Abstract
Objective
To examine associations of orthostatic hypotension (OH) with dementia and long-term cognitive decline and to update previously published results in the same cohort for stroke with an additional 16 years of follow-up.
Methods
We analyzed data from 11,709 participants without a history of coronary heart disease or stroke who attended the baseline examination (1987–1989) of the prospective Atherosclerosis Risk in Communities (ARIC) study. OH was defined as a drop in systolic blood pressure (BP) of at least 20 mm Hg or a drop in diastolic BP of at least 10 mm Hg on standing. Dementia was ascertained via examination, contact with participants or their proxy, or medical record surveillance. Ischemic stroke was ascertained via cohort surveillance of hospitalizations, cohort follow-up, and linkage with registries. Both outcomes were adjudicated. Cognitive function was ascertained via 3 neuropsychological tests administered in 1990 to 1992 and 1996 to 1998 and a full battery of tests in 2011 to 2013. Scores were summarized and reported as SDs. We used adjusted Cox regression and linear mixed models.
Results
Over ≈25 years, 1,068 participants developed dementia and 842 had an ischemic stroke. Compared to persons without OH at baseline, those with OH had a higher risk of dementia (hazard ratio [HR] 1.54, 95% confidence interval [CI] 1.20–1.97) and ischemic stroke (HR 2.08, 95% CI 1.65–2.62). Persons with OH had greater, although nonsignificant, cognitive decline over 20 years (SD 0.09, 95% CI −0.02 to 0.21).
Conclusions
OH assessed in midlife was independently associated with incident dementia and ischemic stroke. Additional studies are needed to elucidate potential mechanisms for these associations and possible applications for prevention.
An estimated 14% of older adults in the United States have dementia,1 and another 22% have cognitive impairment without dementia.2 Cardiovascular disease and its risk factors, including hypertension, have consistently been associated with cognitive decline and dementia, and evidence is rapidly growing regarding the complex association between cardiovascular and cerebrovascular disease.3–5 Orthostatic hypotension (OH), defined as a drop in systolic (SBP) or diastolic blood pressure (DBP) after a postural change,6 is associated with incident cardiovascular disease, all-cause mortality, falls, and syncope.7–11
Repeated drops in BP may result in cerebral hypoperfusion, often manifesting as dizziness, fatigue, and weakness on standing.12 In the few studies that have examined associations of OH with cognitive decline and dementia, the results have been mixed, potentially because of small sample sizes, limited follow-up, or the age at which OH was ascertained.13–16
Our aim is to characterize the association of OH and postural change in BP measured in midlife with incident dementia and 20-year cognitive decline. We also update previously published associations of incident ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study10 with an additional 16 years of follow-up.
Methods
Study population
The ARIC study is a community-based, prospective cohort of middle-aged adults. Adults were recruited from the following 4 US communities with probability sampling: suburbs of Minneapolis, MN; Washington County, Maryland; Forsyth County, North Carolina; and Jackson, MS. The first study visit was 1987 to 1989, with subsequent visits in 1990 to 1992 (visit 2), 1993 to 1995 (visit 3), 1996 to 1998 (visit 4), and 2011 to 2013 (visit 5). Institutional review boards at each study site approved the study, and all participants gave written informed consent.
Postural change in BP was assessed only at visit 1, which is the baseline for the present study. For analyses of incident dementia and stroke, we excluded participants with a history of coronary heart disease or stroke (n = 1,039), those without supine and standing BP measurements (n = 2,385, of whom ≈73% underwent their baseline examination before the protocol for postural BP change was initiated), those with self-reported Parkinson disease (n = 37), participants who identified as neither black nor white and nonwhites in Washington and Minneapolis (n = 41), and those missing covariates (n = 581), for an analytic sample size of 11,709. For analyses of dementia, we excluded 1 person without follow-up information. Persons missing covariates were not appreciably different from those in the analytic sample (data available from Dryad, table 1, doi.org/10.5061/dryad.p7q8t15). For analyses of cognitive decline (cognitive function was first measured at visit 2), we additionally excluded persons with no measurement of cognitive function at visit 2 (n = 1,059), giving an analytic sample size of 10,650.
