Abstract
Patients with non-dialysis-dependent chronic kidney disease (NDD-CKD) are 10 times more likely to die of cardiovascular (CV) diseases than the general population, and dialysis-dependent patients are at even higher risk. Although traditional CV risk factors are highly prevalent in individuals with CKD, these patients were often excluded from studies targeting modification of these risks. While treatment of hypertension is beneficial in CKD, the best target blood pressure has not been established. Trial data showed that renin-angiotensin-aldosterone blockade may prevent CV events in CKD patients. The risks of aspirin may equal the benefits in NDD-CKD samples, and there are no trials testing aspirin in dialysis-dependent patients. Lipid lowering therapy improves CV outcomes in NDD-CKD, but not in dialysis-dependent patients. Strict glycemic control prevents CV events in non-albuminuric individuals, but showed no benefit in those with baseline albuminuria >300 mg/g, and there are no data in dialysis-dependent patients. Data on lifestyle modifications, such as weight loss, physical activity, and smoking cessation, are mostly observational and extrapolated from non-CKD samples. This comprehensive review summarizes the best existing evidence and current clinical guidelines for modification of traditional risk factors for the prevention of CV events in CKD patients and identifies knowledge gaps.
Keywords: chronic kidney disease (CKD), hemodialysis (HD), cardiovascular disease (CVD), hypertension, aspirin, hyperlipidemia, smoking, weight loss, lifestyle interventions, modifiable risk factor, statin, aspirin, dialysis, albuminuria, cardiovascular risk, review, RAAS blockade
INTRODUCTION
Chronic kidney disease (CKD) affects approximately 14% of people in the United States, and of those, more than 100,000 progress to dialysis-dependent CKD (CKD-5D) every year.1 Individuals with non-dialysis-dependent CKD (NDD-CKD) have a disproportionately higher risk of cardiovascular (CV) events compared to age-matched controls and are 5–10 times more likely to die, primarily of CV disease, than to progress to dialysis dependence.1 Of those who reach CKD-5D, approximately 50% will ultimately die of CV causes.1 The manifestations of CV disease and arterial stiffening are unique in CKD; in addition to the intimal plaque atherosclerosis seen in the general population, CKD patients also develop concentric stiffening of the arterial media, which may arise from distinct underlying mechanisms.2
Several traditional CV risk factors, such as hypertension, hyperlipidemia, diabetes mellitus, smoking, physical inactivity, and obesity, were identified in the general population and included in CV risk estimators.3 Although large trials have shown that modification of these risk factors decreases the incidence of CV events, data are limited in individuals with CKD, who were often excluded from such trials. Consequently, guidelines for CV risk management in CKD are predominantly derived from small studies, subgroup analyses, or extrapolation from non-CKD samples, and are not consistently based on robust evidence. Given the differences in the epidemiology and presentation of CV disease in CKD compared to the general population,2 it is critical to understand whether modification of traditional risk factors benefits CKD patients.
Herein, we comprehensively review existing evidence examining whether modification of traditional risk factors decreases CV events in NDD-CKD and CKD-5D patients and identify gaps in our current understanding of risk modification. Randomized trials are evaluated when available; observational data are presented where trials are lacking.
HYPERTENSION
This section focuses on clinical trials addressing the effects of intensive vs. standard blood pressure control and use of specific antihypertensive medications on CV endpoints.
Intensive blood pressure control
Five trials studied the effects of intensive vs. standard blood pressure targets on long-term outcomes (Table 1). The three that included only NDD-CKD patients were designed to study renal outcomes and were underpowered for CV events.4–6 The African American Study of Kidney Disease and Hypertension (AASK) reported that a mean arterial blood pressure (BP) target of <92 mmHg (corresponding to <130/80) vs. 102–107 (<140/90) did not reduce the risk of the composite of 50% reduction in GFR, dialysis-dependence, or death.6 However, effects differed according to baseline proteinuria, with a potential benefit in those with a protein-creatinine ratio >0.22.
Table 1.
Randomized trials of blood pressure target for CV outcomes in NDD-CKD
| Trial | BP Target* | Comparator* | Duration (y) | Sample (mean eGFR±SD) | CV Outcome Events |
|---|---|---|---|---|---|
| MDRD Study4 (1994) | MAP 92a | MAP 107 | 2.2 | 585 w/eGFR 25–55 (38.6±8.9); 255 w/eGFR 13–24 (18.5±3.4) | CV death: NS |
| REIN-25 (2005) | BP <130/80 | BP <140/90 | 1.6 | 338 w/non-DM CKD3-4 (34.1±18.1 vs. 35.9±18.6) | CV death: NS |
| AASK6 (2010) | MAP ≤92 | MAP 102–107 | 3.8 | 1,094 blacks w/HTN and CKD (46.0±12.9 vs. 45.3±13.2) | CV death or CV event rate: NS |
| ACCORD7 (2010) | SBP <120 | SBP <140 | 8.0 | 4,733 T2DM pts; sCr ≤1.5, of which 1,703 w/albuminuria (eGFR NR) | HR for all: 0.88 (95% CI, 0.73–1.06); NDD-CKD subgroup NR |
| SPRINT8 (2015) | SBP <120 | SBP <140 | 5.0 | 9,631 non-DM pts, of which 2,646 w/eGFR 20–60 (71.8±20.7 vs. 71.7±20.5) | HR for all: 0.75 (95% CI, 0.64–0.89) |
| SPRINT CKD subgroup9 (2017) | SBP <120 | SBP <140 | 3.3 | 2,646 non-DM pts w/eGFR 20–60 (47.9±9.5) | CV events HR: 0.81 (95% CI, 0.63–1.05); death HR: 0.72 (95% CI, 0.53–0.99) |
Abbreviations: CI, confidence interval; AASK, African American Study of Kidney Disease and Hypertension; ACCORD, Action to Control Cardiovascular Risk in Diabetes; BP, blood pressure; CKD-5D, dialysis-dependent chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate (in mL/min/1.73); HR, hazard ratio; MAP, mean arterial pressure; MDRD, modification of diet in renal disease; NDD-CKD, non-dialysis-dependent chronic kidney disease; REIN-2, Ramipril Efficacy In Nephropathy-2; RR, relative risk; SBP, systolic blood pressure; sCr, serum creatinine (in mg/dL); SD, standard deviation; SPRINT, Systolic Blood Pressure Intervention Trial; NR, not reported; pts, patients.
A MAP of 92 is lower than the blood pressure target of 130/80 mm Hg, traditionally recommended for CKD patients; a MAP of 107 mm Hg corresponds a blood pressure target of 140/90 mm Hg; HTN, hypertension.
units are mm Hg
Only two trials studied effects of blood pressure target on primary CV endpoints. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial and the Systolic Blood Pressure Intervention Trial (SPRINT) compared a systolic blood pressure target of 120 vs. 140 mmHg in diabetic and non-diabetic samples, respectively, which included participants with NDD-CKD (Table 1). ACCORD excluded patients with a serum creatinine >1.5 mg/dL, but 36% had CKD defined by albuminuria. Results indicated that in diabetics, lower blood pressure did not reduce CV events except for stroke, and was associated with more serious adverse events.7 SPRINT, in which 28% of participants had NDD-CKD (estimated glomerular filtration rate [eGFR] 20–60 mL/min/1.73m2), reported that those randomized to lower blood pressure had fewer CV events and all-cause mortality.8 Among those with NDD-CKD, there was a reduction in all-cause death and a trend toward reduction of CV events with intensive blood pressure control.9 Although there was an increased rate of eGFR decline and more instances of acute kidney injury, hyperkalemia, and hypokalemia in the intensive BP control group, there was no difference in doubling of creatinine and ESRD or in total adverse events.9 Two meta-analyses reported no difference in CV events with intensive vs. standard blood pressure control in CKD individuals,10, 11 but neither included data from SPRINT. The most recent meta-analysis, which included results from SPRINT, showed improved all-cause mortality with lower blood pressure targets, but other CV outcomes were not studied.12
Evidence is even more limited in CKD-5D. Substantial differences in pre-, inter-, and post-dialysis blood pressures, as well as session-to-session variability, complicate diagnosis and treatment.13 Although previous studies reported that the mean of 3–4 weeks of in-center blood pressure readings predict LV mass equally as well as ambulatory measurements, more recent studies indicate that blood pressures measured outside of the dialysis unit, either by the patient at home or by 44-hour ambulatory blood pressure monitoring, prognosticate left ventricular hypertrophy and death more accurately than in-center blood pressures.14, 15 The feasibility of home or ambulatory blood pressure monitoring, such as availability, reproducibility, and patient adherence needs to be better established.15–18
Observational studies revealed that the association between blood pressure and mortality follows a U-shaped curve, with pre–dialysis session systolic blood pressures <120 and >180 mmHg associated with increased risk.19 No randomized trials have addressed the effect of blood pressure target in CKD-5D on hard outcomes. A meta-analysis of studies comparing antihypertensive treatments to lower blood pressure vs. placebo or no treatment favored active treatment for decreasing CV events and deaths. However, it is unclear whether the benefit was from antihypertensive medication class or from blood pressure lowering effect, with a modest weighted mean decrease in blood pressure of 4.5/2.3 mmHg between active and control treatment arms.20
Conclusion
Available evidence is mixed regarding whether intensive blood pressure control reduces CV events in NDD-CKD, but there are no trials specifically designed to address this question. Current knowledge is based on CKD subgroup analyses, and the two major studies addressing this, SPRINT and ACCORD, have discordant results. In both trials, intensive blood pressure control was associated with increase in some adverse events, but there may be mortality and possibly CV event reduction with intensive control in non-diabetics. In CKD-5D, although the optimal blood pressure target remains unknown, data indicate that treating hypertension may reduce CV events.
