Abstract
We examine the role of adipose tissue, typically considered an energy storage site, as a potential site of toxicant accumulation. Although the production of most persistent organic pollutants (POPs) was banned years ago, these toxicants persist in the environment due to their resistance to biodegradation and widespread distribution in various environmental forms (e.g., vapor, sediment, water). As a result, human exposure to these toxicants is inevitable. Largely due to their lipophilicity, POPs bioaccumulate in adipose tissue, resulting in greater body burdens of these environmental toxicants with obesity. POPs of major concern include polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins and furans (PCDDs/PCDFs), and polybrominated biphenyls and diphenyl ethers (PBBs/PBDEs), among other organic compounds. In this review, we 1) highlight the physical characteristics of toxicants that enable them to partition into and remain stored in adipose tissue, 2) discuss the specific mechanisms of action by which these toxicants act to influence adipocyte function, and 3) review associations between POP exposures and the development of obesity and diabetes. An area of controversy relates to the relative potential beneficial versus hazardous health effects of toxicant sequestration in adipose tissue.
Introduction
As the name implies, POPs are organic lipophilic compounds that are resistant to environmental degradation, exhibit considerable stability, and persist in the environment. Due to their low water solubility, POPs can present as vapors in the atmosphere or strongly bind to particulate matter in sediments, where the sediment may serve as a reservoir, removing the POPs from circulation (18). If disturbed, however, the POPs may be released from the sediment and travel far from their origin before being re-deposited. One hallmark characteristic of POPs is their ability to move up the food chain and increase in concentration, or biomagnify, subsequently resulting in widespread environmental and human exposure (18). This is largely due to the high degrees of halogenation, which allows them to resist degradation by metabolizing enzymes. The bioaccumulation potential of these compounds can allow them to biomagnify to potentially dangerous levels (193) (245).
To address the global concern of environmental pollutants, 90 countries signed a United Nations treaty in 2001, known as the Stockholm Convention (18). The intention of the Convention was to severely limit, but preferentially eliminate, the widespread production and use of POPs. Recognizing the potentially toxic effects of POPs on human and environmental health, a preliminary list of chemicals known as the “dirty dozen” was established (18). This original list of 12 key POPs included aldrin, chlordane, dichlorodiphenyltrichloroethane (DDT), dieldrin, endrin, heptachlor, hexachlorobenzene, mirex, toxaphene, polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (dioxins), and polychlorinated dibenzofurans (furans) (18). Since then, this list has expanded to include additional compounds, such as polycyclic aromatic hydrocarbons (PAHs), brominated flame retardants (BFRs), and other compounds that have proven particularly harmful and toxic to humans and animals (see Table 1 for a full list of abbreviations that will be used throughout the text). The primary route of human exposure to POPs is via food contamination, where fatty foods (e.g. meat, fish, and dairy) are important vectors for many classes of POPs, including PCBs, polybrominated flame retardants, dioxins and furans (PCDDs/PCDFs), and other organochlorines (Table 2).
Table 1.
List of Abbreviations
| AhR | Aryl hydrocarbon receptor |
| AR | Androgen receptor |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| AT | Adipose tissue |
| BADGE | Bisphenol A diglycidyl ether |
| BCF | Bioconcentration factor |
| BDE | Brominated diphenyl ether |
| BFDGE | Bisphenol F diglycidyl ether |
| BFRs | Brominated flame retardants |
| β-HCH | β-Hexachlorocyclohexane |
| BMI | Body mass index |
| BP | Blood pressure |
| BPA | Bisphenol A |
| ClBPA | Monochloro-BPA |
| Cl2BPA | Dichloro-BPA |
| Cl3BPA | Trichloro-BPA |
| CYP | Cytochrome P450 |
| DBP | Dibutyl phthalate |
| DDE | Dichlorodiphenyldichloroethylene |
| DDT | Dichlorodiphenyltrichloroethane |
| DEHP | Di(2-ethylhexyl) phthalate |
| DRE | Dioxin response element |
| ER | Estrogen receptor |
| ERE | Estrogen response element |
| ERK | Extracellular signal-regulated kinase |
| Glut-4 | Glucose transporter type 4 |
| HAHs | Halogenated aromatic hydrocarbons |
| HBCD | Hexabromocyclododecane |
| HCB | Hexachlorobenzene |
| HDL | High density lipoprotein |
| HMW | High molecular weight |
| HOMA | Homeostatic model assessment |
| HOMA-B | Homeostatic model assessment-beta |
| HOMA-IR | Homeostatic model assessment- insulin resistance |
| HpCDD | 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin |
| HxCDD | 1,2,3,4,7,8-Hexachlorodibenzo-p-dioxin |
| Kow | Octanol-water partition coefficient |
| LDL | Low density lipoprotein |
| LMW | Low molecular weight |
| MEK | Mitogen-activated protein kinase kinase |
| MBP | Mono-butyl phthalate |
| MBuP | Mono-sec-butyl phthalate |
| MBzP | Monobenzyl phthalate |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MEHHP | Mono(2-ethyl-5-hydroxyhexyl) phthalate |
| MEHP | Mono-(2-ethylhexyl) phthalate |
| MEOHP | Mono(2-ethyl-5-oxohexyl) phthalate |
| MEP | Mono-ethyl phthalate |
| MetS | Metabolic syndrome |
| MiBP | Mono-isobutyl phthalate |
| MnBP, | Mono-n-butyl phthalate |
| NDL | Non-dioxin-like |
| NHANES | National Health and Nutrition Examination Survey |
| OC | Organochlorine |
| OCDD | Octachlorodibenzodioxin |
| PAHs | Polycyclic aromatic hydrocarbons |
| PBBs | Polybrominated biphenyls |
| PBDEs | Polybrominated diphenyl ethers |
| PCBs | Polychlorinated biphenyls |
| PCDDs | Polychlorinated dibenzo-p-dioxins |
| PCDFs | Polychlorinated dibenzofurans |
| PCP | Pentachlorophenol |
| PeCB | Pentachlorobenzene |
| PhIP | 2-amino-1-methyl-6-phenylimidazo[4-5-b]pyridine |
| POPs | Persistent organic pollutants |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RXR | Retinoid X receptor |
| scAT | Subcutaneous adipose tissue |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| TBBPA | Tetrabromobisphenol A |
| TBDD | Tetrabrominated dinenzo-p-dioxin |
| TBT | Tributyltin chloride |
| TCBPA | Tetrachlorobisphenol A |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TNF-α | Tumor necrosis factor alpha |
| TPTO | Bis(triphenyltin) oxide |
| TR | Thyroid receptor |
| vAT | Visceral adipose tissue |
| VLDL | Very low density lipoprotein |
| WC | Waist circumference |
| XRE | Xenobiotic response element |
Table 2.
General Characteristics of Toxicants
| Category | Environmental Chemicals | Exposure | Use | General Characteristics |
|---|---|---|---|---|
| Polychlorinated biphenyls | PCBs | Air, Food | Industrial chemicals; By-products during combustion | PCBs consist of a family of 209 congeners (49), whose toxicity and persistence in the environment often increases with higher degrees of chlorination (half-lives can vary from months to years). PCBs have been banned in the U.S. since the 1970s, but are still evident in environmental and human samples (14). Prior to the ban, PCBs were used in a variety of industrial processes and were also produced as by-products during combustion (14). |
| Polychlorinated dibenzo-p-dioxins/Polychlorinated dibenzofurans | PCDDs/PCDFs | Air, Water, Food | Produced only for research purposes; By- products during combustion and other industrial processes | PCDDs are chemicals produced as by-products during the manufacture of pesticides and other chlorinated substances, like pentachlorophenol (PCP). There are about 75 different dioxins, 7 of which have ignited much concern, including TCDD (313). Like PCDDs, PCDFs are also produced unintentionally and have structural similarities to dioxins. As a result, they share many of the same toxic effects. However, there are about 135 different types of furans and their toxicities vary (313). Dioxins and furans can both exist in the environment for several years and can bioaccumulate in fatty tissue and biomagnify through the food chain (5). Furans are also classified as possible carcinogens. |
| Organochlorines | Aldrin/Dieldrin/Endrin | Soil, Air and Water | Insecticide | Aldrin and dieldrin were used as crop insecticides from 1950-1970 until banned by the Department of Agriculture, but were reintroduced by the EPA and used from 1972-1987 (3). Once aldrin enters the body, it is converted to dieldrin, and both can undergo degradation. Endrin is a chlorinated cyclodiene that was used as an insecticide and pesticide to control rodents and birds until its use was discontinued in the US in 1986. Endrin is a stereoisomer of dieldrin. Although similar in structure to other organochlorines, endrin is rapidly metabolized and does not accumulate in adipose to the same extent as other pesticides with similar structures. |
| Chlordane/Heptachlor | Soil, Air and Water | Insecticide | Chlordane is an organochlorine mixture of over 140 different compounds with its main constituents being cis- and trans-chlordane, cis- and trans-nonachlor, and heptachlor (97). Trans-nonachlor and oxychlordane, the major metabolite of chlordane, are primary contaminants detected in human fat samples (50). Chlordane was used from 1948 -1979 when concerns of toxicity began to emerge. Use of chlordane in the 1980s was largely as an insecticide against termites until the sale and use of chlordane were halted in 1988 (4). However, the half-life for chlordane in soil is 10-20 years; therefore, this pesticide remained in soil surrounding areas treated with chlordane (42). Heptachlor is a component and degradation product of chlordane (10). It was used extensively prior to 1970, but now the only permitted use is for fire ant control. Heptachlor is converted to its epoxide, heptachlor epoxide, upon environmental exposure and human ingestion. The epoxide accumulates and biomagnifies in the food chain (10). | |
| Mirex/Chlordecone | Soil, Air and Water | Insecticide | Mirex is a derivative of cyclopentadiene, and was used in the United States in the 1960s and 1970s as a pesticide against fire ants and also as a flame retardant additive (13). Chlordecone is a transformation product of mirex. It was in use until 1977, but due to its stability, high lipophilicity, and resistance to metabolism it has a high potential to biomagnify in the food chain (111) (165). However, no information on level in humans or adipose is available. | |
| Methoxychlor | Soil, Air and Water | Insecticide | Methoxychlor was used as a pesticide from 1946 - 2000; it tightly binds to soil and exhibits estrogenic activity (12) (88). | |
| Endosulfan | Soil, Air and Water | Insecticide | Endosulfan was used as a pesticide since 1954, however following the 2011 Stockholm Convention, endosulfan use was completely discontinued by July 31, 2016 (8). Endosulfan is a derivative of hexachlorocyclopentadiene, and is chemically similar to aldrin, chlordane, and heptachlor. Technical-grade endosulfan is comprised of a 7:3 mixture of α- and β-endosulfan isomers, which are also known as endosulfan I and II, respectively (8). The β isomer slowly converts to the more stable α-endosulfan. | |
| Toxaphene | Soil, Air and Water | Insecticide | Toxaphene, a product of chlorine gas and camphene, is a pesticide that was used heavily in the southern US to control pests on livestock and crops. Although it was once one of the most heavily used pesticides, it has since been banned for use in the United States (15) (228). | |
| Hexachlorobenzene | Soil, Air and Water | Fungicide; Industrial Chemicals; By- products during combustion and other industrial processes; Impurities in certain pesticides | Hexachlorobenzene was introduced in 1945 as a fungicide to protect food crops. Due to its structural stability and resistance to biodegradation and metabolism, HCB is recognized as one of the most environmental persistent pollutants (11). The estimated half-life in soil is 3 - 6 years (11), and it can exist in the atmosphere and environment long after it is used. Hexachlorobenzene has also been used as a reference compound for BCFs in fish (19). | |
| p,p′-DDT/p,p′-DDE | Soil, Air, Water, and Food | Insecticide | Dichlorodiphenyltrichloroethane (p,p′-DDT) was initially used as an insecticide during WWI to protect against malaria, typhus, and other diseases transmitted by insects (6). It is an extremely persistent pollutant, with approximately 50% remaining in the soil 10-15 years after application. Although it was banned due to its toxic effects on birds, DDT has been detected in food worldwide. As such, food- borne DDT is the greatest source of human exposure. p,p′- Dichlorodiphenyldichloroethylen e (p,p′-DDE) is the primary metabolite produced by the dehydrochlorination of DDT in humans, and is also considered a persistent environmental pollutant that can have adverse effects on human health (33). | |
| Polybrominated bi-/di- phenyl ethers | PBDEs/TBBPA/HBCD | Soil, Air, Food and | Flame retardants; Industrial chemicals; By - products of debromination of other BDEs | While brominated flame retardants are beneficial in numerous materials for their fire-resistant characteristics, some may pose a threat to human and environmental health. Since most flame retardants are not chemically bound to the material, they can leech into the environment, where they resist biodegradation. Of all the brominated flame retardants, PBDE, TBBPA, and HBCD rank highest in global consumption (138). Polybrominated diphenyl ethers (PBDEs) are generally characterized by two brominated biphenyl rings joined by an ether. All PBDEs are lipophilic substances that are very likely to adsorb on particulate matter and not likely to volatilize from water phase. Tetrabromobisphenol A (TBBPA) reactive flame is a retardant used in electric equipment. Although it is a polybrominated compound, it does not share the same toxicity profile as PBDEs. It is produced by brominating bisphenol A, and is rapidly metabolized after exposure (331) (359). TBBPA is not considered a persistent and bioaccumulative toxicant (63). TBBPA has a log Kow of 6.53 at a low pH, but at pH 6-9 TBBPA is in a dissociated form and has a lower log Kow (211). PBDEs, hexabromocyclododecane (HBCD), and, to a lesser extent, TBBPA, are brominated biphenyl ethers that possess fire-resistant and degradation-resistant properties that allow them to bioaccumulate and move up the food chain (242) (281) (92). Note that HBCD is a brominated cyclic aliphatic compound, not a diphenyl ether. |
| Endocrine Disruptors | Phenols/Phthalates | Food, Water | Industrial chemicals | Bisphenol A (BPA) is most commonly used for “binding, plasticizing, or hardening plastics” and as an additive in flame retardants (116). Although BPA is not as persistent as other POPs, due to its common use, BPA is frequently released into the environment, which can lead to indirect human exposure. Phthalates are a class of compounds that are used for a variety of purposes and are generally non-persistent in humans (262) (156). Di (2-ethylhexyl) phthalate (DEHP) is a manufactured chemical that is commonly added to plastics to increase their flexibility (7), and can be absorbed from food and water (351). Mono-(2-ethylhexyl) phthalate (MEHP) is the primary, active metabolite of DEHP. Phthalates are lipophilic and may accumulate in adipose tissue. MEHP accumulation, in particular has been found to impact lipolysis and glucose uptake/glycolysis in fat cells (73). |
| Polycyclic Aromatic Hydrocarbons | PAHs/Benzo(a)pyrene | Air, Water, Food | Industrial chemicals; By- products of erupting volcanoes and forest fires | Polycyclic aromatic hydrocarbons (PAHs) do not refer to a single compound, but instead cover a wide range of complex mixtures, which are often produced during incomplete combustion of organic matter. Benzo[a]pyrene belongs to this group of PAHs and is regarded by the EPA as a priority pollutant (16). Long-term exposure to PAHs, particularly benzo[a]pyrene can result in a number of adverse health effects in humans. PAHs have been detected in adipose samples, and benzo[a]pyrene is regarded as a probable carcinogen (16). |
| Polychlorinated naphthalenes | PCNs | Soil, Air, Water, and Food | Industrial chemicals; By- products during production of PCBs | Polychlorinated naphthalenes (PCNs) are industrial chemicals that until the 1970s were in high production and most commonly used as wood preservatives and insulating coatings for electrical wires and plastic additives (87). In addition, PCNs can be released as by-products of waste incineration or PCB production, and many PCNs still persist, unchanged, in the environment. The family of PCNs consists of approximately 75 chlorinated naphthalenes that are polychlorinated and structurally similar to PCBs. As a result of this structural similarity, PCNs share many physical and chemical properties with PCBs, including high lipophilicity, great stability, and high resistance to biodegradation. As a result, the toxicity profiles of PCNs also resemble those of most coplanar PCB congeners. |
| Pentachlorophenol | PCP | Soil, Air, Water, and Food | Insecticide; By- products of chemical metabolism | Pentachlorophenol (PCP) exists in two forms, one as pure PCP, and the other as its sodium salt. PCP was initially introduced in the 1930s and has been used as an insecticide, herbicide, fungicide, and disinfectant. However, as a consequence of its toxicity profile, PCP use has significantly decreased. Although data is limited on the distribution of PCP in humans, there are a few reports that indicate that PCP can be absorbed by the liver, adipose, and other tissues (133) (259). The binding of PCP to plasma proteins also plays a vital role in PCP distribution (55) (130). Hexachlorobenzene and hexachlorocyclohexane are metabolized to pentachlorophenol. |
| Perfluorinated compounds | PFCs/PFAS | Food | Industrial chemicals | Perfluorinated compounds (PFCs) are a diverse family of compounds containing fluorine atoms. They have a number of applications, including use in textiles, kitchen ware, and food packaging materials. They are highly persistent in the environment and bioaccumulate in people and wildlife (295) (60) (125) (188). |
When considering the toxicity of POPs, it is important to discuss their relationship with adipose tissue (AT), a significant site of toxicant bioaccumulation. AT is a connective tissue that is primarily comprised of white or brown adipocytes, but also contains several other cell types. White adipocytes are the most common type of fat cell, serving as a storage depot for lipids that are released upon need as an energy source. By comparison, brown adipocytes, which are much less prevalent in adults, are enriched with specialized mitochondria that mobilize lipid to produce heat for maintenance of body temperature (347). Of the two primary types of adipocytes, white adipocytes, with a large unilocular lipid droplet, are the prominent storage site of lipophilic POPs. It has been suggested that AT plays a major role in the storage and overall toxicokinetics of hydrophobic xenobiotic POPs (202) (203) (213). In addition, the physical properties that enable certain toxins/toxicants to partition into lipid are important in determining the extent of AT POP sequestration. The octanol:water partition coefficient, for example, offers insight into a toxicant’s ability to partition between water and organic matter, and provides a clearer understanding of a toxicant’s potential biological uptake, accumulation, and storage in AT (213).
The collection and storage of POPs in fatty tissue can have both positive and negative consequences. One beneficial aspect of POP sequestration in AT is that the toxicant concentration in blood is decreased, limiting POP availability to other cells and tissues where they may have hazardous effects (213). In this manner, POP sequestration in AT lipids appears protective against the harsh effects of lipophilic toxicants (24) (27) (137) (399) (154) (123). On the contrary, bioaccumulation of POPs in expanded AT of obese subjects results in a significantly increased body burden (213). The tonic release of these chemicals into the systemic circulation, especially during periods of weight loss (96) (105) (202) (240) (213) (283), can pose tremendous threats on overall human health (202) (154) (316) (38) (39) (231). We review data on accumulation of specific POPs in AT, their mechanism of action, and influence on diseases associated with dysregulation of AT function.
Physico-chemical properties and lipids influence AT POP bioaccumulation
The dynamics of contaminant accumulation in and release from AT depends on their physic-chemical properties. The partition coefficient has proven to be a major parameter governing the uptake of lipophilic toxicants into adipocytes. However, even within a toxicant class, structural determinants dictate physico-chemical properties that determine AT accumulation. For example, different PCB congeners can display distinct uptake and storage dynamics into adipocytes (54) (250) (249). One study compared the accumulation potential in AT of three PCB congeners: PCB −28, −153, and −118 based on each of the congeners physico-chemical features. Results indicated that the dynamics of accumulation varied between the congeners due to molecular size, molecular volume, and lipophilicity (54). Specifically, the degree of halogenation, or number and position of chlorine substituents on the PCBs, influenced their uptake and accumulation in adipocytes. PCB-28 entered adipocytes more rapidly than the other two congeners likely due to its smaller molecular weight, size, and lipophilicity, while PCBs −153 and −118 remained trapped in the lipophilic cell membrane and diffused more slowly into the intracellular, hydrophobic cytoplasm of the adipocyte (54).
The lipophilicity of a compound depends on its chemical structure, where bigger, more complex and halogenated compounds are typically more lipophilic and resistant to biodegradation (206). As early as the 1900s, researchers tested for lipophilicity by studying the uptake of nonpolar compounds using organic solvents, like octanol, as a surrogate for the organic matter present in organisms (345). Although not identical, the extent of chemical uptake from the water into the organic phase is proportional to what is expected and observed in organisms (345). The octanol-water partition coefficient (Kow) is defined by the following equation: Kow = Coctanol/Cwater, where Coctanol is the molar concentration of the compound in the octanol phase, and Cwater is the molar concentration of the compound in the aqueous phase when the system is at equilibrium (345). The adipose-serum partition coefficient determines the extent to which a chemical may accumulate in adipose (319); it is a ratio of the concentration of a chemical in adipose to serum at equilibrium. Typically, the distribution of xenobiotics into AT is dependent on a number of pharmacokinetic factors including tissue volume and blood flow (232). The standard approach assumes that the tissue is “flow limited,” which means that the venous blood leaving the organ is at equilibrium with the “well-stirred” tissue compartment (232). While this approach has proven valid for the distribution of various xenobiotics into tissues and organs, there are a number of chemicals for which this flow limited model has proven invalid, including highly lipophilic POPs (421) (190) (208) (232). These chemicals, along with other organic compounds, act according to a “diffusion-limited” model, which states that diffusion limitation is proportional to the octanol-water partition coefficient (Kow) of a chemical (232).
Ultimately, diffusion limitation increases as Kow increases. In support of the studies by Oberg et al. (294) who simultaneously measured PCB concentrations in rat plasma and adipose tissue, Levitt (232) found that hexachlorobenzene (HCB), hexabromobenzene, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and tetrabrominated dinenzo-p-dioxin (TBDD) have a “similar increase in diffusion limitation with increasing Kow.” Specifically, results of the study showed that the “apparent” rat adipose perfusion rate was smaller for a PCB (0.005kg/min/kg) with a log Kow greater than 7, while significantly larger (0.2 kg/min/kg) for chemicals with log Kow less than 5 (232). Collectively, these studies support the notion that at steady-state conditions, the log Kow, a measure of lipophilicity, can help predict the likelihood of a chemical to diffuse and accumulate into AT and contribute to steady-state body burdens. Table 3 provides an overview of the structures and partition coefficients of numerous POPs.
Table 3.
Summary of Toxicant Structures and Partition Coefficients
| Compound | General Structure | Partition Coefficient (Log Kow or Log P) | References |
|---|---|---|---|
| Hexachlorobutadiene (HCBD) |
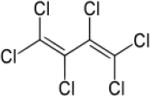
|
4.78 | (141) |
| Aldrin |
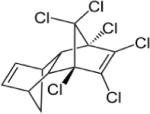
|
5.17–7.40 | (327) |
| Dieldrin |
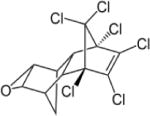
|
3.69–6.20 | (327) |
| Endrin |
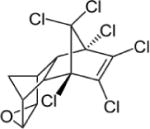
|
3.21–5.34 | (327) |
| Chlordane |
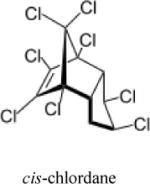
|
6 | (327) |
| Endosulfan |
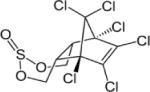
|
3.83 (α isomer); 3.62 (β isomer) | (141) |
| Toxaphene |
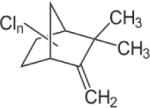
|
3.23–5.50 | (327) |
| Heptachlor |
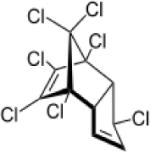
|
4.40–5.50 | (327) |
| Mirex |
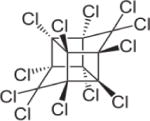
|
6.89 | (412) |
| Chlordecone |
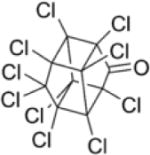
|
5.41 | (141) |
| α-/β-Hexachlorocyclohexane (HCH) |
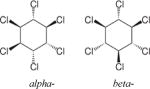
|
3.78 | (141) |
| Lindane (γ-HCH) |
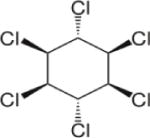
|
3.8 | (141) |
| Pentachlorobenzene (PeCB) |
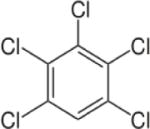
|
5.18 | (141) |
| Hexachlorobenzene(HCB) |
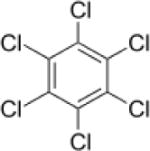
|
3.03–6.42 | (327) |
| Pentachlorophenol (PCP) |
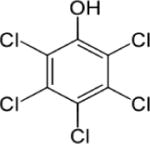
|
5.12; PCP sodium salt: 1.3 at pH 10 | (66) (141) |
| Polycyclic aromatic hydrocarbons (PAHs) |
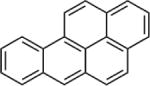
|
3.30–6.84 | (141) (253) |
| Polychlorinated naphthalenes (PCNs) |
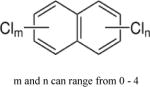
|
3.90–8.3 | (87) |
| Polychlorinated biphenyls (PCBs) |
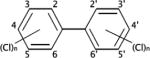
|
4.30–8.26 | (327) |
| Polychlorinated dibenzo-p-dioxins (PCDDs) |
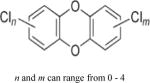
|
4.75–8.20 | (327) |
| Polychlorinated dibenzofurans (PCDFs) |
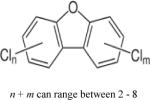
|
4.9–6.92 | (356) |
| Dichlorodiphenyl-trichloroethane (DDT) |
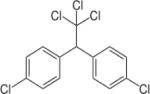
|
4.89–6.91; 3.88–8.18 | (158) (327) |
| Methoxychlor |
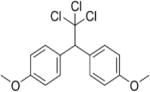
|
4.68–5.08 | (159) |
| Bisphenol A |
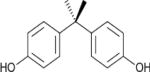
|
3.32 | (141) |
| Bisphenol A diglycidyl ether (BADGE) |
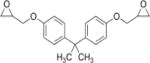
|
3.84 | (268) |
| Tetrabromobisphenol A (TBBPA) |
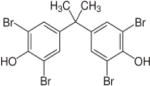
|
||
| Polybrominated diphenyl ethers (PBDEs) |
|
||
| Polybrominated | 6.39 (Hexabromo– | ||
| biphenyls (PBBs) |
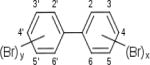
|
biphenyl) | |
| Hexabromo-cyclododecane (HBCD) |
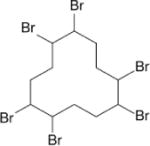
|
5.6 | |
| Di-(2-ethylhexyl) phthalate (DEHP) |
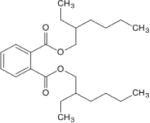
|
7.6 | |
| Mono-(2-ethylhexyl) phthalate (MEHP) |
|
4 | (170) |
| Trifluralin |
|
5.34 | (141) |
| Pendimethalin |
|
5.2 | (392) |
Lipids are generally the primary components of any tissue that determine the movement, distribution, and sequestration of hydrophobic compounds, and play a vital role in toxicant accumulation in tissues (41). The bioaccumulation potential of a toxicant can vary among different lipid classes. For example, phospholipids exhibit moderate polarity, while triglycerides and free fatty acids display neutral polarity, which can influence their tendency to accumulate toxicants (1). Despite the large number of cell types in AT (i.e. preadipocytes, fibroblasts, macrophages, etc.), storage of POPs is believed to primarily occur in adipocytes (54) whose cytoplasm is composed mainly of triglyceride droplets (341). There are reports indicating that TCDD and DDT are transported out of the gut into the triglyceride component of chylomicrons, which are responsible for delivering lipids absorbed from the intestine to AT (416) (205). Another study demonstrated that the extent of PCB accumulation in different adipocyte models directly correlated to the amount of cellular triglycerides (54). However, due to the high cost of analyzing toxicants in different lipid components, toxicants are generally reported as a measure of total lipids in tissues (54).
It appears that the type of fat storage may also contribute to AT toxicant accumulation. There are two major areas where AT deposits: 1) visceral AT (vAT), which surrounds internal organs and is generally considered to contribute to obesity-related diseases (275) (196) (257), and 2) subcutaneous AT (scAT), located beneath the skin. These AT locations can display unique structural features and properties that may influence the kinetics of toxicants. Although one study found no significant difference in POP accumulation between visceral versus subcutaneous AT (256), several studies found that visceral AT contained higher POP concentrations than subcutaneous AT (316). In an obese population in Portugal, endrin and endosulfan I and II were detected in more visceral compared to subcutaneous AT samples (316). Moreover, the total concentration of POPs was significantly higher (p<0.001) in vAT (213.9 + 204.2 ng/g fat) versus scAT (155.1 + 147.4 ng/g fat) (316). Although a greater percentage of the population had detectable levels of endosulfans I and II and methoxychlor in their visceral compared to subcutaneous AT, higher concentrations of each toxicant were detected in subcutaneous AT (316). Similarly, average concentrations of aldrin and median concentrations of lindane were higher in subcutaneous than visceral AT (316).
