Abstract
Background
By the traditional definition of unintended weight loss, cachexia develops in ~80% of patients with pancreatic ductal adenocarcinoma (PDAC). Here, we measure the longitudinal body composition changes in patients with advanced PDAC undergoing 5‐fluorouracil, leucovorin, irinotecan, and oxaliplatin therapy.
Methods
We performed a retrospective review of 53 patients with advanced PDAC on 5‐fluorouracil, leucovorin, irinotecan, and oxaliplatin as first line therapy at Indiana University Hospital from July 2010 to August 2015. Demographic, clinical, and survival data were collected. Body composition measurement by computed tomography (CT), trend, univariate, and multivariate analysis were performed.
Results
Among all patients, three cachexia phenotypes were identified. The majority of patients, 64%, had Muscle and Fat Wasting (MFW), while 17% had Fat‐Only Wasting (FW) and 19% had No Wasting (NW). NW had significantly improved overall median survival (OMS) of 22.6 months vs. 13.0 months for FW and 12.2 months for MFW (P = 0.02). FW (HR = 5.2; 95% confidence interval = 1.5–17.3) and MFW (HR = 1.8; 95% confidence interval = 1.1–2.9) were associated with an increased risk of mortality compared with NW. OMS and risk of mortality did not differ between FW and MFW. Progression of disease, sarcopenic obesity at diagnosis, and primary tail tumours were also associated with decreased OMS. On multivariate analysis, cachexia phenotype and chemotherapy response were independently associated with survival. Notably, CT‐based body composition analysis detected tissue loss of >5% in 81% of patients, while the traditional definition of >5% body weight loss identified 56.6%.
Conclusions
Distinct cachexia phenotypes were observed in this homogeneous population of patients with equivalent stage, diagnosis, and first‐line treatment. This suggests cellular, molecular, or genetic heterogeneity of host or tumour. Survival among patients with FW was as poor as for MFW, indicating adipose tissue plays a crucial role in cachexia and PDAC mortality. Adipose tissue should be studied for its mechanistic contributions to cachexia.
Keywords: Cachexia, Pancreatic cancer, Sarcopenia, FOLFIRINOX, Muscle wasting
Introduction
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States and is on the rise.1 The ductal adenocarcinoma (PDAC) subtype accounts for the majority of cases of pancreatic cancer.1 Patients with PDAC typically present late with advanced, unresectable disease and have a dismal prognosis.2, 3 Gemcitabine‐based chemotherapy regimens have traditionally been the standard treatment protocol for unresectable PDAC. Unfortunately, most patients will not respond and overall median survival (OMS) is only 6–9 months.2, 3 The chemotherapy combination of 5‐fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) has improved survival but the overall prognosis remains exceedingly grim. Recent studies have shown FOLFIRINOX to be superior to gemcitabine, however, OMS still only approaches 15 months.4, 5, 6, 7, 8 Furthermore, FOLFIRINOX is often poorly tolerated and dosing may need to be reduced or completely discontinued due to severe side effects.5, 6, 7, 8, 9
Many patients with PDAC present with involuntary weight loss. The vast majority of patients with PDAC will experience severe wasting during the course of their illness, and cachexia has been implicated as a significant cause of PDAC‐related deaths.10 Cachexia is associated with decreased tolerance of and response to treatment, decreased quality of life, decreased survival, and generally worse overall outcomes in patients with malignancies, including PDAC.11, 12, 13, 14 Thus, although improving antitumour therapies is clearly indicated, anti‐cachexia therapy represents a major unmet need that could promote survival in patients with PDAC.
Cachexia is defined as a complex metabolic derangement characterized by loss of muscle with or without the loss of fat that cannot be reversed by nutritional support.11 Cachexia is not due solely to inadequate food intake, although there are components of anorexia and malabsorption. Rather, cachexia represents a shift in whole body fuel utilization resulting in increased adipose lipolysis and reduced lipogenesis, and increased proteolysis but reduced structural protein synthesis in skeletal muscle (SKM). As well, cachexia influences central mechanisms regulating appetite, activity, and resting energy expenditure, and shifts hepatic gene expression and metabolism. Tissue inflammation and elevated inflammatory cytokines in the circulation are hallmarks of some forms of cachexia.
The consensus definition of cachexia in patients with cancer is weight loss greater than 5%, or weight loss greater than 2% in patients already symptomatic by virtue of having body mass index (BMI) less than 20 kg/m2, or sex‐specific SKM index (SKMI) consistent with sarcopenia.15 In a validation study, weight loss and BMI generally discriminated between patients with and without cachexia and associated with survival.16 Thus, cachexia development and progression can be monitored by tracking weight loss or weight loss with BMI, or via more sophisticated quantitative and imaging modalities. Indeed, body composition measurements obtained from computed tomography (CT) scans have emerged as a powerful, prognostic methodology applicable across many disease states, including PDAC.17, 18, 19, 20 In many countries, CT scans are routinely obtained in patients with unresectable PDAC for staging and to monitor disease response to treatment. Body composition measurements made from these images can equally be used to monitor treatment effects on cachexia. CT measurements can detect low muscle mass, or sarcopenia, as well as fatty muscle infiltration, or myosteatosis. All of these, sarcopenia, sarcopenic obesity, and myosteatosis are associated with decreased survival in patients with malignancy.21, 22, 23, 24
Despite a proliferation of body composition studies in the literature, including in PDAC, fundamental questions regarding cancer cachexia remain. These include whether all cancer cachexia is similar across tumour types, what effects specific treatments might have on cachexia course, and whether muscle and fat are lost equally. Most studies aggregate patients with a variety of tumour types or treatment modalities, obviating any disease or treatment‐specific effects. Moreover, while there are considerable cross‐sectional data particularly on muscle, to date, there has been much less work done to define the longitudinal development of cachexia and much less focus on fat.
We posit that there are disease‐specific, treatment‐specific, and tissue‐specific mechanisms in cancer cachexia, and thus we sought to characterize muscle and fat changes in a controlled and highly homogenous population. We chose patients with advanced PDAC on FOLFIRINOX chemotherapy. The data indicate that there is diversity in cachexia phenotypes even in this population, suggesting underlying diversity in molecular and cellular drivers. Moreover, cachexia phenotypes were unrelated to tumour response. Finally, our data suggest that adipose tissue wasting, not just muscle wasting, is an important influence on mortality from PDAC.