Antihypertensive medication classes
Three points should be considered in practice about the use antihypertensives in CKD before considering effect on CV outcomes: First, evidence favors angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) over other medication classes for improvement of renal outcomes in NDD-CKD patients, particularly in those with albuminuria; second, diuretics are the cornerstone of management in NDD-CKD patients because volume overload is a major driver of hypertension; and third, NDD-CKD and CKD-5D patients often require more than one medication to control blood pressure.
In NDD-CKD, trials investigating the effect of ACEi compared to placebo on CV outcomes have reported mixed results (Table 2). The Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) Study, designed to capture renal endpoints in diabetics with nephropathy, showed that losartan vs. placebo was associated with a decrease in hospitalization for heart failure but no difference in composite CV outcomes or death.21 One post hoc analysis of individuals with serum creatinine between 1.4 and 2.3 mg/dL showed a significant improvement in CV death but not composite CV outcomes in the ACEi group.22 Several other subgroup analyses of individuals with eGFR <60 mL/min./1.73 m2 showed reduced CV outcomes or all-cause death for ACEi vs. placebo.23–25 Three other studies in albuminuric individuals, one of which may have been limited by power and the other two of which were large studies with over 700 CV events, showed no benefit of ACEi compared to placebo.26–28
Table 2.
Randomized trials of antihypertensive medications for the prevention of CV events in CKD
| Trial | Intervention | Comparator | Duration (y) | Event no. in CKD | Sample (mean eGFR±SD) | Outcome events |
|---|---|---|---|---|---|---|
| NDD-CKD | ||||||
| RAAS blockade vs. control | ||||||
| RENAAL21 (2001) | Losartan | Placebo | 3.4 | 515 | 1,513 DM pts w/ACR >30 mg/g ×2 & SCr 1.3–3.0 (eGFR NR) | Secondary outcome (CV death, MI, stroke, CHF, unstable angina, or revascularization): risk reduction, 10% (P=0.26) |
| PREVEND IT26 (2004) | Fosinopril | Placebo | 3.8 | 45 | 864 w/ACR >15 in NL (eGFR NR) | Primary outcome (CV death, MI, CHF, PVD, or stroke): HR, 0.60 (0.33–1.10) |
| DIABHYCAR27 (2004) | Ramipril | Placebo | 4.0 | 739 | 4,912 T2DM pts w/albuminuria ≥20 mg/L (eGFR NR) | Primary outcome (CV death, MI, stroke, CHF, or ESRD): HR, 1.03 (0.89–1.20) |
| HOPE CKD subgroup22 (2001) | Ramipril | Placebo | 4.5 | 218 | 9,287 w/vascular disease or DM, of which 980 w/sCr 1.4–2.3 (eGFR NR) | Primary outcome (CV death, MI, or stroke: HR, 0.80 (0.59–1.09) HRs in CKD: 0.59 (0.39–0.91) for CV death, 0.78 (0.54–1.11) for MI, 0.83 (0.44–1.56) for stroke |
| SAVE CKD subgroup23 (2004) | Captopril | Placebo | 3.5 | 327 | 2,183 post-MI pts w/LVEF ≤40 & sCr<2.5 (70.0±20.7), of which 719 w/eGFR <60 | Primary outcome (CV morbidity & mortality): RR in CKD, 0.69 (0.55–0.86) |
| PEACE CKD subgroup24 (2006) | Trandolapril | Placebo | 5.0 | 161 | 8,290 w/CAD (77.6±19.4), of which 1,355 w/eGFR <60 | Primary outcome (all-cause death): HR in CKD, 0.73 (0.54–1.00); P=0.05 |
| PROGRESS CKD subgroup25 (2007) | Perindopril | Placebo | 5.0 | 400 | 6,105 w/CBVD, of which 1,757 w/CrCl <60 (eGFR NR) | Primary outcome (CV death, MI, or stroke): RR in CrCl <60, 0.70 (0.58–0.86); NNT for 5 y=11 |
| ADVANCE CKD subgroup28 (2007) | Perindopril-indapamide | Placebo | 4.3 | 742 | 11,140 T2DM pts, of which 2,862 w/ACR 30-300, 401 w/ACR >300 (eGFR NR) | Primary outcome: major macrovascular (CV death, MI, stroke) or microvascular (incident albuminuria, Scr doubling, ESRD) events: RRs of 0.92 (0.81–1.04) for total study & 1.01 (0.82–1.23) for those w/history of microvascular disease |
| Calcium channel blockers vs. control | ||||||
| BENEDICT-B35 (2011) | Verapamil + trandolapril | Trandolapril alone | 4.5 | 41 | 281 T2DM pts w/HTN & microalbuminuria (eGFR NR) | Major CV events: HR, 0.93 (0.50–1.72) |
| Head-to-head trials of antihypertensive classes | ||||||
| NEPHRON D30 (2013) | Losartan + lisinopril | Losartan | 2.2 | 270 | 1,448 T2DM pts w/eGFR 30–90 & ACR >300 (53.7±16.2 vs. 53.6±15.5) | Tertiary outcome (MI, CHF, or stroke): HR, 0.97 (0.76–1.23) |
| CASE-J CKD subgroup37 (2009) | Candesartan | Amlodipine | 3.2 | 201 | 2,720 JP pts w/HTN & eGFR <60 or dipstick proteinuria (mean eGFR NR) | Primary outcome (CV death, MI, or stroke): HRs of 0.95 (0.72–1.25) overall, 1.24 (0.61–2.54) for CKD1-2, 1.01 (0.73–1.40) for CKD3, 0.45 (0.20–1.00) for CKD4; P=0.048 |
| ONTARGET subgroup29 (2011) | Telmisartan + ramipril | Telmisartan or ramipril | 4.7 | 1,281 in eGFR <60; 902 in ACR 30-300; 403 in ACR >300 | 5,623 w/eGFR <60, of which 3,809 w/ACR 30-300, 1,287 ACR >300 (50.2±8.1) | Primary outcome (CV death, MI, stroke, or CHF): HRs of 0.99 (0.88-1.12) for eGFR <60, 1.02 (0.89-1.17) for ACR 30-300, 0.90 (0.73-1.12) for ACR >300 |
| ALLHAT CKD subgroup34 (2012) | Amlodipine | Chlorthalidone | 8.8 | 984 CV death, 622 CHD, 1,353 CVD | 31,350 HTN pts w/>=1 other CV risk factor, of which 5,545 w/eGFR <60 (50.6±8.5 vs 50.1±8.7) | HRs of 1.01 (0.82-1.23) for CV death in eGFR <60, 0.86 (0.66-1.11) for CHD in eGFR <60, 1.04 (0.88-1.24) for CVD in eGFR <60 |
| ALLHAT subgroup34 (2012) | Lisinopril | Chlorthalidone | 8.8 | 973 CV death, 643 CHD, 1,534 CVD | 31,350 HTN pts w/>=1 other CV risk factor, of which 5,545 w/eGFR <60 (50.1±8.6 vs. 50.1±8.7) | HRs of 0.90 (0.73-1.11) for CV death in eGFR <60, 0.92 (0.72-1.19) for CHD in eGFR <60, 0.99 (0.83-1.18) for CVD in eGFR <60 |
| COPE subgroup36 (2013) | Beta blocker (various) | ARB (various) | 3.6 | 17 | 3,501 HTN pts on benidipine, of which 834 w/CKD3 (58.7±15.2) | Composite CV secondary outcome (CV death, MI, or stroke): HR in CKD, 0.92 (0.36-2.40) |
| COPE subgroup36 (2013) | ARB (various) | Thiazide diuretic (various) | 3.6 | 16 | 3,501 HTN pts on benidipine, of which 834 w/CKD3 (58.7±15.2) | Composite CV secondary outcome (CV death, MI, or stroke): HR in CKD, 1.19 (0.44-3.20) |
| COPE subgroup36 (2013) | Beta blocker (various) | Thiazide diuretic (various) | 3.6 | 15 | 3,501 HTN pts on benidipine, of which 834 w/CKD3 (58.7±15.2) | Composite CV secondary outcome (CV death, MI, or stroke): HR in CKD, 1.10 (0.40-3.04) |
| OSCAR subgroup38 (2013) | ARB uptitration | ARB plus CCB combination | 3.0 | 46 | 1,164 elderly JP HTN pts (67.2±18.7), of which 353 w/eGFR <60 (47.3±8.9 vs. 48.3±9.3) | Primary outcome (all-cause death and CV events): HR in CKD, 2.25 (1.20-4.20) |
| CKD-5D | ||||||
| RAAS blockade vs. control | ||||||
| Zannad, et al., FOSDIAL42 (2006) | Fosinopril | Placebo | 2.0 | 130 | 397 prevalent HD pts | Primary outcome (CV death, resuscitated death, stroke, CHF, MI, or revascularization): RR, 0.93 (0.68-1.26); BP same between groups |
| Takahashi, et al.39 (2006) | Candesartan | Nothing | 3.0 | 24 | 80 prevalent HD pts | Primary outcome (sudden death, MI, unstable angina, CHF, or severe arrhythmia): OR, 0.23 (0.08-0.67) |
| Suzuki, et al.40 (2008) | Various ARBs | No ARB | 3.0 | 93 | 360 prevalent HD pts | Primary outcome (CV death, MI, stroke, CHF, or revascularization): HR, 0.51 (0.33-0.79); BP same between groups |
