ABSTRACT
A mechanism participating in energy sensing and signalling in plants involves the regulation of sucrose non-fermenting1 (Snf1)-related protein kinase 1 (SnRK1) activity in response to sugar availability. SnRK1 is thought to regulate the activity of both metabolic enzymes and transcription factors in response to changes in energy availability, with trehalose-6-phospate functioning as a signalling sugar that suppresses SnRK1 activity under sugar-replete conditions. Sucrose supplementation increases the elongation of hypocotyls of developing Arabidopsis seedlings, and this response to sucrose involves both the SnRK1 subunit KIN10 and also TREHALOSE-6-PHOSPHATE SYNTHASE1 (TPS1). Here, we measured sucrose-induced hypocotyl elongation in two insertional mutants of KIN10 (akin10 and akin10-2). Under short photoperiods, sucrose supplementation caused great proportional hypocotyl elongation in these KIN10 mutants compared with the wild type, and these mutants had shorter hypocotyls than the wild type in the absence of sucrose supplementation. One interpretation is that SnRK1 activity might suppress hypocotyl elongation in the presence of sucrose, because KIN10 overexpression inhibits sucrose-induced hypocotyl elongation and akin10 mutants enhance sucrose-induced hypocotyl elongation.
KEYWORDS: Arabidopsis, signal transduction, metabolism, development
We reported recently the involvement of a sugar-signalling mechanism in a pathway that causes hypocotyl elongation in response to sucrose.1 Hypocotyl elongation in Arabidopsis thaliana (Arabidopsis) seedlings is caused by cell expansion within the elongating hypocotyl and represents an informative experimental model to study signalling processes that regulate development. In Arabidopsis, hypocotyl length is increased by supplementation of the growth media with sucrose.2-9 We identified that the sugar- and energy-sensing kinase sucrose non-fermenting1 (Snf1)-related protein kinase 1 (SnRK1) regulates sucrose-induced hypocotyl elongation.1 Under short photoperiods, hypocotyls did not elongate in response to exogenous sucrose in seedlings overexpressing the catalytic alpha subunit of SnRK1, termed SNF1-RELATED PROTEIN KINASE1.1 (KIN10/AKIN10/SnRK1.1).1 We also found that TREHALOSE-6-PHOSPHATE SYNTHASE1 (TPS1) is required for sucrose-induced hypocotyl elongation under short photoperiods.1 TPS1 synthesizes the sugar trehalose-6-phosphate (Tre6P), which is a potent inhibitor of SnRK1 activity.10 Tre6P is thought to function as a signalling sugar that provides information about cellular energy availability.10,11
Hypocotyl elongation in response to sucrose might be suppressed in overexpressors of KIN10 (KIN10-ox) because SnRK1 activity is thought to inhibit growth and catabolism under conditions of starvation,12-14 preventing seedlings from taking advantage of the additional sugars.1 We reasoned that the converse might be true when SnRK1 activity is low, as occurs under sugar-replete conditions.10 To investigate this, we measured the elongation of hypocotyls in response to sucrose in two T-DNA mutants of the KIN10 catalytic subunit of SnRK1 (GABI_579E09 or akin1015, and SALKseq_093965, a new allele named here akin10-2 for consistency) (Fig S1A). The full-length KIN10 transcript is absent in these akin10 and akin10-2 T-DNA lines (Fig. S1B). In the akin10 mutant, there is a partial loss of phosphorylation of the SnRK1 target bZIP63, most likely due to reduced SnRK1 activity.15 The remaining phosphorylation of bZIP63 in akin10 is likely due to KIN11 activity.15
Supplementation of wild type seedlings with 3% (w/v) sucrose increased the hypocotyl length under short photoperiods but not under long photoperiods (Fig. 1A, B), as we reported previously.1 Sucrose supplementation also increased the hypocotyl length of two akin10 mutants under both short and long photoperiods (Fig. 1A, B). Under short photoperiods, sucrose caused a greater increase in hypocotyl length in akin10 (6.51 mm longer, 224% increase) and akin10-2 (6.90 mm longer, 286% increase) compared with the wild type (3.75 mm longer, 67% increase) (Fig. 1A). This greater fold-change in hypocotyl length in the akin10 mutants under these conditions is because the mutants had significantly shorter hypocotyls than the wild type in the absence of sucrose (Fig. 1A). Under long photoperiods, sucrose supplementation induced hypocotyl elongation in akin10 mutants, which contrasted the wild type in which sucrose supplementation did not increase hypocotyl length (Fig. 1B). We found previously that sucrose supplementation can decrease the hypocotyl length of the Landsberg erecta background under long photoperiods.1 but this did not occur in the Col-0 background used here (Fig. 1B), suggesting that there is some variation between accessions in this developmental response to sucrose.
Figure 1.
