Abstract
Objective
To undertake a systematic review and meta-analysis to assess the impact of cardiac rehabilitation (CR) on physical activity (PA) levels of patients with heart disease and the methodological quality of these studies.
Methods
Databases (MEDLINE, EMBASE, CENTRAL, CINAHL, PsychINFO and SportDiscus) were searched without language restriction from inception to January 2017 for randomised controlled trials (RCTs) comparing CR to usual care control in adults with heart failure (HF) or coronary heart disease (CHD) and measuring PA subjectively or objectively. The direction of PA difference between CR and control was summarised using vote counting (ie, counting the positive, negative and non-significant results) and meta-analysis.
Results
Forty RCTs, (6480 patients: 5825 CHD, 655 HF) were included with 26% (38/145) PA results showing a statistically significant improvement in PA levels with CR compared with control. This pattern of results appeared consistent regardless of type of CR intervention (comprehensive vs exercise-only) or PA measurement (objective vs subjective). Meta-analysis showed PA increases in the metrics of steps/day (1423, 95% CI 757.07 to 2089.43, p<0.0001) and proportion of patients categorised as physically active (relative risk 1.55, 95% CI 1.19 to 2.02, p=0.001). The included trials were at high risk of bias, and the quality of the PA assessment and reporting was relatively poor.
Conclusion
Overall, there is moderate evidence of an increase in PA with CR participation compared with control. High-quality trials are required, with robust PA measurement and data analysis methods, to assess if CR definitely leads to important improvements in PA.
Keywords: cardiac rehabilitation, coronary artery disease, heart failure, meta-analysis, systemic review
Introduction
Physical activity (PA) is defined as any bodily movement produced by skeletal muscles resulting in energy expenditure beyond resting expenditure.1 The current UK recommendation for PA in adults and older adults is ≥150 min of moderate intensity PA per week.2 This is based on a number of systematic reviews and consensus statement, consistently identifying 150 min/week as providing considerable health benefits, including reduced all-cause mortality, reduced risk factors for chronic diseases, improved cardiovascular fitness and quality of life.2 3 This is also the standard PA recommendation for patients with cardiac disease by the British Association for Cardiovascular Prevention and Rehabilitation and the Scottish Intercollegiate Guidelines Network.4 5
The benefits of cardiac rehabilitation (CR) participation for those with coronary heart disease (CHD) and heart failure (HF) are well established and include reduced cardiovascular mortality, reduced risk of hospital admissions, improved exercise capacity and health-related quality of life.6 7 A key aim of CR is to increase total daily energy expenditure in addition to exercise capacity.2 However, previous observational studies demonstrated that many patients with heart disease (pre-CR and post-CR) are failing to meet recommended daily PA levels8 9 and the extent that CR impacts on PA levels of patients remains unclear.
While two systematic reviews to date have indicated inadequate evidence of an impact of CR participation on PA levels of patients with CHD,10 11 these studies have limitations. Neither included studies involving patients with HF nor attempted meta-analysis due to the heterogeneity of CR interventions. Therefore, an updated systematic review with an improved search strategy and broader population inclusion criteria is justified.
The aim of this systematic review and meta-analysis of randomised controlled trials (RCTs) was twofold. First, to clarify the impact of CR participation on PA levels of patients with CHD and HF. Second, to review the methodological quality of PA outcomes reported in these trials.
Methods
The protocol was registered on PROSPERO (CRD42017055137). We conducted and report this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyse statement.12
Search strategy and inclusion criteria
Details of the search strategy and inclusion criteria are provided in the online Supplementary file 1. The full search strategy is provided in the online Supplementary file 2.
heartjnl-2017-312832supp001.pdf (111.3KB, pdf)
heartjnl-2017-312832supp002.pdf (192.9KB, pdf)
Data extraction and risk of bias assessment
A standardised data extraction form was used to extract study characteristics, patient characteristics, intervention and control details, PA measurement method and outcome data at all follow-up time points. Multiple publications of the same study were assessed for additional data and presented as a single RCT (see online Supplementary file 3).
heartjnl-2017-312832supp003.pdf (272.5KB, pdf)
The Cochrane Collaboration’s tool for assessing risk of bias was used to assess the quality of included studies.13 Data extraction and risk of bias assessment were initially completed by a single reviewer (GD) and then checked for accuracy by one other reviewer (MH, HD or RST). Disagreement was resolved by discussion.
Data synthesis and meta-analysis
Due to the wide range of PA metrics reported across studies, we first summarised the direction of PA results using a vote counting approach13 (quantifying studies on the basis of their positive, negative or non-significant results). Given the wide range of PA measures, we decided against using standardised effect size for meta-analysis and instead conducted meta-analysis where two or more studies reported the same units of PA measurement. Meta-analysis was completed on all follow-up time points apart from one outcome measure (proportion of patients categorised as physically active) where there was sufficient data to separate into short-term (≤12 months post-CR) and long-term (>12 months post-CR) follow-up.
Given the clinical heterogeneity of the included studies, random-effects models were used to pool data. Statistical heterogeneity was assessed using the I2 statistic. Binary outcomes for each study were pooled as relative risks (RR) and continuous outcomes as mean differences (MD). Meta-analysis results were reported as means and 95% CIs. A two-tailed p value of ≤0.05 was considered statistically significant. Analyses were performed in Review Manager (RevMan V.5.3, The Cochrane Collaboration) or Stata V.14.
