Abstract
Growing evidence identifies maternal adiposity as a potentially-modifiable risk factor for adverse neurodevelopment. This retrospective cohort analysis examined whether maternal prepregnancy adiposity and gestational weight gain were associated with behavioral outcomes in 173 rhesus macaque infants at the California National Primate Research Center. Dams conceived indoors, had uncomplicated pregnancies, delivered vaginally and reared infants indoors. Infants underwent standardized bio-behavioral analysis at 90-120 days of age from 3/2001–5/2015. Offspring of mothers with greater baseline adiposity or gestational weight gain exhibited a pattern of poor adaptability characterized by greater emotionality as the assessments proceeded, blunted affective response to a human intruder challenge, and reduced interest in novel stimuli which is associated with poorer social functioning later in life. They also had lower cortisol levels following dexamethasone suppression, perhaps a response to cortisol excess during gestation. These results amplify growing public health concerns implicating maternal adiposity in impaired fetal neurobehavioral programming.
Keywords: Fetal programming, neurodevelopment, obesity, gestational weight gain, pregnancy, gestation, nonhuman primate, rhesus monkey
Obesity is recognized as a global health epidemic by the World Health Organization, with an estimated 38% of adults – over 1.8 billion men and women – considered obese worldwide in 2013 (Ng et al., 2014). In the US in 2014, more than half of pregnant women were overweight or obese (Branum, Kirmeyer, & Gregory, 2016). Obesity’s impact on the gestational environment appears to program fetal development in key ways that are strongly associated with a number of long-term health risks, including cardiovascular disease (Roberts, Frias, & Grove, 2015) and several neuropsychiatric conditions (Rivera, Christiansen, & Sullivan, 2015).
A growing body of evidence has identified maternal prepregnancy body mass index (BMI) (Hinkle et al., 2012; Hinkle, Sharma, Kim, & Schieve, 2013; Huang et al., 2014; Jo et al., 2015) and gestational weight gain (GWG) (Hinkle, Albert, Sjaarda, Grewal, & Grantz, 2016; Keim & Pruitt, 2012) – or both (Pugh et al., 2016a, 2016b; Pugh et al., 2015) – as potentially-modifiable risk factors for impaired child neurocognitive development. Maternal obesity has been associated with attention deficit hyperactivity disorder (Chen et al., 2014; Rodriguez et al., 2008), anxiety and depression (Rodriguez, 2010; Van Lieshout, Robinson, & Boyle, 2013), schizophrenia (Jones, Rantakallio, Hartikainen, Isohanni, & Sipila, 1998; Schaefer et al., 2000) and autism spectrum disorder (ASD). Fetal exposure to maternal pregravid adiposity, measured as obesity at age 18 (Lyall, Pauls, Santangelo, Spiegelman, & Ascherio, 2011), being taller and heavier (Wilkerson, Volpe, Dean, & Titus, 2002), weight ≥90 kg (Dodds et al., 2011), and weight >91kg among non-smokers (Burstyn, Sithole, & Zwaigenbaum, 2010) have each been associated with ASD. Excess GWG has also been associated with ASD (Bilder et al., 2013; Dodds et al., 2011; Gardner et al., 2015).
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is common in obese individuals and a possible mechanism in developmental programming. Elevated cortisol is implicated in obesity causality and plays a significant role in adipocyte biology and weight gain (Bose, Olivan, & Laferrere, 2009; Torres & Nowson, 2007). In pregnancy, maternal preconception obesity and excess GWG demonstrate synergism in their association with elevated third trimester evening cortisol levels compared to women at healthy weight (Aubuchon-Endsley, Bublitz, & Stroud, 2014). Maternal stress is associated with offspring anxiety and HPA responsivity in rodents, suggesting that the developing fetal HPA axis may be susceptible to maternal obesity (Brunton, 2013). It is not clear whether these mechanisms operate in primates.
To avoid methodologic issues inherent in human epidemiology, we explored relationships between maternal adiposity, GWG and neurobehavioral impairment in the indoor colony of rhesus (Macaca mulatta) monkeys at the California National Primate Research Center (CNPRC). Our hypotheses were that prepregnancy maternal adiposity and/or excess gestational weight gain (GWG) would be associated with impairments in infant adaptive development.
The rhesus macaque model is highly relevant to the study of gestational adiposity with strong generalizability to humans. While the majority of CNPRC adults are at healthy weight, many indoor females are overweight or obese, the result of years of inactivity and over-eating, similar to adult humans. Further, the controlled environment in which CNPRC monkeys live limits research confounds arising from lifestyle variations in women. Finally, gestational and postnatal neurobehavioral development in the nonhuman primate closely parallel that of humans, with similar feto-placental maturation, brain organization and postnatal evolution of cognitive, affective, and social functioning (Phillips et al., 2014). Although causal relationships cannot be determined in this type of study, this design has been used to study developmental effects of gestational obesity in humans. Furthermore, retrospective studies like this one can provide important “proof-of-principle” data that can lead to more causally-oriented prospective studies.
METHODS
Because obesity is largely restricted to indoor dams, we restricted our retrospective cohort investigation to the indoor colony. Inclusion criteria were indoor conception and gestation; maternal infant rearing; no hospitalizations; being conventional (i.e., not specific pathogen-free) animals; and having experienced a standardized infant evaluation at 3-4 months of age. Colony health records from 2001-2015 identified 173 dam-infant pairs who met inclusion criteria.
