Abstract
Adeno-associated virus (AAV) vectors have been successfully applied in hemophilia clinical trials. However, this approach is limited to patients without AAV-neutralizing antibodies (NAbs). In this study, we explored the feasibility of AAV re-administration in hemophilia A dogs treated initially 8 years ago with AAV8.canine FVIII. After the re-administration in two NAb-negative dogs with AAV8 vectors carrying human factor VIII (hFVIII), along with the proteasome inhibitor bortezomib, we observed a phenotypic improvement in both dogs that persisted in one dog. Phenotypic improvement disappeared at 59 days after re-administration in the other dog, and specific cytotoxic T lymphocytes (CTLs) to the capsid were detected at day 17, but not to hFVIII. hFVIII inhibitors were observed at day 59 and gradually increased. Mechanistic studies demonstrated an increase in pro-inflammatory cytokines, a decrease in immunomodulatory cytokines, as well as lower Tregs after re-administration. These results suggest that hFVIII inhibitor development may contribute to the therapeutic failure via immune response activation. Interestingly, it takes about 30–50 days for AAV NAb titers to decrease by half. Collectively, this study suggests that re-administration of the same AAV serotype after long-term follow-up is feasible and that the study of AAV NAb kinetics will provide important information for predicating the efficacy of re-administration.
Keywords: AAV, hemophilia, neutralizing antibodies, NAbs, re-administration, human factor VIII, hFVIII, hFVIII inhibitor
Introduction
The management of hemophilia in the last decade has achieved great progress, including extending the half-life of clotting factors, subcutaneously administered hemostatic non-factor therapies, and gene therapy. For gene therapy, adeno-associated virus (AAV) vector-mediated gene delivery has emerged as one of the most promising approaches to make a cure for hemophilia a reality. AAV is a single-stranded DNA virus. Due to its low immunogenicity and long-term transgene expression, as well as rare integration into host chromosomes, AAV vectors have been extensively studied as a vehicle to deliver therapeutic genes in numerous preclinical animal models. Based on the exciting data obtained from pre-clinical studies, over 150 AAV-based clinical trials are underway.
Notably, a therapeutic effect has been achieved for an inherited retinal disease, which very recently received approval from an advisory committee at the US Food and Drug Administration (FDA).1 AAV vectors have also been used for gene therapy in patients with hemophilia A and B. The safety, efficacy, and persistence of AAV-mediated gene transfer following a systemic administration of the vector have been confirmed by the majority of ongoing clinical studies for patients with hemophilia.2, 3, 4, 5, 6, 7 Indeed, from a follow-up more than 7 years after a single AAV vector infusion through the peripheral vein, therapeutic levels of clotting factor IX have been achieved in a successful hemophilia B clinical trial.8, 9, 10 In the human population, as high as 90% of subjects have AAV-neutralizing antibodies (NAbs). In patients with hemophilia, we found AAV NAbs in approximately 50%11 of subjects. Only patients with hemophilia who have little or no NAbs against AAV vectors are eligible to receive AAV gene therapy, so NAbs pose an outstanding challenge for the application of AAV vectors for patients to be eligible for systemic administration, especially for those who have received AAV gene therapy before. Several approaches have been proposed to evade NAbs by engineering a modification of the AAV capsid or decreasing NAb titer in circulation by the depletion of NAbs.
Given that the AAV genome forms a non-integrating episome after viral transduction, the loss or decrease of the transgene and its expression will be an important concern and potential outcome over the long term.12 This might necessitate re-administration of gene therapy vectors at a later time. It has been assumed that the re-administration of the same AAV serotype is impossible due to serotype-specific, anti-AAV NAbs that are produced after the initial AAV treatment.13 Nonetheless, in both mice and non-human primates (NHPs), sequential AAV delivery of serotypes AAV5 and AAV1 have proven safe and successful for re-administration to target the liver tissue, implicating that alternative serotypes could serve as an option in patients with pre-existing immunity toward a certain serotype after the initial treatment.13
It is impossible to study the transduction tropism of different AAV serotypes in humans due to ethical issues, so in clinical trials the AAV serotype has been chosen based solely on empirical experience. For example, AAV8 has been used in several clinical trials for patients with hemophilia since AAV8 has shown to be superior to transduce the mouse liver and NHP and other large animal models.14, 15 Indeed, clinical trials with the AAV8 vector have generated a therapeutic level of clotting factors after a systemic administration of liver targeting. However, generally, AAV vectors do not transduce dog liver as efficiently as in mouse,16 and even in NHPs the liver transduction efficiency is much less than that in the mouse.17, 18 Other serotypes and capsids (AAV5, LK03, etc.) are starting to emerge for human liver targeting.7, 19 In this study, we tested the feasibility of re-administration using the same serotype of AAV vector in a dog model with hemophilia. Previously, we demonstrated that a phenotypic correction was achieved after a systemic administration of AAV8 encoding canine FVIII in hemophilia A dogs.14 After 8 years, the phenotypes measured by whole-blood clotting time (WBCT) and FVIII-specific activated partial thromboplastin time (aPTT) of the two dogs decreased to the levels of the untreated hemophilia A dogs. To study whether we were able to correct the disease after re-administration of the same serotype of AAV vector encoding the therapeutic transgene, two hemophilia A dogs were treated with AAV8 expressing human factor VIII (AAV8/hFVIII). After re-administration, phenotypic improvement was again achieved in both dogs 8 years after the initial infusion of AAV8/canine FVIII.
Results
AAV NAbs Are Undetectable in Dogs Receiving the AAV8 Vector 8 Years Prior
In our prior study, seven dogs with hemophilia A were treated with an AAV8 vector encoding the canine FVIII.14 Before the initial AAV8 administration, no NAbs against AAV8 were detected. After the administration of AAV8 and the proteasome inhibitor bortezomib, the AAV8 NAb titer increased to 1:128 at week 2 for two available dogs (U10 and U11), decreased to 1:64 and 1:32 at week 8, respectively, and was undetectable at year 8 (Table 1). Since the NAb analysis based on an in vitro assay could not predict the actual neutralizing activity of the recipient serum due to the variable dose used, we performed an in vivo NAb assay.20
Table 1.
Neutralizing Antibody to AAV8 after Initial Administration
| Pre-administration | Week 2 after Initial Administration | Week 8 after Initial Administration | 8 Years after Initial Administration | |
|---|---|---|---|---|
| Dog U10 | 0 | 128 | 64 | 0 |
| Dog U11 | 0 | 128 | 32 | 0 |
Anti-AAV8 neutralizing antibody titers were detected from citrated plasma after initial AAV8.cFVIII administration (8 years prior) from hemophilia dogs U10 and U11. The values are expressed as 1:x.
