Abstract
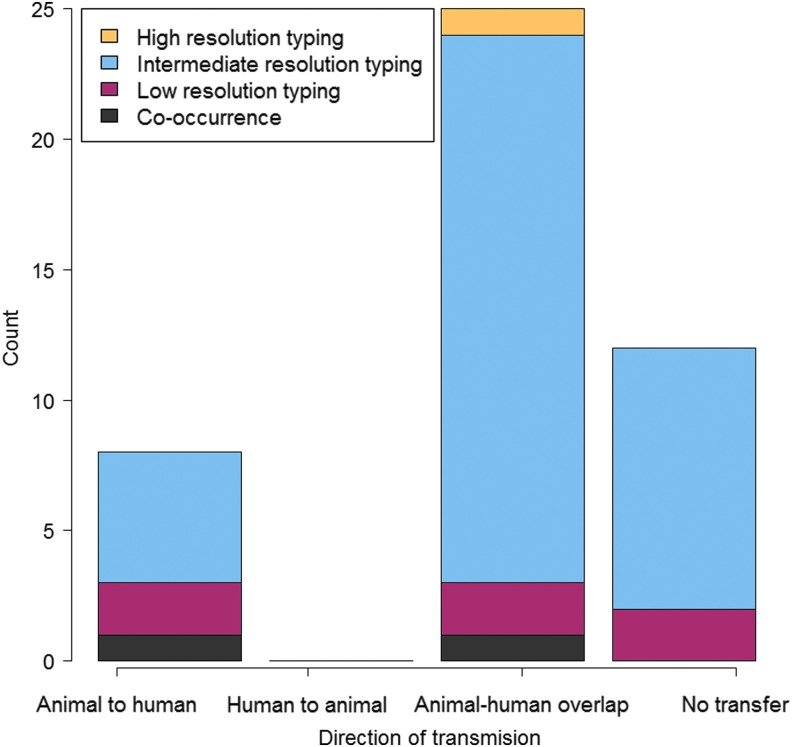
The role of farm animals in the emergence and dissemination of both AMR bacteria and their resistance determinants to humans is poorly understood and controversial. Here, we systematically reviewed the current evidence that food animals are responsible for transfer of AMR to humans. We searched PubMed, Web of Science, and EMBASE for literature published between 1940 and 2016. Our results show that eight studies (18%) suggested evidence of transmission of AMR from food animals to humans, 25 studies (56%) suggested transmission between animals and humans with no direction specified and 12 studies (26%) did not support transmission. Quality of evidence was variable among the included studies; one study (2%) used high resolution typing tools, 36 (80%) used intermediate resolution typing tools, six (13%) relied on low resolution typing tools, and two (5%) based conclusions on co-occurrence of resistance. While some studies suggested to provide evidence that transmission of AMR from food animals to humans may occur, robust conclusions on the directionality of transmission cannot be drawn due to limitations in study methodologies. Our findings highlight the need to combine high resolution genomic data analysis with systematically collected epidemiological evidence to reconstruct patterns of AMR transmission between food animals and humans.
Keywords: : antimicrobial resistance, Escherichia coli, food animals, humans, systematic review
Introduction
The evolution of microbial pathogens that enables them to evade antimicrobial treatment has been regarded as a serious public health threat (Davies, 2011; WHO, 2015; O'Neill, 2016).
At present, the role of farm animals in the emergence and dissemination of both antimicrobial resistance (AMR) bacteria and their resistance determinants to humans is poorly understood and controversial (Marshall and Levy, 2011; Woolhouse et al., 2015). Various studies have suggested that AMR bacteria and their AMR determinants can be transmitted from food animals to humans via direct contact and/or through animal products (Howells and Joynson, 1975; Aminov and Mackie, 2007; Jakobsen et al., 2010; Overdevest et al., 2011; Kluytmans et al., 2013; Voets et al., 2013). However, most of these studies have relied heavily on traditional microbiology and molecular tools, such as pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). These tools may not have sufficient discriminatory power to provide evidence of the transmission (or not) of resistant bacteria and their AMR determinants and, importantly, to infer the direction of the transmission (de Been et al., 2014; Woolhouse et al., 2015). Two key pathways of transfer of resistant bacteria and their AMR determinants from food animals to humans have been hypothesized: (i) horizontal transmission of AMR genes of food animal origin and (ii) clonal transfer of resistant bacteria of food animal origin to humans (Lipsitch et al., 2002; Chang et al., 2015). Evidence from a recent systematic review suggests that a proportion of human cephalosporin-resistant Escherichia coli (E. coli) clones, often associated with human disease, originate from food animals through food products (Lazarus et al., 2015), though these products could have been contaminated elsewhere in the production chain (Wooldridge, 2012).
Evidence either supporting or refuting the claim that dissemination of AMR bacteria or their resistance determinants from food animals to humans is occurring will be key to the development of effective policies on antibiotic stewardship and infection control for both human and animal health. To address this knowledge gap, we performed a systematic review to (i) explore the current evidence that food animals are of the source of resistant E. coli and their AMR determinants in humans, (ii) examine and summarize the kinds of evidence used to support, or not support, transfer of resistant E. coli and their AMR determinants to humans, and (iii) make recommendations for future studies to address this question. E. coli is found in both human and food animal populations (Neidhardt et al., 1996), and it has recently been categorized as one of the priority pathogens that pose the greatest threat to human health due to widespread AMR (WHO, 2017). It is for these reasons that, when considering transmission between hosts, we chose to focus on E. coli.
