Abstract
In the human pathogen Mycoplasma genitalium, homologous recombination is under the control of σ20, an alternative sigma factor that boosts the generation of genetic and antigenic diversity in the population. Under laboratory growth conditions, σ20 activation is rare and the factors governing its intermittent activity are unknown. Two σ20-regulated genes, rrlA and rrlB, showed to be important for recombination of homologous DNA sequences in this bacterium. Herein, we demonstrate that rrlA and rrlB code for two small proteins that participate in a feed-forward loop essential for σ20 function. In addition, we identify novel genes regulated by σ20 and show that several non-coding regions, which function as a reservoir for the generation of antigenic diversity, are also activated by this alternative sigma factor. Finally, we reveal that M. genitalium cells can transfer DNA horizontally by a novel mechanism that requires RecA and is facilitated by σ20 over-expression. This DNA transfer system is arguably fundamental for persistence of M. genitalium within the host since it could facilitate a rapid dissemination of successful antigenic variants within the population. Overall, these findings impose a novel conception of genome evolution, genetic variation and survival of M. genitalium within the host.
Keywords: mycoplasma, sigma factor, homologous recombination, antigenic variation, horizontal gene transfer
1. Introduction
Pathogenic microorganisms have evolved sophisticated strategies to evade or subvert the host immune system.1 Typically, extracellular bacteria modify their surface structures or control the expression of their immunodominant proteins to avoid antibody recognition.2 These two widespread strategies meant to survive and persist within the host are known as antigenic variation (AnV) and phase variation (PhV), respectively. The sexually transmitted pathogens Mycoplasma genitalium, Neisseria gonorrhoeae and Treponema pallidum, generate AnV by means of programmed rearrangements of unique chromosomal sequences.3 These chromosomal rearrangements are essentially facilitated by homologous recombination and accordingly, RecA and other important recombination enzymes play a fundamental role in AnV.4–8 Likewise, the participation of proteins that regulate the capacity of RecA to polymerize or load onto ssDNA in the generation of antigenic variants is substantiated by several reports.9,10 Considering these studies, RecA emerges as the primary target to control sequence variation of major surface antigens by homologous recombination. Remarkably, despite RecA is critical for DNA repair and maintenance of genome stability in bacteria, the sexually transmitted pathogens aforementioned are devoid of a classical SOS system coordinating the concerted activation of recA and other repair genes in response to DNA insults.7,11–14 In light of these data, it has been suggested that the participation of RecA in the generation of genetic variants imposes important restrictions as to the multifaceted mechanisms that bacteria exploit to regulate recombination.15
P140 (MgpB) and P110 (MgpC) are the major cytadhesins and the main surface antigens of M. genitalium. In its chromosome, this bacterium comprises nine DNA repeats, designated as MgPar, that contain sequences with homology to the MG_191 and MG_192 genes, which code for P140 and P110, respectively.12,16 Recombination between the cytadhesin genes and MgPar sequences provides a virtually unlimited collection of genetic and antigenic variants.17,18 Moreover, recombination with particular MgPar regions leads to the expression of truncated P140 or P110 proteins, which evidences the existence of a subjacent and versatile mechanism of PhV.19 Remarkably, P140 and P110 variation is critical for survival and persistence of M. genitalium within the host.20–23 Recently, we identified an alternative sigma factor, herein designated as σ20, that controls the activation of homologous recombination in M. genitalium.24 Of note, σ20 is a major determinant for the generation of genetic variants of the cytadhesin genes.24,25 σ20 regulates transcription of recA (MG_339), ruvA (MG_358) and ruvB (MG_359), plus several genes of unknown function. Bewilderingly, under laboratory growth conditions, the σ20 regulon is only activated in a small subset of cells in response to unidentified factors. These singularities impose single cell analyses as the only suitable techniques to study and characterize σ20 activity. Of note, overexpression of σ20 induces a hyper-recombinogenic phenotype that is highly deleterious.24 Despite these hitches, we recently identified two novel genes under the control of σ20, designated as recombination regulatory loci A and B (rrlA and rrlB), which are intimately related to homologous recombination.24 Null mutants of these genes exhibit severe recombination defects, similar to those observed in a σ20 defective mutant, but the exact role of the RrlA and RrlB proteins in M. genitalium remains obscure.
Overall, in the current study we provide further knowledge regarding the function of σ20 in M. genitalium. We describe the whole regulon and identify several factors controlling σ20 activation and modulating recombination in this bacterium. Moreover, we reveal a link between σ20 and a mechanism of horizontal gene transfer (HGT) that is mediated by the recombination machinery of this bacterium. This novel activity associated to the σ20 pathway is independent of any known mobile genetic elements, which defies traditional HGT systems and might be a valuable tool for genome evolution and adaptation of this small pathogen.
2. Materials and methods
2.1. Bacterial strains, culture conditions and primers
All M. genitalium strains were grown in SP-4 broth at 37°C in a 5% CO2 atmosphere in tissue culture flasks. SP-4 plates were prepared supplementing the medium with 0.8% agar (BD). Tetracycline (3 µg ml−1), chloramphenicol (Cm) (17 µg ml−1) or puromycin (Pm) (3 µg ml−1) were added for mutant selection when necessary. All M. genitalium strains used in this work are listed in Supplementary Table S1. Escherichia coli strain XL-1 Blue was used for cloning and plasmid propagation. The strain was grown in Luria Bertani (LB) or LB agar plates containing 100 μg ml−1 ampicillin, 40 μg ml−1 X-Gal and 24 μg ml−1 Isopropyl β-d-1-thiogalactopyranoside (IPTG) when needed. All primers used in this study are listed in Supplementary Table S2.
2.2. DNA manipulation
Standard techniques for cloning were performed as described in Sambrook and Russell.26 Plasmid DNA was obtained using Fast Plasmid Mini kit (5Prime). PCR products were purified from agarose gels using Nucleospin Gel and PCR Clean-up kit (Macherey-Nagel) and digested with the corresponding restriction enzymes (Fermentas) when necessary. Plasmids for M. genitalium transformation were obtained using the GenElute HP Midiprep kit (Sigma).
2.3. Mutant construction, transformation and screening
A detailed explanation of the steps and methodology, including primers and plasmids, used to construct all the mutants created in this study is supplied as Supplementary Information. Transformation of M. genitalium was carried out as previously described.24 Screening for mutants was performed using cell lysates as template for PCR or sequencing reactions. Cell lysates were obtained by centrifugation of 5 ml cell cultures, disruption of pellets using 30 µl of Lysis Buffer (Tris–HCl 0.1 M pH 8.5, Tween-20 0.05%, proteinase K 0.25 mg ml−1) and incubation for 1 h at 37°C followed by inactivation at 95°C for 10 min.
