Abstract
Context:
Central and peripheral neural adaptations have been identified after anterior cruciate ligament (ACL) injury and reconstruction (ACLR) and are hypothesized to contribute to posttraumatic muscle dysfunction. Limited evidence exists about the temporal nature of neuromuscular adaptations during early and late-term phases of recovery after ACLR, and no researchers have studied patients with posttraumatic osteoarthritis.
Objective:
To compare quadriceps neuromuscular function less than 2 years (early) and more than 2 years (late) after ACLR, including in patients who experienced posttraumatic knee osteoarthritis.
Design:
Cross-sectional study.
Setting:
Laboratory.
Patients or Other Participants:
A total of 72 patients after ACLR, consisting of 34 early (9.0 ± 4.3 months postsurgery), 30 late (70.5 ± 41.6 months postsurgery), and 8 with osteoarthritis (115.9 ± 110.0 months postsurgery), and 30 healthy control volunteers.
Main Outcome Measure(s):
Quadriceps function was measured bilaterally during a single visit to determine normalized Hoffmann reflex (H : M ratio), knee-extension maximal voluntary isometric contraction torque (Nm/kg), central activation ratio (%), fatigue index (% decline), and active motor threshold (%). Comparisons were made using 2-way analyses of variance to identify the effect of limb and group on each outcome measure. We calculated Cohen d effect sizes to assess the magnitude of difference between ACLR and matched control limbs for each group.
Results:
Compared with healthy control limbs, involved-limb maximal voluntary isometric contraction was lower among all patients after ACLR (P < .001, Cohen d values = −1.00 to −1.75). The central activation ratio (P < .001, Cohen d = −1.74) and fatigue index (P = .003, Cohen d = −0.95) were lower among patients only early after ACLR. The active motor threshold was higher among all patients after ACLR (P < .001, Cohen d values = −0.42 to −1.56).
Conclusions:
Neuromuscular impairments were present in patients early and late after ACLR, regardless of osteoarthritis status. Quadriceps strength and corticospinal excitability were impaired at each time point compared with values in healthy control individuals, suggesting the need to address cortical function early after ACLR.
Key Words: corticospinal excitability, muscle activation, spinal-reflex excitability
Key Points
Neuromuscular impairments occurred less than and more than 2 years after anterior cruciate ligament reconstruction in patients with or without knee osteoarthritis.
Quadriceps strength and corticospinal excitability were impaired at each time point for patients after anterior cruciate ligament reconstruction compared with healthy control participants.
Strategies that seem to be maladaptive appear to have both peripheral and central origins.
Clinical outcomes are multifactorial and not likely influenced solely by time.
Anterior cruciate ligament (ACL) injuries are common among young, active individuals and present a specific challenge to long-term joint health. The recommended treatment in this population remains ACL reconstruction (ACLR), yet poor outcomes are well documented.1 As many as one-third of patients will not return to their preinjury levels of activity,2 and among those who do, prospective data support the dramatically increased incidence of a second ACL injury to the ipsilateral or contralateral limb within 2 years of reconstruction.3,4 Of additional concern is the high prevalence of posttraumatic knee-joint osteoarthritis, with radiographic signs appearing as early as the first decade in more than one-third of patients after reconstruction.1 Quadriceps function has been widely studied relative to ACLR, both as a source of persistent impairment and as a contributing factor to subsequent knee-joint injury.5 Posttraumatic quadriceps dysfunction is well described after ACLR and is associated with increased self-reported disability.6 Given that articular cartilage degeneration is irreversible, the hallmark for prevention is early detection with a thorough evaluation of quadriceps neuromuscular function.
Central and peripheral neural adaptations in the muscular, spinal, and supraspinal regions have been identified after ACL injury and ACLR and are hypothesized to contribute to posttraumatic muscle dysfunction.7 The specific origins of impairment may present opportunities to develop a targeted approach to address underlying factors impeding the recovery of quadriceps function after ACLR. In their meta-analysis, Pietrosimone et al8 identified quadriceps weakness and central activation failure more than 4 years after ACLR7,9 and in patients with tibiofemoral osteoarthritis. Neuromuscular adaptations are theorized to arise from alterations in spinal-mediated and corticospinal pathways and, if not addressed, may be a limiting factor in recovery from ACL injury.10 Therefore, it may be necessary to assess each of these unique pathways to build a complete neuromuscular profile after ACLR in order to identify early deficits that may perpetuate muscle dysfunction.
Outcomes after major joint injury and reconstruction often change over time, suggesting that chronicity may be an important factor when identifying patients at risk for long-term consequences associated with altered neuromuscular function. The nature and magnitude of neural adaptations after peripheral joint injury are reported to change over time. For example, decreased spinal-reflex excitability in response to an artificial knee-joint effusion has been identified,11 suggesting an acute neural response to joint injury mediated at the spinal level. However, researchers7,9 have described conflicting results for the presence and direction of spinal-mediated alterations in the context of chronic ACL injury, which may be due to the variability of the naturally occurring injury or related to the phase of recovery. For example, Kuenze et al9 observed no differences in spinal-reflex excitability at an average of 2.5 years after ACLR compared with healthy individuals, whereas Pietrosimone et al7 noted a bilateral up-regulation at an average of 4 years after surgery. In contrast, immediate changes in corticospinal excitability have not been seen in response to an artificial knee-joint effusion12 but have been seen more than 3 years after ACLR.7,9 Collectively, these data provide evidence of the temporal nature of the pathophysiological response of quadriceps neuromuscular function to ACL injury and ACLR. Whereas neuromuscular adaptations appear to be an expected outcome after joint injury, these impairments are treatable and present a way for clinicians to detect centrally mediated impairments early with the intention of promoting optimal long-term joint health.
Evidence of central nervous system adaptations after ACLR is growing.7 However, the temporal relationship of these adaptations is unclear. Despite wide variability in times since surgery, patients are often classified as a single group for comparison with their healthy counterparts, which may prevent early detection of impairments or delay early intervention. To better understand how neuromuscular adaptations progress over time, time from surgery should be evaluated empirically. Paterno et al3,4 highlighted the implications of postoperative time with respect to the occurrence of secondary injury, demonstrating incidence rates that were 15 and 6 times greater than in matched controls at 13 and 24 years, respectively, after ACLR. Further study of this phase of recovery is warranted given the elevated risk for injury and inherent biological adaptations known to occur. It is possible that neuromuscular function within 2 years after ACLR influences long-term patient outcomes, such as the onset or progression of knee osteoarthritis. However, evidence is limited for the specific timing of alterations in quadriceps neuromuscular function less than and more than 2 years after ACLR, and no researchers have included patients with posttraumatic osteoarthritis after ACLR. Therefore, the purpose of our study was to compare quadriceps neuromuscular function at clinically relevant phases of recovery after ACLR, including in patients with posttraumatic knee osteoarthritis. We hypothesized that neuromuscular function would be diminished within 2 years after ACLR compared with healthy control participants but not different in patients without knee osteoarthritis more than 2 years after ACLR.8,13
METHODS
In this cross-sectional study, we investigated patients after ACLR and participants serving as healthy controls. Independent variables were group (<2 years after ACLR [early], >2 years after ACLR [late], osteoarthritis after ACLR [osteoarthritis], and healthy control) and limb (involved, contralateral). Dependent variables were measures of quadriceps neuromuscular function: Hoffmann reflex (H-reflex), knee-extension maximal voluntary isometric contraction (MVIC) torque, central activation ratio (CAR), fatigue index (FI), and active motor threshold (AMT). Quadriceps neuromuscular function was recorded bilaterally, and the order of testing was counterbalanced by limb within each group. Limb dominance was recorded for participants and determined by asking them which limb they would use to kick a ball.