Methods
Data sources and search strategy
A systematic literature search according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Liberati et al., 2009) was performed. Searches were carried out in multiple electronic databases: PubMed, Web of Science, and EMBASE for research articles published between 1940 and 2016; and Scopus for research articles published between 1960 and 2016 without geographical and language restriction. We did initial and subsequent keyword searches with various combinations of search terms: E. coli, AMR terminologies, human, and food animal descriptors (Supplementary Data; Supplementary Data are available online at www.liebertpub.com/fpd).
Selection criteria and data extraction
Articles were included if they comprised an original research published in a peer reviewed journal, and investigated transmission of resistant E. coli and/or AMR determinants between humans and food animals. Articles were excluded if (i) they reported only agents other than E. coli; (ii) they studied nonfood animals; (iii) they focused exclusively on food animals or humans without any overlap between the two populations and/or (iv) they focused exclusively on food of animal origin. Article searches and screening were performed by considering article titles and abstracts for inclusion according to the search criteria. Data extraction from studies was performed by one author (D.M.M.) and independently checked by another author (B.v.B.) using a customized checklist.
Data analysis
For all included studies we categorized the direction of AMR transmission according to the authors' conclusions: (i) studies suggesting to provide evidence of transmission from food animals to humans with direction specified; (ii) studies suggesting to provide evidence of transmission from humans to food animals with direction specified; (iii) studies suggesting overlap indicating the possibility of between-host AMR transmission, with no direction specified; and (iv) studies suggesting no evidence of transmission in either direction.
The quality of evidence was assessed using a customized Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (Godfray et al., 2013). Each article was matched to the following categories: (i) high resolution typing: studies using whole genome sequencing (WGS) and phylogenetic analysis; (ii) intermediate resolution typing: studies carrying out genetic characterisation through molecular tools such as MLST; (iii) low resolution typing: studies using tools such as PFGE; or (iv) co-occurrence of resistances: studies comparing AMR phenotypes between the two populations.
Additionally, we assessed the methodological quality of the articles included in the review by adapting a standardized quality assessment (Centre for Reviews Dissemination, 2009). Each article was evaluated based on two items aimed at assessing potential biases including study design (a ctive, passive) and spatiotemporal matching (no matching, temporal matching only, spatial matching only, and both temporal and spatial matching).
Because of heterogeneity of the studies (regarding typing tools, antibiotics investigated and quality of evidence) we did not perform a meta-analysis. However, we used Fisher's exact tests using R package “stats” (R Core Team, 2017) to describe associations between direction of transmission, selection bias variables and nature of transmission (clonal, determinant or both). We considered p < 0.05 to be statistically significant.
Results
Description of included studies
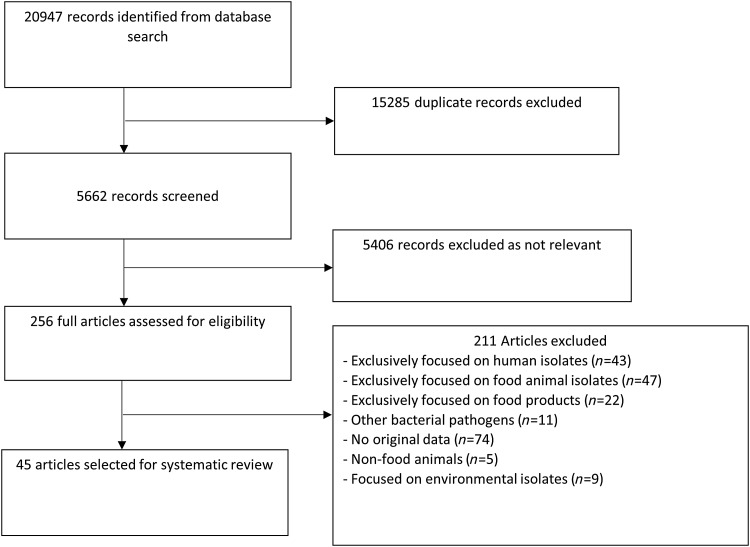
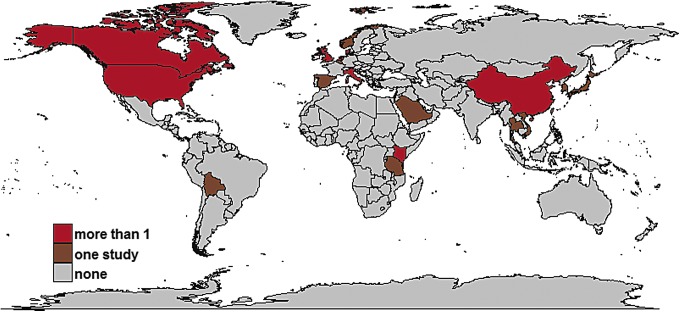
Of the 5662 distinct articles retrieved, 256 studies were reviewed (Fig. 1); and 45 studies met all inclusion criteria (Supplementary Table S1 in the Supplementary Data). The 45 studies were geographically diverse and included 20 countries, with 26 from Europe, 11 from Asia, five from North America, two from Africa, and one from the Middle East (Fig. 2).