2.4. RNA extraction and transcriptional analysis
Mycoplasma genitalium was grown to mid-exponential phase in 25 cm2 tissue culture flasks. Attached mycoplasmas were scrapped off in 1 ml of fresh SP-4, inoculated in two new 25 cm2 tissue culture flasks with fresh SP-4 medium and incubated at 37°C 5% CO2 for 6 h. Then, total RNA was extracted using the miRNeasy Mini Kit (Qiagen) following manufacturer’s instructions. Contaminant DNA was eliminated with the RNase-Free DNase Set (Qiagen).
To conduct the RNAseq study, three independent biological repeats of each strain were submitted to analysis. RNA libraries were prepared with TruSeq Stranded Total RNA Library Prep Kit (Illumina) and analysed using a HiSeq 3000 System (Illumina) at the Genomics Unit from Center for Genomic Regulation (CRG), Barcelona. cDNA clusters were immobilized in sequencing lanes of 2 × 50 reads. Prior to any data analysis, reverse and complementary was computed for sequences coming from Read1 primer. Data analysis and sequence alignment was performed using Bowtie2 tool27 in the End-to-End mode and Forward-Forward paired-ends. Sequences were piled up using SAMtools28 with no limit set to the number of sequences in the alignment. Counts in the different ORFs were performed with a standalone version of featureCounts program29 without counting the multi-mapping reads and disabling multi-overlapping reads.
Counted features were then submitted to the R/Bioconductor package DESeq230,31 for statistical analysis. DESeq2 analysis used a parametric fitType and a zero-mean normal prior on the non-intercept coefficients. Data were sorted by log2 fold change and statistical significance was set at P-value < 0.05. DESeq2 was chosen as the RNAseq normalization method over other widely used procedures, such as RPKM (Reads Per Kilobase per Million mapped reads) or TC (Total Count), since a recent study has shown that DESeq2 normalization can maintain a reasonable false-positive rate in different library sizes and widely different library compositions.32
2.5. Primer extension
Primer extension analyses were performed with 20 μg of total RNA as previously described.24 Total RNA was extracted from mid-log phase cultures using the RNAqueous kit (Life Technologies) and treated using Turbo DNase (Life Technologies) following manufacturer’s instructions. Fragments were analysed using PeakScanner v1.0 software (Applied Biosystems). At least two independent primer extension experiments were performed with each primer.
2.6. Sequencing reactions
DNA sequencing reactions were performed using BigDye® v3.1 Cycle Sequencing kit using 2.5 µl of genomic DNA or M. genitalium lysate, following manufacturer’s instructions. All reactions were analysed in an ABI PRISM 3130xl Genetic Analyzer at the Servei de Genòmica i Bioinformàtica (UAB).
2.7. Quantitative assessment of the recombination capacity
Recombination capacity was calculated using the transformation efficiency by homologous recombination of a suicide plasmid as previously described.24 Results presented in the manuscript correspond to at least three independent biological repeats.
2.8. Phase contrast and fluorescence microscopy
Mycoplasma genitalium was grown in filtered (0.22 µm) SP-4 medium on IBIDI chamber slides for 16 h, washed once with 1 × PBS and visualized on a Nikon Eclipse TE 2000-E microscope. All strains were grown and visualized under the same conditions. Phase contrast and TRITC epifluorescence images were captured with a Digital Sight DS-SMC Nikon camera controlled by NIS-Elements BR software. Images were analysed using Image J software and GDSC plug-in. Fluorescence intensities were determined by quantifying gray levels of individual cells in binary images using Image J software.
2.9. Mating of M. genitalium strains
Mycoplasma genitalium strains were grown separately in 75 cm2 tissue culture flasks until mid-log phase. Then, cells were recovered in 10 ml of fresh SP-4 and passed through 0.45 µm filters. Four millilitre of the cell suspension from each strain were mixed and incubated in a 75 cm2 tissue culture flask with 12 ml of fresh SP-4 without antibiotic selection. After 24 h of co-incubation, cells were scrapped off in 1 ml of fresh SP-4 and 200 µl aliquots were seeded on 0.9% SP-4 agar plates (8.5 cm diameter) supplemented with Cm (34 µg ml−1) and Pm (3 µg ml−1) or used to inoculate 75 cm2 tissue culture flasks with dual antibiotic selection. To exclude transformation as a mechanism of transfer, the mating experiments were performed in the presence of DNase. After 14 days, colonies were picked up and screened for by PCR and sequencing of the resulting amplicons. Mating efficiency was calculated by dividing the number of double resistant colonies obtained on selective medium by the number of mycoplasma colonies obtained on non-selective medium. For the liquid cultures, typically 14–16 days were needed to observe colonization of the flask surface. Dual antibiotic selection was maintained during all the process. Mobilization of the antibiotic markers in the cell pools was also assessed by PCR and sequencing analysis of the resulting amplicons.
3. Results
3.1. Elucidation of the whole σ20 regulon
The recent identification and initial characterization of the σ20 regulator of M. genitalium was not accompanied by a comprehensive transcriptional study. Herein, we conducted a genome-wide RNA-Seq analysis to identify genes controlled by σ20-dependent promoters. To this end, we compared RNA samples from strains lacking or overexpressing σ20 to those of the wild-type strain. We found that transcription of up to thirteen genes increased significantly upon σ20 overexpression (Table 1). In keeping with previous data, we observed activation of recA, ruvAB and the recombination regulatory loci rrlAB.24 Additionally, we identified three novel genes controlled by σ20-dependent promoters: MG_285, MG_286 and MG_412. While the MG_285 and MG_286 genes code for two polypeptides with unknown function, the protein encoded by the MG_412 gene shows sequence homology to the substrate binding subunit of phosphate transport systems.
Table 1.
Differentially transcribed genes upon overexpression (Up) or deletion (ΔMG_428) of σ20
| Mean transcript fold-increase |
||||||
|---|---|---|---|---|---|---|
| New locus | Old locus | Annotation | Function | Up σ20 | ΔMG_428 | |
| MG_RS01295 | MG_220 | rrlA | Sigma accessory protein | 56.57 | 0.63 | |
| MG_RS02205 | MG_358 | ruvA | Recombination and repair | 20.7 | 0.98 | |
| MG_RS02550 | MG_428 | rpo20 | Alternative sigma factor | 20.6 | 0.06 | |
| MG_RS02065 | MG_339 | recA | Recombination and repair | 19.6 | 0.79 | |
| MG_RS02210 | MG_359 | ruvB | Recombination and repair | 18.7 | 0.99 | |
| MG_RS01710 | MG_286 | HP | Unknown | 17.7 | 0.89 | |
| MG_RS01705 | MG_285 | HP | Unknown | 16.3 | 0.93 | |
| MG_RS02495 | MG_414 | HP | Unknown | 15.4 | 0.79 | |
| MG_RS02200 | – | rrlB | Sigma accessory protein | 12.4 | 0.90 | |
| MG_RS02370 | MG_389 | HP | Unknown | 9.7 | 0.90 | |
| MG_RS00050 | MG_010 | HP | Replication (putative) | 3.8 | 0.84 | |
| MG_RS02490 | MG_412 | pstS | Phosphate binding lipoprotein (putative) | 2.8 | 1.0 | |
| MG_RS02375 | MG_390 | sunT | Peptide secretion (putative) | 2.4 | 0.91 | |
| – | – | ncRNA-1 | Unknown | 5.4 | 0.80 | |
| – | – | ncRNA-2 | Unknown | 20.56 | 0.88 | |
| – | – | ncRNA-3/4 | Unknown | 86.82 | 0.02 | |
Differential gene expression compared with the WT strain and judged based on the common arbitrary 2-fold cutoff.
