Abstract
Background
Impulsivity is a core deficit in attention deficit hyperactivity disorder (ADHD). Transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) has been shown to modulate cognitive control circuits and could enhance DLPFC activity, leading to improved impulse control in ADHD.
Objective/Hypothesis
We predicted 2.0 mA anodal stimulation (tDCS) versus sham stimulation applied over the left DLPFC would improve Conners Continuous Performance Task (CPT) scores. Our secondary hypothesis predicted that stop signal task (SST) reaction time would decrease with tDCS (versus sham).
Methods
Thirty-seven participants completed two periods of three tDCS (or sham) sessions two weeks apart in a within-subject, double-blind, counterbalanced order. Participants performed a fractal N-back training task concurrent with tDCS (or sham) stimulation. Participants completed the CPT and SST at the beginning of treatment (baseline), at the end of the treatment, and at a 3-day post-stimulation follow-up.
Results
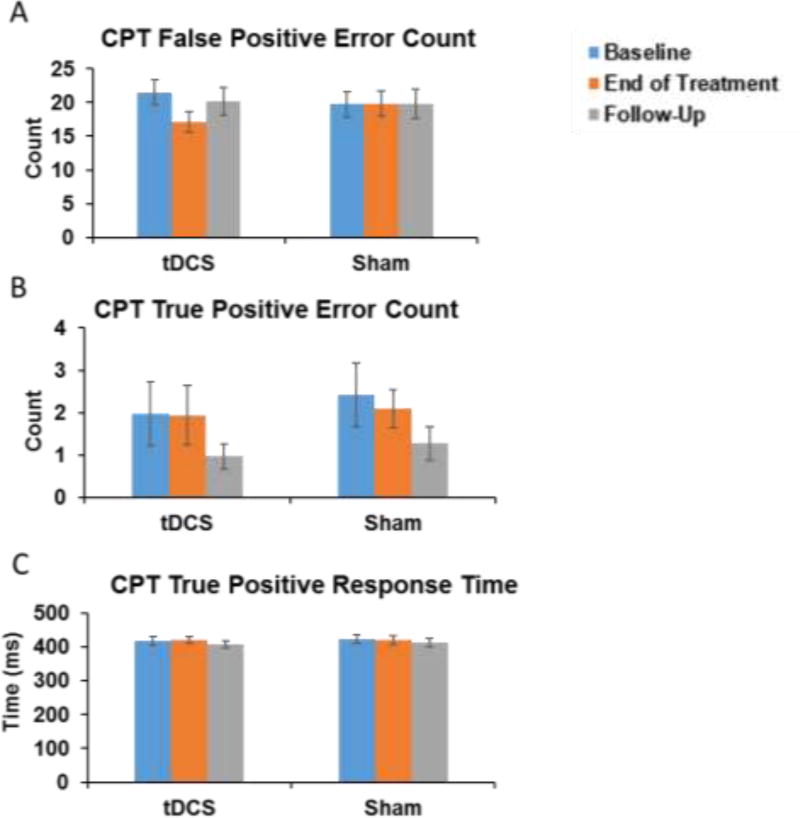
There was a significant stimulation condition by session interaction for CPT false positive scores (χ2 =15.44, p<0.001) driven by a decrease in false positive errors from baseline to end of treatment in the tDCS group (β=−0.36, 95% Confidence Interval (CI) −0.54 to −0.18, p<0.001). This effect did not persist at follow-up (β=−0.13, p>0.05). There was no significant stimulation condition by session interaction effect on CPT true positive errors or response time (ps>0.05). No significant change in SSRT performance was observed (p>0.05).
Conclusion
These findings suggest that stimulation of the left DLPFC with tDCS can improve impulsivity symptoms in ADHD, supporting the therapeutic potential for tDCS in adult ADHD patients.
Keywords: Attention deficit hyperactivity disorder, tDCS, impulsivity, dorsolateral prefrontal cortex, continuous performance task
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a disease characterized by symptoms of impulsivity, inattention, and hyperactivity that emerge in childhood. In up to 60% of cases, these symptoms persist into adulthood and can lead to poorer life outcomes in areas such as employment and interpersonal relationships [1]. Current pharmacological treatments include stimulants such as methylphenidate and amphetamine, and non-stimulant medications such as atomoxetine [2]. These medications can significantly improve ADHD symptoms and life outcomes. For example, in adults with ADHD, pharmacologic treatment for more than two years is associated with improved ADHD symptoms and mental health functioning compared to treatment for two years or less [3]. There is substantial variation in response; dosages must be individually titrated to minimize adverse effects while maintaining efficacy [2] and for more than 50% of adult ADHD patients pharmacotherapy alone is not sufficient treatment [4]. In addition, the long-term risk/benefit profile of these treatments is uncertain. There remains a need for novel treatments for adult ADHD.
Neuroimaging studies in healthy subjects and ADHD subjects have linked cognitive deficits and impulsive decision-making with reduced activity in brain regions sub-serving the cognitive control network [5–8]. A meta-analysis of 55 whole-brain fMRI studies showed significant hypoactivation in ADHD patients relative to controls in bilateral attention networks, including the dorsolateral prefrontal cortex [9]. When performing a response inhibition task, adolescent ADHD patients demonstrated reduced activation in the DLPFC compared to healthy controls [10]. Because cognitive control networks rely heavily on prefrontal cortex function, impairment in these regions can promote impulsivity, a core symptom of ADHD [11]. These deficits can be particularly debilitating for adults diagnosed with ADHD, as they are associated with poor occupational outcomes and difficulty in maintaining relationships [12–14]. Impulsivity in adult ADHD patients can be evaluated using computerized measures such as the Conners Continuous Performance Test (CPT) [15] and Stop Signal Task [16]. Continuous performance tasks are the leading assessment of ADHD symptomology in ADHD research, and the Conners CPT is considered the gold standard of CPTs [17, 18]. CPT outcome measures are associated with ADHD symptomology: false positive errors (i.e., response to a non-target stimulus) are associated with impulsivity, while true positive errors (i.e., non-response to a target stimulus) are associated with inattention [19–21]. ADHD patients make more false positive errors than healthy adults, and these errors are sensitive to the effects of stimulant treatment in ADHD patients [22–25]. Furthermore, performance on this task is sensitive to effects of methylphenidate, an efficacious ADHD treatment [24–27]. Stimulant medications such as methylphenidate decrease false positive error rates following three weeks of treatment, with a medium-to-large effect size (η2=.21) [26]. The Stop Signal Task, which measures an individual’s ability to inhibit a proponent response, is another computerized task which has been used to assess ADHD symptoms. Stop-signal reaction time (SSRT) is longer in patients who endorse more symptoms of impulsivity, and this measure effectively discriminates ADHD patients from healthy control patients [16, 22, 28].