FIG. 1.
Flow diagram showing the selection of studies for inclusion.
FIG. 2.
Geographic distribution of included studies. Different colors show the number of articles from each country. The map was created using several R packages [ggplot2 (Wickham et al., 2013), mapdata (Becker and Wilks, 2016), maps (Becker and Wilks, 2017), and ggmap (Kahle and Wickham, 2013)] in R version 3.4.1. The shapefile with borders of countries is freely available from the Natural Earth data set (www.naturalearthdata.com). Color images available online at www.liebertpub.com/fpd
Although the studies span five decades, there has been an increasing number of studies on this subject in recent years; with 56% of the studies published since 2010 (Supplementary Fig. S1 in the Supplementary Data). Twenty two studies (49%) had both temporal and spatial matching for human and food animal sampling, while seven (16%) had temporal matching only, and 16 (35%) were not temporally or spatially matched. We found no statistical associations between whether direction of transmission was inferred and study design or spatiotemporal matching.
Studies in our review reported different livestock species, either alone or in combination with other species. Of the eight studies that suggested transfer of AMR from food animals to humans, seven studies were based on poultry isolates and one study on pig isolates (Supplementary Fig. S2 in the Supplementary Data). Among the studies, 13 antibiotic classes were reported, either alone or in combination with other classes (Supplementary Fig. S3 in the Supplementary Data).
Overall, eight studies (18%) suggested to have data to support transfer of AMR bacteria and/or their AMR determinants from food animals to humans (Levy, 1978; Al-Ghamdi et al., 1999; van den Bogaard et al., 2001; Hammerum et al., 2006; Johnson et al., 2006; Leverstein-van Hall et al., 2011; Giufre et al., 2012; Dierikx et al., 2013), while 25 studies (56%) presented data showing overlap of AMR bacteria and AMR determinants between food animals and humans, indicating the possibility of between-host AMR transmission but with no direction specified (Jorgensen, 1983; Oppegaard et al., 2001; Winokur et al., 2001; Ho et al., 2009, 2010; Moodley and Guardabassi, 2009; Mulvey et al., 2009; Smet et al., 2009; Zhang et al., 2009; Jakobsen et al., 2010, 2011; Zhao et al., 2010; Deng et al., 2011; Vieira et al., 2011; Stokes et al., 2012; Ciccozzi et al., 2013; Hu et al., 2013; de Been et al., 2014; Hammerum et al., 2014; Valentin et al., 2014; Dahms et al., 2015; Dohmen et al., 2015; Huijbers et al., 2015; Lupindu et al., 2015; Tseng et al., 2015), and 12 studies (26%) did not suggest to find evidence supporting transmission between food animals and humans (Kariuki et al., 1997, 1999; Maynard et al., 2004; Kang et al., 2005; Phongpaichit et al., 2007; Graziani et al., 2009; Schwaiger et al., 2010; Xia et al., 2010; Johnson et al., 2012; Riccobono et al., 2012; Jakobsen et al., 2015; Ueda et al., 2015). No study in our review suggested to provide evidence for AMR transmission from humans to food animals (Fig. 3).
FIG. 3.
Nature of evidence used to infer direction of transmission in each study. Color images available online at www.liebertpub.com/fpd
Only one study (2%) based its conclusion regarding transmission on high resolution typing tools, 36 studies (80%) on intermediate resolution typing tools, six (13%) on low resolution typing tools, and two (5%) on co-occurrence of resistances (Fig. 3). Overall, 18 (40%) studies based their conclusion on transmission of AMR determinants, nine (20%) on transmission of AMR bacteria, and 18 (40%) transmission of AMR bacteria together with AMR determinants (Supplementary Fig. S4 in the Supplementary Data). We found no statistical association between whether direction of transmission was inferred and the nature of transmission (p = 0.33).
Studies suggesting to provide evidence of transmission of AMR from food animals to humans with direction specified
Three studies suggested to find evidence for transfer of AMR bacteria from food animals to humans, two of which concluded there is transfer of resistant clones from poultry to humans (Al-Ghamdi et al., 1999; van den Bogaard et al., 2001). In addition to overlapping clonal patterns, one study reported that human and chicken isolates were resistant to spectinomycin, an antibiotic mostly used in veterinary medicine (Al-Ghamdi et al., 1999). Similarly, one study (van den Bogaard et al., 2001) reported a higher prevalence of ciprofloxacin resistance among food animal isolates compared to human isolates.
One study found identical ciprofloxacin-resistant isolates in chicken and humans, which they concluded was suggestive of food animal to human AMR transmission (Johnson et al., 2006). Two studies suggested to find evidence for horizontal transfer of AMR determinants from food animals to humans (Hammerum et al., 2006; Dierikx et al., 2013). One study found that clonally unrelated poultry and human isolates shared ESBL/AmpC genes located on identical plasmid families (Dierikx et al., 2013). Another study found that sulfonamide-resistant isolates from pigs and healthy humans shared sul1 and sul2 genes (Hammerum et al., 2006).
