Abstract
The objective of this study was to determine if mRNA sequences downstream of the translation initiation codon are important for translation of plastid mRNAs. We have employed a transgenic approach, measuring accumulation of the neomycin phosphotransferase (NPTII) reporter enzyme translationally fused with 14 N-terminal amino acids encoded in the rbcL or atpB plastid genes. NPTII accumulation from wild-type and mutant rbcL and atpB segments was compared. We report that silent mutations in the rbcL segment reduced NPTII accumulation 35-fold. In contrast, mutations in the atpB mRNA reduced NPTII accumulation only moderately from approximately 7% (w/w) to approximately 4% (w/w) of the total soluble cellular protein, indicating that the importance of sequences downstream of the translation initiation codon are dependent on the individual mRNA. Information provided here will facilitate transgene design for high-level expression of recombinant proteins in chloroplasts by translational fusion with the N-terminal segment of highly expressed plastid genes or by introduction of silent mutations in the N-terminal part of the coding region.
Plastids are plant cellular organelles that have their own genome and a prokaryotic-type transcription and translation machinery (Rochaix, 1996; Sugita and Sugiura, 1996; Danon, 1997; Stern et al., 1997; Bruick and Mayfield, 1999; Hess and Börner, 1999). In prokaryotes translation is facilitated by mRNA-rRNA interactions between the Shine-Dalgarno (SD) sequence upstream of the translation initiation codon and the anti-Shine-Dalgarno sequence (ASD) at the 3′ end of the small (16S) ribosomal RNA. A second mRNA element facilitating translation is comprised of sequences downstream of the translation initiation codon (Baneyx, 1999).
In higher plant plastids mRNA sequences in the 5′-untranslated region (UTR) were shown to be important for translation. These sequences are complementary to the 16S rRNA 3′ end and may be SD-like (GGA) as in the rps14 mRNA leader (Hirose et al., 1998) or distinct from SD, such as the RBS1 (AAG) and RBS2 (UGAUGAU) sequences in the psbA leader (Hirose and Sugiura, 1996). Signals for light-dependent psbA mRNA translation (Staub and Maliga, 1993; Staub and Maliga, 1994b) and rbcL mRNA stability (Shiina et al., 1998) are also localized in the 5′-UTR. Alternative processing of the 5′-UTR in barley chloroplasts was shown to regulate the availability of translatable rbcL mRNA (Reinbothe et al., 1993). Although several studies have addressed the role of 5′-UTR, no information is available on the role of sequences downstream of the translation initiation codon in higher plant plastid mRNAs.
The objective of this study was to determine if mRNA sequences downstream of the translation initiation codon are important for translation of plastid mRNAs. We have employed a transgenic approach, comparing accumulation of the neomycin phosphotransferase (NPTII) reporter enzyme translationally fused with 14 N-terminal amino acids encoded in the rbcL or atpB plastid genes. Silent mutations that alter the mRNA sequence without affecting the amino acid sequence probed the rbcL and atpB segments for the importance of mRNA sequence in translation.
We report that silent mutations in the rbcL seqment downstream of AUG have a dramatic effect reducing NPTII accumulation 35-fold. Therefore, the N-terminal coding region and the 5′-UTR will be collectively designated as the 5′-translation control region or 5′-TCR. In contrast, mutagenesis of the atpB segment reduced protein accumulation only about 2-fold from approximately 7% (w/w) to approximately 4% (w/w). The importance of mRNA sequences downstream of AUG is therefore dependent on the individual mRNA. Information provided here will facilitate transgene design for high-level expression of recombinant proteins in chloroplasts.
RESULTS
Experimental Design
The chimeric genes have the same promoter (Prrn), coding region (neo), and 3′-UTR (TrbcL), and differ only with respect to the 5′-TCR. Prrn is the strong plastid rRNA operon promoter (Vera and Sugiura, 1995). The bacterial neo gene encodes NPTII (Beck et al., 1982). TrbcL is the 3′-UTR of the plastid rbcL gene required for the stabilization of the chimeric mRNA (Shinozaki and Sugiura, 1982).