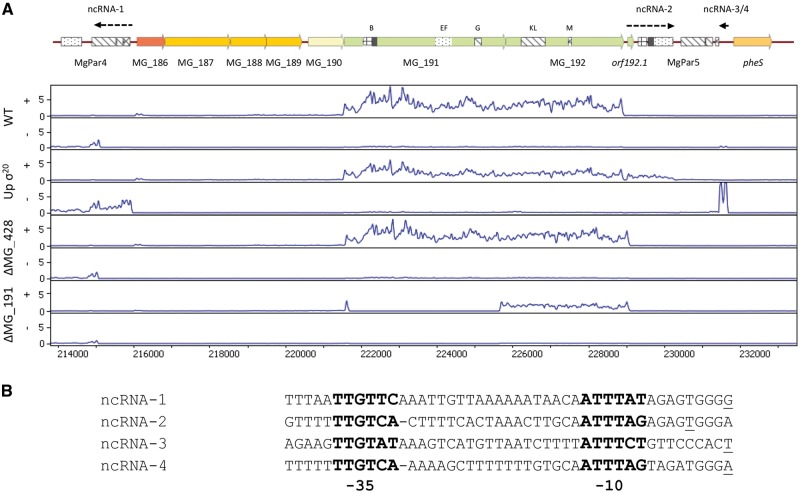
Of note, we found that several transcripts originating from chromosomal regions without annotated genes were markedly upregulated upon σ20 overexpression (Table 1 and Fig. 1A). These transcripts comprise DNA sequences with homology to the cytadhesin genes of M. genitalium. The transcript designated as ncRNA-1 lies within the MgPar-4 region of the G37 chromosome.17 It comprises the repeat G of P140 and the large KLM repeat of P110, both in the antisense orientation. Transcripts ncRNA-2 and ncRNA-3/4 lie within the MgPar-5 region, which is located immediately downstream of the cytadherence operon. ncRNA-2 comprises the repeats B and EF of P140, plus a putative novel ORF located at the 5′ end of the transcript and herein designated as orf192.1. Upregulation of ncRNA-3/4 is particularly apparent and encompasses two short transcripts located immediately upstream of the EF repeat of the MgPar-5. In agreement with the intermittent activation of σ20, these two short transcripts were still detected in the WT strain (Fig. 1A). In contrast, transcription of ncRNA-3/4 was downregulated by 50-fold in strains lacking σ20 (Table 1 and Fig. 1A).
Figure 1.
Chromosomal localization of the ncRNAs activated by σ20. (A) Normalized relative coverage of the RNA-Seq data for the WT strain, a mutant overexpressing σ20, and the MG_428 and MG_191 null mutants, respectively. Only the chromosome section corresponding to the cytadherence operon and its flanking regions is shown. For each strain, the upper and lower panels depict the coverage for the + and – strands, respectively. Different ORFs are coloured according to the different COG families44 (Supplementary Table S3). (B) Alignment of the upstream regions of the identified ncRNAs. Conserved bases corresponding to the -35 and -10 elements recognized by σ20 are highlighted in bold and experimentally identified TSS are underlined.
3.2. Identification of novel σ20-regulated promoters
Primer extension analyses established the presence of σ20-dependent transcriptional start sites (TSS) within the upstream regions of the MG_285 and MG_412 genes (Supplementary Fig. S1). Similarly, we identified TSSs upstream of the three upregulated regions without annotated genes (Supplementary Fig. S1). For the ncRNA-3/4 transcript, we identified two independent TSS. As expected, all the identified TSSs were preceded by σ20 promoter sequences (Fig. 1B), which show the consensus sequence 5′-TTGTCA-N18/19-ATTWAT-3′ in M. genitalium.24
Remarkably, the chromosome of Mycoplasma pneumoniae carries an orthologue of the MG_428 gene and accordingly, σ20-regulated promoters of M. genitalium are conserved in this close related species.24 In keeping with this idea, we identified a putative σ20 promoter in the upstream region of the MPN404 gene, the ortholog of MG_285. Likewise, we found a putative σ20 promoter in the upstream region of MPN143, which codes for a protein of 175 amino acids exclusive of M. pneumoniae. The MPN143 gene is located immediately downstream of the cytadherence genes (MPN141 and MPN142), a chromosomal location that is similar to the putative novel orf192.1 identified in M. genitalium (Supplementary Fig. S2).
3.3. Expression of RrlA, RrlB and ORF192.1 in single cells
To support and expand our transcriptional data, we monitored the expression of RrlA and RrlB in single cells using fluorescent protein fusions. Similarly, we used the same approach to test whether the putative novel ORF identified within the ncRNA-2 transcript, orf192.1, was expressed in M. genitalium. For this purpose, we obtained mutants carrying transcriptional fusions of rrlA, rrlB or orf192.1 to the mcherry fluorescent marker at their respective native loci, which were designated as RrlA:Ch, RrlB:Ch and ORF192.1:Ch. As previously observed for other σ20-regulated genes,24 only a small subset of cells displayed mCherry fluorescence (Table 2 and Fig. 2). Remarkably, the percentage of fluorescent cells observed for each target protein was relatively diverse, which is likely due to a different strength of the transcriptional response plus specific differences in protein turnover.
Table 2.
Quantitative data of mCherry fluorescence
| Strain | % of fluorescent cells | Average fluorescence intensity per cell ± SD |
|---|---|---|
| MG427:Ch | 99.48 | 33.19 ± 17.81 |
| ORF192.1:Ch | 0.54 | 39.97 ± 25.34 |
| RrlA:Ch | 0.32 | 14.62 ± 10.46 |
| RrlA:Ch ΔMG_428 | 0 | – |
| RrlA:Ch ΔMG_428 Tnσ20 | 8.13 | 38.92 ± 41.64 |
| RrlB:Ch | 1.91 | 12.92 ± 9.24 |
| RrlB:Ch ΔMG_428 | 0 | – |
| RrlB:Ch ΔMG_428 Tnσ20 | 34.17 | 58.52 ± 44.22 |
| RecA:Ch | 0.66 | 19.97 ± 16.48 |
| RecA:Ch ΔrrlA | 0 | – |
| RecA:Ch ΔrrlB | 0 | – |
| RecA:Ch ΔMG_390 | 0.51 | 25.84 ± 21.81 |
| RecA:Ch ΔMG_414 | 0.58 | 22.80 ± 11.76 |
| RecA:Ch ΔrrlA TnrrlA | 0.22 | ND |
| RecA:Ch ΔrrlB TnrrlB | 0.24 | ND |
| RecA:Ch Tnσ20:YFP | 3.21 | 57.30 ± 62.76 |
| RecA:Ch Tnσ20:YFP ΔrrlA | 0 | – |
| RecA:Ch Tnσ20:YFP ΔrrlB | 0 | – |
| RecA:Ch Tnσ20:YFP TnrrlA | 2.60 | ND |
| RecA:Ch Tnσ20:YFP TnrrlB | 2.85 | ND |
| σ20:Ch | 0.46 | 6.50 ± 4.09 |
| σ20:Ch ΔrrlA | 0 | – |
| σ20:Ch ΔrrlB | 0 | – |
| σ20:Ch ΔrrlA TnrrlA | 0.13 | ND |
| σ20:Ch ΔrrlB TnrrlB | 0.20 | ND |
| σ20:Ch Tnσ20:YFP | 3.02 | 29.11 ± 16.42 |
SD: standard deviation; ND: not determined.
