Abstract
Background: The development of kidney disease is a serious complication among people with type 2 diabetes mellitus, associated with substantially increased morbidity and mortality. We aimed to summarise the current evidence for the relationship between treatments for type 2 diabetes and long-term kidney outcomes, by conducting a systematic search and review of relevant studies.
Methods: We searched Medline, Embase and Web of Science, between 1st January 1980 and 15th May 2018 for published clinical trials and observational studies comparing two or more classes of oral therapy for type 2 diabetes. We included people receiving oral antidiabetic drugs. Studies were eligible that; (i) compared two or more classes of oral therapy for type 2 diabetes; (ii) reported kidney outcomes as primary or secondary outcomes; (iii) included more than 100 participants; and (iv) followed up participants for 48 weeks or more. Kidney-related outcome measures included were Incidence of chronic kidney disease, reduced eGFR, increased creatinine, ‘micro’ and ‘macro’ albuminuria.
Results: We identified 15 eligible studies, seven of which were randomised controlled trials and eight were observational studies. Reporting of specific renal outcomes varied widely. Due to variability of comparisons and outcomes meta-analysis was not possible. The majority of comparisons between treatment with metformin or sulfonylurea indicated that metformin was associated with better renal outcomes. Little evidence was available for recently introduced treatments or commonly prescribed combination therapies.
Conclusions: Comparative evidence for the effect of treatments for type 2 diabetes on renal outcomes, either as monotherapy or in combination is sparse.
Keywords: Review, Kidney Diseases, Comparative Effectiveness Research, Diabetes Mellitus, Type 2, Hypoglycemic Agents
Introduction
Type 2 diabetes mellitus (DM) increases an individual’s risk for health problems including cardiovascular disease, blindness, chronic kidney disease (CKD), and nerve damage 1– 4. The development of kidney disease is associated with other complications of type 2 diabetes and with poorer outcomes 1, 3, 5. Therefore, slowing the development of, or preventing kidney disease is one aim of therapy 2. Type 2 diabetes drugs are thought to play a major role in protecting the kidneys by controlling blood sugar levels and may confer additional protective effects according to specific drug profiles 3. However, as kidney function declines, type 2 diabetes drug options become limited due to prescribing restrictions 2, 3, 5– 7. This presents a challenge for treating type 2 diabetes in patients with non-diabetic related kidney disease, as well as those with renal diabetic complications.
Treatment choice reflects a complex balancing of expected risks and benefits. A recent systematic review focused on vascular outcomes, glyclated hemoglobin (HbA1c), body weight, hypoglycaemia and common adverse events 8. Here we focus on kidney-related outcomes as another important aspect of clinical care that clinicians must consider when prescribing drugs for type 2 DM. Our aim was to provide a summary of the current evidence of long term kidney outcomes, from comparative, long terms studies of oral antidiabetic drugs. We included the following outcomes: change in kidney function (estimated glomerular filtration rate), progression or development of proteinuria, development of end-stage renal disease (ESRD) and composite outcomes compared between different oral drugs for the treatment of type 2 DM.
Methods
The protocol for this systematic review was submitted, reviewed and approved by PROSPERO (International prospective register of systematic reviews, ref. 2016: CRD42016036646). The study was conducted and is reported in accordance with the PRISMA protocol ( Supplementary File 1) 9.
Data sources and searches
We searched the databases; Medline, Embase and Web of Science for articles published between 1 st January 1980 and 15 th May 2018. The search comprised keywords and MESH terms relating to three broad themes: kidney function, type 2 diabetes drugs and clinical studies. We limited the search to English-language studies, and studies in humans. The search strategies are in Supplementary Table 1 and Supplementary Table 2 ( Supplementary File 2). The reference lists of relevant reviews identified through the search were also screened.
Study selection
One reviewer (SW) screened all citations identified in the searches. Titles and abstracts for all studies were compared to the selection criteria. Then the full-text of selected studies were reviewed against the inclusion and exclusion criteria. Reviewer two (MI) was blinded to the articles selected by reviewer one and screened a 20% sample of the articles selected by reviewer one after the title screen. The studies chosen by the two reviewers were compared.
We defined the search and screening strategies before completing the searches. Studies were eligible for inclusion if they were clinical studies that (i) compared two or more classes of oral therapy for type 2 DM; (ii) reported kidney outcomes as primary or secondary outcomes; (iii) included more than 100 participants, and (iv) followed participants for 48 weeks or more. We restricted the review to oral antidiabetic drugs recommended at the initiation and first intensification of treatment 6.
We did not include studies that reported only placebo-controlled comparisons as we were interested in the difference in effects between active therapy regimes to reflect therapy choices made in routine clinical care; placebo-controlled studies would not estimate this difference. Our definition of a kidney outcome was broad to identify as many studies as possible. We accepted any kidney-related outcome, including the incidence of chronic kidney disease, reduced estimated Glomerular Filtration Rate (eGFR), increased creatinine, ‘micro’ and ‘macro’ albuminuria, proteinuria, end stage renal disease (ESRD) and composite kidney outcomes. We did not include composite microvascular outcomes that combined kidney outcomes with other microvascular outcomes such as retinopathy or neuropathy.
Data extraction and quality assessment
After study selection, using a predefined data collection tool, we extracted data for the following items: number of participants, study design, calendar years covered by the study, length of follow-up, drug comparison, mean age of study population, exclusion criteria for study, kidney measurements taken at baseline, mean duration of diabetes, mean HbA1c at baseline, primary outcome for the study, kidney outcomes reported and results for kidney outcomes reported. Reviewer one (SW) assessed each study for quality, using the GRACE 2014 10 items for observational comparative effectiveness research and the Cochrane Collaboration tool for assessing risk of bias in randomised trials 11 for RCTs.