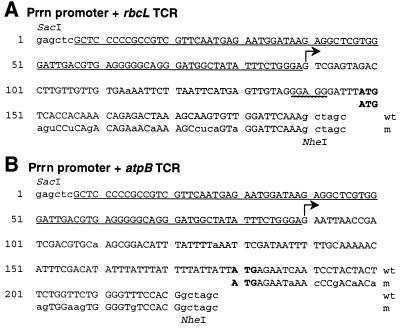
The plastid rbcL gene encodes the large subunit of the Rubisco. Transcription of the tobacco rbcL mRNA initiates 182 nucleotides upstream of the translation initiation codon (Shinozaki and Sugiura, 1982). The primary transcript may be processed to create a mRNA with a 59-nucleotide 5′-UTR (Allison et al., 1996). Two constructs were prepared with the rbcL TCR. One construct contained the wild-type sequence, including the processed 5′-UTR and 42 nucleotides of the coding region N terminus. The second construct was similar, except that it contained silent mutations in codons two to eleven of the rbcL N-terminal segment (Fig. 1A).
Figure 1.
DNA sequence of the chimeric Prrn plastid promoter fragments. A, Prrn promoter with the wild-type and mutant rbcL-TCR. The Prrn promoter sequence is underlined. The transcription initiation site is marked by a horizontal arrow. The translational initiation codon (ATG) is in bold. SD is underlined with a wavy line. Wild-type (wt) sequence is shown on top; mutant sequences (m) are duplicated below. Wild-type nucleotides are in capital letters; mutant nucleotides are in lowercase. B, Prrn promoter with the wild-type and mutant atpB TCR.
The plastid atpB gene encodes the ATP synthase β-subunit. It is transcribed from four distinct promoters initiating 611, 502/488, 289, and 255 nucleotides upstream of the translation initiation codon. The atpB 5′-UTR contains an RNA processing site that creates a transcript with a 90-nucleotide long leader sequence (Orozco et al., 1990). Two constructs were prepared to test the role of atpB TCR for the expression of NPTII. One construct contained the wild-type TCR, including the processed 5′-UTR and 42 nucleotides of the N-terminal coding region. The second construct contained silent mutations in codons three to 12 of the atpB N-terminal segment (Fig. 1B).
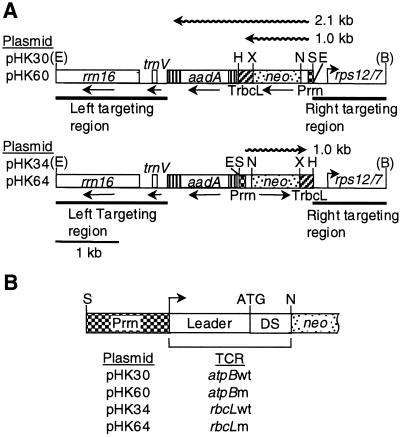
Plastid transformation vectors pHK34 and pHK64, and pHK30 and pHK60 carry chimeric neo genes under control of Prrn fused with the wild-type and mutant rbcL and atpB TCRs, respectively (Fig. 2). The chimeric neo genes were introduced into the tobacco plastid genome in pPRV111 vector derivatives and the transplastomic lines were purified to the homoplastomic stage to ensure that each of the plastid genome copies carried the transgene (data not shown).
Figure 2.
Vectors for insertion of chimeric neo genes into the tobacco plastid genome. A, Targeting region of plastid vectors. Shown are the relative positions of selectable spectinomycin resistance (aadA) and neo passenger genes, flanked by plastid DNA encoding rrn16, trnV, and rps12/7 (Shinozaki et al., 1986). The neo gene is expressed from the Prrn promoter; the rbcL 3′-UTR (TrbcL) stabilizes the mRNA. The pPRV111B (top) and pPRV111A (bottom) vector derivatives differ with respect to the relative orientation of neo genes. Wavy line represents neo transcripts. Restriction sites: E, EcoRI; S, SacI; N, NheI; X, XbaI; H, HindIII; and B, BglII. Restriction sites removed during plasmid construction in parenthesis. B, Listing of plasmids and the schematic map of their promoter and N-terminal coding regions. DS, Sequence downstream of initiation codon.
