Abstract
Sarcopenia is defined as the loss of skeletal muscle mass and function due to age, and represents a major cause of disability in the elderly population. The contributing factors to the onset of sarcopenia are not well defined, but appear to involve age-dependent changes in both the tissue microenvironment and muscle progenitor cell (MPC) population. MPC transplantation has the potential to be a novel therapy for treatment of muscle dysfunction due to aging or injury, but has not shown significant clinical efficacy to date. The goal of this research was to use a rat model of skeletal muscle injury to examine the differential effects of age on MPC survival, differentiation, and tissue regeneration after transplantation. Fluorescently labeled MPCs, derived from young (YMPCs) and adult (AMPCs) donor rats, were transplanted in the injured tibialis anterior (TA) muscles of young, adult, and aged rats. Our results demonstrated that integration and maturation of YMPCs into mature myofibers were dependent on the age of the host microenvironment; whereas, the integration and maturation of AMPCs were less dependent on age and more dependent on intrinsic cellular changes. These data suggest that the age of both the host microenvironment and cells for transplantation must be considered when designing cell therapy regimens.
Keywords: : aging, muscle progenitor cells, skeletal muscle, stem cell therapy, injury
Introduction
Age-associated declines in muscle function and healing are a major cause of disability in the elderly population. Regenerative medicine provides new biological tools and approaches to better understand and potentially remedy this progressive loss of muscle function. Cellular therapies are being developed to replace and/or repair damaged tissues for use when the host stem cell population is impaired or depleted; however, the use of cell therapy in the clinic has resulted in little success to date.1,2
While much emphasis has been devoted to the type and source of cells for efficacious therapy, the research performed examining the role of the microenvironment of the damaged tissue in promoting or repressing tissue regeneration and healing has been controversial. In an article published in Nature over a decade ago, Conboy et al., used heterochronic parabiotic pairings of young and old mice to demonstrate the presence of a “youthful” circulating factor that enhanced muscle regeneration in the old animals,3 which has since been identified as growth differentiation factor-11 (GDF-11).4,5 Several recent publications have been published disputing the ability of GDF-11 to enhance regeneration in older animals,6,7 including data from our group.8 Alternatively, Brack et al., suggested that an increased concentration of circulating Wnt detected in serum from older mice can reduce Notch signaling, which is required for maintenance of muscle progenitor cell (MPC) potential and can push MPCs toward myofibrogenic differentiation,9,10 ultimately resulting in impaired tissue regeneration and poor functional recovery after injury.
In addition to circulating factors, extrinsic components of the tissue microenvironment have been implicated in the performance of MPCs after injury in older animals.11–14 Muscles in aged animals contain a higher percentage of fibrogenic and adipogenic cells that directly influence the outcome of regeneration after injury.15 Fibro/adipogenic precursor cells (FAPs) are connective tissue cells that are directly involved in the deposition of fibrotic and adipogenic tissue in skeletal muscle16–19 and are necessary for normal skeletal muscle regeneration.15,20,21 Moreover, it has been demonstrated that the extracellular matrix (ECM) from aged animals can promote fibrogenic differentiation of MPCs,22 leaving the damaged tissue incompatible to cellular therapies due to acute inflammatory responses and the formation of scar tissue. Furthermore, age-related changes in muscle vascularization and innervation can have direct effects on the regenerative capacity of MPCs after injury.23,24
Successful clinical application of stem cell therapy will rely on a thorough understanding of the molecular and cellular processes that regulate normal tissue regeneration (i.e., wound healing). Successful regeneration or engineering of complex tissues, such as skeletal muscle, requires an understanding of the interactions of multiple tissue components of skeletal muscle, including the ECM, autocrine, and paracrine interactions of diverse resident cell types, inflammatory responses, vascularization, innervation, and proper mechanical transduction. The consideration of these factors during the design of present therapies is generally lacking, and may explain the limited clinical success of cell therapy.
Animal models of muscle tissue loss traditionally utilize arterial ligation, injection of myotoxins, the excision/removal of muscle tissue, or amputation.25–28 However, these models lack the clinical manifestation of most muscle injuries in humans, in which the damaged muscle tissue progressively degenerates and a nonregenerative environment remains. The injured tissue is often incompatible to cellular therapies due to acute inflammation, formation of scar tissue, and lack of vascularity and innervation. We have developed and characterized a model of compression/ischemia reperfusion injury in the hind limbs of rats29 that mimics the damage to the muscular, vascular, and neural components, such as is seen in patients after crush injuries or blunt force trauma. Although several groups have previously examined age-related changes in the microenvironment and satellite cell activation,30–32 our complex injury model provides a unique setting to identify age-dependent changes in the microenvironments (i.e., vasculature, neuromuscular junctions [NMJs], etc.) of uninjured and injured tissue, as well as the interactions between the microenvironment and the progenitor cells used for therapy.