Materials and methods
This retrospective study was approved by the Indiana University Institutional Review Board and was carried out in compliance with the IU Standard Operating Procedures for Research Involving Human Subjects. All data collection occurred between 1 September 2015 and 29 November 2016.
Patient acquisition
All patients presenting to Indiana University Hospital between the dates of 1 July 2010 and 31 August 2015 with advanced PDAC treated with FOLFIRINOX as first‐line therapy with available survival data and adequate CT images for analysis were eligible for inclusion in the study. Advanced PDAC was defined as PDAC that was not amenable to surgical resection. Sixty‐six patients were identified. Upon review, 13 patients were excluded: four with missing CT scans, six with only a single CT scan, two with poor quality CT scans unable to be analysed, and one treated with an alternative chemotherapy regimen prior to presentation at Indiana University. This resulted in 53 total subjects in the study. Demographic, clinical, and survival data along with CT scans were collected on these 53 subjects.
CT tissue mass analysis
Computed tomography images were analysed for cross‐sectional area (cm2) for SKM, intramuscular adipose tissue (IMAT), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SCAT) mass at the level of the 3rd lumbar vertebrae (L3)25 using Slice‐O‐Matic® software V4.3 (Tomovision, Montreal, Quebec, Canada). Hounsfield unit (HU) thresholds were set at −29 to +150 HU for SKM, −30 to −190 HU for IMAT and SCAT, and −50 to −150 HU for VAT.26 Two consecutive images were analysed on all CT scans by a single investigator (J. K. K.). Approximately 25% of those images were also analysed by a second investigator and inter‐observer variance was <1.8%. The mean of the two images was normalized to height in metres2 to establish tissue‐specific indices. Total adipose index (TAI) was calculated by adding IMAT index, VAT index, and SCAT index. Sarcopenia was defined as a SKMI <52.4 cm2/m2 for males and <38.5 cm2/m2 for females.21 Myosteatosis was defined as a mean SKM radiodensity of <33 HU for patients with a BMI ≥25 kg/m2 and <41 HU for patients with a BMI <25 kg/m2.19 Estimates of whole body stores for SKM, total adipose, and each adipose compartment were obtained by applying the following regression equations by Mourtzakis et al.27
Obesity was defined by the World Health Organization criteria.28 Patients were classified as having sarcopenic obesity if they met the criteria for sarcopenia and obesity. Total muscle measurements included the rectus abdominus, external and internal oblique, transversus abdominus, psoas, erector spinae, and quadratus lumborum.
Determination of disease response
Disease response to chemotherapy was determined by using Response Evaluation Criteria in Solid Tumours criteria29 by a single investigator (S. S.). Patients were classified into three groups based on best response: regression of disease, stable disease, and progression of disease.
Determination of cachexia phenotype
Serial CT scans were performed every 3 months per institution protocol. All CT scans from the time of diagnosis until the end of the study were analysed and included in the data analysis. A total of 298 CT scans were analysed. Tissue mass measurements were graphed vs. time to identify any trends. Trends in SKMI and TAI were noted and patients were divided into three categories: No Wasting (NW), Fat‐Only Wasting (FW), and Muscle and Fat Wasting (MFW). Cut‐offs for significant change was set at ≥5% increase or decrease from the initial CT measurement to the final CT measurement. The subjects were then re‐categorized as needed based on this cut‐off threshold. Patients with a significant increase in one category but significant decrease in another category were defined as NW.
Statistical analysis
The primary outcome measure was overall survival, calculated in months from the time of diagnosis to the time of death or last follow‐up. The main prognostic factor was cachexia phenotype, however, age, sex, disease extent, best chemotherapy response, presence of sarcopenia, obesity, sarcopenic obesity, and myosteatosis at diagnosis, and tumour location were also examined. Age was dichotomized at the mean for Kaplan–Meier and Cox proportional analysis. Results of Kaplan–Meier survival analysis are reported as median survival with log base P values. Results of Cox proportional analysis are reported as hazard ratios with 95% confidence intervals.
Mean age, BMI, Eastern Cooperative Oncology Group (ECOG) performance status,30 tissue indices, changes in tissue indices, HU, total number of CT scans, and months between initial and final CT scan were compared between cachexia phenotypes using analysis of variance. Tukey's method was performed when the analysis of variance revealed a difference to identify where the difference occurred. Changes in BMI, tissue indices, and HU were analysed by one‐sample t‐test to evaluate for difference from zero. Sex, disease extent, disease response, tumour location, obesity, sarcopenia, sarcopenic obesity, and myosteatosis were compared between cachexia phenotypes using Pearson's chi‐square test. All continuous variables are reported as the mean with standard deviation, with the exception of total number of CT scans and months between initial and final CT scan, which are reported with the range. All categorical variables are reported as the true measurement with percentage.
Multivariate analysis was performed using generalized linear regression. All variables were included in the multivariate analysis except sarcopenia and obesity. Age was dichotomized at the mean for multivariate analysis. Results are reported as difference in months of survival compared with the stated reference with 95% confidence intervals and P values.
All statistical analysis was performed using IBM SPSS Statistics for Windows version 23.0 (SPSS, Chicago, IL). Images for Kaplan–Meier curves and tissue trend graphs were created using GraphPad Prism version 7 (GraphPad Software, LaJolla, CA). Waterfall plots were created using Microsoft Excel for Windows 2016 (Microsoft Corporation, Redman, WA). Significance was set at a P value <0.05 for all results.
