Abstract
Worldwide statins are considered to be the first-line pharmacological treatment for dyslipidemia and reducing the risk of coronary heart disease. However, recently various studies have shown its adverse effect on glucose control among diabetic patients and the U.S. Food and Drug Administration have revised statin drug labels to include information that increases in fasting serum glucose and glycated hemoglobin levels have been reported. This systematic review objective is to evaluate the risks and benefits of statins in glucose control management of type 2 diabetes patients based on the 44 published journal articles included and obtained through MEDLINE full text, PubMed, Science Direct, Pro Quest, SAGE, Taylor and Francis Online, Google Scholar, High Wire, and Elsevier Clinical Key. Statins were found to affect glucose control through several ways, namely, by affecting insulin production and secretion by β-pancreatic cells, insulin resistance, insulin uptake by the muscles and adipocytes and production of adipokines. Current evidence available shows that most of the statins give unfavorable side effects with regards to glucose control among diabetic patients. A dose-dependent and time-dependent effect was also observed in some statins which may be present among other statins as well.
Keywords: statins, glucose control, diabetes, hypercholesterolemia, insulin secretion, insulin sensitivity, insulin resistance
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors or better known as statins are a class of medications widely known for decreasing low-density lipoprotein cholesterol (LDL-C) and reducing the risk for coronary heart disease and are considered the first-line pharmacological treatment and have become the cornerstone for the management of dyslipidemia today. 1 2 A total of 96.4% of Europe's and 96.1% of America's population, 6.6% of them diabetic, are currently eligible for statins following the 2013 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines lowered threshold rate of atherosclerotic cardiovascular disease 10-year risk (7.5%) required to start statin therapy. 3 4 They are considered to be the number one prescribed class of drugs and ranks third in terms of sales worldwide. In 2013, the Centre for Disease Control and Prevention reported that 32 million people in total or approximately one out of four are consuming statins in the United States. 5
There are currently seven types of statins available in the market: atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. All statins are cholesterol-lowering agents with the primary effect of reducing cardiovascular risks. 6 However, adverse effects such as myopathy, rhabdomyolysis, increased activity shown by liver tests, acute renal failure, and incidence of type 2 diabetes mellitus have been reported. 7
Furthermore, the U.S. Food and Drug Administration recently revised statin drug labels to include information that increases in fasting serum glucose and glycated hemoglobin levels have been reported with the use of statins which was supported by a recent study showing that statin use is associated with increased glycated hemoglobin (HbA 1c ) levels. 8 9 This review aims to determine whether statins incur a cost or a benefit to glucose control.
Materials and Methods
Participants
Studies that had patients diagnosed with type 2 diabetes mellitus and using statins were included. Ideally, diabetes in the studies should be defined following guidelines provided either by the International Diabetic Federation, American Diabetes Association, or PERKENI (Perkumpulan Endokrinologi Indonesia).
Data Source
The databases searched to obtain the articles included MEDLINE full text, PubMed, Science Direct, Pro Quest, SAGE, Taylor and Francis Online, Google Scholar, High Wire, and Elsevier Clinical Key. The search strategies used included availability of full text written in English from January 1, 2000 to till date.
Keywords used were “glucose control or synonyms” OR “diabetes or synonyms” AND “statins.” When multiple articles for a single study was found, the most recent publication was used. Relevance of studies was assessed by using an approach based on title, abstract, and full text.
Study Selection
Studies were included if they were original studies and if they have a randomized controlled trial (RCT) and cohort study design. In addition, studies were included if (1) they were done on patients or animals with continuous statin treatment of any dose, (2) parameters related to glucose control were reported, (3) studies compare patients/animals treated with statins versus placebo, or (4) studies comparing patients/animals treated with one type of statin versus another type with placebo as control. The rest were excluded from this review.
Results
Our initial search resulted in 5,069 articles found, of which 4,968 were excluded on the basis of title and abstract. Of the remaining 101, 57 were excluded because they did not meet the inclusion criteria or were duplicated publications. Finally, 44 articles were found that fulfilled all of our inclusion criteria, 7 of which were experimental studies and the remaining 37 were clinical studies.
Sample sizes vary in between studies ranging from 10 to 6,770 with a follow-up duration in between 4 weeks 10 and 5 years. 11 Majority of them were RCTs with two cohort studies 9 12 included. A total of 27,966 subjects were found in the clinical studies.
The animal studies included had a sample size ranging from 6 to 60 with a follow-up time between 3 and 52 weeks. 13 14 15 A total of 138 subjects were found in the animal studies.
The outcomes of interest were changes in blood glucose and HbA 1c level, reported impacts on diabetes either improving, worsening or no change, the adverse effects reported, diabetes complications and overall effect on mortality as well as morbidity of participants. The majority of studies were published from 2010 onwards and conducted in Western Countries and Japan with one from Taiwan 16 and one from India. 13
It must be noted that the studies included were based on published reports and take into account that studies reporting positive effects are more readily accepted to be published as compared with studies reporting negative effects.
