Short abstract
Chronic alcohol intake causes hepatic steatosis and changes the body composition and glucose metabolism. We examined whether water extracts of mulberry (WMB) and white flower dandelion (Taraxacum coreanum Nakai, WTC) can prevent and/or delay the symptoms of chronic ethanol-induced hepatic steatosis in male Sprague Dawley rats, and explored the mechanisms. Ethanol degradation was examined by orally administering 3 g ethanol/kg bw after giving them 0.3 g/kg bw WMB or WTC. All rats were continuously provided about 7 g ethanol/kg bw/day for four weeks and were given either of 0.1% dextrin (control), WMB, WTC, or water extracts of Hovenia dulcis Thunb fruit (positive-control) in high-fat diets. Area under the curve of serum ethanol levels was lowered in descending order of control, WTC and positive-control, and WMB in acute ethanol challenge. WMB and WTC prevented alcohol intake-related decrease in bone mineral density and lean body mass compared to the control. After glucose challenge, serum glucose levels increased more in the control group than other groups in the first part and the rate of decrease after 40 min was similar among all groups. These changes were associated with decreasing serum insulin levels. WMB had the greatest efficacy for decreasing triglyceride and increasing glycogen deposits. WMB and WTC prevented the disruption of the hepatic cells and nuclei while reducing malondialdehyde contents in rats fed alcohol, but the prevention was not as much as the normal-control. The ratio of Firmicutes to Bacteroidetes in the gut was much higher in the control than the normal-control, but WTC and WMB decreased the ratio compared to the control. WMB and WTC separated the gut microbiota community from the control. In conclusion, WMB and WTC protected against alcoholic liver steatosis by accelerating ethanol degradation and also improved body composition and glucose metabolism while alleviating the dysbiosis of gut microbiome by chronic alcohol intake.
Impact statement
Excessive alcohol consumption is associated with serious pathologies and is common in much of the world. Pathologies include liver damage, glucose intolerance, and loss of lean body mass and bone mass. These pathologies are mediated by changes in metabolism as well as toxic metabolic byproducts, and possibly by gut dysbiosis. In this study, we demonstrate that aqueous extracts of mulberry and dandelion protected rats against ethanol-induced losses in lean body and bone masses, improved glucose tolerance and partially normalized gut bacterial populations, with mulberry extract being generally more effective. This research suggests that mulberry and dandelion extracts may have the potential to improve some of the pathologies associated with excess alcohol consumption, and that further clinical research is warranted.
Keywords: Alcohol, mulberry, dandelion, glucose metabolism, gut microbiome
Introduction
The World Health Organization reported in 2010 that people in cold regions, such as Russia, drank more high-alcohol content liquors, although ethanol consumption varied among persons.1 Koreans drink less ethanol (about 15.1 L/year) than Russians. However, individual drinking by Korean drinkers is reported to be about 27.5 L/year, since 55% of people do not drink at all.1 Excessive alcohol consumption leads to various metabolic diseases and especially liver diseases, with a diverse spectrum from steatosis to hepatitis, fibrosis, and cirrhosis.2 In addition to alcoholic liver steatosis, a high-fat diet and obesity result in non-alcoholic steatosis.3 A high-fat diet exacerbates the liver steatosis caused by excessive alcohol consumption,4 and when people have both increased body fat and high alcohol intake, they are highly susceptible to liver steatosis.
Ethanol is degraded into acetate through acetaldehyde. Increased acetate production by the gut microbiome activates the parasympathetic nervous system to increase glucose-stimulated insulin secretion and hyperphagia, leading to obesity.5 Excessive acetate production due to ethanol intake may lead to similar conditions to increase the deposition of fat, which is consistent with the production of acetate by the microbiome.5 Ethanol and acetaldehyde are toxic substances to the cells and the damage primarily occurs in hepatic and brain cells through increasing the production of reactive oxygen species (ROS).6 The disturbance of the immune system by alcohol consumption is associated with change in the gut microbiome.7 Alcohol consumption is reported to result in intestinal bacterial dysbiosis and bacterial overgrowth in the small intestines in humans and experimental animals.7 The change in gut microbiota alters inflammatory status, and bacterial products such as lipopolysaccharide activate pro-inflammatory signaling pathways and stimulate an innate immune response.7 Bacterial products are translocated from the intestines to the liver through the portal vein, and the liver removes them and is subsequently damaged by them.8 Thus, excessive alcohol consumption induces liver steatosis.
Alcoholic hepatic steatosis (fatty liver) is induced in an early stage of liver diseases and the symptoms are reversible. The hepatic steatosis needs to be prevented and alleviated in order to prevent its progression to other liver diseases.7 Alcohol abstinence can reverse hepatic steatosis, but other effective medical therapies are currently not available. Complementary and alternative medicine has been studied and some herbal treatments such as Milk Thistle are used for relieving alcoholic hepatic steatosis by reducing oxidative stress and inflammation.9,10 New herbal therapies to prevent and/or delay the progression of alcoholic hepatic steatosis with a high-fat diet need to be found. Herbs containing polyphenols, flavonoids, and anthocyanins reduce oxidative stress and lipid deposition, possibly by alleviating alcoholic liver steatosis. Mulberry (Morus alba L.) fruits have been reported to have antioxidant, neuroprotective, antiatherosclerosis, immunomodulatory, antitumor, antihyperglycemic, and hypolipidemic activities.11 Previous studies showed that mulberry extracts acutely expedited ethanol degradation12 and they also attenuate hepatic steatosis while reducing insulin resistance.13 In addition, the water extracts of white flower dandelion (Taraxacum coreanum Nakai) have been reported to alleviate the symptoms of ethanol-induced gastritis by reducing oxidative stress and inflammation.14 Dandelion extracts are reported to protect against liver damage by toxic substances by removing ROS.15,16 Thus, mulberry and white flower dandelion extracts containing flavonoids and anthocyanins may alleviate the alcoholic liver steatosis. However, the direct impact of mulberry extracts to protect against alcoholic liver steatosis and its mechanism has not been examined.
The hypothesis of the present study was that water extracts of mulberry and white flower dandelion may prevent and/or delay the symptoms of chronic ethanol-induced hepatic steatosis in male rats fed a high-fat diet, which accelerates hepatic steatosis. We tested the hypothesis and explored the mechanism of their protection against ethanol-induced hepatic steatosis.
Materials and methods
Water extract of mulberry and dandelion
Dried mulberry fruits (Worldway Inc., Yeongi, Korea) and whole plant of white flower dandelion (Gichan Heenmindeolre Inc., YeongAm, Korea) were separately homogenized and powdered. The homogenates of mulberry fruits and white flower dandelion were extracted in 5-fold volumes of water at 60 and 85°C for 2 h, respectively. Hovenia dulcis Thunb fruits, a major component of commercial hangover products, were also extracted with water at 85°C for 2 h. Each extract was filtered and concentrated up to 50% using a low-pressure rotary evaporator. Each concentrate was freeze-dried to make a powder. The yields of WMB and WTC were 16.7 and 9.6%, respectively. The content of total phenolic compounds was measured using Folin–Ciocalteu reagent, and units were reported as mg gallic acid equivalents g−1.17,18 The total flavonoid content was also assessed using a modified method,19 and was expressed as mg rutin equivalents g−1.
HPLC was performed using a JASCO liquid chromatography instrument (JASCO, Tokyo, Japan) equipped with an autoinjector and an UV detector. Each extract was analyzed with a YMC ODS-AM column (4.6 × 250 nm, 5 µm, Waters). The mobile phase was 0.1% acetic acid aqueous solution (A) and 0.1% acetic acid in acetonitrile (B). The gradients used were as follows: 0 min, A:B 88:12 (v/v); 18 min, A:B 78:22; 28 min, A:B 72:28; 35 min, A:B 62:38; 48 min, A:B 52:48; 54 min, A:B 32:68; 58 min, 0:100 for TCN. The mobile phase flow rate was 1.0 mL/min under the following conditions: column temperature, 35°C; injection volume, 20 μL; and UV detection at 285 nm. The indicated compounds were hydroxybenzoic acid, cyanidin-3-glucoside and rutin for WMB and caffeic acid, chlorogenic acid, and rutin for WTC. Each indicator compound was purchased from Sigma (St. Louise, MO, USA) or ChromaDex (USA).
