Abstract
Objective
Increasing numbers of outbreaks caused by contaminated duodenoscopes used for Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures have been reported, some with fatal outcomes. We conducted a nationwide cross-sectional study to determine the prevalence of bacterial contamination of reprocessed duodenoscopes in The Netherlands.
Design
All 73 Dutch ERCP centres were invited to sample ≥2 duodenoscopes using centrally distributed kits according to uniform sampling methods, explained by video instructions. Depending on duodenoscope type, four to six sites were sampled and centrally cultured. Contamination was defined as (1) any microorganism with ≥20 colony forming units (CFU)/20 mL (AM20) and (2) presence of microorganisms with gastrointestinal or oral origin, independent of CFU count (MGO).
Results
Sixty-seven out of 73 centres (92%) sampled 745 sites of 155 duodenoscopes. Ten different duodenoscope types from three distinct manufacturers were sampled including 69 (46%) Olympus TJF-Q180V, 43 (29%) Olympus TJF-160VR, 11 (7%) Pentax ED34-i10T, 8 (5%) Pentax ED-3490TK and 5 (3%) Fujifilm ED-530XT8. Thirty-three (22%) duodenoscopes from 26 (39%) centres were contaminated (AM20). On 23 (15%) duodenoscopes MGO were detected, including Enterobacter cloacae, Escherichia coli, Klebsiella pneumonia and yeasts. For both definitions, contamination was not duodenoscope type dependent (p values: 0.20 and higher).
Conclusion
In 39% of all Dutch ERCP centres, at least one AM20-contaminated patient-ready duodenoscope was identified. Fifteen per cent of the duodenoscopes harboured MGO, indicating residual organic material of previous patients, that is, failing of disinfection. These results suggest that the present reprocessing and process control procedures are not adequate and safe.
Keywords: endoscopic retrograde pancreatography, endoscopy
Significance of this study.
What is already known on this subject?
In the light of current outbreaks of multidrug-resistant organisms caused by contaminated duodenoscopes, understanding to what extent duodenoscopes are inadequately reprocessed is crucial. Despite current reprocessing procedures, contamination of duodenoscopes continues to occur on a large scale worldwide.
Several studies assessed contamination of duodenoscopes with varying outcomes. However, it is unclear what the true burden on a national level is.
What are the new findings?
This cross-sectional study showed high prevalence rates of duodenoscope contamination in Endoscopic Retrograde Cholangiopancreatography (ERCP) centres in the Netherlands.
In a substantial proportion of the cultured duodenoscopes, organic material of previous patients was still present, as they were contaminated with microorganisms of gastrointestinal or oral origin. These results suggest that the current combination of reprocessing and process control does not suffice.
In this study, contamination occurred with all types of duodenoscopes, independent of type specific design.
How might it impact on clinical practice in the foreseeable future?
Patients undergoing ERCP procedures remain to be at risk of being treated with contaminated equipment.
Efficient surveillance strategies and reprocessing control measures are required to reduce the number of contaminated duodenoscopes, minimising the chance of interpatient transmission of microorganisms.
Introduction
Very recently, an increasing number of infectious outbreaks involving multidrug-resistant organisms (MDRO) caused by contaminated duodenoscopes, used for Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures, have been reported in both Europe and USA.1–5 These include outbreaks of infections with carbapenem-resistant Enterobacteriaceae, such as Escherichia coli and Klebsiella pneumoniae, 3 4 of which some have been associated with fatal outcomes.6 Post-ERCP infections typically range between 2% and 4%.7 It is not clear to what extent these infections are caused by the procedure itself (ie, endogenous infections) or to what extent contaminated duodenoscopes are the source of infection (ie, exogenous infections). For example, in one specific outbreak with a persistently contaminated duodenoscope, 14.4% of all patients who underwent an ERCP were found to be colonised or infected.8 Outbreaks can be traced by bacterial typing. Especially when MDRO strains are involved, detection is easier as laboratories usually store these resistant strains and (retrospective) typing can be performed. This raises the question whether outbreaks with duodenoscopes are a new and emerging problem or whether outbreaks are only detected more frequently because of increased awareness facilitated by recognisable MDRO in patients.3 9
During procedures in the gastrointestinal tract, all flexible endoscopes including duodenoscopes become heavily exposed to gastrointestinal flora.10 Therefore, flexible endoscopes are reprocessed after each procedure: a multistep process involving flushing, manual cleaning, automated cleaning, high-level disinfection and drying. Duodenoscopes are more difficult to reprocess compared with other flexible endoscopes.10 This is due to their complex design, which includes a side viewing tip, forceps elevator and elevator channel. Patient-ready duodenoscopes can be contaminated because of breaches in the reprocessing protocol, inadequate handling or because the current technique of reprocessing may be inadequate for the currently available duodenoscope design.11 Recent outbreaks have been documented to occur even when manufacturers’ Instructions For Use (IFU) for reprocessing were followed to the letter.2 3 9
In the Netherlands, as in many other parts of the world, process control is used. This means that reprocessing is considered to be adequate when it is performed according to the IFU and according to the standard handbook of the Dutch Steering Group for Flexible Endoscope Cleaning and Disinfection (SFERD).12 This handbook is based on regulations applicable in the Netherlands as well as the guidelines of the European Society of Gastrointestinal Endoscopy (ESGE).13 Despite international outbreaks and outbreaks in Dutch ERCP centres,14 15 both the IFU and SFERD do not include microbial surveillance after disinfection as a routine practice. Recently, contamination of duodenoscopes has been assessed in several studies.16–18 Most studies were performed in a single university centre,16 18 making it difficult to extrapolate their results and estimate the true burden on a national level. A study among 21 centres was conducted by Brandabur et al,17 showing contamination rates with a wide variability across centres. To date, no such study has been conducted in a nationwide setting using a uniform sampling and culture method as well as examining all possible contamination sites.
Given the increase in the number of publications pertaining duodenoscope contamination and the potentially severe consequences for patients, there is an urgency to develop a more thorough understanding of the scale of the problem. Therefore, the aim of this study was to determine the prevalence of microbial contamination of patient-ready duodenoscopes in all ERCP centres in the Netherlands.
Materials and methods
Setting
We conducted a prospective nationwide cross-sectional study among all Dutch ERCP centres. In the Netherlands, over 16.000 ERCP procedures are performed in 73 ERCP centres yearly.19 All 73 Dutch ERCP centres were asked to sample at least two duodenoscopes at their own choosing and, if present, to include the newest Olympus TJF-Q180V (Olympus, Zoeterwoude, The Netherlands). Duodenoscopes were eligible for sampling if they were reprocessed and ready for patient use, for example, after high level disinfection or after drying in the storage cabinet. No data were recorded about the moment of sampling, surveillance methods or adherence to reprocessing or sampling protocols. No patient data were included in this study; therefore, there was no need for approval by the Medical Ethical Research Committee.
