Abstract
Estriol (E3) is an endogenous estrogen in females with broad biological activity within diverse tissue types. In the context of certain T-cell–mediated autoimmune inflammatory diseases, E3 can ameliorate disease severity through immunomodulatory mechanisms that decrease tissue inflammation. Severe disease caused by influenza A virus (IAV) infection is also characterized by aberrant inflammation and immunopathology. How E3 might affect the pathogenesis of IAV infection, however, has not been explored. Gonadally intact female C57BL/6 mice that were treated with exogenous E3 during infection with mouse-adapted 2009 H1N1 had reduced total pulmonary inflammation and improved disease outcomes compared with females that received no hormone. Furthermore, compared with no hormone treatment, E3 treatment reduced the induction of genes associated with proinflammatory cytokine and chemokine responses in the lungs, which preceded clinical disease, reductions in innate immune cell recruitment, altered pulmonary T-cell skewing, and reduced antibody titers during IAV infection. Although E3 treatment was associated with reduced local and systemic anti-influenza adaptive immune responses, there was no effect of E3 on viral replication or clearance. Together, these data suggest that exogenous E3 confers protection during IAV infection through immunomodulatory mechanisms and that E3 may have broad therapeutic potential in the context of both infectious and noninfectious inflammatory diseases.
Treatment of female C57BL/6 mice with exogenous estriol during IAV infection decreased lung inflammation but not virus control or clearance, to improve clinical disease outcome.
Estrogens are a group of reproductive hormones that influence diverse biologic processes in both males and females. Estrogens include three endogenously produced, biologically distinct compounds: estrone, estradiol (E2), and estriol (E3). Owing to their varying binding affinities for the two forms of the nuclear estrogen receptor (ER), ERα and ERβ, different estrogens and estrogen combinations mediate variable downstream effects that are both concentration and tissue dependent (1). E3 has relatively weak ER binding affinity but greater binding affinity for ERβ than ERα (2). Because E3 has reduced signaling through ERα (i.e., the dominant ER in the endometrium and glandular breast tissue), exogenous E3 is associated with a lower risk of uterine and breast cancer than E2 (3, 4) and may be preferable over E2 for long-term use. Both oral (4) and vaginal (5) formulations of E3 are available and widely marketed as hormone replacement therapies (HRTs) for reducing the symptoms of menopause in women. Although E3 is not approved by the US Food and Drug Administration for use in the United States or Canada, it is commonly included as a main component of bioidentical HRTs, which are produced and sold by compounding pharmacies and have increased in popularity throughout North America (6). Despite its wide use in such preparations, however, current knowledge of how E3 may influence host response to infectious diseases is limited.
ERs are widely distributed throughout the body, and estrogens have biologic activity in diverse cell and tissue types. Notably, ERs are present within both innate and adaptive immune cells (7, 8), and estrogen signaling can influence the pathogenesis of several inflammatory diseases. In autoimmune inflammatory diseases driven largely by T cells, such as rheumatoid arthritis (RA) and multiple sclerosis (MS), estrogens are protective (7, 8). In animal models of RA, E2-mediated protection is associated with decreased levels of anti–type II collagen antibodies and dampened T-cell proliferation (9). Similarly, in the experimental autoimmune encephalomyelitis (EAE) mouse model of MS, estrogens decrease disease severity by reducing overall inflammatory cell recruitment to the central nervous system (CNS) by reducing the frequency of TNF-producing cells and polarizing resident immune cells toward T helper type 2 (Th2) and tolerogenic phenotypes (10–13). Most recently, the neuroprotective and immunomodulatory effects of E3 have been tested in humans, with oral formulations of E3 showing therapeutic promise in phase II clinical trials by reducing relapses of relapsing-remitting MS in women (14).
Despite the therapeutic potential of E3 for diverse inflammatory disease, little is known of its biologic activity outside of reproductive tissues and the CNS. Furthermore, there are no reports that characterize the role of E3 in the context of infection. Similar to the pathogenesis of RA and MS, pulmonary disease following infection with influenza A virus (IAV) is caused by excessive and aberrant inflammatory responses to the virus, primarily driven by T cells, which leads to immunopathology and tissue damage (15). Effective therapies for limiting severe pulmonary disease following IAV infection include prostaglandins (16) and sphingosine analogs (17), which are aimed at limiting the “cytokine storm” and pulmonary inflammation in addition to controlling viral replication. Furthermore, there is a well-established, albeit poorly understood, bidirectional link between MS and respiratory tract infection, including IAV. Respiratory infections account for a large proportion of deaths of humans with MS (18), and in an EAE mouse model, IAV infection can trigger and exacerbate neuroinflammation and disease (19, 20). Similarly, in mice, the induction of EAE can increase IAV mortality (21), suggesting that there may be shared mechanisms of disease. It is, therefore, plausible that an effective treatment against one condition might be mutually beneficial for the other.
Treatment with E2 during IAV infection of mice is protective, and exogenous E2 given to ovariectomized female C57BL/6 mice improved survival during lethal IAV infection by decreasing proinflammatory cytokines and chemokines in the lungs and increasing the recruitment of pulmonary neutrophils (22, 23). In the current study, we sought to characterize the effects of E3 in the respiratory tract following infection with a sublethal dose of a mouse-adapted 2009 H1N1 IAV. Exogenous E3 treatment of either female or male mice significantly improved the outcome of IAV infection. Furthermore, similar to its role in the CNS, E3 significantly reduced the transcriptional activity of genes associated with proinflammatory cytokines and chemokines during early IAV infection of the respiratory tract, which was associated with reduced recruitment of immune cells into the lungs. Reduced pulmonary inflammation in E3-treated mice correlated with improved pulmonary function and reduced morbidity and clinical disease. E3 treatment, however, did not alter the kinetics of virus replication and clearance, suggesting that E3-treated animals maintained sufficient antiviral immunity despite reduced overall inflammation. Together, these data describe a therapeutic use for E3 in mediating protection against an inflammation-mediated, infectious disease in the respiratory tract.
Materials and Methods
Animals, hormone treatments, and virus infections
Adult (7 to 9 weeks old) female and male C57BL/6 mice were purchased from Charles River Laboratories (Frederick, MD). All animals remained gonad intact for translation to humans, in which exogenous hormone treatments would be administered to gonad-intact individuals, with exogenous sex steroids shutting down endogenous reproductive hormone production and activity through negative feedback mechanisms. Mice were housed five animals per cage under standard Biological Safety Level 2 housing condition with food and water ad libitum. All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee under animal protocol M015H236. Mice were anesthetized with a ketamine (100 mg/kg) and xylazine (10 mg/kg) cocktail, and pellets (21-day release; Innovative Research of America, Sarasota, FL) containing E2 (15 mg), E3 (5 mg), combined E2 + E3, or placebo (cholesterol) were implanted subcutaneously in between the shoulder blades. These doses of E2 and E3 are reported to increase circulating concentrations to levels comparable to pregnant mice (10). Immediately following pellet implantation, anesthetized mice were infected with a mouse-adapted IAV, A/California/04/09 [ma2009 H1N1; generated by Dr. Andrew Pekosz (The Johns Hopkins Bloomberg School of Public Health, Baltimore, MD) from a published sequence (24)] or a vehicle (mock) control. Mice were inoculated intranasally with 10 tissue culture infectious dose 50 (TCID50) units of ma2009 H1N1 suspended in 30 μL DMEM or mock-infected with 30 μL DMEM alone. Mice were monitored daily for changes in body weight as a surrogate of morbidity. The change in body weight for each animal was calculated as a percentage of its weight at the time of infection. At select time points, a clinical score was calculated for each animal based on a 4-point scale for hunched posture, piloerection, dyspnea, and lack of an escape response, as previously described (25). The cumulative score from these parameters represents the clinical score for each animal.