Animals and experimental design
All experimental procedures were conducted according to the guidelines of and with the approval of the Animal Care and Use Review Committee at Hoseo University, Korea (2013–06). Male Sprague Dawley rats with the age of seven to eight weeks were purchased from Daehan Biolink (Eum Sung, Korea) and they were kept in individual stainless steel cages in a controlled environment with temperature (22 ± 1°C), humidity (55 ± 4%), and a 12-h light/dark cycle. After a one-week acclimation in the animal facility, the 40 rats were divided into the following four treatment groups. Each group was provided with the assigned diets supplemented with either dextrin, the water extract of mulberry fruits, whole plant of white flower dandelion, or Hovenia dulcis Thunb fruits, and were designated as the control, WMB, WTC, and positive-control groups, respectively. Additionally, the rats in the normal-control group were given the same diet as the control group.
Acute ethanol metabolism
All rats except those in the normal-control group had oral intakes of 3 g ethanol/kg bw by oral gavage at 30 min after the assigned extracts (0.3 g/kg bw) were orally provided. The amount of ethanol and extracts was equivalent to about 25 and 2.5 g for human, respectively, and blood samples were taken from tail veins at 0.5, 1, 3, and 5 h. The rats were allowed no additional water or food during the blood collection. The rats were provided with the diet containing 0.1% assigned extracts after acute ethanol administration.
Chronic ethanol intake
The next day after acute ethanol treatment, rats were provided 6% vol/vol ethanol as water. The diet was a semi-purified, modified AIN-93 formulation for experimental animals.20 The diet was made of 40 percent energy (En%) from carbohydrates, 20 En% from protein, and 40 En% from fats. The major sources of carbohydrate, protein, and fat were starch plus sugar, casein (milk protein), and lard (CJ Co., Seoul, Korea), respectively. Assigned extracts were mixed with a high-fat diet by 0.1%. The diet and water containing ethanol were freely consumed for four weeks. As based on the amount of daily food and water intake, the consumption of each extract was about 1 g/kg body weight/day (3 g/day as a daily human equivalent) and the ethanol intake was about 7 g ethanol/kg body weight/day.
Y maze tests
At 4 h after ethanol removal during the fourth week, a Y maze test was conducted to check short-term spatial memory and to assess the brain damage caused by chronic ethanol intake.21,22
Body composition
Prior to euthanizing the rats, they were laid in a prone position with their hind legs maintained in external rotation and hip, knee, and ankle articulations in 90° flexion with tape. The body was scanned by dual-energy X-ray absorptiometry (DEXA) method using an absorptiometer (pDEXA Sabre; Norland Medical Systems Inc., Fort Atkinson, WI, USA). The facility was equipped with the appropriate software for assessment in small animals. Bone mineral density (BMD) was measured in the lumbar spine and femur and lean and fat mass were determined in the abdomen and leg.13
Metabolic analysis
Body weight, food and water intake, and overnight-fasted serum glucose levels were measured every Tuesday at 10 a.m. An oral glucose tolerance test (OGTT) was performed in overnight fasted rats by oral injection of 2 g/kg body weight of glucose at the fourth week of the experimental period: serum glucose levels were measured every 10 min for 90 min and 120 min and serum insulin levels were measured at 0, 20, 40, 90, and 120 min.13 The levels of blood glucose and insulin levels were determined using a Glucometer (Accuchek, Roche Diagnostics, Indianapolis, IN) and radioimmunoassay kit (Linco Research, Billerica, MA), respectively. Homeostasis model assessment for insulin resistance index (HOMA-IR) was calculated as fasting serum insulin (μU) × fasting serum glucose (mmol/L)/22.5. After two days from OGTT, a fasting intraperitoneal insulin tolerance test (IPITT) was performed by measuring serum glucose levels every 15 min for 90 min after an intraperitoneal injection of 0.75 IU insulin/kg body weight.
After two days from IPITT, overnight fasted rats were anesthetized with ketamine and xylazine (100 and 10 mg/kg body weight, respectively) and blood for serum was collected by cardiac puncture. Next, human insulin (5 U/kg body weight; Lily) was injected into the inferior vena cava. After 10 min, the liver was collected and stored at −70°C for further assays. Serum was separated after centrifugation of the blood. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GPT), the markers for liver damage, were assessed by colorimetric methods using kits obtained from Asan Pharmaceutical company (Seoul, Korea). Serum triglyceride levels were measured by using colorimetry kits for triglycerides (Asan Pharmaceutical, Seoul, Korea).
The livers were homogenized with 1.5 N perchloric acid and the lysates were treated with α-amyloglucosidase to hydrolyze glycogen. The hydrolysate was neutralized with NaOH to pH 7.4 and centrifuged at 3000 r/min for 10 min and the glucose concentration measured using a glucose oxidase kit (Young Dong Pharm., Seoul, Korea). Liver glycogen was calculated from the glucose concentrations. Triacylglycerol was extracted with chloroform-methanol (2:1, vol/vol) from the livers and brains and resuspended in pure chloroform.21 After evaporating chloroform, the residues were suspended with PBS with 0.1% triton X-100 and the suspension was sonicated and boiled for 5 min. The triacylglycerol contents of the suspensions were assayed using a Trinder kit (Young Dong Pharm., Seoul, Korea). Lipid peroxide levels in the liver and brain were measured using a thiobarbituric acid reactive substance (TBARS) assay kit (Cayman Chemical, Ann Arbor, Michigan, USA).
Histological analysis
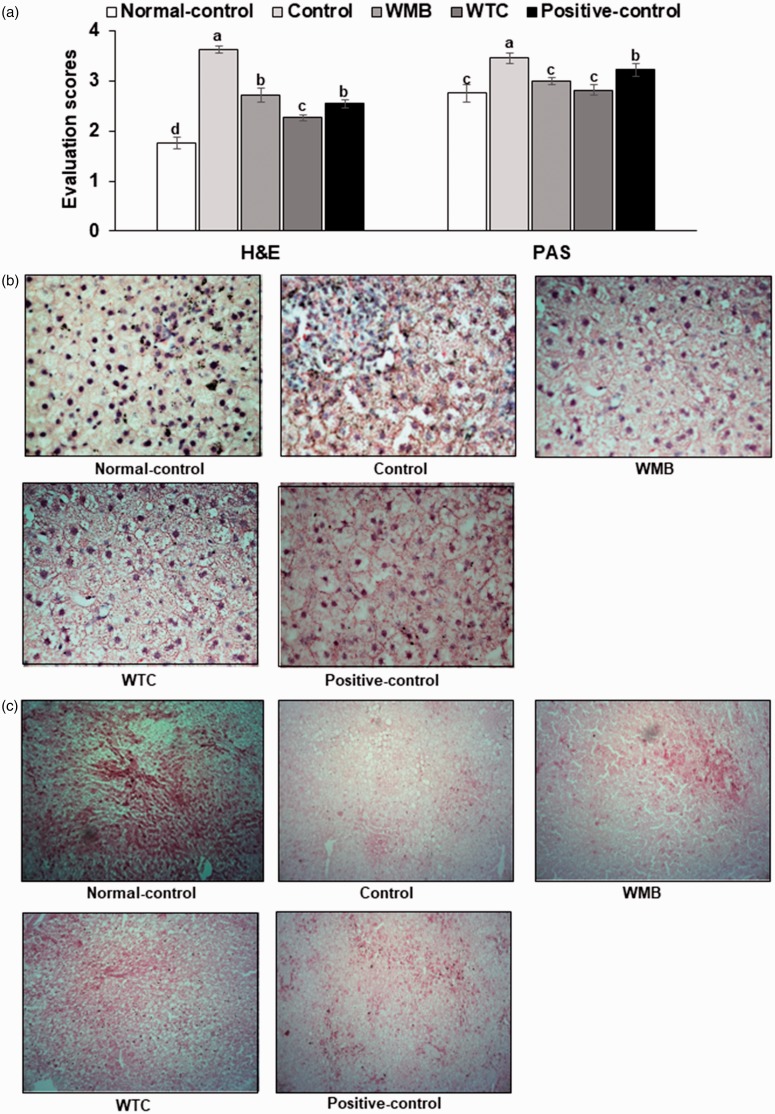
At the end of the experiments, the liver samples were taken and fixed in 10% buffered neutral formaldehyde and embedded in paraffin wax. Histological sections were 6 μm thick and were stained with hematoxylin and eosin (H-E) and used to score the liver damage. The liver damage and glycogen contents were determined in two sections with random selection from six consecutive sections at 400× magnification. The liver damage was scored by summing each item such as the nucleus size and shape, cell size and arrangement, and the number of macrophages. Higher scores indicated more hepatic cell damage. In addition, glycogen contents were determined by red color intensity in periodic acid–Schiff (PAS) staining of the stomach tissues. Lower scores indicated higher glycogen contents.