Three studies suggested to support transmission of both AMR bacteria and their AMR determinants from food animals to humans. Two studies found similar sequence types, plasmid families and ESBL genes in E. coli isolates sourced from poultry and human patients (Leverstein-van Hall et al., 2011; Giufre et al., 2012). A further study reported an increase in tetracycline-resistant E. coli in humans in contact with tetracycline fed chicken and, therefore, suggested that chicken were a reservoir of AMR bacteria and plasmids for humans (Levy, 1978).
We found that studies suggesting to provide evidence of transmission of AMR from food animals to humans did not have distinct features compared to those suggesting overlap of resistance, with regard to study methodologies, food animal species, typing tools, or antibiotics tested. For most of these it is unclear why they suggested evidence of directional transmission when 25 broadly similar studies suggested only overlap of resistance.
Studies suggesting overlap indicating the possibility of between-host AMR transmission, with no direction specified
Four studies suggested there was evidence of overlap of resistant E. coli between humans and food animals. One of these studies found human and avian sequence types associated with multidrug resistance clustered together in a Bayesian phylogenetic tree (Ciccozzi et al., 2013). Another study found indistinguishable PFGE patterns of ampicillin and tetracycline-resistant isolates in cattle and humans (Lupindu et al., 2015). A cluster analysis of E. coli phylogroups found that human, pig, and chicken isolates clustered together (Jakobsen et al., 2010). One extensive ecological study reported a significant correlation between the prevalence of resistance in human and livestock isolates, for both cephalosporins and fluoroquinolones (Vieira et al., 2011).
Thirteen studies suggested there was evidence of overlap of AMR determinants in human and food animal isolates. Of the 13 studies, one study based on WGS and plasmid reconstruction found that clonally unrelated human and poultry isolates carried ESBL genes encoded on genetically identical plasmids (de Been et al., 2014). Eleven studies found that unrelated human and food animal isolates shared identical AMR genes, integrons and plasmids (Oppegaard et al., 2001; Winokur et al., 2001; Ho et al., 2009, 2010; Moodley and Guardabassi, 2009; Mulvey et al., 2009; Smet et al., 2009; Zhang et al., 2009; Stokes et al., 2012; Huijbers et al., 2015; Tseng et al., 2015). One study identified identical plasmids encoding chloramphenicol resistance in unrelated human and food animal isolates (Jorgensen, 1983).
Eight studies suggested there was evidence of overlap of resistant E. coli and AMR determinants, with five of these finding that clonally related human and food animal isolates harbored similar ESBL gene types and plasmid types (Hu et al., 2013; Hammerum et al., 2014; Valentin et al., 2014; Dahms et al., 2015; Dohmen et al., 2015). Likewise, two studies found that clonally related human and food animal isolates carried similar fluoroquinolone AMR genes (Zhao et al., 2010; Deng et al., 2011). In one study, cluster analysis of AMR gene profiles and E. coli pathotypes showed that human and food animal isolates clustered together (Jakobsen et al., 2011).
Studies suggesting no evidence of transmission of AMR between humans and food animals
Two studies found no evidence for transfer of resistant clones, with one of these studies finding that human and avian ciprofloxacin-resistant E. coli strains had distinct phylogenetic compositions (Graziani et al., 2009). Likewise, a PFGE analysis of multidrug-resistant E. coli isolates from sympatric children and chicken found that the isolates were source specific (Kariuki et al., 1999).
Three studies reported no evidence for transfer of AMR determinants between food animals and humans with one of these studies reporting that human and porcine isolates had different distribution patterns of sulfonamide and tetracycline resistance genes (Schwaiger et al., 2010). Two studies (Kariuki et al., 1997; Phongpaichit et al., 2007) reported that human and food animal multidrug-resistant isolates had distinct plasmids and integrons.
Seven studies reported no evidence for transmission of bacterial clones together with AMR determinants between food animals and humans. These studies showed that human and food animal isolates belonged to different phylogenetic groups, and had different AMR genes and plasmid profiles (Maynard et al., 2004; Kang et al., 2005; Xia et al., 2010; Johnson et al., 2012; Riccobono et al., 2012; Jakobsen et al., 2015; Ueda et al., 2015).
Discussion
We performed a systematic review to explore the evidence that food animals are responsible for the transfer of AMR E. coli and their AMR determinants to humans. Some studies in our review suggested to provide evidence for the transfer of AMR from and between food animals and humans, while a larger number did not suggest to provide evidence of transmission in either direction. In addition to the differing nature of methods used to infer direction, studies in our review differed in sampling methodologies and antibiotics tested. These differences may have affected the conclusions made regarding the epidemiological connection between food animals and humans.
