Abstract
Objective
To compare overall survival (OS) in patients with cervical spine metastases between initial radiotherapy followed by surgery and initial surgery followed by radiotherapy.
Methods
The medical records of 36 patients with cervical spine metastases from January 2007 to December 2015 were retrospectively analyzed. These patients were divided into 2 groups. Group 1 included patients who underwent initial radiotherapy followed by surgery, while group 2 included patients who underwent initial surgery followed by radiotherapy. Clinical outcomes, OS, OS after cervical spine metastasis, and OS after surgery were analyzed in both groups. We evaluated whether primary tumor type, initial treatment modality, the modified Tomita score, Eastern Cooperative Oncology Group performance status, Karnofsky performance status, Japanese Orthopedic Association (JOA) score, Nurick grade, Frankel classification, and preoperative symptoms were associated with OS after cervical spine metastasis.
Results
Both groups exhibited improvement in the postoperative visual analogue scale, but only group 2 showed a significant improvement in postoperative JOA score (p=0.03). OS did not differ significantly between groups. However, OS after cervical spine metastasis was only 7.0 months (95% confidence interval [CI], 4.8–9.3) in group 1 versus 15.8 months (95% CI, 8.8–24.0) in group 2, which represented a significant difference (p<0.05). Factors related to OS after cervical spine metastasis were primary tumor type, initial treatment modality, and preoperative symptoms (p<0.05). Patients who presented with only preoperative pain had approximately 3 fold longer OS after cervical spine metastasis than patients with preoperative motor weakness, even in group 2 (p<0.05).
Conclusion
Surgical treatment prior to the onset of motor weakness or radiotherapy may be a good decision in case of cervical spine metastasis.
Keywords: Cervical spine metastasis, Surgery, Surgical treatment, Radiotherapy, Prognostic factor, Overall survival
INTRODUCTION
Spinal epidural metastases occur in more than 50% of patients with malignant disease [1-3], and 10% to 20% of these patients develop neurologic deficits [4]. Cervical spine metastases represent up to 20% of all metastatic spinal tumors. The outcomes of cervical spine metastases, such as paraplegia, quadriplegia, and respiratory failure, can be more serious than the outcomes of thoracic and lumbar metastases [4].
Patients typically undergo nonsurgical treatment at the initial stage of symptomatic cervical spine metastasis [5]. Surgical treatment has been limited to tissue diagnosis, resolving mechanical instability, and as a second-line treatment when radiotherapy fails [6]. Recently, significant advances have been made in the neurosurgical management of spinal metastases, and surgical treatment can rapidly decompress spinal structures [7]. Despite advances in spinal surgery for spinal metastases, few authors have reported the results of surgical treatment for cervical spine metastases [5,8,9], although some larger surgical studies have included results on spine metastases. Few studies have assessed whether initial radiotherapy influenced subsequent surgical treatment.
We analyzed clinical outcomes and survival in patients with cervical spine metastases managed by surgery at 2 different times: initial radiotherapy followed by surgery and initial surgery followed by radiotherapy. We also investigated the clinical factors associated with survival after cervical spine metastasis.
MATERIALS AND METHODS
1. Patient Selection
Medical records from 47 patients who underwent surgery for cervical spinal metastasis at spine tumor center of Samsung Medical Center between January 2007 and December 2015 were reviewed. Patient demographics and clinical characteristics were obtained from electronic medical records. Institutional Review Board approval of Samsung Medical Center was obtained (2016-07-164). The inclusion criteria were as follows: (1) age >18 years, (2) underwent surgery of the cervical spine due to a metastatic tumor, and (3) histologic confirmation of a metastatic tumor. Patients with hematologic neoplasms, such as multiple myeloma and lymphoma, were excluded from this study. Furthermore, patients who underwent vertebroplasty or kyphoplasty were excluded.
Finally, 36 patients were enrolled in the study. The patients were divided into 2 groups according to the first treatment modality used after cervical spine metastasis. Twelve patients underwent initial radiotherapy after the diagnosis of cervical spine metastasis followed by surgery (group 1). Twenty-four patients underwent initial surgery followed by radiotherapy (group 2) (Fig. 1). Clinical outcomes and survival were compared between the 2 groups, and prognostic factors for survival after cervical spine metastasis were analyzed.
Fig. 1.
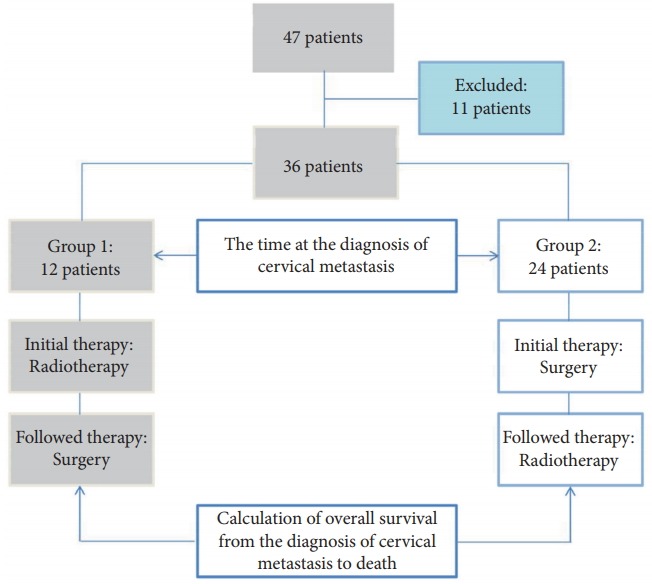
Schematic diagrams of 36 patients with cervical spine metastases who were categorized into groups 1 and 2. Group 1, patients treated with initial radiotherapy followed by surgical treatment; group 2, patients treated with initial surgical treatment followed by radiotherapy.