Sequences Downstream of the rbcL AUG Are Important for NPTII Accumulation
Immunoblot analysis was carried out to determine NPTII levels in the leaves of the transplastomic plants. Since NPTII from wild-type and mutant TCRs has the exact same protein sequence, the rates of protein degradation should be the same. Therefore, protein levels in the plants directly reflect the efficiency of mRNA translation. NPTII levels on the immunoblots were quantified by comparison with commercial NPTII (Fig. 3A). NPTII was also readily detected in some of the samples after staining with Coomassie Brilliant Blue R250 (Fig. 3B).
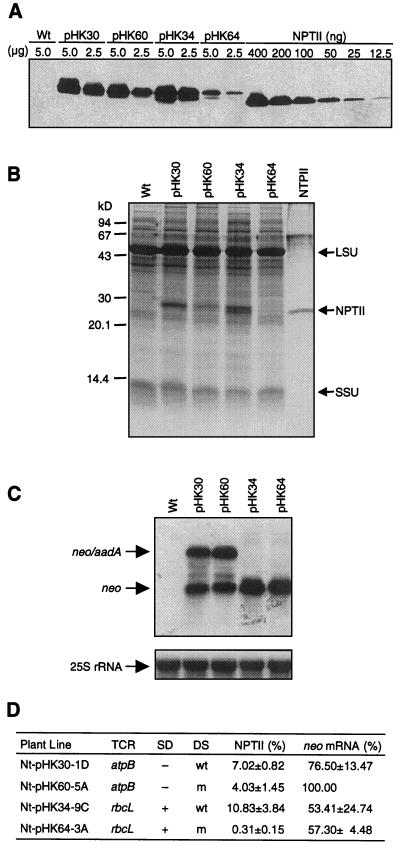
Figure 3.
Expression of neo transgenes in the plastid genome. A, Immunoblot analysis to detect NPTII. Amount of total soluble leaf protein (micrograms) loaded on the SDS-PAGE gel is indicated above the lanes. A commercial NPTII dilution series was loaded for reference. Lanes for plant lines are designated with transforming plasmid; Wt, Wild-type tobacco sample. B, NPTII detected by Coomassie Brilliant Blue R250 in SDS-PAGE gel. Twenty micrograms total soluble protein was loaded per lane. Commercially available NPTII (400 ng) was also loaded. Marked are the Rubisco large (LSU) and small (SSU) subunits. C, The levels of neo mRNA in the transplastomic leaves. Blots were probed for neo (top) and cytoplasmic 25S rRNA as loading control (bottom). D, Levels of NPTII and neo mRNA based on three to six experiments. Highest value was taken as 100%. Plant line, plasmid name (for example pHK30) and number and letter combination; + or −, SD is present or absent at prokaryotic consensus position; wt or m, downstream sequence is wild type or mutant.
We have found that NPTII from the wild-type rbcL 5′-TCR in the Nt-pHK34 line accumulated to approximately 11% (w/w) of the total soluble cellular protein. Mutagenesis of the rbcL sequences had a dramatic effect reducing NPTII accumulation 35-fold from approximately 11% (w/w) to 0.3% (w/w) in the pHK64 plants. Thus sequences downstream of the translation initiation codon are very important for the translation of this chimeric mRNA. It is interesting that immunoblot analysis and Coomassie staining in the protein gels detected two discrete bands. Maturation of the tobacco rbcL gene product involves removal of the two N-terminal amino acids, acetylation of Pro-3 and Nε-trimethylation of Lys-14 (Houtz et al., 1989). Incorporation of the rbcL segment resulted in translationally fusing the 14 N-terminal amino acids of the Rubisco with NPTII. It is likely, therefore, that the two NPTII bands are generated by post-translational modification of the rbcL N-terminal segment.
NPTII from the wild-type atpB TCR accumulated to approximately 7% (w/w) of the total soluble cellular protein. Unlike mutations in the rbcL TCR, mutations in the atpB segment reduced protein accumulation only moderately from approximately 7% (w/w) to approximately 4% (w/w; Fig. 3, A and D). Thus sequences downstream of the translation initiation codon are relatively unimportant for translation of the atpB mRNA.
Silent Mutations Downstream of AUG Do Not Affect mRNA Stability
The NPTII levels depend not only on the efficiency of translation, but also on the mRNA levels. Therefore, RNA gel-blot analysis was carried out to determine if silent mutations in the N-terminal coding region affected mRNA stability.
