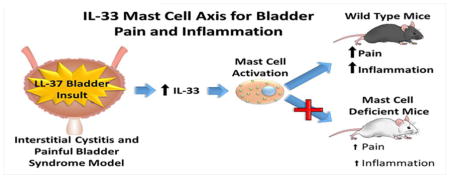
Abstract
Interstitial cystitis (IC), also known as painful bladder syndrome (PBS), is a debilitating chronic condition that afflicts over 3 million women above the age of 18 in the U.S., and most patients fail to respond to current treatment options. Mast cells have previously been implicated as both a diagnostic and prognostic marker in IC/PBS. Patients with IC/PBS have been shown to have elevated levels of IL-33, a cytokine released in response to tissue insult, in their urine. We hypothesize that mast cell-mediated inflammation induced from IL-33 may play an important role in initiating pain and inflammation in IC/PBS. A human cathelicidin, LL-37, which is found at elevated levels in IC/PBS patients, was used to induce an IC/PBS-like state of inflammation and bladder pain in mast cell deficient C-kit (−/−) and wild type C57Bl/6 (WT) mice. Inflammation was quantified using myeloperoxidase (MPO) expression in bladder tissues measured via ELISA. Response rate to suprapubic stimulation from von Frey filaments was used to assess the relative pain and discomfort. Both types of mice increased IL-33 expression in response to LL-37 exposure. However, mast cell deficient mice demonstrated significantly lower levels of inflammation (p<0.001) and reduced pain response (p < 0.001) compared to WT mice. These findings implicate an IL-33-mast cell dependent axis with a potential etiology of pain and inflammation in IC/PBS. Future therapeutics aimed at targeting the IL-33 - mast cell axis could potentially serve as useful targets for treating IC/PBS.
Keywords: Interstitial Cystitis, Painful Bladder Syndrome, IL-33, Mast Cell, Bladder Pain, Inflammation
Graphical Abstract
1.0 Introduction
1.1 Interstitial cystitis/painful bladder syndrome
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a debilitating chronic condition with over 3 million women associated with significant declines in overall well-being and quality of life (QOL).[1] The reduction of QOL results from unrelenting pain, sexual dysfunction, and urinary urgency, and frequency, necessitating frequent trips to the bathroom, sometimes as often as every 10–15 minutes throughout the day and night.[1,2] The direct and indirect costs for U.S. IC/PBS patients and their families, including increased medical expenses and decreased work productivity, is estimated to be on the order of $21.1 billion dollars annually.[3] IC/PBS is a chronic bladder disorder with many patients lacking effective treatment options.[4,5]
The etiology of IC/PBS is not well understood; however, mast cells are hypothesized to play a key role in driving both inflammation and pain. Mast cells are reported to act as modulators of nociceptive neurons via the release of histamine, serotonin, IL-1β, TNF-α, and IL-6, all of which have the ability to independently induce hyperalgesia.[6] Studies have shown mast cells are significantly increased in number and associated with bladder pain and inflammation in IC/PBS patients and animal models.[7–11] There also exists a significant correlation between the number of mast cells and bladder capacity, a key metric associated with the severity of IC/PBS.[12] Furthermore, a significant correlation has been demonstrated between IC/PBS-associated urothelial damage and the increased presence of mast cells.[13]
1.2 LL-37 Murine Model of IC/PBS
The cathelicidin LL-37 is a human anti-microbial peptide found at elevated levels in urine from patients suffering from infection-associated or infection-independent IC/PBS.[14–16] LL-37 is a potent inflammatory amino acid sequence and can trigger apoptotic events within the urothelium, induce leukocyte chemotaxis, stimulate mast cell degranulation, enhance neutrophil function, induce inflammatory chemokines such as IL-8, increase tissue vascularization, and stiffen the bladder wall by stimulating increased extra cellular matrix deposition.[17,18] Based on these key biologic and physiologic components, we have previously generated a mouse IC/PBS model via the intravesical administration of LL-37 into healthy mice. This yields a model which has all of the key physiologic and symptomatic signs of IC/PBS including frequent urination, bladder inflammation, and lower abdominal pain.[16]
1.3 The potential role of IL-33 in IC-PBS
Multiple types of insult can lead to cellular injury and inflammation within the bladder–including mechanical trauma, infection, radiation, ischemia, and exposure to toxins including cationic urinary metabolites.[19,20] Bladder injury and insult can activate adjacent mast cells through the release of a potent cytokine IL-33, which acts as an alarmin released from endothelial, epithelial, and smooth muscle cells.[21–24] Patients with IC/PBS have also been shown to have elevated levels of IL-33 in their urine.[8] Mast cell activation is one of the primary roles of IL-33 and induces them to release pro-inflammatory mediators and further propagate inflammatory responses.[22,25] Furthermore, prior investigations have demonstrated mast cells are increased in IC/PBS and are uniquely poised to recognize rapidly IL-33 released from damaged cells and then exacerbate the inflammatory response.[10,13,16]
We hypothesized that the IL-33 – mast cell axis is implicated in LL-37 induced bladder injury and sought to test this hypothesis by exploiting our mouse model in both wild type (WT) and mast cell deficient (C-kit −/−) mice. We further hypothesized that both bladder inflammation and pain responses are attenuated in mast cell deficient mice, further supporting the key role mast cells play in driving both inflammation and pain. By exploiting our biologically based IC/PBS mouse model, testing these hypotheses would enhance mechanistic insight into the pathophysiology. Ultimately, characterization of an IL-33 – mast cell – bladder inflammation and pain axis would further provide potential therapeutic targets in order to help treat IC/PBS.
2.0 Methods
2.1 Animal Care and Ethics Statement
This research complied with the Institutional Animal Care and Use Committee of the University of Utah (Protocol #: 1106010) and all procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.[26] The animal facilities at the University of Utah are American Association for the Accreditation of Laboratory Animal Care (AAALAC) accredited. All experiments were carried out within procedure rooms within the animal care facility. Animals were kept on a standard 12 hr. light/dark cycle and given food and water ad libitum. Within each experimental group, mice were housed together. Experiments were performed during the light portion of the cycle between 6:00 AM and 6:00 PM. Within each cage, Paperchip® (Shepherd Specialty Papers, Watertown, TN) was used as beading and the mice were provided with an Enviropak Nestpak® filled with envirodri® (Fibercore, Cleveland, OH). The condition of all animals was assessed at least once daily by either a veterinary technician or veterinarian.