Ascertainment of exposure: OH
Supine and standing BPs were measured with a Dinamap-SX oscillometric machine (GE Healthcare, Chicago, IL) immediately after an ultrasound examination procedure. After the participant was lying in the supine position for at least 20 minutes, a technician triggered the device to collect BP measurements automatically for 2 minutes, during which up to 5 readings were collected. Participants who reported a history of falls, dizziness, or syncope before beginning the ultrasound examination were instructed to rest at the edge of the examination table for 15 seconds before standing. Otherwise, participants were instructed to stand quickly, safely, and in 1 smooth motion. Once both feet were planted on the floor, a technician triggered the oscillometric device to automatically collect BP measurements for 2 minutes, during which time up to 5 readings were again collected. Participants who became dizzy while standing were instructed to lean back against the examination table.
Up to 5 measurements of supine BP were used to calculate mean supine SBP and DBP. Per common practice, we excluded the first standing BP measurement and averaged up to 4 measurements to calculate mean standing SBP and DBP. OH was defined as a drop in mean SBP from supine to standing of at least 20 mm Hg or a drop in mean DBP from supine to standing of at least 10 mm Hg.6 Prior analyses of OH data in ARIC17 suggested that the first measurement of BP on standing may be most predictive of certain adverse events. Therefore, as a sensitivity analysis, we also defined OH using the difference between mean supine BP and the first standing BP measurement only.
Ascertainment of outcomes: Dementia, ischemic stroke, and cognitive function
Dementia cases were ascertained from information from study visits, including cognitive testing and interviews with participants or their proxies at visit 5, with expert classification and adjudication of cognitive status. For persons who did not attend visit 5, additional dementia cases were identified via surveillance of hospitalizations and death certificates, including ICD-9 codes for dementia, along with telephone interviews with informants of suspected cases or telephone-based cognitive evaluations for some participants not seen at visit 5.18
Ischemic stroke was ascertained via active surveillance of hospitalizations, cohort follow-up, and linkage with registries through December 31, 2014. Trained personnel abstracted hospital records, and events were adjudicated by committee review.19,20 For our study, we included definite and probable ischemic stroke events. In secondary analyses, we examined ischemic stroke subtype (thrombotic [lacunar, secondary to carotid stenosis, or nonlacunar but not due to carotid disease] or cardioembolic), hemorrhagic stroke, and all definite/probable strokes combined.
Cognitive testing was performed at visits 2, 4, and 5 with the Delayed Word Recall,21 Digit Symbol Substitution of the Wechsler Adult Intelligence Scale–Revised,22 and Word Fluency Test.23 Visit 5 also included additional neuropsychological tests in the memory, executive function, and language domains. To account for differences in testing across time and for maximum use of all the cognitive tests available at visit 5, we used a factor score representing general cognitive performance, which was derived in ARIC with latent variable methods, as recently described.24
Covariates
The following variables were included as covariates in fully adjusted models based on a priori knowledge: age; sex; race-center (categorized into 5 groups as white adults from Minneapolis, Washington County, and Forsyth County or black adults from Forsyth County or Jackson); education (less than high school education; high school graduate, high school equivalency, or vocational school; or college or above); drinking status (current; former; never); cigarette smoking (current; former; never); diabetes mellitus (categorized as yes or no on the basis of self-reported diagnosis or medication use); glucose; hypertension (categorized into hypertension [SBP ≥140 mm Hg, DBP ≥90 mm Hg, or BP-lowering medication], prehypertension [SBP 120–139 mm Hg or DBP of 80–89 mm Hg], or no hypertension); body mass index; APOE genotype, number of ε4 alleles (0 or ≥1 alleles); total cholesterol; and high-density lipoprotein (HDL) cholesterol.
For analyses of stroke and dementia for which the baseline was visit 1, we used variables measured at visit 1. For analyses of cognitive decline, we used variables measured at visit 2 with the exception of education, which was collected at visit 1.
