Abstract
Cerebral ischemia-reperfusion injury (IRI) potentiates existing brain damage and increases mortality and morbidity via poorly understood mechanisms. The aim of our study is to investigate the role of Sirtuin 3 (Sirt3) in the development and progression of cerebral ischemia-reperfusion injury with a focus on mitochondrial fission and the Wnt/β-catenin pathway. Our data indicated that Sirt3 was downregulated in response to cerebral IRI. However, the overexpression of Sirt3 reduced the brain infarction area and repressed IRI-mediated neuron apoptosis. Functional assays demonstrated that IRI augmented mitochondrial fission, which induced ROS overproduction, redox imbalance, mitochondrial pro-apoptotic protein leakage, and caspase-9-dependent cell death pathway activation. However, the overexpression of Sirt3 blocked mitochondrial fission and induced pro-survival signals in neurons subjected to IRI. At the molecular level, our data further illustrated that the Wnt/β-catenin pathway is required for the neuroprotection exerted by Sirt3 overexpression. Wnt/β-catenin pathway activation via inhibiting β-catenin phosphorylation attenuates mitochondrial fission and mitochondrial apoptosis. Collectively, our data show that cerebral IRI is associated with Sirt3 downregulation, Wnt/β-catenin pathway phosphorylated inactivation, and mitochondrial fission initiation, causing neurons to undergo caspase-9-dependent cell death. Based on this, strategies for enhancing Sirt3 activity and activating the Wnt/β-catenin pathway could be therapeutic targets for treating cerebral ischemia-reperfusion injury.
Keywords: Cerebral ischemia-reperfusion (IR) injury, Mitochondrial fission, Apoptosis, Wnt/β-catenin pathways
Introduction
Despite ongoing advances in ischemic stroke therapy, the restoration of vascular oxygen supply via timely reperfusion treatment remains the standard approach for ischemic damage. Interestingly, reperfusion treatment also paradoxically leads to a second attack on the damaged brain; this second attack is called cerebral ischemia-reperfusion injury (IRI) (Gadicherla et al. 2017; Zhou et al. 2018b). IRI is believed to be caused by platelet hyperactivation, microvascular damage, oxidative stress, cellular calcium, and excessive inflammation responses (Zhou et al. 2018c; Zhou et al. 2018d; Zhou et al. 2018g). Subsequent to the occurrence of the IRI, most cells in the brain undergo programmed cell death via apoptosis, which is characterized by cellular swelling and DNA breakage (Tobisawa et al. 2017; Zhu et al. 2018b). In addition, the extent of IRI is clinically associated with mortality and disability during hospitalization. More importantly, although current revascularization approaches have been extensively applied in clinical practice and become highly successful, the incidence of IRI continues to increase considerably. Accordingly, the advancement of our understanding of IRI could obviously improve the effectiveness of reperfusion treatment and increase the clinical benefits for patients with ischemic stroke.
Mitochondrial dysfunction is a critical event leading to the execution of IRI-mediated cell death (Alghanem et al. 2017; Zhou et al. 2018b). For example, IRI activates the caspase-9-dependent mitochondrial apoptotic pathway, which induces caspase-3 activation and DNA cleavage (Ackermann et al. 2017; Zhou et al. 2017a). In addition, mitophagy, a protective mitochondrial repair system, is inhibited by IRI and contributes to the augmentation of mitochondrial apoptosis due to its failure to remove damaged mitochondria (Zhou et al. 2017e; Zhou et al. 2018g). Moreover, IRI-mediated oxidative stress is primarily attributed to the downregulation and inactivation of mitochondrial respiratory complex I (Zhang et al. 2016). Additionally, IRI-induced mitochondrial calcium overload also promotes mitochondrial permeability transition pore (mPTP) opening (Ghaffari et al. 2017; Zhou et al. 2017d), leading to mitochondrial potential dissipation and necrosis in neurons, possibly via the activation of CaMKII pathways (Zhu et al. 2018b). These data indicate that protecting mitochondrial function and structure is key to reducing damage to the reperfused brain.
Recently, mitochondrial fission has been well recognized as an early event in mitochondrial apoptosis; this process is also involved in mitochondrial oxidative stress, mitochondrial calcium overload, and mitophagy modifications in several types of cells (Jin et al. 2018; Zhou et al. 2018a; Zhou et al. 2017c). However, the role of mitochondrial fission in the brain, especially in cerebral IRI, has not yet been fully elucidated. Moreover, the upstream regulatory mechanism for activating mitochondrial fission in response to cerebral IRI remains obscure.
Sirtuin 3 (Sirt3), a type of NAD-dependent deacetylase expressed mainly in mitochondria, has recently been reported to have multiple effects on mitochondrial protection in response to several types of stress, such as oxidative stress(Torrens-Mas et al. 2018), hyperglycemia (Nassir et al. 2018), fatty acid composition (Chabi et al. 2018), and myocardial infarction (Hou et al. 2015). At the molecular level, Sirt3 protects against p53-mediated mitochondrial damage and neuronal death in a deacetylase activity-dependent manner (Lee et al. 2018). With the help of Ku70, Sirt3 also blocks Drp1 translocation from the cytoplasm to the mitochondria, effectively alleviating mitochondrial fission in t-BHP-challenged hepatocytes (Liu et al. 2017a). The overexpression of Sirt3 in cardiac microvascular endothelial cells activates mitophagy via PINK/Parkin pathways to reduce mitochondrial oxidative stress and maintain vessel sprouting and tube formation (Wei et al. 2017). In addition, Sirt3 overexpression also leads to the marked inhibition of cardiac hypertrophy induced by doxorubicin via closing mPTPs, which preserves mitochondrial potential and ensures mitochondrial respiration (Du et al. 2017). Collectively, these findings suggest that Sirt3 could regulate mitochondrial homeostasis via pleiotropic mechanisms. However, the functional role of Sirt3 in reperfusion-mediated mitochondrial fission in the context of cerebral IRI is incompletely understood.
The Wnt pathway is closely associated with cerebral cortex development, and Wnt pathway activation via β-catenin dephosphorylation causes the massive expansion of the cerebral cortex (Chenn 2008). In response to cerebral ischemia-reperfusion injury, Wnt pathway activation is positively correlated with hypoxia-inducible factor-1 (HIF-1) and promotes neural stem cell proliferation and self-renewal in vivo (Chen et al. 2018). Moreover, Wnt pathway activation could increase neuroprotective autophagic flux in ischemia-mediated neuroinjury (Shi et al. 2017). In contrast, inhibiting the Wnt pathway represses angiogenesis and proliferation in brain microvascular endothelial cells via blocking the VEGF/CREB/Egr-3/VCAM1 signaling pathways (Kang et al. 2014). In addition, Wnt signaling has been found to be a downstream effector of the PI3K/Akt pathway, a classical antiapoptotic signaling pathway in cerebral IRI (Xing et al. 2015). These data identify that the Wnt/β-catenin signaling pathway may be required for neuroprotection in cerebral IRI. However, whether the Wnt/β-catenin pathway could protect against IRI-induced mitochondrial fission remains unknown. Moreover, given the causal link between mitochondrial protection and Sirt3, we question whether the protective effects of Sirt3 against cerebral IRI are exerted via inhibiting mitochondrial fission in a Wnt/β-catenin pathway-dependent manner. Accordingly, the aim of our study is to explore the functional role of Sirt3 in cerebral IRI, with a particular focus on mitochondrial fission and the Wnt/β-catenin pathway.
