Abstract
Cerebral amyloid angiopathy (CAA) of the Aβ type is variably present in the brains of patients with Alzheimer’s disease (AD). CAA contributes to cognitive decline and increases the risk of lobar hemorrhage; because both AD-typical dementia and lobar hemorrhage are more common in African-Americans than in Caucasians, we postulated that African-Americans with AD might be particularly susceptible to CAA. To test this hypothesis, we analyzed CAA histopathologically in the large vessels and capillaries of autopsy-derived frontal, temporal, parietal and occipital cortical samples from African-Americans (n=18) and Caucasians (n=19) with end-stage AD. In the combined cohort of 37 subjects, 22% of the subjects had severe CAA in large vessels, and 11% had severe CAA in capillaries. However, the prevalence and histopathologic characteristics of CAA were similar in the African-Americans and Caucasians. This conclusion was substantiated in an independent sample from the National Alzheimer’s Coordinating Center database, in which the degree of CAA was comparable in 1554 Caucasians and 68 African-Americans with end-stage AD. These findings support a growing consensus that the fundamental histopathologic features of Alzheimer’s disease are largely impartial to the race of the afflicted.
Keywords: Abeta, aging, amyloid, cerebrovascular amyloidosis, dementia, ethnicity, race, tauopathy, vascular disease
INTRODUCTION
Although African-Americans are more likely than Caucasians to manifest clinically diagnosed Alzheimer’s disease (AD) [1–7], the lesions that define the disease - senile (Aβ) plaques and neurofibrillary (tau) tangles - are similarly present in both groups [8, 9]. The reasons for this discrepancy are uncertain; AD is the most common form of dementia [10], but cognition can be seriously compromised in at least 50 other medical conditions [11], many of which may interact with AD-type pathology to advance symptom onset or accelerate decline [12, 13]. Ethnoracial differences in the dementia phenotype thus might reflect the influence of factors that differentially modulate or unmask the concurrent pathobiology of AD. Of these, vascular disease is a frequent co-morbid condition [14], and the presence of vascular risk factors in mid-life is associated with increased brain Aβ deposition [15] and dementia [16] in old age.
Aging African-Americans are at a relatively high risk of developing cardiovascular and metabolic disorders[17–19] as well as mixed pathologies in the brain, particularly plaques and tangles along with α-synucleinopathy (Lewy body disease) and/or infarcts[7, 20]. The contribution of cardiovascular disease to dementia has received special attention because African-Americans are especially susceptible to hypertension[19], diabetes/metabolic disorder[1, 5, 21, 22], cerebrovascular disease[7] and stroke[23–25]. Since ethnoracial differences might reflect disparities in modifiable risk factors, it is important to define the underlying pathobiology in differentially vulnerable populations.
Of particular interest is the elevated predisposition of older African-Americans to lobar hemorrhage[25], a disorder that has been linked to cerebral amyloid angiopathy (CAA)[26, 27]. Neuropathologically, CAA is characterized by the deposition of amyloid in and around the walls of cerebral blood vessels[28–30]. Several different proteins are capable of forming cerebrovascular amyloid[29], but in AD, the culpable protein is Aβ, typically a 40- or 42-amino acid cleavage product of the Aβ-precursor protein that also constitutes the cores of senile plaques[31]. Amyloid impairs the integrity of the vascular wall and increases the likelihood that affected vessels will rupture[31]. CAA also is thought to be related to diminished drainage of cerebrospinal fluid along perivascular pathways, a mechanism by which the brain eliminates toxins and waste[32]. CAA contributes to cognitive decline[33–40], and it has been associated with cortical atrophy[41] and disruption of the cerebral connectome[42]. In AD, CAA most often afflicts arterioles coursing through the leptomeninges and parenchyma of the brain; veins and capillaries are less often affected[29, 39], but when present, capillary CAA appears to have distinctive pathobiologic features[43].
Although CAA occurs to some extent in nearly all AD patients[44–46], the level of involvement varies widely, and it is considered severe in approximately 20% of cases[27, 32, 47, 48]. An earlier study provided suggestive evidence that CAA might be more common in African-Americans than in white individuals[9], but the difference between the groups was not statistically significant, and the prevalence of CAA in the white subjects was lower than expected based on the prior literature[9]. A more recent investigation of a larger cohort of subjects with dementia also indicated that CAA is more often present in African-Americans [7]. However, the sample of African-Americans also had a higher prevalence of AD-type lesions, and the severity of CAA was not assessed, leaving open the question of whether CAA differs in African-Americans with advanced AD. In light of the elevated incidence and prevalence of clinical AD[1–5, 7] and lobar hemorrhage[25] among African-Americans, along with the established link between lobar hemorrhage and CAA[31], we sought to test the hypothesis that African-Americans with AD are particularly susceptible to CAA, and that this vulnerability contributes to their elevated predisposition to dementia. Our findings instead indicate that both the prevalence and phenotype of CAA are similar in African-Americans and Caucasians with end-stage AD.
MATERIALS AND METHODS
Subjects
Thirty-seven patients who self-identified (or were identified by their family members) as African-American (n = 18; 12 females, 6 males) or non-Hispanic Caucasian (n = 19; 14 females, 5 males) served as subjects. Brains were collected at autopsy during the years spanning 2003 to 2014 at the Emory University Alzheimer’s Disease Research Center (ADRC) in Atlanta, Georgia. The two ethnoracial groups were matched as closely as possible in terms of age at death, duration of disease, postmortem interval (PMI), ApoE type, sex, and level of education (Table 1); however, the prevalence of hypertension was greater in the African-Americans than in the Caucasians (see Table 1 and the Discussion). In a supplementary analysis, we compared the degree of CAA in 1554 Caucasians and 68 African-Americans with clinically diagnosed and histopathologically confirmed advanced Alzheimer’s disease (Table 3) in a multi-institutional dataset from the National Alzheimer’s Coordinating Center (NACC) (for details, see https://www.alz.washington.edu/). To ensure independence from our primary cohort (above), this analysis excluded data from subjects furnished to NACC by Emory (a contributing institution). Note that this sample of subjects partially overlaps with the NACC sample analyzed by Graff-Radford et al. [7], except that our analysis concentrated only on those with definite advanced AD, and we evaluated the degree of CAA instead of just its presence or absence. Data specifically on capillary CAA were not available for the NACC subjects. This study was conducted under the auspices of the Emory ADRC and was approved by the Emory Institutional Review Board. Informed consent was obtained from all subjects or their family members.
TABLE 1.