Statistical analyses
We compared baseline characteristics by OH status using means (SD) and proportions. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between baseline OH and incident stroke and dementia. We used the Efron method to handle tied failure times and verified the proportional hazards assumption using log-log plots. We present results unadjusted and adjusted with 2 nested models, using confounders selected on the basis of prior information as described above: model 1 was adjusted for demographic variables, including age, race-center, sex, and education; model 2 was the fully adjusted model and adjusted for the variables in model 1 plus lifestyle and cardiovascular risk factors, including cigarette smoking status, drinking status, body mass index, diabetes mellitus, glucose, hypertension, presence of APOE ε4 allele, total cholesterol, and HDL cholesterol. For analyses of incident dementia, we examined the effect of stroke on dementia by censoring follow-up for dementia at the time of the stroke; this should remove any effect that stroke may have on the likelihood of developing dementia. Lastly, we examined postural change in SBP and DBP continuously using restricted cubic splines with knots at the 10th, 50th, and 90th percentiles25; 3 knots fit the data well, and likelihood ratio tests indicated that splines with up to 7 knots were not significantly different from models with 3 knots (data available from Dryad, figure 1, doi.org/10.5061/dryad.p7q8t15).
In sensitivity analyses, we additionally adjusted for seated SBP and DBP, BP-lowering medication use, and their interaction. We also explored the potential influence of kidney function by adjusting for estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.26 We also performed analyses excluding persons with low seated SBP and DBP and, separately, persons with drops greater than the 5th percentile in either SBP (>30 mm Hg) or DBP (>17 mm Hg).
For analysis of cognitive decline, we used linear mixed-effects models. The time axis was time since baseline and was modeled with a linear spline with a knot at the median time between visits 2 and 4 (6 years). Models included a random intercept and random slopes for each time spline term and assumed that the random effects were independent. This model structure was chosen for consistency with prior ARIC studies and based on the likelihood that indicated the model including the random slopes was preferred. To account for participant dropout across visits (data available from Dryad, table 2, doi.org/10.5061/dryad.p7q8t15), we used multiple imputation by chained equations to impute cognitive scores of persons who did not attend study visits. These methods, as previously described,27 are expected to reduce potential attrition bias. Briefly, to impute cognitive scores, we used information collected on participants during study visits, from telephone calls with participants or their proxy, and from cohort surveillance of hospitalizations. We imputed scores for participants at the median visit date if they were alive but did not attend the study visit. We calculated 20 imputations. Because participants may have died after attending visits and contributing cognitive data, the estimand from these models can be interpreted as the average 20-year decline had participants remained alive and under observation for the duration of the study. As a sensitivity analysis, we also estimated the average 20-year decline while participants were alive and under observation. This was achieved by using linear mixed models fit with generalized estimating equations, assuming an independence correlation structure, which removes the implicit imputation of cognitive trajectories past death. We used robust variance estimation to account for the misspecified correlation structure of independent observations. The estimand from these models can be interpreted as the average 20-year decline while participants remained alive and under observation. We also examined models in which we imputed scores for participants who died; these scores were imputed 6 months before death and incorporated death certificate information on dementia status if available.
We also conducted analyses stratified by hypertension status, diabetes status, race, and APOE ε4. All analyses were done with Stata/SE 14.2 (StataCorp, College Station, TX). Values of p < 0.05 were considered statistically significant.
Data availability
The full data are not available publically because of participant privacy and consent. A public-use ARIC dataset is available through BioLINCC, and more information regarding the data is available from the corresponding author on reasonable request.
