar to that of CCK. Bile acid - taurolithocholic acid 3-sulfate (TLC-S), fatty acid - palmitoleic acid (POA) and palmitoleic acid ethyl ester (POAEE) reduced cytosolic ATP. The ATP decrease in response to these substances was observed in cells with either intact or inhibited oxidative phosphorylation.
TLC-S, POA and POAEE reduced mitochondrial ATP, 20pM CCK increased mitochondrial ATP and 10nM CCK produced a biphasic response composed of a small initial decline followed by a stronger increase.
Conclusions
Both glycolysis and oxidative phosphorylation make substantial contributions to ATP production in acinar cells. Calcium-releasing secretagogues increased the ATP level in the cytosol and the mitochondria of intact isolated cells. TLC-S, POA and POAEE reduced cytosolic and mitochondrial ATP in spite of their ability to trigger Ca2+ signals. When cells rely on non-oxidative ATP production Ca-releasing secretagogues as well as TLC-S, POA and POAEE all diminish cytosolic ATP levels.
Introduction
The importance of bioenergetics in physiological and pathophysiological responses of the pancreas was highlighted in a number of studies ((Barrow et al., 2008;Criddle et al., 2006;Halangk et al., 1997;Halangk et al., 1998;Kosowski et al., 1998;Luthen et al., 1994;Schild et al., 1999;Voronina et al., 2004;Odinokova et al., 2009). Stimulation with secretagogues strongly up- regulates energy demand of acinar cells, as revealed by a considerable increase in glucose uptake (Korc et al., 1979). Under pathological conditions the efficiency of the microcirculation declined (Plusczyk et al., 1997;Plusczyk et al., 2001), pancreatic oxygen concentration in severe acute pancreatitis was reduced (Kinnala et al., 2002) and total ATP concentration dropped significantly in some models of pancreatitis (Halangk et al., 1998;Kinnala et al., 2002). Respiratory rate declined following damage of mitochondria by episodes of hypoxia and re-oxygenation (Kosowski et al., 1998). In some cellular models of pancreatic injury mitochondrial transmembrane potential was shown to depolarise (Voronina et al., 2004;Criddle et al., 2006;Gukovskaya et al., 2002;Gerasimenko et al., 2002); this is expected to compromise mitochondrial ATP production.
Important secretagogues (e.g. CCK, ACh) employ Ca2+ signalling to trigger and regulate secretion from the exocrine pancreas. Ca2+ signals are expected to increase ATP use due to activation of energy-consuming Ca2+ -dependent processes like exocytotic secretion, fluid secretion, protein synthesis…In some cell types restoration / maintenance of cytosolic Ca2+ levels could also have significant effects on ATP consumption (Brough et al., 2005). This is particularly relevant considering that almost all known inducers of acute pancreatitis trigger prolonged Ca2+ signals (reviewed in (Petersen & Tepikin, 2008;Halangk & Lerch, 2005)).
On the other hand cytosolic Ca2+ can be sampled by mitochondria, which are located in the vicinity of Ca2+ releasing channels (Rizzuto et al., 1992;Rizzuto et al., 1993;Park et al., 2001) or Ca2+ entry channels (Hoth et al., 1997;Varadi et al., 2004;Park et al., 2001), resulting in an increase in mitochondrial calcium followed by stimulation of Ca2+-sensitive dehydrogenases involved in the Citric Acid cycle (Hajnoczky et al., 1995;Denton & McCormack, 1986). Substances that increase [Ca2+ ]c in pancreatic acinar cells have been shown to either increase (Voronina et al., 2002b) or decrease (Criddle et al., 2006;Voronina et al., 2002b) the NAD(P)H (the important reducing equivalent for the respiratory chain) content in the mitochondria of pancreatic acinar cells. Finally sustained Ca2+ accumulation in pancreatic acinar cells (particularly in conjunction with reactive oxygen species) can trigger the opening of the mitochondrial permeability transition pore (MPTP) (Gerasimenko et al., 2002;Gukovskaya et al., 2002;Odinokova et al., 2009;Schild et al., 1999), followed by a decline in mitochondrial electrochemical potential (ΔΨ), which is essential for ATP synthesis; a recent study from Gukovskayas laboratory indicated that in mitochondria from pancreatic acinar cells mPTP can be induced by relatively low concentrations of extramitochondrial Ca2+ (Odinokova et al., 2009). The contradictory roles of Ca2+ signals which can accelerate ATP consumption, upregulate ATP production or suppress ATP synthesis makes it rather difficult to predict the net effect of these signals on the ATP concentration. On the other hand, ATP itself serves as an important regulator of Ca2+ signals - in pancreatic acinar cells a decrease in cytosolic ATP inhibits Ca2+ release from internal stores (Barrow et al., 2008;Betzenhauser et al., 2008;Park et al., 2008), Ca2+ leak (Hofer et al., 1996) and Ca2+ influx via store-operated Ca2+ channels (Barrow et al., 2008). Consequently ATP should be considered as an important regulator of both physiological and pathological downstream reactions of the Ca2+ signalling cascade, which are essential for the functioning of the exocrine pancreas. Direct ATP measurements are essential for our understanding of the complex relationships between the Ca2+ signalling cascade and the bioenergetics of pancreatic acinar cells.
Luciferase-based bioluminescence assays allow dynamic measurements of ATP content in isolated cells ((Barrow et al., 2008;Jouaville et al., 1999;Kennedy et al., 1999;Koop & Cobbold, 1993)). We utilized viral constructs carrying modified luciferase, which was optimised for ATP measurements (for details see (ref Bell) (Barrow et al., 2008;Kennedy et al., 1999))
In the present study we aimed to utilize this technical development and characterise ATP changes induced in acinar cells due to stimulation with Ca2+ releasing secretagogues and known or putative inducers of acute pancreatitis.
Method
Cell preparation and extracellular solutions
Pancreata were obtained from adult male mice (CD1) that had been killed by cervical dislocation, in accordance with the Animals (Scientific Procedures) Act of 1986. Isolated pancreatic acinar cells, were prepared using collagenase (Worthington Biochemical Corporation, Lakewood, NJ) digestion as previously described (Voronina et al., 2004). The standard extracellular solution used throughout cell isolation and during all experiments contained (in mM): NaCl 140, KCl 4.7, CaCl2 1, MgCl2 1.13, glucose 10, pyruvate 2, HEPES 10 (adjusted to pH 7.2 with NaOH). During ATP measurements the extracellular solution was supplemented with 200μM luciferin. In some experiments, where indicated, glucose was omitted from the extracellular solution. Specified inhibitors of ATP production, secretagoges, activators of pancreatitis and Ca2+ releasers were dissolved in the luciferin-containing extracellular solution to the indicated concentration.
ATP measurements
Bioluminescence measurements of ATP were carried out using adenoviruses that expressed either cytosolic luciferase (cLuc) or mitochondrial-targeted luciferase (mtLuc) (ref, Bell et al., 2007). cLuc was from Vectors BioLabs (Eagleville, PA, USA). The procedure of infection with adenovirus and measurements of bioluminescence was similar to that described in (Barrow et al., 2008). Briefly approximately 100µl of cell suspension was spotted onto glass bottom 35mm Microwell Dishes (MatTek Corporation, Ashland, MA, USA), coated with poly-L lysine and left to spontaneously attach for 5 minutes. The cells were then covered with 2mls of Dulbecco’s modified Eagle’s medium (DMEM; Sigma) supplemented with 10mM glucose, 2mM sodium pyruvate, 10% (v/v) fetal calf serum (Life Technologies), 10µg ml-1 penicillin and 10µg ml-1 streptomycin. Following a 16-24 hour infection period, the media was changed to extracellular solution and the cells were placed on the stage of a Nikon Diaphot microscope (objective Fluor x20, NA 0.75), following this bioluminescent imaging of cytosolic ATP was carried out by time resolved photon counting using a Photek HRPCS 325-18 High Resolution Photon Counting Camera (Photek, East Sussex, UK). Single photon events were integrated for one second intervals.