Discussion
It is widely known that insulin resistance in muscle as well as liver and β-cell failure represent the core pathophysiologic defects in type 2 diabetes (triumvirate). However, other mechanisms have been identified as metabolic defects present in type 2 diabetes representing various roles in the development of glucose intolerance in type 2 diabetic individuals. Together, these eight players consisting of the fat cell (accelerated lipolysis), gastrointestinal tract (incretin deficiency/resistance), α-cell (hyperglucagonemia), kidney (increased glucose reabsorption), brain (insulin resistance) in addition to the triumvirate comprise to form the ominous octet. 17
An ongoing discussion has opened up on whether statins exert any influence on glucose metabolism and the pathophysiology of type 2 diabetes (ominous octet). 17 On this part, numerous health institutions have created clinical guidelines on statin use for patients suffering from type 2 diabetes which are still used today. In Indonesia, PERKENI 2011 clinical guidelines recommend diabetic patients over the age of 40 years the use of statin therapy to reduce levels of LDL-C as much as 30 to 40% of its initial measurement. Patients of less than 40 years of age with acute coronary syndrome or in higher risks of cardiovascular disease which cannot be improved by lifestyle changes are also included in this category provided an observation of the risks of side effects are done. 18
In 2015, the American Diabetes Association revised its guidelines in statin use for diabetic patients to align with the ACC/AHA 2013 guidelines resulting in patients over the age of 40 and beyond, regardless of whether CVD risks or overt CVD are present will be required to take moderate-to-high statin therapy in addition to lifestyle therapy. 19
Potential Mechanisms of Action
Effect of Statins on Insulin Production and Secretion by β-Pancreatic Cells
Statins are responsible for the inhibition of de novo synthesis of cholesterol, which caused the LDL-C receptors present in the pancreatic β cells to increase their uptake of LDL-C which resulted in the increased production of plasma-derived LDL-C which held several key differences as compared with de novo synthesized cholesterol. The oxidation of plasma-derived LDL-C is known to induce the intracellular response system of the body, leading to inflammatory consequences which can lead to impaired insulin secretion. In this case, statins instead provide a beneficial effect by improving insulin resistance through their anti-inflammatory effects. It is believed that the usage of statins increase the amounts of nitric oxides in an effort to improve endothelial function which in turn activates calpain (a calcium-dependent protease) leading to cellular apoptosis. 20 21
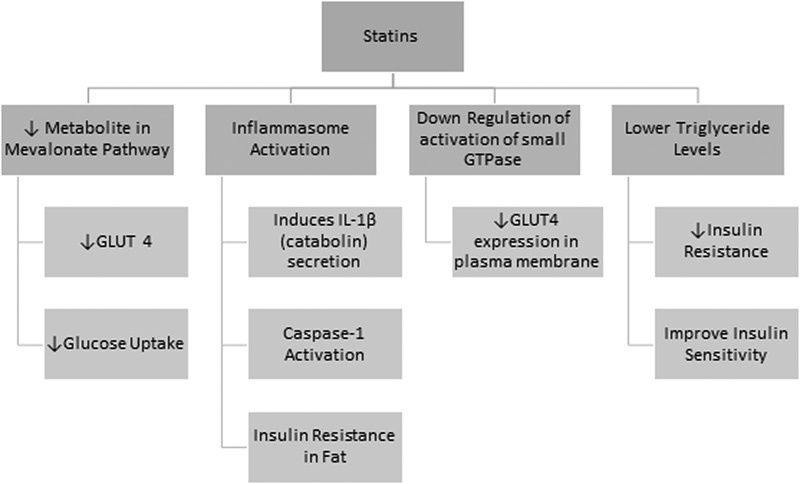
The mevalonate pathway or HMG-CoA reductase pathway is an important cellular metabolic pathway which plays a key role in synthesizing sterol isoprenoids, such as cholesterol and nonsterol isoprenoids. 22 Statins are able to reduce glycemic control as they block the production of several metabolites usually produced during cholesterol synthesis in the mevalonate pathway, such as isoprenoids, geranylgeranyl pyrophosphate (GGPP), farnesyl pyrophosphate (FPP), and ubiquinone (coenzyme Q 10 , CoQ 10 ) which are determining factors in glucose uptake as well as insulin levels. 23 24
Statins also block other products formed in the process of the biosynthetic pathway of cholesterol with the greater impact falling upon dolichol, FPP, GGPP, and CoQ 10 (ubiquinone). Dolichol is a cofactor necessary for intracellular membrane-bound receptor processing. Isoprenoids (FPP and GGPP) are known to improve glucose uptake through glucose transporter 4 (GLUT 4). A downregulation of GLUT4 would impair insulin action and will cause insulin resistance. 25 Under statin, CoQ 10 (ubiquinone), which is responsible in the electron-transfer system in the mitochondria was associated with a decrease in nutrient-related insulin secretion by the cells due to reduced adenosine triphosphate. In addition to these effects, statins are known to decrease insulin secretion due to its consequences on systolic Ca 2+ which has been linked to the endogenous cholesterol functions in β cells by the functional activity of Ca 2+ channels and thus was able to reduce Ca 2+ -dependent insulin secretion by blocking this signaling ( Fig. 1 ). 24
Fig. 1.

Statin and its possible effects on the mevalonate pathway. 21
Effect of Statins on Tissue Insulin Sensitivity (Adipocytes and Adipokines)
The exact role of statins on adipose has been a highly debatable issue, but has mostly demonstrated a major effect on the cellular physiology of the adipocyte in multiple levels. 26 White adipose tissue is responsible for the secretion of an array of signaling molecules termed as adipokines, which include leptin, adiponectin, resistin, and visfatin. Adipokines could lead to an inflammatory response in the adipose tissue which could play a role in the development of insulin resistance. A dysfunction of adipokines can be implicated with obesity, type 2 diabetes, and the increased risk of cardiovascular disease 27 28 ( Table 1 ).
Table 1. Statin's effects on various adipocytes affecting molecules.
| Molecule | Physiological effects | Statin's effects |
|---|---|---|
| Adiponectin | ↓ Hepatic glucose production ↑ Insulin secretion 26 29 |
Decreased by atorvastatin, simvastatin Neutral or increase by atorvastatin, pravastatin, simvastatin, fluvastatin 24 25 |
| Visfatin | Mimics the effects of insulin binding the insulin receptor providing hypoglycemic effects 30 | Downregulation by rosuvastatin, atorvastatin Neutral effect by simvastatin 24 31 |
| Resistin | ↑ Blood glucose ↑ Glycogenolysis ↑ Gluconeogenesis |
Little or no effect by statins (mostly atorvastatin studies) 24 29 |
| Leptin |
Effects on insulin signaling and hepatic glucose production
29
Performs a similar function as compared with insulin 32 |
Suppression by rosuvastatin, simvastatin, and atorvastatin Neutral effect by pravastatin, atorvastatin, simvastatin 24 25 |
Effect of Statins on Adipose Tissue
The plasma membrane of the adipose tissue contains distinct regions of lipid storage known as lipid rafts and caveolae. Caveolae are unique forms of lipid rafts due to their expression of Cav proteins (Cav-1 and Cav-2), a family of integral membrane proteins that are the principal components of the caveolae forming small, bulb-shaped invaginations of the plasma membrane. Caveolae plays a role in fatty acid transport and is linked to the transduction of lipid-linked signaling proteins present in adipocytes. Statins have been proven to provide an inhibitory effect on the caveolae formation through low Cav-1 levels. Although statins increased the number of caveolae present, there is a reduced number of the typical bulb-like caveolar structures and instead a buildup of caveolar vesicles thus affecting adiponectin levels. As adiponectin levels and Cav-1 positively correlate each other, when statins inhibit the Cav-1 expression and proper formation of caveolar structure, the mechanism on the secretion of adiponectin is disrupted thus reducing its circulation levels though studies show that total adiponectin levels (intracellular and in circulation) remain mostly unchanged. 26
Adipocyte maturation/differentiation is a process all preadipocytes must undergo before they can secrete insulin-sensitizing hormones along with GLUT4 translocation and therefore directly affects insulin resistance. An accumulation of undifferentiated adipocyte may lead to an increased insulin resistance and the risk of the development of statin-induced diabetes. Not many studies have been performed on the potential mechanism and the implications of statins toward it. Peroxisome proliferator activated receptor gamma and CCAAT/enhancer-binding protein(C/EBPs) are the two main transcription factors associated with adipocyte differentiation, both which decreased in levels following statin treatment. C/EBPα-deficient cells are linked to increase to normal levels of GLUT4 but reduced expression of insulin receptor (IR) and insulin receptor substrate (IRS-1) which increased upon statin therapy. However, some studies also showed no effect on adipocyte differentiation occurring upon consuming statins. 25
Obesity has been known to give rise to a state of chronic, low-grade inflammation that contributes to insulin resistance and type 2 diabetes. Macrophages present in the adipose tissue possess several proinflammatory cytokines such as tumor necrosis factor (TNF)-α that inhibit insulin action along with adiponectin production in adipocyte thus leading to risks of inflammation. Statins, with their anti-inflammatory properties, is able to reduce macrophage accumulation in atherosclerotic plaque and lower levels of proinflammatory cytokines such as TNF-α mostly through a direct effect through the liver ( Fig. 2 ). 26 33
Fig. 2.