2. Details of Surgical Treatment and Radiotherapy
Indications for surgical treatment were as follows: (1) neurologic complications related to a metastatic tumor, (2) local or referred pain that did not respond to radiotherapy or medical treatment, (3) axial or translational instability due to a pathological fracture, and (4) local tumor progression after radiotherapy.
The surgical approach was chosen according to the site of metastases on the vertebral bodies, the level of the cervical spine, and the number of levels involved [10,11]. A typical anterior approach to the cervical spine with partial or total corpectomy, vertebral body replacement, and subsequent anterior plate stabilization was used. Vertebral bodies were reconstructed with a mesh cage filled with autografts, tricortical iliac allografts, or polymethylmethacrylate, and instrumentation was performed with a cervical plating system (Zephir plate system, Medtronic Sofamor Danek, Memphis, TN, USA). In the posterior approach, spinal cords were decompressed and cervical spine stabilized with a lateral mass screw-rod or pedicle screw-rod fixation system (Vertex system, Medtronic Sofamor Danek) (Fig. 2).
Fig. 2.
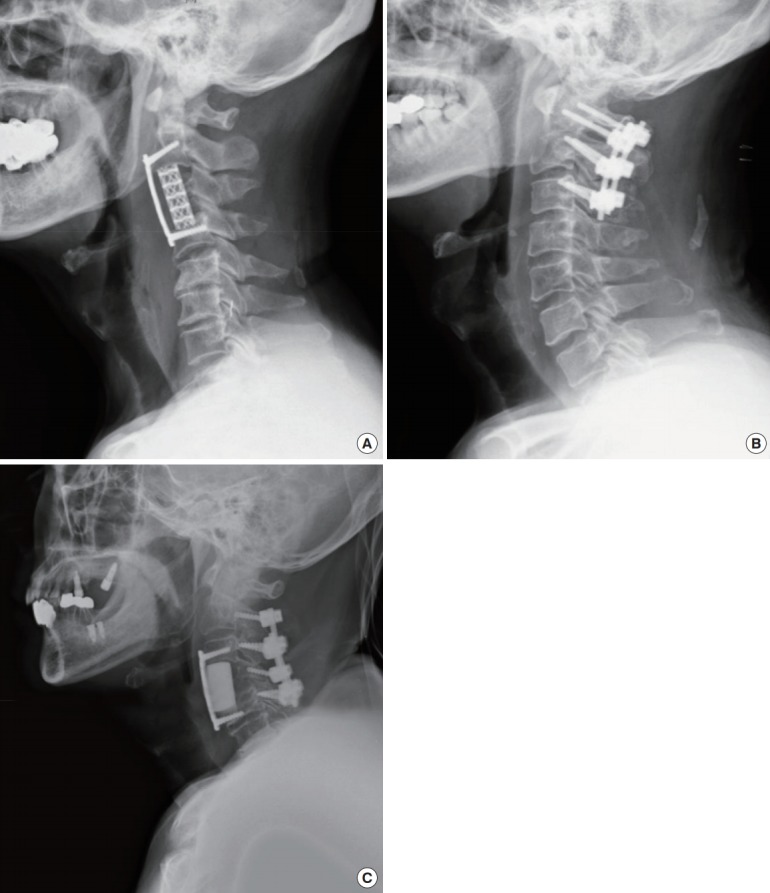
Postoperative radiographs presenting each approach, which was determined according to the tumor location, level of the cervical spine, and number of levels involved. (A) An anterior approach was performed with a mesh cage filled with autograft bone and an anterior plating system. (B) A posterior approach was carried out with decompressive laminectomy and a lateral mass and pedicle screw-rod system. (C) A combined approach was performed with polymethylmethacrylate to reconstruct the vertebral body, an anterior plating system, and a lateral mass screw-rod system.
Conventional radiotherapy considered the tumor location and primary tumor histology. The radiation dose was modulated from 36 Gy/12 Fr to 20 Gy/5 Fr (30 Gy/10 Fr). In group 2, radiotherapy began 2–8 weeks after surgery depending on the patient’s general condition and the surgical wound.