In this study, MPC transplantation was used as a means to examine extrinsic (host age) and intrinsic (transplanted cells) effects on the ability of different aged animals to recover from injury. The characterization of the survival and myogenic capacity of MPCs, as well as their ability to aid in functional recovery after injury, in different-aged animals was performed to identify the key players and processes involved in muscle regeneration that could be further targeted for therapeutic intervention.
Materials and Methods
MPC culture
MPC harvest and culture
MPCs were harvested from young (4 weeks) and adult (11–12 months) female Lewis rats as previously described.29 Cells were expanded in Dulbecco's modified Eagles' medium (Hyclone, Thermo Scientific, Logan, UT) low glucose containing 10% horse serum, 20% fetal bovine serum, 1% chick embryo extract (Sera Laboratories International Ltd., United Kingdom), and 1% penicillin/streptomycin at 37°C with 5% CO2. MPCs (no later than passage 2) were labeled with a GFP-expressing lentivirus immediately before use.
MPC characterization
Flow cytometry of labeled cells was performed to confirm similar infection efficiency and signal strength. An IncuCyte (Essen Bioscience, Ann Arbor, MI) real-time live-cell imager was used to determine the growth curves of MPCs harvested from young (YMPCs) and adult (AMPCs) rats. Cells were seeded in 12-well plates (70,000 cells per well) and placed into the IncuCyte. Plate confluence was measured every 2 h for 8 days, using a software platform supplied by the manufacturer.
RNA and quantitative PCR analyses
Total RNA was isolated from the rat TA muscle using the PerfectPure RNA Fibrous Tissue Kit (5 Prime, Inc., Gaithersburg, MD) as previously described.33Quantitative PCR (qPCR) was performed to measure the transcripts of myogenic, fibrogenic, and FAP markers in both populations of cells. qPCR was performed in 20-mL reactions in 96-well plates using cDNA samples generated from 12.5 ng of total RNA. TaqMan probes (Applied Biosystems) specific for rat genes are as listed: GAPDH (NM_017008.3), Pax7 (NM_001191984.1), PDGFRα (NM_012802.1), Tcf4 (NM_053369.1), and CTGF (NM_022266.2). qPCR was performed using SYBR green (Applied Biosystems/Life Technologies, Carlsbad, CA) in an ABI 7300 Real-Time PCR System. GAPDH was used as an endogenous control for the normalization of gene expression.
Animal studies
Injury model
All animal studies were performed in strict accordance with the Wake Forest University Institutional Animal Care and Use Committee and NIH standards. Young (2–3 months), adult (11–12 months), and aged (18 months) male Lewis rats were acquired from Harlan Laboratories (Indianapolis, IN). Injury was induced in rats as previously described.33,34 In brief, prenatal blood pressure cuffs were secured on the hindlimb proximal to the anterior muscle compartment of anesthetized rats and held at a pressure of 120–140 mmHg for 3 h. Animals were euthanized 14 days after injury for histological analyses. At least three animals were used for each experiment.
Cell injections
Postlabeling, MPCs were resuspended in phosphate-buffered saline (PBS) and a total of 1 × 106 MPCs, in a total 100 μL volume, were injected into the proximal, midbelly, and distal regions of the injured tibialis anterior (TA) muscle of rats under general anesthesia 4 days postinjury. Previous data have shown that this number of cells is sufficient for retention and detection in the muscle 14 days after injury.35 Control animals were injected with 100 μL PBS alone.
In vivo muscle function
Contractile function (i.e., torque–frequency relationship) of the left anterior crural muscles was measured in vivo as previously described.33,34 In brief, the left hindlimb of anesthetized rats was clamped, and the left foot was secured to a custom-made footplate that is attached to the shaft of an Aurora Scientific 305C-LR-FP servomotor, which in turn was controlled using a PC. Electrical stimulus was applied to the left common peroneal nerve. Contractile function of the anterior crural muscles was assessed by measuring maximal isometric torque as a function of stimulation frequency (1–200 Hz). Data were recorded using a PC loaded with a custom-made LabVIEW®-based program (provided by the U.S. Army Institute of Surgical Research).
Histology and microscopy
Injured and the contralateral uninjured TA muscles were harvested from animals 14 days after injury, weighed and then either frozen in OCT for cryosectioning or fixed in 10% neutral buffered formalin, and processed for paraffin embedding. Serial sections (8 μm) were analyzed for tissue morphology using standard H&E staining. All microscopic images were acquired with a Leica DM400B upright fluorescent microscope (Leica Microsystems, Wetzlar, Germany) and a Retiga-2000RV camera (Qimaging, Surrey, BC, Canada).