Materials and methods
Animal study and cerebral IRI model
Sirt3 transgenic (Sirt3-TG) mice purchased from Jackson Laboratory (Bar Harbor, ME, USA) were used to create the cerebral IRI model. WT mice were used as the control group. Cerebral IRI was achieved using transient middle cerebral artery occlusion. After the mice were anesthetized with chloral hydrate (0.3 mg/kg i.p.), the left common carotid artery was identified. Then, a 0.2-mm nylon suture was passed underneath the common carotid artery. The cerebral IRI model was conducted according to the previous study (Zuo et al. 2018). Briefly, after 45 min of ischemia, blood flow was restored by removing the nylon filament to induce reperfusion for 2 h (n = 6/group). After cerebral IRI, the brain tissues were collected, and 2,3,5-triphenyltetrazolium chloride (TTC) staining was used to observe the acute cerebral infarction as described in a previous study. To analyze the protein expression in vivo, the infarction area in the brain was collected.
Cell culture and hypoxia-reoxygenation injury
The normal Neuro-2a (N2a, ATCC® number: ATCC® CCL-131™) neuroblastoma cell line, which has a neuron-like morphology and physiology, was used in this study. The cells were cultured in L-DMEM containing low glucose, 10% serum, and 1% streptomycin and penicillin at 37 °C in CO2 conditions. To induce hypoxia-reoxygenation (HR) injury in vitro, N2a cells were incubated in a hypoxia chamber containing 5% CO2 and 95% N2 for 45 min. Then, the cells were returned to normal culture conditions for 2 h. To inhibit the activity of Wnt/β-catenin pathway, DKK1 (10 mM, Sigma-Aldrich, USA; catalog no. 13380) was used 2-h to induce β-catenin phosphorylated degradation(Das et al. 2017).
ROS detection
Cellular ROS production was analyzed according to a previous study (Murphy et al. 2017). Cells were washed with cold PBS and cultured with ROS probe (1 mg/ml, DCFHDA, Molecular Probes, USA) at 37 °C in the dark for 15 min. After cells were washed with cold PBS three times, the samples were observed under a confocal microscope (Alghanem et al. 2017).
ATP production and mitochondrial potential measured
The cellular ATP levels were measured using a firefly luciferase-based ATP assay kit (Beyotime Institute of Biotechnology) (Zhu et al. 2018a). The mitochondrial transmembrane potential was analyzed using a JC-1 Kit (Beyotime, China). Images were captured using a fluorescence microscope (OLYMPUS DX51; Olympus, Tokyo, Japan) and were analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD) to obtain the mean densities of the regions of interest, which were normalized to those of the control group. Red-orange fluorescence was attributable to potential-dependent dye aggregation in the mitochondria. Green fluorescence, reflecting the monomeric form of JC-1, appeared in the cytosol following mitochondrial membrane depolarization (Nunez-Gomez et al. 2017).
LDH assay and caspase-3/9 activity detection
Lactate dehydrogenase (LDH) is a fairly stable enzyme that is released from the cytosol into the culture medium as a consequence of cellular integrity damage. Thus, we used an LDH assay (Beyotime Institute of Biotechnology) to evaluate the presence of cell injury or damage. The level of LDH released was expressed as a percentage of the control group (Glab et al. 2017; Kalyanaraman 2017). The caspase-3/9 activity kits (Beyotime Institute of Biotechnology, China) were used according to the manufacturer’s protocols. The relative caspase-3/9 activity was calculated from the ratio of treated cells to untreated cells. The assays were repeated three times (Liu et al. 2017b).
Sirt3 overexpression
The pDC315-Sirt3 vector (1336 bp, pDC315-Sirt3-NheI-F, 5′-CTAATGCGTTGCAATACGTGCGTCCTATATG-3′ and pDC315-Sirt31-HindIII-R, 5′-TTGTCCATTGCAAGGCCTCTGATTGAGTCTG-3′) was designed and purchased from Shanghai Gene-Pharma Co. (Shanghai, China) (Brasacchio et al. 2017). Briefly, the plasmid (3.0 μg per 1 × 104 cells/well) was transfected into HEK293 cells. When the cells detached from the plates, the medium supernatant was collected. Then, the viral supernatant was identified and amplified to obtain adenovirus-Sirt3 (Ad-Sirt3). Subsequently, Ad-Sirt3 was transfected into the N2a cells to overexpress Sirt3. Viral transductions were performed by incubating N2a cells with recombinant Ad-Sirt3 in Opti-MEM media supplemented with Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol (Torres-Quesada et al. 2017). Null vector transfection was used as the control group (Ad-ctrl).
GSH, GPx, and SOD detection
GSH, GPx, and SOD are important antioxidants that scavenge free radicals and therefore suppress the extent of oxidative stress and reduce the oxidative stress injury of N2a exposed to HR. SOD/GPx activity and GSH concentration were measured using commercial kits (Beyotime Institute of Biotechnology, China) following the manufacturer’s instructions (Le Cras et al. 2017; Randriamboavonjy et al. 2017).
Western blot
Cells were collected and lysed with RIPA lysis buffer (Beyotime, China) for 30 min. Equal amounts of protein were separated and transferred to a PVDF membrane (Millipore) (Dufour et al. 2017). After being blocked with 5% milk in Tris-buffered saline containing 0.05% Tween20 (TBST) at room temperature for 1 h, the membrane was incubated at 4 °C overnight with the following primary antibodies: caspase-9 (1:1000, Cell Signaling Technology, no. 9504); pro-caspase-3 (1:1000, Abcam, no. ab13847); cleaved caspase-3 (1:1000, Abcam, no. ab49822); Bcl2 (1:1000, Cell Signaling Technology, no. 3498); Bax (1:1000, Cell Signaling Technology, no. 2772); c-IAP (1:1000, Cell Signaling Technology, no. 4952); survivin (1:1000, Cell Signaling Technology, no. 2808); cyt-c (1:1000; Abcam; no. ab90529); Drp1 (1:1000, Abcam, no. ab56788); Fis1 (1:1000, Abcam, no. ab71498); Mff (1:1000, Cell Signaling Technology, no. 86668); Opa1 (1:1000, Abcam, no. ab42364); Mfn1 (1:1000, Abcam, no. ab57602); Sirt3 (1:1000, Abcam, no. ab86671); β-catenin (1:1000, Abcam, no. ab32572); p-β-catenin (1:1000, Abcam, no. ab53050); GAPDH (1:1000, Cell Signaling Technology, no. 5174); β-actin (1:1000, Cell Signaling Technology, no. 4967). After washing with PBST, the membrane was incubated with secondary antibody (1:1000, Cell Signaling Technology, nos. 7076 and 7074) for 1 h at room temperature, followed by enhanced chemiluminescence western blot detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
MTT assay and TUNEL staining
MMT assay was conducted as previously described (Zhou et al. 2017b). Briefly, the cells were seeded in a 96-well plate at 37 °C with 5% CO2. Subsequently, 20 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml; pH 7.4; Sigma-Aldrich) was added to the cells for 4 h (Lin et al. 2017). The supernatants were then discarded and 100 μl dimethyl sulfoxide (Sigma-Aldrich) was added to each well for 10 min. The OD of the samples was measured at an absorbance of 490 nm using a spectrophotometer (Epoch 2; BioTek Instruments, Inc., Winooski, VT, USA). The assay was repeated three times.