Effect of Statins on Insulin Resistance (HOMA-IR Levels)
The homeostatic model assessment (HOMA)-IR is a method used to quantify insulin resistance and β cell function. Insulin resistance is often associated with the progression of atherosclerosis and coronary heart disease. Although, a high majority of studies have shown that statins provide an increment in HOMA-IR, others (rosuvastatin, simvastatin) have also demonstrated a decrease or no change in insulin resistance. The mechanisms by which statins affect insulin resistance are multifactorial. One possibility is the decrease of several important metabolites produced in the mevalonate pathway which has been linked to the protein, GLUT4 in 3T3–L1 adipocytes, thus augmenting glucose uptake. Lipophilic statins have also been argued to penetrate the cell membrane more than its hydrophilic counterpart and are likely to possess more extrahepatic effects. 34
A study has also shown that statins could induce insulin resistance through inflammasome activation. This works by activating NLRP3 inflammasome in the presence of a priming agent lipopolysaccharide, which induces the secretion of interleukin (IL)-1β (catabolin) leading to caspase-1 activation and the subsequent insulin resistance in fat. NLRP3 inflammasome is a protein present in the macrophages as well as the adipose tissue in humans, which plays a role in inflammation and apoptosis. However, it was later proven that caspase-1 activation without the appearance of IL-1β is sufficient to impair insulin signaling in adipocyte causing insulin resistance. 35 However, statins could also reduce insulin resistance through the lowering of triglyceride levels. Triglycerides have been known to impair insulin action during its infusion (activity of the Randle cycle) after 3 to 4 hours. An improvement in insulin sensitivity was detected on the usage of statins due to its hypotriglyceridemic effect. 24
On the other hand, the effect of statin can also be shown to be inhibitory. GLUT4 is an insulin-regulated glucose transporter that moves from its intracellular storage to the plasma membrane through the initiation of insulin-receptor tyrosine kinase phosphorylation. IRS-1 undergoes phosphorylation to insulin or insulin growth factor which binds to the IR which creates a chain reaction through the PI3K/AKT pathway resulting in GLUT4 translocation to the plasma membrane. Studies have shown that statins downregulate the activation of small GTPases, which are involved in GLUT4 expression in the plasma membrane through the altered intracellular insulin signal transduction ( Fig. 3 ). 24 25
Fig. 3.
Effect of Statins on Skeletal Muscle
The most complication in the usage of statins is some degree of skeletal myopathy though the degree of complications can range from myositis (inflammation of the muscles) to rhabdomyolysis (breakdown of muscle tissue that releases the muscle fiber content into the blood). 36
The mechanism of statin-induced myopathy has still been much theorized but it is widely agreed that multiple factors contribute to this. Statins reduce LDL-C by altering the mevalonate pathway at the cost of the formation of several important products, the mitochondrial cofactor and CoQ 10 (ubiquinone) being associated the most with muscle symptoms. The ubiquinone, a metabolite produced in the mevalonate pathway is involved in electron transport during oxidative phosphorylation in the mitochondria (OXPHOS). Statin treatment may result in the impaired mitochondrial oxidative metabolism as well as energy production. A lower capacity of OXPHOS has been linked with the usage of statins in several studies. 37
Other than the mevalonate pathway, the ubiquitin-proteasome pathway (UPP), a principal mechanism for protein catabolism in the mammalian cytosol and nucleus is also adversely affected. The UPP plays an important role in the structural integrity of the skeletal muscle and is responsible for the degradation and repair of many skeletal muscle proteins. One of the enzymes produced in the atrophy process is an ubiquitin protein ligase known as atrogin-1 which increased and is often associated with muscle wasting other than disease or fasting. Statin-induced apoptosis could also occur via calpain (stimulates programmed cell death), the repression of the antiapoptosis gene (Birc4) and the activation of proapoptosis gene (Cflar). Finally, statins alter Ca 2+ homeostasis increasing systolic Ca 2+ which might impair sarcoplasmic reticulum calcium cycling. 38 39
The mechanism of statin-induced myopathy has still been much theorized but it is widely agreed that multiple factors contribute to this as shown in the figure below ( Fig. 4 ).
Fig. 4.

Effects of Various Statins on Glucose Control
The effect of statins on glucose control among diabetic patients has been extensively evaluated by numerous clinical trials.
Atorvastatin
Experimental data: Several studies which evaluate the effect of atorvastatin treatment on glucose control in experimental animal model were found. Both Suzuki et al and Ahmed et al reported that atorvastatin improved glucose control, decreasing plasma glucose levels, plasma insulin levels as well as a reduction in insulin resistance index among diabetic mice models treated with atorvastatin. 13 40 Whereas, Aguirre et al reported no significant change in serum glucose and insulin concentration in his study, however, there was a significant increase in insulin resistance found. 14 Higher glucose levels in atorvastatin-treated diabetic mice as compared to control with no change in insulin secretion were, however, reported by Kanda et al. 41
Clinical data: In a study by Newman et al, 2,838 type 2 diabetic patients were treated with atorvastatin 10 mg/day for 3.9 years, which resulted in a modest change in HbA 1c levels. 42 Whereas, Yokote et al also reported no change in fasting plasma glucose, fasting plasma insulin, and insulin resistance levels in his study with atorvastatin 10 mg/day, however, it was reported that there was a tendency for a higher HbA 1c level among the participants. 43 These results were in agreement with that of Mita et al, Szotowska et al, Huptas et al, Endo et al, Holman et al, Athyros et al, Kryzhanovski et al, Ogawa et al, and Nakata et al. 44 45 46 47 48 49 50 51 52
Other studies found, however, demonstrated unfavorable effects of atorvastatin as shown by Liu et al, in this study 113 type 2 diabetic patients were treated with atorvastatin 10 mg/day for 12 weeks, which resulted in significant negative outcomes in glucose control, namely, increase in glucose levels, insulin levels, insulin resistance as well as HbA 1c levels. 16 Increase in blood glucose levels following atorvastatin therapy was also reported by Yamakawa et al and Takano et al. 53 54 Furthermore, Her et al also reported increase in insulin resistance, fasting insulin with no significant change in HbA 1c levels and fasting glucose in her study. 55 A significant worsening of fasting insulin, fasting glucose, HbA 1c , and insulin-resistance levels was also reported by Sasaki et al. 56
A dose-dependent effect of atorvastatin on glucose control was also shown by Koh et al in his study evaluating varying doses of atorvastatin (10, 20, 40, and 80 mg/day) and their effects on glucose control. His result shows that there was an increase in fasting plasma insulin, HbA 1c levels and decrease in insulin sensitivity with increase doses though it was not linear. 57 Simsek et al in his study also showed that while atorvastatin 20 and 40 mg/day for 6 weeks resulted in no significant change in HbA 1c levels and fasting plasma glucose, a significant increase was reported among atorvastatin 80 mg/day users. 58 Similarly, Stalenhoef et al also reported greater increase in fasting glucose and insulin resistance among atorvastatin 20 mg/day users as compared with 10 mg/day users. 59
Furthermore, a study by Gumprecht et al also suggested that short-term use of atorvastatin will not result in a significant increase in blood glucose, whereas long-term use resulted in a significant increase in blood glucose levels. 60 Owing to the lack of similar studies this result must be considered with caution though it may explain the various short-term studies reporting no effect of atorvastatin on glucose control.