Sample collection
Sampling was performed independently by local staff of the included ERCP centres, using a centrally distributed sample collection kit, according to a strict and uniform sampling protocol (see online supplementary files). This method was developed by a multidisciplinary team of reprocessing staff, medical device experts, infection control professionals, medical microbiologists and gastroenterologists based on the SFERD standard handbook.12 The sampling protocol was explained using 12 instruction videos available online (see online supplementary videos). As examples, the sampling and labelling procedure was shown in detail using one Olympus TJF-160VR and one Pentax ED34-i10T (Pentax, Dodewaard, The Netherlands) duodenoscope. Duodenoscopes were sampled while placed in the Automated Endoscope Reprocessor or on a sterile surface. Depending on the duodenoscopes type, four to six sites were sampled. The four sites present in all duodenoscope types were: (1) a flush of the biopsy channel, (2) a flush of the suction channel, (3) a swab from the forceps elevator and (4) a single brush through the biopsy and suction channel. Type-dependent samples were: (1) a swab of the removable protection cap and (2) a flush of the elevator channel or air/water channel, if these channels were unsealed. Channels were flushed with sterile physiological saline solution of which at least 20 mL was collected at the distal tip in a sterile container. The flush fluid was aspirated with a sterile needle and injected in two 9.5 mL BD Vacutainers without additives (Becton Dickinson, Etten-Leur, The Netherlands). Forceps elevator and protection cap were sampled with ESwabs (Copan Italia S.p.A., Brescia, Italy). Type dependent, Olympus BW-412T or Pentax CS6021T single-use endoscope cleaning brushes were used to brush the biopsy and suction channel. Both ESwabs and the brush tip were transported in ESwab medium. Instructions were to swab first, second to flush the channels and finally to brush the channels. The decision to reprocess the endoscope after sampling was up to the respective centres and was not documented for the purpose of the current study. Samples were sent to the Erasmus MC department of Medical Microbiology and Infectious Diseases for culturing.
Culturing and interpretation
Samples were cultured on the day of receipt. Channel flushes were filtrated over a 0.45 µm filter of which the filtrate was forced on R2A agar. ESwabs and brush tips were vortexed in their ESwab medium of which 0.75 mL was poured on a blood agar. Samples were incubated at 35°C, examined for growth for 72 hours and read at 24 hours, 48 hours and 72 hours. Culture results were presented in colony forming units (CFU)/20 mL per microorganism. Results were sent to the respective ERCP centres without further interpretation: further action was up to the respective ERCP centre and was not documented for the purpose of the current study. At the time of study conduct, Dutch guidelines for endoscopy centres stated that in case of contamination with a subset of indicator microorganisms with ≥20 CFU/20 mL or in case of persistent contamination, endoscopes should be quarantined and possible causes be investigated.12 Cultured microorganisms were categorised depending on their origin into gastrointestinal, oral, skin and waterborne flora. Contamination was defined according to two definitions: (1) microbial growth with ≥20 CFU/20 mL of any type of microorganism (AM20) as used by the ESGE guideline and Dutch SFERD handbook12 13 or (2) presence of microbial growth (≥1 CFU/20 mL) of gastrointestinal and/or oral microorganisms (MGO).
Statistical analysis
Categorical data are presented in percentages. Mean (range) and median (IQR) are given for continuous and skewed data, respectively. The χ² test was used to compare categorical data and Student’s t-test or Mann Whitney U-test was used to compare continuous data. Contamination rates of duodenoscope types and sample sites were compared according to a logistic regression model, using the SAS procedure GENMOD. This model adjusted for the multiple samples of each unique duodenoscope, with each duodenoscope clustered within their respective ERCP centre. Duodenoscope types were compared with the newest Olympus TJF-Q180V type as a reference and sample sites were compared with the flush of the biopsy channel. For both analyses, duodenoscope types or sample sites could be included if there was at least one contamination case and one non-contamination case. Analyses were performed using SAS V.9.4 (SAS, Cary, North Carolina, USA) and SPSS V.21.0 (IBM, Armonk, New York, USA).
Results
Between June 2015 and March 2016, 67 out of 73 (92%) Dutch ERCP centres sampled 745 sites of 155 endoscopes. Five endoscopes were excluded: four duodenoscopes from one centre whose samples were cultured in their own microbiology department and one gastroscope from another centre as this type of endoscope does not have a forceps elevator, that is, no duodenoscope (figure 1). Twenty-six samples from 17 duodenoscopes were excluded, as these sites did not correspond with the specified duodenoscope type. This resulted in an inclusion of 150 duodenoscopes with a total of 701 samples from 66 (92% of all centres) ERCP centres (figure 1). The median time between local sampling and culturing in the Erasmus MC was 1 day (IQR 1–2). Table 1 provides an overview of the contamination prevalence per duodenoscope type and sample site for AM20 and MGO contamination definitions. Contamination according to the AM20 definition was found in 33 (22%) out of the 150 reprocessed and patient-ready duodenoscopes. Duodenoscopes were most often contaminated with skin flora (n=17; 11%) and to a lesser extent with waterborne flora (n=12; 8%), gastrointestinal flora (n=10; 7%) or oral flora (n=4; 3%). Contamination according to the MGO definition was found in 23 (15%) duodenoscopes. Table 2 shows all different microorganisms that were cultured, among others Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa.
Figure 1.
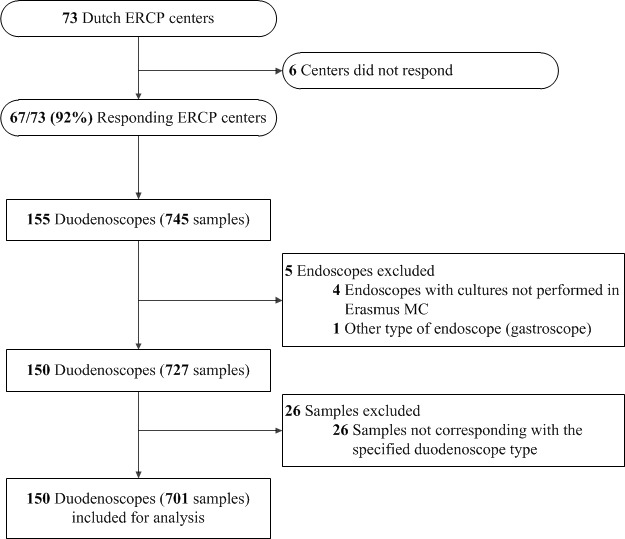
Flow diagram. ERCP, Endoscopic Retrograde Cholangiopancreatography.
