Abstract
Objectives
Biofilm formation is one of the important features of Staphylococcus epidermidis, particularly in nosocomial infections. We aimed to investigate the biofilm production by phenotypic methods and the presence of ica genes in S epidermidis.
Methods
A total of 41 S epidermidis isolates were recovered from different clinical specimens. Biofilm formation was evaluated by microtiter plate, tube method and Congo red agar method. The presence of icaA and icaD genes was investigated by PCR. Validity of methods (sensitivity and specificity), and metrics for test performance (positive/negative predictive value, and positive/negative likelihood ratio) were determined.
Results
By both microtiter plate and tube method, 53.6% of S epidermidis isolates were able to produce biofilm, whilst only 24.4% of isolates provided a biofilm phenotype on Congo red agar plates. icaA and icaD genes were found in 100% and 95.1% of isolates, respectively. Biofilm phenotypes accounted for 4.8% by microtiter plate assay, despite the absence of the ica gene. Congo red agar and PCR exhibited a lower sensitivity (18% and 45.5%, respectively) for identifying the biofilm phenotype in comparison to microtiter plate.
Conclusion
The microtiter plate method remains generally a better tool to screen biofilm production in S epidermidis. In addition, the ability of S epidermidis to form biofilm is not always dependent on the presence of ica genes, highlighting the importance of ica-independent mechanisms of biofilm formation. The use of reliable methods to specifically detect biofilms can be helpful to treat the patients affected by such problematic bacteria.
Keywords: biofilm, congo red, ica, operon, staphylococcus epidermidis
Introduction
Staphylococcus epidermidis is the most abundant coagulase-negative staphylococci (CoNS) isolated from humans. This organism is a part of the normal flora of human skin and mucosa, with a capacity to cause disease in individuals with immune impairment, or in those with injury caused by foreign bodies [1]. In recent years, due to increased medical interventions, such as the use of vascular catheters and prosthetic device implants, the prevalence of infections caused by S epidermidis has considerably increased [2,3]. Consequently, this organism is increasingly isolated and identified as a pathogen causing nosocomial sepsis; it accounts for approximately 30% of all nosocomial bloodstream infections [4]. This organism is also associated with a variety of clinical manifestations, including late sepsis in premature infants, central nervous system shunt infection, endocarditis, urinary tract infection, surgical site infection, and endophthalmitis [1].
Although S epidermidis is an opportunistic organism, this microorganism has several virulence factors, such as hemolysin, lipase, protease, lecithinase, DNase and toxins [5]. One of the special features of S epidermidis is the ability to adhere to polymeric surfaces and subsequently form a biofilm [6]. Strong binding of the bacterial biofilm to polymeric surfaces is the 1st step of intravascular catheter-related bacteremia and other device-associated infections, leading to sepsis [7]. In the biofilm there are layers embedded in a matrix of extracellular polysaccharide (slime), which facilitates the adhesion of bacteria onto surfaces, protects them from the host immune response, and serves as an efficient barrier against antimicrobials. Therefore, eradication of bacteria within biofilms is difficult, as the tolerance to antibiotics eventually leads to the removal of contaminated devices [3,6].
Biofilm formation is regulated by the expression of polysaccharide intercellular adhesion (PIA) [6]. PIA is composed of β -1,6 N- acetyl glucosamine and is responsible for cell to cell adhesion and is necessary for biofilm formation in S epidermidis strains [6,7]. PIA is encoded by the chromosomal intercellular adhesion (ica) locus, consisting of the icaADBC structural and icaR regulatory genes [8]. Amongst them, the icaA and icaD genes have been reported to play a central role in biofilm production [9], having enzymatic activity (N-acetylglucosaminyltransferase). icaA alone has negligible enzymatic activity [7], but simultaneous expression with icaD initiates activity of N-acetylglucosaminyltransferase and produces oligomeres with a length of 20 residues [6,7].