For calibration experiments cells were permeabilized in intracellular-like solution containing (in mM): KCl 130, NaCl 10, HEPES 10, EGTA 10, MgCl2 1.5, CoA, 0.01, 200μM luciferin and 5000 units α-toxin, together with varying concentrations of MgATP. Data analysis was performed using Photek Software. Following addition of α-toxin in the presence of 30mM MgATP to cells transfected with cLuc bioluminescence increased by 90% (here and in other parts of the manuscript 100% corresponds to bioluminescence of resting unstimulated cells). Unlike calibration at room temperature (Barrow et al., 2008) at 35C° (temperature used in this study) it was impossible to achieve stable levels of bioluminescence at intermediate concentrations of ATP (0.3, 1 and 10mM), the reason for this was probably the rapid demise of the cells following α-toxin treatment. The values of Km measured in previous studies (ref, ref) allow us to make estimations of cytosolic ATP concentrations but the method clearly remains only semi-quantitative. The main advantage of this method of ATP measurement is its high time resolution and ability to observe and select cells for experiments (and consequently avoid contamination with unwanted cell types or with cells damaged during the preparatory steps of isolation and culturing). Data were expressed as mean ± standard error of mean.
Results
Effects of inhibitors of oxidative phosphorylation and glycolysis on the cytosolic ATP level of pancreatic acinar cells
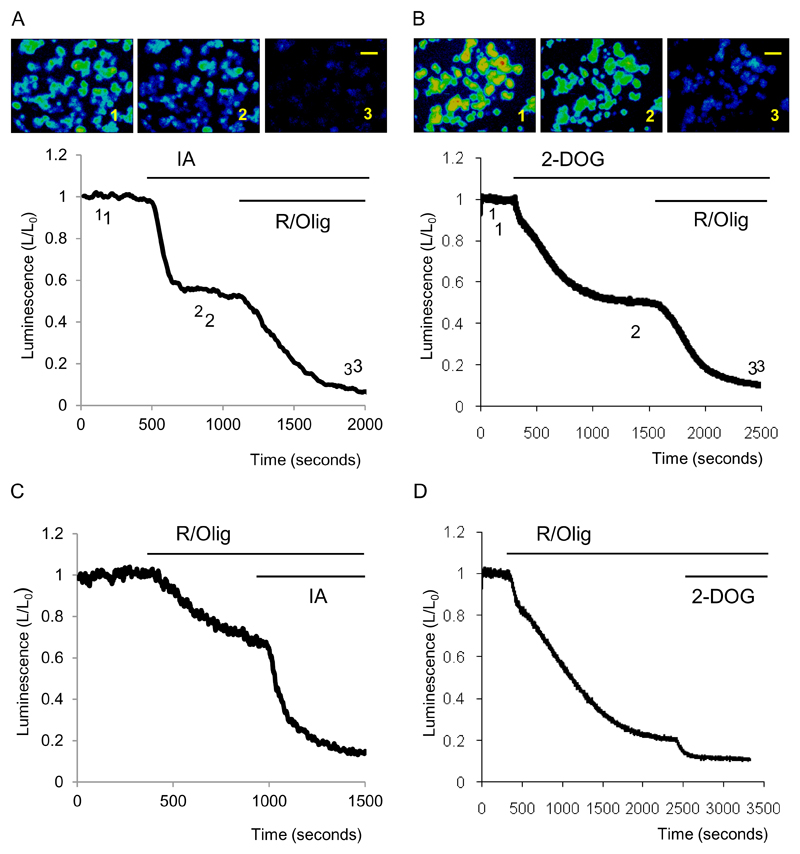
In the presence of pyruvate in the extracellular solution inhibition of glycolysis by IA or 2-DOG resulted in a significant decrease in cellular bioluminescence (Fig.1A,B). Application of IA resulted in a decrease in bioluminescence by 49± 5% of its initial value (n=5) whilst 2-DOG (in the absence of glucose) reduced the bioluminescence by 40± 3% (n=6). Subsequent application of inhibitors of oxidative phosphorylation R and Olyg further decreased the bioluminescence to near zero (Fig.1A, B).
Figure 1. Effects of inhibitors of glycolysis and oxidative phosphorylation on the cytosolic ATP level of pancreatic acinar cells.
Bioluminescence was recorded from cells transfected with cLuc.
A. Images show bioluminescence of pancreatic acinar cells. The images were acquired at time points indicated on the trace. Scale bar corresponds to 100μm Application of IA resulted in a decrease in bioluminescence. R/Olig produced a further strong decrease in bioluminescence. Experiments were conducted with pyruvate (2mM) and glucose (10mM) present in the extracellular solution.
B. The images were acquired at time points indicated on the trace. Application of 2-DOG resulted in a decrease in bioluminescence. Scale bar corresponds to 100μm. R/Olig produced a further strong decrease in bioluminescence. Experiment was conducted with pyruvate (2mM) present in the extracellular solution. Glucose was removed from the extracellular solution just before the beginning of the recording.
C. The trace illustrates decreases of bioluminescence induced by sequential application of R/O and IA. Experiment was conducted with pyruvate (2mM) and glucose (10mM) present in the extracellular solution.
D. The trace illustrates decreases in bioluminescence induced by the sequential application of R/Olig and IA. Experiment was conducted with pyruvate (2mM) present in the extracellular solution. Glucose was removed from the extracellular solution just before the beginning of the recording.
We then reversed the sequence of the inhibitors of ATP synthesis. The inhibitors of oxidative phosphorylation R and Olig also produced a substantial drop in bioluminescence (by 32 ± 2%, n= 17 see Fig.1C) and the subsequent application of IA resulted in nearly complete loss of bioluminescence. These results suggest an approximately equal contribution of glycolysis and oxidative phosphorylation to ATP production in pancreatic acinar cells. When mitochondrial inhibitors R and Olig were added in the absence of glucose in pyruvate-containing extracellular solution the bioluminescence decreased drastically (by 75± 4 % of the initial value, n= 5, see Fig. 1D), suggesting that under these conditions (as expected) oxidative phosphorylation was the main mechanism of ATP production. A small but resolvable decrease in the bioluminescence was observed upon subsequent addition of 2-DOG. Residual glucose could still be present in the cytosol following the removal of glucose from the extracellular solution. The observed small drop in bioluminescence could be attributed to 2-DOG inhibition of metabolism of this residual glucose.