Results
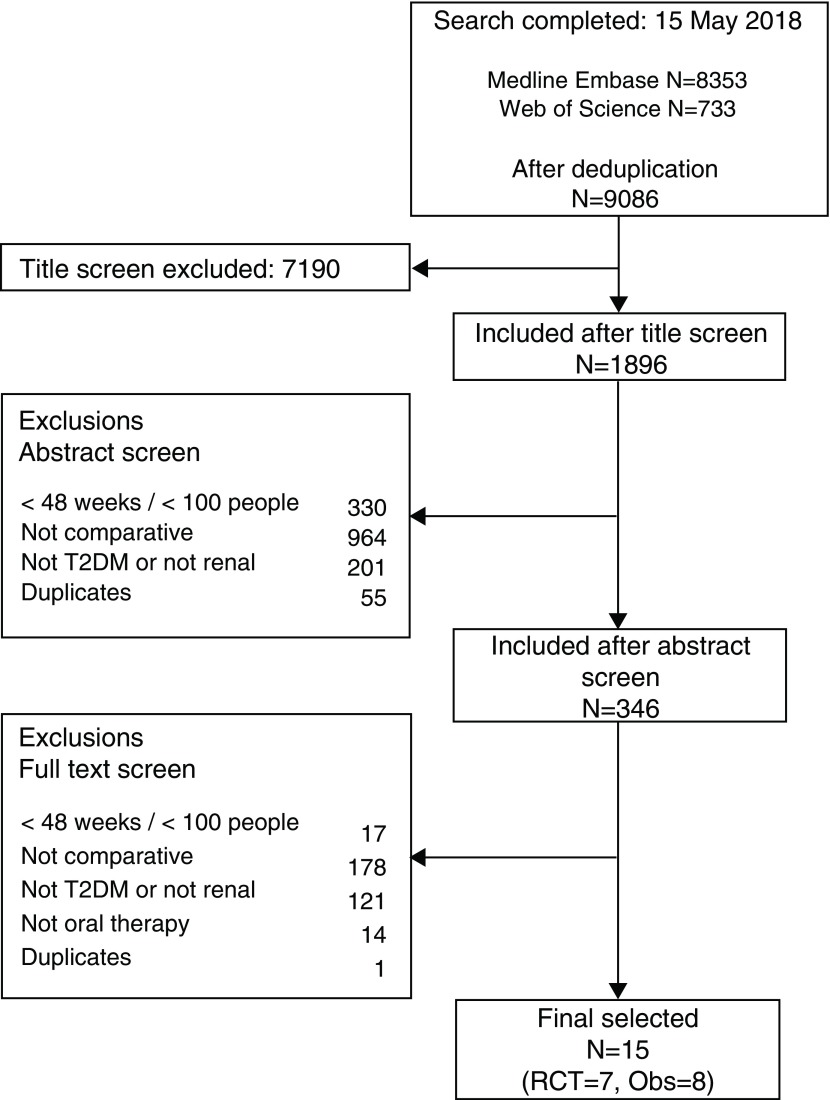
Figure 1 details the study selection process through which we found 9,086 potentially eligible studies. The first reviewer (SW) completed the initial title screen and selected 1,896 articles. The second reviewer (MI) was blinded and reviewed a 20% random sample of these articles. The agreement between reviewers was good, reviewer two selected an additional paper that was rejected after discussion. After subsequent discussions (SW, MI and LT), we selected 15 studies.
Figure 1. Flow diagram of study selection.
Ovid was used to search the Embase and Medline databases.
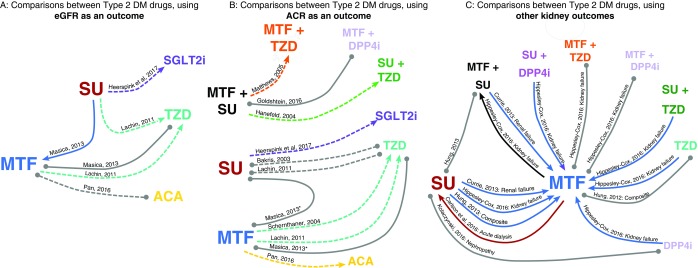
We identified 15 eligible studies, seven of which were randomised controlled trials (RCTs) 12– 18 and eight were observational studies 19– 26. Across the 15 studies, three RCTs 16– 18 and one observational study 22, reported changes in eGFR as an outcome. All seven RCTs 12– 18 and two observational studies 22, 25 investigated albumin-creatinine ratio (ACR) as an outcome. Six observational studies reported kidney endpoints, including kidney failure, nephropathy, acute dialysis and composite endpoints with eGFR 19– 21, 23, 24, 26. Comparisons made, and outcomes studied are summarised graphically in Figure 2. Given the range of the kidney function outcomes reported and the drug class comparisons made we did not complete a meta-analysis of the results, instead we provide a narrative summary of studies. Selected studies and their findings are summarised in Table 1 and Table 2.
Figure 2. Graphical representation of drug comparisons and findings.
Connecting lines indicate where studies have made comparisons between drugs. Lines connect drug names and are labelled with the authors that made the comparison. Dashed line indicates randomised studies, single line indicates non-interventional studies. Findings are indicated by the colour of the line: where one drug appears to be protective, the line is the colour of the protective drug. Grey lines indicate no significant difference. E.g. Blue lines connecting metformin to sulfonylurea indicate that metformin appeared to be protective of kidney function. Arrow heads point towards the drug that appeared to be protective. One further comparison not included here. Hung et al. 2012, as two studies by Hung et al. reported similar comparison using similar data* Also includes dipstick and urine protein tests, † metformin group largely metformin, but some taking TZD or SU. Abbreviations: MTF: metformin, SU: sulfonylurea, TZD: Thiazolidinedione, DPP4i: Dipeptidyl peptidase-4 inhibitor, ACA: acarbose, SGLT: Sodium-glucose Cotransporter 2 inhibitors, GLP1: Glucagon-like peptide-1 receptor agonist, eGFR: estimated Glomerular Filtration Rate, ACR: Albumin creatinine ratio, ARF: Acute renal failure.
Table 1. Summary of study characteristics: Randomised Studies.