There are various methods to evaluate biofilm formation in bacteria, such as qualitative Congo red agar (CRA), the tube method (TM) [10], and analysis on quantitative micro titer plates (MTP) [3,11]. There is controversy amongst scientists regarding which method is the most reliable for assessing biofilm formation [3,6,10]. However, the microtiter plate biofilm formation assay has been identified as a valuable method [3, 8,12]. Furthermore, molecular DNA-based techniques, such as PCR have been used recently to better understand the molecular mechanisms of biofilm formation [6].
A better understanding of the mechanisms of adhesion by microorganisms to produce a microbial biofilm could identify new procedures to counteract the numerous infections associated with biofilm growth. According to previous studies, controversies involve the selection of a reliable approach to identify biofilm formation in bacteria. In this regard, the aim of the present study was to determine biofilm formation and the presence of icaA and icaD genes in S epidermidis clinical isolates, and evaluate the reliability of CRA, TM, and MTP phenotypic methods for detection of a biofilm.
Materials and Methods
1. Bacterial isolates
Sayyad Shirazi hospital is university-affiliated teaching medical center in Gorgan, Northern Iran. In a cross-sectional study between 2013 and 2014, various clinical specimens from patients admitted to Sayyad Shirazi hospital were collected. All specimens were stored in brain-heart infusion broth medium (Merck, Darmstadt, Germany) and taken directly to the diagnostic microbiological laboratory, Department of Microbiology, School of Medicine All specimens were cultured on blood agar (Merck, Darmstadt, Germany) and incubated at 37°C for 24 hours. Colonies that had grown were identified as S epidermidis by using the standard microbiological and biochemical tests, and then confirmed by amplifying and sequencing the tuf gene [13].
2. Microtiter plate method
MTP test was performed according to the published method [14]. Briefly, a 0.5 McFarland standard was prepared from an overnight culture grown in trypticase soy broth (Merck, Darmstadt, Germany) containing 1% glucose (Merck, Darmstadt, Germany), 200 μL aliquots were added to a 96-well tissue culture plate (JET BIOFIL, Guangzhou, China) and incubated for 24 hours at 37°C. The supernatant was removed from each well and the plate was washed 4 times with phosphate buffer saline. The plate was incubated at 65°C for 1 hour until dry. Adherent cells attached to each well were stained with 0.1% crystal violet (Sigma Chemical Co., St Louis, MO, USA). The plate was washed twice with deionized water to remove the excess dye, and then 100 mL of 70% ethanol with 10% isopropyl alcohol, was added. The optical density of the adherent biofilm was read at 570 nm by an ELISA plate reader (BioTek, Bad Friedrichshall, Germany). Each test was performed in triplicate. The results were interpreted based on the criteria shown in Table 1 [15]. As described by Thilakavathy et al [8], strains were categorized as biofilm positive when a strong or moderate biofilm was detected.
Table 1.
Interpretation of biofilm by the microtiter plate method.
| Mean OD value | Biofilm formation |
|---|---|
| OD ≤ ODc * | None |
| ODc < OD ≤ 2× ODc | Weak |
| 2× ODc < OD ≤ 4× ODc | Moderate† |
| 4× ODc < OD | Strong† |
Mean OD of negative control + 3× SD of negative control.
Isolates with strong or moderate biofilm were considered biofilm producers.
OD = optical density; ODc = optical density cut off.
3. Congo red agar method
Detection of biofilm formation in all isolates was studied by the CRA method according to the protocol described by Freeman et al [10]. CRA medium was prepared with brain heart infusion broth (Merck, Darmstadt, Germany) 37 g/L, sucrose (Merck, Darmstadt, Germany) 50 g/L, agar-agar (Pronadisa, Laboratories Conda, S.A., Madrid, Spain) 10 g/L, and Congo red dye (Merck, Darmstadt, Germany) 0.8 g/L. Prepared CRA plates were inoculated with a 0.5 McFarland turbidity standard of microorganism and incubated aerobically at 37°C for 24 hours. For color evaluation of colonies, a 5-colourimetric scale method was used. Biofilm-positive isolates appeared as dry opaque black and bright black colonies, while biofilm-negative variants developed a red, pink with or without a darkening at the center, or Bordeaux red colonies [6].