Results
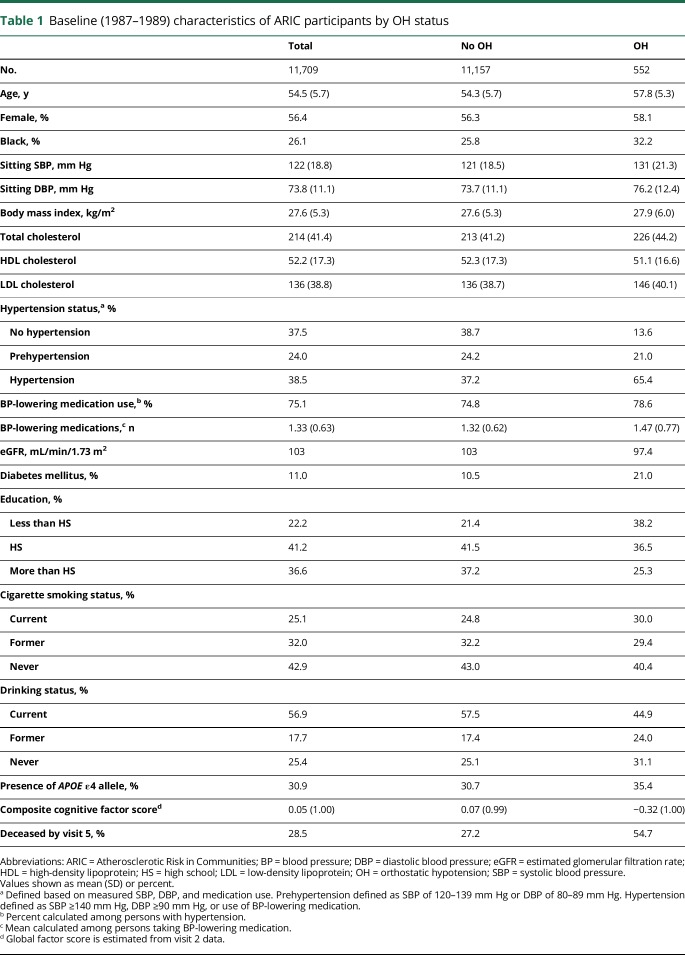
At baseline, 552 (4.7%) participants had OH, the mean age of participants was 54 years, 56% were female, and 26% were black (table 1). Persons with OH tended to be older (58 vs 54 years), were more likely to be black (32% vs 26%), had higher sitting SBP (131 vs 121 mm Hg) and DBP (76 vs 74 mm Hg), and were more likely to have hypertension (65% vs 37%) (table 1). Lastly, persons with OH at baseline had lower cognitive scores at visit 2 (composite score −0.32 compared to 0.07) and were much more likely to die by visit 5 (55% vs 27%).
Table 1.
Baseline (1987–1989) characteristics of ARIC participants by OH status
During a median follow-up of 24 years, 1,068 participants developed dementia. In fully adjusted models, persons with OH were 1.54 times more likely to develop dementia compared to persons without OH (95% CI 1.20–1.97) (table 2). Over a median follow-up of 25 years, 842 participants had an ischemic stroke. In the fully adjusted model, persons with OH had 2.08 times higher risk of an ischemic stroke compared to persons without OH (95% CI 1.65–2.62) (table 2). Accounting for ischemic stroke in the association between OH and dementia explained a small part of the excess risk of dementia in persons with OH (table 2). Associations were similar across stroke subtypes, with indication of stronger associations for thrombotic, nonlacunar stroke due to carotid disease (HR 2.80, 95% CI 1.16–6.76) and weaker associations for lacunar subtype (HR 1.41, 95% CI 0.78–2.55), although sample sizes in subgroups were small (data available from Dryad, table 3, doi.org/10.5061/dryad.p7q8t15). We found no association of OH with hemorrhagic stroke (HR 1.10, 95% CI 0.44–2.74) (data available from Dryad, table 3, doi.org/10.5061/dryad.p7q8t15).
Table 2.