There were two fetal exposures of interest in this study. The first involved a proxy for maternal prepregnancy adiposity in monkeys known as the Body Condition Score (BCS) (Summers, Clingerman, & Yang, 2012). As in humans, weight alone is an inadequate measure of adiposity in monkeys. This subjective semi-quantitative assessment of key anatomic features reliably identifies obese monkeys with body fat that ranges from 30 to 55% (Summers et al., 2012). The BCS ranges from 1 to 5, and a score of 3.5 correlates with 32% body fat, comparable with the World Health Organization classification of obesity in women of 30% body fat or greater. Monkeys are scored on a semi-annual basis, with minimal variation from one measurement to the next in non-pregnant adult females. The BCS recorded immediately prior to conception was used for these analyses.
The second fetal exposure was GWG, measured as steepness of weight change over the interval from the last weight prior to pregnancy (W1) to the first weight after birth of the infant (W2). We accounted for variation in time interval between the measurements and computed steepness of weight change using the formula: S(W1, W2) = 1,000 × ln(W2/W1)/(D2 – D1), where W2 and W1 are weights in kilograms (kg) at dates D2 and D1, respectively. Steeper growth curves identified dams with accelerated or excess adjusted GWG.
Outcomes were a series of measurements recorded in standardized infant BioBehavioral Assessment (BBA) testing (J. P. Capitanio, Mason, W.A., Mendoza, S.P., Del Rosso, L.A., Roberts, J.A, 2006). Nearly all available indoor-colony infants participate in a 25-hour program designed to characterize behavioral and physiological responsiveness. Measurements have been described in detail previously (J. P. Capitanio, Kyes, & Fairbanks, 2006; J. P. Capitanio, Mason, W.A., Mendoza, S.P., Del Rosso, L.A., Roberts, J.A, 2006; Golub, Hogrefe, Germann, Capitanio, & Lozoff, 2006; Golub, Hogrefe, Widaman, & Capitanio, 2009; Sclafani et al., 2016), and are reviewed briefly below. Offspring 90-120 days of age were separated from their mothers, relocated to individual standard-sized indoor cages (referred to as holding cages) and tested in cohorts of 5-8 infants. Each holding cage had a cloth towel, a stuffed toy and a novel 4×9 cm object containing an activity sensor. Food, water and enrichment (pieces of apple, banana, grapes) were provided. For specific assessments, animals were manually captured and transported to test cages in an adjacent room. Observers were blind to maternal adiposity status. Inter- and/or intra-observer reliability data were collected annually for the two behavioral observers, one of whom tested every BBA animal since 2001 and the second of whom tested all animals since 2005. For the behavioral data, observers scored the same animals, either contemporaneously or at different times by rescoring video from earlier years. For the rating data, technicians shadowed each other for animal cohorts, insuring they had comparable experiences, after which they independently rated each animal. Reliability values, calculated as percent agreement, always exceeded 85%. All procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee and were conducted in accordance with the Guidelines for Use and Care of Laboratory Animals of the National Research Council. The CNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
BioBehavioral Assessment Components
Holding Cage Observations
Within 15 minutes of arrival to the testing area (0915 on D1) and again ~22 h later (0700 on Day 2), each animal was observed in a predetermined random order (which remained constant for all assessments) for 5 minutes. Recorded behaviors included activity, agonistic, and anxiety behaviors, and vocalizations. Exploratory and confirmatory factor analyses revealed that the behavioral data could be described by two latent factors: Activity (time spent locomoting or not hanging from the side of the cage; rate of environmental exploration; and whether the animal ate food, drank water, and crouched on the floor of the cage) and Emotionality (rates of cooing and barking; and whether the animal scratched itself, displayed threats, and displayed lipsmacks) were constructed separately for each day during the 2-day testing period (Golub et al., 2009). Factor scores for each animal were z-scored within assessment year.
Response to Novel Objects
The novel object in the cage was a black hollow cylinder with ridges on the surface, approximately 4 cm × 9 cm, and its white replacement provided at 1400 h. It contained an actimeter (Philips Respironics, Bend, Oregon) that estimated the amount of contact throughout the day and night, adjusted for inadvertent contacts from moving monkeys for other assessments. Contact was computed as the number of 15-s periods in 5-min blocks of time in which there was any force exerted on the object. Data were averaged for four time periods.
Visual Recognition Memory
The goal of this test was to determine whether animals would show a preference for novel visual stimuli. Each animal was presented with 7 problems via color videotape, each of which began with a 20-second familiarization trial, with presentation of a pair of identical pictures of an unknown monkey side-by-side (Sclafani et al., 2016), followed by a white screen for 5-seconds. Next, an 8-second test trial displayed the original picture alongside a novel picture, followed by a 5-sec delay, and a second 8-sec test trial with the same two stimuli with positions reversed. All stimuli for the seven problems can be found online (https://doi.org/10.1371/journal.pone.0165401.s002). Measures of interest were: a) proportion of looking time during the test trials spent focused on the novel stimulus, and b) the number of problems (maximum=7) with which the animal engaged. If an animal did not look at the initial familiarization trial, there was no basis to judge which stimulus was familiar or novel, and so the looking time data were dropped for that problem.
Human Intruder
This test assessed responsiveness of animals under graded conditions of challenge. In the test cage each animal received four 1-min trials: a) technician 1 m in front of the animal’s cage (far position), presenting left profile; b) technician ~0.5 m (near position) with left profile; c) far position while making direct eye contact with the animal; d) near position, direct eye contact. We recorded the behaviors described above, plus position information. Exploratory and confirmatory factor analyses revealed a four-factor structure to the data (Gottlieb & Capitanio, 2013) labelled Activity (active, cage-shake, environment explore), Emotionality (convulsive jerk, fear grimace, self-clasp, coo vocalization), Aggression (threat, bark vocalization, other vocalization), and Displacement (i.e., Anxiety) (tooth grind, yawn). Factor scores for each animal were z-scored within assessment year.
