Abstract
Closure of persistent foramen ovale (PFO) to avoid cryptogenic strokes is performed globally with enthusiasm but lacks prove of efficacy. We present a 79-year-old man who had had a PFO device introduced nine years previously because of cryptogenic strokes presenting as syncopes. The patient was referred from his general practitioner with two new syncopes. Transthoracic echocardiography revealed no cardiac causes of embolism. Transesophageal echocardiography (TEE) revealed a misplaced device like an umbrella in a storm, but no septum defects. Holter revealed seconds-long episodes of atrial fibrillation (AF). The patient was successfully treated with anticoagulation.
A literature review showed that: (i) the efficacy of PFO closure devices has not been proven in any trial, but was demonstrated in a meta-analysis comparing three different devices; (ii) PFO devices are rarely controlled by TEE during or after insertion; (iii) residual shunts are detected in up to 45% of cases; (iv) there is an increased rate of post-arrhythmic complications; (v) the risk of AF in congenital heart disease increases with increasing age, with a 13% risk of transient ischemic attacks and stroke; and (vi) surgical treatment of PFO was found to have a 4.1% risk of complications including stroke.
The question to be asked is whether device closure of PFO should be avoided, considering that PFO is a congenital heart defect with risks of AF and (cryptogenic) stroke? Heart surgery should be a treatment option for symptomatic PFO.
Keywords: Atrial septum defect, paradoxical embolism, cryptogenic stroke, stroke, transesophageal echocardiography, atrial fibrillation
Introduction
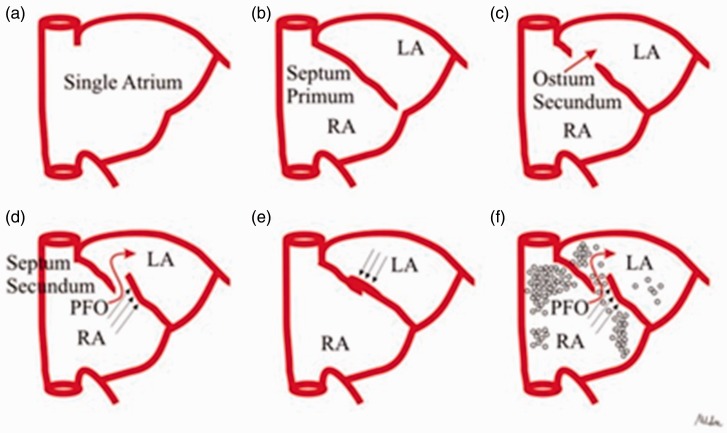
To bypass the maternal oxygenated blood from fetal lung circulation, the blood passes through the foramen ovale. The onset of respiration after birth causes an increase in Qp/Qs, hence pulmonary vascular resistance decreases, leading to higher left atrial pressures and closure of the foramen ovale flap against the septum secundum. The contact between the septum primum flap and the septum secundum leads to fusion of these tissues and permanent closure of the foramen ovale (Fig. 1).
Fig. 1.
Interatrial septal development. The primitive atrium is a single cavity (a) subsequently divided by the septum primum which grows down from the roof of the atrium, toward the developing endocardial cushions (b). Thus, small perforations begin to develop superiorly resulting in the ostium secundum (c). The atrial roof grows down along the right side of the septum primum, the septum secundum, which comes to lie over the ostium secundum; however, an opening remains between septa, the PFO (d). At birth, lung pressures drop and the blood pressure in the left atrium exceeds that of the right atrium, so that the septum primum is shoved against the septum secundum, obtaining septa fusion (e). If this final step does not occur, PFO results (f). With permission. Courtesy of Contaldi et al. Cardiovascular Ultrasound 2012;10:16.
Transesophageal studies (TEE) reveal that 9–12% of the general population have a persistent foramen ovale (PFO) (1), increasing to 29% with catheter probing during ablation for atrial fibrillation (AF) (2,3). Autopsy studies reveal that 27% of PFOs have diameters in the range of 1–10 mm and that size increases with age (4); the size of the PFO predicts the risk of paradoxical embolism (5).
PFO is reported to be the cause of cryptogenic stroke in 31–77% of cases, while atrial septal aneurysms are the cause in 4–25% of cases (6,7). Cryptogenic stroke through a PFO was first described in 1877 by Julius Cohnheim during an autopsy of a young woman with a fatal occlusion of a cerebral artery. He observed that the patient had a significant lower limb thrombus and a large PFO. He hypothesized that the latter served as a conduit for an arterial embolism that paradoxically started in the venous circulation. The Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study confirmed that patients with cryptogenic stroke have an increased prevalence (20%) of pelvic deep venous thrombosis (8). Cryptogenic stroke also seems to be associated with vigorous or strenuous exercise, decompression illness, sneezing, coughing, obstructive sleep apnea, and even migraine (9–17).
A transcatheter approach to close the most common congenital heart defect in adults, ostium secundum atrial septal defect (ASD), was successfully introduced in uncomplicated cases four decades ago due to its minimally invasive nature compared to open heart surgery (18,19). Inspired by the success in treating atrial septal secundum defects, closure of PFO to avoid cryptogenic stroke and hence transient ischemic attacks (TIA) (6,20) is performed globally with enthusiasm but lacks proof of efficacy (21–24).