Emerging evidence suggests that activity in cognitive control circuits can be modulated using noninvasive direct current transcranial stimulation (tDCS) [29–31]. TDCS treatment consists of a weak electric current (1–2 mA) applied to the scalp through conductive electrodes [32]. A single session of tDCS targeting the left DLPFC has been shown to improve memory, planning ability, inhibitory control, and neural efficiency during cognitive processing with minimal side effects [33–35]. Some findings suggest that performance improvements may be related to current density; studies utilizing a 1mA dose have shown mixed results in an ADHD population [36, 37], whereas a higher dosage (i.e., 2mA compared to 1mA or sham tDCS) has been shown to improve cognitive performance in both healthy samples and neuropsychiatric populations [31, 38, 39]. Furthermore, concurrent performance of a challenging task to engage the targeted control circuits may offer synergistic effects on tDCS-induced neuroplastic changes, promoting greater functional connectivity between large-scale brain networks and improved neural efficiency resulting in improved performance on objective measures of cognitive control [40–43]. The fractal N-back is a working memory task which has been shown to robustly activate the DLPFC, and co-administration of this task with tDCS results in greater DLPFC activation than when the task is performed alone [11, 29, 30, 44, 45]. Finally, multiple tDCS sessions with concurrent cognitive training may provide greater benefits than a single session [26, 46].
Although many studies have reported positive results for cognitive enhancement with tDCS, studies investigating tDCS treatment specifically for ADHD are limited. In a study of adolescent ADHD patients, tDCS over the left DLPFC with a concurrent N-back task revealed that active stimulation (compared to sham) led to greater activation of the working memory network, including the left DLPFC [45]. A second study of adolescents found that 5 days of anodal tDCS over the left DLPFC caused a significant reduction in inattention and impulsivity at end of treatment and 7 days post stimulation [47]. In adults, anodal tDCS to the right DLPFC resulted in improved inattention scores [48] and anodal tDCS over the inferior frontal gyrus reported that tDCS treatment reduced false positive errors on an interference task in male adolescents with ADHD [37]. However, tDCS applied over the left DLPFC in adults with ADHD did not reveal significant differences on a go/no go task following one stimulation session [36].
Based on the rationale above, we hypothesized that modulating activation in the cognitive control network using tDCS with a concurrent training task would transfer to improved performance on objective measures of cognitive control and impulsivity. We conducted a within-subject crossover study to examine whether three sessions of anodal 2mA tDCS applied over the left DLPFC during working memory training (versus working memory training with sham stimulation) would attenuate the cognitive symptoms of ADHD in adults. We predicted 2.0 mA anodal tDCS (versus sham) applied over the left DLPFC would improve Conners Continuous Performance Task (CPT) scores (false positive errors, true positive errors, and true positive response time). Our secondary hypothesis predicted that stop signal task (SST) reaction time, a measure of response inhibition, would decrease with tDCS (versus sham).
Materials and Methods
Participants
Adults between the ages of 18 and 65 with a prior diagnosis of ADHD were identified through referrals from the University of Pennsylvania’s Adult ADHD Treatment & Research Program or recruited by mass media. ADHD diagnosis and comorbid medical conditions were assessed by an experienced clinician using a brief medical history interview and the Structured Clinical Interview for DSM-V (SCID-V; [49]). Individuals who met criteria for DSM-V Axis I psychiatric (schizophrenia, mania, bipolar disorder, and major depression) or substance disorders (except nicotine dependence) on the SCID-V and those taking psychotropic medications (other than stimulant medications for ADHD) were excluded. Participants with a history of major depression who had been in remission for the past 6 months were considered eligible. Participants who reported taking daily stimulant medication for the treatment of ADHD were asked to continue their prescribed regime for the duration of the study. Exclusion criteria included neurological conditions including history of epilepsy, seizure disorder, stroke, and tumors of the brain or spinal cord. Additional exclusion criteria were: pregnancy, planned pregnancy or breastfeeding; tDCS application contraindication (e.g. metallic implants in the head or history of seizure); estimated IQ <90 on Shipley Institute of Living Scale [50]; and any vision impairment or other disability that would prevent task performance.
Participants were assigned to a treatment order (tDCS first versus sham first) using a simple randomization with replacement. Prior to each session, participants completed a urine drug screen, pregnancy screen (women only), and provided exhaled carbon monoxide (smokers only) and breath alcohol content measures. All participants provided consent. All procedures were approved by the University of Pennsylvania Institutional Review Board and carried out in accordance with the Declaration of Helsinki.
Thirty-seven participants completed both study periods and thirty-five participants attended all sessions. The sample was predominantly male (n=26, 70.1%), and white (n=29, 78.4%). Approximately half the sample completed high school or some college (n=18, 48.6%). The mean age was 31.7 years old. At intake, 17 participants reported taking stimulant medication to treat ADHD. Twenty-one participants were of the primarily inattentive ADHD subtype; 16 participants were combined (inattentive and hyperactive) subtype. There were no significant differences in performance between ADHD subtypes. There were no differences in age, gender, or education level between participants on and off medication.
Overview of procedures
This study utilized a within-subject, cross-over design consisting of two treatment periods: active 2mA tDCS and sham. Periods were separated by a two-week washout and period order was randomized, double-blind and counterbalanced [32, 51]. During each period, participants attended four visits: three stimulation visits on days one, three, and five, and a follow-up visit on day eight. On days one, three, and five, participants received twenty minutes of stimulation (tDCS or sham) while concurrently performing a working memory training task (see below). Participants missing more than one treatment session were withdrawn (n = 4), leaving a final sample of 37.
tDCS Treatment
A neuroConn DC-Stimulator Plus delivered a constant direct current via two 5cm × 5cm electrodes covered in saline-soaked sponges. Electrode placement used the international 10–20 system developed for EEG [52]. The anodal electrode was placed at F3 for stimulation over the left DLPFC and the cathode was placed over the right supra-orbital area. Our montage choice was based on previously reported tDCS modulation of the DLPFC [30, 39, 44, 53–56] and results from a pilot feasibility study conducted in our lab (unpublished data). This montage allowed for effective blinding, ease of administration, and tolerable participant comfort. Stimulation with the neuroConn DC stimulator allows for double-blinding: a collection of five digit codes are assigned to each treatment condition, and the randomization procedure supplies the tDCS administrator with a code that can be input into the tDCS device. With this approach, neither the administrator nor the participant know which treatment condition is being applied. During the active condition, current was ramped up over 30 seconds until 2.0 mA was reached, maintained for 19 minutes and ramped down over 30s at the end of stimulation (total stimulation period 20 min). For the sham treatment session, current was ramped up over 30 seconds until 2mA was reached and then immediately ramped down over 30 seconds at the beginning and end of a 20 minute period to mimic the skin sensations experienced during tDCS [57].