2.2 IL-33 expression in response to LL-37 induced bladder injury
IL-33 up regulation in LL-37 induced bladder injury was assessed via ELISA and fluorescent immunohistochemistry (IHC). Adult female C57Bl/6 mice (Jackson Laboratory, Strain: C57BL/6J, Stock Number: 000664) 8–10 weeks of age were randomly assigned to groups by cage cohort at the time they were received in the animal facility. Each group was assigned a treatment receiving 10, 20, 40, 80, 160, 320 μM of LL-37 and sterile normal saline as a control (n=5 for each group). LL-37 (Single letter amino acid sequence: LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) was synthesized by the DNA/Peptide Synthesis Core at the University of Utah and purified using preparative high performance liquid chromatography. LL-37 was synthesized and given via intravesical administration to the bladder for 1 hour as previously described.[14,16] Animals were sacrificed using CO2 asphyxiation 24 hours post LL-37 installation and samples were taken for IL-33 ELISA and fluorescent immunohistochemistry. The bladder was removed and hemisected along the median plane. Half of the bladder was fixed in 4% paraformaldehyde (Sigma Aldrich, St. Louis, MO) while the other half was flash frozen in liquid nitrogen and stored at −75 ± 5°C until processing.
2.3 IL-33 ELISA
For ELISA frozen bladder tissues were first thawed on ice and immersed in ice cold lysis buffer (pH 7.4) that contained 200 mM NaCl, 10 mM Tris, 10% glycerin, 5 mM EDTA, and were supplemented with Halt protease inhibitor cocktail (Thermo Fisher Scientific, IL). The tissue was homogenized using silica beads (BioSpec, Products inc., OK) at 4°C. The homogenate was then centrifuged and the supernatant was used in the Quantikine ELISA kit (R&D Systems, inc., Minneapolis, MN) according to the manufacturer’s directions.
2.4 Fluorescent Immunohistochemistry for IL-33
Bladder tissue was fixed in 4% paraformaldehyde overnight, then dehydrated within graded ethanol and xylene prior to being embedded with paraffin. Tissue was then sectioned at 5 μm. Endogenous peroxidase activity was blocked with 1% hydrogen peroxide in tris-buffered saline (TBST) for 20 minutes and washed 3X in TBST for 3′. Antigen retrieval was performed using antigen unmasking solution (Vector laboratories, CA). To minimize non-specific anti- body binding, sections were incubated for 60 min in 5% fetal bovine serum in TBST with 0.3% Triton X-100 (Sigma Aldrich, St. Louis, MO). Sections were incubated overnight at 4 °C with rabbit anti-mouse IL-33 at a 1:400 in blocking solution (Santa Cruz Biotech, Inc., CA). Following incubation, slides were washed three times in TBST for 3 min. Sections were then incubated for 60 min. with DyeLight633 conjugated secondary antibody (goat anti-rabbit IgG 1:3000 in blocking solution (Thermo Scientific, IL) at room temperature, sections were washed three times for 3 min in TBST. Coverglass was added after one to two drops of FluoMount-G (Southern Biotech, AL) premixed with Hoechst 33342 (Invitrogen, CA) at 1:200 dilution for staining cellular DNA. Negative controls included incubation with TBST in place of the primary antibody and no immuno- reactivity was observed. Images were obtained on an Olympus BX40 fluorescent microscope.
2.5 Animal model for evaluating the role of Mast Cells in IC/PBS
C-kit (−/−) mice(Jackson Laboratory, Strain: STOCK KitW-sh/HNihrJaeBsmJ, Stock Number: 005051), also known as KitW-sh/W-sh mice, arose from a spontaneous inversion mutation in an upstream regulatory domain to the C-kit element in the C57Bl/6 strain which rendered them mast cell deficient.[27] The paired use of C-kit (−/−) and WT C57Bl/6 mice provided an optimal strain specific model for assessing the role of mast cells in inflammation and pain as genetic differences, other than the C-Kit mutation, were minimized. Female mice 8–10 weeks in age were used. Estrous cycle of the mice was not considered, as the impact to both pain response and inflammation should be minimal compared to the expected contributions from LL-37 tissue insult based on previous experience.[28] LL-37 was administered as described in section 2.2.
2.6 Assessment of Pain Responses in Mast Cell Deficient and WT Mice
Naive mice were placed onto a wire mesh floor inside of transparent enclosures to allow for observation. After at least 10 minutes of acclimatization, their degree of mechanical allodynia in the suprapubic region was assessed using von Frey filaments corresponding to 0.04, 0.16, 0.4, 1.0 and 4.0 g of stimulation as described previously.[11,29–31] Each mouse was stimulated for 1 sec for aproximately 10 successive stimulations at 3 second intervals. A sharp retraction of the abdomen, immediate licking or scratching of the stimulated area, or a jump was considered a positive indication of pain.[29] Baseline measurements for each mouse were taken prior to saline or LL-37 administration, then 24 hours later when the relative sensitization should be at its peak.[31] Thus, the probability of observing potential differences between mast cell deficient and WT mice would be at its greatest. Mice were randomly assigned to groups (n=4) that received either a sham treatment consisting of a saline injection or exposure to 80 μM, 160 μM, or 320 μM LL-37. The change in response rate was calculated as the percentage of positive responses post challenge minus the percentage of positive responses during the baseline assessment for each animal. The average change in response rate at each of the 5 stimuli levels for each treatment group was used for comparison between varying treatments and mouse strains.
2.7 Tissue Assessment
Tissue was collected as described section 2.2. Histology samples were stained with hematoxylin and eosin (H&E) as described previously.[30] Tissue myeloperoxidase (MPO) was assessed using a mouse MPO kit (Hycult®Biotech Inc., Plymouth Meeting, PA). The total protein content in the supernatant of the tissue homogenate was determined using a Bradford assay with Coomassie Plus Protein Assay (Thermo Fisher Scientific, IL). The concentration of MPO was normalized to the total protein content in each of the samples.