Probing of total cellular RNA from plants expressing neo from the wild-type and mutant rbcL TCR (constructs PrrnLrbcLwt and PrrnLrbcLm in plants Nt-pHK34 and Nt-pHK64) identified a 1.0-kb mRNA species (Fig. 3C). This mRNA is the predicted monocistronic neo mRNA marked in Figure 2A. Mutagenesis of the rbcL segment did not significantly affect mRNA stability, as indicated by the accumulation of mRNA to comparable levels from the genes with the wild-type and mutant sequences (Fig. 3, C and D).
Probing of total cellular RNA from plants expressing neo from the PrrnLatpBwt and PrrnLatpBm constructs (plants Nt-pHK30 and Nt-pHK60) yielded two signals. The 1.0-kb mRNA in Figure 3C is the monocistronic neo message initiated from the Prrn promoter and terminated within the TrbcL; the 2.1-kb mRNA is a dicistronic neo-aadA read-through transcript (see Fig. 2A). The stability of the mRNAs was not significantly affected by the atpB TCR mutations, indicated by the accumulation of neo mRNA to comparable levels in the leaves of transplastomic plants (Fig. 3, C and D).
DISCUSSION
The rbcL 5′-UTR contains an SD sequence at the prokaryotic consensus position, whereas the atpB 5′-UTR lacks one. Therefore, it is likely that the atpB 5′-UTR has mechanisms other than SD-ASD interactions in the 5′-UTR to facilitate translation initiation. The mechanism of translational activation is not known. A candidate for translational activation of the atpB mRNA is the general S1 ribosomal protein mediated mechanism with affinity for AU-reach sequences in the 5′-UTR (Franzetti et al., 1992; Alexander et al., 1998). A specific control factor could be encoded in a maize atp1 gene homolog (McCormac and Barkan, 1999). Our mutagenesis study indicates that the role of sequences downstream of AUG in plastid translation is highly dependent on the individual mRNA. The consequence of mutagenesis on rbcL translation was a dramatic 35-fold drop in NPTII levels from 11% (w/w) to 0.3% (w/w). It is apparent that translation of the chimeric mRNA is highly dependent on sequences directly downstream of AUG. In contrast to the rbcL TCR, mutagenesis of the atpB segment only slightly affected NPTII accumulation, reducing it approximately 2-fold from approximately 7% (w/w) to approximately 4% (w/w; Fig. 3D).
In Escherichia coli, complementarity of the 16S rRNA with sequences downstream of the AUG was used to define the 15-bp downstream box (DB) region in the mRNA (Sprengart et al., 1996). Improved complementarity resulted in up to 34-fold increase in protein level depending on the individual mRNA. It was assumed, therefore, that direct mRNA-rRNA interactions are the mechanism by which DB facilitates translation (Faxén et al., 1991; Etchegaray and Inouye, 1999). Recent data based on the mutagenesis of the E. coli 16S rRNA (O'Connor et al., 1999) and considerations of mRNA positioning within the small ribosomal RNA subunit (McCarthy and Brimacombe, 1994) argue against direct DB (mRNA) and anti-DB (rRNA) interactions during translation initiation. Thus the specific mechanism by which sequences downstream of the translation initiation codon enhance mRNA translation in E. coli remains to be determined.
The frequency of use of synonymous codons usually reflects the abundance of their cognate tRNAs. Replacement of frequently used codons with rare codons in E. coli mRNAs results in reduced protein accumulation (Kane, 1995; Makrides, 1996). Most dramatic is the consequence of the AGA/AGG codons in the heterologous mRNA, which occur at the frequency of 1.4/2.1 per 1,000 codons. Incorporation of rare codons (up to 4.3 per 1,000 codons) may have a deleterious effect on translation efficiency and/or accuracy when present in clusters or in large numbers. However, no impairment of translation efficiency or accuracy was shown for any codons more frequent than 4.6 per 1,000 codons (Kane, 1995). In plastids, codon usage preference in somewhat dependent on the plastid gene type. For example, the highly-expressed photosynthetic genes have a higher overall GC content and higher GC preference at the third codon position than do other plastid genes (Shimada and Sugiura, 1991). Therefore, codon usage frequency was calculated for the highly expressed photosynthetic genes rbcL, psaA, psaB, psaC, psbA, psbB, psbC, psbD, psbE, and psbF (Nakamura et al., 1999). None of the changes made involved replacing a frequently used codon with a codon that would be considered rare by the E. coli criteria (≤4.6 per 1,000 codons; Table I). Therefore, we believe that reduced NPTII accumulation from the mutagenized rbcL sequence is due to the change in mRNA sequence downstream of AUG. It is possible that maintaining the native rbcL sequence downstream of AUG is important for efficient translation of the mRNA (Bonham-Smith and Bourque, 1989). In an alternate manner, silent mutagenesis may have created an mRNA sequence that interferes with the translation of the mRNA. Further experiments are needed to identify nucleotides responsible for reduced translation efficiency from this construct.