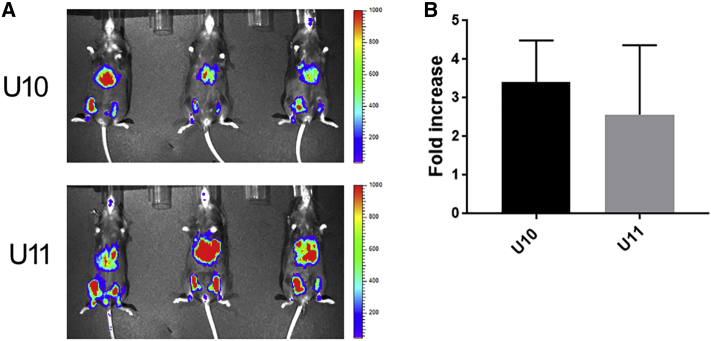
In this study, we took into consideration the planned re-administration dose of 4 × 1012 particles/kg of AAV8/hFVIII, which was estimated based on the current body weight of the dogs and the anticipated potency of the available AAV8/hFVIII vectors. Assuming a plasma volume of 4% body weight, the concentration of the AAV vector in the blood should be 1 × 108 particles/μL plasma following the injection of 4 × 1012 particles/kg AAV8/hFVIII. To mimic the actual ratio of the AAV8 vector-to-dog serum volume (1 × 108/μL) in dogs with re-administration, we incubated 3 × 109 particles of the AAV8/luciferase with 30 μL of either dog plasma or PBS for 2 hr at 4°C, and then the mixture was injected into the hind legs of the C57BL/6 mice. One leg received AAV8 incubated with dog plasma and the contralateral leg was injected with AAV8 incubated with PBS as an internal control. After 2 weeks, the luciferase imaging was performed (Figure 1; Figure S3). The inhibition or enhancement of the dog plasma was calculated against the luciferase expression from the AAV8 incubated with PBS (Figure 1). The plasma from both dogs had no detectable neutralizing activity, but actually it had an enhanced effect (about 3-fold) on AAV8 transduction. These results suggest that re-administration is feasible in these dogs after a long-term follow-up from the first AAV administration.
Figure 1.
AAV-Neutralizing Antibody Analysis In Vivo
3 × 109 particles of AAV8/firefly luciferase were incubated with 30 μL of either canine plasma (dog U10 and dog U11) or PBS for 2 hr at 4°C. Then the mixture of AAV8 and canine plasma was injected into the left hind legs of C57BL/6 male mice, and the contralateral leg was injected with AAV8 and PBS as an internal control. (A) After 2 weeks, the luciferase imaging was performed; scale was represented as photons per second. (B) The average fold increase from 3 mice treated with AAV8.canine plasma versus AAV8/PBS was calculated. The capture and measurement of the signal intensities from regions of interest are also shown in Figure S3. Data are presented as average ± SE.
Re-administration of AAV8 Expressing Human FVIII Results in a Phenotypic Improvement of Hemophilia A Dogs
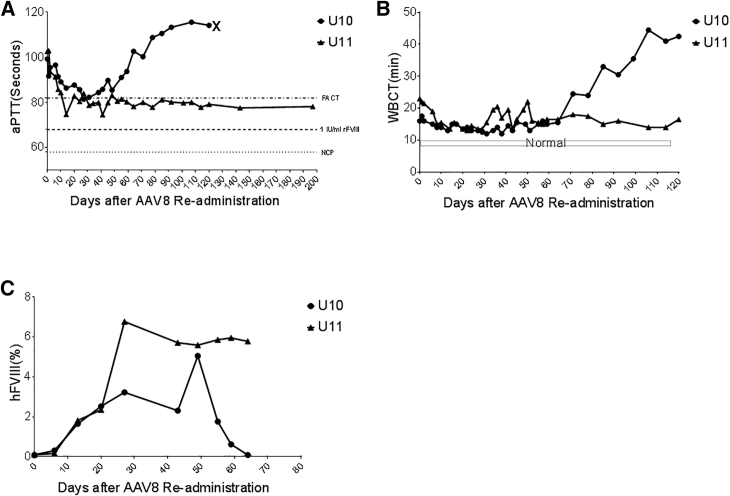
To study whether re-administration of the same serotype of AAV vector induces therapeutic transgene expression in hemophilia A dogs that received AAV8 vector encoding canine FVIII 8 years ago, a hFVIII cassette was chosen for easily differentiating transgene expression. For re-administration, we co-injected 4 × 1012 particles/kg AAV8/hFVIII vector, along with bortezomib at a dose of 1.3 mg/m2, into two dogs via the peripheral vein, since proteasome inhibitor bortezomib has demonstrated the ability to enhance AAV transduction in our previous studies.14 Correction of the plasma-clotting potential was quantified using an FVIII-specific aPTT (Figure 2A), the WBCT (Figure 2B), hFVIII antigen quantification by ELISA (Figure 2C), and thromboelastography (TEG; Figure S1) assay. For aPTT analysis, three FVIII-containing products were used as controls, including normal human plasma (factor assay control plasma [FACT]), 1 IU/mL recombinant hFVIII, and normal canine plasma (NCP).
Figure 2.
Dynamic aPTT Improvement after an AAV8/hFVIII Re-administration
The hemophilia dogs U10 and U11 were treated with the dose of 4 × 1012 particles/kg of AAV8/hFVIII, with bortezomib, at a dose of 1.3 mg/m2 via the peripheral vein. At the indicated time points after re-administration, aPTT analysis from citrated plasma was performed. (A) FVIII-specific aPTT. FACT, normal human plasma; NCP, normal canine plasma; X, U10 died at day 120. (B) Whole-blood clotting time (WBCT). Normal range of 6–10 min in hemostatically normal dogs is indicated in the box region as normal. Hemophilic dogs had baseline WBCT > 20 min. (C) FVIII antigen protein level in blood at different time points up to day 64 post-AAV8/hFVIII re-administration was measured by hFVIII-specific ELISA.
After AAV8/hFVIII administration, a shortened aPTT time was observed beginning at day 2, followed by further shortening over time, and the shortest aPTT time was observed at days 14 and 27 after vector re-administration for dogs U10 and U11, respectively. The peak aPTT clotting time in U11 was shorter than the FACT (around 80 s), a value corresponding to about 20% activity under a standard prepared from a recombinant hFVIII product. For the dog U10, aPTT remained stable until day 59 and then became prolonged. The dog U10 died from a fatal spontaneous soft tissue hemorrhage at day 120 post-AAV injection. For the dog U11, after reaching its peak, the aPTT remained unchanged during the ongoing follow-up period. A slight decrease of the WBCT was observed for the dog U10 (16 min pre-AAV8/hFVIII and 12 min after AAV8), and the WBCT was 42.5 min before death. The improvement of the WBCT in the dog U11 was consistent with the result for aPTT analysis. The WBCT decreased from 23 min before the AAV8 injection to 13.5 min after the AAV administration. Although there was a variation of the TEG parameters, the overall outcomes were consistent with the aPTT and WBCT values (Figure S1). We also examined the hFVIII expression in the blood. The increased concentration of plasma hFVIII level was observed in both dogs after a systemic administration of the AAV8/hFVIII. In the dog U10, after the FVIII peaked at day 49, it started to drop starting at day 55, and it declined to the baseline at day 64. In the dog U11, FVIII levels peaked at day 27 and remained persistent thereafter (Figure 2C).
AAV Capsid CTL Response after AAV8/hFVIII Administration
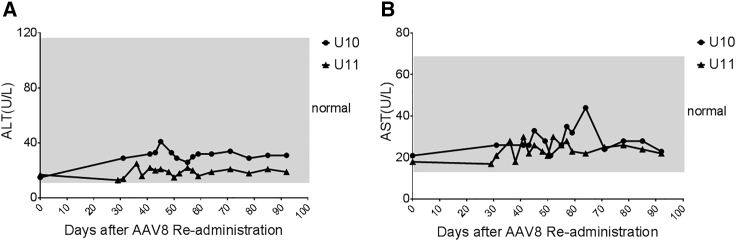
For the dog U10, starting from day 59 post-AAV8/hFVIII injection, the aPTT and WBCT were gradually prolonged until death at day 120. There are three possibilities for this: a cytotoxic T lymphocyte (CTL) response to the AAV8 capsid, a CTL response to the hFVIII protein, or a hFVIII inhibitor development. It has been suggested that the AAV capsid is able to induce a capsid-specific CTL response via antigen cross-presentation after AAV transduction and the capsid-specific CTLs eliminate the AAV-transduced cells in clinical trials for patients with hemophilia. At first, we investigated whether the liver was damaged after AAV transduction in these dogs by the detection of elevated liver enzymes in the blood. There was no obvious change of the alanine transaminase (ALT) level in the plasma (<2-fold) in the dog U11 before and at multiple time points after the AAV8/hFVIII administration. For the dog U10, the ALT level was persistently higher after AAV8/hFVIII administration, and the ALT level was 40 IU/mL at 45 days post-AAV injection, when compared to pre-injection levels (15 IU/mL) (Figure 3). Other biochemistry parameters (Table S1) after AAV8 vector re-administration were not altered. The liver function analysis suggests that the CTL response may contribute to the elimination of AAV8-transduced hepatocytes, which would lead to lower FVIII function in the blood.