We explored the effect of various potential treatment effect modifiers by stratifying the vote counting results, that is, setting of CR (centre vs home based), patient group (CHD vs HF), publication date (pre-1990, representing the time of major changes in drug and device management of CHD and HF), dose of exercise intervention (dose=number of weeks of exercise training×average sessions/week×average duration of session in minutes. Dose ≥2000 units (median) vs dose <2000 units); objective versus subjective PA measures and method of PA statistical analysis. Studies lacking enough information to calculate dose were omitted from the analysis.
Results
Study selection
Figure 1 summarises the screening process resulting in 47 publications across 40 RCTs included in the review.
Figure 1.
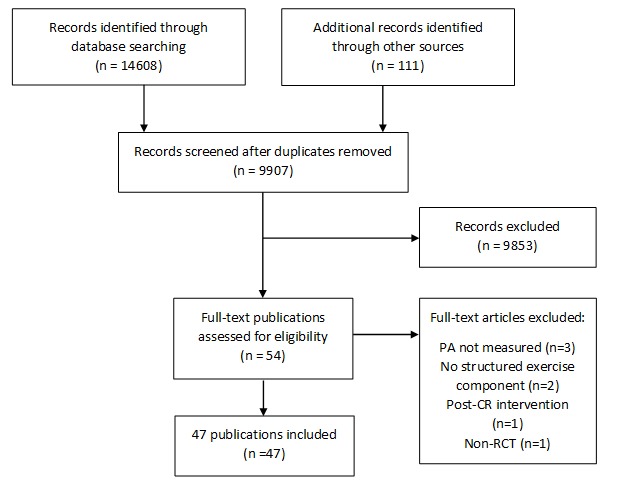
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow chart of search process. CR, cardiac rehabilitation; PA, physical activity; RCT, randomised controlled trial.
Characteristics of included studies
The 40 RCTs, all published in English, included a total of 6480 patients with cardiac disease (5825 CHD, 655 HF). A summary of study characteristics is shown in table 1. Individual study characteristics are detailed in the online Supplementary file 4.
Table 1.
Summary of study characteristics
| Characteristic | Number of studies (%) or median (range) |
| Multicentre randomised controlled trial | 10 (25) |
| Exercise only | 17 (42.5) |
| Cardiac rehabilitation location | |
| Home based | 10 (25) |
| Centre based | 23 (57.5) |
| Both | 7 (17.5) |
| Sample size | 89.5 (19–1813) |
| <50 | 10 (25) |
| 51–100 | 14 (35) |
| >100 | 16 (40) |
| Publication date | |
| 1970–1979 | 1 (2.5) |
| 1980–1989 | 5 (12.5) |
| 1990–1999 | 12 (30) |
| 2000–2009 | 10 (25) |
| 2010–2017 | 12 (30) |
| Study location | |
| Europe | 25 (62.5) |
| North America | 10 (25) |
| Asia/Australia | 5 (12.5) |
| Sex | |
| Male only | 6 (15) |
| Female only | 1 (2.5) |
| Both | 32 (80) |
| Not reported | 1 (2.5) |
| Age (years)* | 58.3 (47–81) |
| Diagnosis | |
| Coronary heart disease | 28 (70) |
| Heart failure | 10 (25) |
| Both | 2 (5) |
| Follow-up (months) | 12 (1.5–120) |
*Median of study means.
heartjnl-2017-312832supp004.pdf (359KB, pdf)
PA measures reported
In total, 28 studies measured PA using subjective approaches, 10 studies used objective methods and two studies used a combination of both. Across all studies, 45 different PA metrics were used (median 1.5, range 1–10). Details of individual study PA measurement methods including a summary is presented in the online Supplementary file 5.
heartjnl-2017-312832supp005.pdf (334.1KB, pdf)
Risk of bias assessment
Risk of bias assessments for each study are summarised in figure 2. All studies were assigned high risk in blinding of participants and personnel due to the nature of CR. The most prevalent methodological issues were non-adequate description of randomisation (25/40, 62.5%), allocation concealment (27/40, 67.5%) and blinding of PA outcome assessment (26/40, 65%). There was high risk of bias in 50% (20/40) trials for incomplete outcome data. Most trials were low risk for selective reporting (33/40, 82.5%), balanced groups at baseline (34/40, 85%) and were free of cointerventions (35/40, 87.5%).
Figure 2.
