Abstract
Abnormal cholesterol metabolism is an established feature of Alzheimer’s disease (AD). Cerebrospinal fluid (CSF) is the fluid surrounding the central nervous system, and the protein and lipid content alterations in the CSF could be biomarkers for degenerative changes in the brain. The laboratory diagnosis of AD is limited to the analysis of three biomarkers in CSF: Aβ42, total tau, and phospho-tau. The purpose of this analysis is to systematically analyze the available data describing the biomarkers of cholesterol and its metabolites in the CSF of subjects with AD. MEDLINE, EMBASE, and the Cochrane Central database were systematically queried to collect studies that have evaluated the markers of cholesterol and its metabolites in the CSF of subjects with mild cognitive impairment (MCI) or AD and age-matched controls. Analysis of the published data shows that the levels of cholesterol are increased in MCI subjects; 24-hydroxycholesterol and 27-hydroxycholesterol are elevated in AD and MCI subjects compared to controls. There is a significant dysfunction of cholesterol metabolism in the CSF of AD subjects. This analysis indicates that in addition to the available biomarkers in the CSF, such as Aβ42, total tau, and phospho-tau, 24-hydroxycholesterol, 27-hydroxycholesterol, and cholesterol appear to be sensitive biomarkers for the evaluation of MCI and AD.
Keywords: Alzheimer’s disease, cerebrospinal fluid, cholesterol, 24-hydroxycholesterol, 27-hydroxycholesterol
INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disorder resulting in cognitive impairment. A diagnosis of AD is made according to the clinical symptoms of the patients; laboratory diagnosis is limited to the analysis of three biomarkers in the cerebrospinal fluid (CSF): amyloid-β42 (Aβ42), total tau, and phospho-tau [1]. CSF Aβ42 shows the best diagnostic accuracy among the CSF biomarkers at a sensitivity of 85%, and the specificity in the differentiation of AD dementia ranged from 42% to 77% [2].
The human brain contains approximately 25% of the total body cholesterol, which is involved in neuronal structure and function and is an essential component of the neuronal membranes required for membrane lipid organization, regulating the fluidity of membranes and the structural disposition of membrane proteins. Cholesterol metabolism plays a key role in amyloidogenesis in the brain [3]. Experimental in vivo and in vitro studies suggest that excess cholesterol in the brain increases amyloid production [4, 5] via tau phosphorylation, aggregation, and deposition [6]. Aberrant cholesterol metabolism has been implicated in the development of AD as one of the main drivers of AD mechanisms [4].
The blood-brain barrier prevents direct cholesterol uptake from the circulation; de novo cholesterol synthesis is responsible for almost all cholesterol present in the brain. Therefore, the cholesterol levels in the peripheral blood are regulated differently than the cholesterol levels in the central nervous system and the CSF. In the brain, 24-hydroxycholesterol (24OHC) is the end product of cholesterol elimination by the neurons; its levels in plasma are proportional to the degree of brain atrophy and the loss of active grey matter, and the levels of CSF 24OHC are related to the amount of Aβ, tau, and phospho-tau in AD subjects [7]. The correlation between CSF and plasma 27-hydroxycholesterol (27-OHC) is hypothesized to be related to hypercholesterolemia and AD [7].
In this analysis, we summarize the studies of cholesterol and its metabolites 24-OHC and 27-OHC in the CSF of mild cognitive impairment (MCI) and AD patients. Our findings provide an overview of lipid biomarkers in AD, which may be used for risk assessment, diagnosis, and the determination of therapeutic implications.
METHODS
The data search was completed on 1 December 2014. Studies were identified from systematic the searching of MEDLINE, EMBASE, and the Cochrane Central database. The search strategy used Mesh phrases and words such as “Alzheimer’s disease, cholesterol, hydroxycholesterols, 24-hydroxycholesterol, 27-hydroxycholesterol, oxysterols, and cerebrospinal fluid,” as well as a review of the reference lists of included articles and previous systematic reviews for additional relevant citations.