| Author (Year) | Number | Follow-
up |
Drug
comparison * |
Mean
age (yrs) |
Exclusions † | Inclusions † | Measures at baseline | Primary
outcomes of study |
Kidney outcomes
recorded |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney measures
Proteinuria/ Mean ACR/ eGFR |
Yrs
with T2DM Mean (SD) |
Mean
HbA1c(%, SD) |
|||||||||
| Bakris
et al
(2003) 12 |
121 a | 52w | SU, TZD
(GLY, RSG) |
55.6 | Prior use of ACEI,
ARBs, BB or CCBs |
40–80 yrs with type 2 DM | 28% micro-
albuminuria b Baseline ACR NR |
NR | GLY: 9.5 (1.6)
RSG: 9.1 (1.7) |
Change in left
ventricular mass index |
52 w
Microalbuminuria b resolved in: RSG: 43%, GLY: 6% ACR mean % change: RSG: -23, GLY: -8 |
| Hanefeld
et al
(2004) 13 |
639 | 52w | SU+TZD,
SU+MTF (SU+PGZ, SU+MTF) |
60 | Previous cardiac
events, malignant disease in 6 months before study. Previous treatment with MTF or TZD |
35–75yrs with type 2
diabetes inadequately managed with SU monotherapy with HbA1c 7.5-11.0% |
28% albuminuria
c
Mean ACR (SD) SU+PGZ: 0.07 (0.25) SU+MTF: 0.11(0.56) |
7 | SU+PGZ:
8.8 (0.98) SU+MTF: 8.8 (0.97) |
HbA1c at
week 52, FPG, Insulin and lipid profiles. |
52 w
Microalbuminuria c resolved in: SU+ PGZ: 10.2%, SU+MTF: 7.7% ACR mean % change: SU+ PGZ: -15, SU+MTF: +2 |
| Schernthaner
et al (2004) 14 |
1199 | 12m | MTF, TZD (
MTF, PGZ ) |
56.5 | Use of thiazides
but other antihypertensives allowed |
People inadequately
treated with di et alone, or HbA1c 7.5–11% |
NR | 3.3 | PGZ: 8.7 (1)
MTF: 8.7 (1) |
HbA1c | 52 w
ACR mean % change: PGZ: -19, MTF -1 |
| Matthews
et al (2005) 15 |
630 | 52w | MTF+TZD,
MTF+SU ( MTF+PGZ, MTF+GLZ ) |
56.5 | Ketoacidosis,
MI, TIA, stroke in the previous 6m; symptomatic heart failure; acute malabsorption or chronic pancreatitis; familial polyposis coli; malignant disease in past 10ys; substance abuse |
Previously not managed
with MTF monotherapy, HbA1c 7.5–11%. No previous treatment with insulin, gliclazide, pioglitazone, SU/ TZD |
Mean ACR (SD)
MTF+PGZ: 0.06 (0.14) MTF+GLZ: 0.05(0.16) |
5.7 | SU+Pio:
8.7 (0.1) SU+MTF: 8.53 (0.9) |
HbA1c | 52 w
ACR mean % change: MTF+ PGZ: -10, MTF+GLZ: +6 |
| ADOPT
Lachin et al (2011) 16 |
4351 | 5yrs | TZD, MTF, SU
(RSG, MTF, GLY) |
56.9 | Significant liver
disease, kidney impairment (serum creatinine males: >1.3mg, females: >1.2mg), history of lactic acidosis, angina, congestive heart failure uncontrolled hypertension |
≥3yrs history of type 2
DM, FPG 7-10mmol/L. |
16%
albuminuria c Mean ACR (log transformed) RSG 9.9 (180), MTF 9.3 (172), GLY 9.4 (172) Mean eGFR (geometric): RSG 98.0 (24.6), MTF 97.1 (25.6), GLY 95.7 (27.6) |
RSG: 7.36
(0.93) MTF: 7.36 (0.93) GLY: 7.35 (0.92) |
Time to
drug failure, using FPG |
4 yr
Albuminuriad resolved in: RSG: 69.5%, MTF: 64%, GLY: 64% ACR mean change (95% CI): RSG 2.1 (-4.2, 8.8), MTF 20.9 (13.3, 28.9), GLY 6.1 (-1.2, 14.0) eGFR mean change % (95% CI): RSG: 5.1 (3.6-6.7), MTF: 1.4 (0.0, 2.9), GLY: -0.4 (-2, 1.2) |
|
| Pan
et al
(2016) 18 |
762 | 48w | ACA, MTF | 50 | History of cardiac
disease, kidney disease, uncontrolled hypertension, urinary infection |
Newly diagnosed type 2
diabetes within 1 yr: >1 month of treatment with type 2 diabetes in previous 12m and no treatment 3 months prior. |
Elevated ACRe
ACA 20%, MTF 24% Median ACR (IQR) ACA: 12.5 (4.9- 25.8), MTF 11.6 (5.3-28.8) Mean eGFR (SD) ACA: 109.6 (29.8), MTF 114.9 (32.3) |
ACA:
1.6, MTF: 1.7 |
ACA: 7.49
(1.25) MTF: 7.6 (1.23) |
ACR, eGFR | Elevated ACRe
Median ACR (IQR) ACA: 5.80 (0.9-13.2), MTF 7.31 (2.2-18.7) Mean eGFR (SD): ACA: 112.8 (32.6), MTF 114.6 (32.8) |
| CANTATA-SU
Heerspink et al (2017) 17 |
1450 | 104w | SGLT, SU
(CNG, GLM) |
56.2 | eGFR >60, last
6 months severe hypoglycaemia, serum creatinine (μmol/L) (men >124, women >115), TZD in last 16 weeks |
18-80 yrs with type 2
DM, HbA1c 7-9.5 %. managed with MTF therapy |
Mean ACR
(25th, 75), CNG 100mg: -2.7 (-3.5, -1.9), CNG 300mg: percentile) GLM: 8.2 (5.75, 17.98), CNG 100mg: 8.7 (5.74, 17.52), CNG 300mg: 8.6 (5.28, 20.64) Mean eGFR (SD) GLM: 89.5 (17.5), CNG 100mg: 89.7 (19.3), CNG 300mg: 91.4 (19.4) |
6.6 | GLM: 7.8 (0.8)
CNG 100mg: 7.8 (0.8) CNG 300mg: 7.8 (0.8) |
Change in
albuminuria and kidney function |
104w ACR mean %
change, relative to GLM (SD): CNG 100mg: -5.7 (2.2, -13.1), CNG 300mg: -11.2 (-3.6, -18.3) eGFR Mean change (95 CI): GLM: -5.4 (-6.2, -4.5), CNG 100mg: -2.7 (-3.5, -1.9), CNG 300mg: -3.9 (-4.7, -3.0) Incidence of 30% eGFR decline HR (95% CI) Referent GLM CNG 100mg: 0.66 (0.42, 1.04), CNG 300mg:0.93 (0.62, 1.42) |
Abbreviations: ACA: acarbose, ACEI: ACE Inhibitor, ACR: Albumin:Creatinine Ratio, ARB: Angiotensin receptor blocker, BB: beta-blocker, CCB: calcium channel blocker, CI: confidence interval, CNG: Canagliflozin, CV: coefficient of variation [100x(exp[SD-mean])], eGFR: estimated glomerular filtration rate, FPG: Fasting plasma glucose, GLY: glyburide, GLZ: Gliclazide, GLM: Glimepiride, IQR: Inter Quartile Range, MI myocardial infarction, MTF: metformin, NR: not reported , PGZ: Pioglitazone, RSG: Rosiglitazone, SU: sulfonylurea, SGLT: SGLT2i, SD: Standard deviation, TZD: thiazolidinedione, TIA: transient ischaemic attack
Notes: *Oral type 2 diabetes drugs only, †Summary inclusion and exclusion criteria only, a: N with ACR at baseline and by 52w, b: Defined as ACR 30 µg/mg or below [or 30mg/g], c: Not defined, d: ACR greater than or equal to 30mg/g, e: elevated ACR included ‘micro’ albuminuria (30-300mg/g) and ‘macro’ albuminuria (≥300mg/g)
Table 2. Summary of study characteristics: Observational Studies.
| Author
(Year) |
Number | Data source
(Country) |
Yrs of
study |
Drug
comparison |
Age (yrs) | Kidney
related exclusions |
Measures at baseline | Primary
outcomes of study |
Follow-up
(yrs) |
Kidney
outcomes recorded HR (95% CI) c |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | Years with
T2DM |
HbA1c % | ||||||||||
| Hung
et al.