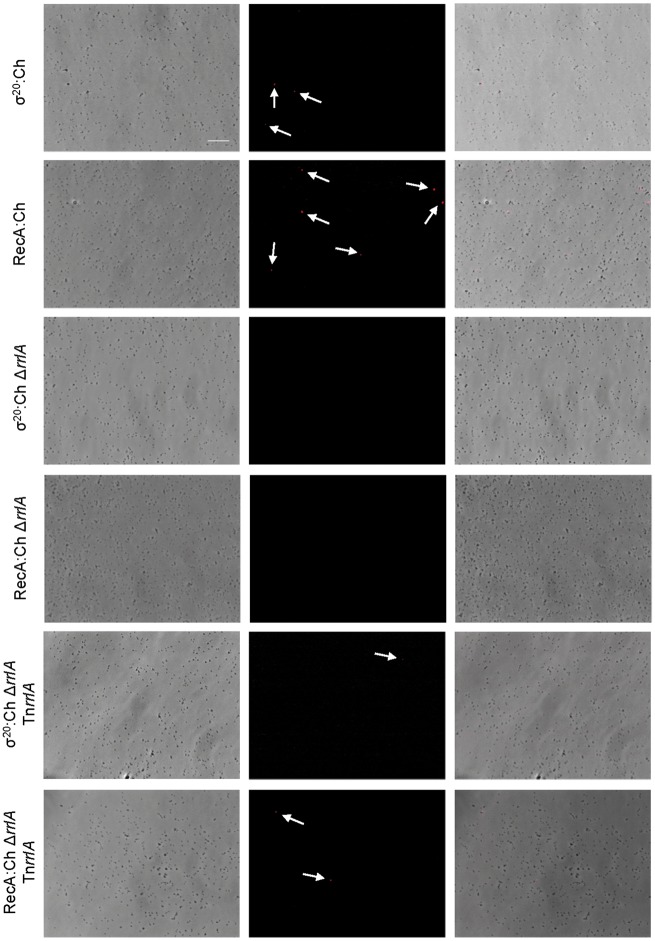
Figure 2.
Single cell analysis of RrlA, RrlB and ORF192.1 expression in different mutant backgrounds. Each row contains a series of three fluorescence microscopy images corresponding to the phase contrast, the TRITC channel and the resulting overlay of the different mutants analysed. White arrows indicate the presence of mCherry fluorescent cells in strains where fluorescence is rare. Scale bar is 10 µm.
In agreement with the σ20-controlled expression of rrlA and rrlB, we found that deletion of the MG_428 gene completely abrogated mCherry fluorescence in the population (Table 2, Fig. 2 and Supplementary Fig. S3). Next, we reintroduced the MG_428 gene by transposon delivery (Tnσ20) to the mcherry mutants lacking σ20 to assess complementation. Transcription of the transposon-encoded copy of the MG_428 gene was driven by the MG_427 promoter,24,33 which according to our transcriptional data is strong and not regulated by σ20. Expression of the ectopic copy of the MG_428 gene reestablished mCherry fluorescence to all mutants (Fig. 2 and Supplementary Fig. S3). In particular, the presence of cells with detectable levels of RrlA and RrlB increased by 25- and 18-fold, respectively, as compared with the parental strains where σ20 was expressed from its native locus (Table 2). Yet, the majority of cells from the complemented mutants were non-fluorescent. This was unexpected because transcriptional fusions of the MG_427 gene to the mcherry marker at its native locus indicate that the MG_427 promoter is constitutive (Supplementary Fig. S3). Altogether, these findings suggest that transcription of the target genes is still regulated in the complemented strains. Therefore, either the expression of rrlAB is under the control of additional factors beyond σ20 or the activity of σ20 is regulated at the post-transcriptional level in M. genitalium.
3.4. RrlA and RrlB are necessary for the activation of the σ20-regulon
Aiming to better understand the role of rrlA and rrlB in the recombination pathway, we deleted one of these genes from strains carrying MG_428- or recA-mcherry fusions at their respective native loci. In both mutant backgrounds, σ20:Ch and RecA:Ch, the absence of rrlA or rrlB completely abrogated the presence of fluorescent cells (Table 2, Fig. 3 and Supplementary Fig. S4). In contrast, deletion of other genes under the control of σ20 such as MG_390 or MG_414, had very little or no impact on RecA expression (Table 2). Reintroduction of the rrlA or rrlB genes by transposon delivery (TnrrlA or TnrrlB) reestablished the presence of fluorescent cells to their respective mutant backgrounds (Table 2, Fig. 3 and Supplementary Fig. S4). Hence, our data indicate an important role for RrlAB in the activation of σ20-dependent recombination.
Figure 3.
Single cell analysis of RecA and σ20 expression in different mutant backgrounds. Each row contains a series of three fluorescence microscopy images corresponding to the phase contrast, the TRITC channel and the resulting overlay of the different mutants analysed. White arrows indicate the presence of mCherry fluorescent cells in strains where fluorescence is rare. Scale bar is 10 µm in all images.
