Abstract
The current study sought to evaluate the effects of post-exercise ingestion of a high molecular weight glucose polymer solution (HMW) compared to an isocaloric low molecular weight solution (LMW) or placebo (PLA) on subsequent cycling performance in female athletes. In a randomized, double-blind, placebo-controlled, cross-over design, 10 competitive female cyclists (Mean ± SD; Age = 25.7 ± 5.0 yrs; VO2peak = 49.7 ± 4.3 ml·kg−1·min−1) completed three testing sessions separated by 7–10 days. Visits consisted of a ride-to-exhaustion (RTE) at 75% VO2peak, followed by immediate consumption of 700 mL containing either: 1.2 g·kg−1 LMW (maltodextrin/dextrose/fructose); 1.2 g·kg−1 HMW (Vitargo®); or 0.066 g·kg−1 PLA (noncaloric flavoring). After 2 hours rest, participants performed a 15-minute time-trial (TT). Respiratory exchange ratio (RER) was assessed via indirect calorimetry during exercise. Total body water (TBW) was measured using bioelectrical impedance to assess fluid balance. When covaried for estrogen, there was no treatment effect on distance (km; p=0.632) or power output (watts; p=0.974) during the 15-minute TT. RER was not significantly different during the LMW and HWM TTs (p>0.999), but both were significantly higher than PLA (p=0.039, p=0.001, respectively). Changes in TBW pre- to post-exercise were not significantly different between trials (p=0.777). Despite benefits of HMW on cycling performance previously reported in males, current results demonstrate no ergogenic effect of HMW or LMW in females. Sex differences in substrate utilization may account for the discrepancy, and further research involving performance nutrition for female athletes is merited.
Keywords: cycling, carbohydrate metabolism, endurance training, sports nutrition, sex
INTRODUCTION
Carbohydrate (CHO) is a critical source of energy during endurance exercise (13). Consequently, the sports supplement market is saturated with carbohydrate-based products claiming to enhance endurance performance and recovery (i.e. glycogen repletion). The preservation and restoration of glycogen, a primary fuel source for skeletal muscle during endurance activities, is important for endurance events, such as cycling (15). Post-exercise ingestion of CHO has been consistently shown to facilitate glycogenesis and aid recovery, prompting investigations surrounding the dosage, timing, and form of CHO (8).
While most conventional sports drinks contain a mixture of low molecular weight carbohydrates (LMW), research has demonstrated that ingesting a solution of high molecular weight glucose polymers (HMW) facilitates faster gastric emptying rates than LMW, particularly within the first 10 minutes following consumption (11,18). As the molecular weight of a solution increases, the relative decrease in osmolality increases the rate of passage from the stomach into the duodenum (34). Theoretically, earlier arrival to the intestinal brush border may accelerate glucose absorption and energy availability to speed glycogen resynthesis. Previous data has demonstrated significantly greater glycogen repletion in men with HMW compared to LMW following an exhaustive running and cycling bout (25). However, research investigating whether HMW carbohydrates render actual ergogenic benefits remains equivocal (23). Stephens et al. (2008) reported that HMW consumption following an exhaustive endurance cycle ride improved total work output during a subsequent 15-min time trial in trained males. In contrast, sprint capacity was not different between post-exercise ingestion of a HMW or LMW recovery beverage in male athletes (20). Such discrepancies in these findings, potentially due to variance in exercise protocols or the timing and dose of CHO supplementation, suggest a need for further research evaluating CHO molecular weight.
Studies have consistently shown that females rely more heavily on fat oxidation and may utilize glycogen at different rates than males during endurance exercise of equal relative intensity (%VO2max; 15,29,32), likely due to inherent hormonal differences, mainly as a function of estrogen. Previous data has reported significant differences between men and women in glycogen storage and subsequent cycling performance following a CHO loading protocol, with females demonstrating a much lower capacity for glycogen super-compensation, and only marginal improvement in exercise capacity compared to males (33). Specifically, previous data reported no significant difference in cycling sprint performance between a CHO supplement and non-caloric placebo in trained females(16). Although apparent sex differences exist in fuel selection during exercise, few studies have explored factors affecting glycogen repletion in female athletes. Initial evidence suggests CHO with varied molecular weights may be beneficial in male cyclists (30), however to our knowledge, no study has assessed the influence of CHO molecular weight on recovery and subsequent performance in trained female cyclists. Therefore, the current study aimed to evaluate the effects of post-exercise ingestion of a HMW glucose polymer solution compared to an isocaloric LMW or placebo (PLA) on cycling performance in competitive female cyclists.