Figure 3.
Liver Enzyme Changes after AAV8.hFVIII Re-administration
Serum alanine transaminase (ALT) (A) and aspartate transaminase (AST) (B) were monitored at different time points post-AAV8/hFVIII re-administration in hemophilia dogs U10 and U11. Normal canine ALT range is 12–118 U/L and AST level is 15–66 U/L, as displayed in the gray area.
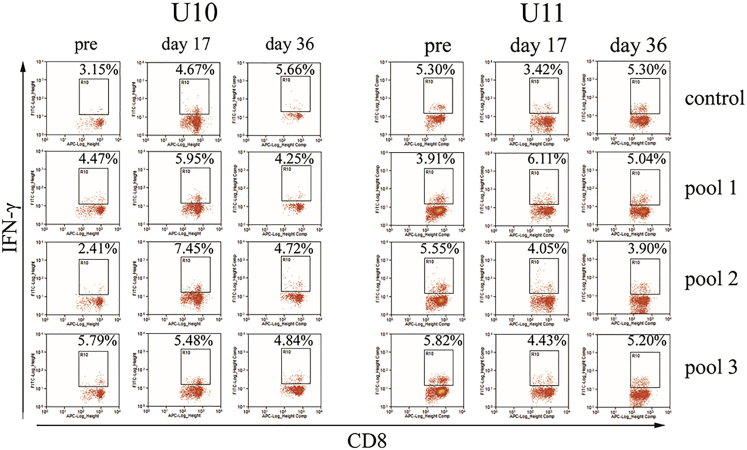
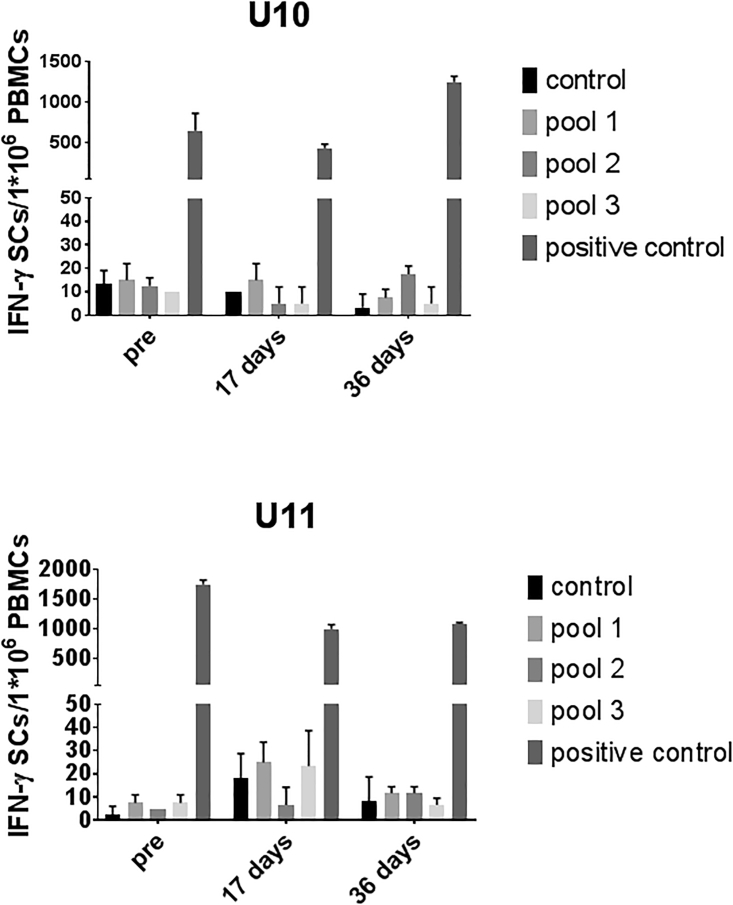
Next, we assayed the CTL response to the AAV capsid. After peptides derived from AAV8 capsid protein were incubated with fresh peripheral blood mononuclear cells (PBMCs) from the dogs, it was noted that a much higher percentage of CD8 cells expressing interferon (IFN)-γ (7.45%) was observed at day 17 when compared to that of the pre-AAV8 injection (2.41%) for peptide pool 2 incubated with the dog U10. There was no obvious difference (less than 2-fold) in the percentage of CD8 cells expressing IFN-γ in both dogs regardless of the peptide pools or detection at different time points (Figure 4). Although the time at which higher capsid-specific CTLs (day 17) were detected was different from that for the detection of increased aPTT time (day 59), it is impossible to rule out the effect of capsid CTL-mediated elimination of AAV8-transduced hepatocytes on decreased FVIII activity in the blood.
Figure 4.
Capsid-Specific CTL Detection after AAV8/hFVIII Re-administration
PBMCs separated from the whole blood of the hemophilia dogs U10 and U11 were cultured in the presence of different AAV8 capsid peptide pools for 48 hr. Then PBMCs were stained with CD8 and IFN-γ antibodies and analyzed by flow cytometry. After the gating of CD8+ cells, the percentage of IFN-γ+ cells in CD8 cells is shown.
CTL Response to Human FVIII after AAV8/hFVIII Administration
For dogs, hFVIII is a foreign protein, which can potentially elicit a CTL response when expressed endogenously from an AAV vector cassette. To study the CTL response induced from hFVIII expression after a systemic administration of AAV8/hFVIII, we performed an IFN-γ ELISPOT assay. Dog PBMCs were cultured with peptides derived from hFVIII protein, and 48 hr later IFN-γ-secreting cells were detected. As shown in Figure 5, when compared to the control group with PBS, there was no increase in IFN-γ-expressing cells in either dog, regardless of the time points for detection or peptide pools used for stimulation. This result indicates that no hFVIII-specific CTL response could be mounted after hepatocyte targeting in the dogs.
Figure 5.
Transgene-Specific CTL Response Detection after AAV8/hFVIII Administration
The FVIII-specific CTL responses were measured by IFN-γ ELISPOT. The PBMCs from either dog U10 or U11 blood was cultured in the presence of different pools of the FVIII peptides for 48 hr in an ELISPOT plate. The combination of PMA and ionomycin was used as positive controls. IFN-γ-secreting cells (IFN-γ SCs) were detected by ELISPOT assay. The data represented the average and SD (error bar) from triplicates. Control used 10% DMSO in PBS, which was the solvent for the peptides. Data are presented as average ± SE.