A TUNEL assay was performed using a one-step TUNEL kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s instructions (Jovancevic et al. 2017). TUNEL staining was performed with fluorescein-dUTP (Invitrogen; Thermo Fisher Scientific, Inc.) to stain apoptotic cell nuclei, and DAPI (5 mg/ml) was used to stain all cell nuclei at room temperature for 3 min. Cells in which the nucleus was stained with fluorescein-dUTP were defined as TUNEL positive. The slides were then imaged under a confocal microscope (Zhang et al. 2017).
Immunofluorescence confocal microscopy
Cells were fixed in 3.7% paraformaldehyde for 10 min at room temperature and permeabilized in 100% prechilled acetone (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Following blocking with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in PBS for 1 h at room temperature, the cells were incubated with primary antibodies for 4 h at room temperature (Pickard et al. 2017). Subsequently, the cells were incubated with secondary antibody (1:1000; cat. no. A-21206; Invitrogen; Thermo Fisher Scientific, Inc.) at 37 °C for 1 h in the dark. The primary antibodies used for cell immunofluorescence were as follows: Sirt3 (1:1000, Abcam, no. ab86671); p-β-catenin (1:1000, Abcam, no. ab53050); cyt-c (1:1000; Abcam; no. ab90529); Tom20 (1:1000, Abcam, no. ab186734) (Nunez-Gomez et al. 2017). The mitochondria were stained using Tom20 and the mitochondrial length was measured via laser confocal microscope (TcS SP5; Leica Microsystems, Inc., Buffalo Grove, IL, USA) according to the previous study (Zhou et al. 2018e).
Statistical analysis
All analyses were performed with SPSS 20.0 software (IBM Corp., Armonk, NY, USA). All experiments were repeated three times. The data are presented as the mean ± standard deviation and statistical significance for each variable was estimated by a one-way analysis of variance followed by Tukey’s test for the post hoc analysis. P < 0.05 was considered to indicate a statistically significant difference.
Results
Sirt3 overexpression regulates cerebral IRI
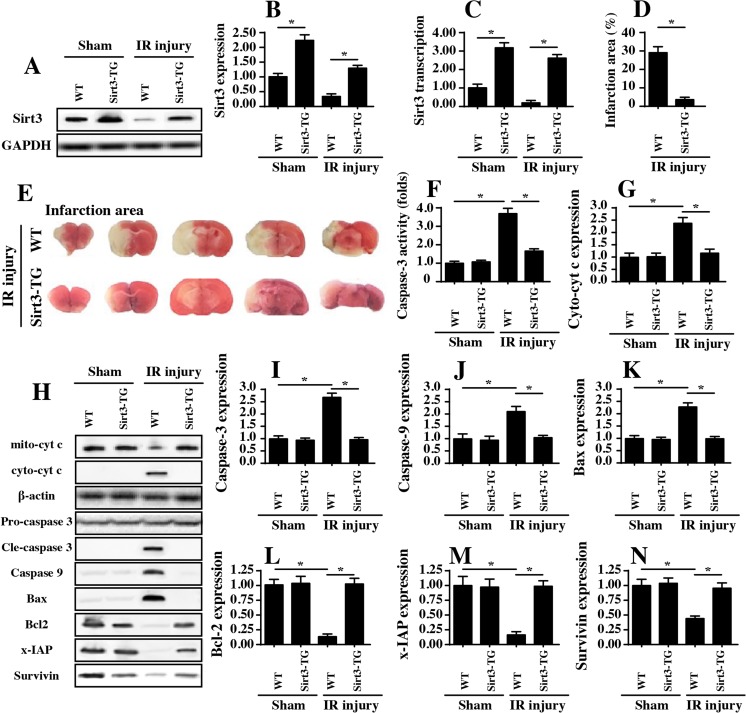
To address the functional role of Sirt3 in cerebral IRI, an in vivo model of 45 min of ischemia and 2 h of reperfusion was created. Subsequently, proteins were isolated from the brain, and the expression of Sirt3 was examined via western blots and qPCR. The results shown in Fig. 1a–c demonstrate that Sirt3 expression and transcription were significantly downregulated in the cerebral IRI group compared to the sham group. To explain the neuroprotective role of Sirt3 in cerebral IRI, Sirt3 transgenic (Sirt3-TG) mice were subjected to the ischemia-reperfusion process. Compared to the WT mice, Sirt3-TG mice had higher Sirt3 expression levels after IRI (Fig. 1a–c). In addition, compared to the sham group, the IRI group had a significantly increased infarction area in the brain (Fig. 1d–e), and this effect was significantly inhibited in Sirt3-TG mice, suggesting that IRI-mediated infarction expansion was negated by Sirt3 overexpression (Fig. 1d–e). Similarly, the caspase-3 activity was also increased in response to IRI and was reversed to near normal levels with Sirt3 overexpression (Fig. 1f).
Fig. 1.
Sirt3 reintroduction protects the brain from IRI. a–c Ischemia for 45 min and reperfusion for 2 h were used to induce cerebral IRI. Then, western blotting and qPCR were performed to analyze Sirt3 expression. d–e After cerebral IRI, TTC staining was used to observe the infarction size in WT mice and Sirt3-TG mice. f After cerebral IRI, proteins were isolated and caspase-3 activity was measured via ELISA assay. g–n Proteins were isolated from the brain tissues of WT mice and Sirt3-TG mice after cerebral IRI, and apoptotic proteins were analyzed by western blotting. *P < 0.05
In light of the central role of cellular apoptosis in cerebral IRI progression (Nunez-Gomez et al. 2017), western blotting was carried out to analyze the expression of apoptotic proteins in Sirt3-TG mice and WT mice after IRI or sham surgery. Compared to the sham group, the IRI group had significantly increased expression levels of pro-apoptotic proteins, such as Bax, cyto-cyt c, caspase-3, and caspase-9 (Fig. 1g–n). In comparison, the expression of antiapoptotic factors, such as Bcl2, x-IAP, and survivin, was markedly inhibited by IRI. These data confirmed that IRI was accompanied by increased cellular apoptosis. Interestingly, the restoration of Sirt3 in Sirt3-TG mice reversed the balance between pro- and antiapoptotic proteins, identifying the indispensable role of Sirt3 in blocking neuron apoptosis induced by IRI (Fig. 1g–n). Altogether, these data highlighted that Sirt3 is required for neuroprotection in cerebral IRI because it suppresses neuron apoptosis.