Table I.
Codon frequency in wild-type and mutant rbcL and atpB segments
| rbcL wild type | ||||||||||||||
| Codon | AUGa | UCA | CCA | CAA | ACA | GAG | ACU | AAA | GCA | AGU | GUU | GGA | UUC | AAA |
| Amino acid | Met | Ser | Pro | Gln | Thr | Glu | Thr | Lys | Ala | Ser | Val | Gly | Phe | Lys |
| Fraction | 1.00 | 0.21 | 0.24 | 0.57 | 0.23 | 0.38 | 0.37 | 0.60 | 0.29 | 0.14 | 0.35 | 0.24 | 0.40 | 0.60 |
| Triplet/1,000 | 24.6 | 13.5 | 10.6 | 21.0 | 11.7 | 12.4 | 18.4 | 22.0 | 18.1 | 9.3 | 24.9 | 17.9 | 22.5 | 22.0 |
| rbcL mutant | ||||||||||||||
| Codon | AUGa | agu | CCu | CAg | ACA | GAa | ACa | AAA | GCc | uca | GUa | GGA | UUC | AAA |
| Amino acid | Met | Ser | Pro | Gln | Thr | Glu | Thr | Lys | Ala | Ser | Val | Gly | Phe | Lys |
| Fraction | 1.00 | 0.14 | 0.30 | 0.43 | 0.23 | 0.62 | 0.23 | 0.60 | 0.16 | 0.21 | 0.31 | 0.24 | 0.40 | 0.60 |
| Triplet/1,000 | 24.6 | 9.3 | 13.5 | 15.5 | 11.7 | 20.7 | 11.7 | 22.0 | 10.1 | 13.5 | 21.8 | 17.9 | 22.5 | 22.0 |
| atpB wild type | ||||||||||||||
| Codon | AUGa | AGA | AUC | AAU | CCU | ACU | ACU | UCU | GGU | UCU | GGG | GUU | UCC | ACG |
| Amino acid | Met | Arg | Ile | Asn | Pro | Thr | Thr | Ser | Gly | Ser | Gly | Val | Ser | Thr |
| Fraction | 1.00 | 0.22 | 0.27 | 0.61 | 0.30 | 0.37 | 0.37 | 0.31 | 0.38 | 0.31 | 0.26 | 0.35 | 0.14 | 0.15 |
| Triplet/1,000 | 24.6 | 7.8 | 15.5 | 18.1 | 13.5 | 18.4 | 18.4 | 20.2 | 28.2 | 20.2 | 19.2 | 24.9 | 9.1 | 7.5 |
| atpB mutant | ||||||||||||||
| Codon | AUGa | AGA | AUa | AAc | CCg | ACa | ACa | agU | GGa | agU | GGG | GUg | UCC | ACG |
| Amino acid | Met | Arg | Ile | Asn | Pro | Thr | Thr | Ser | Gly | Ser | Gly | Val | Ser | Thr |
| Fraction | 1.00 | 0.22 | 0.29 | 0.39 | 0.30 | 0.23 | 0.23 | 0.14 | 0.24 | 0.14 | 0.26 | 0.21 | 0.14 | 0.15 |
| Triplet/1,000 | 24.6 | 7.8 | 16.6 | 11.4 | 13.2 | 11.7 | 11.7 | 9.3 | 17.9 | 9.3 | 19.2 | 15.3 | 9.1 | 7.5 |
AUG, Translation initiation codon.