Next generation sequencing gut microbiome
The gut microbiome composition was measured from feces by analyzing metagenome sequencing using next-generation sequencing. Bacterial DNA was extracted from the samples of each rat using a Power Water DNA Isolation Kit (MoBio, Carlsbad, CA) according to the manufacturer’s instructions. Each library was prepared using polymerase chain reaction (PCR) products according to the GS FLX plus library prep guide. The emPCR, corresponding to clonal amplification of the purified library, was carried out using the GS-FLX plus emPCR Kit (454 Life Sciences, Branford, CT). Libraries were immobilized onto DNA capture beads. The library-beads were added to the amplification mix and oil and the mixture was vigorously shaken on a Tissue Lyser II (Qiagen, Valencia, CA) to create “micro-reactors” containing both amplification mix and a single bead. Emulsion was dispensed into a 96-well plate and the PCR amplification program was run with 16S universal primers in the FastStart High Fidelity PCR System (Roche, Basel, Switzerland) according to the manufacturer's recommendations. Sequencing of bacterial DNA in the feces was performed by the Macrogen Ltd. (Seoul, Korea) by a Genome Sequencer FLX plus (454 Life Sciences) as previously reported.23
Statistical analysis
Statistical analysis was performed using SAS software, and all results were expressed as mean±standard deviation. The variables related to the metabolic changes were compared among control, WMB, WTC, positive-control, and normal-control by one-way analysis of variance in cell-based and animal studies. Multiple comparisons among the groups were conducted by Tukey's test at P < 0.05.
Results
Total polyphenolic compounds and flavonoids of WMB and WTC and their bioactive components
The polyphenol and flavonoid contents of WMB and WTC are shown in Table 1, and were higher in WTC than in WMB. WMB and WTC had similar contents of rutin and WTC and WMB were rich in caffeic acid cyaniding 3-glucoside, respectively (Table 1).
Table 1.
Contents of total phenols and flavonoids and bioactive components in water extracts of mulberry and white flower dandelion.
| Total polyphenols | Total flavonoids | Rutin | Chlorogenic acid | Caffeic acid | |
|---|---|---|---|---|---|
| White flower dandelion | 99.9 ± 0.7 | 20.7 ± 0.5 | 8.81 ± 0.48 | 1.44 ± 0.06 | 12.8 ± 0.01 |
| Mulberry | 34.4 ± 0.6 | 24.3 ± 0.7 | 8.6 ± 0.01 | Hydroxybenzoic acid | Cyanidin-3-glucoside |
| 3.66 ± 0.18 | 6.45 ± 0.03 |
Note: Values represent means±standard deviation (n = 3). Units: mg/100 g extract.
Ethanol metabolism
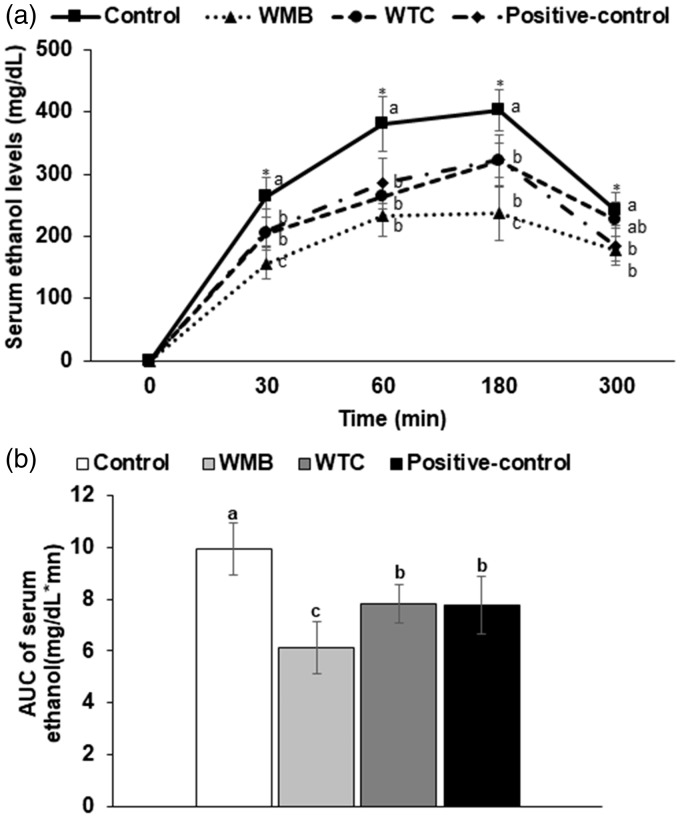
After acute ethanol challenge (3 g ethanol/kg bw), serum ethanol levels increased until 180 min and then decreased in all groups. The levels were much higher in the control group at 30, 60, 180, and 300 min after ethanol intake. WMB reduced the levels the most compared to the other groups (Figure 1(a)). WTC and positive-control showed similar levels at 30, 60, and 180 min, but the levels quickly decreased in positive-control at 300 min (Figure 1(a)). The area under the curve of serum ethanol levels was lowered in descending order of control, WTC, and positive-control and WMB (Figure 1(b)).
Figure 1.
Serum ethanol concentrations after ethanol administration with water extracts of mulberry and dandelion. Male rats had oral intakes of 3 g ethanol/kg bw by oral gavage at 30 min after the assigned extracts (0.3 g/kg bw) were orally provided. Blood was collected at 30, 60, 190, and 300 min after ethanol administration without providing additional water and food and serum ethanol levels were measured by colorimetry method. Changes in serum ethanol concentrations (a) and area under the curve of serum ethanol concentrations (b) were provided.
Body composition
Body weight did not differ between the control and normal-control groups and WMB, WTC, and positive-control also did not affect body weight (Table 2). However, visceral fat, the sum of the epididymal fat, and retroperitoneal fat were much higher in the control group than the normal-control group (Table 2). WMB lowered the epididymal and retroperitoneal fat mass to less than the control group and it was similar to the normal-control group (Table 2). Food intake was higher in the normal-control than the control, but caloric intake, summing food, and ethanol intake in the normal-control tended to be lower than the control (Table 2). Food intake and ethanol intakes were not significantly different among the treatment groups in comparison to the control group (Table 2). Thus, the changes in metabolic parameters were associated with ethanol itself, and not with the differences in energy intake.
Table 2.