Tissue analyses
Immunohistochemistry against GFP was used to identify transplanted cells (GFP antibody [B2], Santa Cruz Biotechnology, Santa Cruz, CA). Laminin (L9393; Millipore-Sigma, St. Louis, MO) and DAPI were used to label cell membranes and nuclei, respectively, for fluorescent imaging. Adobe Photoshop (Adobe, San Jose, CA) was used to measure myofiber cross-sectional area (CSA) from H&E and/or GFP stained sections. The numbers of GFP+ regenerating fibers, GFP+ degenerating fibers, and nonintegrated GFP+ MPCs were counted from GFP-stained sections and identified as previously described.29 In brief, degenerating fibers were counted as myofibers containing three or more centrally located nuclei, angular in shape, and/or anucleated. Regenerating fibers were identified as having two or less centrally located nuclei. ImageJ was used to quantitate fibrosis using Masson's trichrome-stained sections. At least 10 slides from at least 3 animals were used for each analysis.
Statistics
All functional data were analyzed using GraphPad Prism Software (GraphPad Software, San Diego, CA) or Microsoft Excel (Redmond, WA). Each functional and morphological measure was compared among groups using two-way ANOVA. In the event of a significant ANOVA, post hoc means comparison testing was performed with Fisher's LSD correction or with Tukey's HSD tests. Statistical significance was achieved at p < 0.05. Data are presented as mean ± SEM.
Results
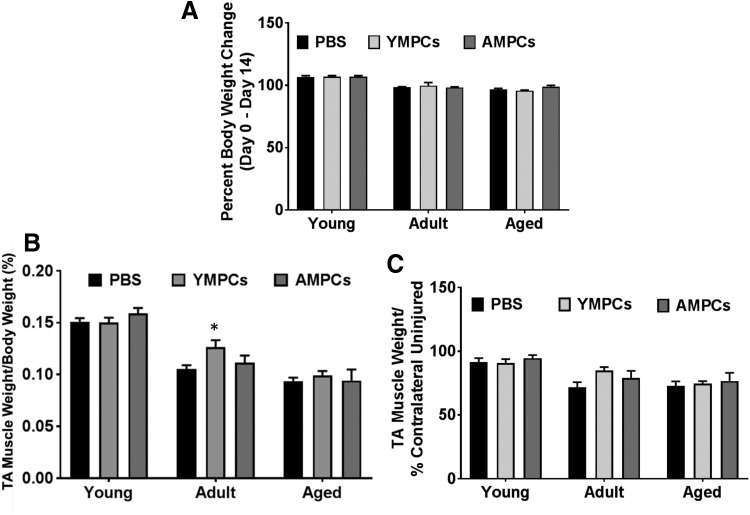
The time line and experimental design are outlined in Figure 1. The MPCs used in this study were derived from whole limb muscles of different-aged rats and therefore are a heterogeneous population of cells. More than 70% of the harvested cells express the myogenic marker Pax7+ (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). The growth curve and cell markers (myogenic and fibrogenic) of YMPCs and AMPCs were assessed to identify potential differences in growth and cellular composition that could affect survival after transplantation. No differences were detected in the growth curves of YMPCs and AMPCs, determined using an IncuCyte real-time live-cell imager, up to 8 days after plating (Fig. 2A). Moreover, no differences were detected in Pax7 (myogenic), CTGF (fibrogenic), Tcf4 (FAPs), and PDGFRα (FAPs) transcripts, suggesting that both populations of cells are similar (Fig. 2A–D).
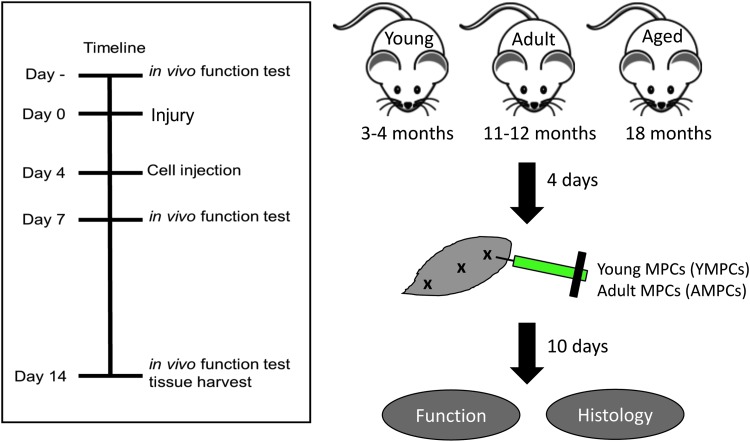
FIG. 1.
Timeline and experimental design. Baseline isometric torque was determined before injury. MPCs were transplanted 4 days after injury and muscle function was measured 14 days after injury before tissue harvest. MPC, muscle progenitor cell.
FIG. 2.
Characterization of MPCs. (A) The growth curve of YMPCs and AMPCs measured as percent plate coverage using IncuCyte real-time live-cell imaging. Quantitative PCR analysis of Pax7 (B), CTGF (C), Tcf4 (D), and PDGFRα (E). RNA was harvested from cells isolated from two different rats per group (young and adult). AMPC, MPCs derived from adults; YMPCs, MPCs derived from young.