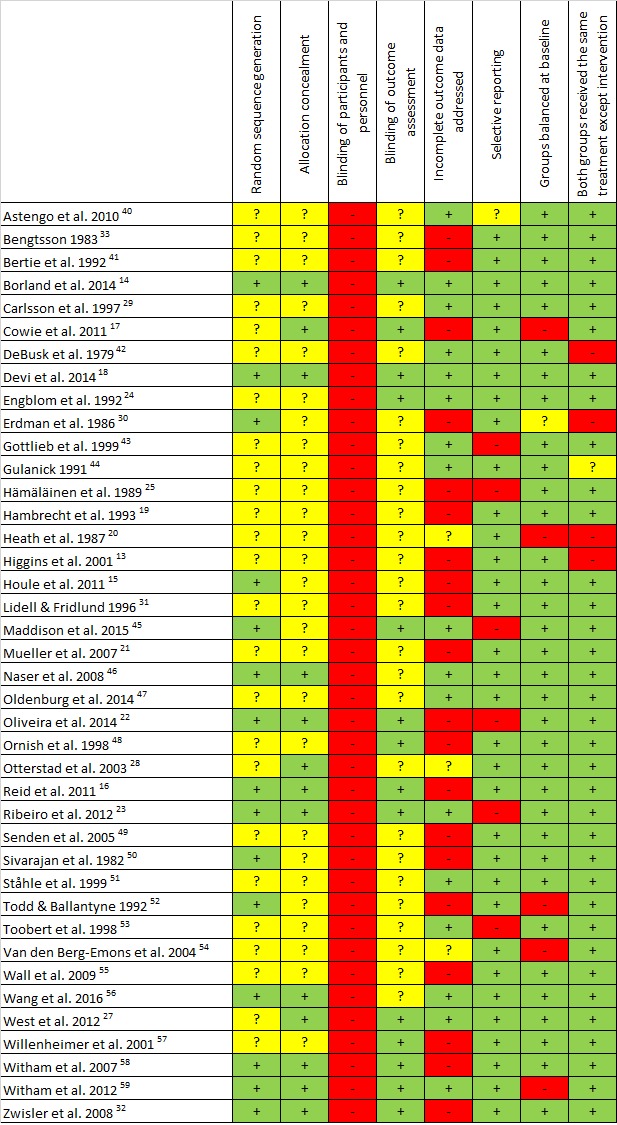
Quality appraisal. + (green), low risk of bias; ? (yellow), unclear risk of bias; − (red), high risk of bias.40–59
Impact of CR participation on PA levels
Vote counting
A total of 145 CR versus control PA comparisons were reported across all studies (online Supplementary file 6). Overall, 26% of results showed a statistically significant improvement in PA with CR (table 2).
Table 2.
Vote counting
| Direction of result | Number of results (%) |
| PA in CR same as control (p>0.05) | 100 (69%) |
| PA in CR higher than control (p≤0.05) | 38 (26%) |
| PA in control higher than CR (p≤0.05) | 2 (1%) |
| PA difference between CR and control not clear (no p value reported) | 5 (3%) |
| Total | 145 |
CR, cardiac rehabilitation; PA, physical activity.
heartjnl-2017-312832supp006.pdf (227.8KB, pdf)
Stratified analysis
The pattern of results was similar whether PA measurement was objective or subjective (online Supplementary file 7). The statistical methods used across the studies were varied. The majority reported a p value for between-group differences. Comparing the direction of results by statistical method showed a greater number of positive results reported when the p value for interaction time×group was used (online Supplementary file 8). As numbers were small, this is unlikely to be of significance.
heartjnl-2017-312832supp007.pdf (259.6KB, pdf)
heartjnl-2017-312832supp008.pdf (261.3KB, pdf)
There was a higher proportion of non-significant results (86% vs 63%) and fewer positive results (10% vs 32%) in studies including patients with HF compared with studies with CHD (online Supplementary file 9). Removing the results from studies conducted prior to 1990 or those based on exercise frequency did not affect the direction of results (online Supplementary file 10).
heartjnl-2017-312832supp009.pdf (259.2KB, pdf)
heartjnl-2017-312832supp010.pdf (262KB, pdf)
CR intervention
Table 3 shows an increased number of positive results with home-based CR interventions compared with centre-based interventions. Studies with a higher exercise dose also produced a slightly increased number of positive results compared with studies with a lower exercise dose (online Supplementary file 11). The pattern of results was similar when comparing studies of comprehensive CR to exercise-only CR studies (online Supplementary file 12).
Table 3.
Vote counting—comparing centre-based CR to home-based CR and combined RCTs
| Direction of result | Number of results | ||
| Centre-based CR intervention | Home-based CR intervention | Combined centre and home based or RCT included both | |
| PA in CR same as control (p>0.05) | 63 (77%) | 22 (51%) | 15 (75%) |
| PA in CR higher than control (p≤0.05) | 15 (18%) | 19 (44%) | 4 (20%) |
| PA in control higher than CR (p≤0.05) | 1 (1%) | 0 | 1 (5%) |
| PA difference between CR and control not clear (no p value reported) | 3 (4%) | 2 (5%) | 0 |
| Total | 82 | 43 | 20 |
CR, cardiac rehabilitation; PA, physical activity; RCT, randomised controlled trial.
heartjnl-2017-312832supp011.pdf (260.4KB, pdf)
heartjnl-2017-312832supp012.pdf (260.2KB, pdf)
Meta-analyses
Steps/day
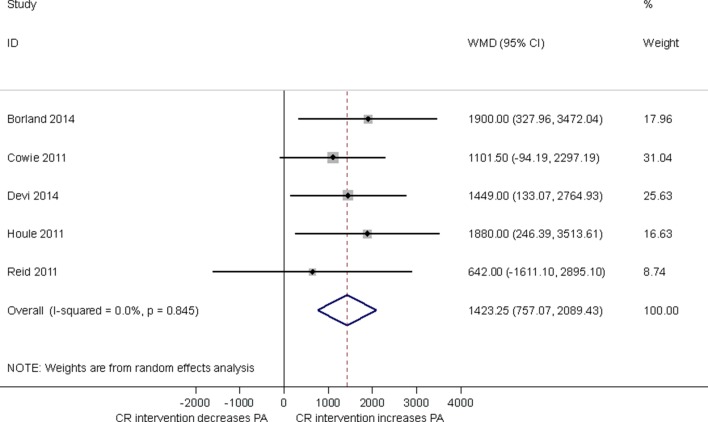
Five studies used mean steps/day as a measure of PA assessed by either pedometer 14–16 or accelerometer.17 18 Pooling results across studies showed compared with control, CR participation was associated with an increase in mean steps/day (1423, 95% CI 757.07 to 2089.43, p<0.0001; figure 3) at short-term follow-up (median 3, range 1.5–12 months). With no evidence of statistical heterogeneity (I2=0%, p=0.845).