Reintroduction of Sirt3 enhances N2a cell survival via inhibiting mitochondrial apoptosis
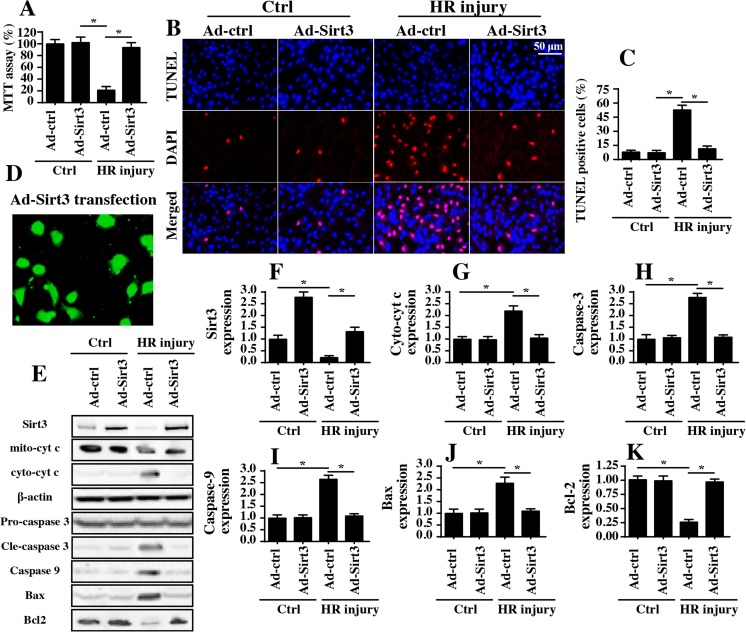
More evidence was obtained in vitro using a hypoxia-reoxygenation (HR) model in N2a cells. Subsequently, cellular viability and apoptotic rates were measured via MTT assays and TUNEL staining, respectively. As illustrated in Fig. 2a–c, HR treatment reduced cell viability and thus enhanced the cellular apoptotic index; these effects were significantly inhibited by Sirt3 overexpression via adenovirus-based technology (Ad-Sirt3) (Fig. 2a–c). The transfection efficiency of Sirt3 was shown in Fig. 2d and the overexpression efficiency was demonstrated in Fig. 2e–f. These data, consistent with the results observed in the animal study, confirmed that Sirt3 is an endogenous protector against neuron damage in response to IRI.
Fig. 2.
Sirt3 overexpression promotes N2a cell survival via blocking mitochondrial apoptosis in vitro. a Hypoxia for 45 min and reoxygenation (HR) for 2 h were used to mimic cerebral IRI in N2a cells. To overexpress Sirt3, Ad-Sirt3 was transfected into N2a cells, and a null vector was used for the control group (Ad-ctrl). After HR injury, cellular viability was analyzed via MTT assay. The relative cellular viability was normalized to the control group. b, c After HR injury, the cells were subjected to TUNEL assays, and the TUNEL-positive cells were recorded. d The transfection efficiency of Ad-Sirt3. e–k Proteins were isolated from N2a cells after HR injury; then, western blotting was used to analyze apoptotic protein expression. *P < 0.05
Subsequently, western blots were performed to analyze changes in apoptotic protein levels. Similar to the data obtained in the in vivo experiments, HR injury upregulated pro-apoptotic proteins related to mitochondria damage and downregulated antiapoptotic proteins (Fig. 2e–k). However, the transfection of Ad-Sirt3 could inhibit this imbalance between anti- and pro-apoptotic proteins (Fig. 2e–k). Collectively, these data indicate the role of Sirt3 in suppressing reperfusion-mediated neuron apoptosis.
Sirt3 blocks IRI-activated mitochondrial fission
Next, experiments were carried out to investigate the mechanism by which Sirt3 alleviated neuron apoptosis. To address this question, we investigated mitochondrial fission since it has been well-documented as an inducer of mitochondrial apoptosis in heart and liver reperfusion (Zhou et al. 2018a; Zhou et al. 2018e). As shown in Fig. 3a, HR treatment induced the division of strip-shaped mitochondria into several round fragments, and this effect was strongly inhibited by Ad-Sirt3 transfection. These data hinted that mitochondrial fission was activated by HR and inhibited by Sirt3 overexpression. Subsequently, the average length of mitochondria was measured, and the results indicated that mitochondrial length was decreased to 2.3 ± 0.3 μm by HR treatment and reversed to 8.7 ± 0.9 μm after Ad-Sirt3 transfection (Fig. 3b); these data suggest that Sirt3 can block HR-induced mitochondrial fission. Besides, western blots were performed to analyze the expression levels of proteins related to mitochondrial fission. Compared to the control group, the HR treatment group had increased expression levels of proteins involved in mitochondrial fission, such as Drp1, Fis1, and Mff (Fig. 3c–f). In comparison, the expression levels of mitochondrial fusion proteins, such as Mfn1 and Opa1 (Fig. 3d–h), were reduced by HR injury. Interestingly, the restoration of Sirt3 reversed the balance between fission- and fusion-associated factors. Considering that mitochondrial fusion protects against excessive mitochondrial fission (Ligeza et al. 2017; Ronchi et al. 2017), we concluded that Sirt3 deficiency triggers mitochondrial fission in neurons upon HR injury. Lastly, to explore whether mitochondrial fission is responsible for HR-mediated neuron apoptosis, a fission activator and inhibitor were used. We blocked mitochondrial fission in HR-treated cells via Mdivi-1 and reactivated fission in Sirt3-overexpressing cells via FCCP. Subsequently, caspase-3 activity was measured via ELISA. Compared to that in the control group, caspase-3 activity was increased by HR treatment and reduced by Sirt3 overexpression or Mdivi-1 pretreatment (Fig. 3i). In contrast, the reactivation of mitochondrial fission via FCCP abolished the inhibitory effect of Sirt3 overexpression on caspase-3 activation (Fig. 3i). Similarly, TUNEL assay also demonstrated that Sirt3 reduced HR-mediated cell apoptosis via suppressing mitochondrial fission (Fig. 3j–k). Through loss- and gain-of-function assays for mitochondrial fission, we confirmed that mitochondrial fission, which is strongly inhibited by Sirt3, was the upstream trigger for lethal mitochondrial apoptosis in the cerebral IRI model.
Fig. 3.
Sirt3 overexpression inhibits mitochondrial fission. a, b Hypoxia for 45 min and reoxygenation (HR) for 2 h were used to mimic cerebral IRI in N2a cells. To overexpress Sirt3, Ad-Sirt3 was transfected into N2a cells, and a null vector was used for the control group (Ad-ctrl). After IRI, the cells were stained for Tom20, a special mitochondrial marker. Then, the average length of the mitochondria was recorded. c–h Proteins were isolated from N2a cells after HR injury; then, western blotting was carried out to determine mitochondrial fission- and fusion-related protein expression. i To activate mitochondrial fission, FCCP was added to the medium for 2 h. To inhibit mitochondrial fission, Mdivi1 was added to N2a cells 4 h before HR injury. Then, caspase-3 activity was analyzed via ELISA. j, k After HR injury, the cells were subjected to TUNEL assays, and the TUNEL-positive cells were recorded. *P < 0.05
Mitochondrial fission accounts for neuron apoptosis via triggering the caspase-9-dependent mitochondrial apoptosis pathway
Subsequently, we determined how mitochondrial fission promoted neuron apoptosis. Previous findings indicated that mitochondrial fission initiated caspase-9-dependent mitochondrial apoptosis via inducing oxidative stress and pro-apoptotic factor leakage in a myocardial reperfusion injury model (Jin et al. 2018; Zhou et al. 2017a). Based on this, we first examined cellular oxidative stress with Sirt3 overexpression. As shown in Fig. 4a, b, HR increased ROS generation, the effects of which were inhibited by Sirt3 overexpression. Interestingly, the reactivation of mitochondrial fission via FCCP restored ROS generation in Sirt3-overexpressing cells (Fig. 4a–b). Subsequently, we found that excessive ROS levels were accompanied by a decrease in cellular antioxidant factors, such as SOD, GSH, and GPX (Fig. 4c–e). In comparison, Sirt3 overexpression maintained the expression of SOD, GSH, and GPX (Fig. 4c–e), and this effect was negated by FCCP. Moreover, the mitochondrial potential was reduced by HR stress and was reversed to normal levels by Sirt3 overexpression because mitochondrial fission was prevented (Fig. 4f, g).