Table 1.
Prevalence of AM20 and MGO contamination for duodenoscopes and sample sites
| Duodenoscope type | N | AM20 | MGO | ||
| Contam. | Not contam. | Contam. | Not contam. | ||
| All duodenoscopes | 150 | 33 (22%) | 117 (78%) | 23 (15%) | 127 (85%) |
| Olympus TJF-Q180V | 69 | 15 (22%) | 54 (78%) | 15 (22%) | 54 (78%) |
| Olympus TJF-160VR | 43 | 13 (30%) | 30 (70%) | 6 (14%) | 37 (86%) |
| Olympus TJF-160R | 8 | 1 (13%) | 7 (87%) | 0 | 8 |
| Olympus TJF-140R | 2 | 0 | 2 | 0 | 2 |
| Olympus TJF-145 | 2 | 0 | 2 | 0 | 2 |
| Pentax ED34-i10T | 11 | 3 (27%) | 8 (73%) | 0 | 11 |
| Pentax ED-3490TK | 8 | 0 | 8 | 0 | 8 |
| Pentax ED-3680TK | 1 | 0 | 1 | 1 (100%) | 0 |
| Fujifilm ED-530XT8 | 5 | 0 | 5 | 0 | 5 |
| Fujifilm ED-530XT | 1 | 1 (100%) | 0 | 1 (100%) | 0 |
| Sample site | AM20 | MGO | |||
| Contam. | Not contam. | Contam. | Not contam. | ||
| All sample sites | 701* | 47 (7%) | 654 (93%) | 35 (5%) | 666 (95%) |
| Biopsy channel | 146 | 5 (3%) | 141 (97%) | 6 (4%) | 140 (96%) |
| Suction channel | 137 | 4 (3%) | 133 (97%) | 5 (4%) | 132 (96%) |
| Forceps elevator | 148 | 14 (10%) | 134 (90%) | 7 (5%) | 141 (95%) |
| Brush | 139 | 17 (12%) | 122 (89%) | 14 (10%) | 125 (90%) |
| Protection cap | 56 | 6 (11%) | 50 (89%) | 3 (5%) | 53 (95%) |
| Elevator channel | 53 | 0 | 53 | 0 | 53 |
| Air/water channel | 26 | 1 (5%) | 21 (95%) | 0 | 22 |
*Sampling of all possible sites would have yielded 745 samples: 44 (6%) sites were not sampled. This included 4/150 (3%) biopsy channel, 13/150 (9%) suction channel, 2/150 (1%) forceps elevator, 11/150 (7%) brush, 9/65 (13%) protection cap, 2/55 (4%) elevator channel and 3/25 (12%) air/water channel samples.
AM20, microbial growth with ≥20 CFU/20 mL of any type of microorganism; Contam., contaminated; MGO, presence of any microbial growth of gastrointestinal or oral microorganisms; Not contam., not contaminated.
Table 2.
Cultured microorganisms of 150 duodenoscopes
| Gastrointestinal flora independent of CFU count | Oral flora independent of CFU count | ||||
| No. of duodenoscopes | Quantity range | No. of duodenoscopes | Quantity range | ||
| Yeasts | 7 | 6–100 CFU | Moraxella spp. | 4 | 1 CFU |
| Klebsiella pneumoniae | 4 | 100 - >100 CFU | Streptococcus salivarius | 4 | 1–15 CFU |
| Enterobacter cloacae | 3 | 100 - >100 CFU | Moraxella osloensis | 3 | 1 CFU – 100 CFU |
| Escherichia coli | 2 | 50 and 100 CFU | Streptococcus mitis | 2 | 30 and 50 CFU |
| Klebsiella oxytoca | 2 | 100 CFU- >100 CFU | Neisseria flavescens | 1 | 1 CFU |
| Enterococcus faecium | 1 | 1 CFU | Rothia spp. | 1 | 10–30 CFU |
| Enterococcus faecalis | 1 | 100 | Streptococcus mutans | 1 | 2 CFU |
| Pseudomonas aeruginosa | 1 | 100 CFU | Streptococcus oralis | 1 | 5 CFU |
| Staphylococcus aureus | 1 | >100 CFU | Streptococcus spp. | 1 | 10 CFU |
| Skin flora ≥20 CFU | Waterborne flora ≥20 CFU | ||||
| No. of duodenoscopes | Quantity range | No. of duodenoscopes | Quantity range | ||
| Bacillus spp. | 5 | 40–100 CFU | Stenotrophomonas maltophilia | 3 | 100 - >100 CFU |
| Micrococcus luteus | 5 | 100 CFU | Acinetobacter spp. | 2 | 80 and 100 CFU |
| Staphylococcus epidermidis | 4 | 50–100 CFU | Agrobacterium radiobacter | 2 | 20 and 100 CFU |
| Kocuria spp. | 2 | 25 and 100 CFU | Paracoccus yeeii | 2 | 30 and 100 CFU |
| Staphylococcus hominis | 2 | 25 and 100 CFU | Achromobacter xylosoxidans | 1 | 100 CFU |
| Staphylococcus warneri | 2 | 50 and 80 CFU | Alternaria spp. | 1 | >100 CFU |
| Kocuria rhizophila | 1 | >100 CFU | Pseudomonas monteilii | 1 | 100 CFU |
| Micrococcus spp. | 1 | 30 CFU | Pseudomonas putida | 1 | 100 CFU |
| Staphylococcus auricularis | 1 | >100 CFU | Sphingomonas paucimobilis | 1 | 100 CFU |
| Staphylococcus spp. (CNS) | 1 | 60 CFU | Rhizobium spp. or Sphingobium spp. | 1 | >100 CFU |
CFU, colony forming units; CNS, coagulase - negative staphylococci.
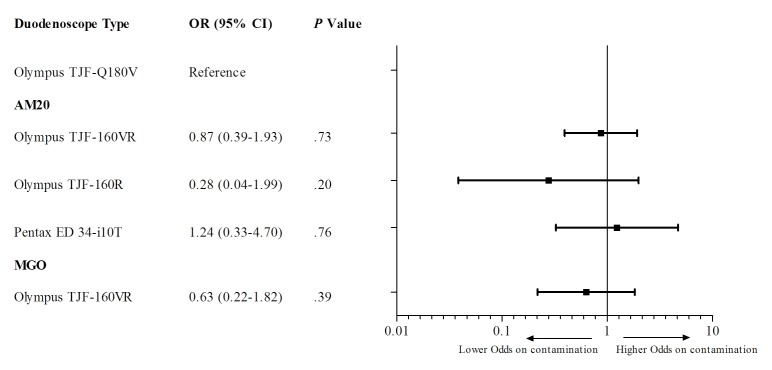
Ten different duodenoscope types from three distinct manufacturers (ie, Olympus, Pentax and Fujifilm) were sampled. Contamination as defined by AM20 was identified in five different duodenoscope types and contamination as defined by MGO was identified in four different types. As shown in figure 2, contamination for AM20 (four duodenoscope types included) as well as MGO (two duodenoscope types included) was shown not to be type-dependent (all p>0.05).