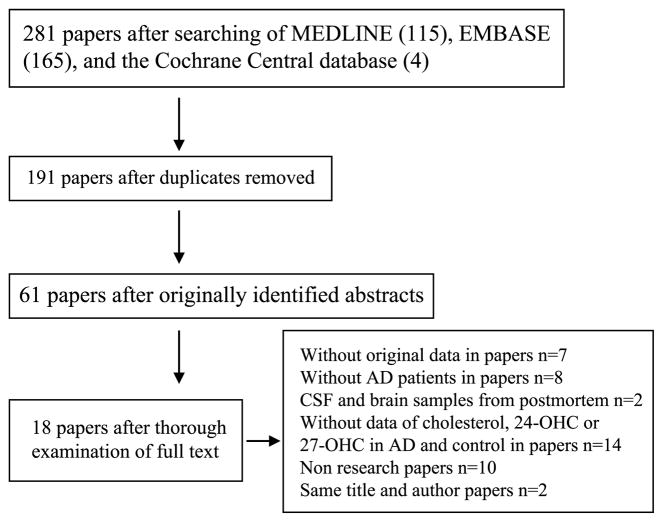
Studies on human subjects were considered for inclusion (studies including case and control subjects; CSF collected by lumbar puncture; data expressed as the mean and SD), and a quality assessment of the included studies was conducted using the Newcastle-Ottawa scale (Table 1). Of the 284 originally identified abstracts, 61 met the initial inclusion criteria. Following a thorough examination, we excluded 43 of the 61 studies (without original data in papers, n = 7; without AD patients in papers, n = 8; postmortem CSF and brain samples, n = 2; without cholesterol data, 24-OHC data or 27-OHC data in AD and control subjects, n = 14; non-research papers, n = 10; same title and author, n = 2); the remaining 18 studies were included in this analysis (Fig. 1). Important details regarding the subjects, methods and measurements were extracted from the selected articles and summarized (Table 2).
Table 1.
Quality assessment of included studies using the Newcastle-Ottawa scale. High-quality choices were identified with a star. The more stars allocated to a study, the better quality it was. A study could be awarded a maximum of one star for each numbered item within the “Selection” and “Exposure/Outcome” categories. A maximum of two stars could be given for the “Comparability” category. The definitions of controls underwent minor revisions. MMSE, Mini-Mental State Examination
| Reference | Selection | Comparability | Exposure/Outcome | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Cases definition adequate | Representativeness of cases | Selection of controls | Definition of controls | Age and gender | Additional factors (MMSE) | Ascertainment of exposure | Same method for case and controls | Non-response rate | ||
| Papassotiropoulos 2002 [8] | * | * | * | * | * | * | * | 7 | ||
| Martínez-Morillo 2014 [9] | * | * | * | * | * | * | * | 7 | ||
| Kölsch 2006 [10] | * | * | * | * | * | * | * | 7 | ||
| Kölsch 2009 [11] | * | * | * | * | * | * | * | 7 | ||
| Kölsch 2009 [12] | * | * | * | * | * | * | * | 7 | ||
| Kölsch 2010 [13] | * | * | * | * | * | * | * | 7 | ||
| Qureischie 2008 [14] | * | * | * | * | * | * | * | 7 | ||
| Mateos 2011 [15] | * | * | * | * | * | * | * | 7 | ||
| Wollmer 2003 [16] | * | * | * | * | * | * | * | 7 | ||
| Wollmer 2003 [17] | * | * | * | * | * | * | * | 7 | ||
| Shafaati 2007 [18] | * | * | * | * | * | * | 6 | |||
| Schönknecht 2002 [19] | * | * | * | * | * | * | * | 7 | ||
| Popp 2012 [20] | * | * | * | * | * | * | * | 7 | ||
| Popp 2013 [21] | * | * | * | * | * | * | * | 7 | ||
| Vanmierlo 2011 [22] | * | * | * | * | * | * | * | 7 | ||
| Leoni 2004 [23] | * | * | * | * | * | 5 | ||||
| Leoni 2006 [24] | * | * | * | * | * | * | * | 7 | ||
| Leoni 2013 [25] | * | * | * | * | * | * | * | 7 | ||
Fig. 1.
Flow diagram of selection of studies focusing on cholesterol, 24-hydroxycholesterol, 27-hydroxycholesterol in the CSF of Alzheimer’s disease subjects.
Table 2.
Eighteen studies including details regarding the subjects, methods and measurement were extracted and summarized.