(2012) 19 |
93577 | Veterans
Administration (US) |
2001–
2008 |
Incident MTF,
SU or RSG, excluding combination users |
Median (IQR)
MTF: 60 (55, 69) SU: 62 (56, 72) RSG: 64 (57, 72) |
eGFR <60 | Microalbuminuria
b %:
MTF: 3, SU: 3, RSG: 4 [only available for 15,065 people] Median eGFR (IQR) MTF: 81 (72, 93), SU: 80 (70, 93), RSG: 79 (69, 91) |
NR | Median (IQR):
MTF: 7.1 (6.5, 7.9) SU: 7.3 (6.6, 8.4) RSG: 6.8 (6.2, 7.6) |
1 eGFR (≥25%
decline) 2 ESRD (eGFR<15, ICD-9 codes for dialysis or renal transplant) 3 Mortality |
Median
(IQR): MTF: 0.9 (0.5, 1.8) SU: 0.8 (0.4, 1.7) RSG: 0.7 (0.3, 1.5) |
eGFR event
or ESRD Referent MTF SU: 1.20, (1.13, 1.28), RSG: 0.92, (0.71, 1.18) eGFR event, ESRD or mortality Referent MTF: SU 1.20, (1.13, 1.28), RSG: 0.89, (0.69, 1.12) |
| Currie
et al.
(2013) 21 |
84,622 | CPRD GOLD
datalink (UK) |
2000–
2010 |
MTF, SU,
MTF+SU |
Mean
(median) 61.9 (12.8) |
None stated | Creatinine
>130 µmol/L: 4.5% |
Mean:
2.3 (SD 3.0) |
Mean (SD):
8.7 (1.9) |
Renal failure
(Read codes) |
Mean: 2.8 |
Renal failure
Referent: MTF SU: 2.63 (2.20, 3.15), MTF+SU: 1.39 (1.12, 1.72) |
| Hung
et al.
(2013) 20 |
13238 | Veterans
Administration (US) |
1999–
2008 |
MTF, SU,
MTF+ SU |
Median (IQR)
MTF: 59 (54, 67) SU: 60 (54, 71) MTF+SU: 58 (53, 65) |
Serum
creatinine >1.5 mg/dL or eGFR < 60 |
eGFR Median (IQR)
MTF: 81 (72, 93) SU: 80 (71, 93) MTF+SU: 82 (73, 97) |
NR | Median (IQR)
MTF: 7.1 (6.5, 7.9) SU: 7.3 (6.6, 8.4) MTF+SU: 7.9 (6.8, 10) |
1 eGFR (≥25%
decline) 2 ESRD (eGFR<15, ICD-9 codes for dialysis or renal transplant) 3 Mortality |
Mean: 1.2 |
eGFR event
or ESRD Referent: SU MTF: 0.85 (0.72, 1.01), SU+MTF: 1.01 (0.75, 1.37) eGFR event, ESRD or mortality Referent: SU MTF: 0.82 (0.70, 0.97), SU+MTF 1.05 (0.79, 1.40) |
| Masica
et al.
(2013) 22 |
Proteinuria analysis:
N=798 eGFR analysis: N=977 [IPW cohort] |
Clinical data
from primary care networks (US) |
1998–
2009 |
Exposure to
drug (≥90d) MTF, SU, TZD, or combo |
Mean (SD)
MTF: 53.9 (11.9) SU: 53.7 (13.0) TZD: 53.9 (12.0) [Age at diagnosis, IPW cohort] |
Baseline
proteinuria or MDRD eGFR<60 |
eGFR Mean (SD)
Proteinuria analysis: MTF: 82.3 (20) SU: 79.5 (23) TZD: 75.6 (16) eGFR analysis: MTF: 86.8 (18) SU: 86.2 (21) TZD: 91.4 (34) |
NR | 8.0 % IPW
group |
1 New proteinuria
(24-hour albumin/protein, spot protein, spot ACR, or dipstick) 2 New eGFR <60 |
Proteinuria
analysis: Mean: 3.2 eGFR analysis: Mean: 2.8 |
9% (72/798)
developed proteinuria Incidence of proteinuria MTF referent SU: 1.27 (0.93, 1.74), TZD: 1.00 (0.70, 1.42) Fall in eGFR to <60 (2) MTF referent SU: 1.41 (1.05, 1.91), TZD: 1.04 (0.71, 1.50) |
| Hippisley-
Cox and Coupland (2016) 23 |
274,324
[N for kidney analysis not reported] |
QResearch (UK) | 2007 –
2015 |
DPP4i, TZD,
MTF, SU, ‘other agents’ |
Mean (SD)
TZD: 63 (12) DPP4I: 63 (12) MTF: 64 (13) SU: 66 (13) Other: 60 (12) |
Kidney
disease at baseline, and severe kidney disease |
NR for kidney
analysis: prior to kidney baseline exclusions: Creatinine µmol/L mean (SD) TZD: 87 (34), DPP4I: 85 (33), MTF: 85 (30), SU: 92 (48) |
% 1–3yrs
since diagnosis: TZD: 28 DPP4I: 26 MTF: 25 SU: 24 |
Mmol/mol
Mean (SD) TZD: 67 (19) DPP4i: 68 (18) MTF: 61 (19) SU: 65 (20) Other: 71 (20) |
Incident severe
kidney failure (Read codes for dialysis & transplantation, or CKD stage 5 based on serum creatinine values) |
NR |
Incident
severe kidney failure MTF referent TZD: 2.55 (1.13, 5.74), DPP4i: 3.52 (2.04, 6.07), SU: 2.63 (2.25, 3.06), MTF+SU: 0.76 (0.62, 0.92), MTF+TZD: 0.71 (0.33, 1.50), MTF+DPP4i: 0.59 (0.28, 1.25), SU+TZD: 2.14 (1.27, 3.61), SU+DPP4I: 3.21 (2.08, 4.93) |
| Kolaczynski
et al. (2016) 24 |
5436
matched sample |
IMS Lifelink
(Germany) |
2007–
2013 |
SU, DPP4i | Mean (SD)
SU: 63.7 (10.7) DPP4I: 64.6 (10.9) |
History of
nephropathy |
Renal failure %
(ICD-10 code) DPP4I: 13 SU: 11.1 |
Mean (SD)
DPP4I: 3.1 (3.4) SU: 3.2 (3.4) |
Mean (SD)
DPP4i: 7.61 (1.47), SU: 7.64 (1.37) |
Incident
nephropathy (ICD-10 code) |
Mean (SD)
DPP4I: 3.48 (3.75) SU: 2.49 (3.46) |
Incidence of
nephropathy Referent SU DPP4i 0.90 (0.72, 1.14) |
| Goldshtein
et al. (2016) 25 |
564
matched sample |
Maccabi
Health Service diabetes registry (Israel) |
2008–
2014 |
MTF+SU,
MTF+DPP4i |
Mean (SD)
SU: 58.5 (11) DPP4I: 59.1 (11.2) |
Dialysis,
eGFR <45 or ACE/ARB in 90 day post index |
ACR mg/g
mean (SD) SU: 122.4 (194.5) DPP4I: 139.9 (261.9) eGFR mean (SD) SU: 84 (19.5), DPP4I: 82.4 (19.1) |
Mean (SD)
SU: 5 (3.5), DPP4I: 5.2 (3.5) |
Mean (SD)
SU: 8.6 (1.5), DPP4i: 8.5 (1.5) |
Improvements
in urinary ACR (≥20% improvement in ACR and change in KDIGO category) |