Figure 2.
OR for each duodenoscope type on contamination. AM20, microbial growth with ≥20 CFU/20 mL of any type of microorganism; CFU, colony forming units; MGO, presence of any microbial growth of gastrointestinal or oral microorganisms.
The AM20 contaminated duodenoscopes originated from 26 (39%) centres across the Netherlands. No difference (p=0.10) was shown in contamination prevalence between academic tertiary medical centres (n=3/8; 38%), specialised peripheral medical centres (n=13/23; 57%) or general peripheral medical centres (n=10/35; 29%). This was also the case for MGO-contaminated duodenoscopes originating from 19 (28%) centres. No difference was found (p=0.25) between academic tertiary medical centres (n=3/8; 38%), specialised peripheral medical centres (n=9/23; 39%) and general peripheral medical centres (n=7/35; 20%).
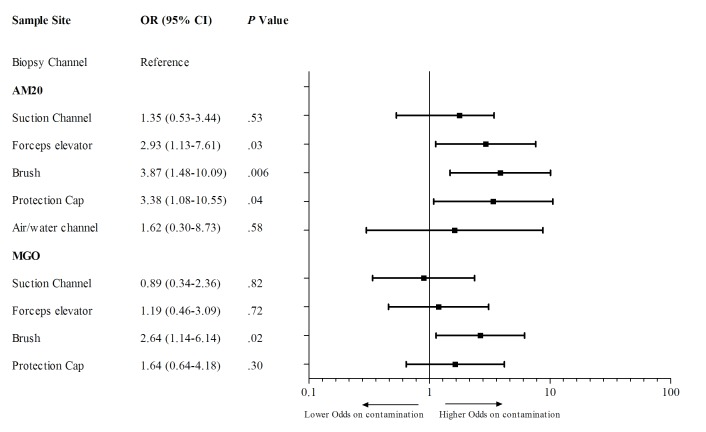
Microorganisms were cultured from 166 (24%) sample sites of 97 (65%) duodenoscopes. Additionally, 54 (8%) sample sites of 41 (27%) duodenoscopes contained two or more microorganisms, in some cases up to five different microorganisms. As shown in table 1, all sample sites, except the flush of the elevator channel, were found positive for AM20 or MGO contamination. The flush of the biopsy channel was used as a reference to compare the contamination prevalence of all sample sites. Three sample sites had a higher probability of being contaminated (figure 3). According to the AM20 definition, the swab of the elevator (OR 2.93, 95% CI 1.13 to 7.61; p=0.03) and the swab of the protection cap (3.38, 95% CI 1.08 to 10.55; p=0.04) were more often contaminated. The brush of the biopsy/suction channel was more often contaminated for both AM20 (OR 3.87, 95% CI 1.13 to 7.61; p=0.006) and MGO (OR 2.64, 95% CI 1.14 to 6.14; p=0.02) definitions.
Figure 3.
OR for each sample site on contamination. AM20, microbial growth with ≥20 CFU/20 mL of any type of microorganism; CFU, colony forming units; MGO, presence of any microbial growth of gastrointestinal or oral microorganisms.
Discussion
In our nationwide prevalence study, we found that over one-fifth of sampled duodenoscopes were contaminated according to AM20 definition, with 39% of Dutch ERCP centres having at least one contaminated duodenoscope intended to be ready for patient use. Furthermore, MGO were cultured on 15% of the sampled duodenoscopes, indicating the presence of organic residue of previously treated patients. Our observations coincide with worldwide reported outbreaks indicating that exogenous transmission of bacteria and associated infections and even viral infections related to contaminated duodenoscopes continue to threaten patients undergoing ERCP.1–4 20 Therefore, stringent measures are required to lower the number of contaminated duodenoscopes in order to minimise the risk of interpatient microbial transmission during ERCP and to prevent future outbreaks.
The prevalence of duodenoscope contamination in this study was in line with reports from several retrospective single tertiary centre studies.18 21 22 Recent studies by Brandabur et al and Ross et al performing postprocedure or everyday morning cultures reported remarkably lower contamination rates.16 17 This could be explained by the fact that continuous feedback of microbial surveillance resulted in a raised alertness, resulting in lower contamination rates over time. In the centres included in the present study, it is not common practice to perform surveillance cultures, especially no daily or postprocedure cultures, as Dutch guidelines do not demand these.12 23 Other contributing factors could be differences in sampling and culture methods. For example, we used a more sensitive contamination cut-off and a longer incubating time than Brandabur et al and Ross et al.16 17 The present study was conducted in 2015–2016 after multiple MDRO-outbreaks were reported (inter)nationally, including reports of outbreaks in Dutch ERCP centres as early as 2009 and 2012.14 15 Despite current national awareness about the potential consequences of contamination, our results were concordant with a cross-sectional multicentre (n=37) Canadian study published in 2002 in which a contamination prevalence of 30% was reported using a contamination cut-off of 10 CFU/mL.24
The most recent duodenoscope types introduced into the market have distinct design changes, including sealing of the elevator channel and a sealed protection cap, aimed at preventing contamination and the need for reprocessing at these locations. In 2012, an outbreak in our hospital was linked to the newest Olympus TJF-Q180V duodenoscope.2 After the outbreak, the duodenoscope was investigated by Olympus and an independent expert. One of the conclusions was that TJF-Q180V’s specific design features hampered adequate cleaning and disinfection.15 To further investigate these matters, we asked participating centres to include the TJF-Q180V duodenoscope, if present. The current study shows that contamination for both AM20 and MGO were not restricted to certain duodenoscope types. This is in line with outbreaks that have been reported involving various duodenoscope types from all three manufacturers.6 Moreover, Brandabur et al also reported that culture positivity was not affected by scope type.17 Despite differences in design, none of the available duodenoscope types seem excluded from the risk of contamination.