It is worth noting that a possible explanation for the varying concentrations of POPs in different types of AT may depend on the individual exposed to the toxicant and toxicant exposure duration (434). Orban et al. (303) noted that the effect of age was also a significant factor for the detection of nine PCDDs/PCDFs in AT, while no significant differences were associated with sex or race.
Numerous toxicants have been detected in different AT samples from various populations (Table 4). In humans, two of the most predominant chlordane-related contaminants (trans-nonachlor and oxychlordane) were detected in breast milk and AT (50). In addition, levels of PAHs, including anthracene, pyrene, benzo[e]pyrene, benzo[k]fluoranthene, benzo[a]pyrene, and benzo[g,h,i]perylene, were detected in AT samples in the range of 11 to 2,700 ng/g tissue (293). PAH refers to a ubiquitous group of over 100 environmental POPs that are composed of multiple aromatic rings containing only carbon and hydrogen. Total concentrations of PAHs in AT from Korean women ranged from 15 to 361 ng/g lipid (277), and levels of dioxin-like PCBs ranged from 4.1 to 125 ng/g lipid in a Chinese population (353). Mean levels of chlorobenzenes, including pentachlorobenzene (PeCB), in human milk and adipose tissue samples ranged from undetectable to 146 ng/g (178). Some of the highest levels of polybrominated diphenyl ethers (PBDEs) in AT were found in a New York population, with concentrations ranging from 17 to an astounding 9,630 ng/g lipid weight (183).
Table 4.
Toxicant Levels in Human and Aquatic Samples
| Compound | Toxicant Concentrations | Study Population | References | |||
|---|---|---|---|---|---|---|
| Adipose | Breast Milk | Blood Serum | BCF | |||
| Aldrin | 0.048 ppb | 0.003 ppb | 0.004 ppb | — | Women in Delhi | (286) |
| Mean: 7.2 ng/g fat vAT; Mean: 22.7 ng/g fat scAT | — | — | — | Obese men and women in Portugal undergoing bariatric surgery | (389) | |
| — | — | — | 350 44,600 | Freshwater Fish | (184) | |
| 0.0014 mg/g | — | — | — | Men and women in Korea | (307) | |
| 0.042–0.173 ppm | — | — | — | Men and women in Jordan (ages | (25) | |
| 0–60yrs old) | ||||||
| Mean: 25.56 ng/g lipid Max.: 137. 2 ng/g lipid | — | Mean: 2.17 ng/mL Max.: 14.16 ng/mL | — | Post-menopausal women in Spain | (52) | |
| Dieldrin | — | — | — | 2,385–68,286 | Freshwater Fish | (17) |
| 0.099 ppb | 0.060 ppb | 0.002 ppb | — | Women in Delhi | (286) | |
| Mean: 65.2 ng/g fat vAT; Mean: 45.1 ng/g fat scAT |
— | — | — | Obese men and women in Portugal undergoing bariatric surgery | (317) | |
| — | 0.54 mg/kg | — | — | Women in Canada (1975) | (119) | |
| Mean: 0.029 mg/kg scAT; Max.: 145 ng/g scAT | — | — | — | Men and women in Canada (1983–1984) | (119) | |
| 0.0001 mg/g | — | — | — | Men and women in Korea | (307) | |
| 0.04–0.27 ppm | — | — | — | Men and women in Jordan (ages 0–60yrs old) | (25) | |
| Mean: 17.01 ng/g lipid Max.: 84.05 ng/g lipid | — | Mean: 1.21 ng/mL Max.: 6.35 ng/mL | — | Post–menopausal women in Spain | (52) | |
| 0.24 ppm | — | — | — | US individuals with non-Hodgkin’s Lymphoma | (323) | |
| 0–15 ng/g fat | — | — | — | Women in Hong Kong | (322) | |
| Chlordane | — | — | — | 3,000–12.000 | Marine Fish | (435) |
| — | — | — | 18,500 | Freshwater | (301) | |
| Fish | ||||||
| Chlordane: 4.5–49 ng/g fat;
Oxy-chlordane: 11–75 ng/g fat; Nonachlor: 29–230 ng/g fat |
— | — | — | Men and women in Japan | (153) | |
| Mean: 0.012 mg/kg scAT; Max.: 91 ng/g scAT | — | — | — | Men and women in Canada | (119) | |
| Oxy-chlordane: Mean: 200 ng/g fat; Trans-nonachlor: Mean: 140 ng/g fat | — | — | — | Men and women in Ontario, Canada | (97) | |
| Oxy-chlordane: 40.9 ng/g fat; Trans-nonachlor: 45.9 ng/g fat | — | — | — | Women in Long Island, New York without breast cancer | (372) | |
| Oxy-chlordane: 12 ng/g lipid; Trans-nonachlor: 32 ng/g lipid | — | — | — | Men and women in Finland | (24) | |
| Oxy-chlordane: 0.20 ppm | — | — | — | US individuals with non-Hodgkin’s Lymphoma | (323) | |
| Endrin | — | — | — | 2,000–10,000 | Freshwater Fish | (9) |
| — | — | — | 4,800–6,000 | Saltwater Fish | (9) | |
| Mean: 284.8 ng/g fat vAT; Mean: 146.4ng/g fat scAT | — | — | — | Obese individuals in Portugal | (317) | |
| 0.003 μg/g fat | — | — | — | Men and women in Korea | (307) | |
| 0.061–0.358 ppm | — | — | — | Men and women in Jordan (ages 0–60yrs old) | (25) | |
| Mean: 47.43 ng/g lipid, Max.: 148.13 ng/g lipid | — | Mean: 2.25 ng/mL; Max.: 6.24 ng/mL | — | Post-menopausal women in Spain | (52) | |
| Heptachlor | — | — | — | 10,630 | Clam Fat | (10) |
| — | — | — | 2,570 | Soft Clams | (10) | |
| — | — | — | 8,511 | Oysters | (10) | |
| — | Heptachlor epoxide: 0.60 mg/kg | — | — | Women in Canada (1975) | (119) | |
| Mean: 0.083 mg/kg fat Max.:116 ng/g fat | — | — | — | Men and women in Canada (1983–1984) | (119) | |
| Heptachlor epoxide: 41 ng/g fat | — | — | — | Men and women in Ontario, Canada | (97) | |
| 0.086–0.173 ppm | — | — | — | Men and women in Jordan (ages 0–60yrs old) | (25) | |
| Heptachlor epoxide 0.14 ppm | — | — | — | US individuals with non-Hodgkin’s Lymphoma | (323) | |
| Heptachlor epoxide: 0–11 ng/g fat | — | — | — | Women in Hong Kong | (322) | |
| Mirex | — | — | — | 15,000 | Rainbow trout | (13) |
| 0.04 mg/kg fat scAT | — | — | — | Men and women in Canada (1980–1981) (ages 0–102yrs old) | (119) | |
| Mean: 116 mg/kg lipid scAT Mean: 126 mg/kg lipid omental fat |
— | — | — | Men and women in Greenland | (103) | |
| Toxaphene | — | — | — | 4,247–76,000 | Fish | (15) |
| Compounds detected include toxaphene congeners 2, 26, 38, 40/41, 44, 50, and 62 | Sum of toxaphenes: 0.82–17 ng/g lipid | — | — | — | Swedish population (n=8) | (112) |
| Methoxychlor | — | — | — | 195–1500 | Fish | (12) |
| 0.68 μg/kg | — | — | — | Harp Seals (blubber) | (12) | |
| Mean: 21.3 ng/g fat vAT; Mean: 40.6 ng/g fat scAT |
— | — | — | Obese men and women in Portugal undergoing bariatric surgery | (317) | |
| Mean: 39.86 ng/g lipid; Max.: 155.58 ng/g lipid |
— | Mean: 0.38 ng/mL; Max.: 0.39 ng/mL |
— | Post-menopausal women in Spain | (52) | |
| 347.73 ng/g fat | — | — | — | Women undergoing C-section in southern Spain | (181) | |
| Endosulfans (alpha/beta isomers) | — | — | — | 17.1–11,583 | Fish | (8) |
| Endosulfan I: 2.8 ng/g fat vAT; 47 ng/g fat in scAT Endosulfan II: 1.8 ng/g fat vAT 2.2ng/g fat scAT |
— | — | — | Obese men and women in Portugal | (317) | |
| Endosulfans I/II: Mean: 21.37 ng/g lipid; Max.: 417.5 9 ng/g lipid | — | Endo-sulfans I/II: Mean: 8.85 ng/mL; Max.: 210.99 ng/mL | — | Post-menopausal women in Spain | (52) | |
| Endosulfan I: Mean 75.46 ng/g fat Endosulfan II: Mean: 51.68 ng/g fat | — | Endo-sulfan I: Mean: 1.27 ng/mL Endo-sulfan II: Mean: 76.38 ng/mL | — | Women undergoing C-section in southern Spain | (181) | |
| Chlordecone | — | — | — | >60,000 | Fish | (13) |
| α-Hexachlorocyclohexane (α-HCH) | Mean: 0.016 mg/kg fat | — | — | — | Men and women in Poland | (251) |
| β-Hexachlorocyclohexane (β-HCH) | 34.25 + 43.2 mg/kg fat | — | — | — | Obese population | (72) |
| Median: 64.7 ng/g vAT Median: 72.2 ng/g scAT | — | — | — | Obese men and women in Portugal | (317) | |
| Mean: 0.228 mg/kg fat Median: 0.120 mg/kg fat | — | — | — | Men and women in Poland | (251) | |
| 189, 424, 253 ng/g fat | — | — | — | Men and women from Southeast China | (419) | |
| Hexachlorobenzene (HCB) | 0.553 ppb | — | — | — | USA Population | (330) |
| 23.4 + 3.17 mg/kg | — | — | — | Obese population | (72) | |
| — | — | — | 17,000,0 00; 21, 900 | Lichens; Fish | (282) (412) | |
| Mean: 0.310 | — | — | — | Men and | (251) | |
| mg/kg fat Median: 0.120 mg/kg fat | women in Poland | |||||
| 16.3, 39.4, 23.9 ng/g fat | — | — | — | Men and women from Southeast China | (419) | |
| Dichlorodiphenyl-trichloroethane (P,P′-DDT)/Dichlorodiphenyl-dichloroethylene (p,p′-DDE) | p,p′-DDE: 512.2 + 284 mg/kg | — | — | — | Obese population | (72) |
| p,p′-DDE: 610 ng/g lipid | — | — | — | Men and women in Finland | (24) | |
| p,p′-DDE: Mean: 5.745 mg/kg fat Median: 4.382 mg/kg fat Max.: 35.850 mg/kg fat | — | — | — | Men and women in Poland | (251) | |
| p,p′-DDT : Mean: 0.537 mg/kg
fat Median: 0.478 mg/kg fat |
— | — | — | Men and women in Poland | (251) | |
| p,p′-DDE: 18.3, 11.5, 9.86 ng/g fat | — | — | — | Men and women from Southeast China | (419) | |
| p. p′-DDE: Median: 93.0 ng/g lipid | — | 175.7 ng/g lipid | — | Men and women in southern Spain | (33) | |
| p,p′-DDE: Median: 22.6 ng/g
vAT Median: 2.8 ng/g scAT |
— | — | — | Obese men and women in Portugal | (317) | |
| Pentachlorobenzene (PeCB) | 0–70 ng/kg | — | — | — | Canadian Men and Women | (261) |
| 0–146 mg/kg | 0–25 mg/kg | — | — | Men and Women in the Republic of Slovenia (between ages 20–60) | (178) | |
| Lindane (γ-HCH) | Median: 19.0 ng/g vAT Median: 31.0 ng/g scAT | — | — | — | Obese men and women in Portugal | (317) |
| Mean: 0.074 mg/kg fat | — | — | — | Men and women in Poland | (251) | |
| 0.210, 0.130, 0.620 ng/g fat | — | — | — | Men and women from Southeast China | (419) | |
| Hexachlorobutadiene (HCBD) | 0.004 mg/g | — | — | — | Canada | (267) |
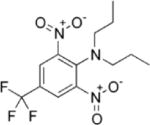
| Trifluralin | 0.170, 0.620, 7.17 ng/g fat | — | — | — | Men and women from Southeast China | (419) |
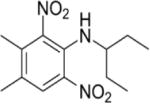
| Pendimethalin | 0.9 ppm | — | — | — | Royal Hart Wistar Rats | (438) |
| Polycyclic Aromatic Hydrocarbons (PAHs) | 11–2,700 ng/g | — | — | — | General population (n=4) | (293) |
| Compounds detected: anthracene, pyrene, benzo[e]pyrene, benzo[k] fluoranthene, benzo[a]pyrene, and benzo[g,h,i]perylene | 15–361 ng/g lipid | — | — | — | Women in Korea | (277) |
| Tetrabromobisphenol A (TBBPA) | not detected | 0.06–37.34 ng/g lipid | 154 pg/g fresh weight (maternal serum) 199 pg/g fresh weight (cord serum) | — | French women and their newborns | (64) |
| Polychlorinated dibenzo-p-dioxins (PCDD)/Polychlorinated dibenzofurans (PCDF) | 33.9–504 pg/g lipid Mean: 108 pg/g lipid | — | — | — | General Population in China | (353) |
| Fertile men: 3.0–15.8 pg/g fat Infertile men: 2.8–17.2 pg/g | — | — | — | Men in Ankara, Turkey | (81) | |
| 16–56 pg/g, fat weight | — | — | — | Women in India | (210) | |
| 14–46 pg/g, fat weight | — | — | — | Men in India | (210) | |
| — | — | 0.1–3,270 ppt | — | Adult men and women in the US | (147) | |
| Bisphenol F diglycidyl ether (BFDGE) | 19.1–4,500 ng/g wet weight | — | — | — | Men and women in the US | (418) |
| Polybrominated diphenyl ethers (Compounds detected included hexa-, hepta-, tetra-, and penta-BDEs) | 17–9,630 ng/g lipid weight Mean: 399 ng/g lipid weight | — | — | — | New York, NY population | |
| — | 6.2–419 ng/g (or ppb) lipid Mean: 73.9 ng/g lipid | — | — | U.S. Mothers | (342) | |
| — | — | — | 115,000–1,440.00 0 (based on lipid weights) | Guppies | (129) | |
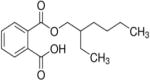
| Mono-(2-ethylhexyl)phthalate (MEHP)–(metabolite of DEHP) | — | — | Max.: 19,740 ±1,670 (one sample) | — | Rats | (78) |
| Polychlorinated naphthalenes (PCN) (Compounds detected included tetra-, penta-, and hexa-, congeners) | 0.9–34.6 ng/g fat | — | — | — | Children in Germany, Russia, and Kazakhstan | (427) |
| 0.04–1.09 ng/g lipid | — | — | — | Men and women in Sweden | (425) | |
| — | 483–3081 | — | — | Women in Sweden | (252) | |
| ng/kg | ||||||
| — | — | 1,150–30,400 ng/kg | — | Individuals in Taiwan after exposure incident | Values reported by (87) | |
| (Compounds detected included mono- to tetra-, congeners) | — | — | — | 0–33,884 | Fish | Values reported by (87) |
| Hexabromobiphenyl | 1–2 ppb | — | — | — | General Population in US | (233) |
| Perirenal fat: 475 ng/g | — | — | — | Individuals in US after exposure incident | (270) | |
| — | — | 18,100 | Fathead Minnows | (412) | ||
| Hexabromo-cyclododecane (HBCD) | — | — | — | 708,000 (lipid weight-based) | Guppies | (129) |
| 2.4–38.1 ng/g, lipid weight | — | — | — | Fish | (352) | |
| Blubber: 7.38 ng/g, lipid weight | — | — | — | Dolphin | (182) | |
| 0.333 ± 0.571 ng/g, lipid weight | — | — | — | Men and women in the US | (182) | |
| Pentachlorophenol (PCP) | Median: 0.013 mg/g | — | 0.005–0.069 mg/mL | — | Men and Women in Northern Bavaria | (133) |
| — | 884 mg/L | — | Men, women, and children (ages 8–60) | (259) | ||
| Polychlorinated biphenyls (PCBs) (Compounds detected include bi- to deca-chlorobiphenyls) | — | — | — | 2.64–5.97 (log BCF) | Fish | Reported by (162) as published by (118) (129) (375) (338) (287) |
| Range: 97–768 ng/g fat Median: 235 | — | — | — | Stillbirths in the US | (219) | |
| ng/g fat | ||||||
| Mean: 0.856 mg/kg fat Max.: 36 mg/kg fat | — | — | — | Men and women in Poland | (251) | |
| PCB-153: 310 ng/g lipid | — | — | — | Men and women in Finland | (24) | |
| Dioxin-like PCBs: 4.1–125 ng/g lipid Mean 32.8 ng/g lipid | — | — | — | General population in China | (353) | |
| Bisphenol A (BPA) Chlorinated derivatives detected: monochloro-BPA (ClBPA), dichloro-BPA (Cl2BPA), and trichloro-BPA (Cl3BPA) | BPA: 5.83 ± 3.48 ng/g; ClBPA: 3.05 ± 0.28 ng/g; Cl2BPA: 9.21 ± 9.26 ng/g; Cl3BPA: 0.74 ± 0.15 ng/g | — | — | — | Women in Spain | (116) |
| Di-(2-ethylhexyl) phthalate (DEHP) | — | — | — | 3, 173 ± 3,149 | Algae | (288) |
| — | — | — | 1,4693 ± 949 | Mollusks | (288) | |
| — | — | — | 1,164 ± 1,182 | Crustaceans | (288) | |
| — | — | — | 1,058 ± 772 | Insects | (288) | |
| — | — | — | 422 | Polychaetes | (288) | |
| — | — | — | 280 ± 230 | Fish | (288) | |
| — | — | — | 605 | Amphibians | (288) | |
| 0.25 to 9.85 mg/kg | — | — | — | Chicks | (179) | |
Blood levels of POPs (as shown in Table 4) are commonly used to assess point exposures (33) (279) (55) (363) (416) (309) (319). Hydrophobic toxicants in blood often bind to lipoproteins and proteins. Many PCBs and other organochlorine pesticides found in blood, for example, are associated with the protein fraction and all major lipoprotein compartments, including very low density lipoprotein (VLDL), low density lipoprotein (LDL), and high density lipoprotein (HDL) (416). Aldrin and dieldrin bind to VLDL and LDL to distribute preferentially to fat, while chlordecone and mirex preferentially bind albumin and HDL (363), and pentachlorophenol (PCP) strongly binds to plasma proteins (55). Furthermore, Ljunggren et al. (243) found that POP concentrations in LDL/VLDL were more associated with cancer, while POPs in HDL were more associated with cardiovascular disease. Although most PCDD/PCDF congeners are found in lipoproteins of blood, liver, and fat tissues (319), the more highly chlorinated congeners (penta-through octa-substituted) do not partition between the lipoprotein and protein fractions of blood (309). In addition, less than 20% of dichlorodiphenyldichloroethylene (DDE) or DDT was distributed in erythrocytes, but greater than 40% of dieldrin was detected in these blood cells (279).
Measurements taken in whole blood, serum and/or plasma are minimally invasive, but may add difficulty in comparing toxicant concentrations between blood samples and other tissues. For example, Teixeira et al. (389) reported that plasma levels of aldrin did not reflect levels accumulated in tissues, and Archibeque-Engle et al. (30) found no correlation between levels of 15 of 17 compounds in breast AT compared to serum. Thus, preferential binding of some toxicants to lipoproteins and various other lipid compartments in serum and AT may contribute to reported variance of blood levels versus toxicant concentrations in other tissues (434).
When discussing toxicants in AT, it is important to define the process of bioaccumulation, which refers to the build-up of substances in the body because the substance is not readily metabolized and excreted. Many organic compounds not only resist environmental degradation, but may also bypass liver biotransformation enzymes and diffuse into AT. Highly lipid soluble POPs can disseminate through the food chain by collecting in body fat and biomagnifying, or increasing in concentration as they move from one organism to another. For example, the northern elephant seal is a “marine mammal predator” at the top of the food chain that contains massive concentrations of environmental contaminants that are primarily stored in AT (249) (99) (250) (318) (2). Measurements of bioaccumulation in fish and other aquatic organisms is often reported as the bioconcentration factor (BCF), which is defined as the extent to which a chemical concentration in an aquatic organism exceeds the chemical concentration in surrounding water (206). BCFs also correlate with octanol-water partition coefficients (345). Table 4 summarizes various bioaccumulation studies in aquatic organisms.
Once sequestered in adipose, toxicants are not released until lipolysis occurs, often through weight loss, diet, and exercise. It has been well documented that throughout episodes of fasting or weight loss, AT serves as a source of PCBs due to lipid mobilization (202) (250) (249). With lipolysis, PCBs and other toxicants are not only released into blood but they also concentrate into remaining AT (72) (202) (250) (249). Although no link has been established, one hypothesis for the “heterogeneous release of PCBs” from AT is that during lipolysis, fatty acids are differentially mobilized from AT and may influence the release of some PCBs versus others (98).
The release of PCBs and other toxicants into systemic circulation can potentially expose an individual to various known hazardous effects. Therefore, measuring chemical contaminants (and/or their metabolites) in adipose and blood samples can provide great insight into overall exposures and body burden, which can strengthen the ability to determine associations between chemical exposures and the development of adverse health effects.
Effects of POPs on AT function
While epidemiological studies indicate an association between systemic POP concentrations and metabolic diseases (228) (382) (386), mechanisms mediating impairment of metabolism by POPs remain unclear. In addition to the central role that AT plays in maintenance of metabolism and energy homeostasis through storage of excess fuels as fat (lipogenesis) and mobilization of fatty acids for use as fuel (lipolysis), adipocytes secrete a multitude of adipokines that contribute to metabolic regulation and inflammatory responses. In addition, a major role of AT is expansion in response to metabolic excess, which is achieved through both an increase in adipocyte size (hypertrophy) and increased differentiation of preadipocytes to mature adipocytes (adipocyte differentiation). Given the central role of AT in regulation of body weight and metabolism, POP-mediated disruption of AT function may contribute to the development of obesity and related metabolic diseases. A summary of the effects of POPs on AT function, organized by pollutant class, is presented in Table 5, and a summary of the major mechanisms by which POPs are purported to influence AT function is presented in Table 6.
Table 5.
Summary of the Effects of POPs on AT Function
| Class | Compound | Effect on Adipocyte Differentiati on | Effect on Lipogenesis | Effect on Adipokine Release | Effect on Glucose Uptake | Effect on Lipolysis |
|---|---|---|---|---|---|---|
| Organochlori nes | TCDD | Low dose increase, high dose decrease (34) Decrease (56) (71) (354) (161) | Decrease (56) (302) Decrease (195) | Increase inflammatory (195) (235) (292) (204) Increase leptin, adiponectin (385) | Decrease (195) (292) | Increase (235) (157) |
| PCBs | Low dose increase, high dose decrease (PCB-77) (34) Decrease (PCB-126) (121) Increase (PCB-153) (69) (385) | Increase inflammation (PCB-77) (34) (204) Increase leptin, adiponectin (PCB-153) (385) | Decrease (336) Decrease (37) | |||
| PCDDs PCDFs | No effect (336) | |||||
| DDT/DDE | Increase (DDT) (278) Increase (DDE) (69) | Increase (160) | Increase leptin, adiponectin, resistin (DDE) (160) Increase leptin, adiponectin (385) | Decrease (336) | ||
| HCB | Decrease in BAT (29) | |||||
| Oxy- chlordane, dieldrin | Increase (160) | |||||
| BFRs | HBCD | Decrease (431) | ||||
| PDBE | Increase (397) | Decrease (155) | Increase (155) | |||
| BDE-47 | Increase (185) | |||||
| Phthalates | DEHP | Increase (62) | Increase (373) | Increase inflammatory (62) | ||
| MEHP | Increase (142) (115) (385) | Increase (142) | Increase leptin, adiponectin (385) | Increase (73) | Increase (73) | |
| BPA/BADGE | BADGE | Increase (67) | ||||
| BPA | Increase (67) (433) (297) (385) | Increase (258) (433) (32) | Increase inflammatory (433) (32) (404) Decrease adiponectin (264) (166) |
Decrease (404) (32) | ||
| PAH | Benzo[a]pyrene | Decrease (173) (172) | ||||
| Air pollution | Increase (377) | |||||
| PAH | Decrease (198) |
Table 6.
POP Mechanisms of Action in AT
| Pathway | Normal function in adipose tissue | Purported disruptors | Impacts of disruption |
|---|---|---|---|
| PPARγ | Increase adipogenesis, increase lipogenesis, increase glucose uptake | Phthalates Organotins BFRs? |
Promote adipogenesis |
| AhR | Xenosensor, regulation of lipogenesis | Dioxins PCBs PAHs BFRs? |
Wasting syndrome Increased body weight, increased fat mass, increased inflammatory response, impaired glucose tolerance |
| ER | Inhibit lipogenesis, reduce body weight/fat mass, maintain glucose homeostasis | BPA | Prenatal exposure linked to increased body weight and adiposity in adults |
| AhR interaction | Inhibition of normal estrogen function to reduce body weight and adiposity | ||
| AR | Promote glucose uptake | DDE, PCBs | Insulin resistance |
| TR | Regulation of lipid mobilization and storage | BFRs AhR interaction | Unknown |
Adipocyte differentiation
Mechanisms regulating fat accumulation have been a major focus of research given the increased prevalence of obesity and associated health risks. Several studies have characterized the effects of POPs on the differentiation of progenitor cells and/or preadipocytes to mature, lipid-laden adipocytes but findings have been contradictory. Findings are further complicated by use of a variety of models. For example, use of preadipocyte cell lines with restricted potential to differentiate into other cell types (e.g. 3T3-L1 cells) versus use of multi- or pluri-potent stem cells (mesenchymal stem cells or stromal-vascular cells) can influence the experimental outcome. The effects of TCDD and dioxin-like PCBs on adipocyte differentiation have been the most heavily studied. TCDD has consistently been shown to decrease adipocyte differentiation in vitro in 3T3-L1 cells (354) (161) and from stromal vascular cells (56). Further, TCDD (in an aryl hydrocarbon receptor (AhR)-dependent manner) was demonstrated to suppress hormone-induced adipogenesis in mouse embryonic fibroblasts that exhibited proliferative expansion, but did not exit the cell cycle when exposed to the toxicant, suggesting that TCDD is an early regulator of adipocyte differentiation (26). However, effects appear to be dose-dependent. Low doses of both TCDD and dioxin-like PBC-77 induced differentiation of 3T3-L1 adipocytes, while high doses had an inhibitory effect (34). These results suggest that the reported effect of TCDD at high doses to induce wasting syndrome (348) may relate to an ability of the toxicant to decrease adipocyte differentiation, while lower exposures may contribute to an obesogenic phenotype. By comparison, studies examining effects of various organochlorine pesticides such as the non-dioxin-like PCB-153 (69), DDE (69) in human preadipocytes, and DDT in 3T3-L1 cells (278) report increased adipocyte differentiation. An ability of low dose POPs to induce adipocyte differentiation are consistent with increased body burden of these toxicants with obesity (332) (333) (104).
In addition to organochlorine pesticides, phthalates have also been associated with increased BMI and waist circumference (WC) in humans (370). Moreover, di(2-ethylhexyl) phthalate (DEHP) or its metabolite mono-(2-ethylhexyl) phthalate (MEHP) increased adipocyte differentiation in 3T3-L1 cells, (142) (115), murine mesenchymal stem cells (44), and in vivo (142) (62). Few human studies have addressed effects of BFRs on obesity and metabolic syndrome; however, adipocyte differentiation was increased by the BFRs PDBE (397) and BDE-47 (185). These findings are supported by a recent study which reported increased body weight of obese mice treated with hexabromocyclododecane (HBCD) (431). Other environmental toxicants (bisphenol A, BPA, bisphenol A diglycidyl ether, BADGE) have also been reported to increase adipocyte differentiation in 3T3-L1 cells (258), in vivo, (433) and in adipose stromal stem cells (297) (67).