Fluvastatin
Experimental data: Only one study by Aguirre et al that showed unfavorable effects, namely, increase in serum glucose concentration, insulin resistance followed by no change in insulin concentration among 10 obese Zucker rats treated with fluvastatin 0.6 mg/kg/day for 6 weeks was found. 14
Clinical data: The effect of fluvastatin on glucose control among diabetic patients has not been extensively studied, only one study was found that fit this criterion. In the study by Derosa et al, showed that extended release fluvastatin 80 mg/day for 12 months among 48 diabetic patients with dyslipidemia and coronary heart disease did not significantly alter fasting or postprandial glucose levels. In addition, a trend toward reduction in HbA 1c levels by 7% was shown to occur. 61 These possibly favorable effects on glucose control should be noted by caution and supported by more studies before conclusions can be made.
Lovastatin
Experimental data: Similar to fluvastatin, there exists a lack of evidence reporting the effects of lovastatin among diabetic patients. Only one experimental study was found which reported that the use of lovastatin 0.6 mg/kg/day for 6 weeks on obese Zucker rats resulted in a significant increase in serum glucose concentration, insulin resistance while subsequently showing no change in insulin concentration. 14
Clinical data: No clinical data reporting lovastatin use among diabetic patients was found which followed our criteria.
Pitavastatin
Experimental data: From 2000 to date, no published experimental studies were found which assess the effect of pitavastatin on glucose control among diabetic patients.
Clinical data: Pitavastatin is the newest statin found in the market to date. However, ample clinical studies have been found documenting its effects on glucose control among diabetic patients. A report by Gumprecht et al on 279 patients aged 18 to 75 years with type 2 diabetes treated with pitavastatin 4 mg/day for 12 weeks resulted in a significant increase in blood glucose levels (7.2%). A further 44-week extended study was also done, which on the contrary, showed a less significant increase in blood glucose (3.5%). 60
Studies evaluating the use of pitavastatin 2 mg/day were reported by Sasaki et al among 103 patients, which reported increases in fasting insulin, fasting glucose, HbA 1c , and insulin resistance. 56 This result was in agreement with that of Liu et al which evaluated pitavastatin 2 mg/day use among 112 patients with diabetes, which showed significant increases in glucose levels, insulin levels, insulin resistance, and HbA 1c . 16 Further negative effects were shown by Daido et al, which reported falling insulin secretion though accompanied by no change in HbA 1c levels, fasting plasma glucose levels, and insulin resistance. 62 Similar results were obtained by Takano et al as well, with regard to blood glucose and HbA 1c levels. 54
However, a study by Yamakawa et al reported no significant effect of pitavastatin on both blood glucose and HbA 1c levels. 53 It was in agreement with the results reported by Kawai et al which showed the use of pitavastatin 1 to 2 mg/day resulted in no significant change in fasting plasma glucose and HbA 1c levels. 63 On a large-scale 5-year study by Teramoto et al evaluating the use of pitavastatin 1, 2, or 4 mg/day among 1,843 patients with diabetes and hypercholesterolemia, improvements in HbA 1c levels were reported. There was a reduction in mean HbA 1c levels from 7.2 (±1.5) to 6.8 (±1.3). 11 A small comparison study with 28 patients equally divided among atorvastatin and pitavastatin conducted by Mita et al also showed that pitavastatin had a more favorable effect on glucose metabolism, including HbA 1c , glycoalbumin, fasting blood glucose, and insulin resistance as compared with atorvastatin. 44
Pravastatin
Experimental data: Prior experimental studies support a potential favorable effect of pravastatin on glucose control. Treatment with pravastatin of rats bound to develop diabetes mellitus reported increase glucose metabolism as well as reduction in glucose intolerance as compared with untreated rats following a 20 and 30 week follow-up. 64 Similarly favorable results were reported by Otani et al, pravastatin therapy resulted in a lower fasting glucose serum concentration, lower insulin serum concentration, and no change in insulin resistance. 15 Different outcomes were, however, reported by Kanda et al wherein significantly higher glucose levels and no change in insulin secretion were found. Aguirre et al reported a neutral effect of pravastatin on serum glucose concentration and insulin concentration with a detrimental effect on insulin resistance. 14 41
Clinical data: In agreement with the above experimental data, several studies on the effect of pravastatin on glucose control have reported its favorable or otherwise neutral effects among diabetic patients. Beneficial effects of pravastatin on glucose control include a modest lowering of glucose levels, insulin levels combined with no change on HbA 1c and insulin resistance as reported by Koh et al, Yamakawa et al, and Mita et al. 53 65 66
Rosuvastatin
Experimental data: Miller et al reported that 5 weeks of rosuvastatin therapy on insulin-resistant rats resulted in a moderate reduction in insulin concentrations though it remains elevated (did not return to normal). However, no significant changes in fasting glucose were reported. 67 Unfavorable effects of rosuvastatin were however reported by Aguirre et al wherein 6 weeks of rosuvastatin therapy in obese Zucker rats resulted in an increase in serum glucose concentration, insulin resistance with no change in insulin concentrations. 14
Clinical data: Varying unfavorable effects of rosuvastatin on glycemic control in varying degrees have been reported by numerous clinical trials. A study by Stalenhoef et al among 244 patients with metabolic syndrome treated by either rosuvastatin 10 or 20 mg/day for 6 weeks showed that low-dose rosuvastatin resulted in an insignificant increase in fasting glucose while showing a modest increase in insulin resistance, the higher dose resulted in a modest increase fasting glucose accompanied by a large increase in insulin resistance (12.1%). 59 Simsek et al also reported that high doses of rosuvastatin (40 mg/day) for 6 weeks resulted in a significant increase in HbA 1c levels (7.9 ± 1.2%) and mean fasting plasma glucose. 58