| Cice, et al.41 (2010) | Telmisartan | Placebo | 3.0 | 149 all-cause death, 123 CV death | 332 prevalent HD pts already taking ACEi | Primary outcomes (all-cause death; CV death; CHF): HRs of 0.51 (0.32-0.82) for All-cause death, 0.42 (0.38-0.61) for CV death |
| Iseki, et al., OCTOPUS43 (2013) | Olmesartan | No ACEi/ARB | 3.5 | 135 | 469 prevalent JP HD pts | Primary outcome (death, stroke, MI, or coronary revascularization): HR, 1.00 (0.71-1.40); BP same between groups |
| Matsumoto, et al.44 (2014) | Spironolactone | Nothing | 3.0 | 32 | 309 prevalent HD pts | Primary outcome (CV death or hospitalization for CV event): HR, 0.38 (0.17-0.83) |
| Lin, et al.45 (2016) | Spironolactone | Placebo | 2.0 | 32 | 253 prevalent HD pts | Primary outcome (CV death or aborted cardiac arrest): HR, 0.42 (0.26-0.78) |
| Beta blockers vs. control | ||||||
| Cice, et al.46 (2003) | Carvedilol | Placebo | 2.0 | 71 All-cause death, 55 CV death, 1 MI | 114 prevalent HD pts w/dilated cardiomyopathy | Primary outcomes: LV end systolic and diastolic volume and EF Secondary outcomes: HRs of 0.51 (0.32-0.82) for All-cause death, 0.32 (0.18-0.57) for CV death, 0.81 (0.61-1.34) for MI BP lower in carvedilol vs placebo |
| Calcium channel blockers vs. control | ||||||
| Tepel, et al.47 (2008) | Amlodipine | Placebo | 4.0 | 51 | 251 prevalent HD pts w/HTN | Primary outcome: all-cause death HR for CV event or all-cause death: 0.55 (0.31-0.97) BP lower in amlodipine vs placebo |
| Head-to-head trials of antihypertensive classes | ||||||
| Agarwal, et al.16(2014) | Lisinopril | Atenolol | 1.0 | 34 | 200 prevalent HD pts w/HTN and LVH | Primary outcome: change in LVMI Serious adverse events: HR of 2.29 (1.07-5.21) for MI, stroke, CV death, CHF (outcome worse in lisinopril group) |
Abbreviations: OR, odds ratio (values in parenthese are 95% confidence intrvals); ACEi, angiotensin converting enzyme inhibitor; ACR, urinary albumin-creatinine ratio (in mg/g); ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation; ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ARB, angiotensin receptor blocker; BENEDICT-B, Bergamo Nephrologic Diabetes Complications Trial-B; BP, blood pressure; CAD, coronary artery disease; CASE-J, Candesartan Antihypertensive Survival Evaluation in Japan; CHD, coronary heart disease; CHF, congestive heart failure; CKD-5D, dialysis-dependent chronic kidney disease; COPE, Combination Therapy of Hypertension to Prevent Cardiovascular Events; CrCl, creatinine clearance; CV, cardiovascular; DIABHYCAR, Non-insulin dependent diabetes, hypertension, microalbuminuria or proteinuria, cardiovascular events, and ramipril; eGFR, estimated glomerular filtration rate (in mL/min/1.73 m2); ESRD, end-stage renal disease; FOSDIAL, Fosinopril in Dialysis; HD, hemodialysis; HOPE, Heart Outcomes and Prevention Evaluation; HR, hazard ratio (values in parentheses are 95% confidence intervals); LVEF, left ventricular ejection fraction; MI, myocardial infarction; NDD-CKD, non-dialysis-dependent chronic kidney disease; NNT, number needed to treat; NR, not reported; OCTOPUS, Olmesartan Clinical Trial in Okinawan Patients Under OKIDS; OSCAR, Olmesartan and Calcium Antagonists Randomized; PEACE, Prevention of Events with ACE inhibition; PREVEND IT, Prevention of Renal and Vascular Endstage Disease Intervention Trial; PROGRESS, Perindopril Protection against Recurrent Stroke Study; PVD, peripheral vascular disease; RR, relative risk (values in parenthese are 95% confidence intrvals); SAVE, Survival and Ventricular Enlargement; sCr, serum creatinine (in mg/dL); SD, standard deviation; CBVD, cerebrovascular disease; pts, patients; NL, Netherlands; T2DM, type 2 diabetes mellitus; HTN, hypertension; JP, Japanese; LV, left ventricular; EF, ejection fraction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index
Importantly, 2 large randomized controlled trials have reported that dual blockade of the renin-angiotensin-aldosterone (RAAS) system is not efficacious for improvement of hard outcomes in NDD-CKD patients and results in adverse events. A secondary analysis of the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) reported no improvement in CV events in NDD-CKD patients treated with both telmisartan and ramipril compared to either alone, and an increased risk of serum creatinine doubling and hyperkalemia.29 The Veterans Affairs Nephropathy in Diabetes (NEPHRON-D) study showed that therapy with both losartan and lisinopril increased the risk of acute kidney injury and hyperkalemia, with no benefit for mortality or CV events compared to losartan alone.30 It is important to note that the study was terminated early due to adverse events and low conditional power to detect a treatment effect on the primary endpoint.
Multiple trials have compared different classes of antihypertensives in various samples, including diabetics and individuals with stable CAD, for prevention of CV outcomes.31–33 Even though these studies included individuals with either albuminuria or eGFR <60 mL/min/1.73 m2, the majority did not report a CKD subgroup, and no trials compared classes of anti-hypertensives specifically in NDD-CKD samples. A subgroup analysis of the Antihypertensive and Lipid-Lowering Treatment To Prevent Heart Attack Trial (ALLHAT) stratified by eGFR showed no difference in CV outcomes between chlorthalidone, amlodipine, and lisinopril in those with eGFR <60 mL/min/1.73 m2.34 Several other studies also reported no differences in CV events between classes of antihypertensives in NDD-CKD samples.35–38 Only one open-label study showed worse CV outcomes over 3 years with up-titration of ARB compared to adding a calcium channel blocker (CCB) to an ARB for hypertension control.38 A 2013 meta-analysis of trials comparing various antihypertensive classes to either placebo or head-to-head in individuals with eGFR <60 mL/min/1.73 m2 showed that CV outcomes were improved in studies of ACEi vs. placebo and either ACEi or CCB vs. placebo. Trials of ACEis vs. diuretics, beta-blockers, or CCBs, and studies of CCBs vs. diuretics or beta-blockers showed no difference in CV outcomes between groups (Table 2).11
In hemodialysis samples, three studies comparing RAAS blockers – ACEis, ARBs, or aldosterone receptor blockers – to either placebo or open-label control showed improvement in CV outcomes in the RAAS blocker groups,39–41 but two others showed no difference.42, 43 Two studies showed benefit of spironolactone over placebo in preventing CV events in hemodialysis patients, but there was a high dropout rate in the spironolactone arm in both, primarily due to gynecomastia.44, 45 Other studies reported benefit from carvedilol or amlodipine over placebo in hemodialysis patients.46, 47 A study comparing treatment with lisinopril vs. atenolol did not show a difference in left ventricular mass regression, but was terminated early due to increased CV events and hyperkalemia in the lisinopril arm.16
Conclusions
RAAS blockade as compared with placebo has been shown to decrease CV events in some studies in NDD-CKD patients, but head-to-head studies have not reported benefit of one antihypertensive drug class over another, and large comparative effectiveness trials are lacking. Trials in hemodialysis patients have shown mixed results for CV event reduction, but most favor RAAS blockade.