Lipid Storage and Mobilization
Uptake of circulating fatty acids for storage as triglycerides is a major function of AT, with excess lipid accumulation a hallmark of expanded AT mass with obesity. In 3T3-L1 adipocytes, the organochlorine pesticides DDE, oxychlordane, and dieldrin have been reported to increase basal fatty acid uptake (160) and BPA was demonstrated to increase lipid accumulation (32). Administration of PCB-77 to mice resulted in greater body weight and adipocyte hypertrophy (34). Similarly, in utero exposure of mice to BPA (433) or the phthalates DHEP and MEHP increased adult fat mass, lipid accumulation and body weight (142) (373). However, in general, reports of direct effects of POPs to increase lipid accumulation are limited. As discussed below, and summarized in Table 6, it is likely that increases in lipogenesis or fat deposition can be attributed to impaired metabolism through endocrine disrupting effects of POPs.
In contrast, TCDD has been reported to reduce lipid accumulation and/or promote lipid mobilization (235) from adipocytes. This effect of TCDD has been attributed to inhibition of lipoprotein lipase (LPL), a key enzyme in the pathway for adipocyte uptake of fatty acids for storage as triglycerides (302) (195).
Adipokine secretion
A major function of AT is the secretion of a wide range of signals and factors termed adipokines. These include inflammatory cytokines (TNF-α, interleukins) and chemokines (MCP-1), as well as hormones that participate in body weight regulation and glucose and lipid homeostasis (adiponectin, leptin, resistin) (396). Reduced adiponectin levels (237) and increased resistin levels (177) with obesity are associated with insulin resistance and inflammation (237). Several studies have linked BPA exposure with regulation of AT adipokines. A study in obese children reported an association of urinary BPA concentration with insulin resistance, and incubation of AT explants from these patients with BPA increased gene expression of resistin and decreased gene expression of adiponectin (264). Similarly, incubation of adult AT explants with BPA inhibited adiponectin (166). In contrast, one study reported an increase in leptin and adiponectin expression in 3T3-L1 cells incubated with BPA (385). As BPA was also reported to increase adipocyte differentiation, it is possible that the increase in leptin and adiponectin reflected an increase in the mature adipocyte population. In addition to BPA (404) (32) (433), gene expression and secretion of inflammatory factors from adipocytes has been reported to increase in response to TCDD (195) (235) (34) (292) (203), PCB-77 (34) PCB-126 (203), and DEHP (62). Moreover, infiltration of macrophages into AT, a pathway associated with obesity-induced insulin resistance, has been reported as a result of exposure to DEHP (a phthalate) (62) HBCD (a BFR) (431), and TCDD (415).
Glucose uptake
Glucose uptake by adipocytes contributes to whole body glucose homeostasis and impaired glucose uptake is associated with insulin resistance. A vast majority of data indicate that POPs impair glucose uptake in adipocytes. Specifically, treatment of adipocytes with TCDD, PCBs, DDT, BFRs, BPA, and PAHs impaired glucose uptake. Exposure of animals to PCBs (38), phthalates (142) (374), BFRs (431), or mixtures of POPs (336) impaired insulin sensitivity. Mechanisms for these effects are not fully understood, but may include reduced AT levels of Glut-4 mRNA or increased expression of inflammatory markers (both AT and circulating levels) associated with impaired glucose uptake or insulin resistance.
Mechanisms of POPs to impair AT function
1. AhR
AhR is a basic-helix-loop-helix Per-ARNT-SIM (bHLH-PAS) ligand-activated transcription factor (136). Evolutionarily well-conserved, and expressed across a diverse number of mammalian species, AhR is a prominent mediator of the biological response to synthetic and naturally occurring chemicals (102). Ligand binding results in translocation of AhR from the cytoplasm to the nucleus and subsequent dimerization with its binding partner, aryl hydrocarbon receptor nuclear translocator (ARNT). The activated AhR/ARNT heterodimer complex binds to DNA at specific response elements (typically dioxin or xenobiotic response elements; DRE or XRE) to activate the expression of AhR target genes, such as cytochrome P450s (CYP1A1) (140). This classical activation of AhR has been described in response to halogenated aromatic hydrocarbons (HAHs), such as PCDDs, TCDD (being the best characterized and most potent), PCDFs, several PCBs, and PAHs (339).
AhR is expressed in adipocytes (354), and the adipocyte AhR has recently garnered increased attention for its role not only in the xenobiotic response of AT, but also as a regulator of body weight, fat mass, and lipid homeostasis (194) (39) (430). Several studies provide evidence of AhR-mediated regulation of AT function, but results have not been consistent. AhR activation was reported to suppress de novo lipogenesis, as mouse embryonic fibroblasts isolated from AhR deficient mice displayed enhanced triglyceride synthesis (26). These data are consistent with results from mice with adipocyte-specific deficiency in AhR, where mice displayed increased body weight, fat mass, AT inflammation, and decreased glucose tolerance compared to wild type mice when fed a high fat diet (39). In direct contrast, it was recently reported that high-fat fed mice with whole body deficiency of AhR were protected from obesity, insulin resistance, and adipose inflammation (430). In another study, aged, but not young mice with whole body AhR deficiency were reported to have impaired glucose tolerance compared to wild type controls, without concomitant differences in body weight between genotype (46). Differences in findings from mice with whole body AhR deficiency versus those with cell-specific deletions may result from diverging effects of AhR across multiple cell types. Taken together, data implicate AhR in the regulation of AT function, body weight and lipid homeostasis.
As a wide spectrum of ligands are capable of binding and activating AhR, its activation by various POPs may contribute to their observed effects on obesity and fat mass in human populations. The best characterized AhR agonists capable of eliciting effects in AT or adipocyte cell lines are TCDD and TCDD-like PCBs, which can be abolished by AhR antagonists (34) (121). Moreover, TCDD-induced impairment of adipogenesis in mouse embryonic fibroblasts was abolished when cells were isolated from AhR deficient mice (26). Further, effects of TCDD and TCDD-like PCBs to regulate the inflammatory response (204) (37) and glucose uptake in adipocytes (37) were AhR-mediated. In mice with whole body AhR deficiency, administration of PCB-77 resulted in adipocyte hypertrophy and increased body weight compared to wild-type mice (34). Moreover, effects of PCB-77 to impair glucose homeostasis and AT inflammation were abolished in mice with adipocyte-specific AhR deficiency (39).
PAHs are also high-affinity ligands for AhR (102). However, limited studies have defined effects of PAHs to regulate AT function and development of obesity. Exposure of mice to air pollution (a major source of PAHs) (377) or to benzo-[a]-pyrene (173) increased visceral AT, circulating inflammatory factors, AT macrophage infiltration, fat mass, body weight, and impaired whole body glucose tolerance (377). In humans, prenatal exposure to air pollution has been associated with increased body size in children (335) (260). Additionally, a consistent association exists between exposure to cigarette smoke (another PAH source) in utero and increased risk of overweight and or obesity in adulthood (300) (171). Whether PAH molecules impair AT function through AhR-mediated activation is unknown.
2. PPARγ
PPARγ, a ligand-activated nuclear transcription factor, is a central regulator of AT function. Specifically, the PPARγ2 isoform is predominantly expressed in adipose tissue, especially very early in adipose cell differentiation (365), and activation of PPARγ2 stimulates adipogenesis (393). Upon activation and formation of a heterodimer with the co-activator retinoid × receptor (RXR), PPARγ binds to PPARγ response elements to stimulate transcription of genes involved in adipogenesis, lipid metabolism, and glucose homeostasis (22). It is thought that inappropriate activation of PPARγ by some POPs may contribute to obesity. In particular, phthalates have been identified as modulators of PPARγ. In 3T3-L1 adipocytes, activation of endogenous PPARγ target genes has been demonstrated by MEHP, monobenzyl phthalate (MBzP), and mono-sec-butyl phthalate (MBuP) (167). Moreover, direct activation of PPARγ by MEHP in 3T3-L1 adipocytes promoted adipocyte differentiation, albeit to a lesser extent than the known PPARγ agonist, rosiglitazone (115). Interestingly, compared to rosiglitazone, MEHP resulted in promotion of only a subset of PPARγ coregulators, indicating differential effects of MEHP versus rosiglitazone on PPARγ transcriptional activation of adipocyte gene expression (115).
Evidence suggests a link between AhR binding by POPs and PPARγ activation. Reduced differentiation of 3T3-L1 cells to adipocytes by TCDD (71) (161) (320), PCBs (121), or DDT (278) was associated with decreased PPARγ gene expression. Further, TCDD suppressed PPARγ and adipogenesis via a MEK/ERK mechanism (79). In contrast, in mouse embryonic fibroblasts, AhR-mediated inhibition of adipogenesis preceded suppression of PPARγ activity (26). Thus, changes in PPARγ expression in response to TCDD may reflect reduced adipocyte differentiation rather than a direct effect of AhR activation to decrease PPARγ.
One additional class of POPs that may influence PPARγ activity is organotins. Compounds such as tributyltin chloride (TBT) and bis(triphenyltin) oxide (TPTO) have been demonstrated to promote adipogenesis in 3T3-L1 cells through activation of PPARγ (186) (236). The effects of these compounds to influence PPARγ-mediated regulation of adipocyte function are thought to be through binding of the PPARγ binding partner, RXR (186) (221).
Although some BFRs have been associated with increased adipocyte differentiation, the mechanism has not been reported. The brominated analogs of BPA, TBBPA and TCBPA were demonstrated to bind to and activate PPARγ in reporter cell lines. It was observed that the bulkier the brominated BPA analogs, the greater their capacity to activate PPARγ (328). 3. Endocrine hormone receptors. Endocrine hormone receptors may be the target of many POPs. Endocrine disruption resulting from inappropriate interactions with these receptors can negatively influence obesity and AT function. Several POPs that accumulate in AT are reported to be xenoestrogens (environmental ligands capable of binding and influencing ER signaling) (334) (380) (220) (350), including BPA, DDT/DDE, methoxychlor, the PAH 2-amino-1-methyl-6-phenylimidazo[4–5-b]pyridine (PhIP), TCDD, PCBs, polybrominated biphenyls (PBBs), and phthalate esters. Estrogen receptor (ER) α and β are primary mediators of the effects of estrogens. Estrogen binding to these nuclear receptors results in the formation of homodimer complexes that bind to the promoter regions (termed estrogen response elements, or EREs) of estrogen-responsive genes, many of which contribute to regulation of metabolism. In post-menopausal women or ovariectomized rodents, where estrogen is low, white adipose tissue mass, body weight, and insulin resistance are increased (387). ERα signaling is purported to modulate the beneficial metabolic effects of estrogens, such as anti-lipogenesis, insulin sensitivity and glucose tolerance, and reduction of body weight and adipose mass, whereas ERβ is thought to play a larger role in the maintenance of normal glucose and lipid homeostasis (117). ERα deficient mice are prone to obesity, exhibit increased visceral fat mass, decreased insulin sensitivity, and impaired glucose tolerance (149). ERs are expressed in AT (311) and adipocyte-specific deletion of ERα resulted in increased adiposity, AT inflammation and fibrosis (93).
In particular, BPA has garnered significant interest as an estrogenic compound. Despite having a relatively low affinity for the ER compared to that of estrogen (209), BPA is widely accepted to mimic the effects and potency of estrogen (410). However, BPA has been associated with increased adipocyte differentiation, body weight and fat mass, effects which are inconsistent with known ER-mediated reductions in adiposity as described above. A potential explanation for this discrepancy relates to a developmental window for the effects of BPA, as rodent studies have demonstrated a significant effect of prenatal BPA exposure to increase body weight, adipocyte hypertrophy, and adiposity in adults (410) (364) (272). In a recent study, BPA-mediated differentiation of human preadipocytes could be inhibited by an ERα antagonist (53). Estrogens contribute to an increase in adipocyte number (84). Thus, exposure to estrogen during a critical period of development may predispose for AT expansion, especially when children or adults are faced with a metabolic challenge, or in combination with exposure to other obesogenic environmental chemicals. The brominated BPA analogs TBBPA and TCBPA have also been described as ligands for ERs (329), but further mechanisms examining the role of these BFRs to modulate AT function through ERs have not been defined.
AhR has been reported to interact with endocrine hormone receptors, thus one mechanism by which AhR may mediate body weight and fat mass is through modulation of ER-signaling pathways. Ligand-bound AhR/ARNT has been demonstrated to directly associate with ERs (299); however, consequences of AhR/ER interaction are complex and not well understood. In general, crosstalk between ER and AhR is thought to be inhibitory with respect to ER signaling. An inhibitory effect of AhR on ER signaling is consistent with reports demonstrating AhR agonists increase body weight and adiposity, since deficiency of estrogen (387) or ERα (149) are associated with increased obesity and adipose mass.
Several mechanisms may contribute to inhibition of ER signaling as a result of ER/AhR crosstalk. First, TCDD-mediated expression of CYP1A1 and CYP1B1 has been reported to enhance metabolism of estrogen in some cell types (368) (366) (367). Although circulating estrogen levels were not altered in TCDD-treated rodents (355), no study has examined the relationship between AhR activation and adipose ER expression/activity. Moreover, ligand-bound AhR/ARNT prevents ER promoter binding and downregulation of ER target genes (reviewed in (340)). Also, ligand-bound AhR has been demonstrated to participate in an E3 ubiquitin ligase complex targeting the ER to proteasomal degradation (298). Finally, AhR and ERα interact with several common nuclear coregulatory factors (340), including ARNT (57), suggesting competition for these factors could influence activation of either pathway.
Inhibition versus potentiation of ER signaling may depend on the presence or absence of estrogen. For example, AhR coactivation of ERα resulted in transcriptional activity from ERE-regulated genes in the absence of estradiol (299). Alternatively, in the presence of estradiol, ARNT was recruited to estrogen-responsive promoters leading to increased ER transcription (379).
In addition to disruption of ER signaling, some lipophilic organochlorines may interfere with androgen receptor (AR) signaling. For example, DDT/DDE (191) and some PCBs (344) have been reported to act as AR antagonists. Testosterone has been shown to stimulate glucose uptake in adipocytes (271). Additionally, a positive correlation between insulin sensitivity and testosterone levels has been reported in males (321). Thus, antagonism of the AR by certain POPs may impair AT function through inhibition of glucose uptake.
AT is also a target of thyroid hormones, and thyroid hormone signaling through thyroid receptors (TRs) regulates lipid mobilization and storage. Disruption of TR signaling by certain POPs may contribute to dysregulation of AT function. For example, BPA has been reported to inhibit TR signaling through enhanced recruitment of corepressors (280); however, this effect of BPA has not been localized to AT. Exposure of rats to PDBEs resulted in increased circulating levels of thyroxine and altered glucose metabolism of isolated primary adipocytes, however there was no effect on body weight (155). Additionally, TCDD and certain PCBs have been suggested to be repressors of thyroid function, as exposed rodents (291) and humans (310) demonstrate compensatory increases in circulating levels of thyroid stimulating hormone. Future studies should address whether these TR-mediated effects are manifest in AT.
Associations between POP exposures and the development of obesity and diabetes
Obesity
The prevalence of obesity and its related comorbidities has been rising rapidly over the last three decades and is reaching epidemic proportions in the Western world, most notably in the US (244) (296). The rising prevalence of obesity becomes more alarming when considering that the comorbidities of overweight and obesity include an increased risk of type II diabetes and cardiovascular diseases (82) (273) (274), two leading causes of rising medical costs and poor prognosis in the US (266) (207). Interestingly, in parallel with the increased prevalence of obesity, the use and environmental levels of synthetic organic and inorganic chemicals has risen dramatically (35). Therefore, in addition to the importance of diet and exercise in the etiology of obesity (152), the hypothesis that exposure to environmental contaminants such as POPs contributes to the development of obesity is gaining popularity (35). The “obesogen hypothesis” proposes that exposure to environmental xenobiotic chemicals either in utero or throughout life contributes to the development of obesity (148) (109) (289). “Obesogens” have been defined as “molecules that inappropriately regulate lipid metabolism and adipogenesis to promote obesity” (135).
Previous reviews have investigated potential links between POPs and obesity (212) (228) (382); however, findings are often inconsistent between different POP classes and even within chemical congeners. Associations between POPs and obesity may be complicated by the lipophilicity of these compounds; obese individuals possess greater adiposity in which these lipophilic chemicals may be stored. Furthermore, obese individuals may consume more fatty foods rich in lipophilic chemicals and may, therefore, experience higher dietary exposure to POPs. Findings are further complicated by the dose and timing of exposure as well as the gender of the individual exposed. Although in vitro and animal studies have typically investigated very high doses of POPs in their research, there is evidence that physiological changes can occur at much lower doses of POPs, and that these effects do not exhibit monotonic dose-response relationships (426). Therefore, conflicting findings between a particular POP and obesity may be explained by the level of exposure within the population. For example, toxic, high-dose exposures may result in weight loss while lower levels of exposure, which are characteristic of the typical population and may be considered “safe,” might promote obesity (134) (148) (35). Additionally, the effects of POPs on parameters of obesity often vary by gender (Tables 7 and 8), which may be due to the POP’s ability to disrupt endocrine function and mimic estrogenic effects (51). Lastly, the time of exposure poses an important consideration for investigating associations between POPs and obesity. Many POPs, including PCBs, DDE and HCB have shown contrasting effects on body mass index (BMI) in prenatal versus adult exposures (212). As such, associations between POPs and obesity will be investigated separately based on the timing of exposure: prenatal and early life, or adult exposure. Regardless of these interactions and individual effects of the chemicals, the net result of POP mixtures appears to be weight gain.
Table 7.
Prenatal POP Exposure and Associations with Obesity
| Class | Compound(s) Studied | Population | Finding | Reference |
|---|---|---|---|---|
| Organochlorines | PCBs, DDE | Mothers of Michigan fisheaters cohort and their daughters | Prenatal exposure to DDE associated with increased offspring BMI. Prenatal PCB had no effect. | (189) |
| PCBs, DDE, HCB | Rhea study of pregnant women and their children in Greece. | Prenatal exposure to HCB associated with BMI, obesity, abdominal obesity, greater skinfold thickness, and systolic BP. Prenatal DDE associated with BMI, abdominal obesity, and diastolic BP. PCBs not associated with offspring obesity. | (402) | |
| PCB, DDE | Mother-child pairs from ENRIECO cohort | Postnatal NDL PCB-153 associated with a decrease in weight-for-age z-score. Prenatal DDE associated with increased weight-for- age z-score. | (174) | |
| Dioxins, PCBs, lead, HCB, DDE, HCH | Russian Children’s Study: young boys. Background exposure. | Early exposure. Serum HCB, β-HCH, DDE negatively associated with 4 year follow-up BMI in boys. | (58) | |
| PBB and PCB | Daughters of women in the Michigan PBB cohort. | Prenatal PCB exposure negatively associated with weight for height females. | (47) | |
| PCBs, PBB, DDT | Women and children in Michigan at risk for PCB exposure | Prenatal PCB associated with lower weight at 4 years. | (176) | |
| PCB, DDE, DDT | Mothers and African American children of National Collaborative Perinatal Project (NCPP). Background exposure. | Maternal levels of dioxin-like PCBs negatively
associated with girl’s weight. Non-dioxin-like PCBs (PCB 15) not associated with girl’s weight. Maternal levels of dioxin-like PCBs marginally associated with boy’s weight. |
(214) | |
| PCBs | Pregnant women of CHDS prospective cohort
study. Background exposure. |
Maternal PCBs associated with lower birth weight in males. | (151) | |
| PCBs, DDE, DDT, HCB | AMICS-INMA Spanish cohort of pregnant women and children. Background exposure. | Maternal PCB and DDE associated with overweight in females but not in males. DDT associated with overweight in males but not associated in females. | (408) | |
| PCBs, DDE | Mothers and newborns in Belgium. Background exposure. | Maternal DDE and PCBs associated with BMI 1-3 years. | (414) | |
| DDE, DDT | Mothers and male children with normal birth weights in Mexico. Background exposure. | Prenatal DDE exposure no association with BMI in males. | (89) | |
| PCBs and DDE | North Carolina Infant Feeding Study children. Background exposure. | Maternal transplacental DDE positively associated with weight in boys but not girls at 14yrs old. Lactational and trasnsplacental PCBs and lactational DDE not associated with weight. | (127) | |
| DDE, HCB, β- HCH, NDL PCB | INMA cohort in Spain. Background exposure. | Maternal serum: Prenatal DDE associated with
BMI z-scores at 14 months and rapid growth (stronger association in
boys). Other OCs (HCB, β-HCH, and NDL PCB) not associated with BMI. |
(265) | |
| PCBs, DDE | Mothers of Michigan fisheaters cohort and their daughters. | Prenatal DDE associated with BMI and BW in adult female offspring. Prenatal PCBs not associated with BMI in adult female offspring. | (189) | |
| DDE, DDT | CPP study mothers and male offspring. Background exposure. | Prenatal DDE and DDT from breastmilk not associated with BMI in boys. | (126) | |
| DDT, DDE | CHAMACOS cohort of pregnant women and children
in California. Background exposure. |
Maternal DDE and DDT not significantly associated with BMI and did not significantly increase odds of overweight and obesity. | (422) | |
| HCB, PCBs, DDE, DDT | Mothers and children in Asthma Multicenter
Infants Cohort in Spain. Background exposure. |
Maternal HCB associated with higher BMI and increased risk of being overweight and obese at age 6.5yrs old. | (362) | |
| PCB, DDE | Mother-child pairs fromFaroe Islands. Background exposure. | Prenatal PCB associated with BMI and waist circumference in 7yr old girls with overweight mothers. In girls with normal weight mothers, PCBs were associated with increased WC but not associated with BMI. In girls with overweight mothers, DDE was associated with WC at 7yrs old. No associations between PCB or DDE and BMI in 5yr old girls. No associations in boys. | (383) | |
| PCB 153, DDE | European birth cohorts. Background exposure. | PCB-153 cord serum inversely associated with birth weight. DDE not associated with birth weight. | (131) | |
| Phthalates, BPA, PCBs, HCH, HCB, PBDE | Pregnant Spanish birth cohort study of Environment and Childhood project. Background exposure. | Exposure to HCB, β-HCH, PCB 138 (NDL), PCB 180 (NDL) associated with increased BMI at age 7. DDE not significantly associated with increased BMI. HCB, β-HCH, NDL PCBs, and DDE associated with increase in overweight at age 7. | (21) | |
| PCBs, PCDD, PCDF, | Caucasian mother- infant pairs in
Netherlands. Background exposure. |
NDL PCBs in cord plasma and maternal plasma negatively associated with birth weight and growth rate 0-3 months in formula fed babies but not body fat. | (308) | |
| NDL PCBs, DL PCBs, DDE, HCB | 14-15yr old Flemish
adolescents. Background exposure. |
Serum DL PCBs associated with increased BMI and NDL PCBs and HCB associated with decreased BMI at puberty in males and females. DDE not associated with BMI. | (104) | |
| DDE, HCB, NDL PCBs | INMA birth cohort in Spain. Background exposure. | Prenatal DDE and HCB associated with rapid growth 0-6 months and overweight at 14 months. PCBs not associated with rapid growth or overweight. | (405) | |
| DDE, phthalates, NDL PCB | Dutch mother- daughter pairs. Background exposure. | Maternal DDE associated with increased BMI 6-11 months. NDL PCB not associated with BMI. | (95) | |
| DDE, HCB, NDL PCBs | Spanish mother-child pairs | Maternal HCB and DDE associated with rapid growth and overweight. PCBs not associated with postnatal growth. | (407) | |
| DDE, DDT, PCBs, HCH | CPP mother-child pairs in US. Background exposure. | Prenatal HCB, heptachlor, β-HCH, DDE, total PCBs, trans-nonachlor, and oxychlordane not associated with obesity or BMI at age 7. Dieldrin associated with obesity but not BMI. | (90) | |
| NDL PCBs, DDE, HCB | Flemish mother-child pairs from FLEHS I | Prenatal DDE associated with WC and waist/height ratio in girls. PCBs, dioxins, HCB not associated with WC. | (101) | |
| DDE, DDT | American mother- child pairs in CHAMACOS study | In boys, prenatal DDT associated with BMI, WCz-scores, and overweight/obesity at 9yrs old. DDE not associated. Girls not associated. | (423) | |
| PCB, DDE, HCB | Danish children in EYHS study | PCBs, DDE, HCB in 8-22yr olds not associated with obesity. | (384) | |
| DDT, DDE, HCB, β-HCH, total PCBs | Mother-child pairs in Spain. Background exposure. | Prenatal DDT and DDE associated with decreased birth weight. HCB, β-HCH, PCB not associated. | (248) | |
| PCBs | Mother-child pairs in NY. Background exposure. | Preconception PCBs associated with reduced birth weight. | (284) | |
| Poly-brominated flame retardants (BFRs) | PBDE | Pregnant Long-Evans hooded rats dosed with 1 or 10mg/kg bodyweight PBDE- 99 | Prenatal exposure to PBDE-99 increased rat offspring birth weight. | (238) |
| PBB and PCB | Daughters of women in the Michigan PBB cohort. | Moderate (but not high) prenatal PBB exposure associated with increased weight for height in females. | (47) | |
| Phthalates, BPA, PCBs, HCH, HCB, PBDE | Pregnant Spanish birth cohort study of Environment and Childhood project. Background exposure. | PBDE not associated with child weight status. | (21) | |
| PBDE | Mexican-American mother-child pair of CHAMACOS study | Maternal PBDE associated with decreased BMI
z-scores in girls but NS in boys. Child’s serum BDE-153 negatively associated with BMI and WC at 7yrs old in both sexes. |
(113) | |
| PBDE | Mexican-American mother-child pairs of CHAMACOS | Maternal PBDEs associated with lower birth weight, but effect is nonsignificant when maternal weight gain is included. | (144) | |
| Polycyclic Aromatic Hydrocarbons (PAHs) | PAH | MOCEH study in Korea without diabetes | Consumption of foods high in PAH (i.e. grilled or roasted meat) associated with reduced birth weight. | (215) |
| PAH | Birth cohort in Poland, nonsmoking mothers. | Maternal dietary and airborne PAH exposure associated with reduced birth weight. | (180) | |
| PAH | Birth cohort in Poland. Background exposure. | Newborn PAH-DNA adduct levels associated with reduced birth weight. | (315) | |
| PAH | Birth cohort in Poland. Background exposure. | Newborn PAH-DNA adduct levels associated with reduced birth weight. | (314) | |
| PAH | Krakow Caucasians, NYC African Americans, NYC Dominicans | Relatively low levels of prenatal PAH exposure associated with reduced birth weight. | (76) | |
| PAH | NHANES 2003-2008 16-18yr olds | PAH metabolites associated with BMI and WC in 6-18yr olds. High exposure to both PAH and environmental tobacco smoke associated with increased obesity as compared to low-PAH, low-ETS. | (197) | |
| PAH | African-Americans and Hispanic children and mothers in NY | Prenatal PAH exposure associated with higher childhood BMI and risk for obesity at 5-7yrs old. | (335) | |
| PAH | NHANES 2001-2006 6-19yr olds | Early exposure: mass sum of PAH associated with BMI z-score, WC, and obesity. | (346) | |
| Phthalate Esters | Phthalates, BPA | Korean girls 6-14yrs old | Early exposure 6-14yr old female: urine MEP not associated with childhood obesity. Urine PA associated with obesity. Serum MEP, PA, and DBP associated with childhood obesity. | (77) |
| Phthalates | NHANES 1999-2002. Background exposure. | Early exposure: serum MEP associated with BMI and WC in 12-19yr old females, but not associated in 6-11yr old males or females, or in 12-19yr old males. MEHP associated with decreased BMI in 12-19yr old females, not associated in 6-11 males and females, or in 12-19yr old males. MBP and MEOHP were not associated with BMI. | (145) | |
| Phthalates, BPA | Girls in US | MEP, MEHHP, MEHP, MECPP, MBzP, MiBP, MCPP, MBP, and MEOHP not associated with BMI in 6-9yr old girls. | (428) | |
| Phthalates, BPA, PCBs, HCH, HCB, PBDE | Pregnant Spanish birth cohort study of Environment and Childhood project. Background exposure. | Phthalates inversely associated with overweight. | (21) | |
| Phthalates | INMA Spanish birth cohort. Background exposure. | High MW phthalate metabolites associated with lower weight gain and BMI z-score in boys and higher BMI in girls. Low MW metabolites not associated with BMI or weight gain. | (406) | |
| Phthalates | Hispanic and Black NY children 6-8yrs old | Early exposure: MEP and low MWP associated with BMI and WC in overweight children but not normal weight children. | (388) | |
| Phthalates | NHANES 2003-2008 | Early exposure: Low MW metabolites (Mn BP, MEP, and MiBP) associated with obesity in male children and adolescents. Not associated for high MW and obesity | (59) | |
| DDE, phthalates, NDL PCB | Dutch mother-daughter pairs. Background exposure. | Maternal exposure to low exposure of MEOHP (DEHP metabolite) associated with higher BMI. | (95) | |
| Phthalates | Mother-child pairs in China | Prenatal DBP associated with low birth weight. | (436) | |
| Phthalates | Children 4-9yrs old in Denmark without diabetes | Urinary MEHP (percent of DEHP metabolites excreted as MEHP) associated with wetght. | (48) | |
| Bisphenol A and Bisphenol A diglycidyl ether (BADGE) | BPA | RHEApregnancy cohort in Greece | BPA levels at 4 years associated with BMI and WC. Prenatal BPA negatively associated with BMI and adiposity in girls and positively in boys. | (403) |
| Phthalates, BPA | Girls in US | BPA associated with decreased BMI in 6- 9yr old girls. | (428). | |
| Phthalates, BPA, PCBs, HCH, HCB, PBDE | Pregnant Spanish birth cohort study of Environment and Childhood project. Background exposure. | BPA not associated with child weight status. | (21) | |
| BPA | CHAMACOS study in California birth cohort. Background exposure. | Prenatal urinary BPA inversely associated with BMI and %body fat at 9 years in girls but not boys. Urinary BPA at 5 years not associated with BMI. Urinary BPA at 9 years positively associated with BMI, body fat, and overweight/obesity. | (143) | |
| BPA | INMA birth cohort in Spain. Background exposure. | BPA weakly associated with increased WC at 4 years but not associated with BMI or WC at earlier ages. | (405) | |
| Phthalates, BPA | Korean girls 6-14yrs old | Early exposure 6-14yr old females: urine BPA not associated with childhood obesity. | (77) | |
| BPA | Chinese school children 8-15yrs old. Background exposure. | Early exposure: BPA associated with BMI in males and females. | (417) | |
| BPA | 2003-2008 NHANES children 6-9yrs old | Early Exposure: BPA associated with obesity and BMI z-score. | (394) | |
| BPA | Mother-child pairs with or without occupational BPA exposure | Maternal exposure to BPA in workplace associated with decreased birth weight. | (269) |
Table 8.