The differences in the type of cultured flora can give an indication where in the reprocessing process the duodenoscopes were contaminated. Several guidelines that advocate active microbiological surveillance give guidance on how to interpret culture results.13 25 In this study, a substantial number of duodenoscopes were contaminated with skin and waterborne flora. Contamination with skin flora is thought to arise from handling and therefore could potentially easily be reduced by improved handling during reprocessing and transport. However, the presence of skin flora could be due to contamination during sampling. We cannot rule out this cause as sampling on site was not audited. Dutch centres have to use filtered water for reprocessing facilities and process control involves quarterly microbiological control of the rinse water.12 In our view, persistent contamination with waterborne flora demands a thorough investigation as it can be caused by several factors, including contamination of the water supply, inadequate filtering of the water supply and inadequate drying of the endoscope during storage. Contamination with MGO indicates inadequate reprocessing as originating from the gastrointestinal tract. This type of contamination could be due to a breach in the reprocessing procedure or because the reprocessing procedure cannot be adequately performed due to reprocessing, endoscopic or procedure specific risk factors. Currently, we are working on a Dutch guideline in which actions following positive cultures will be described extensively. The guideline will be submitted for international publication in the near future. Differences in Automated Endoscope Reprocessors, endoscope hang time and different reprocessing methods do not seem to affect contamination rates.17 26 27 Beside the complex design of the duodenoscope,2 6 28 endoscope age has also been suggested as a risk factor,2 18 26 with Brandabur et al proposing the number of procedures as a better indicator for endoscope usage.17 Contamination does not seem to be confined to duodenoscopes: single-centre studies show that coloscopes and gastroscopes can have similar contamination rates.18 22 However, compared with duodenoscopes, other gastrointestinal endoscopes are far less the reason of recent reported outbreaks.5 We hypothesise that this could be due to differences between types of procedures as ERCP procedures tend to be more invasive, entering sterile body cavities and could have a more compromised patient population. The latter defines the more serious and therefore detectable clinical outcome of transmission of microorganisms by ERCP compared with other gastrointestinal endoscopes.
In the present study, the brush, the forceps elevator and the protection cap had the highest probability of detection of contamination. The forceps elevator is a site known to be prone to persistent contamination.2 3 16 17 The brush is also noted as a site that can harbour the involved microorganism during an outbreak.16 Borescope channel inspections of gastroscopes and coloscopes performed by Ofstead et al revealed that all reprocessed endoscope channels contained fluid, discoloration and debris.29 This underlines that the biopsy channel is subject to heavy wear and tear: devices are introduced frequently, causing soiling of the channel which adds to the risk of contamination.30 Remarkably, in the present study, the elevator channel was not contaminated in any duodenoscope and the air/water channel in only one duodenoscope. Sampling of these specific channels is often not performed during surveillance and often not even in the case of an outbreak.16
In current guidelines and studies, there is no international consensus on a uniform sampling and culturing method, although several differences could potentially affect culture outcomes. The location and the number of sample sites differ greatly: in some instances, a channel brush18 31 or swab of the forceps elevator12 24 is omitted. When the channel brush or the forceps elevator would not be cultured in the present series, 19% (6/32) or 9% (3/32), respectively, of the AM20 contaminated duodenoscopes would have been missed. Some studies and guidelines advocate a different order of sampling, such as retrograde sampling or the flush-brush-flush method, as it might have a higher sensitivity.14 25 31 32 The cleaning brush that is used for sampling could disrupt present biofilms and affect subsequent samples. However, in this study, the brush sample was performed last. A sample flush with a neutraliser instead of saline solution can prevent false negative outcomes due to the biocidal activity of residual disinfectants33 34 and is advocated by the French guideline and several French studies.18 21 33 35 The toxicity of the neutralisers might also cause false negatives,36 and theoretically the endoscope should not contain any residual disinfectant after a successful reprocessing cycle. Other guidelines including the Dutch guideline, according to which our sampling protocol was designed, do not require a neutraliser based on current evidence.12 13 25 31 However, if a neutraliser effectively prevents false negative outcomes, the contamination rates in this study could be even higher. A longer incubation time is associated with a higher culture positivity rate. Saliou et al state that endoscope samples should be incubated for at least 1 week. In their study, after 48 hours only 55.5% of the final number of contaminated endoscopes were found positive.18 Some studies and guidelines use an incubation time of 48 hours.16 17 25 31 In this study, we have chosen for a 72 hours period: the microorganisms of concern would be detected and the study results could by compared with the centres’ previous microbiological surveillance results. Also, the choice of growing media for incubation of flush samples can affect the culture positivity rate. R2A agar, as used in this study, has a high sensitivity, especially for slower growing microorganisms.37 38 To be able to compare test results and omit false negative test results, standardised and uniform instructions for sampling, culturing and interpretation of culture results should be devised which, based on results in this study, should include a channel brush and a swab of the forceps elevator as these sites pose the highest risk of contamination.
To the best of our best knowledge, this is the first study assessing contamination of duodenoscopes nationwide. Another strength of our study is that we cultured all samples in one microbiology laboratory using a standardised protocol. Finally, because of the extensive sampling method we were able to analyse all possible contamination sites. This study has some limitations. This study could only be conducted nationwide as a cross-sectional study without follow-up samples of the duodenoscopes: improvement of contamination rates or persistent contamination was not assessed. Furthermore, sampling was conducted independently by local staff. Although we provided strict sampling protocols with clear video instructions on how the culture procedure should be performed, we were not able to check for adherence to the sampling protocol. Also the conditions in which the endoscopes were sampled (ie, just disinfected or after drying with or without alcohol flush or positive air flow) were not recorded. Potential differences in culture outcomes between sampling post-disinfection or postdrying, differences in drying times or other storage or reprocessing parameters could not be assessed. However, all assessed duodenoscopes were ready for use in patients and should not be contaminated, regardless of the moment of reprocessing. We hypothesise that the effect of these factors on the presence of especially gastrointestinal and or oral flora is rather small, as we see this as a failure of the reprocessing process. Last, a small amount of sites were not sampled, which could cause underestimation of the total number of contaminated duodenoscopes.
The observed nationwide high prevalence of contamination of patient-ready duodenoscopes is a clear indication that the current combination of reprocessing and process control is not sufficient. All participating hospitals are dedicated endoscopy centres following the national guideline that underlines process control. This includes reprocessing exactly according to the manufacturer’s instructions and extensive yearly audits.12 23 As adherence to reprocessing protocols was not observed, this study shows real-life outcomes of patient-ready duodenoscopes with little bias. Regardless of whether the precise cause of contamination was a breach in the reprocessing process or the complex duodenoscope design, process control was not able to identify and prevent such large-scale inadequate reprocessing. This calls for concerted action by all parties involved, that is,: manufacturers, regulatory bodies, government agencies, gastroenterologists and medical microbiologists. Nowadays, ERCP has evolved into a minimally invasive interventional procedure having replaced more invasive and complicated surgical procedures. It is an essential procedure practiced all over the world with over 650 000 procedures performed in USA annually.39 During revision of the market clearance of the Olympus TJF-Q180V duodenoscope, the U.S. Food and Drug Administration (FDA) stated that a decrease in ERCP capacity would be unacceptable.40 However, contaminated duodenoscopes put patients at risk of developing clinically relevant infections by transmission of microorganisms.