Hormone measurements
Approximately 300 to 500 μL plasma was obtained from heparinized blood collected via cardiac puncture at euthanasia, and samples were stored at −80°C. Steroid hormones were extracted from plasma using a liquid-liquid extraction method. Briefly, plasma samples were mixed with methyl-tert-butyl-ether at a 5:1 solvent/sample ratio. The solvent layer was allowed to separate for 5 minutes, then transferred into a clean tube and evaporated to dryness in a fume hood overnight. The extracted hormone was suspended in 500 μL assay buffer and vortexed. The concentrations of E2 and E3 were quantified by competitive immunoassay (Enzo Life Sciences, Farmingdale, NY), according to the manufacturer’s instructions.
Tissue harvesting and processing of whole lung
Lungs were homogenized in 500 μL sterile PBS using Lysing Matrix D tubes (MP Biomedical, Santa Ana, CA) in an MP Fast-prep 24 5G instrument. Supernatants from lung homogenates were stored at −80°C. Total RNA was extracted from the remaining tissue homogenate using the RNeasy Lipid Tissue Mini Kit (Qiagen, Germantown, MD), according to the manufacturer’s instructions.
Quantification of infectious virus titers
Infectious virus was measured from lung homogenate supernatants by a TCID50 assay, as previously described (24). Briefly, 10-fold dilutions of lung homogenate supernatants were plated onto a monolayer of Madin-Darby canine kidney cells in replicates of 6 for 6 days at 32°C. Cells were stained with naphthol blue black (Sigma-Aldrich, St. Louis, MO) and scored for cytopathic effects. The TCID50 titer was calculated according to the Reed-Muench method.
Histopathology
Following euthanasia, the left bronchus was ligated, and the left lung lobe was removed. The right lung lobes were then inflated with zinc-buffered formalin (Z-fix; Anatech, Battle Creek, MI) delivered for 2 minutes at a fixed pressure (25 cmH2O). The trachea was tied under pressure and the lungs dissected free and placed in fixative for 48 hours. Tissues were subsequently embedded in paraffin, cut into 5-μm sections, and mounted on glass slides. Slides were stained with hematoxylin and eosin and used to evaluate lung inflammation. Histopathological scoring was performed by a single blinded observer using a 0 to 3 scale (0, no inflammation; 1, mild inflammation; 2, moderate inflammation; and 3, severe inflammation) for bronchiolitis, perivasculitis, alveolitis, and edema, as previously described (26). The sum of these parameters represents the cumulative inflammation score. Images were taken using a Nikon Eclipse E800 camera.
Fluorescence-activated cell sorting and RNA extraction
CD45+ and CD45− cell populations were sorted from whole lungs by fluorescence-activated cell sorting. Briefly, lungs were excised following a dispase-agarose infusion, and single-cell suspensions were generated following serial filtration and red blood cell lysis, as previously described (27). The total numbers of viable cells were determined using a hemocytometer and trypan blue (Invitrogen, Carlsbad, CA) exclusion. The cell suspensions were treated with anti-CD16/32 (BD Biosciences, Franklin Lakes, NJ) and subsequently stained with anti–CD45-PE-Cy7 (clone 30-F11; eBiosciences, San Diego, CA) (28) and propidium iodide. Live cells (propidium iodide negative) were sorted using a MoFlo cell sorter (Beckman Coulter, Pasadena, CA) based on CD45 expression into DMEM. A minimum of 106 cells of each population was collected from each animal. RNA was immediately isolated from cell pellets using the RNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions.
Analysis of proinflammatory gene expression
Total RNA isolated from either whole lung or sorted CD45− and CD45+ cell populations was quantified using a nanodrop spectrophotometer, and cDNA was prepared with the RT2 First Strand Kit (Qiagen) using 1 µg input RNA and the manufacturer’s instructions. Quantitative PCR for 84 genes was performed using the Mouse Inflammatory Response and Autoimmunity RT2 Profiler PCR Array (Qiagen), according to the manufacturer’s instructions. All reactions were run on the StepOnePlus Applied Biosystems Real-time PCR machine under the following conditions: 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute for 40 cycles. Relative gene expression levels were determined by normalizing the target gene threshold cycle (CT) value to the CT value of the endogenous housekeeping gene, Hsp90ab1 (∆CT), and then normalizing the ∆CT value of infected animals to the average ∆CT value of the respective mock-inoculated controls (∆∆CT).
Flow cytometry
Lungs were excised and single-cell suspensions were generated following red blood cell lysis. The total numbers of viable cells were determined using a hemocytometer and trypan blue (Invitrogen) exclusion, and the cells were suspended at 1 × 106 cells/mL in RPMI 1640 (Cellgro) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) and 1% penicillin-streptomycin. T cells were stimulated ex vivo with influenza peptide (CD8: NP366–374 or CD4: NP311–325) (ProImmune, Sarasota, FL) in media containing Brefeldin A (GolgiPlug; BD Biosciences) for 5 hours at 37°C. Fc receptors were blocked using anti-CD16/32 (clone 2.4G2; BD Biosciences; catalog no. 553141) (29). Leukocyte populations were stained with the following antibodies (eBiosciences): CD45-PE-Cy7 (clone 30-F11; Thermo Fisher Scientific; catalog no. 25-0451) (28), CD3-APC (clone 17A2; Thermo Fisher Scientific; catalog no.17-0032-82) (30), CD4-PerCP-Cy5.5 (clone RM4-5; Thermo Fisher Scientific; catalog no. A14785) (31), CD8-AF700 (clone 53-6.7; Thermo Fisher Scientific; catalog no. 56-0081-82) (32), CD11b-PerCP-Cy5.5 (clone M1/70; Thermo Fisher Scientific; catalog no. 45-0112-80) (33), CD11c-APC (clone N418; Thermo Fisher Scientific; catalog no. 17-0114-81) (34), Ly6G-FITC (clone Gr-1; Thermo Fisher Scientific; catalog no. 11-5931-81) (35), and PE-conjugated tetramer for ma2009 (ASNENVETM; NIH Tetramer Core Facility). The cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) and subsequently stained for interferon γ (IFNγ)–Pacific Blue (clone XMG1.2; Thermo Fisher Scientific; catalog no. 48-7311-80) (36) and IL-4–PE (clone 11B11; Thermo Fisher Scientific; catalog no. 12-7041-71) (37). Intracellular staining with Foxp3-PE (clone MF23; BD Biosciences; catalog no. 560408) (38) was performed following fixation and permeabilization with the Transcription Factor Staining Buffer Set (eBiosciences). The viability of cells was determined by use of a fixable Live/Dead aqua viability dye (Invitrogen). Data were acquired using a Fortessa fluorescence-activated cell sorter (with FACSDiva software) and analyzed using FlowJo (v.10) software (Tree Star, Inc., Ashland, OR). Total cell counts were determined based on the percentages of live cells in the live-cell gate multiplied by the total live-cell counts acquired prior to staining by the trypan blue exclusion counts obtained on a hemocytometer.
Anti-influenza ELISA
ELISA plates (Microlon 96-well high-binding plates; Greiner Bio-One) were coated with 100 ng purified IAV overnight at 4°C in carbonate buffer (pH 9.6). For total IgG and IgG isotype ELISAs, the plates were washed three times with 1× PBS plus 0.1% Tween 20 and blocked for at least 1 hour at 37°C with 10% dry milk powder in 1× PBS. The plates were washed three times prior to the addition of serially diluted serum, and they were incubated for 1 hour at 37°C. The plates were then washed three more times, and the secondary antibody was added as follows: anti-mouse horseradish peroxidase (HRP) conjugated secondary IgG (1:250; Thermo Fisher Scientific), anti-mouse HRP IgG2c (1:20,000; Thermo Fisher Scientific), or anti-mouse HRP IgG1 (1:6000, Thermo Fisher Scientific) was added, and the plates were incubated for 1 hour at 37°C. The plates were washed three times with 1× PBS plus 0.1% Tween 20, and the reactions were developed with tetramethylbensidine (BD Biosciences) and subsequently stopped with 1 N HCl. The absorbance at 450 nm was read on a plate reader. To determine the antibody titer, a cutoff value was determined by multiplying the average optical density values for the negative controls at each dilution by 3, and the titer for the sample was calculated as the highest serum dilution with an optical density value above the cutoff.