Adult POP Exposure and Associations with Obesity
| Class | Compound(s) Studied | Population | Finding | Reference |
|---|---|---|---|---|
| Organochlorines | OC pesticides, PCBS, PBB, DDE, DDT | CARDIA prospective study in young adults without diabetes. Background exposure. | Serum p, p’-DDE, p, p’- DDT, and some PCB congeners predicted BMI. Several PCB congeners nonlinearly associated with increased BMI. Oxychlordane, trans-nonachlor, HCB, β- HCH, Mirex not associated with BMI. | (230) |
| Dioxins, dioxin-like PCBs, non-dioxin-like PCBs, DDE, β-HCH, OC pesticides | NHANES 1999- 2002. Adults without diabetes. Background exposure. | PCDD, DDE, β-HCH and PCBs associated with waist circumferences. NDL PCB inverted U-shaped association with WC. | (223) | |
| Dioxin, PCBs | Cross-sectional study of general population in Japan with and without diabetes | Dioxins and PCBs associated with MS. | (399) | |
| PCDDs, PCDFs, PCBs, OC pesticides | NHANES 1999- 2002 adults with background exposure. | HpCDD, OCDD, and DDE positively associated with BMI. PCB 153 negatively associated with BMI. Oxychlordane, trans- nonachlor not associated with BMI. | (224) | |
| HCH, HCB, OC pesticides, TCDD, DDE/DDT | Obese adults undergoing bariatric surgery in Portugal. Background exposure. | Adipose methoxychlor associated with LDL. Adipose methoxychlor and HCH lindane associated with Framingham CVD risk score. | (317) | |
| PCBs, DDE | Obese adults without diabetes. | Adipose PCBs and DDE associated with weight, BMI, WC, and CT-VAT. Adipose PCBs and DDE associated with visceral adipose and visceral/subcutaneous ratio. | (107) | |
| HCH, endosulfans, Aldrin, dieldrin, DDT, DDE | Adults with and without MetS. Background exposure. | β-HCH and Aldrin associated with MetS. | (391) | |
| PCB, OC pesticides, dioxin, HCB, DDE, BDE | PIVUS older adults, background exposure. Cross-sectional and prospective | Cross sectional: Trans- nonachlor positively associated with WC in males, no association in females. DDE positively associated with WC in males and females. HCB positively associated with WC in males but not in females. OCDD not associated with WC in males or females. PCB associations were positive, negative, or null based on congener and gender. Prospective: trans- nonachlor not associated with WC. OCDD associated with WC in females but not in males. DDE associated with WC in males but not in females. PCB associations were positive, negative, or null based on congener and gender. | (226) | |
| PCBs, OC pesticides | PIVUS older adults, background exposure. | Sum of OC pesticides and of less-chlorinated PCBs were positively associated with weight gain. Sum of highly- chlorinated PCBs were negatively associated with weight gain. | (241) | |
| PCBs | Non-obese adults in the Seguimiento Universidad de Navarra (SUN) Project | PCBs associated with increased risk of becoming obese. | (108) | |
| PCBs, DDE, β-HCH | Obese and normal weight individuals in Belgium. Case- control. Background exposure. | β-HCH positively associated and NDL PCBs negatively associated with BMI, WC, fat mass percentage, and total adipose tissue. DDE not associated. | (106) | |
| Dioxin, oxychlordane, trans-nonachlor, DDT | NHANES 1999- 2002 adults. Cross-sectional. Background exposure. | DDT positively associated with WC in females but negatively associated in males. OC pesticides positively associated with BMI in males and negatively associated in females. | (110) | |
| NDL PCB, DL PCB, HCB, DDE | Flemish adults. Background. | In men and women, NDL PCB negatively associated with BMI and HCB positively associated with BMI. Also in women, DDE and DL PCB positively associated with BMI. | (104) | |
| PCBs, dioxin, BDE, DDE, OC pesticides | PIVUS older adults, background exposure. | OCDD, PCBS 74, 99, 105, 118, HCB, and DDE positively associated with fat mass. PCBs 156, 157, 169, 170, 180, 189, 194, 206, and 209 negatively associated with fat mass. | (332) | |
| PCBs, DDE, HCB | Adults in highly polluted Eastern Slovakia. | PCBs, DDE, HCB associated with BMI. | (218) | |
| PCBs, DDE, HCB, OC pesticides | PIVUS older adults, background exposure. | Less chlorinated PCBs (105, 118), DDE, HCB, trans-nonachlor were positively related to visceral and subcutaneous adipose tissue (vAT and scAT). More highly chlorinated PCBs were negatively associated with vAT and scAT. PCB 189 had inverted U-shaped association with vAT/scAT. | ||
| PCBs, DDE, HCB, β- HCH, trans-nonachlor, oxychlordane | Cross-sectional study of Swedish women. Background exposure. | Some DL PCB congeners (PCB-105, PCB-118), DDE, HCB, and β-HCH positively associated with BMI. NDL PCBs (PCB-156 and PCB-180) negatively associated with BMI. OC pesticides not associated. | (128) | |
| PCBs, β-HCH, DDT, DDE, HCB, OC pesticides | Canadian males. Cross-sectional. Background exposure. | Total organochlorines not associated with BMI. β-HCH, DDE, and oxychlordane positively associated with BMI. PCBs, HCB, mirex, trans-nonachlor, and oxychlordane not associated with BMI. | (164) | |
| PCBs, β-HCH, DDT, DDE, HCB, OC pesticides | Canadian males. Cross-sectional. Background exposure. | Total organochlorines, NDL PCBs, DDE, HCB, β-HCH, trans- nonachlor, oxychlordane positively associated with BMI. DDT and mirex not associated with BMI. | (312) | |
| DDE | African American women in U.S. Cross-sectional. Background exposure. | DDE positively associated with BMI but not waist-hip ratio. | (343) | |
| Polybrominated flame retardants (BFRs) | PBBs and PBDEs | NHANES 2003- 2004 adults. Background exposure. | PBB-153 nonlinearly associated with MetS and WC. PBDE-153 inverted U shaped associated with MetS. | (239) |
| PBDEs | PIN study pregnant women in US. Background exposure. | Milk levels PBDEs associated with BMI in female moms. | (91) | |
| Polycyclic Aromatic Hydrocarbons (PAHs) | PAH metabolites | NHANES 2001- 2008 adults. Background exposure. | Urinary 2-phenanthrene positively associated with obesity. 1− naphthalene negatively associated with obesity. 2-naphthalene, 1− phenanthrene and 2− phenanthrene positively associated with 3+ risk factors for MetS. | (324) |
| Phthalate Esters | Phthalates | NHANES 2003- 2008 | High MW metabolites (MECPP, MEHHP, MEOHP, MEHP, MBzP, MCNP, and MCOP) associated with obesity in male and female adults. DEHP metabolites associated with obesity in female adults. DEHP and high MW metabolites associated with obesity in males 60+ yrs old. | (59) |
| Phthalates | NHANES 1999- 2002. Background exposure. | MEP associated with BMI in adult males and females. MBP inversely associated with BMI and WC in elderly, non- significant positive trend in males but inverse in females. MBzP positively associated with BMI and WC in adult males, not associated in females. MEHP inversely related to BMI and WC in adult females. MEHHP associated with adult males, not associated in females. | (145) | |
| Phthalates | NHANES 1999-2002 male adults | MBzP, MEHHP, MEOHP, and MEP associated with WC. | (370) | |
| Bisphenol A (BPA) and Bisphenol A diglycidyl ether (BADGE) | BPA | In CHIANTI Italian adults | BPA associated with WC and weight | (122) |
| BPA | NHANES 2003-2006 adults. | BPA associated with general and abdominal obesity. | (65) | |
| BPA | Cross-sectional study in Chinese adults. | BPA associated with general and abdominal obesity. | (420) | |
| BPA | NHANES 2003- 2004 adults. | BPA not associated with BMI. | (216) |
Prenatal and Early Exposure to POPs
Amidst the growing prevalence of obesity, rampantly rising rates of childhood obesity has emerged as a critical public health concern. Prevalence of childhood obesity worldwide has risen sharply since the 1980s, reaching 42 million in 2013 with estimates that this number will increase to 70 million infants and children in 2025 (305). The heightened concern over the childhood obesity crisis stems from both short-term and long-term effects on health and longevity. In the short term, obese children possess a higher risk for cardiovascular disease (120) and prediabetes (234). However, and perhaps even more frightening, is the association between childhood obesity and future risk of obesity (255) (36) (139), cardiovascular disease, type II diabetes, and greater morbidity and mortality (255) (36) (139) (285) (369). Together, these findings support the hypothesis of the fetal origins of adult disease, which proposes that the environment of the fetus can determine health and disease outcomes later in life (360).
Therefore, in accordance with the obesogen hypothesis, prenatal and early life exposure represents a critical window during which children are particularly vulnerable to both the short-term and long-term effects of POPs on obesity (134) (325) (290) (192). Furthermore, prenatal and early life contexts represent particularly vulnerable periods for environmental exposures. The fetus is sensitive to chemical exposures, which can cross the fetal-maternal blood barrier (175). This increased susceptibility continues into early life, as children have different levels and sources of exposure. Children can experience the effects of maternal exposure to POPs even after birth, as chemicals appear in breastmilk and represent a source of exposure during breastfeeding (31). Furthermore, children are not simply “tiny adults”; they differ not only in size but also in metabolism and physiological function, such as higher caloric consumption consumed per kg body weight and higher minute ventilation (361) (74), which can put them at increased risk of exposure to environmental chemicals. We describe below evidence regarding exposures to specific POPs and the development of obesity and/or diabetes.
1. PCBs and Dioxins
The effect of prenatal PCBs on obesity is inconclusive. Twelve of the 25 studies investigating prenatal PCBs found no association between prenatal and early exposure to PCBs and obesity (Table 7). However, of the 12 studies that did find an association between prenatal PCBs and obesity, 9 found that PCBs had an inverse association on measures of obesity, including birth weight (131, 151, 284, 308), growth rate (308), weight (174) (47) (176) (214), and BMI (104). Conversely, 5 studies found positive associations between prenatal and early PCB exposure and measures of obesity (104) (414) (383) (21) (408).
A factor that may complicate interpretation of these studies is differing effects of TCDD-like PCBs and non-TCDD-like PCBs, as well as different effects in males versus females. Two studies found differing effects between toxicants based on their similarity to TCDD within the same study population. In a study of early exposure in Flemish adolescents aged 14–15 years, serum TCDD-like PCBs were positively associated with increased BMI while non-TCDD-like PCBs were negatively associated with BMI in males and females (104). In a second study, which investigated prenatal PCBs and obesity in African American children of the National Collaborative Perinatal Project (NCPP), maternal levels of TCDD-like PCBs were negatively associated with girl’s weight and marginally positively associated with boy’s weight (214). Conversely, maternal levels of non-TCDD-like PCBs were not associated with weight (214). This study highlights a complexity in the relationship between prenatal PCBs and obesity: gender. Of the 14 studies that found some association between prenatal or early exposure to PCBs and obesity in study populations containing both males and females, 5 studies found different effects between males and females. For example, negative associations between PCB exposure and weight were reported in males with no effect in females (151) (174). Conversely, a positive association was reported between PCB exposures and obesity in females but not males (383) (408). Therefore, diverging effects of prenatal and early PCB exposure and obesity in males versus females may contribute to differences in findings between studies. In summary, study findings should consider gender of the population under study, as well as the type (e.g., TCDD-like) and/or individual congeners which are screened.
2. DDE and DDT
Fifteen of 24 studies that investigated DDE exposures found positive associations between prenatal and early exposure to DDE and measures of obesity including BMI (189) (402) (414) (265) (189) (95) (423), weight (174) (189) (127), overweight (21, 408), rapid growth (265) (407), and waist circumference (383, 402) (101, 423). Only one 4-year study on Russian boys aged 6–8 years found a negative association between DDE and BMI (58). Similar to PCBs and TCDD, there appear to be different effects between genders, as some studies found associations with obesity in females but not in males(95) (101) (408). Conversely, two studies found effects on BMI, waist circumference, and overweight and obesity at 9 years (423) and weight at 14 years (127) in males but not in females. Additionally, an association between prenatal DDE and BMI z-score and rapid growth that was seen in both sexes was stronger in boys (265). Therefore, the evidence supporting a link between prenatal DDE exposure and obesity is compelling despite potentially different effects between genders. By comparison, the effect of prenatal DDE on birth weight is less conclusive. One study showed an association between prenatal DDE and birth weight (248) while another showed no association with birth weight (131). Therefore, while there is a strong argument for exposure to prenatal DDE and obesity in later life, the effects of DDE on birth weight are inconclusive and might be weakly associated with decreased birth weight.
Five studies investigating prenatal exposure to DDT and obesity outcomes were analyzed. Two studies in childhood and adolescent males found no association between prenatal DDT exposure and BMI in boys (126) (423). However, another study in 6.5-year-old children found a positive association between prenatal DDT and overweight in males but not in females (408). In a study of 7.5-year-old children of both genders, a nonsignificant association was found between maternal DDT and BMI and increased odds of overweight and obesity (422). Lastly, prenatal DDT was associated with decreased birth weight (248). Therefore, prenatal exposure to DDT may decrease birth weight while being weakly associated with overweight in childhood, particularly among boys.
3. Hexachlorobenzene (HCB), β-Hexachlorocyclohexane (HCH), and OC pesticides
Of the 11 studies which investigated the role of prenatal or early exposure to HCB and obesity, 5 found positive associations with HCB exposure and obesity. Four of the 5 studies found association between prenatal and maternal exposure to HCB and parameters of obesity including BMI (402) (362) (21), rapid growth (405) (407), and increased overweight and obesity (362) (21) (407). One study investigating early exposure to HCB and obesity found that in 7 year olds, HCB exposure was associated with increased BMI and overweight. However, a study investigating early exposure to HCB in 8–22 year olds found no association between HCB exposure and obesity (384). Furthermore, there were 4 studies that found no association between prenatal HCB and BMI (265) (90), obesity (90), birth weight (248), or waist circumference (101). One study found that exposure to HCB in 14–15 year olds decreased BMI. Studies appear to support an association between prenatal HCB exposure and rapid growth and increased BMI and overweight, particularly in childhood. However, the relationship between HCB exposure and obesity in adolescents is inconclusive.
Three studies investigating the effect of prenatal exposure to HCH failed to support an association between HCH and BMI (265) (90), obesity (90), or birth weight (248). However, conflicting associations were found when studying early exposure to HCH and obesity. There was an inverse association between β-HCH exposure and BMI in Russian boys aged 8–9 over a 4-year study (58). Conversely, Spanish children of both genders exhibited positive associations between β-HCH and BMI and increased overweight at 7 years (21). These conflicting findings might be due to gender, as boys exhibit a negative association between β-HCH and obesity while a positive association was seen when children of both sexes were studied, of which 51.3% were female. Furthermore, the two populations might differ in dose, type, or duration of exposure, as the two studies differed in which chemicals were included in their screening in addition to β-HCH.
A study investigating OC pesticides found no association between prenatal trans-nonachlor and oxychlordane and obesity or BMI at 7 years (90). However, the same study did find a positive association between prenatal dieldrin exposure and obesity but no association with BMI.
4. Polybrominated flame retardants (BFRs)
As compared to the organochlorines, fewer studies have investigated the prenatal or early exposure effects of BFRs on obesity, and the results are inconsistent. Of the 5 studies analyzed for the effects of BFRs, two found positive associations while two studies found negative associations between BFRs and obesity. A study in pregnant Long-Evans hooded rats found that prenatal dosing with PBDE-99 increased rat offspring birth weight (238). Furthermore, studies in humans have found the prenatal PBB exposure (above 5 ppb) was associated with increased weight for height in females (47). Conversely, studies in Mexican-Americans from the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) found negative associations between maternal PBDE levels and BMI, waist circumference, and birth weight (113) (144). However, the association seen between PBDE and lower birth weight became nonsignificant when maternal weight gain was included in the analysis (144).
5. Polycyclic Aromatic Hydrocarbons (PAHs)
Maternal and early exposure to PAHs appears to have contrasting effects on birth weight and childhood obesity. Several studies have shown that maternal dietary (215) (180) and airborne PAH (215) exposure as well as PAH-DNA adduct levels in newborns (314) (315) are associated with reduced birth weight. Conversely, prenatal and early exposure to PAHs is positively associated with increased BMI, obesity, and waist circumference in childhood (197) (335) (346). Therefore, it appears that prenatal exposure to PAHs may reduce birth weight but increase the risk of childhood obesity.
6. Phthalate Esters
The findings of the relationship between prenatal and early exposure to phthalates and obesity is inconsistent and complicated by different effects between the two sexes and also between low molecular weight (LMW) and high molecular weight (HMW) phthalate metabolites. LMW metabolites, including MnBP, MEP, and MiBP, have been associated with childhood obesity (77) (145) (388) (59) (48). Conversely, HMW metabolites have been associated with lower weight gain and lower BMI z-scores in boys but higher BMI in girls (406).
7. Bisphenol A (BPA)
The effects of BPA seem to vary depending if the exposure was prenatal or early in life. Prenatal exposure to BPA has been shown to be negatively associated with BMI, adiposity, and percent body fat in young girls (403) (143) as well as associated with decreased birth weight (269). On the other hand, early exposure to BPA in ages 4–15 has been associated with increased BMI, obesity, and waist circumference (403) (143) (405) (417) (394); however, one study did show a negative association between BPA levels and BMI in 6–9 year old girls (428). Therefore, prenatal exposure to BPA seems to decrease the risk of obesity later in life while postnatal early exposure in childhood seems to increase the risk of childhood obesity.
Adult Exposure to POPs
In addition to the growing burden of obesity in children, more than 1.9 billion adults (39%) age 18 years and older were overweight in 2014, of which over 600 million (13%) were obese (306). Because adults experience a longer duration and type of exposure to POPs, they are presented separately from prenatal exposure. Furthermore, because maternal levels of POPs can determine offspring health, adult women of the childbearing age are of particular interest in terms of obesity outcomes.
1. PCBs and Dioxins
The studies of PCBs and dioxins and obesity are, by far, the most complex of the POPs. Complications when studying PCBs and potential effects on obesity arise from different effects between congeners and genders and non-linear and inverted U-shaped associations. While dioxins have consistently shown positive associations with BMI (224), waist circumference (223), fat mass (332), and metabolic syndrome (399), PCBs have shown positive, negative, or null associations between studies. These consistencies appear to be due to different effects between congeners and between genders.
TCDD-like PCBs have been positively associated with BMI (312) (128) (104). However, the non-TCDD-like PCBs have produced more inconsistent associations. For example, non-TCDD-like PCBs have been both negatively (224) (106) (104) (128) and positively (312) (223) associated with BMI and waist circumference. Furthermore, less-chlorinated PCBs have been positively associated with weight gain (241) and fat mass, while highly-chlorinated PCBs have been negatively associated with weight gain (241) and fat mass . Furthermore, several studies have reported conflicting associations with PCBs and obesity within the same study that varied by congener (226) (332). When PCBs were grouped together, positive associations were found with an increased risk of becoming obese (108), BMI (218), and waist circumference (223). Furthermore, in a study of obese adults without diabetes, adipose levels of PCBs were positively associated with weight, BMI, waist circumference, and visceral adipose tissue (107).
Additionally, studies have found different effects of PCBs between genders. In the PIVUS study by Lee et al. on older adults, women had positive associations between waist circumference and the PCB congeners 74, 99, 118, 138, 153, and 156, but negative associations with congeners 105 and 126 (226). Similar inconsistencies in waist circumference were present in men, with PCBs 156, 157, 169, 180, 189, and 209 positively associated and PCBs 74, 99, 106, 118, 126, 138, 153, 170, 194, and 206 negatively associated with waist circumference. As discussed previously, study results must be considered with attention to the specific congeners studied and the gender of the study population. Lastly, the effects of PCBs also vary by dose, with several studies reporting nonmonotonic and inverted U-shaped associations (226) (230) (96) (333) (223), indicating that lower doses of PCBs might produce greater effects on obesity development than higher doses.
2. DDE and DDT
Of the fourteen studies that measured DDE in regards to obesity outcomes, thirteen found positive associations and only one study found no association. Therefore, DDE has consistently been positively associated with BMI (230) (224) (107) (104) (218) (128) (164) (312) (343), waist circumference (223) (107) (226), and visceral and subcutaneous adipose tissue (107). Only two studies found sex differences in the relationship between DDE exposure and obesity. In a study of the NHANES 1999-2002 data set, DDE was positively associated with waist circumference in women but negatively associated in men (110). On the other hand, in the PIVUS study of older adults, Lee et al. found that DDE was associated with waist circumference in males but not in females (226). Therefore, the literature supports a positive association between DDE and obesity, although gender differences may exist.
Fewer studies have investigated a relationship between DDT and obesity. In the Coronary Artery Risk Development in Young Adults (CARDIA), a prospective study in young adults without diabetes, DDT positively predicted BMI (230). On the other hand, in a cross-sectional study on Canadian males, DDT was not associated with BMI (312). However, these inconsistent results may be a result of gender differences, as a cross-sectional study on the 1999–2002 NHANES dataset found that DDT was positively associated with waist circumference in females, but negatively associated in males (110).
3. HCB, β-HCH, and OC pesticides
The effects of HCB, β-HCH, and the remaining organochlorine pesticides, such as oxychlordane, trans-nonachlor, mirex, and aldrin, are less clear than the strongly positive associations of DDE with obesity. However, the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study in older adults found that the sum of OC pesticides was positively associated with weight gain (241). Of these pesticides, studies on HCB and β-HCH tend to support positive associations with obesity. HCB has been positively associated with BMI (104) (218) (128) (312), waist circumference (226) and fat mass (332) (230). Similarly, β-HCH has been positively associated with BMI (312) (106, 164), metabolic syndrome (391), waist circumference (106), and fat mass (106). The Chlordane constituents oxychlordane and trans-nonachlor have been positively associated with BMI (164), waist circumference (226), and visceral and subcutaneous adipose tissue (96). However, several studies have also shown no association between chlordane and measures of obesity (230) (224) (164). The other OC pesticides have not been studied as extensively; however, three studies have reported no association between mirex and BMI (230) (164) (312). On the other hand, aldrin has been positively associated with metabolic syndrome (MetS) (391).
4. BFRs
Two studies have investigated PBBs and PBDEs levels in adults, and both supported positive associations between BFR exposure and obesity. In a study of the 2003-2004 NHANES data set, PBB-153 was nonlinearly associated with MetS and WC; furthermore, PBDE-153 exhibited an inverted U-shaped association with MetS (239). These results indicate that potential associations between BFRs and obesity in adults may be complicated by nonmonotonic dose response curves. Additionally, a second study in pregnant women found that milk levels of PBDEs were associated with the mom’s BMI (91). Therefore, not only were PBDE levels associated with maternal BMI, but these levels in milk also represent a source of early life exposure to BFRs in offspring, which represents a potential factor in health outcomes later in life.
5. PAHs
One study investigated urinary PAH metabolites and obesity; however, the results were inconsistent and varied between metabolites (324). While urinary 2-phenanthrene was positively associated with obesity, 1-naphthalene was negatively associated with obesity in NHANES adults (324). Additionally, 2-naphthalene, 1-phenanthrene and 2-phenanthrene were positively associated with MetS. Furthermore, studies on smoking cigarettes, which can be a source of exposure to PAHs, have found positive associations with central obesity (75).
6. Phthalate Esters
As in prenatal exposure, the relationship between phthalates and adult obesity is complicated by gender, age, and differences between the groups of phthalate metabolites; however, studies generally support a positive association between phthalate exposure and adult obesity. In a study of the 1999–2002 NHANES data set, although females had higher urinary levels of phthalate metabolites, the strongest positive associations with BMI and WC were found in 20–59-year old males with MBzP, MEHHP, and MEOHP (145). For LMW metabolites, MEP was positively associated with BMI in adult males (20–59 and 60–80 years old) and for adolescent and adult (20–59 years old) females; however, no association was found in adolescent males and an inverse relationship was found in older females (145). Furthermore, while MBP showed inverse trends with BMI and WC in 60–80 year olds and female adults (20–59 years old), positive trends existed in 20–59-year old males (145). Furthermore, while the high molecular weight metabolite MBzP had a positive association with BMI and WC of 20–59-year old males, no associations were seen in females (145). Of urinary DEHP metabolites, MEHP was inversely associated with BMI and WC in adolescent and adult females, but no associations were found in males. On the other hand, MEHHP was positively associated with BMI in males, but no associations were found in females (145). Again, apparent inconsistencies between studies on phthalates and obesity might be explained by the particular metabolites studied and the age and gender of study participants.
7. BPA
Few studies have investigated relationships between BPA and obesity. Three studies support positive associations between BPA exposure and abdominal obesity (65) (420) as well as WC (122).
Diabetes
The global prevalence of diabetes is staggering; the number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014, corresponding to an increase from 4.7% to 8.5% of adults over 18 years of age (304). Type 1 diabetes (T1D) is an autoimmune disease characterized by insulin deficiency as a result of beta-cell failure. On the other hand, Type 2 diabetes (T2D) is characterized by insulin resistance and includes an alarming number of children and adolescents (424).
There has been a growing interest in the environmental contribution to the etiology of diabetes and obesity. In 2011, the US National Toxicology Program (NTP) and the National Institute of Environmental Health Sciences (NIEHS) conducted a workshop titled “Role of Environmental Chemicals in the Development of Diabetes and Obesity” to investigate the science studying POPs and their association to these two diseases. The workshop concluded that there is a strong argument for a role of POPs in the etiology of diabetes and obesity (390) (386). The importance of studying the influence of environmental pollutants on the development of these diseases has been acknowledged by the National Institutes of Health (NIH) (146), the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) (83) (24), and the White House Task Force on Childhood Obesity (40).
The ubiquitous exposure to low and chronic levels of POPs presents a complicating factor in linking these toxicants to diabetes, as there is no true reference population with zero exposure to POPs. Moreover, the majority of studies that have attempted to link POP exposures to diabetes have focused on background levels within the general population, as opposed to occupational exposures. Background level POP exposures within the general population are present as mixtures of chemicals. Therefore, findings from studies investigating individual POPS must be analyzed in reference to not only the other POPs studied, but also the total mixture of chemicals to which the population is exposed. Two prospective studies have investigated the effect of POPs as mixtures on diabetes incidence, and both studies found that exposure to POP mixtures is associated with a 3–5 time higher risk of developing T2D (227) (229). In the Coronary Artery Risk Development in Young Adults (CARDIA) study, an inverted U-shaped association between exposure to POP mixtures and diabetes was found in young adults (229). The inverted U-shaped association indicates a nonmonotonic dose-response relationship between POPs and diabetes. Similarly, in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study, an association between POP exposure and T2D in the elderly was found (227). However, while this association suggested nonlinearity (227), it was not a clear inverted U-shaped association as in the CARDIA study.