Figure 3.
Impact of cardiac rehabilitation on mean steps/day at short-term follow-up (median 3 months, range 1.5–12 months). CR, cardiac rehabilitation, PA, physical activity; WMD, weighted mean difference.
Energy expenditure
Energy expenditure (kcal/week) was estimated via questionnaire in three studies (median follow-up time 12 months, range 32 weeks–72 months).19–21 Meta-analysis showed that CR participation was associated with an increase in energy expenditure compared with control (878.4, 95% CI 433.83 to 1323.01, p=0.0001). Test for statistical heterogeneity was significant (I2=70%, p=0.04).
Sedentary time, light PA and moderate–vigorous PA (min/day)
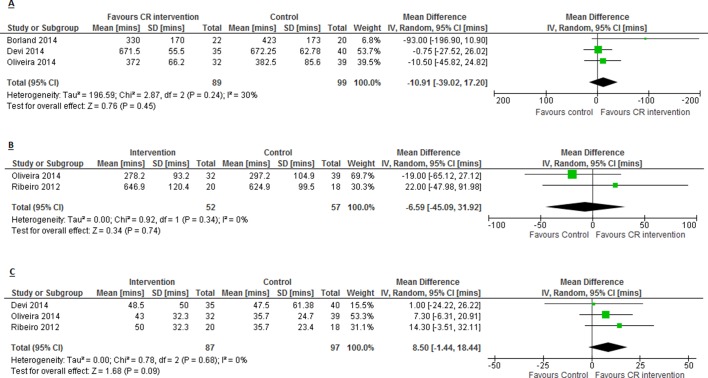
There was no impact on mean min/day spent sedentary or sitting between CR and control (−10.9, 95% CI −39.02 to 17.20, p=0.45; figure 4A) based on two studies estimating this objectively via accelerometer14 18 and subjectively via International Physical Activity Questionnaire (IPAQ),22 at 9 weeks follow-up (median, range 6–12 weeks). There was no evidence of a difference in mean min/day spent in light intensity PA in CR compared with control (−6.6, 95% CI −45.09 to 31.92, p=0.74; figure 4B) based on two studies reporting this outcome via accelerometer23 and IPAQ,22 at 9.5 weeks follow-up (median, range 9–10 weeks). There was no difference in mean min/day spent in moderate–vigorous PA in CR compared with control (8.5, 95% CI −1.44 to 18.44, p=0.09; figure 4C), measured via accelerometer18 23 and IPAQ,22 at 9 weeks follow-up (median, range 6–10 weeks).
Figure 4.
Impact of cardiac rehabilitation on (A) min/day spent sedentary or sitting; (B) min/day spent in light intensity PA and (C) min/day spent in moderate–vigorous PA. CR, cardiac rehabilitation.
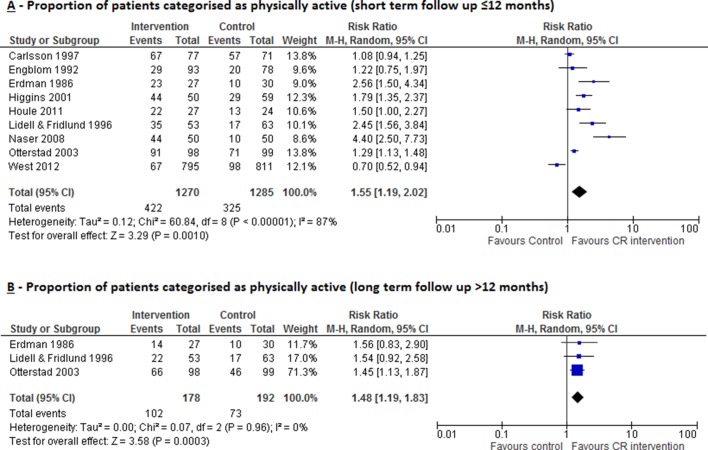
Proportion of patients categorised as physically active (short-term follow-up ≤12 months)
CR increased the proportion of patients categorised as ‘physically active’, measured at short-term follow-up (median 6 months, range 0–12 months) across nine studies (RR 1.55, 95% CI 1.19 to 2.02, p=0.001; figure 5A). There was evidence of substantial statistical heterogeneity (I2=87%, p<0.00001). The definition of ‘physically active’ varied across studies: that is, exercise frequency ≥3×/week,24 exercising ≥3×/week for 20 min,25 26 exercising >100 kcal/day,27 average daily steps >7500,15 exercising for >1 hour/week,28 regularly training (defined as either walking or cycling ≥30 min daily, sport activities once weekly or vigorous physical training)29 and two studies did not provide any definition.30 31
Figure 5.
Impact of cardiac rehabilitation on proportion of patients categorised as physically active measured at (A) short-term follow-up (≤12 months) and (B) long-term follow-up (>12 months). CR, cardiac rehabilitation.