Temperament Rating
At the conclusion of the 25-hr assessment, the technician responsible for testing the animals rated each animal on an instrument composed of 16 trait adjectives using a 1-7 scale for each item. This provides ‘thumbnail” indicators of the animal’s overall functioning during the BioBehavioral Assessment. Exploratory and confirmatory factor analyses(Golub et al., 2009) revealed a four-factor structure to the data. Scales were created to measure Confidence (confident, bold, active, curious, playful), Vigilance (vigilant, depressed, tense, timid; italicized adjectives were reverse-scored), Nervousness (nervous, fearful, timid, calm, confident), and Gentleness (gentle, calm, flexible, curious). Factor scores were z-scored within each assessment year.
Blood Samples
Four blood samples were obtained, two on Day 1 at 1100 h and 1600 h and another two on Day 2 at 0830 h and 0900 h. Following the second sample, each animal was injected with 500 μg/kg dexamethasone; this is roughly equivalent to the “very high-dose” overnight dexamethasone suppression test in humans. Dexamethasone is a glucocorticoid receptor agonist that acts at the level of the hypothalamus (particularly the paraventricular nucleus (Herman, McKlveen, Solomon, Carvalho-Netto, & Myers, 2012), to suppress corticotropin-releasing hormone and adrenocorticotropic hormone, leading to decreased circulating concentrations of cortisol. Sample 3 reflects the dexamethasone-suppressed cortisol concentrations. Immediately after Sample 3, each animal was injected with 2.5 IU of ACTH to stimulate the adrenal, and Sample 4 was collected 30-min later. Samples were collected, processed, stored and assayed as described previously(Golub et al., 2009).
Statistics
Pregestational maternal adiposity and gestational weight gain were examined in relation to offspring developmental outcomes. Factor scores for each BBA assessment were computed for each animal. To account for missing BCS (104 of 173) and weight (1 of 173) data, we employed the Markov Chain Monte Carlo method (JL, 1997) with a single chain to create 50 imputations (i.e., 50 imputed data sets). For continuous outcome variables, linear regression models were fit to each imputed data set. A proportional odds model was used for the categorized ordinal outcome variable for number of problems contacted successfully in the Visual Recognition Memory task. We generated 50 models to achieve final composite results, controlling for infant sex, birth year, age in days and weight at BBA testing. While season of birth appears to have an impact on BBA outcomes in outdoor colony animals (Vandeleest, Blozis, Mendoza, & Capitanio, 2013; Vandeleest & Capitanio, 2012), it did not exert an appreciable effect on this analysis of animals conceived, born and raised indoors and was not included in final analyses. Similarly, neither maternal age nor parity had a negligible impact and were not included in final models. All analyses were performed with SAS v9.4 (SAS Institute, Cary, NC). A p-value ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics for dams and infants are displayed in Table 1. There was a slight predisposition for female offspring (55%). Table 2 provides descriptive statistics for the BBA measures assessed in this study. Adjusted estimates for associations between maternal prepregnancy BCS, GWG, BBA and cortisol are depicted in Table 3 and described below. Significant findings are portrayed in Figure 1. For those measures, over the continuum of BCS or GWG, each incremental increase in maternal adiposity was associated with a compensatory change in the outcome of interest.
Table 1.
Dam and Infant Characteristics
| N | Mean | SD | SE | Median | QI | Q3 | Min | Max | IQR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dam | ||||||||||
| Age in years at Delivery | 173 | 10.77 | 3.24 | 0.25 | 10.19 | 8.23 | 13.04 | 4.43 | 20.98 | 4.81 |
| Prior Live Births | 173 | 4.06 | 2.63 | 0.20 | 4 | 2 | 6 | 1 | 13 | 4 |
| Prepregnancy Body Condition Score | 69 | 2.75 | 0.73 | 0.09 | 3 | 2.5 | 3.5 | 1 | 4 | 1 |
| Gestational Weight Gain in kg | 172 | 1.22 | 0.79 | 0.06 | 1.19 | 0.67 | 1.75 | −0.69 | 3.62 | 1.08 |
| Infant | ||||||||||
| Birthweight | 172 | 0.64 | 0.14 | 0.01 | 0.62 | 0.53 | 0.74 | 0.33 | 1.11 | 0.21 |
| Age in days at BBA testing | 173 | 106.21 | 9.28 | 0.71 | 106 | 100 | 112 | 90 | 128 | 12 |
| Weight in kg at BBA testing | 173 | 1.03 | 0.16 | 0.01 | 1.04 | 0.94 | 1.16 | 0.49 | 1.43 | 0.22 |
N = number; SD = Standard Deviation; SE = Standard Error, Q1 = 25th percentile; Q3 = 75th percentile; Min = Minimum; Max = Maximum; IQR = Interquartile Range. Weight Gain = S(W1, W2) = 1000 × ln(W2/W1)/(D2 – D1), where W1 is the last weight prior to pregnancy, W2: the first weight after pregnancy, and D1 and D2: dates of each measurement.
Table 2.