3. Clinical Evaluations
All patients were evaluated at hospital admission and at follow-up every 3 months after cervical spine metastases by a neurosurgeon in the outpatient department. A visual analogue scale (VAS) was used to evaluate pain. The degree of myelopathy was assessed with the Nurick grade [12], Japanese Orthopedic Association (JOA) score [13], and Frankel classification [14]. To analyze changes in Frankel classification, each category of Frankel classification was converted into a number (e.g., grade E=5 and grade A=1). The growth rate of the primary tumor and extent of metastasis were assessed with the modified Tomita scoring system reported by Kim et al. [15] This system categorized hepatobiliary cancer into a moderate growing tumor instead of rapid growing tumor, and readjusted it from 4 to 2 points in primary tumor section of Tomita scoring system. General functional status was assessed with the Eastern Cooperative Oncology Group (ECOG) status and Karnofsky performance status (KPS) [16,17]. The stability of the cervical spinal metastasis was assessed with the spinal instability neoplastic score (SINS) [18].
4. Survival Analysis and Prognostic Factors
Survival was calculated from three different starting points. Overall survival (OS) was defined as the time from diagnosis of the primary tumor to death. OS after cervical spine metastasis was defined as the time from diagnosis of cervical spine metastasis to death. OS after surgery was defined as the time from postoperative day 1 until death. OS, OS after cervical spine metastasis, and OS after surgery were analyzed in each group. OS after cervical spine metastasis was used to identify prognostic factors.
Patients were divided into 2 groups according to initial treatment modality (radiotherapy vs. surgery), primary tumor type divided by modified Tomita score system (rapid growing vs. moderate and slow growing tumor), and modified Tomita score (> 5 vs. ≤5). A modified Tomita score of 5 was used to determine whether a patient was an appropriate surgical candidate. Patients were also categorized by general performance status, ECOG status (> 2 vs. ≤2) and KPS (< 80 vs. ≥80); by preoperative neurologic deficit, JOA score (< 14 vs. ≥14), Nurick grade (> 1 vs. ≤1), and Frankel grade (≤ D vs. E); by instability, SINS (≥ 13 vs. <13); and by preoperative symptoms (motor weakness vs. pain only). To determine whether these factors affected OS after cervical spine metastasis, a Cox proportional hazards model was used.
5. Statistical Analysis
Chi-square and Fisher exact tests were used to analyze categorical variables, and Student t-test, paired t-test, and Mann-Whitney U-test were used to analyze continuous and ordinal variables, as appropriate. Survival curves, which were created by using Kaplan-Meier survival analysis with the log-rank test, were used to compare survival between groups. Significant prognostic factors from univariate analysis were included in the multivariate analysis with the Cox proportional hazards model. A p-value <0.05 (2-tailed) was considered statistically significant. Data were analyzed with commercial software (IBM SPSS Statistics ver. 23.0, IBM Co., Armonk, NY, USA).
RESULTS
1. Clinical Information
Patient demographics and clinical data are summarized in Table 1. Mean age in both groups was 57.6 years (range, 38–73 years). The study population included 30 men and 6 women. The last evaluation was performed in December 2015 with a mean follow-up of 42 months. Fifteen patients had rapid growing tumors, 17 had moderate growing tumors, and 4 had slow growing tumors. In total, 39 cervical spine levels were involved. Eight of these (20.4%) were atlantoaxial cervical spines, and 31 (79.6%) were subaxial. Three patients in group 2 were alive at the end of the study. VAS, modified Tomita score, ECOG status, and KPS showed similar results in both groups. JOA score, Nurick grade, SINS were better in group 1 than group 2, but the differences were not significant (p>0.05).
Table 1.
Comparison of clinical information between groups
| Variable | Group 1 | Group 2 | p-value |
|---|---|---|---|
| Age (yr) | 56.5 ± 7.9 | 58.3 ± 9.2 | 0.568 |
| Sex, male:female | 1:11 | 5:19 | - |
| Type of primary tumor | |||
| Lung cancer | 4 | 6 | - |
| Gastrointestinal | 2 | 3 | - |
| Hepatobiliary | 2 | 12 | - |
| Bladder cancer | 1 | 0 | - |
| Renal cell carcinoma | 2 | 0 | - |
| Prostate cancer | 1 | 1 | - |
| Breast cancer | 0 | 2 | - |
| Cervical spine level | |||
| Atlantoaxial:subaxial spine | 4:10 | 4:21 | - |
| Survival | |||
| Dead:alive | 12/0 | 21/3 | - |
| VAS | 7.3 ± 2.4 | 7.3 ± 2.1 | 0.915 |
| JOA score | 14.8 ± 2.3 | 12.7 ± 3.3 | 0.053 |
| Nurick grade | 1.9 ± 1.0 | 2.5 ± 1.4 | 0.194 |
| Frankel classification | 4.3 ± 0.7 | 4.0 ± 0.8 | 0.212 |
| Modified Tomita score | 5.6 ± 2.2 | 4.6 ± 1.7 | 0.159 |
| ECOG scale | 1.8 ± 0.6 | 1.8 ± 1.1 | 0.772 |
| KPS | 71.7 ± 9.4 | 71.3 ± 13.6 | 0.925 |
| SINS | 12.4 ± 2.7 | 12.6 ± 2.3 | 0.848 |
Values are presented as mean±standard deviation or number.
Group 1, patients treated with initial radiotherapy followed by surgical treatment; group 2, patients treated with initial surgical treatment followed by radiotherapy; VAS, visual analogue scale; JOA score, Japanese Orthopedic Association score; ECOG, Eastern Cooperative Oncology group; KPS, Karnofsky Performance Status; SINS, spinal instability neoplastic score.