Concurrent tDCS Task
While receiving tDCS (or sham), participants performed a visual working memory training task with complex geometric figures (fractals) [58, 59]. Participants viewed complex fractals under four conditions (0, 2, 3, and 4-back): in the 0-back condition, participants responded with a button press (dominant hand) to a specified target fractal; for the 2-back condition, participants responded if the current fractal was identical to the item presented two trials back; etc. Each condition was presented three times in 20-trial blocks (33% targets; 60s). Each fractal was presented for 500 ms, with a 2500 ms inter-stimulus interval. The task was synchronized with tDCS administration and began with a 3-minute baseline rest period to allow participants to become accustomed to the sensations produced by the stimulation.
Outcome Measures
The primary outcome measures were CPT false positive errors, true positive errors, and true positive response time. The secondary outcome measure was stop signal reaction time (SSRT).
Cognitive Assessment
Participants completed a computerized cognitive assessment battery at baseline, end of treatment, and at a follow-up session 3 days post-treatment. Tasks were administered in a fixed order that prioritized our primary outcome [60]. All tasks were presented on a standardized computer monitor. The timing of the cognitive battery relative to stimulation was different at each session: the cognitive assessment was performed prior to stimulation at the baseline session in each period, immediately following stimulation at the end of treatment sessions, and prior to the N-back task (without concurrent tDCS) at the follow-up session. Participants were seated approximately 50 inches from the monitor and responded to stimuli with their dominant hand by pressing labeled keys on a standard keyboard.
Conners Continuous Performance Task (CPT)
The Conners CPT (Multi-Health Systems, North Tonawanda, NY) is a well-validated attention task with excellent internal consistency for both normative and clinical groups and a median test-retest correlation of .67 [15]. In this task, participants are shown a series of stimuli (letters) on a computer screen and are asked to press the spacebar in response to target stimuli, but to withhold responding to other stimuli. The letters (approximately 1 inch in size) are presented one at a time and each letter is displayed for 250 ms. The task consists of 6 blocks with 60 trials each; each block contained three sub-blocks of 20 trials each. The sub-blocks differ in terms of interstimulus interval (1, 2 or 4s). Performance variables of interest are false positive errors (commission errors) and true positive errors (omission errors), as well as true positive response time. (Task duration: ~14 min).
Stop Signal Task (SST)
The SST is a measure of the ability to inhibit a prepotent response that involves two tasks: the “go task” and the “stop task” [16, 28]. The go task is a two-choice visual discrimination task that instructs participants to press labeled keyboard keys as quickly and as accurately as possible to indicate the direction of the right or left-facing arrowed present on the screen (“z” for left; “/” for right). Following a 32-trial practice, stop signals (an 800-Hz, 100-ms, 70-dB tone) were presented on 25% of trials for three task blocks of 64 trials each. The initial stop delay in each block was 250 ms and adjusted by 50 ms increments depending on whether the participant was able to successfully inhibit a response [16]. All trials consisted of a 500-ms warning stimulus followed by a 1,000-ms go signal (left- or right-facing arrow) and 1,000-ms intertrial interval blank screen. The timing of the stop signal adjusts dynamically based on performance on earlier stop trials to yield approximately 50% inhibition. Mean RT for each block was calculated based on valid responses (i.e., RT greater than 200 ms), and only blocks with 20–80% inhibition and at least 80% accuracy were analyzed. SST reaction time (SSRT) was calculated by subtracting the mean stop delay from the mean RT on go-trials (Task duration: ~10 minutes).
tDCS Side Effects
Side effects of tDCS were assessed at the end of each tDCS (or sham) session using the tDCS Effects Questionnaire [61]. This questionnaire asks participants to indicate to what extent they experienced symptoms both during and after tDCS administration using an 11-point Likert-like scale (0 = “None” to 10 = “Severe”).
Analysis
Descriptive statistics were obtained for all variables. Performance outliers were identified as values 2.5 SD above the mean for error rates and 2.5SD above the mean for reaction times, and were excluded from analysis. Stimulation condition (tDCS vs. sham) by session (baseline, end of treatment, and follow-up) interaction effects for primary outcomes were analyzed using separate linear mixed effects models with subject-level random effects estimated using maximum likelihood techniques (Stata; StataCorporation, College Station, TX, USA). We used an adjusted alpha of 0.02 to correct for multiple hypothesis testing, based on 3 primary outcome measures with an average correlation of r=−0.26 [62]. Education level (high school/some college versus college graduate), period order (tDCS first vs. sham first), sex, age, and current medication usage were included as covariates in the multiple regression models. Similar models were used to examine the secondary outcome (SSRT). An exploratory analysis used similar models to examine stimulation condition by session interaction effects within the sub-groups of participants who were taking stimulant medications and those who were not. Reported side effects of tDCS were examined for statistical differences between the active and sham conditions using t-tests for side effect rating during and following tDCS.
Results
Primary Outcomes
There was a significant stimulation condition by session interaction effect on CPT false positive scores, after correcting for three primary outcomes (χ2=15.44, p<0.001; Figure 1A). Post-hoc examination suggests that this effect was driven by the decrease in false positive errors from baseline to end of treatment in the tDCS group (β=−0.36, 95% Confidence Interval (CI) −0.54 to −0.18, p<0.001). The effect did not persist at follow-up after tDCS had been discontinued (β=−0.13, p>0.05). There were no significant baseline differences between conditions in any measures, no condition by order interactions, and no stimulation condition by session interaction effects for CPT true positive errors or hit response time (p>0.05; Figure 1B–C).
Figure 1. CPT Performance by Session.
There was a significant stimulation condition by session interaction for CPT false positive scores (χ2 =15.44, p<0.001; Figure 1A) driven the decrease in commission errors from baseline to end of treatment in the tDCS group (β=−0.36, 95% Confidence Interval (CI) −0.54 to −0.18, p<0.001). This effect did not persist at follow-up (β=−0.13, p>0.05). There was no significant stimulation condition by session interaction effect on true positive errors or response time (p>0.05; Figure 1B–C).
Secondary Outcome
There was no significant stimulation condition by session interaction for SSRT (p>0.05). In the Stop Signal Task, there was no significant difference observed between task performance measure at baseline between sham and active condition (p>0.05). Absolute stop signal reaction time is included in Table 2.