A large increase in fasting insulin (16 ± 53%), insulin resistance (19 ± 56%) with a modest increase in fasting glucose and HbA 1c was also reported by Her et al. 55 A modest increase in fasting serum insulin and serum insulin levels were also reported Moutzori et al and Koh et al, respectively. 34 65 Whereas Bellia et al in her two studies reported no change in insulin sensitivity and falling insulin levels. In addition, insulin secretion was also reported to be markedly reduced (21.9%). 10 68 No significant change in HbA 1c levels were shown in studies by Moutzori et al, Ogawa et al, Koh et al, and Bellia et al. 10 34 51 65
Simvastatin
Experimental data: Few animal studies were found which reported simvastatin's effects on glucose control, furthermore they present conflicting results. In one study, Aguirre et al reported that simvastatin use (0.6mg/kg/day) for 6 weeks on obese Zucker rats resulted in a significant decrease in serum glucose concentration with no change reported in insulin concentration and insulin resistance. 14 Its result was in disagreement with a study by Wang et al among 16 diabetic Wistar rats treated with simvastatin (10 mg/kg/day) for 12 weeks which resulted in a significant increase in fasting plasma glucose. 69
Clinical data: Differing results showing either a detrimental or neutral effect were shown among the several studies included. Results from a large-scale study done by the Heart Protection Study Collaborative Group to find out the various long-term effects of simvastatin, which included 5,963 adults with diabetes and were given simvastatin 40 mg/day therapy for a period of 5 years showed a nonsignificant trend toward a worsening development of diabetes. 70 No significant increase in fasting serum insulin and HbA 1c was also reported by Moutzori et al. 34 Furthermore, a study by Krysiak et al among patients with impaired glucose tolerance treated by simvastatin 20 mg/day for 12 weeks showed no significant changes in fasting glucose, HbA 1c , and insulin resistance, in addition the study reported a modest reduction in 2-hour postglucose load plasma. 71
In another study, Bellia et al reported in her study using simvastatin 20 mg/day a modest increase in insulin levels accompanied by no significant change in glucose levels. 10 Further longer term study made by her with simvastatin 20 mg/day, however, reported a significant increase in fasting glucose (8.32 ± 0.28 mmol/L) accompanied by a modest increase in HbA 1c levels (0.8 ± 0.2%) as well as a 21.9% decrease in insulin secretion. No change in insulin sensitivity was found. 68
The use of high doses of simvastatin (80 mg/day) in a study by Szendroedi et al among type 2 diabetic patients with a known duration of disease between 3 and 10 years reported a significant increase in HbA 1c levels (6.7 ± 0.6%) followed by a modest increase in insulin resistance (2.7 ± 0.6%). 72 The modest increase in insulin resistance was also reported by Kater et al among patients treated by simvastatin 20 mg/day though to a much lesser degree. 73 Taking into account the varying doses used in the above studies and their effects, it may be possible to infer that simvastatin shows a dose-dependent effect on glucose control ( Table 2 ).
Table 2. Effects of different statins on glucose metabolism, insulin signaling, and specific tissues.
| Statin | Dose (mg) | Glucose metabolism | Insulin signaling | Tissue-specific effects/toxicity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | FPG | 2-h OGTT | HbA 1c | Insulin level | IS | FPI | HOMA-IR | |||
| Atorvastatin | 10 |
↑
53
54
Neutral 16 45 66 ↓ 75 |
↑
56
59
Neutral 43 52 ↓ 46 |
↓ 46 |
↑
16
42
43
52
53
54
56
Neutral 46 47 51 57 66 ↓ 75 |
↑
16
Neutral 66 ↓ 46 |
↓ 57 |
↑
56
57
Neutral 43 |
↑
16
56
59
Neutral 43 66 ↓ 46 |
Conflicting effects on hepatic glucose production
24
25
Causes insulin resistance among peripheral tissues especially adipocytes 24 25 31 |
| 20 |
↑
50
55
60
↓ 75 |
↑ 55 58 59 |
↑
55
57
75
Neutral 48 58 59 ↓ 75 |
↓ 57 | ↑ 55 57 | ↑ 55 59 | ||||
| 40 | ↑ 60 | Neutral 58 |
↑
57
Neutral 58 |
↓ 57 | ↑ 57 | |||||
| 80 | ↑ 58 | ↑ 57 58 | ↓ 57 | ↑ 57 58 | ||||||
| Fluvastatin | 80 | Neutral 61 | ↓ 61 | Reduces or neutral effect on hepatic glucose production 24 25 | ||||||
| Lovastatin | No clinical studies were found | |||||||||
| Pitavastatin | 1 | Neutral 63 |
Neutral
63
↓ 11 |
No reports were found | ||||||
| 2 |
↑
16
56
Neutral 53 |
↑
56
Neutral 62 63 ↓ 43 |
↑
16
62
Neutral 43 53 63 ↓ 11 |
↑
16
↓ 62 |
↑
56
↓ 43 |
↑
16
56
↓ 43 62 |
||||
| 4 | Neutral 50 60 | ↓ 11 | ||||||||
| Pravastatin | 10 | Neutral 53 54 66 | Neutral 53 54 66 | Neutral 66 | Neutral 66 |
Reduces or neutral effect on hepatic glucose production
24
25
Does not cause insulin resistance among peripheral tissues 24 25 |
||||
| 20 | Neutral 65 | Neutral 47 65 | Neutral 65 | |||||||
| Rosuvastatin | 5 | Neutral 51 | Causes insulin resistance among peripheral tissues especially adipocytes 24 31 | |||||||
| 10 |
Neutral
55
65
↓ 58 |
↑ 55 59 |
↑
55
Neutral 34 58 65 |
Neutral 65 | ↑ 34 55 | ↑ 55 59 | ||||
| 20 | Neutral 10 |
↑
59
68
Neutral 58 |
↑
68
Neutral 58 |
Neutral
10
↓ 68 |
Neutral 68 | ↑ 59 | ||||
| 40 | ↑ 58 | ↑ 58 | ||||||||
| Simvastatin | 20 | Neutral 10 |
↓
71
↑ 68 |
↓ 71 |
↑
68
↓ 71 |
↑
10
↓ 68 |
Neutral 68 |
↑
73
↓ 71 |
Conflicting effects on hepatic glucose production
24
25
Causes insulin resistance among peripheral tissues especially adipocytes 24 25 Inhibits caveolae and adiponectin formation on adipocytes 26 Reduces OXPHOS among myocytes and myotubules leading to myopathy 37 |
|
| 40 | Neutral 34 | ↑ 34 | ||||||||
| 80 | ↑ 72 | ↑ 72 | ||||||||
Abbreviations: 2-h OGTT, 2 hours post-oral glucose load tolerance test; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HbA 1c , glycated hemoglobin; HOMA-IR, homeostasis model assessment insulin resistance; IS, insulin sensitivity; OXPHOS, oxidative phosphorylation.