Proportion of patients categorised as physically active(long-term follow-up >12 months)
CR increased the proportion of patients considered physically active, measured at long-term follow-up (median 5 years, range 2–5 years) in three studies28 30 31 (RR 1.48, 95% CI 1.19 to 1.83, p=0.0003; figure 5B) with no evidence of statistical heterogeneity (I2=0.0%, p=0.96).
Proportion of patients categorised as sedentary or not physically active
Five studies reported the proportion of patients considered sedentary,29 exercising <4 hours per week32 or undertaking no exercise,24 28 33 at 12 months (median, range 12–24 months) follow-up. There was a reduction in CR participants categorised as sedentary or not physically active(RR 0.76, 95% CI 0.61 to 0.95, p=0.02), with no evidence of statistical heterogeneity (I2=36%, p=0.18).
Discussion
This systematic review of RCTs shows moderate evidence of an increase in PA with CR participation with 26% (38/145) of comparisons reporting a statistically significant result in favour of CR compared with control. This pattern of results appear consistent regardless of whether studies assessed PA using subjective or objective methods, or the CR intervention was comprehensive or exercise only. Studies involving patients with HF appeared less likely to have positive results in favour of CR. There was an increased proportion of positive results with higher doses of CR suggesting that higher doses of exercise training may be more effective in improving PA levels. Similarly, results suggest that home-based interventions may be more effective in improving PA levels.
Meta-analyses showed that CR participation compared with control is associated with an increase in some PA outcomes: steps/day at short-term follow-up, energy expenditure (kcal/week) at short-term follow-up, proportion of patients categorised as physically active both at short-term and long-term follow-up and reduced proportion of patients categorised sedentary or not physically active at short-term follow-up. CR was not shown to have a significant impact on minutes/day spent sedentary or in light or moderate-vigorous PA at short-term follow-up.
It remains uncertain if the mean increase of 1423 steps/day that we observed with CR is clinically meaningful. In patients with chronic obstructive pulmonary disease undergoing rehabilitation, the minimal clinically important difference (MCID) was calculated to lie between 600 and 1100 steps/day and resulted in a reduction in hospital admissions.34 However, we know of no published MCID for patients with CHD or HF.
We believe there are two potential reasons why we saw improvements in some outcomes, but not others. First, categorising continuous PA data to PA categories (eg, sedentary, light moderate or vigorous) may have resulted in a loss of sensitivity to change. Second, some studies may have been susceptible to measurement bias as they used subjective PA measures.
Comparison of findings to previous studies
Our results build on previous systematic reviews10 11 that found some evidence to indicate that CR positively impacts on PA in patients with CHD, but little evidence in long term and recommended CR programmes place more emphasis on improving the long-term PA levels of patients.10 Ter Hoeve et al concluded that centre-based CR was not sufficient to improve and maintain PA levels and suggested home-based CR programmes may be more successful; however, literature is limited in this area.11 In accord with recent Cochrane systematic reviews of CR,9 10 the participating patients were relatively young (<60 years), predominantly male, with large differences in the programme location, duration, intensity, modality and length of follow-up.
Strengths and limitations
We believe this to be the first meta-analysis to assess the impact of CR on PA levels of patients with both CHD and HF. Strengths of this review include extensive literature searches, use of RCTs and inclusion of both subjective and objective PA assessment. Compared with the previous systematic reviews, we identified an additional 23 RCTs (2432 additional patients), 10 of which specifically involved patients with HF (655 patients).
However, this review has limitations. With the wide range of PA outcomes reported across the studies, at various follow-up time points, we were limited in the extent of meta-analysis we were able to complete. That only small numbers of studies were suitable for inclusion in the meta-analysis, limits our ability to draw firm conclusions from these pooled results. Vote counting was done to give a quantitative overview of the results. However, this method has limitations: (1) large and small studies carry the same weight, (2) studies reporting multiple PA outcome results contribute more weight and (3) results from multiple outcomes within study may not be independent. Furthermore, judgements by the authors on levels of PA were not based on national recommendations, leading to uncertainty about the clinical meaningfulness of PA improvements.
Key issues raised in risk of bias assessments were insufficiently described randomisation and allocation concealment procedures, leading to difficulty rating the quality of the RCTs. Additionally, 65% of studies had unclear risk of bias with regard to blinding of outcome assessment. This is particularly important in PA measurement since awareness of being assessed may cause both the intervention and control group patients to alter their behaviour and increase their PA on assessment days, potentially introducing bias to results.
There were numerous limitations in approaches studies took to assessing PA. Where questionnaires were used, few had been evidently validated for use in cardiac populations. Self-report commonly considered the frequency of exercise sessions undertaken as opposed to overall PA per se. Self-reported measures of PA are less valid and reliable than direct measures in patients with CR, generally overestimating PA and relying on patient recall.35 Despite accelerometers being the most commonly used objective PA measurement method, a variety of devices were used, with sensors placed at different body sites, and a wide range of outcome metrics reported across studies, limiting the ability to meta-analyse these data. Additionally, data handling methods were poorly reported; no studies adequately explained the minimum wear time requirement for inclusion in data analysis or data reduction techniques. Where accelerometer thresholds were used to estimate intensity, they were derived from studies in young, healthy adults which may mean the PA level is underestimated in patients with cardiac disease.36 Resting metabolic rate in patients with cardiac disease has been previously demonstrated to be significantly lower (23%–36%) than the typically utilised value of 3.5 mL/kg/min,37 which may have implications in underestimating energy expenditure during higher intensity activities. Therefore, researchers should consider using thresholds specifically established for patients with cardiac disease.