Microneutralization assay
Serially diluted serum was mixed with 100 TCID50 units of IAV suspended in DMEM for 1 hour at room temperature. The serum-virus mixture was used to infect confluent Madin-Darby canine kidney cells for 24 hours at 37°C. All infections were performed in quadruplicate on flat-bottom 96-well plates. After 16 to 18 hours of incubation, the inoculum was removed, the cells were washed with 1× PBS (with calcium and magnesium), and fresh DMEM was added. The cells were incubated for 6 days at 32°C and then fixed with 4% formaldehyde and stained with naphthol blue black for at least 6 hours. The infectious virus titer was calculated as the highest serum dilution that eliminated virus cytopathic effects in 50% (i.e., two of four wells) per dilution.
Statistical analyses
Longitudinal morbidity measures, including body weight and clinical score data, were analyzed with repeated-measures two-way ANOVAs. Other longitudinal measures with unmatched samples, including virus titers, pulmonary inflammation, and the kinetic flow cytometry data (i.e., total CD11b+ cells, neutrophils, eosinophils, alveolar macrophages, monocytes/macrophages, and total CD3+ T cells), were analyzed using unmatched two-way ANOVAs with Bonferroni post hoc correction for multiple comparisons. Single time point analyses, including T-cell phenotyping and antibody titers, were analyzed using Student t tests. PCR array data were analyzed using multiple t tests, where P values were corrected for a false discovery rate of 5%. Mean differences were considered statistically significant if the corrected P < 0.05. All statistical analyses were performed using GraphPad Prism 7 software (GraphPad Software, La Jolla, CA).
Results
E3 improves the outcome of IAV infection in female mice
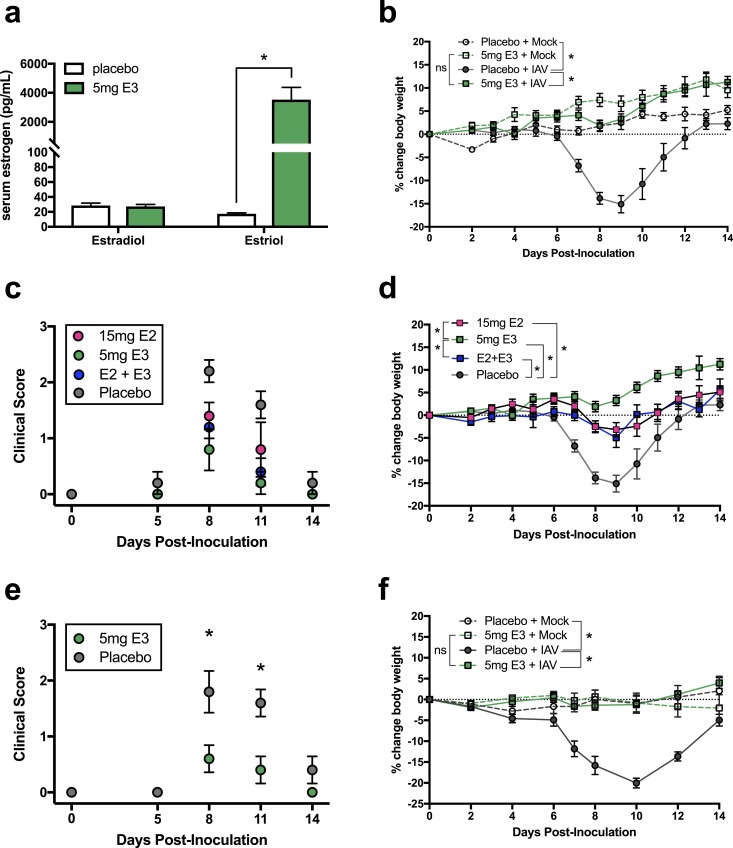
To determine the effect of exogenous E3 treatment on the pathogenesis of IAV infection, pellets containing either E3 or cholesterol (placebo) were implanted subcutaneously into adult female C57BL/6 mice. Following implantation, these pellets continuously released hormone into the circulation, and this dose of E3 significantly increased concentrations of plasma E3 but had no effect on concentrations of plasma E2 (Fig. 1a). Following pellet implantation, mice were intranasally inoculated with either ma2009 H1N1 IAV or vehicle control and monitored for changes in body weight and evidence of clinical disease. In placebo-treated females, IAV infection resulted in significant body weight loss, characterized by a ∼15% reduction from baseline to 8 days postinoculation (dpi). In contrast, E3-treated females did not experience any significant change in body weight compared with their respective mock-infected controls, and they maintained body weight at or above baseline levels throughout the course of infection (Fig. 1b). Furthermore, clinical IAV-associated disease—defined by alterations in breathing, posture, and activity—which was evident at 8 and 11 dpi, was significantly reduced in E3-treated compared with placebo-treated females (Fig. 1c). To compare the effects of E3 with those of E2, the predominant circulating estrogen in cycling females (2), female mice were treated with pellets containing E2, a combination of E2 and E3, or cholesterol (placebo). Similar to the effects of E3 alone, both E2 and the combined E2 + E3 treatment resulted in significant clinical protection during IAV infection. Compared with placebo-treated controls, both E2 and combined E2 + E3 protected against clinical disease (Fig. 1c) and body weight loss (Fig. 1d), but the protective effects of treatment with E3 alone were greater than those of either E2 or combined E2 + E3. Because the therapeutic benefit of E3 in ameliorating disease in the EAE mouse model has also been demonstrated in males (10, 11), we evaluated the efficacy of E3 treatment of IAV-infected male mice. Similar to the phenotype observed for females, E3 conferred significant protection against clinical disease and body weight loss in males (Fig. 1e and 1f), suggesting that E3 mediates effects independent of biological sex.
Figure 1.
Treatment of female mice with exogenous E3 improves the outcome of influenza. Female or male C57BL/6 mice received a subcutaneous implant containing either 5 mg E3, 15 mg E2, combined E2 and E3, or a placebo (cholesterol) control. (a) Serum estrogens, including E2 and E3, were measured from E3- and placebo-treated females 8 dpi by ELISA (n = 5 animals per treatment). (b) Placebo- and E3-treated female mice were infected with a sublethal dose of IAV or vehicle (mock) and monitored over the course of infection for the percent change in body weight (n = 10 to 15 animals per treatment). Placebo- and estrogen-treated female mice (n = 5 to 15 animals per treatment) were infected with a sublethal dose of IAV or vehicle (mock) and monitored over (c) the course of infection for clinical disease and (d) the percent change in body weight. Placebo- and E3-treated male mice (n = 5 animals per treatment) were infected with a sublethal dose of IAV or vehicle (mock) and monitored over (e) the course of infection for clinical disease and (f) the percent change in body weight. Bars represent the mean and SEM. ELISA data were analyzed using Student t tests, and body weight and clinical score data were analyzed using matched two-way ANOVAs with Bonferroni post hoc correction, with *P < 0.05. ns, nonsignificant.
E3 decreases pulmonary inflammation during IAV infection
To determine if the protective effects of E3 were related to altered IAV replication, infectious viral titers were quantified in lung tissue at select time points following infection. Despite significant differences in clinical disease between placebo- and E3-treated females (Fig. 1b and 1c), there were no differences in virus replication kinetics. Regardless of hormone treatment, infectious virus titers peaked at 5 dpi and were cleared by 14 dpi (Fig. 2a). To determine if reduced influenza severity in E3-treated females was instead due to differences in pulmonary inflammation, total pulmonary CD45+ leukocytes were quantified by flow cytometry. Corresponding with peak clinical disease (Fig. 1b and 1c), frequencies of total pulmonary leukocytes were greatest at 8 dpi but were significantly lower in the lungs of E3-treated compared with placebo-treated females (Fig. 2b). Congruent with total inflammatory cell numbers, the histologic distribution (Fig. 2c) and severity (Fig. 2d) of pulmonary inflammation was also greater at 8 dpi than either 5 (before peak disease) or 14 (after peak disease) dpi. However, at 5 and 8 dpi, the severity of inflammation was significantly reduced in E3-treated females, which was characterized primarily by reduced perivasculitis and bronchiolitis compared with placebo-treated females (Fig. 2d and 2e).