Much of the evidence regarding transfer of AMR was based on the demonstration that AMR E. coli clones and AMR determinants were indistinguishable in both food animal and human isolates. However, the demonstration of overlapping patterns should be interpreted with care as the direction of transmission is difficult to infer, and co-colonization from a shared source is also possible. Demonstrating the direction of transmission and thus the epidemiological history of pathogens and their determinants requires a quantitative description of relatedness, including phylogenetic analysis (Grad and Lipsitch, 2014).
Molecular techniques, such as MLST and PCR, used in most studies in our review, are limited in resolution (Didelot et al., 2014). In one study, E. coli isolates were considered genetically indistinguishable based on MLST suggesting clonal transfer (Leverstein-van Hall et al., 2011); however, subsequent WGS revealed that the isolates were genetically distinct (de Been et al., 2014), highlighting the need for sequencing the entire genome, rather than only a few loci. WGS provides the current “gold standard” resolution for studying genetic relatedness, but as it is a technology that has only recently become routinely available it was used in just one study in our review. Future studies in this area could benefit from combining phylogeographic methods with WGS, which yields the potential for quantitative hypothesis testing for inferring pathogen movement between host populations (De Maio et al., 2015; Woolhouse et al., 2015).
Just over half of the studies in our review did not consider spatiotemporal relationships between human and food animal isolates, a fundamental requirement for investigating transmission (Singer et al., 2006). Future research on the directionality of transmission will benefit from designing studies in which epidemiologically linked human and food animal populations are systematically sampled, preferably longitudinally (Woolhouse et al., 2015). Moreover, there is considerable diversity within both human populations (i.e., healthy individuals vs. hospitalized patients) and food animals (i.e., free range vs. intensive farming) and the specific population considered may impact their exposure to diverse groups of bacteria; thus we recommend that future studies investigating transmission of AMR between humans and food animals clearly clarify the subpopulations studied. In addition, inclusion of detailed data on antibiotic usage in these populations should be considered.
None of the included studies provided a detailed overview of antibiotic usage in either human or food animal populations, or association between antibiotic usage and subsequent development of AMR. A recent systematic review has indicated that interventions that limit antibiotic use in food animals are associated with a reduction of AMR development in humans (Tang et al., 2017), and therefore further research is warranted to explore this complex association.
Although transfer of AMR from humans to food animals is likely (Barber, 2001; Wooldridge, 2012), none of the studies in our review suggested to find evidence to support transmission from humans to animals. In many instances, responsibility for the burden of AMR has been placed on food animals (Barber, 2001; Woolhouse et al., 2015; Mendelson et al., 2017), and thus study bias may exist in terms of source attribution. Therefore, more research is needed to provide evidence for this potential route of transfer and, importantly, the relative magnitude of that spread.
Akin to the studies in our review, most AMR studies focus on a single bacterial type; however, rapid dissemination of AMR determinants frequently occurs between bacterial species, making it hard to track infection source (Sheppard et al., 2016). Tracking these determinants, frequently located on plasmids, using traditional molecular techniques may be limited. Using long read sequencing technologies such as Pacbio can overcome this by accurately generating plasmid structures (Orlek et al., 2017).
Our systematic review excluded studies focusing on transmission of resistant bacteria and/or their AMR determinants through food animal-sourced food products. However, we acknowledge the potentially significant role played by food products of food animal origin in dissemination of AMR as reported in a recent systematic review (Lazarus et al., 2015).
We have highlighted studies that suggest to provide evidence for transfer of resistant E. coli and their AMR determinants from food animals to humans. However, differences in study methodologies, such as lack of spatiotemporal overlap in sample collection, and the quality of typing tools used, suggest that transmission may occur, the evidence used to support the hypothesis is rarely compelling. The underlying problem is that demonstrating similarity or identity of AMR bacteria and/or AMR resistance determinants does not, by itself, provide information on directionality of transfer; this could be in either direction, or both, or neither but from a different source. Information on differential prevalence of resistance, and consumption of antibiotics, in the two populations may make stronger inference possible, but these data are rarely available.
Taken together, by combining genomic data analysis and epidemiological approaches it may be possible to reconstruct the complex transmission dynamics of resistant bacteria and their AMR determinants between human and food animal populations. Although we still have some way to go before a truly comprehensive integration of data—differential antibiotic usage data, detailed denominator data, information about the origin of the samples, human-food animal contact data, and pathogen sequence data—is available, disentangling and quantifying transmission of resistant bacteria and their AMR determinants between humans and food animals may still be an attainable goal.