1. PCBs and Dioxins
High-dose, toxic levels of chemical exposure have been the focus of both animal and human studies to investigate associations between exposures and effects on human health. For example, early findings on the human effects of TCDD were studied in populations with occupational and accidental exposure, such as U.S. Air Force veterans exposed to Agent Orange in Vietnam (150) (200) (187) and workers and residents exposed to TCDD following accidents or spills at chemical plants (43) (437) (371). Unfortunately, these early findings were inconsistent, as positive (150) (200) (187), inverse (371) (437), and null associations (381) (413) were observed between TCDD and T2D.
The findings from the Air Force Study on Vietnam War veterans of Operation Ranch Hand exposed to Agent Orange highlight the perplexing relationship between POPs and T2D. Compared to those not exposed to TCDD-contaminated Agent Orange, US Air Force veterans exposed to Agent Orange had glucose abnormalities and a higher risk of T2D (150). However, a follow-up study excluded veterans exposed to Agent Orange and only considered veterans who did not come into contact with TCDD-contaminated herbicides and, therefore, had exposure levels consistent with background levels in the United States (247). In this second study, the dose-response relationships between TCDD and T2D were surprisingly stronger in veterans with background levels of exposure as compared to high occupational exposure to TCDD, indicating that not only do POPs have low level effects on T2D, but also that these associations might be unexpectedly stronger at lower, background exposure levels (247).
This puzzling association between lower doses of TCDD and T2D was reinforced by a study of occupational exposure following a chemical plant accident in Italy (43). Of residents living in high-, medium, and low-exposure areas surrounding the accident, there was higher T2D mortality in the medium-exposure area as compared to residents from the high-exposure area (43). Furthermore, in occupational exposure studies on individuals with very high levels of TCDD exposure, no associations were found between TCDD exposure and T2D (61) (381) (437). In contrast, recent studies in heavily contaminated areas, such as Superfund sites, demonstrate associations between serum TCDD and PCB levels and T2D (357) (411) (401), as well as elevated glucose (218) (86). While it remains debatable whether direct associations exist between exposure levels and T2D, reports demonstrating that lower level POP exposures are associated with diabetes are alarming, as these levels can be found in most individuals, and certainly in the obese population. A summary of POPs and their links to T2D are presented in Table 9.
Table 9.
POP Exposures and Associations with Diabetes
| Class | Compound(s) Studied | Population | Finding | Reference |
|---|---|---|---|---|
|
| ||||
| Organochlorines | OC pesticides, PCBS, PBB | CARDIA young adults without diabetes, age 27.2+/−3.3yrs old, BMI 29.1+/− 6.7 kg/m2. Background exposure. | p,p’-DDE and PCBs predicted HOMA-IR. | (230) |
|
| ||||
| PCDDs, PCDFs, dioxin- like PCBs, non- dioxin-like PCBs, OC pesticides | NHANES 1999-2002. Adults without diabetes. Background exposure. | Oxychlordane, trans-nonachlor, PCB170, PCB187 strongly associated with higher HOMA-IR. | (222) | |
|
|
||||
| PCDDs, PCDFs, dioxin- like PCBs, non- dioxin-like PCBs, OC pesticides | NHANES 1999-2002. Adults without diabetes. Background exposure. |
OC pesticides and PCBs associated with high fasting glucose | (223) | |
|
|
||||
| PCB, OC pesticides, DDE, Dioxins | NHANES 1999-2002. Adults with background exposure. |
PCB-153, trans-nonachlor, oxychlordane, DDE, OCDD and PCDD 73 strongly associated with T2D prevalence. TCDD unrelated to diabetes prevalence. | (224) | |
|
|
||||
| PCB, OC pesticides, dioxin, HCB, DDE, BDE | Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Older adults, background exposure. Cross-sectional and prospective | PCBs, sum of OC pesticides, and trans-nonachlor associated with incident T2D. Dioxin not associated with incident diabetes. | (227) | |
|
|
||||
| PCB, PCDD, PCDF, OC pesticides | NHANES 1999-2002 Adults with background exposure. |
Dioxin-like PCBs and OC pesticides associated
with diabetes. PCDDs and non-dioxin-like PCBs were not associated with diabetes. PCDFs were weakly associated with diabetes. |
(225). | |
|
|
||||
| PCB, HxCDD, DDT | NHANES 1999-2002. Adults with background exposure. |
Serum 1, 2, 3, 4, 6, 7, 8-PCDD, PCB 126, DDT levels associated with diabetes. | (114) | |
|
|
||||
| OC pesticides, DDE, DTT | HHANES 1982-1984: Mexican-American Adults with background exposure. |
Serum trans-nonachlor, β- hexachlorocyclohexane, oxychlordane, and highest exposure to DDT and DDE associated diabetes. Trans-nonachlor and β- hexachlorocyclohexane associated with elevated serum glucose. | (85) | |
|
|
||||
| PCB, DDE, OC pesticides, HCB | Mohawk Nation at Akwesasne adults | Serum PCB, DDE, HCB associated with diabetes. Mirex negatively associated with diabetes. | (80) | |
|
|
||||
| PCB, DDE, | Great Lakes Consortium for the | DDE and dioxin-like mono-ortho | (398) | |
|
|
||||
| PBDE, BDE | Health Assessment of Great Lakes Sport Fish Consumption adults. | PCBs associated with diabetes. Non- dioxin like PCBs and PBDEs were not associated with diabetes. | ||
|
|
||||
| OC pesticides, PCBs, PDBE, PBB | CARDIA(Coronary Artery Risk Development in
Young Adults) 18- 30yrs old in US without diabetes. Prospective study, background exposure. |
Low dose trans-nonachlor, oxychlordane, mirex, highly chlorinated PCBs, and PBB 153 associated with increased risk diabetes incidence. | (229) | |
|
|
||||
| PCBs, DDE | Obese adults without diabetes. Background exposure. | Serum PCBs associated with fasting glucose and abnormal GTT, while negatively associated with HOMA-B. Serum PCB180 negative associated with fasting insulin. Adipose PCBs associated with HbA1C and fasting glucose. Serum and adipose DDE associated with glucose levels during GTT and abnormal GTT. | (107) | |
|
|
||||
| HCH, HCB, OC pesticides, TCDD, DDE/DDT | Obese adults undergoing bariatric surgery in Portugal. Background exposure. | Adipose total POPs associated with HOMA-IR and dysglycemia. Adipose methoxychlor associated with glycemia, HbA1c. Adipose DDE associated with glucose metabolism and HbA1c. | (317) | |
|
|
||||
| PCB, DDT, DDE, HCB | Nurse’s Health Study: female adults in US. Background exposure. Prospective study | Plasma HCB positively associated with incident T2D. PCBs not significantly associated with increased T2D risk. | (429) | |
|
|
||||
| Oxychlordane, trans-nonachlor, p,p’-DDE, PCB 153, BDE 153 | Adults in Finland. Background exposure. | Oxychlordane, trans-nonachlor, p-p’-DDE, and PCB 153 positively associated with prevalent T2D. | (24) | |
|
|
||||
| PCB, DDE | Elderly adults in Faroese Islands. Background exposure | Elderly adults with T2D had higher PCB levels. In nondiabetics, increased PCB levels associated with increased fasting glucose and decreased fasting insulin. | (132) | |
|
|
||||
| PCBs, PCDDs, PCDFs | Healthy adults in Japan. Background exposure. | Accumulated toxic equivalents (TEQs) of PCDDs, PCDFs, dioxin-like PCBs, and total dioxins associated with HbA1c. | (400) | |
|
|
||||
| HCB, DDE | Swedish fishermen and their wives. | PCB-153 and p,p’-DDE associated with diabetes prevalence. | (337) | |
|
High levels of exposure | ||||
| TCDD | Healthy adults without diabetes around Superfund site in Arkansas | TCDD associated with higher plasma insulin at fasting and 30, 60, and 120 min during GTT | (86) | |
|
|
||||
| PCBs, DDE, OC pesticides | Anniston Community Health Survey adults near PCB- contaminated area. | PCB associated with diabetes. When sex-stratified, PCBs had a positive association in women and an inverse association in men. DDE associated with diabetes prevalence in women. | (357) | |
|
|
||||
| PCB, PBB | Michigan PBB cohort exposed to contaminated food, prospective study. | PCB associated with increased incidence of diabetes in women. | (411) | |
|
|
||||
| PCBs, DDE, HCB | Adults in highly polluted Eastern Slovakia. | Circulating PCBs, DDE, HCB correlated with fasting glucose and seruminsulin. | (218) | |
|
|
||||
| OC pesticides | Agricultural Health Study: pesticide applicators and spouses in US without diabetes. Occupational exposure | Having ever used and cumulative use of aldrin, chlordane, and heptachlor increased odds of diabetes incidence. | (276) | |
|
|
||||
| PCB, DDE, DDT, HCB, β-HCH | PCBRISK cross- sectional survey of heavily polluted area of Eastern Slovakia | PCBs, DDE, DDT, HCB, and β-HCH associated with prediabetes, but only PCB, DDT, and DDE associated with diabetes. | (401) | |
|
|
||||
| TCDD | US Air Force veterans of Operation Ranch Hand (Air Force Health Study) from Vietnam War 1961-1971. Occupational exposure | Agent Orange associated with higher risk of glucose abnormalities and T2D. | (150) | |
|
|
||||
| TCDD | US Air Force veterans of Operation Ranch Hand (Air Force Health Study) from Vietnam War 1961-1971. Occupational exposure. | Dose-response relationship between TCDD-contaminated Agent Orange and T2D in veterans with background levels of exposure. | (247) | |
|
|
||||
| TCDD | Residents surrounding Seveso, Italy, industrial accident. | Residents living in medium-exposure areas to TCDD had higher T2D mortality as compared to high-exposure areas. | (43) | |
|
Maternal/PrenatalExposure | ||||
| PCB and DDE | Swedish mothers and their children. Background exposure. | Nonsignificant trend of maternal exposure to PCB-135 or DDE and decreased T1D development in the offspring. | (326) | |
|
|
||||
| PCBs | Collaborative Perinatal Project (CPP): mothers with and without diabetes and their children | Maternal exposure to PCBs monotonically associated with T1D in offspring. | (246) | |
|
|
||||
| DDE, PCB, HCB, HCH, Organophospha te pesticides | PELAGIE cohort of pregnant women in Brittany | Prenatal exposure to DDE was associated with a decrease in insulin in girls but not boys. Prenatal exposure PCB153 was associated with decreased insulin. | (100) | |
|
|
||||
| PCBs, DDE, HCB | Adults frompolluted area of Slovakia whose mothers experienced high level s of exposure. | Maternal exposure to PCBs associated with impaired fasting glucose. | (217) | |
|
| ||||
| Polybrominated flame retardants (BFRs) | PBBs and PBDEs | NHANES 2003-2004, adults, BMI 28.3+/- 5.9kg/m2 | PBB-153 and PBDE-153 positively associated
with prevalent diabetes. PBB-153 associated with glycemia. PBDE-99 and PBDE-100 nonsignificant positive association with diabetes. PBDE-28 and -47 not associated with diabetes. |
(239) |
|
|
||||
| PCB, DDE, PBDE, BDE | Great Lakes Consortium for the Health Assessment of Great Lakes Sport Fish Consumption adults. | PBDEs not associated with diabetes. | (398) | |
|
|
||||
| OC pesticides, PCBs, PDBE, PBB | CARDIA(Coronary Artery Risk Development in Young Adults) 18-30y olds in US without diabetes. Prospective study, background exposure. | Low dose PBB153 associated with increased risk of diabetes incidence. | (229) | |
|
|
||||
| PCB, OC pesticides, dioxin, HCB, DDE, BDE | Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Older adults, background exposure. Cross-sectional and prospective | BDE not associated with T2D prevalence or risk of T2D incidence. | (227) | |
|
|
||||
| PCB, PBB | Michigan PBB cohort exposed to contaminated food, prospective study. | PBB not a risk factor for diabetes incidence. | (411) | |
|
|
||||
| Oxychlordane, trans-nonachlor, p,p’-DDE, PCB 153, BDE 47, BDE 153 | Adults in Finland. Background exposure. | BDE 47 and BDE 153 not associated with T2D. | (24) | |
|
| ||||
| Polycyclic Aromatic Hydrocarbons (PAHs) | PAHs | NHANES merged 2001-2006. Adults, background exposure. | Urinary PAH biomarkers positively associated with diabetes. 0 | (28) |
| PAHs | Chinese adults, background exposure. | Urinary PAH metabolites had dose-response association to increased risk of diabetes. | (432) | |
|
|
||||
| 8 PAH metabolites | NHANES 2001-2008 adults. Background exposure. | 1-naphthalene, 2-naphthalene, 2- phenanthrene and 1-pyrene associated with T2D. | (324) | |
|
| ||||
| Phthalate Esters | BPA and phthalate metabolites | Nurses’ Health Study (NHS) and NHSII female adults. Background exposure. | In NHSII- urine butyl phthalates (MBP and MiBP) and total phthalate metabolites positively associated with incident T2D. In older NHS cohort, no significant association between phthalates and incident T2D. | (376) |
| Phthalates | NHANES 2001-2008 adults without diabetes. Background exposure. | Urinary MnBP, MiBP, MCPP, and DEHP (sum of MEHP, MEHHP, MEOHP) positively associated with fasting blood glucose, fasting insulin, and HOMA-IR | (163) | |
|
|
||||
| Phthalates | ElderlyKorean adults. | Sum of DEHP metabolites (sum of MEHHP and MEOHP) associated with HOMA. No association between MnBP and HOMA. | (199) | |
|
|
||||
| Phthalates and BPA | NHANES 2003-2008 adolescents (12-19yrs old). Background exposure. | DEHP metabolites (MEHP, MECPP, MEHHP, and MEOHP) associated with HOMA-IR and insulin resistance. Lower molecular weight phthalates (MEP, MBP, MiBP, MBP) (found in cosmetics and personal use items) not associated with HOMA- IR or insulin resistance. | (395) | |
|
|
||||
| Phthalates | Healthy Mexican women. Background exposure. | Higher levels of DEHP, MEHHP, MEOHP, and MECPP are positively associated with diabetes. MBzP negatively associated with diabetes. | (378) | |
|
|
||||
| Phthalates | NHANES 1999-2002 | MBP, MBzP, and MEP associated with increased HOMA, but only MBzP and MEP remained significant after adjustment for renal and hepatic function. | (370) | |
|
| ||||
| Bisphenol A and Bisphenol A diglycidyl ether (BADGE) | BPA | National Health Examination Survey of Thai
adults. Background exposure. |
Serum BPA positively associated with diabetes and impaired fasting glucose. | (20) |
|
|
||||
| BPA | Adults in Iran. Background exposure. | Urine BPA positively associated with diabetes prevalence and HbA1c. | (23) | |
|
|
||||
| BPA | Adults in Korea. Background exposure. | Urine BPA not significantly associated with T2D. | (201) | |
|
|
||||
| BPA | NHANES 2003-2008 adults. Background exposure. | Urine BPA positively associated with diabetes. | (349) | |
|
|
||||
| BPA and phthalate metabolites | Nurses’ Health Study (NHS) and NHSII female adults. Background exposure. | In NHSII- urine BPA positively associated with incident T2D. In older NHS cohort, no significant associations between BPA and incident T2D. | (376) | |
|
|
||||
| BPA | NHANES 2003-2004 adults. | BPA associated with diabetes (adjusted for BMI, WC). | (216) | |
|
|
||||
| BPA | NHANES 2005-2006 adults. | BPA associated with diabetes, but association lost in fully adjusted models (age, sex, race, education, income, smoking, BMI, WC, urinary creatinine). | (263) | |
Williamson, JR, Lacy, PE. Structural aspects of adipose tissue: A summary attempting to synthesize the information contained in the preceding chapters. Compr Physiol 2011, Supplement 15: Handbook of Physiology, Adipose Tissue: 201-210. First published in print 1965. doi: 10.1002/cphy.cp050120
Jeanrenaud, B. Lipid components of adipose tissue. Compr Physiol 2011, Supplement 15: Handbook of Physiology, Adipose Tissue: 169-176. First published in print 1965. doi: 10.1002/cphy.cp050115
Napolitano, L. The fine structure of adipose tissues. Compr Physiol 2011, Supplement 15: Handbook of Physiology, Adipose Tissue: 109-124. First published in print 1965. doi: 10.1002/cphy.cp050112
Chalmers, TM. Lipid-mobilizing activity during fasting. Compr Physiol 2011, Supplement 15: Handbook of Physiology, Adipose Tissue: 549-555. First published in print 1965. doi: 10.1002/cphy.cp050156
Street, JC, Sharma, RP. Accumulation and Release of Chemicals by Adipose Tissue. Compr Physiol 2011, Supplement 26: Handbook of Physiology, Reactions to Environmental Agents: 483-493. First published in print 1977. doi: 10.1002/cphy.cp090130
Scow, RO. Perfusion of isolated adipose tissue: FFA release and blood flow in rat parametrial fat body. Compr Physiol 2011, Supplement 15: Handbook of Physiology, Adipose Tissue: 437-453. First published in print 1965. doi: 10.1002/cphy.cp050145
2. DDE and DDT
In adults with both background and occupational exposure, DDE and DDT have been linked with diabetes and related phenotypes. For adults with higher than background exposure levels to environmental contaminants, two studies investigating a heavily polluted area of Eastern Slovakia found associations between DDE and DDT and diabetes (218) (401). In one study, DDE and DDT were associated with both prediabetes and diabetes in these heavily exposed Slovakian adults (401). In a second study in this Slovakian population, DDE correlated with fasting glucose and serum insulin (218). Furthermore, DDE was also found to have a positive association with diabetes prevalence in women in the Anniston Superfund site (357). In studies in adults with background levels of exposure to POPs, DDE has been associated with T2D prevalence (224) (80) (398) (24) (337) (85), glucose abnormalities (107), and HOMA-IR (230). Additionally, studies have found associations between DDT and diabetes (114) (85).
Of particular interest are two studies performed in obese adults with background exposure who underwent bariatric surgery, which allowed for the researchers to obtain fat depots and evaluate associations between adipose levels of DDE and diabetes (107) (316). Both serum and adipose levels of DDE were associated with glucose levels and an abnormal glucose tolerance test (GTT) (107). In a second study by Pestana et al., (316), not only were total POPs in adipose tissue associated with HOMA-IR and dysglycemia, but adipose levels of DDE were associated with glucose metabolism and HbA1c. Similar findings across studies, as well as associations between both serum and AT levels to diabetes, support potential contributions of DDE exposures to the development of diabetes.
3. HCB, HCH, and OC pesticides
HCB, HCH, and the OC pesticides, such as oxychlordane, heptachlor, trans-nonachlor, and aldrin, have consistently shown positive associations with diabetes. When grouped together as OC pesticides, studies have found associations with high fasting glucose (223), diabetes prevalence (225), and incident T2D (227). When investigated individually, studies of these pesticides have largely indicated positive associations between exposure levels and diabetes. HCH has been associated with prediabetes (401), diabetes (85), and elevated serum glucose (85), while HCB was associated with diabetes prevalence (80) (337) and incidence (429). Chlordane and its constituents and metabolites heptachlor, trans-nonachlor, and oxychlordane have found positive associations with diabetes. Of the chlordane constituents, trans-nonachlor and oxychlordane have been the most heavily studied. These constituents have been positively associated with T2D prevalence (224) (85) (24), diabetes incidence (227) (229), and HOMA-IR (222). In contrast, while low doses of mirex were found to be associated with diabetes incidence in young adults of the Coronary Artery Risk Development in Young Adults (CARDIA) study (229), mirex was negatively associated with diabetes in adults of the Mohawk Nation at Akwesasne (80). Despite these inconsistent findings for mirex, which might be due to differences in study population and outcomes (diabetes prevalence versus diabetes incidence), OC pesticides appear to be consistently associated with diabetes.
4. BFRs
Literature on polybrominated flame retardants and diabetes are inconsistent, with studies showing either positive or no associations. Two main categories of BFRs have been investigated-PBDEs and PBBs. Studies on PBDEs have produced inconsistent findings in relationship to obesity, which may represent differing effects between PBDE congeners. In NHANES 2003-2004, PBDE-153 showed positive associations with diabetes prevalence and glycemia (239). In the same study, PBDE-99, PBDE-100, PBDE-28, and PBDE-47 were not significantly associated with diabetes. However, in a study of a cohort of sport fish consumers, the sum of PBDEs and the PBDE congeners PBDE-47 and PBDE-153 were not associated with diabetes (398). As described previously for other POP classes, individual congeners of PBDE may have some association with obesity and/or diabetes, but generalizations from mixed exposures cannot be assumed.
The findings for PBBs are similarly inconsistent. While PBB-153 was found to be positively associated with prevalent diabetes and glycemia in adults within NHANES 2003–2004 (239), prospective studies on PBB exposures and diabetes incidence have been inconsistent in their findings. In the CARDIA prospective study, low doses of PBB-153 were found to be associated with an increased risk of diabetes incidence (229); however, a prospective study of a Michigan cohort found no association between PBB and diabetes incidence (411).
5. PAHs
Although PAHs have not been as extensively studied as other environmental contaminants for their potential relationship to diabetes, the studies reviewed here show positive associations between PAH exposure and diabetes. In studies of merged 2001–2006 NHANES data, urinary PAH biomarkers, 1-naphthalene, 2-naphthalene, 2-phenanthrene, and 1-pyrene were associated with diabetes in adults (28) (324). Furthermore, in a study of Chinese adults, urinary PAH metabolites had a dose-response association with an increased risk of diabetes (432). Therefore, although the literature for PAH exposure and diabetes is not as extensive as other POPs, results support an association between PAHs and diabetes prevalence.
6. Phthalate Esters
While studies have often found positive associations between phthalates and diabetes, there are inconsistent findings that may be attributable to differing effects between phthalate metabolites, categorized as DEHP metabolites, low molecular weight metabolites, or high molecular weight metabolites. Urinary DEHP metabolites (MEHP, MECPP, MEHHP, MEOHP) have been positively associated with diabetes (378), HOMA-IR (395) (163), insulin resistance (395), and fasting levels of blood glucose and insulin (163). However, studies on LMW metabolites (MEP, MBP, MiBP, and MBP) have yielded inconsistent results. In an analysis on the Nurses’ Health Study (NHS) and NHSII female adults, MBP, MiBP, and total phthalate metabolites were positively associated with incident T2D in the younger NHSII cohort; however, no association was found between phthalates and incident T2D in the older NHS cohort (376). In data from NHANES 2001–2008 in adults without diabetes, MnBP and MiBP were positively associated with fasting blood levels of glucose and insulin and HOMA-IR (163). Furthermore, adult males in NHANES 1999–2002 had positive associations between MEP and HOMA (370). In contrast, results from studies in adults outside of NHANES have failed to show associations between LMW metabolites and diabetes. In NHANES adolescents and elderly Korean adults, no associations were found between LMW phthalates and HOMA-IR (201) (395). The high molecular weight metabolites (MBzP, MCPP) have yielded perhaps the most inconsistent results. While MECPP positively associated with fasting blood levels of glucose and insulin and HOMA-IR (163), MBzP has been both positively and negatively associated with diabetes. In 1999–2002 NHANES, MBzP was associated with increased HOMA in adult males (370). However, in a study of healthy Mexican women, MBzP was negatively associated with diabetes (378). Therefore, the effect of phthalates on diabetes may differ depending on the particular metabolite examined, but also depending on the gender of the exposed individual. 7. BPA. Results from studies examining BPA exposures have generally supported a positive association between both serum and urine BPA and diabetes incidence (376), diabetes prevalence (20) (23) (349) (216), HbA1c (23), and impaired fasting glucose (20). However, two studies found no association between BPA and diabetes (201) (376). Differences in the relationship between BPA and diabetes between these studies could potentially be explained by the age of the population or the amount or duration of exposure.
In summary, in general, evidence supports an association between POPs, obesity and diabetes, particularly among the extensively-studied organochlorines. The relationship between POPs and obesity and diabetes may be complicated by differing effects between individual congeners, between genders, and between ages. Age may add complexity to the study of the role of environmental pollutants on diabetes due to the two phases of diabetes pathogenesis: insulin resistance and beta cell dysfunction. The age at the time of study and the age of diabetes onset may determine the relative importance of each phase of pathogenesis, as age is associated with greater beta cell dysfunction and insulin secretory effects (68). The stage of diabetes pathogenesis of the individuals within a study may, therefore, result in distinctive overall associations with POPs and dose-response relationships. Furthermore, an interaction of age on the association between adiposity and diabetes risk has been suggested (45), which deserves particular attention considering the complexity that obesity adds to the study of diabetes.
The consistent findings of nonmonotonic and inverted U-shaped associations between POPs (particularly PCBs) and disease outcomes are of particular interest. As compared to a linear dose response, in which the sign of the slope (i.e. negative or positive) does not change, a nonmonotonic relationship is characterized by a dose response curve with a slope that changes sign over the range of concentrations studied. These nonmonotonic dose response relationships indicate that lower levels of POPs, such as those in background levels of the general population, may be more harmful in terms of the development of diabetes and obesity than high exposures experienced in chemical spills. An inverted U-shaped association indicates that the maximal effect (i.e. highest disease incidence or prevalence) is observed at intermediate concentrations of POPs and drops off at increasing doses. Inverted U-shaped associations could explain the seemingly conflicting results of studies investigating the effects of POPs on obesity and diabetes. Different studies may indeed investigate different portions of the U-shaped curve based on the range of exposure concentrations studied. For example, studies in a population with low exposures may observe the initial positive linear portion of the inverted U, while studies in populations with intermediate and high exposure concentrations could experience null or inverse associations. Furthermore, studies are complicated by the lack of a true reference population, as all individuals are exposed to some level of POPs. Therefore, low, chronic exposure to POPs may be more detrimental to health than previously thought. The effects at background levels of exposure may not be properly predicted by studies performed in populations with high, occupational exposure in POPs exhibiting nonmonotonic dose responses.
Potential mechanisms to explain the inverted U-shaped associations observed in studies of the effect of exposure to POPs on obesity and diabetes may stem from the effects these toxicants have as endocrine-disrupting chemicals, including cytotoxicity, cell-specific receptors, differences in receptor selectivity, receptor downregulation, desensitization, and receptor competition (409). Cytotoxicity, perhaps the simplest explanation for nonmonotonic responses, could explain how disease prevalence is increased at low concentrations, at which the toxicant can exert physiological effects, but disease prevalence drops off at higher concentrations at which the POP becomes acutely toxic (409). Furthermore, dose response to POPs is complicated by cell-specific receptors that can activate different pathways. Different receptors may have different responses to a POP, and a single cell might exhibit different responses at different concentrations of exposure; as such, these overlapping responses can create an nomonotonic association overall (409). Furthermore, a response to a particular POP may decrease at increasing concentrations due to receptor downregulation, degradation, and desensitization (409). Therefore, an inverted U-shape curve may result from a decreased number of receptors or a decreased receptor response at increasing concentrations of POPs. An additional factor that may contribute to nonmontonic associations between exposure levels and disease is the ability of some POPs to induce enzymes involved in their own metabolism and detoxification. Lastly, nonmonotonic responses can occur due to receptor competition, which may be particularly relevant given that exposure, particularly background levels of exposure, to POPs occurs as a mixture and not as a single toxicant.
Obesity contributes to the development of diabetes; furthermore, as previously discussed, increased consumption of fatty foods can increase the body burden of lipophilic POPs, and expanded adipose tissue in obesity serves as a reservoir of POPs. Just as obesity and weight loss can alter the balance between sequestration in fat and release of POPs into the bloodstream, diabetes development and progression may influence the circulating levels of POPs, which raises issues of potential reverse causality. Just as circulating POP levels are dictated not only by exposure, but also by the individual’s history of weight gain and loss, serum levels of POPs at the time of study may be influenced by the progression of diabetes, complicating any potential associations. Simply put, are higher serum levels of POPs contributing to the development and progression of diabetes or is diabetes pathogenesis altering the metabolism of POPs, leading to an increase in serum levels? These are important considerations to be made when analyzing the existing literature and when formulating new studies on POPs and health outcomes.