4. Tube method
An overnight culture, grown in nutrient agar (Merck, Darmstadt, Germany) was inoculated in trypticase soy broth supplemented with 1% glucose contained in 2 mL plastic tubes. The cultures were incubated overnight at 37°C, following which the tubes were decanted gently, washed with phosphate buffer saline and air-dried. The tubes were stained using 0.1% crystal violet (Sigma Chemical Co., St Louis, MO, USA), washed with deionized water to remove the unbound stain, and air dried in an inverted position. Scoring for the tube method was performed using the control strains. The criteria for production of biofilm in isolates was when a visible film lined the wall and the bottom of the tube. The amount of biofilm formed was categorized as negative, weak, moderate, or strong [12]. A biofilm positive strain had a strong or moderate biofilm.
5. Polymerase chain reaction (PCR)
The presence of icaA and icaD genes were analyzed by PCR using specific primers for each. The PCR primers for icaA gene were as follows: forward, 5’-ACACTTGCTGGCGCAGTCAA-3’; reverse, 5’-TCTGGAACCAACATCCAACA-3’. The primer sequences for amplification of the icaD gene were as follows: forward, 5’-ATGGTCAAGCCCAGACAGAG-3’; reverse, 5’-AGTATTTTCAATGTTTAAAGCAA-3’ [16]. DNA extraction was performed by phenol chloroform/isoamyl alcohol method as described previously [17]. The reaction volume was 25 μL, and the reaction mixture contained 1× buffer [10 mM Tris-HCl (pH = 8.3), 50 mM KCl, 0.01% Triton X-100], 0.2 mM of each dNTP, 2 mM MgCl2, 1 μM of each primer, 1 U Taq polymerase, and 0.1 μg of template DNA. Amplification of both genes was performed in a Mastercycler (Eppendorf, Hamburg, Germany) using the following thermal conditions: initial denaturation at 94°C for 4 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 10 minutes. PCR products were electrophoresed on 1.5% agarose gel (Sigma Chemical Co., St Louis, MO, USA), stained with SYBR Safe DNA Gel Stain (Thermo Fisher Scientific, USA) and visualized by UV illuminator (SABZ Biomedical, Tehran, Iran).
6. Statistical analysis
Data were analyzed by SPSS16.0 software. Chi-square test was used to determine significance. In all cases, p < 0.05 was considered statistically significant. The validity of studied methods (sensitivity and specificity), and metrics for test performance, including positive (negative) predictive value (PPV/NPV), and positive (negative) likelihood ratio (PLR/NLR), were compared against the MTP gold standard method.
Results
1. Bacterial isolates
There were 41 S epidermidis isolates obtained from various biological materials, including blood (61%, 25/41), tracheal aspirate (14.6%, 6/41), eye exudates (14.6%, 6/41), and urine (9.7%, 4/41). The majority of S epidermidis was isolated from patients hospitalized in ICU (31.7%, 13/41) and pediatric units (24.4%, 10/41). The percentage of isolates recovered from males and females were 39% (16/41) and 61% (25/41), respectively. The highest proportions of isolates (46.3%, 19/41) were from patients 20 years or younger, followed by patients 45 years and older (36.5%, 15/41).
2. Detection of the biofilm-producing phenotype
Table 2 shows the results of biofilm formation in S epidermidis isolates using 3 methods: MTP, TM and CRA . The percentage of biofilm-producing S epidermidis in both MTP and TM assays was 53.6% (22/41), 19.5% of isolates were identified as strong biofilm producers by MTP assay compared to 14.6% of those detected by TM.
Table 2.