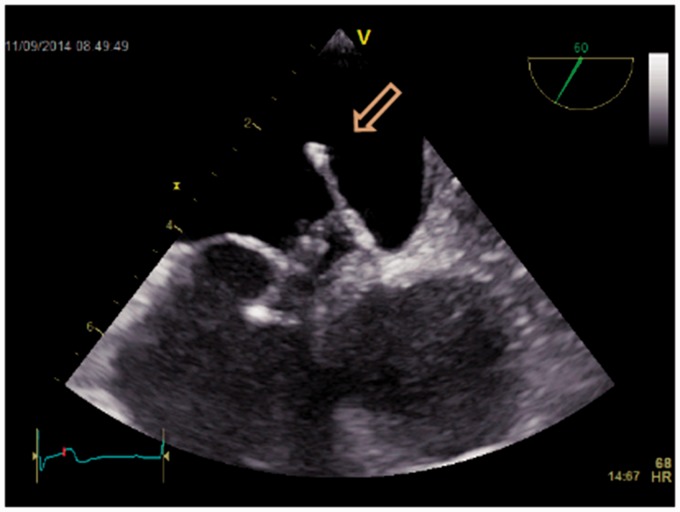
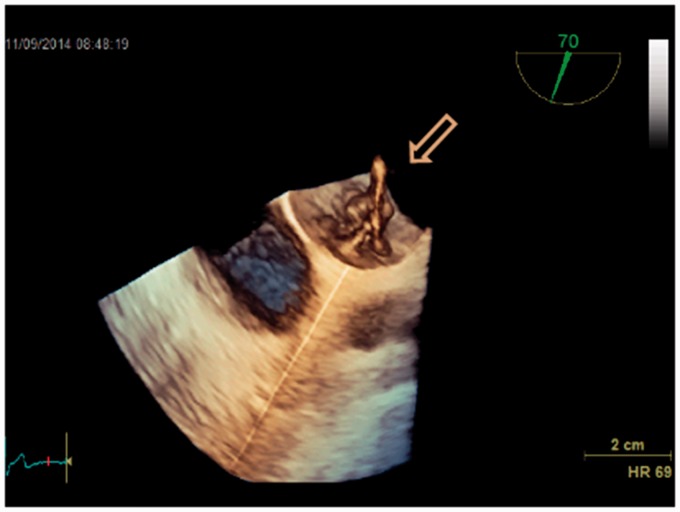
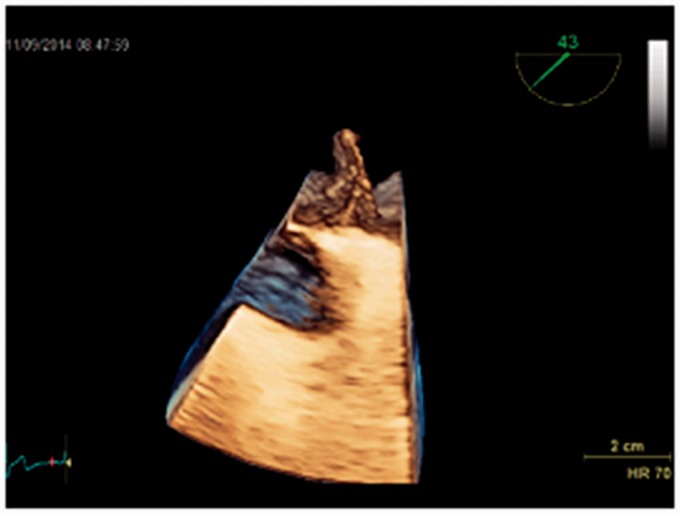
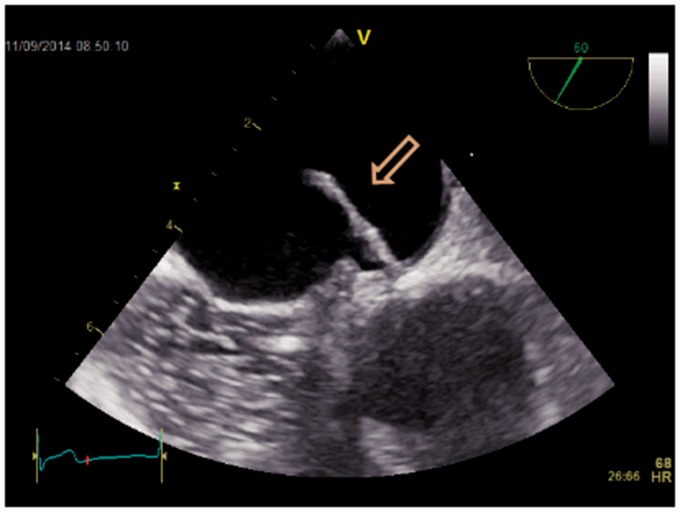
This case drew our attention to find the best evidence documented treatment of PFO: a 79-year-old man who had had a PFO device introduced nine years previously, because of three cryptogenic strokes presenting as syncopes, was referred by his general practitioner to our department with two new syncopes. Initial Holter and biochemistry were normal (including screening for coagulopathies). Transthoracic echocardiography (TTE) did not reveal any cardiac causes of embolism. TEE revealed a misplaced device (Figs. 2–5) like an umbrella in a storm. Using the method described by Johansson et al. (25) with prerequisite Valsalva maneuvers before contrast injections, repeated five times, we found no septum defects (Figs. 6 and 7). A second Holter revealed paroxysmal episodes of AF lasting several seconds. The patient was treated with anticoagulation and has had no symptoms since. The patient gave verbal and written consent to the publication of this case report.
Fig. 2.
2D transesophageal echocardiography of a displaced atrial septal occluder (arrow).
Fig. 3.
3D transesophageal echocardiography of a displaced atrial septal occluder.
Fig. 4.
3D transesophageal echocardiography of a displaced atrial septal occluder (arrow).
Fig. 5.
2D transesophageal echocardiography of a displaced atrial septal occluder (arrow). Test with isotonic solution of agitated saline water.