2.8 Immunohistochemistry for Tryptase
Freshly harvested bladder tissues were fixed and imbedded as described in section 2.2. Using xylene and graded ethanol solutions, 5 μm tissue sections were deparaffinized then rehydrated. Endogenous peroxidase activity was blocked with 1% hydrogen peroxide in TBST for 20 min and then washed in TBST for 3 min three times. Antigen retrieval was performed using a tris-based antigen unmasking solution (Vector Laboratories, Inc., Burlingame, CA). Non-specific anti-body binding was reduced by incubating sections for 60 min in 5% fetal bovine serum in TBST with 0.3% Triton X-100(Sigma Aldrich, St. Louis, MO). Tissue samples were then incubated overnight at 4 °C with a primary polyclonal Rabbit Anti Mouse mast cell tryptase (Santa Cruz Biotech Inc., Santa Cruz, CA) 1:800 in blocking solution and then washed three times in TBST. Samples were then incubated for 60 min with a biotinylated goat anti-rabbit IgG, 1:2000 in blocking solution (Santa Cruz Biotech Inc., Santa Cruz, CA). Vectastatin Elite® ABC-HRP kit (Vector Laboratories, Inc., Burlingame, CA) was used to develop the slides to show the presence of tryptase. Sections were washed in double distilled water, counterstained, and mounted. Negative controls included incubation with TBST in place of the primary antibody and no immuno- reactivity was observed. Images were taken on an Olympus BX40 light microscope and white balanced.
2.9 Statistical Analysis
A Shapiro-Wilks Test was used to assess if the data was viable to be analyzed using parametric statistics that assume a normal distribution. A Grubbs Test was used to identify and exclude any outliers. Paired T-test was used to assess the difference between two groups and one-way analysis of variance (ANOVA) with Bonferroni’s Multiple Comparison Test was used to make comparisons between multiple sets of data. A one sample two tailed T-test was used to test data sets against theoretical values. An F-test was used to assess the significance of linear least squares regression results. P-values of less than 0.05 were considered statistically significant, with p-values of less than 0.01 and 0.005 considered highly significant and very highly significant, respectively. Statistical analysis was performed using GraphPad Prism ™ 5.0 (GraphPad Software, CA).
3.0 Results
3.1 IL-33 Levels Increase due to LL-37 exposure in a Dose-Dependent Fashion
Initially, after bladder exposure to LL-37 in WT mice, IL-33 expression rose in a dose dependent fashion. As the intravesical concentrations of LL-37 were increased the observed expression of IL-33 increased proportionally (see Figure 1). IL-33 elevation became highly significant at 320 μM of LL-37 challenge when compared to background levels. The relative increase in expression level compared to background at 80 μM, 160 μM, and 320 μM was 25%, 191%, and 284%, respectively. The linear regression (r2=0.925) gave a slope of 0.049 ± 0.006 pg/mg IL-33 per μM LL-37 and a y intercept of 4.5 ± 0.9 pg/mg IL-33. An F-test showed that the observed slope was highly significant (p<0.001) compared to the null hypothesis that there was no effect on IL-33 expression in response to LL-37 exposure. At concentrations below 80 μM of LL-37 challenge, IL-33 levels were not significantly distinguishable from the sham procedural controls. Therefore, in subsequent testing only concentrations of 80 μM of LL-37 challenge or higher were utilized.
Figure 1. IL-33 expression rises linearly in response to increasing concentrations of LL-37 challenge.
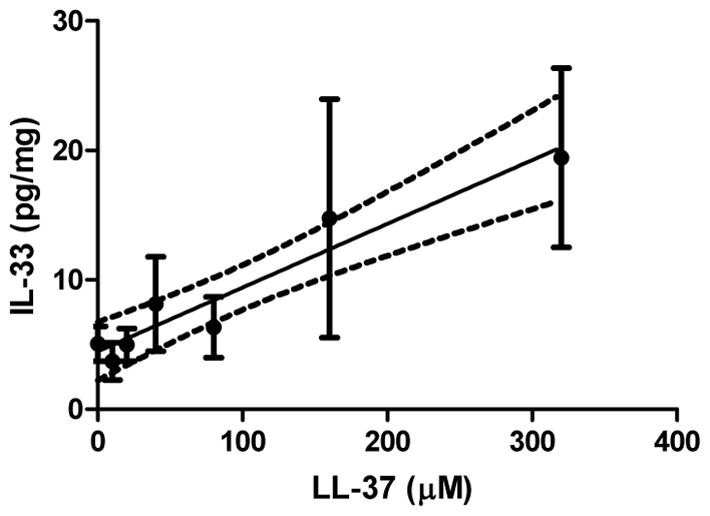
The solid line represents the linear least squares regression of the IL-33 expression level against the concentration of LL-37 administered intravesically in wild type mice. Points represent the mean, while the error bars indicate the standard deviation. The dashed lines represent the 95% confidence interval of the linear regression.
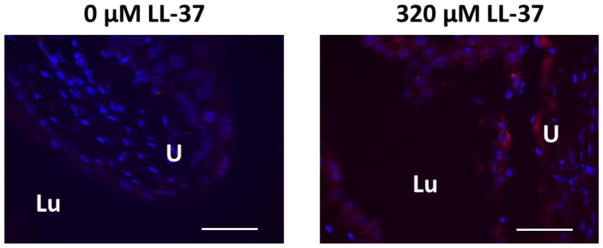
From our fluorescent IHC experiments, increased IL-33 expression secondary to LL-37 exposure occurred primarily within the urothelial compartment (see Figure 2). Very limited IL-33 was observed in the nuclei of the endothelial cells within the mucosa of the control group. This is opposed to the group challenged with 320 μM of LL-37 where substantial IL-33 expression drastically increased. The staining pattern also changed from being associated with the nucleus to also being observed within the cytoplasm of urothelial cells (see Figure 2). Non-inflamed lung tissue, which is known to constitutively express IL-33, was used as a control and stained positive for IL-33.[23]
Figure 2. Immunohistochemistry for IL-33 in the urothelium demonstrating increased IL-33 expression in response to LL-37 in wild type mice.