HR (95% CI) for OH with incident dementia and incident ischemic stroke
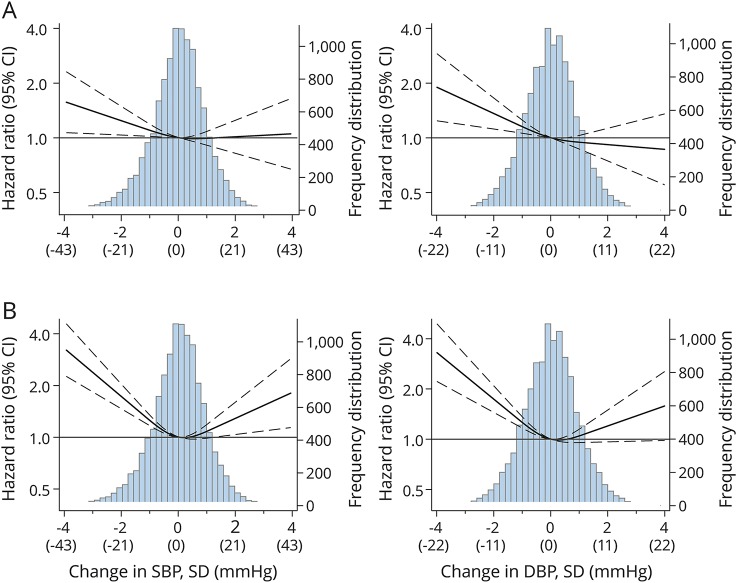
When they were modeled continuously using restricted cubic splines, we observed a nearly linear association between a drop in SBP and DBP from supine to standing and higher risk of dementia (figure 1A) and ischemic stroke (figure 1B). There were no statistically significant interactions between OH and hypertension, diabetes mellitus, race, or APOE ε4 for either ischemic stroke or dementia (figure 2). However, the point estimates indicated that OH was associated with higher risk of stroke in black persons (HR 2.63) compared to white participants (HR 1.69, p for interaction = 0.083) and with higher risk of dementia among persons with hypertension (HR 1.86) compared to those with prehypertension (HR 0.95) or no hypertension (HR 1.12, p for interaction = 0.076) and in persons with diabetes mellitus (HR 2.32) compared to those without (HR 1.37, p for interaction = 0.064) (figure 2). Results were not appreciably different with additional adjustment for seated BP or kidney function, with exclusion of persons with large drops in BP or exclusion of persons with low seated or standing BP (data available from Dryad, table 4, doi.org/10.5061/dryad.p7q8t15).
Figure 1. HR (95% CI) for Incident ischemic stroke and incident dementia by change in SBP and DBP from supine to standing.
(A) Incident dementia. (B) Incident stroke. Hazard ratios (HRs) (95% confidence intervals [CIs]) are from Cox proportional hazards regression with adjustment for age, race, sex, education, smoking status (current, former, never), drinking status (current, former, never), body mass index, diabetes mellitus (yes/no), glucose, APOE ε4 (presence of ε4 allele, 0 or ≥1 alleles), high-density lipoprotein cholesterol, and total cholesterol. Change in systolic (SBP) and diastolic (DBP) blood pressure from supine to standing was modeled with restricted cubic splines with knots at the 10th, 50th, and 90th percentiles. These correspond to z scores of −1.225, −0.05, and 1.17. All graphs truncated at ±4 SDs for SBP and DBP. Change in BP is calculated as mean standing BP (excluding the first standing measurement) minus mean supine BP.
Figure 2. HRs (95% CIs) for (A) incident dementia and (B) incident ischemic stroke in persons with OH compared to those without, stratified by hypertension, diabetes mellitus, race, and APOE ε4.
Hazard ratios (HRs) (95% confidence intervals [CIs]) are from stratified Cox proportional hazards regression with adjustment for age, sex, education, smoking status (current, former, never), drinking status (current, former, never), body mass index, glucose, APOE ε4 (presence of ε4 allele, 0 or ≥1 alleles), high-density lipoprotein cholesterol, total cholesterol, and, as applicable, hypertension, diabetes mellitus, and race. To calculate p values for interaction, we fit Cox models that included all categories (of hypertension, diabetes mellitus, race, or APOE ε4) with an interaction term between the categories and orthostatic hypotension (OH). The p values for interaction are from χ2 tests that test the interaction terms that are not significantly different from 1.