Results
Demographic and disease data
Demographic and disease data are summarized in Table 1. Overall mean age was 59.5 (SD = 9.9) years. The majority of the patients were male, 62.3%. Tumour, lymph node, and metastasis staging convention in PDAC is such that locally advanced disease is stage III, T4NxM0, due to vascular involvement. For patients with metastatic disease, primary tumour and node status is frequently not assessed because metastasis status informs stage, prognosis, and treatment; that is, M1 is stage IV, irrespective of T and N stage. Here, patients presented equally with locally advanced and metastatic disease, 49% and 51%, respectively. At diagnosis, 33% of patients showed ECOG performance status 0, and 67% ECOG 1. The majority of patients had positive response to chemotherapy with 43% having tumour regression and 40% having stable disease compared with 17% with tumour progression. The pancreatic head (51%) was the most common place for the primary tumour followed by the body (38%) then the tail (11%). Overall patients were less likely to be obese (44%) or have sarcopenic obesity (11%) and equally likely to have sarcopenia (49%) at presentation. A total of 296 CT scans were analysed for an overall mean of 5.6 (range = 2.0–18.0) scans per subject. Mean time between initial CT scan and final CT scan analysed was 11.1 months. OMS was 14.7 months for the entire cohort (Figure 1A). History of weight loss prior to presentation was not recorded for the entire cohort and thus was uninformative.
Table 1.
Patient characteristics (N = 53)
| Variable | Overall | No Wasting (19%) | Fat‐Only Wasting (17%) | Muscle and Fat Wasting (64%) | P value |
|---|---|---|---|---|---|
| Age, years (SD) | 59.5 (9.9) | 58.8 (7.7) | 61.0 (12.4) | 59.4 (10.0) | 0.88 |
| Sex | |||||
| Male | 33 | 7 | 5 | 13 | 0.81 |
| Female | 20 | 3 | 4 | 21 | |
| Disease extent | |||||
| Locally advanced | 26 | 3 | 6 | 17 | 0.28 |
| Metastatic | 27 | 7 | 3 | 17 | |
| Disease response | |||||
| Regression | 23 | 6 | 5 | 12 | 0.52 |
| Stable | 21 | 2 | 3 | 16 | |
| Progression | 9 | 2 | 1 | 6 | |
| Tumour location | |||||
| Head | 27 | 2 | 6 | 19 | 0.22 |
| Body | 20 | 6 | 3 | 11 | |
| Tail | 6 | 2 | 0 | 4 | |
| Obesity at diagnosis | |||||
| Yes | 18 | 2 | 4 | 12 | 0.51 |
| No | 35 | 8 | 5 | 22 | |
| Sarcopenia at diagnosis | |||||
| Yes | 26 | 5 | 7 | 14 | 0.15 |
| No | 27 | 5 | 2 | 20 | |
| Sarcopenic obesity at diagnosis | |||||
| Yes | 6 | 0 | 4 | 2 | 0.002 |
| No | 47 | 10 | 5 | 32 | |
| Myosteatosis | |||||
| Yes | 28 | 6 | 7 | 15 | 0.18 |
| No | 25 | 4 | 2 | 19 | |
| Mean number of CT scans (range) | 5.6 | 6.9 (2–18) | 4.2 (2–7) | 5.6 (2–13) | 0.21 |
| Mean months between initial and final CT scan (range) | 11.1 | 13.1 (6.7–24.7) | 11.2 (0.9–45.6) | 10.5 (0.7–28.2) | 0.71 |
CT, computed tomography.
Figure 1.
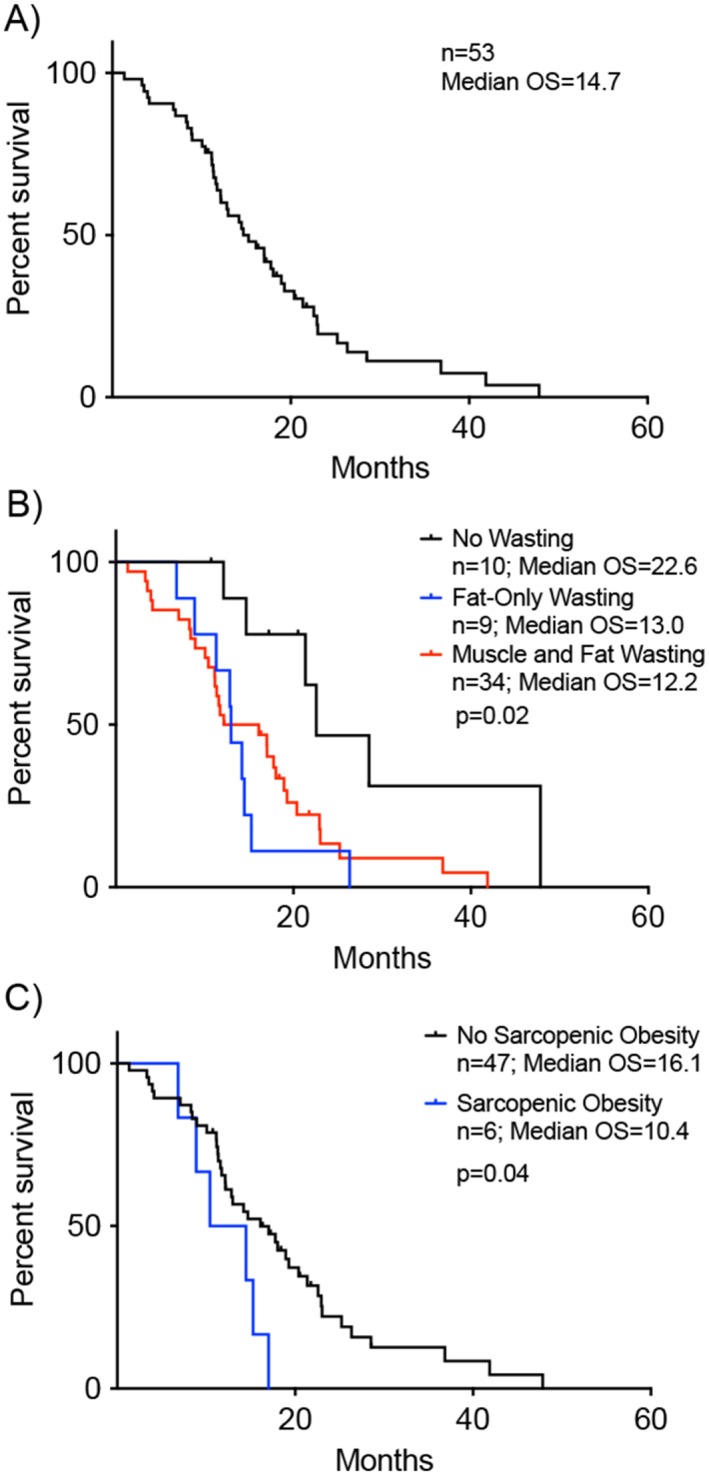
Univariate survival analysis reveals variables associated with decreased median overall survival (OS). Survival on x‐axis plotted as time from date of tissue‐confirmed diagnosis for all plots. (A) Kaplan–Meier curve showing OS for entire patient cohort, which was 14.7 months (n = 53). (B) OS differed by cachexia phenotype. No Wasting OS was 22.6 months (n = 10) vs. Fat‐Only Wasting OS 13.0 months (n = 9) vs. Muscle and Fat Wasting 12.2 months (n = 34)(log rank P = 0.02). (C) OS for patients without sarcopenic obesity at time of initial computed tomography scan was 16.1 months (n = 47) vs. those with sarcopenic obesity 10.4 months (n = 6)(log rank P = 0.04).