2. Clinical Outcomes
All 8 patients with cervical spine metastases at the occipitocervical junction were treated with a posterior approach. Of the 28 patients with cervical spine metastases at the subaxial cervical spine, 25 were treated with an anterior approach, 2 with a posterior approach, and 1 with a combined approach. Surgical complications occurred in 7 patients (19.4%): screw pull-out in 1 patient, pneumonia in 2 patients, wound dehiscence in 2 patients, and infection in 2 patients.
Both groups had improved VAS scores after surgical treatment. VAS scores decreased from 7.3±2.4 to 2.4±1.6 in group 1 and from 7.3±2.1 to 1.8±1.2 in group 2. The difference between pre- and postoperative VAS scores were statistically significant (p<0.01) in each group, but the improvement in VAS did not differ significantly between groups. In group 1, pre- and postoperative JOA scores were 14.3±1.6 and 14.9±1.3, respectively, which were not significantly different (p=0.089). Preand postoperative JOA scores in group 2 were 12.7±3.3 and 14.0±2.2 points, which showed a statistically significant change (p=0.03). JOA score at 6, 9, and 12 months decreased slightly in both groups from 12.1±3.4 to 10.6±4.1 and 9.6±3.9, respectively. Nurick grade did not improve significantly after surgical treatment. In group 1, it changed from 1.9±1.0 to 2.1±1.3 and from 2.5±1.4 to 2.2±1.5 in group 2. Frankel classification also did not improve significantly after surgery. In group 1, it changed from 4.3±0.7 to 4.3±0.5 and from 4.0±0.8 to 4.1±0.6 in group 2.
This trend maintained in subgroup analysis based on modified Tomita scoring system (Table 2). Both groups divided into 2 subgroups (longer and shorter life expectancy group) according to five points of Tomita score, both subgroups also showed significant improvement of VAS (p<0.033). However, group 1 failed to showed significant improvement of JOA score in both subgroups. Especially, patients with Tomita score ≤5 in group 2 showed significant improvement of JOA score. Patients with Tomita score >5 in group 2 also showed improvement of JOA score, but it was not significant. As previously stated, Nurick grade and Frankel classification did not show the significant improvement in subgroup analysis.
Table 2.
Comparison of clinical outcomes between groups after surgical treatment
| Variable | Group 1 |
Group 2 |
||||
|---|---|---|---|---|---|---|
| Preop | Postop | p-value | Preop | Postop | p-value | |
| Modified Tomita score > 5 | ||||||
| VAS | 8.14 ± 0.38 | 3.14 ± 1.21 | 0.001* | 7.18 ± 1.9 | 2.0 ± 1.34 | 0.001* |
| JOA score | 14.0 ± 1.53 | 14.57 ± 1.99 | 0.172 | 12.27 ± 2.8 | 13.64 ± 3.96 | 0.007 |
| Nurick grade | 2.0 ± 1.15 | 2.3 ± 1.38 | 0.356 | 2.55 ± 1.44 | 2.27 ± 1.42 | 0.588 |
| Frankel classification | 4.29 ± 0.76 | 4.29 ± 0.49 | 1.000 | 3.91 ± 0.83 | 4.27 ± 0.47 | 0.104 |
| Modified Tomita score ≤ 5 | ||||||
| VAS | 6.2 ± 3.63 | 1.4 ± 1.67 | 0.033* | 7.31 ± 2.32 | 1.62 ± 1.04 | 0.001* |
| JOA score | 14.8 ± 1.64 | 15.0 ± 1.87 | 0.887 | 13.08 ± 3.75 | 14.48 ± 2.90 | 0.002* |
| Nurick grade | 1.8 ± 0.84 | 1.8 ± 1.30 | 1.000 | 2.38 ± 1.45 | 2.08 ± 1.50 | 0.455 |
| Frankel classification | 4.4 ± 0.55 | 4.4 ± 0.35 | 1.000 | 4.08 ± 0.76 | 4.12 ± 0.64 | 0.436 |
Values are presented as mean±standard deviation.
Group 1, patients treated with initial radiotherapy followed by surgical treatment; group 2, patients treated with initial surgical treatment followed by radiotherapy; preop, preoperative; postop, postoperative; VAS, visual analogue scale; JOA score, Japanese Orthopedic Association score.
p<0.05, statistically significance was determined by the paired t-test.