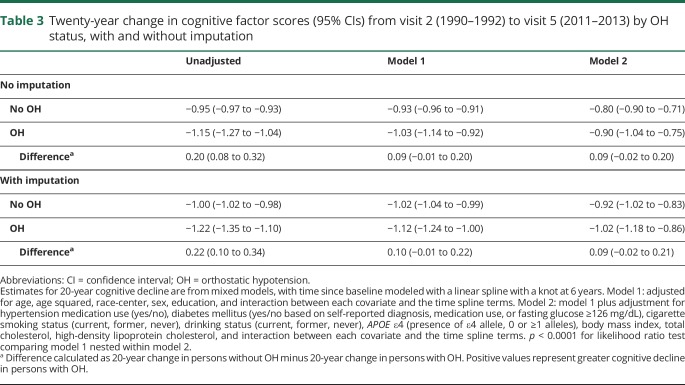
In unadjusted models, persons with OH had 0.22 points greater decline in their cognitive factor score compared to persons without OH (Table 3). However, in fully adjusted models, this result was attenuated to 0.09 and was no longer statistically significant (p = 0.118). Using generalized estimating equations with an independent correlation structure to model cognitive decline and imputing scores for persons who died during follow-up yielded similar differences in cognitive decline between persons with and without OH data (available from Dryad, table 5, doi.org/10.5061/dryad.p7q8t15). Using the alternative definition of OH that included the first measurement on standing yielded similar results (data not shown).
Table 3.
Twenty-year change in cognitive factor scores (95% CIs) from visit 2 (1990–1992) to visit 5 (2011–2013) by OH status, with and without imputation
Discussion
In this large, community-based cohort of middle-aged adults without coronary heart disease or stroke at baseline, OH was independently associated with an increased risk of dementia and ischemic stroke during ≈25 years of follow-up. We also found OH to be associated with greater long-term cognitive decline, although this result was not significant in adjusted models.
The association between OH and cognition could be the result of recurring cerebral hypoperfusion, which may have long-term effects on cognitive function.28,29 A similar interpretation may apply to studies of hypoxia-inducing conditions such as sleep apnea30,31 and chronic obstructive pulmonary disease,32,33 which show an increased risk of mild cognitive impairment and dementia. For dementia, our finding that OH may be particularly detrimental in persons with hypertension and diabetes mellitus adds support to the idea that OH operates primarily via vascular processes, perhaps through microinfarcts or other manifestations of small vessel disease. Notably, censoring stroke events before dementia had only a modest effect on the association between OH with dementia. This is expected in view of current knowledge of the overwhelming influence on cognitive outcomes of small vessel disease measured by brain imaging and microinfarcts that are observed only at autopsy.34 Recurrent cerebral hypoperfusion could also explain the observed association with stroke; this is further supported by the particularly strong association seen with thrombotic strokes due to carotid stenosis. Low-flow states such as from OH might particularly increase risk of strokes through a narrow carotid artery.
Because most dementia in the general population is thought to be primarily related to Alzheimer disease (AD), it might be surprising that the association of OH with dementia (HR 1.54) was fully half as strong as that with stroke (HR 2.08). This may suggest that vascular disease contributes to both vascular and Alzheimer dementias. It is possible that OH and the vascular processes associated with it have a role in the pathogenesis of AD, as has been previously suggested.35,36 Alternatively, they may contribute to only the cognitive impairment associated with AD rather than to its underlying pathology. Studies, in fact, have documented that for a given level of AD pathology, the presence of vascular risk factors results in reduced cognitive ability.37
Our results are similar to results from studies in which OH was measured in older adults.38,39 Studies of OH and cognitive decline to date have generally reported no association when OH was examined in older adults (mean age 72–80 years),14,15 although the shorter duration of the studies (mean follow-up 2 years) may play a role in the lack of associations. Furthermore, reported associations of OH with incident cardiovascular events are stronger in populations <65 years old,7 which is consistent with research on risk factors for cognitive decline that has generally found weaker associations when risk factors are measured in late life.40,41
A prior study in ARIC examining the association of OH with 6-year cognitive decline did not find significant associations after multivariate adjustment,42 potentially because only modest declines occur within middle age. Our study extended the follow-up period by an additional 14 years, but a number of factors may explain the persisting lack of a significant association. First, there was substantial mortality during follow-up in persons with OH (55%) compared to those without OH (27%), and only a fraction of participants with OH returned to visit 5 to have their cognitive function measured. While we attempted to account for missingness through imputation, the small sample of survivors with OH may have limited our ability to adequately account for the effects of attrition. Alternatively, the OH associations with dementia but not with cognitive decline may be a result of the association we saw between OH and lower baseline cognitive scores. That is, persons whose cognitive scores were lower at baseline would be more likely to “cross the threshold” for dementia diagnosis earlier compared to persons with higher cognitive scores at baseline.