Mean:
9 months, max 52 weeks |
ACR
reductions Referent MTF+SU MTF+DPP4i: 1.20 (0.99,1.47) |
| Carlson
et al. (2016) 26 |
168,443 | All Danish
citizens |
2000–
2012 |
MTF, SU | Mean (SD)
MTF: 65.7 (9.4) SU: 69.2 (10.8) |
ESRD or
eGFR <30 ml/min/1.73m 2 |
eGFR Median (IQR)
MTF: 74 (63–87) SU: 69 (57–82) |
NR | NR | 1 Acute dialysis | 1y
following treatment initiation |
Acute
dialysis Referent: SU MTF: 1.51 (1.06–2.17) |
Abbreviations: ACR: Albumin: Creatinine Ratio, eGFR: estimated glomerular filtration rate, ESRD: End Stage Renal Disease, ICD: International Classification of Diseases, MTF: metformin, SU: sulfonylurea, TZD: Thiazolidinedione, DPP4i: Dipeptidyl peptidase-4 inhibitor, RSG: Rosiglitazone, STG: Sitagliptin, EXE: Exenatide. IPW: Inverse Probability Weight, FU: Follow-up, SD: Standard deviation, ARF: Acute Renal Failure, CKD: Chronic Kidney Disease, IQR: Inter Quartile Range, p-yr: person-years, NR: Not reported, DB: Database, KDIGO: Kidney Disease: Improving Global Outcomes Notes: a: MACE: Major adverse cardiac event: non-fatal MI, non-fatal stroke, or cardiovascular death, b: microalbuminuria if ACR was >30 mg/g, c: Hazard Ratio (HR), Mantel Haenszel (MH) or Odds Ratio (OR), eGFR units: mL/min/1.73m 2
In total, we identified 32 direct comparisons between oral drugs for the treatment of type 2 DM: 22 comparisons between monotherapies, three comparisons between dual therapy combinations, and seven comparisons between dual therapies and monotherapies, outlined in Table 3. One study compared many combination therapy options to metformin; we did not include the triple therapy combinations from this study in our results, details of the comparisons are in Supplementary Table 3 ( Supplementary File 2) 23.
Table 3. Results summary.
| RCTs | Observational | |||||
|---|---|---|---|---|---|---|
| Number | Results | Number | Results | |||
| ACR | ||||||
| Monotherapy | ||||||
| MTF vs ACA | 1 | Favours ACA | 0 | |||
| MTF vs SU | 0 | 1 | No difference | |||
| MTF vs TZD | 2 | Both favour TZD | 1 | No difference | ||
| SU vs SGLT | 1 | Favours SGLT | 0 | |||
| SU vs TZD | 2 | Both no difference | 0 | |||
| Dual therapy | ||||||
| MTF+SU vs MTF+DPP4i | 0 | 1 | No difference | |||
| MTF+TZD vs MTF+SU | 1 | Favours MTF+TZD | 0 | |||
| SU+TZD vs SU+MTF | 1 | Favours SU+TZD | 0 | |||
| eGFR | ||||||
| Monotherapy | ||||||
| MTF vs ACA | 1 | No difference | 0 | |||
| MTF vs SU | 0 | 1 | Favours MTF | |||
| MTF vs TZD | 1 | Favours TZD | 1 | No difference | ||
| SU vs SGLT | 1 | Favours SGLT | 0 | |||
| SU vs TZD | 1 | Favours TZD | 0 | |||
|
KIDNEY
OUTCOMES |
||||||
| Monotherapy | ||||||
| MTF vs DPP4i | 0 | 1 | Favours MTF | |||
| MTF vs SU | 0 | 4 | 3 favour MTF, 1 favours SU | |||
| MTF vs TZD | 0 | 2 | 1 no difference, 1 favours MTF | |||
| SU vs DPP4i | 0 | 1 | No difference | |||
| Mono vs. dual therapy | ||||||
| MTF vs MTF+DPP4i | 0 | 1 | No difference | |||
| MTF vs MTF+SU | 0 | 2 |
1 favours MTF, 1 favours
MTF+SU |
|||
| MTF vs MTF+TZD | 0 | 1 | No difference | |||
| MTF vs SU+DPP4i | 0 | 1 | Favours MTF | |||
| MTF vs SU+TZD | 0 | 1 | Favours MTF | |||
| SU vs MTF+SU | 0 | 1 | No difference | |||
Abbreviations: ACR: Albumin: Creatinine Ratio, eGFR: estimated glomerular filtration rate, MTF: metformin, SU: sulfonylurea, TZD: Thiazolidinedione, DPP4i: Dipeptidyl peptidase-4 inhibitor, ACA: acarbose, , EXE: Exenatide. SGLT: SGLT2i, GLP1: Glucagon-like peptide-1 receptor anonist, IPW: Inverse Probability Weight, FU: Follow-up, SD: Standard deviation, ARF: Acute Renal Failure, CKD: Chronic Kidney Disease, IQR: Inter Quartile Range, p-yr: person-years, NR: Not reported, DB: Database, KDIGO: Kidney Disease: Improving Global Outcomes. One further comparison not included here. Hung et al. 2012, as two studies by Hung et al. reported similar comparison using similar data
Monotherapy comparisons
Metformin monotherapy vs. thiazolidinedione monotherapy. The most common drug comparison was metformin monotherapy vs. thiazolidinedione monotherapy (five studies made seven comparisons) 14, 16, 19, 22, 23. Two RCTs found that thiazolidinediones were associated with improved kidney outcomes (reduced proteinuria or improved eGFR) compared to metformin 14, 16 while two observational studies found no differences between the two drug classes 19, 22. One observational cohort study showed that thiazolidinediones were associated with a higher risk for development of kidney failure (a composite of kidney dialysis, kidney transplant and CKD stage five) compared to metformin 23.