Figure 2.
E3 treatment during influenza decreases pulmonary inflammation without altering viral replication or clearance. Female C57BL/6 mice received a subcutaneous implant containing either 5 mg E3 or a placebo (cholesterol) control, followed by infection with a sublethal dose of IAV. (a) At select dpi, mice were euthanized and infectious virus titers were quantified from lung homogenates by TCID50 assay (n = 5 to 10 animals per treatment per timepoint). (b) Total pulmonary CD45+ leukocytes were quantified by flow cytometry. (c) Total pulmonary inflammation and (d) the percentage of the lesioned area were quantified from hematoxylin and eosin–stained fixed lung tissue from both placebo- and E3-treated females (n = 5 to 8 animals per treatment per timepoint). Pulmonary inflammation was assessed using a 0 to 3 scoring system for perivasculitis, bronchiolitis, alveolitis, and edema, where (c) the sum of each score is presented, and (d) the percentage of the lesioned area within each lung section was calculated using ImageJ software. (e) Representative photomicrographs from lung tissue collected at 8 dpi from both placebo- and E3-treated females (magnification: top images, ×4; bottom images, ×20), demonstrating perivasculitis (arrow), bronchiolitis (arrowhead), and alveolitis/edema (asterisk). Bars represent the mean and SEM. All data were analyzed using two-way ANOVAs with Bonferroni post hoc correction, with *P < 0.05 between treatment groups and #P < 0.05 within a treatment compared with baseline. LOD, limit of detection.
E3 reduces proinflammatory transcriptional activity in the lung during IAV infection
Reduced pulmonary inflammation following IAV infection may be the result of reduced immune cell recruitment and/or accelerated immune cell egress from the lungs. Because the differences in pulmonary inflammation and total pulmonary leukocytes between placebo- and E3-treated females were observed prior to and during peak disease (i.e., 5 and 8 dpi, respectively) (Fig. 2), we hypothesized that E3 may play a larger role in dampening immune cell recruitment through reduced induction of pulmonary chemotactic signals. To test this hypothesis, the relative expression of 84 inflammatory genes in whole lung tissue was compared between placebo- and E3-treated females at 5 and 8 dpi. Following IAV infection, there was an overall greater induction of inflammatory gene expression in placebo-treated compared with E3-treated females at both 5 and 8 dpi (Fig. 3a and Supplemental Table 1). At 5 dpi, 36 genes, including genes that code for CC chemokines and receptors (Fig. 3b), CXC chemokines and receptors (Fig. 3c), interleukins and receptors (Fig. 3d), Toll-like receptors (Fig. 3e), TNF superfamily genes (Fig. 3f), adhesion molecules (Fig. 3g), and IFNγ (Fig. 3h), were significantly induced (i.e., greater than fourfold over mock) by IAV infection. Of these 36 genes, 35 were induced in placebo-treated females, whereas only 10 were induced in E3-treated females (Fig. 3b–3h and Supplemental Table 1). Only one gene, Ccl22, was induced in E3-treated females but not in placebo-treated females (Fig. 3b), and all but three genes (Ccl12, Ccl22, and Il10) were differentially regulated between placebo- and E3-treated females (Fig. 3b–3h). At 8 dpi, 42 genes were significantly induced (i.e., greater than fourfold over mock) in placebo-treated females, whereas only 24 genes were similarly induced in E3-treated females, including 2 genes, Ccl19 and Ccl22, that were not induced in placebo-treated females (Supplemental Table 1). Together, these data suggest that E3 treatment during IAV infection was associated with an overall global reduction of inflammatory gene expression in the lungs.
Figure 3.
E3 decreases the induction of proinflammatory gene expression in the lungs prior to the onset of influenza-associated disease. Female C57BL/6 mice received a subcutaneous implant containing either 5 mg E3 or a placebo (cholesterol) control, followed by infection with a sublethal dose of IAV or vehicle (mock). At 5 dpi, mice were euthanized, and quantitative PCR was performed on RNA extracted from whole lung homogenates. (a) The heat map represents the log2 fold change in gene expression relative to respective mock-inoculated controls for both placebo- and E3-treated female mice (n = 4 animals per treatment). Relative induction of (b) CC chemokines and receptors, (c) CXC chemokines and receptors, (d) interleukins and receptors, (e) Toll-like receptors, (f) TNF superfamily genes, (g) adhesion molecules, and (h) IFNγ was compared between placebo- and E3-treated females. Data were analyzed using multiple t tests, corrected for a false discovery rate of 5%, with *P < 0.05 (adjusted).
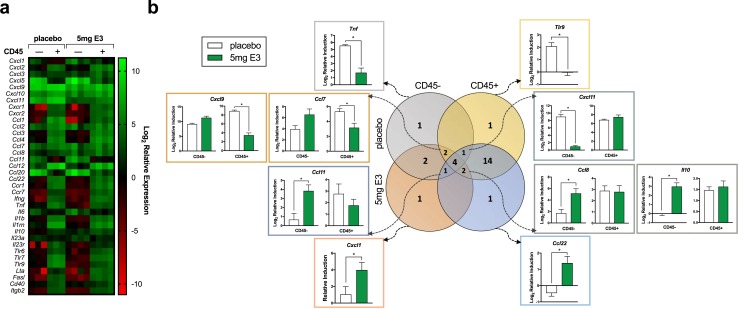
Cells from both hematopoietic and nonhematopoietic lineages are responsible for the production of proinflammatory cytokines and chemokines, which propagate immune cell recruitment and shape the overall immunologic milieu in the lungs. To differentiate the transcriptional activity of the resident nonhematopoietic (i.e., epithelial, endothelial, and mesenchymal) cells from the activity of the resident and recruited hematopoietic cells (i.e., leukocytes), the transcriptional profiles of CD45− (i.e., nonhematopoietic) and CD45+ (i.e., hematopoietic) cells isolated from lungs at 5 dpi were compared. IAV infection of both placebo- and E3-treated females resulted in differential expression of inflammatory genes in CD45− compared with CD45+ cells (Fig. 4a). Most genes (22 of 36) were more highly expressed in CD45+ cells, whereas a minority of genes (4 of 36) were more highly expressed in CD45− cells (Fig. 4b and Supplemental Table 1). Furthermore, select genes were identified that were differentially regulated by E3 treatment: within CD45− cells, Tnf was induced to a greater degree in placebo-treated females, whereas Cxcl1 was induced to a greater degree in E3-treated females; within CD45+ cells, Tlr9 was induced to a greater degree in placebo-treated females, whereas Ccl22 was induced to a greater degree in E3-treated females. A portion of genes (10 of 30) was similarly expressed in both CD45− and CD45+ cells at 5 dpi, and within this subset, six genes were identified to be dichotomously regulated by E3 treatment in CD45− compared with CD45+ cells. For example, although E3 treatment did not alter Cxcl9 induction within CD45− cells, it significantly reduced Cxcl9 induction in CD45+ cells. In contrast, Cxcl11 induction was not altered by E3 treatment in CD45+ cells, but it was significantly reduced by E3 treatment in CD45− cells (Fig. 4b). Together, these data suggest that E3 signals through diverse cell types within the lung and can have differential and sometimes opposing effects on gene regulation within different cell populations.
Figure 4.