Results of three biofilm formation detection assays and ica genes detection among the 41 S epidermidis clinical isolates.
| Characteristics (No.) | No. (%) of biofilm formation | No. (%) of gene present | |||
|---|---|---|---|---|---|
| MTP | TM | CRA | icaA | icaD | |
| Clinical specimen | |||||
|
| |||||
| Blood (25) | 15 (60) | 15 (60) | 6 (24) | 25 (100) | 23 (92) |
| Tracheal aspirate (6) | 3 (50) | 4 (67) | 1 (17) | 6 (100) | 6 (100) |
| Eye exudate (6) | 3 (50) | 3 (50) | 2 (33) | 6 (100) | 6 (100) |
| Urine (4) | 1 (25) | 0 | 1 (25) | 4 (100) | 4 (100) |
| p | 0.5 | 0.15 | 0.12 | 0.36 | |
|
| |||||
| Hospital ward | |||||
|
| |||||
| ICU (13) | 11 (84.6) | 6 (46.1) | 3 (23) | 13 (100) | 12 (92.3) |
| Pediatric (10) | 4 (40) | 5 (50) | 3 (30) | 10 (100) | 10 (100) |
| Internal medicine (7) | 3 (42.8) | 5 (71.4) | 1 (14.2) | 7 (100) | 6 (86) |
| Neurology (5) | 2 (40) | 3 (60) | 2 (40) | 5 (100) | 5 (100) |
| Infectious diseases (4) | 2 (50) | 2 (50) | 0 | 4 (100) | 4 (100) |
| Women’s surgical (2) | 0 | 1(50) | 1 (50) | 2 (100) | 2 (100) |
| p | 0.04 | 0.075 | 0.074 | 0.11 | |
|
| |||||
| Patient age (y) | |||||
|
| |||||
| < 20 (19) | 11 (57.9) | 9 (47.3) | 6 (31.6) | 19 (100) | 18 (95) |
| 20 – 45 (7) | 4 (57.1) | 6 (85.7) | 1 (14.2) | 7 (100) | 7 (100) |
| > 45 (15) | 7 (46.7) | 7 (46.7) | 3 (20) | 15 (100) | 14 (93) |
| p | 0.915 | 0.218 | 0.703 | 0.373 | |
|
| |||||
| Patient gender | |||||
|
| |||||
| Female (25) | 13 (52) | 12 (48) | 9 (36) | 25 (100) | 24 (96) |
| Male (16) | 9 (56.2) | 10 (62.5) | 1 (6.2) | 16 (100) | 15 (94) |
| p | 0.79 | 0.36 | 0.06 | 0.48 | |
|
| |||||
| Total (41) | 22 (53.6) | 22 (53.6) | 10 (24.4) | 41 (100) | 39 (95.1) |
CRA = congo red agar; MTP = microtiter plate; TM = tube method.
Blood isolates were the highest proportion with a biofilm phenotype (60%, 15/25) in both MTP and TM assays. In addition, 84.6% (11/13) and 71.4% (5/7) of isolates obtained from ICU and internal medicine wards, were biofilm-positive by MTP and TM, respectively. Using the CRA method, 24.4% (10/41) of isolates had a biofilm phenotype: 19.5% (8/41) of isolates produced bright black colonies and 4.8% (2/41) isolates produced dry opaque colonies; whereas 75.6% (31/41) isolates were identified as having a non-biofilm phenotype, compared with 29.3% (12/41), 14.6% (6/41), and 31.7% (13/41) isolates that produced red, pink, and Bordeaux red colonies, respectively. However, the CRA method did not correlate with the MTP assay, where only 9.7% (4/41) of isolates had a biofilm phenotype identified by both CRA and MTP.
In addition, biofilm-forming ability in S epidermidis isolates by 3 phenotypic assays did not correlate with patient demographics and characteristics, including clinical specimen type, and patient’s age and gender. However, there was a significant correlation between the ability for biofilm formation using the MTP method and the hospital ward (p = 0.04).