FVIII-Neutralizing Inhibitory Antibodies after AAV8/hFVIII Re-administration
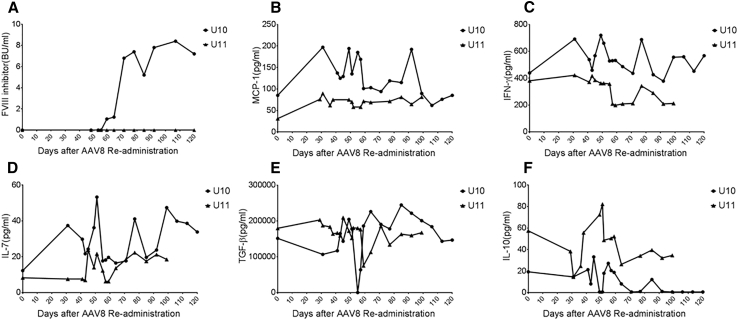
For the dog U11, over a follow-up of 7 months, no inhibitors to hFVIII were found. However, canine anti-hFVIII inhibitors were detectable at day 59 post-AAV injection in the dog U10, and they gradually increased. These inhibitors reached a peak at day 71 post-AAV treatment, and they remained unchanged at the level of 8 Bethesda units (BUs)/mL (Figure 6A). These data indicate that the hFVIII inhibitor development prevented the correction of hemophiliac coagulopathy in the dog U10.
Figure 6.
Dynamic Changes in FVIII Inhibitory Antibodies in Hemophilia A Dog U10 and the Cytokine Profile after AAV8/hFVIII Re-administration
(A) FVIII inhibitory antibodies were measured by Bethesda assay in units per milliliter. Canine plasma at different dilutions was mixed with normal human plasma at the ratio of 1:1 and incubated at 37°C for 2 hr. Naive hemophilia A canine plasma served as the control. The mixture was assayed using standard aPTT reagents, and the remaining activities were calculated against the control. Dynamic changes in pro-inflammatory cytokines MCP-1 (B), IFN-γ (C), and IL-7 (D) and immunomodulatory cytokines TGF-β (E) and IL-10 (F) were measured on Luminex MAGPIX system from the plasma of hemophilia dogs U10 and U11 after AAV8/hFVIII re-administration.
Elevated Pro-inflammatory Cytokines MCP-1, IFN-γ, and IL-7 and Decreased Anti-inflammatory Cytokines TGF-β and IL-10 in the Hemophilia Dog with FVIII Inhibitor Formation
In hemophilia A mice administered AAV8-mediated gene therapy, we have found that there are elevated monocyte-derived pro-inflammatory cytokines or chemokines, together with decreased anti-inflammatory cytokine transforming growth factor β (TGF-β) at an early time point. This may contribute to the persistent inflammatory environment in favor of an immune response toward FVIII inhibitor development.21 In this study, we also detected the kinetics of these cytokines or chemokines in the blood of these dogs with AAV8/hFVIII treatment. Strikingly, the level of anti-inflammatory cytokine TGF-β (Figure 6E) dropped sharply to undetectable levels 4 days before FVIII inhibitor development was observed at day 55 in the dog U10. This was not seen in the dog U11, who did not develop FVIII inhibitors. Generally, interleukin (IL)-10 levels in the dog U10 were lower (Figure 6F), while monocyte chemoattractant protein (MCP)-1, IFN-γ, and IL-7 were higher than in the dog U11 (Figures 6B–6D). There was no difference in the levels for other measured cytokines, including IL-6 and IL-8, between the two dogs, and the cytokines IL-2, IL-18, IFN-γ, and tumor necrosis factor alpha (TNF-α) were undetectable at most of the time points in both dogs (Figure S2).
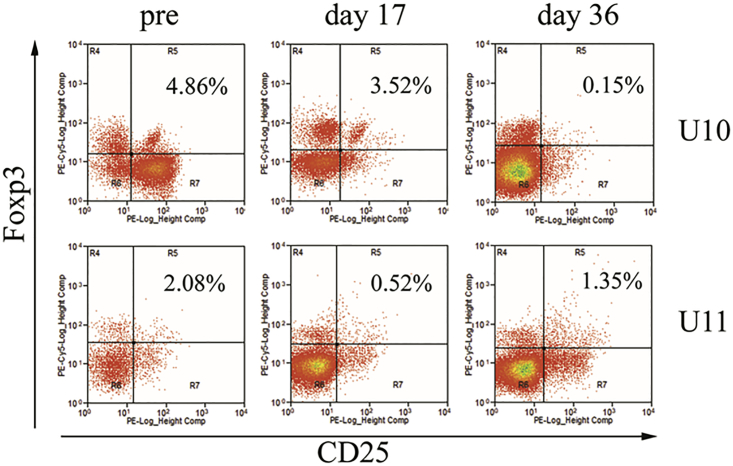
Regulatory T Cell Response in Dogs after AAV8/hFVIII Administration
Regulatory T cells play a critical role in immune tolerance in treatment of hemophilia. It has been demonstrated that Treg cells are induced in mice after AAV8 vector administration via systemic administration. We performed Treg analysis before re-administration and at days 17 and 36 after re-administration of AAV8/hFVIII. A dramatic drop in the level of Treg cells was observed at day 36 post-AAV8 injection in the dog U10 (0.15% versus 4.86% pre-AAV and 3.52% at day 17 post-AAV treatment; Figure 7). However, a low level of Treg cells was detected at day 17 after AAV8/hFVIII administration in the dog U11 (0.52% versus 2.08% pre-AAV and 1.35% at day 36 post-AAV; Figure 7).
Figure 7.
The Profile of T Regulatory Cells after AAV8/hFVIII Re-administration
PBMCs separated from the whole blood of the hemophilia dogs U10 and U11 were stained with CD4, CD25, and Foxp3 antibodies, and then they were analyzed by flow cytometry. Gated on CD4+ cells, Treg cells (CD4+ CD25+ Foxp3+) were shown in Gate R5. The controls were set up to include CD4+, CD25+, Foxp3+, and CD4+CD25+.
Kinetics of AAV8-NAbs after Re-administration
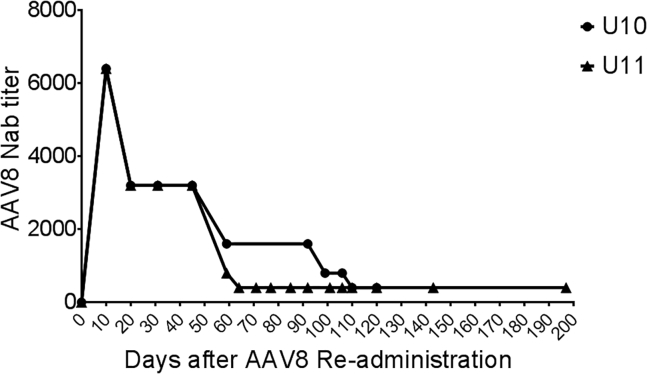
It is interesting to note that AAV8 NAbs were generated at a high titer of 1:128 and then decreased to an undetectable level at 8 years after the initial AAV8 administration. This observation has not previously been reported. Unfortunately, due to the unavailability of serum samples from these dogs, we could not investigate the kinetics of the NAbs after the initial AAV8 administration. In this study, after AAV8 vector re-administration, a robust NAb against AAV8 developed. The titer peaked at 1:6,400 10 days post-AAV re-administration in both dogs, then gradually dropped to 1:400 at day 110 in dog U10 and 1:400 at day 64 in dog U11 and remained at this level up to day 197 (Figure 8). The NAb titer level in both dogs after re-administration was nearly 50-fold higher than that after the initial AAV8 vector administration (Table 1), indicating that memory B cells might still exist even at 8–9 years after the AAV8 initial administration. We suspect memory B cells are activated after re-administration of the AAV8 capsid even though NAbs against AAV8 are undetectable before its re-administration. Although there was a slight difference in the NAb-level kinetics between the two dogs after the AAV8 re-administration, the trend of gradual NAb decrease was similar. It took about 30–50 days after the second administration for the NAbs to decrease by half. This finding may explain why no NAbs were detected at years 8–9 after the initial AAV administration in these dogs.