Table 2. Mean Ratings for Side Effects Reported during tDCS.
The average side effect ratings were mild. Ratings for tingling, itching sensation, burning sensation, and pain were significantly different between active and sham stimulation.
| Side effect during tDCS |
Sham M(SD) | tDCS M(SD) |
|---|---|---|
| Tingling | 1.4(1.4) | 1.9(1.4)* |
| Itching Sensation | 1.8(1.3) | 1.1 (1.3)* |
| Burning Sensation | 1.5 (2.0) | 2.8 (2.0)* |
| Pain | 0.2(0.4) | 0.5(0.7)* |
| Fatigue | 1.3(1.9) | 1.4(1.8) |
| Nervousness | 0.2(0.4) | 0.3(0.8) |
| Difficulty concentrating | 2.1(2.0) | 2.0(1.9) |
| Mood change | 0.4(0.8) | 0.5(0.8) |
| Change in vision | 0.2(0.6) | 0.3(0.7) |
| Headache | 0.3(0.6) | 0.3(0.6) |
| Visual sensation | 0.3(0.7) | 0.6(0.8) |
p<0.05
Concurrent tDCS Task Performance
There was no significant stimulation condition by session interaction for total true positives or true positive reaction time on the N-back task (χ2=4.92, p>0.05). Overall, task performance was typical for the N-back task with a parametric decrease in true positives as memory load increased and overall performance for this sample was comparable to previous studies [63]. Absolute error rates and reaction times for baseline, end of treatment, and follow-up for tDCS and sham condition are included in Table 1.
Table 1. Cognitive Task Performance Outcomes.
Stimulation condition by session interaction is significant for CPT false positive errors only (p<0.001). There were no significant differences by condition in baseline performance measures.
| tDCS | Sham | ||||
|---|---|---|---|---|---|
| CPT False Positive Error (Primary) | Mean | SEM | Mean | SEM | |
| Baseline | 21.5 | 1.9 | 19.8 | 1.9 | |
| End of Treatment | 17.1 | 1.5 | 19.8 | 1.8 | |
| Follow-up | 20.2 | 2.0 | 19.8 | 2.2 | |
| CPT True Positive Error | |||||
| Baseline | 2.0 | 0.8 | 2.4 | 0.8 | |
| End of Treatment | 1.9 | 0.7 | 2.1 | 0.4 | |
| Follow-up | 1.0 | 0.3 | 1.3 | 0.4 | |
| CPT Response Time | |||||
| Baseline | 416.7 | 12.2 | 422.6 | 12.2 | |
| End of Treatment | 420.9 | 10.4 | 419.7 | 12.0 | |
| Follow-up | 407.2 | 10.3 | 411.9 | 12.7 | |
| SST Reaction Time | |||||
| Baseline | 284.3 | 11.0 | 300.8 | 11.3 | |
| End of Treatment | 288.4 | 12.5 | 291.5 | 11.2 | |
| Follow-up | 268.1 | 9.3 | 267.6 | 11.0 | |
| N-back True Positive Response Count | |||||
| Baseline | 45.5 | 1.1 | 43.5 | 1.5 | |
| End of Treatment | 43.3 | 1.3 | 44.9 | 1.4 | |
| Follow-up | 46.0 | 1.3 | 47.6 | 1.7 | |
| N-back True Positive Response Time | |||||
| Baseline | 727.9 | 24.1 | 725.4 | 29.3 | |
| End of Treatment | 744.1 | 26.0 | 744.6 | 29.5 | |
| Follow-up | 709.1 | 23.8 | 715.6 | 27.1 | |
| N-back False Positive Count | |||||
| Baseline | 20.6 | 1.9 | 19.5 | 2.5 | |
| End of Treatment | 13.9 | 1.6 | 14.8 | 2.2 | |
| Follow-up | 16.9 | 1.9 | 17.1 | 2.5 | |
| N-back False Positive Reaction Time | |||||
| Baseline | 955.0 | 55.6 | 955.9 | 49.0 | |
| End of Treatment | 1021.9 | 49.0 | 984.1 | 53.3 | |
| Follow-up | 982.4 | 44.8 | 1016.9 | 48.6 | |
Exploratory Analysis of Effects of Medication Status
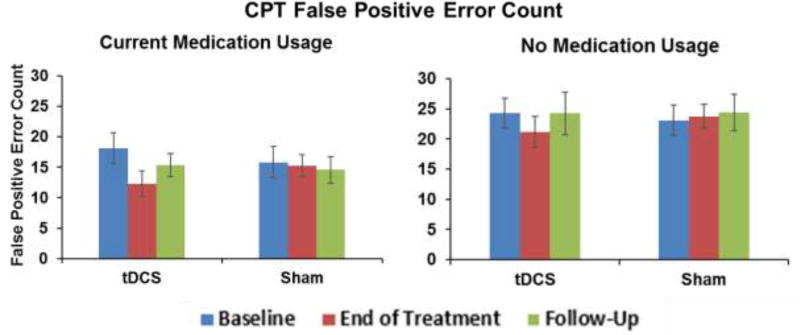
CPT false positive errors, but not true positive errors or reaction time, were significantly different by medication status at each session (Figure 2; p < .05 for medicated vs. non-medicated participants at each time point). Medication status was included as a covariate in the analytical models, and significantly predicted CPT false positives (β = −0.69; p=0.001). Although our sample size was too small to test for a three-way condition by session by medication status interaction effect, separate exploratory analyses within each group revealed significant condition by session interaction at end of treatment for both medicated (χ2 =12.15; p<0.001) and non-medicated participants (χ2=4.97; p<0.03), suggesting that our overall effect was not driven by one of these sub-groups.
Figure 2. CPT Performance by Medication Status.
Medication status was a significant covariate in the overall model. Exploratory analysis reveals a significant condition by session interaction at end of treatment for those currently using ADHD medication (χ2 =12.15; p<0.001) There is also a significant interaction at end of treatment for those currently not using ADHD medication (χ2 =4.97; p<0.03) Overall, there is no significant condition by current medication interaction (p>0.05).
Side Effects
There were significant differences in reported side effects during stimulation in the tDCS period compared to sham for burning, itching, and tingling (Table 2). However, there were no differences in reported side effects following the stimulation. Participants were able to correctly identify active tDCS stimulation during period 1 and period 2 (OR=8.56, P<0.0001).