| Reference | Participants categorization | Method | Measurement | ||
|---|---|---|---|---|---|
|
| |||||
| AD | MCI | Controls | |||
| Papassotiropoulos 2002 [8] | N = 32 | n = 7 | n = 7 | gas | cholesterol |
| Age: 69 ± 8 | Age: 65 ± 8 | Age: 55 ± 10 | chromatography | 24-hydroxycholesterol | |
| Female: 62% | Female: 57% | Female: 29% | mass spectrometry | ||
| Martínez-Morillo 2014 [9] | n = 38 | – | n = 37 | mass spectrometry | cholesterol |
| Age: from (43)# | Age: from (43)# | based assay | |||
| 78 (60–94)& | Female: from (43) 53% | ||||
| Female: from (43) 64% | 61 (43–80)& | ||||
| Kölsch 2006 [10] | n = 75 | – | n = 39 | gas | cholesterol |
| Age: 68.4 ± 7.9 | Age: 65.9 ± 11.4 | chromatography | |||
| Female: 63.4% | Female: 53.8% | mass spectrometry | |||
| Kölsch 2009 [11] | n = 118 | – | n = 62 | gas | cholesterol |
| Age: 68.5 ± 7.9 | Age: 70.4 ± 7.1 | chromatography | 24-hydroxycholesterol | ||
| Female: 61.9% | Female: 57.9% | mass spectrometry | |||
| Kölsch 2009 [12] | n = 149 | – | n = 86 | gas | cholesterol |
| Age: from (405)# 74.1 ± 7.9 | Age: from (405)# 72.8 ± 7.6 | chromatography | 24-hydroxycholesterol | ||
| Female: from (405)# 69.1% | Female: from (405)# 53.5% | mass spectrometry | |||
| Kölsch 2010 [13] | n = 90 | – | n = 57 | gas | cholesterol |
| Age:70.6 ± 8.3 | Age:69.3 ± 6.8 | chromatography | |||
| Female: 63.9% | Female: 51.6% | flame ionization | |||
| Qureischie 2008 [14] | n = 104 | – | n = 49 | gas | cholesterol |
| Age: from (351)# 72.5 ± 8.8 | Age: from (388)# 72.4 ± 7.9 | chromatography | 24-hydroxycholesterol | ||
| Female: from (351)# 68% | Female: from (351)# 53.4% | mass spectrometry | |||
| Mateos 2011 [15] | n = 21 | n = 10 | n = 28 | isotope | cholesterol |
| Age: 67.3 ± 1.70 | Age: 61.2 ± 2.33 | Age: 57.8 ± 1.27 | dilution | 24-hydroxycholesterol | |
| Female: 66.7% | Female: 30% | Female: 67.9% | mass spectrometry | 27-hydroxycholesterol | |
| Wollmer 2003 [16] | n = 24 | – | n = 22 | gas | cholesterol |
| Age: from (169)# 73.5 ± 5.5 | Age: from (166)# 70.1 ± 6.3 | chromatograph | |||
| Female: from (169) 58% | Female: from (166) 49.4% | ||||
| Wollmer 2003 [17] | n = 24 | – | n = 22 | gas | cholesterol |
| Age: 71 from (309)# 71.7 ± 7.8 | Age: 65.6 from (356)# | chromatography | |||
| Female: ? | 68.7 ± 8.6 | mass spectrometry | |||
| Female: ? | |||||
| Shafaati 2007 [18] | n = 17 | n = 20 | n = 43 | isotope | cholesterol |
| Age:73 (62–83)& | Age: 56.5(45–83)& | Age: 50.5 (18–85)& | dilution-mass | 24-hydroxycholesterol | |
| Female: 41.2% | Female: 50% | Female: 65.1% | spectrometry | 27-hydroxycholesterol | |
| Schönknecht 2002 [19] | n = 17 | – | n = 55 | gas | cholesterol |
| Age: 75.4 ± 10.3 | Age: 69.0 ± 5.8 | chromatography | 24-hydroxycholesterol | ||
| Female: 42.9% | Female: 40% | mass spectrometry | |||
| Popp 2012 [20] | n = 53 | – | n = 43 | gas | cholesterol |
| Age: 71.23 ± 8.29 | Age: 67.33 ± 9.04 | chromatography | 24-hydroxycholesterol | ||
| Female: 62.3% | Female: 51.2% | mass spectrometry | 27-hydroxycholesterol | ||
| Popp 2013 [21] | n = 106 | – | n = 87 | gas | cholesterol |
| Age: 71.1 ± 7.87 | Age: 67.7 ± 9.13 | chromatography–mass | 24-hydroxycholesterol | ||
| Female: 64.2% | Female: 49.4% | spectrometry | 27-hydroxycholesterol | ||
| Vanmierlo 2011 [22] | n = 67 | – | n = 29 | gas | cholesterol |
| Age: 71.8 ± 7.5 | Age: 69.0 ± 6.9 | chromatography–mass | |||
| Female: 44.8% | Female: 62.7% | spectrometry | |||
| Leoni 2004 [23] | n = 54 | – | n = 58 | isotope-dilution | 24-hydroxycholesterol |
| Age: 72 | Age: 39 | mass | 27-hydroxycholesterol | ||
| Female: 55.6% | Female: 72.4% | spectrometry | |||
| Leoni 2006 [24] | n = 18 | n = 20 | n = 35 | isotope | 24-hydroxycholesterol |
| Age: 76.6 ± 4.2 | Age: 61.3 ± 10.4 | Age: 58.4 ± 7.4 | dilution-mass | 27-hydroxycholesterol | |
| Female: 44.4% | Female: 50% | Female: 57.1% | spectrometry | ||
| Leoni 2013 [25] | n = 24 | n = 27 | n = 28 | isotope | cholesterol |
| Age: 66.8 ± 8.0 | Age: 60.6 ± 9.5 | Age: 68.6 ± 2.85 | dilution-mass | 24-hydroxycholesterol | |
| Female: 70.8% | Female: 44% | Female: 65.8% | spectrometry | ||
indicated the original sample.
indicated that data are presented as median, min-maximal intervals.