The prevalence of OH in adults has been estimated as 5% in middle-aged individuals, similar to what we estimated in our study, and ≈30% in persons ≥70 years of age.12 While the prevalence is low in younger individuals, the increase in prevalence with age, assuming a causal mechanism by which OH affects cognition, may provide the opportunity for targeting interventions earlier to improve cognitive health.
Our study has a few limitations to consider. First, OH was measured at a single time point and may not represent a participant's typical postural change in BP over time. Second, we are unable to rule out the possibility of residual confounding; other unmeasured multimorbid conditions or associated sympathetic and parasympathetic dysfunction are possibilities. However, we excluded participants with cardiovascular disease at baseline and adjusted for a number of potential confounders to attempt to address this issue, and neurodegenerative conditions that affect both OH and cognitive function are rare in middle age (other than Parkinson disease, which we excluded), when OH was ascertained. Furthermore, OH may in part elevate some of the cardiovascular risk factor levels. If so, our fully adjusted models may potentially be overadjusted, resulting in our estimates being conservative. Third, because this was an observational study, we were unable to identify indications and potential indication biases to examine the effect of BP-lowering medication in these associations. Lastly, we lacked CSF and imaging biomarkers (e.g., amyloid) in the cohort to define dementia subtypes, so we cannot determine whether OH preferentially increases the risk of vascular or mixed dementia rather than AD, which might provide some specificity to the evidence for a causal pathway. Our study has a number of important strengths, including the large sample size of middle-aged adults, >24 years of follow-up, alternative measures of OH, and comprehensive assessment of potential confounders.
Postural drops in SBP and DBP are associated with substantial risk for both ischemic stroke and dementia, particularly in those with hypertension and diabetes mellitus. The presence of orthostasis, especially in midlife, might identify individuals in whom more careful monitoring or risk factor management might be warranted. Additional studies are needed to elucidate potential mechanisms for these associations and possible applications for prevention.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- HR
hazard ratio
- ICD-9
International Classification of Diseases, 9th revision
- OH
orthostatic hypotension
- SBP
systolic blood pressure
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Andreea Rawlings designed the study, researched the data, performed the statistical analysis, and drafted the manuscript. Stephen P. Juraschek, Gerardo Heiss, Tim Hughes, Michelle L. Meyer, and Elizabeth Selvin interpreted the data and provided meaningful contributions and revisions to the manuscript. A. Richey Sharrett designed the study, researched the data, and drafted the manuscript. B. Gwen Windham interpreted the data and provided meaningful contributions and revisions to the manuscript. Rebecca F. Gottesman designed the study, researched the data, and drafted the manuscript.
Study funding
The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917, with previous brain MRI examinations funded by R01-HL70825.
Disclosure
A. Rawlings was supported by NIH/NHLBI grant T32 HL007024. S. Juraschek was supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant T32DK007732-20 and NIH/NHLBI grant K23HL13527301. G. Heiss, T. Hughes, and M. Meyer report no disclosures relevant to the manuscript. E. Selvin was supported by NIH/N grants R01DK089174 and K24DK106414. R. Sharrett reports no disclosures relevant to the manuscript. B. Windham has previously received reimbursements from ACADIA Pharmaceuticals. R. Gottesman was supported by K24AG052573. Go to Neurology.org/N for full disclosures.