Participants
A total of 102 individuals between the ages of 15 and 65 years volunteered for this study, including 72 patients assigned to the early (n = 34; 9.0 ± 4.3 months postsurgery), late (n = 30; 70.5 ± 41.6 months postsurgery), or osteoarthritis group (n = 8; 115.9 ± 110.0 months postsurgery) and 30 individuals serving as healthy controls (Table 1). Patients were recruited from our orthopaedic clinic, university, and community. To be eligible, patients must have undergone a primary, unilateral reconstruction with no postoperative complications. Patients were not excluded based on graft type or concomitant meniscal procedure. However, we excluded patients with a history of lower extremity injury other than ACLR within 6 months of the study, multiligament knee injury, lower extremity orthopaedic surgery before ACLR, or concussion within 6 months of the study. Patients assigned to the knee osteoarthritis group had documented radiographic evidence of tibiofemoral or patellofemoral compartment involvement (Kellgren-Lawrence grade > 1) at a minimum of 12 months after ACLR. We excluded patients with a diagnosis of osteoarthritis before ACLR. A convenience sample of healthy individuals with no history of lower extremity injury, joint surgery, or concussion within 6 months of the study was recruited from the community. Control participants were matched by mass and height. All participants were screened for the use of transcranial magnetic stimulation (TMS) according to the safety and ethical guidelines proposed by the International Federation of Clinical Neurophysiology.14 All participants or their parent or guardian provided written informed consent or assent, respectively, and the University of Virginia Institutional Review Board for Health Sciences Research approved this study.
Table 1.
Participant Demographics and Patient-Reported Outcomes
| Variable |
Group |
|||
| Time After Anterior Cruciate Ligament Reconstruction |
||||
| Healthy Control (n = 30) |
Early (<2 y; n = 34) |
Late (>2 y; n = 30) |
Osteoarthritis (n = 8) |
|
| Sex, no. (male/female)a | 12/18 | 20/14 | 10/20 | 2/6 |
| Time from diagnosis to surgery, y (range) | NA | NA | NA | 5.0 (1.0–18.0) |
| Graft type, % (n)a | ||||
| Patellar tendon | NA | 51.5 (18) | 27.6 (8) | 37.5 (3) |
| Hamstrings | NA | 42.4 (14) | 48.3 (15) | 62.5 (6) |
| Allograft | NA | 6.1 (2) | 24.1 (7) | 0.0 (0) |
| Meniscectomy, % (n)a | NA | 35.5 (12) | 37.0 (11) | 75.0 (6) |
| Meniscus repair, % (n)a | NA | 19.4 (7) | 14.8 (4) | 12.5 (1) |
| Mean ± SD |
||||
| Age, y | 22.7 ± 4.6 | 22.5 ± 6.3c | 24.9 ± 5.9c | 45.4 ± 7.4 d–f |
| Height, cm | 174.8 ± 11.8 | 174.1 ± 11.0 | 171.7 ± 11.8 | 170.0 ± 9.7 |
| Mass, kg | 75.1 ± 16.2 | 73.9 ± 16.9 | 74.9 ± 16.2 | 85.2 ± 24.8 |
| Time since surgery, mob | NA | 9.0 ± 4.3c,f | 70.5 ± 41.6c,e | 115.9 ± 110.0e,f |
| International Knee Documentation Committee Subjective Knee Evaluation Form score | 98.2 ± 4.2 | 81.5 ± 13.4c,d | 86.4 ± 10.4c,d | 62.9 ± 15.2d–f |
| Knee Injury and Osteoarthritis Outcome Knee Form score | ||||
| Totalb | 98.7 ± 2.5 | 87.5 ± 9.3d | 92.1 ± 6.0c,d | 76.4 ± 10.8d,f |
| Pain | 98.6 ± 3.7 | 90.1 ± 8.3c,d,f | 94.1 ± 5.8c–e | 79.2 ± 8.1d–f |
| Symptoms | 98.0 ± 4.1 | 84.8 ± 12.1c,d | 86.3 ± 10.1c,d | 71.4 ± 11.5d–f |
| Activities of Daily Livingb | 99.8 ± 0.7 | 95.8 ± 6.4d | 97.8 ± 3.1d | 90.1 ± 9.1d |
| Sportb | 97.8 ± 6.3 | 76.5 ± 21.1d | 88.5 ± 13.7c,d | 53.8 ± 29.4d,f |
| Quality of life | 96.5 ± 9.8 | 64.5 ± 20.6c,d,f | 78.1 ± 18.5c–e | 49.2 ± 27.4d–f |
| Western Ontario and McMaster Universities Arthritis Index score | ||||
| Totalb | 0.3 ± 0.8 | 4.9 ± 6.20c,d | 3.2 ± 2.9c,d | 10.9 ± 7.7d |
| Painb | 19.8 ± 0.6 | 19.3 ± 1.0c | 19.4 ± 0.9c | 17.6 ± 1.4d–f |
| Stiffnessb | 8.0 ± 0.0 | 7.0 ± 1.4d | 7.0 ± 1.4d | 6.5 ± 1.5d |
| Functionb | 67.9 ± 0.3 | 65.3 ± 4.4d | 66.5 ± 2.1d | 61.4 ± 6.3d |
| Tegner Activity Scale score | ||||
| Preinjury | NA | 8.4 ± 1.4c | 8.4 ± 1.1c | 6.3 ± 1.5e,f |
| Current | 7.2 ± 1.4 | 6.1 ± 1.9c,d | 6.9 ± 1.6c | 4.3 ± 1.7d–f |
| Visual analog scale, cmb | 0.1 ± 0.2 | 0.7 ± 0.9d,f | 0.2 ± 0.5c,e | 1.2 ± 0.8d,f |
| Tampa Scale for Kinesiophobia score | 28.6 ± 5.8 | 34.4 ± 5.7d,f | 32.1 ± 6.5c,e | 36.0 ± 6.0d,f |
| Veterans RAND 12-Item Health Survey score | 86.0 ± 7.6 | 80.4 ± 10.0c,d | 82.4 ± 6.7c | 68.9 ± 14.2d–f |
Abbreviation: NA, not applicable.
Fisher exact test.
Non-normally distributed (Kruskal-Wallis test).
Indicates different from the osteoarthritis group (P ≤ .05).
Indicates different from the healthy control group (P ≤ .05).
Indicates different from the early group (P ≤ .05).