E3 differentially regulates proinflammatory gene expression in hematopoietic and nonhematopoietic cells in the lung during influenza. Female C57BL/6 mice received a subcutaneous implant containing either 5 mg E3 or a placebo (cholesterol) control, followed by infection with a sublethal dose of IAV or vehicle (mock). At 5 dpi, mice were euthanized, and CD45− and CD45+ cells were isolated using fluorescence-activated cell sorting from the lungs. Quantitative PCR was performed on RNA extracted from sorted cell populations. (a) The heat map represents the log2 fold change in gene expression relative to whole lung from respective mock-inoculated controls for both placebo- and E3-treated female mice (n = 3 to 4 animals per treatment). (b) The Venn diagram shows the number of genes that were differentially expressed between CD45− and CD45+ cell populations at 5 dpi and, within those categories, the number of genes that were differentially expressed between placebo- and E3-treated females. The graphs show the gene induction (relative to respective mock-inoculated controls) at 5 dpi in CD45− and/or CD45+ cell populations. All data were analyzed using multiple t tests, corrected for a false discovery rate of 5%, with *P < 0.05 (corrected).
E3 reduces pulmonary immune cell recruitment and polarizes pulmonary T cells during IAV infection
Proinflammatory cytokine and chemokine expression profiles can influence the character, magnitude, and kinetics of the local inflammatory response. To determine whether E3 altered pulmonary immune cell recruitment during IAV infection, immunophenotyping of lungs from placebo- and E3-treated females was conducted using flow cytometry at 0, 5, 8, and 14 dpi. Total CD45+ leukocytes (Fig. 2b) were broadly stratified into CD11b+ myeloid cells (including monocytes/macrophages, neutrophils, and eosinophils) and CD3+ T cells, respectively. In both placebo- and E3-treated females, CD11b+ myeloid cells were increased as early as 5 dpi and remained significantly elevated at 8 dpi before returning to baseline at 14 dpi (Fig. 5a). Recruitment of pulmonary CD3+ T cells occurred later, with total numbers peaking 8 dpi in both placebo- and E3-treated females and remaining elevated at 14 dpi in placebo-treated but not E3-treated females (Fig. 5b). Although the kinetics of pulmonary CD11b+ and CD3+ immune cells were similar for both treatment groups, the magnitude of the response was significantly reduced in E3-treated compared with placebo-treated females (Fig. 5a and 5b). When evaluating the relative proportions of pulmonary CD45+ leukocytes, CD11b+ leukocytes represented a significantly greater percentage of cells in E3-treated compared with placebo-treated females at baseline (0 dpi), but the proportions were similar at all time points following infection (Fig. 5c). Further characterization of CD11b+ immune cells (39) (Fig. 5d) revealed greater frequencies and percentages of Ly6G+ neutrophils in E3-treated compared with placebo-treated females at baseline (0 dpi) but reduced numbers in E3-treated compared with placebo-treated females at 8 dpi (two-way ANOVA, interaction, P = 0.0007) (Fig. 5e and 5f). Eosinophils were increased in the lungs at 8 dpi in both E3- and placebo-treated females compared with baseline (0 dpi) and remained elevated at 14 dpi in placebo-treated females but not in E3-treated females (Fig. 5g). Pulmonary monocytes and macrophages represented the largest fraction of CD11b+ cells both prior to and during IAV infection (Fig. 5e), and cell frequencies were significantly lower in E3-treated compared with placebo-treated females at both 5 and 8 dpi (Fig. 5h).
Figure 5.
E3 alters the baseline pulmonary immune cell profile and reduces immune cell recruitment during influenza. Female C57BL/6 mice received a subcutaneous implant containing either 5 mg E3 or a placebo (cholesterol) control, followed by infection with a sublethal dose of IAV or vehicle (mock). At select dpi, mice were euthanized and pulmonary immune cells were profiled using flow cytometry (n = 5 to 10 animals per treatment per timepoint). CD11b and CD3 surface markers were used to quantify (a) total myeloid and (b) T-cell populations, respectively, as well as their percentages within (c) total CD45+ leukocytes. Additional surface markers were used to further stratify CD11b+ myeloid cells into neutrophils, eosinophils, and monocyte/macrophage lineages, with (d) a sample gating schematic shown. The (e) percentages and the total numbers of (f) neutrophils, (g) eosinophils, and (h) monocyte/macrophages were quantified based on total CD45+ cells over the course of infection. All data were analyzed using two-way ANOVAs with Bonferroni post hoc correction, with *P < 0.05 between treatment groups and where #P < 0.05 within a treatment compared with baseline.
Polarization of adaptive immune responses following IAV infection can be a significant determinant of disease pathogenesis and recovery (40, 41). To determine how E3 treatment and the associated inflammatory gene profile modified the local adaptive immune responses, pulmonary T cells from both placebo-treated and E3-treated females at 8 dpi [i.e., during peak disease (Fig. 1b) and peak CD3+ T-cell recruitment (Fig. 5b)] were further characterized following ex vivo stimulation with influenza peptide. Stratification of CD3+ T cells into CD4+ and CD8+ compartments at 8 dpi revealed significantly reduced total numbers of CD8+ T cells but similar total numbers of CD4+ T cells, which contributed to an increased CD4/CD8 T-cell ratio in E3-treated compared with placebo-treated females (Fig. 6a and 6b). Correspondingly, influenza-specific (NP+) CD8+ T cells were also reduced in E3-treated compared with placebo-treated females at 8 dpi (Fig. 6c).
Figure 6.
E3 alters the pulmonary T-cell profile and polarizes T-cell responses during influenza. Female C57BL/6 mice received a subcutaneous implant containing either 5 mg E3 or a placebo (cholesterol) control, followed by infection with a sublethal dose of IAV or vehicle (mock). At 8 dpi, mice were euthanized and pulmonary T cells were profiled using flow cytometry following ex vivo stimulation with IAV peptide (n = 5 to 10 animals per treatment). The (a) total numbers and (b) ratio of CD4+ and CD8+ T cells, as well as (c) the total numbers of tetramer (NP)+ CD8+ T cells, were quantified from the lungs of placebo- and E3-treated females. Pulmonary CD4+ T cells that were stimulated ex vivo with IAV peptide were phenotyped by intracellular staining for cytokines and transcription factors, where the production of IFNγ, IL-4, and FoxP3 identified T helper type 1 (Th1), Th2, and Treg populations, respectively. The (d) percentages and (e) ratios were quantified from total CD4+ cells. At 14 dpi, serum was collected for measurement of total (f) anti-IAV IgG and (g) neutralizing antibody responses by ELISA and microneutralization assays, respectively (n = 4 to 5 animals per treatment). The serum titers for anti-IAV IgG subtypes, (h) IgG2a/c and (i) IgG1, were also measured by ELISA, and (j) the ratios of IgG2a/c/IgG1 are shown. All data were analyzed using Student t tests, with *P < 0.05.
In the context of EAE, treatment with estrogens has been associated with polarization of T-cell responses toward Th2 (42) and T regulatory (Treg) (43) phenotypes, which corresponds with reduced disease severity. Following IAV infection, E3 treatment resulted in a similar skewing of CD4+ T cells, and both the percentage (Fig. 6d) and ratio (Fig. 6e) of Th2 and Treg compared with proinflammatory T helper type 1 cells in the lungs at 8 dpi were increased. The reduced numbers of CD8+ T cells in the lungs of E3-treated females mirrored the systemic humoral responses, where E3 treatment similarly reduced both total anti-2009 H1N1 IgG and neutralizing antibody titers (Fig. 6f and 6g). Evaluation of anti-2009 H1N1 IgG isotypes, however, revealed a proportionate reduction of both IgG1 and IgG2a/c titers, with an equal ratio of IgG1/IgG2a/c in both placebo- and E3-treated females (Fig. 2h–2j), which suggests that skewing of adaptive immune responses in response to E3 treatment may be tissue specific.