Three cachexia phenotypes are identified
Based on SKMI and TAI trend analysis (Figure 2), three distinct wasting patterns were identified: The majority of patients developed MFW, 64%, followed by NW, 19%, and FW, 17%. There was no difference between phenotypes for age, sex, ECOG performance status, disease stage, disease response, tumour location, obesity, sarcopenia, myosteatosis, mean number of CT scans, or time between CT scans. Sarcopenic obesity was more likely to be present in the FW phenotype.
Figure 2.
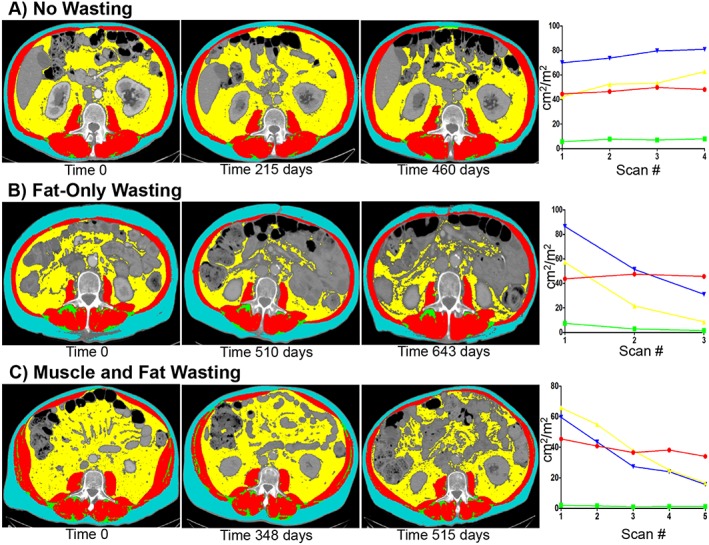
Selective representative computed tomography (CT) images with tag overlay and corresponding tissue mass vs. time graphs from individual patients in each cachexia phenotype at the L3 level. (A) No changes in any tissue mass can be visual appreciated on serial CT scans for the No Wasting phenotype. Graphing tissue masses vs. time shows that there is an initial increase in total adipose and visceral adipose mass with return to starting values at the end of the study. (B) Decreases in subcutaneous adipose and visceral adipose can be visually appreciated on serial CT scans in the Fat‐Only Wasting phenotype. Graphic representation of this patient shows trends towards decreased subcutaneous, visceral, and total adipose mass by the end of the study. Statistical analysis would confirm these changes to be significant (≥5%). (C) Significant decrease in skeletal muscle, subcutaneous, and visceral adipose tissue can be visually appreciated on serial CT scans in the Muscle and Fat Wasting phenotype. Graphic representation of tissue mass vs. time shows trends towards decreases in all tissues. Statistically analysis would confirm this patient to have significant losses in skeletal muscle, subcutaneous, visceral, and total adipose masses (≥5%).
Morphometric analysis
Initial body composition measurements and changes are summarized in Table 2. There was no difference in initial BMI, IMAT index, VAT index, SCAT index, or TAI. A difference was observed in initial SKMI and SKM HU between the groups (P = 0.036 and P = 0.045). Post hoc analysis revealed the FW phenotype started with lower SKMI and SKM HU measurements than the MFW phenotype (Table S1). Figure 3 illustrates the tissue changes for each patient. The NW phenotype showed no significant losses in any measured variable and actually showed significant increases in BMI, IMAT, SCAT, and TAI. The FW phenotype showed significant losses in all adipose tissue compartments and in total adipose tissue. The MFW phenotype showed significant losses in all measured variables including SKM HU.
Table 2.