In 2015, the FDA issued a warning that some parts of duodenoscopes may be extremely difficult to access and adequate cleaning of all areas may not be possible.28 Since then additional measures have been suggested,11 including alternative reprocessing methods or implementation of microbial surveillance as proposed by Centres for Disease Control and Prevention.10 31 Eventually, radical changes in the design of duodenoscopes should ensure thorough cleaning and disinfection. However, development and market introduction of such newly designed duodenoscopes will require substantial time. A complicating factor is that standardised procedures to test duodenoscopes in their ability to be adequately cleaned and disinfected are not available. Therefore, on the short term, we should not solely rely on process control as there is no scientific proof that this serves as a reliable proxy for safe and clean duodenoscopes. Uniform guidelines and instructions for microbial surveillance should be developed. Also, an international registry for contaminated scopes should be instituted in order to truly estimate the scale of the problem and track its impact and revolution over time.
To conclude, this nationwide cross-sectional study shows high prevalence rates of contamination of duodenoscopes in Dutch ERCP centres. The recent reports on infections due to contaminated endoscopes will probably be due to involvement and alertness on highly resistant microorganisms, but also the more and more complex designs of endoscopes can play a role in this emergence. Additional preventive measures including microbial surveillance strategies are needed to reduce the number of contaminated duodenoscopes.
gutjnl-2017-315082supp001.pdf (3.1MB, pdf)
gutjnl-2017-315082supp002.pdf (1.6MB, pdf)
gutjnl-2017-315082supp003.m4v (22.6MB, m4v)
gutjnl-2017-315082supp004.m4v (26.6MB, m4v)
gutjnl-2017-315082supp005.m4v (59.3MB, m4v)
gutjnl-2017-315082supp006.m4v (54.5MB, m4v)
gutjnl-2017-315082supp007.m4v (64.9MB, m4v)
gutjnl-2017-315082supp008.m4v (63MB, m4v)
gutjnl-2017-315082supp009.m4v (29.4MB, m4v)
gutjnl-2017-315082supp010.m4v (20.1MB, m4v)
gutjnl-2017-315082supp011.m4v (46.2MB, m4v)
gutjnl-2017-315082supp012.m4v (48.9MB, m4v)
gutjnl-2017-315082supp013.m4v (58.2MB, m4v)
gutjnl-2017-315082supp014.m4v (82.3MB, m4v)
Acknowledgments
The authors would like to thank the reprocessing staff, medical devices experts, infection control professionals, medical microbiologists and gastroenterologists at the following Dutch ERCP centres for their participation and effort for this study: Academic Medical Center (AMC), Amsterdam; Albert Schweitzer Hospital, Dordrecht; Alrijne Hospital, Leiden/Leiderdorp; Amphia Hospital, Breda; Antoni van Leeuwenhoek, Amsterdam; Antonius Zorggroep, Sneek; Beatrix Hospital, Gorinchem; Bernhoven, Uden; Bravis Hospital, Roosendaal; Canisius Wilhelmina Hospital, Nijmegen; Catharina Hospital Eindhoven, Eindhoven; Deventer Hospital, Deventer; Diakonessenhuis, Utrecht; Elkerliek Hospital, Helmond; Erasmus University Medical Center (Erasmus MC), Rotterdam; FlevoHospital, Almere; Groene Hart Hospital, Gouda; Hospital Amstelland, Amstelveen; Hospital De Tjongerschans, Heerenveen; Hospital Gelderse Vallei, Ede; Hospital St. Jansdal, Harderwijk; IJsselland Hospital, Capelle aan Den IJssel; Ikazia, Rotterdam; Isala Diaconessenhuis, Meppel; Jeroen Bosch Hospital, ’s Hertogenbosch; Laurentius Hospital, Roermond; Leiden University Medical Center (LUMC), Leiden; Maasstad Hospital, Rotterdam; Maastricht University Medical Center (MUMC), Maastricht; Martini Hospital, Groningen; Maxima Medical Center, Veldhoven; Meander Medical Center, Amersfoort; Medical Center Alkmaar, Alkmaar; Medical Center Haaglanden, Den Haag; Medical Center Leeuwarden, Leeuwarden; Medical Center Slotervaart, Amsterdam; Medical Center Zuiderzee, Lelystad; Medisch Spectrum Twente, Enschede; Nij Smellinghe Hospital, Drachten; Ommelander Hospital Group, Delfzijl; Onze Lieve Vrouwe Gasthuis—Location East/West, Amsterdam; Radboud University Medical Center (Radboudumc), Nijmegen; Reinier de Graaf Hospital, Delft; Rijnstate Hospital, Arnhem; Rode Kruis Hospital, Beverwijk; Slingeland Hospital, Doetinchem; St. Anna Hospital, Eindhoven; St. Antonius Hospital, Nieuwegein; St. Elisabeth Hospital, Tilburg; St. Franciscus Gasthuis, Rotterdam; Tergooi Hospital, Hilversum; Treant Zorggroep, Location Scheper, Emmen; TweeSteden Hospital, Tilburg/Waalwijk; University Medical Center Groningen (UMCG), Groningen; University Medical Center Utrecht (UMCU), Utrecht; VieCuri Medical Center, Venlo; VU Medical Center, Amsterdam; Westfriesgasthuis, Hoorn; Wilhelmina Hospital Assen, Assen; Zaans Medical Center, Zaandam; Ziekenhuisgroep Twente, Almelo; ZorgSaam Hospital, Terneuzen; Zuwe Hofpoort Hospital, Woerden; Zuyderland Medical Center, Heerlen.
Footnotes
MJB and MCV contributed equally.
Contributors: AWR: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. AFV in ‘t holt: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. JGB: study concept and design; critical revision of the manuscript for important intellectual content. RDG: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content. BEH: analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. MJB: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; study supervision. MCV: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding; study supervision.
Funding: This study was supported by grant 324841 from the Dutch Ministry of Health, Wellbeing and Sports (Professor Dr MCV).