3. Survival Analysis
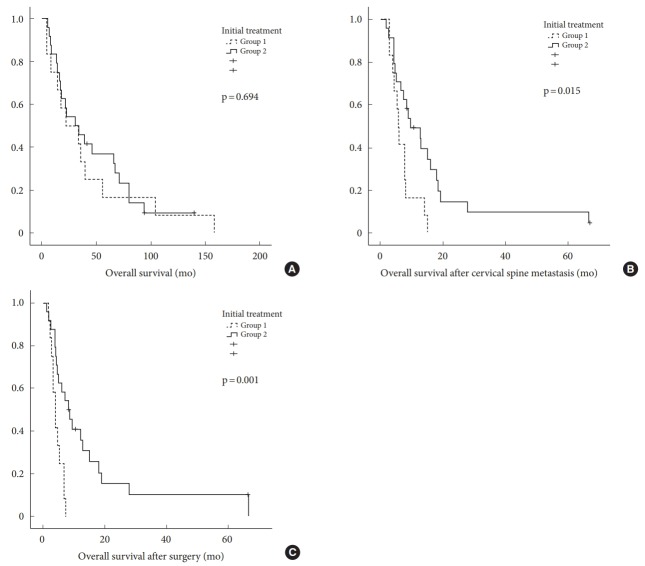
Thirty-three patients died during follow-up. Three patients were alive at the final follow-up visit. The mean OS was 41.4 months in group 1 (95% confidence interval [CI], 15.3–67.5) and 46.2 months in group 2 (95% CI, 30.6–63.1). OS did not differ significantly between groups (p=0.694). The mean OS after cervical spine metastasis was 7.0 months in group 1 (95% CI, 4.8–9.3) and 15.8 months in group 2 (95% CI, 8.8–24.0), which was significantly different (p=0.015). The mean OS after surgery was 4.5 months in group 1 (95% CI, 3.4–5.5) and 15.3 months in group 2 (95% CI, 7.1–23.4) (Fig. 3). Group 2 had significantly longer survival after cervical spine metastasis and after surgery than did group 1 (p<0.05) (Fig. 4). The 1-year survival rate after cervical spine metastasis was 16.7% in group 1 and 41.7% in group 2.
Fig. 3.
Comparison of overall survival, overall survival after cervical spine metastasis, and overall survival after surgery between groups by using Kaplan-Meier survival curves. (A) Overall survival. (B) Overall survival after cervical spine metastasis. (C) Overall survival after surgery.
Fig. 4.
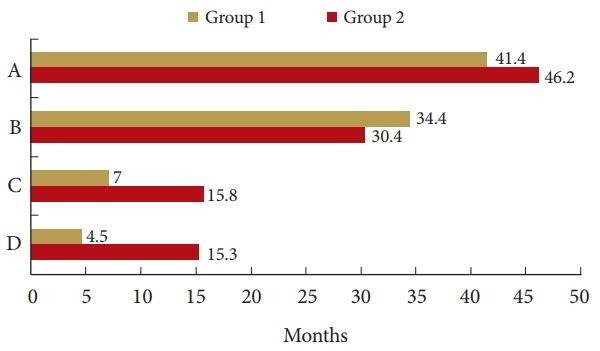
Comparison of each survival period between groups. (A) The mean overall survival (p= 0.694). (B) The period from diagnosis of the primary caner to cervical spine metastasis (p = 0.976). (C) Overall survival after cervical spine metastasis (p = 0.015). (D) Overall survival after surgery (p = 0.001).
Of the 36 patients, 13 presented with pain only, 1 presented with motor weakness only, and 22 presented with both symptoms. We compared OS after cervical spine metastasis according to preoperative symptoms among patients who underwent initial surgical treatment (group 2). In group 2, 33.3% of patients presented with pain only. We tried to evaluate whether OS after cervical spine metastasis differed according to the presence or absence of motor weakness before surgery. In group 2, patients who presented with pain only lived longer than those who presented with motor weakness (p=0.001). OS after cervical spine metastasis was 30.4 months (95% CI, 22.8–38) in patients who presented with pain only and 8.0 months (95% CI, 4.1–11.9) in patients who presented with motor weakness, a 3-fold increase in patients who presented with pain only.
4. Prognostic Factors for Survival
In a univariate analysis, OS after cervical spine metastasis was significantly associated with primary tumor type (p=0.004), initial treatment modality (p=0.019), Modified Tomita score (p=0.003), KPS score (p=0.04), and preoperative symptoms (p=0.002) (Table 3).
Table 3.
Prognostic factors of overall survival after cervical spine metastasis
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Modified Tomita score | ||||
| >5 vs. ≤5 | 2.993 (1.436–6.237) | 0.003* | 1.116 (0.443–2.809) | 0.816 |
| ECOG status | ||||
| >2 vs. ≤2 | 1.100 (0.516–2.344) | 0.805 | - | - |
| KPS | ||||
| <80 vs. ≥80 | 2.268 (1.038–4.955) | 0.040* | 1.642 (0.721–3.740) | 0.238 |
| JOA score | ||||
| <14 vs. ≥14 | 1.455 (0.725–2.919) | 0.291 | - | - |
| Nurick grade | ||||
| >1 vs. ≤1 | 1.085 (0.528-2.231) | 0.824 | - | - |
| Frankel classification | ||||
| ≤D vs. E | 1.332 (0.644–2.757) | 0.440 | - | - |
| Primary tumor type | ||||
| Rapid growth tumor vs. moderate-slow growth tumor | 2.836 (1.390–5.788) | 0.004* | 2.265 (1.023–5.015) | 0.044* |
| Initial treatment | ||||
| Radiotherapy vs. surgery | 2.529 (1.162–5.504) | 0.019* | 3.012 (1.242–7.305) | 0.015* |
| SINS | ||||
| ≥13 vs. <13 | 1.904 (0.941–3.856) | 0.073 | - | - |
| Preoperative symptom | ||||
| Motor weakness vs. pain | 3.278 (1.555–6.910) | 0.002* | 4.009 (1.435–11.200) | 0.008* |
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology group; KPS, Karnofsky performance status; JOA score, Japanese Orthopedic Association score; SINS, spinal instability neoplastic score.
p<0.05, statistically significance was determined with the Cox’s proportional hazards model.