Cytosolic ATP changes induced by CCK
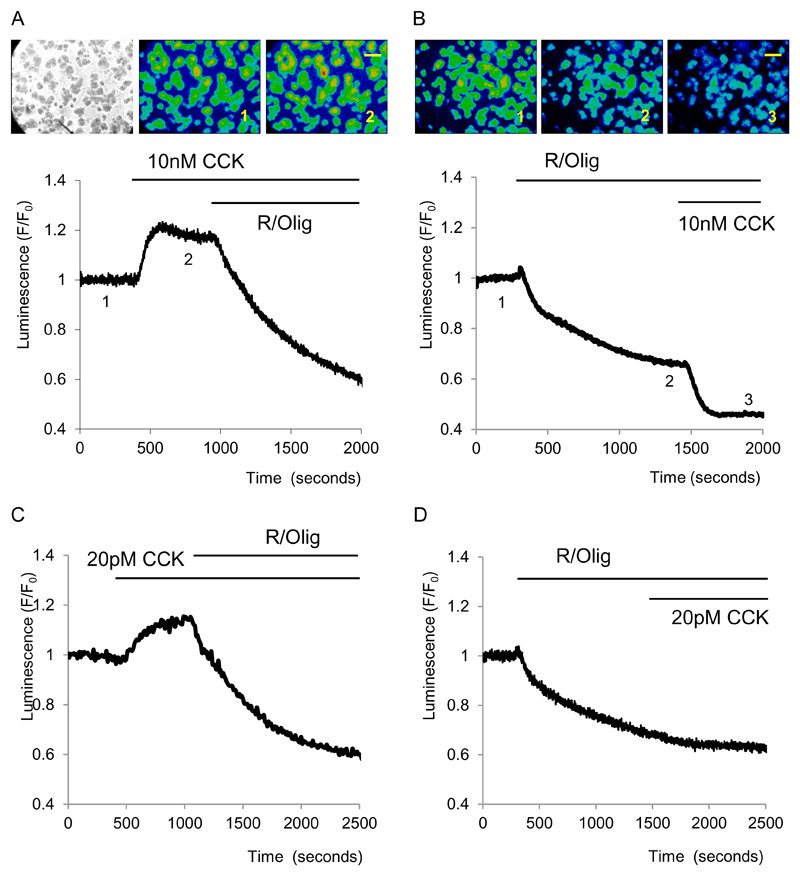
A supramaximal concentration of CCK (10nM) increased bioluminescence by 19 ± 1%, n=6 see Fig.2A). The increase was smaller than the maximal rise in bioluminescence, observed during calibration with high ATP levels (see Methods), suggesting that the cytosolic ATP level following CCK stimulation does not saturate the assay and that the bioluminescence continues to reflect the balance of ATP production and consumption. The response to CCK was drastically different after inhibition of oxidative phosphorylation with R and Olig (Fig.2B). Following treatment with R/Olig the application of CCK resulted in a substantial decrease of bioluminescence (by 23±2%, n= 6 see Fig. 2B). The additional loss of bioluminescence most probably reflects CCK – induced acceleration of ATP consumption.
Figure 2. Effects of CCK on cytosolic ATP level.
Bioluminescence was recorded from cells transfected with cLuc. Extracellular solution contained pyruvate (2mM) and glucose (10mM).
A. Increase of bioluminescence in cells stimulated with 10nM CCK. The left panel shows transmitted light image of isolated pancreatic acinar cells and clusters of pancreatic acinar cells. Central and right panels show images of bioluminescence recorded at time points indicated on the trace. Scale bar corresponds to 100μm
B. Following inhibition of mitochondrial ATP production with R/Olig, a high concentration of CCK triggers a further decrease in ATP level. Images of bioluminescence were recorded at time points indicated on the trace. Scale bar corresponds to 100μm
C. Increase of bioluminescence induced by a low (20pM) concentration of CCK.
D. Following inhibition of mitochondrial ATP production with R/Olig a low concentration of CCK does not produce a further decrease in bioluminescence.
A low near-physiological concentration of CCK (20pM) also produced an increase in ATP levels (bioluminescence increased by 11±2%, n= 7, see Fig. 2C). Following the inhibition of the mitochondrial component of ATP production an application of 20pM CCK did not produce resolvable changes in ATP level (Fig. 2D, n=3).
CCK increases the rate of ATP consumption
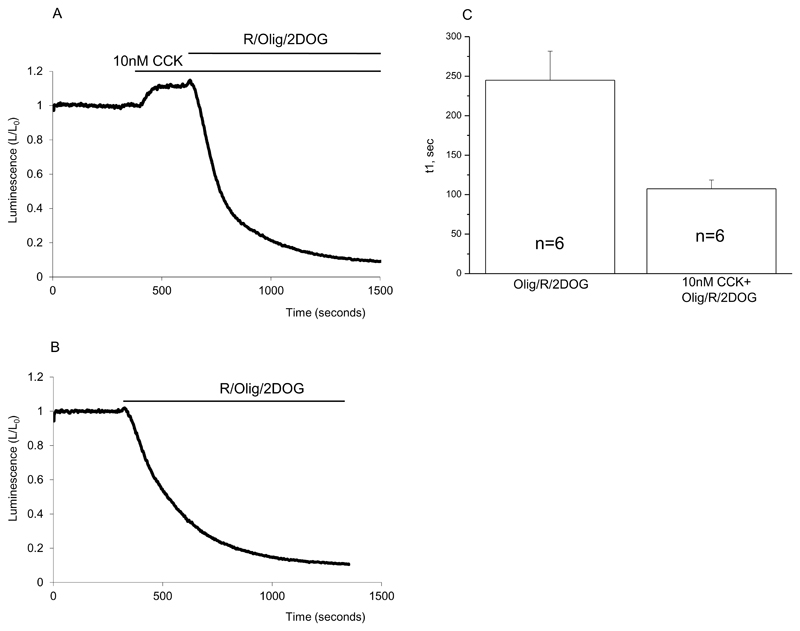
The combined application of inhibitors of oxidative phosphorylation and glycolysis results in a rapid decline in bioluminescence, which reflects the ATP consumption ((Barrow et al., 2008) and Fig.3). It is important to note that the rate of consumption, evaluated as the τ of the exponential fit, was substantially (approximately 2.3 times) increased under conditions of CCK stimulation (Fig.3). Similar increase (approximately 2.6 times) was found by comparing the rates of changes in normalised bioluminescence (L/L0) between values 0.9 and 0.6 (Fig 3A and B). Paradoxically the high concentration of CCK (10nM) increased both the rate of ATP consumption and the ATP content of pancreatic acinar cells.
Figure 3. CCK increases the rate of cytosolic ATP consumption.
Bioluminescence was recorded from cells transfected with cLuc.
A. Bioluminescence changes induced by CCK and subsequent inhibition of both oxidative phosphorylation and glycolysis.
B. Bioluminescence changes induced by inhibition of oxidative phosphorylation and glycolysis.
C. The decline of bioluminescence in experiments illustrated in A and B was approximated by an exponential function. The averaged time constants for the decline in the presence and absence of CCK are shown as bar graphs (± standard errors, number of experiments indicated on the bars).