Body weight, body composition, and glucose metabolism.
| Normal-control (n = 12) | Control (n = 12) | WMB (n = 12) | WTC (n = 12) | Positive-control (n = 12) | |
|---|---|---|---|---|---|
| Body weight (g) | 409 ± 38 | 410 ± 39 | 406 ± 38 | 407 ± 40 | 403 ± 39 |
| Epididymal fat (g) | 4.4 ± 0.5c | 6.2 ± 0.8a | 4.1 ± 0.6c | 5.6 ± 0.8b | 5.4 ± 0.8b |
| Retroperitoneal fat (g) | 6.4 ± 0.7b | 7.9 ± 1.0a | 5.2 ± 0.7c | 6.0 ± 0.9b | 6.5 ± 0.9b |
| Visceral fat (g) | 10.8 ± 1.2c | 14.1 ± 1.8a | 9.3 ± 1.4c | 11.6 ± 1.7b | 11.9 ± 1.7b |
| Food intake (g/day) | 15.7 ± 0.9 | 12.8 ± 1.2 | 12.3 ± 1.0 | 13.0 ± 1.0 | 13.2 ± 1.1 |
| Ethanol intake (g/day) | 0b | 2.8 ± 0.3a | 2.9 ± 0.4a | 3.0 ± 0.4a | 3.0 ± 0.5a |
| Calorie intake | 74.1 ± 4.3 | 77.2 ± 7.3 | 75.6 ± 6.1 | 79.6 ± 6.1 | 80.4 ± 7.3 |
| Serum glucose (mg/dL) | 90.1 ± 7.2 | 92.9 ± 6.7 | 91.2 ± 6.4 | 85.9 ± 6.5 | 87.0 ± 7.1 |
| Serum insulin (ng/mL) | 1.79 ± 0.20a | 1.74 ± 0.18a | 1.28 ± 0.13c | 1.40 ± 0.14bc | 1.47 ± 0.16b |
| HOMA-IR | 9.0 ± 1.0a | 9.0 ± 0.9a | 6.5 ± 0.6b | 6.7 ± 0.6b | 7.1 ± 0.8b |
| % of right turns in Y maze test | 72.2 ± 6.8a | 48.9 ± 7.5c | 56.7 ± 6.8b | 73.5 ± 5.4a | 70.2 ± 6.9a |
Note: Male rats were provided 6% vol/vol ethanol instead of water and 0.1% dextrin (control), water extract of mulberry (WMB), dandelion (WTC), or Hovenia dulcis Thunb fruits (positive-control) in high-fat diet for four weeks. Normal-control had no ethanol with a high-fat diet. Values are means ± standard deviation. Different letters on the same row represented significant difference among the groups by Tukey test at P < 0.05.
HOMA-IR: homeostasis model assessment for insulin resistance index.
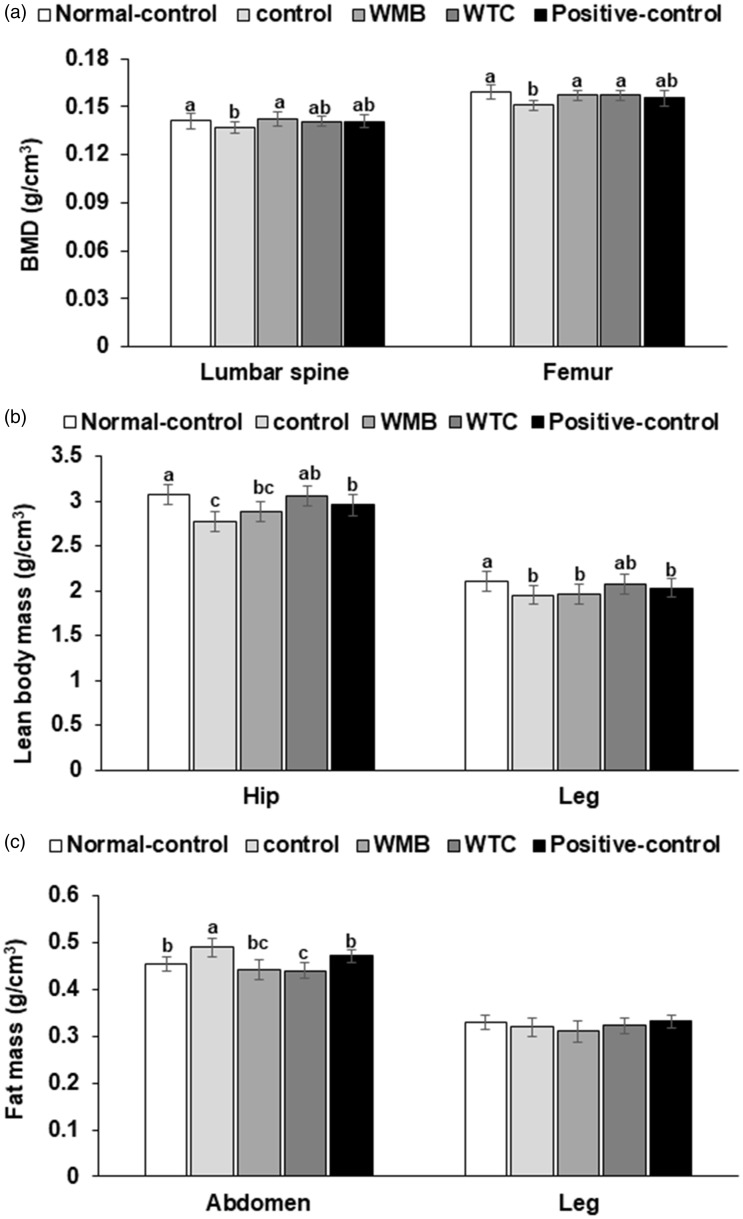
Although body weight was not different among groups, ethanol intake had a large effect on body composition. Ethanol intake decreased BMD in the lumbar spine and femur, and its decrease in the femur was greater than the lumbar spine (Figure 2(a)). WMB protected against the decrease of BMD in the lumbar spine and femur, and WTC prevented the BMD decrease in the femur (Figure 2(a)). The lean mass in the hip and leg was also decreased in the control group compared to the normal-control, but it was restored by WTC the most (Figure 2(b)). On the contrary, the fat mass in the abdomen was higher in the control than the normal-control group, and WTC and WMB protected against abdominal fat accumulation (Figure 2(c)). However, fat mass in the leg was not different between the control and normal-control groups and other treatments did not modulate it (Figure 2(c)). These results suggested that ethanol intake changed the body composition without changing the body weight.
Figure 2.
Bone mineral density (BMD) and lean body mass (LBM). Male rats were provided 6% vol/vol ethanol instead of water and 0.1% dextrin (control), water extract of mulberry (WMB), dandelion (WTC), or Hovenia dulcis Thunb fruits (positive-control) in high-fat diet for four weeks. Normal-control had no ethanol with a high-fat diet. At the end of the experimental period, BMD in the lumbar spine and femur (a), LBM in the hip and leg regions (b) and fat mass in the abdomen and leg (c) were measured by DEXA. Each bar and error bar represents means ± SD (n=12). The different letters on the bars represent significant differences among the groups by Tukey’s test at P<0.05.
Glucose metabolism
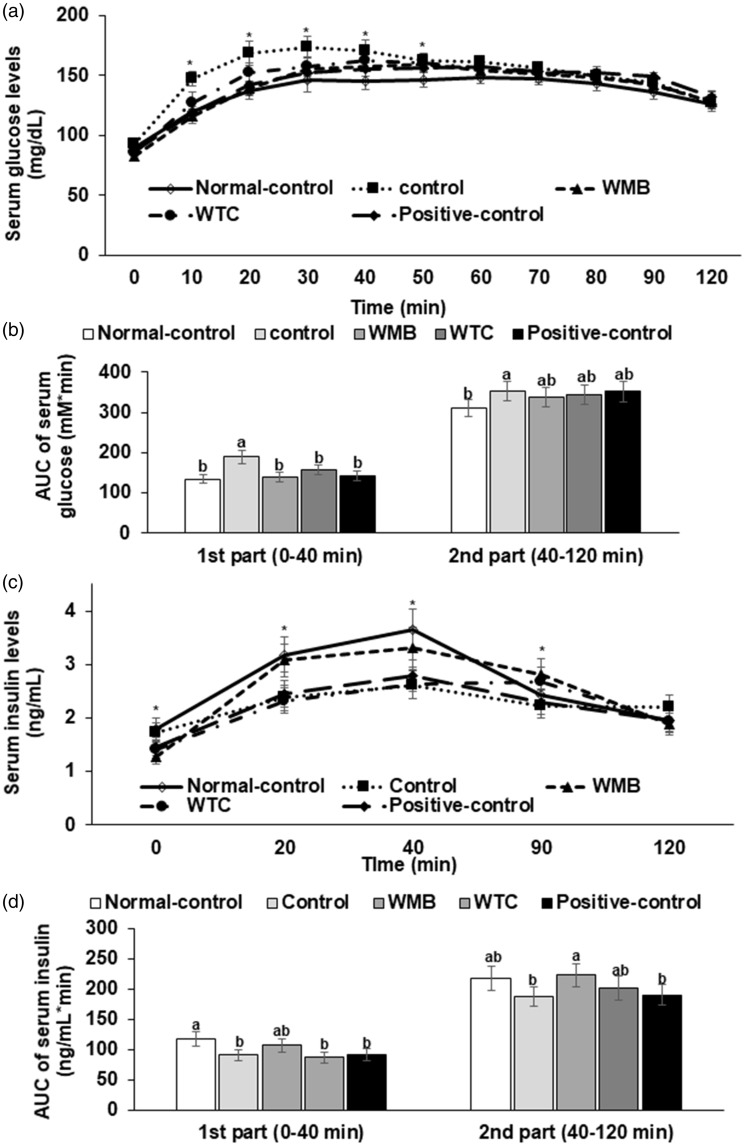
Serum glucose levels in overnight fasting states were not significantly different among the groups but serum insulin levels were lowered in WTC, WMB, and positive-control groups than the control group (Table 2). However, HOMA-IR, an index of insulin resistance, was not significantly different between the control and normal-control, but WTC, WMB, and positive-control lowered the index (Table 2).