Metformin monotherapy vs. sulfonylurea monotherapy. Six observational studies 19– 23, 26 compared metformin monotherapy to sulfonylurea monotherapy. Though two of these studies ( 19 and 20) reported similar findings from the same source population, we have therefore only reported one of the results, making six comparisons. Four comparisons favoured metformin. One study found the risk of eGFR falling to below 60 mL/min/1.73m 2 was greater in the sulfonylurea group compared to the metformin group 22. Three found higher risks of kidney failure outcomes (various composites of codes for nephropathy, dialysis, renal transplant, ESRD, and reductions in eGFR) for sulfonylurea compared to metformin 20, 21, 23. One study, using proteinuria as an outcome, found no difference between drug classes 22. One further study reported higher rates of acute dialysis for people initiating metformin compared to sulfonylureas 26.
Sulfonylurea monotherapy vs. thiazolidinedione monotherapy. Findings from two RCTs showed differences in ACR that were not statistically significant 12, 16. However, one of these studies also showed an increase in mean eGFR among patients treated with a TZD, but a fall in the SU group 16.
Sulfonylurea monotherapy vs. SGLT2i monotherapy. One RCT showed canagliflozin slowed kidney function decline, and reduced albuminuria, compared to glimepiride 17.
Combination therapy comparisons
Only three studies compared combination therapies.
Metformin plus sulfonylurea vs. metformin plus thiazolidinedione. One RCT compared metformin plus sulfonylurea to metformin plus a thiazolidinedione 15. They reported that ACR decreased in the metformin plus thiazolidinedione group and increased in the metformin plus sulfonylurea group 15.
Sulfonylurea plus metformin vs. sulfonylurea plus thiazolidinedione. One RCT compared sulfonylurea plus metformin to sulfonylurea plus thiazolidinedione 13. The study found that the ACR increased in the sulfonylurea plus metformin group, and decreased in the sulfonylurea plus thiazolidinedione group 13.
Metformin plus sulfonylurea vs. metformin plus gliptin (DPP4i). One observational study compared metformin plus sulfonylurea combination therapy to metformin plus sitagliptin 25. The results showed weak evidence that metformin plus sitagliptin improved the likelihood of reductions in ACR, with an odds ratio of 1.20 (95% CI: 0.99–1.47, P = 0.063) 25.
Dual therapy vs. monotherapy
Three observational studies made seven comparisons between monotherapy options and combination therapy 20, 21, 23. One study indicated that people taking metformin were at a lower risk of renal failure compared to people taking metformin plus sulfonylurea 21. Another study found the opposite, people taking metformin plus sulfonylurea were at lower risk of kidney failure compared to metformin 23. The same study found no differences in the risk of kidney failure compared to metformin in people prescribed; i) metformin plus thiazolidinedione, and ii) metformin plus gliptin. They also reported that people prescribed sulfonylurea plus thiazolidinedione, and a sulfonylurea plus DPP4i were at higher risk for kidney failure compared to metformin 23.
Another observational study found no difference in eGFR outcomes between sulfonylurea monotherapy and metformin plus sulfonylurea combination therapy 20.
Study quality
We assessed each study for quality, using the GRACE 2014 10 items for observational comparative effectiveness research and the Cochrane Collaboration risk of bias tool for RCTs 11 Supplementary Table 5 and Supplementary Table 6 ( Supplementary File 2) detail the results. For the RCTs, we assessed study quality as good, though few studies reported details of randomisation techniques. Of the observational studies, reporting was reasonable, according to the GRACE criteria. However, many of the studies made comparisons between drugs used at different stages of drug intensification, or between monotherapy and combination therapy. For example, two observational studies 21, 23 used metformin monotherapy as the baseline in comparisons with combination therapy. As metformin monotherapy is the most common drug for initiating treatment, and the addition of other drugs to metformin is likely to be associated with progression or poor control of type 2 DM, comparing metformin to drug prescribed at the first stage of intensification is problematic, particularly for renal outcomes. Those people receiving treatment intensification will tend to be sicker, and distinguishing between the effects of treatment and the effects of the underlying disease may not always be possible.
Conclusion
Key findings
Overall, we have found a lack of consistent evidence of long-term differences in kidney outcomes between T2DM drugs. In comparisons of treatments for type 2 DM, for thiazolidinediones vs metformin, there is some evidence of reduced proteinuria - of four comparisons with ACR as an outcome (in combination or monotherapy), three favoured TZD and one showed no difference. Most evidence from observational research also suggested that metformin is associated with better kidney outcomes than sulfonylureas.
Despite frequent use of combination therapies for the treatment of diabetes, we found few studies that compared commonly used dual therapies that investigated renal outcomes.
Previous work
The finding that thiazolidinediones may reduce proteinuria compared with metformin is aligned with observations of other authors and supported by animal studies 27, 28. Though previous evidence is limited, other work suggests that TZDs could exert reno-protective effects via a number of pathways, including reducing blood pressure 28. TZDs may also act directly in the kidneys via proliferator-activated receptor gamma (PPARg), found in the kidney (and in other tissue) 27, 28. However, changes in estimated GFR may reflect changes in fluid status rather than true changes in renal function, which was not measured directly in any study 29.
Strengths
To our knowledge, this is the first systematic review of the comparative research literature that investigated the effects of type 2 diabetes drug regimens on renal function. We have conducted an extensive and detailed search, with broad definitions of renal function.