Conclusion
It is clear that POPs have physical characteristics that enable their bioaccumulation in adipose tissue, resulting in greater body burdens of a wide array of environmental toxicants with distinct mechanisms of action in the setting of expanded AT mass. It is also clear that accumulating evidence supports a role for various POPs in the development of obesity, and in obesity-associated conditions such as type 2 diabetes. Association of POPs with obesity and/or diabetes have indicated that low level exposures, as would be experienced by the majority of US citizens, may influence not only the development of diabetes in adults, but also exert gestational influences that influence the health of offspring. There are many unanswered questions that warrant further investigation. Below, we summarize several unresolved issues and questions of significance related to POPs, AT, and disease development.
Do POPs contribute to the development of obesity and diabetes?
In 2013, at a National Toxicology Program Workshop, an evaluation of the literature in terms of consistency, strengths and weaknesses of the clinical diagnosis, exposure assessment and study population characteristics was performed to evaluate the area of POP exposures and diabetes outcomes (382). While the authors found that there was insufficient evidence to conclude a positive association of some organochlorine POPs with type 2 diabetes, strongest positive correlations occurred for DDE, PCBs, TCDD and TCDD-like chemicals. Within the appendix of this analysis, the authors provide an extensive list of data gaps and research recommendations that if performed, would provide more definitive information related to POP exposures and type 2 diabetes. It is unclear if any of these issues have been resolved to move this field forward.
Is bioaccumulation of POPs in AT helpful or harmful?
The physical chemical properties of POPs result in their bioaccumulation within adipocyte lipids. When trapped in triacylglycerol lipid droplets, POPs are sequestered away from target effectors, suggesting that bioaccumulation in adipocyte lipids may minimize harmful effects of POPs. However, sequestered POPs dynamically equilibrate between adipocyte lipids and the intracellular/extracellular environment, most likely resulting in low level tonic stimulation of effectors. Moreover, given that the total body burdens of lipophilic POPs increase with their bioaccumulation in an expanded AT mass of obese subjects, and that many POPs exert inflammatory actions that could contribute to the development of insulin resistance, low level POP exposures may contribute to the development of diabetes and other inflammatory-related conditions. Alternatively, when lipids are mobilized from adipocyte stores, POPs also mobilize and can act at effector targets to negatively influence health. As an example, given that 66% of the adult population are overweight and/or obese and attempting to lose weight, lipolysis-mediated release of POPs may negatively influence AT (and other target organs), mitigating the positive health benefits of weight loss. Therefore, areas of additional investigation would be identification of therapies and/or approaches that mitigate the harmful effects of liberated POPs. Moreover, it would be informative to understand ramifications of rapid versus slow weight loss as a means of influencing the bioaccumulation, actions and elimination of POPs from the body.
What are the implications of mixtures of POPs and their influence on AT function?
Our body burden of POPs reflects mixtures we are exposed to through the environment. As discussed, mechanisms of POPs to influence AT function vary, and this is complicated by not only differences in mechanism of action between parent compounds within mixtures, but also influences of metabolic bi-products of POP metabolism. Moreover, even within a given class of POPs, interactions with target effectors may occur through the same binding site, or through allosteric mechanisms, suggesting additive and/or synergistic effects of POP mixtures at a given target effector. An advantage of experimental models is that they enable investigators to determine effects of individual POPs at exposure levels that hopefully mimic those experienced by humans. However, humans are exposed to and bioaccumulate a broad array of POPs that are further influenced by not only the level of adiposity, but also influenced by the regional deposition of AT. Thus, further studies are needed to define molecular interactions of mixtures at specific target effectors, integrated whole body responses to POP mixtures, and the influence of regional AT deposition on bioaccumulation and health-related effects of POPs.
Is biomedical remediation of POPs possible?
Considerable efforts are underway to remediate POPs from our environment. In contrast, aside from reducing environmental exposures, there are few avenues available to remediate POP burdens from humans experiencing chronic low level exposures, or to treat acutely exposed populations. This is alarming, as obesity prevalence continues to increase in children and adults, resulting in greater body burdens of POPs. Moreover, a preponderance of evidence suggests that prenatal POP exposures negatively influence the health of offspring, and an alarming number of child-bearing women are overweight and/or obese and would predictably have increased body burdens of POPs during gestation. Thus, biomedical remediation is needed not only to influence the health of the mother, but to minimize potential harmful effects of POPs on future generations. Additional studies should identify potential therapies, including lifestyle interventions and/or pharmacologic approaches, that can mitigate the harmful effects of liberated or tonically released POPs that decrease the bioaccumulation of POPs in AT lipids, or that hasten their elimination.
Are there variables that influence the bioaccumulation within and/or toxicity of POPs at AT?
Results from human exposure studies suggest that dose-response relationships (e.g., nonmonotonic dose-response), gender, and POP chemical class influence their bioaccumulation in AT, effector mechanisms, and toxicity. However, mechanisms for these relationships are not well defined. For example, it is unclear why some studies indicate more prevalent associations between POP exposures and obesity/diabetes in females compared to males, or vice versa. Additional studies are warranted at the experimental level to dissect potential mechanisms for these variables and their influence on POP toxicity.
In conclusion, physical/chemical characteristics of lipophilic POPs result in their bioaccumulation in AT, a site where these toxicants not only are sequestered away from effector targets, but also where they may exert actions that influence metabolism. Given that almost all humans harbor some level of POPs, further studies are warranted to define their contribution to diseases of increasing prevalence (obesity, diabetes), mechanisms of action, effects of POP mixtures, and the development of biomedical remediation therapies.
Acknowledgments
The authors wish to acknowledge support from the National Institute of Environmental Health Sciences (P42 ES007380-21) and the National Institute of Diabetes, Kidney and Digestive Diseases (T32 DK007778) of the National Institutes of Health.
References
- 1.Dioxin and Related Compounds. Springer International Publishing; 2016. p. XVI, 462. [Google Scholar]
- 2.Persistent Organic Pollutants. Springer US; 2001. p. IX, 272. [Google Scholar]
- 3.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Aldrin/Dieldrin. Atlanta, GA: 2002. [Google Scholar]
- 4.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for chlordane. Atlanta, GA: 1994. [Google Scholar]
- 5.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Chlorinated Dibenzo-p-dioxins (CDDs) Atlanta, GA: 1998. [Google Scholar]
- 6.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for DDT, DDE, and DDD. Atlanta, GA: 2002. [Google Scholar]
- 7.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Di(2-ethylhexyl) phthlate. Atlanta, GA: 2002. [Google Scholar]
- 8.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Endosulfan. Atlanta, GA: 2013. [Google Scholar]
- 9.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Endrin. Atlanta, GA: 1996. [Google Scholar]
- 10.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Heptachlor and Heptachlor Epoxide. Atlanta, GA: 2007. [Google Scholar]
- 11.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Hexachlorobenzene. Atlanta, GA: 2013. [Google Scholar]
- 12.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Methoxychlor. Atlanta, GA: 2002. [Google Scholar]
- 13.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Mirex and Chlordecone. Atlanta, GA: 1995. [Google Scholar]
- 14.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Polychlorinated Biphenyls (PCBs) Atlanta, GA: 2000. [Google Scholar]
- 15.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Toxaphene. Atlanta, GA: 2014. [Google Scholar]
- 16.U.S. Department of Health and Human Services PHS, editor. (ATSDR) AfTSaDR. Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs) Atlanta, GA: 1995. [Google Scholar]
- 17.(EPA) USEPA. Ambient Water Quality Criteria for Aldrin/Dieldrin. Washington, DC: Environmental Protection Agency; 1980. edited by 440.5-80-0191980 E. [Google Scholar]
- 18.(EPA) USEPA. Persistent Organic Pollutants: A Global Issue, A Global Response. https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response. [March 15, 2016]
- 19.Adolfsson-Erici M, Åkerman G, McLachlan MS. Measuring bioconcentration factors in fish using exposure to multiple chemicals and internal benchmarking to correct for growth dilution. Environ Toxicol Chem. 2012;31:1853–1860. doi: 10.1002/etc.1897. [DOI] [PubMed] [Google Scholar]
- 20.Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B. Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes. 2015;7:240–249. doi: 10.1111/1753-0407.12159. [DOI] [PubMed] [Google Scholar]
- 21.Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagana X, Robinson O, Casas M, Sunyer J, Vrijheid M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ Health Perspect. 2015;123:1030–1037. doi: 10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadkhaniha R, Mansouri M, Yunesian M, Omidfar K, Jeddi MZ, Larijani B, Mesdaghinia A, Rastkari N. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng. 2014;12:64. doi: 10.1186/2052-336X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care. 2011;34:1972–1979. doi: 10.2337/dc10-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alawi MA, Tamimi S, Jaghabir M. Storage of organochlorine pesticides in human adipose tissues of Jordanian males and females. Chemosphere. 1999;38:2865–2873. doi: 10.1016/s0045-6535(98)00488-3. [DOI] [PubMed] [Google Scholar]
- 26.Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 28.Alshaarawy O, Zhu M, Ducatman AM, Conway B, Andrew ME. Urinary polycyclic aromatic hydrocarbon biomarkers and diabetes mellitus. Occup Environ Med. 2014;71:437–441. doi: 10.1136/oemed-2013-101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez L, Randi A, Alvarez P, Kolliker Frers R, Kleiman de Pisarev DL. Effect of hexachlorobenzene on NADPH-generating lipogenic enzymes and L-glycerol-3-phosphate dehydrogenase in brown adipose tissue. J Endocrinol Invest. 1999;22:436–445. doi: 10.1007/BF03343587. [DOI] [PubMed] [Google Scholar]
- 30.Archibeque-Engle SL, Tessari JD, Winn DT, Keefe TJ, Nett TM, Zheng T. Comparison of organochlorine pesticide and polychlorinated biphenyl residues in human breast adipose tissue and serum. J Toxicol Environ Health. 1997;52:285–293. doi: 10.1080/00984109708984065. [DOI] [PubMed] [Google Scholar]
- 31.Arendt M. Communicating human biomonitoring results to ensure policy coherence with public health recommendations: analysing breastmilk whilst protecting, promoting and supporting breastfeeding. Environ Health. 2008;7(Suppl 1):S6. doi: 10.1186/1476-069X-7-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, Cimmino I, Longo M, Beguinot F, Formisano P, Valentino R. Low-Dose Bisphenol-A Impairs Adipogenesis and Generates Dysfunctional 3T3-L1 Adipocytes. PLoS One. 2016;11:e0150762. doi: 10.1371/journal.pone.0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arrebola JP, Pumarega J, Gasull M, Fernandez MF, Martin-Olmedo P, Molina-Molina JM, Fernández-Rodríguez M, Porta M, Olea N. Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ Res. 2013;122:31–37. doi: 10.1016/j.envres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 36.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker NA, English V, Sunkara M, Morris AJ, Pearson KJ, Cassis LA. Resveratrol protects against polychlorinated biphenyl-mediated impairment of glucose homeostasis in adipocytes. J Nutr Biochem. 2013;24:2168–2174. doi: 10.1016/j.jnutbio.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, Sunkara M, Morris AJ, Swanson HI, Cassis LA. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121:105–110. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker NA, Shoemaker R, English V, Larian N, Sunkara M, Morris AJ, Walker M, Yiannikouris F, Cassis LA. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ Health Perspect. 2015;123:944–950. doi: 10.1289/ehp.1408594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes M. White House Task Force on Childhood Obesity Report to the President. Washington, DC: 2010. Solving the problem of childhood obesity within a generation. [DOI] [PubMed] [Google Scholar]
- 41.Barrett JR. POPs vs. fat: persistent organic pollutant toxicity targets and is modulated by adipose tissue. Environ Health Perspect. 2013;121:a61. doi: 10.1289/ehp.121-a61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett GW, Ballee DL, Hall RC, Fahey JE, Butts WL, Osmun JV. Persistence and distribution of chlordane and dieldrin applied as termiticides. Bull Environ Contam Toxicol. 1974;11:64–69. doi: 10.1007/BF01685030. [DOI] [PubMed] [Google Scholar]
- 43.Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. The Seveso studies on early and long-term effects of dioxin exposure: a review. Environ Health Perspect. 1998;106(Suppl 2):625–633. doi: 10.1289/ehp.98106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biemann R, Navarrete Santos A, Navarrete Santos A, Riemann D, Knelangen J, Bluher M, Koch H, Fischer B. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem Biophys Res Commun. 2012;417:747–752. doi: 10.1016/j.bbrc.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Biggs ML, Mukamal KJ, Luchsinger JA, Ix JH, Carnethon MR, Newman AB, de Boer IH, Strotmeyer ES, Mozaffarian D, Siscovick DS. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA. 2010;303:2504–2512. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biljes D, Hammerschmidt-Kamper C, Kadow S, Diel P, Weigt C, Burkart V, Esser C. Impaired glucose and lipid metabolism in ageing aryl hydrocarbon receptor deficient mice. EXCLI J. 2015;14:1153–1163. doi: 10.17179/excli2015-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanck HM, Marcus M, Rubin C, Tolbert PE, Hertzberg VS, Henderson AK, Zhang RH. Growth in girls exposed in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology. 2002;13:205–210. doi: 10.1097/00001648-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, Juul A, Main KM. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118:1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodin J, Stene LC, Nygaard UC. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? Biomed Res Int. 2015;2015:208947. doi: 10.1155/2015/208947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bondy G, Armstrong C, Coady L, Doucet J, Robertson P, Feeley M, Barker M. Toxicity of the chlordane metabolite oxychlordane in female rats: clinical and histopathological changes. Food Chem Toxicol. 2003;41:291–301. doi: 10.1016/s0278-6915(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 51.Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 52.Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Boucher JG, Boudreau A, Atlas E. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr Diabetes. 2014;4:e102. doi: 10.1038/nutd.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourez S, Van den Daelen C, Le Lay S, Poupaert J, Larondelle Y, Thomé JP, Schneider YJ, Dugail I, Debier C. The dynamics of accumulation of PCBs in cultured adipocytes vary with the cell lipid content and the lipophilicity of the congener. Toxicol Lett. 2013;216:40–46. doi: 10.1016/j.toxlet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 55.Braun WH, Young JD, Blau GE, Gehring PJ. The pharmacokinetics and metabolism of pentachlorophenol in rats. Toxicol Appl Pharmacol. 1977;41:395–406. doi: 10.1016/0041-008x(77)90041-2. [DOI] [PubMed] [Google Scholar]
- 56.Brodie AE, Azarenko VA, Hu CY. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibition of fat cell differentiation. Toxicol Lett. 1996;84:55–59. doi: 10.1016/0378-4274(95)03537-0. [DOI] [PubMed] [Google Scholar]
- 57.Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, Pongratz I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc Natl Acad Sci U S A. 2003;100:6517–6522. doi: 10.1073/pnas.1136688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burns JS, Williams PL, Sergeyev O, Korrick SA, Lee MM, Revich B, Altshul L, Del Prato JT, Humblet O, Patterson DG, Turner WE, Starovoytov M, Hauser R. Serum concentrations of organochlorine pesticides and growth among Russian boys. Environ Health Perspect. 2012;120:303–308. doi: 10.1289/ehp.1103743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007-2010. Int J Hyg Environ Health. 2014;217:687–694. doi: 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butt CM, Berger U, Bossi R, Tomy GT. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ. 2010;408:2936–2965. doi: 10.1016/j.scitotenv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Calvert GM, Sweeney MH, Deddens J, Wall DK. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med. 1999;56:270–276. doi: 10.1136/oem.56.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diabetes. 2014;4:e115. doi: 10.1038/nutd.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canada Go. In: Screening Assessment Report. Environment Canada HC, editor. Canada: Government of Canada; 2013. [Google Scholar]
- 64.Cariou R, Antignac JP, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–1041. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 65.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res. 2011;111:825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cavender F, O’Donohue J. Phenols and Phenolics. In: Bingham E, John Cohrssen B, editors. Patty’s Toxicology. Wiley & Sons Inc; 2012. [Google Scholar]
- 67.Chamorro-Garcia R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ Health Perspect. 2012;120:984–989. doi: 10.1289/ehp.1205063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 69.Chapados NA, Casimiro C, Robidoux MA, Haman F, Batal M, Blais JM, Imbeault P. Increased proliferative effect of organochlorine compounds on human preadipocytes. Mol Cell Biochem. 2012;365:275–278. doi: 10.1007/s11010-012-1268-0. [DOI] [PubMed] [Google Scholar]
- 70.Chemicals NRCUSoF-R. Toxicological Risks of Selected Flame-Retardant Chemicals. Washington, DC: National Academic Press (US); 2000. [PubMed] [Google Scholar]
- 71.Chen CL, Brodie AE, Hu CY. CCAAT/enhancer-binding protein beta is not affected by tetrachlorodibenzo-p-dioxin (TCDD) inhibition of 3T3-L1 preadipocyte differentiation. Obes Res. 1997;5:146–152. doi: 10.1002/j.1550-8528.1997.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 72.Chevrier J, Dewailly E, Ayotte P, Mauriège P, Després JP, Tremblay A. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int J Obes Relat Metab Disord. 2000;24:1272–1278. doi: 10.1038/sj.ijo.0801380. [DOI] [PubMed] [Google Scholar]
- 73.Chiang HC, Kuo YT, Shen CC, Lin YH, Wang SL, Tsou TC. Mono(2-ethylhexyl)phthalate accumulation disturbs energy metabolism of fat cells. Arch Toxicol. 2016;90:589–601. doi: 10.1007/s00204-014-1446-9. [DOI] [PubMed] [Google Scholar]
- 74.Children CoPitDoIa. Pesticides in the Diets of Infants and Children. Washington (DC): 1993. [Google Scholar]
- 75.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 76.Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, Tsai WY, Perera FP. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi J, Eom J, Kim J, Lee S, Kim Y. Association between some endocrine-disrupting chemicals and childhood obesity in biological samples of young girls: a cross-sectional study. Environ Toxicol Pharmacol. 2014;38:51–57. doi: 10.1016/j.etap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Chu I, Villeneuve DC, Secours V, Franklin C, Rock G, Viau A. Metabolism and tissue distribution of mono-2-ethylhexyl phthalate in the rat. Drug Metab Dispos. 1978;6:146–149. [PubMed] [Google Scholar]
- 79.Cimafranca MA, Hanlon PR, Jefcoate CR. TCDD administration after the pro-adipogenic differentiation stimulus inhibits PPARgamma through a MEK-dependent process but less effectively suppresses adipogenesis. Toxicol Appl Pharmacol. 2004;196:156–168. doi: 10.1016/j.taap.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Codru N, Schymura MJ, Negoita S, Akwesasne Task Force on E. Rej R, Carpenter DO. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect. 2007;115:1442–1447. doi: 10.1289/ehp.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cok I, Donmez MK, Satiroğlu MH, Aydinuraz B, Henkelmann B, Shen H, Kotalik J, Schramm KW. Concentrations of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and dioxin-like PCBs in adipose tissue of infertile men. Arch Environ Contam Toxicol. 2008;55:143–152. doi: 10.1007/s00244-007-9094-1. [DOI] [PubMed] [Google Scholar]
- 82.Collins S. Overview of clinical perspectives and mechanisms of obesity. Birth Defects Res A Clin Mol Teratol. 2005;73:470–471. doi: 10.1002/bdra.20140. [DOI] [PubMed] [Google Scholar]
- 83.Committee DMIC. Advances and Emerging Opportunities in Diabetes Research: A Stragegic Planning Report of the Diabetes Mellitus Interagency Coordinating Committee. US Department of Health and Human Services, National Institutes of Health, Diabetes Mellitus Interagency Coordinating Committee; 2011. [Google Scholar]
- 84.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 2004;229:1127–1135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 85.Cox S, Niskar AS, Narayan KM, Marcus M. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: Hispanic health and nutrition examination survey, 1982–1984. Environ Health Perspect. 2007;115:1747–1752. doi: 10.1289/ehp.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cranmer M, Louie S, Kennedy RH, Kern PA, Fonseca VA. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol Sci. 2000;56:431–436. doi: 10.1093/toxsci/56.2.431. [DOI] [PubMed] [Google Scholar]
- 87.Crookes MJ, Howe PD. In: Environmental hazard assessment: halogenated naphthalenes. Department of the Environment BRE, Directorate for Air, Climate and Toxic Substances, Toxic Substances Division, editor. Garston, United Kingdom: 1993. [Google Scholar]
- 88.Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- 89.Cupul-Uicab LA, Hernandez-Avila M, Terrazas-Medina EA, Pennell ML, Longnecker MP. Prenatal exposure to the major DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and growth in boys from Mexico. Environ Res. 2010;110:595–603. doi: 10.1016/j.envres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cupul-Uicab LA, Klebanoff MA, Brock JW, Longnecker MP. Prenatal exposure to persistent organochlorines and childhood obesity in the US collaborative perinatal project. Environ Health Perspect. 2013;121:1103–1109. doi: 10.1289/ehp.1205901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daniels JL, Pan IJ, Jones R, Anderson S, Patterson DG, Jr, Needham LL, Sjodin A. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ Health Perspect. 2010;118:155–160. doi: 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis KE, M DN, Sun K, W MS, J DB, J AZ, Zeve D, L DH, D WC, L MG, Xu Y, Z VW, S AK, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Bruijn J, Busser F, Seinen W, Hermens J. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environmental Toxicology and Chemistry. 1989;8:499–512. [Google Scholar]
- 95.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors - a Dutch prospective cohort study. Int J Environ Res Public Health. 2014;11:7001–7021. doi: 10.3390/ijerph110707001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Roos AJ, Ulrich CM, Sjodin A, McTiernan A. Adiposity, body composition, and weight change in relation to organochlorine pollutant plasma concentrations. J Expo Sci Environ Epidemiol. 2012;22:617–624. doi: 10.1038/jes.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dearth MA, Hites RA. Complete analysis of technical chlordane using negative ionization mass spectrometry. Environmental Science and Technology. 1991;25:245–254. [Google Scholar]
- 98.Debier C, Chalon C, Le Boeuf BJ, de Tillesse T, Larondelle Y, Thomé JP. Mobilization of PCBs from blubber to blood in northern elephant seals (Mirounga angustirostris) during the post-weaning fast. Aquat Toxicol. 2006;80:149–157. doi: 10.1016/j.aquatox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Debier C, Crocker DE, Houser DS, Vanden Berghe M, Fowler M, Mignolet E, de Tillesse T, Rees JF, Thomé JP, Larondelle Y. Differential changes of fat-soluble vitamins and pollutants during lactation in northern elephant seal mother-pup pairs. Comp Biochem Physiol A Mol Integr Physiol. 2012;162:323–330. doi: 10.1016/j.cbpa.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Debost-Legrand A, Warembourg C, Massart C, Chevrier C, Bonvallot N, Monfort C, Rouget F, Bonnet F, Cordier S. Prenatal exposure to persistent organic pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ Res. 2016;146:207–217. doi: 10.1016/j.envres.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 101.Delvaux I, Van Cauwenberghe J, Den Hond E, Schoeters G, Govarts E, Nelen V, Baeyens W, Van Larebeke N, Sioen I. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ Res. 2014;132:24–32. doi: 10.1016/j.envres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 102.Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- 103.Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ Health Perspect. 1999;107:823–828. doi: 10.1289/ehp.99107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dhooge W, Den Hond E, Koppen G, Bruckers L, Nelen V, Van De Mieroop E, Bilau M, Croes K, Baeyens W, Schoeters G, Van Larebeke N. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int. 2010;36:330–337. doi: 10.1016/j.envint.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Dirinck E, Dirtu AC, Jorens PG, Malarvannan G, Covaci A, Van Gaal LF. Pivotal Role for the Visceral Fat Compartment in the Release of Persistent Organic Pollutants During Weight Loss. J Clin Endocrinol Metab. 2015;100:4463–4471. doi: 10.1210/jc.2015-2571. [DOI] [PubMed] [Google Scholar]
- 106.Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, Van Gaal L. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring) 2011;19:709–714. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- 107.Dirinck EL, Dirtu AC, Govindan M, Covaci A, Van Gaal LF, Jorens PG. Exposure to persistent organic pollutants: relationship with abnormal glucose metabolism and visceral adiposity. Diabetes Care. 2014;37:1951–1958. doi: 10.2337/dc13-2329. [DOI] [PubMed] [Google Scholar]
- 108.Donat-Vargas C, Gea A, Sayon-Orea C, Carlos S, Martinez-Gonzalez MA, Bes-Rastrollo M. Association between dietary intakes of PCBs and the risk of obesity: the SUN project. J Epidemiol Community Health. 2014;68:834–841. doi: 10.1136/jech-2013-203752. [DOI] [PubMed] [Google Scholar]
- 109.Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes. 2008;15:403–408. doi: 10.1097/MED.0b013e32830ce95c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elobeid MA, Padilla MA, Brock DW, Ruden DM, Allison DB. Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999–2002 data. Int J Environ Res Public Health. 2010;7:2988–3005. doi: 10.3390/ijerph7072988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Epstein SS. Kepone–hazard evaluation. Sci Total Environ. 1978;9:1–62. doi: 10.1016/0048-9697(78)90002-5. [DOI] [PubMed] [Google Scholar]
- 112.Ericson I, van Bavel B, Lindström G. Screening of persistent halogenated compounds in human adipose tissue and blood from Sweden. Man-Technology-Environment (MTM) Research Centre, Örebro University; SE-701 82 Örebro, Sweden: 2008. [Google Scholar]
- 113.Erkin-Cakmak A, Harley KG, Chevrier J, Bradman A, Kogut K, Huen K, Eskenazi B. In utero and childhood polybrominated diphenyl ether exposures and body mass at age 7 years: the CHAMACOS study. Environ Health Perspect. 2015;123:636–642. doi: 10.1289/ehp.1408417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Everett CJ, Frithsen IL, Diaz VA, Koopman RJ, Simpson WM, Jr, Mainous AG., 3rd Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2007;103:413–418. doi: 10.1016/j.envres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 115.Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 116.Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007;24:259–264. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 117.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 118.Fox K, Zauke GP, Butte W. Kinetics of bioconcentration and clearance of 28 polychlorinated biphenyl congeners in zebrafish (Brachydanio rerio) Ecotoxicol Environ Saf. 1994;28:99–109. doi: 10.1006/eesa.1994.1038. [DOI] [PubMed] [Google Scholar]
- 119.Frank R, Rasper J, Smout MS, Braun HE. Organochlorine Residues in Adipose Tissues, Blood and Milk from Ontario Residents, 1976–1985. Canadian Journal of Public Health. 1988;79:150–158. [PubMed] [Google Scholar]
- 120.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17 e12. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 121.Gadupudi G, Gourronc FA, Ludewig G, Robertson LW, Klingelhutz AJ. PCB126 inhibits adipogenesis of human preadipocytes. Toxicol In Vitro. 2015;29:132–141. doi: 10.1016/j.tiv.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gauthier MS, Rabasa-Lhoret R, Prud’homme D, Karelis AD, Geng D, van Bavel B, Ruzzin J. The metabolically healthy but obese phenotype is associated with lower plasma levels of persistent organic pollutants as compared to the metabolically abnormal obese phenotype. J Clin Endocrinol Metab. 2014;99:E1061–1066. doi: 10.1210/jc.2013-3935. [DOI] [PubMed] [Google Scholar]