Infant BioBehavioral Assessment Outcomes
| N | Mean | SD | SE | Median | QI | Q3 | Min | Max | IQR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Holding Cage Observations | ||||||||||
| Day 1 Activity | 173 | 0.46 | 0.89 | 0.07 | 0.49 | −0.17 | 1.11 | −1.33 | 2.38 | 1.28 |
| Day 1 Emotionality | 173 | 0.20 | 0.77 | 0.06 | −0.01 | −0.40 | 0.63 | −0.88 | 3.00 | 1.03 |
| Day 2 Activity | 172 | 0.28 | 0.90 | 0.07 | 0.31 | −0.23 | 0.92 | −2.18 | 2.24 | 1.15 |
| Day 2 Emotionality | 172 | 0.12 | 0.71 | 0.05 | 0.06 | −0.40 | 0.48 | −1.04 | 3.80 | 0.87 |
| Visual Recognition Memory | ||||||||||
| Novelty Preference | 172 | 0.57 | 0.12 | 0.01 | 0.58 | 0.49 | 0.66 | 0.19 | 0.823 | 0.16 |
| Number Problems Contacted | 172 | 6.50 | 0.81 | 0.06 | 7.00 | 6.00 | 7.00 | 3.00 | 7.00 | 1.00 |
| Human Intruder Response | ||||||||||
| Activity | 173 | 1.20 | 1.18 | 0.09 | 1.24 | 0.30 | 1.98 | −0.84 | 5.08 | 1.69 |
| Emotionality | 173 | 0.44 | 1.09 | 0.08 | 0.00 | −0.39 | 1.00 | −0.54 | 7.40 | 1.39 |
| Aggression | 173 | −0.02 | 1.03 | 0.08 | −0.47 | −0.53 | −0.02 | −0.63 | 7.53 | 0.52 |
| Displacement | 173 | 0.38 | 1.43 | 0.11 | −0.39 | −0.45 | 0.49 | −0.60 | 5.07 | 0.94 |
| Temperament Rating | ||||||||||
| Confidence | 173 | 0.26 | 0.76 | 0.06 | 0.33 | −0.30 | 0.73 | −1.90 | 2.22 | 1.03 |
| Vigilance | 173 | 0.34 | 0.62 | 0.05 | 0.45 | 0.11 | 0.78 | −2.01 | 1.50 | 0.67 |
| Nervousness | 173 | 0.16 | 1.10 | 0.08 | 0.05 | −0.62 | 0.87 | −1.92 | 3.08 | 1.49 |
| Gentleness | 173 | 0.19 | 0.87 | 0.07 | 0.27 | −0.42 | 0.72 | −2.08 | 2.58 | 1.15 |
| Blood Cortisol Levels (ug/dL) | ||||||||||
| Sample 1 | 172 | 87.98 | 23.93 | 1.82 | 85.14 | 72.10 | 102.18 | 45.751 | 161.04 | 30.08 |
| Sample 2 | 172 | 88.21 | 31.13 | 2.37 | 81.03 | 65.23 | 104.69 | 35.520 | 185.84 | 39.46 |
| Sample 3 | 171 | 57.99 | 41.65 | 3.18 | 50.86 | 37.21 | 67.17 | 7.634 | 376.02 | 29.96 |
| Sample 4 | 170 | 94.31 | 37.87 | 2.90 | 86.87 | 72.02 | 104.26 | 48.42 | 370.35 | 32.25 |
N = number; SD = Standard Deviation; SE = Standard Error; Q1 = 25th percentile; Q3 = 75th percentile; Min = Minimum; Max = Maximum; IQR = Interquartile Range
Table 3.
Adjusted Estimates with 95% Confidence Intervals for associations between Maternal Adiposity or Gestational Weight Gain and Biobehavioral Assessment Findings*
| BBA Measures | Maternal Adiposity | Gestational Weight Gain | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Est | 95% CI | Est | 95% CI | |||
| Holding Cage Observations | ||||||
| Day 1 Activity | −0.08 | −0.47 | 0.31 | 0.02 | −0.22 | 0.27 |
| Day 1 Emotionality | 0.01 | −0.29 | 0.31 | −0.06 | −0.25 | 0.14 |
| Day 2 Activity | 0.04 | −0.31 | 0.39 | 0.18 | −0.05 | 0.41 |
| Day 2 Emotionality | 0.27 | 0.03 | 0.51 | 0.06 | −0.11 | 0.24 |
| Visual Recognition Memory | ||||||
| Novelty Preference | −0.05 | −0.10 | −0.00 | −0.02 | −0.06 | 0.01 |
| Number Problems Contacted | −1.80 | −3.08 | −0.52 | −1.23 | −2.14 | −0.32 |
| Human Intruder Response | ||||||
| Activity | −0.07 | −0.47 | 0.34 | 0.05 | −0.23 | 0.34 |
| Emotionality | −0.51 | −0.91 | −0.10 | −0.30 | −0.59 | −0.01 |
| Aggression | 0.41 | −0.15 | 0.97 | 0.26 | −0.04 | 0.56 |
| Displacement | −0.19 | −0.89 | 0.51 | 0.04 | −0.35 | 0.44 |
| Temperament Rating | ||||||
| Confidence | −0.45 | −0.70 | −0.21 | 0.10 | −0.27 | 0.08 |
| Vigilance | −0.22 | −0.44 | 0.01 | −0.07 | −0.23 | 0.08 |
| Nervousness | 0.11 | −0.29 | 0.52 | 0.00 | −0.28 | 0.28 |
| Gentleness | −0.17 | −0.49 | 0.14 | −0.05 | −0.26 | 0.16 |
| Blood Cortisol Levels (ug/dL) | ||||||
| Sample 1 | 3.49 | −4.82 | 11.80 | 0.37 | −5.29 | 6.04 |
| Sample 2 | 1.51 | −10.90 | 13.93 | −0.57 | −8.42 | 7.28 |
| Sample 3 | −22.81 | −39.43 | −6.19 | −12.38 | −22.50 | −2.26 |
| Sample 4 | −5.04 | −29.69 | 19.62 | −8.40 | −20.76 | 3.97 |
Infant sex, birth year, age in days and weight at BBA testing were adjusted when regression analyses were performed.