Data were extracted and compiled as summary statistics (N, mean, and SD) and then pooled using an inverse-variance method. Heterogeneity between the studies was assessed using Cochran’s Chi-squared test for homogeneity (Chi2), and the amount of variation due to heterogeneity was estimated by calculating the I2. As heterogeneity was invariably high, random-effects meta-analyses were performed on the estimates to generate summary values (Review Manager Version 5.2, The Nordic Cochrane Centre, Copenhagen, Denmark). The results were presented as forest plots and determined to be significant when p < 0.05.
RESULTS
Study characteristics and heterogeneity
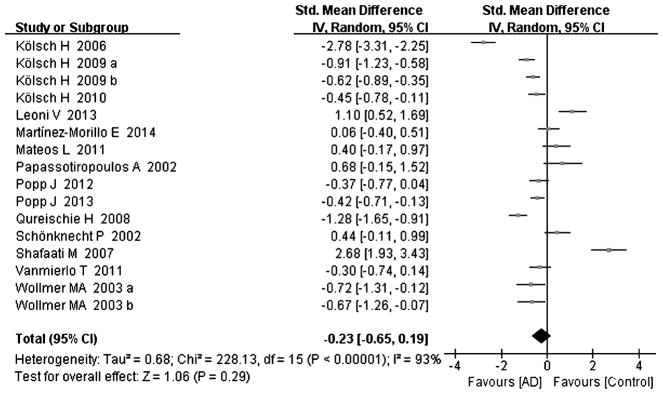
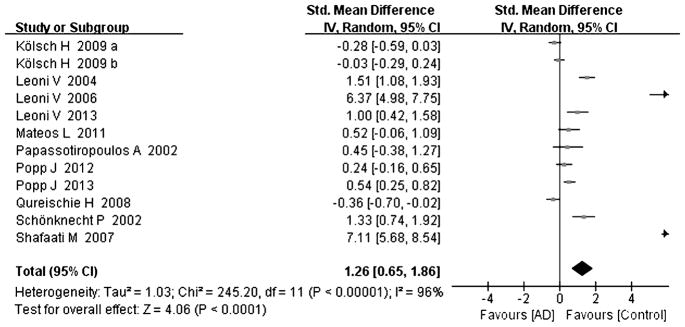
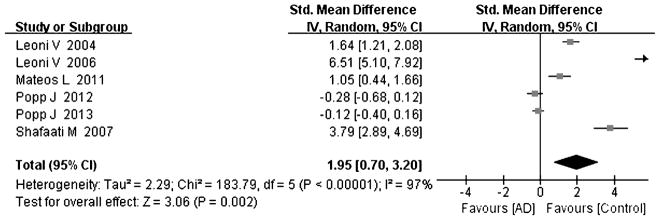
Tables 1 and 2 summarize the 18 studies included in this analysis [8–25]. All studies were judged to be of good quality using the Newcastle–Ottawa scale (Table 1). All studies examined the concentration of cholesterol and its metabolites with gas chromatography or/and mass-based methods. The diagnosis of AD was made according to the DSM IV (Diagnostic and Statistical Manual of Mental Disorders), and/or the criteria of the NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer Disease and Related Disorders Association). The diagnosis of MCI was conducted according to the reference [8, 15, 18, 24, 25]. Heterogeneity between the studies was assessed (AD versus controls: Fig. 2, cholesterol, Chi2 = 228.13, I2 = 93%; Fig. 3, 24-OHC, Chi2 = 245.20, I2 = 96%; Fig. 4, 27-OHC, Chi2 = 183.79, I2 = 97%; and MCI versus controls: Fig. 5, cholesterol, Chi2 = 18.2, I2 = 83%; Fig. 6, 24-OHC, Chi2 = 67.78, I2 = 94%; Fig. 7, 27-OHC, Chi2 = 104.55, I2 = 98%). As heterogeneity was invariably high, random-effects meta-analyses were performed in this study.
Fig. 2.
Forest plot comparing CSF cholesterol concentrations in subjects with Alzheimer’s disease and controls.
Fig. 3.
Forest plot comparing CSF 24-hydroxycholesterol concentrations in subjects with Alzheimer’s disease and controls.
Fig. 4.
Forest plot comparing CSF 27-hydroxycholesterol concentrations in subjects with Alzheimer’s disease and controls.
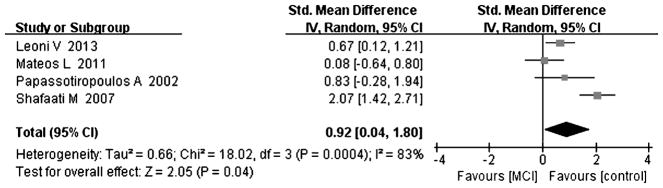
Fig. 5.