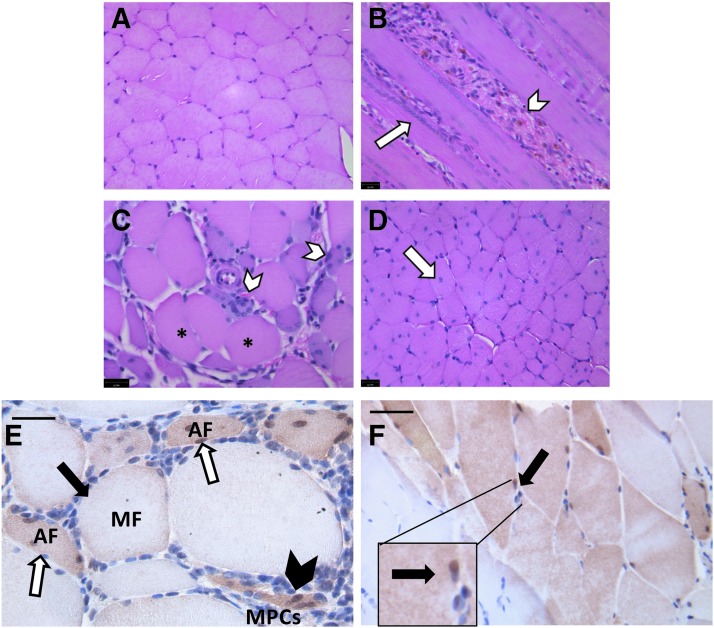
MPCs were infected with a lentivirus expressing GFP before injection into the injured TA muscles of young, adult, and aged rats 4 days after injury. Control animals were injured and treated with saline. Flow cytometry was used to determine the labeling efficiency for each experiment, which was found to be consistent for both the YMPCs and AMPCs (Supplementary Fig. S2). Rats were weighed at the beginning and end of the experiment and the percent weight change over that time did not differ between the treatment groups (PBS, YMPCs, AMPCs; Fig. 3A). TA muscles were excised 14 days after injury (10 days postinjection), weighed, and examined for GFP expression. When corrected for body weight, the muscles from the young animals were heavier than those of adult or aged rats, regardless of treatment (Fig. 3B). When corrected for the contralateral uninjured muscle, treatment had no effect on muscle weight (Fig. 3C). Actual body and muscle weights are listed in Supplementary Tables S1 and S2.
FIG. 3.
TA wet weight and rat body weight analysis. (A) Rat body weight was measured as percent change from day 0 to 14 after injury. (B) TA wet muscle weight corrected for rat body weight. Adult muscles injected with YMPCs were significantly heavier than adult muscles injected with PBS or AMPCs. *p < 0.02 (C) TA wet muscle weight corrected to uninjured contralateral muscle. PBS, phosphate-buffered saline.
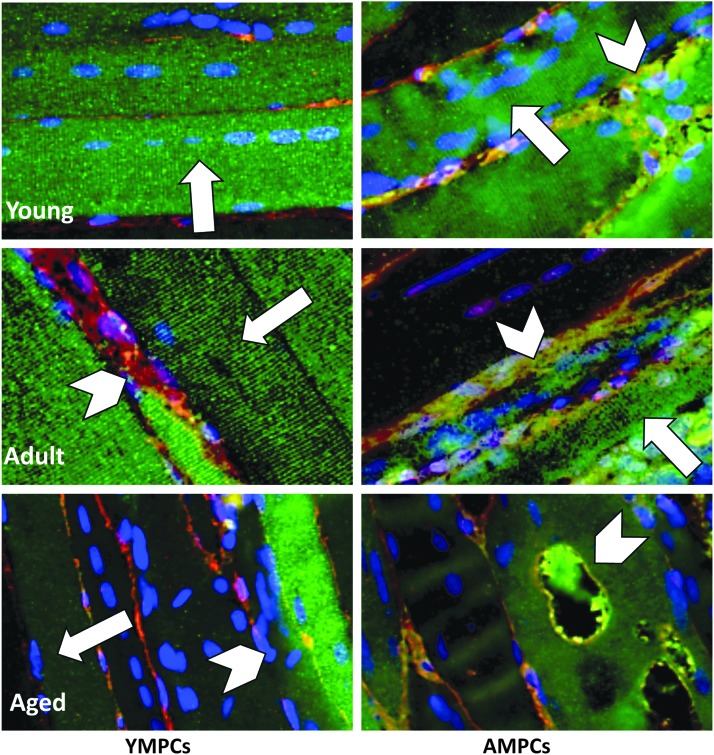
A heterogeneously mixed morphology of GFP+ and host myofibers were detected in the TA muscles of all experimental groups (Fig. 4), with evidence of large mature myofibers (peripherally located nuclei, black arrows), smaller regenerating myofibers (centrally located nuclei, white arrows), and single MPCs (black arrowheads), regardless of host age (Fig. 4A–F). The presence of myofibers containing both GFP+ and GFP− nuclei demonstrated the ability of transplanted MPCs to integrate with the host tissue to form chimeric fibers (Fig. 4F, arrows and inset). However, qualitative differences in the GFP+ myofibers from the different experimental groups were noted, despite the ability of YMPCs and AMPCs to form myofibers after transplantation (Fig. 5). Longitudinal sectioning and fluorescent staining showed many striated GFP+ myofibers, with centrally localized nuclei, were found in the muscles injected with YMPCs, regardless of the age of the host (Fig. 5, left panels, arrows). However, muscle from adult and aged rats injected with YMPCs also contained many necrotic GFP+ myofibers (Fig. 5, left panels, arrowheads). Transplanted AMPCs were also able to form striated myofibers in all animals (Fig. 5, right panels, arrows), however, an increase in necrotic and/or vacuolated myofibers was present in all muscles receiving AMPCs (Fig. 5, right panels, arrowheads).