Limitations
We have focused on renal outcomes only but recognize this is just one of many safety and effectiveness factors to be considered when deciding treatment options. Despite the importance of careful monitoring and maintenance of kidney function for people with diabetes, we identified just 15 long-term studies reporting renal outcomes. Renal complications of type 2 diabetes take many years to develop after the onset of diabetes and studies may not be adequately powered or have sufficient length of follow-up to detect differences. Therefore, many studies have used the surrogate marker of changes in proteinuria as a marker of clinical renal outcomes. Further, initial changes in kidney function may be misleading. One included study indicates benefits of canagliflozin over glimipiride for kidney function decline at 104 weeks: however these benefits were not apparent until 52 weeks 17, 30. This and the EMPA-REG study 31 have indicated initial acute falls in eGFR with better outcomes compared to placebo only observed over the longer term so this would not be apparent in short-term studies.
Our review included both randomised and non-interventional studies. Whilst the unique inferential advantages of randomization are clear, our review highlights a large overall difference in population size depending on study type: randomised trials generally included hundreds of patients, whilst non-interventional studies often had tens of thousands of participants. Rarer outcomes such as ESRD are therefore more likely to be detected in non-interventional settings. This highlights their important role, but the evidence generated from them needs to be evaluated cautiously due to the potential for bias and confounding.
The available evidence does not reflect drugs currently prescribed in routine care. In our review, 69% (22/32) of the comparisons, contrasted different monotherapies, with just three comparisons between dual therapy combinations. In clinical practice, metformin is the most common first-line therapy, and GPs now rarely prescribe thiazolidinediones (EU marketing authorization for Rosiglitazone was suspended in 2010 32, following concern regarding increased heart failure risk) 33.
In the UK, NICE guidance recommends the addition of sulfonylureas, Dipeptidyl peptidase-4 inhibitors (DPP4is) Sodium-glucose Cotransporter 2 Inhibitors (SGLT2is), or TZDs to metformin, yet, just one study compared these combinations (MTF+SU vs MTF+DPP4i) 25, 33– 35. Recent studies that have shown potentially exciting improvements in renal outcomes for patients treated with SGLT2is were conducted against placebo and so were not eligible for this study 36, 37.
We found that definitions of kidney outcomes were not consistent across studies. Definitions of renal decline in the observational studies relied upon either codes for kidney disease (e.g. diabetic nephropathy, acute renal failure), surrogate markers (e.g. eGFR or proteinuria) or a combination of codes and tests, summarised in Supplementary Table 4 ( Supplementary File 2). For the albuminuria data, which has a skewed distribution, most studies used logarithmic transformation to approximate normal, yet not all studies applied this method 18. Such differences between outcomes will limit future opportunities for pooling effect estimates in meta-analyses. Different approaches to study design may also limit the validity of findings. We found two observational studies that made the same comparisons yet found different effects. Both examined renal failure, using UK primary care data, (QResearch 23 and Clinical Practice Research Datalink 21). They found comparable effect sizes when comparing the use of sulfonylurea monotherapy to metformin monotherapy, for renal failure (2.63, 95% CI: 2.25, 3.06 23 and 2.63, 95% CI: 2.19, 3.15 21). However, when comparing sulfonylurea plus metformin dual therapy to metformin monotherapy, estimates of the risk of kidney failure were in opposite directions (0.76, 95% CI: 0.62, 0.92 23 and 1.39, 95% CI: 1.12, 1.72 21). Difficulties in adjusting for levels of diabetic control or change in renal function that led to these treatment choices (confounding by indication), may explain these conflicting results.
In the randomised controlled studies, we found that eligibility criteria were strict. Many studies excluded people most at risk of kidney outcomes e.g. those with reduced kidney function or cardiovascular disease 12, 13, 15– 18. These restrictions limit the generalisability of study findings to routine clinical settings where people presenting with diabetes have complex comorbidities 38. Further, as most individuals with type 2 diabetes will receive treatment for other comorbid conditions, prescribers need to know how diabetic therapies interact with concomitant drugs, yet this is not addressed by the studies identified in this review.
Clinical relevance
In clinical practice, kidney function is one of many considerations for treatment choice in type 2 DM. Some of the differences we found for albuminuria and eGFR between people taking different oral therapies for type 2 diabetes were statistically significant, but the clinical importance of these findings may be limited. Some surrogate outcomes such as a doubling of creatinine or 30% decline in eGFR are closely associated with risk of future ESRD 39, 40 while ACR is not 39, 41, 42. Outcomes that are clinically relevant need to be assessed in future studies. Ideally, these should include hard outcomes such as hospital admission with acute kidney injury or the development of ESRD. Therefore, large, well-designed studies with long follow up, including individuals that represent the typical type 2 diabetes population, will be required. However, the incidence of kidney outcomes is likely to be low in most randomised trials and therefore high-quality observational studies will also be needed.
Our review highlights a lack of rigorous studies comparing the effects of oral type 2 diabetes drugs on kidney outcomes, in particular, for the newer drug intensification options where prescribing is rapidly increasing.
Data availability
All data underlying the results are available as part of the article and supplementary material no additional source data are required.
Funding Statement
This work was was supported by the Wellcome Trust through a Wellcome Trust intermediate clinical fellowship to LAT [101143] and a Wellcome Trust Senior Research Fellowship in Clinical Science to LS [098504] This review was also supported by GlaxoSmithKline (GSK), through a PhD scholarship for SW. HS-F is a full-time employee of GSK. MI is supported by the Honjo International Scholarship Foundation. IJD is paid by an unrestricted grant from GSK.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
Supplementary material
Supplementary File 1 – Completed PRISMA checklist
Supplementary File 2 – File contain the following supplementary tables.