Discussion
Consistent with our primary hypothesis, we found that three treatment sessions with active anodal tDCS over the left DLPFC (with cathodal placement over the right supra-orbital area) significantly improved performance on the Conners Continuous Performance Task. Specifically, participants in this within-subject cross-over study showed significant reductions in false positive errors on the Conners CPT during the active tDCS period (compared to sham treatment) at the end of treatment time point. However, these effects were not present at the follow-up session conducted three days after the final stimulation session. The improvement in performance following tDCS (versus sham) observed in the current study (d=0.5) is similar to effect sizes previously noted for methylphenidate on false positive errors [26, 64]. We did not observe an effect of tDCS on CPT true positive error or CPT response time, which is also similar to findings reported for methylphenidate treatment [26, 65]. False positives, unlike true positive errors, are specifically believed to probe impulsivity and are among the most reported outcomes for continuous performance task results [26]. This suggests that repeated tDCS may be a novel treatment for impulsivity in ADHD, though additional research is necessary to determine whether an optimized treatment approach could induce persistent effects.
Impulsivity is a core deficit in adult ADHD, and is one of the primary diagnostic criteria [11]. Impulsive behaviors such as blurting out answers without thinking, having difficulty awaiting a turn, or interrupting others can lead to poor occupational performance and difficulty in maintaining relationships [12]. The Conners CPT task is considered a gold standard of measuring ADHD symptoms such as impulsivity and sustained attention [15]. Specifically, false positive errors on the CPT task provide a continuous quantitative measure that can effectively distinguish ADHD patients versus healthy controls and has been associated with genetic factors that are also associated with ADHD [22, 23, 66]. A decrease in false positive errors on the CPT may reflect reduced impulsivity symptoms in ADHD patients [23, 67, 68]. False positive errors in children with ADHD were found to be positively correlated with parental ratings of impulsive behavior [69]. This pattern provides support for a model of poor cognitive control contributing to underactive behavioral inhibition and increased impulsivity in adults with ADHD [70]. In a study conducted by Boonstra et al., methylphenidate treatment resulted in a significant decrease in false positive errors [26]. Furthermore, this study found that the decrease in false positive errors during the medication phase compared to placebo provided a moderate predictive value for clinical response to treatment; positive predictive power of the decrease in false positive errors on medication response was 78%. In addition, associations have been identified between false positive errors and the dopamine receptor D2 gene (DRD2; rs207654, rs1079596), which may contribute to the pathology of ADHD [66].
Although the precise mechanisms underlying the effects we observed were not tested in this study, we propose that tDCS treatment targeting the DLPFC network may enhance top-down control by enhancing DLPFC activity, as frontal dysfunction in ADHD patients may be involved in generating impulsive behavior [71, 72]. The DLPFC is a crucial site for dopaminergic effects on cognitive function, and current stimulant treatments for ADHD rely on increases in dopaminergic activity to improve ADHD symptomology [73–75]. It is possible that modulation of DLPFC activity increases the level of inhibitory control over impulsive behaviors [76]. Therefore, novel treatments, such as tDCS administered with the N-back training task, which enhance DLPFC activity and reduce impulsivity may be beneficial for ADHD patients.
Our findings are consistent with previous reports that tDCS may be beneficial for ADHD and other conditions marked by deficits in cognitive control, such as addiction and obesity. A recent meta-analysis of studies utilizing tDCS or repetitive transcranial magnetic stimulation (rTMS) found that stimulation of the DLPFC reduced craving for nicotine, alcohol, and marijuana in addicted individuals, and reduced craving for food in subjects who normally experienced strong food cravings [77]. High definition tDCS stimulation over the left DLPFC specifically was found to reduce subject impulsivity on an intertemporal choice task, another measure of impulsive behavior [78]. Indeed, multiple studies targeting regions involved in executive control functions have observed improvements in cognitive deficits that characterize ADHD, such as impulsive responding, memory, and planning, and have shown increases in brain connectivity and neural efficiency following treatment [79–81]. For example, anodal tDCS over the left DLPFC with contralateral cathodal tDCS resulted in more cautious decision-making behavior [82]. Boggio et al. reported that active anodal stimulation to the DLPFC (compared to sham stimulation) enhanced inhibitory responses in a go/no-go task [54]. Differences in paradigms, such as differences in stimulation amplitude or lack of training task, may explain why some studies have failed to find an effect of tDCS targeting the DLPFC on impulsivity [36].
CPT false positive errors were unrelated to working memory and SST performance outcomes, suggesting that CPT false positive errors may assess a specific component of impulsivity in ADHD patients (Pearson’s r for false positives vs: N-back true positive count r=−0.11, p=0.19; N-back true positive reaction time r=0.08, p=0.54; SSRT r=−0.02, p=0.81). Lack of treatment response in the SSRT is not unexpected; previous studies have found smaller methylphenidate effects on SSRT [26]. This may be due to differences in the nature of the auditory stop signal used in the SST compared to visual signals like those in the CPT, or even differences in neural systems underlying the SST compared to other response inhibition tasks [71]. The go/no-go task is similar to the CPT in that the visual cue indicates when a participant should act or not, so that participants must restrain a primed action. In comparison, the SST presents an auditory stop cue after the visual go cue has been presented; therefore, participants are required to cancel an action that has already begun. In direct comparisons of generic stop signal tasks and go/no-go, tasks increased BOLD signal was observed in left DLPFC, medial, and parietal cortices during the go/no-go task, presumably reflecting a left frontoparietal specialization for response selection [83]. Performance on the go/no-go is not associated with SST performance in children with ADHD [84], and in adults, tDCS treatment targeting the left DLPFC increased the proportion of correct responses in the “go stage” of the go/no-go test compared to sham [85]. It is possible that impulsivity consists of multiple components, and component-specific assessment of impulse control in healthy participants has revealed different activation patterns of the neural impulse control network [86, 87]. Therefore, the absence of tDCS effects on other CPT outcomes, such as true positive errors and reaction time, may be due to differences in inhibitory processes for false positive versus true positive errors. Similar to studies using methylphenidate, there was no effect of tDCS treatment on overall mean CPT reaction time, and correlation studies suggest that mean reaction time is minimally related to ADHD symptoms as a whole [26, 69]. Differences may also be due to the fixed task order and fatigue experienced as a result of performing the N-back before or after cognitive tasks. However, findings by Erdodi et al. suggest that a standardized administration sequence minimizes order effects in the CPT [60]. Lastly, we did not observe changes in performance for the N-back training task (true positive count or true positive reaction time) during tDCS. The effects of tDCS on concurrent working memory performance are mixed; studies often fail to replicate previous reported effects [36, 38, 53, 55, 85, 88]. In a meta-analysis of 12 studies, meta-regressions showed that tDCS presented only an improvement in faster response times, not in accuracy. Other studies showing improvement in working memory performance measured performance following stimulation [44, 89, 90]. Studies showing positive effects of tDCS in ADHD have primarily been conducted in adolescents [37, 45, 47, 91], and it is possible that adults with ADHD respond differently. Differences may also be due to differences in study design such as dosage and treatment duration, or to participant experiences of side effects during stimulation.