METHODS
Experimental Approach to the Problem
In a double-blind, placebo-controlled, cross-over design, qualifying participants underwent three randomized testing sessions, each separated by 7–10 days and occurring within two hours of the same time of day (Figure 1). Participants completed a 24-hour diet log preceding each trial and were instructed to replicate dietary intake across the 3 trials, while also abstaining from caffeine, alcohol, and vigorous exercise ≥24 hours prior to testing. Trials consisted of a glycogen-depleting ride-to-exhaustion (RTE) at a workload set at 75% of their VO2peak power output (PPO) in watts (W) (7,31), followed by immediate consumption of one of three solutions (700 mL of: 1.2 g·kg−1 LMW starch blend; 700 mL of: 1.2 g·kg−1 HMW glucose polymer; flavored non-caloric PLA). The order in which supplements were administered to each participant was determined using random allocation software (Version 1.0.0, Isfahan, Iran). After a subsequent 2-hour rest period, participants completed a 15-min time trial (TT) at a self-selected pace and resistance to maximize work output. All exercise was performed on participants’ personal bicycle, rear-wheel mounted to a CompuTrainer® PRO indoor cycling trainer (RacerMate, Seattle, WA, USA). Using indirect calorimetry, respiratory gases were collected and analyzed every 15 minutes during the RTE, throughout the entire TT, and for an additional 15 minutes post-TT to assess respiratory exchange ratio (RER). Salivary estradiol levels were assessed in order to account for potential fuel utilization differences between the exercise sessions based on menstrual cycle phase. Total body water (TBW) was measured using bioelectrical impedance spectroscopy (BIS; SFB7 ImpediMed, Queensland, Australia) before and after the RTE bout to assess fluid balance. Gastrointestinal discomfort, including nausea, bloating, and abdominal cramping, was evaluated prior to exercise and after the 15 minute TT using a visual analog scale (VAS) that ranged from 0 (nothing), 2 (mild, annoying pain), 4 (nagging, uncomfortable pain), 6 (distressing, miserable pain), (8 intense, dreadful pain), and 10 (unbearable, excruciating pain) (26). Treatment palatability was assessed during consumption with a 9-point hedonic scale.
Figure 1.
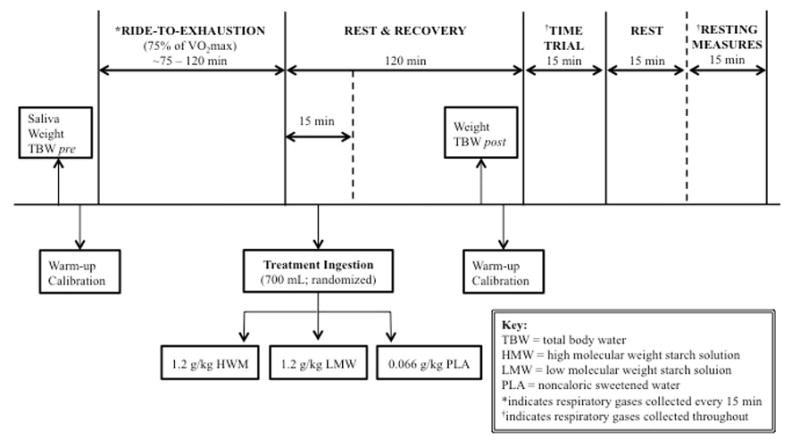
Testing protocol schematic for the ride to exhaustion (RTE) and time trial (TT).
Subjects
A total of 13 competitive female cyclists were enrolled in the present study; one participant did not attain the minimum criteria for maximal oxygen consumption (VO2peak; ≥45 ml·kg−1·min−1), one participant did not return following baseline testing and therefore was never randomized, and one participant decided to discontinue after one treatment session due to time constraints and fasting requirements. Therefore, 10 participants (Mean ± SD; age = 25.7 ± 5.0 yrs; height = 167.7 ± 6.0 cm; weight = 62.6 ± 4.2 kg) completed the study. Prior to all testing, participants provided written informed consent, completed a medical history questionnaire, and underwent an exercise clearance screening that included a physical examination and 12-lead electrocardiogram. For inclusion, participants must have been currently involved in a high-volume cycling training program (minimum of 8 hours per week), participated in ≥1 competitive cycling race in the previous 12 months, and achieved a VO2peak value of at least 45 ml·kg−1·min−1 on a preliminary maximal cycling exercise test. To access VO2peak, participants began pedaling at a power output of 20 watts (W), then workload automatically increased 1 W every 3 seconds while a pedal cadence between 60–80 rpms was maintained until volitional fatigue. Respiratory gases were analyzed via indirect calorimetry (True One 2400 Metabolic Measurement System, Parvo-Medics Inc., Provo, UT). VO2 values were averaged every 15 seconds, with the highest 15 second VO2 value was recorded as the VO2peak. Participants obtained a mean relative VO2peak of 49.7 ± 4.3 ml·kg−1·min−1 and absolute PPO of 272.9 ± 34.2 W, classifying the cyclists as Performance Level 3 according to recently published guidelines for categorizing participant groups in female cycling research (9). The University’s Biomedical Institutional Review Board approved all procedures.