Characteristics of African-Americans and Caucasians in the Emory cohort of 37 subjects with clinically diagnosed and pathologically confirmed AD.
| Variable | African-Americans (n=18) | Caucasians (n=19) | p value |
|---|---|---|---|
| Age at death (yrs)+ | 73.8 ± 12.4 | 75.1 ± 10.4 | 0.75‡ |
| Duration (yrs)+^ | 9.4 ± 4.2 | 10.8 ± 4.1 | 0.34‡ |
| PMI (hrs)+ | 17.6 ± 13.0 | 12.2 ± 6.8 | 0.12‡ |
| Gender n (%) | 0.64‡ | ||
| Male | 6 (33%) | 5 (26%) | |
| Female | 12 (67%) | 14 (74%) | |
| APOE genotype n (%)^^ | |||
| ε4/4 | 6 (33%) | 3 (17%) | |
| ε3/4 | 8 (44%) | 10 (56%) | |
| ε3/3 | 4 (22%) | 4 (22%) | |
| ε2/4 | 0 | 1 (6%) | |
| APOEε4-positive n (%)^^ | 0.61‡ | ||
| Yes | 14 (78%) | 14 (78%) | |
| No | 4 (22%) | 4 (22%) | |
| Hypertension n (%)䫨 | 14/16 (88%) | 2/15 (13%) | <0.001 |
| Education (yrs)§ | 14.6 ± 3.3 | 14.9 ± 2.4 | 0.77‡ |
All percentages are rounded to the nearest whole number
APOE: apolipoprotein E
PMI: postmortem interval
Mean ± standard deviation
Time from diagnosis to death; Data not available for 2 Caucasians
Data not available for 1 Caucasian
Data not available for 2 African-Americans and 4 Caucasians
Data not available for 6 African-Americans and 6 Caucasians
Statistically non-significant
TABLE 3.
Characteristics of African-Americans and Caucasians in the NACC cohort of 1622 subjects with clinically diagnosed and pathologically confirmed AD.
| Variable | African-Americans (n=68) | Caucasians (n=1554) | p value |
|---|---|---|---|
| Age at death (yrs)+ | 81.9 ± 9.5 | 79.9 ± 10.5 | 0.13‡ |
| Duration (yrs)+^ | 10.5 ± 4.0 | 10.3 ± 4.0 | 0.62‡ |
| PMI (hrs)+^^ | 16.4 ± 18.2 | 11.7 ± 10.2 | 0.21‡ |
| Gender n (%) | 0.02 | ||
| Male | 27 (40%) | 838 (54%) | |
| Female | 41 (60%) | 716 (46%) | |
| APOE genotype n (%)^^^ | |||
| ε4/4 | 11 (20%) | 205 (15%) | |
| ε3/4 | 24 (44%) | 597 (43%) | |
| ε3/3 | 14 (26%) | 488 (35%) | |
| ε2/4 | 1 (2%) | 46 (3%) | |
| ε2/3 | 4 (7%) | 43 (3%) | |
| ε2/2 | 0 (0%) | 2 (0.1%) | |
| APOEε4-positive n (%)^^^ | 0.44‡ | ||
| Yes | 36 (67%) | 848 (61%) | |
| No | 18 (33%) | 533 (39%) | |
| Hypertension n (%)䫨 | 51/62 (82%) | 753/1418 (53%) | <0.001 |
| Education (yrs)+ 䫨䫨 | 13.4 ± 3.4 | 15.3 ± 2.9 | <0.001 |
All percentages except ε2/2 incidence in Caucasians are rounded to the nearest whole number
APOE: apolipoprotein E
PMI: postmortem interval
Mean ± standard deviation
Time from diagnosis to death; Data not available for 1 African-American and 13 Caucasians
Data not available for 43 African-Americans and 950 Caucasians
Data not available for 14 African-Americans and 173 Caucasians
Data not available for 6 African-Americans and 136 Caucasians
Data not available for 1 African-American and 13 Caucasians
Statistically non-significant
Histopathology and Quantitation of CAA
The brains were immersion-fixed for 1–2 weeks in phosphate-buffered, 4% (w/v) de-polymerized paraformaldehyde. For histopathological diagnosis and staging of the cases, tissue blocks from multiple brain areas were embedded in paraffin, cut at 8 μm thickness, and sections stained with hematoxylin and eosin, the Bielschowsky silver stain, and immunohistochemically labeled with antibodies to Aβ (4G8; Biolegend, San Diego, CA), tau (PHF-1; courtesy of Peter Davies, The Feinstein Institute for Medical Research), TDP-43 (Cosmo Bio USA, Carlsbad, CA), ubiquitin (Millipore, Temecula, CA), and α-synuclein (Wako, Mountain View, CA). In all subjects, Thal phases of Aβ-proteopathy[49], Braak stages of tauopathy[50], CERAD neuritic plaque scores[51], and the combined Aβ (Amyloid), Braak and CERAD (ABC) scores for the degree of AD-type neuropathology[52] were determined; the presence of comorbid brain conditions also was noted (summarized in Table 2).
TABLE 2.
CAA scores (overall mean scores based on a scale of 0–5), neuropathologic stages, secondary (2o) neuropathologic diagnoses, and prior hypertension for subjects in the Emory cohort. African-Americans are denoted by §.