Only minimal IL-33 is observed in controls (left image) compared to bladders challenged with 320 μM LL-37 (right image). IL-33 was observed to be specifically within urothelial cells. Urothelial tissue stained with Hoechst 3342 is shown in blue and for IL-33 in red. The scale bar represents 100 μm. LU: lumen of the bladder. U: urothelium.
3.2 Pain Responses in Mast Cell Deficient and Wild Type Mice
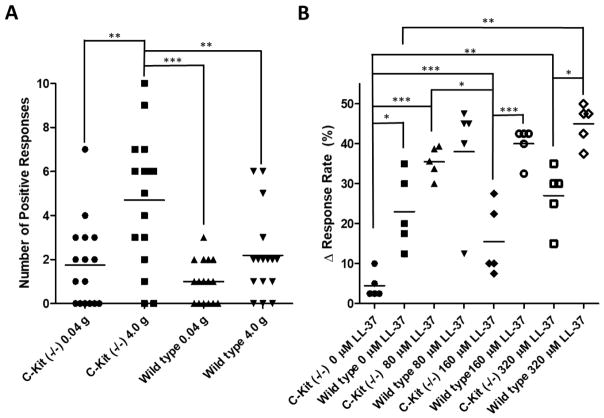
At baseline, mast cell deficient mice demonstrated highly variable pain responses to suprapubic stimuli compared to WT mice. The WT mice responded in a typical logarithmic response pattern to stimuli from 0.04 g to 4 g of stimulation, as has been observed previously in the LL-37 induced models of IC/PBS.[31] In contrast, mast cell deficient C-kit (−/−) mice demonstrated highly erratic behavior in response to suprapubic mechanical stimuli compared to WT mice (Figure 3A). As a result of this increased variability, we evaluated the change in pain response rate from baseline for each individual. The change in response rate for each treatment group was then used to assess changes in suprapubic mechanical sensitivity between the mast cell deficient C-kit (−/−) and WT mice in response to LL-37.
Figure 3. Pain responses in C-kit (−/−) and wild type mice to Von Frey Filament stimulation.
A) C-Kit (−/−) mice exhibited both a higher variability in response and higher mean response during baseline pain response assessment. As the level of stimuli was increased, C-kit (−/−) demonstrated a highly significant elevation in pain responses (n=16). However, while there was an elevation in response rate the degree of the elevation was not as great and was not observed as significant in the wild type mice (n=16). Each marker represents the number of stimulations out of ten to which each mouse yielded a positive response. B) Exposure to LL-37 highly significantly elevated the sensitivity of wild type and C-kit (−/−) mice. The degree of sensitization was lower in C-kit (−/−) compared to wild type mice. Each marker indicates the average change in response rate from baseline measurements at 0.04, 0.16, 0.4, 1.0, or 4.0 g stimulation from Von Frey filaments within each group. The horizontal bar represents the mean of the data. *, **, and *** indicate p-values of less than 0.05, 0.01, and 0.001, respectively, as determined from a 1-way ANOVA with Bonferroni posttest.
C-kit (−/−) mice were less sensitized to mechanical stimulation after LL-37 induced bladder insult compared to WT mice. As the Von Frey filament stimulation increased from 0.04 g to 4.0 g both the C-Kit (−/−) and WT mice increased their rate of response. Both groups of mice showed increased response rate after the sham treatment with PBS indicating that the catheterization of the urethra did impact sensitization (see Figure 3B). This sensitization was significant from the null hypothesis that there would be no change from baseline, meaning Δ response rate equaled 0.0%, for the sham treatment groups for both WT and C-kit (−/−) mice with p-values of 0.0051 and 0.0367, respectively.
Sensitization was greater in WT mice compared to C-kit (−/−) mice and was positively correlated with increasing levels of LL-37. However the observed increase in response rate was greater in WT mice compared to C-kit (−/−) mice. The C-kit (−/−) demonstrated an unexpected drop in sensitization when LL-37 challenge was increased from 80 μM and 160 μM, and the reason for this was not clear from the data. In all cases the C kit (−/−) demonstrated less change in their response rate compared to WT mice. These results illustrated that mast cell deficient C-kit (−/−) mice were less sensitized by LL-37 induced bladder insult compared to WT mice.
3.3 LL-37 Induced Bladder Inflammation
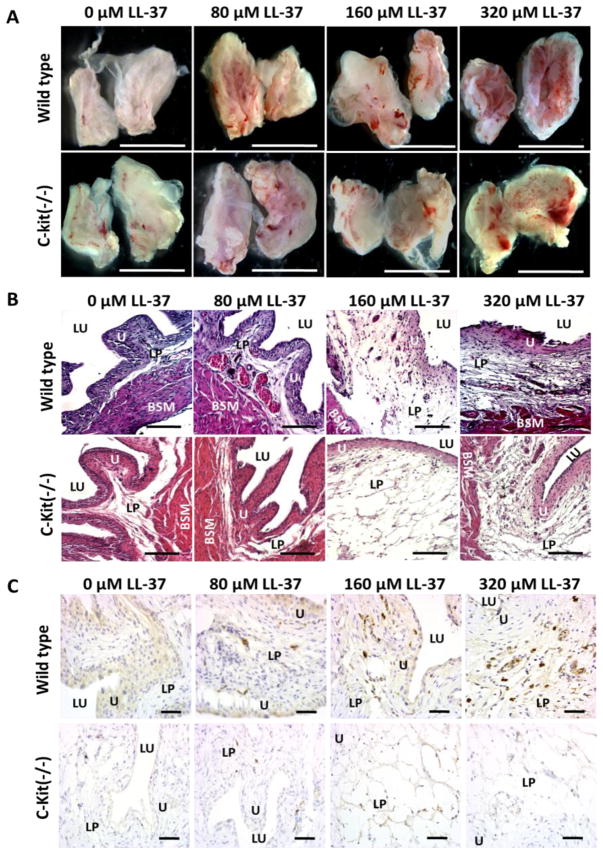
Gross anatomical examination of C-kit (−/−) and WT mice demonstrated increasing signs of inflammation with increasing concentrations of LL-37 challenge (see Figure 4A). WT sham controls had no signs of inflammation while the C-kit (−/−) had minor erythema and edema. The cause of this minor inflammation in C-kit (−/−) was not clear from gross examination, but was present in all 4 of the mice in the sham control group. At 80 μM of LL-37 challenge there was a minor increase in erythema and edema for the WT group. The level of edema increased in the 160 μM LL-37 challenge group, but the degree of erythema demonstrated only a mild increase. At 320 μM of LL-37 challenge the inflammation observed in both C-kit (−/−) and WT mice became severe as indicated by the pronounced erythema across the bladder urothelium, severe edema, and evidence of hemorrhage. These same signs of inflammation were observed in the C-kit (−/−) mice, albeit less pronounced.