Procedures
Participants fasted for at least 2 hours prior to laboratory testing. If food was eaten during the morning of the ride before the 2h fast, it was a low-CHO snack and kept consistent across trials. Participants were asked to be euhydrated; hydration status was not assessed. There were no significant differences in the CHO (211.8 ± 77.6 g; p=0.409), PRO (83.5 ± 2.9 g; p=0.902), FAT (88.3 ± 12.0 g; p=0.119), or total caloric (1939.6 ± 505.9 cal; p=0.095) content of participants’ meals in the 24-hours prior to testing sessions. Weight was measured with participants wearing cycling singlet without shoes or socks, and TBW was assessed using BIS. Following 5 minutes of supine rest, pairs of single-tab electrodes were placed 5 cm apart on the participant’s right wrist and hand, and on the right ankle and foot. Measurements were taken while the participant lay with ≥30° between their arm and torso and between legs; the average of 2 trials was used to represent TBW. Estradiol concentrations were determined by a 1.0 mL passive drool saliva sample taken prior to each exercise bout, using an ELISA assay for salivary estradiol-β;-17 (estrogen) (Salivary 17β;-Estradiol Enzyme Immunoassay Kit, Salimetrics, LLC, State College, PA, USA). To prevent potential contamination, participants were asked to avoid brushing teeth within 45 minutes of the visit and instructed to rinse their mouths with water 10 minutes prior to collection. All samples were maintained at 4°C no longer than necessary before freezing them at −20°C.
To reach a state of glycogen depletion, participants performed a cycling ride-to-exhaustion at a workload set at 75% of PPO (W). Following a 10-minute warm-up ride and CompuTrainer calibration, participants were asked to breathe through a mouthpiece and tube connected to a metabolic cart (Parvo Medics TrueMax 2400, Salt Lake City, UT). Respiratory gases were collected for the first four minutes of exercise, and then collected in 4-minute intervals to capture data at every 15 minute interval (0–4 min; 13–17 min; 28–32 min; 43–47 min, 58–62 min; 73–77min, 88–92 min; 116–120 min) to assess substrate utilization via RER. Substrate utilization from RER is estimated by a value of 0.70 of all fat oxidation; a value of 1.00 for exclusively carbohydrate oxidation (29). Average RER values were calculated for the first two respiratory collections (min 0:00–0:04 and 0:13–0:17) and the final two respiratory collections of each individual’s ride to allow for start-to-finish comparisons of substrate utilization.. Participants were allowed to drink water ad libitum, and fans were provided to help maintain body temperature during the ride. The test was ended when the participant dropped below a pedal cadence of 60 revolutions·min−1 for ≥ 30 sec, or they decided they could not continue.
Following the RTE, participants were asked to consume 700mL of HMW, LMW, or PLA in entirety within 15 minutes of dismounting their bike. The HMW contained a glucose polymer derived from barley (Vitargo®, Swecarb AB, Kalmar, Sweden) with a molecular weight of ~500,000–700,000 g·mol−1, and osmolality of 34 mOsmol·kg−1. The LMW contained an equal-ratio blend of maltodextrin, dextrose, and fructose representative of a standard exercise recovery drink (900 g·mol−1; 124 mOsmol·kg−1). The HMW and LMW were isoenergetic (~1150 kJ) and mixed at a concentration of 1.2 g per kg bodyweight; PLA contained noncaloric flavoring (0.066 g·kg−1). The supplement provider blinded the treatments beforehand, and researchers prepared all beverages. Following consumption of the respective supplement, participants rested quietly for two hours and during the final 15 minutes of the recovery period, weight and TBW were measured. One participant did not have TBW data at the HMW visit due to equipment availability and therefore was excluded in TBW statistical analysis for all trials (n=9).
Following the recovery period, participants completed a 15-minute TT, after a 5-minute self-selected warm-up ride and CompuTrainer recalibration. For the TT, starting resistance was set at a self-selected workload. Participants were allowed to manually adjust resistance throughout the ride as desired using a controller closely mounted to the handlebars. Participants were informed of time remaining every minute to allow for pacing. Instantaneous power output (W) was collected every second and total distance traveled (km) was recorded. Participants wore headgear and a mouthpiece connected via a breathing tube to a metabolic cart to evaluate RER. Following the TT, participants cooled down for approximately 5 minutes, dismounted the bike, and rested for 15 minutes. Respiratory gases were then collected again for 15 minutes while participants sat quietly breathing through a mouthpiece and tube connected to a metabolic cart. RER values were averaged every 15 seconds to determine substrate utilization during recovery.