Fig. 4.
Mitochondrial fission regulates neuron apoptosis upon HR injury. a, b Hypoxia for 45 min and reoxygenation (HR) for 2 h were used to mimic cerebral IRI in N2a cells. To overexpress Sirt3, Ad-Sirt3 was transfected into N2a cells, and a null vector was used for the control group (Ad-ctrl). Then, DCFHDA, a type of probe, was used to stain for cellular ROS, and ROS production was observed. FCCP was used to activate mitochondrial fission. c–e The cellular antioxidant factors SOD, GSH, and GPX were analyzed via ELISA. f–g Mitochondrial potential was measured via JC-1 staining. h, i Immunofluorescence assay for cyt-c. DAPI was used to stain the nuclei. j Caspase-9 activity was analyzed via ELISA, and the relative caspase-9 activity was normalized to that of the control group. *P < 0.05
In response to the destruction of mitochondrial potential, mitochondria released cyt-c into the nucleus (Fig. 4h, i), and this effect was largely repressed by Sirt3 transfection in a mitochondrial fission-dependent manner. Cyt-c, a pro-apoptotic factor primarily located in mitochondria, could activate caspase-9 and thus increase caspase-3 activity to induce apoptosis in cells. Based on this, we measured the activity of caspase-9. The results showed that HR increased caspase-9 activity, whereas Ad-Sirt3 transfection reduced caspase-9 activity (Fig. 4j). Interestingly, FCCP treatment restored the increased caspase-9 activity despite transfection with Ad-Sirt3. Altogether, these data indicated that neuron apoptosis induced by HR stress can be attributed to activated mitochondrial fission, which is strongly inhibited by Sirt3.
The Wnt/β-catenin pathway is activated by Sirt3 and contributes to mitochondrial homeostasis
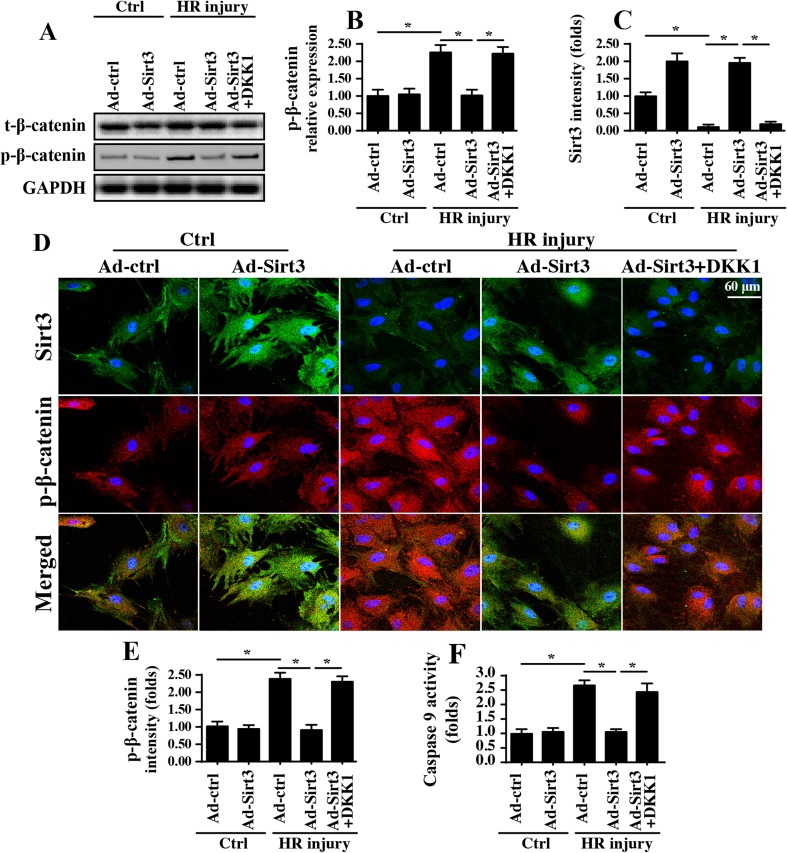
Next, experiments were conducted to explore the downstream effector of Sirt3 that is responsible for mitochondrial fission inhibition. Given the neuroprotective role of the Wnt/β-catenin pathway in cerebral IRI (Lee et al. 2017b; Torres-Estay et al. 2017), we questioned whether Sirt3 modified mitochondrial fission via the Wnt/β-catenin pathway. First, western blots for β-catenin displayed that the Wnt/β-catenin pathway was inhibited by HR treatment, as evidenced by increased β-catenin phosphorylation (Fig. 5a, b), which indicates that the Wnt/β-catenin pathway is inactive. Interestingly, the reintroduction of Sirt3 via Ad-Sirt3 transfection repressed HR-mediated β-catenin phosphorylation (Fig. 5a, b), indicative of β-catenin activation. This concept was further verified via immunofluorescence assays costaining for Sirt3 and p-β-catenin (Fig. 5c–e). These data indicated that the Wnt/β-catenin pathway is inhibited by HR and activated in response to Sirt3 activation. To establish the causal role of the Wnt/β-catenin pathway in Sirt3-mediated neuroprotection, pathway blocker (DKK1) was used to inhibit the activity of β-catenin in Sirt3-overexpressing cells. The inhibitory efficiency is shown in Fig. 5a, b. After blocking Wnt/β-catenin pathway, caspase-9 activity was measured again. Similar to the above results, HR-augmented caspase-9 activity was mostly suppressed by Ad-Sirt3 transfection, and this effect was fully inhibited by DKK1 (Fig. 5f). Overall, these data firmly establish the central role of Sirt3-induced Wnt/β-catenin pathway activation in inducing mitochondrial homeostasis in HR-treated neurons.
Fig. 5.