Figure 4. Gross anatomy, histology, and IL-33 immunohistochemistry for C Kit (−/−) and wild type mice after LL-37 challenge.
A) Examination of the gross anatomy of bladders 24 hours after LL-37 challenge demonstrated increasing inflammation within the inner mucosa for both C-kit (−/−) and wild type mice in a dose response fashion. Bladders were hemisected to allow for inspection of the urothelium. Scale bar represents 5 mm. B) Histological examination demonstrated more pronounced inflammation in the wild type mice compared to the C-kit (−/−) group. Edema, polymorphonuclear cell infiltrate, and microabscesses were more pronounced in the normal mast cell intact mice compared to the C-kit (−/−) group. Scale Bar represents 250 μm. C) Immunohistochemistry demonstrated increasing tryptase expression, which is indicatory of mast cells, in the lamina propria and urothelium as exposure to concentrations of LL-37 increased in only the WT mice. No tryptase expression was observed in the C-kit (−/−) mice at all concentrations of LL-37 challenge. Scale bar represents 100 μm. LU: lumen of the bladder. U: urothelium. SM: submucosa. LP: lamina propria. BSM: bladder smooth muscle layer.
Histology qualitatively confirmed the observations made during gross anatomical examination. The control group of the WT mice demonstrated no evidence of inflammation, while there was some minor edema noted in the C-kit (−/−) mice but otherwise no other signs of inflammation (see Figure 4b). At 80 μM of LL-37 challenge, the WT mice demonstrated moderate polymorphonuclear leukocytes (PMNL) infiltrates, along with moderate edema. PMNL infiltrates were not noted in the C-kit (−/−) mice at 80 μM LL-37. At 160 and 320 μM of LL-37 challenge, the edema became highly evident and disrupted the lamina propria layer in both the C-kit (−/−) and WT mice. In the WT mice the number of PMNLs increased dramatically, while this was not as evident in the C-kit (−/−) mice.
In order to detect mast cell activity, tryptase IHC demonstrated that LL-37 induced bladder insult produced a strong mast cell response in WT mice as expected and was non-detectable in the mast cell deficient C-kit (−/−) mice (see figure 4C). In the control groups for both the WT mice and C-kit (−/−) mice, there was notably no substantial evidence for tryptase activity in either group. Although as dose escalation with LL-37 challenge occurred, increased tryptase activity was observed in the WT mice in a dose response fashion, with higher concentrations of LL-37 challenge yielding increased tryptase activity. However, this same trend and pattern was not observed in the C-kit (−/−) mice, which demonstrated no evidence of tryptase activity regardless of the degree of LL-37 exposure.
3.4 Tissue IL-33 and MPO expression
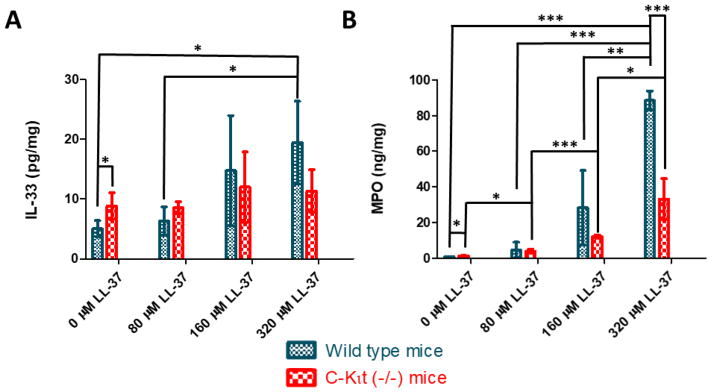
With increasing concentrations of LL-37 challenge, IL-33 levels rose significantly in the WT mice group (see Figure 5A). A similar trend of IL-33 increase occurred in the C-kit (−/−) mice, although not as substantial when compared to the WT mice. In addition, these results demonstrated C-kit (−/−) mice yielded significantly higher baseline levels of IL-33 expression, 8.8 ± 2.2 pg/mg, compared to the WT mice, 5.1 ± 1.4 pg/mg. Overall though, as a result of LL-37 challenge, the trend observed with increased IL-33 in the WT mice when compared to the C-kit (−/−) mice suggested that the WT mice could mount a stronger expression pattern for IL-33 than the mast cell deficient C-kit (−/−) mice.
Figure 5. Quantitative assessment of inflammation in wild type and mast cell deficient C-kit (−/−) mice.
Both A) IL-33 and B) MPO were measured using ELISA. In the sham control, mast cell deficient mice expressed a modestly higher level of IL-33, but did not exhibit as strong of a response as the wild type mice to LL-37 challenge. After LL-37 exposure, wild type mice demonstrated similar levels of IL-33 to C-kit (−/−) mice. For MPO activity, the wild type mice produced higher levels of MPO when compared to C-kit (−/−) mice after the administration of LL-37. The plot is of the mean ± standard deviation. *, **, and *** indicate P-values of <0.05, <0.01 and <0.001, respectively.