Indicates different from the late group (P ≤ .05).
Procedures
Testing was conducted during 1 visit and always followed the same order: H-reflex, knee-extension MVIC torque, CAR, FI, and AMT. The same researcher (G.E.N.) performed all testing.
Patient-Reported Outcomes
Regional knee function was assessed using the 2000 International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form and Knee Injury and Osteoarthritis Outcome Score (KOOS). The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is advocated for patients with osteoarthritis and was used to assess pain, stiffness, and function. The combination of measurement tools for regional knee function was used to ensure appropriate assessment of all patients over a long duration after ACLR (range = 4–301 months). Pain, physical activity, fear, and perceived health status are likely to be influenced by ACL injury. Therefore, pain at rest was assessed using a visual analog scale; preinjury and current activity levels were assessed using the Tegner Activity Scale; fear of movement and reinjury was assessed using the Tampa Scale of Kinesiophobia; and global health perception was assessed using the Veterans RAND 12-Item Health Survey.
Quadriceps Spinal-Reflex Excitability
The H-reflex was used to quantify the spinal-reflex excitability of the vastus medialis as previously described.15 The area of the greatest bulk over the vastus medialis was cleaned, shaved, and debrided before placement of two 10-mm pregelled Ag-AgCl recording electrodes (model EL503; BIOPAC Systems, Inc, Goleta, CA). Electromyography (EMG) signals were sampled at 2000 Hz, band-pass filtered from 10 to 500 Hz, and processed using AcqKnowledge 4.2 software (BIOPAC Systems, Inc). The recording electrodes were outlined to ensure similar placement during corticospinal excitability testing. Testing was completed in a quiet, dimly lit room in which participants lay supine with a bolster under the knees to maintain approximately 15° of knee flexion and with hands folded over their chests. Participants wore earplugs (Aero Technologies, Indianapolis, IN) and were instructed, “Close your eyes, and clear your mind” during testing. The femoral artery was palpated, and a 4-mm, round, stimulating electrode (model EL254; BIOPAC Systems, Inc) was placed in the inguinal fold over the femoral nerve. A series of 10-millisecond square-wave electrical stimuli ranging from 10 to 200 V was delivered via a stimulator module (model STM100A; BIOPAC Systems, Inc) and a current isolation unit (model STMISOC; BIOPAC Systems, Inc) with a minimum of 10 seconds between stimuli. Three maximal H-reflexes were averaged and normalized to the average of 3 maximal muscle responses (M-wave) to calculate the H : M ratio.
Quadriceps Strength and Voluntary Activation
Knee-extension MVIC torque and quadriceps CAR were used to quantify quadriceps strength and voluntary activation. Participants were seated in a dynamometer (model System 3; Biodex Medical Systems, Inc, Shirley, NY) with their hips and knees flexed to 85° and 90°, respectively. They completed a standardized acclimatization protocol, in which a series of submaximal trials (25%, 50%, and 75% of perceived effort) was performed before 3 maximal-effort isometric trials were recorded. Trials that differed by more than 10 V were repeated until 3 consistent MVIC trials were recorded. Accessory motion was discouraged by using a lap belt and having participants cross their upper extremities over their chests while keeping their heads and shoulders back against the rest. To ensure maximal effort, we provided oral encouragement in a similar manner for all participants. We supplied participants with visual feedback via a 43-in (109-cm) liquid crystal display monitor and encouraged them to kick harder than in each previous trial. Participants rested for a minimum of 60 seconds between trials.
The superimposed-burst technique was used as previously described9 to estimate quadriceps CAR during the third MVIC trial only. A square-wave stimulator (model S88; GRASS-TeleFactor, West Warwick, RI) and isolation unit (model SIU8T; GRASS-TeleFactor) were used to manually deliver a supramaximal percutaneous electrical stimulus to the anterior thigh via two 3- × 5-in (8- × 13-cm) self-adhesive electrodes applied over the proximal vastus lateralis and distal vastus medialis. Force data were sampled at 125 Hz, low-pass filtered at 10 Hz, and normalized to body mass. A 100-millisecond epoch was recorded from a central stable region during trials 1 and 2 and immediately before the superimposed-burst torque (TSIB) during trial 3. The mean MVIC torque from the 3 trials was used for analysis, and the value from trial 3 was used to calculate the CAR (Equation 1).
Equation 1:
Quadriceps Fatigue Index
Quadriceps fatigue was quantified using a previously described16 index of torque decline during a 30-second knee-extension MVIC. Participants were instructed to kick out as hard as possible and to maintain the contraction while seated in a dynamometer—similar to the quadriceps strength testing. Oral encouragement and visual feedback were not used in order to mitigate the risk of transient aberrant increases in torque, which were observed during pilot testing. Force data were sampled at 125 Hz and low-pass filtered at 10 Hz. The mean torque was recorded from a series of 1-second epochs during the first 5 seconds, and the greatest torque epoch within this time frame was recorded as the maximum torque (TMax). The FI was calculated using the area under the force-time curve (AUFC) for the entire contraction period (range, 0–30 seconds), which began at the time of maximal muscle torque (TPM; Equation 2).
Equation 2:
Quadriceps Corticospinal Excitability
We used the TMS to quantify quadriceps corticospinal excitability as measured by the AMT. Participants were instructed to abstain from caffeine consumption and intense exercise for a minimum of 12 hours before testing. They were seated in the dynamometer and instructed to rest their hands in their laps and minimize accessory motion. Two surface EMG electrodes were re-placed over the vastus medialis and used to record activity in a manner similar to the H-reflex testing. Participants wore earplugs and an elastic swim cap with straight lines drawn in the sagittal and frontal planes to aid us in determining the appropriate location for the TMS coil over the primary motor cortex.17 A 1- × 1-cm grid was drawn on the swim cap to improve the precision of the delivered stimuli. Motor-evoked potentials (MEPs) were elicited in the vastus medialis using a magnetic stimulator (model Rapid; MagStim Company, Ltd, Wales, United Kingdom) with a 110-mm double-cone coil. The MEP signals were sampled at 2000 Hz and band-pass filtered from 1 to 5000 Hz. The coil was positioned over the contralateral cortical hemisphere in the area of M1 and shifted by 0.5 cm in the anterior, posterior, medial, and lateral directions to identify the optimal stimulating location (hotspot). When the hotspot was determined, the stimulus intensity was sequentially lowered by 5% until no MEP was detected and then increased by 1% until the MEP returned. The AMT was defined as the lowest intensity required to evoke a measurable MEP (>100 μV) during a tonic quadriceps contraction.14 Participants were instructed to maintain an isometric contraction at 5% of their previously determined MVIC, which was depicted by a solid line displayed in real time on a liquid crystal display monitor, and to relax immediately after each stimulus.