Figure 8.
Dynamic Change in Neutralizing Antibodies against the AAV8 Capsid after AAV8/hFVIII Re-administration
After the AAV8/hFVIII re-administration, neutralizing antibodies against AAV8 capsid were measured in the hemophilia dogs U10 and U11.
Discussion
Two dogs with hemophilia A were initially treated 8 years ago with AAV8 vectors encoding canine FVIII. AAV-specific NAbs were produced and decreased to an undetectable level 8 years later. This observation supports our rationale to re-administer the same serotype of AAV for these dogs. In this study, these dogs received AAV8 vectors encoding hFVIII via a systemic administration, and a therapeutic effect was achieved in both dogs. Over the 5 months of follow-up, one dog (U11) survived with a stable level of FVIII expression. In contrast, the dog U10 showed an initial phenotypic correction but died 4 months after AAV injection when a Bethesda FVIII inhibitor developed against the foreign hFVIII protein. In the dog U10, a capsid-specific CTL response was detected at an early time point after vector re-administration. Subsequently, FVIII inhibitors developed, a much lower level of Treg cells were found circulating in the blood, and an imbalance of both proinflammatory cytokines and immunosuppressive cytokines were also observed in this dog. To the best of our knowledge, this is the first report where the same serotype of the initial AAV-FVIII vector was re-administered to achieve hemostasis improvement in a hemophilia A dog. Given the safety and the efficacy of the current hemophilia clinical trials,2, 3, 4, 5, 7 the feasibility of an AAV vector re-administration would potentially expand the application to patients who lose therapeutic transgene expression later on.
Pre-existing circulating NAbs22 against AAV are the biggest barrier23 for a systemic application of AAV-mediated gene therapy, especially for liver targeting to treat patients with hemophilia. Numerous approaches have been proposed to evade NAb activity or decrease NAb titers in the blood. It has also been assumed that re-administration of the same AAV serotype is not feasible. In this study, re-administration was successful in two hemophilia A dogs that received the same serotype of an AAV vector 8 years prior. We believe two factors determine whether success will be achieved after re-administration. The first factor is the titer of NAbs in the recipient’s blood and the second factor is the dose of the AAV vector proposed. If the NAb titer is very high and the dose of the AAV vector is low, no therapeutic effect will be obtained. If the NAb titer is low and a relatively high dose of AAV vector is proposed, there is a chance to achieve therapeutic effect. Careful examination of the ratio of NAb titer to AAV vector concentration in blood will help to figure out the actual dose needed for individual subjects.
For re-administration of the same AAV serotype, it is very important to understand the kinetics of NAbs in human blood. In these two dogs, we found that it takes 30–50 days for the NAb titer to decrease by 50%. There are no data about the kinetics of NAbs in patients with hemophilia after a systemic administration of AAV vector. However, in the clinical trial for patients with Duchenne muscular dystrophy, the NAb titer reached a peak and slightly decreased over a 1-year follow-up after an intramuscular injection of the AAV2.5 vector.24 The NAb titer level generated is related to the virus doses, and the route of vector delivery may also influence NAb titer. It is possible that the kinetics of NAb decline is related to the magnitude of NAbs generated and the balance of the continuous generation of NAbs from activated B cells and NAb half-life. Therefore, studies focusing on the kinetics of NAbs in future clinical trials with different administration routes and doses are warranted.
In this study, therapeutic hFVIII expression was achieved at an early time point after AAV8 re-administration, and then it decreased to the baseline in one of the dogs. There are several possibilities contributing to the failure of this therapy: the AAV capsid-specific CTL response mediated the elimination of AAV-transduced hepatocytes, hFVIII-specific CTLs mediated clearance of the transgene product expressed from liver cells, and/or hFVIII inhibitor development. It has been suggested that capsid-specific CTLs might attribute to the decreased FIX expression in clinical trials for patients with hemophilia B after a systemic administration of AAV vectors.25 Although a high number of the capsid-specific CTLs was detected at day 17 after AAV administration, but not at a later time (day 36), and in combination with the finding of slightly high liver enzyme ALT at day 43, it is still impossible to rule out the possibility that capsid-specific CTL response mediated liver cell clearance. Consistent with this study in the dog U10, a prolonged mild increase in ALT levels has also been reported in some participants in the AAV5-FVIII gene therapy trial.7 There was no obvious CTL response specific to the transgene hFVIII after its re-administration, perhaps due to utilization of the liver-specific promoter in the AAV cassette, which could not induce efficient hFVIII expression and antigen presentation in antigen-presenting cells (APCs).
The development of NAbs or inhibitors against the exogenously administered FVIII concentrates is the most significant therapeutic complication of hemophilia A, affecting up to 30%–40% of previously untreated patients (PUPs) treated with FVIII.26 By rendering a conventional replacement therapy ineffective, inhibitor formation results in increased morbidity and mortality,27 high cost, and a decreased quality of life.28 The development of inhibitors is a complex interplay of host and environmental factors. Among the network, the interaction and collaboration among the T, B, and APCs are mainly regulated by biochemical signals and cytokines.29, 30 In a hemophilia A dog treated with gene therapy expressing hFVIII, the FVIII inhibitor formation, if any, usually arises before week 4 post-administration (unpublished data). The dog U10 developed an FVIII inhibitor at day 59 after re-administration. It is our speculation that a co-administration of a proteasome inhibitor might delay the FVIII inhibitor formation. Further regimen optimization for proteasome inhibitor application would require future investigation to determine if inhibitor formation could be decreased in certain patients with high risks.31, 32 Interestingly, the dog that developed an FVIII inhibitor showed lower IL-10 and higher MCP-1 levels when compared with the dog that did not develop an FVIII inhibitor. More remarkably, 4 days before the FVIII inhibitor formation, the TGF-β dropped significantly to below the detection limit. IL-10 and TGF-β are recognized as immunomodulatory cytokines, while MCP-1 is a pro-inflammatory cytokine. Both animal models33, 34 and clinical findings35, 36 show that the imbalance of pro-inflammatory and anti-inflammatory cytokines is involved in the development of FVIII inhibitors. It warrants further investigation whether changes in cytokine patterns can be used as biomarkers to predict inhibitor formation and whether the modulation of cytokines can be used to decrease the development of inhibitor formation.
Numerous studies, including ours,37, 38 have shown that proteasome inhibitors can enhance AAV transduction in vitro and in vivo39, 40 by increasing virus intracellular trafficking. We have taken advantage via co-administration of a proteasome inhibitor to increase AAV transduction efficacy in this report. It should be noted that there are other potential effects of the proteasome inhibitor, which might be relevant to our findings. The proteasome inhibitor bortezumib has been successfully used for the induction of long-term immune tolerance in patients with infantile Pompe disease.41 TGF-β1 and IL-10 expression in cutaneous T cell lymphoma (CTCL) cells can be suppressed by bortezomib (BZ), which has shown promising results in the treatment of CTCL.42 It is worthwhile to study the role of bortezomib in AAV NAb kinetics in the future.