| Subject | lvCAA | capCAA | Thal | Braak | CERAD | ABC | 2o NP Diagnoses | Hyperten |
|---|---|---|---|---|---|---|---|---|
| AD1 § | 1.1 | 0 | 5 | VI | C | high | LBD | Yes |
| AD2 § | 0.4 | 0.4 | 5 | VI | C | high | TDP, meningioma, HV | No |
| AD3 § | 0.2 | 0.1 | 5 | V | C | high | LBD, TDP | nd |
| AD4 § | 0.3 | 0.4 | 5 | VI | C | high | LBD, sm infarcts | Yes |
| AD5 § | 1.3 | 0.3 | 5 | VI | C | high | Yes | |
| AD6 § | 1.0 | 0.8 | 4 | III | C | intermed | LBD | Yes |
| AD7 § | 4.3 | 3.0 | 5 | VI | C | high | TDP, LBD | Yes |
| AD8 § | 1.9 | 2.3 | 4 | VI | C | high | LBD, sm infarcts | Yes |
| AD9 § | 2.2 | 0.1 | 5 | VI | C | high | No | |
| AD10 § | 0.9 | 0 | 5 | VI | C | high | Yes | |
| AD11 § | 0.7 | 1.1 | 5 | VI | C | high | LBD, sm infarcts | nd |
| AD12 § | 3.6 | 5.0 | 4 | VI | C | high | LBD, TDP, TL scler | Yes |
| AD13 § | 0.5 | 0 | 5 | V | C | high | sm-med infarcts, TDP, LBD | Yes |
| AD14 § | 0.3 | 0 | 4 | V | C | high | LBD, TDP, sm-med infarcts | Yes |
| AD15 § | 0.3 | 0 | 5 | VI | C | high | LBD | Yes |
| AD16 § | 0.8 | 0.1 | 5 | VI | C | high | TL scler, TDP | Yes |
| AD17 § | 2.6 | 1.3 | 5 | VI | C | high | meningitis, LBD | Yes |
| AD18 § | 0.3 | 0.1 | 5 | VI | C | high | LBD | No |
| AD19 | 0.3 | 0 | 5 | VI | C | high | No | |
| AD20 | 0.3 | 0.5 | 5 | VI | C | high | No | |
| AD21 | 0.3 | 0.3 | 5 | VI | C | high | LBD, TDP | No |
| AD22 | 0.3 | 0.1 | 5 | VI | C | high | TDP | nd |
| AD23 | 0.2 | 0.6 | 5 | V | C | high | LBD | No |
| AD24 | 1.0 | 0.1 | 5 | VI | C | high | LBD | nd |
| AD25 | 0.4 | 0 | 5 | VI | C | high | No | |
| AD26 | 0.5 | 0 | 5 | VI | C | high | No | |
| AD27 | 3.7 | 0.1 | 5 | VI | C | high | TDP | No |
| AD28 | 1.8 | 1.3 | 4 | V | C | high | TL scler, TDP | Yes |
| AD29 | 0.5 | 0 | 5 | V | C | high | LBD, TDP | nd |
| AD30 | 1.6 | 0 | 5 | VI | C | high | TL scler, TDP, LBD, sm infarcts | nd |
| AD31 | 3.7 | 0.1 | 5 | VI | C | high | No | |
| AD32 | 3.8 | 3.9 | 4 | VI | C | high | sm infarcts | No |
| AD33 | 1.3 | 0 | 5 | VI | C | high | med-large infarcts | No |
| AD34 | 0.7 | 0 | 5 | VI | C | high | LBD, TDP | No |
| AD35 | 2.5 | 0 | 5 | VI | C | high | sm-med infarcts, TDP | No |
| AD36 | 0.3 | 0 | 5 | V | C | high | LBD, TDP, arterioscl (severe) | Yes |
| AD37 | 0.5 | 0.1 | 5 | VI | C | high | LBD, TDP | No |
ABC: Amyloid-Braak-CERAD neuropathology score; arterioscl: arteriosclerosis; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease (pathology); Hyperten: hypertension; HV: Hypertensive Vasculopathy; intermed: intermediate; LBD: Lewy Body Disease; nd: no data; sm: small; med: medium; TDP: TAR-DNA-binding protein 43KDa (TDP-43); TL scler - Temporal Lobe sclerosis
Aβ-CAA was assessed in four distinct neocortical regions: the frontal lobe (primarily Brodmann area 8), temporal lobe (primarily Brodmann areas 21/22), parietal lobe (Brodmann area 7) and occipital lobe (primarily Brodmann areas 17/18). Aβ-immunostained tissue sections from each of the four regions were examined independently by two investigators (DMK and LCW) who were incognizant of the ethnoracial and other characteristics of the subjects. The prevalence of CAA in all large vessels (arteriolar and venous; lvCAA) was determined in both the brain parenchyma and the superficial vessels of the leptomeninges; in addition, the degree of CAA in parenchymal capillaries (vessels of apparent diameter of ~10 μm; capCAA) was evaluated. Using a modified quantitative scale similar to that of Olichney et al.[48, 53], each type of CAA was assigned a score of 0 (none) to 5 (most severe) in each brain area. The CAA scores were standardized by comparison to a prepared set of photographs illustrating the different levels of involvement, with half-step intermediate scores (1.5, 2.5, etc.) permitted. In cases where three or fewer positive vessels were evident in a given tissue section, a score of 0.5 was assigned. By a priori agreement of the two raters, cortical regions with CAA scores of 0 to 1.0 were considered to have no-to-light CAA, regions with scores of >1.0 to 2.0 were considered to have moderate CAA, and regions with scores >2.0 were considered to have severe CAA. The expanded scale in the severe range (>2.0 to 5.0) allowed us to capture the full breadth of severity in the context of spatial variation in the extent of CAA within a given sample. Cohen’s kappa coefficient indicated satisfactory inter-rater concordance in assignment of categorical severity levels (none-light, moderate, or severe) for lvCAA (kappa = 0.91) and capCAA (kappa = 0.70), so the means of the two raters’ scores were calculated as the reported values. Final lvCAA and capCAA scores then were assigned to each brain area of all 37 cases.
Statistical Analysis
In the primary cohort at Emory, patient characteristics were compared using the two-sample t-test for continuous variables, or, for categorical variables, the Chi-square test or Fisher’s exact test. Because the scores for lvCAA and capCAA were not normally distributed, the Wilcoxon rank-sum test was used to compare the scores between the ethnoracial groups, genders, APOEε4 carriers/non-carriers, APOEε4 homozygotes/heterozygotes, presence of hypertension, and the quartiles of age at death and duration of disease. Spearman’s rank correlation coefficient (rho) was used to evaluate correlations involving lvCAA and capCAA.
In the supplemental dataset from NACC, age at death, disease duration, and education level were analyzed using two-sample t-tests assuming equal variances. Owing to dissimilar variances, a t-test assuming unequal variances was used to assess PMIs in the two groups. Gender, APOEε4 status and the presence or absence of hypertension where analyzed by Chi-square. The degree of CAA (ranked by evaluators at the NACC-contributing institutions) was assessed by comparing the distribution of the 4 scores (0,1,2,3) in African-American and Caucasian subjects using the Chi-square test.
RESULTS
General Findings in the Unified Emory Cohort
Diagnostic neuropathologic findings
All 37 subjects in the Emory cohort had CERAD neuritic plaque scores and Thal Aβ plaque levels indicative of a neuropathologic diagnosis of AD (Table 2). With the exception of case AD6 (Braak stage III), the cases also exhibited marked tau deposition in the frontal, temporal and parietal cortices, with more variable deposition in the occipital cortex, consistent with Braak tauopathy stages V and VI (Table 2).