3. Detection of ica genes
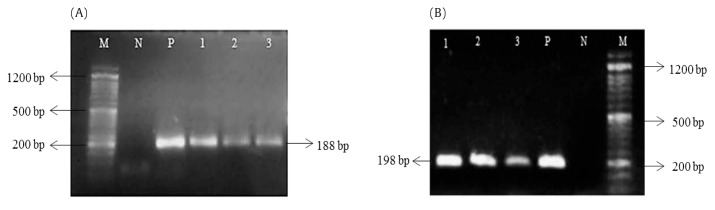
Generally, S epidermidis isolates were positive for icaA [100% (41/41)] and icaD genes [95.1% (39/41)] and had bands of 188 bp and 198 bp, respectively (Figure 1). With regard to correlation of phenotypic characteristics with the presence of icaAD genes, isolates were non-biofilm forming when assessed by MTP [46.3% (19/41)], TM [43.9% (18/41)] and CRA [70.7% (29/41)], but were positive for icaA or icaD genes. On the other hand, only 4.8% (2/41) and 2.4% (1/41) of isolates were biofilm forming by MTP and TM, respectively, in spite of the absence of ica gene.
Figure 1.
Agarose gel electrophoresis of icaA (A) and icaD (B) genes in S epidermidis isolates. Lanes 1–3 = PCR products of the corresponding genes; M= 50 bp DNA marker; N = negative control; P = positive control.
4. Validity of tests
The specificity and sensitivity of TM, CRA and PCR methods in comparison to the MTP assay are shown in Table 3. Other parameters, such as PPV, NPV, PLR, and NLR were also calculated. Sensitivity and specificity of TM was estimated to be 64% and 58%, respectively. Although CRA and PCR had a relatively acceptable specificity (each 68%), they exhibited low sensitivity (18% and 45.5%, respectively) to identify biofilm phenotype, when compared to MTP.
Table 3.
Performance indices used for the evaluation of biofilm formation using TM, CRA and PCR methods.
| Method | % of statistical parameter | |||||
|---|---|---|---|---|---|---|
| SN | SP | PPV | NPV | PLR | NLR | |
| TM | 64 | 58 | 64 | 58 | 1.52 | 0.62 |
| CRA | 18 | 68 | 40 | 42 | 0.56 | 1.2 |
| PCR | 45.5 | 68 | 62.5 | 52 | 1.42 | 0.8 |
CRA = congo red agar; NLR = negative likelihood ratio; NPV = negative predictive value; PCR = polymerase chain reaction; PLR = positive likelihood ratio; PPV = positive predictive value; SN = sensitivity; SP = specificity; TM = tube method.
Discussion
The ability to adhere to the surfaces of biomaterials, and consequently form a biofilm is one of the most important factors for S epidermidis to induce serious nosocomial infections [3,7]. So, detection of biofilm-forming strains of S epidermidis by an appropriate method and suppression of their adhesive mechanisms may be useful for development of anti-adhesive coatings for prosthetic medical devices and in drug development.
The MTP is a quantitative gold standard method for detection of biofilm formation [12]. In this study, 53.6% of S epidermidis were found with a biofilm phenotype, which is comparable to results by Ebrahimi et al in Iran [18], and Ninin et al in France [19], but lower than rates reported in Egypt [20] and Thailand [21]. These discrepancies could be due to spectrophotometric procedures and clinical specimen sources [15].
In the present study, only 24.4% of isolates tested, showed a biofilm phenotype on CRA plates, which was consistent with the study by El–Mahallawy et al [22], where 23.5% of S epidermidis isolated from blood cultures formed a biofilm. In contrast, El-Khier et al [15], reported that 43.6% of isolates from orthopedic prosthetic implants, were biofilm-producers. As previously reported [15] such contradictions may be due to multifactorial reasons, and can result from the differences in the origin of the specimens, CRA composition, incubation conditions, and also the interpretation of the color of the colonies. In addition, with respect to the specificity and sensitivity of methods used for assessing the biofilm formation, the results in this study are consistent with those reported by Melo et al [23] and Oliveira et al [6], where MTP and TM showed higher sensitivity and specificity than CRA.