Supplementary Material
Acknowledgments
This study was supported by the UK Medical Research Council, Biotechnology and Biological Science Research Council (UK), the Economic and Social Research Council (UK), the Natural Environment Research Council (UK), through the Environmental & Social Ecology of Human Infectious Diseases Initiative (ESEI), Grant Reference: G1100783/1. This work also received support from the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). We also acknowledge the CGIAR Fund Donors (www.cgiar.org/who-we-are/cgiar-fund/fund-donors-2). D.M was supported by the Darwin Trust of Edinburgh and Centre for Immunology, Infection and Evolution (CIIE). M.J.W. was supported by a Sir Henry Wellcome Postdoctoral Fellowship from the Wellcome Trust (WT103953MA). B.A.D.vB was funded through the project “Selection and Transmission of Antimicrobial Resistance in Complex Systems [STARCS]” in the Joint Programming Initiative on Antimicrobial Resistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Al-Ghamdi MS, El-Morsy F, et al. . Antibiotic resistance of Escherichia coli isolated from poultry workers, patients and chicken in the eastern province of Saudi Arabia. Trop Med Int Health 1999;4:278–283 [DOI] [PubMed] [Google Scholar]
- Aminov RI, Mackie RI. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol Lett 2007;271:147–161 [DOI] [PubMed] [Google Scholar]
- Barber DA. New perspectives on transmission of foodborne pathogens and antimicrobial resistance. J Am Vet Med Assoc 2001;218:1559–1561 [DOI] [PubMed] [Google Scholar]
- Becker RA, Wilks AR. mapdata: Extra Map Databases, version 2.30 2016
- Becker RA, Wilks AR. maps: Draw Geographical Maps version 3.3.0 2017
- Centre for Reviews Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York, UK: Centre for Reviews and Dissemination, University of York, 2009 [Google Scholar]
- Chang Q, Wang W, et al. . Antibiotics in agriculture and the risk to human health: How worried should we be? Evol Appl 2015;8:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccozzi M, Giufre M, et al. . Phylogenetic analysis of multidrug-resistant Escherichia coli clones isolated from humans and poultry. New Microbiol 2013;36:385–394 [PubMed] [Google Scholar]
- Dahms C, Hubner NO, et al. . Occurrence of ESBL-Producing Escherichia coli in Livestock and Farm Workers in Mecklenburg-Western Pomerania, Germany. PLoS One 2015;10:e0143326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SC. Infections and the Rise of Antimicrobial Resistance–Annual Report of the Chief Medical Officer, ed. Vol. 2 London: Department of Health, 2011 [DOI] [PubMed] [Google Scholar]
- de Been M, Lanza VF, et al. . Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet 2014;10:e1004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio N, Wu C-H, et al. . New routes to phylogeography: A bayesian structured coalescent approximation. PLoS Genet 2015;11:1005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Zeng Z, et al. . Dissemination of IncFII plasmids carrying rmtB and qepA in Escherichia coli from pigs, farm workers and the environment. Clin Microbiol Infect 2011;17:1740–1745 [DOI] [PubMed] [Google Scholar]
- Didelot X, Gardy J, et al. . Bayesian inference of infectious disease transmission from whole-genome sequence data. Mol Biol Evol 2014;31:1869–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierikx CM, van der Goot JA, et al. . Presence of ESBL/AmpC -Producing Escherichia coli in the broiler production pyramid: A descriptive study. PLoS One 2013;8:e79005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen W, Bonten MJM, et al. . Carriage of extended-spectrum beta-lactamases in pig farmers is associated with occurrence in pigs. Clin Microbiol Infect 2015;21:917–923 [DOI] [PubMed] [Google Scholar]
- Giufre M, Graziani C, et al. . Escherichia coli of human and avian origin: Detection of clonal groups associated with fluoroquinolone and multidrug resistance in Italy. J Antimicrob Chemother 2012;67:860–867 [DOI] [PubMed] [Google Scholar]
- Godfray HCJ, Donnelly CA, et al. . A restatement of the natural science evidence base relevant to the control of bovine tuberculosis in Great Britain. Proc Biol Sci 2013;280:20131634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad YH, Lipsitch M, Epidemiologic data and pathogen genome sequences: A powerful synergy for public health. Genome Biol 2014;15:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani C, Luzzi I, et al. . Phylogenetic background and virulence genotype of ciprofloxacin-susceptible and ciprofloxacin-resistant Escherichia coli strains of human and avian origin. J Infect Dis 2009;199:1209–1217 [DOI] [PubMed] [Google Scholar]
- Hammerum AM, Larsen J, et al. . Characterization of extended-spectrum [beta]-lactamase (ESBL)-producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third- or fourth-generation cephalosporins. J Antimicrob Chemother 2014;69:2650–2657 [DOI] [PubMed] [Google Scholar]
- Hammerum AM, Sandvag D, et al. . Detection of sul1, sul2 and sul3 in sulphonamide resistant Escherichia coli isolates obtained from healthy humans, pork and pigs in Denmark. Int J Food Microbiol 2006;106:235–237 [DOI] [PubMed] [Google Scholar]
- Ho P-L, Wong RC, et al. . Genetic identity of aminoglycoside-resistance genes in Escherichia coli isolates from human and animal sources. J Med Microbiol 2010;59:702–707 [DOI] [PubMed] [Google Scholar]
- Ho PL, Wong RC, et al. . Distribution of integron-associated trimethoprim-sulfamethoxazole resistance determinants among Escherichia coli from humans and food-producing animals. Lett Appl Microbiol 2009;49:627–634 [DOI] [PubMed] [Google Scholar]