Statistical Analysis
A sample-size estimate was based on the minimal detectable change (MDC95) for each dependent variable, assuming an α level ≤ .05 and power of 1 − β = 0.80. The MDC95 was calculated as √2 × 1.96 × standard error of the mean. Accordingly, the MDC95 and required sample per group, respectively, for each dependent variable were 0.30 and 11 for the H : M ratio,18 47.8 Nm and 24 for MVIC,19 6.0% and 16 for CAR,19 11.0% and 11 for FI,20 and 8.4% and 14 for AMT.21
All data were assessed for normality using the Shapiro-Wilk test before analysis. Separate 1-factor analyses of variance were used to compare demographics and patient-reported outcomes among groups (4 levels). Separate mixed-model 2 × 4 (limb × group) analyses of variance were used to assess differences between the involved and contralateral limbs for each measure of quadriceps function across all groups. In healthy participants, the nondominant limb was initially treated as the involved limb. However, 57% of ACLR limbs were dominant. Therefore, a random-number generator (Excel 2016; Microsoft Corp, Redmond, WA) was used to reassign an equal proportion of dominant to nondominant limbs in the healthy control group compared with the ACLR groups (ie, the dominant limb was now treated as the involved limb in 57% of the participants in each group). Where appropriate, separate 1-factor analyses of variance were performed to compare groups (at 4 levels) for each limb when we observed main effects. We used Dunnett post hoc comparisons to compare each ACLR group with the healthy control group. Post hoc Fisher least significant difference comparisons were used to determine group differences among patients with ACLR only. Planned comparisons between limbs were made using paired-samples t tests. Cohen d effect sizes with associated 95% confidence intervals (CIs) were calculated to determine the magnitude of difference between the involved limbs in patients with ACLR and healthy individuals for each outcome measure. Effect sizes were interpreted as small (≤0.3), moderate (0.4–0.7), or large (≥0.08). Effect sizes with CIs that did not cross zero were interpreted as clinically meaningful.
As an exploratory analysis, the bivariate Pearson product moment correlation coefficient (r) was used to assess the relationship among measures of quadriceps neuromuscular function for each limb among patients with ACLR. The α level was set a priori at ≤ .05. All statistical analyses were performed using SPSS (version 20.0; IBM Corp, Armonk, NY).
RESULTS
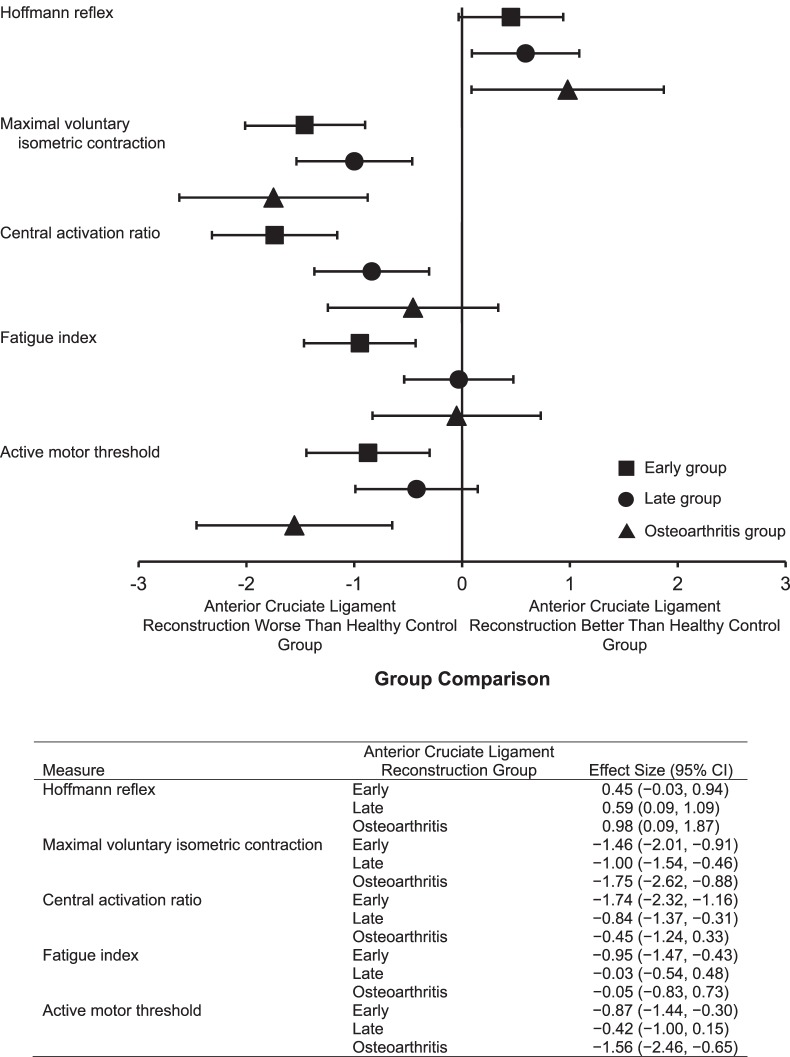
All data were normally distributed. Group demographics and patient-reported functional data are presented in Table 1. Age was different among groups (F3,97 = 36.1, P < .001); patients with osteoarthritis were older than all others (P < .001). Group means and standard deviations are presented for the H : M ratio, MVIC torque, CAR, FI, and AMT in Table 2. Effect sizes demonstrating the magnitude of difference between the involved ACLR and matched healthy limbs are presented for each dependent variable in Figure 1.
Table 2.
Quadriceps Neuromuscular Function in Patients With Anterior Cruciate Ligament Reconstruction (ACLR) and Healthy Control Participants
| Measure |
ACLR Group |
ACLR Limb |
Healthy Control Limb |
||||
| Mean ± SD |
Mean ± SD |
||||||
| Involveda |
Contralateralb |
P Value |
Matched Involved |
Matched Contralateral |
P Value |
||
| Hoffmann reflex (H : M ratioc) | Earlyd | 0.19 ± 0.19 | 0.16 ± 0.15 | .15 | 0.14 ± 0.12 | 0.14 ± 0.12 | .99 |
| Latee | 0.21 ± 0.19 | 0.19 ± 0.16 | .16 | ||||
| Osteoarthritis | 0.26 ± 0.23 | 0.30 ± 0.32 | .67 | ||||
| Maximal voluntary isometric contraction torque, Nm/kg | Early | 1.9 ± 0.6f | 2.6 ± 0.7 | <.001g | 2.7 ± 0.6 | 2.8 ± 0.6 | .18 |
| Late | 2.2 ± 0.6f | 2.3 ± 0.6f | .03g | ||||
| Osteoarthritis | 1.7 ± 0.7f | 1.9 ± 0.9f,h | .33 | ||||
| Central activation ratio, % | Early | 85.5 ± 11.4f,i,j | 88.1 ± 9.4f,i,j | .06 | 93.8 ± 6.6 | 95.2 ± 5.6 | .18 |
| Late | 90.5 ± 8.4 | 91.0 ± 10.3 | .70 | ||||
| Osteoarthritis | 92.6 ± 6.8 | 92.9 ± 7.6 | .90 | ||||
| Fatigue index, % | Early | 14.3 ± 9.7f,i | 19.6 ± 9.0f,i | .002g | 22.2 ± 8.2 | 22.0 ± 8.0 | .72 |
| Late | 21.7 ± 7.6 | 21.1 ± 6.8 | .56 | ||||
| Osteoarthritis | 21.6 ± 6.8 | 19.7 ± 7.3 | .30 | ||||
| Active motor threshold, % 2.0 T | Early | 45.8 ± 7.9f,i | 45.1 ± 7.4f,i | .60 | 39.0 ± 3.4 | 39.0 ± 4.1 | .92 |
| Late | 42.8 ± 9.1f,j | 42.3 ± 9.5f,j | .60 | ||||
| Osteoarthritis | 50.8 ± 7.6f,i | 48.3 ± 7.1f,i | .11 | ||||
The involved limb of the patients with anterior cruciate ligament reconstruction was compared with the matched involved limb of the healthy control participants.