Distribution of CAA
In the 37 subjects as a whole, CAA was most prominent in the occipital lobe, in agreement with the findings of others[30, 54–56]. The mean overall scores (rounded to the nearest tenth) for lvCAA were 1.5 (occipital), 1.2 (parietal), 1.1 (temporal), and 1.2 (frontal); for cap CAA the scores were 1.1 (occipital), 0.6 (parietal), 0.3 (temporal) and 0.4 (frontal).
Large-vessel CAA
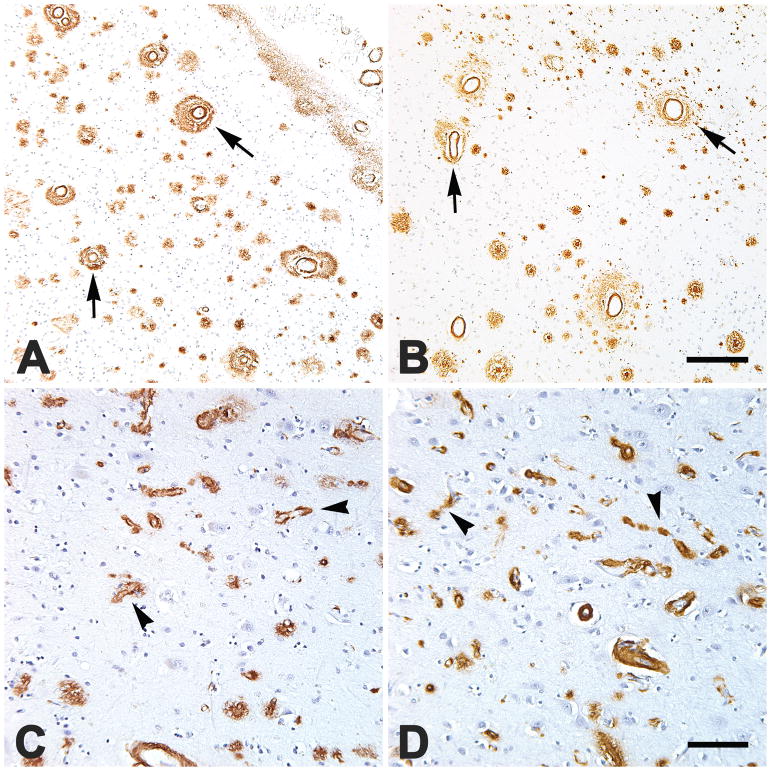
The mean scores for parenchymal lvCAA correlated strongly with the mean scores for leptomeningeal lvCAA (rho = 0.86, p <0.001); these scores therefore were combined into a single mean lvCAA score for each brain area in each subject. The degree of lvCAA varied among the subjects, with the lvCAA scores in the total cohort skewing toward light-to-moderate involvement. Specifically, the mean composite lvCAA scores (i.e., the mean of the scores for all 4 cortical regions) were less than 2 (light-to-moderate) in 29 of the cases (78%) and greater than 2 (severe) in 8 cases (22%) (Table 2). Within subjects, the lvCAA scores correlated significantly across the 4 cortical regions (rho = 0.81, p <0.001); for this reason, and because of the sometimes patchy distribution of CAA within the cortex[55], the mean of the lvCAA scores for the 4 cortical regions was used for subsequent analyses. The extent of lvCAA was not significantly related to age at death, duration of disease, sex of the subjects, presence of hypertension, or APOEε4 positivity, although there was a trend toward greater lvCAA in APOEε4/4 cases than in APOEε3/4 cases (Wilcoxon rank-sum test, p = 0.09). Four cases (two African-Americans and two Caucasians) presented pronounced dyshoric lvCAA, a condition in which the Aβ deposition extends beyond the vascular wall into the surrounding parenchyma (Figures 1A,B; 2). One other case (a Caucasian) had conspicuous dyshoric lvCAA only in the occipital cortical sample. In all of these instances, the dyshoric vessels often were surrounded by profuse tau-immunoreactive processes that spatially overlapped with the perivascular Aβ (Figure 2). Diffuse CAA-related inflammation/angiitis[30] was not seen in any of the subjects.
FIGURE 1.
Severe, dyshoric large-vessel CAA (A,B; four vessels are marked by arrows) in occipital (A) and parietal (B) neocortical sections, and severe dyshoric capillary CAA (C,D; four are marked by arrowheads) in the parietal neocortices of two African-Americans (A,C) and two Caucasians (B,D). Areas of heavy capCAA generally had relatively few parenchymal Aβ plaques (C,D). Nissl counterstain. Scale bar in B = 200 μm for A&B, scale bar in D = 100 μm for C&D.
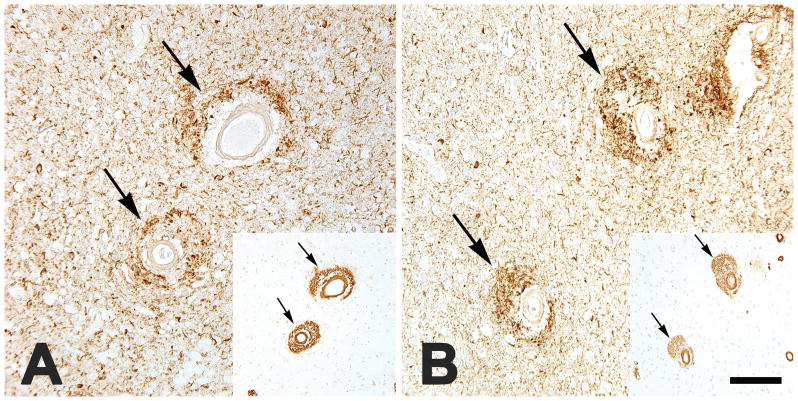
FIGURE 2.
Large cortical blood vessels with dyshoric CAA are surrounded by profuse neuritic tauopathy (antibody PHF-1), here shown in the parietal neocortex of an APOEe3/4-bearing African-American man (AD7; A) and the occipital neocortex of an APOEe3/3-bearing Caucasian woman (AD27; B). Insets show intramural and perivascular Aβ immunoreactivity in the same vessels in nearby tissue sections (antibody 4G8). Note the spatial overlap of tauopathic neurites and perivascular Aβ. Nissl counterstain. Bar = 200 μm for the insets, and 80 μm for the main panels.