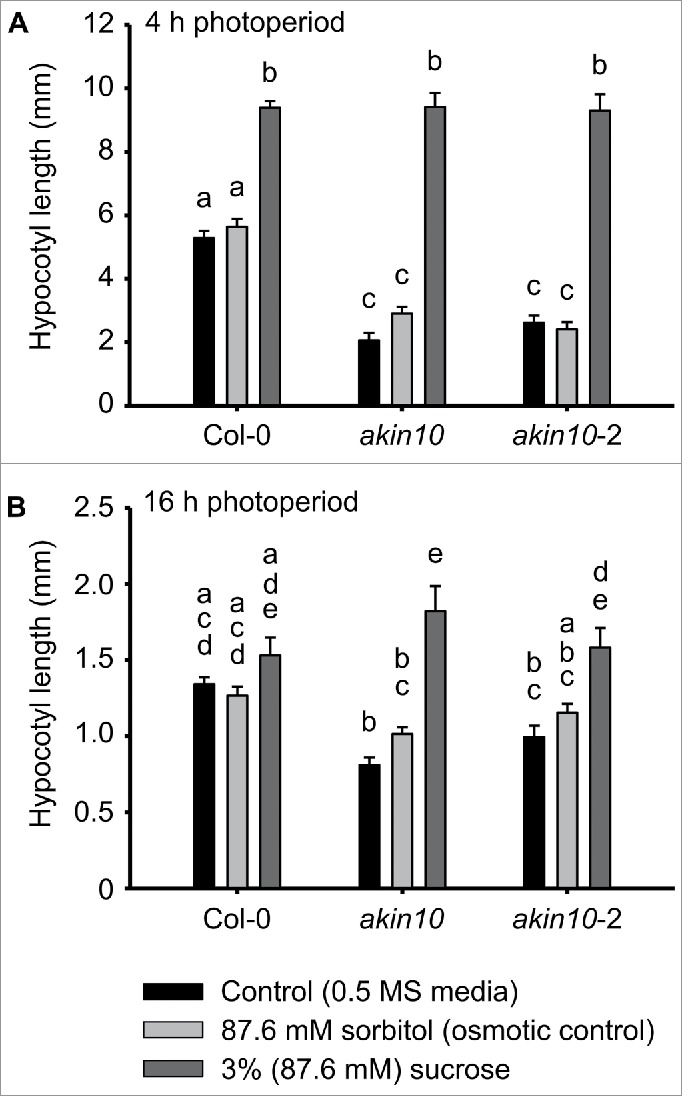
Sucrose-induced hypocotyl elongation in wild type and akin10 seedlings measured under (A) 4 h and (B) 16 h photoperiods. Seedlings were cultivated on control media (half strength Murashige and Skoog medium with 0.8% (w/v) agar; 0.5 MS), an equimolar osmotic control (sorbitol), or 3% (w/v) sucrose. Measurement of hypocotyl elongation was conducted as described by Simon et al. 2018. Statistical significance indicated for comparison between seedlings supplemented with 3% (w/v) sucrose and 87.6 mM sorbitol (osmotic control); analysis by univariate ANOVA followed by post-hoc Tukey analysis. Different letters indicate statistically-significant differences between means (p < 0.05); n = 20 +/- s.e.m.
Hypocotyls of akin10 and akin10-2 mutants were significantly shorter than the wild type when cultivated in the absence of sucrose on 0.5MS meda (4 h photoperiods, akin10 p < 0.001; akin10-2 p < 0.001; 16 h photoperiods, akin10 p < 0.006; akin10-2 p < 0.001). In addition to changes in phytohormone signalling, the reduced hypocotyl elongation of akin10 mutations might derive from altered seed quality,16 attenuated seedling development as occurs in tps1 knockouts,17 altered circadian regulation,18 or altered carbohydrate utilization.12,19
The greater proportional increase in hypocotyl length that was caused by sucrose in akin10 mutants compared with the wild type suggests that SnRK1 activity might contribute to suppression of hypocotyl elongation in response to sucrose. This is because KIN10 forms a catalytic subunit of SnRK1, and in the absence of this catalytic subunit there was an increase in the magnitude of sucrose-induced hypocotyl elongation. Although KIN10 and KIN11 are thought to confer kinase activity to the SnRK1 complex,12, 15 akin10 single mutants change the response of elongating hypocotyls to sucrose (Fig. 1). This indicates that KIN11 cannot completely replace KIN10 within the mechanisms underlying sucrose-induced hypocotyl elongation. This is consistent with the loss of SnRK1 kinase activity in the akin10 single mutant.15 An alternative interpretation is that there is some suppression of hypocotyl elongation in the akin10 mutants in the absence of sucrose, and that this phenotype is lost in the presence of sucrose supplementation (Fig. 1A). Under long photoperiods, sucrose does not cause hypocotyl elongation in the wild type (Fig. 1B), which appears to be due to a combination of photoperiod and daily light input.1 In comparison, there was sucrose-induced hypocotyl elongation in two akin10 mutants under long photoperiods. However, under 16 h photoperiods sucrose induced a smaller increase in hypocotyl length in the akin10 mutants than in akin10 mutants under 4 h photoperiods. Therefore, as with the wild type,1 photoperiod and/or daily light input influence the magnitude of sucrose-induced hypocotyl elongation in akin10 mutants. This suggests that mechanisms additional to KIN10 activity within SnRK1 contribute to the photoperiod/daily light input within the response of elongating hypocotyls to sucrose. Such additional mechanisms could include the circadian oscillator, phototransduction pathways, and additional energy-sensing mechanisms.