3.5. Positive autoregulation by σ20
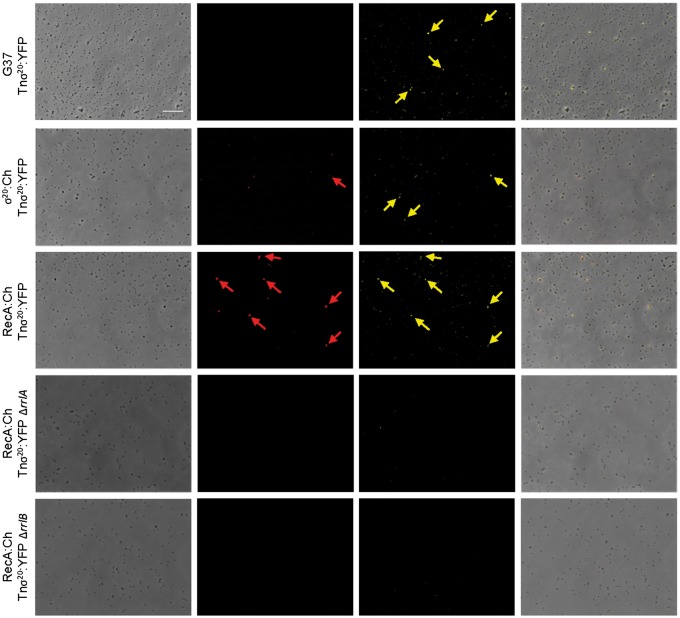
Next, we wondered whether σ20 expression was autoregulated. To ascertain this question, we delivered an ectopic copy of the MG_428 gene fused to the eyfp fluorescent marker under the control of a constitutive promoter of M. genitalium (Tn σ20:YFP) into the σ20:Ch and RecA:Ch mutants. In both cases, we observed a 6-fold increase in the percentage of cells displaying mCherry fluorescence (Table 2 and Fig. 4). In addition, the average mCherry fluorescence intensity of the population was incremented 3–6-fold by the presence of the Tnσ20:YFP transposon (Table 2). Altogether, these data demonstrate the existence of a positive feedback during the activation of the σ20 pathway.
Figure 4.
Single cell analysis of RecA and σ20 expression upon σ20 overexpression. Each row contains a series of four fluorescence microscopy images corresponding to the phase contrast, the TRITC channel, the eYFP channel and the resulting overlay of the different mutants analysed. Yellow arrows point to cells showing an intense YFP fluorescence. Red arrows point to mCherry fluorescent cells that show also intense YFP fluorescence. Scale bar is 10 µm in all images.
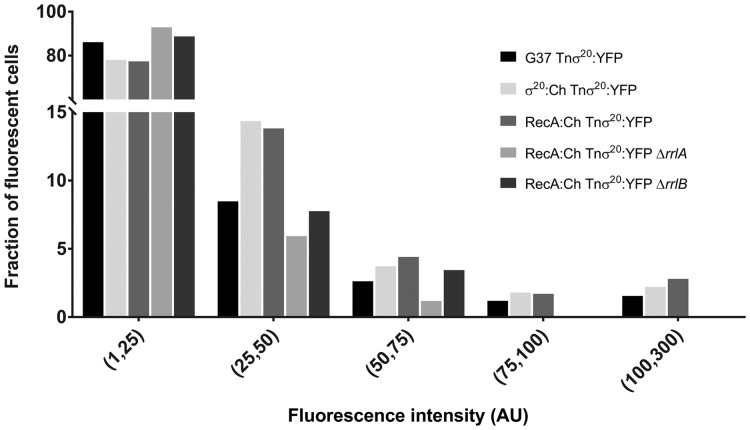
On the other hand, despite the majority of cells carrying the σ20: YFP fusion showed yellow fluorescence (74.9 ± 2.3%), cells without fluorescence were also present (Fig. 4). Moreover, YFP fluorescence intensity was exceptionally heterogeneous and we observed individual cells (∼2%) with an intensity 10–20-fold higher than the average of the population (Fig. 5). Of note, in the RecA-mCherry background, we observed a strong correlation between mCherry positive cells and cells with intense YFP fluorescence (Fig. 4), suggesting that σ20 is more stable coinciding with the activation of the σ20 regulon.
Figure 5.
Fluorescence intensity distribution in the population of different mutants carrying a σ20:YFP fusion. Distribution was obtained by analysing at least 150 YFP fluorescent cells from each strain. AU denotes arbitrary units.
3.6. Role of RrlA and RrlB in σ20 stability
To get further knowledge on the role of RrlAB in the activation of the σ20 regulon, we deleted the rrlA or rrlB genes from the RecA:Ch Tnσ20:YFP reporter strain. This strain was chosen because it allows the study of σ20 expression and activity at the same time. As described earlier, mCherry fluorescence was not observed when RrlA or RrlB were absent (Table 2 and Fig. 4). Concurrently, the presence of cells with YFP fluorescence was substantially reduced and cells with intense YFP fluorescence were no longer observed (Fig. 5). These data reinforce the role of RrlAB in σ20 activation and suggest that these two small proteins likely stabilize σ20. On the other hand, RrlA or RrlB overexpression using the TnrrlA and TnrrlB minitransposons did not modify the percentage of RecA-mCherry or σ20-YFP fluorescent cells (Table 2 and Supplementary Fig. S5). Remarkably, the majority of clones (∼70%) recovered from these transformation experiments showed a non-adherent phenotype, which is indicative of an increased frequency of generation of phase variants.
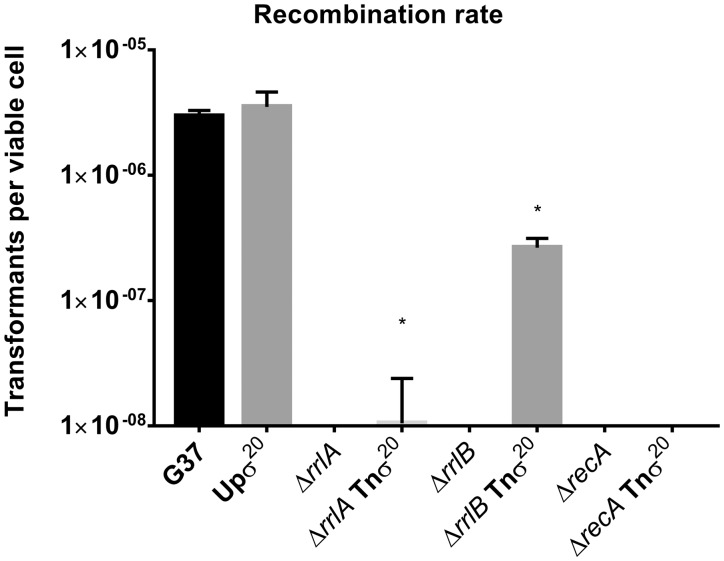
3.7. σ20 overexpression reestablishes recombination to rrlA- and rrlB- mutants
On the basis of our findings, the participation of RrlAB in the homologous recombination pathway is expected to be indirect. To support this notion, we overexpressed σ20 in mutants lacking RrlA, RrlB or RecA using the Tnσ20 minitransposon (Fig. 6). As expected, in agreement with the central role of RecA in the recombination pathway, σ20 overexpression did not restore recombination to recA mutants. In contrast, σ20 overexpression reestablished recombination to the rrlB mutant, though the recombination levels were moderate as compared with the wild-type strain. The recombination defects were still pronounced in the rrlA mutants overexpressing σ20, but unlike in the parental strain, some recombination events could be detected.
Figure 6.
Recombination capacity of different mutants. Graphic showing the recombination capacity of different M. genitalium mutants. Bars represent the averages and the standard deviations of three independent biological repeats. The recombination capacity of the ΔrrlA, ΔrrlB, ΔrecA and ΔrecA Tnσ20 mutants was below the limit of detection. Asterisks mark differences that are statistically significant compared with the WT. Statistical significance was assessed using a standard Student’s t test (p < 0.05).