- 124.Geyer HJ, Rimkus GG, Scheunert I, Kaune A, Schramm K-W, Kettrup A, Zeeman M, Muir DCG, Hansen LG, Mackay D. Bioaccumulation-New Aspects and Developments. Springer; Berlin Heidelberg: 2000. Bioaccumulation and Occurrence of Endocrine-Disrupting Chemicals (EDCs), Persistent Organic Pollutants (POPs), and Other Organic Compounds in Fish and Other Organisms Including Humans; pp. 1–166. [Google Scholar]
- 125.Giesy JP, Kannan K. Perfluorochemical surfactants in the environment. Environ Sci Technol. 2002;36:146A–152A. doi: 10.1021/es022253t. [DOI] [PubMed] [Google Scholar]
- 126.Gladen BC, Klebanoff MA, Hediger ML, Katz SH, Barr DB, Davis MD, Longnecker MP. Prenatal DDT exposure in relation to anthropometric and pubertal measures in adolescent males. Environ Health Perspect. 2004;112:1761–1767. doi: 10.1289/ehp.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gladen BC, Ragan NB, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136:490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- 128.Glynn AW, Granath F, Aune M, Atuma S, Darnerud PO, Bjerselius R, Vainio H, Weiderpass E. Organochlorines in Swedish women: determinants of serum concentrations. Environ Health Perspect. 2003;111:349–355. doi: 10.1289/ehp.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gobas FAPC, Clark KE, Shiu WY, MacKay D. Bioconcentration of polybrominated benzenes and biphenyls and related superhydrophobic chemicals in fish: Role of bioavailability and elimination into the feces. Environmental Toxicology and Chemistry. 1989;8:231–245. [Google Scholar]
- 130.Gómez-Catalán J, To-Figueras J, Rodamilans M, Corbella J. Transport of organochlorine residues in the rat and human blood. Arch Environ Contam Toxicol. 1991;20:61–66. doi: 10.1007/BF01065329. [DOI] [PubMed] [Google Scholar]
- 131.Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, Chevrier C, Eggesbo M, Guxens M, Kramer U, Legler J, Martinez D, Palkovicova L, Patelarou E, Ranft U, Rautio A, Petersen MS, Slama R, Stigum H, Toft G, Trnovec T, Vandentorren S, Weihe P, Kuperus NW, Wilhelm M, Wittsiepe J, Bonde JP, Obelix, and Enrieco Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect. 2012;120:162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grandjean P, Henriksen JE, Choi AL, Petersen MS, Dalgard C, Nielsen F, Weihe P. Marine food pollutants as a risk factor for hypoinsulinemia and type 2 diabetes. Epidemiology. 2011;22:410–417. doi: 10.1097/EDE.0b013e318212fab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grimm HG, Schellmann B, Schaller KH, Gossler K. [Pentachlorophenol concentrations in tissues and body fluids of normal persons (author’s transl)] Zentralbl Bakteriol Mikrobiol Hyg B. 1981;174:77–90. [PubMed] [Google Scholar]
- 134.Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304:19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 136.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 137.Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environ Health Perspect. 2007;115:1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 139.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 141.Hansch C, Leo A, Hoekman D. Exploring QSAR-Hydrophobic, Electronic, and Steric Constants. American Chemical Society; 1995. p. 557. [Google Scholar]
- 142.Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012;32:619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121:514–520. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harley KG, Chevrier J, Aguilar Schall R, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174:885–892. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Health UDo, Services H, and Health NIo. Strategic plan for NIH obesity research. 2013. (A report of the NIH Obesity Research Task Force (NIH publication no. 11-5493) 2011). [Google Scholar]
- 147.Hedgeman E, Chen Q, Hong B, Chang CW, Olson K, Ladronka K, Ward B, Adriaens P, Demond A, Gillespie BW, Lepkowski J, Franzblau A, Garabrant DH. The University of Michigan Dioxin Exposure Study: population survey results and serum concentrations for polychlorinated dioxins, furans, and biphenyls. Environ Health Perspect. 2009;117:811–817. doi: 10.1289/ehp.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol. 2009;304:90–96. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 149.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8:252–258. doi: 10.1097/00001648-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 151.Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology. 2005;16:648–656. doi: 10.1097/01.ede.0000173043.85834.f3. [DOI] [PubMed] [Google Scholar]
- 152.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 153.Hirai Y, Tomokuni K. Levels of chlordane, oxychlordane, and nonachlor in human adipose tissues. Bull Environ Contam Toxicol. 1991;47:173–176. doi: 10.1007/BF01688636. [DOI] [PubMed] [Google Scholar]
- 154.Hong NS, Kim KS, Lee IK, Lind PM, Lind L, Jacobs DR, Lee DH. The association between obesity and mortality in the elderly differs by serum concentrations of persistent organic pollutants: a possible explanation for the obesity paradox. Int J Obes (Lond) 2012;36:1170–1175. doi: 10.1038/ijo.2011.187. [DOI] [PubMed] [Google Scholar]
- 155.Hoppe AA, Carey GB. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring) 2007;15:2942–2950. doi: 10.1038/oby.2007.351. [DOI] [PubMed] [Google Scholar]
- 156.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Houlahan KE, Prokopec SD, Sun RX, Moffat ID, Linden J, Lensu S, Okey AB, Pohjanvirta R, Boutros PC. Transcriptional profiling of rat white adipose tissue response to 2,3,7,8-tetrachlorodibenzo-rho-dioxin. Toxicol Appl Pharmacol. 2015;288:223–231. doi: 10.1016/j.taap.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 158.Howard P, Meylan W. Handbook of physical properties of organic chemicals. Lewis Publishers; 1997. [Google Scholar]
- 159.Howard PH, editor. Handbook of environmental fate and exposure data for organic chemicals. Chelsea, MI: Lewis Publishers Inc; 1991. pp. 502–504. [Google Scholar]
- 160.Howell G, 3rd, Mangum L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol In Vitro. 2011;25:394–402. doi: 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hsu HF, Tsou TC, Chao HR, Kuo YT, Tsai FY, Yeh SC. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J Hazard Mater. 2010;182:649–655. doi: 10.1016/j.jhazmat.2010.06.081. [DOI] [PubMed] [Google Scholar]
- 162.Hu H, Xu F, Li B, Cao J, Dawson R, Tao S. Prediction of the Bioconcentration Factor of PCBs in Fish Using the Molecular Connectivity Index and Fragment Constant Models. Water Environment Research. 2005;77:87–97. doi: 10.2175/106143005x41663. [DOI] [PubMed] [Google Scholar]
- 163.Huang T, Saxena AR, Isganaitis E, James-Todd T. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environ Health. 2014;13:6. doi: 10.1186/1476-069X-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hue O, Marcotte J, Berrigan F, Simoneau M, Dore J, Marceau P, Marceau S, Tremblay A, Teasdale N. Plasma concentration of organochlorine compounds is associated with age and not obesity. Chemosphere. 2007;67:1463–1467. doi: 10.1016/j.chemosphere.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 165.Huff JE, Gerstner HB. Kepone: a literature summary. J Environ Pathol Toxicol. 1978;1:377–395. [PubMed] [Google Scholar]
- 166.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 168.Information NCfB. Compound Summary for CID 6618 (Tetrabromobisphenol A) PubChem Compound Database. 2005 [Google Scholar]
- 169.Information NCfB. Compound Summary for CID 8343 PubChem Compound Database. 2004 [Google Scholar]
- 170.Information NCfB. Compound Summary for CID 20393 PubChem Compound Database. 2004 [Google Scholar]
- 171.Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52:94–99. doi: 10.1111/j.1442-200X.2009.02883.x. [DOI] [PubMed] [Google Scholar]
- 172.Irigaray P, Lacomme S, Mejean L, Belpomme D. Ex vivo study of incorporation into adipocytes and lipolysis-inhibition effect of polycyclic aromatic hydrocarbons. Toxicol Lett. 2009;187:35–39. doi: 10.1016/j.toxlet.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 173.Irigaray P, Ogier V, Jacquenet S, Notet V, Sibille P, Mejean L, Bihain BE, Yen FT. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice. A novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006;273:1362–1372. doi: 10.1111/j.1742-4658.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 174.Iszatt N, Stigum H, Verner MA, White RA, Govarts E, Murinova LP, Schoeters G, Trnovec T, Legler J, Pele F, Botton J, Chevrier C, Wittsiepe J, Ranft U, Vandentorren S, Kasper-Sonnenberg M, Klumper C, Weisglas-Kuperus N, Polder A, Eggesbo M, Obelix Prenatal and Postnatal Exposure to Persistent Organic Pollutants and Infant Growth: A Pooled Analysis of Seven European Birth Cohorts. Environ Health Perspect. 2015;123:730–736. doi: 10.1289/ehp.1308005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 176.Jacobson JL, Jacobson SW, Humphrey HE. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol Teratol. 1990;12:319–326. doi: 10.1016/0892-0362(90)90050-m. [DOI] [PubMed] [Google Scholar]
- 177.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jan J. Chlorobenzene residues in human fat and milk. Bull Environ Contam Toxicol. 1983;30:595–599. doi: 10.1007/BF01610180. [DOI] [PubMed] [Google Scholar]
- 179.Jarosova A, Harazim J, Suchy P, Kratka L, Stancova V. The distribution and accumulation of phthalates in the organs and tissues of chicks after the administration of feedstuffs with different phthalate concentrations. Veterinarni Medicina. 2009;54:427–434. [Google Scholar]
- 180.Jedrychowski W, Perera FP, Tang D, Stigter L, Mroz E, Flak E, Spengler J, Budzyn-Mrozek D, Kaim I, Jacek R. Impact of barbecued meat consumed in pregnancy on birth outcomes accounting for personal prenatal exposure to airborne polycyclic aromatic hydrocarbons: Birth cohort study in Poland. Nutrition. 2012;28:372–377. doi: 10.1016/j.nut.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jimenez Torres M, Campoy Folgoso C, Cañabate Reche F, Rivas Velasco A, Cerrillo Garcia I, Mariscal Arcas M, Olea-Serrano F. Organochlorine pesticides in serum and adipose tissue of pregnant women in Southern Spain giving birth by cesarean section. Sci Total Environ. 2006;372:32–38. doi: 10.1016/j.scitotenv.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 182.Johnson-Restrepo B, Adams DH, Kannan K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 2008;70:1935–1944. doi: 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 183.Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ Sci Technol. 2005;39:5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
- 184.Jorgenson JL. Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ Health Perspect. 2001;109(Suppl 1):113–139. doi: 10.1289/ehp.01109s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kamstra JH, Hruba E, Blumberg B, Janesick A, Mandrup S, Hamers T, Legler J. Transcriptional and epigenetic mechanisms underlying enhanced in vitro adipocyte differentiation by the brominated flame retardant BDE-47. Environ Sci Technol. 2014;48:4110–4119. doi: 10.1021/es405524b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol. 2005;67:766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- 187.Kang HK, Dalager NA, Needham LL, Patterson DG, Jr, Lees PS, Yates K, Matanoski GM. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49:875–884. doi: 10.1002/ajim.20385. [DOI] [PubMed] [Google Scholar]
- 188.Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, Aldoust KM. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 189.Karmaus W, Osuch JR, Eneli I, Mudd LM, Zhang J, Mikucki D, Haan P, Davis S. Maternal levels of dichlorodiphenyl-dichloroethylene (DDE) may increase weight and body mass index in adult female offspring. Occup Environ Med. 2009;66:143–149. doi: 10.1136/oem.2008.041921. [DOI] [PubMed] [Google Scholar]
- 190.Kedderis LB, Mills JJ, Andersen ME, Birnbaum LS. A physiologically based pharmacokinetic model for 2,3,7,8-tetrabromodibenzo-p-dioxin (TBDD) in the rat: tissue distribution and CYP1A induction. Toxicol Appl Pharmacol. 1993;121:87–98. doi: 10.1006/taap.1993.1132. [DOI] [PubMed] [Google Scholar]
- 191.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 192.Kelishadi R, Poursafa P, Jamshidi F. Role of environmental chemicals in obesity: a systematic review on the current evidence. J Environ Public Health. 2013;2013:896789. doi: 10.1155/2013/896789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FA. Food web-specific biomagnification of persistent organic pollutants. Science. 2007;317:236–239. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- 194.Kerley-Hamilton JS, Trask HW, Ridley CJ, Dufour E, Ringelberg CS, Nurinova N, Wong D, Moodie KL, Shipman SL, Moore JH, Korc M, Shworak NW, Tomlinson CR. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Environ Health Perspect. 2012;120:1252–1259. doi: 10.1289/ehp.1205003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kern PA, Dicker-Brown A, Said ST, Kennedy R, Fonseca VA. The stimulation of tumor necrosis factor and inhibition of glucose transport and lipoprotein lipase in adipose cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Metabolism. 2002;51:65–68. doi: 10.1053/meta.2002.28088. [DOI] [PubMed] [Google Scholar]
- 196.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 197.Kim HW, Kam S, Lee DH. Synergistic interaction between polycyclic aromatic hydrocarbons and environmental tobacco smoke on the risk of obesity in children and adolescents: The U.S. National Health and Nutrition Examination Survey 2003–2008. Environ Res. 2014;135:354–360. doi: 10.1016/j.envres.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 198.Kim HW, Song WJ, Li Q, Han SM, Jeon KO, Park SC, Ryu MO, Chae HK, Kyeong K, Youn HY. Canine adipose tissue-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating T cells in rats. J Vet Sci. 2016 doi: 10.4142/jvs.2016.17.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim JH, Park HY, Bae S, Lim YH, Hong YC. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One. 2013;8:e71392. doi: 10.1371/journal.pone.0071392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim JS, Lim HS, Cho SI, Cheong HK, Lim MK. Impact of Agent Orange exposure among Korean Vietnam veterans. Ind Health. 2003;41:149–157. doi: 10.2486/indhealth.41.149. [DOI] [PubMed] [Google Scholar]
- 201.Kim K, Park H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int J Hyg Environ Health. 2013;216:467–471. doi: 10.1016/j.ijheh.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 202.Kim MJ, Marchand P, Henegar C, Antignac JP, Alili R, Poitou C, Bouillot JL, Basdevant A, Le Bizec B, Barouki R, Clément K. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clement K, Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clément K, Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kohan AB, Vandersall AE, Yang Q, Xu M, Jandacek RJ, Tso P. The transport of DDT from chylomicrons to adipocytes does not mimic triacylglycerol transport. Biochim Biophys Acta. 2013;1831:300–305. doi: 10.1016/j.bbalip.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kohlmeier L, Kohlmeier M. Adipose tissue as a medium for epidemiologic exposure assessment. Environ Health Perspect. 1995;103(Suppl 3):99–106. doi: 10.1289/ehp.95103s399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Korbel L, Spencer JD. Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the United States. Journal of diabetes and its complications. 2015;29:192–195. doi: 10.1016/j.jdiacomp.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kramer HJ, Drenth H, vandenBerg M, Seinen W, DeJongh J. Physiologically based pharmacokinetic model for tetrachlorobenzyltoluenes in rat: comparison of in vitro and in vivo metabolic rates. Toxicol Sci. 2001;63:22–28. doi: 10.1093/toxsci/63.1.22. [DOI] [PubMed] [Google Scholar]
- 209.Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- 210.Kumar KS, Kannan K, Paramasivan ON, Shanmuga Sundaram VP, Nakanishi J, Masunaga S. Polychlorinated dibenzo-p-dioxins, dibenzofurans, and polychlorinated biphenyls in human tissues, meat, fish, and wildlife samples from India. Environ Sci Technol. 2001;35:3448–3455. doi: 10.1021/es010555+. [DOI] [PubMed] [Google Scholar]
- 211.Kuramochi H, Kawamoto K, Miyazaki K, Nagahama K, Maeda K, Li XW, Shibata E, Nakamura T, Sakai S. Determination of physicochemical properties of tetrabromobisphenol A. Environ Toxicol Chem. 2008;27:2413–2418. doi: 10.1897/07-472.1. [DOI] [PubMed] [Google Scholar]
- 212.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78:22–48. doi: 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Birnbaum LS, Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121:162–169. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lamb MR, Taylor S, Liu X, Wolff MS, Borrell L, Matte TD, Susser ES, Factor-Litvak P. Prenatal exposure to polychlorinated biphenyls and postnatal growth: a structural analysis. Environ Health Perspect. 2006;114:779–785. doi: 10.1289/ehp.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lamichhane DK, Leem JH, Kim HC, Lee JY, Park MS, Jung DY, Ko JK, Ha M, Kim Y, Hong YC, Ha EH. Impact of prenatal exposure to polycyclic aromatic hydrocarbons from maternal diet on birth outcomes: a birth cohort study in Korea. Public Health Nutr. 2016;19:2562–2571. doi: 10.1017/S1368980016000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 217.Langer P, Kocan A, Tajtakova M, Koska J, Radikova Z, Ksinantova L, Imrich R, Huckova M, Drobna B, Gasperikova D, Sebokova E, Klimes I. Increased thyroid volume, prevalence of thyroid antibodies and impaired fasting glucose in young adults from organochlorine cocktail polluted area: outcome of transgenerational transmission? Chemosphere. 2008;73:1145–1150. doi: 10.1016/j.chemosphere.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 218.Langer P, Ukropec J, Kocan A, Drobna B, Radikova Z, Huckova M, Imrich R, Gasperikova D, Klimes I, Trnovec T. Obesogenic and diabetogenic impact of high organochlorine levels (HCB, p,p′-DDE, PCBs) on inhabitants in the highly polluted Eastern Slovakia. Endocr Regul. 2014;48:17–24. doi: 10.4149/endo_2014_01_17. [DOI] [PubMed] [Google Scholar]
- 219.Lanting CI, Huisman M, Muskiet FA, van der Paauw CG, Essed CE, Boersma ER. Polychlorinated biphenyls in adipose tissue, liver, and brain from nine stillborns of varying gestational ages. Pediatr Res. 1998;44:222–225. doi: 10.1203/00006450-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 220.le Maire A, Bourguet W, Balaguer P. A structural view of nuclear hormone receptor: endocrine disruptor interactions. Cell Mol Life Sci. 2010;67:1219–1237. doi: 10.1007/s00018-009-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, Bourguet W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009;10:367–373. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 223.Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007;50:1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- 224.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 225.Lee DH, Lee IK, Steffes M, Jacobs DR., Jr Extended analyses of the association between serum concentrations of persistent organic pollutants and diabetes. Diabetes Care. 2007;30:1596–1598. doi: 10.2337/dc07-0072. [DOI] [PubMed] [Google Scholar]
- 226.Lee DH, Lind L, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Int. 2012;40:170–178. doi: 10.1016/j.envint.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 227.Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–1784. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee DH, Porta M, Jacobs DR, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118:1235–1242. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lerch S, Guidou C, Thome JP, Jurjanz S. Non-dioxin-like Polychlorinated Biphenyls (PCBs) and Chlordecone Release from Adipose Tissue to Blood in Response to Body Fat Mobilization in Ewe (Ovis aries) J Agric Food Chem. 2016;64:1212–1220. doi: 10.1021/acs.jafc.5b05817. [DOI] [PubMed] [Google Scholar]
- 232.Levitt DG. Quantitative relationship between the octanol/water partition coefficient and the diffusion limitation of the exchange between adipose and blood. BMC Clin Pharmacol. 2010;10:1. doi: 10.1186/1472-6904-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lewis RG, Sovocool GW. Identification of polybrominated biphenyls in the adipose tissues of the general population of the United States. J Anal Toxicol. 1982;6:196–198. doi: 10.1093/jat/6.4.196. [DOI] [PubMed] [Google Scholar]
- 234.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care. 2009;32:342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li W, Vogel CF, Matsumura F. Studies on the cell treatment conditions to elicit lipolytic responses from 3T3-L1 adipocytes to TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Biochem. 2007;102:389–402. doi: 10.1002/jcb.21303. [DOI] [PubMed] [Google Scholar]
- 236.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011;127:9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 238.Lilienthal H, Hack A, Roth-Harer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2, 4,4, 5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lim JS, Lee DH, Jacobs DR., Jr Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003-2004. Diabetes Care. 2008;31:1802–1807. doi: 10.2337/dc08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lim JS, Son HK, Park SK, Jacobs DR, Lee DH. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int J Obes (Lond) 2011;35:744–747. doi: 10.1038/ijo.2010.188. [DOI] [PubMed] [Google Scholar]
- 241.Lind PM, Lee DH, Jacobs DR, Salihovic S, van Bavel B, Wolff MS, Lind L. Circulating levels of persistent organic pollutants are related to retrospective assessment of life-time weight change. Chemosphere. 2013;90:998–1004. doi: 10.1016/j.chemosphere.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 242.Lindberg P, Sellström U, Häggberg L, de Wit CA. Higher brominated diphenyl ethers and hexabromocyclododecane found in eggs of peregrine falcons (Falco peregrinus) breeding in Sweden. Environ Sci Technol. 2004;38:93–96. doi: 10.1021/es034614q. [DOI] [PubMed] [Google Scholar]
- 243.Ljunggren SA, Helmfrid I, Salihovic S, van Bavel B, Wingren G, Lindahl M, Karlsson H. Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environ Int. 2014;65:93–99. doi: 10.1016/j.envint.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 244.Lobstein T, Frelut ML. Prevalence of overweight among children in Europe. Obes Rev. 2003;4:195–200. doi: 10.1046/j.1467-789x.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 245.Lohmann R, Breivik K, Dachs J, Muir D. Global fate of POPs: current and future research directions. Environ Pollut. 2007;150:150–165. doi: 10.1016/j.envpol.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 246.Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Collaborative Perinatal P Polychlorinated biphenyl serum levels in pregnant subjects with diabetes. Diabetes Care. 2001;24:1099–1101. doi: 10.2337/diacare.24.6.1099. [DOI] [PubMed] [Google Scholar]
- 247.Longnecker MP, Michalek JE. Serum dioxin level in relation to diabetes mellitus among Air Force veterans with background levels of exposure. Epidemiology. 2000;11:44–48. doi: 10.1097/00001648-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 248.Lopez-Espinosa MJ, Murcia M, Iniguez C, Vizcaino E, Llop S, Vioque J, Grimalt JO, Rebagliato M, Ballester F. Prenatal exposure to organochlorine compounds and birth size. Pediatrics. 2011;128:e127–134. doi: 10.1542/peds.2010-1951. [DOI] [PubMed] [Google Scholar]
- 249.Louis C, Covaci A, Crocker DE, Debier C. Lipophilicity of PCBs and fatty acids determines their mobilisation from blubber of weaned northern elephant seal pups. Sci Total Environ. 2016;541:599–602. doi: 10.1016/j.scitotenv.2015.09.094. [DOI] [PubMed] [Google Scholar]
- 250.Louis C, Dirtu AC, Stas M, Guiot Y, Malarvannan G, Das K, Costa DP, Crocker DE, Covaci A, Debier C. Mobilisation of lipophilic pollutants from blubber in northern elephant seal pups (Mirounga angustirostris) during the post-weaning fast. Environ Res. 2014;132:438–448. doi: 10.1016/j.envres.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 251.Ludwicki JK, Góralczyk K. Organochlorine pesticides and PCBs in human adipose tissues in Poland. Bull Environ Contam Toxicol. 1994;52:400–403. doi: 10.1007/BF00197828. [DOI] [PubMed] [Google Scholar]
- 252.Lundén A, Norén K. Polychlorinated naphthalenes and other organochlorine contaminants in Swedish human milk, 1972-1992. Arch Environ Contam Toxicol. 1998;34:414–423. doi: 10.1007/s002449900338. [DOI] [PubMed] [Google Scholar]
- 253.Mabey WR, Smith JH, Podoll RT, Johnson HL, Mill T, Chou T-W, Gates J, Waight Partridge I, Jaber H, Vandenberg D. In: Aquatic Fate Process Data for Organic Priority Pollutants. Standards. MaDSDOoWRa, editor. Washington, D.C.: Environmental Protection Agency; 1981. [Google Scholar]
- 254.MacGregor JA, Nixon WB. Hexabromocyclododecane (HBCD): Determination of n-Octanol/Water Partition Coefficient. Arlington, VA: Wildlife International LTD; 1997. Project No. 439C-104. [Google Scholar]
- 255.Maffeis C, Tato L. Long-term effects of childhood obesity on morbidity and mortality. Horm Res. 2001;55(Suppl 1):42–45. doi: 10.1159/000063462. [DOI] [PubMed] [Google Scholar]
- 256.Malarvannan G, Dirinck E, Dirtu AC, Pereira-Fernandes A, Neels H, Jorens PG, Gaal LV, Blust R, Covaci A. Distribution of persistent organic pollutants in two different fat compartments from obese individuals. Environ Int. 2013;55:33–42. doi: 10.1016/j.envint.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 257.Marette A. Molecular mechanisms of inflammation in obesity-linked insulin resistance. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S46–48. doi: 10.1038/sj.ijo.0802500. [DOI] [PubMed] [Google Scholar]
- 258.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 259.McConnachie PR, Zahalsky AC. Immunological consequences of exposure to pentachlorophenol. Arch Environ Health. 1991;46:249–253. doi: 10.1080/00039896.1991.9937456. [DOI] [PubMed] [Google Scholar]
- 260.McConnell R, Gilliland FD, Goran M, Allayee H, Hricko A, Mittelman S. Does near-roadway air pollution contribute to childhood obesity? Pediatr Obes. 2016;11:1–3. doi: 10.1111/ijpo.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.McDonald M. NTP technical report on the toxicity studies of Pentachlorobenzene in F344/N Rats and B6C3F1 Mice (Feed Studies) (CAS No. 608-93-5) Toxic Rep Ser. 1991;6:1–48. [PubMed] [Google Scholar]
- 262.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Menale C, Grandone A, Nicolucci C, Cirillo G, Crispi S, Di Sessa A, Marzuillo P, Rossi S, Mita DG, Perrone L, Diano N, Miraglia Del Giudice E. Bisphenol A is associated with insulin resistance and modulates adiponectin and resistin gene expression in obese children. Pediatr Obes. 2016 doi: 10.1111/ijpo.12154. [DOI] [PubMed] [Google Scholar]
- 265.Mendez MA, Garcia-Esteban R, Guxens M, Vrijheid M, Kogevinas M, Goni F, Fochs S, Sunyer J. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ Health Perspect. 2011;119:272–278. doi: 10.1289/ehp.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health affairs. 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 267.Mes J, Davies DJ, Turton D. Polychlorinated biphenyl and other chlorinated hydrocarbon residues in adipose tissue of Canadians. Bull Environ Contam Toxicol. 1982;28:97–104. doi: 10.1007/BF01608420. [DOI] [PubMed] [Google Scholar]
- 268.Meylan WM, Howard PH. Atom/fragment contribution method for estimating octanol-water partition coefficients. J Pharm Sci. 1995;84:83–92. doi: 10.1002/jps.2600840120. [DOI] [PubMed] [Google Scholar]
- 269.Miao M, Yuan W, Zhu G, He X, Li DK. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod Toxicol. 2011;32:64–68. doi: 10.1016/j.reprotox.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 270.Miceli JN, Nolan DC, Marks B, Hariharan M. Persistence of polybrominated biphenyls (PBB) in human post-mortem tissue. Environ Health Perspect. 1985;60:399–403. doi: 10.1289/ehp.8560399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Mitsuhashi K, Senmaru T, Fukuda T, Yamazaki M, Shinomiya K, Ueno M, Kinoshita S, Kitawaki J, Katsuyama M, Tsujikawa M, Obayashi H, Nakamura N, Fukui M. Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine. 2016;51:174–184. doi: 10.1007/s12020-015-0666-y. [DOI] [PubMed] [Google Scholar]
- 272.Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 273.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 274.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 275.Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 276.Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993-2003. Am J Epidemiol. 2008;167:1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Moon HB, Lee DH, Lee YS, Kannan K. Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere. 2012;86:485–490. doi: 10.1016/j.chemosphere.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 278.Moreno-Aliaga MJ, Matsumura F. Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p,p′-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem Pharmacol. 2002;63:997–1007. doi: 10.1016/s0006-2952(01)00933-9. [DOI] [PubMed] [Google Scholar]
- 279.Morgan DP, Roan CC, Paschal EH. Transport of DDT, DDE, and dieldrin in human blood. Bull Environ Contam Toxicol. 1972;8:321–326. doi: 10.1007/BF01684768. [DOI] [PubMed] [Google Scholar]
- 280.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 281.Morris S, Allchin CR, Zegers BN, Haftka JJ, Boon JP, Belpaire C, Leonards PE, Van Leeuwen SP, De Boer J. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ Sci Technol. 2004;38:5497–5504. doi: 10.1021/es049640i. [DOI] [PubMed] [Google Scholar]
- 282.Muir DCG, Segstro MD, Welbourn PM, Toom D, Eisenreich SJ, Macdonald CR, Whelpdale DM. Patterns of accumulation of airborne organochlorine contaminants in lichens from the upper Great Lakes region of Ontario. Environmental Science and Technology. 1993;27:1201–1210. [Google Scholar]
- 283.Müllerová D, Matějková D, Dvořáková J, Müller L, Rosmus J, Kovářová K. PERSISTENT ORGANOCHLORINE POLLUTANTS IN OBESE WOMEN AFTER DIET INDUCED WEIGHT LOSS: FIVE YEARS FOLLOW UP STUDY. Cent Eur J Public Health. 2015;23:214–217. doi: 10.21101/cejph.a4100. [DOI] [PubMed] [Google Scholar]
- 284.Murphy LE, Gollenberg AL, Buck Louis GM, Kostyniak PJ, Sundaram R. Maternal serum preconception polychlorinated biphenyl concentrations and infant birth weight. Environ Health Perspect. 2010;118:297–302. doi: 10.1289/ehp.0901150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 286.Nair A, Dureja P, Pillai MK. Aldrin and dieldrin residues in human fat, milk and blood serum collected from Delhi. Hum Exp Toxicol. 1992;11:43–45. doi: 10.1177/096032719201100106. [DOI] [PubMed] [Google Scholar]