Supplementary Material
Funding Statement
This work was supported by the Biotechnology and Biological Sciences Research Council (grant BB/J014400/1).
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Acknowledgments
We thank BBSRC (UK) for funding (South-West Doctoral Training Partnership grant BB/J014400/1). We thank Prof. Alistair Hetherington for donating akin10 mutants and Dr. Jean-Charles Isner for discussion about reference transcripts.
References
- 1.Simon NML, Kusakina J, Fernandez-Lopez A, Chembath A, Belbin FE, Dodd AN. The energy-signaling hub SnRK1 is important for sucrose-induced hypocotyl elongation. Plant Physiol. 2018;176:1299–310. doi: 10.1104/pp.17.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurata T, Yamamoto KT. petit1, a conditional growth mutant of Arabidopsis defective in sucrose-dependent elongation growth. Plant Physiol. 1998;118:793. doi: 10.1104/pp.118.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H. Sugar-induced adventitious roots in Arabidopsis seedlings. J Plant Res. 2003;116:83–91. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Liu Z, Wang L, Zheng S, Xie J, Bi Y. Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J Plant Physiol. 2010;167:1130–6. doi: 10.1016/j.jplph.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Zhang Y, Liu R, Hao H, Wang Z, Bi Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J Plant Physiol. 2011;168:1771–9. doi: 10.1016/j.jplph.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JL, Maloof JN, Nemhauser JL. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS One. 2011;6:e19894. doi: 10.1371/journal.pone.0019894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart Lilley JL, Gee CW, Sairanen I, Ljung K, Nemhauser JL. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012;160:2261–70. doi: 10.1104/pp.112.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Zhu J-Y, Roh J, Marchive C, Kim S-K, Meyer C, Sun Y, Wang W, Wang ZY. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr Biol. 2016;26:1854–60. doi: 10.1016/j.cub.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Liu Z, Wang J, Chen Y, Bi Y, He J. Brassinosteroid is required for sugar promotion of hypocotyl elongation in Arabidopsis in darkness. Planta. 2015;242:881–93. doi: 10.1007/s00425-015-2328-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. Inhibition of SNF1-related protein kinase 1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009;149:1860–71. doi: 10.1104/pp.108.133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, et al.. The sucrose–trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot. 2014;65:1051–68. doi: 10.1093/jxb/ert457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baena-González E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–42. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 13.Baena-González E, Sheen J. Convergent energy and stress signaling. Trends Plant Sci. 2008;13:474–82. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delatte TL, Sedijani P, Kondou Y, Matsui M, de Jong GJ, Somsen GW, Wiese-Klinkenberg A, Primavesi LF, Paul MJ, Schluepmann H. Growth arrest by trehalose-6-phosphate: An astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiol. 2011;157:160. doi: 10.1104/pp.111.180422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mair A, Pedrotti L, Wurzinger B, Anrather D, Simeunovic A, Weiste C, Valerio C, Dietrich K, Kirchler T, Nägele T, et al.. SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. Elife. 2015;4:e05828. doi: 10.7554/eLife.05828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radchuk R, Radchuk V, Weschke W, Borisjuk L, Weber H. Repressing the expression of the SUCROSE NONFERMENTING-1-RELATED PROTEIN KINASE gene in pea embryo causes pleiotropic defects of maturation similar to an abscisic acid-insensitive phenotype. Plant Physiol. 2006;140:263–78. doi: 10.1104/pp.105.071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA. AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J. 2010;64:1–13. [DOI] [PubMed] [Google Scholar]
- 18.Shin J, Sánchez-Villarreal A, Davis AM, Du S-x, Berendzen KW, Koncz C, Ding Z, Li C, Davis SJ. The metabolic sensor AKIN10 modulates the Arabidopsis circadian clock in a light-dependent manner. Plant Cell Environ. 2017;40:997–1008. doi: 10.1111/pce.12903. [DOI] [PubMed] [Google Scholar]
- 19.Jossier M, Bouly J-P, Meimoun P, Arjmand A, Lessard P, Hawley S, Grahame Hardie D, Thomas M. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 2009;59:316–28. doi: 10.1111/j.1365-313X.2009.03871.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