ASPIRIN
We previously reviewed the evidence for anti-platelet agents in NDD-CKD.48 This section focuses on the most commonly prescribed antiplatelet agent, aspirin, and the evidence about its use for primary or secondary prevention in NDD-CKD and CKD-5D.
Primary prevention in NDD-CKD
Because CKD patients are at increased baseline risk of both bleeding and thrombosis,48, 49 the use of aspirin for primary prevention deserves consideration. Data from 3 randomized trials address the efficacy and/or safety of low-dose aspirin for primary prevention in NDD-CKD (Table 3). The United Kingdom Heart and Renal Protection (UK-HARP-I) study, not powered to evaluate CV outcomes, showed no increased risk of major bleeding with aspirin use vs. placebo. However, minor bleeding was increased 3-fold.50 Subsequently, a subgroup analysis of the Hypertension Optimal Treatment (HOT) study showed that among individuals with hypertension, low-dose aspirin decreased CV events in those with eGFR <45 mL/min/1.73 m2, but also doubled the risk of major bleeding events.51 Finally, an open-label comparison between low-dose aspirin and no aspirin in Japanese diabetics showed no difference in CV events (29 vs. 19 events, respectively) or major bleeding risk in those with eGFR <60 mL/min/1.73 m2, but did show CV benefit in participants with eGFR 60–89 mL/min/1.73 m2.52 A meta-analysis of these 3 trials demonstrated no difference in CV events, but a significant increase in both major and minor bleeding.53
Table 3.
Studies of the safety and efficacy of aspirin for prevention of CV events in CKD
| Study | Study Design | Predictor Variable | Comparator | Duration (y) | Sample (eGFR mean ±SD or median[IQR]) | Outcome, RR (95% CI) |
|---|---|---|---|---|---|---|
| NDD-CKD | ||||||
| UK-HARP-I50 (2005) | RCT | Aspirin 100 mg daily | Placebo (2×2 design w/simvastatin) | 1.0 | 242 NDD-CKD pts (SCr≥1.7, eGFR NR), 73 HD pts, 133 KTRs w/no known CAD | CV events: not assessed; Major bleeding: 0.66 (0.19-2.31); Minor bleeding: 2.8 (1.5-5.3) |
| HOT post hoc51 (2010) | RCT | Aspirin 75 mg daily | Placebo | 3.8 | 18,597 w/diastolic HTN w/no known CAD, of which 3,083 w/eGFR 45-59 (55 [52-58] vs. 55 [52-58]), 536 w/eGFR <45 (40 [34-43] vs. 39 [32-43]) | CV events: 0.85 (0.73-0.98) overall, 0.91 (0.76-1.09) in eGFR ≥60, 0.85 (0.61-1.17) in eGFR 45-59, 0.34 (0.17-0.67) in eGFR <45; Major bleeding: 2.04 (1.05-3.96) |
| JPAD52 (2011) | RCT | Aspirin 81 or 100 mg daily (physician discretion) | Nothing (open label) | 4.4 | 2,523 JP DM pts w/no known CAD (74±31), of which 632 w/eGFR <60 (mean eGFR NR) | CV events: 1.36 (0.73-2.54) overall, SS in eGFR 60-89, NS in eGFR <60; Major bleeding: 1.70 (0.43-6.72) |
| Major, et al.53 (2016) | MA | Aspirin | Control | N/A | 3 trials, all w/no known CAD (eGFR NR) | CV events: 0.92 (0.49-1.73); CHD: 0.79 (0.34-1.87); ; major bleed: 1.98 (1.11-3.52); Minor bleed: 2.70 (1.66-4.39) |
| Palmer, et al.54 (2012) | MA | Anti-platelet therapy | Control | N/A | 31 trials in pts w/or at risk for CAD (eGFR NR) | Fatal/nonfatal MI: 0.66 (0.51-0.87); CV death: 0.91 (0.60-1.36); Major bleeding: 1.29 (CI 0.69-2.42); Minor bleeding: 1.70 (CI 1.44-2.02) |
| CKD-5D | ||||||
| DOPPS56 (2007) | Nested case-control | Aspirin | No aspirin | 1.9 | 28,320 prevalent HD pts | CV events: 1.08 (1.02-1.14); Fatal/nonfatal MI: 1.21 (1.06-1.38); Stroke: 0.82 (0.69-0.98); GI bleed: 1.01 (0.88-1.17); Subdural hematoma: 0.56 (0.30-1.07) |
Relative risks reported for the DOPPS study were calculated using adjusted models.
Abbreviations: CAD, coronary artery disease; CHD, coronary heart disease; CKD-5D, dialysis-dependent chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate (in mL/min/1.73 m2); GI, gastrointestinal HD, hemodialysis; HOT, Hypertension Optimal Treatment; IQR, interquartile range; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirin in Diabetics; MI, myocardial infarction; NDD-CKD, non-dialysis-dependent chronic kidney disease; NR, not reported; NS, non-significant; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; SS, statistically significant; UK-HARP-I, United Kingdom Heart and Renal Protection-I; KTRs, kidney transplant recipients; JP, Japanese; DM, diabetes mellitus; pts, patients; MA, meta-analysis
Secondary prevention in NDD-CKD
There is even less robust data for aspirin use for secondary prevention in CKD. A meta-analysis of antiplatelet therapy for prevention of CV events in NDD-CKD patients with or at risk for coronary artery disease showed a decrease in myocardial infarction, no difference in CV death, all-cause death, or major bleeding, but a 1.7-fold increase in minor bleeding risk.54 This meta-analysis included the HOT and UK-HARP-I studies, as well as other studies reporting fewer than 30 CV events per group, so it was limited by the quality of the underlying evidence and did not distinguish those with known coronary artery disease from those being treated for primary prevention. More data exist from larger studies of aspirin in patients with acute coronary syndrome or undergoing percutaneous coronary intervention; multiple studies showed a non-significant trend toward benefit for MI, revascularization, or all-cause death, and an increase in major and minor bleeding events in these samples.54 Of the trials included in the meta-analysis, only one reported more than 100 outcome events.55
Primary and secondary prevention in CKD-5D
No randomized trials have studied the safety and efficacy of aspirin for primary or secondary prevention of CV events in CKD-5D. Observational data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) cohort shows that hemodialysis patients taking aspirin had a higher risk of CV events or MI and a lower risk of stroke than those not taking aspirin, but this could be biased by indication. This study reported no difference in gastrointestinal bleed or subdural hematoma between groups.56
Conclusions
Overall, existing data do not support the use of aspirin for primary prevention of CV events in NDD-CKD, as the bleeding risk may equal or outweigh the uncertain benefits. Data regarding aspirin for secondary prevention in NDD-CKD are limited, but suggest that there may be some preventive effect on myocardial infarction. Based on the HOT results, the 2013 KDIGO (Kidney Disease: Improving Global Outcomes) clinical practice guideline recommends aspirin for secondary prevention in those without excessive bleeding risk.57 There is insufficient evidence to support routine aspirin use in dialysis-dependent patients. Importantly, for CKD individuals presenting with acute coronary syndrome, the AHA recommends administering aspirin to reduce the risk of death and CV events.58 Further studies are needed to clarify which CKD patients may benefit from aspirin use for secondary prevention, and whether those appropriately treated with antiplatelet therapy in the setting of an acute event should be prescribed lifelong vs. time-limited therapy.
HYPERLIPIDEMIA
Evidence
Three large randomized, placebo-controlled trials studied the effect of lipid-lowering therapy using HMG-CoA (3-hydroxy-3-methylglutaryl–coenzyme A) reductase inhibitors (statins) on CV outcomes in NDD-CKD and CKD-5D (Table 4). The Study of Heart and Renal Protection (SHARP) compared a fixed dose combination of simvastatin and ezetimibe to placebo in >9,000 participants with CKD, about 30% of whom were dialysis-dependent. Lipid-lowering therapy significantly reduced the primary composite CV outcome and did not result in excess risk of adverse events, such as myopathy or hepatitis.59 The reduction in risk was most robust in the NDD-CKD subgroup and did not reach statistical significance in the CKD-5D subgroup.
Table 4.