The contralateral limb of the patients with anterior cruciate ligament reconstruction was compared with the matched contralateral limb of the healthy control participants.
To calculate the H : M ratio, we averaged and normalized 3 maximal Hoffmann reflexes to the average of 3 maximal muscle responses (M-wave).
Less than 2 years after ACLR.
Greater than 2 years after ACLR.
Different from the healthy control group (P ≤ .05).
Indicates between-limbs difference (P ≤ .05).
Indicates different from the early group (P ≤ .05).
Indicates different from the late group (P ≤ .05).
Indicates different from the osteoarthritis group (P ≤ .05).
Figure 1.
Cohen d effect sizes with 95% confidence intervals (CIs) for patients early (<2 y), late (>2 y), and with osteoarthritis after anterior cruciate ligament reconstruction comparing the Hoffmann reflex, maximal voluntary isometric contraction torque, central activation ratio, fatigue index, and active motor threshold of the involved limb with the matched limbs of healthy control participants. Effect sizes were interpreted as small (≤0.3), moderate (0.4–0.7), or large (≥0.08).
Patient-Reported Outcomes
Each ACLR group reported a lower level of knee function (IKDC, KOOS, WOMAC) than the healthy control group (all P values < .001). The IKDC and WOMAC scores were better in patients without osteoarthritis than in patients with osteoarthritis after ACLR but did not differ between the early and late ACLR groups. Among all patients with ACLR, those in the late group reported the highest KOOS scores, followed by the early and then the osteoarthritis group (Table 1).
Spinal-Reflex Excitability
The H : M ratio did not differ by limb (F1,176 = 0.01, P = .92) or group (F3,176 = 2.5, P = .07). Between-limbs differences were not detected for any group. Effect sizes were moderate for the early (Cohen d = 0.45 [95% CI = −0.03, 0.94]) and late (Cohen d = 0.59 [95% CI = 0.09, 1.09]) groups and large for the osteoarthritis group (Cohen d = 0.98 [95% CI = 0.09, 1.87]).
Strength and Voluntary Activation
A group main effect (F3,194 = 11.6, P < .001) indicated that each ACLR group demonstrated less knee-extension MVIC torque than the healthy control group. A limb main effect (F1,194 = 5.5, P = .02) indicated that the involved ACLR limb was weaker in the early (Cohen d = −1.46 [95% CI = −2.01, −0.91]), late (Cohen d = −1.00 [95% CI = −1.54, −0.46]), and osteoarthritis (Cohen d = −1.75 [95% CI = −2.62, −0.88]) groups than in the healthy control group. However, we observed differences among ACLR groups. Contralateral limb MVIC torque was less in the late and osteoarthritis groups than in the healthy control group and less in the osteoarthritis than in the early group. A limb-by-group interaction (F3,194 = 4.8, P = .003) indicated that the involved limb was weaker than the contralateral limb in the early (P < .001) and late (P = .03) groups but not in the osteoarthritis group (P = .33). We found no difference between limbs in the healthy control group (P = .18).
A group main effect (F3,193 = 6.8, P < .001) indicated that the quadriceps CAR was lower in the early group (Cohen d = −1.74 [95% CI = −2.32, −1.16]) but not lower in the late (Cohen d = −0.84 [95% CI = −1.37, −0.31]) or osteoarthritis (Cohen d = −0.45 [95% CI = −1.24, 0.33]) group than in the healthy control group. The CAR was lower in the early than in the late and osteoarthritis groups but did not differ by limb (F1,193 = 0.1, P = .71) or display a limb-by-group interaction (F3,193 = 0.4, P = .78). We observed no differences between limbs for any group.
Fatigue Index
A group main effect (F3,193 = 4.9, P = .003) showed that patients in the early group fatigued less than did patients in the late group and healthy control group (Cohen d = −0.95 [95% CI = −1.47, −0.43]) but did not differ from the osteoarthritis group. The FI did not differ by limb (F1,193 = 0.2, P = .62) or demonstrate a limb-by-group interaction (F3,193 = 1.8, P = .15). Planned comparisons between limbs revealed that the early group fatigued less in the involved than in the contralateral limb (P = .002). We observed no differences between limbs for any other group.
Corticospinal Excitability
A group main effect (F3,173 = 9.7, P < .001) indicated that the AMT was higher bilaterally in the early (Cohen d = −0.87 [95% CI = −1.44, −0.30]), late (Cohen d = −0.42 [95% CI = −1.00, 0.15]), and osteoarthritis (Cohen d = −1.56 [95% CI = −2.46, −0.65]) groups than in the healthy control group (decreased corticospinal excitability). The early and osteoarthritis groups had higher AMTs than the late group. The AMT did not differ by limb (F1,173 = 1.0, P = .32) or display a limb-by-group interaction (F3,173 = 0.2, P = .88). We observed no differences between limbs for any group.