3.8. σ20 overexpression facilitates HGT
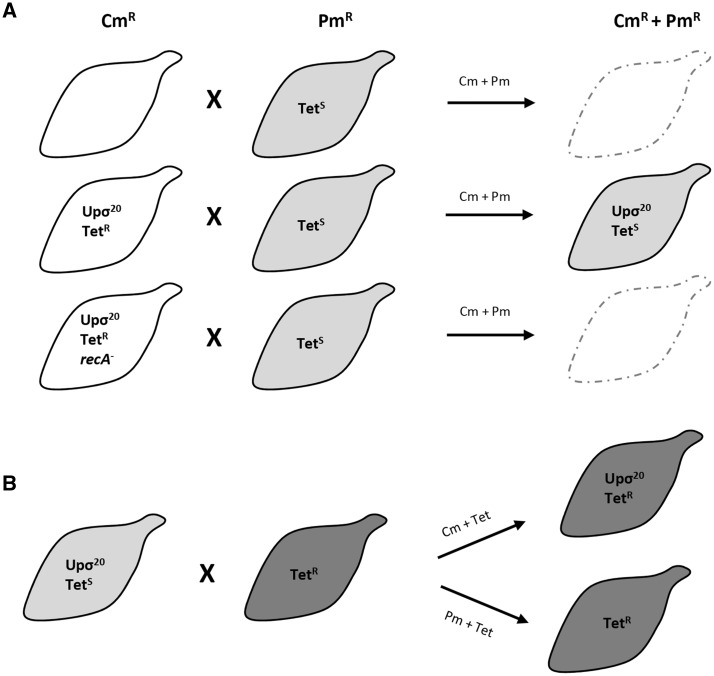
Previously, we described that cells with an active σ20 pathway were often observed in pairs in single cell analyses.24 On the basis of this observation, we wondered whether the activation of σ20-recombination could be associated to a mechanism of HGT. To ascertain this question, we mixed strains carrying two different antibiotic resistance markers in the presence of high concentrations of DNase and assessed the generation of double resistant mutants. In the first attempts, we mixed strains where σ20 expression was driven from its natural locus. We used combinations of different antibiotic resistance markers and strains, but we repeatedly failed to isolate double resistant mutants. Then, we initiated experiments using strains over-expressing σ20 by means of the Tnσ20 minitransposon (Fig. 7). This time, we successfully isolated mutants resistant to Cm and Pm upon incubation of a strain carrying the cat and tetM markers, and a strain carrying the pac gene. The calculated mating efficiency was 1.1 ± 0.2 × 10−8 transconjugants per viable cell, which is in agreement with the transfer efficiencies reported in other mollicutes.34,35 The presence of the cat and pac markers in the isolated mutants was confirmed by PCR and sequencing (Supplementary Fig. S6). Of note, we did not detect the presence of the tetM marker in the transconjugant cells, suggesting the transfer of the cat gene from the strain overexpressing σ20 (donor) to the Pm resistant strain (recipient). The location of the cat gene in the recipient strain was the same as in the donor strain, suggesting that the cat marker was integrated into the chromosome by homologous recombination. Similarly, we also observed the mobilization of the pac gene from a donor strain overexpressing σ20 to a recipient strain carrying the cat marker (data not shown). In addition, we also found that recipient strains receiving the ectopic MG_428 gene, responsible for σ20 overexpression, become donor strains capable to transfer selectable markers (Fig. 7B).
Figure 7.
Schematic representation of representative mating experiments. Qualitative assessment of recovery of double resistant mutants in mating experiments using M. genitalium strains carrying different antibiotic gene markers. (A) Mating experiments using donor strains (depicted as white mycoplasma cells) carrying the chloramphenicol (CmR) and tetracycline (TetR) resistance markers and recipient strains (depicted as light gray mycoplasma cells) carrying the puromycin (PmR) resistance marker. Donor strains overexpressing σ20 are indicated as ‘Upσ20.’ One of the donor strains used, carried a deletion of the recA gene (recA-). Unsuccessful DNA transfers are indicated as empty, dash-lined mycoplasma cells. (B) Mating experiments using a previous transconjugant mutant carrying the chloramphenicol and puromycin resistance markers as a donor strain (depicted as a light gray mycoplasma cell) and a recipient strain (depicted as a dark gray mycoplasma cell) carrying the tetracycline resistance marker. Selection with tetracycline and chloramphenicol results in the isolation of a new transconjugant strain bearing the corresponding antibiotic markers and overexpressing σ20. Selection with puromycin and tetracycline allows the isolation of another transconjugant strain with the corresponding antibiotic markers but without the ectopic copy of σ20. All the transconjugant strains were genotyped by PCR and sequencing.
On the other hand, we found that the absence of RecA or the expression of a truncated σ20 protein in the donor strain, prevented the isolation of transconjugant strains (Fig. 7A). Similarly, heat inactivation of the donor strains prevented the isolation of double resistant mutants. Moreover, we could not isolate double resistant mutants upon incubation of the recipient strains with chromosomal DNA from potential donors or plasmid DNA carrying different antibiotic markers. Altogether, these data indicate that activation of σ20 facilitates cell-to-cell transfer of DNA sequences by homologous recombination by an uncharacterized mechanism of HGT of M. genitalium.
4. Discussion
In M. genitalium, homologous recombination is controlled by the alternative sigma factor σ20.24 Under laboratory growth conditions, σ20 activity is only observed in a small subset of cells and so far, it is unknown whether this intermittent activation is elicited by means or transcriptional or post-transcriptional factors. Herein, we assessed the expression in single cells of a σ20-YFP fusion protein under the control of a constitutive, endogenous promoter of M. genitalium. We found that σ20 levels were highly heterogeneous in the population and σ20 expression could not be detected in numerous cells. Although we cannot rule out the existence of transcriptional factors controlling σ20-activation, our results demonstrate a conspicuous post-transcriptional regulation of σ20 function. On the other hand, we found a direct correlation between the presence of rrlAB, two genes subject to σ20-regulation, and σ20 activity. Moreover, σ20 expression was negligible when RrlAB were absent, indicating that these small proteins act synergistically to stabilize and therefore prolong the activity of σ20 in M. genitalium. In keeping with this idea, we demonstrate that σ20 positively autoregulates its own activity, which reinforces the participation of RrlAB in a positive feed-back loop enabling σ20-activation.