After orally giving 2 g glucose/kg body weight, serum glucose concentrations were elevated and reached the highest concentration at 20–30 min earlier than the normal-control group and then the concentrations decreased, but not as much as the normal-control (Figure 3(a)). Rats in the WMB, WTC, and positive-control groups exhibited lower serum glucose concentrations at 20–30 min, to as much as those of the normal-control group but the concentrations did not decrease as fast as the normal-control group (Figure 3(a)). Area under the curve of serum glucose levels (AUCG) in the first part (0–40 min) of the control group was much higher than that of the normal-control group (Figure 3(b)). AUCG in the second part (40–120 min) tended to be higher in the control than the normal-control group, but it was not significantly different (Figure 3(b)). Other treatments did not differ from the control. The differences in AUCG were partly associated with insulin secretion during OGTT. Serum insulin concentrations were much higher in the normal-control than the control group between 20 and 90 min during OGTT (Figure 3(c)). WMB treatment increased serum insulin concentrations as much as the normal-control group but not WTC and positive-control (Figure 3(c)). AUC of serum insulin concentrations (AUCI) in the first (0–40 min) and second parts (40–120 min) was lower in the control group than the normal-control and WMB also increased AUCI of the first and second parts as much as the normal-control group (Figure 3(d)).
Figure 3.
Serum glucose levels and areas under the curve of glucose and insulin during the oral glucose tolerance test (OGTT). At the fourth week of administration of 6% vol/vol ethanol instead of water and 0.1% dextrin (control), water extracts of mulberry (WMB), dandelion (WTC) or Hovenia dulcis Thunb fruits (positive-control) in high-fat diet, 2 g of glucose/kg body weight were orally administered at the overnight fasting state. Normal-control had no ethanol with a high-fat diet. The changes in the serum glucose levels (a) and serum insulin levels (c) were measured during the OGTT. The averages of the areas under the curve (AUC) of glucose (b) and insulin (d) were calculated for the first part (0–40 min) and second part (40–120 min) of the OGTT. Each dot and bar represents the mean ± SD (n=12). *Significantly different among all groups in one-way ANOVA at P<0.05. The different letters on the bars represent significant differences among the groups by Tukey’s test at P<0.05.
The impairment of insulin secretion might be related to increased malondialdehyde (MDA) in the pancreas which increased apoptosis of β-cells (Table 3). The concentrations of MDA in the control group were higher than those in the normal-control, and WMB and WTC reduced the concentrations. WMB reduced the levels more than WTC and the positive-control (Table 3).
Table 3.
Cell damage and glycogen and fat accumulation in the liver.
| Normal-control (n = 12) | Control (n = 12) | WMB (n = 12) | WTC (n = 12) | Positive-control (n = 12) | |
|---|---|---|---|---|---|
| Serum ALT (U/L) | 13.2±1.2c | 19.2±2.3a | 11.5±1.3d | 11.7±1.3d | 16.4±1.5b |
| Serum AST (U/L) | 49.1±4.7b | 58.3±5.4a | 40.8±4.5c | 41.1±4.3c | 43.2±4.6c |
| Serum triglyceride (mg/dL) | 67.7±6.8b | 77.8±7.1a | 69.4±6.5b | 75.6±6.3a | 79.8±7.2a |
| MDA in the liver (nM) | 20.5±1.6c | 27.3±1.9a | 19.5±1.5c | 23.6±1.7b | 24.4±1.8b |
| MDA in the brain (nM) | 17.5±1.4b | 21.5±1.9a | 15.8±1.5c | 18.3±1.7b | 19.5±1.6ab |
| MDA in the pancreas (nM) | 21.8±1.9c | 29.5±2.5a | 20.7±1.9c | 24.3±2.5b | 25.9±2.6b |
| Triglyceride in the liver (mg/g tissue) | 1.8±0.2b | 2.5±0.2a | 1.2±0.1c | 1.4±0.1c | 1.7±0.2b |
| Triglyceride in the brain (mg/g tissue) | 0.44±0.04b | 0.54±0.07a | 0.41±0.05b | 0.53±0.06a | 0.52±0.05a |
| Glycogen in the liver (mg/g tissue) | 0.99±0.09a | 0.84±0.79b | 1.05±0.10a | 1.01±0.09a | 0.96±0.09ab |
ALT: alanine aminotransferase; APT: aspartate aminotransferase; MDA: malondialdehyde.
Note: Male rats were provided 6% vol/vol ethanol instead of water and 0.1% dextrin (control), water extract of mulberry (WMB), dandelion (WTC), or Hovenia dulcis Thunb fruits (positive-control) in high-fat diet for four weeks. Normal-control had no ethanol with a high fat diet. Values are means ± standard deviation. Different letters on the same row represented significant difference among the groups by Tukey test at P < 0.05.
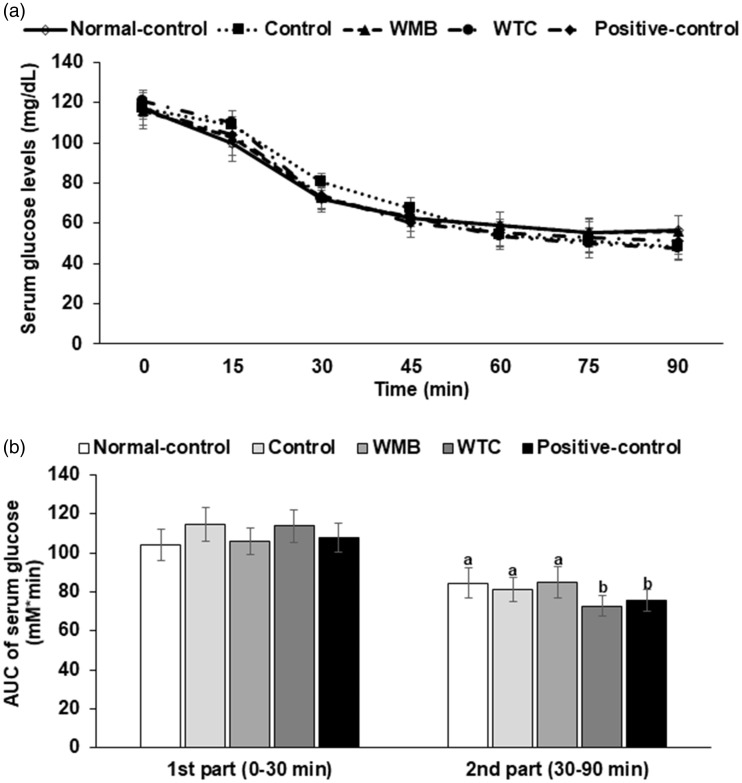
After the intraperitoneal injection of insulin, serum glucose concentrations decreased more slowly in the the control group than in the normal-control group than in the normal-control group in the first part (0–30 min) of the IPITT, and WMB and positive control groups decreased serum glucose concentrations as fast as in the normal-control group (Figure 4(a)). In the second part (30–90 min), AUCG was not significantly different between the control and normal-control groups, but WTC and positive-control reduced the AUCG of the second part of IPITT (Figure 4(b)).