Correlations
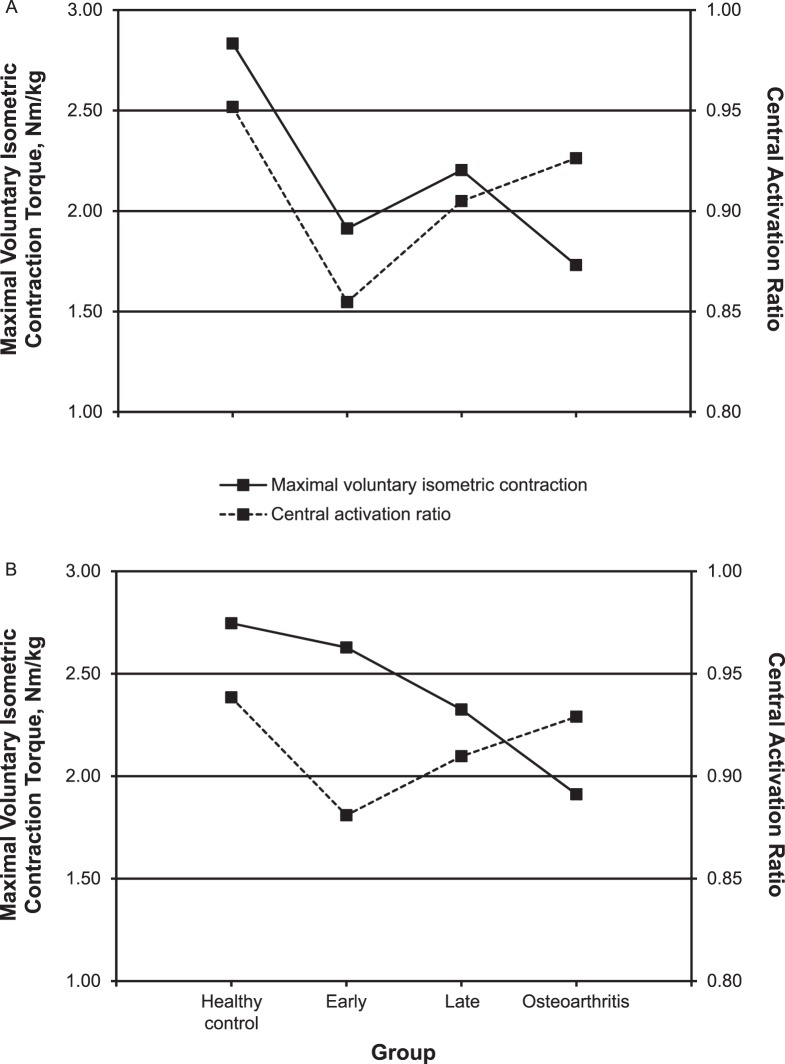
The involved-limb MVIC torque and CAR were correlated in the early (r = 0.546, P = .001) and late (r = 0.486, P = .006) groups but not in the osteoarthritis group (r = −0.135, P = .75). Contralateral limb MVIC torque and CAR were correlated in the late group (r = 0.388, P = .03) but not in the early (r = 0.200, P = .26) or osteoarthritis (r = −0.020, P = .96; Figure 2) group. Among all patients with ACLR, the involved-limb MVIC torque was positively correlated with the CAR (r = 0.452, P < .001) and negatively correlated with the AMT (r = −0.273, P = .02). In the contralateral limb, the FI was positively correlated with the AMT (r = 0.298, P = .01). No outcome measure was associated with time since surgery or age (all P > .05).
Figure 2.
Maximal voluntary isometric contraction torque and quadriceps central activation ratio of the A, involved, and B, contralateral limb in healthy control participants and patients early (<2 y), late (>2 y), and with osteoarthritis after anterior cruciate ligament reconstruction.
DISCUSSION
Our results indicated that the early ACLR group demonstrated reduced quadriceps strength in the involved limb and bilateral reductions in voluntary activation, fatigue, and corticospinal excitability compared with the healthy control group. Less symmetric quadriceps strength and FIs were also observed in the early group. Patients in the late and osteoarthritis groups demonstrated bilateral reductions in quadriceps strength and corticospinal excitability compared with the healthy control group. Less symmetric quadriceps strength was also observed in the late group but not in the osteoarthritis group. To our knowledge, we are the first to conduct a cross-sectional study to examine comprehensive quadriceps neuromuscular function at distinct times (early and late) after ACLR and to include patients who experienced posttraumatic knee osteoarthritis. To better understand the temporal nature of posttraumatic adaptations, a spectrum of postoperative outcomes is warranted, toward which we provided an initial step. Collectively, these data offer supporting evidence of the temporal nature of neuromuscular adaptations after ACLR.
Early Postoperative Outcomes
Large-magnitude and clinically meaningful reductions in unilateral quadriceps strength, voluntary activation, fatigue, and corticospinal excitability were observed in the early group (average = 9 months) compared with the healthy control group. Whereas spinal-reflex excitability did not differ from that in the uninvolved limbs, a moderate effect size was calculated for the involved limbs. This may begin to suggest a clinically meaningful up-regulation in quadriceps motoneuron-pool excitability. However, the CI of this measure crossed zero (−0.03, 0.94) and should therefore be interpreted with caution. Our findings agree with the longitudinal data for neural function in patients 6 months after ACLR.22 Lepley et al22 reported that spinal-reflex excitability increased from 2 weeks to 6 months after ACLR even though it was not different from that in healthy controls. In contrast, corticospinal excitability did not differ from that in healthy controls at 2 weeks but was reduced by 6 months after ACLR. These data, in conjunction with our findings, suggest that a temporal, yet reciprocal, relationship exists between spinal-mediated and cortically driven signaling to muscle, which supports the theoretical attempt to preserve muscle function.
Persistent quadriceps weakness has been reported10 to manifest from aberrant sensory information arising from damaged periarticular tissue, which is an inherent consequence of ACLR. Early after joint trauma, the presence of pain and swelling stimulates nociceptors and articular mechanoreceptors, which has been noted to result in an ongoing reflexive inhibition of uninjured musculature due to a net reduction in spinal-mediated excitability.10 On average, patients reported minimal pain (range, 0–3.0 out of 10 mm). Whereas we did not observe clinical signs of swelling and inflammation in our cohort, the integration of sensory information into the central nervous system may have remained altered and negatively influenced muscle function despite the resolution of pain and swelling. Spinal-reflex excitability was not different from that in our healthy control group, but moderate to large effect sizes suggested that clinically meaningful up-regulations occurred in patients in the late and osteoarthritis groups, which may have begun to occur as the clinical signs of injury resolved. If true, this may suggest that persistent sensory aberrations inhibit peripheral musculature via both supraspinal- and spinal-mediated mechanisms. We demonstrated a decrease in corticospinal excitability at each time point after ACLR, indicating that alterations in the threshold of neuronal depolarization in the motor cortex may be a mediating factor contributing to the observed reductions in quadriceps strength and voluntary activation.
Interestingly, quadriceps fatigue was less in the early than in the healthy control group. Snyder-Mackler et al23 hypothesized that selective type II fiber atrophy of the injured limb may explain this phenomenon, thereby increasing the proportion of type I fibers and the subsequent ability to resist fatigue. We did not assess muscle atrophy and fiber type. However, we speculate that atrophy of type II fibers may occur early after ACL injury due to disuse, which may persist through the first year of recovery and explain the observed reduction in quadriceps strength and activation. In contrast, quadriceps fatigue was greater in the late than in the early group but did not differ from that in the healthy control group. This finding may indicate a partial recovery of type II muscle fibers if atrophied during early recovery, but the presence of persistent weakness suggests incomplete morphologic recovery. Chronic ACL injury may result in a combination of type I and II fiber atrophy,24 thereby explaining the long-term persistence of muscle weakness despite an increase in fatigability. Muscle dysfunction during early recovery is likely a product of combined neuromuscular and morphologic factors.