Randomized trials of lipid-lowering therapy for prevention of CV events in CKD
| Study | Intervention | Comparator | Duration (y) | Sample (mean eGFR±SD) | Outcome |
|---|---|---|---|---|---|
| NDD-CKD | |||||
| SHARP59 (2011) | Simvastatin 20 mg/d + ezetimibe 10 mg/d | Placebo | 5.0 | 6,247 NDD-CKD pts (26.6±12.9 vs. 26.6±13.1); 3,023 CKD-5D pts | Primary outcome (composite of MI, coronary death, non-hemorrhagic stroke, or arterial revascularization): RR, 0.78 (0.67-0.91) in NDD-CKD |
| CKD-5D | |||||
| 4D60 (2005) | Atorvastatin 20 mg/d | Placebo | 4.0 | 1,255 prevalent HD pts w/DM | Primary outcome (composite of CV death, MI, or stroke): RR, 0.92 (0.77-1.10) |
| AURORA61 (2009) | Rosuvastatin 10 mg/d | Placebo | 5.0 | 2,776 prevalent HD pts | Primary outcome (composite of CV death, MI, or stroke): HR, 0.96 |
| SHARP59 (2011) | Simvastatin 20 mg/d + ezetimibe 10mg/d | Placebo | 5.0 | 6,247 NDD-CKD pts; 3,023 CKD-5D pts (2,527 HD; 496 PD) | Primary outcome (composite of MI, coronary death, non-hemorrhagic stroke, or arterial revascularization): RRs of 0.90 (0.75-1.08) in CKD-5D, 0.95 (0.78-1.15) in HD, 0.70 (0.46-1.08) in PD |
Abbreviations: 4D, Deutsche Diabetes Dialyse Studie; AURORA, Assessment of Survival and Cardiovascular Events; CKD-5D, dialysis-dependent chronic kidney disease; CV, cardiovascular; HD, hemodialysis; MI, myocardial infarction; NDD-CKD, non-dialysis-dependent chronic kidney disease; PD, peritoneal dialysis; RR, relative risk (values in parentheses are 95% confidence intervals); SD, standard deviation; SHARP, Study of Heart and Renal Protection; HR, hazard ratio.
The efficacy of statins vs. placebo in reducing CV events in hemodialysis patients was also studied in 4D (Die Deutsche Diabetes Dialyse Studie) and AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis). In both, although statin therapy lowered low-density lipoprotein cholesterol, there was no effect on the primary composite of CV death, MI, or stroke.60, 61 4D did report a nominally significant effect on reduction of all cardiac events combined.60
Conclusions
There is trial evidence to support the use of lipid-lowering therapy with statins for CV event reduction in NDD-CKD, without increasing risk of adverse events. Existing evidence suggests that lowering cholesterol with statins does not decrease risk of events in CKD-5D patients.
DIABETES
Evidence
There are no trials investigating the efficacy of target glycemic control for CV event reduction in diabetics with NDD-CKD or CKD-5D. Current recommendations for targeting a glycated hemoglobin of 7.0% are based on the reduction in microvascular events such as progression of albuminuria, doubling of creatinine, or worsening of diabetic retinopathy.57 Two large clinical trials comparing intensive vs. standard glucose control showed no difference in the composite of CV death, MI, or stroke. ACCORD showed no difference between a target hemoglobin A1c of ≤6.0% vs. 7.0–7.9% for improving CV outcomes, but the incidence of all-cause death and hypoglycemia increased with the lower target, which led to the termination of the intensive strategy arm. This study excluded those with a serum creatinine >1.5 mg/dL and did not report the subgroup with moderately increased albuminuria.62 The ADVANCE (Action in Diabetes and Cardiovascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) study compared a target hemoglobin A1c of ≤6.5% vs. standard control (achieved HbA1c=7.3%) for the same composite outcome, and included 3,261 (29.3%) participants with moderately increased albuminuria. The trial did not detect a difference, but the subgroup analysis showed a decrease in composite microvascular (development or worsening of diabetic nephropathy or retinopathy) and macrovascular (CV death, nonfatal MI, or stroke) events in those without prior microvascular disease (albuminuria >300 mg/g or proliferative retinopathy). No difference was seen in those with preexisting microvascular disease.63 A recent Cochrane meta-analysis reviewing glycemic targets in diabetics confirmed that intensive glycemic control did not improve CV outcomes.64
Conclusions
The current recommendation for a target HbA1c of 7.0% is based on extrapolation of decreased risk of microvascular events, such as worsening nephropathy or retinopathy. Subgroup analyses indicated that intensive glycemic control may be beneficial in those without preexisting microvascular disease. However, there are no trials of glycemic control intensity in individuals with albuminuria or early stage diabetic nephropathy for hard CV outcomes. The evidence suggests that at least in those with albuminuria >300 mg/g, more aggressive glycemic control does not prevent CV events. No studies have examined glycemic control intensity in CKD-5D patients.
LIFESTYLE MODIFICATION
Cigarette Smoking
Observational data suggest that smoking cigarettes increases risk for CV events in NDD-CKD, and that smoking cessation may attenuate that risk (Table 5). Two large cohort studies showed that current smokers with NDD-CKD are at higher risk of CV events than never smokers, and this relationship remained significant in a subgroup with prior CV disease or diabetes.65, 66 However, a study of diabetics comparing 10,128 current vs. 30,175 never smokers reported that the hazard ratio decreased and became non-significant in those with lower eGFR of 15–44 mL/min/1.73 m2.67 These studies also reported that former smokers had lower risk of CV events than current smokers.65, 66 Of the two studies comparing CV risk in former vs. never smokers, one showed that CV risk was higher in former smokers, and the other showed that it was higher in NDD-CKD samples with eGFR 45–59 or ≥60, but not 30–44 or 15–29 mL/min/1.73 m2.65, 67
Table 5.
Studies of smoking and CV events in CKD
| Study | Study Design | Predictor Variable | Comparator | Duration (y) | Sample (mean eGFR±SD) | Outcome |
|---|---|---|---|---|---|---|
| NDD-CKD | ||||||
| Cea Soriano, et al.67 (2015) | PC | Current or prior smokers | Never smokers | 6.8 | 57,946 T2DM pts, categorized by eGFR (mean eGFR NR) | Primary outcome (all-cause death): aHRs for Current vs. never smokers of 1.60 (1.48-1.74), 1.33 (1.18-1.50), 1.09 (0.84-1.40) for eGFR 45-49, 30-44, 15-29, respectively; risk for death higher in former vs never smokers w/eGFR 45-59 Secondary outcome (MI): aHRs for current vs. never smokers of 1.41 (1.17-1.70), 1.23 (0.91-1.66), 0.84 (0.42-1.70) for eGFR 45-59, 30-44, 15-29, respectively; Risk for MI same for former & never smokers for all eGFR |
| Ricardo, et al.66 (2015) | PC | Prior or never smokers | Current smokers | 4.0 | 3,006 in CRIC w/eGFR 20-70 (43±14) | Primary outcome (atherosclerotic events): aHRs vs current of 0.73 (0.54-0.99) for prior, 0.55 (0.40-0.75) for Never |
| Staplin, et al.65 (2016) | PC | Current or prior smokers | Never smokers | 4.0 | 9,270 in SHARP, of which 6,245 NDD-CKD (27.3±12.9 vs. 26.6±13.2 vs. 26.4±13.1), 3,023 CKD-5D | Primary outcome (all vascular events): aRRs vs never of 1.35 for current, 1.12 for prior; Atherosclerotic events: aRRs vs never or 1.49 for current, 1.09 for prior; No NDD-CKD subgroup analysis |
| CKD-5D | ||||||
| Cheung, et al.72 (2000) | CS | Current or prior smokers | Never smokers | N/A | 936 maintenance HD pts in HEMO study | CBVD: OR,1.79; PVD: OR, 1.77; CHD: OR, 1.11, NS |
| Zoccali, et al.70 (2002) | PC | Smokers | Non-smokers | 2.8 | 228 prevalent HD pts | Unadjusted HR NR (P=0.03); CV events per 1-pack more cigarettes per mo: aHR, 1.01 (0.99-1.02) |
| Mallamaci, et al.73 (2002) | PC | Smokers | Non-smokers | 2.4 | 175 anuric prevalent HD pts | CV events per 1-pack more cigarettes per mo: aHR 1.00 (0.99-1.02) |
| Huybrechts, et al.68 (2005) | PC | Smokers | Non-smokers | 4.1 | 179 prevalent HD pts | Composite CV events for nonsmokers: aHR, 0.67 (P=0.02) |
| Schwaiger, et al.69 (2006) | PC | Smokers | Never smokers | 5.5 | 154 prevalent HD pts | CV events: aHR, 1.91 (1.06-3.44) |
| Liebman, et al.74 (2011) | MA | Smokers | Non-smokers | N/A | 5 HD cohorts | CV events in HD: pooled aHR, 1.01 (0.98-1.05) |
| Staplin, et al.65 (2016) | PC | Current or prior smokers | Never smokers | 4.0 | 9,270 in SHARP, of which 2,527 on HD & 496 on PD | Results as detailed above in combined NDD-CKD & CKD-5D; no CKD-5D subgroup analysis done |
Adjusted hazard ratios and relative risks are presented where available (values in parenthese are 95% confidence intervals).