Sigma factors direct the activity of the RNA polymerase complex to specific promoter sequences. When a bacterium expresses simultaneously more than one sigma factor, these transcription factors compete to bind to the RNA polymerase enzyme. Bacteria use diverse strategies to ensure the concurrent action of different sigma factors and to enforce transcription transition from the primary to alternative sigma factors.36 On the basis of this notion, we hypothesize that RrlAB could be important to redirect transcription from σ70 to σ20-dependent promoters in M. genitalium. Specifically, these two small proteins could facilitate binding of σ20 to the RNA polymerase complex, which in turn could prevent the rapid degradation of the otherwise free σ20 particles. The proposed role for RrlAB as sigma auxiliary proteins that assist σ20 in the activation of the recombination regulon is not unprecedented. Accordingly, the positive effect of RrlAB on σ20 function resembles the activity of the curli fimbriae formation regulator Crl of E. coli and Salmonella typhimurium.37,38 Crl is a small protein that binds to the alternative sigma factor σS and enhances the formation of a σS-RNA polymerase complex. This step is critical for σS activity as it aids overcoming the low affinity of the alternative sigma factor for the RNA polymerase core enzyme as compared with the primary sigma particle. Alternatively, the activity of the RrlAB could be related to anti-sigma factors, which control the availability of alternative sigma factors. In this case, RrlAB could hamper de formation of a σ70-RNA polymerase complex, facilitating the activity of σ20. Neither RrlA nor RrlB shows sequence homology to known anti-sigma factors, but these regulatory proteins usually exhibit low sequence conservation.39
In this study, we demonstrate that strains overexpressing σ20 can act as donor cells for HGT. In mycoplasmas, HGT has only been described in some ruminant species that encode integrative conjugative elements (ICE) or ICE-like sequences in their chromosomes.40,41 Recent studies have documented the transfer of large chromosomal regions between different mycoplasma strains by homologous recombination.35 A similar mechanism was described years ago in Spiroplasma citri, a species phylogenetically related to the mycoplasmas. Barroso and coworkers reported the recombination-dependent chromosomal transfer of genetic traits conferring antibiotic resistance between spiroplasma cells.34 Remarkably, these DNA transfer events were independent of known mobile genetic elements and relied on stable cell membrane fusions. Genetic fluxes in wall-less bacteria are not extraordinary, as similar chimeric genomes obtained by membrane fusions have been observed in L-forms of different bacteria.42 As M. genitalium seems to be devoid of ICE or ICE-like elements, gene transfer could be catalyzed by intrinsic non-mobilizable factors encoded in the chromosome. At the present time, it is unknown whether genes unrelated to recombination under the control of σ20 are involved in HGT. However, several σ20-regulated genes code for proteins with putative transmembrane domains that could be implicated in the establishment of cell-to-cell contacts and ultimately facilitate DNA exchange between cells. The ability to exchange DNA through inter- and intra-chromosomal recombination increases the potential to generate genetic diversity and the versatility of the M. genitalium chromosome.
It is expected the σ20 recombination pathway will be triggered by factors positioned hierarchically upstream from RrlAB in the cascade of σ20-activation. Remarkably, we have strong evidence that σ20 activation is inhibited or severely impaired in non-adherent strains. Indeed, transcriptional analysis of a MG_191 mutant reveals that the ncRNA-3/4 is downregulated 13-fold as compared with the wild-type strain (Fig. 1A), which denotes a decreased σ20 activity. Of note, the main cytadhesins of M. genitalium are reciprocally stabilized and neither P140 nor P110 is expressed in the MG_191 mutant.19 As the biological role of σ20 seems intimately related to the generation of antigenic variants, it is reasonable to think that σ20 activity will be less relevant in strains lacking these two immunodominant proteins. Of note, non-adherent mutants of M. genitalium arise spontaneously at relatively high-rates by means of an exquisite PhV mechanism.19,43 For this reason, it is thought that non-adherent cells play an important role in infection and survival of M. genitalium within the host. The inhibition of the σ20 system in non-adherent mutants could represent an attempt to arrest this phenotype, which can often be reversed by homologous recombination. Whether P140 or other adherence-related factors are directly involved in the activation of σ20, remains to be investigated. On the other hand, the prominent upregulation of the ncRNA-3/4 transcript as well as other non-coding RNAs by σ20 is puzzling. In the case of the ncRNA-1, which carries homologous regions to the cytadhesins genes in the antisense orientation, we hypothesize that the activation of this non-coding RNA could interfere with the translation of P140 and P110. Silencing of the main cytadhesins could hinder the recognition of M. genitalium cells by the host immune system during a coordinated response to boost the generation of antigenic variants.
Overall, in this work we have identified two factors that regulate σ20 activity and are key to generate AnV catalyzed by this alternative sigma factor. Moreover, the discovery of a form of HGT in M. genitalium opens a new avenue in the understanding of dissemination of effective antigenic variants and provides some important clues as to the rapid emergence of antibiotic resistance in urogenital pathogens.
Supplementary Material
Acknowledgements
We are grateful to the staff of the Servei de Genòmica I Bioinformàtica (UAB) and the Genomics Unit (CRG) for performing Sanger sequencing and RNA-Seq analysis, respectively. We are also very grateful to Dr Eva Yus (CRG) for her valuable advice on performing RNA preparation and RNA-Seq analysis. ST and CM want to acknowledge a PIF fellowship from the Universitat Autònoma de Barcelona. This work was presented in part at the first Microbe Meeting of the American Society for Microbiology held in Boston, Massachusetts, in June 2016.
Conflict of interest
None declared.
Funding
This work was supported by Grants BIO2013-48704-R and BFU2013-50176-EXP from the Ministerio de Economía y Competitividad.