Capillary CAA
Capillary CAA was less prevalent than was large-vessel CAA in the overall Emory cohort. Fourteen subjects had no detectable capCAA, 19 had light-to-moderate capCAA (all had mean scores of 1.3 or less), and 4 had severe capCAA (scores of 2.3, 3.0, 3.9 and 5.0) (Table 2). As with lvCAA, the capCAA scores correlated significantly across the 4 cortical regions (rho = 0.77, p <0.001), so the mean capCAA scores across the 4 sites was used as the representative value for further analysis. The degree of capCAA was not significantly related to duration of disease, age at death, or presence of hypertension, but males tended to have more capCAA than females (Wilcoxon rank-sum test, p = 0.054); capCAA also tended to be more abundant in APOEε4-positive subjects, although the difference from APOEε4-negative subjects was not statistically significant (Wilcoxon rank-sum test, p = 0.25). Of the 7 moderate-to-severe capCAA cases, 4 were APOEε4/4-homozygous, and 3 were APOEε3/4-heterozygous. Because of the relatively small proportion of males (30%) as well as APOEε3/3- and APOEε4/4-bearing subjects (22% and 25%, respectively) in the inclusive cohort, comparisons of the genders and APOE groups here should be interpreted cautiously. In agreement with other reports[43, 57], the presence of copious capCAA in a given sub-region of cortex was associated with sparse parenchymal Aβ plaques within the same space (Figure 1 C,D). Dyshoric capCAA sometimes was associated with peri-capillary tau-immunoreactive processes (not shown). The degree of capCAA in the total cohort showed a trend toward correlation with the degree of lvCAA (rho = 0.32, p = 0.057). While severe capCAA was present only in patients with moderate-to-severe lvCAA, several cases of moderate-to-severe lvCAA had little or no capCAA (Table 2).
CAA in African-Americans and Caucasians with AD
Emory cohort
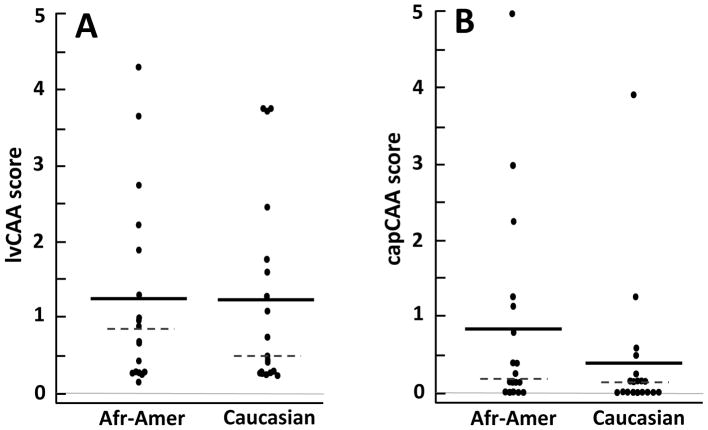
The amount of CAA in the 37 Emory subjects did not differ significantly between African-Americans and Caucasians (lvCAA: Wilcoxon rank-sum test, p = 0.94; capCAA: Wilcoxon rank-sum test, p = 0.13) (Figures 1–3). Large-vessel CAA was severe (mean lvCAA scores above 2.0) in 4 subjects in each ethnoracial group (22% of African-Americans and 21% of Caucasians). Capillary CAA was severe in 3 African-Americans (17%) and in 1 Caucasian (5%) (Table 2 and Figure 3).
FIGURE 3.
Distribution of CAA scores in 18 African-American (Afr-Amer) and 19 Caucasian subjects. Each dot represents the mean score of the 4 brain regions for lvCAA (A) and capCAA (B) in each subject. The group means are indicated by the solid bars, and the medians by the dashed lines. There were no statistically significant differences in the amount of lvCAA (p = 0.94) or capCAA (p = 0.13) between the two ethnoracial groups.
Comorbid brain conditions were slightly more common in the African-American group (30 comorbidities in 18 subjects) than in the Caucasian group (25 comorbidities in 19 subjects) (Table 2). Of these, only the proportion of patients with Lewy body disease trended toward a difference between African-Americans (72%) and Caucasians (42%) (Chi-square = 3.3, p = 0.07). Five of the 18 African-Americans had evidence of infarcts, as did 3 of 19 Caucasians (Fisher’s Exact Test, p = 0.45). Of the 31 subjects for which sufficient clinical data were available, the proportion of African-Americans with hypertension (14/16: 88%) was significantly greater than in Caucasians (2/15: 13%) (Fisher’s Exact Test, p <0.001). However, in the combined Emory cohort, the presence of hypertension was not significantly related to the degree of lvCAA (p = 0.62) or capCAA (p = 0.38) (Table 2).
NACC cohort
To further assess the degree of CAA in the two ethnoracial groups, we evaluated data from a large, independent sample of subjects (1554 Caucasians and 68 African-Americans) with clinicopathologically confirmed AD from the NACC database (Table 3). In the combined NACC cohort, CAA was severe (score of 3 on a scale of 0–3) in 18% of the NACC subjects. Unlike the Emory cohort (in which the two ethnoracial groups were deliberately matched on several variables), the proportion of males and females differed significantly in the Caucasian and African-American NACC groups, and mean educational attainment was significantly greater in Caucasians (Table 3). Similar to the Emory cohort, African-Americans in the NACC cohort were significantly more likely to have been diagnosed with hypertension during life (Chi-square = 20.35, p <0.001); however, the distribution of CAA scores did not differ significantly in the two groups (Chi-square = 2.72, p = 0.44) (Table 4).
TABLE 4.
Degree of CAA (on a scale of 0–3) in African-Americans and Caucasians in the NACC cohort who were clinically diagnosed and pathologically confirmed to have AD. The frequency of CAA scores did not differ significantly between the two groups (Chi-square = 2.72, p = 0.44).
| Group | CAA Score (n, %*) | Total (n) | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| African-Americans | 13 (19%) | 23 (34%) | 24 (35%) | 8 (12%) | 68 |
| Caucasians | 313 (20%) | 527 (34%) | 434 (28%) | 280 (18%) | 1554 |
Percentages are rounded to the nearest whole number
DISCUSSION
Despite a relatively high incidence of AD-type dementia[1–5, 7] and lobar hemorrhage[25], African-Americans with AD appear not to be disproportionately vulnerable to cerebral β-amyloid angiopathy. This finding supports the conclusions of Wilkins et al.[9] and Riudavets et al.[8], and indicates that CAA does not contribute significantly to the differential susceptibility to AD among aging African-Americans.