Sirt3 activates the Wnt/β-catenin pathway to sustain mitochondrial homeostasis. a–b Western blotting was performed to analyze the levels of β-catenin phosphorylation, an indicator of Wnt/β-catenin inactivation. To inhibit the Sirt3-mediated β-catenin activation, DKK1 was administrated into Sirt3-overexpressing cell. Then, the proteins were isolated, and protein expression levels were analyzed. c–e Immunofluorescence for p-β-catenin and Sirt3. f Caspase-9 activity was analyzed via ELISA. *P < 0.05
The Wnt/β-catenin pathway is also involved in mitochondrial fission
To validate whether the Wnt/β-catenin pathway is involved in HR-induced mitochondrial fission and neuron apoptosis, we observed mitochondrial morphology and the neuron apoptotic index in the presence of Wnt/β-catenin pathway blocker. As shown in Fig. 6a, b, mitochondrial fragmentation was stimulated by HR treatment, and this configuration change was recused by Sirt3 overexpression. However, inhibition of Wnt/β-catenin pathway via DKK1 restored the formation of mitochondrial fragments. This observation was further supported via measuring the average length of the mitochondria (Fig. 6a, b). This information confirmed that Wnt/β-catenin pathway activation is associated with mitochondrial fission inhibition. Lastly, we used TUNEL staining to observe the role of the Wnt/β-catenin pathway in neuron death. The results in Fig. 6c, d show that the number of TUNEL-positive cells was increased in response to HR and was reversed to normal levels by Sirt3 overexpression. In comparison, inhibition of Wnt/β-catenin pathway restored the ratio of TUNEL-positive cells (Fig. 6c, d), even with Sirt3 overexpression. Altogether, our data illustrated that the Wnt/β-catenin pathway is involved in mitochondrial fission and neuron apoptosis in response to HR injury.
Fig. 6.
The Wnt/β-catenin pathway is also involved in mitochondrial fission. a, b DKK1 was used to inhibit the Wnt/β-catenin pathway. Mitochondria were stained with Tom20, and the average length of the mitochondria was recorded. c, d After HR injury, the cells were subjected to TUNEL assays, and the TUNEL-positive cells were recorded. *P < 0.05
Discussion
The pathogenesis of cerebral IRI involves a range of complex processes, including cellular oxidation, calcium overload, and excess inflammation responses. Interestingly, mitochondrial fission is noted in the above pathophysiological process. Moreover, the role and upstream regulatory mechanism of mitochondrial fission in cerebral IRI remain unknown. In the present study, we found that (1) Sirt3 was significantly downregulated in response to cerebral IRI in vivo and in vitro; (2) functional investigations indicated that the reintroduction of Sirt3 attenuated IRI-mediated neuron apoptosis, possibly through blocking caspase-9-dependent mitochondrial apoptotic signals; (3) at the molecular level, mitochondrial apoptosis was triggered by excessive mitochondrial fission; (4) Sirt3 overexpression strongly suppressed mitochondrial fission via activating the Wnt/β-catenin pathway; and (5) the loss of β-catenin abolished the neuroprotective effects of Sirt3 overexpression. Taken together, our present study comprehensively describes the pathogenesis of cerebral IRI, which involves Sirt3 downregulation, Wnt/β-catenin pathway inactivation, mitochondrial fission initiation, and caspase-9-dependent apoptosis in neurons. To the best of our knowledge, this is the first study to establish that Sirt3 protects against cerebral IRI via blocking mitochondrial fission and normalizing Wnt/β-catenin signaling.
Mitochondrial fission is a physiological response occurring in numerous biological processes, such as cellular proliferation, cancer mobility, and metabolism reprogramming (Griffiths et al. 2017). The aim of normal mitochondrial fission is to provide more daughter mitochondria to meet cellular energy requirements (Brasacchio et al. 2017; Oanh et al. 2017). However, excessive mitochondrial fission causes the formation of mitochondrial fragments, which are harmful to cellular homeostasis. In heart ischemia reperfusion (Zhou et al. 2017a; Zhou et al. 2017d), excessive mitochondrial fission has been reported to mediate the uneven division of mitochondrial DNA (mtDNA) into daughter mitochondria, which impairs mtDNA copy and transcription. Moreover, excessive mitochondrial fission induces extensive mitochondrial oxidative stress via XO-dependent (Zhang et al. 2016) or COX1-related (Zhou et al. 2018f) ROS generation; this process results in cell death because mPTP opening is promoted (Zhu et al. 2018a). In the present study, we found that mitochondrial fission is the primary contributor to cerebral IRI-induced neuron death; excessive mitochondrial fission was associated activating caspase-9-dependent mitochondrial apoptosis as evidenced by reduced mitochondrial potential, augmented ROS production and more cyt-c release into the nucleus. These findings identify mitochondrial fission as a potential target for protecting the brain from reperfusion injury. Accordingly, strategies to prevent mitochondrial fission could be considered an effective therapeutic approach for treating cerebral IRI in clinical practice.
In the present study, we determined that IRI-induced mitochondrial fission is primarily regulated by Sirt3 and the Wnt/β-catenin pathway. Our results illustrated that Sirt3 was downregulated by cerebral IRI, which was followed by increased mitochondrial fission. However, the overexpression of Sirt3 effectively inhibited the mitochondrial fission, and this mechanism was achieved via the Wnt/β-catenin pathway. Inactivation of the Wnt/β-catenin pathway via pathway blocker abolished the pro-survival effects exerted by Sirt3 in neurons during cerebral IRI. These data identified Sirt3 as the upstream regulator of mitochondrial fission inhibition via Wnt/β-catenin pathway activation. In fact, ample evidence has shown the role of Sirt3 in mitochondrial homeostasis and ROS management. For example, Sirt3 is associated with mitochondrial cardiolipin expression (Chabi et al. 2018), mPTP opening regulation (Nassir et al. 2018), mitochondrial energy production (Wu et al. 2018), autophagy activity (Takakura et al. 2017), cellular oxidative stress (Lee et al. 2018), and metabolic reprogramming (Lee et al. 2017a). More importantly, the protective effects of Sirt3 on mitochondria have been verified in different disease models, including Alzheimer’s disease (Lee et al. 2018), gastric cancer (Lee et al. 2017a), fatty liver disease (Liu et al. 2017a), and heart failure (Du et al. 2017). These findings indicate that Sirt3 is an endogenous protector against mitochondrial dysfunction in many diseases (Garcia-Nino et al. 2017). In the present study, our data further support the beneficial impact of Sirt3 on mitochondria, especially in cerebral IRI via inhibiting mitochondrial fission. These findings provide new insights into the mechanisms of mitochondrial damage during cerebral IRI.
We observed that the Wnt/β-catenin pathway is necessary for the brain protection provided by Sirt3. Notably, previous studies have noted the causal relationship between the sirtuin family and the Wnt/β-catenin pathway (Zhou et al. 2016). Sirt1 represses adipogenesis by activating the Wnt/β-catenin pathway. In comparison, Sirt3 deficiency is associated with the inactivation of the Wnt/β-catenin pathway, which contributes to hearing loss and neurological disease (Kwon et al. 2015). These data indicate that Sirt3 might be the upstream inducer of the Wnt/β-catenin pathway. Regarding the Wnt/β-catenin pathway, its crucial role in brain development has been described by several reports; this pathway is necessary for cerebral cortex expansion (Chenn 2008), neuronal connectivity, and synapse formation (Oliva et al. 2013). However, in response to cerebral IRI, the Wnt/β-catenin pathway has been found to be inactivated through mechanisms that are poorly understood (Couto et al. 2017; Schock et al. 2017). In the current study, we describe these mechanisms. Our data show that the Wnt/β-catenin pathway is phosphorylated inactivation due to Sirt3 downregulation in cerebral IRI; the overexpression of Sirt3 could reverse the activity of the Wnt/β-catenin pathway. Notably, the mechanism by which Sirt3 activates the Wnt/β-catenin pathway remains unclear, and whether Sirt3 directly interacts with β-catenin to stabilize its expression and activity or promotes its transcription with the assistance of other proteins have not yet been determined(Lassen et al. 2017; Merjaneh et al. 2017). Accordingly, further investigations are required to obtain the full role of Sirt3 in activating the Wnt/β-catenin pathway in cerebral IRI.