Concerns and Practical Implications to Type 2 Diabetes Mellitus
The worsening glucose control among diabetic patients during the use of statin is clinically concerning due to the large number of diabetic patients using statins and the possibility of increasing mortality, morbidity, and complications among diabetic patients following statins use. Thus, it is of great concern to evaluate the various effects of the different types of statins among diabetic patients as their use has become a regular regiment for diabetic patients to prevent cardiovascular diseases.
Statins have been associated with unfavorable effects on glucose control as shown by the cohort retrospective study by Liew et al evaluating the use of various statins on glucose control, which reported significantly higher change in HbA 1c levels among diabetic statin users as compared with nondiabetic statin users. 9 In a recent study by Mansi et al, similar results were obtained in which diabetic statin users have more than double the odds of developing diabetic complications as compared with nonstatin users. 12 Increase in fasting plasma glucose levels were also reported in a RCT by Sukhija et al among 6,770 diabetic statins users treated with a variety of statins for 2 years. 74 However, it is important to evaluate whether all statins confer an unfavorable effect on glucose control among diabetic patients or only some of them do.
Our review shows that rosuvastatin has shown a tendency for unfavorable effects on glucose control. Conflicting results exists for atorvastatin use though the use of high doses of atorvastatin has been shown to negatively impact glucose control. Similarly, a dose-dependent effect was also observed regarding simvastatin use. Pitavastatin was also reported to be related to worsening in glucose control though to a lesser degree as compared with atorvastatin. Lovastatin was also shown to result in unfavorable effects in experimental studies, however, further studies are required to confirm it.
On the other side, pravastatin was shown to give neutral or possibly favorable results on glucose control. Fluvastatin was also shown to beneficially effect glucose control nevertheless the lack of evidence meant further studies are required to ascertain it surely.
Limitations of the Review
This review article suffers limitations in only being able to synthesize journal articles and trials previously published which are accessible to the authors.
Conclusions
Current evidence available shows that most of the statins give unfavorable side effects with regard to glucose control among diabetic patients. A dose-dependent effect was also observed in some statins wherein higher doses are associated with greater worsening side effect to glucose control. Furthermore, a time-dependent effect was also observed with regards to atorvastatin use which may be present among other statins.
However, given the overwhelming life-saving benefits of statin use on cardiovascular health further concrete evidences from large-scale long-term studies evaluating the side effects of statins use on glucose control as well as complications among diabetic patients are still required to further confirm these findings. Future research should also aim on not only evaluating statin's effect on cardiovascular morbidity but also on the patient's overall morbidity as well. Only then should the current guidelines be reviewed to accommodate those findings.
With regard to these findings, it is important to evaluate the widespread use of statins for primary prevention and use them only when clear and strict indications for their use are met. It is also important to inform patients of the possible risks and motivate them to adhere to lifestyle changes, such as losing weight, exercising, stop smoking, etc., to avoid taking statins. For now, it may be best to be prudent and encourage greater caution in prescribing statins especially with elderly patients suffering from chronic diabetes with low-cardiovascular risk category.
References
- 1.Chinwong D, Patumanond J, Chinwong S et al. Statin therapy in patients with acute coronary syndrome: low-density lipoprotein cholesterol goal attainment and effect of statin potency. Ther Clin Risk Manag. 2015;11:127–136. doi: 10.2147/TCRM.S75608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tziomalos K. Clinical controversies in lipid management. Panminerva Med. 2015;57(02):65–70. [PubMed] [Google Scholar]
- 3.Maddox T M, Borden W B, Tang F et al. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64(21):2183–2192. doi: 10.1016/j.jacc.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Kavousi M, Leening M JG, Nanchen D et al. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311(14):1416–1423. doi: 10.1001/jama.2014.2632. [DOI] [PubMed] [Google Scholar]
- 5.Wehrwein P.(Harvard Health Publications). Statin use is up, cholesterol levels are down: Are Americans' hearts benefiting? Available at:http://www.health.harvard.edu/blog/statin-use-is-up-cholesterol-levels-are-down-are-americans-hearts-benefiting-201104151518. Accessed February 15, 2015
- 6.Zhou Q, Liao J K. Pleiotropic effects of statins. - Basic research and clinical perspectives - Circ J. 2010;74(05):818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Šimić I, Reiner Ž. Adverse effects of statins - myths and reality. Curr Pharm Des. 2015;21(09):1220–1226. doi: 10.2174/1381612820666141013134447. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration.FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugsAvailable at:http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed February 15, 2015
- 9.Liew S M, Lee P Y, Hanafi N S et al. Statins use is associated with poorer glycaemic control in a cohort of hypertensive patients with diabetes and without diabetes. Diabetol Metab Syndr. 2014;6:53. doi: 10.1186/1758-5996-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellia A, Rizza S, Galli A et al. Early vascular and metabolic effects of rosuvastatin compared with simvastatin in patients with type 2 diabetes. Atherosclerosis. 2010;210(01):199–201. doi: 10.1016/j.atherosclerosis.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Teramoto T, Urashima M, Shimano H, Yokote K, Saito Y, LIVES Study Extension Group.A large-scale survey on cardio- cerebrovascular events during pitavastatin (LIVALO tablet) therapy in Japanese patients with hypercholesterolemia Jpn Pharmacol Ther 20113909789–803. [Google Scholar]
- 12.Mansi I, Frei C R, Wang C P, Mortensen E M. Statins and New-Onset Diabetes Mellitus and Diabetic Complications: A Retrospective Cohort Study of US Healthy Adults. J Gen Intern Med. 2015;30(011):1599–1610. doi: 10.1007/s11606-015-3335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed D, Sharma M, Pillai K K. The effects of triple vs. dual and monotherapy with rosiglitazone, glimepiride, and atorvastatin on lipid profile and glycemic control in type 2 diabetes mellitus rats. Fundam Clin Pharmacol. 2012;26(05):621–631. doi: 10.1111/j.1472-8206.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre L, Hijona E, Macarulla M T et al. Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J Physiol Pharmacol. 2013;64(03):281–288. [PubMed] [Google Scholar]