MPO ELISA was used to quantify the presence of an acute inflammatory response in both WT and mast cell deficient C-kit (−/−) mice (see Figure 5B). The controls for both groups of mice demonstrated as expected, very minimal MPO activity. In the sham control group, which received saline instillation, the WT mice had significantly less MPO, 0.91 ± 0.08 ng/mg, compared to the matched C-kit (−/−) group, 1.29 ± 0.17 ng/ml. However, as LL-37 exposure was increased, both groups demonstrated statistically significant rising levels of inflammation via assessment of MPO expression. WT mice demonstrated substantially higher levels of MPO, particularly at 320 μM of LL-37 challenge, 88.5 ± 5.3 ng/mg, when compared to the mast cell deficient C-kit (−/−) group, 33.2 ± 11.5 ng/mg. These results were highly suggestive that mast cells may play a significant role in the mediation and propagation of inflammation within this LL-37 induced cystitis model.
4.0 Discussion
We hypothesized that IL-33, a potent pro-inflammatory cytokine, is upregulated in LL-37 induced bladder injury. This was confirmed when WT, C57Bl/6 mice demonstrated a positive dose-response profile, with increased levels of inflammation observed with increased concentrations of LL-37 challenge. In addition, IL-33 levels rose in a similar dose response fashion. This response was mediated in part by insult to urothelial cells and fibroblasts, inducing localized release of IL-33. IL-33 is a known trigger for mast cell degranulation and accumulation within damaged tissues.[22,32] Previous experiments, have shown that blocking the primary receptor of IL-33, ST2, prevents mast cell injury in response to damage in epithelial tissues.[33] These observations suggests that IL-33 was at least partially responsible for past observations of mast cell involvement in LL-37 induced bladder inflammation.[14,16,34] The observation that IL-33 was observed to distribute to the cytoplasm after LL-37 administration is of interest as typically IL-33 is observed to be localized in the nucleus.[23,35] There are also numerous reports where IL-33 has been observed within the cytoplasm.[36,37] In the cases where IL-33 was observed within the cytoplasm there was either an experimentally induced state of inflammation or the derived tissue was in a chronic inflammatory state.[38] Based on these observations we postulate that IL-33 redistribution from the nucleus to the cytoplasm is a key step in its response to epithelial insult either from mechanical or chemical insult. The nature of this redistribution and its mechanism warrants future investigation and may occur due to activation of alternative splicing during translation, alterations to nuclear pore structure, or other yet undescribed mechanisms.[37,38]
C-kit (−/−) mice represents a viable model for evaluating the role of mast cells within the context of our LL-37 induced cystitis model. C-kit (−/−) mice differ from WT C57Bl/6 mice by a single inversion mutation (Kit W-Sh) otherwise both are genetically identical. The white spotting locus, associated with tyrosine kinase dependent signaling, results in changes beyond mast cell deficiency. Other phenotypes associated with similar mutations at this locus include impaired melanogenesis, anemia, and sterility.[27] It is unclear if there may be secondary factors at play beyond simple mast cell depletion. It is assumed in the presented research that all observed changes between C-kit (−/−) and WT mice were due to the lack of mast cells, but this may not be the case as there are many phenotypes affected by the C-kit mutation. However, C-kit(−/−) mice, also referred to as KitW-sh/W-sh mice, have been observed to return to wild type behavior, inflammatory responses, and tissue associated mast cells after the transplantation of bone marrow from wild type mice.[27,33] This supports that the lack of mast cells may be predominantly responsible for the observed differences in pain and inflammation that we observed. LL-37 exposure is also limited as a single dose and results in an acute condition of IC/PBS that eventually self resolves.[31] In humans, IC/PBS is a chronic condition and only diagnosed late after onset. The findings in this study focused on the acute inflammatory state induced by LL-37. Further studies are needed to assess the role of mast cells in chronic IC/PBS. While mast cells are typically best known for their effector role in promoting inflammation and immune responses, they can also have important anti-inflammatory and immunosuppressive roles under certain conditions.[39] This lack of mast cells in an anti-inflammatory capacity, may be the source of the higher baseline levels of inflammation, heightened variability in baseline pain responses, and in histological evaluation. However, this also requires further investigation.
Mast cell deficient mice demonstrated reduced pain and inflammation despite having increases in IL-33 expression after LL-37 bladder insult. IL-33 immunohistochemistry demonstrated fewer strongly stained cells in mast cell deficient C-kit (−/−) mice compared to their WT counterparts. While IL-33 expression differences between the strains in response to LL-37 challenge were not statistically different, there was strong statistical significance indicating that the WT mice were more sensitive to LL-37 induced injury from both the MPO assays and pain sensitization assessments. We propose that this difference in sensitivity is likely due to lack of mast cells in C-kit (−/−) mice.
In spite of mast cell deficiency and reduced IL-33 expression, C-kit (−/−) mice still demonstrated sensitization and inflammation, albeit at a reduced level compared to WT mice in response to LL-37 insult. This demonstrates the presence of other pathways that might impact the inflammatory and pain responses. In particular, biopsies of IC/PBS patient samples or urine samples have implicated IL-6, IL-10, iNOS, Vascular Endothelial Growth Factor (VEGF), CXCL10, CCL21 and IL-17A in addition to IL-33 in IC/PBS.[40–42] Some of these cytokines have been previously implicated in sensitizing nociceptive neurons IL-6, CCL21, and IL-17A, while the others may play secondary or ancillary signaling roles in IC/PBS-associated nociception.[42–44] While these cytokines contribute to the pain response, we still observed significant differences between mast cell deficient and WT mice, indicating that there is an important role mediated exclusively through a mast cells associated signaling axis. Further research is needed to elucidate the function of other cytokines with respect to IC/PBS pain and inflammation.
The lack of mast cells may potentially be interrupting the normal MyD88 signaling cascade initiated by IL-33 mast cell activation, resulting in fewer pro-inflammatory cytokines being released, particularly IL-33, NF-κB, IL-13, histamine and IL-8.[24] This hypothesized reduction in pro-inflammatory molecules, in part explains the observed significant decreases in MPO activity and pain responses. At 320 μM of LL-37 challenge, the MPO concentration was reduced by 62% and pain response rates were reduced by 40% in C-kit (−/−) mice compared to WT animals. These reductions in pain and inflammation are not trivial. For example, in a chemically induced visceral pain model, a clinically pertinent dose of 1.9 ± 0.1 mg/kg of morphine is needed to reduce pain responses by 50% in mice.[45] The reduction in MPO activity between mast cell deficient C-kit (−/−) and WT mice is similar to the observed reductions in LL-37-induced inflammation treated with common clinically used therapies for IC/PBS including heparin, chondroitin, or pentosan polysulfate.[46,47]
This data supports the hypothesis that LL-37 induced bladder inflammation and pain is associated with an IL-33-mast cell axis. However, only the acute phase of LL-37 induced cystitis was evaluated. Further investigation is necessary to evaluate the influence of mast cells during a chronic disease state. This newly identified IL-33-mast cell axis in the bladder offers potential insight towards a pathophysiologic process in patients afflicted with IC/PBS.[21] Ultimately, with this added mechanistic insight, innovative therapeutic approaches could be developed to target and abrogate the IL-33-mast cell axis to help treat patients afflicted with IC/PBS.