Statistical Analyses
Data was evaluated for normality using the Shapiro-Wilk test; all data was normally distributed. All variables were co-varied for estrogen levels. A series of one-way analyses of covariance (ANCOVAs) were performed to evaluate differences in absolute measures of RTE distance, RTE time, TT distance, TT average power, when controlling for estrogen. Repeated measures ANOVAs were used to evaluate TT RER, and post-exercise recovery RER between treatments. For RTE respiratory data, initial and final RER values were compared using a two-way mixed factorial ANOVA (time × trial). Pre- and post-exercise TBW were also analyzed using a two-way mixed factorial ANOVA (time × trial). Absolute hedonic scores, as well as VAS change scores pre- to post-, were analyzed using repeated measures ANOVAs. The alpha level was set at 0.05, with Bonferroni-corrected post-hoc comparisons used to further assess significant findings. Partial eta squared was calculated to assess effect size (ES). All data were analyzed using SPSS (Version 23.0 Chicago, IL, USA). In addition, magnitude based inferences were calculated for RTE time using a published spreadsheet using the unequal variances t-statistic (2). The precision of the magnitude inference was set 90% confidence limits using the p value corresponding to the t-statistic.
RESULTS
Ride-to-Exhaustion
There was no significant difference in RTE total distance (p=0.632, ES=0.035) or duration (p=0.332, ES=0.081) across all trials (Table 1). Magnitude based inferences suggested cyclists rode an average of 7 and 10 minutes longer during the PLA and LMW trials, respectively, then at the HMW trial, suggesting a likely beneficial effect of LMW on RTE (1.5, ± 1.8) (Table 2). For RTE respiratory data, there was no significant time × trial interaction (p=0.863, ES=0.016). There was no main effect for trial (p=0.958, ES=0.002), but there was a significant main effect for time (p=0.001, ES=0.714), with all three trials demonstrating lower final RER measures than baseline RER [mean difference=−0.054]. Group mean RER time series plots for each treatment are presented in Figure 2.
Table 1.
Characteristics of each trial (Mean ± SD). Values are reported as adjusted means when covaried for salivary estradiol.
| PLA | LMW | HMW | |
|---|---|---|---|
| Ride-to-Exhaustion | |||
| Distance (km) | 22.0 ± 3.0 | 23.4 ± 2.9 | 21.5 ± 3.0 |
| Time (min) | 98.3 ± 11.4 | 103.2 ± 10.3 | 92.3 ± 10.2 |
| Initial RER (L/L) | 0.88 ± 0.05 | 0.89 ± 0.04 | 0.89 ± 0.04 |
| Final RER (L/L) | 0.83 ± 0.03 | 0.83 ± 0.04 | 0.83 ± 0.03 |
| Time Trial | |||
| Power (watts) | 161.4 ± 21.8 | 161.7 ± 21.6 | 164.5 ± 21.4 |
| Distance (km) | 7.5 ± 1.1 | 6.8 ± 1.8 | 7.1 ± 1.1 |
| RER (L/L) | 0.88 ± 0.03* | 0.92 ± 0.03 | 0.93 ± 0.04 |
| Resting Recovery | |||
| RER (L/L) | 0.70 ± 0.03 | 0.71 ± 0.04 | 0.72 ± 0.02 |
| Hormonal Status | |||
| Salivary Estradiol (pg/mL) | 1.82 ± 0.64 | 1.45 ± 0.39 | 1.55 ± 0.61 |
| Hydration Status | |||
| TBW (ΔL) | −0.67 ± 0.60 | −0.53 ± 0.58 | −0.63 ± 0.52 |
indicates significant difference between treatments (p<0.05); Δ = change
Table 2.
Magnitude Based Inferences for RTE time
| RTE Time | Beneficial or Substantially +ive | Negligible or Trivial | Harmful or Substantially −ive |
|---|---|---|---|
| LMW-PLA | 53.0% | 31.5% | 15.4% |
| HMW-PLA | 3.7% | 12.9% | 83.5% |
| LMW-HMW | 85.1% | 11.6% | 3.2% |
Figure 2.