- 15.Otani M, Yamamoto M, Harada M, Otsuki M. Effect of long- and short-term treatments with pravastatin on diabetes mellitus and pancreatic fibrosis in the Otsuka-Long-Evans-Tokushima fatty rat. Br J Pharmacol. 2010;159(02):462–473. doi: 10.1111/j.1476-5381.2009.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu P Y, Lin L Y, Lin H J et al. Pitavastatin and Atorvastatin double-blind randomized comPArative study among hiGh-risk patients, including thOse with Type 2 diabetes mellitus, in Taiwan (PAPAGO-T Study) PLoS ONE. 2013;8(10):e76298. doi: 10.1371/journal.pone.0076298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defronzo R A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(04):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PB PERKENI Indonesia.Konsensus Pengelolaan dan Pencegahan Diabetes Melilitus Tipe 2 di Indonesia 2011 Indonesia: Perkumpulan Endokrinologi; 2011 [Google Scholar]
- 19.American Diabetes Association.Standards of medical care in diabetes 2015: Clinical guideline Diabetes Care 20153801S1–S94. [Google Scholar]
- 20.O'Keefe J H, DiNicolantonio J J, Lavie C J, Bell D S. The influence of statins on glucose tolerance and incipient diabetes. US Endocrinology. 2014;10(01):68–74. [Google Scholar]
- 21.Sattar N, Taskinen M R. Statins are diabetogenic—myth or reality? Atheroscler Suppl. 2012;13(01):1–10. doi: 10.1016/j.atherosclerosissup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Buhaescu I, Izzedine H.Mevalonate pathway: a review of clinical and therapeutical implications Clin Biochem 200740(9–10):575–584. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki J, Iwashita M, Kono S. Statins: beneficial or adverse for glucose metabolism. J Atheroscler Thromb. 2006;13(03):123–129. doi: 10.5551/jat.13.123. [DOI] [PubMed] [Google Scholar]
- 24.Kostapanos M S, Liamis G L, Milionis H J, Elisaf M S. Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol. 2010;8(05):612–631. doi: 10.2174/157016110792006879. [DOI] [PubMed] [Google Scholar]
- 25.Brault M, Ray J, Gomez Y H, Mantzoros C S, Daskalopoulou S S. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63(06):735–745. doi: 10.1016/j.metabol.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Khan T, Hamilton M P, Mundy D I, Chua S C, Scherer P E. Impact of simvastatin on adipose tissue: pleiotropic effects in vivo. Endocrinology. 2009;150(12):5262–5272. doi: 10.1210/en.2009-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon H, Pessin J E.Adipokines mediate inflammation and insulin resistance Front Endocrinol (Lausanne) 20134(June):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antuna-Puente B, Feve B, Fellahi S, Bastard J P. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34(01):2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Wanders D, Plaisance E P, Judd R L. Pharmacological effects of lipid-lowering drugs on circulating adipokines. World J Diabetes. 2010;1(04):116–128. doi: 10.4239/wjd.v1.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul. 2010;44(01):25–36. doi: 10.4149/endo_2010_01_25. [DOI] [PubMed] [Google Scholar]
- 31.Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15(18):1851–1862. doi: 10.2174/092986708785133004. [DOI] [PubMed] [Google Scholar]
- 32.Benoit S C, Clegg D J, Seeley R J, Woods S C. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 33.Abe M, Matsuda M, Kobayashi H et al. Effects of statins on adipose tissue inflammation: their inhibitory effect on MyD88-independent IRF3/IFN-beta pathway in macrophages. Arterioscler Thromb Vasc Biol. 2008;28(05):871–877. doi: 10.1161/ATVBAHA.107.160663. [DOI] [PubMed] [Google Scholar]
- 34.Moutzori E, Liberopoulos E, Mikhailidis D P et al. Comparison of the effects of simvastatin vs. rosuvastatin vs. simvastatin/ezetimibe on parameters of insulin resistance. Int J Clin Pract. 2011;65(11):1141–1148. doi: 10.1111/j.1742-1241.2011.02779.x. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell P, Marette A. Statin-induced insulin resistance through inflammasome activation: sailing between Scylla and Charybdis. Diabetes. 2014;63(11):3569–3571. doi: 10.2337/db14-1059. [DOI] [PubMed] [Google Scholar]
- 36.Meador B M, Huey K A. Statin-associated myopathy and its exacerbation with exercise. Muscle Nerve. 2010;42(04):469–479. doi: 10.1002/mus.21817. [DOI] [PubMed] [Google Scholar]
- 37.Smith R, Solberg R, Jacobsen L L et al. Simvastatin inhibits glucose metabolism and legumain activity in human myotubes. PLoS ONE. 2014;9(01):e85721. doi: 10.1371/journal.pone.0085721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker B A, Thompson P D. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40(04):188–194. doi: 10.1097/JES.0b013e31826c169e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Stasi S L, MacLeod T D, Winters J D, Binder-Macleod S A. Effects of statins on skeletal muscle: a perspective for physical therapists. Phys Ther. 2010;90(10):1530–1542. doi: 10.2522/ptj.20090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M, Kakuta H, Takahashi A et al. Effects of atorvastatin on glucose metabolism and insulin resistance in KK/Ay mice. J Atheroscler Thromb. 2005;12(02):77–84. doi: 10.5551/jat.12.77. [DOI] [PubMed] [Google Scholar]
- 41.Kanda M, Satoh K, Ichihara K. Effects of atorvastatin and pravastatin on glucose tolerance in diabetic rats mildly induced by streptozotocin. Biol Pharm Bull. 2003;26(12):1681–1684. doi: 10.1248/bpb.26.1681. [DOI] [PubMed] [Google Scholar]
- 42.Newman C B, Szarek M, Colhoun H M et al. The safety and tolerability of atorvastatin 10 mg in the Collaborative Atorvastatin Diabetes Study (CARDS) Diab Vasc Dis Res. 2008;5(03):177–183. doi: 10.3132/dvdr.2008.029. [DOI] [PubMed] [Google Scholar]
- 43.Yokote K, Saito Y; CHIBA Study Investigators.Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study) J Atheroscler Thromb 20091603297–298. [DOI] [PubMed] [Google Scholar]
- 44.Mita T, Nakayama S, Abe H et al. Comparison of effects of pitavastatin and atorvastatin on glucose metabolism in type 2 diabetic patients with hypercholesterolemia. J Diabetes Investig. 2013;4(03):297–303. doi: 10.1111/jdi.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szotowska M, Czerwienska B, Adamczak M, Chudek J, Wiecek A. Effect of low-dose atorvastatin on plasma concentrations of adipokines in patients with metabolic syndrome. Kidney Blood Press Res. 2012;35(04):226–232. doi: 10.1159/000332403. [DOI] [PubMed] [Google Scholar]
- 46.Huptas S, Geiss H C, Otto C, Parhofer K G. Effect of atorvastatin (10 mg/day) on glucose metabolism in patients with the metabolic syndrome. Am J Cardiol. 2006;98(01):66–69. doi: 10.1016/j.amjcard.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 47.Endo K, Miyashita Y, Saiki A et al. Atorvastatin and pravastatin elevated pre-heparin lipoprotein lipase mass of type 2 diabetes with hypercholesterolemia. J Atheroscler Thromb. 2004;11(06):341–347. doi: 10.5551/jat.11.341. [DOI] [PubMed] [Google Scholar]