Forest plot comparing CSF cholesterol concentrations in subjects with MCI and controls.
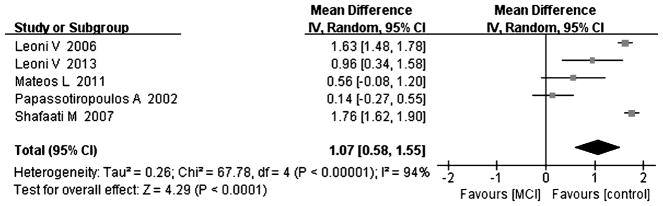
Fig. 6.
Forest plot comparing CSF 24-hydroxycholesterol concentrations in subjects with MCI and controls.
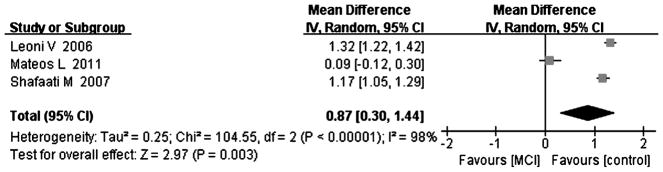
Fig. 7.
Forest plot comparing CSF 27-hydroxycholesterol concentrations in subjects with MCI and controls.
Cholesterol and its metabolites in the CSF of AD and control subjects
Sixteen studies reporting cholesterol levels were included in this meta-analysis, including 959 subjects with AD and 694 controls; cholesterol levels were not different between the AD subjects and controls [8–22, 25], with an effect size of −0.23 (95% CI −0.65, 0.19, p = 0.29, Fig. 2), omitting one similar study by Wollmer [16], where the overall effect size had no significant influence (95% CI −0.65, 0.25, p = 0.38, Supplement Figure 1).
24-OHC in AD subjects has been described in twelve studies including 713 AD subjects and 581 controls [8, 11, 12, 14, 15, 18–21, 23–25]. Cumulatively, 24-OHC was significantly increased in the CSF of AD subjects compared to that of controls, with an effect size of 1.26 (95% CI 0.65, 1.86, p < 0.05, Fig. 3); nine of twelve studies had effect sizes that were positive [8, 15, 18–21, 23–25], ranging from 0.24 to 7.11, and the other three studies had effect sizes ranging from −0.03 to −0.36 [11, 12, 14].
Six studies describing 27-OHC in the CSF of subjects (including 269 AD cases and 294 controls) [15, 18, 20, 21, 23, 24] were conducted, and the data suggested that there was a significant increase in 27-OHC in the CSF of AD subjects (effect size 1.95, 95% CI 0.7, 3.20, p < 0.05, Fig. 4) compared to controls; four of six studies had a positive effect size ranging from 1.05 to 6.51 [15, 18, 23, 24], and the other two studies had an effect size ranging from −0.12 to −0.28 [20, 21].
Cholesterol and its metabolites in the CSF in MCI and control subjects
Compared with controls, four studies indicated that cholesterol levels were elevated in MCI subjects [8, 15, 18, 25], and the overall effect size was 0.92 (95% CI 0.04, 1.80, p < 0.05, Fig. 5, including 64 subjects with MCI and 106 controls). Five studies including 84 MCI subjects and 141 controls tested for 24-OHC in the CSF [8, 15, 18, 24, 25]; the results of the above studies indicated that there was a significant increase in 24-OHC in MCI subjects compared to controls, with an effect size of 1.07 (95% CI 0.58, 1.55, p < 0.05, Fig. 6). Three studies including 50 MCI subjects and 106 controls and showed increased levels of 27-OHC in MCI subjects [15, 18, 24] compared to controls, with an overall effect size of 0.87 (95% CI 0.30, 1.44, p < 0.05, Fig. 7). All of above studies had a positive effect size (cholesterol: 0.08 to 2.07, 24-OHC: 0.14 to 1.76, 27-OHC: 0.09 to 1.32), indicating that MCI cases had higher cholesterol, 24-OHC, and 27-OHC concentrations in their CSF compared to controls.