The PrrnLrbcLwt and PrrnLatpB promoters described here will find many uses driving the expression of selectable marker genes (Khan and Maliga, 1999), and of genes encoding proteins with agronomic, industrial, or pharmaceutical importance. Furthermore, information provided here will facilitate transgene design for high-level protein expression in chloroplasts by translational fusion or introduction of silent mutations in the coding region N terminus.
MATERIALS AND METHODS
Plasmid Construction
The chimeric Prrn-TCR sequences are contained in SacI-NheI fragments. PrrnLatpBwt (Prrn promoter with wild-type atpB TCR) is carried by plasmid pHK10, a pUC118 plasmid derivative. PrrnLatpBm (Prrn promoter with mutant atpB TCR) is available in plasmid pHK50, a pBluescript II KS+ plasmid derivative. PrrnLrbcLwt (Prrn promoter with wild-type rbcL TCR) is available in plasmid pHK14 (Bluescript II KS+ derivative). PrrnLrbcLm (Prrn promoter with mutant rbcL TCR) is available in plasmid pHK54 (pBluescript II KS+ derivative). The promoter fragments were constructed by PCR. Construction details are available upon request.
The rbcL or atpB N-terminal amino acids included in the Prrn promoter fragments (Fig. 1) were translationally fused with the neo coding region via an engineered NheI site. The engineered neo gene derives from plasmid pSC1, and was obtained by inserting the NheI restriction site (GCTAGC) between the ATG and the first codon (ATT) of the neo coding region (Chaudhuri and Maliga, 1996). The neo genes have the plastid rbcL gene 3′-UTR (TrbcL) to stabilize the mRNAs (Staub and Maliga, 1994a). Plastid vectors pHK34 and pHK64 were obtained by cloning the neo gene from plasmids pHK14 and pHK54 as a SacI-HindIII fragment into plastid vector pPRV111A (Zoubenko et al., 1994). Plastid vectors pHK30 and pHK60 were obtained by cloning the neo gene from plasmids pHK10 and pHK50 as a SacI-HindIII fragments into plastid vector pPRV111B (Zoubenko et al., 1994). The map of the targeting region of the plastid transformation vectors is shown in Figure 2.
Plastid Transformation and Regeneration of Transgenic Plants
DNA for plastid transformation was prepared using the Qiagen Plasmid Maxi Kit (Qiagen, Valencia, CA). Transforming DNA was introduced into leaf chloroplasts on the surface of tungsten particles (1 μm) using the Du Pont PDS1000He Biolistic gun. Transplastomic plants were selected on RMOP medium containing 500 mg L−1 spectinomycin dihydrochloride. A uniform population of transformed plastid genome copies was confirmed by DNA gel-blot analysis. The transgenic plants were grown on Murashige-Skoog medium (Murashige and Skoog, 1962) containing 3% (w/v) Suc and 0.6% (w/v) agar in sterile culture condition. The protocol was described in more detail elsewhere (Svab and Maliga, 1993).
RNA Gel-Blot Analysis
RNA gel-blot analysis was carried out as described loading 4 μg total cellular RNA per lane (Silhavy and Maliga, 1998). Double-stranded DNA probes were prepared by random-primed 32P-labeling. The template for probing neo was a gel-purified NheI-XbaI fragment excised from plasmid pHK30. The template for probing the tobacco cytoplasmic 25S rRNA was a fragment PCR amplified from total tobacco cellular DNA with primers 5′-TCACCTGCCGAATCAACTAGC-3′ and 5′-GACTTCCCTTGCCTACATTG-3′. RNA hybridization signals were quantified using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and normalized to the cytoplasmic 25S rRNA signal.
SDS-PAGE and Immunoblot Analysis
Leaves for protein extraction were taken from plants grown in sterile culture. To obtain total soluble leaf protein, about 200 mg of leaf was homogenized in 1 mL of buffer containing 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]/KOH (pH 7.5), 10 mm potassium acetate, 5 mm magnesium acetate, 1 mm EDTA, 1 mm dithiothreitol, and 2 mm phenylmethanesulfonyl fluoride. Protein concentrations were determined by the Bradford Protein Assay reagent kit (Bio-Rad, Hercules, CA). Immunoblot analysis of NPTII accumulation was carried out as described (Carrer et al., 1993). NPTII was quantified on the immunoblots by densitometric analysis with the DensoSpot program of Alpha Imager 2000 (Alpha Innotech, San Leandro, CA) by comparison of the experimental samples with a dilution series of commercial NPTII (5Prime→3Prime, Inc., Boulder, CO).