The disruption of immune cytokine or chemokine balance may also be related to a Treg alteration. In coincidence to inhibitor development, the percent of circulating Treg was gradually decreased in dog U10, but not in dog U11. Whether Tregs plays a role in lowering immune modulation cytokine levels in the blood and in hFVIII inhibitor development warrants further investigation. The liver microenvironment is able to induce a strong regulatory immune response, and immune tolerance to transgene products has been demonstrated after the administration of AAV vectors.43, 44 The ability of AAV to induce immune tolerance is serotype dependent, as well as mouse strain dependent. For example, AAV8 induces a much stronger immune tolerance than AAV2 in mouse models, and inhibitors to FIX develop in C3H mice, but not in C57BL mice.45 It would be important to develop a transgene-specific Treg to induce and maintain the tolerance for blocking the transgene inhibitor occurrence in a clinical setting.
In summary, in the hemophilia canine model, we validated the feasibility of re-administration of AAV vectors in gene therapy for hemophilia, which is translationally significant given the successful clinical trials using AAV-mediated gene therapy.6, 7, 46, 47
Materials and Methods
Cell Lines and Virus
HEK293 cells and Huh7 cells were maintained at 37°C in 5% CO2 in DMEM (Mediatech, Manassas, VA, USA) with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin (Corning, distributed by Mediatech, Manassas, VA, USA). The hFVIII expression cassette with a synthesized poly A48 was kindly provided by St. Jude Children’s Research Hospital and flanked by AAV2 inverted terminal repeats (ITRs), in which hFVIII was driven by the liver-specific promoter HLP. The firefly luciferase gene in the AAV cassette with bovine growth factor poly A was driven by the chicken β-actin promoter. All vectors with single-stranded AAV genome were packaged into an AAV8 capsid (pXR8 with AAV2 rep) and produced using a triple-transfection protocol by the transient transfection of HEK293 cells, and vector was purified from clarified cell lysates by ion exchange chromatography, as described by Grieger et al.49 Titer was determined by dot blot at the Virus Vector Core Facility at the University of North Carolina at Chapel Hill.
Animal Care
Two female mixed-breed hemophilia A dogs, U10 (Marjorie) and U11 (Sprocket), that survived about 8 years post-treatment of the seven total hemophilia dogs treated, were initially housed at the University of Alabama at Birmingham (UAB) Medical School, and then transferred and maintained at the Francis Owen Blood Research Laboratory at the University of North Carolina (UNC) at Chapel Hill, a U.S. Department of Agriculture-approved facility. C57BL/6 mice were purchased from the Jackson ImmunoResearch Laboratories and housed and fed ad libitum at UNC at least 2 weeks before recruitment into the study. Mice and hemophilia dogs were maintained in the USDA-approved facilities as described before.14 All studies were approved by the UNC Institutional Animal Care and Use Committee.
Dog Experiment
Dogs U10 and U11 were initially treated by AAV8.canine factor VIII (cFVIII) in 2008 via portal vein administration,14 and then they were re-administered AAV8/hFVIII at the dose of 4 × 1012/kg body weight in 2017. The dogs were given a single intravenous dose of 1.3 mg/m2 of the proteasome inhibitor bortezomib (Millennium Pharmaceuticals, Cambridge, MA) that was diluted in normal saline 5 min prior to vector administration. The AAV vector was diluted with normal saline to 15 mL and infused within 5 min.
NAb against AAV8 Capsid by In Vitro and In Vivo Analysis
Inhibition of AAV8 transduction by NAbs was assessed in vitro according to published methods as described.48, 50 Briefly, Huh7 cells were seeded in a 48-well plate at 1 × 105 cells/well, and they were cultured with 1 × 108 particles AAV8/luciferase that had been pre-incubated with a serial dilution of dog sera from dogs U10 and U11 for 2 hr at 4°C. First, we used a 10-fold dilution with the start concentration of undiluted sera to obtain the NAb range. Then 2-fold dilution within the NAb range was used to determine the actual NAb titer. Luciferase activity in the cell lysate was measured with a Wallac-1420 Victor 2 automated plate reader 48 hr post-AAV transduction. NAb titers were defined to be the highest dilution of dog serum that reduced luciferase activity by 50% in comparison to that in cells transduced with AAV8/luciferase vectors that had been pre-incubated with PBS.
For in vivo analysis of NAbs, 3 × 109 particles of AAV8/ luciferase vector were incubated with 30 μL undiluted dog sera or PBS for 2 hr at 4°C. The vectors were then injected directly into the hind leg muscle of C57BL/6 male mice that were 6–8 weeks old. Transgene expression was assessed by imaging 2 weeks thereafter48 using a Xenogen IVIS Lumina imaging system (Caliper Lifesciences, Hopkinton, MA). Mice were first anesthetized using 2.5% isoflurane in a gas chamber and then intraperitoneally injected with an excess of d-luciferin (5 mg in PBS, Caliper Lifesciences). At 5 min after the injection, image analysis was carried out using the Living Image software (Caliper Lifesciences). The signal intensities from regions of interest were expressed as total photon flux (photons per second), which has been used widely in studies.51, 52
FVIII-Specific ELISA, aPTT and Bethesda Assays, and Coagulation in Dogs
Blood samples were obtained from normal controls, untreated FVIII controls, and treated hemophilia A dogs as described previously.51 FVIII-specific aPTTs were recorded on the STart 4 coagulation analyzer (Diagnostica Stago) by incubating 50 μL canine plasma samples diluted in Owren-Koller buffer (Diagnostica Stago), 50 μL hFVIII-deficient plasma (George King Bio-Medical, Overland Park, KS), and 50 μL aPTT reagents for 3 min, then adding 50 μL 0.25 M calcium chloride. aPTT clotting times were generated based on normal human plasma (FACT, George King Bio-Medical, Overland Park, KS), normal pooled canine plasma (NCP), and 1 U/mL recombinant hFVIII (Advate, Baxter, Westlake Village, CA) to compare the efficacy achieved after vector re-administration. The Bethesda titer of the anti-hFVIII inhibitor was measured as previously reported48 with a slight modification. Briefly, the test plasma (at serial dilutions from 1:2 to 1:16, if applicable) were mixed with normal human plasma at a ratio of 1:1 and incubated for 2 hr at 37°C. Naive hemophilia A canine plasma mixed with normal human plasma (1:1) served as the control. The incubated mixture was analyzed using aPTT reagents, and the remaining activities were calculated against the control. FVIII protein level in blood was measured by the FVIII antigen kit from Affinity Biological (ON, Canada). WBCT and TEG were performed using whole-blood samples, as previously described.51, 52
PBMC Isolation
Fresh peripheral blood was diluted with 2 times the volume of 1640 medium (Gibco). 30 mL diluted cell suspension was carefully layered over 15 mL Ficoll and centrifuged at 2,000 rpm for 30 min at room temperature without any stops. Then the PBMCs were transferred to another tube and washed with 1640 medium and re-suspended in 1640 medium with 10% fetal bovine serum (FBS).
Flow Cytometry
For regulatory T cell detection, PBMCs isolated from the canine peripheral blood were stained with a PE/Cy7-CD4 antibody (eBioscience) and a PE-CD25 antibody (eBioscience) after being blocked by 5% FBS. After 30 min, the PBMCs were fixed and permeabilized following the manufacturer’s recommendation for the Foxp3 Transcription Factor Staining Buffer Kit (Thermo Fisher Scientific). Then the PBMCs were stained by PE/Cy5.5-conjugated Foxp3 antibody (eBioscience) and analyzed by the Beckman Coulter CyAn ADP.