Discussion
The host response to IAV infection is based on the timing, magnitude, and character of the inflammatory response. Although innate antiviral and virus-specific adaptive immune responses are required for IAV recognition and clearance, excessive or persistent inflammatory responses can exacerbate disease indirectly through inflammation-mediated tissue damage (15, 44, 45). In uncomplicated cases of influenza, disease severity is most closely related to the early cytokine release, termed the “cytokine storm,” rather than tissue viral burden (46, 47). Concordant with this, novel therapeutic strategies for combatting severe influenza are focused on the development of compounds that decrease pulmonary inflammation without interfering with virus control and clearance (48). In the current study, we demonstrated that E3 treatment of both female and male mice conferred significant protection during infection with ma2009 H1N1 IAV. Clinical protection in females was characterized by early mitigation of pulmonary inflammation and corresponding abatement of systemic disease. Furthermore, E3-mediated clinical protection was associated with broad reductions in the induction of transcriptional activity of genes coding for cytokines and chemokines in both hematopoietic and nonhematopoietic cells in the lungs. Moreover, these transcriptional changes corresponded with reduced pulmonary recruitment of both innate and adaptive immune cells, as well as a skewing of pulmonary CD4+ T-cell responses toward Th2 and Treg phenotypes. The reduced inflammatory responses associated with E3 treatment, however, were not associated with differences in the kinetics of viral replication, suggesting that sufficient pulmonary immune responses were maintained to clear the infection.
In both humans and mice, different types of estrogens bind with different affinities to ERs, which underlie their variable downstream effects. Estrogenic activity within a given tissue or cell type is complex and depends on the type and concentration of estrogen, the type of receptor, and the presence of regulatory proteins, including coactivators and corepressors that bind the ER complex. Compared with E2, E3 is a relatively weak estrogen, with higher binding affinity for ERβ than ERα. Unlike other types of estrogens, E3 cannot be converted into E2 (49), and therefore, the effects of exogenous E3 treatment can be attributed to the effects of E3 alone. Treatment of female mice with either E2 or E3 revealed that E3 treatment alone conferred the greatest protection against IAV-associated disease. This finding corroborates those from similar studies of the role of estrogens in protecting against EAE, where E3 is more protective than E2 in female mice (10). The observation that E3 treatment alone was still more protective than combined E2 + E3 treatment suggests that E2 may even inhibit the effects of E3 through competitive receptor binding (50) or cross-modulation of ER complexes (51). It is also possible that the phenotype observed following treatment with E2 and E2 + E3 was the result of differences in dose, as biphasic dose effects of E2 and E3 have been reported in the context of human T-cell responses (52). Thus, high doses of estrogens in mice may similarly oppose some of the protective effects observed at lower doses. E3 also conferred protective effects in males during IAV infection, suggesting that because males also express ERs, they can respond similarly to exogenous estrogen treatment. This is consistent with reports of exogenous estrogen treatment mediating protection against EAE in male mice (10, 12) and suggests that the protective effects of E3 in the lungs are independent of biological sex.
In the EAE mouse model, estrogens, including E2 and E3, confer clinical protection by reducing inflammation of the CNS. Specifically, estrogen treatment decreases TNF-α and chemokine ligands and receptors both locally and systemically to reduce immune cell recruitment to the CNS (11, 53). In the current study, E3 administered during IAV infection reduced pulmonary cytokine and chemokine induction prior to the onset of clinical disease, suggesting that the corresponding reduction in pulmonary inflammation in E3-treated females may be due to reduced immune cell recruitment rather than increased resolution of pulmonary inflammation. One important question that remains is if and how the timing and kinetics of E3 dosing may influence the inflammatory response to IAV. In the present studies, E3 administration corresponded with infection; however, this is unlikely to be the case in a clinical setting. Instead, patients are more likely to have been exposed to E3 as a component of HRT or other therapy prior to IAV infection. Our pulmonary immunophenotyping data from uninfected animals show that E3 alone can alter the pulmonary immune profile, which suggests that individuals receiving long-term E3 therapy may be “primed” to respond differently to certain respiratory insults. Additional studies are, therefore, warranted to determine how E3 pretreatment or dynamic E3 dosing kinetics may differentially affect response to IAV infection. In the same vein, studies to determine how quickly following IAV infection E3 needs to be administered to confer clinical protection will be informative for evaluating E3 as a candidate IAV therapeutic.
Similar to other estrogens, E3 can influence gene transcription through both direct genomic and indirect nongenomic mechanisms. The direct effects of estrogens are dependent on the presence of specific sequences in gene promoters, termed “estrogen-responsive elements” (EREs). Binding of ER complexes on EREs can either induce or repress transcription, based on the presence of other transcription factors and other upstream regulatory proteins. One important inflammatory mediator that is known to contain EREs in its promoter is TNF, and in human monocytic U937 cells, genomic binding of the E2-ER complex can either activate or repress TNF transcription, depending on the presence of other regulatory proteins (54). In the context of IAV, TNF is produced early during infection and is central to the influenza cytokine storm. In both humans (15, 55) and mice (56, 57), TNF has been associated with heightened disease severity following infection with highly pathogenic IAVs. Similar to humans, Tnf was induced during early IAV infection in female mice, and this induction was significantly blunted by treatment with E3. This corroborates evidence from similar studies showing that E2 reduced TNF protein during both IAV infection (22, 23) and carrageenan-induced lung inflammation (58) in mice. Genomic control of early Tnf induction by E3 may, therefore, be an important mechanism for protecting against severe IAV disease. In addition to Tnf, we identified 32 other inflammatory genes that were broadly upregulated during acute IAV infection and differentially regulated by E3 treatment. Unlike Tnf, however, these genes have not been reported to contain EREs in their promoters, and differential expression of these factors was more likely to have been mediated through nongenomic mechanisms. Studies using human peripheral blood mononuclear cells showed that E3, to a greater degree than E2, regulated production of proinflammatory cytokines, including TNF, IFNγ, and IL-10, through inhibition of the nuclear transcription factor, nuclear factor κB (59). Collectively, these data suggest that the activity of E3 in the lungs is mediated through both direct and indirect mechanisms to reduce cytokine and chemokine induction following IAV infection. Future studies in vivo should be aimed at differentiating the effects of E3 from E2 and combined estrogen treatment on the pulmonary transcriptome.
Based on our transcriptional analysis, the effects of E3 on gene expression in whole lung following IAV infection appeared to be broadly anti-inflammatory, with E3 treatment significantly attenuating the induction of 33 of the 36 genes that were upregulated in placebo-treated females at 5 dpi. These included the genes encoding for IL-6, CCL2, CCL4, CXCL-9, and CXCL-10, which are associated with pathogenicity and mortality in humans infected with H1N1 IAV (60). Pulmonary inflammation following IAV infection is initiated by both hematopoietic and nonhematopoietic resident cell populations and then further propagated and shaped by the recruited immune cells. Each cell population in the lung has a variable expression pattern of ERs and thus a different response to IAV infection; although immune cells are reported to express and respond to estrogens primarily through ERα (7), pulmonary epithelial and endothelial cells signal primarily through ERβ (61, 62). Owing to this variability, along with the complexities of ER signaling within a cell, different cell populations within the lungs are likely to respond to E3 treatment in diverse ways. In the current study, the transcriptional profiles of hematopoietic and nonhematopoietic cells in the lungs during IAV infection were different, with hematopoietic cells being overall greater contributors to proinflammatory cytokine and chemokine induction. Moreover, although E3l affected gene expression in both hematopoietic and nonhematopoietic cell populations, it had different and sometimes opposing effects with respect to certain genes. For example, E3 reduced the expression of Cxcl9 in hematopoietic but not in nonhematopoietc cells, and E3 reduced the expression of Cxcl11 in nonhematopoietic cells but not in hematopoietic cells. The significance of this dichotomous gene regulation in the context of IAV infection is unclear; the CXCL9, CXCL10, CXCL11 axis, however, is important for selective T-cell recruitment and shaping the balance of CD4+ T-cell subsets in the context of autoimmune disease (63). Additional mechanistic studies will be useful for determining if similar mechanisms mediate T-cell skewing in the lungs during IAV infection.