Footnotes
This research was supported by the National Science Foundation (grant no. MCB 96–30763).
LITERATURE CITED
- Alexander C, Faber N, Klaff P. Characterization of protein-binding to the spinach chloroplast psbA mRNA 5′ untranslated region. Nucleic Acids Res. 1998;26:2265–2272. doi: 10.1093/nar/26.10.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison LA, Simon LD, Maliga P. Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J. 1996;15:2802–2809. [PMC free article] [PubMed] [Google Scholar]
- Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10:411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Beck E, Ludwig G, Auerswald EA, Reiss B, Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982;19:327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Bonham-Smith PC, Bourque DP. Translation of chloroplast-encoded mRNA: potential initiation and termination signals. Nucleic Acids Res. 1989;17:2057–2080. doi: 10.1093/nar/17.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK, Mayfield SP. Light-activated translation of chloroplast mRNAs. Trends Plant Sci. 1999;4:190–195. doi: 10.1016/s1360-1385(99)01402-8. [DOI] [PubMed] [Google Scholar]
- Carrer H, Hockenberry TN, Svab Z, Maliga P. Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet. 1993;241:49–56. doi: 10.1007/BF00280200. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S, Maliga P. Sequences directing C to U editing of the plastid psbL mRNA are located within a 22 nucleotide segment spanning the editing site. EMBO J. 1996;15:5958–5964. [PMC free article] [PubMed] [Google Scholar]
- Danon A. Translational regulation in the chloroplast. Plant Physiol. 1997;115:1293–1298. doi: 10.1104/pp.115.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Inouye M. Translational enhancement by an element downstream of the initiation codon in Escherichia coli. J Biol Chem. 1999;274:10079–10085. doi: 10.1074/jbc.274.15.10079. [DOI] [PubMed] [Google Scholar]
- Faxén M, Plumbridge J, Isaksson LA. Codon choice and potential complementarity between mRNA downstream of the initiation codon and bases 1471–1480 in 16S ribosomal RNA affects expression of glnS. Nucleic Acids Res. 1991;19:5247–5251. doi: 10.1093/nar/19.19.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzetti B, Carol P, Mache R. Characterization and RNA-binding properties of a chloroplast S1-like ribosomal protein. J Biol Chem. 1992;267:19075–19081. [PubMed] [Google Scholar]
- Hess WR, Börner T. Organellar RNA polymerases of higher plants. Int Rev Cytol. 1999;190:1–59. doi: 10.1016/s0074-7696(08)62145-2. [DOI] [PubMed] [Google Scholar]
- Hirose T, Kusumegi T, Sugiura M. Translation of tobacco chloroplast rps14 mRNA depends on a Shine-Dalgarno-like sequence in the 5′-untranslated region but not on internal RNA editing in the coding region. FEBS Lett. 1998;430:257–260. doi: 10.1016/s0014-5793(98)00673-5. [DOI] [PubMed] [Google Scholar]
- Hirose T, Sugiura M. Cis-acting elements and trans-acting factors for accurate translation of chloroplast psbA mRNAs: development of an in vitro translation system from tobacco chloroplasts. EMBO J. 1996;15:1687–1695. [PMC free article] [PubMed] [Google Scholar]
- Houtz RL, Stults JT, Mulligan RM, Tolbert NE. Post-translational modifications in the large subunit of ribulose bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci USA. 1989;86:1855–1859. doi: 10.1073/pnas.86.6.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:494–500. doi: 10.1016/0958-1669(95)80082-4. [DOI] [PubMed] [Google Scholar]
- Khan MS, Maliga P. Fluorescent antibiotic resistance marker to track plastid transformation in higher plants. Nat Biotechnol. 1999;17:910–915. doi: 10.1038/12907. [DOI] [PubMed] [Google Scholar]
- Makrides SC. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60:512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JE, Brimacombe R. Prokaryotic translation: the interactive pathway leading to initiation. Trends Genet. 1994;10:402–407. doi: 10.1016/0168-9525(94)90057-4. [DOI] [PubMed] [Google Scholar]