For CTL detection, synthesized AAV8 capsid peptides were divided into 3 peptide pools: peptide pool 1, 1–130 amino acid (aa); peptide pool 2, 131–260 aa; and peptide pool 3, 261–390 aa. The PBMCs were cultured in the presence of different peptide pools at a dose of 20 μg/mL for 48 hr. Before harvest, the cells were treated by Brefeldin A for 5 hr (BD Biosciences). Then the cells were stained with the APC-CD8a antibody for 30 min. The Fixation/Permeabilization Solution Kit (BD Biosciences) was used for cell fixation and permeabilization. Cells were incubated in 250 μL Fixation/Permeabilization solution for 20 min at 4°C and washed twice in 1 mL 1× BD Perm/Wash buffer. Then PBMCs were stained by fluorescein isothiocyanate (FITC)-conjugated IFN-γ antibody (Bio-Rad) in the Perm/Wash buffer.
ELISPOT
PBMCs were seeded to an IFN-γ antibody-coated ELISPOT plate. Synthesized FVIII peptides (GenScript) were divided into 3 peptide pools: peptide pool 1, 1–250 aa; peptide pool 2, 251–510 aa; and peptide pool 3, 511–750 aa. PBMCs were cultured in the presence of the different peptide pools at a dose of 20 μg/mL. The combination of phorbol 12-myristate 13 acetate (PMA) and ionomycin (Thermo Fisher Scientific) was used as a positive control. After 48 hr, the IFN-γ-secreting cells were detected by following the protocol of the ELISPOT assay kit (R&D Systems).
Liver Enzyme Analysis
Detection of serum ALT and aspartate transaminase (AST) levels was performed at the Histopathology Facility at UNC at Chapel Hill. Serum chemistry tests for dog samples were performed at ANTECH.
Multiplex Cytokine Measurement
Multiple cytokines, including TGF-β, IL-2, IL-6, IL-7, IL-8, IL-10, IL-18, IFN-γ, MCP-1, and TNF-α, were measured. Cytokines were measured on a Luminex MAGPIX system (Luminex, Austin, TX, USA), equipped with LUMINEX xPONENT software using custom kits (Bio-Rad, Hercules, CA). Cytokine levels were expressed in picograms per milliliter (pg/mL). Levels below the detection limit of each cytokine were defined as 0 pg/mL.
Author Contributions
Conceptualization, P.E.M., R.J.S., T.C.N., and C.L.; Methodology, J.S., W.S., X.C., E.P.M., L.W., and C.L.; Investigation, J.S., W.S., X.C., E.P.M., L.W., and C.L.; Writing – Original Draft, J.S. and C.L.; Writing – Review & Editing, Y.L.A., G.P.N., P.E.M., R.J.S., and T.C.N.; Funding Acquisition, P.E.M., R.J.S., and C.L.; Resources, T.C.N.; Supervision, T.C.N. and C.L.
Conflicts of Interest
R.J.S. is the founder and a shareholder of Asklepios BioPharmaceutical. He receives research support through the University of North Carolina from Asklepios BioPharmaceutical. He holds patents that have been licensed by UNC to Asklepios Biopharmaceutical, for which he receives royalties. He has consulted for Baxter Healthcare and has received payment for speaking. P.E.M., during a portion of the conduct of these studies, received research support through the University of North Carolina from Asklepios BioPharmaceutical and Novo Nordisk, and he has received research support in the past from Baxter Healthcare, Pfizer, and Prolor. During a portion of the conduct of these studies, he was an employee of Baxalta (now a part of Shire). He holds patents licensed to Asklepios, for which he receives royalties. He has received payment for consultation, services, and for speaking for Asklepios, Chatham LLC, Baxter Healthcare, and Pfizer, and he has additionally consulted for Bayer, Novo Nordisk, and Biogen.
Acknowledgments
We would like to thank Kelly Rigsbee, Charles Askew, and Amanda Dobbins for their critical proofreading of the manuscript. The authors acknowledge the UNC Biomedical Research Imaging Center (BRIC) Small Animal Imaging (SAI) facility for its assistance with mouse imaging. This work was supported by NIH grants R01AI117408 (to C.L.), R01HL125749 (to T.C.N. and C.L.), P01HL112761 (to R.J.S., P.E.M., and C.L.), HL086944 (to C.D.L.), P30-CA016086-35-37, and U54-CA151652-01-04 (to the BRIC SAI facility) and a research grant from Asklepios BioPharmaceutical (to P.E.M. and J.S.).
Footnotes
Supplemental Information includes three figures and one table and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.07.011.
Supplemental Information
References
- 1.Smalley E. First AAV gene therapy poised for landmark approval. Nat. Biotechnol. 2017;35:998–999. doi: 10.1038/nbt1117-998. [DOI] [PubMed] [Google Scholar]
- 2.Dolgin E. Early clinical data raise the bar for hemophilia gene therapies. Nat. Biotechnol. 2016;34:999–1001. doi: 10.1038/nbt1016-999. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann J., Croteau S.E. 2017 Clinical trials update: Innovations in hemophilia therapy. Am. J. Hematol. 2016;91:1252–1260. doi: 10.1002/ajh.24543. [DOI] [PubMed] [Google Scholar]
- 4.Herzog R.W. A Cure For Hemophilia: the Promise Becomes a Reality. Mol. Ther. 2016;24:1503–1504. doi: 10.1038/mt.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S.R., Markusic D.M., Biswas M., High K.A., Herzog R.W. Clinical development of gene therapy: results and lessons from recent successes. Mol. Ther. Methods Clin. Dev. 2016;3:16034. doi: 10.1038/mtm.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 8.Nienhuis A.W., Nathwani A.C., Davidoff A.M. Gene Therapy for Hemophilia. Mol. Ther. 2017;25:1163–1167. doi: 10.1016/j.ymthe.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C., Narkbunnam N., Samulski R.J., Asokan A., Hu G., Jacobson L.J., Manco-Johnson M.J., Monahan P.E., Joint Outcome Study Investigators Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- 12.Naso M.F., Tomkowicz B., Perry W.L., 3rd, Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majowicz A., Salas D., Zabaleta N., Rodríguez-Garcia E., González-Aseguinolaza G., Petry H., Ferreira V. Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1. Mol. Ther. 2017;25:1831–1842. doi: 10.1016/j.ymthe.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monahan P.E., Lothrop C.D., Sun J., Hirsch M.L., Kafri T., Kantor B., Sarkar R., Tillson D.M., Elia J.R., Samulski R.J. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol. Ther. 2010;18:1907–1916. doi: 10.1038/mt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatino D.E., Lange A.M., Altynova E.S., Sarkar R., Zhou S., Merricks E.P., Franck H.G., Nichols T.C., Arruda V.R., Kazazian H.H., Jr. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol. Ther. 2011;19:442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathwani A.C., Gray J.T., McIntosh J., Ng C.Y., Zhou J., Spence Y., Cochrane M., Gray E., Tuddenham E.G., Davidoff A.M. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathwani A.C., Gray J.T., Ng C.Y., Zhou J., Spence Y., Waddington S.N., Tuddenham E.G., Kemball-Cook G., McIntosh J., Boon-Spijker M. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulk N.K., Pekrun K., Zhu E., Nygaard S., Li B., Xu J., Chu K., Leborgne C., Dane A.P., Haft A. Bioengineered AAV Capsids with Combined High Human Liver Transduction In Vivo and Unique Humoral Seroreactivity. Mol. Ther. 2018;26:289–303. doi: 10.1016/j.ymthe.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Sun J., Crosby A., Woodard K., Hirsch M.L., Samulski R.J., Li C. Direct interaction of human serum proteins with AAV virions to enhance AAV transduction: immediate impact on clinical applications. Gene Ther. 2017;24:49–59. doi: 10.1038/gt.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J., Yuan Z., Abajas Y.L., Szollosi D.E., Hu G., Hua B., Xiao X., Li C. A retrospective study of the cytokine profile changes in mice with FVIII inhibitor development after adeno-associated virus-mediated gene therapy in a hemophilia A mouse model. Hum. Gene Ther. 2018;29:381–389. doi: 10.1089/hum.2017.094. [DOI] [PubMed] [Google Scholar]