Older AD patients in general are more likely than younger patients to manifest comorbid brain disorders such as α-synucleinopathy and microinfarcts[7, 58], and such mixed brain pathologies have been reported to be more common in blacks than whites with clinical AD[20]. In our primary cohort of 37 subjects, the prevalence of comorbidities was slightly higher among African-Americans (30 comorbidities in 18 subjects) compared to Caucasians (25 comorbidities in 19 subjects). In particular, African-Americans tended to have more α-synucleinopathy than did Caucasians, consistent with previous investigations[7, 20]. Several systemic risk factors for age-associated cognitive decline also are more prevalent among African-Americans, such as cardiovascular and metabolic disorders[17, 18], including hypertension[19]; as expected, more African-American subjects in both the Emory and NACC cohorts had been diagnosed with hypertension than had Caucasians, although both groups had similar levels of CAA. It is therefore plausible that the deleterious effects of Aβ- and tau-accumulation on brain function are more likely to be unmasked by comorbid conditions in African-Americans, thereby increasing the probability of dementia. Imaging of cerebral β-amyloid in living subjects using florbetapir positron-emission tomography has indicated that older, cognitively normal African-Americans have a greater Aβ burden than a comparable sample of whites[59]. Longitudinal studies employing brain imaging and/or sensitive biomarkers for AD and comorbid conditions in living patients are needed to more fully evaluate the contribution of comorbidity to cognitive deterioration in differentially vulnerable populations.
Large vessel CAA was severe in approximately one-fifth of the subjects in both the Emory and NACC combined cohorts of African-Americans and Caucasians, in agreement with previous analyses[27, 32, 47, 48, 60]. We also confirm[29, 39] that CAA is less common in capillaries than in large vessels (11% of the Emory subjects had severe capCAA, and 38% had none). Severe capCAA was present only in patients with moderate-to-severe lvCAA, although many cases of moderate-to-severe lvCAA had little capCAA (Table 2). There was a tendency for African-Americans to have more capCAA (Figure 3), an observation that warrants further study in a larger group of subjects. In addition, all subjects were analyzed at the end-stage of AD; whether the prevalence of CAA differs in the two groups at earlier stages of pathogenesis remains unknown. It will also be important to determine if CAA in the absence of AD lesions is more common in different ethnoracial groups.
Earlier reports have noted that regions of severe capillary CAA tend to have few parenchymal Aβ plaques in the same space[43, 57], and our observations confirm this (Figure 1C,D). Extant evidence thus suggests that the deposition of Aβ in capillaries may involve a mechanism that differs from that in large vessels and plaques, possibly with different functional consequences for the brain[43]. The degree of capCAA can be strongly associated with dementia in AD[61], and dyshoric capCAA has in some instances been linked to pericapillary inflammation and a rapidly progressive form of dementia[62]. Pericapillary inflammation was not evident in any of the 37 subjects in which the degree of capCAA was specifically investigated.
The APOEε4 allele is associated with an increased risk of both AD[63–65] and CAA[56, 66], and the ε4 allele has been reported to be more common in African-Americans than Caucasians with dementia[7]. In the present primary sample of 37 subjects (in which the presence of APOEε4 was roughly matched in the two ethnoracial groups), moderate-to-severe capCAA was found only in APOEε4-bearing subjects, confirming previous findings[43, 67]; although lvCAA was more abundant in APOEε4-bearing subjects compared to those lacking APOEε4, the group difference did not reach statistical significance, possibly owing to the relatively small percentage of APOEε4-negative subjects (22%) in this sample. In addition, the effect of the APOEε4 allele on CAA risk has been reported to be greater for males than for females[8], and most of the subjects that we analyzed (70%) were females. In the context of previous observations [56, 66], our results generally substantiate the importance of APOEε4 as a genetic risk factor for CAA.
A prior analysis of two APOEε4/4 homozygotes with severe CAA described marked perivascular neuritic tauopathy associated with dyshoric Aβ deposition[68]. We also found profuse tau-immunoreactive processes surrounding dyshoric amyloidotic vessels, but this was seen both in APOEε3/3- and APOEε3/4-bearing subjects (Figure 2). Interestingly, the spatial distribution of the tauopathic neurites overlapped considerably with that of the perivascular Aβ, and in cases of severe non-dyshoric CAA, little perivascular tauopathy was evident. The region of tau and Aβ co-deposition around dyshoric vessels thus could yield profitable insights into how the two proteins interact in the AD brain.
We matched the two ethnoracial groups in the Emory sample as closely as possible on a number of variables, although there is unavoidable selection bias in autopsy-based studies[58], and willingness to undergo autopsy is subject to cultural differences[69–71]. In addition, both ‘African-American’ and ‘Caucasian’ are broad terms that include people from diverse geographic and cultural backgrounds[1, 72]. Even among people of African origin, dementia has been noted to be significantly more common in African-Americans living in Indianapolis than in age-matched Africans in Ibadan, Nigeria[73]. The causes of this disparity are unclear[1], but the findings emphasize the potential pitfalls that attend the multifaceted concept of race[74]. In addition, it is likely that the pathobiology of dementia is influenced by social and environmental factors that differ among different ethnoracial populations[75]. To the extent that ethnoracial differences in the vulnerability to certain disease processes can be characterized, it may be possible to identify group-specific, modifiable risk factors that can be targeted to reduce the burden of disease[7]. Of these, cardiovascular and metabolic risk factors should command special attention.
We conclude that both the degree and type of cerebrovascular Aβ deposition are largely similar in African-Americans and Caucasians with end-stage AD. This finding underscores the overall pathologic similarity of AD-type lesions in these groups[8, 9, 76], and supports the broader view that, despite the normal inter-individual heterogeneity in the disease phenotype[77], the fundamental neuropathologic features of AD are comparable in humans of differing ethnoracial backgrounds.
Acknowledgments
We thank Anil Mehta, David Lynn, and Yury Chernoff for helpful discussions, and Kaylor Kelly and Deborah Cooper for expert technical assistance. This work was supported by National Institutes of Health (NIH) grants P50 AG025688, RR00165, and OD11132. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Conflict of Interest/Disclosure Statement: The authors have no conflict of interest to report.