- McCormac DJ, Barkan A. A nuclear gene in maize required for the translation of the chloroplast atpB/E mRNA. Plant Cell. 1999;11:1709–1716. doi: 10.1105/tpc.11.9.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for the growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nakamura Y, Gojobori T, Ikemura T. Codon usage tabulated from the international DNA sequence databases: its status 1999. Nucleic Acids Res. 1999;27:292. doi: 10.1093/nar/27.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M, Asai T, Squires CL, Dahlberg AE. Enhancement of translation by the downstream box does not involve base pairing of mRNA with the penultimate stem sequence of the 16S rRNA. Proc Natl Acad Sci USA. 1999;96:8973–8978. doi: 10.1073/pnas.96.16.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco EMJ, Chen LJ, Eilers RJ. The divergently transcribed rbcL and atpB genes of tobacco plastid DNA are separated by nineteen base pairs. Curr Genet. 1990;17:65–71. doi: 10.1007/BF00313250. [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Heintzen C, Seidenbecher C, Parthier B. A methyl jasmonate-induced shift in the length of the 5′ untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J. 1993;12:1505–1512. doi: 10.1002/j.1460-2075.1993.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix JD. Post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Plant Mol Biol. 1996;32:327–341. doi: 10.1007/BF00039389. [DOI] [PubMed] [Google Scholar]
- Shiina T, Allison L, Maliga P. rbcL transcript levels in tobacco plastids are independent of light: reduced dark transcription rate is compensated by increased mRNA stability. Plant Cell. 1998;10:1713–1722. doi: 10.1105/tpc.10.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H, Sugiura M. Fine structural features of the chloroplast genome: comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991;19:983–995. doi: 10.1093/nar/19.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsabayashi T, Zaita N, Chungwongse J, Obokata J, Yamaguchi-Shinozaki K, Deno H, Kamogashira T, Yamada K, Kasuda J, Takaiwa F, Kato A, Todoh N, Shimada H, Sugiura M. The complete sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982;20:91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Silhavy D, Maliga P. Mapping of the promoters for the nucleus-encoded plastid RNA polymerase (NEP) in the iojap maize mutant. Curr Genet. 1998;33:340–344. doi: 10.1007/s002940050345. [DOI] [PubMed] [Google Scholar]
- Sprengart ML, Fuchs E, Porter AG. The downstream box: an efficient and independent translation initiation signal in Escherichia coli. EMBO J. 1996;15:665–674. [PMC free article] [PubMed] [Google Scholar]
- Staub JM, Maliga P. Accumulation of D1 polypeptide in tobacco plastids is regulated via the untranslated region of the psbA mRNA. EMBO J. 1993;12:601–606. doi: 10.1002/j.1460-2075.1993.tb05692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub JM, Maliga P. Extrachromosomal elements in tobacco plastids. Proc Natl Acad Sci USA. 1994a;91:7468–7472. doi: 10.1073/pnas.91.16.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub JM, Maliga P. Translation of psbA mRNA is regulated by light via the 5′-untranslated region in tobacco plastids. Plant J. 1994b;6:547–553. doi: 10.1046/j.1365-313x.1994.6040547.x. [DOI] [PubMed] [Google Scholar]
- Stern DB, Higgs DC, Yang JJ. Transcription and translation in chloroplasts. Trends Plant Sci. 1997;2:308–315. [Google Scholar]
- Sugita M, Sugiura M. Regulation of gene expression in chloroplasts of higher plants. Plant Mol Biol. 1996;32:315–326. doi: 10.1007/BF00039388. [DOI] [PubMed] [Google Scholar]
- Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera A, Sugiura M. Chloroplast rRNA transcription from structurally different tandem promoters: an additional novel-type promoter. Curr Genet. 1995;27:280–284. doi: 10.1007/BF00326161. [DOI] [PubMed] [Google Scholar]
- Zoubenko OV, Allison LA, Svab Z, Maliga P. Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res. 1994;22:3819–3824. doi: 10.1093/nar/22.19.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]