FIG. 4.
GFP+ myofiber morphology. H&E stain of a cross-sectional cut of uninjured (A) and injured (B–D) skeletal muscle. Regenerating myofibers (white arrow) and degenerating myofibers (white arrowhead) are present. Immunohistochemistry using an antibody against GFP and DAB counterstain showed mature GFP+ myofibers (MF, black arrow), angulated myofibers (AF, white arrow), and single MPCs (arrowheads) were detected alongside mature host fibers (E, F) in all experimental groups. Image is of young mouse muscle injected with YMPCs, but is representative of all groups. (F) Chimeric myofibers derived from host tissue and transplanted cells were identified by the presence of GFP+ and GFP− nuclei (inset, arrow).
FIG. 5.
Immunofluorescent images of GFP+ myofibers. GFP-immunofluorescence of longitudinally cut muscle tissue was used to indicate the morphology of GFP+ myofibers derived from YMPCs (left column) and AMPCs (right column) in the injured TA muscle of young, adult, and aged rats. Myofibers containing centrally localized nuclei (arrows) and necrotic myofibers (arrowheads) were noted. Green (GFP+ myofibers), red (laminin), blue (DAPI). 400 × magnification. TA, tibialis anterior.
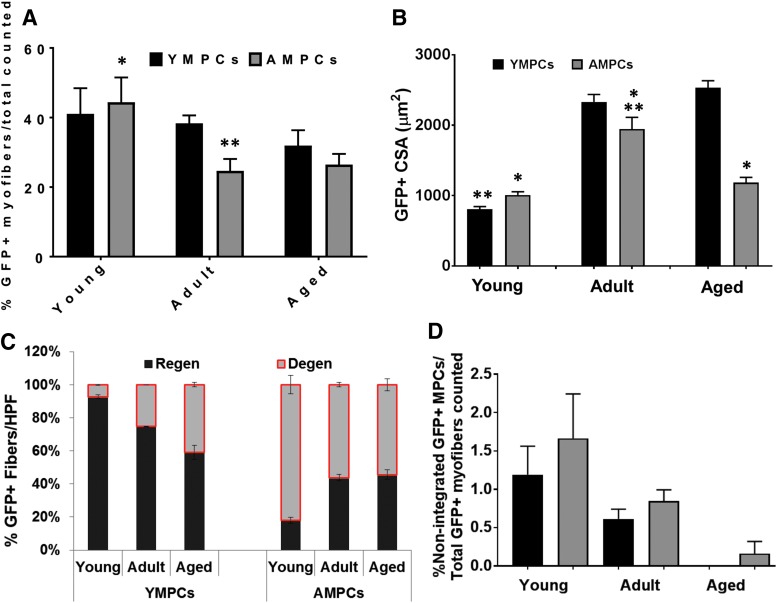
Quantitative analyses of GFP+ myofibers confirmed these observations (Fig. 6). The number of GFP+ myofibers derived from YMPCs was the same regardless of age of host (Fig. 6A). However, there was a significant increase in GFP+ myofibers derived from AMPCs in young rats compared with adult and aged rats (Fig. 6A). The CSA of GFP+ myofibers derived from YMPCs was significantly smaller in young rats compared with adult and aged rats, even when differences in animal weight were considered (Fig. 6B). However, the CSA of GFP+ myofibers derived from AMPCs remained the same regardless of the age of the host and was significantly different from the CSA of YMPC derived myofibers in aged rats (Fig. 6B). Moreover, a greater number of GFP+ myofibers derived from AMPCs were found to be degenerating, regardless of host age, at 14 days postinjury (Fig. 6C). It is of interest to note the decrease or lack of nonintegrated YMPCs in the adult and aged rats, respectively (Fig. 6D), which may be a reflection of the inability of these cells to survive in the older tissue microenvironment.
FIG. 6.