Late Postoperative Outcomes
Large-magnitude reductions in quadriceps strength were seen bilaterally in the late group compared with the healthy control group, which was supported by a large and clinically meaningful effect size calculated for the involved limb. Quadriceps CAR was higher in the late than in the early group (90.5% versus 85.5%) but not different from the healthy control (95.2%) or osteoarthritis (92.6%) group. This could be interpreted as an improvement in voluntary activation over time. However, a large and clinically meaningful effect size was calculated between the involved and matched healthy control limbs, suggesting the presence of persistent central activation failure beyond what is considered normal during late recovery. A bilateral reduction in corticospinal excitability was observed compared with the healthy control group. Whereas this difference was statistically significant, the effect size was moderate, and the CI crossed zero, suggesting clinical uncertainty. Interestingly, a pattern of up-regulated spinal-reflex excitability was observed similar to that in the early group, which was supported by a moderate and clinically meaningful effect size, despite the absence of a statistical difference. The combination of a meaningful increase in spinal-reflex and corticospinal excitability may have contributed to the improved voluntary activation observed in the late group. Pietrosimone et al7 identified increased spinal-reflex excitability bilaterally, which is theorized to be a shunting response to maintain voluntary activation, at an average of 4 years after ACLR. This could explain the improved activation but may still be inadequate to fully restore quadriceps strength.
Knee Osteoarthritis
The osteoarthritis group demonstrated reductions in quadriceps strength and corticospinal excitability bilaterally, with large and clinically meaningful effect sizes calculated for the involved limb compared with the matched limb of the healthy control group. Quadriceps strength in the involved limb did not differ among patients with ACLR at any time point, which may reflect an ongoing inhibitory process that prevents complete recovery of quadriceps function. Notably, both the involved and contralateral limbs were weaker in the osteoarthritis group than in all other groups. The reduction in contralateral limb strength likely contributed to the symmetry observed in this group and may provide a false sense of successful outcomes if evaluated by symmetry alone.
The relationship between quadriceps strength and voluntary activation has been hypothesized to have a greater association early after ACLR.25 Our data partially supported this hypothesis because involved-limb MVIC torque and CAR were correlated in the early and late groups but not in the osteoarthritis group (Figure 2). The osteoarthritis group demonstrated a bilateral reduction in MVIC torque compared with the healthy control group, but this was within the normal limits of healthy voluntary activation. This divergence between quadriceps strength and voluntary activation may suggest that a point exists along the spectrum from late recovery to the onset of osteoarthritis when patients must adapt to using a larger proportion of an already diminished motoneuron pool. This could explain why patients with knee osteoarthritis exhibited relatively normal activation despite being weaker than healthy individuals. Compared with the healthy control group, the osteoarthritis group also demonstrated the lowest corticospinal excitability coupled with the highest spinal-reflex excitability (not different), each of large magnitude and with a clinically meaningful difference. To maintain adequate muscle activation, a reorganization of the central nervous system may occur in patients with chronic joint degeneration after ACLR. A differentiation in organization of the motor cortex has been identified in patients with and without knee osteoarthritis,13 further highlighting the importance of addressing cortical adaptations early after ACL injury and ACLR. It remains unclear how improving corticospinal function would influence muscle strength and clinical outcomes. However, our results warrant further investigation of the role of posttraumatic cortical function.
Patients in the osteoarthritis group were older than individuals in all other groups. However, age was not correlated with any of the primary outcome measures; therefore, we did not control for it in our statistical analyses. Knee-extension strength is reportedly highest between the ages of 25 and 35 years, whereas a 15% decline per decade may be expected from ages 50 to 70 years.26 In our study, the average age of patients with knee osteoarthritis was 45.4 years, and ages ranged from 36 to 59 years. The involved limb of the osteoarthritis group demonstrated more than a 38% decline in MVIC torque compared with the healthy control group, which appears to exceed the natural response to aging. The osteoarthritis group also reported lower levels of physical activity (Tegner Activity Scale) and perceived health status (Veterans RAND 12-Item Health Survey) than the other groups, which may have contributed to a decline in neuromuscular function. It is unclear whether physical activity was reduced because of impaired muscle function or was simply a product of patient goals. Whereas we did not observe a difference in body mass, patients in the osteoarthritis group were approximately 10 kg heavier than all other participants on average, which may also have contributed to reduced physical activity. These patients reported lower preinjury activity levels than patients in the early and late groups, which may simply reflect different lifestyles.
Clinical Implications
It is unclear if early changes in strength, endurance, voluntary activation, and corticospinal excitability perpetuate long-term muscle dysfunction, but the temporal relationships of these neuromuscular measures may be a contributing factor. Although we cannot support this theory due to the cross-sectional design of this study, time since surgery and age were not correlated with any measure of quadriceps function discussed, suggesting that the underlying cause of persistent muscle dysfunction is multifactorial and not related to time alone. As such, the influence of surgical characteristics (concomitant meniscal procedure and graft type) was assessed among all patients with ACLR despite not being different between groups. Having undergone a partial meniscectomy or meniscal repair did not influence any outcome measure. Interestingly, the FI was the only outcome influenced by graft type, with patients who received a patellar tendon graft fatiguing 47.9% less than patients who received a hamstrings graft. Quadriceps strength did not differ by graft type for any ACLR group and was not correlated with time since surgery. Whereas a patellar tendon graft was not associated with more quadriceps weakness than a hamstrings graft or allograft in this study, quadriceps weakness has been associated with decreased muscle fatigue27 and theorized to be an adaptive strategy for preserving muscle function. In our study, the AMT demonstrated a weak negative correlation with MVIC torque among all patients with ACLR. As expected, decreased corticospinal excitability was consistently observed in conjunction with quadriceps weakness, which may further suggest the role of addressing alterations originating from the brain early after ACL injury.
Clinically accessible treatments, such as transcutaneous electrical nerve stimulation and cryotherapy, have been used in conjunction with traditional therapeutic exercise to improve quadriceps central activation in patients with ACLR28 and tibiofemoral osteoarthritis.29 Although the mechanisms involved in these treatments are not completely understood, improvements in muscle function are theorized to be mediated at the spinal level. Less is known about treating impairments that originate at the cortical level. In the future, researchers must also examine the functional implications of chronic alterations in cortical drive to muscle. From our study, we do not know whether corticospinal drive decreased in response to a progressive joint degeneration or if quadriceps function (and subsequent joint health) decreased due to a progressive reduction in corticospinal drive. Investigators should attempt to identify the short-term and long-term functional implications of neurologic alterations in patients after ACLR. Time is often used as a primary consideration when determining readiness for return to activity, but Myer et al30 reported that recovery after ACLR was independent of time, which our results support. Therefore, the progression of many factors (eg, neural adaptations, graft type, activity level, perceived health) other than time may be driving outcomes after ACLR.
Limitations
To further understand the effect of ACL injury and subsequent ACLR on the natural history of quadriceps neuromuscular function, longitudinal data from injured and uninjured cohorts are necessary. Our groups were not equally matched by age. Whereas age may influence outcomes after ACLR, no measures of quadriceps function were correlated with age, supporting our decision to include these patients. To achieve a realistic case series, having different age distributions among groups is reasonable. Researchers may consider matching by age to determine if the same relationships exist.
Patients in the study did not complete a uniform rehabilitation program. Most patients were enrolled from the same orthopaedic center and were provided with similar postoperative instructions. However, surgical procedures varied, and concomitant meniscal repair would have affected early weight-bearing status (eg, 50% weight bearing for 6 weeks versus weight bearing as tolerated). Despite these inherent differences during early rehabilitation, within the first 2 years after surgery, quadriceps strength did not differ between patients with a meniscectomy or meniscus repair and those with an isolated ACL rupture.