Baseline body composition and changes
| Variable (SD) | Overall (SD) | No Wasting | P valuea | Fat‐Only Wasting | P valuea | Muscle and Fat Wasting | P valuea | P value among cachexia phenotypes |
|---|---|---|---|---|---|---|---|---|
| Initial BMI (kg/m2) | 28.8 (7.3) | 26.3 (4.8) | 28.7 (12.4) | 29.5 (6.1) | 0.48 | |||
| BMI change (kg/m 2 ) | −2.9 (5.9) | 2.2 (2.7) | 0.028 | −5.5 (11.2) | 0.129 | −3.7 (3.5) | <0.001 | 0.005 |
| BMI % change | −8.3 (15.0) | 8.3 (9.4) | 0.021 | −12.5 (19.5) | 0.058 | −12.1 (11.7) | <0.001 | <0.001 |
| Initial SKMI (cm 2 /m 2 ) | 46.9 (9.5) | 44.4 (8.3) | 40.8 (7.1) | 49.2 (9.6) | 0.036 | |||
| SKM change (kg) | −3.5 (5.9) | 3.7 (5.4) | 0.056 | 0.7 (1.4) | 0.144 | −6.8 (3.9) | <0.001 | <0.001 |
| SKMI % change | −7.2 (13.3) | 10.1 (14.6) | 0.056 | 2.2 (3.5) | 0.911 | −14.8 (6.5) | <0.001 | <0.001 |
| Initial IMATI (cm2/m2) | 4.1 (2.6) | 4.5 (2.9) | 4.1 (2.9) | 3.9 (2.6) | 0.85 | |||
| IMAT change (kg) | −0.09 (0.3) | 0.1 (0.1) | 0.003 | −0.2 (0.2) | 0.034 | −0.1 (0.3) | 0.006 | 0.005 |
| IMATI % change | −13.0 (78.2) | 44.6 (52.6) | 0.025 | −40.0 (25.5) | 0.001 | −22.7 (86.1) | 0.133 | 0.027 |
| Initial VATI (cm2/m2) | 49.6 (34.6) | 48.5 (41.8) | 31.9 (18.0) | 54.6 (34.9) | 0.22 | |||
| VAT change (kg) | −2.1 (2.8) | 0.8 (2.0) | 0.240 | −2.0 (1.9) | 0.016 | −2.9 (2.7) | <0.001 | <0.001 |
| VATI % change | −28.5 (64.9) | 50.3 (103.0) | 0.157 | −49.6 (33.9) | 0.001 | −46.1 (33.8) | <0.001 | <0.001 |
| Initial SCATI (cm2/m2) | 71.3 (46.2) | 57.5 (28.4) | 53.0 (30.4) | 80.2 (51.8) | 0.169 | |||
| SCAT change (kg) | −2.8 (3.9) | 1.3 (1.0) | 0.003 | −2.0 (2.2) | 0.026 | −4.3 (4.0) | <0.001 | <0.001 |
| SCATI % change | −27.8 (47.9) | 36.0 (62.8) | 0.103 | −34.3 (27.4) | 0.002 | −44.8 (28.6) | <0.001 | <0.001 |
| Initial TAI (cm2/m2) | 120.9 (61.7) | 106.0 | 84.9 (41.3) | 134.8 (63.4) | 0.066 | |||
| TA change (kg) | −5.0 (5.9) | (59.1) | 0.028 | −4.1 (4.0) | 0.015 | −7.3 (5.2) | <0.001 | <0.001 |
| TAI % change | −29.5 (49.8) | 2.2 (2.6) | 0.118 | −46.2 (27.1) | <0.001 | −46.2 (27.1) | <0.001 | <0.001 |
| 37.8 (69.1) | ||||||||
| Initial SKM HU | 35.0 (7.1) | 34.3 (6.5) | 30.0 (7.3) | 36.5 (6.8) | 0.045 | |||
| SKM HU change | −0.7 (8.3) | −1.6 (5.0) | 0.34 | 9.6 (12.5) | 0.089 | −3.2 (5.3) | 0.001 | <0.001 |
| SKM HU % change | −0.04 (29.3) | −6.3 (16.2) | 0.247 | 38.1 (48.7) | 0.078 | −8.3 (15.1) | 0.003 | <0.001 |
Abbreviations: BMI, body mass index; SKMI, skeletal muscle index; IMATI, intramuscular adipose tissue index; VATI, visceral adipose tissue index; SCATI, subcutaneous adipose tissue index; TA, total adipose; TAI, total adipose index; SKM HU, skeletal muscle Hounsfield units.
Bolded values are statistically significant (P < 0.05).
This is the P value for in group comparison and represents whether the changes are significant within the group.
Figure 3.
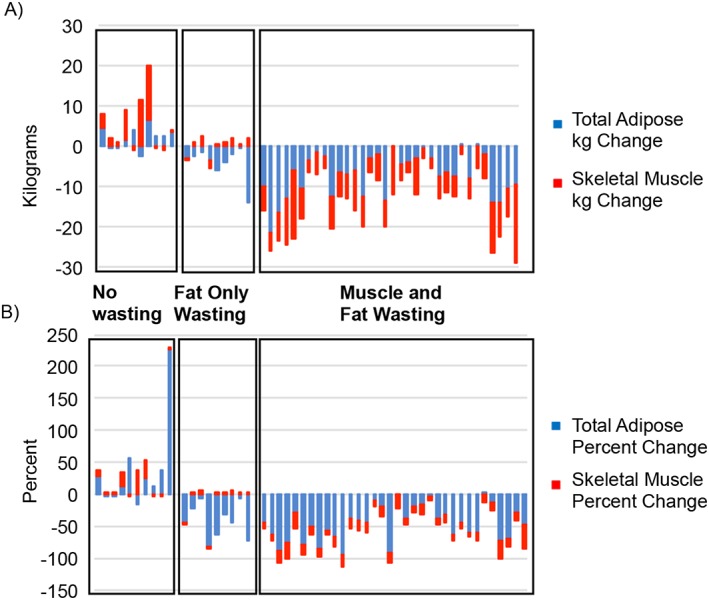
Cachexia phenotypes show distinct patterns of tissue wasting. (A) Breakdown of skeletal muscle index (SKMI) and total adipose index (TAI) changes. Mean SKMI and TAI changes for the No Wasting (NW) phenotype were +4.3 cm2/m2 (P = 0.056) and +16.8 cm2/m2 (P = 0.03) vs. mean SKMI and TAI change for the Fat‐Only Wasting (FW) phenotype were +0.8 cm2/m2 (P = 0.788) and −37.7 cm2/m2 (P = 0.014) and Muscle and Fat Wasting (MFW) phenotype mean SKMI and TAI changes of −7.5 cm2/m2 (P < 0.001) and −58.6 cm2/m2 (P < 0.001). (B) Percent changes in SKMI and TAI for each cachexia phenotype. NW had increases in SKMI and TAI percent change of +10.1% (P = 0.056) and +37.8% (P = 0.118) while FW showed SKMI percent change of +2.2% (P = 0.911) and a decrease in TAI of −46.2% (P < 0.001) and MFW's SKMI and TAI percent change were −14.8% (P < 0.001) and −46.2% (P < 0.001). Skeletal muscle is represented in red, intramuscular adipose tissue in green, visceral adipose tissue in yellow, and subcutaneous adipose tissue in blue.
Variables associated with overall survival
Univariate analysis showed that cachexia phenotype, chemotherapy response, and tumour location were associated with survival. FW phenotype had an OMS of which was significantly longer than FW and MFW (Figure 1B). FW and MFW were associated with increased mortality when compared with NW (Table 3). Presence of sarcopenic obesity on initial CT scan was associated with a significantly decreased OMS (Figure 1C). Other variables associated with decreased OMS and increased risk of mortality were progression of disease while on FOLFIRINOX therapy and primary tumours located in the tail of the pancreas (Table 3).