Figure 4.
Changes in the serum glucose levels during an intraperitoneal insulin tolerance test (IPITT).At the fourth week of administration of 6% vol/vol ethanol instead of water and 0.1% dextrin (control), water extracts of mulberry (WMB), dandelion (WTC) or Hovenia dulcis Thunb fruits (positive-control) in high-fat diet, an IPITT was conducted by intraperitoneally injecting insulin (1 U/kg body weight) after a 6 h fast. Normal-control had no ethanol with a high-fat diet. The serum glucose levels (a) and area under the curve of serum glucose levels (b) were measured. Each dot and bar represents the mean ± SD (n=12). *Significantly different among all groups in one-way ANOVA at P<0.05. The different letters on the bars represent significant differences among the groups by Tukey’s test at P<0.05.
Liver damage
Serum ALT and AST levels, indexes of liver damage, were higher in the control group than the normal-control, and WMB and WTC lowered the levels (Table 3). The decreases by WMB and WTC were better than the positive-control (Table 3). Serum triglyceride levels were higher in control group than the normal-control group (Table 3). In addition, the contents of MDA (an index of oxidative stress) in the liver, brain, and pancreas were much higher in the control group than the normal-control group (Table 3). WMB protected against the increase of MDA contents by alcohol, and was lower than even the normal-control. WTC and positive-control lowered the contents, but they did not decrease them as much as normal-control (Table 3).
The H-E staining showed that the liver cells in the control group had irregular nuclei and cell shapes in comparison to the normal-control (Figure 5(a)). The cellular shape and nucleus in the WMB and positive-control exhibited more normal shapes than those of the control. The WTC-treated group also had greater deterioration of cellular and nucleus shapes than WMB but its deterioration was less than the control (Figure 5(a) and (b) ). The control group has less PAS staining than the normal-control, indicating that glycogen deposition in the control group was much less than the normal-control group (Figure 5(a) and (c)). WMB and WTC increased glycogen deposition more than the control.
Figure 5.
Hematoxylin-eosin (H-E) and periodic acid–Schiff (PAS) staining in the liver tissue. After collecting the liver, it was paraffin-embedded and the liver sections were stained with H-E and PAS staining. The evaluation scores for H-E were calculated by summing of each item such as the nucleus size and shape, cell size and arrangement and the number of macrophages and those for PAS staining were determined by the glycogen storage (red staining) (a). The images of H-E staining (b) and PAS (c) are provided.
The contents of triglyceride and glycogen were reflected by the histology of the liver staining. The contents of glycogen were higher in the normal-control group than the control group and were similar to the normal-control group in the positive-control and WMB groups (Table 3). WTC also prevented the decreased glycogen compared to the control, but it was not as much as the normal-control. In contrast to the glycogen contents, the contents of triglyceride were higher in the control than the normal-control. The hepatic triglyceride contents were reduced the most in WMB group (Table 3). The triglyceride contents were also decreased in the WTC and positive-control groups, and were similar to the normal-control group.
Gut microbiome
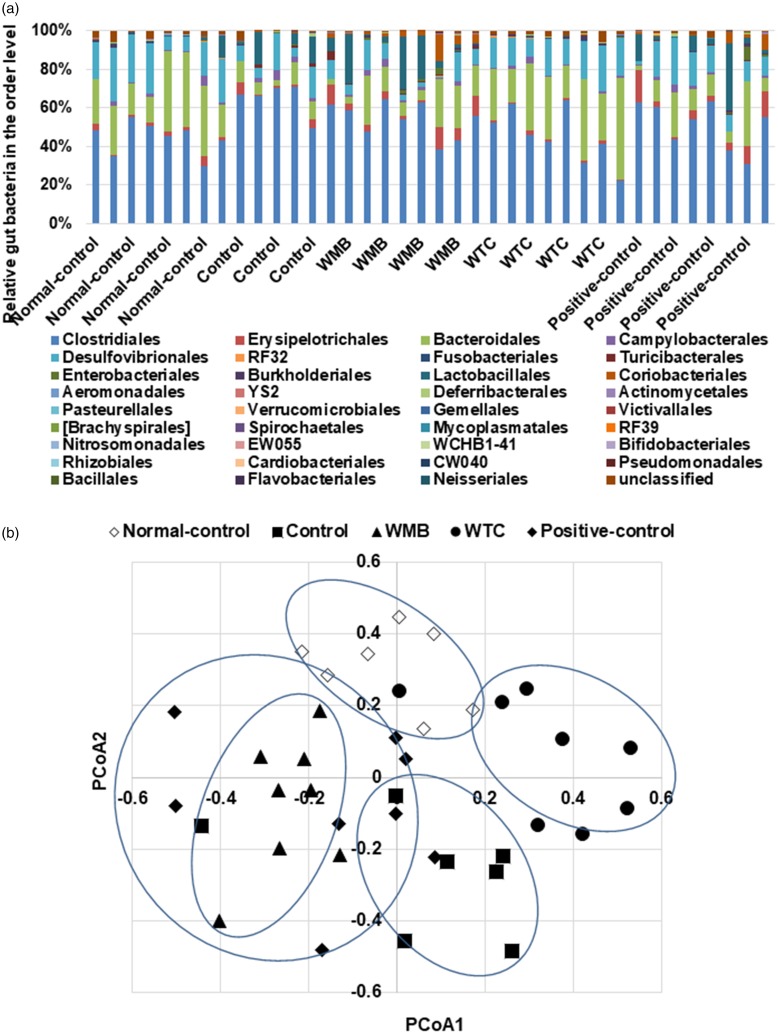
The gut microbiota community was compared among the groups by analysis of molecular variance (AMOVA). The gut bacterial community among the groups was significantly different by AMOVA (P < 0.001). Alcohol intake altered the bacterial distribution to increase Firmicutes and to decrease Bacteroidetes compared to the normal-control. The gut microbiota in the control group was different from that of the normal-control: the ratio of Firmicutes to Bacteroidetes was much higher in the control (13.2 ± 4.1) than the normal-control (1.93 ± 0.42) at the phylum level. The ratio was lowered by WMB (8.82 ± 3.39) and positive-control (9.81 ± 4.27) but it was higher than the normal-control. WTC decreased the ratio (1.87 ± 0.50) to as many as the normal-control. At the order level, Clostridiales was higher in the control than the normal-control and it was lowered by the positive-control and WTC in alcohol-administered rats. WTC showed a similar gut microbiota to the normal-control at the phylum level and WMB and WTC changed the distribution at the order level (Figure 6(a)). The Bacteroidales content was lower in the control group than the normal-control group, and WTC increased the contents to as many as the normal-control group (Figure 6(a)). WMB and positive control also increased Bacteroidales content but it was less than the normal-control group (Figure 6(a)).
Figure 6.
The profiles of gut microbiomes. At the fourth week of administration of 6% vol/vol ethanol instead of water and 0.1% dextrin (control), water extracts of mulberry (WMB), dandelion (WTC) or Hovenia dulcis Thunb fruits (positive-control), feces were collected and the bacterial DNA was analyzed. Proportion of taxonomic assignments [order] for gut microbiomes (a) was analyzed. Normal-control had no ethanol with a high-fat diet. The fecal bacterial community was shown in principal coordinate analysis (PCoA) (b).