Lastly, we could not verify the severity of knee osteoarthritis at the time of enrollment or confirm that patients did not have osteoarthritis before surgery. Yet we confirmed that no patient was given a medical diagnosis of osteoarthritis before ACLR. It is possible that heterogeneity in the time from surgery to osteoarthritis diagnosis, as well as the location and severity of osteoarthritis, influenced neuromuscular profiles differently. Similarly, we could not verify the absence of osteoarthritis in the early and late groups (ie, those without diagnosed osteoarthritis). Radiographic changes are likely to precede the onset of clinical symptoms, which may have inflated the presence of osteoarthritis in patients more than 2 years after ACLR. Investigators may consider assessing neuromuscular function based on a clinical diagnosis in addition to radiographic evidence.
CONCLUSIONS
Neuromuscular impairments manifested early and persisted more than 2 years after ACLR in patients with and those without knee osteoarthritis. Quadriceps strength and corticospinal excitability were impaired at each time point compared with healthy individuals, suggesting the role of addressing cortical function early after ACLR. Seemingly maladaptive strategies appear to have both peripheral and central origins. Whereas time may play a role in the manifestation of specific neuromuscular adaptions after ACLR, clinical outcomes are multifactorial and not likely to be influenced by time alone.
ACKNOWLEDGMENTS
We thank Lindsay Slater, PhD; John Goetschius, PhD, ATC; Meaghan McMillen, MEd, LAT, ATC; and Stephan Bodkin, MEd, ATC, for their assistance in data collection.
REFERENCES
- 1.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. doi: 10.1136/bjsports-2013-093398. [DOI] [PubMed] [Google Scholar]
- 3.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sports Med. 2012;22(2):116–121. doi: 10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oiestad BE, Juhl CB, Eitzen I, Thorlund JB. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(2):171–177. doi: 10.1016/j.joca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Kuenze C, Hertel J, Saliba S, Diduch DR, Weltman A, Hart JM. Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015;24(1):36–46. doi: 10.1123/jsr.2013-0110. [DOI] [PubMed] [Google Scholar]
- 7.Pietrosimone BG, Lepley AS, Ericksen HM, Clements A, Sohn DH, Gribble PA. Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(6):665–674. doi: 10.4085/1062-6050-50.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrosimone BG, Hertel J, Ingersoll CD, Hart JM, Saliba SA. Voluntary quadriceps activation deficits in patients with tibiofemoral osteoarthritis: a meta-analysis. PM R. 2011;3(2):153–162. doi: 10.1016/j.pmrj.2010.07.485. [DOI] [PubMed] [Google Scholar]
- 9.Kuenze CM, Hertel J, Weltman A, Diduch D, Saliba SA, Hart JM. Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(3):303–312. doi: 10.4085/1062-6050-49.5.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 11.Hopkins JT, Ingersoll CD, Krause BA, Edwards JE, Cordova ML. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33(1):123–126. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Lepley AS, Bahhur NO, Murray AM, Pietrosimone BG. Quadriceps corticomotor excitability following an experimental knee joint effusion. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1010–1017. doi: 10.1007/s00167-013-2816-1. [DOI] [PubMed] [Google Scholar]
- 13.Shanahan CJ, Hodges PW, Wrigley TV, Bennell KL, Farrell MJ. Organisation of the motor cortex differs between people with and without knee osteoarthritis. Arthritis Res Ther. 2015;17:164. doi: 10.1186/s13075-015-0676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39(3):268–277. [PMC free article] [PubMed] [Google Scholar]
- 16.Surakka J, Romberg A, Ruutiainen J, Virtanen A, Aunola S, Maentaka K. Assessment of muscle strength and motor fatigue with a knee dynamometer in subjects with multiple sclerosis: a new fatigue index. Clin Rehabil. 2004;18(6):652–659. doi: 10.1191/0269215504cr781oa. [DOI] [PubMed] [Google Scholar]
- 17.Norte GE, Pietrosimone BG, Hart JM, Hertel J, Ingersoll CD. Relationship between transcranial magnetic stimulation and percutaneous electrical stimulation in determining the quadriceps central activation ratio. Am J Phys Med Rehabil. 2010;89(12):986–996. doi: 10.1097/PHM.0b013e3181f1c00e. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins JT, Wagie NC. Intrasession and intersession reliability of the quadriceps Hoffmann reflex. Electromyogr Clin Neurophysiol. 2003;43(2):85–89. [PubMed] [Google Scholar]
- 19.Park J, Hopkins JT. Within- and between-session reliability of the maximal voluntary knee extension torque and activation. Int J Neurosci. 2013;123(1):55–59. doi: 10.3109/00207454.2012.725117. [DOI] [PubMed] [Google Scholar]
- 20.Poulsen JB, Rose MH, Moller K, Perner A, Jensen BR. A novel noninvasive method for measuring fatigability of the quadriceps muscle in noncooperating healthy subjects. BioMed Res Int. 2015;2015:193493. doi: 10.1155/2015/193493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luc BA, Lepley AS, Tevald MA, Gribble PA, White DB, Pietrosimone BG. Reliability of corticomotor excitability in leg and thigh musculature at 14 and 28 days. J Sport Rehabil. 2014;23(4):330–338. doi: 10.1123/jsr.2013-0069. [DOI] [PubMed] [Google Scholar]
- 22.Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25(6):828–839. doi: 10.1111/sms.12435. [DOI] [PubMed] [Google Scholar]
- 23.Snyder-Mackler L, Binder-Macleod SA, Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 1993;25(7):783–789. doi: 10.1249/00005768-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Lorentzon R, Elmqvist LG, Sjostrom M, Fagerlund M, Fuglmeyer AR. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989;17(3):423–429. doi: 10.1177/036354658901700318. [DOI] [PubMed] [Google Scholar]
- 25.Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7–11. doi: 10.1016/j.jsams.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev. 1993;21:65–102. [PubMed] [Google Scholar]
- 27.Kuenze C, Hertel J, Hart JM. Effects of exercise on lower extremity muscle function after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013;22(1):33–40. doi: 10.1123/jsr.22.1.33. [DOI] [PubMed] [Google Scholar]
- 28.Hart JM, Kuenze CM, Diduch DR, Ingersoll CD. Quadriceps muscle function after rehabilitation with cryotherapy in patients with anterior cruciate ligament reconstruction. J Athl Train. 2014;49(6):733–739. doi: 10.4085/1062-6050-49.3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrosimone BG, Hart JM, Saliba SA, Hertel J, Ingersoll CD. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med Sci Sports Exerc. 2009;41(6):1175–1181. doi: 10.1249/MSS.0b013e3181982557. [DOI] [PubMed] [Google Scholar]
- 30.Myer GD, Martin L, Jr, Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. Am J Sports Med. 2012;40(10):2256–2263. doi: 10.1177/0363546512454656. [DOI] [PMC free article] [PubMed] [Google Scholar]