There was inconsistency in statistical methods used across the studies. Baseline adjusted regression methods are recommended for analysis of RCTs.38 However, only 35% reported a p value that took the baseline PA level into account. Although many studies showed between group differences in fitness outcomes, 26% of results demonstrated a statistically significant difference in PA outcomes. This is likely because individual studies were often small and underpowered to detect small differences in PA. Only 13 (32%) of the included studies included formal sample size calculations and of these only 4 (31%) were based on PA outcomes.
Implications for clinical practice and future research
That our results showed no difference in PA outcomes in studies that employed comprehensive CR compared with exercise-only CR suggest that improvements in PA with CR are the result of exercise training rather than components of education and psychosocial interventions. Additionally, improvement in exercise capacity may not be directly related to increases in PA levels. CR programmes should consider supplementing their existing exercise-training intervention with interventions that specifically aim to increase PA level. For example, the ongoing PATHway I trial, where the basis of the CR intervention is PA promotion and the primary outcome is objectively measured PA level.39
Further research is required to validate interventions that promote PA in cardiac populations. Furthermore, objective measurement of PA requires population-specific calibration studies to establish intensity thresholds. The use of inconsistent PA measures and units made formal pooling of data problematic. We therefore recommend that future studies use objective measures of PA such as accelerometers, be statistically powered to detect small differences in PA, use appropriate data handling and analysis methods, and PA outcomes are reported in relation to national PA recommendations. Studies should assess PA outcomes over the long term.
Conclusions
This systematic review and meta-analysis provides moderate evidence of an increase in PA with CR participation compared with control. However, the included trials were at risk of bias, and the quality of PA assessment and reporting was relatively poor. It is unclear whether increases in PA with CR are clinically meaningful. Further high-quality trials are required to assess if CR leads to important improvements in PA, such as the UK recommended target of 150 min of moderate intensity PA per week, especially in long term.
Acknowledgments
We would like to thank Catriona Organ, librarian at the Royal Cornwall Hospital Library, Knowledge Spa, Truro, and the PenCLARHC Evidence Synthesis Team at University of Exeter Medical School for their assistance throughout the process of this review. We also thank Dr Joe Mills (immediate past president of BACPR) for encouraging us to write this review and facilitating its submission.
Footnotes
Contributors: GOD, HMD, RST and MH contributed to the conception, design, planning, conduct and reporting of the work described in this article. All authors contributed to the critical revision of the manuscript.
Funding: This study was supported by a University of Exeter Postgraduate Studentship Grant.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 2. Chief Medical Officers. Start active, stay active. A report on physical activity for health from the four home countries. 2011. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216370/dh_128210.pdf (accessed Oct 2017).
- 3. O’Donovan G, Blazevich AJ, Boreham C, et al. The ABC of physical activity for health: a consensus statement from the British association of sport and exercise sciences. J Sports Sci 2010;28:573–91. 10.1080/02640411003671212 [DOI] [PubMed] [Google Scholar]
- 4. British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and rehabilitation. 2017. http://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf (accessed Oct 2017).
- 5. Scottish Intercollegiate Guidelines Network. Cardiac rehabilitation. A national clinical guideline http://www.sign.ac.uk/assets/sign150.pdf (accessed October 2017). [Google Scholar]
- 6. Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;7 10.1002/14651858.CD003331.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dontje ML, van der Wal MH, Stolk RP, et al. Daily physical activity in stable heart failure patients. J Cardiovasc Nurs 2014;29:218–26. 10.1097/JCN.0b013e318283ba14 [DOI] [PubMed] [Google Scholar]
- 9. Yates BC, Pozehl B, Kupzyk K, et al. Are heart failure and coronary artery bypass surgery patients meeting physical activity guidelines? Rehabilitation Nursing 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jolliffe J, Taylor R. Physical activity and cardiac rehabilitation: a critical review of the literature. Coronary Health Care 1998;2:179–86. 10.1016/S1362-3265(98)80015-1 [DOI] [Google Scholar]
- 11. ter Hoeve N, Huisstede BM, Stam HJ, et al. Does cardiac rehabilitation after an acute cardiac syndrome lead to changes in physical activity habits? Systematic review. Phys Ther 2015;95:167–79. 10.2522/ptj.20130509 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Green S, The Cochrane Collaboration Cochrane handbook for systematic reviews of interventions version 5.1.0 2011. [Google Scholar]