- 48.Holman R R, Paul S, Farmer A, Tucker L, Stratton I M, Neil H A; Atorvastatin in Factorial with Omega-3 EE90 Risk Reduction in Diabetes Study Group.Atorvastatin in Factorial with Omega-3 EE90 Risk Reduction in Diabetes (AFORRD): a randomised controlled trial Diabetologia 2009520150–59. [DOI] [PubMed] [Google Scholar]
- 49.Athyros V G, Papageorgiou A A, Athyrou V V, Demitriadis D S, Kontopoulos A G. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25(07):1198–1202. doi: 10.2337/diacare.25.7.1198. [DOI] [PubMed] [Google Scholar]
- 50.Kryzhanovski V, Gumprecht J, Zhu B, Yu C Y, Hounslow N, Sponseller C A. Atorvastatin but not pitavastatin significantly increase plasma glucose in patients with type 2 diabetes and combined dyslipedemia. J Am Coll Cardiol. 2011;57(14):1113–1279. [Google Scholar]
- 51.Ogawa H, Matsui K, Saito Y et al. Differences between rosuvastatin and atorvastatin in lipid-lowering action and effect on glucose metabolism in Japanese hypercholesterolemic patients with concurrent diabetes. Lipid-lowering with highly potent statins in hyperlipidemia with type 2 diabetes patients (LISTEN) study –. Circ J. 2014;78(10):2512–2515. doi: 10.1253/circj.cj-14-0810. [DOI] [PubMed] [Google Scholar]
- 52.Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49(08):1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 53.Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2008;15(05):269–275. doi: 10.5551/jat.e562. [DOI] [PubMed] [Google Scholar]
- 54.Takano T, Yamakawa T, Takahashi M, Kimura M, Okamura A. Influences of statins on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13(02):95–100. doi: 10.5551/jat.13.95. [DOI] [PubMed] [Google Scholar]
- 55.Her A Y, Kim J Y, Kang S M et al. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. J Cardiovasc Pharmacol Ther. 2010;15(02):167–174. doi: 10.1177/1074248409357922. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki J, Ikeda Y, Kuribayashi T et al. A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance. Clin Ther. 2008;30(06):1089–1101. doi: 10.1016/j.clinthera.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Koh K K, Quon M J, Han S H, Lee Y, Kim S J, Shin E K. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55(12):1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simsek S, Schalkwijk C G, Wolffenbuttel B H. Effects of rosuvastatin and atorvastatin on glycaemic control in Type 2 diabetes---the CORALL study. Diabet Med. 2012;29(05):628–631. doi: 10.1111/j.1464-5491.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 59.Stalenhoef A FH, Ballantyne C M, Sarti C et al. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. Eur Heart J. 2005;26(24):2664–2672. doi: 10.1093/eurheartj/ehi482. [DOI] [PubMed] [Google Scholar]
- 60.Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20-40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab. 2011;13(11):1047–1055. doi: 10.1111/j.1463-1326.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 61.Derosa G, Cicero A E, Bertone G, Piccinni M N, Ciccarelli L, Roggeri D E. Comparison of fluvastatin + fenofibrate combination therapy and fluvastatin monotherapy in the treatment of combined hyperlipidemia, type 2 diabetes mellitus, and coronary heart disease: a 12-month, randomized, double-blind, controlled trial. Clin Ther. 2004;26(10):1599–1607. doi: 10.1016/j.clinthera.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Daido H, Horikawa Y, Takeda J. The effects of pitavastatin on glucose metabolism in patients with type 2 diabetes with hypercholesterolemia. Diabetes Res Clin Pract. 2014;106(03):531–537. doi: 10.1016/j.diabres.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 63.Kawai T, Tokui M, Funae O et al. Efficacy of pitavastatin, a new HMG-CoA reductase inhibitor, on lipid and glucose metabolism in patients with type 2 diabetes. Diabetes Care. 2005;28(12):2980–2981. doi: 10.2337/diacare.28.12.2980-a. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y, Ohmori K, Chen Y et al. Effects of pravastatin on progression of glucose intolerance and cardiovascular remodeling in a type II diabetes model. J Am Coll Cardiol. 2004;44(04):904–913. doi: 10.1016/j.jacc.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 65.Koh K K, Quon M J, Sakuma I et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int J Cardiol. 2013;166(02):509–515. doi: 10.1016/j.ijcard.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 66.Mita T, Watada H, Nakayama S et al. Preferable effect of pravastatin compared to atorvastatin on beta cell function in Japanese early-state type 2 diabetes with hypercholesterolemia. Endocr J. 2007;54(03):441–447. doi: 10.1507/endocrj.k06-198. [DOI] [PubMed] [Google Scholar]
- 67.Miller A W, Tulbert C D, Busija D W. Rosuvastatin treatment reverses impaired coronary artery vasodilation in fructose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(01):R157–R160. doi: 10.1152/ajpregu.00647.2003. [DOI] [PubMed] [Google Scholar]
- 68.Bellia A, Rizza S, Lombardo M F et al. Deterioration of glucose homeostasis in type 2 diabetic patients one year after beginning of statins therapy. Atherosclerosis. 2012;223(01):197–203. doi: 10.1016/j.atherosclerosis.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, Duan G, Lu Y et al. The effect of simvastatin on glucose homeostasis in streptozotocin induced type 2 diabetic rats. J Diabetes Res. 2013;2013:274986. doi: 10.1155/2013/274986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial Lancet 2003361(9374):2005–2016. [DOI] [PubMed] [Google Scholar]
- 71.Krysiak R, Okopien B. Different effects of simvastatin on ex vivo monocyte cytokine release in patients with hypercholesterolemia and impaired glucose tolerance. J Physiol Pharmacol. 2010;61(06):725–732. [PubMed] [Google Scholar]
- 72.Szendroedi J, Anderwald C, Krssak M et al. Effects of high-dose simvastatin therapy on glucose metabolism and ectopic lipid deposition in nonobese type 2 diabetic patients. Diabetes Care. 2009;32(02):209–214. doi: 10.2337/dc08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kater A L, Batista M C, Ferreira S R. Improved endothelial function with simvastatin but unchanged insulin sensitivity with simvastatin or ezetimibe. Metabolism. 2010;59(06):921–926. doi: 10.1016/j.metabol.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 74.Sukhija R, Prayaga S, Marashdeh M et al. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J Investig Med. 2009;57(03):495–499. doi: 10.2310/JIM.0b013e318197ec8b. [DOI] [PubMed] [Google Scholar]
- 75.Pollak A W, Kramer C M. LDL lowering in peripheral arterial disease: are there benefits beyond reducing cardiovascular morbidity and mortality? Clin Lipidol. 2012;7(02):141–149. doi: 10.2217/clp.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