These questions were sought to be answered in the literature: (i) Is the position of atrial septum devices in PFO to avoid cryptogenic stroke monitored by TEE during or after insertion? (ii) What is the complication rate? (iii) Is insertion of atrial septum devices in PFO to avoid cryptogenic stroke beneficial compared with anticoagulation or open heart surgery? (iv) Is the rate of post-arrhythmic complications known? (v) Is there an increased risk of AF in patients with PFO?
Methods and Results
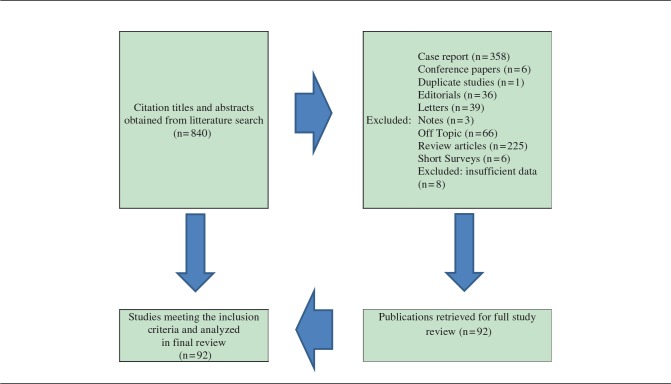
To answer the above questions, the following analysis and a review were performed in MEDLINE and the Cochrane Collaboration and Cochrane Register of Controlled Trials for relevant studies. The following search terms were used: “PFO” AND “cryptogenic stroke” resulted in 454 articles, while “PFO” AND “atrial fibrillation” resulted in 156 articles, and “PFO device” AND “heart surgery” resulted in 230 articles. Searches were not limited, but irrelevant articles and articles in languages other than English were excluded (Table 1). The main author performed the literature search. All studies that appeared to fit the inclusion criteria were identified for full review. The retrieved and selected articles were approved by all authors. Of those, 92 articles were selected as relevant for the review.
Table 1.
Literature search strategy.
Discussion
Complication rate and control of the PFO device position
Atrial septum device misplacements are reported as rare, but the devices are rarely controlled by TEE post insertion (26–30). TTE may reveal transient changes in left atrial passive emptying and strain (31), while left ventricular function is not affected when assessed by magnetic resonance imaging (MRI) (32). However, as in our case, TTE may not reveal displaced devices or even remaining defects in the PFO. Accordingly, a recent follow-up study concluded that devices evaluated > 5 years after closure with TTE were well placed (33), but two patients in this population had their devices surgically removed, and up to 45% had residual shunting when evaluated with contrast. While intracardiac ultrasound may be of help during the insertion of PFO devices (34–39), TEE remains the diagnostic “gold standard” for evaluating cardioembolic sources of stroke (40–44), and repeated prerequisite Valsalva maneuvers followed by contrast injections and careful evaluations are important to avoid misinterpretations and diagnostic failure (25).
PFO treatment: device closure versus medical therapy
Jean-Louis Mas et al. (45) reported that patients with both a patent foramen ovale and an atrial septal aneurysm who had had a cryptogenic stroke had a higher risk of recurrent stroke while taking aspirin than did patients with no septal abnormality or either septal abnormality alone. While overall causes for recurrent stroke (8%/year) seem equally protected by aspirin compared to warfarin (46), device closure of PFO has not yet been proven more effective than medical therapy in preventing recurrent cryptogenic stroke (47–49). The RESPECT trial, funded by St. Jude Medical, showed in the primary intention-to-treat analysis that there was no significant benefit associated with closure of a patent foramen ovale in adults who had had a cryptogenic stroke, but in pre-specified per-protocol and as-treated analyses, device closure was superior to medical therapy, with a low rate of associated risks (50); these results from the RESPECT trial were later confirmed in a sub-analysis excluding patients (who actually had the device inserted) without indications for device closure and presented at a conference in Washington in November 2016, but have not yet been published. In the CLOSURE I trial, the first randomized clinical trial evaluating the effects of PFO closure on recurrent cryptogenic stroke and TIAs versus medical therapy (51), 909 patients were randomized to percutaneous PFO closure with a STARFlex® PFO device versus medical therapy with warfarin (target international normalized ratio of 2.0–3.0), aspirin 325 mg alone, or aspirin 81 mg plus warfarin. The results did not demonstrate a benefit of PFO closure with the STARFlex® device compared with medical therapy. Device closure was successful in only 87% of cases, which is similar to previous device closure studies with the STARFlex® device (52), but lower than that reported for other PFO closure devices (53–56). AF and major vascular complications were significantly higher in the device group, although the latter had no further implications for the patients, which was confirmed in other trials (57,58). The population in the CLOSURE I trial had several atherosclerotic risk factors, including increased body mass index, diabetes, hypertension, and history of ischemic heart disease predicting recurrent ischemic neurologic events; however, a diagnosis of AF after trial enrollment portended the greatest risk of recurrent events (59).
Each trial on its own failed to significantly improve its primary endpoint in the intention-to-treat analyses. However, they all point in the same direction: all three trials were subjects of a recent meta-analysis showing a significant benefit of PFO closure with a > 40% relative risk reduction for recurrent stroke or TIAs and a 33% reduction of death or vascular events (60). The benefit of PFO closure over medical therapy was even more pronounced when only the trials using the Amplatzer® device were included in the analysis (61). Also, a single-center study (28) reporting on very long-term follow-up data on PFO closure or medical therapy showed significantly improved survival (the most compelling endpoint) in the PFO closure group, with a relative risk reduction for all-cause mortality of 60% (P = 0.03). Risks of thrombus formation at the devices seem comparable to other devices, decreasing to negligible after one year (62–64). However, concluding that device closure is safe by comparing different devices subject to different study designs and analyzing all data in one pool seems too ambitious, and as explained below, the complications following device closure point in another direction. Percutaneous closure of PFO with the indication obstructive sleep apnea or migraine also lacks proof of efficacy (65–68).