ATP changes induced by non-agonist dependent elevation of [Ca2+]c
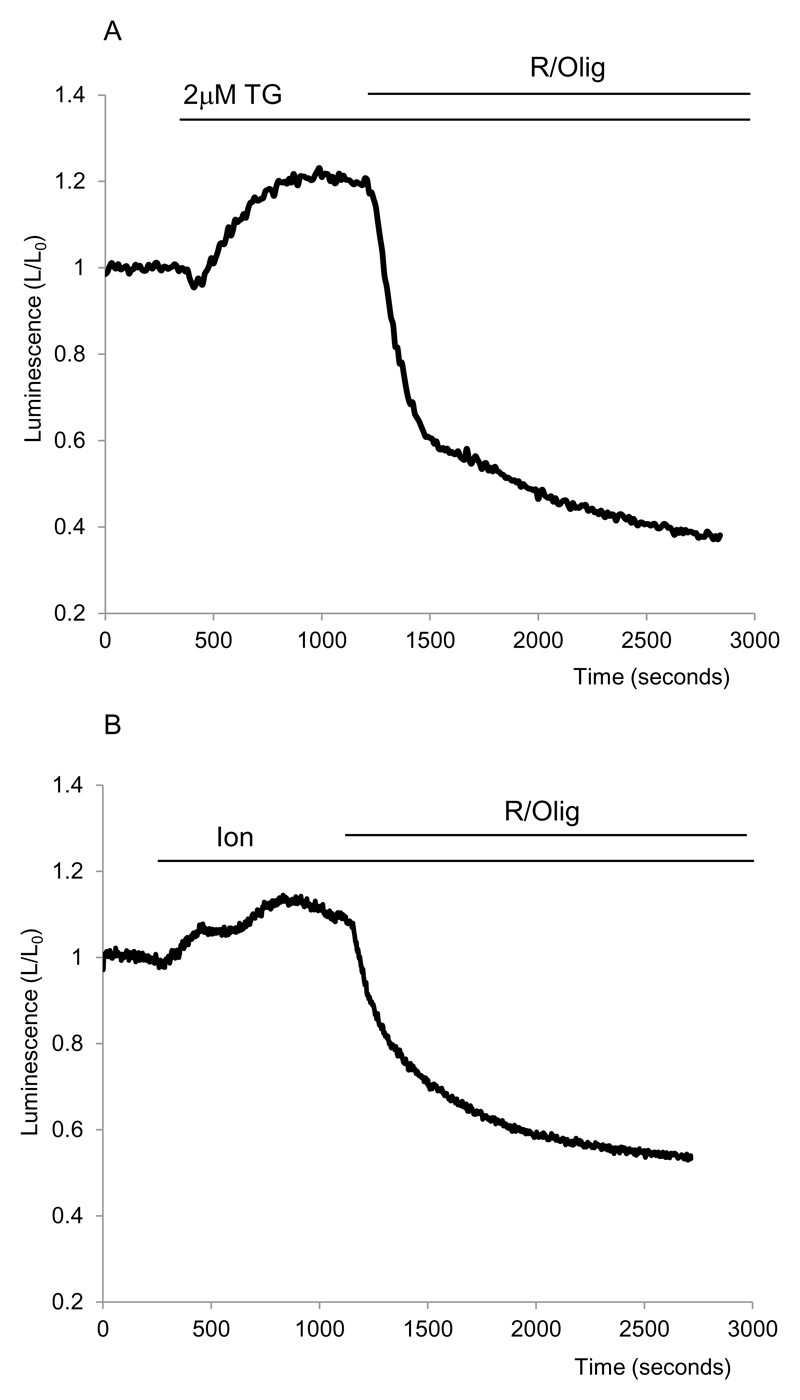
The rise in [Ca2+]c is an important consequence of CCK stimulation. Indeed we observed that CCK – induced [Ca2+]c responses were retained in pancreatic acinar cells following transfection and overnight incubation with the luciferase - encoding replication deficient adenovirus (n= 9 for 20pM CCK, which produced oscillatory responses and n=8 for 10nM CCK, which produced a large transient followed by a plateau, not shown). Increases in [Ca2+]c has previously been shown to increase the cytosolic ATP content (Jouaville et al., 1999;Dumollard et al., 2008). This effect is at least partially mediated by the increase of mitochondrial [Ca2+] ([Ca2+]m) and stimulation of Ca2+ - sensitive dehydrogenases involved in the Citric acid cycle (Denton & McCormack, 1986;Hajnoczky et al., 1995;Jouaville et al., 1999;Voronina et al., 2002b). We therefore hypothesised that CCK stimulates ATP production due to its activation of the Ca2+-signalling cascade. To verify this hypothesis we used alternative mechanisms of [Ca2+]c elevation and studied the effects of a [Ca2+]c rise on the ATP level. Tg (Fig.4A) or Ionomycin (Fig.4B) were used to elevate the [Ca2+]c; effects of these two substances on [Ca2+]c in pancreatic acinar cells have been documented in previous studies (Craske et al., 1999;Gerasimenko et al., 2006) and were not further investigated here. Similar results to CCK were observed upon Tg application. A sizable increase of bioluminescence of 26± 6% (n= 5, Fig.4A) was observed in experiments with Tg, indicating a rise in cytosolic ATP. Ionomycin also induced an increase in bioluminescence of 11±1% (Fig. 4B, n=5). Note that we used a submaximal concentration of Ionomycin which in this cell type releases Ca2+ from the ER but is insufficient to calibrate Ca2+ indicators (Craske et al., 1999). Experiments with Tg and Ionomycin suggest that CCK-induced increase inATP level occurs as a result of Ca2+-dependent stimulation of mitochondrial ATP production.
Figure 4. Thapsigargin and Ionomycin induce an increase in cytosolic ATP.
Bioluminescence was recorded from cells transfected with cLuc.
A. Rise of bioluminescence induced by TG.
B. Rise of bioluminescence induced by Ion.
Effect of other Ca2+ - releasing secretagogues on cytosolic ATP
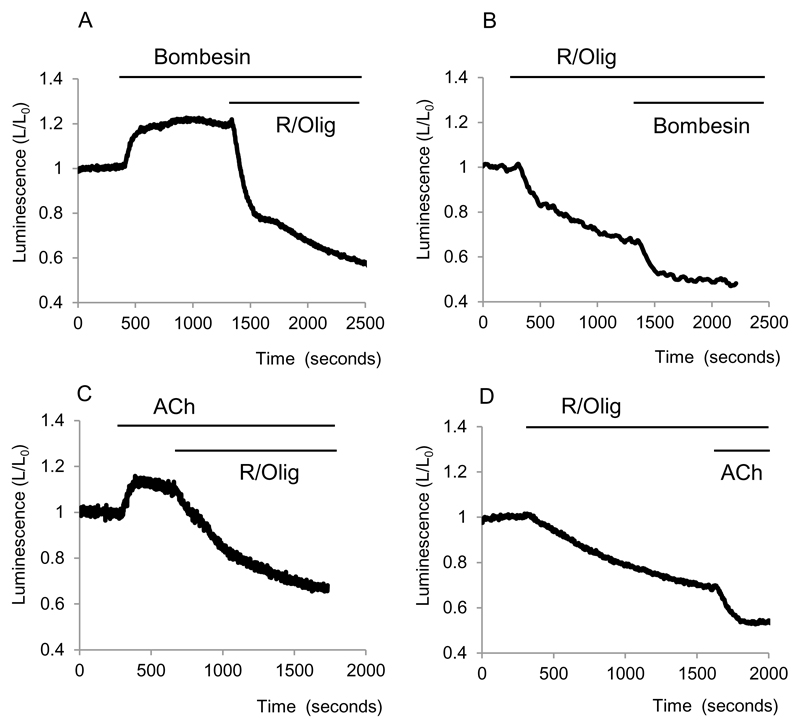
Bombesin (100pM) had a very similar effect to a high concentration of CCK. The increase in bioluminescene was 15±4% (n= 3, Fig.5A) and similar to that observed in experiments with high concentrations of CCK. Following treatment with mitochondrial inhibitors bombesin induced a clear decrease in bioluminescence (further decrease of 16 ± 2%, n= 3, Fig.5B).
Figure 5. Effects of Bombesin and ACh on cytosolic ATP.
Bioluminescence was recorded from cells transfected with cLuc.
A and B show effects of Bombesin on cytosolic bioluminescence in cells with intact and inhibited (by R/Olig) mitochondrial ATP production respectively.
C and D show effects of ACh on cytosolic bioluminescence in cells with intact and inhibited (by R/Olig) mitochondrial ATP production respectively.
The effect of ACh (10μM) on the bioluminescence of cells with intact oxidative phosphorylation was similar to that of other calcium-releasing agonists – the bioluminescence increased by 18±1% (n=4, Fig. 5C). ACh in a similar manner to CCK and Bombesin decreased the bioluminescence in cells with inhibited oxidative phosphorylation (decrease of a further 15±1%, n= 8, Fig.5Bii).