Disclaimer: The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Competing interests: AWR, AFV in ‘t holt, JGB and RDG reported no relevant financial activities. BEH has had the following relevant financial activities outside the submitted work: Consultant for Intercept Pharmaceuticals Inc.; Consultant for Novartis; Consultant for Albiero; Received grants from Intercept Pharmaceuticals Inc.; Received grants from Roche International. MJB has had the following relevant financial activities outside the submitted work: Consultant for 3M; Grant from 3M for an investigator initiated study for the benefit of the Department of Gastroenterology and Hepatology, Erasmus MC University Medical Center, Rotterdam, The Netherlands; Consultant and lecturer for Boston Scientific; Grants from Boston Scientific for investigator initiated studies and industry initiated studies for the benefit of the Department of Gastroenterology and Hepatology, Erasmus MC University Medical Center, Rotterdam, The Netherlands; Consultant and lecturer for Cook Medical; Grants from Cook Medical for investigator initiated studies and industry initiated studies for the benefit of the Department of Gastroenterology and Hepatology, Erasmus MC University Medical Center, Rotterdam, The Netherlands; Grant of Pentax Medical for the benefit of the Department of Gastroenterology and Hepatology, Erasmus MC University Medical Center, Rotterdam, The Netherlands. MCV has had the following relevant financial activities outside the submitted work: Grant from 3M for an investigator initiated study.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Presented at: Spring meeting Dutch Society of Gastroenterology. Veldhoven, The Netherlands, 17–18 March 2016. Title: Bacterial contamination of reprocessed ERCP duodenoscopes in The Netherlands is widespread (abstract number: 4317) Oral presentation. Session Title: President Select 26th ECCMID Congress, the European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, The Netherlands, 9–12 April 2016. Title: ERCP duodenoscopes in Dutch ERCP centres: high prevalence of bacterial contamination despite reprocessing (abstract number: O384) Oral presentation. Session Title: New insights in the control of multi-resistant Gram-negatives Digestive Disease Week. San Diego, USA, 21–24 May 2016. Title: ERCP Duodenoscopes in Dutch ERCP Centres: High Prevalence of Bacterial Contamination Despite Reprocessing (abstract number: 2442341) Oral presentation. Session Title: Bugs on Duodenoscope: Pressing Need for Pest Control (ASGE) 8th Steering Group Flexible Endoscopes Cleaning and Disinfection (SFERD) Symposium, Veenendaal, the Netherlands, 14 September 2016. Title: Contamination of duodenoscopes, where are we now? Oral presentation.
References
- 1. Kola A, Piening B, Pape UF, et al. . An outbreak of carbapenem-resistant OXA-48 - producing Klebsiella pneumonia associated to duodenoscopy. Antimicrob Resist Infect Control 2015;4:8 10.1186/s13756-015-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verfaillie CJ, Bruno MJ, Voor in ’t Holt AF, et al. . Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy 2015;47:493–502. 10.1055/s-0034-1391886 [DOI] [PubMed] [Google Scholar]
- 3. Epstein L, Hunter JC, Arwady MA, et al. . New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014;312:1447–55. 10.1001/jama.2014.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alrabaa SF, Nguyen P, Sanderson R, et al. . Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control 2013;41:562–4. 10.1016/j.ajic.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 5. Kovaleva J. Infectious complications in gastrointestinal endoscopy and their prevention. Best Pract Res Clin Gastroenterol 2016;30:689–704. 10.1016/j.bpg.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 6. United States Senate. Preventable Tragedies: superbugs and how ineffective monitoring of medical device safety fails patients. Washington, United States: United States Senate, 2016. https://www.help.senate.gov/imo/media/doc/Duodenoscope_Investigation_FINAL_Report.pdf (accessed 13 Jan 2016). [Google Scholar]
- 7. Kovaleva J, Peters FT, van der Mei HC, et al. . Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev 2013;26:231–54. 10.1128/CMR.00085-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S, Russell D, Mohamadnejad M, et al. . Risk factors associated with the transmission of carbapenem-resistant Enterobacteriaceae via contaminated duodenoscopes. Gastrointest Endosc 2016;83:1121–9. 10.1016/j.gie.2016.03.790 [DOI] [PubMed] [Google Scholar]
- 9. Wendorf KA, Kay M, Baliga C, et al. . Endoscopic retrograde cholangiopancreatography-associated AmpC Escherichia coli outbreak. Infect Control Hosp Epidemiol 2015;36:634–42. 10.1017/ice.2015.66 [DOI] [PubMed] [Google Scholar]
- 10. Rutala WA, Weber DJ. Outbreaks of carbapenem-resistant Enterobacteriaceae infections associated with duodenoscopes: What can we do to prevent infections? Am J Infect Control 2016;44:e47–e51. 10.1016/j.ajic.2015.10.037 [DOI] [PubMed] [Google Scholar]
- 11. Petersen BT, Koch J, Ginsberg GG. Infection Using ERCP Endoscopes. Gastroenterology 2016;151:46–50. 10.1053/j.gastro.2016.05.040 [DOI] [PubMed] [Google Scholar]
- 12. Advisory Board Cleaning and Disinfection Flexible Endoscopes (SFERD). Professional standard handbook. Flexible endoscopes - Cleaning and disinfection, 2016. https://www.infectiepreventieopleidingen.nl/kennisbank/kennisbank/sferd-handboek-4-0 (accessed 1 Sep 2016).