References
- 1.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, Szapocznik J. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann Epidemiol. 2003;13:472–478. doi: 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- 3.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S, Social B Diversity Research Workgroup of the AsA. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimers Dement. 2008;4:305–309. doi: 10.1016/j.jalz.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Steenland K, Goldstein FC, Levey A, Wharton W. A Meta-Analysis of Alzheimer’s Disease Incidence and Prevalence Comparing African-Americans and Caucasians. J Alzheimers Dis. 2016;50:71–76. doi: 10.3233/JAD-150778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohman TJ, Cooke-Bailey JN, Reitz C, Jun G, Naj A, Beecham GW, Liu Z, Carney RM, Vance JM, Cuccaro ML, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue MW, Baldwin CT, Green RC, Barnes LL, Cantwell LB, Fallin MD, Go RC, Griffith P, Obisesan TO, Manly JJ, Lunetta KL, Kamboh MI, Lopez OL, Bennett DA, Hardy J, Hendrie HC, Hall KS, Goate AM, Lang R, Byrd GS, Kukull WA, Foroud TM, Farrer LA, Martin ER, Pericak-Vance MA, Schellenberg GD, Mayeux R, Haines JL, Thornton-Wells TA Alzheimer Disease Genetics C. Global and local ancestry in African-Americans: Implications for Alzheimer’s disease risk. Alzheimers Dement. 2016;12:233–243. doi: 10.1016/j.jalz.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alzheimers Dement. 2016;12:669–677. doi: 10.1016/j.jalz.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol. 2006;65:1143–1148. doi: 10.1097/01.jnen.0000248548.20799.a3. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol. 2006;63:87–90. doi: 10.1001/archneur.63.1.87. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vonsattel JP, Hedley-White ET. Pathology of the Aging Human Nervous System. Oxford University Press; New York: 2001. Dementia; pp. 156–169. [Google Scholar]
- 12.Abner EL, Kryscio RJ, Schmitt FA, Fardo DW, Moga DC, Ighodaro ET, Jicha GA, Yu L, Dodge HH, Xiong C, Woltjer RL, Schneider JA, Cairns NJ, Bennett DA, Nelson PT. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol. 2017;81:549–559. doi: 10.1002/ana.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, Uyehara-Lock JH, Gelber RP, Ross GW, Petrovitch H, Masaki KH, Lim KO, Launer LJ, Montine TJ. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86:1000–1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy MP, Corriveau RA, Wilcock DM. Vascular contributions to cognitive impairment and dementia (VCID) Biochim Biophys Acta. 2016;1862:857–859. doi: 10.1016/j.bbadis.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy RE, Cutter GR, Wang G, Schneider LS. Challenging Assumptions About African American Participation in Alzheimer Disease Trials. Am J Geriatr Psychiatry. 2017 doi: 10.1016/j.jagp.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst) 2017;7:69–87. doi: 10.1016/j.dadm.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams SK, Ravenell J, Seyedali S, Nayef S, Ogedegbe G. Hypertension Treatment in Blacks: Discussion of the U.S. Clinical Practice Guidelines. Prog Cardiovasc Dis. 2016;59:282–288. doi: 10.1016/j.pcad.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA, Schneider JA. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85:528–534. doi: 10.1212/WNL.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes LL, Bennett DA. Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff (Millwood) 2014;33:580–586. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain Aging in African-Americans: The Atherosclerosis Risk in Communities (ARIC) Experience. Curr Alzheimer Res. 2015;12:607–613. doi: 10.2174/1567205012666150701102445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorelick PB. Cerebrovascular disease in African Americans. Stroke. 1998;29:2656–2664. doi: 10.1161/01.str.29.12.2656. [DOI] [PubMed] [Google Scholar]
- 24.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 26.Auriel E, Charidimou A, Gurol ME, Ni J, Van Etten ES, Martinez-Ramirez S, Boulouis G, Piazza F, DiFrancesco JC, Frosch MP, Pontes-Neto OV, Shoamanesh A, Reijmer Y, Vashkevich A, Ayres AM, Schwab KM, Viswanathan A, Greenberg SM. Validation of Clinicoradiological Criteria for the Diagnosis of Cerebral Amyloid Angiopathy-Related Inflammation. JAMA Neurol. 2016;73:197–202. doi: 10.1001/jamaneurol.2015.4078. [DOI] [PubMed] [Google Scholar]
- 27.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 28.Kawai M, Kalaria RN, Cras P, Siedlak SL, Velasco ME, Shelton ER, Chan HW, Greenberg BD, Perry G. Degeneration of vascular muscle cells in cerebral amyloid angiopathy of Alzheimer disease. Brain Res. 1993;623:142–146. doi: 10.1016/0006-8993(93)90021-e. [DOI] [PubMed] [Google Scholar]
- 29.Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, Holton JL. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003;62:885–898. doi: 10.1093/jnen/62.9.885. [DOI] [PubMed] [Google Scholar]
- 30.Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 2015;17:17–30. doi: 10.5853/jos.2015.17.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012;14:343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- 32.Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- 33.Haan J, Lanser JB, Zijderveld I, van der Does IG, Roos RA. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Arch Neurol. 1990;47:965–967. doi: 10.1001/archneur.1990.00530090035010. [DOI] [PubMed] [Google Scholar]
- 34.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62:1287–1301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 35.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 36.Case NF, Charlton A, Zwiers A, Batool S, McCreary CR, Hogan DB, Ismail Z, Zerna C, Coutts SB, Frayne R, Goodyear B, Haffenden A, Smith EE. Cerebral Amyloid Angiopathy Is Associated With Executive Dysfunction and Mild Cognitive Impairment. Stroke. 2016;47:2010–2016. doi: 10.1161/STROKEAHA.116.012999. [DOI] [PubMed] [Google Scholar]
- 37.Schrag M, Kirshner H. Neuropsychological Effects of Cerebral Amyloid Angiopathy. Curr Neurol Neurosci Rep. 2016;16:76. doi: 10.1007/s11910-016-0674-1. [DOI] [PubMed] [Google Scholar]
- 38.Xiong L, Boulouis G, Charidimou A, Roongpiboonsopit D, Jessel MJ, Pasi M, Reijmer YD, Fotiadis P, Ayres A, Merrill E, Schwab K, Blacker D, Gurol ME, Greenberg SM, Viswanathan A. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17700435. 271678X17700435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 40.Zekry D, Duyckaerts C, Belmin J, Geoffre C, Moulias R, Hauw JJ. Cerebral amyloid angiopathy in the elderly: vessel walls changes and relationship with dementia. Acta Neuropathol. 2003;106:367–373. doi: 10.1007/s00401-003-0738-6. [DOI] [PubMed] [Google Scholar]