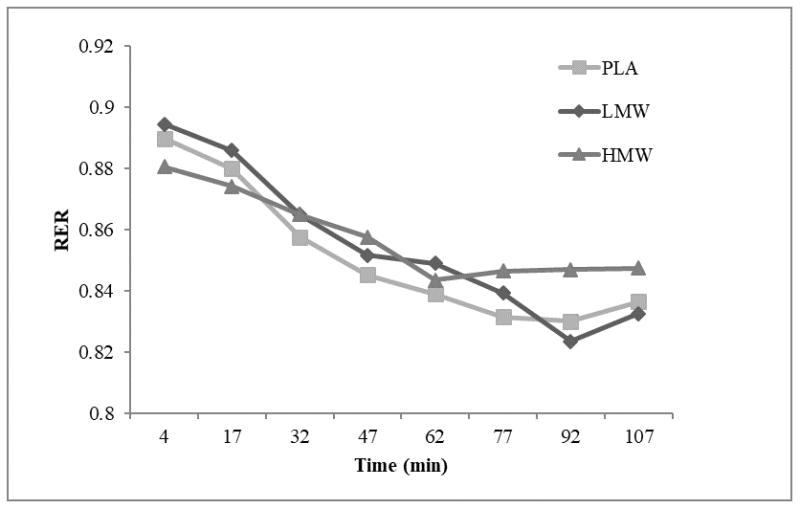
Group mean RER responses throughout RTE for PLA (square), LMW (diamond), and HMW (triangle).
Time Trial
There was no significant difference between treatments in TT distance traveled (km; p=0.607, ES=0.038) or TT average power output (W; p=0.955, ES=0.001). There was a significant treatment effect on RER during the 15-minute TT (p=0.001, ES=0.551). Time trial RER for LMW and HWM were both significantly higher than PLA (p=0.039, p=0.001), with no statistically significant difference between RER values for LMW and HMW (p>0.999) (Table 1).
Recovery RER
Respiratory exchange ratios during 15 minutes of post-exercise recovery were not significantly different among treatments (p=0.121, ES=0.209) (Table 1).
Salivary Estradiol
There was no significant difference (p=0.350) between estradiol concentrations for each treatment (Table 1). Values ranged from 0.83–2.84 pg/mL. Estradiol concentrations were significantly different across visit days (p=0.009, ES=0.406). Values for visit 1=1.92 ± 0.70 pg/mL; visit 2=1.56 ± 0.42 pg/mL; and visit 3=1.32 ± 0.37 pg/mL.
Total Body Water, Hedonic, and VAS
There was no significant time × trial interaction for TBW (p=0.844), though a main effect for time pre- to post- (p=0.001) was observed. There were no significant differences in TBW changes between trials (p=0.777). Hedonic scores for PLA, LMW, and HMW solutions were 5.9 ± 1.8, 4.9 ± 1.4, and 5.3 ± 1.9 respectively, with no significant difference in palatability between the three (p=0.724, ES=0.35). Change scores for VAS pre-and post- were not significantly different (p=0.407, ES=0.095), indicating no effect on GI distress between treatments.
DISCUSSION
A large majority of available research exploring CHO administration and glycogen repletion has exclusively evaluated males, leaving a gap in literature regarding optimal recovery nutrition for female athletes. The aim of the present study was to determine the effects of post-exercise ingestion of HMW CHO versus LWM and placebo on subsequent exercise performance in trained female cyclists. Time trial RER was significantly higher following both HMW and LMW compared to PLA, reflecting an expected increase in CHO availability and utilization, though RER values were not significantly different between HMW and LMW treatments. Furthermore, there was no difference in TT power output or total distance traveled across all three treatments. These findings contrast a Stephens et al. (2008), with a nearly identical protocol, who reported a 20% increase in TT work output with HMW in eight endurance-trained males. Other investigations exploring the potential advantages of HMW compared to conventional LMW beverages have not been conclusive (20,22,27). Sex differences likely played a primary role in discrepancies from previous studies, as there are well-documented distinctions in substrate utilization (see below) between males and females during exercise (35).
Although the present study did not directly assess muscle glycogen content, the RTE was similar to a previously published protocol (30), which was validated with muscle biopsies (7). However, it should be noted that the previous protocol (30) did allow for 5-minute rest increments throughout the ride to exhaustion, potentially altering the total glycogen depletion. RER demonstrated a consistent decrease from the RTE’s initiation to the point of exhaustion, likely indicating a shift from CHO to fat utilization as glycogen was depleted (Figure 2). Due to the length and rigor of the RTE protocol, no familiarization trial was included in the current study and must be considered a limitation. Consideration should be given to the magnitude based inferences demonstrating that the PLA and LMW groups rode longer during the RTE, which may have affected potential performance outcomes during the TT. However, there was no significant difference between visits on final RER, duration, or total distance cycled during the RTEs. Though this suggests that post-RTE glycogen content was similar across all three trials, it remains possible that glycogen was not the primary limiting factor to performance outcomes. The lack of a familiarization trial may have also confounded the results.