Characterization of the morphology of GFP+ myofibers. (A) The number of myofibers was determined by counting GFP+ and nonlabeled myofibers from all tissue sections that contained GFP+ staining and graphed as the percent of GFP+ myofibers per non-GFP labeled myofibers. Young rats injected with YMPCs had a significant increase in GFP+ myofibers over adult and aged rats injected with YMPCs (*p < 0.002). There was a trend in decreased myofibers derived from AMPCs compared with myofibers derived from YMPCs in adult and aged rats (**p < 0.06). (B) CSA of GFP+ myofibers was determined as the averaged GFP-CSA controlled to rat weight and then the CSA of host myofibers. Young rats with YMPCs had a significantly smaller CSA compared with adult and aged rats (**p < 0.002). AMPCs showed significantly larger CSA compared with adult rats and compared AMPCs in young and aged rats (**p < 0.01) and YMPCs in adult rats (*p < 0.01). Aged rats injected with AMPCs showed significantly smaller CSA compared with aged rats with YMPCs (*p < 0.001). (C) The numbers of healthy, regenerating, degenerating myofibers, and (D) nonintegrated GFP+ MPCs were counted from at least 10 high powered fields (HPFs) as described in Materials and Methods section. For each experimental condition, data were acquired from three or more rats. Data are expressed as means ± SEM. CSA, cross-sectional area.
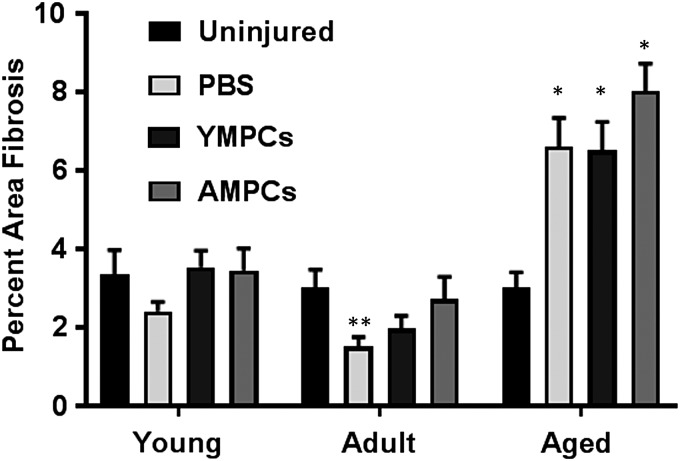
We have previously shown that the muscles of aged rats contain a significant amount of fibrosis after injury compared with similarly treated muscles from young and adult rats.33 To determine the effect of MPC transplantation on tissue fibrosis, we quantitated the fibrotic areas represented on Masson's trichrome-stained slides. No change in fibrosis was detected between the muscles compared with uninjured muscles from young and adult animals in any of the cell-injected groups, but an unexpected significant decrease in fibrosis was noted in the PBS-treated muscles (Fig. 7). Muscles from aged animals showed a significant increase in fibrosis compared with uninjured muscle regardless of the treatment group (Fig. 7). These data are consistent with our previous results.33
FIG. 7.
Quantification of fibrosis in cell-injected injured muscle. Masson's trichrome stained slides were used to calculate area fibrosis. Total area fibrosis was determined 14 days after injury as described in Materials and Methods section. Data were normalized to fibrosis of the contralateral uninjured muscles. For each experimental condition, data were acquired from three or more rats. Data are expressed as means ± SEM. *p < 0.001 versus all other treatment groups and ages, **p < 0.06 versus uninjured and AMPCs.
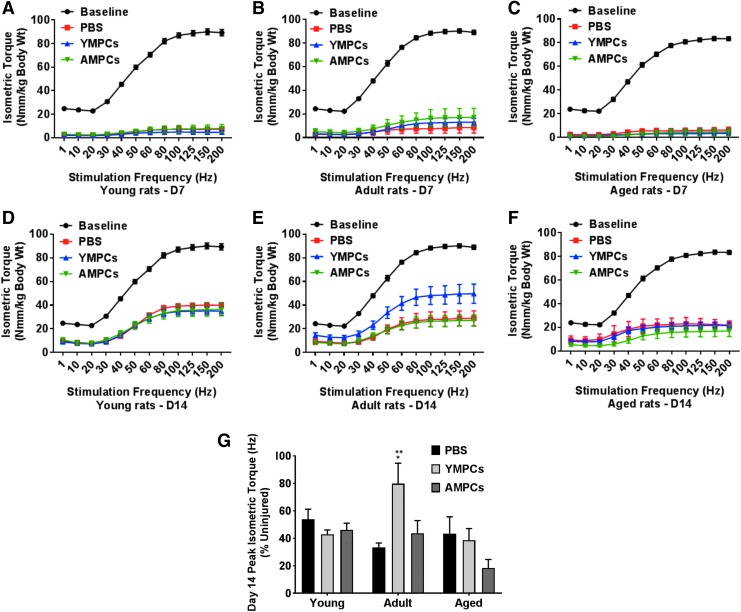
Finally, assessment of the in vivo torque production of the anterior muscle compartment was used as a measure of recovery of muscle function after injury (Fig. 8). Isometric torque curves for 7 and 14 days after injury are shown in Figure 8A. At day 14 postinjury, adult rats injected with YMPCs showed a significant improvement of peak isometric torque and greater to that detected in the other age groups (Fig. 8B). It is of interest to note that transplantation of AMPCs in the aged animals seem to result in decreased peak isometric torque at 14 days postinjury, although this is not statistically significant.