Figure 1. Maternal prepregnancy obesity or excess gestational weight gain and statistically-significant infant behavioral outcomes, N=173.
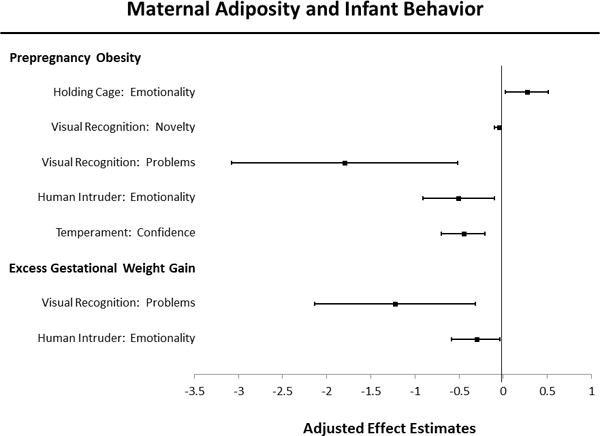
Linear regression models were adjusted for infant sex, birth year, age in days and weight at BBA testing. The Y-axis lists behavioral measures that reached significance, separated into two categories according to the parent analysis predictor in bolded print. Adjusted effect estimates are denoted by black squares, and 95% confidence intervals by error bars (Tukey whiskers) that extend laterally from each box.
Holding Cage Observations
Higher prepregnancy BCS was associated with greater Day 2 Emotionality (p=0.027). No effects were evident for either of the Day 1 measures, or for Day 2 Activity, and GWG had no impacts.
Response to Novel Objects
There were no significant effects for either prepregnancy BCS or GWG on the response to novel objects.
Visual Recognition Memory
Higher maternal BCS was significantly associated with reduced interest in the novel face stimuli in the test of visual recognition memory: these infants spent less time looking at the novel stimuli (p=0.038) and had lower odds of completing the facial recognition test (i.e., more trials in which their data were dropped because of not looking at the stimuli) (p=0.006). Higher maternal GWG was independently and significantly associated with lower odds of completing the facial recognition test (p=0.017).
Human Intruder
Higher prepregnancy BCS and GWG were independently and significantly associated with reduced emotionality in the Human Intruder Test, (p=0.015) and (p=0.045), respectively.
Temperament Rating
Higher prepregnancy BCS was significantly associated with lower Confidence on the temperament rating scale assessed at the end of the 25-hour test period (p<0.001). We found no effects of BCS on the other three temperament scales. GWG was not significantly associated with any of the temperament scales.
Blood Samples
Higher prepregnancy BCS and greater GWG were each associated with lower cortisol concentrations in response to dexamethasone suppression (p=0.008 for each comparison). Maternal variables were unrelated to cortisol concentrations from the other three blood samples.
DISCUSSION
Our exploration of rhesus macaque maternal adiposity measures revealed a pattern of greater behavioral disturbance during the BBA testing for infants of dams that had higher prepregnancy adiposity and/or that gained excess weight. Importantly, these effects were not evident immediately upon relocation to the testing area – no effects were found for Day 1 Activity and Day 1 Emotionality – when all animals likely responded similarly to the separation and relocation. Rather, the pattern for the infants of obese mothers reflected poor adaptability, as indicated by a number of results from the later assessments, during which infants exhibited enhanced holding-cage Emotionality, reduced display of affective behaviors during the human intruder test, and completion of fewer problems and less interest in novel stimuli during the test for visual recognition memory compared with control infants. We note that reduced interest in the novel stimuli has been associated with poorer social functioning later in life(Sclafani et al., 2016). Undoubtedly, this pattern of behavior contributed to the ratings by the behavioral observer of infants of obese mothers as less Confident (i.e., less confident, active, bold, curious, playful).
We found no differences in morning or afternoon cortisol concentrations in infants from obese or excessive GWG mothers, nor were differences seen in response to an ACTH challenge stimulus, suggesting that the ability of the adrenal gland to synthesize cortisol was not a target of maternal or gestational adiposity. Interestingly, however, these infants had significantly lower cortisol levels in response to dexamethasone suppression, indicating that the cortisol feedback loop in these animals is more sensitive to negative regulation by glucocorticoids.
The origins of these behavioral and glucocorticoid findings are unclear, as is speculation as to whether they are all related to the same underlying mechanisms. Certainly, gestational environments complicated by obesity and over-nutrition are characterized by baseline insulin resistance enhanced by demands of the growing fetus and placenta. Progressive hyperglycemia and hyperlipidemia are common (“ACOG Practice Bulletin No 156: Obesity in Pregnancy,” 2015; Retnakaran et al., 2016). Immune activation leads to a chronic state of low-grade systemic inflammation (Bremer, Devaraj, Afify, & Jialal, 2011), stimulation of the transcription factor nuclear factor kappa B (NFkB) pathway (Shi et al., 2006) and cellular damage associated with oxidative stress (Brownlee, 2005; Sada et al., 2016). The role of maternal-fetal immune dysfunction in the development of neurobehavioral impairment has numerous strong lines of supporting evidence reviewed elsewhere (Meltzer & Van de Water, 2016).