Post-arrhythmic complications after device closure
The incidence of AF following device closure is not reported by Inglessis et al. (69); it is excluded in all three trials with device closure (60). Interestingly, Rengifo-Moreno et al. reported a small, but statistically significant increased risk of developing new-onset AF with the transcatheter closure of PFO when compared with medical therapy alone (2.7% vs. 0.5%, odds ratio [OR] = 5.7, P< 0.001). In the CLOSURE I trial, it was associated with significantly higher rates of AF than was medical therapy (5.7% vs. 0.7%; P < 0.001) (51) and similar results were observed in trials with the Gore® septal occluder, the Intrasept® device, and the Spider® PFO occluder (70–72). By contrast, AF occurred at similar rates after PFO closure with Amplatzer® devices (St. Jude Medical, Plymouth, MN, USA) compared with medical therapy in the RESPECT (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment) trial (2.9% vs. 1.0%; P = 0.16) and in the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism) (3% vs. 1.5%; P = 0.13) (50,73). However, uniformly comparable heart rhythm monitoring before device closure was not available in any of the studies, and recent follow-up studies of “atrial septum defects closed with devices” demonstrate a high risk (6.6–17.9%) of AF in the first year after the procedure, decreasing to negligible (0–3.9%), but with only 76–82% of the original population included in one study (33,74,75).
The risk of AF in patients with PFO and other congenital heart diseases
AF, which occurs in 1–2% of the general population and is probably underestimated with an expected doubling in the coming decades, confers a fivefold risk of stroke and one in five of all strokes is attributed to this arrhythmia (76). The AF detection rate with 12-lead ECG or 24-h Holter is in the range of 2–6% after ischemic stroke or transient ischemic stroke (77,78). However, the CRYSTAL-AF (CRYptogenic STroke And underLying AF) trial conducted in Europe, Canada, and the United States used an insertable cardiac monitor (ICM) from Medtronic (REVEAL XT) and the EMBRACE study (30-Day Cardiac Event Monitor Belt for Recording AF After a Cerebral Ischemic Event) in Canada investigated a non-invasive, 30-day event-triggered loop recorder from Braemar (ER910AF Cardiac Event Monitor®) (79,80) to show that up to 36 months of heart rhythm monitoring increased the detection rate of AF from 8.9% after six months to 12.4% after 12 months and up to 30.0% after 36 months. The aforementioned results confirm the findings of observational studies that had demonstrated a high rate of undetected AF in patients after cryptogenic stroke (81).
PFO is considered to be a structural (congenital) heart disease (82) and as a result of structural heart disease, atrial arrhythmias increase with increasing age to up to 38% in 50-year-old patients. Yet, these numbers do not allow the exclusion of the risk associated with corrective or palliative surgical procedures (83,84). Catheter ablation for paroxysmal AF with co-existing PFO may increase procedure time, while success seems to be unaffected (2,3). Surgical correction of PFO, even with minimally invasive (robotic) surgery, is successful with a low risk of residual shunting or any other complications and with a lower recurrence of stroke compared with device closure and is considered to be the gold standard (85–90) in smaller studies: after a mean follow-up time of 24 months, only eight patients (all in one of the four studies) of a total of 194 were reported to have a TIA as a complication of the direct suture closure of PFO (Table 3). This is equivalent to a total risk of 4.1% and hence considerably lower than with device closure of PFO.
Table 3.
Complication rate after surgical closure of PFO.
| Reference | Patients (n) | Complications reported |
|---|---|---|
| Cujec B et al. Can J Cardiol 1999;15:57–64 (75) | 14 | No neurological recurrences during a mean follow-up of 43 months (crude incidence rate difference 12%/patient/year, 95% CI = 6.6–17.9, P < 0.02) |
| Dearani JA et al. Circulation 1999;100(19 Suppl):II171–II175 (74) | 91 | Follow-up totaled 176.3 patient-years and mean follow-up was 2.0 years. 8 had a TIA during follow-up. The overall freedom from TIA recurrence during follow-up was 92.5 ± 3.2% at 1 year and 83.4 ± 6.0% at 4 years |
| Devuyst G et al. Neurology 1996;47:1162–1166 (73) | 30 | After a mean follow-up of 2 years without antithrombotic treatment, no recurrent cerebrovascular event (stroke or TIA) and no new lesion on MRI had developed |
| Ruchat P et al. Eur J Cardiothorac Surg 1997;11:824–827 (76) | 32 | All patients were followed-up corresponding to a cumulative time of 601 patient-months. This revealed no recurrent vascular events nor silent new brain lesions on brain MRI |
| Sabata RA et al. World J Pediatr Congenit Heart Surg 2014;5:527–533 (77) | 27 | Follow-up: mean = 1.5 years, maximum = 4.2 years. No recurrence of neurological events |
A recent retrospective longitudinal multicenter study of 199 patients, “DysrhythmiAs in patieNts with congenitAl heaRt diseAse” (DaNaRA), demonstrated that patients with congenital heart disease, particularly patients with complex defects, develop AF at a young age and progress frequently from paroxysmal AF to (long-standing) persistent/permanent AF (91). Sixteen patients (8%) experienced a cerebrovascular event 14 (2–33) years before the first documented AF. The total incidence of TIA/stroke in the population was 13%. Coexistence of episodes of AF and regular atrial tachycardia occurred in a considerable number of patients in this study; most of them initially presented with regular atrial tachycardia, hence the authors suggest that aggressive therapy and close follow-up of congenital heart disease patients with atrial tachyarrhythmia is justified (91). A large retrospective study comparing the general Danish population to > 4400 patients (age range = 1–49 years) with almost five decades of follow-up (92) demonstrated that: (i) AF is more prevalent in patients with ASD diagnosed and closed in childhood, with a cumulative incidence of 9.8% at the end of 50 years of follow-up; and (ii) the risk of arrhythmia is independent of whether the ASD was closed by catheter or surgery. Yet the authors of this important study ask: does closure of an ASD at a young age reduce AF later in life (92)?