- 13. Beilenhoff U, Neumann CS, Rey JF, et al. . ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy 2007;39:175–81. 10.1055/s-2006-945181 [DOI] [PubMed] [Google Scholar]
- 14. Kovaleva J, Meessen NE, Peters FT, et al. . Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy 2009;41:913–6. 10.1055/s-0029-1215086 [DOI] [PubMed] [Google Scholar]
- 15. Loeve AJ. Investigational report on a TJF-Q180V duodenoscope following contamination after cleaning and disinfection. Available on request from the Dutch Healthcare Inspectorate/National Institute for Public Health and the Environment. Bilthoven, The Netherlands, 2012. [Google Scholar]
- 16. Ross AS, Baliga C, Verma P, et al. . A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest Endosc 2015;82:477–83. 10.1016/j.gie.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 17. Brandabur JJ, Leggett JE, Wang L, et al. . Surveillance of guideline practices for duodenoscope and linear echoendoscope reprocessing in a large healthcare system. Gastrointest Endosc 2016;84:392–9. 10.1016/j.gie.2016.03.1480 [DOI] [PubMed] [Google Scholar]
- 18. Saliou P, Le Bars H, Payan C, et al. . Measures to improve microbial quality surveillance of gastrointestinal endoscopes. Endoscopy 2016;48:704–10. 10.1055/s-0042-107591 [DOI] [PubMed] [Google Scholar]
- 19. van Turenhout ST, Terhaar sive Droste JS, Meijer GA, et al. . Anticipating implementation of colorectal cancer screening in The Netherlands: a nation wide survey on endoscopic supply and demand. BMC Cancer 2012;12:46 10.1186/1471-2407-12-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tennenbaum R, Colardelle P, Chochon M, et al. . [Hepatitis C after retrograde cholangiography]. Gastroenterol Clin Biol 1993;17:763–4. [PubMed] [Google Scholar]
- 21. Saliou P, Cholet F, Jézéquel J, et al. . The use of channel-purge storage for gastrointestinal endoscopes reduces microbial contamination. Infect Control Hosp Epidemiol 2015;36:1100–2. 10.1017/ice.2015.139 [DOI] [PubMed] [Google Scholar]
- 22. Saviuc P, Picot-Guéraud R, Shum Cheong Sing J, et al. . Evaluation of the quality of reprocessing of gastrointestinal endoscopes. Infect Control Hosp Epidemiol 2015;36:1017–23. 10.1017/ice.2015.123 [DOI] [PubMed] [Google Scholar]
- 23. Working party on infection prevention (WIP). Heat-sensitive flexible endoscopes. Leiden, the Netherlands, 2015. http://www.rivm.nl/dsresource?objectid=4c1522b5-9aa2-4a3e-b812-4e08abace739&type=org&disposition=inline (accessed 1 Jan 2016). [Google Scholar]
- 24. Alfa MJ, Olson N, DeGagne P, et al. . A survey of reprocessing methods, residual viable bioburden, and soil levels in patient-ready endoscopic retrograde choliangiopancreatography duodenoscopes used in Canadian centers. Infect Control Hosp Epidemiol 2002;23:198–206. 10.1086/502035 [DOI] [PubMed] [Google Scholar]
- 25. Gastroenterological Society of Australia / Gastroenterological Nurses College of Australia. Infection control in endoscopy. Mulgrave, Australia: Gastroenterological Society of Australia, 2010. http://cart.gesa.org.au/membes/files/Clinical_Guidelines_and_Updates/Infection_Control_in_Endoscopy_Guidelines_2014.pdf (accessed 13 Jan 2016). [Google Scholar]
- 26. Heroux R, Sheppard M, Wright SB, et al. . Duodenoscope hang time does not correlate with risk of bacterial contamination. Am J Infect Control 2017;45 10.1016/j.ajic.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 27. Snyder GM, Wright SB, Smithey A, et al. . Randomized comparison of 3 high-level disinfection and sterilization procedures for duodenoscopes. Gastroenterology 2017;153:1018–25. 10.1053/j.gastro.2017.06.052 [DOI] [PubMed] [Google Scholar]
- 28. US Food and Drug Administration (FDA). Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) Duodenoscopes May Impede Effective Cleaning: FDA Safety Communication. USA, 2015. http://wayback.archive-it.org/7993/20170722213105/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm434871.htm (Updated 4 Mar 2015). [Google Scholar]
- 29. Ofstead CL, Wetzler HP, Heymann OL, et al. . Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures. Am J Infect Control 2017;45:e26–e33. 10.1016/j.ajic.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 30. Lee DH, Kim DB, Kim HY, et al. . Increasing potential risks of contamination from repetitive use of endoscope. Am J Infect Control 2015;43:e13–e17. 10.1016/j.ajic.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention. Interim protocol for healthcare facilities regarding surveillance for bacterial contamination of duodenoscopes after reprocessing. Atlanta, United States, 2015. https://www.cdc.gov/hai/pdfs/cre/interim-duodenoscope-surveillance-protocol.pdf (accessed 19 Aug 2015). [Google Scholar]
- 32. Buss AJ, Been MH, Borgers RP, et al. . Endoscope disinfection and its pitfalls--requirement for retrograde surveillance cultures. Endoscopy 2008;40:327–32. 10.1055/s-2007-995477 [DOI] [PubMed] [Google Scholar]
- 33. Aumeran C, Thibert E, Chapelle FA, et al. . Assessment on experimental bacterial biofilms and in clinical practice of the efficacy of sampling solutions for microbiological testing of endoscopes. J Clin Microbiol 2012;50:938–42. 10.1128/JCM.06221-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Espigares E, Bueno A, Fernández-Crehuet M, et al. . Efficacy of some neutralizers in suspension tests determining the activity of disinfectants. J Hosp Infect 2003;55:137–40. 10.1016/S0195-6701(03)00238-X [DOI] [PubMed] [Google Scholar]
- 35. Ministere des Affaires sociales et de la sante. Guide Technique: traitement des endoscopes souples thermosensibles a canaux. France, 2016. http://nosobase.chu-lyon.fr/recommandations/Ministere_Sante/2016_EndoscopeSouple_Ministere.pdf [Google Scholar]
- 36. Sutton SV, Proud DW, Rachui S, et al. . Validation of microbial recovery from disinfectants. PDA J Pharm Sci Technol 2002;56:255–66. [PubMed] [Google Scholar]
- 37. van der Linde K, Lim BT, Rondeel JM, et al. . Improved bacteriological surveillance of haemodialysis fluids: a comparison between Tryptic soy agar and Reasoner’s 2A media. Nephrol Dial Transplant 1999;14:2433–7. 10.1093/ndt/14.10.2433 [DOI] [PubMed] [Google Scholar]
- 38. Massa S, Caruso M, Trovatelli F, et al. . Comparison of plate count agar and R2A medium for enumeration of heterotrophic bacteria in natural mineral water. World J Microbiology and Biotechnology 1998;14:727–30. 10.1023/A:1008893627877 [DOI] [Google Scholar]
- 39. US Food and Drug Administration (FDA). Executive summary: effective reprocessing of endoscopes used in Endoscopic Retrograde Cholangiopancreatography (ERCP) Procedures. USA, 2015. www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/medicaldevices/medicaldevicesadvisorycommittee/gastroenterology-urologydevicespanel/ucm445592.pdf (accessed 14 May 2015). [Google Scholar]
- 40. US Food and Drug Administration (FDA). Updated information for healthcare providers regarding duodenoscopes. USA, 2015. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofReusableMedicalDevices/UCM436588.pdf (accessed 4 Mar 2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2017-315082supp001.pdf (3.1MB, pdf)
gutjnl-2017-315082supp002.pdf (1.6MB, pdf)
gutjnl-2017-315082supp003.m4v (22.6MB, m4v)
gutjnl-2017-315082supp004.m4v (26.6MB, m4v)
gutjnl-2017-315082supp005.m4v (59.3MB, m4v)
gutjnl-2017-315082supp006.m4v (54.5MB, m4v)
gutjnl-2017-315082supp007.m4v (64.9MB, m4v)
gutjnl-2017-315082supp008.m4v (63MB, m4v)
gutjnl-2017-315082supp009.m4v (29.4MB, m4v)
gutjnl-2017-315082supp010.m4v (20.1MB, m4v)
gutjnl-2017-315082supp011.m4v (46.2MB, m4v)
gutjnl-2017-315082supp012.m4v (48.9MB, m4v)
gutjnl-2017-315082supp013.m4v (58.2MB, m4v)
gutjnl-2017-315082supp014.m4v (82.3MB, m4v)