The metabolic programming effects of maternal obesity on the fetal/infant HPA axis, childhood growth and obesity risk have been reported extensively in humans and underlying mechanisms have been explored in rodent models (Hunter, Minnis, & Wilson, 2011). Exposure to longstanding stress with consequent over-stimulation of the HPA axis can evolve into a state of generally low HPA-axis activity (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Heim, Ehlert, & Hellhammer, 2000; Miller, Chen, & Zhou, 2007). This state has been referred to as hypocortisolism and has been associated with adult depression (Maripuu, Wikgren, Karling, Adolfsson, & Norrback, 2014; Oldehinkel et al., 2001; Penninx et al., 2007; Wikgren et al., 2012) and adiposity in humans (Ljung, Andersson, Bengtsson, Bjorntorp, & Marin, 1996; Pasquali et al., 2002; Rask et al., 2001), and with chronic stress in monkeys (J. P. Capitanio, Mendoza, Lerche, & Mason, 1998). Fetal programming of the HPA-axis may promote compensatory responses in the infant brain that suppress cortisol and stress responses via enhanced negative feedback.
Postnatal experiences – particularly maternal-infant bonding and early social experiences – have been implicated in further shaping of infant temperament and HPA axis regulation. For example, there is substantial evidence that nursery-rearing of rhesus monkeys results in an altered regulation of the HPA axis (J. P. Capitanio, Mendoza, Mason, & Maninger, 2005; Feng et al., 2011). Other primate studies have also demonstrated that postnatal social experience can re-organize the HPA axis (J. P. Capitanio et al., 1998). It’s possible that maternal behaviors may differ by level of adiposity, leading the offspring of obese mothers to develop a less adaptable phenotype. Clearly, careful assessment of the social development of offspring of obese vs. non-obese animals is required to better understand the role of possible postnatal factors in the development of this maternal adiposity-related phenotype of low adaptability.
Together, our findings provide a unique contribution to the NHP literature. Previous NHP studies have characterized the fetal metabolic programming effects of maternal obesity generally as a result of feeding an experimental high-fat diet (Kahr et al., 2016; McCurdy et al., 2016; Pound, Comstock, & Grove, 2014; Sullivan et al., 2017). Similarly, feeding high sugar (Golub, Hogrefe, & Vandevoort, 2014) or high-fat (Sullivan et al., 2010; Sullivan, Nousen, Chamlou, & Grove, 2012; Sullivan, Smith, & Grove, 2011; Thompson JR, 2017) diets to dams prior to or during pregnancy can lead to offspring behavioral alterations. In contrast to these experimental models, the animal population examined in this study developed and maintained their obesity over a period of years during their adult lives as a result of inactivity and over-eating the standard CNPRC high-protein monkey diet (Lab Diet, 30% protein, 13% fat and 56% carbohydrate). Food enrichment included 1-2 tablespoons of oats and dried legumes daily and produce twice weekly. It is notable that pregnant dams received a 25% increase in chow once pregnancy was diagnosed, which may have enhanced GWG. The offspring behavior and cortisol responses reported in this study were associated with maternal adiposity and GWG alone without alteration to dietary composition, suggesting that there may be independent effects of adiposity, GWG and specific nutritional constituents on fetal neurodevelopmental programming.
These results have critical implications for the long-term management of captive primate colonies. Although it is not recommended that obese animals be used in research as control or experimental subjects (Bauer, Arndt, Leslie, Pearl, & Turner, 2011), elevated prepregnancy body condition score does not exclude dams from many pregnancy studies. The unintended effects of maternal adiposity on offspring reported in this study suggest that obese dams should be excluded from participation in experimental pregnancy designs or – at the very least – that prepregnancy body condition score should be considered as a covariate in analytic models. Our findings underscore the importance of preventing both baseline adiposity and excess GWG in the captive breeding colony, as these conditions regardless of dietary composition can impact fetal development adversely.
Limitations of this study include that it was a retrospective analysis of cohort data amassed for alternative purposes. We do not have biological information that would allow for exploration of the role of specific molecular mechanisms in the mother that may be involved in behavioral programming. Further, because data on maternal parenting behaviors are not available, it is not possible to determine with certainty the timing of behavioral programming. Alterations in the composition of breastmilk and maternal parenting behaviors of obese dams may differ in important ways from those of healthy-weight mothers, meaning that programming could have occurred postnatally. These are all issues for further study using prospective experimental designs. An important strength of our study is that maternal adiposity was the natural result of excess eating and limited activity – similar to what is seen in human populations – rather than introduction of an experimental nutritionally-manipulated diet.
The findings of this study add to accumulating human and animal evidence regarding the negative impacts of maternal obesity on fetal development and long-term health. It is imperative that we identify interventions that reverse aberrant underlying physiology of obesity during pregnancy. While the current study identifies numerous behavior changes in infants exposed to maternal adiposity during fetal development, these findings need to be assessed in a prospective study, given the propensity for retrospective studies to capitalize on chance associations. The NHP cohorts identified in this study would be excellent models for elucidation of fetal programming mechanisms that link maternal obesity and high GWG with adverse offspring behaviors.
Acknowledgments
Funding/Support: This publication was made possible by NIH/NICHD grant 1R01HD084203-01A1 (CKW) and grants R24OD010962 (JPC) and P51OD011107 (California National Primate Research Center).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
DR. CHERYL K. WALKER (Orcid ID : 0000-0003-4854-9695)
AUTHOR CONTRIBUTIONS
Dr. Walker had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Walker. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Walker. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Li. Obtained funding: Walker, VandeVoort.