- Howells CH, Joynson DH. Possible role of animal feeding-stuffs in spread of antibiotic-resistant intestinal coliforms. Lancet Infect Dis 1975;1:156–157 [DOI] [PubMed] [Google Scholar]
- Hu YY, Cai JC, et al. . Molecular typing of CTX-M-Producing Escherichia coli isolates from environmental water, swine feces, specimens from healthy humans, and human patients. Appl Environ Microbiol 2013;79:5988–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers PMC, van Hoek AHAM, et al. . Methicillin-resistant Staphylococcus aureus and extended-spectrum and AmpC beta-lactamase-producing Escherichia coli in broilers and in people living and/or working on organic broiler farms. Vet Microbiol 2015;176:120–125 [DOI] [PubMed] [Google Scholar]
- Jakobsen L, Bortolaia V, et al. . Limited similarity between plasmids encoding CTX-M-1 beta-lactamase in Escherichia coli from humans, pigs, cattle, organic poultry layers and horses in Denmark. J Global Antimicrob Resist 2015;3:132–136 [DOI] [PubMed] [Google Scholar]
- Jakobsen L, Garneau P, et al. . Microarray-based detection of extended virulence and antimicrobial resistance gene profiles in phylogroup B2 Escherichia coli of human, meat and animal origin. J Med Microbiol 2011;60:1502–1511 [DOI] [PubMed] [Google Scholar]
- Jakobsen L, Kurbasic A, et al. . Escherichia coli isolates from broiler chicken meat, broiler chickens, pork, and pigs share phylogroups and antimicrobial resistance with community-dwelling humans and patients with urinary tract infection. Foodborne Pathogens Dis 2010;7:537–547 [DOI] [PubMed] [Google Scholar]
- Jakobsen L, Spangholm DJ, et al. . Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol 2010;142:264–272 [DOI] [PubMed] [Google Scholar]
- Johnson JR, Kuskowski MA, et al. . Similarity between human and chicken Escherichia coli isolates in relation to ciprofloxacin resistance status. J Infect Dis 2006;194:71–78 [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Logue CM, et al. . Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathogens Dis 2012;9:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen ST. Relatedness of chloramphenicol resistance plasmids in epidemiologically unrelated strains of pathogenic Escherichia coli from man and animals. J Med Microbiol 1983;16:165–173 [DOI] [PubMed] [Google Scholar]
- Kahle D, Wickham H. ggmap: Spatial Visualization with ggplot2. The R Journal 2013;5:144–161 [Google Scholar]
- Kang HY, Jeong YS, et al. . Characterization of antimicrobial resistance and class 1 integrons found in Escherichia coli isolates from humans and animals in Korea. J Antimicrob Chemother 2005;55:639–644 [DOI] [PubMed] [Google Scholar]
- Kariuki S, Gilks C, et al. . Genotype analysis of Escherichia coli strains isolated from children and chickens living in close contact. Appl Environ Microbiol 1999;65:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki S, Gilks CF, et al. . Plasmid diversity of multi-drug-resistant Escherichia coli isolated from children with diarrhoea in a poultry-farming area in Kenya. Ann Trop Med Parasitol 1997;91:87–94 [DOI] [PubMed] [Google Scholar]
- Kluytmans JAJW, Overdevest ITMA, et al. . Extended-spectrum beta-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis 2013;56:478–487 [DOI] [PubMed] [Google Scholar]
- Lazarus B, Paterson DL, et al. . Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis 2015;60:439–452 [DOI] [PubMed] [Google Scholar]
- Leverstein-van Hall MA, Dierikx CM, et al. . Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 2011;17:873–880 [DOI] [PubMed] [Google Scholar]
- Levy SB. Emergence of antibiotic-resistant bacteria in the intestinal flora of farm inhabitants. J Infect Dis 1978;137:689–690 [PubMed] [Google Scholar]
- Liberati A, Altman DG, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Singer RS, et al. . Antibiotics in agriculture: When is it time to close the barn door? Proc Natl Acad Sci USA 2002;99:5752–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupindu AM, Dalsgaard A, et al. . Transmission of antibiotic-resistant Escherichia coli between cattle, humans and the environment in peri-urban livestock keeping communities in Morogoro, Tanzania. Prev Vet Med 2015;118:477–482 [DOI] [PubMed] [Google Scholar]
- Marshall BM, Levy SB. Food animals and antimicrobials: Impacts on human health. Clin Microbiol Rev 2011;24:718–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard C, Bekal S, et al. . Heterogeneity among virulence and antimicrobial resistance gene profiles of extraintestinal Escherichia coli isolates of animal and human origin. J Clin Microbiol 2004;42:5444–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson M, Balasegaram M, et al. . Antibiotic resistance has a language problem. Nature 2017;545:23–25 [DOI] [PubMed] [Google Scholar]
- Moodley A, Guardabassi L. Transmission of IncN plasmids carrying bla(CTX-M-1) between commensal Escherichia coli in pigs and farm workers. Antimicrob Agents Chemother 2009;53:1709–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey MR, Susky E, et al. . Similar cefoxitin-resistance plasmids circulating in Escherichia coli from human and animal sources. Vet Microbiol 2009;134:279–287 [DOI] [PubMed] [Google Scholar]
- Neidhardt FC, Ingraham JL, et al. . Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd edition. Washington, DC: American Society for Microbiology, 1996 [Google Scholar]
- O'Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance, 2016