Abbreviations: 95% CI, 95% confidence interval; aHR, adjusted hazard ratio; aRR, adjusted relative risk; CKD-5D, dialysis-dependent chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HD, hemodialysis; HEMO, Hemodialysis; HR, hazard ratio; MI, myocardial infarction; NDD-CKD, non-dialysis-dependent chronic kidney disease; NS, not statistically significant OR, odds ratio; RR, relative risk; SD, standard deviation; SHARP, Study of Heart and Renal Protection; CBVD, cerebrovascular disease; PVD, peripheral vascular disease; CHD, coronary heart disease; PC, prospective cohort; MA, meta-analysis; CS, cross-sectional.
Observational studies show even more conflicting results in CKD-5D patients. Four showed that smokers had a higher risk of CV events and death than non-smokers,68–71 and 2 others showed no difference.72, 73 A meta-analysis of these studies concluded that smoking was not associated with increased CV event risk but was associated with all-cause death in hemodialysis patients.74
Weight loss
Although there are no prospective trials investigating whether weight loss improves outcomes in CKD, epidemiologic studies show an inverse paradoxical relationship between body mass index (BMI) and survival in NDD-CKD and CKD-5D patients, such that BMI in the overweight or obese range is associated with a survival benefit. In NDD-CKD samples, a BMI of 25–<30 vs. 20-<25 kg/m2 was associated with fewer atherosclerotic events, but BMI ≥30 vs. 20-<25 was not significant.66 Another observational study in diabetics showed lower risk of death in individuals with BMI 25–29 and ≥30 vs. 20–24 for all eGFR strata.67 The risk of MI in this study did not reach significance for any category of BMI or eGFR. Two other studies showed no difference in CV death with BMI categories.75, 76
In CKD-5D samples, multiple epidemiologic studies have confirmed an overall survival benefit associated with overweight or obese BMI.77–80 Several other studies, including a recent meta-analysis, reported decreased CV death associated with higher BMI.81–86 Proposed mechanisms include more stable hemodynamic status in obesity, differences in cytokine or neuroendocrine profiles such as tumor necrosis factor α, survival bias, or protection against frank protein energy malnutrition.87 Importantly, these observational studies are limited by confounding, given that multiple variables, including weight, blood pressure, and cholesterol, all demonstrate similar U-shaped associations with mortality, as is also seen in other chronic diseases such as heart failure.
Physical Activity
Few studies have investigated whether level of physical activity affects CV outcomes in CKD samples. In NDD-CKD, one large cohort study showed no difference in atherosclerotic events in those who self-reported physical inactivity, a less than ideal level physical activity, or an ideal level of physical activity.66 However, another study showed that individuals who enrolled in a weight management program had a lower risk of all-cause death or CV events than those who did not enroll.88 This study was limited by few events and may be confounded by motivation bias. In CKD-5D samples, two large cohort studies reported that individuals who exercised minimally had higher mortality than those who engaged in some regular physical activity.89, 90 Preexisting CV disease or overall poor health may limit physical activity, confounding the results. It is unclear whether prospectively engaging in physical activity would be safe or efficacious for CKD-5D patients.
Conclusions
There are no definitive prospective trials addressing the benefit of lifestyle modification in CKD patients. Subgroup analyses suggest that smoking cessation is likely beneficial in preventing CV events in NDD-CKD, with diminishing returns as eGFR declines and patients reach dialysis-dependence. Given the many other risks associated with smoking, it is likely worth encouraging smoking cessation in these patients. There may be a reverse epidemiological association between obesity and CV outcomes in CKD patients, particularly in those with CKD-5D. Some data supports that physical activity may be beneficial for reducing CV outcomes, but existing studies are not consistent.
SUMMARY AND PERSPECTIVE
Data examining whether modification of traditional CV risk factors decreases CV events and death in NDD-CKD and CKD-5D patients are limited. Although evidence for lipid-lowering therapy is robust, existing data for blood pressure targets and antihypertensive medication classes other than RAAS blockers, aspirin use, and tight glycemic control primarily comes from subgroup or post hoc analyses of trials conducted in non-CKD samples that were not designed to detect a difference in the CKD subgroup. Lack of statistical significance in CKD subgroups may be due to limited power or perhaps suggest that competing nontraditional risks increase CV events in those with advanced CKD, and attenuate the effects of traditional risk factor modifications. Data in CKD-5D can even less precisely be extrapolated from subgroup analyses, as dialysis patients were generally excluded from such trials. The few existing studies in this population are limited by noticeable biases. As practicing nephrologists, we are, therefore, left with the challenging conundrum of whether evidence to support such management is lacking because robust trials don’t yet exist, whether our patients are different from the general population, or, worse yet, is it just too late to intervene? Until more data become available, clinical management of CKD patients has to rely on clinical guidelines published by well-respected organizations which, understandably, are not always consistent and often have to be based on less than strong evidence and expert opinion. These guidelines are compared in Table 6. Ultimately, existing evidence does suggest that few of the interventions proven to reduce CV risk in the general population have an impact in individuals with NDD-CKD or CKD-5D, as detailed in Figure 1. However, adequately powered clinical trials designed to address these important knowledge gaps in CKD patients are desperately needed.
Table 6.
Clinical guidelines for modification of CV risk factors in CKD patients.
| Risk Factor | ACC/AHA | KDIGO | KDOQI |
|---|---|---|---|
| NDD-CKD | |||
| BP target | <130/80 (B)91 | ≤140/90 if no albuminuria (1B)92 ≤130/80 if albuminuria ≥30 mg/24 h (2D)92 | <130/80 (B)93 |
| BP medication choice | Use ACEi/ARB (B)91 | ACEi/ARB in DM pts with albuminuria ≥30 mg/24 h (2D)92 ACEi/ARB in non-DM pts with albuminuria ≥300 mg/24 h or equivalent (1B)92 | Other than ACEi/ARB for albuminuric pts, RCT evidence does not support specific recommendations (NR)93 |
| Aspirin | Low-dose aspirin should be used in DM pts with albuminuria or other CV risk factors (B)94 | Aspirin is indicated for secondary but not primary prevention (2B)92 | – |
| Lipid management | No specific recommendation for CKD; initiate high intensity statin if ≤75 y for secondary prevention, including for CKD subgroup (low)95 | Give statin if ≥50 y (1A-1B)96 Give statin if known CAD, DM, prior ischemic stroke, or estimated 10-y CVD risk >10% (2A)96 | Add to KDIGO guidelines to treat pts with <50 y and LDL ≥190 mg/dL with high intensity statins, as recommended by ACC/AHA (NR)97 |
| Glycemic control | Target HbA1c to < or ~7.0% (A)98 | Target HbA1c to ~7.0% (1A)92 | Target HbA1c <7.0% (A)99 |
| Smoking | No specific recommendations for CKD; smoking avoidance and cessation for all (NR) | Smoking cessation is an important modifiable CV risk factor (1D)92 | No evidence in CKD, but recommend smoking cessation as it is a CV risk factor (NR)93 |
| Weight loss | No specific recommendations for CKD; advise overweight and obese adults that the greater the BMI, the greater risk of CV disease, T2DM, and death (A)100 | Target BMI 20-25 (1D)92 | – |
| Physical activity | No specific recommendations for CKD; moderate to vigorous aerobic exercise 3–4×/wk, lasting ~40 min per session (B)101 | Goal physical activity 30 min 5×/wk as tolerated by CV health (1D)92 | RCTs in general population support exercise and there is little evidence that NDD-CKD patients may respond differently (NR)93 |
| CKD-5D | |||
| BP target | – | Aggressively treat pre-dialysis SBP ≥200; BP associated with minimal risk unknown; only study published showed best outcome for home SBP 120-145 (NR)102 | Pre-dialysis BP <140/90, post-dialysis BP <130/80 (C)103 |
| BP medication choice | – | No compelling evidence to recommend one class of anti-HTN agents over another (not rated)102 | ACEi/ARB preferred (greater LVH regression; reduce sympathetic nerve activity and PWV; may improve endothelial function and reduce oxidative stress) (C)103 |
| Aspirin | – | – | – |
| Lipid management | – | Statins should not be initiated, but should be continued if the pt is already treated (2C)96 | Add to KDIGO guidelines to consider statin initiation if recent acute coronary event, young age or long life expectancy, or on transplant wait-list (not rated)97 |
| Glycemic control | – | – | Dialysis pts with DM should follow the ADA guidelines (C)103 |
| Smoking | – | – | All dialysis pts should be counseled and regularly encouraged to stop smoking (A)103 |
| Weight loss | – | – | Safety and efficacy of weight loss in the overweight dialysis pt is unknown, as is the potential benefit to CV outcomes (NR)103 |
| Physical activity | – | – | Counsel and encourage all dialysis pts to increase their physical activity (B)103 Goal for activity is for CV exercise at moderate intensity for 30 min most, if not all, d/wk (C)103 |
The information in this table was adapted in summary form from published guidelines from the American College of Cardiology and American Heart Association (ACC/AHA), Kidney Disease: Improving Global Outcomes (KDIGO), and Kidney Disease Outcomes Quality Initiative (KDOQI). Quality of evidence is presented in parentheses
ACC/AHA: A, data from multiple randomized clinical trials; B, data from one randomized trial or nonrandomized studies; C, expert opinion
KDIGO: 1, recommended; 2, suggested; A, high quality; B, moderate quality; C, low quality; D, very low quality
KDOQI: A, strongly recommended and based on strong evidence; B, recommended and based on moderately strong evidence; C, recommended based on weak evidence or expert opinion
Abbreviations: ACC, American College of Cardiology; ACEi, angiotensin converting enzyme inhibitor; ADA, American Diabetes Association AHA, American Heart Association; ARB, angiotensin receptor blocker; BMI, body mass index (in kg/m2); CAD, coronary artery disease; CKD-5D, dialysis-dependent chronic kidney disease; CV, cardiovascular; HbA1c, hemoglobin A1c; KDIGO, Kidney Disease: Improving Global Outcomes; KDOQI, Kidney Disease Outcomes Quality Initiative; LDL, low density lipoprotein; LVH, left ventricular hypertrophy; NDD-CKD, non-dialysis-dependent chronic kidney disease; SBP, systolic blood pressure; NR, not rated; BP, blood pressure (given in mm Hg); DM, diabetes mellitus; T2DM, type 2 diabetes mellitus; PWV, pulse wave velocity; HTN, hypertension; pts, patients; CVD, cardio vascular disease
Figure 1. Evidence-based modification of traditional risk factors to mitigate CV disease and current knowledge gaps.