Some Actinomycetales, Desulfovibrionales, Neisseriales, and Campylobacterales were higher in the normal-control fed high-fat diet group than the control group. WMB and WTC increased Desulfovibrionales and Campylobacterales, but the positive-control did not alter them (Figure 6(a)). However, the WMB, WTC, and positive-control groups exhibited increased Actinomycetales levels, but not to as many as the normal-control. Lactobacillales were not altered by alcohol intake, but interestingly, WMB markedly elevated Lactobacillales (Figure 6(a)). Thus, alcohol changes the gut microbiota community and WMB and WTC prevented the gut microbiota dysbiosis that might be associated with metabolic dysfunction.
Principal coordinate analysis (PCoA) shows the clustering of gut bacterial community in the groups (Figure 6(b)). The control group exhibited a separation from the normal-control and WMB and WTC were separated from the control group (Figure 6(b)). The PcoA of the gut microbiome in the WTC and WMB was separated from the control, but the separation was in the opposite directions. Thus, alcohol intake changed the gut bacterial community and WTC altered the community to reduce the alcohol-induced changes in gut microbiota.
Discussion
Chronic alcohol consumption induces liver diseases such as fatty liver, cirrhosis, liver cancer, and liver failure and it causes a significant increase in morbidity and mortality.24 The major risk factors for liver steatosis are alcohol consumption, hepatitis B and C virus infection, and obesity. Alcohol consumption and obesity are the major triggers of liver steatosis currently, although their mechanisms are different. Excessive alcohol consumption and obesity often occur together and the conditions additively contribute to the development of liver steatosis. Alcohol abstinence and losing weight can protect against liver steatosis. However, it is difficult to achieve those goals by commonly used interventions, and functional foods can help the process. In the present study, we determined the efficacy of WMB and WTC to prevent and/or delay the symptoms of chronic ethanol-induced hepatic steatosis in male rats fed high-fat diets and explored the mechanism involved in relieving ethanol-induced hepatic steatosis. This study demonstrated that WMB and WTC may be useful as functional foods for alcoholic liver steatosis by reducing decreasing fat deposition in the liver while decreasing the ratio of Firmicutes to Bacteroidetes in the large intestine.
Excessive alcohol intake increases oxidative stress and inflammation which damages the body, especially, the liver. ROS are mainly produced by the mitochondria and reduce bioenergetics, leading to hepatocyte death.25 During the process, lipid clearance is dysregulated to increase triglyceride accumulation. The present study showed a consistent result that chronic alcohol intake increased MDA (lipid peroxides) and facilitated the disarrangement of hepatocytes and nuclei in comparison to the normal-control group. WTC decreased oxidative stress to as much as the normal-control, and WMB was even more effective for lowering hepatic lipid peroxide levels. In addition to increasing oxidative stress, the enhancement of ethanol degradation reduces the hepatic damage by alcohol even during the chronic consumption. Ethanol degradation was faster in WMB, WTC, and positive-control than the control. WMB resulted in greater ethanol utilization than WTC and positive-control. Thus, WMB and WTC both protected against hepatic cell damage and WMB provided better protection than WTC.
Fat accumulation is associated not only with hepatic damage but also cellular energy state. Alcohol is metabolized into acetate in the liver and is then oxidized to generate ATP in the mitochondria or used to make fatty acids. The high level of cellular ATP dephosphorylates AMP kinase, a central energy regulator, which inhibits energy production and activates the synthesis of fatty acids and cholesterol.26 Thus, chronic ethanol intake increases triglyceride deposition in the liver. The present study showed that rats in the control group developed fat deposition in the liver and WMB and WTC treatments similarly prevented hepatic fat accumulation and increased glycogen deposition.
Although heavy alcohol drinking is associated with the development of type 2 diabetes in humans,27 the mechanism is not fully understood. Type 2 diabetes is developed, when insulin secretion cannot compensate for insulin resistance.28 Chronic alcohol intake is involved in both insulin resistance and insulin secretory capacity.27 Liver steatosis is involved in increased hepatic insulin resistance. Chronic alcohol intake is associated with cytokines and oxidative stress that cause cellular apoptosis and deposition of triglyceride.29 Alcohol intake also inhibits insulin and insulin-like growth factor signaling in the liver and brain to induce insulin resistance.30 The present study showed that insulin sensitivity slightly decreased in the control group compared to the normal-control, and WMB prevented the decrease, but it was not significantly different. The slight decreases in insulin resistance might be related to the relatively short-term treatment of alcohol (four weeks). Thus, chronic alcohol intake increased hepatic insulin resistance and it may influence glucose tolerance.
Alcohol intake also affects insulin secretion capacity. In cross-sectional studies in Europe and Asia, alcohol consumption is negatively associated with both insulin resistance and β-cell function,31,32 which are the crucial factors in the development of type 2 diabetes.28 These results suggest that heavy alcohol drinking may increase susceptibility to type 2 diabetes in Asians since Asians have lower β-cell function. A recent Japanese cohort study demonstrated that moderate and heavy alcohol consumption impairs insulin secretion and also shows a tendency to increase insulin resistance.33 However, no prospective studies have been conducted in humans, and there are few experimental studies. The present study showed that chronic alcohol intake decreased serum insulin levels in response to a 2 g glucose/kg body weight challenge, in comparison to no alcohol intake. WMB protected against the decrease in serum insulin levels during OGTT, but WTC and the positive-control did not. Previous studies have demonstrated that anthocyanins in mulberry extracts protect against pancreatic β-cell apoptosis induced by oxidative stress and glucotoxicity and maintains glucose-stimulated insulin secretion.34,35 Thus, chronic alcohol intake increased oxidative stress which impaired β-cell function, and mulberry extracts alleviated the β-cell dysfunction by decreasing oxidative stress.
The metabolic changes induced by chronic alcohol intake may be associated with changes in the gut microbiota. After alcohol intake, alcohol in the blood circulation affects the gut microbiome by the ways to increase intestinal hyperpermeability to luminal bacterial products and oxidative metabolites. The changes lead to the development of dysbiosis in the intestinal microbiota.36 The dysbiosis resulting from alcohol intake may influence the development of, or exacerbate alcoholic liver disease. Recent studies have shown that the proportion of Bacteriodetes species relative to Firmicutes is lower in obese persons and animals in comparison to lean people and animals, and microbial diversity is also decreased.4,37 This might be related to changes in nutrients in the gut which enhance the growth of certain microbiomes and the microbiota produces inflammatory factors such as lipopolysaccharides and short chain fatty acids37 The microbial byproducts affect inflammation and immune activation which modulate brain-gut axis to change appetite and energy expenditure that alter body weight and fat mass. Their changes also modify hepatic insulin resistance and insulin secretion which modulate glucose metabolism. Thus, the changes in gut microbiota modify energy metabolism and glucose metabolism. Alcohol intake is reported to increase intestinal permeability to luminal bacterial products that go to the liver causing the development of alcoholic liver disease.36 Alcohol intake also modulates the bile acid pool size and composition to increase the production of deoxycholic acid38 which reduces the diversity of the gut microbiota and elevates the number of Firmicutes.38 The present study showed a comparable result, that alcohol intake increased the Firmicutes population, especially Clostridiales, and it was associated with increased visceral fat in the rats fed alcohol. WMB and WTC altered the gut microbial community in rats with chronic alcohol intake, and WTC was most protective against the changes induced by alcohol intake in the present study. The modification of gut microbiome might be associated with the protection of alcoholic hepatic steatosis and glucose dysregulation.38
In conclusion, water extracts of mulberry fruits and white flower dandelion protected against fatty liver induced by alcohol intake and also alleviated the impairment of glucose metabolism. They also prevented the decrease in BMD and LBM and the increase in fat mass in rats consumed alcohol. Water extracts of mulberry fruits had better efficacy for improving glucose metabolism than WTC, whereas white flower dandelion increased LBM more than water extracts of mulberry fruits. The changes in glucose and lipid metabolism might be involved in gut microbiome modification. Thus, mulberry fruits and white flower dandelion may be useful functional foods for preventing or alleviating alcoholic liver steatosis, and for improving glucose metabolism while protecting against the dysbiosis of the gut microbiome community. Mulberry fruits had better efficacy for improving alcoholic liver steatosis than white flower dandelion. Human studies are needed to confirm the functionality.