The prognostic factors determined by univariate analysis were included in a multivariate analysis. In the multivariate analysis, OS after cervical spine metastasis was significantly associated with primary tumor type (rapid growing vs. moderate and slow growing tumor; HR, 2.27; 95% CI, 1.02–5.02; p=0.044), initial treatment modality (radiotherapy vs. surgical treatment; HR, 3.01; 95% CI, 1.24–7.31; p=0.015), and preoperative symptoms (motor weakness only vs. pain only; HR, 4.01; 95% CI, 1.44–11.20; p=0.008) (Table 3).
DISCUSSION
Since the 1980s, advances in surgical technique and the advent of spinal implants have allowed for better removal of tumors with immediate spinal cord decompression, while stabilizing and reconstructing the spine. Decompressive surgery and spine stabilization are widely and effectively used in patients with spinal metastases [5,8,9]. Furthermore, these procedures are associated with less pain, improved neurological function, and better OS than with nonsurgical treatment [19].
1. Clinical Outcomes
In the present study, surgical treatment effectively controlled pain, and radiotherapy before surgical treatment did not seem to prevent this improvement. JOA scores after surgical treatment, however, differed significantly between groups. JOA scores only improved significantly after surgical treatment in group 2, especially patients with Tomita score ≤5. This difference could be explained by results from previous studies. One study reported that preoperative radiotherapy could prevent neurological recovery after surgical treatment. Chaichana et al. [20] stated that preoperative radiation may directly (through radiation-induced myelitis) and indirectly (through reactive gliosis, fibrosis, and compromised spinal cord blood supply) prevent neurological recovery after surgery. These untoward effects of radiotherapy might have affected postoperative neurologic status in group 1. Nurick grade and Frankel classification did not improve significantly after surgical treatment in either group. The improvement in JOA score in group 2 was slight, about 2 points. Neither Nurick grade nor Frankel classification is sensitive enough to identify slight clinical changes between grades. A detailed grading system may be necessary to assess patients with cervical spine metastasis. Twenty-one patients (58.3%) in the current study had a measurable neurological improvement in JOA score.
Neurological function tended to decrease during follow-up. JOA score suddenly decreased between 6 and 9 months after surgery. These decreases could be explained by recurrence of metastatic tumors at the surgical site or new metastases at other sites.
2. Surgical Treatment Related Complication
Surgical complications occurred in 7 patients (19.4%). One pneumonia and screw pull out were observed in 2 patients of group 1. One pneumonia, 2 wound dehiscence, and 2 wound infections were checked in 5 patients of group 2. Wound problems occurred with frequency in group 2. This incidence of complication is similar to those of earlier studies. Previous studies have reported complication rates of 10% to 30% [21,22]. No patients experienced injuries of the vertebral artery or death due to surgical complications. One patient who had a screw pull out underwent revision surgery.
3. Survival Analysis
Improvements in spinal surgery for spinal metastatic disease have been shown to resolve intractable pain and improve neurologic deficit, but whether they can extend life expectancy remains an open question [1,23,24]. Modified Tomita score, ECOG status and KPS were similar in both groups, and OS did not differ significantly between groups in the present study. OS is mainly influenced by the histology of the primary tumor and visceral metastases of major organ and surgical outcomes of primary cancer, not the initial treatment modality or timing of surgical treatment for spinal metastases [1,4,23]. However, OS after cervical spine metastasis differed significantly between groups in our series (Fig. 4).
This phenomenon might be a result of the relatively long period from diagnosis of the primary cancer to cervical spine metastasis. The mean for this period was about 30.1±35.8 months in our study, which was longer than OS after cervical spine metastasis. This period accounted for most part of OS and was determined by the histology of the primary tumor and visceral metastases of major organ and surgical outcomes of primary cancer. Thus, we thought that surgical treatment would have an effect on OS after cervical spine metastasis, but its effect on OS was minimal. In the present study, OS after cervical spine metastasis differed significantly between groups, but the significance disappeared when the period from diagnosis of the primary cancer to cervical spine metastasis was added in.
4. Impact of Cervical Spine Surgery
Some authors have reported that early surgical decompression before irreversible neural injury may reduce damage to the spinal cord and nerve roots, allowing good recovery of neurologic function [25,26]. Radiation therapy cannot affect epidural cord compression with progressive vertebral destruction and posterior vertebral body retropulsion. Thus, the neurological status of patients usually worsened during radiotherapy, which might lead to reduced OS after cervical spine metastasis.
Moreover, surgical difficulties with anterior decompression, surgical morbidities, and postoperative recovery in the cervical spine differ from those in the thoracic and lumbar spine [27,28]. An anterior approach for the cervical spine, which was used in 25 cases, is relatively easy and safe with regard to soft tissue dissection and visualizing and removing infiltrated vertebral bodies [6,29,30]. An anterior approach for the cervical spine also provided more decompression than did a posterior approach. These features of the cervical spine might increase the effect of surgical treatment.