- 22.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Pogoda J.M., Provost R., Guerrero J., Hajjar R.J., Zsebo K.M. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016;23:313–319. doi: 10.1038/gt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowles D.E., McPhee S.W., Li C., Gray S.J., Samulski J.J., Camp A.S., Li J., Wang B., Monahan P.E., Rabinowitz J.E. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 26.Miller C.H., Rice A.S., Boylan B., Payne A.B., Kelly F.M., Escobar M.A., Gill J., Leissinger C., Soucie J.M., Hemophilia Inhibitor Research Study Investigators Characteristics of hemophilia patients with factor VIII inhibitors detected by prospective screening. Am. J. Hematol. 2015;90:871–876. doi: 10.1002/ajh.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain S., Gupta S., Ambrusko S.J., Shapiro A. Successful management of refractory haematuria with recombinant porcine factor VIII replacement for paediatric congenital haemophilia A with high-titre inhibitor. Haemophilia. 2017;23:e358–e361. doi: 10.1111/hae.13240. [DOI] [PubMed] [Google Scholar]
- 28.Ragni M.V. New and Emerging Agents for the Treatment of Hemophilia: Focus on Extended Half-Life Recombinant Clotting Proteins. Drugs. 2015;75:1587–1600. doi: 10.1007/s40265-015-0451-5. [DOI] [PubMed] [Google Scholar]
- 29.Hartholt R.B., Peyron I., Voorberg J. Hunting down factor VIII in the immunopeptidome. Cell. Immunol. 2016;301:59–64. doi: 10.1016/j.cellimm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Scott D.W. Inhibitors - cellular aspects and novel approaches for tolerance. Haemophilia. 2014;20(Suppl 4):80–86. doi: 10.1111/hae.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouw S.C., van den Berg H.M. The multifactorial etiology of inhibitor development in hemophilia: genetics and environment. Semin. Thromb. Hemost. 2009;35:723–734. doi: 10.1055/s-0029-1245105. [DOI] [PubMed] [Google Scholar]
- 32.Gunasekera D., Ettinger R.A., Nakaya Fletcher S., James E.A., Liu M., Barrett J.C., Withycombe J., Matthews D.C., Epstein M.S., Hughes R.J., Pratt K.P., Personalized Approaches to Therapies for Hemophilia (PATH) Study Investigators Factor VIII gene variants and inhibitor risk in African American hemophilia A patients. Blood. 2015;126:895–904. doi: 10.1182/blood-2014-09-599365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaitonde P., Peng A., Straubinger R.M., Bankert R.B., Balu-Iyer S.V. Downregulation of CD40 signal and induction of TGF-β by phosphatidylinositol mediates reduction in immunogenicity against recombinant human Factor VIII. J. Pharm. Sci. 2012;101:48–55. doi: 10.1002/jps.22746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ragni M.V., Wu W., Liang X., Hsieh C.C., Cortese-Hassett A., Lu L. Factor VIII-pulsed dendritic cells reduce anti-factor VIII antibody formation in the hemophilia A mouse model. Exp. Hematol. 2009;37:744–754. doi: 10.1016/j.exphem.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding K.Y., Ji W.C., Wu J.S., Li T., Sheng Y.Y. Higher frequency of CD4(+)CD25(high) Treg cells in hemophilia patients with factor VIII inhibitor. Genet. Mol. Res. 2014;13:1774–1781. doi: 10.4238/2014.March.17.5. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira C.A., Velloso-Rodrigues C., Machado F.C., Carvalho B.N., Gentz S.H., Martins-Filho O.A., Chaves D.G. Cytokine profile and FVIII inhibitors development in haemophilia A. Haemophilia. 2013;19:e139–e142. doi: 10.1111/hae.12096. [DOI] [PubMed] [Google Scholar]
- 37.Berry G.E., Asokan A. Chemical Modulation of Endocytic Sorting Augments Adeno-associated Viral Transduction. J. Biol. Chem. 2016;291:939–947. doi: 10.1074/jbc.M115.687657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaanine A.H., Nonnenmacher M., Kohlbrenner E., Jin D., Kovacic J.C., Akar F.G., Hajjar R.J., Weber T. Effect of bortezomib on the efficacy of AAV9.SERCA2a treatment to preserve cardiac function in a rat pressure-overload model of heart failure. Gene Ther. 2014;21:379–386. doi: 10.1038/gt.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell A.M., Samulski R.J. Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors. J. Virol. 2013;87:13035–13041. doi: 10.1128/JVI.01826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C., He Y., Nicolson S., Hirsch M., Weinberg M.S., Zhang P., Kafri T., Samulski R.J. Adeno-associated virus capsid antigen presentation is dependent on endosomal escape. J. Clin. Invest. 2013;123:1390–1401. doi: 10.1172/JCI66611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazi Z.B., Prater S.N., Kobori J.A., Viskochil D., Bailey C., Gera R., Stockton D.W., McIntosh P., Rosenberg A.S., Kishnani P.S. Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses. JCI Insight. 2016;1:e86821. doi: 10.1172/jci.insight.86821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang T.P., Poltoratsky V., Vancurova I. Bortezomib inhibits expression of TGF-β1, IL-10, and CXCR4, resulting in decreased survival and migration of cutaneous T cell lymphoma cells. J. Immunol. 2015;194:2942–2953. doi: 10.4049/jimmunol.1402610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper M., Nayak S., Hoffman B.E., Terhorst C., Cao O., Herzog R.W. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum. Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LoDuca P.A., Hoffman B.E., Herzog R.W. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr. Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markusic D.M., Hoffman B.E., Perrin G.Q., Nayak S., Wang X., LoDuca P.A., High K.A., Herzog R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Berg H.M. A Cure for Hemophilia within Reach. N. Engl. J. Med. 2017;377:2592–2593. doi: 10.1056/NEJMe1713888. [DOI] [PubMed] [Google Scholar]
- 48.Sun J., Hua B., Chen X., Samulski R.J., Li C. Gene Delivery of Activated Factor VII Using Alternative Adeno-Associated Virus Serotype Improves Hemostasis in Hemophiliac Mice with FVIII Inhibitors and Adeno-Associated Virus Neutralizing Antibodies. Hum. Gene Ther. 2017;28:654–666. doi: 10.1089/hum.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grieger J.C., Soltys S.M., Samulski R.J. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol. Ther. 2016;24:287–297. doi: 10.1038/mt.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M., Crosby A., Hastie E., Samulski J.J., McPhee S., Joshua G., Samulski R.J., Li C. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22:984–992. doi: 10.1038/gt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du L.M., Nurden P., Nurden A.T., Nichols T.C., Bellinger D.A., Jensen E.S., Haberichter S.L., Merricks E., Raymer R.A., Fang J. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat. Commun. 2013;4:2773. doi: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shetty K.A., Merricks E.P., Raymer R., Rigsbee N., Nichols T.C., Balu-Iyer S.V. Soy Phosphatidylinositol-Containing Lipid Nanoparticle Prolongs the Plasma Survival and Hemostatic Efficacy of B-domain-Deleted Recombinant Canine Factor VIII in Hemophilia A Dogs. J. Pharm. Sci. 2016;105:2459–2464. doi: 10.1016/j.xphs.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.