Conflict of Interest Disclosures: There are no relevant conflicts of interest for any of the authors.
Disclaimer: The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the funding agencies. Furthermore, the funders do not endorse the purchase of any commercial products mentioned in the publication.
References
- ACOG Practice Bulletin No 156: Obesity in Pregnancy. Obstet Gynecol. 2015;126(6):e112–126. doi: 10.1097/AOG.0000000000001211. [DOI] [PubMed] [Google Scholar]
- Aubuchon-Endsley NL, Bublitz MH, Stroud LR. Pre-pregnancy obesity and maternal circadian cortisol regulation: Moderation by gestational weight gain. Biol Psychol. 2014;102:38–43. doi: 10.1016/j.biopsycho.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer SA, Arndt TP, Leslie KE, Pearl DL, Turner PV. Obesity in rhesus and cynomolgus macaques: a comparative review of the condition and its implications for research. Comp Med. 2011;61(6):514–526. [PMC free article] [PubMed] [Google Scholar]
- Bilder DA, Bakian AV, Viskochil J, Clark EA, Botts EL, Smith KR, Coon H. Maternal prenatal weight gain and autism spectrum disorders. Pediatrics. 2013;132(5):e1276–1283. doi: 10.1542/peds.2013-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M, Olivan B, Laferrere B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):340–346. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branum AM, Kirmeyer SE, Gregory EC. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016;65(6):1–11. [PubMed] [Google Scholar]
- Bremer AA, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(11):E1782–1788. doi: 10.1210/jc.2011-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Brunton PJ. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction. 2013;146(5):R175–189. doi: 10.1530/REP-13-0258. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30(4):125–134. [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, Fairbanks LA. Considerations in the selection and conditioning of Old World monkeys for laboratory research: animals from domestic sources. ILAR J. 2006;47(4):294–306. doi: 10.1093/ilar.47.4.294. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. Nursery rearing and biobehavioral organization. In: R G, Sackett GP, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York: Springer; 2006. [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci U S A. 1998;95(8):4714–4719. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sjolander A, Langstrom N, Rodriguez A, Serlachius E, D’Onofrio BM, Larsson H. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. Int J Epidemiol. 2014;43(1):83–90. doi: 10.1093/ije/dyt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Hu X. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci U S A. 2011;108(34):14312–14317. doi: 10.1073/pnas.1010943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Lee BK, Magnusson C, Rai D, Frisell T, Karlsson H, Dalman C. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol. 2015;44(3):870–883. doi: 10.1093/ije/dyv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28(1):3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Vandevoort CA. Binge drinking prior to pregnancy detection in a nonhuman primate: behavioral evaluation of offspring. Alcohol Clin Exp Res. 2014;38(2):551–556. doi: 10.1111/acer.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP. Latent variables affecting behavioral response to the human intruder test in infant rhesus macaques (Macaca mulatta) Am J Primatol. 2013;75(4):314–323. doi: 10.1002/ajp.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45(4):292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle SN, Albert PS, Sjaarda LA, Grewal J, Grantz KL. Trajectories of maternal gestational weight gain and child cognition assessed at 5 years of age in a prospective cohort study. J Epidemiol Community Health. 2016;70(7):696–703. doi: 10.1136/jech-2014-205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle SN, Schieve LA, Stein AD, Swan DW, Ramakrishnan U, Sharma AJ. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int J Obes (Lond) 2012;36(10):1312–1319. doi: 10.1038/ijo.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle SN, Sharma AJ, Kim SY, Schieve LA. Maternal prepregnancy weight status and associations with children’s development and disabilities at kindergarten. Int J Obes (Lond) 2013;37(10):1344–1351. doi: 10.1038/ijo.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Yu X, Keim S, Li L, Zhang L, Zhang J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int J Epidemiol. 2014;43(3):783–792. doi: 10.1093/ije/dyu030. [DOI] [PubMed] [Google Scholar]
- Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14(6):614–626. doi: 10.3109/10253890.2011.577848. [DOI] [PubMed] [Google Scholar]
- JL S. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall; 1997. [Google Scholar]
- Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, Lind JN. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics. 2015;135(5):e1198–1209. doi: 10.1542/peds.2014-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155(3):355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- Kahr MK, Antony KM, DelBeccaro M, Hu M, Aagaard KM, Suter MA. Increasing maternal obesity is associated with alterations in both maternal and neonatal thyroid hormone levels. Clin Endocrinol (Oxf) 2016;84(4):551–557. doi: 10.1111/cen.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim SA, Pruitt NT. Gestational weight gain and child cognitive development. Int J Epidemiol. 2012;41(2):414–422. doi: 10.1093/ije/dyr229. [DOI] [PubMed] [Google Scholar]