In conclusion, PFO is a common heart defect associated with stroke that has been treated for decades with device closure, although the efficacy has never been proven. Device closure of PFO is associated with a high risk of remaining defects, including displacement, and an undetermined increased risk of AF; the latter may even be the natural cause of so-called cryptogenic strokes.
We interpret our review of the literature as follows (Table 2): (i) the efficacy of PFO closure devices has not been proven in any trial (however, efficacy was proven in a meta-analysis comparing three different devices); (ii) PFO device positions are rarely controlled by TEE during or after insertion; (iii) residual shunts can be demonstrated in up to 45% of cases; (iv) there is an increased rate of post-arrhythmic complications, especially AF, which was recently concluded to be an underdiagnosed and hence increasingly important cause of cryptogenic strokes; (v) the risk of AF in congenital heart disease (which PFO should be considered to be) increases with increasing age, with a 13% risk of TIAs and stroke; and (vi) surgery, both minimally invasive and open heart, is a proven treatment of congenital heart defects causing AF, with a 4.1% risk of complications.
Table 2.
Key points in the review.
| Key point | Conclusion/review of literature | References |
|---|---|---|
| Is insertion of atrial septum devices in PFO to avoid cryptogenic stroke beneficial compared to anticoagulation or open heart surgery? | The efficacy of PFO closure devices has not been proven in any published trial | (21–24,26,36–49,73–78) |
| Is the position controlled with TEE before or after insertion? | The positions are rarely controlled with TEE before or after insertion | (26–27,30–33) |
| What is the complication rate? | Residual shunts can be demonstrated in up to 45% of cases | (30–33,41–44) |
| Is the rate of post-arrhythmic complications known? | The rate of post-arrhythmic complications increases, especially AF, recently concluded as an underdiagnosed and hence increasingly important cause of cryptogenic strokes | (30,40,41,58–60,62,63) |
| Is the risk of AF in patients with PFO increased? | (Considering PFO as a congenital heart disease) the risk of AF in congenital heart disease is increasing with increasing age, with a 13% risk of TIAs and stroke | (65–72) |
| How should we treat patients with cryptogenic stroke and PFO? | Literature points towards surgery as evident treatment of congenital heart defects including PFO causing AF | (2,3,36–49,73–80) |
The question to be asked is whether device closure of PFO should be avoided, considering that PFO is a congenital heart defect with the associated risks of AF and (cryptogenic) stroke? Heart surgery should be a treatment option for symptomatic PFO.
Supplemental Material
Supplemental material, sj-vid-1-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental Material
Supplemental material, sj-vid-2-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental Material
Supplemental material, sj-vid-3-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental Material
Supplemental material, sj-vid-4-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental Material
Supplemental material, sj-vid-5-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental Material
Supplemental material, sj-vid-6-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material is available for this article online.
References
- 1.Fisher DC, Fisher EA, Budd JH, et al. The incidence of patent foramen ovale in 1,000 consecutive patients. A contrast transesophageal echocardiography study. Chest 1995; 107: 1504–1509. [DOI] [PubMed] [Google Scholar]
- 2.Knecht S, Wright M, Lellouche N, et al. Impact of a patent foramen ovale on paroxysmal atrial fibrillation ablation. J Cardiovasc Electrophysiol 2008; 19: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 3.Gate-Martinet A, Da CA, Romeyer-Bouchard C, et al. Does a patent foramen ovale matter when using a remote-controlled magnetic system for pulmonary vein isolation? Arch Cardiovasc Dis 2014; 107: 88–95. [DOI] [PubMed] [Google Scholar]
- 4.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984; 59: 17–20. [DOI] [PubMed] [Google Scholar]
- 5.Wessler BS, Kent DM, Thaler DE, et al. The RoPE Score and right-to-left shunt severity by transcranial Doppler in the CODICIA Study. Cerebrovasc Dis 2015; 40: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000; 58: 1172–1179. [DOI] [PubMed] [Google Scholar]
- 7.Bogousslavsky J, Garazi S, Jeanrenaud X, et al. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46: 1301–1305. [DOI] [PubMed] [Google Scholar]
- 8.Cramer SC, Rordorf G, Maki JH, et al. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study. Stroke 2004; 35: 46–50. [DOI] [PubMed] [Google Scholar]
- 9.Guerin P, Lambert V, Godart F, et al. Transcatheter closure of patent foramen ovale in patients with platypnea-orthodeoxia: results of a multicentric French registry. Cardiovasc Intervent Radiol 2005; 28: 164–168. [DOI] [PubMed] [Google Scholar]
- 10.Walsh KP, Wilmshurst PT, Morrison WL. Transcatheter closure of patent foramen ovale using the Amplatzer septal occluder to prevent recurrence of neurological decompression illness in divers. Heart 1999; 81: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silver B, Greenbaum A, McCarthy S. Improvement in sleep apnea associated with closure of a patent foramen ovale. J Clin Sleep Med 2007; 3: 295–296. [PMC free article] [PubMed] [Google Scholar]
- 12.Wertman B, Azarbal B, Riedl M, et al. Adverse events associated with nickel allergy in patients undergoing percutaneous atrial septal defect or patent foramen ovale closure. J Am Coll Cardiol 2006; 47: 1226–1227. [DOI] [PubMed] [Google Scholar]