5.0 Conclusion
Our findings demonstrate an inflammatory and pain axis implicating IL-33 and mast cell activation. Our work provides innovative insight that after a bladder insult, IL-33 liberation occurs which could lead to mast cell activation and the propagation of potent inflammatory and pain responses. We demonstrated that both bladder inflammation and pain are substantially reduced in mast cell deficient mice further highlighting the importance of mast cells in IC/PBS disease pathogenesis and etiology. Future therapeutics aimed at targeting the IL-33-mast cell axis may provide approaches in treating inflammatory and pain conditions involving the bladder.
HIGHLIGHTS.
Mast cells play a key role in IC/PBS & in our murine model of LL-37 induced cystitis.
IL-33 appears to be a key cytokine in mast cell activation within the bladder.
Mast cell deficient mice demonstrate diminished bladder inflammation and pain.
IL-33 is upregulated in the bladder after LL-37 exposure.
Targeting the IL-33 – mast cell axis may be promising for future therapeutics.
Acknowledgments
6.0 Funding:
This work was supported by a NIH NIDDK R01 grant DK100868 (SO), Primary Children’s Hospital Integrated Science Award (SO), NIH K12 grant UL1RR025764 (SO), University of Utah Nanotechnology Training Program Fellowship (MMJ), and a National Science Foundation Graduate Research Fellowship program grant 1256065 (MMJ).
Footnotes
7.0 Declarations of interest:
SO and GP consult, own stock, and have patents licensed to GlycoMira Therapeutics, Inc. However, no technology associated with GlycoMira Therapeutics, Inc. was evaluated in this manuscript. MJ, WJ, XY, and AS were responsible for conducting the experiments, analyzing data, and preparing the manuscript and have no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasudevan V, Moldwin R. Addressing quality of life in the patient with interstitial cystitis/bladder pain syndrome. Asian J Urol. 2017;4:50–54. doi: 10.1016/J.AJUR.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens JQ, Markossian T, Calhoun EA. Comparison of Economic Impact of Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Interstitial Cystitis/Painful Bladder Syndrome. Urology. 2009;73:743–746. doi: 10.1016/j.urology.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, FitzGerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L, Payne CK, Wesselmann U, Faraday MM. AUA Guideline for the Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. J Urol. 2011;185:2162–2170. doi: 10.1016/j.juro.2011.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanno PM, Erickson D, Moldwin R, Faraday MM. American Urological Association, Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjea D, Martinov T. Mast cells: versatile gatekeepers of pain. Mol Immunol. 2015;63:38–44. doi: 10.1016/j.molimm.2014.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518–27. doi: 10.1016/S0022-5347(01)62606-5. [DOI] [PubMed] [Google Scholar]
- 8.Kochiashvili G, Kochiashvili D. Urinary IL-33 and galectin-3 increase in patients with interstitial cystitis/bladder pain syndrome. J Med Biomed Appl Sci. 2018;6:14–16. doi: 10.15520/jmbas.v6i3. [DOI] [PubMed] [Google Scholar]
- 9.Bjorling DE, Jerde TJ, Zine MJ, Busser BW, Saban MR, Saban R. Mast cells mediate the severity of experimental cystitis in mice. J Urol. 1999;162:231–6. doi: 10.1097/00005392-199907000-00073. [DOI] [PubMed] [Google Scholar]
- 10.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69:34–40. doi: 10.1016/j.urology.2006.08. [DOI] [PubMed] [Google Scholar]
- 11.Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One. 2008;3:e2096. doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shie J-H, Liu H-T, Kuo H-C. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79:484.e7–13. doi: 10.1016/j.urology.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57:47–55. doi: 10.1016/S0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- 14.Oottamasathien S, Jia W, McCoard L, Slack S, Zhang J, Skardal A, Job K, Kennedy TP, Dull RO, Prestwich GD. A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides. J Urol. 2011;186:1684–1692. doi: 10.1016/j.juro.2011.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, Hökfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 16.Oottamasathien S, Jia W, Roundy LM, Zhang J, Wang L, Ye X, Hill AC, Savage J, Lee WY, Hannon AM, Milner S, Prestwich GD. Physiological relevance of LL-37 induced bladder inflammation and mast cells. J Urol. 2013;190:1596–1602. doi: 10.1016/j.juro.2013.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roundy LM, Jia W, Zhang J, Ye X, Prestwich GD, Oottamasathien S. LL-37 induced cystitis and the receptor for advanced glycation end-products (RAGE) pathway. Adv Biosci Biotechnol. 2013;4:1–8. doi: 10.4236/abb.2013.48A2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard PS, Renfrow D, Schechter NM, Kucich U. Mast cell chymase is a possible mediator of neurogenic bladder fibrosis. Neurourol Urodyn. 2004;23:374–382. doi: 10.1002/nau. [DOI] [PubMed] [Google Scholar]
- 19.Chen MC, Blunt LW, Pins MR, Klumpp DJ. Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol. 2006;175:754–9. doi: 10.1016/S0022-5347(05)00171-0. [DOI] [PubMed] [Google Scholar]
- 20.Elbadawi A. Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology. 1997;49:14–40. doi: 10.1016/S0022-5347(01)63337-8. [DOI] [PubMed] [Google Scholar]