DISCUSSION
The brain is the most cholesterol-rich organ in the human body. Cholesterol is an essential component of the neuronal membranes required for membrane lipid organization and also participates in signal transduction, neurotransmitter release, synaptogenesis, and membrane trafficking [26, 27]. It has been shown that a small amount of cholesterol from the periphery can enter the brain through the blood-brain barrier [28] and could play a role in hypercholesterolemia and the CSF levels of 24-OHC and 27-OHC. Refolo et al. also find that diet-induced hypercholesterolemia significantly increase beta-amyloid load in the central nervous system in a transgenic mouse model [28]. In our previous study, we find that abnormal cholesterol metabolism occurs during normal aging and in the process of aging in an AD mouse model [29, 30]. There is evidence of dysfunction of the cholesterol metabolism in AD patients, and Wolozin et al. find that therapy with statins is beneficial for reducing the prevalence of probable AD [31], however, the exact mechanism and effect of statins to AD remains to be explored [32, 33]. Puglielli et al. report that disordered lipid metabolism is one of the main mechanisms of AD; both the generation and clearance of Aβ are regulated by cholesterol, and the variant of the apolipoprotein E gene is a major genetic risk factor for AD, consistent with a role for cholesterol levels [4]. In this analysis, cholesterol was elevated in MCI subjects. In Bennett’s study, over an average of 4.5 years of follow-up, 34% persons with MCI develop AD at a rate 3.1 times higher than those without cognitive impairment [34]. An epidemiologic study by Kivipelto et al. examined 1,409 patients over 20 years and find that peripheral hypercholesterolemia (among other risk factors such as hypertension) in middle age is a risk factor for the development of AD [35]. In another study conducted over a 30-year period, mid-life serum total cholesterol level is associated with an increased risk for AD [36]. Pappolla et al. review autopsy cases of patients and find that cholesterolemia is correlated with the presence of amyloid deposition in the youngest subjects (40 to 55 years) with early amyloid deposition [37]. In this analysis, cholesterol was altered in MCI subjects but was normal in AD subjects, suggest that cholesterol may play a role in preclinical AD, and we can use cholesterol metabolism in combination with the other three biomarkers to obtain a more accurate diagnosis and/or stratify patients according to their cholesterol status.
Interestingly, the changes in cholesterol levels occurred in the CSF of MCI subjects and not in AD subjects compared to controls. In four studies [8, 15, 18, 25], cholesterol levels were significantly elevated in MCI subjects compared to controls, and in the above studies, cholesterol levels were also tested, and it was found that the metabolites were all elevated in AD subjects compared to controls. Thus, we speculate that this difference in CSF cholesterol is associated with a sub-group of AD subjects with dysregulated lipid metabolism.
In this analysis, compared with the controls, convincing evidence of both the concentrations of 24-OHC and 27-OHC in CSF were increased in AD. 24-OHC has been found to regulate the amyloid precursor protein via the production of the amyloidogenic fragment [6]; 27-OHC is able to pass through the blood-brain barrier in the CSF and may thus contribute to amyloid deposition, linking hypercholesterolemia and AD pathogenesis [38]. Higher levels of the metabolites 24-OHC and 27-OHC in the CSF suggest an increase in cerebral cholesterol load, which, along with senile plaques and neurofibrillary tangles deposits, is a common feature of AD brains in postmortem examinations [39]. Certain polymorphisms in the gene that encodes for 24-OHC (the enzyme that metabolizes cholesterol into 24-OHC) have been associated with a higher risk of dementia and AD [40, 41]. Several reviews and studies have concluded that 24-OHC and 27-OHC may be appropriate biomarkers, followed by Aβ42, total tau, and phospho-tau, for AD screening [20, 25, 42, 43], and our analysis supports such a conclusion.
A previous systematic review concludes that 24-OHC in the blood may be an important potential biomarker for cholesterol metabolism in the brain and risk of AD [42], and we come to a similar conclusion regarding 24-OHC in the CSF. In another systematic review, there is association between high mid-life total serum cholesterol and an increased risk of AD; however, there is no evidence supporting an association with late-life total serum cholesterol [44]. The above result regarding the cholesterol levels in the serum is consistent with the levels of cholesterol in the CSF in our analysis.
In the context of this data, previous studies and reviews, we propose a mechanism in which cholesterol homeostasis is disturbed in preclinical AD, whereas metabolite dysregulation occurs throughout the disease process; cholesterol and its metabolite changes might serve as additional biomarkers for the diagnosis and screening of AD. However, most importantly, they might help to identify a sub-group of AD patients with lipid metabolism dysregulation who might have different clinical presentations and clinical courses.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31400969), Young Talents Program of Hebei Universities (BJ2014044), and Key basic research projects, applied basic research program of Hebei Province (14967725D), and Natural Science Foundation of Hebei Province (H2015206238).
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0734r2).
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150734.