Despite global dampening of the inflammatory transcriptome in whole lung, E3 mediated selective induction of certain genes, including Cxcl1 in nonhematopoietic cells. CXCL1 is important for chemotaxis of neutrophils, and its heightened expression in E3-treated females may underlie the greater numbers of pulmonary neutrophils observed in these animals at baseline. Although the role of neutrophils during IAV infection is controversial, recent studies have identified a subset of neutrophils that are responsible for T-cell suppression and can reduce influenza disease in mice (64). Furthermore, early recruitment of neutrophils to the lungs following IAV infection in mice can be protective (65) and is partially mediated through estrogen signaling (22). Although it is known that neutrophils express both ERα and ERβ (66), how E3 may alter the phenotype and function of pulmonary neutrophils has not been reported.
In hematopoietic cells, E3 upregulated the expression of Ccl22, which is an important factor for selective T-cell homing, including Th2 and Treg subsets (67, 68). Consistent with this, upregulation of Ccl22 in E3-treated females preceded the increase in proportions of Th2 and Treg cells in the lungs, which is congruent with the reported effects of estrogen-mediated induction of CCL22 at other mucosal sites (69). Together, our data demonstrate variable activity of E3 across different cell lineages that collectively contribute to the inflammatory milieu in the lungs. Future studies should be aimed at determining how differential receptor expression between different cell populations influences the observed downstream effects.
One important phenomenon that remains unexplained by the data presented in this study is the association between E3, pregnancy, and IAV pathogenesis. E3 is a placenta-derived estrogen that achieves its highest concentrations during late pregnancy. Pregnant women, however, experience overall greater influenza disease severity compared with the general population, and it is estimated that women in their third trimester are three to seven times more likely to be hospitalized and twice as likely to die of IAV-associated disease (70, 71). It has been hypothesized that high circulating estrogens during pregnancy mediate Th2 and Treg immune biases that are detrimental during viral infection (72), resulting in impaired or delayed viral clearance. Several groups have tested this hypothesis in mice treated with exogenous E2 (73, 74), but the results have been conflicting and inconclusive. Although these studies have confirmed that E2 modulates the local and systemic immune response, they have not definitively linked this immune modulation with heightened influenza disease, and most reports (22, 23, 73) have instead shown that E2 and the associated immune modulation are protective during influenza disease. To our knowledge, the current study is the first to characterize the effect of E3 on the pathogenesis of IAV infection, which corroborates related studies using E2. Although translation of observations in hormone-treated mice to pregnant women is complicated by species differences in the concentration and kinetic profile of estrogens during pregnancy, collectively, the literature suggests that estrogen-mediated immune modulation does not contribute to heightened IAV disease severity. It is also possible that other reproductive hormones (e.g., progesterone) that are similarly elevated during late pregnancy may mask or antagonize the protective effects of estrogens. Furthermore, other physiologic changes during pregnancy, including cardiopulmonary and metabolic changes, may also contribute to severe IAV disease. Moreover, other factors that influence IAV pathogenesis, including virus strain, preexisting immunity, or secondary bacterial infections, may have an impact on the effect of estrogens and should be considered in future studies.
As the roles of estrogens in modulating immune response have been characterized in the context of several different infectious and noninfectious inflammatory diseases, it is becoming clear that the activities of estrogens underlie some of the differences in disease incidence and severity between males and females. The mechanisms of various estrogens on disease pathogenesis have proven multifactorial and tissue dependent, but the predominant effects are mediated through immunomodulatory mechanisms. Targeting of estrogen signaling pathways has therefore gained traction as a promising therapeutic avenue for treating autoimmune inflammatory disease, for which estrogens and selective ER modulators are currently being developed and tested as investigational new drugs (14, 75). Here we have described a comprehensive study of the effects of exogenous E3 in the respiratory tract during IAV infection and demonstrate a therapeutic role for E3 in mitigating virus-induced immunopathology in the lungs that is mediated through similar mechanisms as those described for other inflammatory disease processes. Despite significant immune modulation, however, E3 did not interfere with viral control and clearance, which is important for individuals currently receiving E3 through HRT or otherwise. Together, these data suggest that E3 may have broad therapeutic potential in the context of both infectious and noninfectious inflammatory disease.
Supplementary Material
Acknowledgments
We thank the members of the Klein, Davis, and Pekosz laboratories for detailed discussions about these data. We thank Cory Brayton for assistance with the development of the pulmonary histopathology scoring system, Erin Shirk for assistance with the flow cytometry, Hao Zhang for assistance with the cell-sorting experiments, Anne Jedlicka for assistance with the PCR arrays, and Alan Scott for providing valuable immunology expertise and helping with interpretation of the data.
Financial Support: This work was supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases Center of Excellence in Influenza Research and Surveillance contract HHS N272201400007C (to S.L.K.), the NIH T32 CA009110 Training in Areas Fundamental to Cancer Research (to R.L.U.), the NIH 5T32 HL007534 Multidisciplinary Training Program in Lung Disease (to S.E.A.), and the NIH T32 OD011089 Grant for Training Veterinarians for Careers in Biomedical Research (to M.S.V.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CNS
central nervous system
- CT
cycle threshold
- dpi
days postinoculation
- E2
estradiol
- E3
estriol
- EAE
experimental autoimmune encephalomyelitis
- ER
estrogen receptor
- ERE
estrogen-responsive element
- HRP
horseradish peroxidase
- HRT
hormone replacement therapy
- IAV
influenza A virus
- IFNγ
interferon γ
- MS
multiple sclerosis
- RA
rheumatoid arthritis
- TCID50
tissue culture infectious dose 50
- Th2
T helper type 2
- Treg
T regulatory
References
- 1. Perkins MS, Louw-du Toit R, Africander D. A comparative characterization of estrogens used in hormone therapy via estrogen receptor (ER)-α and -β. J Steroid Biochem Mol Biol. 2017;174:27–39. [DOI] [PubMed] [Google Scholar]
- 2. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 3. Lemon HM. Clinical and experimental aspects of the anti-mammary carinogenic activity of estriol. Front Horm Res. 1977;5:155–173. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi K, Okada M, Ozaki T, Kurioka H, Manabe A, Kanasaki H, Miyazaki K. Safety and efficacy of oestriol for symptoms of natural or surgically induced menopause. Hum Reprod. 2000;15(5):1028–1036. [DOI] [PubMed] [Google Scholar]
- 5. Delgado JL, Estevez J, Radicioni M, Loprete L, Moscoso Del Prado J, Nieto Magro C. Pharmacokinetics and preliminary efficacy of two vaginal gel formulations of ultra-low-dose estriol in postmenopausal women. Climacteric. 2015;19(2):172–180. [DOI] [PubMed] [Google Scholar]
- 6. Sites CK. Bioidentical hormones for menopausal therapy. Womens Health (Lond). 2008;4(2):163–171. [DOI] [PubMed] [Google Scholar]
- 7. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 9. Holmdahl R, Jansson L, Meyerson B, Klareskog L. Oestrogen induced suppression of collagen arthritis: I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin Exp Immunol. 1987;70(2):372–378. [PMC free article] [PubMed] [Google Scholar]
- 10. Bebo BF Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166(3):2080–2089. [DOI] [PubMed] [Google Scholar]
- 11. Ito A, Bebo BF Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167(1):542–552. [DOI] [PubMed] [Google Scholar]
- 12. Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;149(1–2):84–89. [DOI] [PubMed] [Google Scholar]