FIG. 8.
Measurement of peak isometric torque in young, adult, and aged rats after MPC transplantation. Peak isometric torque was measured as described in Materials and Methods section before and 7 and 14 days after injury. (A–C) Isometric torque force curves 7 days after injury and (D–F) isometric torque force curves 14 days after injury. (G) Force measurements were normalized to peak isometric torque of uninjured muscle. For each experimental condition, the number of rats used was three or more. Data are expressed as means ± SEM. Adult injected with YMPCs versus young and aged injected with YMPCs, *p < 0.002. Adult injected with YMPCs versus PBS and AMPCs, **p < 0.003.
Discussion
Sarcopenia is the age-dependent loss of muscle mass and function, and is a common cause of mobility disabilities in the elderly population. There is a plethora of current research examining the causes of sarcopenia using rodent models of aging and injury, but many of these models do not accurately mimic the human pathology of muscle dysfunction in a clinically relevant manner. This is especially important for muscle injury models to assess stem and progenitor cell therapy as well as the role of the microenvironment. We have recently demonstrated a decreased ability of older animals to recover muscle mass and function using a rat model of compression injury.33 The data presented herein further utilized this model of injury to examine the ability of different aged MPCs to integrate into the skeletal muscle of young, adult, and aged rats and to aid in the recovery of muscle function after injury.
The role of the muscle microenvironment has been shown to play a major role in regulating efficient tissue regeneration. For example, grafting experiments showed better regeneration when tissues from older animals were transplanted into younger animals,36 and heterochronic parabiosis experiments indicated the importance of “young” circulating factors for tissue regeneration.3,35–37 In addition, successful integration of transplanted donor satellite cells (young and aged) into their natural niche was shown to be dependent on the preservation of the native microenvironmental niche, as well as the properties of the host satellite cells.38 Our data further support these studies indicating that the host microenvironment's age has a profound effect on the success of cellular therapies via support of cell survival and tissue integration. Transplanted MPCs derived from both young (YMPCs) and adult (AMPCs) donors were able to integrate into damaged muscle and form mature striated myofibers, regardless of host age (Figs. 5 and 6). The number of GFP+ myofibers formed from YMPCs did not change regardless of host age. However, transplanted AMPCs formed significantly fewer mature myofibers, in comparison to YMPC-injected muscles and young rats, in both adult and aged rats suggesting that the microenvironment of these animals, unlike the microenvironment of the young rats, was not able to sustain MPC survival, differentiation, integration, and maturation of myofibers. However, the significant increase in the CSA of YMPC-derived myofibers suggests either faster growth of the surviving myofibers or myofiber hypertrophy.
Among the many factors implicated in the development of sarcopenia are decreased capillary to fiber ratios, decreased numbers of NMJs per fiber, muscle fiber-type switching, increased chronic inflammation, increased intramuscular collagen deposition, and myosteatosis.31,35,37,39,40 All of these factors are “external” influences that effect the function of muscle tissue, and their roles in the functional decline seen in the elderly have not been clearly defined. When we examined the regenerative status of the MPCs transplanted into the different-aged animals, we found that the majority of GFP+ myofibers derived from YMPCs were regenerating as indicated by centrally located nuclei with few degenerating fibers, regardless of host age. In contrast, GFP+ myofibers derived from AMPCs were mostly degenerating in all aged animals (Fig. 6D). These data suggest that the age of the transplanted cells is an additional factor for consideration in the regenerative ability of the tissue.
Age-dependent changes in satellite cell proliferation and differentiation have also been identified as contributing factors to sarcopenia. Previous reports revealed that a balance between Notch and Wnt signaling is required for the proper balance of satellite cell proliferation and differentiation, respectively,3,9,41 and that the reduction in Notch activation in aged muscle resulted in decreased proliferation of MPCs with subsequent decreased ability to repair damaged muscle.41 TGFβ is thought to regulate impaired satellite cell activation and muscle repair through the Wnt/Notch pathways and has been shown to be antimyogenic and more active in aging muscle.36 Although misregulation of these pathways has implicated a satellite cell-specific role in decreased tissue healing in the aged, these pathways do not address the other phenomenon seen in older tissue, such as denervation, fiber-type switching, chronic inflammation, and increased collagen and adipose deposition.