Although both LMW and HMW resulted in a significantly higher TT RER compared to PLA, there were no differences in substrate utilization between the two forms of CHO. Additionally, as a result of the shorter RTE for the HMW, it would be suspected that concomitant significant improvements in the TT would result; however this was not the case. These findings are similar to previous findings demonstrating no effect of molecular weight on CHO utilization when ingested at a rate of 1.8 g·min−1 throughout a prolonged cycling effort (26). After concluding that CHO oxidation occurred at a maximal rate of 1.0–1.1 g·min−1, the effects of 0.8 g·min−1 HMW CHO (Vitargo®; 21 mosm·kg−1), a dose below the saturation rate for exogenous glucose absorption, was evaluated (26). Contrary to hypotheses, the HWM solution was oxidized at a significantly lower rate than a glucose solution (469 mosm·kg−1) and led to a lower plasma glucose response. These results conflict with those who previously reported an elevated blood glucose response with HMW (30), while other studies have found no difference in blood glucose following HMW or LMW ingestion, despite differences in glycogen repletion (18,25). It is plausible that brush-border enzyme hydrolysis activity may limit HMW absorption speed of the highly branched polymer, regardless of faster gastric emptying rates; the enhanced absorption is also typically only reported within the first 10-minutes post consumption (26).
Higher TT RER values with LMW and HMW did not translate into improved time trial performance over PLA. In evaluating a CHO recovery beverage in endurance-trained females, Jarvis et al. (1999) reported an augmented blood glucose response with CHO compared to PLA, yet found no difference in subsequent sprint performance between the two beverages. The lack of treatment effect in women contrasts findings from two previous studies (1,3), who reported significant sprint performance improvements with CHO using the same protocol in males only. Jarvis cited sex differences in CHO metabolism as a likely cause of the discrepancy. This same theory could be proposed when interpreting the contrasting findings between the current study and those of Stephens et al. (2008). The influence of hormones on substrate selection is well-documented, with females demonstrating greater relative fat utilization and lower CHO utilization than men (32,35). Investigators have demonstrated that 17β-estradiol increases the activity of lipolytic enzymes (17,19) and suppresses the metabolic clearance rate (MCR) of glucose (6) during exercise, which may delay glycogen storage. Considering hormonal involvement in fuel selection, the current study assessed participants’ estradiol fluctuations across the menstrual cycle, but did not observe any effect of estradiol on performance. Previous studies suggest muscle glycogen storage capacity may vary across phases (12,21), however CHO supplementation before (24) or during exercise (4) appear to eliminate the effects of menstrual cycle phase. It is likely that sex plays a stronger determining role in glucose MCR and glycogen utilization (10); other factors to consider are training status, chronic dietary consumption, and age. Carper et al. (2013) found that after a glycogen-depleting bout of cycling, males resynthesized 84% of resting level-glycogen in 3 hours, while females remained 51% below pre-exercise levels. Thus, it is possible that the current study’s 2-hr recovery window was insufficient for glycogen restoration to influence TT performance. Additionally, glycogen may not be the primary limiting factor to performance, particularly in females.
Practical Applications
In conclusion, the current study demonstrated post-exercise ingestion of HMW had no effect on subsequent cycling performance in females compared to LMW or PLA. These findings agree with previous data evaluating HMW solutions (20,22,27), and only contrast one previous study (30). Based on the current results, and others (20,22,27), any glucose solution may be useful for female endurance athletes to support glycogen needs. Future research should focus on distinguishing the physiological characteristics of female endurance athletes and what is required to optimize performance nutrition recommendations.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The products used in this study were donated by Vitargo®.
Footnotes
Disclosure: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The products used in this study were donated by Vitargo®.