- 21.Jhang J-F, Kuo H-C. Pathomechanism of Interstitial Cystitis/Bladder Pain Syndrome and Mapping the Heterogeneity of Disease. Int Neurourol J. 2016;20:S95–104. doi: 10.5213/inj.1632712.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang TY, Kim YH. Interleukin-33 and Mast Cells Bridge Innate and Adaptive Immunity: From the Allergologist’s Perspective. Int Neurourol J. 2015;19:142–150. doi: 10.5213/inj.2015.19.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moussion C, Ortega N, Girard J-P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel “alarmin”? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. 2015;5:33. doi: 10.1186/s13601-015-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai W-H, Wu C-H, Yu H-J, Chien C-T. l-Theanine inhibits proinflammatory PKC/ERK/ICAM-1/IL-33 signaling, apoptosis, and autophagy formation in substance P-induced hyperactive bladder in rats. Neurourol Urodyn. 2017;36:297–307. doi: 10.1002/nau. [DOI] [PubMed] [Google Scholar]
- 26.Council National Council. Guide for the Care and Use of Laboratory Animals. 8. National Academies Press; Washington, DC: 2011. [DOI] [Google Scholar]
- 27.Grimbaldeston MA, Chen C-C, Piliponsky AM, Tsai M, Tam S-Y, Galli SJ. Mast Cell-Deficient W-sash c-kit Mutant KitW-sh/W-sh Mice as a Model for Investigating Mast Cell Biology in Vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicotra L, Tuke J, Grace PM, Rolan PE, Hutchinson MR. Sex differences in mechanical allodynia: how can it be preclinically quantified and analyzed? Front Behav Neurosci. 2014;8:40. doi: 10.3389/fnbeh.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudick CN, Schaeffer AJ, Klumpp DJ. Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis. BMC Urol. 2009;9:1. doi: 10.1186/1471-2490-9-16. doi:101186/1471-2490-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MMMM, Jia W, Isaacson KJKJKJ, Schults A, Cappello J, Prestwich GDGD, Oottamasathien S, Ghandehari H. Silk-elastinlike protein polymers enhance the efficacy of a therapeutic glycosaminoglycan for prophylactic treatment of radiation-induced proctitis. J Control Release. 2017;263:46–56. doi: 10.1016/j.jconrel.2017.02. [DOI] [PubMed] [Google Scholar]
- 31.Jia W, Schults AJ, Jensen MM, Ye X, Alt JA, Prestwich GD, Oottamasathien S. Bladder pain in an LL-37 interstitial cystitis and painful bladder syndrome model. [accessed November 25, 2017];Am J Clin Exp Urol. 2017 5:10–17. http://www.ncbi.nlm.nih.gov/pubmed/29034266. [PMC free article] [PubMed] [Google Scholar]
- 32.Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63:80–5. doi: 10.1016/j.molimm.2014.06. [DOI] [PubMed] [Google Scholar]
- 33.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, Stassen M, Oyoshi MK, Finkelman FD, Geha RS. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016;138:1356–1366. doi: 10.1016/j.jaci.2016.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Liu W, O’Donnell M, Lutgendorf S, Bradley C, Schrepf A, Liu L, Kreder K, Luo Y. Evidence for the Role of Mast Cells in Cystitis-Associated Lower Urinary Tract Dysfunction: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Animal Model Study. PLoS One. 2016;11:e0168772. doi: 10.1371/journal.pone.0168772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carriere V, Roussel L, Ortega N, Lacorre D-A, Americh L, Aguilar L, Bouche G, Girard J-P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci. 2007;104:282–287. doi: 10.1073/pnas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci. 2010;107:8017–8022. doi: 10.1073/PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–8. doi: 10.1074/jbc.M111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon ED, Simpson LJ, Rios CL, Ringel L, Lachowicz-Scroggins ME, Peters MC, Wesolowska-Andersen A, Gonzalez JR, MacLeod HJ, Christian LS, Yuan S, Barry L, Woodruff PG, Ansel KM, Nocka K, Seibold MA, Fahy JV. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci U S A. 2016;113:8765–70. doi: 10.1073/pnas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalesnikoff J, Galli SJ. Antiinflammatory and immunosuppressive functions of mast cells. Methods Mol Biol. 2011;677:207–20. doi: 10.1007/978-1-60761-869-0_15. [DOI] [PubMed] [Google Scholar]
- 40.Logadottir Y, Delbro D, Fall M, Gjertsson I, Jirholt P, Lindholm C, Peeker R. Cytokine Expression in Patients with Bladder Pain Syndrome/Interstitial Cystitis ESSIC Type 3C. J Urol. 2014;192:1564–1568. doi: 10.1016/j.juro.2014.04. [DOI] [PubMed] [Google Scholar]
- 41.Furuta A, Yamamoto T, Suzuki Y, Gotoh M, Egawa S, Yoshimura N. Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder. Int Urogynecol J. 2018 doi: 10.1007/s00192-017-3547-5. [DOI] [PubMed] [Google Scholar]
- 42.Offiah I, Didangelos A, Dawes J, Cartwright R, Khullar V, Bradbury EJ, O’Sullivan S, Williams D, Chessell IP, Pallas K, Graham G, O’Reilly BA, McMahon SB. The Expression of Inflammatory Mediators in Bladder Pain Syndrome. Eur Urol. 2016;70:283–290. doi: 10.1016/j.eururo.2016.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y-Q, Liu Z, Liu Z-H, Chen S-P, Li M, Shahveranov A, Ye D-W, Tian Y-K. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13:141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller RE, Miller RJ, Malfait A-M. Osteoarthritis joint pain: The cytokine connection. Cytokine. 2014;70:185–193. doi: 10.1016/J.CYTO.2014.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laird JMAA, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 46.Lee WY, Savage JR, Zhang J, Jia W, Oottamasathien S, Prestwich GD. Prevention of Anti-microbial Peptide LL-37-Induced Apoptosis and ATP Release in the Urinary Bladder by a Modified Glycosaminoglycan. PLoS One. 2013;8:e77854. doi: 10.1371/journal.pone.0077854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang P, Auclair B, Beechinor D, Diment M, Einarson TR. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: A meta-analysis. Urology. 1997;50:39–43. doi: 10.1016/S0090-4295(97)00110-6. [DOI] [PubMed] [Google Scholar]