- 13. Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, Shawler T, Whitacre CC. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J Immunol. 2011;186(6):3346–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, Itoh N, Bardens J, Bernard JT, Corboy JR, Cross AH, Dhib-Jalbut S, Ford CC, Frohman EM, Giesser B, Jacobs D, Kasper LH, Lynch S, Parry G, Racke MK, Reder AT, Rose J, Wingerchuk DM, MacKenzie-Graham AJ, Arnold DL, Tseng CH, Elashoff R. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(1):35–46. [DOI] [PubMed] [Google Scholar]
- 15. Peiris JS, Hui KP, Yen HL. Host response to influenza virus: protection versus immunopathology. Curr Opin Immunol. 2010;22(4):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cloutier A, Marois I, Cloutier D, Verreault C, Cantin AM, Richter MV. The prostanoid 15-deoxy-Δ12,14-prostaglandin-j2 reduces lung inflammation and protects mice against lethal influenza infection. J Infect Dis. 2012;205(4):621–630. [DOI] [PubMed] [Google Scholar]
- 17. Walsh KB, Teijaro JR, Wilker PR, Jatzek A, Fremgen DM, Das SC, Watanabe T, Hatta M, Shinya K, Suresh M, Kawaoka Y, Rosen H, Oldstone MB. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci USA. 2011;108(29):12018–12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26(2):102–107. [DOI] [PubMed] [Google Scholar]
- 19. Blackmore S, Hernandez J, Juda M, Ryder E, Freund GG, Johnson RW, Steelman AJ. Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2017;114(30):E6107–E6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Q, Liu Y, Lu A, Ni K, Xiang Z, Wen K, Tu W. Influenza virus infection exacerbates experimental autoimmune encephalomyelitis disease by promoting type I T cells infiltration into central nervous system. J Autoimmun. 2017;77:1–10. [DOI] [PubMed] [Google Scholar]
- 21. Glenn JD, Smith MD, Xue P, Chan-Li Y, Collins S, Calabresi PA, Horton MR, Whartenby KA. CNS-targeted autoimmunity leads to increased influenza mortality in mice. J Exp Med. 2017;214(2):297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17β-Estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88(9):4711–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7(7):e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hale BG, Steel J, Manicassamy B, Medina RA, Ye J, Hickman D, Lowen AC, Perez DR, García-Sastre A. Mutations in the NS1 C-terminal tail do not enhance replication or virulence of the 2009 pandemic H1N1 influenza A virus. J Gen Virol. 2010;91(7):1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol. 2016;311(6):L1234–L1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016;12(9):e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gereke M, Autengruber A, Gröbe L, Jeron A, Bruder D, Stegemann-Koniszewski S. Flow cytometric isolation of primary murine type II alveolar epithelial cells for functional and molecular studies. J Vis Exp. 2012;(70):4322. [DOI] [PMC free article] [PubMed]
- 28.RRID:AB_469625.
- 29.RRID:AB_394656.
- 30.RRID:AB_10597589.
- 31.RRID:AB_2534300.
- 32.RRID:AB_494005.
- 33.RRID:AB_953560.
- 34.RRID:AB_469345.
- 35.RRID:AB_465313.
- 36.RRID:AB_1834367.
- 37.RRID:AB_466154.
- 38.RRID:AB_1645251.
- 39. Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180(4):1273–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180(3):579–585. [DOI] [PubMed] [Google Scholar]
- 42. Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70(2):238–248. [DOI] [PubMed] [Google Scholar]
- 43. Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170(1–2):85–92. [DOI] [PubMed] [Google Scholar]
- 44. Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teijaro JR. The role of cytokine responses during influenza virus pathogenesis and potential therapeutic options. Curr Top Microbiol Immunol. 2015;386:3–22. [DOI] [PubMed] [Google Scholar]
- 46. Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, García-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci USA. 2009;106(9):3455–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cillóniz C, Shinya K, Peng X, Korth MJ, Proll SC, Aicher LD, Carter VS, Chang JH, Kobasa D, Feldmann F, Strong JE, Feldmann H, Kawaoka Y, Katze MG. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5(10):e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2015;13(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tofovic SP. Estrogens and development of pulmonary hypertension: interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol. 2010;56(6):696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brecher PI, Wotiz HH. Competition between estradiol and estriol for end organ receptor proteins. Steroids. 1967;9(4):431–442. [DOI] [PubMed] [Google Scholar]
- 51. Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci USA. 2000;97(11):5936–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161(7):3365–3374. [PubMed] [Google Scholar]
- 53. Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65(6):529–542. [DOI] [PubMed] [Google Scholar]
- 54. An J, Ribeiro RC, Webb P, Gustafsson JA, Kushner PJ, Baxter JD, Leitman DC. Estradiol repression of tumor necrosis factor–alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc Natl Acad Sci USA. 1999;96(26):15161–15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perrone LA, Szretter KJ, Katz JM, Mizgerd JP, Tumpey TM. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J Infect Dis. 2010;202(8):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2006;81(6):2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vegeto E, Cuzzocrea S, Crisafulli C, Mazzon E, Sala A, Krust A, Maggi A. Estrogen receptor-alpha as a drug target candidate for preventing lung inflammation. Endocrinology. 2010;151(1):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zang YC, Halder JB, Hong J, Rivera VM, Zhang JZ. Regulatory effects of estriol on T cell migration and cytokine profile: inhibition of transcription factor NF-kappa B. J Neuroimmunol. 2002;124(1-2):106–114. [DOI] [PubMed] [Google Scholar]
- 60. Betakova T, Kostrabova A, Lachova V, Turianova L. Cytokines induced during influenza virus infection. Curr Pharm Des. 2017;23(18):2616–2622. [DOI] [PubMed] [Google Scholar]
- 61. Ivanova MM, Mazhawidza W, Dougherty SM, Minna JD, Klinge CM. Activity and intracellular location of estrogen receptors alpha and beta in human bronchial epithelial cells. Mol Cell Endocrinol. 2009;305(1–2):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, Chaudry IH. Salutary effects of estrogen receptor–beta agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L1004–L1009. [DOI] [PubMed] [Google Scholar]
- 63. Karin N, Wildbaum G. The role of chemokines in shaping the balance between CD4(+) T cell subsets and its therapeutic implications in autoimmune and cancer diseases. Front Immunol. 2015;6:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tak T, Rygiel TP, Karnam G, Bastian OW, Boon L, Viveen M, Coenjaerts FE, Meyaard L, Koenderman L, Pillay J. Neutrophil-mediated suppression of influenza-induced pathology requires CD11b/CD18 (MAC-1). Am J Respir Cell Mol Biol. 2018;58(4):492–499. [DOI] [PubMed] [Google Scholar]
- 65. Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183(11):7441–7450. [DOI] [PubMed] [Google Scholar]
- 66. Molero L, García-Durán M, Diaz-Recasens J, Rico L, Casado S, López-Farré A. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: regulation by estrogen. Cardiovasc Res. 2002;56(1):43–51. [DOI] [PubMed] [Google Scholar]
- 67. Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194(6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64(7):995–1002. [DOI] [PubMed] [Google Scholar]
- 69. Goode D, Aravantinou M, Jarl S, Truong R, Derby N, Guerra-Perez N, Kenney J, Blanchard J, Gettie A, Robbiani M, Martinelli E. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PLoS One. 2014;9(5):e97767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ; Novel Influenza A (H1N1) Pregnancy Working Group . H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–458. [DOI] [PubMed] [Google Scholar]
- 71. Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–1102. [DOI] [PubMed] [Google Scholar]
- 72. Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Davis SM, Sweet LM, Oppenheimer KH, Suratt BT, Phillippe M. Estradiol and progesterone influence on influenza infection and immune response in a mouse model. Am J Reprod Immunol. 2017;78(4):e12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pazos MA, Kraus TA, Muñoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS One. 2012;7(7):e40502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Itoh N, Kim R, Peng M, DiFilippo E, Johnsonbaugh H, MacKenzie-Graham A, Voskuhl RR. Bedside to bench to bedside research: estrogen receptor beta ligand as a candidate neuroprotective treatment for multiple sclerosis. J Neuroimmunol. 2017;304:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.