- 14. Borland M, Rosenkvist A, Cider A. A group-based exercise program did not improve physical activity in patients with chronic heart failure and comorbidity: a randomized controlled trial. J Rehabil Med 2014;46:461–7. 10.2340/16501977-1794 [DOI] [PubMed] [Google Scholar]
- 15. Houle J, Doyon O, Vadeboncoeur N, et al. Innovative program to increase physical activity following an acute coronary syndrome: randomized controlled trial. Patient Educ Couns 2011;85:e237–e244. 10.1016/j.pec.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 16. Reid RD, Morrin LI, Beaton LJ, et al. Randomized trial of an internet-based computer-tailored expert system for physical activity in patients with heart disease. Eur J Prev Cardiol 2012;19:1357–64. 10.1177/1741826711422988 [DOI] [PubMed] [Google Scholar]
- 17. Cowie A, Thow MK, Granat MH, et al. A comparison of home and hospital-based exercise training in heart failure: immediate and long-term effects upon physical activity level. Eur J Cardiovasc Prev Rehabil 2011;18:158–66. 10.1177/1741826710389389 [DOI] [PubMed] [Google Scholar]
- 18. Devi R, Powell J, Singh S. A web-based program improves physical activity outcomes in a primary care angina population: randomized controlled trial. J Med Internet Res 2014;16:e186 10.2196/jmir.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hambrecht R, Niebauer J, Marburger C, et al. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol 1993;22:468–77. 10.1016/0735-1097(93)90051-2 [DOI] [PubMed] [Google Scholar]
- 20. Heath GW, Maloney PM, Fure CW. Group exercise versus home exercise in coronary artery bypass graft patients: effects on physical activity habits. J Cardiopulmonary Rehabil 1987;7:190–5. [Google Scholar]
- 21. Mueller L, Myers J, Kottman W, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil 2007;21:923–31. 10.1177/0269215507079097 [DOI] [PubMed] [Google Scholar]
- 22. Oliveira NL, Ribeiro F, Teixeira M, et al. Effect of 8-week exercise-based cardiac rehabilitation on cardiac autonomic function: A randomized controlled trial in myocardial infarction patients. Am Heart J 2014;167:753–61. 10.1016/j.ahj.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 23. Ribeiro F, Alves AJ, Teixeira M, Miranda F, et al. Exercise training increases interleukin-10 after an acute myocardial infarction: a randomised clinical trial. Int J Sports Med 2012;33:192–8. 10.1055/s-0031-1297959 [DOI] [PubMed] [Google Scholar]
- 24. Engblom E, Hietanen EK, Hämäläinen H, et al. Exercise habits and physical performance during comprehensive rehabilitation after coronary artery bypass surgery. Eur Heart J 1992;13:1053–9. 10.1093/oxfordjournals.eurheartj.a060313 [DOI] [PubMed] [Google Scholar]
- 25. Hämäläinen H, Luurila OJ, Kallio V, et al. Long-term reduction in sudden deaths after a multifactorial intervention programme in patients with myocardial infarction: 10-year results of a controlled investigation. Eur Heart J 1989;10:55–62. 10.1093/oxfordjournals.eurheartj.a059381 [DOI] [PubMed] [Google Scholar]
- 26. Higgins HC, Hayes RL, McKenna KT. Rehabilitation outcomes following percutaneous coronary interventions (PCI). Patient Educ Couns 2001;43:219–30. 10.1016/S0738-3991(00)00164-6 [DOI] [PubMed] [Google Scholar]
- 27. West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012;98:637–44. 10.1136/heartjnl-2011-300302 [DOI] [PubMed] [Google Scholar]
- 28. Otterstad JE. Vestfold Heartcare Study Group. Influence on lifestyle measures and five-year coronary risk by a comprehensive lifestyle intervention programme in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil 2003;10:429–37. 10.1097/01.hjr.0000107024.38316.6a [DOI] [PubMed] [Google Scholar]
- 29. Carlsson R, Lindberg G, Westin L, et al. Influence of coronary nursing management follow up on lifestyle after acute myocardial infarction. Heart 1997;77:256–9. 10.1136/hrt.77.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erdman RA, Duivenvoorden HJ, Verhage F, et al. Predictability of beneficial effects in cardiac rehabilitation: a randomized clinical trial of psychosocial variables. J Cardiopulm Rehabil 1986;6:206–13. [Google Scholar]
- 31. Lidell E, Fridlund B. Long-term effects of a comprehensive rehabilitation programme after myocardial infarction. Scand J Caring Sci 1996;10:67–74. 10.1111/j.1471-6712.1996.tb00314.x [DOI] [PubMed] [Google Scholar]
- 32. Zwisler AD, Soja AM, Rasmussen S, et al. Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. Am Heart J 2008;155:1106–13. 10.1016/j.ahj.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 33. Bengtsson K. Rehabilitation after myocardial infarction. A controlled study. Scand J Rehabil Med 1983;15:1–9. [PubMed] [Google Scholar]
- 34. Demeyer H, Burtin C, Hornikx M, et al. The minimal important difference in physical activity in patients with COPD. PLoS One 2016;11:e0154587 10.1371/journal.pone.0154587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alharbi M, Bauman A, Neubeck L, et al. Measuring overall physical activity for cardiac rehabilitation participants: a review of the literature. Heart Lung Circ 2017;26:1008–25. 10.1016/j.hlc.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 36. Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. 10.1097/00005768-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 37. Savage PD, Toth MJ, Ades PA. A re-examination of the metabolic equivalent concept in individuals with coronary heart disease. J Cardiopulm Rehabil Prev 2007;27:143–8. 10.1097/01.HCR.0000270693.16882.d9 [DOI] [PubMed] [Google Scholar]