Effects of a bile acid, ethanol, fatty acid and fatty acid ethyl ester on cytosolic ATP levels of pancreatic acinar cells
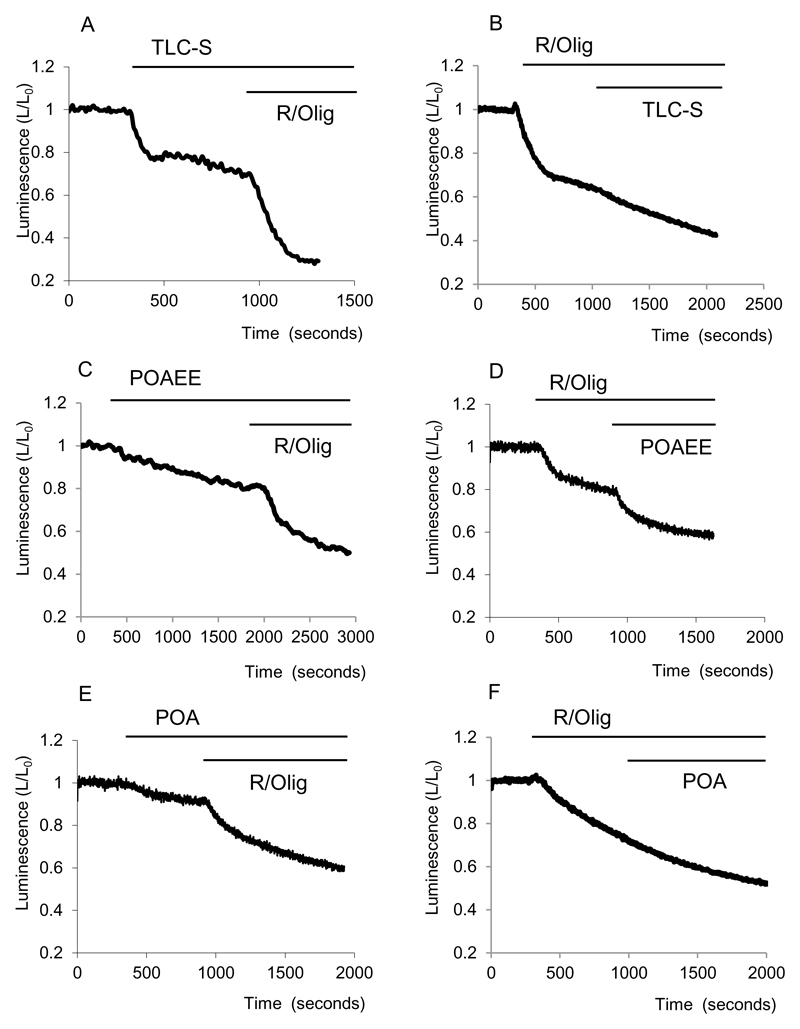
The bile acid TLC-S has been shown to increase [Ca2+]c in pancreatic acinar cells (Fischer et al., 2007;Voronina et al., 2002a). TLC-S – induced [Ca2+]c responses were retained in pancreatic acinar cells following transfection and overnight incubation with a luciferase – encoding, replication-deficient, adenovirus (n= 10, not shown).The effect of this bile acid on the cytosolic ATP level in pancreatic acinar cells was however quite different from that of the calcium-releasing secretagogues CCK or ACh. TLC-S (0.5mM) strongly reduced cellular bioluminescence (reduction by 26±2%, n=5), suggesting considerable decrease in the ATP level (Fig.6 Ai).). In a previous study we reported a substantial mitochondrial depolarisation induced by TLC-S (Voronina et al., 2004). It is therefore possible that the depolarisation is so strong that this has a negative effect on ATP production which overrides the stimulatory effect expected from the TLC-S - induced [Ca2+]c elevation. It is however important to note that a substantial further decrease in fluorescence in TLC-S treated cells was observed following inhibition of mitochondria with R and Olig (42±2%, n=5), suggesting that at least some of the mitochondrial ATP production is maintained in presence of TLC-S. TLC-S also reduced fluorescence in cells with inhibited mitochondria (Fig. 6Aii).
Figure 6. Effects of TLC-S, POAEE and POA on cytosolic ATP.
Bioluminescence was recorded from cells transfected with cLuc.
A and B show effects of TLC-S on cytosolic bioluminescence in cells with intact and inhibited (by R/Olig) mitochondrial ATP production respectively.
C and D show effects of POAEE on cytosolic bioluminescence in cells with intact and inhibited (by R/Olig) mitochondrial ATP production respectively.
E Illustrates a decrease of bioluminescence induced by POA.
F Following inhibition of mitochondrial ATP production by R/Olig we have not observed further changes in bioluminescence upon application of POA.
Another putative activator of acute pancreatitis POAEE induced a decrease in ATP concentration – the bioluminescence declined by 14±2 % (n=5 see Fig. 6Bi). POAEE also diminished ATP levels when added following the application of R/Olig (bioluminescence declined by a further 19±6% (n=3 see Fig.6Bii))
The fatty acid POA induced a small decrease in bioluminescence of 13±2% (n=11 see Fig6Ci) when applied to cells untreated with R/Olig (Fig 6Ci). We have not been able to resolve any clear effect of POA in cells which were treated beforehand with R/Olig (Fig Cii, n= 3).
Finally ethanol at a concentration of 200mM failed to produce measurable changes in bioluminescence (n=6, not shown).
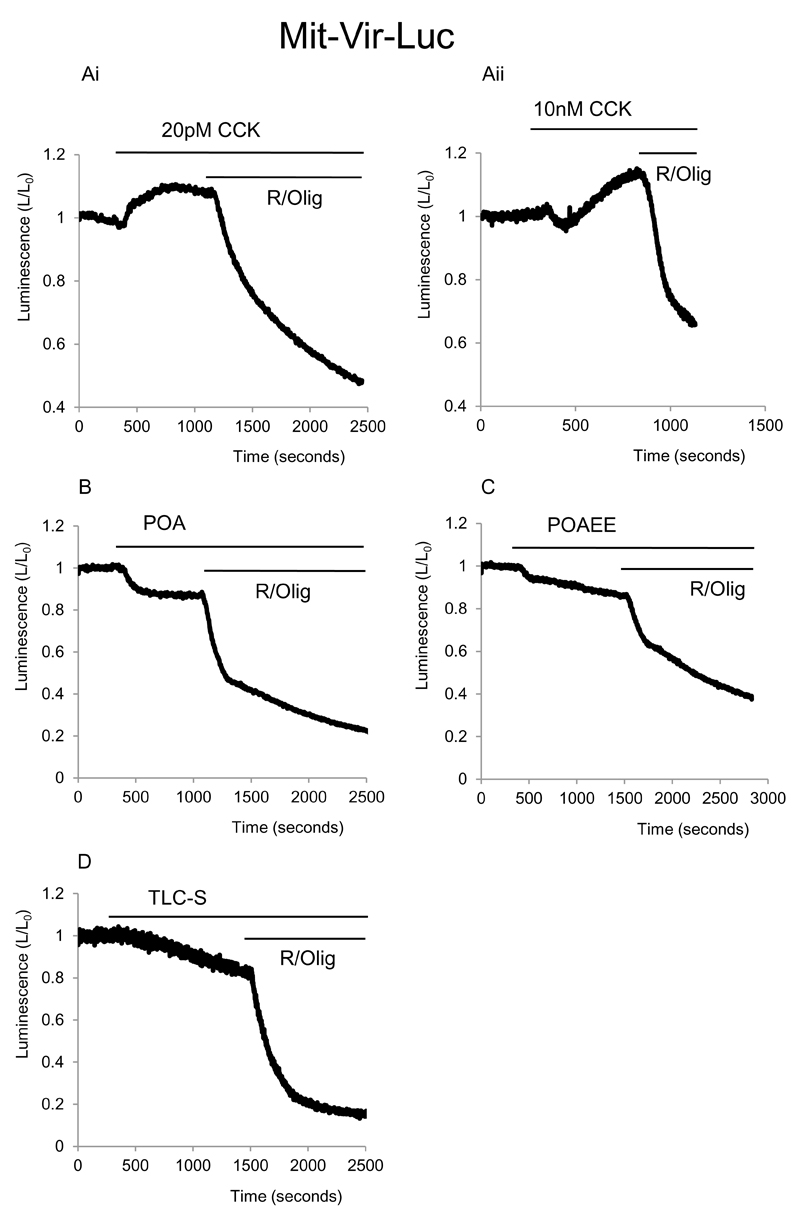
Mitochondrial ATP changes in response to CCK, TLC-S, POA and POAEE
Mitochondrial targeting of luciferase allowed us to observe changes in ATP in the matrix of these organelles. A low near-physiological concentration CCK 20pM induced small increase in mitochondrial ATP levels (of 4±1%, n=10) which was observed in all experiments (Fig.7Ai). High supramaximal concentrations of CCK (10nM) produced a complex biphasic response, composed of an initial small decline (by 8±1%, n=11), followed by a more substantial increase in bioluminescence of 16±2 % (n=11, see Fig.7Aii,). The initial decline in fluorescence was not observed in cytosolic ATP measurements upon stimulation with 10nM CCK. The initial decline in the mitochondrial ATP could reflect an increased rate of ATP transfer from the mitochondria to the cytosol, which is later overcompensated by an accelerated mitochondrial ATP production.