Supplementary Table 1: First Ovid Medline search
Supplementary Table 2: First search Web of science
Supplementary Table 3: Report of further comparisons from Hippisley-Cox and Coupland (2016) paper
Supplementary Table 4: Detailed definitions of composite renal outcomes for observational studies
Supplementary Table 5: GRACE 2014 items for observational studies
Supplementary Table 6: Cochrane items for quality of RCT studies
References
- 1. Thomas MC, Cooper ME, Zimmet P: Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. 10.1038/nrneph.2015.173 [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. : Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9. 10.2337/dc14-2441 [DOI] [PubMed] [Google Scholar]
- 3. Bailey CJ, Day C: Diabetes therapies in renal impairment. Br J Diabetes Vasc Dis. 2012;12(4):167–171. 10.1177/1474651412458811 [DOI] [Google Scholar]
- 4. USRDS: USRDS annual data report: Epidemiology of kidney disease in the United States. National Institute of Health,2016. [Google Scholar]
- 5. National Kidney Foundation: KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–86. 10.1053/j.ajkd.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence (NICE): Type 2 diabetes in adults: management Clinical Guideline Update (NG28). N.I.f.H.a.C. Excellence, Editor.2015. [Google Scholar]
- 7. Nag S, Bilous R, Kelly W, et al. : All-cause and cardiovascular mortality in diabetic subjects increases significantly with reduced estimated glomerular filtration rate (eGFR): 10 years' data from the South Tees Diabetes Mortality study. Diabet Med. 2007;24(1):10–7. 10.1111/j.1464-5491.2007.02023.x [DOI] [PubMed] [Google Scholar]
- 8. Maruthur NM, Tseng E, Hutfless S, et al. : Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164(11):740–51. 10.7326/M15-2650 [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 10. Dreyer NA, Velentgas P, Westrich K, et al. : The GRACE checklist for rating the quality of observational studies of comparative effectiveness: a tale of hope and caution. J Manag Care Spec Pharm. 2014;20(3):301–8. 10.18553/jmcp.2014.20.3.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Altman DG, Gøtzsche PC, et al. : The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakris G, Viberti G, Weston WM, et al. : Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens. 2003;17(1):7–12. 10.1038/sj.jhh.1001444 [DOI] [PubMed] [Google Scholar]
- 13. Hanefeld M, Brunetti P, Schernthaner GH, et al. : One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27(1):141–7. 10.2337/diacare.27.1.141 [DOI] [PubMed] [Google Scholar]
- 14. Schernthaner G, Matthews DR, Charbonnel B, et al. : Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89(12):6068–76. 10.1210/jc.2003-030861 [DOI] [PubMed] [Google Scholar]
- 15. Matthews DR, Charbonnel BH, Hanefeld M, et al. : Long-term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with type 2 diabetes: a randomized, comparative study. Diabetes Metab Res Rev. 2005;21(2):167–74. 10.1002/dmrr.478 [DOI] [PubMed] [Google Scholar]
- 16. Lachin JM, Viberti G, Zinman B, et al. : Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol. 2011;6(5):1032–40. 10.2215/CJN.09291010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heerspink HJ, Desai M, Jardine M, et al. : Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J Am Soc Nephrol. 2017;28(1):368–375. 10.1681/ASN.2016030278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan Q, Xu Y, Yang N, et al. : Comparison of Acarbose and Metformin on Albumin Excretion in Patients With Newly Diagnosed Type 2 Diabetes: A Randomized Controlled Trial. Medicine (Baltimore). 2016;95(14):e3247. 10.1097/MD.0000000000003247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hung AM, Roumie CL, Greevy RA, et al. : Comparative effectiveness of incident oral antidiabetic drugs on kidney function. Kidney Int. 2012;81(7):698–706. 10.1038/ki.2011.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hung AM, Roumie CL, Greevy RA, et al. : Kidney function decline in metformin versus sulfonylurea initiators: assessment of time-dependent contribution of weight, blood pressure, and glycemic control. Pharmacoepidemiol Drug Saf. 2013;22(6):623–31. 10.1002/pds.3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Currie CJ, Poole CD, Evans M, et al. : Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013;98(2):668–77. 10.1210/jc.2012-3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masica AL, Ewen E, Daoud YA, et al. : Comparative effectiveness research using electronic health records: impacts of oral antidiabetic drugs on the development of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2013;22(4):413–422. 10.1002/pds.3413 [DOI] [PubMed] [Google Scholar]
- 23. Hippisley-Cox J, Coupland C: Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: Open cohort study in primary care. BMJ. 2016;352: i1450. 10.1136/bmj.i1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kolaczynski WM, Hankins M, Ong SH, et al. : Microvascular Outcomes in Patients with Type 2 Diabetes Treated with Vildagliptin vs. Sulfonylurea: A Retrospective Study Using German Electronic Medical Records. Diabetes Ther. 2016;7(3):483–496. 10.1007/s13300-016-0177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldshtein I, Karasik A, Melzer-Cohen C, et al. : Urinary albumin excretion with sitagliptin compared to sulfonylurea as add on to metformin in type 2 diabetes patients with albuminuria: A real-world evidence study. J Diabetes Complications. 2016;30(7):1354–9. 10.1016/j.jdiacomp.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 26. Carlson N, Hommel K, Olesen JB, et al. : Metformin-associated risk of acute dialysis in patients with type 2 diabetes: A nationwide cohort study. Diabetes Obes Metab. 2016;18(12):1283–1287. 10.1111/dom.12764 [DOI] [PubMed] [Google Scholar]
- 27. Sarafidis PA, Stafylas PC, Georgianos PI, et al. : Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis. 2010;55(5):835–47. 10.1053/j.ajkd.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 28. Sarafidis PA, Bakris GL: Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int. 2006;70(7):1223–33. 10.1038/sj.ki.5001620 [DOI] [PubMed] [Google Scholar]
- 29. Guan Y, Hao C, Cha DR, et al. : Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11(8):861–6. 10.1038/nm1278 [DOI] [PubMed] [Google Scholar]
- 30. Cefalu WT, Leiter LA, Yoon KH, et al. : Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–50. 10.1016/S0140-6736(13)60683-2 [DOI] [PubMed] [Google Scholar]
- 31. Wanner C, Inzucchi SE, Lachin JM, et al. : Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–34. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 32. Agency EM: European Medicines Agency recommends suspension of Avandia, Avandmet and Avaglim.2010. Reference Source [Google Scholar]
- 33. Sharma M, Nazareth I, Petersen I: Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(1):e010210. 10.1136/bmjopen-2015-010210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Health and Social Care Information Centre (HSCIC): Prescribing for Diabetes England 2005/06 to 2014/15. Prescribing and Medicines Team, Health and Social Care Information Centre.2015. Reference Source [Google Scholar]
- 35. NICE (National Institute for Health and Care Excellence): Type 2 diabetes in adults. management NG28 December 2015. National Institute for Health and Care Excellence,2015. Reference Source [Google Scholar]
- 36. Neal B, Perkovic V, Mahaffey KW, et al. : Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 37. Wanner C, Inzucchi SE, Zinman B: Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(18):1801–2. 10.1056/NEJMc1611290 [DOI] [PubMed] [Google Scholar]
- 38. Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71. 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- 39. Carrero JJ, Grams ME, Sang Y, et al. : Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91(1):244–251. 10.1016/j.kint.2016.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coresh J, Turin TC, Matsushita K, et al. : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–31. 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inker LA, Levey AS, Pandya K, et al. : Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis. 2014;64(1):74–85. 10.1053/j.ajkd.2014.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stoycheff N, Pandya K, Okparavero A, et al. : Early change in proteinuria as a surrogate outcome in kidney disease progression: a systematic review of previous analyses and creation of a patient-level pooled dataset. Nephrol Dial Transplant. 2011;26(3):848–57. 10.1093/ndt/gfq525 [DOI] [PMC free article] [PubMed] [Google Scholar]