- 13.Wilmshurst PT, Nightingale S, Walsh KP, et al. Clopidogrel reduces migraine with aura after transcatheter closure of persistent foramen ovale and atrial septal defects. Heart 2005; 91: 1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimoldi SF, Ott S, Rexhaj E, et al. Patent foramen ovale closure in obstructive sleep apnea improves blood pressure and cardiovascular function. Hypertension 2015; 66: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 15.Mirzada N, Ladenvall P, Hansson PO, et al. Recurrent stroke in patients with patent foramen ovale: An observational prospective study of percutaneous closure of PFO versus non-closure. Int J Cardiol 2015; 195: 293–299. [DOI] [PubMed] [Google Scholar]
- 16.Rigatelli G, Cardaioli P, Dell’avvocata F, et al. Early post-procedural migraine attack predicts migraine resolution after patent foramen ovale transcatheter closure. Minerva Cardioangiol 2008; 56: 461–465. [PubMed] [Google Scholar]
- 17.Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke 2002; 33: 706–711. [DOI] [PubMed] [Google Scholar]
- 18.King TD, Mills NL. Nonoperative closure of atrial septal defects. Surgery 1974; 75: 383–388. [PubMed] [Google Scholar]
- 19.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52: e143–e263. [DOI] [PubMed] [Google Scholar]
- 20.Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105: 2625–2631. [DOI] [PubMed] [Google Scholar]
- 21.Piana RN, Kirshner HS. Closure of the patent foramen ovale: because we can, should we? And in whom? JACC Cardiovasc Interv 2013; 6: 1184–1185. [DOI] [PubMed] [Google Scholar]
- 22.Sievert H, Babic UU, Hausdorf G, et al. Transcatheter closure of atrial septal defect and patent foramen ovale with ASDOS device (a multi-institutional European trial). Am J Cardiol 1998; 82: 1405–1413. [DOI] [PubMed] [Google Scholar]
- 23.Alushi B, Biasco L, Orzan F, et al. Patent foramen ovale treatment strategy: an Italian large prospective study. J Cardiovasc Med (Hagerstown) 2014; 15: 761–768. [DOI] [PubMed] [Google Scholar]
- 24.Furlan AJ, Jauss M. Patent foramen ovale and cryptogenic stroke: the hole story. Stroke 2013; 44: 2676–2678. [DOI] [PubMed] [Google Scholar]
- 25.Johansson MC, Eriksson P, Guron CW, et al. Pitfalls in diagnosing PFO: characteristics of false-negative contrast injections during transesophageal echocardiography in patients with patent foramen ovales. J Am Soc Echocardiogr 2010; 23: 1136–1142. [DOI] [PubMed] [Google Scholar]
- 26.Abaci A, Unlu S, Alsancak Y, et al. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: meta-analysis of 28,142 patients from 203 studies. Catheter Cardiovasc Interv 2013; 82: 1123–1138. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann WJ, Heinisch C, Majunke N, et al. Patent foramen ovale closure with the SeptRx device initial experience with the first “In-Tunnel” device. JACC Cardiovasc Interv 2010; 3: 963–967. [DOI] [PubMed] [Google Scholar]
- 28.Wahl A, Juni P, Mono ML, et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation 2012; 125: 803–812. [DOI] [PubMed] [Google Scholar]
- 29.von Bardeleben RS, Richter C, Otto J, et al. Long term follow up after percutaneous closure of PFO in 357 patients with paradoxical embolism: Difference in occlusion systems and influence of atrial septum aneurysm. Int J Cardiol 2009; 134: 33–41. [DOI] [PubMed] [Google Scholar]
- 30.Bruch L, Parsi A, Grad MO, et al. Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism: single-center experience. Circulation 2002; 105: 2845–2848. [DOI] [PubMed] [Google Scholar]
- 31.Vavuranakis M, Kavouras C, Vlasseros I, et al. Assessment of left atrial function after percutaneous closure of patent foramen ovale. Echocardiography 2013; 30: 765–771. [DOI] [PubMed] [Google Scholar]
- 32.Stern H, Baurecht H, Luechinger R, et al. Does the amplatzer septal occluder device alter ventricular contraction pattern? A ventricular motion analysis by MR tagging. J Magn Reson Imaging 2012; 35: 949–956. [DOI] [PubMed] [Google Scholar]
- 33.Snijder RJ, Suttorp MJ, Berg JM, et al. Percutaneous closure of secundum type atrial septal defects: More than 5-year follow-up. World J Cardiol 2015; 7: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigna C, Marchese N, Zanchetta M, et al. Echocardiographic guidance of percutaneous patent foramen ovale closure: head-to-head comparison of transesophageal versus rotational intracardiac echocardiography. Echocardiography 2012; 29: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 35.Cao QL, Zabal C, Koenig P, et al. Initial clinical experience with intracardiac echocardiography in guiding transcatheter closure of perimembranous ventricular septal defects: feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc Interv 2005; 66: 258–267. [DOI] [PubMed] [Google Scholar]
- 36.Rigatelli G, Bortolazzi A, Cardaioli P, et al. Intracardiac echocardiography-aided diagnosis of superior caval sinus defect in case of contraindications to non-invasive imaging tools. Minerva Cardioangiol 2008; 56: 703–704. [PubMed] [Google Scholar]
- 37.Rigatelli G, Dell'avvocata F, Braggion G, et al. Persistent venous valves correlate with increased shunt and multiple preceding cryptogenic embolic events in patients with patent foramen ovale: an intracardiac echocardiographic study. Catheter Cardiovasc Interv 2008; 72: 973–976. [DOI] [PubMed] [Google Scholar]
- 38.Rigatelli G, Cardaioli P, Dell'avvocata F, et al. The association of different right atrium anatomical-functional characteristics correlates with the risk of paradoxical stroke: an intracardiac echocardiographic study. J Interv Cardiol 2008; 21: 357–362. [DOI] [PubMed] [Google Scholar]