References
- 1.Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2009;18:413–417. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 2.Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, Jessen F, Herukka SK, Soininen H, Maetzler W, Leyhe T, Burger K, Taniguchi M, Urakami K, Lista S, Dubois B, Blennow K, Hampel H. CSF biomarkers for the differential diagnosis of Alzheimer’s disease. A large-scale international multicenter study. Alzheimers Dement. 2015;11:1306–1315. doi: 10.1016/j.jalz.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, Beel AJ, Sanders CR. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 5.Mendoza-Oliva A, Ferrera P, Fragoso-Medina J, Arias C. Lovastatin differentially affects neuronal cholesterol and amyloid-beta production in vivo and in vitro. CNS Neurosci Ther. 2015;21:631–641. doi: 10.1111/cns.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djelti F, Braudeau J, Hudry E, Dhenain M, Varin J, Bieche I, Marquer C, Chali F, Ayciriex S, Auzeil N, Alves S, Langui D, Potier MC, Laprevote O, Vidaud M, Duyckaerts C, Miles R, Aubourg P, Cartier N. CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain. 2015;138:2383–2398. doi: 10.1093/brain/awv166. [DOI] [PubMed] [Google Scholar]
- 7.Leoni V, Caccia C. Potential diagnostic applications of side chain oxysterols analysis in plasma and cerebrospinal fluid. Biochem Pharmacol. 2013;86:26–36. doi: 10.1016/j.bcp.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Papassotiropoulos A, Lutjohann D, Bagli M, Locatelli S, Jessen F, Buschfort R, Ptok U, Bjorkhem I, von Bergmann K, Heun R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res. 2002;36:27–32. doi: 10.1016/s0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Morillo E, Hansson O, Atagi Y, Bu G, Minthon L, Diamandis EP, Nielsen HM. Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls. Acta Neuropathol. 2014;127:633–643. doi: 10.1007/s00401-014-1266-2. [DOI] [PubMed] [Google Scholar]
- 10.Kolsch H, Lutjohann D, Jessen F, Urbach H, von Bergmann K, Maier W, Heun R. Polymorphism in neuropeptide Y influences CSF cholesterol levels but is no major risk factor of Alzheimer’s disease. J Neural Transm. 2006;113:231–238. doi: 10.1007/s00702-005-0319-z. [DOI] [PubMed] [Google Scholar]
- 11.Kolsch H, Lutjohann D, Jessen F, Popp J, Hentschel F, Kelemen P, Schmitz S, Maier W, Heun R. CYP46A1 variants influence Alzheimer’s disease risk and brain cholesterol metabolism. Eur Psychiatry. 2009;24:183–190. doi: 10.1016/j.eurpsy.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kolsch H, Lutjohann D, Jessen F, Popp J, Hentschel F, Kelemen P, Friedrichs S, Maier TA, Heun R. RXRA gene variations influence Alzheimer’s disease risk and cholesterol metabolism. J Cell Mol Med. 2009;13:589–598. doi: 10.1111/j.1582-4934.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolsch H, Heun R, Jessen F, Popp J, Hentschel F, Maier W, Lutjohann D. Alterations of cholesterol precursor levels in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:945–950. doi: 10.1016/j.bbalip.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Qureischie H, Heun R, Lutjohann D, Popp J, Jessen F, Ledschbor-Frahnert C, Thiele H, Maier W, Hentschel F, Kelemen P, Kolsch H. CETP polymorphisms influence cholesterol metabolism but not Alzheimer’s disease risk. Brain Res. 2008;1232:1–6. doi: 10.1016/j.brainres.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Mateos L, Ismail MA, Gil-Bea FJ, Leoni V, Winblad B, Bjorkhem I, Cedazo-Minguez A. Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. J Alzheimers Dis. 2011;24:669–679. doi: 10.3233/JAD-2011-101512. [DOI] [PubMed] [Google Scholar]
- 16.Wollmer MA, Streffer JR, Tsolaki M, Grimaldi LM, Lutjohann D, Thal D, von Bergmann K, Nitsch RM, Hock C, Papassotiropoulos A. Genetic association of acyl-coenzyme A: Cholesterol acyltransferase with cerebrospinal fluid cholesterol levels, brain amyloid load, and risk for Alzheimer’s disease. Mol Psychiatry. 2003;8:635–638. doi: 10.1038/sj.mp.4001296. [DOI] [PubMed] [Google Scholar]
- 17.Wollmer MA, Streffer JR, Lutjohann D, Tsolaki M, Iakovidou V, Hegi T, Pasch T, Jung HH, Bergmann K, Nitsch RM, Hock C, Papassotiropoulos A. ABCA1 modulates CSF cholesterol levels and influences the age at onset of Alzheimer’s disease. Neurobiol Aging. 2003;24:421–426. doi: 10.1016/s0197-4580(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 18.Shafaati M, Solomon A, Kivipelto M, Bjorkhem I, Leoni V. Levels of ApoE in cerebrospinal fluid are correlated with Tau and 24S-hydroxycholesterol in patients with cognitive disorders. Neurosci Lett. 2007;425:78–82. doi: 10.1016/j.neulet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Schonknecht P, Lutjohann D, Pantel J, Bardenheuer H, Hartmann T, von Bergmann K, Beyreuther K, Schroder J. Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett. 2002;324:83–85. doi: 10.1016/s0304-3940(02)00164-7. [DOI] [PubMed] [Google Scholar]