Figure 7. Effect of CCK, POA, POAEE and TLC-S on mitochondrial ATP level in pancreatic acinar cells.
Bioluminescence was recorded from cells transfected with mLuc.
A Effect of different concentrations of CCK on the bioluminescence. A (i) shows changes of bioluminescence induced by 20pM CCK. A (ii) depicts changes of bioluminescence induced by 10nM CCK. Note biphasic response of bioluminescence. At the end of each experiment combination of mitochondrial inhibitors R/Olig was applied, note the abrupt and strong decrease in bioluminescence upon the application of R/Olig in all experiments shown on Fig.7.
B Shows the effect of POA on bioluminescence.
C Illustrates effect of POAEE on bioluminescence.
D Effect of TLC-S on bioluminescence.
POA, POAEE and TLC-S all produced clearly resolvable decreases in bioluminescence (Fig.7 B, C, D) The bioluminescence declined by 14±1%, n=3 ; 10±1%, n= 6 and 19±5% n= 3 respectively. In all experiments subsequent inhibition of mitochondrial ATP production with R / Olig resulted in a very substantial decline in the bioluminescence. This suggests that at least within the time frame of our experiments inhibition of the mitochondrial ATP production by these substances, although measurable, is certainly incomplete.
Discussion
We were not able to calibrate ATP changes. However, assuming the 90% increase in bioluminescence above resting level (observed in the presence of α-toxin and 30mM Mg-ATP, see Methods) as the maximal attainable bioluminescence one can make an estimation of ATP changes. Assuming the Km for the reaction to be 2mM (ref, ref) we can estimate that the resting cytosolic ATP concentration corresponded to approximately 2.2mM, inhibition of mitochondria with R/Olig reduced ATP to 1.1mM (approximately two fold), inhibition of glycolysis with IA reduced ATP to 0.7mM. Application of 10nM CCK increase the ATP concentration from 2.2 to 3.5mM whilst 20pM CCK increased ATP to 2.8mM. A combination of R/Olig and 10nM CCK resulted in a decrease in ATP concentration to 0.6mM. TLC-S on its own decreased ATP to 1.3mM and following R/Olig treatment to 0.3mM. Note that the reaction involving luciferase and luciferin reports Mg-ATP rather that total ATP (ref). Other values of Km have been reported (ref, ref) and in absence of actual calibration the above values should be treated as only estimations.
Both resting ATP level and sensitivity to R/Olig seems to be different in cells incubated at 35C° (this study) and cells maintained at room temperature (ref).
We were somewhat surprised by the considerable contribution that glycolysis can make to the overall ATP balance of the pancreatic acinar cell. We expected cells to be much more reliant on oxidative phosphorylation. However an analysis of the previous publications showed that the results of these studies can be reconciled with our findings. For example the paper by Bauduing and colleagues indicated that 90% of the ATP in the pancreas is produced by oxidative phosphorylation (Bauduin et al., 1969). However in this paper (Bauduin et al., 1969) inhibition of ATP synthase with a high concentration of oligomycin resulted in only a 60% reduction in ATP concentration. Antimicin indeed produced a much stronger reduction in ATP (Bauduin et al., 1969) but the antimicin-induced collapse of ΔΨ is expected to trigger the reversal of ATP synthase and consequently a fast additional consumption of ATP by mitochondria (ref). In this study we selected R and Olig to ensure inhibition of oxidative phosphorylation under conditions where ATP synthase is also inhibited (and can not be converted into an additional powerful ATP consumer). We found that mitochondrial oxidative phosphorylation is responsible for approximately half of the ATP content in pancreatic acinar cells.
Upon stimulation with Ca2+ releasing secretagogues (CCK, ACh and Bombesin) we observed an increase in cytosolic ATP concentration. Importantly this increase occurred under conditions when ATP consumption was also significantly increased. In addition to Ca2+ -dependent activation of dehydrogenases involved in the Citric Acid Cycle, mentioned above, another phenomenon known as long-term metabolic priming (Jouaville et al., 1999) could contribute to this effect. The long-term metabolic priming is defined as an increase in ATP production which is induced by [Ca2+] elevation but persists long after the agonist washout and recovery of [Ca2+] to resting level. Considering the duration of the ATP increase, observed in our study, Ca2+-dependent metabolic priming seems to be a likely contributor.
An increase in the rate of ATP production and of the net ATP content to compensate for the potential energy loss induced by Ca2+ signals is a useful protective mechanism. A similar “proactive” response of bioenergetics to Ca2+ signals was reported for a number of primary isolated cell types (Dumollard et al., 2008;Ainscow & Rutter, 2001). The results of our study indicate that this mechanism is operational in exocrine secretory cells – pancreatic acinar cells.
A recent study suggested that the Ca2+ - dependent acceleration of glycolysis could be responsible for the net increase in cytosolic ATP content (Zamaraeva et al., 2007). In our investigation we have not observed an increase in cytosolic ATP levels when cells with inhibited oxidative phosphorylation were stimulated by Ca2+ releasing secretagogues, on the contrary, the prevaling response was the decrease in ATP (Fig.2B, Fig. 5B)). Therefore Ca2+ -dependent stimulation of glycolysis in pancreatic acinar cells is either absent or is masked by a more pronounced increase in ATP consumption.