- 39.Zanchetta M, Pedon L, Olivieri A, et al. Randomized study comparing mechanical with electronic 2-dimensional intracardiac ultrasound monitoring (MEDIUM) during percutaneous closure of patent foramen ovale in adult patients with cryptogenic stroke. Echocardiography 2008; 25: 496–503. [DOI] [PubMed] [Google Scholar]
- 40.Adams HP, Jr, del ZG, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007; 115: e478–e534. [DOI] [PubMed] [Google Scholar]
- 41.Harloff A, Handke M, Reinhard M, et al. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37: 859–864. [DOI] [PubMed] [Google Scholar]
- 42.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol 2007; 50: 187–204. [DOI] [PubMed] [Google Scholar]
- 43.Schuchlenz HW, Weihs W, Beitzke A, et al. Transesophageal echocardiography for quantifying size of patent foramen ovale in patients with cryptogenic cerebrovascular events. Stroke 2002; 33: 293–296. [DOI] [PubMed] [Google Scholar]
- 44.Van CG, Schulze D, Cosyns B, et al. Relation between patent foramen ovale and unexplained stroke. Am J Cardiol 1993; 71: 596–598. [DOI] [PubMed] [Google Scholar]
- 45.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345: 1740–1746. [DOI] [PubMed] [Google Scholar]
- 46.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001; 345: 1444–1451. [DOI] [PubMed] [Google Scholar]
- 47.Schwerzmann M, Windecker S, Wahl A, et al. Percutaneous closure of patent foramen ovale: impact of device design on safety and efficacy. Heart 2004; 90: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004; 44: 750–758. [DOI] [PubMed] [Google Scholar]
- 49.Taaffe M, Fischer E, Baranowski A, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder). Am J Cardiol 2008; 101: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 50.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 51.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366: 991–999. [DOI] [PubMed] [Google Scholar]
- 52.Chan KC, Godman MJ, Walsh K, et al. Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (Amplatzer septal occluder): multicentre UK experience. Heart 1999; 82: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krizanic F, Sievert H, Pfeiffer D, et al. Clinical evaluation of a novel occluder device (Occlutech) for percutaneous transcatheter closure of patent foramen ovale (PFO). Clin Res Cardiol 2008; 97: 872–877. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee A, Bengur AR, Li JS, et al. Echocardiographic characteristics of successful deployment of the Das AngelWings atrial septal defect closure device: initial multicenter experience in the United States. Am J Cardiol 1999; 83: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 55.Luani B, Markovic S, Krumsdorf U, et al. Efficacy of different devices for transcatheter closure of patent foramen ovale assessed by serial transoesophageal echocardiography and rates of recurrent cerebrovascular events in a long-term follow-up. EuroIntervention 2015; 11: 85–91. [DOI] [PubMed] [Google Scholar]
- 56.Hornung M, Bertog SC, Franke J, et al. Long-term results of a randomized trial comparing three different devices for percutaneous closure of a patent foramen ovale. Eur Heart J 2013; 34: 3362–3369. [DOI] [PubMed] [Google Scholar]
- 57.Baglini R, Baldari D, Amaducci A, et al. The new patent foramen ovale occluder FIGULLA in complex septal anatomy: a case series. Ther Adv Cardiovasc Dis 2013; 7: 21–26. [DOI] [PubMed] [Google Scholar]
- 58.Nyboe C, Olsen MS, Nielsen-Kudsk JE, et al. Atrial fibrillation and stroke in adult patients with atrial septal defect and the long-term effect of closure. Heart 2015; 101: 706–711. [DOI] [PubMed] [Google Scholar]
- 59.Elmariah S, Furlan AJ, Reisman M, et al. Predictors of recurrent events in patients with cryptogenic stroke and patent foramen ovale within the CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale) trial. JACC Cardiovasc Interv 2014; 7: 913–920. [DOI] [PubMed] [Google Scholar]
- 60.Rengifo-Moreno P, Palacios IF, Junpaparp P, et al. Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34: 3342–3352. [DOI] [PubMed] [Google Scholar]
- 61.Capodanno D, Milazzo G, Vitale L, et al. Updating the evidence on patent foramen ovale closure versus medical therapy in patients with cryptogenic stroke: a systematic review and comprehensive meta-analysis of 2,303 patients from three randomised trials and 2,231 patients from 11 observational studies. EuroIntervention 2014; 9: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 62.Krumsdorf U, Ostermayer S, Billinger K, et al. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol 2004; 43: 302–309. [DOI] [PubMed] [Google Scholar]
- 63.Poddar KL, Nagarajan V, Krishnaswamy A, et al. Risk of cerebrovascular events in patients with patent foramen ovale and intracardiac devices. JACC Cardiovasc Interv 2014; 7: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 64.Schwerzmann M, Salehian O. Hazards of percutaneous PFO closure. Eur J Echocardiogr 2005; 6: 393–395. [DOI] [PubMed] [Google Scholar]
- 65.Pickett CA, Villines TC, Ferguson MA, et al. Percutaneous closure versus medical therapy alone for cryptogenic stroke patients with a patent foramen ovale: meta-analysis of randomized controlled trials. Tex Heart Inst J 2014; 41: 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickett CA, Villines TC, Ferguson MA, et al. Cost effectiveness of percutaneous closure versus medical therapy for cryptogenic stroke in patients with a patent foramen ovale. Am J Cardiol 2014; 114: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 67.Dowson A, Mullen MJ, Peatfield R, et al. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008; 117: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 68.Rigatelli G, Dell’avvocata F, Cardaioli P, et al. Migraine-patent foramen ovale connection: role of prominent eustachian valve and large Chiari network in migrainous patients. Am J Med Sci 2008; 336: 458–461. [DOI] [PubMed] [Google Scholar]