- Ljung T, Andersson B, Bengtsson BA, Bjorntorp P, Marin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes Res. 1996;4(3):277–282. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Lyall K, Pauls DL, Santangelo SL, Spiegelman D, Ascherio A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. J Autism Dev Disord. 2011;41(5):618–627. doi: 10.1007/s10803-010-1079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF. Relative hypo- and hypercortisolism are both associated with depression and lower quality of life in bipolar disorder: a cross-sectional study. PLoS One. 2014;9(6):e98682. doi: 10.1371/journal.pone.0098682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Schenk S, Hetrick B, Houck J, Drew BG, Kaye S, Friedman JE. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight. 2016;1(16):e86612. doi: 10.1172/jci.insight.86612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer A, Van de Water J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel AJ, van den Berg MD, Flentge F, Bouhuys AL, ter Horst GJ, Ormel J. Urinary free cortisol excretion in elderly persons with minor and major depression. Psychiatry Res. 2001;104(1):39–47. doi: 10.1016/s0165-1781(01)00300-6. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, Study Group on Obesity of the Italian Society of, E Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab. 2002;87(1):166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Beekman AT, Bandinelli S, Corsi AM, Bremmer M, Hoogendijk WJ, Ferrucci L. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am J Geriatr Psychiatry. 2007;15(6):522–529. doi: 10.1097/JGP.0b013e318033ed80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Voytko ML. Why primate models matter. Am J Primatol. 2014;76(9):801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Comstock SM, Grove KL. Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. Am J Physiol Endocrinol Metab. 2014;307(1):E115–123. doi: 10.1152/ajpendo.00131.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, Bodnar LM. Child academic achievement in association with pre-pregnancy obesity and gestational weight gain. J Epidemiol Community Health. 2016a;70(6):534–540. doi: 10.1136/jech-2015-206800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, Bodnar LM. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG. 2016b;123(13):2094–2103. doi: 10.1111/1471-0528.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh SJ, Richardson GA, Hutcheon JA, Himes KP, Brooks MM, Day NL, Bodnar LM. Maternal Obesity and Excessive Gestational Weight Gain Are Associated with Components of Child Cognition. J Nutr. 2015;145(11):2562–2569. doi: 10.3945/jn.115.215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86(3):1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- Retnakaran R, Ye C, Kramer CK, Connelly PW, Hanley AJ, Sermer M, Zinman B. Evaluation of Circulating Determinants of Beta-Cell Function in Women With and Without Gestational Diabetes. J Clin Endocrinol Metab. 2016;101(7):2683–2691. doi: 10.1210/jc.2016-1402. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:194. doi: 10.3389/fnins.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts VH, Frias AE, Grove KL. Impact of maternal obesity on fetal programming of cardiovascular disease. Physiology (Bethesda) 2015;30(3):224–231. doi: 10.1152/physiol.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51(2):134–143. doi: 10.1111/j.1469-7610.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A, Jarvelin MR. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond) 2008;32(3):550–557. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- Sada K, Nishikawa T, Kukidome D, Yoshinaga T, Kajihara N, Sonoda K, Araki E. Hyperglycemia Induces Cellular Hypoxia through Production of Mitochondrial ROS Followed by Suppression of Aquaporin-1. PLoS One. 2016;11(7):e0158619. doi: 10.1371/journal.pone.0158619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer CA, Brown AS, Wyatt RJ, Kline J, Begg MD, Bresnahan MA, Susser ES. Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophr Bull. 2000;26(2):275–286. doi: 10.1093/oxfordjournals.schbul.a033452. [DOI] [PubMed] [Google Scholar]
- Sclafani V, Del Rosso LA, Seil SK, Calonder LA, Madrid JE, Bone KJ, Parker KJ. Early Predictors of Impaired Social Functioning in Male Rhesus Macaques (Macaca mulatta) PLoS One. 2016;11(10):e0165401. doi: 10.1371/journal.pone.0165401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30(10):3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Nousen EK, Chamlou KA, Grove KL. The Impact of Maternal High-Fat Diet Consumption on Neural Development and Behavior of Offspring. Int J Obes Suppl. 2012;2:S7–S13. doi: 10.1038/ijosup.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Rivera HM, True CA, Franco JG, Baquero K, Dean TA, Kievit P. Maternal and Postnatal High-Fat Diet Consumption Programs Energy Balance and Hypothalamic Melanocortin Signaling in Nonhuman Primate Offspring. Am J Physiol Regul Integr Comp Physiol. 2017 doi: 10.1152/ajpregu.00309.2016. ajpregu 00309 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93(1):1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers L, Clingerman KJ, Yang X. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): assessment of body composition by using dual-energy X-ray absorptiometry. J Am Assoc Lab Anim Sci. 2012;51(1):88–93. [PMC free article] [PubMed] [Google Scholar]
- Thompson JR, V J, Barling AN, Franco JG, DeCapo J, Bagley JL, Sullivan EL. Exposure to a High-Fat Diet during Early Development Programs Behavior and Impairs the Central Serotonergic System in Juvenile Non-Human Primates. Front Endocrinol. 2017 doi: 10.3389/fendo.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Robinson M, Boyle MH. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can J Psychiatry. 2013;58(3):151–159. doi: 10.1177/070674371305800305. [DOI] [PubMed] [Google Scholar]
- Vandeleest JJ, Blozis SA, Mendoza SP, Capitanio JP. The effects of birth timing and ambient temperature on the hypothalamic-pituitary-adrenal axis in 3-4 month old rhesus monkeys. Psychoneuroendocrinology. 2013;38(11):2705–2712. doi: 10.1016/j.psyneuen.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleest JJ, Capitanio JP. Birth timing and behavioral responsiveness predict individual differences in the mother-infant relationship and infant behavior during weaning and maternal breeding. Am J Primatol. 2012;74(8):734–746. doi: 10.1002/ajp.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71(4):294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Wilkerson DS, Volpe AG, Dean RS, Titus JB. Perinatal complications as predictors of infantile autism. Int J Neurosci. 2002;112(9):1085–1098. doi: 10.1080/00207450290026076. [DOI] [PubMed] [Google Scholar]