- 41.Fotiadis P, van Rooden S, van der Grond J, Schultz A, Martinez-Ramirez S, Auriel E, Reijmer Y, van Opstal AM, Ayres A, Schwab KM, Hedden T, Rosand J, Viswanathan A, Wermer M, Terwindt GM, Sperling RA, Polimeni JR, Johnson KA, van Buchem MA, Greenberg SM, Gurol ME Alzheimer’s Disease Neuroimaging Initiative A. Cortical atrophy in patients with cerebral amyloid angiopathy: a case-control study. Lancet Neurol. 2016;15:811–819. doi: 10.1016/S1474-4422(16)30030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reijmer YD, Fotiadis P, Riley GA, Xiong L, Charidimou A, Boulouis G, Ayres AM, Schwab K, Rosand J, Gurol ME, Viswanathan A, Greenberg SM. Progression of Brain Network Alterations in Cerebral Amyloid Angiopathy. Stroke. 2016;47:2470–2475. doi: 10.1161/STROKEAHA.116.014337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard E, Carrano A, Hoozemans JJ, van Horssen J, van Haastert ES, Eurelings LS, de Vries HE, Thal DR, Eikelenboom P, van Gool WA, Rozemuller AJ. Characteristics of dyshoric capillary cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2010;69:1158–1167. doi: 10.1097/NEN.0b013e3181fab558. [DOI] [PubMed] [Google Scholar]
- 44.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease--lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim Biophys Acta. 2016;1862:878–886. doi: 10.1016/j.bbadis.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinters HV. Emerging concepts in Alzheimer’s disease. Annu Rev Pathol. 2015;10:291–319. doi: 10.1146/annurev-pathol-020712-163927. [DOI] [PubMed] [Google Scholar]
- 47.Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 48.Olichney JM, Ellis RJ, Katzman R, Sabbagh MN, Hansen L. Types of cerebrovascular lesions associated with severe cerebral amyloid angiopathy in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:493–497. doi: 10.1111/j.1749-6632.1997.tb48511.x. [DOI] [PubMed] [Google Scholar]
- 49.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 50.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 51.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 52.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers & Dementia. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olichney JM, Hansen LA, Hofstetter CR, Grundman M, Katzman R, Thal LJ. Cerebral infarction in Alzheimer’s disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol. 1995;52:702–708. doi: 10.1001/archneur.1995.00540310076019. [DOI] [PubMed] [Google Scholar]
- 54.Attems J, Jellinger KA, Lintner F. Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110:222–231. doi: 10.1007/s00401-005-1064-y. [DOI] [PubMed] [Google Scholar]
- 55.Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makela M, Paetau A, Polvikoski T, Myllykangas L, Tanskanen M. Capillary amyloid-beta protein deposition in a population-based study (Vantaa 85+) J Alzheimers Dis. 2016;49:149–157. doi: 10.3233/JAD-150241. [DOI] [PubMed] [Google Scholar]
- 57.Oshima K, Akiyama H, Tsuchiya K, Kondo H, Haga C, Shimomura Y, Iseki E, Uchikado H, Kato M, Niizato K, Arai H. Relative paucity of tau accumulation in the small areas with abundant Abeta42-positive capillary amyloid angiopathy within a given cortical region in the brain of patients with Alzheimer pathology. Acta Neuropathol. 2006;111:510–518. doi: 10.1007/s00401-006-0070-z. [DOI] [PubMed] [Google Scholar]
- 58.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottesman RF, Schneider AL, Zhou Y, Chen X, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH., Jr The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87:473–480. doi: 10.1212/WNL.0000000000002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esiri MM, Wilcock GK. Cerebral amyloid angiopathy in dementia and old age. J Neurol Neurosurg Psychiatry. 1986;49:1221–1226. doi: 10.1136/jnnp.49.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Attems J, Lintner F, Jellinger KA. Amyloid beta peptide 1-42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol. 2004;107:283–291. doi: 10.1007/s00401-004-0822-6. [DOI] [PubMed] [Google Scholar]
- 62.Eurelings LS, Richard E, Carrano A, Eikelenboom P, van Gool WA, Rozemuller AJ. Dyshoric capillary cerebral amyloid angiopathy mimicking Creutzfeldt-Jakob disease. J Neurol Sci. 2010;295:131–134. doi: 10.1016/j.jns.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 64.Ohm TG, Kirca M, Bohl J, Scharnagl H, Gross W, Marz W. Apolipoprotein E polymorphism influences not only cerebral senile plaque load but also Alzheimer-type neurofibrillary tangle formation. Neuroscience. 1995;66:583–587. doi: 10.1016/0306-4522(94)00596-w. [DOI] [PubMed] [Google Scholar]
- 65.Roses AD. Apolipoprotein E and Alzheimer’s disease. A rapidly expanding field with medical and epidemiological consequences. Ann N Y Acad Sci. 1996;802:50–57. doi: 10.1111/j.1749-6632.1996.tb32598.x. [DOI] [PubMed] [Google Scholar]
- 66.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 67.Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:282–293. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- 68.Vidal R, Calero M, Piccardo P, Farlow MR, Unverzagt FW, Mendez E, Jimenez-Huete A, Beavis R, Gallo G, Gomez-Tortosa E, Ghiso J, Hyman BT, Frangione B, Ghetti B. Senile dementia associated with amyloid beta protein angiopathy and tau perivascular pathology but not neuritic plaques in patients homozygous for the APOE-epsilon4 allele. Acta Neuropathol. 2000;100:1–12. doi: 10.1007/s004010051186. [DOI] [PubMed] [Google Scholar]
- 69.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9:734–745. doi: 10.2174/156720512801322627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonner GJ, Darkwa OK, Gorelick PB. Autopsy recruitment program for African Americans. Alzheimer Dis Assoc Disord. 2000;14:202–208. doi: 10.1097/00002093-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Darnell KR, McGuire C, Danner DD. African American participation in Alzheimer’s disease research that includes brain donation. Am J Alzheimers Dis Other Demen. 2011;26:469–476. doi: 10.1177/1533317511423020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Gao S, Evans RM, Ogunseyinde AO, Adeyinka AO, Musick B, Hui SL. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 74.Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care. 2006;44:S10–16. doi: 10.1097/01.mlr.0000245427.22788.be. [DOI] [PubMed] [Google Scholar]
- 75.Ighodaro ET, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, Bardach SH, Hord DC, Glover CM, Jicha GA, Van Eldik LJ, Byrd AX, Fernander A. Challenges and Considerations Related to Studying Dementia in Blacks/African Americans. J Alzheimers Dis. 2017;60:1–10. doi: 10.3233/JAD-170242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiol Aging. 2001;22:169–175. doi: 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 77.Vinters HV. Cerebral amyloid angiopathy. In: Barnett H, Mohr J, Stein B, Yatsu F, editors. Stroke: Pathophysiology, Diagnosis and Management. Churchill Livingstone; New York: 1992. pp. 821–858. [Google Scholar]