References
- 1.Ball TC, Headley SA, Vanderburgh PM, Smith JC. Periodic carbohydrate replacement during 50 min of high-intensity cycling improves subsequent sprint performance. Int J Sport Nutr. 1995;5:151–8. doi: 10.1123/ijsn.5.2.151. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7670454. [DOI] [PubMed] [Google Scholar]
- 2.Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform. 2006;1:50–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19114737. [PubMed] [Google Scholar]
- 3.Below PR, Mora-Rodríguez R, González-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc. 1995;27:200–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7723643. [PubMed] [Google Scholar]
- 4.Campbell SE, Angus DJ, Febbraio MA. Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am J Physiol - Endocrinol Metab. 2001;281 doi: 10.1152/ajpendo.2001.281.4.E817. [DOI] [PubMed] [Google Scholar]
- 5.Carper M, Acree LD, GMRSWS, et al. Muscle Glycogen Restoration in Females and Males Following Moderate Intensity Cycling Exercise in Differing Ambient Temperatures. J Exerc Physiol. 2013;16:1–18. Available from: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=10979751&AN=91532673&h=HlfLmEBV84Z6ZV/Kbe/aQEBJ6ZG1NrkAbeiwjjaT0/EXd5eR3Dt9THdqBxPVrCgVlHqnaRHtrlrjHnwQhMI58g==&crl=c. [Google Scholar]
- 6.Carter S, McKenzie S, Mourtzakis M, Mahoney DJ, Tarnopolsky MA, Bergman B, et al. Short-term 17beta-estradiol decreases glucose R(a) but not whole body metabolism during endurance exercise. J Appl Physiol. 2001;90:139–46. doi: 10.1152/jappl.2001.90.1.139. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11133904. [DOI] [PubMed] [Google Scholar]
- 7.Casey A, Short AH, Hultman E, Greenhafft PL. Glycogen resynthesis in human muscle fibre types following exercise-induced glycogen depletion. J Physiol. 1995;483:265–271. doi: 10.1113/jphysiol.1995.sp020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cermak NM, van Loon LJC. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013;43:1139–55. doi: 10.1007/s40279-013-0079-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23846824. [DOI] [PubMed] [Google Scholar]
- 9.Decroix L, De Pauw K, Foster C, Meeusen R. Guidelines to Classify Female Subject Groups in Sport-Science Research. Int J Sports Physiol Perform. 2016;11:204–13. doi: 10.1123/ijspp.2015-0153. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26182438. [DOI] [PubMed] [Google Scholar]
- 10.Devries MC, Hamadeh MJ, Phillips SM, Tarnopolsky MA. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol - Regul Integr Comp Physiol. 2006;291 doi: 10.1152/ajpregu.00700.2005. [DOI] [PubMed] [Google Scholar]
- 11.Foster C, Costill DL, Fink WJ. Gastric Emptying Characteristics of Glucose and Glucose Polymer Solutions. Res Q Exerc Sport. 1980;51:299–305. doi: 10.1080/02701367.1980.10605198. Available from: http://www.tandfonline.com/doi/abs/10.1080/02701367.1980.10605198. [DOI] [PubMed] [Google Scholar]
- 12.Hackney A. Effects of the Menstrual Cycle on Resting Muscle Glycogen Content. Horm Metab Res. 1990;22:647–647. doi: 10.1055/s-2007-1004994. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-2007-1004994. [DOI] [PubMed] [Google Scholar]
- 13.Hawley JA, Leckey JJ. Carbohydrate Dependence During Prolonged, Intense Endurance Exercise. Sports Med. 2015;45(Suppl 1):S5–12. doi: 10.1007/s40279-015-0400-1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26553495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO, Blatchford FK, Knowlton RG, et al. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1998;85:1823–32. doi: 10.1152/jappl.1998.85.5.1823. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9804587. [DOI] [PubMed] [Google Scholar]
- 15.Ivy JL. Regulation of muscle glycogen repletion, muscle protein synthesis and repair following exercise. J Sports Sci Med. 2004;3:131–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24482590. [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis AT, Felix SD, Sims S, JONES MT, COUGHLIN MA, Headley SA. Carbohydrate supplementation fails to improve the sprint performance of female cyclists. J Exerc Physiol Online. 1999;2:16–23. Available from: http://faculty.css.edu/tboone2/asep/headley2.pdf%5Cnpapers2://publication/uuid/7D1BE706-7673-417F-8B0C-345339539260. [Google Scholar]
- 17.Kendrick ZV, Steffen CA, Rumsey WL, Goldberg DI. Effect of estradiol on tissue glycogen metabolism in exercised oophorectomized rats. J Appl Physiol. 1987;63:492–6. doi: 10.1152/jappl.1987.63.2.492. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3654408. [DOI] [PubMed] [Google Scholar]
- 18.Leiper JB, Aulin KP, Söderlund K. Improved gastric emptying rate in humans of a unique glucose polymer with gel-forming properties. Scand J Gastroenterol. 2000;35:1143–9. doi: 10.1080/003655200750056600. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11145284. [DOI] [PubMed] [Google Scholar]