References
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory study. Neuroepidemiology 2007;29:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008;148:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors: a review. Int J Stroke 2012;7:61–73. [DOI] [PubMed] [Google Scholar]
- 4.Kovacic JC, Castellano JM, Fuster V. The links between complex coronary disease, cerebrovascular disease, and degenerative brain disease. Ann NY Acad Sci 2012;1254:99–105. [DOI] [PubMed] [Google Scholar]
- 5.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer's disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement (Amst) 2017;7:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy: the Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- 7.Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015;36:1609–1617. [DOI] [PubMed] [Google Scholar]
- 8.Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens 2014;32:1562–1571. [DOI] [PubMed] [Google Scholar]
- 9.Rose KM, Eigenbrodt ML, Biga RL, et al. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2006;114:630–636. [DOI] [PubMed] [Google Scholar]
- 10.Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the Atherosclerosis Risk in Communities (ARIC) study, 1987–1996. Stroke 2000;31:2307–2313. [DOI] [PubMed] [Google Scholar]
- 11.Hickler RB. Orthostatic hypotension and syncope. N Engl J Med 1977;296:336–337. [DOI] [PubMed] [Google Scholar]
- 12.Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol 2015;66:848–860. [DOI] [PubMed] [Google Scholar]
- 13.Schoon Y, Lagro J, Verhoeven Y, Rikkert MO, Claassen J. Hypotensive syndromes are not associated with cognitive impairment in geriatric patients. Am J Alzheimers Dis Other Demen 2013;28:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap PLK, Niti M, Yap KB, Ng TP. Orthostatic hypotension, hypotension and cognitive status: early comorbid markers of primary dementia? Dement Geriatr Cogn Disord 2008;26:239–246. [DOI] [PubMed] [Google Scholar]
- 15.Viramo P, Luukinen H, Koski K, Laippala P, Sulkava R, Kivela SL. Orthostatic hypotension and cognitive decline in older people. J Am Geriatr Soc 1999;47:600–604. [DOI] [PubMed] [Google Scholar]
- 16.Mehrabian S, Duron E, Labouree F, et al. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci 2010;299:45–48. [DOI] [PubMed] [Google Scholar]
- 17.Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017;21287:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study (ARIC-NCS). Alzheimer's Dement (Amst) 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years' experience. J Clin Epidemiol 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 21.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Manual for the Wechsler Adult Intelligence Scale, Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 23.Benton A, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City: AJA Associates; 1989. [Google Scholar]
- 24.Gross AL, Power MC, Albert MS, et al. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology 2015;26:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE. Regression Modeling Strategies. New York: Springer New York; 2001. [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawlings AM, Sang Y, Sharrett AR, et al. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol 2017;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017;136:719–728. [DOI] [PubMed] [Google Scholar]
- 29.De La Torre JC. Critical threshold cerebral hypoperfusion causes Alzheimer's disease? Acta Neuropathol 1999;98:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015:84:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic obstructive pulmonary disease and asthma and the risk of mild cognitive impairment and dementia: a population based CAIDE study. Curr Alzheimer Res 2013;10:549–555. [DOI] [PubMed] [Google Scholar]
- 33.Liao KM, Ho CH, Ko SC, Li CY. Increased risk of dementia in patients with chronic obstructive pulmonary disease. Medicine 2015;94:e930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Torre JC. Alzheimer's disease: how does it start? J Alzheimers Dis 2002;4:497–512. [DOI] [PubMed] [Google Scholar]
- 36.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res 2017;95:943–972. [DOI] [PubMed] [Google Scholar]
- 37.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA 1997;277:813–817. [PubMed] [Google Scholar]
- 38.Cremer A, Soumaré A, Berr C, et al. Orthostatic hypotension and risk of incident dementia: results from a 12-year follow-up of the Three-City Study cohort. Hypertension 2017;70:44–49. [DOI] [PubMed] [Google Scholar]
- 39.Wolters FJ, Mattace-Raso FUS, Koudstaal PJ, Hofman A, Ikram MA; Heart Brain Connection Collaborative Research Group. Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med 2016;13:e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitmer R, Sidney S, Selby J, Johnston S, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277–282. [DOI] [PubMed] [Google Scholar]
- 41.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose KM, Couper D, Eigenbrodt ML, Mosley TH, Sharrett AR, Gottesman RF. Orthostatic hypotension and cognitive function: the Atherosclerosis Risk in Communities study. Neuroepidemiology 2010;34:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full data are not available publically because of participant privacy and consent. A public-use ARIC dataset is available through BioLINCC, and more information regarding the data is available from the corresponding author on reasonable request.