Our sample of 37 individuals provided 80% power to detect an effect size of d ≈ 0.6, similar to effect sizes seen for methylphenidate treatment in adult ADHD, and the inclusion of ~30% women is representative of the general ADHD population. Strengths of our paradigm include the within-subject design, multiple stimulation sessions, and the use of a concurrent working memory training task during stimulation. A limitation of this study is the lack of CPT performance data immediately following stimulation at Session 1. Because our outcomes were not assessed after Session 1, we cannot be certain that treatment effect on false positive errors was a cumulative effect of three stimulation sessions, rather than an acute effect of stimulation at Session 3. However, multiple tDCS sessions have been shown to produce a cumulative increase in cortical excitability, and combining tDCS with a training task over time may result in greater gain on a non-trained test than tDCS alone [46, 92]. Sham stimulation may not be the optimal method for blinding participants during tDCS treatment [93, 94]. As a contribution to this discussion, we found that our participants were able to correctly identify tDCS during period 1 and period 2 (OR=8.56, P<0.0001). This may be related to the significant differences in side effects ratings between conditions; although side effects in both conditions were generally mild (rated <3 out of 10), participants endorsed higher ratings during the tDCS condition compared to sham (Table 2). It is possible that order of stimulation in a within-subject design could influence outcomes. However, prior studies suggest that a two-week washout period is sufficient to minimize carry over effects, and treatment order did not significantly contribute to our model (ps>0.05; [32]), suggesting that any carry over effects were minimal. Another potential limitation is that our sample included participants who were taking stimulant medications as well as those who were not. However, our within subject design reduces the chance that our results are confounded by medication status. Medication status was included as a covariate in our analysis. Although there was a significant difference in performance by medication status at each time point, our exploratory analysis revealed a significant condition by session interaction at end of treatment for those currently using ADHD medication as well as those who were not. However, it is possible that tDCS could be more effective when used in combination with stimulant medication, because stimulants increase dopamine in the executive function circuitry (such as the DLPFC) targeted by tDCS [73]. Many ADHD symptoms persist despite current medication usage and future research with adequate sample size is needed to assess the effects of tDCS with and without current medication usage. Additionally, approximately half of our participants met criteria for the primarily inattentive subtype of ADHD, and half-met criteria for the combined inattentive and hyperactive/impulsive subtype. ADHD subtype may influence performance and task-related brain activation on attention and response inhibition tasks [71, 95]. Finally, the dose-response curve for tDCS effects on cognitive outcomes is not fully understood and may be non-linear [96]. Building on results from this study, further research conducted examining dose-response curves for tDCS on cognitive performance would be very useful.
Our findings that active anodal tDCS over the left DLPFC with cathodal tDCS over the right supra-orbital area significantly decreased false positive errors in the Conners CPT suggests that tDCS may offer promise as a novel treatment for impulsivity in ADHD. This treatment was well tolerated; reported side effects were mild and subsided immediately following tDCS administration. Future studies employing different standardized training tasks (such as ones more specific response inhibition) may be useful in order to optimize outcomes, and additional studies would benefit from a larger sample size sufficiently powered to test differences in treatment by current medication status. Furthermore, repeated dose administration over a longer time period may provide more persistent performance outcomes following treatment. These data support advancing to a larger study to optimize treatment course for more durable potential benefits.
Acknowledgments
We thank Lofton Harris and Dr. Anita Hole for sharing their clinical expertise; Dr. Mario Cristancho for his support as study physician; Dr. Roy Hamilton for advice and tDCS training; and Dr. Rebecca Ashare for study support.
Funding source
This research was supported by a gift from Robert and Judy Levine and a grant from the National Institutes of Health (T32GM008076 to Dr. Julie Blendy). The funding source had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the article for publication.
Footnotes
Conflicts of Interest
None
References
- 1.Volkow ND, Swanson JM. Clinical practice: Adult attention deficit-hyperactivity disorder. N Engl J Med. 2013;369(20):1935–44. doi: 10.1056/NEJMcp1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD) Ann Pharmacother. 2014;48(2):209–25. doi: 10.1177/1060028013510699. [DOI] [PubMed] [Google Scholar]
- 3.Lensing MB, Zeiner P, Sandvik L, Opjordsmoen S. Four-year outcome in psychopharmacologically treated adults with attention-deficit/hyperactivity disorder: a questionnaire survey. J Clin Psychiatry. 2013;74(1):e87–93. doi: 10.4088/JCP.12m07714. [DOI] [PubMed] [Google Scholar]
- 4.Spencer T, Biederman J, Wilens T. Pharmacotherapy of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2000;9(1):77–97. [PubMed] [Google Scholar]
- 5.Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160–7. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz N, Parsons A, Whelan R, Brennan K, Agan ML, O'Connell R, et al. Decreased frontal, striatal and cerebellar activation in adults with ADHD during an adaptive delay discounting task. Acta Neurobiol Exp (Wars) 2015;75(3):326–38. [PubMed] [Google Scholar]
- 7.Francx W, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, Hoekstra PJ, et al. The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex. 2015;73:62–72. doi: 10.1016/j.cortex.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Salavert J, Ramos-Quiroga JA, Moreno-Alcazar A, Caseras X, Palomar G, Radua J, et al. Functional Imaging Changes in the Medial Prefrontal Cortex in Adult ADHD. J Atten Disord. 2015 doi: 10.1177/1087054715611492. [DOI] [PubMed] [Google Scholar]
- 9.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169(10):1038–55. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181(1):36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Wender PH, Wolf LE, Wasserstein J. Adults with ADHD. An overview. Ann N Y Acad Sci. 2001;931:1–16. [PubMed] [Google Scholar]
- 13.Rohlf H, Jucksch V, Gawrilow C, Huss M, Hein J, Lehmkuhl U, et al. Set shifting and working memory in adults with attention-deficit/hyperactivity disorder. J Neural Transm (Vienna) 2012;119(1):95–106. doi: 10.1007/s00702-011-0660-3. [DOI] [PubMed] [Google Scholar]
- 14.Matthies S, Philipsen A, Svaldi J. Risky decision making in adults with ADHD. J Behav Ther Exp Psychiatry. 2012;43(3):938–46. doi: 10.1016/j.jbtep.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Conners CK, Staff M. Conners' Continuous Performance Test Third Edition (Conners CPT 3) North Tonwanda, NY: Multi-Health Systems Inc.; 2015. [Google Scholar]