The increase in the rate of ATP consumption due to CCK stimulation reported in our study corresponds well to previous findings of changes in glucose uptake induced by Ca2+-releasing secretagogues (Korc et al., 1979). The ability of these cells to completely compensate (and even to overcompensate) for the rapid increase in energy demand is quite remarkable and probably explains why changes in ATP level were not observed in perfused pancreas stimulated with ACh (Matsumoto et al., 1988) and in a few models of acute pancreatitis (Nordback et al., 1991). On the other hand Halangk and colleagues reported that under conditions of cerulein-induced pancreatitis the rate of phosphorylating respiration and ATP concentration are substantially reduced (Halangk et al., 1998); this effect probably develop slowly - on a very different time scale to our measurements. Considering the results of our experiments it seems unlikely that ATP deprivation is responsible for the cell damage at the early stages of cerulein-induced pancreatitis, on the contrary an other mechanism probably damage mitochondria and consequently exacerbates the disease. The situation could be different for other inducers of acute pancreatitis, which decrease ATP content in acinar cells. POA, POAEE and TLC-S are all Ca2+ releasing agonists; the fact that we see a net decrease in ATP content in both the mitochondria and the cytosol means that the ability of these substances to up-regulate ATP production by a Ca2+ dependent mechanism is overwhelmed by the negative effects of these compounds on the cell’s bioenergetics. The bile acid TLC-S was particularly efficient in reducing ATP concentration. One reason for this could be an increase in the demand for ATP since in addition to activating Ca2+ -dependent currents TLC-S also activates a significant cationic current, conducted by non-selective channels (Voronina et al., 2005). This should increase the turnover of both Na+ / K+ and Ca2+ pumps and accelerate ATP consumption. Another possibility could be the depolarising effect of TLC-S on the mitochondria of pancreatic acinar cells (Voronina et al., 2004). Considering the outcome of our experiments (cytosolic and mitochondrial ATP measurements and ATP measurements in cells with inhibited mitochondria) it is likely that both mechanisms are involved. In terms of calcium toxicity bile is a particularly dangerous substance. Whilst Ca2+ responses to “physiological” calcium-releasing secretagogues (particularly ACh) are inhibited by ATP depletion, TLC-S is capable of both releasing Ca2+ from the ER and triggering Ca2+ influx even when ATP is depleted (Barrow et al., 2008). A further decrease of ATP could incapacitate pumping mechanisms and exacerbate Ca2+ toxicity.
Mitochondrial depolarisation in pancreatic acinar cells was reported for POA and POAEE (Criddle et al., 2006). This could be responsible for observed (Fig. 6) decreases in cytosolic ATP levels induced by these substances. The decrease in ATP content could be a contributing factor to cell damage triggered by POA and POAEE.
POA, POAEE and TLC-S induce decreases in mitochondrial ATP concentration (Fig. 7). This finding is in line with the cytosolic effects of these agonists and suggests that at least part of the effect of these substances on cellular bioenergetics is due to the inhibition of mitochondrial ATP production. The effect of near-physiological concentration of CCK (20pM) on mitochondrial ATP was also similar to that on cytosolic ATP (Fig.2 and Fig.7) supporting the notion that the effect is mediated by the acceleration the oxidative phosphorylation. The effect of 10nM CCK on mitochondrial ATP was somewhat unexpected. The initial decrease of ATP content (observed in mitochondria) was not seen in cytosolic ATP measurements (Fig. 2 and Fig.7). A possible explanation of this phenomenon is that mitochondria compensate for the initial increase in the demand of cytosolic ATP and because of their small volume, display substantial decrease in ATP concentration. Later the Ca2+ - dependent acceleration of the Citric Acid Cycle and oxidative phosphorylation overcompensates for this initial drop in ATP content and both mitochondrial and cytosolic ATP levels become up-regulated. This phenomenon is potentially interesting and deserves further investigation, which in our opinion would be better conducted as a separate study. It is interesting to note that a remarkably similar biphasic changes in mitochondrial ATP were recently reported in cardiomyocytes (Bell et al., 2006). These changes were also interpreted as a manifestation of the initial increase in ATP demand (as an explanation of the decline in mitochondrial ATP) followed by up-regulation of ATP production Ca2+ signals which are important for both physiological and pathophysiological responses in pancreatic acinar cells strongly upregulate ATP production in this cell type. This should allow cells to better fulfil their physiological functions of enzyme and electrolyte secretion and probably provides some protection against damage by overstimulation with “physiological” secretagogues (e.g. CCK and ACh). This is particularly relevant considering that the cells might need an increase in ATP production and an increase in ATP level to successfully undergo apoptosis (Zamaraeva et al., 2005) and remove damaged cells without inducing further damage to their neighbours. Efficient apoptosis has been shown to reduce the severity of acute pancreatitis (ref). Unfortunately the situation is different with bile acid, and non-oxidative ethanol metabolites (POA, POAEE) which in spite of their ability to increase Ca2+ are incapable of increasing cytosolic or mitochondrial ATP levels, which makes these substances particularly dangerous inducers of acute pancreatitis.
Contributor Information
Svetlana Voronina, The Physiological Laboratory, School of Biomedical Sciences, The University of Liverpool, Crown Street, Liverpool, L69 3BX, UK.
Stephanie Barrow, The Physiological Laboratory, School of Biomedical Sciences, The University of Liverpool, Crown Street, Liverpool, L69 3BX, UK.
Alec Simpson, Department of Human Anatomy and Cell Biology, School of Biomedical Sciences, The University of Liverpool, Crown Street, Liverpool, L69 3BX, UK.
Oleg Gerasimenko, The Physiological Laboratory, School of Biomedical Sciences, The University of Liverpool, Crown Street, Liverpool, L69 3BX, UK.
Gabriela da Silva Xavier, Section of Cell Biology, Division of Medicine, Imperial College, London, Sir Alexander Fleming Building, Exhibition Road, London SW7 2AZ, UK.
Guy Rutter, Section of Cell Biology, Division of Medicine, Imperial College, London, Sir Alexander Fleming Building, Exhibition Road, London SW7 2AZ, UK.
Ole Petersen, The Physiological Laboratory, School of Biomedical Sciences, The University of Liverpool, Crown Street, Liverpool, L69 3BX, UK.