Collectively, our data explain the functional role of Sirt3 in cerebral reperfusion injury. Sirt3 is downregulated by cerebral IRI and subsequently inactivates the Wnt/β-catenin pathway, which induces excess mitochondrial fission. Extensive mitochondrial fragmentation promotes cellular oxidation and increases caspase-9-related mitochondrial apoptosis, thus causing apoptosis in neurons. These findings shed new light on cerebral IRI development and progression and may provide a new therapeutic approach for treating cerebral reperfusion injury.
Author contributions
HZ, YCL, and LHC conceived the research; ZHZ, CSS, YJL, and RXX performed the experiments; all authors participated in discussing and revising the manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (grant No. 81100917) and Beijing Nova program (Z171100001117096).
Ethics approval and consent to participate
The animal study was performed in accordance with the Declaration of Helsinki. All experimental protocols were approved by the Ethics Committee of Department of neurosurgery, PLA army general hospital, Beijing, China, Beijing, China. The ethics reference number: SCSSJN20112.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ackermann M, et al. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis. 2017;20:359–372. doi: 10.1007/s10456-017-9543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghanem AF, et al. RCAN1.4 regulates VEGFR-2 internalisation, cell polarity and migration in human microvascular endothelial cells. Angiogenesis. 2017;20:341–358. doi: 10.1007/s10456-017-9542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasacchio D, et al. Epigenetic control of mitochondrial cell death through PACS1-mediated regulation of BAX/BAK oligomerization. Cell Death Differ. 2017;24:961–970. doi: 10.1038/cdd.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B et al (2018) Skeletal muscle overexpression of short isoform Sirt3 altered mitochondrial cardiolipin content and fatty acid composition. J Bioenerg Biomembr. 10.1007/s10863-018-9752-1 [DOI] [PubMed]
- Chen X, et al. Peroxynitrite enhances self-renewal, proliferation and neuronal differentiation of neural stem/progenitor cells through activating HIF-1alpha and Wnt/beta-catenin signaling pathway. Free Radic Biol Med. 2018;117:158–167. doi: 10.1016/j.freeradbiomed.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Chenn A. Wnt/beta-catenin signaling in cerebral cortical development. Organogenesis. 2008;4:76–80. doi: 10.4161/org.4.2.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto JA, et al. A somatic GNA11 mutation is associated with extremity capillary malformation and overgrowth. Angiogenesis. 2017;20:303–306. doi: 10.1007/s10456-016-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N et al (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J Pineal Res 62. 10.1111/jpi.12404 [DOI] [PubMed]
- Du Q, Zhu B, Zhai Q, Yu B. Sirt3 attenuates doxorubicin-induced cardiac hypertrophy and mitochondrial dysfunction via suppression of Bnip3. Am J Transl Res. 2017;9:3360–3373. [PMC free article] [PubMed] [Google Scholar]
- Dufour F, et al. N-glycosylation of mouse TRAIL-R and human TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ. 2017;24:500–510. doi: 10.1038/cdd.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadicherla AK, et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol. 2017;112:27. doi: 10.1007/s00395-017-0618-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Nino WR, et al. Cardioprotective kinase signaling to subsarcolemmal and interfibrillar mitochondria is mediated by caveolar structures. Basic Res Cardiol. 2017;112:15. doi: 10.1007/s00395-017-0607-4. [DOI] [PubMed] [Google Scholar]
- Ghaffari S, Leask RL, Jones EAV. Blood flow can signal during angiogenesis not only through mechanotransduction, but also by affecting growth factor distribution. Angiogenesis. 2017;20:373–384. doi: 10.1007/s10456-017-9553-x. [DOI] [PubMed] [Google Scholar]
- Glab JA, et al. DR5 and caspase-8 are dispensable in ER stress-induced apoptosis. Cell Death Differ. 2017;24:944–950. doi: 10.1038/cdd.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths HR, Gao D, Pararasa C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017;12:50–57. doi: 10.1016/j.redox.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Zeng H, He X, Chen JX. Sirt3 is essential for apelin-induced angiogenesis in post-myocardial infarction of diabetes. J Cell Mol Med. 2015;19:53–61. doi: 10.1111/jcmm.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovancevic N, et al. Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res Cardiol. 2017;112:13. doi: 10.1007/s00395-017-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B. Teaching the basics of cancer metabolism: developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–842. doi: 10.1016/j.redox.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Zhu H, Luan H, Han F, Jiang W. Curculigoside A induces angiogenesis through VCAM-1/Egr-3/CREB/VEGF signaling pathway. Neuroscience. 2014;267:232–240. doi: 10.1016/j.neuroscience.2014.02.050. [DOI] [PubMed] [Google Scholar]
- Kwon DN, Park WJ, Choi YJ, Gurunathan S, Kim JH. Oxidative stress and ROS metabolism via down-regulation of sirtuin 3 expression in Cmah-null mice affect hearing loss. Aging (Albany NY) 2015;7:579–594. doi: 10.18632/aging.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen TR, Nielsen JM, Johnsen J, Ringgaard S, Botker HE, Kristiansen SB. Effect of paroxetine on left ventricular remodeling in an in vivo rat model of myocardial infarction. Basic Res Cardiol. 2017;112:26. doi: 10.1007/s00395-017-0614-5. [DOI] [PubMed] [Google Scholar]
- Le Cras TD, Mobberley-Schuman PS, Broering M, Fei L, Trenor CC, 3rd, Adams DM. Angiopoietins as serum biomarkers for lymphatic anomalies. Angiogenesis. 2017;20:163–173. doi: 10.1007/s10456-016-9537-2. [DOI] [PubMed] [Google Scholar]
- Lee DY, Jung DE, Yu SS, Lee YS, Choi BK, Lee YC. Regulation of SIRT3 signal related metabolic reprogramming in gastric cancer by Helicobacter pylori oncoprotein CagA. Oncotarget. 2017;8:78365–78378. doi: 10.18632/oncotarget.18695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J et al (2018) SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell 17. 10.1111/acel.12679 [DOI] [PMC free article] [PubMed]
- Lee MS, Yin TC, Sung PH, Chiang JY, Sun CK, Yip HK (2017b) Melatonin enhances survival and preserves functional integrity of stem cells: a review. J Pineal Res 62. 10.1111/jpi.12372 [DOI] [PubMed]
- Ligeza J, et al. MCPIP1 contributes to clear cell renal cell carcinomas development. Angiogenesis. 2017;20:325–340. doi: 10.1007/s10456-017-9540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Hoffmann K, Gao C, Petrulionis M, Herr I, Schemmer P (2017) Melatonin promotes sorafenib-induced apoptosis through synergistic activation of JNK/c-jun pathway in human hepatocellular carcinoma. J Pineal Res 62. 10.1111/jpi.12398 [DOI] [PubMed]
- Liu J, Li D, Zhang T, Tong Q, Ye RD, Lin L. SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity. Cell Death Dis. 2017;8:e3158. doi: 10.1038/cddis.2017.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gan L, Luo D, Sun C (2017b) Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J Pineal Res 62. 10.1111/jpi.12383 [DOI] [PubMed]