Table 3.
Survival analysis (overall median survival = 14.7 months)
| Survival | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Median OS (months) | P value | Hazard ratio | 95% CI | P value | Months difference | 95% CI | P value |
| Age | ||||||||
| <59.5 years | 16.1 | 0.066 | Reference | Reference | Reference | |||
| ≥59.5 years | 12.8 | 1.76 | 0.96–3.23 | 0.07 | −1.41 | −5.5–2.7 | 0.50 | |
| Gender | ||||||||
| Female | 18.9 | 0.56 | Reference | Reference | Reference | |||
| Male | 13.0 | 1.2 | 0.65–2.21 | 0.56 | −0.94 | −5.2–3.4 | 0.67 | |
| Disease extent | ||||||||
| Locally | 17.0 | 0.23 | Reference | Reference | Reference | |||
| Advanced metastatic | 13.0 | 1.43 | 0.80–2.64 | 0.23 | 0.30 | −4.7–5.3 | 0.91 | |
| Chemotherapy response | ||||||||
| Regression | 18.0 | 0.001 | Reference | Reference | Reference | −3.3 | 0.53 | |
| Stable | 16.1 | 1.3 | 0.7–2.5 | 0.46 | 1.56 | –2.5 | 0.02 | |
| Progression | 8.3 | 2.1 | 1.4–3.3 | 0.001 | −6.98 | −12.8 to −1.1 | ||
| Sarcopenia | ||||||||
| No | 17.0 | 0.32 | Reference | Reference | N/I | |||
| Yes | 13.0 | 1.35 | 0.74–2.46 | 0.32 | ||||
| Obesity | ||||||||
| No | 14.2 | 0.90 | Reference | Reference | N/I | |||
| Yes | 17.0 | 0.96 | 0.50–1.85 | 0.90 | ||||
| Sarcopenic obesity | ||||||||
| No | 16.1 | 0.04 | Reference | Reference | 0.05 | Reference | −8.7–7.3 | 0.87 |
| Yes | 10.4 | 2.47 | 1.0–6.1 | −0.69 | ||||
| Myosteatosis | ||||||||
| No | 17.8 | 0.21 | Reference | Reference | 0.21 | Reference | ||
| Yes | 14.2 | 1.48 | 0.8–2.74 | −3.9 | −8.3–0.54 | 0.09 | ||
| Tumour location | ||||||||
| Head | 17.8 | 0.02 | Reference | Reference | 0.58 | Reference | −7.5 | 0.28 |
| Body | 14.7 | 1.2 | 0.6–2.3 | 0.01 | −2.7 | –2.2 | 0.06 | |
| Tail | 10.4 | 2.1 | 1.2–3.8 | −7.1 | −14.5–0.34 | |||
| Cachexia phenotype | ||||||||
| No Wasting | 22.6 | 0.02 | Reference | Reference | 0.008 | Reference | −18.5 to −2.2 | 0.01 |
|
Fat‐Only Wasting
Muscle and Fat Wasting |
13.0
12.2 |
5.2
1.8 |
1.5–17.3
1.1–2.9 |
0.02 |
−10.4
−9.9 |
−15.6 to −4.3 | 0.001 | |
Abbreviations: OS, overall survival; CI, confidence interval; F test for model significance = 0.009.
Cachexia phenotype and chemotherapy response are independent predictors of survival
Multivariate analysis showed cachexia phenotype, chemotherapy response, and tumour location to be independently associated with overall survival. Development of FW is associated with a mean decrease in survival of 9.9 months while development of MFW is associated with a mean decrease in survival of 8.8 months when compared with NW. Progression of disease while on FOLFIRINOX was associated with a mean decrease in survival of 7.0 months when compared with regression of disease (Table 3).
Rate of cachexia is not associated with survival
Median time to onset of cachexia was 71.5 days. Comparing survival of patients who had early onset cachexia, before the median, and those that had late onset cachexia, after the median, showed no difference, 16.1 vs. 12.2 months (P = 0.88), or risk of mortality (HR = 1.06; 95% confidence interval = 0.50–2.24).
Discussion
This study is the first to examine the longitudinal changes in body composition and the associations of these changes with survival in a highly homogeneous population—patients with advanced PDAC undergoing FOLFIRINOX chemotherapy as first‐line treatment. Using the traditional definition of >5% body weight loss to define cachexia, only 56.6% of patients in this cohort developed cachexia over the course of their treatment. However, using a definition of ≥5% loss of muscle and/or fat mass, the prevalence of cachexia was 81%. This is consistent with previously published data and provides further evidence that cachexia treatment is a major unmet need of patients with advanced PDAC 14. The study also identified three different cachexia phenotypes, NW, FW, and MFW. The study identifies a subset of patients, NW, who appear resistant to cachexia and as a result have significantly improved survival. In addition, the study showed fat loss to be an equally important factor on survival as muscle loss.
To the best of the authors' knowledge, this is the first paper to demonstrate different wasting phenotypes within a homogenous disease/treatment population. The identification of these phenotypes is important because their existence suggests molecular and/or genetic heterogeneity among the hosts or the tumours as the driving force for each wasting pattern. Although we cannot posit specific mechanisms from this limited analysis, we speculate that differences in molecular drivers could be responsible. For example, various cytokines have been implicated in cancer cachexia, including tumour necrosis factor‐alpha, interleukin‐1, interleukin‐6 (IL‐6), interferon‐gamma, ciliary neurotrophic factor, and activin.31, 32, 33, 34, 35, 36, 37 Tumours express varying levels of such cytokines, one potential source of variation. Furthermore, germline nucleotide polymorphisms in genes linked to cytokine production rates are associated with the development of cachexia,38 specifically in regard to IL‐6 in the setting of pancreatic cancer,39 suggesting that patient genetics might be at the root of cachexia phenotype. Future studies focusing on molecular and genetic differences between PDAC tumours or patients and associated with specific cachexia phenotypes are indicated, as therapies targeting the precise cause of cachexia are essential to achieve optimal outcomes.