- Oppegaard H, Steinum TM, et al. . Horizontal transfer of a multi-drug resistance plasmid between coliform bacteria of human and bovine origin in a farm environment. Appl Environ Microbiol 2001;67:3732–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlek A, Stoesser N, et al. . Plasmid classification in an era of whole-genome sequencing: Application in studies of antibiotic resistance epidemiology. Front Microbiol 2017;8:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdevest I, Willemsen I, et al. . Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis 2011;17:1216–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phongpaichit S, Liamthong S, et al. . Prevalence of class 1 integrons in commensal Escherichia coli from pigs and pig farmers in Thailand. J Food Prot 2007;70:292–299 [DOI] [PubMed] [Google Scholar]
- R Core Team R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2017 [Google Scholar]
- Riccobono E, Pallecchi L, et al. . Carriage of antibiotic-resistant Escherichia coli among healthy children and home-raised chickens: A household study in a resource-limited setting. Microbial Drug Resist 2012;18:83–87 [DOI] [PubMed] [Google Scholar]
- Schwaiger K, Hoelzel C, et al. . Resistance gene patterns of tetracycline resistant Escherichia coli of human and porcine origin. Vet Microbiol 2010;142:329–336 [DOI] [PubMed] [Google Scholar]
- Sheppard AE, Stoesser N, et al. . Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 2016;60:3767–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RS, Ward MP, et al. . Can landscape ecology untangle the complexity of antibiotic resistance? Nat Rev Microbiol 2006;4:943–952 [DOI] [PubMed] [Google Scholar]
- Smet A, Martel A, et al. . Comparative analysis of extended-spectrum-β-lactamase-carrying plasmids from different members of Enterobacteriaceae isolated from poultry, pigs and humans: Evidence for a shared β-lactam resistance gene pool? J Antimicrob Chemother 2009;63:1286–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MO, Cottell JL, et al. . Detection and characterization of pCT-like plasmid vectors for bla(CTX-M-14) in Escherichia coli isolates from humans, turkeys and cattle in England and Wales. J Antimicrob Chemother 2012;67:1639–1644 [DOI] [PubMed] [Google Scholar]
- Tang KL, Caffrey NP, et al. . Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet Health 2017;1:e316–e327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S-P, Wang S-F, et al. . Characterization of fosfomycin resistant extended-spectrum beta-lactamase-producing Escherichia coli isolates from human and pig in Taiwan. PLoS One 2015;10:e0135864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Bui Thi Kim N, et al. . Limited transmission of bla(CTX-M-9)-type-positive Escherichia coli between humans and poultry in vietnam. Antimicrob Agents Chemother 2015;59:3574–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin L, Sharp H, et al. . Subgrouping of ESBL-producing Escherichia coli from animal and human sources: An approach to quantify the distribution of ESBL types between different reservoirs. Int J Med Microbiol 2014;304:805–816 [DOI] [PubMed] [Google Scholar]
- van den Bogaard AE, London N, et al. . Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother 2001;47:763–771 [DOI] [PubMed] [Google Scholar]
- Vieira AR, Collignon P, et al. . Association Between Antimicrobial Resistance in Escherichia coli Isolates from Food Animals and Blood Stream Isolates from Humans in Europe: An Ecological Study. Foodborne Pathogens Dis 2011;8:1295–1301 [DOI] [PubMed] [Google Scholar]
- Voets GM, Fluit AC, et al. . Identical plasmid AmpC beta-lactamase genes and plasmid types in E. coli isolates from patients and poultry meat in the Netherlands. Int J Food Microbiol 2013;167:359–362 [DOI] [PubMed] [Google Scholar]
- WHO. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: World Health Organization, 2015 [Google Scholar]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva, Switzerland: World Health Organization, 2017 [Google Scholar]
- Wickham H, Chang W, Wickham M. ggplot2: Elegant Graphics for Data Analysis, version 2.2.1. Heidelberg, Germany: Springer; 2013 [Google Scholar]
- Winokur PL, Vonstein DL, et al. . Evidence for transfer of CMY-2 AmpC beta-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob Agents Chemother 2001;45:2716–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge M. Evidence for the circulation of antimicrobial-resistant strains and genes in nature and especially between humans and animals. Rev Sci Tech 2012;31:231–247 [DOI] [PubMed] [Google Scholar]
- Woolhouse M, Ward M, et al. . Antimicrobial resistance in humans, livestock and the wider environment. Philos Trans R Soc B Biol Sci 2015;370:20140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L-N, Li L, et al. . A Survey of plasmid-mediated fluoroquinolone resistance genes from Escherichia coli isolates and their dissemination in Shandong, China. Foodborne Pathogens Dis 2010;7:207–215 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Ding LJ, et al. . Occurrence and characteristics of class 1 and class 2 integrons in resistant Escherichia coli isolates from animals and farm workers in northeastern China. Microb Drug Resist 2009;15:323–328 [DOI] [PubMed] [Google Scholar]
- Zhao J, Chen Z, et al. . Prevalence and Dissemination of oqxAB in Escherichia coli Isolates from Animals, Farmworkers, and the Environment. Antimicrob Agents Chemother 2010;54:4219–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.