It has been demonstrated that there are some correlations between the biofilm-forming capacity of bacteria, and patient demographics and characteristics [24–26]. In the case of S epidermidis, previous studies found a significant correlation between the type of biological samples, and the biofilm production. Sanchez et al [27] in the United States reported that clinical strains isolated from non-fluid sites, including superficial/deep tissue, bone, and respiratory tract, on average had a significantly higher proportion of biofilm positive strains, compared to those isolates from host fluids, including blood or urine. Solati et al [28] also reported that urinary isolates more frequently formed biofilms compared with isolates from other clinical samples. Thummeepak et al [29] reported that there were significant differences in the biofilm producing capacity amongst isolates from various hospital wards. Similarly, this study detected a high capacity to form a biofilm (using the MTP method) which correlated with the hospital wards. However, other factors, such as gender, presence of invasive devices, and prior or prolonged hospitalization have also been shown to be associated with biofilm production by microbes [24–26].
Consistent with Gad et al [20], icaAD genes were detected in 95.1% of S epidermidis isolates in this current study. In addition, there are some studies to support the appearance of biofilm-negative variants with both icaA and icaD genes [1,30]. Similarly, we also found such variants amongst the S epidermidis isolates studied. Presence of these genes without biofilm production can be explained by chromosomal point mutations or a negative translational or post-translational regulation, affecting the production of biofilm-associated proteins [9]. In addition, where 2 isolates were identified as biofilm-producer by the phenotypic method but the ica gene was absent, is suggesting that this may be due to an ica gene-independent control of biofilm formation/adhesion process in staphylococci [31].
This study may be limited by the small number of isolates, the lack of comprehensive clinical data of the patients, as well as the lack of evaluation of icaB and icaC genes. Furthermore, this study indicates that determination of expression levels of ica genes by quantitative real-time PCR may help assess the role of each corresponding gene in the production of biofilm.
In conclusion, although the TM method has the ability to detect biofilm-producing S epidermidis to the same level as the MTP, the latter would be more appropriate, as it is less costly as well as less likely that results would be misinterpreted. In contrast, CRA, although easier and faster to perform, showed low sensitivity, and therefore, cannot be recommended as a screening test for identifying biofilm production by S epidermidis. Moreover, our results revealed that the presence of ica genes alone does not lead to biofilm formation. On the other hand, the biofilm-forming ability of some isolates in the absence of ica gene emphasizes the importance of ica-independent mechanisms of biofilm formation. Due to increased biomaterial-related infections caused by biofilm-forming pathogens, the use of a reliable method to specifically detect biofilms would help to identify potentially infectious bacteria and aid the therapeutic decision-making in affected patients.
Acknowledgements
This study was carried out as part of a Master’s thesis of Ms. Maryam Kord. We are specifically thankful to the laboratory sciences research center and the department of research and technology, Golestan University of Medical Sciences, for their financial support.
Footnotes
Conflicts of Interest
The author has no conflicts of interest to declare.