- 16.Logan GD, Schahar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8(1):60–6. [Google Scholar]
- 17.Raz S, Bar-Haim Y, Sadeh A, Dan O. Reliability and validity of the online continuous performance test among young adults. Assessment. 2014;21(1):108–18. doi: 10.1177/1073191112443409. [DOI] [PubMed] [Google Scholar]
- 18.Nichols SL, Waschbusch DA. A review of the validity of laboratory cognitive tasks used to assess symptoms of ADHD. Child Psychiatry Hum Dev. 2004;34(4):297–315. doi: 10.1023/B:CHUD.0000020681.06865.97. [DOI] [PubMed] [Google Scholar]
- 19.Egeland J, Kovalik-Gran I. Measuring several aspects of attention in one test: the factor structure of conners's continuous performance test. J Atten Disord. 2010;13(4):339–46. doi: 10.1177/1087054708323019. [DOI] [PubMed] [Google Scholar]
- 20.Egeland J, Kovalik-Gran I. Validity of the factor structure of Conners' CPT. J Atten Disord. 2010;13(4):347–57. doi: 10.1177/1087054709332477. [DOI] [PubMed] [Google Scholar]
- 21.Teicher MH, Polcari A, Fourligas N, Vitaliano G, Navalta CP. Hyperactivity persists in male and female adults with ADHD and remains a highly discriminative feature of the disorder: a case-control study. BMC Psychiatry. 2012;12:190. doi: 10.1186/1471-244X-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–55. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 23.Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18(3):485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- 24.Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/ hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13(4):693–8. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Jaen A, Fernandez-Mayoralas DM, Pardos A, Calleja-Perez B, Munoz Jareno N. Clinical and cognitive response to extended-release methylphenidate (Medikinet) in attention deficit/hyperactivity disorder: efficacy evaluation. Adv Ther. 2009;26(12):1097–110. doi: 10.1007/s12325-009-0083-9. [DOI] [PubMed] [Google Scholar]
- 26.Boonstra AM, Kooij JJ, Oosterlaan J, Sergeant JA, Buitelaar JK. Does methylphenidate improve inhibition and other cognitive abilities in adults with childhood-onset ADHD? J Clin Exp Neuropsychol. 2005;27(3):278–98. doi: 10.1080/13803390490515757. [DOI] [PubMed] [Google Scholar]
- 27.Bedard AC, Stein MA, Halperin JM, Krone B, Rajwan E, Newcorn JH. Differential impact of methylphenidate and atomoxetine on sustained attention in youth with attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2015;56(1):40–8. doi: 10.1111/jcpp.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–91. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 29.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–78. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166(1):23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 31.Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul. 2016;9(4):501–17. doi: 10.1016/j.brs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Dockery CA, Hueckel-Weng R, Birbaumer N, Plewnia C. Enhancement of planning ability by transcranial direct current stimulation. J Neurosci. 2009;29(22):7271–7. doi: 10.1523/JNEUROSCI.0065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJ, et al. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage. 2011;56(4):2249–57. doi: 10.1016/j.neuroimage.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 35.Meinzer M, Lindenberg R, Antonenko D, Flaisch T, Floel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J Neurosci. 2013;33(30):12470–8. doi: 10.1523/JNEUROSCI.5743-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosmo C, Baptista AF, de Araujo AN, do Rosario RS, Miranda JG, Montoya P, et al. A Randomized, Double-Blind, Sham-Controlled Trial of Transcranial Direct Current Stimulation in Attention-Deficit/Hyperactivity Disorder. PLoS One. 2015;10(8):e0135371. doi: 10.1371/journal.pone.0135371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breitling C, Zaehle T, Dannhauer M, Bonath B, Tegelbeckers J, Flechtner HH, et al. Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (tDCS) Front Cell Neurosci. 2016;10:72. doi: 10.3389/fncel.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. J Neurol Sci. 2006;249(1):31–8. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 39.Hoy KE, Emonson MR, Arnold SL, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia. 2013;51(9):1777–84. doi: 10.1016/j.neuropsychologia.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Hunter MA, Coffman BA, Trumbo MC, Clark VP. Tracking the neuroplastic changes associated with transcranial direct current stimulation: a push for multimodal imaging. Front Hum Neurosci. 2013;7:495. doi: 10.3389/fnhum.2013.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res. 2012;219(3):363–8. doi: 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- 42.Elmasry J, Loo C, Martin D. A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neurol Neurosci. 2015;33(3):263–78. doi: 10.3233/RNN-140473. [DOI] [PubMed] [Google Scholar]
- 43.Trumbo MC, Matzen LE, Coffman BA, Hunter MA, Jones AP, Robinson CS, et al. Enhanced working memory performance via transcranial direct current stimulation: The possibility of near and far transfer. Neuropsychologia. 2016;93(Pt A):85–96. doi: 10.1016/j.neuropsychologia.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. 2011;31(43):15284–93. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M. Transcranial Direct Current Stimulation Modulates Neuronal Networks in Attention Deficit Hyperactivity Disorder. Brain Topogr. 2017 doi: 10.1007/s10548-017-0552-4. [DOI] [PubMed] [Google Scholar]
- 46.Ho KA, Taylor JL, Chew T, Galvez V, Alonzo A, Bai S, et al. The Effect of Transcranial Direct Current Stimulation (tDCS) Electrode Size and Current Intensity on Motor Cortical Excitability: Evidence From Single and Repeated Sessions. Brain Stimul. 2016;9(1):1–7. doi: 10.1016/j.brs.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Soff C, Sotnikova A, Christiansen H, Becker K, Siniatchkin M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm (Vienna) 2017;124(1):133–44. doi: 10.1007/s00702-016-1646-y. [DOI] [PubMed] [Google Scholar]
- 48.Cachoeira CT, Leffa DT, Mittelstadt SD, Mendes LS, Brunoni AR, Pinto JV, et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder - A pilot randomized controlled study. Psychiatry Res. 2017;247:28–32. doi: 10.1016/j.psychres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 49.First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5--Research Version. Arlington, VA: American Pyschiatric Assocation; 2015. [Google Scholar]
- 50.Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles: Western Psyschological Services; 1986. [Google Scholar]
- 51.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 52.Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalography and clinical neurophysiology Supplement. 1999;52:3–6. [PubMed] [Google Scholar]
- 53.Brunoni AR, Vanderhasselt MA. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn. 2014;86:1–9. doi: 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Boggio PS, Bermpohl F, Vergara AO, Muniz AL, Nahas FH, Leme PB, et al. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. J Affect Disord. 2007;101(1–3):91–8. doi: 10.1016/j.jad.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Teo F, Hoy KE, Daskalakis ZJ, Fitzgerald PB. Investigating the Role of Current Strength in tDCS Modulation of Working Memory Performance in Healthy Controls. Front Psychiatry. 2011;2:45. doi: 10.3389/fpsyt.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohn SH, Park CI, Yoo WK, Ko MH, Choi KP, Kim GM, et al. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport. 2008;19(1):43–7. doi: 10.1097/WNR.0b013e3282f2adfd. [DOI] [PubMed] [Google Scholar]