5. Prognostic Factors for Survival
Previous studies reported that the life expectancy of patients with spinal metastasis depends on the primary tumor type [31-33], and primary tumor type is a significant prognostic variable [34,35]. Heidecke et al. [36] conducted a study similar to ours but up to 21% of patients had lymphoma or multiple myeloma. They found that the primary tumor histology, extent of metastasis, and baseline general condition as measured by KPS score were the most important prognostic factors. We obtained analogous results in the current study excluding lymphoma and hematologic malignancy. Good prognostic factors affecting OS after cervical spine metastasis in univariate analysis were moderate and slow-growing tumor types, a modified Tomita score of less or equal than 5, KPS of greater than 80, initial surgical treatment, and preoperative pain without motor weakness (Table 3). In multivariate analysis, modified Tomita score and KPS were no longer statistically significant. Moderate and slow growing tumor type, initial surgical treatment, and preoperative pain remained statistically significant (Table 3). Our results support the findings from previous reports that primary tumor type and preoperative symptoms were prognostic factors [22,37]. We also found that initial surgical treatment in patients with cervical spine metastasis increased OS after cervical spine metastasis by the limited methods of comparison between groups.
Earlier studies found that preoperative motor function at the diagnosis of spinal metastasis is the most important predictor of postoperative neurologic status [20,32,38]. Furthermore, preoperative motor weakness was a negative predictor of OS after cervical spine metastasis in our study (p=0.002). OS after cervical spine metastasis differed significantly depending on the presence or absence of preoperative motor weakness in patients who received initial surgical treatment (group 2). In clinical practice, most motor weakness results from epidural cord compression [39]. Some authors have stated that 60%–85% of patients with metastatic epidural spinal cord compression have motor weakness [35]. If epidural cord compression on cervical magnetic resonance imaging was found in patients without motor weakness, surgical treatment of cervical spine metastasis could be considered.
For these reasons, surgical intervention, instead of radiotherapy, could be preferred for patients with cervical spine metastasis. The optimal timing of surgical treatment for cervical spine metastasis is difficult to determine and remains a topic of debate. Nevertheless, patients with cervical spine metastasis who do not have motor weakness and have not received radiotherapy may be good candidates for surgical treatment.
6. Limitation
This study is limited by its retrospective design and small sample size. This study may be affected by selection bias because our institution is one of many spine centers that have different concentrations of patients with cervical spine metastasis. However, Modified Tomita score, JOA score, pain VAS, Nurick grade, and Frankel classification were similar between groups. Another limitation is that surgeons from a single institution decided on the surgical approach. An anterior approach accounted for 25 cases in the present study (69.4%). Finally, this study did not have an even distribution of primary tumor types; slow growing tumors were small, respectively. These factors should be kept in mind when interpreting the results.
CONCLUSIONS
Patients who underwent initial surgical treatment showed good improvement in postoperative neurologic recovery. Surgical treatment instead of radiotherapy could be advantageous in patients with cervical spine metastasis. The optimal timing of surgical treatment for cervical spine metastasis is difficult to determine and remains a topic of debate. Nevertheless, surgical treatment prior to motor weakness or radiotherapy may be a good decision in case of cervical spine metastasis.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12 Suppl 2:S202–13. doi: 10.1007/s00586-003-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenlee RT, Murray T, Bolden S, et al. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer. 1975;36:590–4. doi: 10.1002/1097-0142(197508)36:2<590::aid-cncr2820360240>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Jenis LG, Dunn EJ, An HS. Metastatic disease of the cervical spine. A review. Clin Orthop Relat Res. 1999;359:89–103. doi: 10.1097/00003086-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Rao S, Badani K, Schildhauer T, et al. Metastatic malignancy of the cervical spine. A nonoperative history. Spine (Phila Pa 1976) 1992;17(10 Suppl):S407–12. doi: 10.1097/00007632-199210001-00011. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki T, McLoughlin GS, Patel S, et al. Feasibility and safety of en bloc resection for primary spine tumors: a systematic review by the Spine Oncology Study Group. Spine (Phila Pa 1976) 2009;34(22 Suppl):S31–8. doi: 10.1097/BRS.0b013e3181b8b796. [DOI] [PubMed] [Google Scholar]
- 7.Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Williston Park) 2000;14:1013–24. [PubMed] [Google Scholar]