Alexei V. Tepikin, The Physiological Laboratory, School of Biomedical Sciences, The University of Liverpool, Crown Street, Liverpool, L69 3BX, UK
Reference List
- Ainscow EK, Rutter GA. Mitochondrial priming modifies Ca2+ oscillations and insulin secretion in pancreatic islets. Biochem J. 2001;353:175–180. doi: 10.1042/0264-6021:3530175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow SL, Voronina SG, da S X, Chvanov MA, Longbottom RE, Gerasimenko OV, Petersen OH, Rutter GA, Tepikin AV. ATP depletion inhibits Ca2+ release, influx and extrusion in pancreatic acinar cells but not pathological Ca2+ responses induced by bile. Pflugers Arch. 2008;455:1025–1039. doi: 10.1007/s00424-007-0360-x. [DOI] [PubMed] [Google Scholar]
- Bauduin H, Colin M, Dumont JE. Energy sources for protein synthesis and enzymatic secretion in rat pancreas in vitro. Biochim Biophys Acta. 1969;174:722–733. doi: 10.1016/0005-2787(69)90301-3. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J Biol Chem. 2006;281:28058–28067. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Manfredi G, Griffiths EJ, Rutter GA. Luciferase expression for ATP imaging: application to cardiac myocytes. Methods Cell Biol. 2007;80:341–352. doi: 10.1016/S0091-679X(06)80017-8. [DOI] [PubMed] [Google Scholar]
- Betzenhauser MJ, Wagner LE, Iwai M, Michikawa T, Mikoshiba K, Yule DI. ATP modulation of Ca2+ release by type-2 and type-3 inositol (1, 4, 5)-triphosphate receptors. Differing ATP sensitivities and molecular determinants of action. J Biol Chem. 2008;283:21579–21587. doi: 10.1074/jbc.M801680200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough D, Schell MJ, Irvine RF. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J. 2005;392:291–297. doi: 10.1042/BJ20050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M, Takeo T, Gerasimenko O, Vaillant C, Torok K, Petersen OH, Tepikin AV. Hormone-induced secretory and nuclear translocation of calmodulin: oscillations of calmodulin concentration with the nucleus as an integrator. Proc Natl Acad Sci U S A. 1999;96:4426–4431. doi: 10.1073/pnas.96.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Denton RM, McCormack JG. The calcium sensitive dehydrogenases of vertebrate mitochondria. Cell Calcium. 1986;7:377–386. doi: 10.1016/0143-4160(86)90040-0. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Campbell K, Halet G, Carroll J, Swann K. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev Biol. 2008;316:431–440. doi: 10.1016/j.ydbio.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Fischer L, Gukovskaya AS, Penninger JM, Mareninova OA, Friess H, Gukovsky I, Pandol SJ. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G875–G886. doi: 10.1152/ajpgi.00558.2005. [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Gerasimenko OV, Palejwala A, Tepikin AV, Petersen OH, Watson AJ. Menadione-induced apoptosis: roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J Cell Sci. 2002;115:485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci. 2006;119:226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem. 2002;277:22595–22604. doi: 10.1074/jbc.M202929200. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Halangk W, Lerch MM. Early events in acute pancreatitis. Clin Lab Med. 2005;25:1–15. doi: 10.1016/j.cll.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Halangk W, Matthias R, Nedelev B, Schild L, Meyer F, Schulz HU, Lippert H. Modification of energy supply by pancreatic mitochondria in acute experimental pancreatitis. Zentralbl Chir. 1997;122:305–308. [PubMed] [Google Scholar]
- Halangk W, Matthias R, Schild L, Meyer F, Schulz HU, Lippert H. Effect of supramaximal cerulein stimulation on mitochondrial energy metabolism in rat pancreas. Pancreas. 1998;16:88–95. doi: 10.1097/00006676-199801000-00014. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Curci S, Machen TE, Schulz I. ATP regulates calcium leak from agonist-sensitive internal calcium stores. FASEB J. 1996;10:302–308. doi: 10.1096/fasebj.10.2.8641563. [DOI] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA. Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J Biol Chem. 1999;274:13281–13291. doi: 10.1074/jbc.274.19.13281. [DOI] [PubMed] [Google Scholar]
- Kinnala PJ, Kuttila KT, Gronroos JM, Havia TV, Nevalainen TJ, Niinikoski JH. Splanchnic and pancreatic tissue perfusion in experimental acute pancreatitis. Scand J Gastroenterol. 2002;37:845–849. [PubMed] [Google Scholar]
- Koop A, Cobbold PH. Continuous bioluminescent monitoring of cytoplasmic ATP in single isolated rat hepatocytes during metabolic poisoning. Biochem J. 1993;295(Pt 1):165–170. doi: 10.1042/bj2950165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M, Williams JA, Goldfine ID. Stimulation of the glucose transport system in isolated mouse pancreatic acini by cholecystokinin and analogues. J Biol Chem. 1979;254:7624–7629. [PubMed] [Google Scholar]
- Kosowski H, Schild L, Kunz D, Halangk W. Energy metabolism in rat pancreatic acinar cells during anoxia and reoxygenation. Biochim Biophys Acta. 1998;1367:118–126. doi: 10.1016/s0005-2728(98)00143-1. [DOI] [PubMed] [Google Scholar]
- Luthen RE, Niederau C, Grendell JH. Glutathione and ATP levels, subcellular distribution of enzymes, and permeability of duct system in rabbit pancreas following intravenous administration of alcohol and cerulein. Dig Dis Sci. 1994;39:871–879. doi: 10.1007/BF02087436. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kanno T, Seo Y, Murakami M, Watari H. Phosphorus nuclear magnetic resonance in isolated perfused rat pancreas. Am J Physiol. 1988;254:G575–G579. doi: 10.1152/ajpgi.1988.254.4.G575. [DOI] [PubMed] [Google Scholar]
- Nordback IH, Clemens JA, Chacko VP, Olson JL, Cameron JL. Changes in high-energy phosphate metabolism and cell morphology in four models of acute experimental pancreatitis. Ann Surg. 1991;213:341–349. doi: 10.1097/00000658-199104000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odinokova IV, Sung KF, Mareninova OA, Hermann K, Evtodienko Y, Andreyev A, Gukovsky I, Gukovskaya AS. Mechanisms regulating cytochrome c release in pancreatic mitochondria. Gut. 2009;58:431–442. doi: 10.1136/gut.2007.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Betzenhauser MJ, Won JH, Chen J, Yule DI. The type 2 inositol (1,4,5)-trisphosphate (InsP3) receptor determines the sensitivity of InsP3-induced Ca2+ release to ATP in pancreatic acinar cells. J Biol Chem. 2008;283:26081–26088. doi: 10.1074/jbc.M804184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- Plusczyk T, Westermann S, Bersal B, Menger M, Feifel G. Temporary pancreatic duct occlusion by ethibloc: cause of microcirculatory shutdown, acute inflammation, and pancreas necrosis. World J Surg. 2001;25:432–437. doi: 10.1007/s002680020041. [DOI] [PubMed] [Google Scholar]
- Plusczyk T, Westermann S, Rathgeb D, Feifel G. Acute pancreatitis in rats: effects of sodium taurocholate, CCK-8, and Sec on pancreatic microcirculation. Am J Physiol. 1997;272:G310–G320. doi: 10.1152/ajpgi.1997.272.2.G310. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Schild L, Matthias R, Stanarius A, Wolf G, Augustin W, Halangk W. Induction of permeability transition in pancreatic mitochondria by cerulein in rats. Mol Cell Biochem. 1999;195:191–197. doi: 10.1023/a:1006988625831. [DOI] [PubMed] [Google Scholar]
- Varadi A, Cirulli V, Rutter GA. Mitochondrial localization as a determinant of capacitative Ca2+ entry in HeLa cells. Cell Calcium. 2004;36:499–508. doi: 10.1016/j.ceca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Voronina S, Longbottom R, Sutton R, Petersen OH, Tepikin A. Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J Physiol. 2002a;540:49–55. doi: 10.1113/jphysiol.2002.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina S, Sukhomlin T, Johnson PR, Erdemli G, Petersen OH, Tepikin A. Correlation of NADH and Ca2+ signals in mouse pancreatic acinar cells. J Physiol. 2002b;539:41–52. doi: 10.1113/jphysiol.2001.013134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina SG, Barrow SL, Gerasimenko OV, Petersen OH, Tepikin AV. Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J Biol Chem. 2004;279:27327–27338. doi: 10.1074/jbc.M311698200. [DOI] [PubMed] [Google Scholar]
- Voronina SG, Gryshchenko OV, Gerasimenko OV, Green AK, Petersen OH, Tepikin AV. Bile acids induce a cationic current, depolarizing pancreatic acinar cells and increasing the intracellular Na+ concentration. J Biol Chem. 2005;280:1764–1770. doi: 10.1074/jbc.M410230200. [DOI] [PubMed] [Google Scholar]
- Zamaraeva MV, Sabirov RZ, Maeno E, ndo-Akatsuka Y, Bessonova SV, Okada Y. Cells die with increased cytosolic ATP during apoptosis: a bioluminescence study with intracellular luciferase. Cell Death Differ. 2005;12:1390–1397. doi: 10.1038/sj.cdd.4401661. [DOI] [PubMed] [Google Scholar]
- Zamaraeva MV, Sabirov RZ, Manabe K, Okada Y. Ca(2+)-dependent glycolysis activation mediates apoptotic ATP elevation in HeLa cells. Biochem Biophys Res Commun. 2007;363:687–693. doi: 10.1016/j.bbrc.2007.09.019. [DOI] [PubMed] [Google Scholar]