- 57.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, et al. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16(3):370–9. [PMC free article] [PubMed] [Google Scholar]
- 59.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 60.Erdodi LA, Lajiness-O'Neill R, Saules KK. Order of Conners' CPT-II administration within a cognitive test battery influences ADHD indices. J Atten Disord. 2010;14(1):43–51. doi: 10.1177/1087054709347199. [DOI] [PubMed] [Google Scholar]
- 61.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5(2):155–62. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16(22):2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 63.Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, et al. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatr. 2009;14(8):820–6. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shiozawa P, Fregni F, Bensenor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(9):1443–52. doi: 10.1017/S1461145714000418. [DOI] [PubMed] [Google Scholar]
- 65.Bron TI, Bijlenga D, Boonstra AM, Breuk M, Pardoen WF, Beekman AT, et al. OROS-methylphenidate efficacy on specific executive functioning deficits in adults with ADHD: a randomized, placebo-controlled cross-over study. Eur Neuropsychopharmacol. 2014;24(4):519–28. doi: 10.1016/j.euroneuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Kollins SH, Anastopoulos AD, Lachiewicz AM, FitzGerald D, Morrissey-Kane E, Garrett ME, et al. SNPs in dopamine D2 receptor gene (DRD2) and norepinephrine transporter gene (NET) are associated with continuous performance task (CPT) phenotypes in ADHD children and their families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1580–8. doi: 10.1002/ajmg.b.30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barkley RA. The ecological validity of laboratory and analogue assessment methods of ADHD symptoms. J Abnorm Child Psychol. 1991;19(2):149–78. doi: 10.1007/BF00909976. [DOI] [PubMed] [Google Scholar]
- 68.Inoue K, Nadaoka T, Oiji A, Morioka Y, Totsuka S, Kanbayashi Y, et al. Clinical evaluation of attention-deficit hyperactivity disorder by objective quantitative measures. Child Psychiatry Hum Dev. 1998;28(3):179–88. doi: 10.1023/a:1022885827086. [DOI] [PubMed] [Google Scholar]
- 69.Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between Continuous Performance Test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31(5):543–54. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- 70.Quay HC. Theories of ADDH. J Am Acad Child Adolesc Psychiatry. 1988;27(2):262–3. doi: 10.1097/00004583-198803000-00030. [DOI] [PubMed] [Google Scholar]
- 71.Shang CY, Sheng C, Yang LK, Chou TL, Gau SS. Differential brain activations in adult attention-deficit/ hyperactivity disorder subtypes: a counting Stroop functional MRI study. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9749-0. [DOI] [PubMed] [Google Scholar]
- 72.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26(4):379–95. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Engert V, Pruessner JC. Dopaminergic and noradrenergic contributions to functionality in ADHD: the role of methylphenidate. Curr Neuropharmacol. 2008;6(4):322–8. doi: 10.2174/157015908787386069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11(5 Pt 1):424–46. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- 75.Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24(2):547–53. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 77.Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2472–80. doi: 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Shen B, Yin Y, Wang J, Zhou X, McClure SM, Li J. High-definition tDCS alters impulsivity in a baseline-dependent manner. Neuroimage. 2016;143:343–52. doi: 10.1016/j.neuroimage.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Metzuyanim-Gorlick S, Mashal N. The effects of transcranial direct current stimulation over the dorsolateral prefrontal cortex on cognitive inhibition. Exp Brain Res. 2016;234(6):1537–44. doi: 10.1007/s00221-016-4560-5. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira JF, Zanao TA, Valiengo L, Lotufo PA, Bensenor IM, Fregni F, et al. Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neurosci Lett. 2013;537:60–4. doi: 10.1016/j.neulet.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 81.Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol Psychiatry. 2013;73(7):646–51. doi: 10.1016/j.biopsych.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27(46):12500–5. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 84.Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35(2):229–38. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- 85.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of Anodal and Cathodal Transcranial Direct Current Stimulation on DLPFC on Modulation of Inhibitory Control in ADHD. J Atten Disord. 2015 doi: 10.1177/1087054715618792. [DOI] [PubMed] [Google Scholar]
- 86.Sebastian A, Pohl MF, Kloppel S, Feige B, Lange T, Stahl C, et al. Disentangling common and specific neural subprocesses of response inhibition. Neuroimage. 2013;64:601–15. doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 87.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–65. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 88.Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: leveraging tDCS to advance cognitive research. Front Psychol. 2014;5:800. doi: 10.3389/fpsyg.2014.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulquiney PG, Hoy KE, Daskalakis ZJ, Fitzgerald PB. Improving working memory: exploring the effect of transcranial random noise stimulation and transcranial direct current stimulation on the dorsolateral prefrontal cortex. Clin Neurophysiol. 2011;122(12):2384–9. doi: 10.1016/j.clinph.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012;521(2):148–51. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 91.Nejati V, Salehinejad MA, Nitsche MA, Najian A, Javadi AH. Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility. J Atten Disord. 2017 doi: 10.1177/1087054717730611. 1087054717730611. [DOI] [PubMed] [Google Scholar]
- 92.Martin DM, Liu R, Alonzo A, Green M, Loo CK. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp Brain Res. 2014;232(10):3345–51. doi: 10.1007/s00221-014-4022-x. [DOI] [PubMed] [Google Scholar]
- 93.O'Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GL, et al. Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PLoS One. 2012;7(10):e47514. doi: 10.1371/journal.pone.0047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallace D, Cooper NR, Paulmann S, Fitzgerald PB, Russo R. Perceived Comfort and Blinding Efficacy in Randomised Sham-Controlled Transcranial Direct Current Stimulation (tDCS) Trials at 2 mA in Young and Older Healthy Adults. PLoS One. 2016;11(2):e0149703. doi: 10.1371/journal.pone.0149703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Congdon E, Altshuler LL, Mumford JA, Karlsgodt KH, Sabb FW, Ventura J, et al. Neural activation during response inhibition in adult attention-deficit/hyperactivity disorder: preliminary findings on the effects of medication and symptom severity. Psychiatry Res. 2014;222(1–2):17–28. doi: 10.1016/j.pscychresns.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591(7):1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]