- 69.Inglessis I, Elmariah S, Rengifo-Moreno PA, et al. Long-term experience and outcomes with transcatheter closure of patent foramen ovale. JACC Cardiovasc Interv 2013; 6: 1176–1183. [DOI] [PubMed] [Google Scholar]
- 70.Knerr M, Bertog S, Vaskelyte L, et al. Results of percutaneous closure of patent foramen ovale with the GORE((R)) septal occluder. Catheter Cardiovasc Interv 2014; 83: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 71.Edwards-Lehr T, Franke J, Bertog SC, et al. Safety and performance of the Spider patent foramen ovale occluder. Catheter Cardiovasc Interv 2013; 81: 317–323. [DOI] [PubMed] [Google Scholar]
- 72.Luermans JG, Post MC, Schrader R, et al. Outcome after percutaneous closure of a patent foramen ovale using the Intrasept device: a multi-centre study. Catheter Cardiovasc Interv 2008; 71: 822–828. [DOI] [PubMed] [Google Scholar]
- 73.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 74.Eeckhout E, Martin S, Delabays A, et al. Very long-term follow-up after percutaneous closure of patent foramen ovale. EuroIntervention 2015; 10: 1474–1479. [DOI] [PubMed] [Google Scholar]
- 75.Lakkireddy D, Rangisetty U, Prasad S, et al. Intracardiac echo-guided radiofrequency catheter ablation of atrial fibrillation in patients with atrial septal defect or patent foramen ovale repair: a feasibility, safety, and efficacy study. J Cardiovasc Electrophysiol 2008; 19: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 76.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 77.Liao J, Khalid Z, Scallan C, et al. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke 2007; 38: 2935–2940. [DOI] [PubMed] [Google Scholar]
- 78.Lazzaro MA, Krishnan K, Prabhakaran S. Detection of atrial fibrillation with concurrent Holter monitoring and continuous cardiac telemetry following ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2012; 21: 89–93. [DOI] [PubMed] [Google Scholar]
- 79.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 80.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 81.Schmiegel W, Jähnert A, Dickmann B. Paroxysmal tachycardias - Cardiac event recorder implantation after cryptogenic stroke – First in a series. E-Journal of Cardiology Practice 2015; 13: 22–22. [Google Scholar]
- 82.Schernthaner C, Danmayr F, Daburger A, et al. High incidence of echocardiographic abnormalities of the interatrial septum in patients undergoing ablation for atrial fibrillation. Echocardiography 2013; 30: 402–406. [DOI] [PubMed] [Google Scholar]
- 83.Triedman JK. Arrhythmias in adults with congenital heart disease. Heart 2002; 87: 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bouchardy J, Therrien J, Pilote L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009; 120: 1679–1686. [DOI] [PubMed] [Google Scholar]
- 85.Devuyst G, Bogousslavsky J, Ruchat P, et al. Prognosis after stroke followed by surgical closure of patent foramen ovale: a prospective follow-up study with brain MRI and simultaneous transesophageal and transcranial Doppler ultrasound. Neurology 1996; 47: 1162–1166. [DOI] [PubMed] [Google Scholar]
- 86.Dearani JA, Ugurlu BS, Danielson GK, et al. Surgical patent foramen ovale closure for prevention of paradoxical embolism-related cerebrovascular ischemic events. Circulation 1999; 100: II171–II175. [DOI] [PubMed] [Google Scholar]
- 87.Cujec B, Mainra R, Johnson DH. Prevention of recurrent cerebral ischemic events in patients with patent foramen ovale and cryptogenic strokes or transient ischemic attacks. Can J Cardiol 1999; 15: 57–64. [PubMed] [Google Scholar]
- 88.Ruchat P, Bogousslavsky J, Hurni M, et al. Systematic surgical closure of patent foramen ovale in selected patients with cerebrovascular events due to paradoxical embolism. Early results of a preliminary study. Eur J Cardiothorac Surg 1997; 11: 824–827. [DOI] [PubMed] [Google Scholar]
- 89.Sabate RA, Burkhart HM, Suri RM, et al. Minimally invasive video-assisted surgical closure of atrial septal defects: a safe approach. World J Pediatr Congenit Heart Surg 2014; 5: 527–533. [DOI] [PubMed] [Google Scholar]
- 90.Torracca L, Ismeno G, Quarti A, et al. Totally endoscopic atrial septal defect closure with a robotic system: experience with seven cases. Heart Surg Forum 2002; 5: 125–127. [PubMed] [Google Scholar]
- 91.Teuwen CP, Ramdjan TT, Gotte M, et al. Time course of atrial fibrillation in patients with congenital heart defects. Circ Arrhythm Electrophysiol 2015; 8: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 92.Karunanithi Z, Nyboe C, Hjortdal VE. Long-term risk of atrial fibrillation and stroke in patients with atrial septal defect diagnosed in childhood. Am J Cardiol 2017; 119: 461–465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-vid-1-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental material, sj-vid-2-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental material, sj-vid-3-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental material, sj-vid-4-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental material, sj-vid-5-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open
Supplemental material, sj-vid-6-arr-10.1177_2058460118793922 for Patent foramen ovale and atrial fibrillation as causes of cryptogenic stroke: is treatment with surgery superior to device closure and anticoagulation? A review of the literature by Thomas Kjeld, Tem S Jørgensen, Gitte Fornitz, Jan Roland and Henrik C Arendrup in Acta Radiologica Open