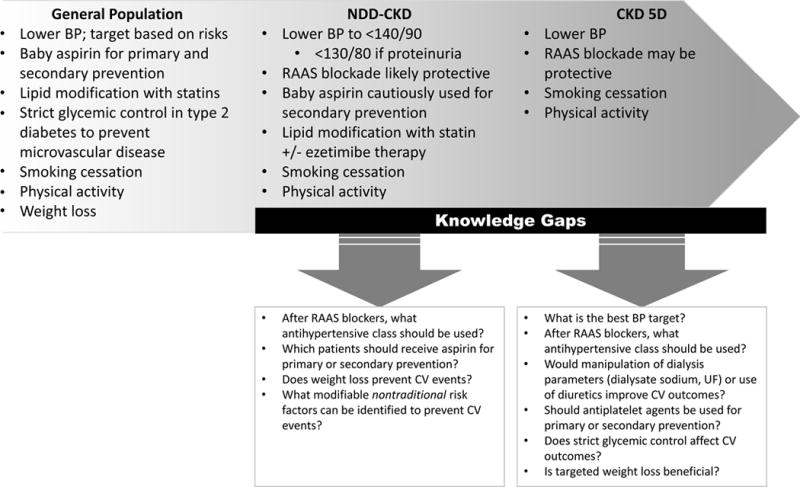
Abbreviations: BP, blood pressure; CKD-5D, dialysis-dependent chronic kidney disease; CV, cardiovascular; NDD-CKD, non-dialysis-dependent chronic kidney disease; RAAS, renin angiotensin aldosterone system; UF, ultrafiltration
Our understanding of the pathophysiology of CV disease in CKD is poor, and the ineffectiveness of most traditional risk factor modification strategies in these patient populations suggests that different metabolic pathways may underlie the development of medial arterial calcification seen in CKD, versus intimal plaque atherosclerosis seen in the general population. It may also suggest that these interventions need to be initiated earlier, because by the time our patients reach dialysis-dependence, or even stage 3 CKD, it may be too late to attenuate their excessive CV risk by these means. Although the management of traditional CV risk factors may prevent plaque atherosclerosis as it does in the general population, it is possible that these interventions do not attenuate medial arterial calcification in CKD or other CV pathogenic pathways, highlighting the critical importance of identifying nontraditional CV risk factors that may arise in the setting of kidney disease.
Acknowledgments
Support: This publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 to the University of Texas Southwestern Medical Center, as well as grant T32DK007257 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Dr Gregg). Support was also provided by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (NIDDK, P30DK079328).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no revelant financial interests.
References
- 1.United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2016. [Google Scholar]
- 2.Mathew RO, Bangalore S, Lavelle MP, et al. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: a review. Kidney Int. 2016;91(4):797–807. doi: 10.1016/j.kint.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365(9463):939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung AK, Rahman M, Reboussin DM, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol. 2017;28(9):2812–2823. doi: 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 11.Blood Pressure Lowering Treatment Trialists’ Collaboration. Ninomiya T, Perkovic V, Turnbull F, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ. 2013;347:f5680. doi: 10.1136/bmj.f5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra R, Nguyen HA, Benavente O, et al. Association Between More Intensive vs Less Intensive Blood Pressure Lowering and Risk of Mortality in Chronic Kidney Disease Stages 3 to 5: A Systematic Review and Meta-analysis. JAMA Intern Med. 2017;177(10):1498–1505. doi: 10.1001/jamainternmed.2017.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014;25(8):1630–1646. doi: 10.1681/ASN.2013060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2(6):1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47(1):62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29(3):672–681. doi: 10.1093/ndt/gft515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conion PJ, Walshe JJ, Heinle SK, Minda S, Krucoff M, Schwab SJ. Predialysis systolic blood pressure correlates strongly with mean 24-hour systolic blood pressure and left ventricular mass in stable hemodialysis patients. J Am Soc Nephrol. 1996;7(12):2658–2663. doi: 10.1681/ASN.V7122658. [DOI] [PubMed] [Google Scholar]
- 18.Zoccali C, Mallamaci F, Tripepi G, et al. Prediction of left ventricular geometry by clinic, pre-dialysis and 24-h ambulatory BP monitoring in hemodialysis patients: CREED investigators. J Hypertens. 1999;17(12 Pt 1):1751–1758. doi: 10.1097/00004872-199917120-00013. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(4):811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 20.Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373(9668):1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 22.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 23.Tokmakova MP, Skali H, Kenchaiah S, et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation. 2004;110(24):3667–3673. doi: 10.1161/01.CIR.0000149806.01354.BF. [DOI] [PubMed] [Google Scholar]
- 24.Solomon SD, Rice MM, Jablonski AK, et al. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation. 2006;114(1):26–31. doi: 10.1161/CIRCULATIONAHA.105.592733. [DOI] [PubMed] [Google Scholar]
- 25.Perkovic V, Ninomiya T, Arima H, et al. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. J Am Soc Nephrol. 2007;18(10):2766–2772. doi: 10.1681/ASN.2007020256. [DOI] [PubMed] [Google Scholar]
- 26.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110(18):2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 27.Marre M, Lievre M, Chatellier G, et al. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study) BMJ. 2004;328(7438):495. doi: 10.1136/bmj.37970.629537.0D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 29.Tobe SW, Clase CM, Gao P, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation. 2011;123(10):1098–1107. doi: 10.1161/CIRCULATIONAHA.110.964171. [DOI] [PubMed] [Google Scholar]
- 30.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 31.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):713–720. [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356(9227):359–365. doi: 10.1016/s0140-6736(00)02526-5. [DOI] [PubMed] [Google Scholar]
- 33.The ALLHAT Officers, Coordinators for the Antihypertensive Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Furberg CD, Wright JT, Davis BR, et al. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 34.Rahman M, Ford CE, Cutler JA, et al. Long-term renal and cardiovascular outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012;7(6):989–1002. doi: 10.2215/CJN.07800811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggenenti P, Fassi A, Ilieva A, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens. 2011;29(2):207–216. doi: 10.1097/hjh.0b013e32834069bd. [DOI] [PubMed] [Google Scholar]
- 36.Rakugi H, Ogihara T, Umemoto S, et al. Combination therapy for hypertension in patients with CKD: a subanalysis of the Combination Therapy of Hypertension to Prevent Cardiovascular Events trial. Hypertens Res. 2013;36(11):947–958. doi: 10.1038/hr.2013.63. [DOI] [PubMed] [Google Scholar]
- 37.Saruta T, Hayashi K, Ogihara T, et al. Effects of candesartan and amlodipine on cardiovascular events in hypertensive patients with chronic kidney disease: subanalysis of the CASE-J Study. Hypertens Res. 2009;32(6):505–512. doi: 10.1038/hr.2009.44. [DOI] [PubMed] [Google Scholar]
- 38.Kim-Mitsuyama S, Ogawa H, Matsui K, Jinnouchi T, Jinnouchi H, Arakawa K. An angiotensin II receptor blocker-calcium channel blocker combination prevents cardiovascular events in elderly high-risk hypertensive patients with chronic kidney disease better than high-dose angiotensin II receptor blockade alone. Kidney Int. 2013;83(1):167–176. doi: 10.1038/ki.2012.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi A, Takase H, Toriyama T, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis–a randomized study. Nephrol Dial Transplant. 2006;21(9):2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Kanno Y, Sugahara S, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52(3):501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 41.Cice G, Di Benedetto A, D’Isa S, et al. Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56(21):1701–1708. doi: 10.1016/j.jacc.2010.03.105. [DOI] [PubMed] [Google Scholar]
- 42.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70(7):1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 43.Iseki K, Arima H, Kohagura K, et al. Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28(6):1579–1589. doi: 10.1093/ndt/gfs590. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto Y, Mori Y, Kageyama S, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63(6):528–536. doi: 10.1016/j.jacc.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 45.Lin C, Zhang Q, Zhang H, Lin A. Long-Term Effects of Low-Dose Spironolactone on Chronic Dialysis Patients: A Randomized Placebo-Controlled Study. J Clin Hypertens (Greenwich) 2016;18(2):121–128. doi: 10.1111/jch.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 47.Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23(11):3605–3612. doi: 10.1093/ndt/gfn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain N, Hedayati SS, Sarode R, Banerjee S, Reilly RF. Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence? Clin J Am Soc Nephrol. 2013;8(4):665–674. doi: 10.2215/CJN.06790712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burlacu A, Genovesi S, Ortiz A, et al. The quest for equilibrium: exploring the thin red line between bleeding and ischaemic risks in the management of acute coronary syndromes in chronic kidney disease patients. Nephrol Dial Transplant. 2017;32(12):1967–1976. doi: 10.1093/ndt/gfx041. [DOI] [PubMed] [Google Scholar]