References
- 1.Abu Taleb AMF, Mohamed MS, Abdel-Latif RS, Gouda M. The role of ica operon and biofilm formation in coagulase negative staphylococcal infection. Egyptian J Med Microbiol. 2012;21(1):21–32. doi: 10.12816/0004864. [DOI] [Google Scholar]
- 2.Rupp ME, Fey PD, Heilmann C, Götz F. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J Infect Dis. 2001;183(7):1038–42. doi: 10.1086/319279. [DOI] [PubMed] [Google Scholar]
- 3.Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of Staphylococci: an evaluation of three different screening methods. Indian J Med Microbiol. 2006;24(1):25–9. doi: 10.4103/0255-0857.19890. [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Chao X, Fei M, Dai Y, Liu B. Analysis of S. epidermidis icaA and icaD genes by polymerase chain reaction and slime production: a case control study. BMC Infect Dis. 2013;13:242. doi: 10.1186/1471-2334-13-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Cunha ML, Rugolo LM, Lopes CA. Study of virulence factors in coagulase-negative staphylococci isolated from newborns. Mem Inst Oswaldo Cruz. 2006;101(6):661–8. doi: 10.1590/S0074-02762006000600014. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira A, de Cunha ML. Comparison of methods for the detection of biofilm production in coagulase-negative staphylococci. BMC Res Notes. 2010;3:260. doi: 10.1186/1756-0500-3-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberto MC, Matera G, Quirino A, et al. Phenotypic and genotypic evaluation of slime production by conventional and molecular microbiological techniques. Microbiol Res. 2009;164(5):522–8. doi: 10.1016/j.micres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Thilakavathy P, Priyan RV, Jagatheeswari P, et al. Evaluation of ica gene in comparison with phenotypic methods for detection of biofilm production by coagulase negative staphylococci in a tertiary care hospital. J Clin Diagn Res. 2015;9(8):DC16–9. doi: 10.7860/JCDR/2015/11725.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darwish SF, Asfour HA. Investigation of biofilm forming ability in Staphylococci causing bovine mastitis using phenotypic and genotypic assays. ScientificWorldJournal. 2013;2013:378492. doi: 10.1155/2013/378492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman D, Falkiner F, Keane C. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42(8):872–4. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deka N. Comparison of tissue culture plate method, tube method and Congo red agar method for the detection of biofilm formation by coagulase negative Staphylococcus isolated from non-clinical isolates. Int J Curr Microbiol App Sci. 2014;3(10):810–5. [Google Scholar]
- 13.Carpaij N, Willems RJ, Bonten MJ, Fluit AC. Comparison of the identification of coagulase-negative staphylococci by matrix-assisted laser desorption ionization time-of-flight mass spectrometry and tuf sequencing. Eur J Clin Microbiol Infect Dis. 2011;30(10):1169–72. doi: 10.1007/s10096-011-1204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasannejad Bibalan M, Javid N, Samet M, Shakeri F, Ghaemi EA. Biofilm formation in Staphylococcus aureus and its relation to phenotypic and genotypic criteria. Med Lab J. 2014;8(3):1–7. in Persian. [Google Scholar]
- 15.Abou El-Khier NT, El-Kazzaz SS, Elganainy AE. Phenotypic and genotypic detection of biofilm formation in Staphylococcus epidermidis isolates from retrieved orthopaedic implants and prostheses. British Microbiol Res J. 2015;9(4):1–10. doi: 10.9734/BMRJ/2015/18650. [DOI] [Google Scholar]
- 16.Szweda P, Schielmann M, Milewski S, Frankowska A, Jakubczak A. Biofilm production and presence of ica and bap genes in Staphylococcus aureus strains isolated from cows with mastitis in the eastern Poland. Pol J Microbiol. 2012;61(1):65–9. [PubMed] [Google Scholar]
- 17.Mehri H, Jahanbakhsh R, Shakeri F, et al. Investigation of glycopeptide susceptibility of coagulase-negative staphylococci (CoNS) from a tertiary care hospital in Gorgan, northern Iran. Arch Pediatr Infect Dis. 2017;5(1):e37264. [Google Scholar]