- 20.Popp J, Lewczuk P, Kolsch H, Meichsner S, Maier W, Kornhuber J, Jessen F, Lutjohann D. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J Neurochem. 2012;123:310–316. doi: 10.1111/j.1471-4159.2012.07893.x. [DOI] [PubMed] [Google Scholar]
- 21.Popp J, Meichsner S, Kolsch H, Lewczuk P, Maier W, Kornhuber J, Jessen F, Lutjohann D. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem Pharmacol. 2013;86:37–42. doi: 10.1016/j.bcp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Vanmierlo T, Popp J, Kolsch H, Friedrichs S, Jessen F, Stoffel-Wagner B, Bertsch T, Hartmann T, Maier W, von Bergmann K, Steinbusch H, Mulder M, Lutjohann D. The plant sterol brassicasterol as additional CSF biomarker in Alzheimer’s disease. Acta Psychiatr Scand. 2011;124:184–192. doi: 10.1111/j.1600-0447.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- 23.Leoni V, Masterman T, Mousavi FS, Wretlind B, Wahlund LO, Diczfalusy U, Hillert J, Bjorkhem I. Diagnostic use of cerebral and extracerebral oxysterols. Clin Chem Lab Med. 2004;42:186–191. doi: 10.1515/CCLM.2004.034. [DOI] [PubMed] [Google Scholar]
- 24.Leoni V, Shafaati M, Salomon A, Kivipelto M, Bjorkhem I, Wahlund LO. Are the CSF levels of 24S-hydroxycholesterol a sensitive biomarker for mild cognitive impairment? Neurosci Lett. 2006;397:83–87. doi: 10.1016/j.neulet.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Leoni V, Solomon A, Lovgren-Sandblom A, Minthon L, Blennow K, Hansson O, Wahlund LO, Kivipelto M, Bjorkhem I. Diagnostic power of 24S-hydroxycholesterol in cerebrospinal fluid: Candidate marker of brain health. J Alzheimers Dis. 2013;36:739–747. doi: 10.3233/JAD-130035. [DOI] [PubMed] [Google Scholar]
- 26.Dietschy JM, Turley SD. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Pfrieger FW. Outsourcing in the brain: Do neurons depend on cholesterol delivery by astrocytes? Bioessays. 2003;25:72–78. doi: 10.1002/bies.10195. [DOI] [PubMed] [Google Scholar]
- 28.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Lian K, Han B, Wang Y, Kuo SH, Geng Y, Qiang J, Sun M, Wang M. Age-related alterations in the metabolic profile in the hippocampus of the senescence-accelerated mouse prone 8: A spontaneous Alzheimer’s disease mouse model. J Alzheimers Dis. 2014;39:841–848. doi: 10.3233/JAD-131463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Geng Y, Han B, Qiang J, Li X, Sun M, Wang Q, Wang M. Repetitive transcranial magnetic stimulation applications normalized prefrontal dysfunctions and cognitive-related metabolic profiling in aged mice. PLoS One. 2013;8:e81482. doi: 10.1371/journal.pone.0081482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer’s disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 32.Wolozin B, Manger J, Bryant R, Cordy J, Green RC, McKee A. Re-assessing the relationship between cholesterol, statins and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:63–70. doi: 10.1111/j.1600-0404.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolozin B. Statins and therapy of Alzheimer’s disease: Questions of efficacy versus trial design. Alzheimers Res Ther. 2012;4:3. doi: 10.1186/alzrt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 35.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 36.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra Garcia M, Manjon M, Girones X, Henry TL, Matsubara E, Zambon D, Wolozin B, Sano M, Cruz-Sanchez FF, Thal LJ, Petanceska SS, Refolo LM. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- 38.Bjorkhem I, Heverin M, Leoni V, Meaney S, Diczfalusy U. Oxysterols and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca AC, Resende R, Oliveira CR, Pereira CM. Cholesterol and statins in Alzheimer’s disease: Current controversies. Exp Neurol. 2010;223:282–293. doi: 10.1016/j.expneurol.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Wollmer MA. Cholesterol-related genes in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:762–773. doi: 10.1016/j.bbalip.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Moncaster JA, Pineda R, Moir RD, Lu S, Burton MA, Ghosh JG, Ericsson M, Soscia SJ, Mocofanescu A, Folkerth RD, Robb RM, Kuszak JR, Clark JI, Tanzi RE, Hunter DG, Goldstein LE. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One. 2010;5:e10659. doi: 10.1371/journal.pone.0010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes TM, Rosano C, Evans RW, Kuller LH. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis. 2013;33:891–911. doi: 10.3233/JAD-2012-121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leoni V. Oxysterols as markers of neurological disease–a review. Scand J Clin Lab Invest. 2009;69:22–25. doi: 10.1080/00365510802651858. [DOI] [PubMed] [Google Scholar]
- 44.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.