- 287.Neely WB, Branson DR, Blau GE. Partition coefficient to measure bioconcentration potential of organic chemicals in fish. Environmental Science and Technology. 1974;8:1113–1115. [Google Scholar]
- 288.Neff JM. Bioaccumulation in Marine Organisms: Effect of Contaminants from Oil Well Produced Water. Elsevier Science; 2002. p. 468. [Google Scholar]
- 289.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 290.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res. 2007;51:912–917. doi: 10.1002/mnfr.200600259. [DOI] [PubMed] [Google Scholar]
- 291.Nishimura N, Miyabara Y, Sato M, Yonemoto J, Tohyama C. Immunohistochemical localization of thyroid stimulating hormone induced by a low oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Sprague-Dawley rats. Toxicology. 2002;171:73–82. doi: 10.1016/s0300-483x(01)00559-5. [DOI] [PubMed] [Google Scholar]
- 292.Nishiumi S, Yoshida M, Azuma T, Yoshida K, Ashida H. 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs an insulin signaling pathway through the induction of tumor necrosis factor-alpha in adipocytes. Toxicol Sci. 2010;115:482–491. doi: 10.1093/toxsci/kfq052. [DOI] [PubMed] [Google Scholar]
- 293.Obana H, Hori S, Kashimoto T, Kunita N. Polycyclic aromatic hydrocarbons in human fat and liver. Bull Environ Contam Toxicol. 1981;27:23–27. doi: 10.1007/BF01610981. [DOI] [PubMed] [Google Scholar]
- 294.Oberg M, Sjödin A, Casabona H, Nordgren I, Klasson-Wehler E, Håkansson H. Tissue distribution and half-lives of individual polychlorinated biphenyls and serum levels of 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl in the rat. Toxicol Sci. 2002;70:171–182. doi: 10.1093/toxsci/70.2.171. [DOI] [PubMed] [Google Scholar]
- 295.Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BA, Olofsson P, Ivarsson SA, Lindh CH, Rignell-Hydbom A. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS One. 2014;9:e95891. doi: 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 297.Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol. 2014;53:345–353. doi: 10.1530/JME-14-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ohtake F, Fujii-Kuriyama Y, Kato S. [Transcription factor AhR is a ligand-dependcnt E3 ubiquitin ligase] Tanpakushitsu Kakusan Koso. 2007;52:1973–1979. [PubMed] [Google Scholar]
- 299.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 300.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Oliver BG, Niimi AJ. Bioconcentration factors of some halogenated organics for rainbow trout: limitations in their use for prediction of environmental residues. Environ Sci Technol. 1985;19:842–849. doi: 10.1021/es00139a013. [DOI] [PubMed] [Google Scholar]
- 302.Olsen H, Enan E, Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin mechanism of action to reduce lipoprotein lipase activity in the 3T3-L1 preadipocyte cell line. J Biochem Mol Toxicol. 1998;12:29–39. doi: 10.1002/(sici)1099-0461(1998)12:1<29::aid-jbt5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 303.Orban JE, Stanley JS, Schwemberger JG, Remmers JC. Dioxins and dibenzofurans in adipose tissue of the general US population and selected subpopulations. Am J Public Health. 1994;84:439–445. doi: 10.2105/ajph.84.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Organization WH. Diabetes; Fact sheet 312. 2016 [Google Scholar]
- 305.Organization WH. Facts and figures on childhood obesity. 2014 [Google Scholar]
- 306.Organization WH. Obesity and Overweight Fact Sheet No 311. 2016 [Google Scholar]
- 307.Park MJ, Lee SK, Yang JY, Kim KW, Lee SY, Lee WT, Chung KH, Yun YP, Yoo YC. Distribution of organochlorines and PCB congeners in Korean human tissues. Arch Pharm Res. 2005;28:829–838. doi: 10.1007/BF02977350. [DOI] [PubMed] [Google Scholar]
- 308.Patandin S, Koopman-Esseboom C, de Ridder MA, Weisglas-Kuperus N, Sauer PJ. Effects of environmental exposure to polychlorinated biphenyls and dioxins on birth size and growth in Dutch children. Pediatr Res. 1998;44:538–545. doi: 10.1203/00006450-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 309.Patterson DGJ, Fürst P, Henderson LO, Isaacs SG, Alexander LR, Turner WE, Needham LL, Hannon H. Partitioning of in vivo bound PCDDs/PCDFs among various compartments in whole blood. Chemosphere. 1989;19:135–142. [Google Scholar]
- 310.Pavuk M, Schecter AJ, Akhtar FZ, Michalek JE. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) levels and thyroid function in Air Force veterans of the Vietnam War. Ann Epidemiol. 2003;13:335–343. doi: 10.1016/s1047-2797(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 311.Pedersen SB, Bruun JM, Hube F, Kristensen K, Hauner H, Richelsen B. Demonstration of estrogen receptor subtypes alpha and beta in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Mol Cell Endocrinol. 2001;182:27–37. doi: 10.1016/s0303-7207(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 312.Pelletier C, Despres JP, Tremblay A. Plasma organochlorine concentrations in endurance athletes and obese individuals. Med Sci Sports Exerc. 2002;34:1971–1975. doi: 10.1249/01.MSS.0000040820.48707.99. [DOI] [PubMed] [Google Scholar]
- 313.Pereira MdS. Polychlorinated dibenzo-p-dioxins (PCDD), dibenzofurans (PCDF) and polychlorinated biphenyls (PCB): main sources, environmental behaviour and risk to man and biota. Química Nova. 2004;27:934–943. [Google Scholar]
- 314.Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107(Suppl 3):451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Perera FP, Whyatt RM, Jedrychowski W, Rauh V, Manchester D, Santella RM, Ottman R. Recent developments in molecular epidemiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- 316.Pestana D, Faria G, Sa C, Fernandes VC, Teixeira D, Norberto S, Faria A, Meireles M, Marques C, Correia-Sa L, Cunha A, Guimaraes JT, Taveira-Gomes A, Santos AC, Domingues VF, Delerue-Matos C, Monteiro R, Calhau C. Persistent organic pollutant levels in human visceral and subcutaneous adipose tissue in obese individuals–depot differences and dysmetabolism implications. Environ Res. 2014;133:170–177. doi: 10.1016/j.envres.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 317.Pestana D, Faria G, Sá C, Fernandes VC, Teixeira D, Norberto S, Faria A, Meireles M, Marques C, Correia-Sá L, Cunha A, Guimarães JT, Taveira-Gomes A, Santos AC, Domingues VF, Delerue-Matos C, Monteiro R, Calhau C. Persistent organic pollutant levels in human visceral and subcutaneous adipose tissue in obese individuals–depot differences and dysmetabolism implications. Environ Res. 2014;133:170–177. doi: 10.1016/j.envres.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 318.Peterson SH, Peterson MG, Debier C, Covaci A, Dirtu AC, Malarvannan G, Crocker DE, Schwarz LK, Costa DP. Deep-ocean foraging northern elephant seals bioaccumulate persistent organic pollutants. Sci Total Environ. 2015;533:144–155. doi: 10.1016/j.scitotenv.2015.06.097. [DOI] [PubMed] [Google Scholar]
- 319.Petreas M, Smith D, Hurley S, Jeffrey SS, Gilliss D, Reynolds P. Distribution of persistent, lipid-soluble chemicals in breast and abdominal adipose tissues: lessons learned from a breast cancer study. Cancer Epidemiol Biomarkers Prev. 2004;13:416–424. [PubMed] [Google Scholar]
- 320.Phillips M, Enan E, Liu PC, Matsumura F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Sci. 1995;108(Pt 1):395–402. doi: 10.1242/jcs.108.1.395. [DOI] [PubMed] [Google Scholar]
- 321.Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–1642. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- 322.Poon BH, Leung CK, Wong CK, Wong MH. Polychlorinated biphenyls and organochlorine pesticides in human adipose tissue and breast milk collected in Hong Kong. Arch Environ Contam Toxicol. 2005;49:274–282. doi: 10.1007/s00244-004-0111-3. [DOI] [PubMed] [Google Scholar]
- 323.Quintana PJ, Delfino RJ, Korrick S, Ziogas A, Kutz FW, Jones EL, Laden F, Garshick E. Adipose tissue levels of organochlorine pesticides and polychlorinated biphenyls and risk of non-Hodgkin’s lymphoma. Environ Health Perspect. 2004;112:854–861. doi: 10.1289/ehp.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ranjbar M, Rotondi MA, Ardern CI, Kuk JL. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons Are Associated with Cardiometabolic Health Risk. PLoS One. 2015;10:e0137536. doi: 10.1371/journal.pone.0137536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rignell-Hydbom A, Elfving M, Ivarsson SA, Lindh C, Jonsson BA, Olofsson P, Rylander L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants in relation to risk of type 1 diabetes. PLoS One. 2010;5:e11281. doi: 10.1371/journal.pone.0011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ritter L, Solomon KR, Forget J, Stemeroff M, O’Leary C. A review of selected persistent organic pollutants DDT-Aldrin-Dieldrin-Endrin-Chlordane-Heptachlor-Hexachlorobenzene-Mirex-Toxaphene-Polychorintaed biphenyls-Dioxins and Furans. Canada: 1995. [Google Scholar]
- 328.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ Health Perspect. 2011;119:1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, Balaguer P, Zalko D. Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci. 2011;122:372–382. doi: 10.1093/toxsci/kfr132. [DOI] [PubMed] [Google Scholar]
- 330.Robinson PE, Leczynski BA, Kutz FW, Remmers JC. An evaluation of hexachlorobenzene body-burden levels in the general population of the USA. IARC Sci Publ. 1986:183–192. [PubMed] [Google Scholar]
- 331.Ronen Z, Abeliovich A. Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl Environ Microbiol. 2000;66:2372–2377. doi: 10.1128/aem.66.6.2372-2377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ronn M, Lind L, van Bavel B, Salihovic S, Michaelsson K, Lind PM. Circulating levels of persistent organic pollutants associate in divergent ways to fat mass measured by DXA in humans. Chemosphere. 2011;85:335–343. doi: 10.1016/j.chemosphere.2011.06.095. [DOI] [PubMed] [Google Scholar]
- 333.Roos V, Ronn M, Salihovic S, Lind L, van Bavel B, Kullberg J, Johansson L, Ahlstrom H, Lind PM. Circulating levels of persistent organic pollutants in relation to visceral and subcutaneous adipose tissue by abdominal MRI. Obesity (Silver Spring) 2013;21:413–418. doi: 10.1002/oby.20267. [DOI] [PubMed] [Google Scholar]
- 334.Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans–a review. Med Sci Monit. 2009;15:RA137–145. [PubMed] [Google Scholar]
- 335.Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, Reyes M, Quinn J, Camann D, Perera F, Whyatt R. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175:1163–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rylander L, Rignell-Hydbom A, Hagmar L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health. 2005;4:28. doi: 10.1186/1476-069X-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sabljic A. On the prediction of soil sorption coefficients of organic pollutants from molecular structure: application of molecular topology model. Environ Sci Technol. 1987;21:358–366. doi: 10.1021/es00158a004. [DOI] [PubMed] [Google Scholar]
- 339.Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit Rev Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- 340.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 341.Sbarbati A, Accorsi D, Benati D, Marchetti L, Orsini G, Rigotti G, Panettiere P. Subcutaneous adipose tissue classification. Eur J Histochem. 2010;54:e48. doi: 10.4081/ejh.2010.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Schildkraut JM, Demark-Wahnefried W, DeVoto E, Hughes C, Laseter JL, Newman B. Environmental contaminants and body fat distribution. Cancer Epidemiol Biomarkers Prev. 1999;8:179–183. [PubMed] [Google Scholar]
- 344.Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod Toxicol. 2003;17:15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- 345.Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Organic Chemistry. John Wiley & Sons, Inc; 2003. [Google Scholar]
- 346.Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001-2006) Environ Health Perspect. 2014;122:299–303. doi: 10.1289/ehp.1307234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes. 2009;58:1482–1484. doi: 10.2337/db09-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Seefeld MD, Corbett SW, Keesey RE, Peterson RE. Characterization of the wasting syndrome in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1984;73:311–322. doi: 10.1016/0041-008x(84)90337-5. [DOI] [PubMed] [Google Scholar]
- 349.Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3822–3826. doi: 10.1210/jc.2011-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sharman M, Read WA, Castle L, Gilbert J. Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk, cream, butter and cheese. Food Addit Contam. 1994;11:375–385. doi: 10.1080/02652039409374236. [DOI] [PubMed] [Google Scholar]
- 352.Shaw SD, Berger ML, Brenner D, Kannan K, Lohmann N, Päpke O. Bioaccumulation of polybrominated diphenyl ethers and hexabromocyclododecane in the northwest Atlantic marine food web. Sci Total Environ. 2009;407:3323–3329. doi: 10.1016/j.scitotenv.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 353.Shen H, Han J, Tie X, Xu W, Ren Y, Ye C. Polychlorinated dibenzo-p-dioxins/furans and polychlorinated biphenyls in human adipose tissue from Zhejiang Province, China. Chemosphere. 2009;74:384–388. doi: 10.1016/j.chemosphere.2008.09.094. [DOI] [PubMed] [Google Scholar]
- 354.Shimba S, Todoroki K, Aoyagi T, Tezuka M. Depletion of arylhydrocarbon receptor during adipose differentiation in 3T3-L1 cells. Biochem Biophys Res Commun. 1998;249:131–137. doi: 10.1006/bbrc.1998.9100. [DOI] [PubMed] [Google Scholar]
- 355.Shiverick KT, Muther TF. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on serum concentrations and the uterotrophic action of exogenous estrone in rats. Toxicol Appl Pharmacol. 1982;65:170–176. doi: 10.1016/0041-008x(82)90375-1. [DOI] [PubMed] [Google Scholar]
- 356.Sijm DTHM, Wever H, de Vries PJ, Opperhuizen A. Octan-1-ol/water partition coefficients of polychlorinated dibenzo-p-dioxins and dibenzofurans: Experimental values determined with a stirring method. Chemosphere. 1989;19:263–266. [Google Scholar]
- 357.Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120:727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sjödin A, Patterson DG, Bergman A. Brominated flame retardants in serum from U.S. blood donors. Environ Sci Technol. 2001;35:3830–3833. doi: 10.1021/es010815n. [DOI] [PubMed] [Google Scholar]
- 359.Sjödin A, Patterson DG, Bergman A. A review on human exposure to brominated flame retardants–particularly polybrominated diphenyl ethers. Environ Int. 2003;29:829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 360.Skogen JC, Overland S. The fetal origins of adult disease: a narrative review of the epidemiological literature. JRSM Short Rep. 2012;3:59. doi: 10.1258/shorts.2012.012048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod Toxicol. 2011;31:297–301. doi: 10.1016/j.reprotox.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Smink A, Ribas-Fito N, Garcia R, Torrent M, Mendez MA, Grimalt JO, Sunyer J. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr. 2008;97:1465–1469. doi: 10.1111/j.1651-2227.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- 363.Soine PJ, Blanke RV, Guzelian PS, Schwartz CC. Preferential binding of chlordecone to the protein and high density lipoprotein fractions of plasma from humans and other species. J Toxicol Environ Health. 1982;9:107–118. doi: 10.1080/15287398209530146. [DOI] [PubMed] [Google Scholar]
- 364.Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Huppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009;117:1549–1555. doi: 10.1289/ehp.11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 366.Spink DC, Eugster HP, Lincoln DW, 2nd, Schuetz JD, Schuetz EG, Johnson JA, Kaminsky LS, Gierthy JF. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys. 1992;293:342–348. doi: 10.1016/0003-9861(92)90404-k. [DOI] [PubMed] [Google Scholar]
- 367.Spink DC, Hayes CL, Young NR, Christou M, Sutter TR, Jefcoate CR, Gierthy JF. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J Steroid Biochem Mol Biol. 1994;51:251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 368.Spink DC, Lincoln DW, 2nd, Dickerman HW, Gierthy JF. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes an extensive alteration of 17 beta-estradiol metabolism in MCF-7 breast tumor cells. Proc Natl Acad Sci U S A. 1990;87:6917–6921. doi: 10.1073/pnas.87.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45:235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 370.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Natl Cancer Inst. 1999;91:779–786. doi: 10.1093/jnci/91.9.779. [DOI] [PubMed] [Google Scholar]
- 372.Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, White A, Kemeny M, Busch E, Nafziger AN. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 1998;7:489–496. [PubMed] [Google Scholar]
- 373.Strakovsky RS, Lezmi S, Shkoda I, Flaws JA, Helferich WG, Pan YX. In utero growth restriction and catch-up adipogenesis after developmental di (2-ethylhexyl) phthalate exposure cause glucose intolerance in adult male rats following a high-fat dietary challenge. J Nutr Biochem. 2015;26:1208–1220. doi: 10.1016/j.jnutbio.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, Lezmi S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol. 2015;284:101–112. doi: 10.1016/j.taap.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sugiura K, Aoki M, Kaneko S, Daisaku I, Komatsu Y, Shibuya H, Suzuki H, Goto M. Fate of 2,4,6-trichlorophenol, pentachlorophenol, p-chlorobiphenyl, and hexachlorobenzene in an outdoor experimental pond: Comparison between observations and predictions based on laboratory data. Archives of Environmental Contamination and Toxicology. 1984;13:745–758. [Google Scholar]
- 376.Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122:616–623. doi: 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Svensson K, Hernandez-Ramirez RU, Burguete-Garcia A, Cebrian ME, Calafat AM, Needham LL, Claudio L, Lopez-Carrillo L. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ Res. 2011;111:792–796. doi: 10.1016/j.envres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology. 2010;268:132–138. doi: 10.1016/j.tox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 380.Swedenborg E, Ruegg J, Makela S, Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43:1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- 381.Sweeney MH, Calvert GM, Egeland GA, Fingerhut MA, Halperin WE, Piacitelli LA. Review and update of the results of the NIOSH medical study of workers exposed to chemicals contaminated with 2,3,7,8-tetrachlorodibenzodioxin. Teratog Carcinog Mutagen. 1997;17:241–247. [PubMed] [Google Scholar]
- 382.Tang-Peronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 383.Tang-Peronard JL, Heitmann BL, Andersen HR, Steuerwald U, Grandjean P, Weihe P, Jensen TK. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: a prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr. 2014;99:5–13. doi: 10.3945/ajcn.113.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Tang-Peronard JL, Jensen TK, Andersen HR, Ried-Larsen M, Grontved A, Andersen LB, Timmermann CA, Nielsen F, Heitmann BL. Associations between Exposure to Persistent Organic Pollutants in Childhood and Overweight up to 12 Years Later in a Low Exposed Danish Population. Obes Facts. 2015;8:282–292. doi: 10.1159/000438834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, Boergesen M, Mandrup S, Vinggaard AM. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol. 2012;361:106–115. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 386.Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, Jacobs D, Kohrle J, Lee DH, Rylander L, Rignell-Hydbom A, Tornero-Velez R, Turyk ME, Boyles AL, Thayer KA, Lind L. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron Artery Dis. 1998;9:503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 388.Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, Silva MJ, Brenner BL, Wolff MS. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res. 2012;112:186–193. doi: 10.1016/j.envres.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Teixeira D, Pestana D, Santos C, Correia-Sá L, Marques C, Norberto S, Meireles M, Faria A, Silva R, Faria G, Sá C, Freitas P, Taveira-Gomes A, Domingues V, Delerue-Matos C, Calhau C, Monteiro R. Inflammatory and cardiometabolic risk on obesity: role of environmental xenoestrogens. J Clin Endocrinol Metab. 2015;100:1792–1801. doi: 10.1210/jc.2014-4136. [DOI] [PubMed] [Google Scholar]
- 390.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Tomar LR, Agarwal MP, Avasthi R, Tyagi V, Mustafa M, Banerjee BD. Serum organochlorine pesticide levels in patients with metabolic syndrome. Indian J Endocrinol Metab. 2013;17:S342–344. doi: 10.4103/2230-8210.119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Tomlin CDS. The e-Pesticide Manual. UK: British Crop Protection Council; 2004–2005. Pendimethalin. [Google Scholar]
- 393.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 394.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 395.Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132:e646–655. doi: 10.1542/peds.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines–energy regulation from the human perspective. J Nutr. 2006;136:1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- 397.Tung EW, Boudreau A, Wade MG, Atlas E. Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells. PLoS One. 2014;9:e94583. doi: 10.1371/journal.pone.0094583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p′-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009;75:674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 399.Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Dakeshita S, Nii K, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Environ Health Perspect. 2009;117:568–573. doi: 10.1289/ehp.0800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Uemura H, Arisawa K, Hiyoshi M, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T. Associations of environmental exposure to dioxins with prevalent diabetes among general inhabitants in Japan. Environ Res. 2008;108:63–68. doi: 10.1016/j.envres.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 401.Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, Drobna B, Trnovec T, Susienkova K, Labudova V, Gasperikova D, Langer P, Klimes I. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia. 2010;53:899–906. doi: 10.1007/s00125-010-1683-2. [DOI] [PubMed] [Google Scholar]
- 402.Vafeiadi M, Georgiou V, Chalkiadaki G, Rantakokko P, Kiviranta H, Karachaliou M, Fthenou E, Venihaki M, Sarri K, Vassilaki M, Kyrtopoulos SA, Oken E, Kogevinas M, Chatzi L. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother-Child Cohort (Crete, Greece) Environ Health Perspect. 2015;123:1015–1021. doi: 10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Vafeiadi M, Roumeliotaki T, Myridakis A, Chalkiadaki G, Fthenou E, Dermitzaki E, Karachaliou M, Sarri K, Vassilaki M, Stephanou EG, Kogevinas M, Chatzi L. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. doi: 10.1016/j.envres.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 404.Valentino R, D’Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, Perruolo G, Oriente F, Beguinot F, Formisano P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS One. 2013;8:e82099. doi: 10.1371/journal.pone.0082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Valvi D, Casas M, Mendez MA, Ballesteros-Gomez A, Luque N, Rubio S, Sunyer J, Vrijheid M. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology. 2013;24:791–799. doi: 10.1097/EDE.0b013e3182a67822. [DOI] [PubMed] [Google Scholar]
- 406.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123:1022–1029. doi: 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goni F, Grimalt JO, Llop S, Marina LS, Vizcaino E, Sunyer J, Vrijheid M. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity (Silver Spring) 2014;22:488–496. doi: 10.1002/oby.20603. [DOI] [PubMed] [Google Scholar]
- 408.Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect. 2012;120:451–457. doi: 10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 2006;17:352–359. doi: 10.1097/01.ede.0000220553.84350.c5. [DOI] [PubMed] [Google Scholar]
- 412.Veith GD, Defoe DL, Bergstedt BV. Measuring and estimating the bioconcentration factor of chemicals in fish. Journal of the Fisheries Research Board of Canada. 1979;36:1040–1048. [Google Scholar]
- 413.Vena J, Boffetta P, Becher H, Benn T, Bueno-de-Mesquita HB, Coggon D, Colin D, Flesch-Janys D, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Pesatori AC, Saracci R, Steenland K, Kogevinas M. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998;106(Suppl 2):645–653. doi: 10.1289/ehp.98106645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Verhulst SL, Nelen V, Hond ED, Koppen G, Beunckens C, Vael C, Schoeters G, Desager K. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117:122–126. doi: 10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Vogel CF, Chang WL, Kado S, McCulloh K, Vogel H, Wu D, Haarmann-Stemmann T, Yang G, Leung PS, Matsumura F, Gershwin ME. Transgenic Overexpression of Aryl Hydrocarbon Receptor Repressor (AhRR) and AhR-Mediated Induction of CYP1A1, Cytokines, and Acute Toxicity. Environ Health Perspect. 2016;124:1071–1083. doi: 10.1289/ehp.1510194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Vost A, Maclean N. Hydrocarbon transport in chylomicrons and high-density lipoproteins in rat. Lipids. 1984;19:423–435. doi: 10.1007/BF02537404. [DOI] [PubMed] [Google Scholar]
- 417.Wang HX, Zhou Y, Tang CX, Wu JG, Chen Y, Jiang QW. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health. 2012;11:79. doi: 10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wang L, Xue J, Kannan K. Widespread occurrence and accumulation of bisphenol A diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives in human blood and adipose fat. Environ Sci Technol. 2015;49:3150–3157. doi: 10.1021/acs.est.5b00096. [DOI] [PubMed] [Google Scholar]
- 419.Wang N, Shi L, Kong D, Cai D, Cao Y, Liu Y, Pang G, Yu R. Accumulation levels and characteristics of some pesticides in human adipose tissue samples from Southeast China. Chemosphere. 2011;84:964–971. doi: 10.1016/j.chemosphere.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 420.Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:E223–227. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- 421.Wang X, Santostefano MJ, Evans MV, Richardson VM, Diliberto JJ, Birnbaum LS. Determination of parameters responsible for pharmacokinetic behavior of TCDD in female Sprague-Dawley rats. Toxicol Appl Pharmacol. 1997;147:151–168. doi: 10.1006/taap.1997.8242. [DOI] [PubMed] [Google Scholar]
- 422.Warner M, Aguilar Schall R, Harley KG, Bradman A, Barr D, Eskenazi B. In utero DDT and DDE exposure and obesity status of 7-year-old Mexican-American children in the CHAMACOS cohort. Environ Health Perspect. 2013;121:631–636. doi: 10.1289/ehp.1205656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Warner M, Wesselink A, Harley KG, Bradman A, Kogut K, Eskenazi B. Prenatal exposure to dichlorodiphenyltrichloroethane and obesity at 9 years of age in the CHAMACOS study cohort. Am J Epidemiol. 2014;179:1312–1322. doi: 10.1093/aje/kwu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Weigensberg MJ, Goran MI. Type 2 diabetes in children and adolescents. Lancet. 2009;373:1743–1744. doi: 10.1016/S0140-6736(09)60961-2. [DOI] [PubMed] [Google Scholar]
- 425.Weistrand C, Norén K. Polychlorinated naphthalenes and other organochlorine contaminants in human adipose and liver tissue. J Toxicol Environ Health A. 1998;53:293–311. doi: 10.1080/009841098159295. [DOI] [PubMed] [Google Scholar]
- 426.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 427.Witt K, Niessen KH. Toxaphenes and chlorinated naphthalenes in adipose tissue of children. J Pediatr Gastroenterol Nutr. 2000;30:164–169. doi: 10.1097/00005176-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 428.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, Sun Q. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses’ health study and meta-analysis. Environ Health Perspect. 2013;121:153–161. doi: 10.1289/ehp.1205248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Xu CX, Wang C, Zhang ZM, Jaeger CD, Krager SL, Bottum KM, Liu J, Liao DF, Tischkau SA. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int J Obes (Lond) 2015;39:1300–1309. doi: 10.1038/ijo.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yanagisawa R, Koike E, Win-Shwe TT, Yamamoto M, Takano H. Impaired lipid and glucose homeostasis in hexabromocyclododecane-exposed mice fed a high-fat diet. Environ Health Perspect. 2014;122:277–283. doi: 10.1289/ehp.1307421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yang L, Zhou Y, Sun H, Lai H, Liu C, Yan K, Yuan J, Wu T, Chen W, Zhang X. Dose-response relationship between polycyclic aromatic hydrocarbon metabolites and risk of diabetes in the general Chinese population. Environ Pollut. 2014;195:24–30. doi: 10.1016/j.envpol.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 433.Yang M, Chen M, Wang J, Xu M, Sun J, Ding L, Lv X, Ma Q, Bi Y, Liu R, Hong J, Ning G. Bisphenol A Promotes Adiposity and Inflammation in a Nonmonotonic Dose-response Way in 5-week-old Male and Female C57BL/6J Mice Fed a Low-calorie Diet. Endocrinology. 2016;157:2333–2345. doi: 10.1210/en.2015-1926. [DOI] [PubMed] [Google Scholar]
- 434.Yu GW, Laseter J, Mylander C. Persistent organic pollutants in serum and several different fat compartments in humans. J Environ Public Health. 2011;2011:417980. doi: 10.1155/2011/417980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zaroogian GE, Heltshe JF, Johnson M. Estimation of bioconcentration in marine species using structure-activity models. Environmental Toxicology and Chemistry. 1985;4:3–12. [Google Scholar]
- 436.Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge RS. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J Pediatr. 2009;155:500–504. doi: 10.1016/j.jpeds.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 437.Zober A, Ott MG, Messerer P. Morbidity follow up study of BASF employees exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) after a 1953 chemical reactor incident. Occup Environ Med. 1994;51:479–486. doi: 10.1136/oem.51.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zulalian J. Study of the absorption, excretion, metabolism, and residues in tissues of rats treated with carbon-14-labeled pendimethalin, PROWL herbicide. Journal of Agricultural and Food Chemistry. 1990;38:1743–1754. [Google Scholar]