The second major finding of this study is the lack of difference in survival between patients who lost muscle and fat and patients who lost only fat. The prominent theory in cachexia is that muscle loss is the major complication in cancer cachexia and to date cachexia research has mainly focused on muscle. The data presented here suggest that fat has an equivalent role, as developing the FW phenotype conferred a decreased survival that was not significantly different than the MFW phenotype.
Adipose tissue has long been seen as an energy regulating tissue with additional responsibilities of mechanical protection and temperature regulation. This changed with the discovery of leptin production by adipose tissue, and it was recognized that fat had important endocrine functions as well.40 Since this discovery, adipose tissue has been shown to express and secrete a number of different signalling molecules including the cachexia‐associated cytokines TNF‐α and IL‐6. The loss of adipose tissue in cancer cachexia has also been shown to be accompanied by changes in gene expression pathways regulating energy turnover.41 Although it has been shown that high fat loss rate is associated with decreased survival in patients with PDAC undergoing a variety of treatment regimens,42 the data presented here show that fat loss is equivalent to muscle loss for mortality risk.
Previous studies show that sarcopenia, sarcopenic obesity, and myosteatosis at diagnosis are associated with decreased survival in pancreatic cancer.22, 23, 24 Of these variables, in our study, only sarcopenic obesity associated with mortality although it was not significant in the multivariate analysis (Table 3). While not statistically significant, all of these variables did trend in the direction of having an impact on survival. In a large patient cohort, these variables would likely show statistical significance and be in line with previously published data. Our data show that development of FW or MFW over the treatment course was associated with decreased survival, independent of tumour response to treatment. Thus, cachexia response and phenotype could be independent of tumour response to therapy, suggesting mechanisms of cachexia that are separable from tumour growth.
Finally, this study also gives perspective onto how we should diagnose cachexia. The traditional definition of cachexia is unintentional weight loss of >5% over 6 months. Such information is not always captured at presentation and relies upon accurate patient recall, which is always subject to bias. Here, using CT scans and data collected after treatment onset, 56.6% of patients experienced >5% body weight loss over treatment course, while 81% of patients exhibited ≥5% loss of muscle or fat mass. No patient in the NW phenotype had >5% body weight loss. Applying the >5% body weight loss criterion to the FW and MFW phenotypes would have classified 5/9 and 25/34 patients as developing cachexia. This would have missed 13/43 or 30% of patients with significant muscle or fat wasting. One explanation could be that patients with advanced PDAC can develop significant tumour burden and metastases, growth of liver and spleen, and production of ascites, all of which would obscure fat and muscle tissue wasting if only weight change is monitored. Thus, use of CT body morphometric measurements is a more accurate way of determining a patient's cachexia status.
Of course, our study has limitations. It is a retrospective study and therefore is subject to all limitations associated with retrospective studies. While the CT scans were reliable and available for objective analysis, certain potentially important clinical data were incompletely recorded (e.g. history of weight loss at presentation) or not collected (e.g. cytokine levels and CRP). This analysis was also performed on an intent‐to‐treat basis. The precise dose modifications/reductions and reasons for altering dose (e.g. adverse events vs. patient choice), while aggressively sought, were insufficiently detailed in the charts to include in this analysis. Additionally, complete accountings of co‐morbidities and concomitant medications were not available, and the authors recognize that some patients may have had significant co‐morbidities that contributed to the outcome. However, ECOG performance status was no different across cachexia phenotypes, and all patients were ECOG 0 or 1 at treatment onset consistent with prescribing practices for FOLFIRINOX. Thus, it seems unlikely that those patients exhibiting either cachexia phenotype had significantly more other disease burden than patients with NW. Additionally, this study is susceptible to length time bias. This is an unavoidable bias because there is no accurate screening test for PDAC and there is no way to know exactly when the disease process began. All patients in the current study presented with advanced PDAC. Given the high rate of cachexia in PDAC, it can be assumed that the initial CT scan was obtained after some degree of cachexia developed. However, the authors do not believe this would have an effect on the overall results. Finally, this study evaluated 53 patients and this sample size may have limited the study's power to detect the effects of some variables. While this sample size is large enough to safely say that the differences found are truly present, the authors recognize that it is not large enough to eliminate type II error and that some real differences were likely not detected.
Our data identify three distinct systemic responses to PDAC treated with FOLFIRINOX. All patients with the NW phenotype show all the patients had significant increase in muscle, fat, or both tissues during the course of treatment, thus establishing a cachexia‐resistant phenotype. Patients with the FW phenotype presented with lower SKMI, which was possibly due to muscle wasting prior to the initial scan. This group, however, did not lose any additional muscle throughout the course of the disease, while patients with the MFW phenotype lost muscle and fat congruently. Moreover, analysis of days from diagnosis to measurable tissue lost showed no difference between FW and MFW (P = 0.67). Therefore, we conclude that these are two distinct phenotypes of cachexia, each contributing to PDAC mortality.
We studied only patients with PDAC who received FOLFIRINOX as first‐line treatment, and perhaps this treatment influenced cachexia presentation. Preclinical data indicate that certain chemotherapy regimens, including FOLFIRI, can induce cachexia in the absence of cancer.43, 44 Currently, there are no published experimental data linking FOLFIRINOX to the development of cachexia, and cachexia develops in the vast majority of patients with PDAC regardless of treatment.10 Thus, it is unlikely that FOLFIRINOX was solely responsible for the cachexia observed. As well, patients with the NW phenotype also received FOLFIRINOX, strengthening the conclusion that the cachexia was disease‐driven and not treatment‐driven. Nevertheless, similar studies involving patients undergoing other chemotherapy regimens and in other malignancies must be done before these results can be generalized.
To the best of our knowledge, this study uniquely shows distinct cachexia phenotypes in PDAC, a single cancer diagnosis, treated with FOLFIRINOX, a reasonably homogeneous regimen. The measurements described here will be used locally to guide clinical trial design for anti‐cachexia trials in the setting of FOLFIRINOX. Our preliminary power analyses suggest that adipose wasting will be more sensitive an indicator than muscle loss, requiring fewer patients. As well, the fact that multiple phenotypes emerged in this uniform population raises suspicion that molecular and/or genetic heterogeneity is present in the tumours or in the hosts, and furthermore, that discovery of these differences could lead to more targeted therapies. As such, identifying differences and developing individualized therapy could be critical to optimize care of patients with cachexia. Finally, our study also emphasizes the important role of adipose wasting in PDAC mortality. Research focusing on fat and fat loss in cachexia will be vital to understanding the disease process. Future studies should focus on parsing molecular subtypes of PDAC cachexia and tumour, host, and treatment factors modulating the systemic response.