Authors’ contributions
SP conceived of the study and contributed to the experimental design and coordination of the study. XW and QJY performed the animal procedures. DSK carried out biochemical assays. SP wrote the initial draft of the manuscript and each author read, made suggestions and approved the final version.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially funded by a grant from the National Research Foundation of Korea, the Ministry of Science, ICT & Future Planning for convergent research in Development program for convergence R&D over traditional culture and current technology (NRF – 2016M3C1B5907152).
References
- 1.Organization WH. Global health observatory data. Prevalence of alcohol use disorders. Washington DC, United States: World Health Organization, 2010 [Google Scholar]
- 2.Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Rev Gastroenterol Hepatol 2007; 4:24–34 [DOI] [PubMed] [Google Scholar]
- 3.Lin C-W, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XX, Yin X-M. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol 2013; 58:993–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shearn CT, Smathers RL, Jiang H, Orlicky DJ, Maclean KN, Petersen DR. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J Nutr Biochem 2013; 24:1436–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016; 534:213–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, Qiang X, Zhang M, Ma D, Zhao Z, Zhou C, Liu X, Li R, Chen H, Zhang Y. Demethyleneberberine, a natural mitochondria-targeted antioxidant, inhibits mitochondrial dysfunction, oxidative stress, and steatosis in alcoholic liver disease mouse model. J Pharmacol Exp Ther 2015; 352:139–47 [DOI] [PubMed] [Google Scholar]
- 7.Lowe PP, Gyongyosi B, Satishchandran A, Iracheta-Vellve A, Ambade A, Kodys K, Catalano D, Ward DV, Szabo G. Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PLoS One 2017; 12:e0174544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo C, Ma J, Zhong Q, Zhao M, Hu T, Chen T, Qiu L, Wen L. Curcumin improves alcoholic fatty liver by inhibiting fatty acid biosynthesis. Toxicol Appl Pharmacol 2017; 328:1–9 [DOI] [PubMed] [Google Scholar]
- 9.Ma Z, Zhang Y, Li Q, Xu M, Bai J, Wu S. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1α expression and mitochondrial ROS production. PLoS One 2017; 12:e0183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko BS, Hong SM, Kim DW, Sung SR, Park HR, Lee JE, Jeon WK, Park S. Effect of new remedies mainly comprised of Hovenia dulcis Thunb on alcohol degradation and liver protection in Sprague Dawley male rats. Kor Soc Food Sci Nutr 2006; 35:828–34 [Google Scholar]
- 11.Yuan Q, Zhao L. The mulberry (Morus alba L.) fruit-a review of characteristic components and health benefits. J Agric Food Chem 2017; 65:10383–94 [DOI] [PubMed] [Google Scholar]
- 12.Yang HJ, Kim MJ, Kang ES, Kim DS, Park S. Red mulberry fruit aqueous extract and silk proteins accelerate acute ethanol metabolism and promote the antioxidant enzyme systems in rats. Mol Med Rep 2018; 18:1197–1205 [DOI] [PubMed] [Google Scholar]
- 13.Song H, Lai J, Tang Q, Zheng X. Mulberry ethanol extract attenuates hepatic steatosis and insulin resistance in high-fat diet-fed mice. Nutr Res 2016; 36:710–8 [DOI] [PubMed] [Google Scholar]
- 14.Yang HJ, Kim MJ, Kwon DY, Kang ES, Kang S, Park S. Gastroprotective actions of Taraxacum coreanum Nakai water extracts in ethanol-induced rat models of acute and chronic gastritis. J Ethnopharmacol 2017; 208:84–93 [DOI] [PubMed] [Google Scholar]
- 15.Hfaiedh M, Brahmi D, Zourgui L. Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ Toxicol 2016; 31:339–49 [DOI] [PubMed] [Google Scholar]
- 16.Gulfraz M, Ahamd D, Ahmad MS, Qureshi R, Mahmood RT, Jabeen N, Abbasi KS. Effect of leaf extracts of Taraxacum officinale on CCl4 induced hepatotoxicity in rats, in vivo study. Pak J Pharm Sci 2014; 27:825–9 [PubMed] [Google Scholar]
- 17.Abdel-Hameed E-SS. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem 2009; 114:1271–7 [Google Scholar]
- 18.Yang HJ, Lim JH, Park KJ, Kang S, Kim da S, Park S. Methyl jasmolate treated buckwheat sprout powder enhances glucose metabolism by potentiating hepatic insulin signaling in estrogen-deficient rats. Nutrition 2016; 32:129–37 [DOI] [PubMed] [Google Scholar]
- 19.AOAC. Official methods of analysis. Method association of official analytical communities. 19th edition Arlington: AOAC International, 2012 [Google Scholar]
- 20.Park S, Kim DS, Kang S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-gamma expression in nonobese Type 2 diabetic rats. J Nutr Biochem 2016; 27:257–65 [DOI] [PubMed] [Google Scholar]
- 21.Golub HM, Zhou QG, Zucker H, McMullen MR, Kokiko-Cochran ON, Ro EJ, Nagy LE, Suh H. Chronic alcohol exposure is associated with decreased neurogenesis, aberrant integration of newborn neurons, and cognitive dysfunction in female mice. Alcohol Clin Exp Res 2015; 39:1967–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin BK, Kang S, Kim DS, Park S. Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer's disease-induced estrogen deficient rats. Exp Biol Med 2018; 243:334–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Kim DS, Kang ES, Kim DB, Kang S. Low dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am J Physiol Endocrinol Metab 2018;315:E99–109 [DOI] [PubMed]
- 24.Stickel F, Datz C, Hampe J, Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver 2017; 11:173–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King AL, Mantena SK, Andringa KK, Millender-Swain T, Dunham-Snary KJ, Oliva CR, Griguer CE, Bailey SM. The methyl donor S-adenosylmethionine prevents liver hypoxia and dysregulation of mitochondrial bioenergetic function in a rat model of alcohol-induced fatty liver disease. Redox Biol 2016; 9:188–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Wang C, Wang C, Zhao H, Zhao C, Chen Y, Wang Y, McClain C, Feng W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J Nutr Biochem 2015; 26:337–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015; 38:1804–12 [DOI] [PubMed] [Google Scholar]
- 28.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015; 1:15019. [DOI] [PubMed] [Google Scholar]
- 29.Hellerbrand C. Pathophysiological similarities and synergisms in alcoholic and non-alcoholic steatohepatitis. Dig Dis 2010; 28:783–91 [DOI] [PubMed] [Google Scholar]
- 30.de la Monte S, Derdak Z, Wands JR. Alcohol, insulin resistance and the liver-brain axis. J Gastroenterol Hepatol 2012; 27(Suppl 2):33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DY, Yoo MG, Kim HJ, Jang HB, Kim JH, Lee HJ, Park SI. Association between alcohol consumption pattern and the incidence risk of type 2 diabetes in Korean men: a 12-years follow-up study. Sci Rep 2017; 7:7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonnet F, Disse E, Laville M, Mari A, Hojlund K, Anderwald CH, Piatti P, Balkau B. Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 2012; 55:3228–37 [DOI] [PubMed] [Google Scholar]
- 33.Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, Miyamoto Y, Izawa S, Ohkubo T. Association between alcohol consumption and incidence of impaired insulin secretion and insulin resistance in Japanese: the Saku study. Diabetes Res Clin Pract 2018; 135:11–7 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Xiang L, Wang C, Tang C, He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One 2013; 8:e71144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JS, Kim YR, Song IG, Ha SJ, Kim YE, Baek NI, Hong Ek. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic beta-cells against oxidative stress-induced apoptosis. Int J Mol Med 2015; 35:405–12 [DOI] [PubMed] [Google Scholar]
- 36.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res 2015; 37:223–36 [PMC free article] [PubMed] [Google Scholar]
- 37.Sanmiguel C, Gupta A, Mayer EA. Gut Microbiome and obesity: a plausible explanation for obesity. Curr Obes Rep 2015; 4:250–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut. Dig Dis 2015; 33:338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]