- 19.Maher AC, Akhtar M, Tarnopolsky MA, Ambros V, Berthon P, Howlett R, et al. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics. 2010;42:342–7. doi: 10.1152/physiolgenomics.00016.2010. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20484157. [DOI] [PubMed] [Google Scholar]
- 20.Mcglory C, Morton JP. The Effects of Postexercise Consumption of High-Molecular-Weight Versus Low-Molecular-Weight Carbohydrate Solutions on Subsequent High-Intensity Interval-Running Capacity. Int J Sport Nutr Exerc Metab. 2010;20:361–369. doi: 10.1123/ijsnem.20.5.361. [DOI] [PubMed] [Google Scholar]
- 21.Nicklas B, Hackney A, Sharp R. The Menstrual Cycle and Exercise: Performance, Muscle Glycogen, and Substrate Responses. Int J Sports Med. 1989;10:264–269. doi: 10.1055/s-2007-1024913. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2606593. [DOI] [PubMed] [Google Scholar]
- 22.Oliver JM, Almada AL, Van Eck LE, Shah M, Mitchell JB, Jones MT, et al. Ingestion of High Molecular Weight Carbohydrate Enhances Subsequent Repeated Maximal Power: A Randomized Controlled Trial. PLoS One. 2016;11:e0163009. doi: 10.1371/journal.pone.0163009. Available from: http://dx.plos.org/10.1371/journal.pone.0163009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ormsbee MJ, Bach CW, Baur DA. Pre-exercise nutrition: the role of macronutrients, modified starches and supplements on metabolism and endurance performance. Nutrients. 2014;6:1782–808. doi: 10.3390/nu6051782. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24787031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul DR, Mulroy SM, Horner JA, Jacobs KA, Lamb DR. Carbohydrate-loading during the follicular phase of the menstrual cycle: effects on muscle glycogen and exercise performance. Int J Sport Nutr Exerc Metab. 2001;11:430–41. doi: 10.1123/ijsnem.11.4.430. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11915778. [DOI] [PubMed] [Google Scholar]
- 25.Piehl Aulin K, Söderlund K, Hultman E. Muscle glycogen resynthesis rate in humans after supplementation of drinks containing carbohydrates with low and high molecular masses. Eur J Appl Physiol. 2000;81:346–51. doi: 10.1007/s004210050053. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10664095. [DOI] [PubMed] [Google Scholar]
- 26.Rowlands DS, Clarke J. Lower oxidation of a high molecular weight glucose polymer vs. glucose during cycling. Appl Physiol Nutr Metab. 2011;36:298–306. doi: 10.1139/h11-006. Available from: http://www.nrcresearchpress.com/doi/abs/10.1139/h11-006. [DOI] [PubMed] [Google Scholar]
- 27.Rowlands DS, Wallis GA, Shaw C, Jentjens RLPG, Jeukendrup AE. Glucose polymer molecular weight does not affect exogenous carbohydrate oxidation. Med Sci Sports Exerc. 2005;37:1510–6. doi: 10.1249/01.mss.0000177586.68399.f5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16177602. [DOI] [PubMed] [Google Scholar]
- 28.Ruby BC, Coggan AR, Zderic TW, Blatchford F, Knowlton R, Schneider D, et al. Gender differences in glucose kinetics and substrate oxidation during exercise near the lactate threshold. J Appl Physiol. 2002;92:1125–32. doi: 10.1152/japplphysiol.00296.2001. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11842049. [DOI] [PubMed] [Google Scholar]
- 29.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990;258:E399–412. doi: 10.1152/ajpendo.1990.258.3.E399. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2180312. [DOI] [PubMed] [Google Scholar]
- 30.Stephens FB, Roig M, Armstrong G, Greenhaff PL. Post-exercise ingestion of a unique, high molecular weight glucose polymer solution improves performance during a subsequent bout of cycling exercise. J Sports Sci. 2008;26:149–54. doi: 10.1080/02640410701361548. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17852670. [DOI] [PubMed] [Google Scholar]
- 31.Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol. 1990;68:302–8. doi: 10.1152/jappl.1990.68.1.302. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2179207. [DOI] [PubMed] [Google Scholar]
- 32.Tarnopolsky MA. Gender differences in substrate metabolism during endurance exercise. Can J Appl Physiol = Rev Can Physiol Appl. 2000;25:312–27. doi: 10.1139/h00-024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10953068. [DOI] [PubMed] [Google Scholar]
- 33.Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol. 1995;78:1360–8. doi: 10.1152/jappl.1995.78.4.1360. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7615443. [DOI] [PubMed] [Google Scholar]
- 34.Vist GE, Maughan RJ. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J Physiol. 1995;486:523–531. doi: 10.1113/jphysiol.1995.sp020831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wismann J, Willoughby D. Gender differences in carbohydrate metabolism and carbohydrate loading. J Int Soc Sports Nutr. 2006;3:28–34. doi: 10.1186/1550-2783-3-1-28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18500960. [DOI] [PMC free article] [PubMed] [Google Scholar]