- 8.Tancioni F, Navarria P, Lorenzetti MA, et al. Multimodal approach to the management of metastatic epidural spinal cord compression (MESCC) due to solid tumors. Int J Radiat Oncol Biol Phys. 2010;78:1467–73. doi: 10.1016/j.ijrobp.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Tabouret E, Cauvin C, Fuentes S, et al. Reassessment of scoring systems and prognostic factors for metastatic spinal cord compression. Spine J. 2015;15:944–50. doi: 10.1016/j.spinee.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Fehlings MG, David KS, Vialle L, et al. Decision making in the surgical treatment of cervical spine metastases. Spine (Phila Pa 1976) 2009;34(22 Suppl):S108–17. doi: 10.1097/BRS.0b013e3181bae1d2. [DOI] [PubMed] [Google Scholar]
- 11.Bilsky MH, Boakye M, Collignon F, et al. Operative management of metastatic and malignant primary subaxial cervical tumors. J Neurosurg Spine. 2005;2:256–64. doi: 10.3171/spi.2005.2.3.0256. [DOI] [PubMed] [Google Scholar]
- 12.Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. doi: 10.1093/brain/95.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Vitzthum HE, Dalitz K. Analysis of five specific scores for cervical spondylogenic myelopathy. Eur Spine J. 2007;16:2096–103. doi: 10.1007/s00586-007-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel HL. Ascending cord lesion in the early stages following spinal injury. Paraplegia. 1969;7:111–8. doi: 10.1038/sc.1969.21. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Lee SH, Park SJ, et al. Analysis of the predictive role and new proposal for surgical strategies based on the modified Tomita and Tokuhashi scoring systems for spinal metastasis. World J Surg Oncol. 2014;12:245. doi: 10.1186/1477-7819-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 17.Karnofsky DA. Chemotherapy of neoplastic disease; trends in experimental cancer therapy. N Engl J Med. 1948;239:260–70. doi: 10.1056/NEJM194808122390705. [DOI] [PubMed] [Google Scholar]
- 18.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–7. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 19.Walcott BP, Cvetanovich GL, Barnard ZR, et al. Surgical treatment and outcomes of metastatic breast cancer to the spine. J Clin Neurosci. 2011;18:1336–9. doi: 10.1016/j.jocn.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Chaichana KL, Woodworth GF, Sciubba DM, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62:683–92. doi: 10.1227/01.neu.0000317317.33365.15. [DOI] [PubMed] [Google Scholar]
- 21.Oda I, Abumi K, Ito M, et al. Palliative spinal reconstruction using cervical pedicle screws for metastatic lesions of the spine: a retrospective analysis of 32 cases. Spine (Phila Pa 1976) 2006;31:1439–44. doi: 10.1097/01.brs.0000219952.40906.1f. [DOI] [PubMed] [Google Scholar]
- 22.Cho W, Chang UK. Neurological and survival outcomes after surgical management of subaxial cervical spine metastases. Spine (Phila Pa 1976) 2012;37:E969–77. doi: 10.1097/BRS.0b013e31824ee1c2. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8:271–8. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 24.North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564–73. doi: 10.3171/spi.2005.2.5.0564. [DOI] [PubMed] [Google Scholar]
- 25.Klimo P, Jr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–8. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 27.Sayama CM, Schmidt MH, Bisson EF. Cervical spine metastases: techniques for anterior reconstruction and stabilization. Neurosurg Rev. 2012;35:463–74. doi: 10.1007/s10143-012-0388-z. [DOI] [PubMed] [Google Scholar]
- 28.Quan GM, Vital JM, Pointillart V. Outcomes of palliative surgery in metastatic disease of the cervical and cervicothoracic spine. J Neurosurg Spine. 2011;14:612–8. doi: 10.3171/2011.1.SPINE10463. [DOI] [PubMed] [Google Scholar]
- 29.Cabanela ME, Ebersold MJ. Anterior plate stabilization for bursting teardrop fractures of the cervical spine. Spine (Phila Pa 1976) 1988;13:888–91. doi: 10.1097/00007632-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Placantonakis DG, Laufer I, Wang JC, et al. Posterior stabilization strategies following resection of cervicothoracic junction tumors: review of 90 consecutive cases. J Neurosurg Spine. 2008;9:111–9. doi: 10.3171/SPI/2008/9/8/111. [DOI] [PubMed] [Google Scholar]
- 31.Hoskin PJ, Grover A, Bhana R. Metastatic spinal cord compression: radiotherapy outcome and dose fractionation. Radiother Oncol. 2003;68:175–80. doi: 10.1016/s0167-8140(03)00191-9. [DOI] [PubMed] [Google Scholar]
- 32.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–67. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen S, Børgesen SE, Rohde K, et al. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer. 1990;65:1502–8. doi: 10.1002/1097-0142(19900401)65:7<1502::aid-cncr2820650709>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58:245–59. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 35.Helweg-Larsen S, Sørensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46:1163–9. doi: 10.1016/s0360-3016(99)00333-8. [DOI] [PubMed] [Google Scholar]
- 36.Heidecke V, Rainov NG, Burkert W. Results and outcome of neurosurgical treatment for extradural metastases in the cervical spine. Acta Neurochir (Wien) 2003;145:873–80. doi: 10.1007/s00701-003-0107-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee MH, Lee SH, Kim ES, et al. Survival-related factors of spinal metastasis with hepatocellular carcinoma in current surgical treatment modalities: a single institute experience. J Korean Neurosurg Soc. 2015;58:448–53. doi: 10.3340/jkns.2015.58.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rades D, Rudat V, Veninga T, et al. A score predicting posttreatment ambulatory status in patients irradiated for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2008;72:905–8. doi: 10.1016/j.ijrobp.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459–66. doi: 10.1016/S1474-4422(08)70089-9. [DOI] [PubMed] [Google Scholar]