References
- 1. Finlay B.B., McFadden G.. 2006, Anti-immunology: evasion of the host immune system by bacterial and viral pathogens, Cell, 124, 767–82. [DOI] [PubMed] [Google Scholar]
- 2. Van Der Woude M.W., Bäumler A.J.. 2004, Phase and antigenic variation in bacteria, Clin. Microbiol. Rev., 17, 581–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vink C., Rudenko G., Seifert H.S.. 2012, Microbial antigenic variation mediated by homologous DNA recombination, FEMS Microbiol. Rev., 36(5), 917–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koomey M., Gotschlich E.C., Robbins K., Bergström S., Swanson J.. 1987, Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae, Genetics, 117, 391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehr I.J., Seifert H.S.. 1998, Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair, Mol. Microbiol., 30, 697–710. [DOI] [PubMed] [Google Scholar]
- 6. Centurion-Lara A., LaFond R.E., Hevner K., et al. 2004, Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection, Mol. Microbiol., 52, 1579–96. [DOI] [PubMed] [Google Scholar]
- 7. Burgos R., Wood G.E., Young L., Glass J.I., Totten P.A.. 2012, RecA mediates MgpB and MgpC phase and antigenic variation in Mycoplasma genitalium, but plays a minor role in DNA repair, Mol. Microbiol., 85, 669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burgos R., Totten P.A.. 2014, Characterization of the operon encoding the holliday junction helicase RuvAB from Mycoplasma genitalium and its role in mgpB and mgpC gene variation, J. Bacteriol., 196, 1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stohl E.A., Seifert H.S.. 2001, The recX gene potentiates homologous recombination in Neisseria gonorrhoeae, Mol. Microbiol., 40, 1301–10. [DOI] [PubMed] [Google Scholar]
- 10. Skaar E.P., Lazio M.P., Seifert H.S.. 2002, Roles of the recJ and recN genes in homologous recombination and DNA repair pathways of Neisseria gonorrhoeae, J. Bacteriol., 184, 919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Black C.G., Fyfe J.A.M., Davies J.K.. 1998, Absence of an SOS-like system in Neisseria gonorrhoeae, Gene, 208, 61–6. [DOI] [PubMed] [Google Scholar]
- 12. Fraser C.M., Gocayne J.D., White O., et al. 1995, The minimal gene complement of Mycoplasma genitalium, Science, 270, 397–403. [DOI] [PubMed] [Google Scholar]
- 13. Fraser C.M. 1998, Complete genome sequence of Treponema pallidum, the syphilis spirochete, Science, 281, 375–88. [DOI] [PubMed] [Google Scholar]
- 14. Schook P.O.P., Stohl E.A., Criss A.K., Seifert H.S.. 2011, The DNA-binding activity of the Neisseria gonorrhoeae LexA orthologue NG1427 is modulated by oxidation, Mol. Microbiol., 79, 846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruenig M.C., Stohl E.A., Chitteni-Pattu S., Seifert H.S., Cox M.M.. 2010, Less is more: neisseria gonorrhoeae RecX protein stimulates recombination by inhibiting RecA, J. Biol. Chem., 285, 37188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peterson S.N., Bailey C.C., Jensen J.S., et al. 1995, Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation, Proc. Natl. Acad. Sci. U. S. A., 92, 11829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iverson-Cabral S.L., Astete S.G., Cohen C.R., Totten P.A.. 2007, mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences, Mol. Microbiol., 66, 55–73. [DOI] [PubMed] [Google Scholar]
- 18. Ma L., Jensen J.S., Myers L., et al. 2007, Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome, Mol. Microbiol., 66, 220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burgos R., Pich O.Q., Ferrer-Navarro M., Baseman J.B., Querol E., Pinol J.. 2006, Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development, J. Bacteriol., 188, 8627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wood G.E., Iverson-Cabral S.L., Patton D.L., Cummings P.K., Cosgrove Sweeney Y.T., Totten P.A.. 2013, Persistence, immune response, and antigenic variation of Mycoplasma genitalium in an experimentally infected pig-tailed macaque (Macaca nemestrina), Infect. Immun., 81, 2938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wood G.E., Patton D.L., Cummings P.K., Iverson-Cabral S.L., Totten P.A.. 2017, Experimental infection of pig-tailed macaques (Macaca nemestrina) with Mycoplasma genitalium, Infect. Immun., 85, e00738–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma L., Mancuso M., Williams J.A., et al. 2014, Extensive variation and rapid shift of the MG192 Sequence in Mycoplasma genitalium strains from patients with chronic infection, Infect. Immun., 82, 1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma L., Jensen J.S., Mancuso M., Myers L., Martin D.H.. 2016, Kinetics of genetic variation of the Mycoplasma genitalium MG192 gene in experimentally infected chimpanzees, Infect. Immun., 84, 747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torres-Puig S., Broto A., Querol E., Piñol J., Pich O.Q.. 2015, A novel sigma factor reveals a unique regulon controlling cell-specific recombination in Mycoplasma genitalium, Nucleic Acids Res., 43, 4923–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burgos R., Totten P.A.. 2014, MG428 is a novel positive regulator of recombination that triggers mgpB and mgpC gene variation in Mycoplasma genitalium, Mol. Microbiol., 94, 290–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sambrook J., Fritsch E.F., Maniatis T.. 1989, Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press: New York. [Google Scholar]
- 27. Langmead B., Salzberg S.L.. 2012, Fast gapped-read alignment with Bowtie 2, Nat. Methods, 9, 357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H., Handsaker B., Wysoker A., et al. 2009, The sequence alignment/map format and SAMtools, Bioinformatics, 25, 2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao Y., Smyth G.K., Shi W.. 2014, featureCounts: an efficient general purpose program for assigning sequence reads to genomic features, Bioinformatics, 30, 923–30. [DOI] [PubMed] [Google Scholar]
- 30. Anders S., Huber W.. 2010, Differential expression analysis for sequence count data, Genome Biol., 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Love M.I., Huber W., Anders S.. 2014, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2, Genome Biol., 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dillies M.-A., Rau A., Aubert J., et al. 2013, A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis, Brief. Bioinform., 14, 671–83. [DOI] [PubMed] [Google Scholar]
- 33. Zhang W., Baseman J.B.. 2014, Functional characterization of osmotically inducible protein C (MG_427) from Mycoplasma genitalium, J. Bacteriol., 196, 1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barroso G., Labarere J.. 1988, Chromosomal gene transfer in Spiroplasma citri, Science, 241, 959–61. [DOI] [PubMed] [Google Scholar]
- 35. Dordet-Frisoni E., Sagné E., Baranowski E., et al. 2014, Chromosomal transfers in mycoplasmas: when minimal genomes go mobile, mBio, 5, e01958–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Österberg S., Peso-Santos T.D., Shingler V.. 2011, Regulation of alternative sigma factor use, Annu. Rev. Microbiol., 65, 37–55. [DOI] [PubMed] [Google Scholar]
- 37. Gaal T., Mandel M.J., Silhavy T.J., Gourse R.L.. 2006, Crl facilitates RNA polymerase holoenzyme formation, J. Bacteriol., 188, 7966–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Typas A., Barembruch C., Possling A., Hengge R.. 2007, Stationary phase reorganisation of the Escherichia coli transcription machinery by Crl protein, a fine-tuner of σS activity and levels, EMBO J., 26, 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paget M.S. 2015, Bacterial sigma factors and anti-sigma factors: structure, function and distribution, Biomolecules, 5, 1245–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tardy F., Mick V., Dordet-Frisoni E., et al. 2015, Integrative conjugative elements are widespread in field isolates of Mycoplasma Species pathogenic for ruminants, Appl. Environ. Microbiol., 81, 1634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dordet Frisoni E., Marenda M.S., Sagné E., et al. 2013, ICEA of Mycoplasma agalactiae: a new family of self-transmissible integrative elements that confers conjugative properties to the recipient strain, Mol. Microbiol., 89, 1226–39. [DOI] [PubMed] [Google Scholar]
- 42. Errington J. 2013, L-form bacteria, cell walls and the origins of life, Open Biol., 3, 120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mernaugh G.R., Dallo S.F., Holt S.C., Baseman J.B.. 1993, Properties of adhering and nonadhering populations of Mycoplasma genitalium, Clin. Infect. Dis., S1, S69–78. [DOI] [PubMed] [Google Scholar]
- 44. Galperin M.Y., Makarova K.S., Wolf Y.I., Koonin E.V.. 2015, Expanded Microbial genome coverage and improved protein family annotation in the COG database, Nucleic Acids Res., 43, D261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.