- 50.Baigent C, Landray M, Leaper C, et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis. 2005;45(3):473–484. doi: 10.1053/j.ajkd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56(12):956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 52.Saito Y, Morimoto T, Ogawa H, et al. Low-dose aspirin therapy in patients with type 2 diabetes and reduced glomerular filtration rate: subanalysis from the JPAD trial. Diabetes Care. 2011;34(2):280–285. doi: 10.2337/dc10-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Major RW, Oozeerally I, Dawson S, Riddleston H, Gray LJ, Brunskill NJ. Aspirin and cardiovascular primary prevention in non-endstage chronic kidney disease: A meta-analysis. Atherosclerosis. 2016;251:177–182. doi: 10.1016/j.atherosclerosis.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Palmer SC, Di Micco L, Razavian M, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;156(6):445–459. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 55.Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppresion Using Integrilin Therapy Trial. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998;339(7):436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 56.Ethier J, Bragg-Gresham JL, Piera L, et al. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2007;50(4):602–611. doi: 10.1053/j.ajkd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 58.Washam JB, Herzog CA, Beitelshees AL, et al. Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the American Heart Association. Circulation. 2015;131(12):1123–1149. doi: 10.1161/CIR.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 59.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 61.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 62.The Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 64.Ruospo M, Saglimbene VM, Palmer SC, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev. 2017;6:CD010137. doi: 10.1002/14651858.CD010137.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staplin N, Haynes R, Herrington WG, et al. Smoking and Adverse Outcomes in Patients With CKD: The Study of Heart and Renal Protection (SHARP) Am J Kidney Dis. 2016;68(3):371–380. doi: 10.1053/j.ajkd.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ricardo AC, Anderson CA, Yang W, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65(3):412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cea Soriano L, Johansson S, Stefansson B, Rodriguez LA. Cardiovascular events and all-cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. doi: 10.1186/s12933-015-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huybrechts KF, Caro JJ, London GM. Modeling the implications of changes in vascular calcification in patients on hemodialysis. Kidney Int. 2005;67(4):1532–1538. doi: 10.1111/j.1523-1755.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 69.Schwaiger JP, Neyer U, Sprenger-Mahr H, et al. A simple score predicts future cardiovascular events in an inception cohort of dialysis patients. Kidney Int. 2006;70(3):543–548. doi: 10.1038/sj.ki.5001589. [DOI] [PubMed] [Google Scholar]
- 70.Zoccali C, Mallamaci F, Parlongo S, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105(11):1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 71.Foley RN, Herzog CA, Collins AJ. Smoking and cardiovascular outcomes in dialysis patients: the United States Renal Data System Wave 2 study. Kidney Int. 2003;63(4):1462–1467. doi: 10.1046/j.1523-1755.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 72.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58(1):353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 73.Mallamaci F, Zoccali C, Tripepi G, et al. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002;61(2):609–614. doi: 10.1046/j.1523-1755.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 74.Liebman SE, Lamontagne SP, Huang LS, Messing S, Bushinsky DA. Smoking in dialysis patients: a systematic review and meta-analysis of mortality and cardiovascular morbidity. Am J Kidney Dis. 2011;58(2):257–265. doi: 10.1053/j.ajkd.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iff S, Wong G, Webster AC, et al. Relative energy balance, CKD, and risk of cardiovascular and all-cause mortality. Am J Kidney Dis. 2014;63(3):437–445. doi: 10.1053/j.ajkd.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 76.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 77.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80(2):324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 78.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55(4):1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 79.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56(3):1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 80.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13(4):1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 81.Ogawa T, Ishida H, Akamatsu M, et al. Progression of aortic arch calcification and all-cause and cardiovascular mortality in chronic hemodialysis patients. Int Urol Nephrol. 2010;42(1):187–194. doi: 10.1007/s11255-009-9574-5. [DOI] [PubMed] [Google Scholar]
- 82.Wanner C, Zimmermann J, Schwedler S, Metzger T. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl. 2002;(80):99–102. doi: 10.1046/j.1523-1755.61.s80.18.x. [DOI] [PubMed] [Google Scholar]
- 83.Postorino M, Marino C, Tripepi G, Zoccali C, Credit Working Group Abdominal obesity modifies the risk of hypertriglyceridemia for all-cause and cardiovascular mortality in hemodialysis patients. Kidney Int. 2011;79(7):765–772. doi: 10.1038/ki.2010.493. [DOI] [PubMed] [Google Scholar]
- 84.Beddhu S, Cheung AK, Larive B, et al. Inflammation and inverse associations of body mass index and serum creatinine with mortality in hemodialysis patients. J Ren Nutr. 2007;17(6):372–380. doi: 10.1053/j.jrn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fung F, Sherrard DJ, Gillen DL, et al. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis. 2002;40(2):307–314. doi: 10.1053/ajkd.2002.34509. [DOI] [PubMed] [Google Scholar]
- 86.Ladhani M, Craig JC, Irving M, Clayton PA, Wong G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32(3):439–449. doi: 10.1093/ndt/gfw075. [DOI] [PubMed] [Google Scholar]
- 87.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 88.MacLaughlin HL, Hall WL, Condry J, Sanders TA, Macdougall IC. Participation in a Structured Weight Loss Program and All-Cause Mortality and Cardiovascular Morbidity in Obese Patients With Chronic Kidney Disease. J Ren Nutr. 2015;25(6):472–479. doi: 10.1053/j.jrn.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 89.O’Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41(2):447–454. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 90.Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45(4):690–701. doi: 10.1053/j.ajkd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 91.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017 Nov 13;2017 doi: 10.1161/HYP.0000000000000065. [DOI] [Google Scholar]
- 92.Summary of Recommendation Statements. Kidney Int Suppl (2011) 2013;3(1):5–14. doi: 10.1038/kisup.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taler SJ, Agarwal R, Bakris GL, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62(2):201–213. doi: 10.1053/j.ajkd.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation. 2010;121(24):2694–2701. doi: 10.1161/CIR.0b013e3181e3b133. [DOI] [PubMed] [Google Scholar]
- 95.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 96.Summary of Recommendation Statements. Kidney Int Suppl (2011) 2013;3(3):263–265. doi: 10.1038/kisup.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarnak MJ, Bloom R, Muntner P, et al. KDOQI US commentary on the 2013 KDIGO Clinical Practice Guideline for Lipid Management in CKD. Am J Kidney Dis. 2015;65(3):354–366. doi: 10.1053/j.ajkd.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119(2):351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 99.National Kidney F. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 102.Levin NW, Kotanko P, Eckardt KU, et al. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2010;77(4):273–284. doi: 10.1038/ki.2009.469. [DOI] [PubMed] [Google Scholar]
- 103.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]