- Merjaneh M, Langlois A, Larochelle S, Cloutier CB, Ricard-Blum S, Moulin VJ. Pro-angiogenic capacities of microvesicles produced by skin wound myofibroblasts. Angiogenesis. 2017;20:385–398. doi: 10.1007/s10456-017-9554-9. [DOI] [PubMed] [Google Scholar]
- Murphy PS, Wang J, Bhagwat SP, Munger JC, Janssen WJ, Wright TW, Elliott MR. CD73 regulates anti-inflammatory signaling between apoptotic cells and endotoxin-conditioned tissue macrophages. Cell Death Differ. 2017;24:559–570. doi: 10.1038/cdd.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassir F, Arndt JJ, Johnson SA, Ibdah JA (2018) Regulation of mitochondrial trifunctional protein modulates nonalcoholic fatty liver disease in mice. J Lipid Res. 10.1194/jlr.M080952 [DOI] [PMC free article] [PubMed]
- Nunez-Gomez E, Pericacho M, Ollauri-Ibanez C, Bernabeu C, Lopez-Novoa JM. The role of endoglin in post-ischemic revascularization. Angiogenesis. 2017;20:1–24. doi: 10.1007/s10456-016-9535-4. [DOI] [PubMed] [Google Scholar]
- Oanh NTK, Park YY, Cho H. Mitochondria elongation is mediated through SIRT1-mediated MFN1 stabilization. Cell Signal. 2017;38:67–75. doi: 10.1016/j.cellsig.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Oliva CA, Vargas JY, Inestrosa NC. Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front Cell Neurosci. 2013;7:224. doi: 10.3389/fncel.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard JM, Burke N, Davidson SM, Yellon DM. Intrinsic cardiac ganglia and acetylcholine are important in the mechanism of ischaemic preconditioning. Basic Res Cardiol. 2017;112:11. doi: 10.1007/s00395-017-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamboavonjy V, Kyselova A, Elgheznawy A, Zukunft S, Wittig I, Fleming I. Calpain 1 cleaves and inactivates prostacyclin synthase in mesenteric arteries from diabetic mice. Basic Res Cardiol. 2017;112:10. doi: 10.1007/s00395-016-0596-8. [DOI] [PubMed] [Google Scholar]
- Ronchi C, Torre E, Rizzetto R, Bernardi J, Rocchetti M, Zaza A. Late sodium current and intracellular ionic homeostasis in acute ischemia. Basic Res Cardiol. 2017;112:12. doi: 10.1007/s00395-017-0602-9. [DOI] [PubMed] [Google Scholar]
- Schock SN, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell Death Differ. 2017;24:615–625. doi: 10.1038/cdd.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZY, et al. Protective effect of autophagy in neural ischemia and hypoxia: negative regulation of the Wnt/beta-catenin pathway. Int J Mol Med. 2017;40:1699–1708. doi: 10.3892/ijmm.2017.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura K, et al. Cordyceps militaris improves the survival of Dahl salt-sensitive hypertensive rats possibly via influences of mitochondria and autophagy functions. Heliyon. 2017;3:e00462. doi: 10.1016/j.heliyon.2017.e00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobisawa T, et al. Insufficient activation of Akt upon reperfusion because of its novel modification by reduced PP2A-B55alpha contributes to enlargement of infarct size by chronic kidney disease. Basic Res Cardiol. 2017;112:31. doi: 10.1007/s00395-017-0621-6. [DOI] [PubMed] [Google Scholar]
- Torrens-Mas M, Hernandez-Lopez R, Oliver J, Roca P, Sastre-Serra J (2018) Sirtuin 3 silencing improves oxaliplatin efficacy through acetylation of MnSOD in colon cancer. J Cell Physiol doi:10.1002/jcp.26443 [DOI] [PubMed]
- Torres-Estay V, et al. Androgens modulate male-derived endothelial cell homeostasis using androgen receptor-dependent and receptor-independent mechanisms. Angiogenesis. 2017;20:25–38. doi: 10.1007/s10456-016-9525-6. [DOI] [PubMed] [Google Scholar]
- Torres-Quesada O, Mayrhofer JE, Stefan E. The many faces of compartmentalized PKA signalosomes. Cell Signal. 2017;37:1–11. doi: 10.1016/j.cellsig.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Wei T et al (2017) Sirtuin 3 deficiency accelerates hypertensive cardiac remodeling by impairing angiogenesis. J Am Heart Assoc 6. 10.1161/JAHA.117.006114 [DOI] [PMC free article] [PubMed]
- Wu Y et al. (2018) SIRT3 aggravates metformin-induced energy stress and apoptosis in ovarian cancer cells. Exp Cell Res. doi:10.1016/j.yexcr.2018.03.030 [DOI] [PubMed]
- Xing XS, Liu F, He ZY. Akt regulates beta-catenin in a rat model of focal cerebral ischemia-reperfusion injury. Mol Med Rep. 2015;11:3122–3128. doi: 10.3892/mmr.2014.3000. [DOI] [PubMed] [Google Scholar]
- Zhang W, Tao A, Lan T, Cepinskas G, Kao R, Martin CM, Rui T. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol. 2017;112:16. doi: 10.1007/s00395-017-0603-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 2016;95:278–292. doi: 10.1016/j.freeradbiomed.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Zhou H et al. (2018a) Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res 64. doi:10.1111/jpi.12450 [DOI] [PubMed]
- Zhou H et al. (2017a) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc 6. doi:10.1161/JAHA.116.005328 [DOI] [PMC free article] [PubMed]
- Zhou H et al. (2017b) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res 63. doi:10.1111/jpi.12438 [DOI] [PubMed]
- Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018b) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res doi:10.1111/jpi.12471 [DOI] [PubMed]
- Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y (2018c) BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis doi:10.1007/s10456-018-9611-z [DOI] [PubMed]
- Zhou H, Wang J, Zhu P, Hu S, Ren J. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 2018;45:12–22. doi: 10.1016/j.cellsig.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Zhou H, et al. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol. 2018;113:23. doi: 10.1007/s00395-018-0682-1. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2017;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Yue Y, Wang J, Ma Q, Chen Y. Melatonin therapy for diabetic cardiomyopathy: a mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal. 2018;47:88–100. doi: 10.1016/j.cellsig.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Zhou H et al. (2017d) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63. doi:10.1111/jpi.12413 [DOI] [PMC free article] [PubMed]
- Zhou H, et al. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018g) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 10.1038/s41418-018-0086-7 [DOI] [PMC free article] [PubMed]
- Zhou Y, et al. SIRT1 suppresses adipogenesis by activating Wnt/beta-catenin signaling in vivo and in vitro. Oncotarget. 2016;7:77707–77720. doi: 10.18632/oncotarget.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, et al. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 2018;23:101–113. doi: 10.1007/s12192-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, et al. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018;16:157–168. doi: 10.1016/j.redox.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo G, et al. Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Mol Brain. 2018;11:9. doi: 10.1186/s13041-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]