- 18.Ebrahimi A, Ghasemi M, Ghasemi B. Some virulence factors of staphylococci isolated from wound and skin infections in Shahrekord, IR Iran. Jundishapur J Microbiol. 2014;7(4):e9225. doi: 10.5812/jjm.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ninin E, Caroff N, Espaze E, et al. Assessment of ica operon carriage and biofilm production in Staphylococcus epidermidis isolates causing bacteraemia in bone marrow transplant recipients. Clin Microbiol Infect. 2006;12(5):446–52. doi: 10.1111/j.1469-0691.2006.01382.x. [DOI] [PubMed] [Google Scholar]
- 20.Gad GF, El-Feky MA, El-Rehewy MS, Hassan MA, Abolella H, El-Baky RM. Detection of icaA, icaD genes and biofilm production by Staphylococcus aureus and Staphylococcus epidermidis isolated from urinary tract catheterized patients. J Infect Dev Ctries. 2009;3(5):342–51. doi: 10.3855/jidc.241. [DOI] [PubMed] [Google Scholar]
- 21.Saising J, Singdam S, Ongsakul M, Voravuthikunchai SP. Lipase, protease, and biofilm as the major virulence factors in staphylococci isolated from acne lesions. Biosci Trends. 2012;6(4):160–4. doi: 10.5582/bst.2012.v6.4.160. [DOI] [PubMed] [Google Scholar]
- 22.El-Mahallawy HA, Loutfy SA, El-Wakil M, El-Al AK, Morcos H. Clinical implications of icaA and icaD genes in coagulase negative staphylococci and Staphylococcus aureus bacteremia in febrile neutropenic pediatric cancer patients. Pediatr Blood Cancer. 2009;52(7):824–8. doi: 10.1002/pbc.21964. [DOI] [PubMed] [Google Scholar]
- 23.de Castro Melo P, Ferreira LM, Ferreira LM, Filho AN, Zafalon LF, Vicente HI, de Souza V. Comparison of methods for the detection of biofilm formation by Staphylococcus aureus isolated from bovine subclinical mastitis. Braz J Microbiol. 2013;44(1):119–24. doi: 10.1590/S1517-83822013005000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhai M, Malik A, Shahid M, Malik A, Rawat V. Colonization of peripheral intravascular catheters with biofilm producing microbes: Evaluation of risk factors. Nigerian Med J. 2012;53(1):37–41. doi: 10.4103/0300-1652.99830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves MJ, Barreira JC, Carvalho I, et al. Propensity for biofilm formation by clinical isolates from urinary tract infections: developing a multifactorial predictive model to improve antibiotherapy. J Med Microbiol. 2014;63(Pt 3):471–7. doi: 10.1099/jmm.0.071746-0. [DOI] [PubMed] [Google Scholar]
- 26.Cha JO, Yoo JI, Yoo JS, et al. Investigation of biofilm formation and its association with the molecular and clinical characteristics of methicillin-resistant Staphylococcus aureus. Osong Public Health Res Perspect. 2013;4(5):225–32. doi: 10.1016/j.phrp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez CJ, Jr, Mende K, Beckius ML, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solati SM, Tajbakhsh E, Khamesipour F, Gugnani HC. Prevalence of virulence genes of biofilm producing strains of Staphylococcus epidermidis isolated from clinical samples in Iran. AMB Express. 2015;5(1):134. doi: 10.1186/s13568-015-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thummeepak R, Kongthai P, Leungtongkam U, Sitthisak S. Distribution of virulence genes involved in biofilm formation in multi-drug resistant Acinetobacter baumannii clinical isolates. Int Microbiol. 2016;19(2):121–9. doi: 10.2436/20.1501.01.270. [DOI] [PubMed] [Google Scholar]
- 30.Los R, Sawicki R, Juda M, et al. A comparative analysis of phenotypic and genotypic methods for the determination of the biofilm-forming abilities of Staphylococcus epidermidis. FEMS Microbiol Lett. 2010;310(2):97–103. doi: 10.1111/j.1574-6968.2010.02050.x. [DOI] [PubMed] [Google Scholar]
- 31.Bartoszewicz M, Nowicka J, Janikowska E, Przondo-Mordarska A. Adhesion dependence of coagulase-negative staphylococci to biomaterials and PIA polysacchapjde synthesis on icaADBC operon presence. Med Dosw Mikrobiol. 2004;56(3):225–30. in Polish. [PubMed] [Google Scholar]