Conflict of Interest
None of the authors have any conflicts of interest or anything to disclose.
Supporting information
Table S1. Tukey's Method For Post‐Hoc Analysis
Acknowledgement
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.45
This work was funded by grants to T.A.Z. from National Institutes of Health (grant numbers R01CA122596, R01CA194593, and R01GM092758), the IU Simon Cancer Center, and the Lustgarten Foundation, and by grants to L.G.K. from National Institutes of Health (grant number R01DK096167) and the Lilly Endowment, Inc.
Kays, J. K. , Shahda, S. , Stanley, M. , Bell, T. M. , O'Neill, B. H. , Kohli, M. D. , Couch, M. E. , Koniaris, L. G. , and Zimmers, T. A. (2018) Three cachexia phenotypes and the impact of fat‐only loss on survival in FOLFIRINOX therapy for pancreatic cancer. Journal of Cachexia, Sarcopenia and Muscle, 9: 673–684. 10.1002/jcsm.12307.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. The Lancet 2011;378:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niederhuber JE, Brennan MF, Menck HR. The national cancer data base report on pancreatic cancer. Cancer 1995;76:1671–1677. [DOI] [PubMed] [Google Scholar]
- 4. Conroy T, Gavoille C, Samalin E, Ychou M, Ducreux M. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep 2013;15:182–189. [DOI] [PubMed] [Google Scholar]
- 5. Paniccia A, Edil B, Schulick R, Byers JT, Meguid C, Gajdos C, et al. Neoadjuvant FOLFIRINOX application in borderline resectable pancreatic adenocarcinoma: a retrospective Cohort study. Medicine 2014;93:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boone BA, Steve J, Krasinskas AM, Zureikat AH, Lembersky BC, Gibson MK, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouam Y, et al. FOLFIRINOX versus Gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 8. Peddi PF, Lubner S, McWilliams R, Tan BR, Picus J, Sorscher SM, et al. Multi‐institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. Journal of the Pancreas 2012;13:497–501. [DOI] [PubMed] [Google Scholar]
- 9. Assaf E, Verlinde‐Carvalho M, Delbaldo C, Grenier J, Sellam Z, Pouessel D, et al. 5‐Fluorouracil/leucovorin combined with irinotecan adn oxaliplatin (FOLFIRINOX) as second‐line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011;80:301–306. [DOI] [PubMed] [Google Scholar]
- 10. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med 2006;69:491–497. [DOI] [PubMed] [Google Scholar]
- 11. Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the consensus‐definition for cancer cachexia and evaluation of a classification model‐a study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol 2014;25:1635–1642. [DOI] [PubMed] [Google Scholar]
- 12. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan BH, Fearon KCH. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care 2008;11:400–407. [DOI] [PubMed] [Google Scholar]
- 14. Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 16. Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the consensus‐definition for cancer cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol 2014;25:1635–1642. [DOI] [PubMed] [Google Scholar]
- 17. Choi Y, Oh D‐Y, Kim T‐Y, Lee K‐H, Han S‐W, Im S‐A, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLOSOne 2015;10:e0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sjoblom B, Gronberg BH, Wentzel‐Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non‐small cell lung cancer. Clin Nutr 2016;35:1386–1393. [DOI] [PubMed] [Google Scholar]
- 19. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 20. Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res 2011;170:e75–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with soldi tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 22. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–6979. [DOI] [PubMed] [Google Scholar]
- 23. Zalite IO, Zykus R, Francisco‐Gonzalez M, Saygili F, Pukitis A, Gaujoux S, et al. Influence of cachexia and sarcopenia on survival in pancratic ductal adenocarcinoma: a systematic review. Pancreatology 2015;15:19–24. [DOI] [PubMed] [Google Scholar]
- 24. Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr 2016;35:1103–1109. [DOI] [PubMed] [Google Scholar]
- 25. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge M‐P, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 26. Baracos V, Sawyer M, Beaumont C, Esfandiari N, Lieffers J, Murphy R. Quality assurance and training manual: body composition analysis using computed tomography (CT) imaging version 1.4. In Uo A, ed. 2014.
- 27. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 28. Organization WH . Obesity and overweight. 2016. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed August 26, 2016.
- 29. Schwartz LH, Litiere S, Ed V, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1—update and clarification: from the RECIST committee. Eur J Cancer 2016;62:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 31. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science 2002;296:1486–1488. [DOI] [PubMed] [Google Scholar]
- 32. Klimek MEB, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin‐family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun 2010;391:1548–1554. [DOI] [PubMed] [Google Scholar]
- 33. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World Journal of Gastrointestinal Oncology 2015;7:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Argiles JM, Busquets S, Moore‐Carrasco R, Lopez‐Soriano FJ. Cachexia and Wasting: A Modern Approach. Milan: Springer; 2006. [Google Scholar]
- 35. Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 2016;54:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL‐6 and in experimental cancer cachexia. Endocrinol Metab 2012;303:E410–E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLOSOne 2011; 10.1371/journal.pone.0022538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan BH, Fearon KCH. Cytokine gene polymorphisms and susceptibility to cachexia. Curr Opin Support Palliat Care 2010;4:243–248. [DOI] [PubMed] [Google Scholar]
- 39. Zhang D, Zhou Y, Wu L, Wang S, Zheng H, Yu B, et al. Association of IL‐6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann Clin Lab Sci 2008;38:113–119. [PubMed] [Google Scholar]
- 40. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537–1545. [DOI] [PubMed] [Google Scholar]
- 41. Dahlman I, Mejhert N, Linder K, Agustsson T, Mutch DM, Kulyte A, et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer 2010;102:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Di Sebastiano KM, Yang L, Zbuk K, Wong RK, Chow T, Koff D, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr 2013;109:302–312. [DOI] [PubMed] [Google Scholar]
- 43. Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 2016;7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy‐related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 2016;7:43442–43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Tukey's Method For Post‐Hoc Analysis