- 38. Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Med Res Methodol 2014;14:49 10.1186/1471-2288-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Claes J, Buys R, Woods C, et al. PATHway I: design and rationale for the investigation of the feasibility, clinical effectiveness and cost-effectiveness of a technology-enabled cardiac rehabilitation platform. BMJ Open 2017;7:e016781 10.1136/bmjopen-2017-016781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Astengo M, Dahl A, Karlsson T, et al. Physical training after percutaneous coronary intervention in patients with stable angina: effects on working capacity, metabolism, and markers of inflammation. Eur J Cardiovasc Prev Rehabil 2010;17:349–54. 10.1097/HJR.0b013e3283336c8d [DOI] [PubMed] [Google Scholar]
- 41. Bertie J, King A, Reed N, et al. Benefits and weaknesses of a cardiac rehabilitation programme. J R Coll Physicians Lond 1992;26:147–51. [PMC free article] [PubMed] [Google Scholar]
- 42. DeBusk RF, Houston N, Haskell W, et al. Exercise training soon after myocardial infarction. Am J Cardiol 1979;44:1223–9. 10.1016/0002-9149(79)90433-8 [DOI] [PubMed] [Google Scholar]
- 43. Gottlieb SS, Fisher ML, Freudenberger R, et al. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. J Card Fail 1999;5:188–94. 10.1016/S1071-9164(99)90002-7 [DOI] [PubMed] [Google Scholar]
- 44. Gulanick M. Is phase 2 cardiac rehabilitation necessary for early recovery of patients with cardiac disease? A randomized, controlled study. Heart Lung 1991;20:9–15. [PubMed] [Google Scholar]
- 45. Maddison R, Pfaeffli L, Whittaker R, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. Eur J Prev Cardiol 2015;22:701–9. 10.1177/2047487314535076 [DOI] [PubMed] [Google Scholar]
- 46. Naser A, Jafar S, Kumar GV, et al. Cardiac risk factor changes through an intensive multifactorial life style modification program in CHD patients: results from a two year follow up. J Biol Sci 2008;8:248–57. [Google Scholar]
- 47. Oldenburg B, Martin A, Greenwood J, et al. A controlled trial of a behavioral and educational intervention following coronary artery bypass surgery. J Cardiopulm Rehabil 1995;15:39–46. 10.1097/00008483-199501000-00006 [DOI] [PubMed] [Google Scholar]
- 48. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001–7. 10.1001/jama.280.23.2001 [DOI] [PubMed] [Google Scholar]
- 49. Senden PJ, Sabelis LW, Zonderland ML, et al. The effect of physical training on workload, upper leg muscle function and muscle areas in patients with chronic heart failure. Int J Cardiol 2005;100:293–300. 10.1016/j.ijcard.2004.10.039 [DOI] [PubMed] [Google Scholar]
- 50. Sivarajan ES, Bruce RA, Lindskog BD, et al. Treadmill test responses to an early exercise program after myocardial infarction: a randomized study. Circulation 1982;65:1420–8. 10.1161/01.CIR.65.7.1420 [DOI] [PubMed] [Google Scholar]
- 51. Ståhle A, Nordlander R, Rydén L, et al. Effects of organized aerobic group training in elderly patients discharged after an acute coronary syndrome. A randomized controlled study. Scand J Rehabil Med 1999;31:101–7. [DOI] [PubMed] [Google Scholar]
- 52. Todd IC, Ballantyne D. Effect of exercise training on the total ischaemic burden: an assessment by 24 hour ambulatory electrocardiographic monitoring. Br Heart J 1992;68:560–6. 10.1136/hrt.68.12.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toobert DJ, Glasgow RE, Nettekoven LA, et al. Behavioral and psychosocial effects of intensive lifestyle management for women with coronary heart disease. Patient Educ Couns 1998;35:177–88. 10.1016/S0738-3991(98)00074-3 [DOI] [PubMed] [Google Scholar]
- 54. van den Berg-Emons R, Balk A, Bussmann H, et al. Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? Eur J Heart Fail 2004;6:95–100. 10.1016/j.ejheart.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 55. Wall HK, Ballard J, Troped P, et al. Impact of home-based, supervised exercise on congestive heart failure. Int J Cardiol 2010;145:267–70. 10.1016/j.ijcard.2009.09.478 [DOI] [PubMed] [Google Scholar]
- 56. Wang W, Jiang Y, He HG, et al. A randomised controlled trial on the effectiveness of a home-based self-management programme for community-dwelling patients with myocardial infarction. Eur J Cardiovasc Nurs 2016;15:398–408. 10.1177/1474515115586904 [DOI] [PubMed] [Google Scholar]
- 57. Willenheimer R, Rydberg E, Cline C, et al. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int J Cardiol 2001;77:25–31. 10.1016/S0167-5273(00)00383-1 [DOI] [PubMed] [Google Scholar]
- 58. Witham MD, Argo IS, Johnston DW, et al. Long-term follow-up of very old heart failure patients enrolled in a trial of exercise training. Am J Geriatr Cardiol 2007;16:243–8. 10.1111/j.1076-7460.2007.06488.x [DOI] [PubMed] [Google Scholar]
- 59. Witham MD, Fulton RL, Greig CA, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2012;5:209–16. 10.1161/CIRCHEARTFAILURE.111.963132 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2017-312832supp001.pdf (111.3KB, pdf)
heartjnl-2017-312832supp002.pdf (192.9KB, pdf)
heartjnl-2017-312832supp003.pdf (272.5KB, pdf)
heartjnl-2017-312832supp004.pdf (359KB, pdf)
heartjnl-2017-312832supp005.pdf (334.1KB, pdf)
heartjnl-2017-312832supp006.pdf (227.8KB, pdf)
heartjnl-2017-312832supp007.pdf (259.6KB, pdf)
heartjnl-2017-312832supp008.pdf (261.3KB, pdf)
heartjnl-2017-312832supp009.pdf (259.2KB, pdf)
heartjnl-2017-312832supp010.pdf (262KB, pdf)
heartjnl-2017-312832supp011.pdf (260.4KB, pdf)
heartjnl-2017-312832supp012.pdf (260.2KB, pdf)