It is interesting to note the lack of nonintegrated GFP+ MPCs in aged muscle transplanted with YMPCs and the trend of decreased numbers of both cell types in the adult and aged muscle (Fig. 6D). These data suggest that the YMPCs may be more sensitive to the host microenvironmental factors rather than intrinsic cellular factors, whereas the AMPCs are more limited by intracellular factors than the microenvironment of the host. In other words, the microenvironment of the young and adult, but not aged, animals is permissive of YMPC survival and integration. The YMPCs that survived the aged “microenvironment” were those that were able to fully integrate into the host tissue, resulting in many fully differentiated GFP+ myofibers and no nonintegrated MPCs. In contrast, AMPCs had a decreased ability to form stable mature myofibers in any aged host. This may be due to the increased and persistent fibrosis seen in the injured muscles from aged animals (Fig. 7), which is consistent with our previously published data.33
Functional improvement is the goal of any regenerative medicine therapy. To date, satellite cell (MPC) therapy for muscle dysfunction has shown little clinical success.42–46 We have previously shown that adult and aged rats show a delayed and compromised regenerative response after injury, respectively.33 Young rats have a remarkable ability to regenerate after injury and therefore are a poor model for testing therapeutic regimens. This is demonstrated in Figure 8, where the young rats, regardless of treatment, have recovered 40% peak isometric torque 14 days after injury. Adult rats transplanted with YMPCs showed a significant improvement in peak isometric torque compared with young and aged animals and compared with PBS or AMPC treatment (Fig. 8B). Aged rats showed a trend in decreased peak torque in muscle transplanted with AMPCs, which may be due to increased inflammation or persistent collagen deposition.
Conclusion
Our data demonstrated that the loss of regenerative capacity in skeletal muscle due to aging is dependent on both microenvironmental and intrinsic cellular changes. For effective cell therapy, both these factors must be taken into consideration, and they may be the overriding reasons for the current clinical failures.
Supplementary Material
Acknowledgment
Supported by the Department of Defense (USAMRAA AFIRM W81XWH-08-2-0032), and the National Institute of General Medical Sciences of the National Institutes of Health (T32GM099606).
Disclosure Statement
No competing financial interests exist.
References
- 1.Sanchez A., Schimmang T., and Garcia-Sancho J. Cell and tissue therapy in regenerative medicine. Adv Exp Med Biol 741, 89, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Tedesco F.S., and Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol 25, 597, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., and Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Poggioli T., Vujic A., Yang P., et al. Circulating growth differentiation factor 11/8 levels decline with age. Circ Res 118, 29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha M., Jang Y.C., Oh J., et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egerman M.A., Cadena S.M., Gilbert J.A., et al. GDF11 Increases with age and inhibits skeletal muscle regeneration. Cell Metab 22, 164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodgers B.D., and Eldridge J.A. Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology 156, 3885, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y., Sharma N., Dukes D., et al. GDF11 treatment attenuates the recovery of skeletal muscle function after injury in older rats. AAPS J 19, 431, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Brack A.S., Conboy M.J., Roy S., et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Brack A.S., and Rando T.A. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3, 226, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Blau H.M., Cosgrove B.D., and Ho A.T. The central role of muscle stem cells in regenerative failure with aging. Nat Med 21, 854, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brack A.S., and Munoz-Canoves P. The ins and outs of muscle stem cell aging. Skelet Muscle 6, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont N.A., Bentzinger C.F., Sincennes M.C., and Rudnicki M.A. Satellite cells and skeletal muscle regeneration. Compr Physiol 5, 1027, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Eliazer S., and Brack A.S. Stem cells: cause and consequence in aged-muscle decline. Nature 540, 349, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Heredia J.E., Mukundan L., Chen F.M., et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras O., and Brandan E. Fibro/adipogenic progenitors safeguard themselves: a novel mechanism to reduce fibrosis is discovered. J Cell Commun Signal 11, 77, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras O., Rebolledo D.L., Oyarzun J.E., Olguin H.C., and Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res 364, 647, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Lemos D.R., Babaeijandaghi F., Low M., et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21, 786, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Natarajan A., Lemos D.R., and Rossi F.M. Fibro/adipogenic progenitors: a double-edged sword in skeletal muscle regeneration. Cell Cycle 9, 2045, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Fiore D., Judson R.N., Low M., et al. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res 17, 161, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Joe A.W., Yi L., Natarajan A., et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stearns-Reider K.M., D'Amore A., Beezhold K., et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16, 518, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong J.O., Kim M.O., Kim H., et al. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation 119, 699, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyle A.N., Joseph G., Fan A.E., Weiss D., Landazuri N., and Taylor W.R. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler Thromb Vasc Biol 32, 1383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza J., and Gottfried C. Muscle injury: review of experimental models. J Electromyogr Kinesiol 23, 1253, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Kaariainen M., and Kauhanen S. Skeletal muscle injury and repair: the effect of disuse and denervation on muscle and clinical relevance in pedicled and free muscle flaps. J Reconstr Microsurg 28, 581, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Walters T.J., Kragh J.F., Kauvar D.S., and Baer D.G. The combined influence of hemorrhage and tourniquet application on the recovery of muscle function in rats. J Orthop Trauma 22, 47, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Sabido F., Milazzo V.J., Hobson R.W., 2nd, and Duran W.N. Skeletal muscle ischemia-reperfusion injury: a review of endothelial cell-leukocyte interactions. J Invest Surg 7, 39, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Criswell T.L., Corona B.T., Wang Z., et al. The role of endothelial cells in myofiber differentiation and the vascularization and innervation of bioengineered muscle tissue in vivo. Biomaterials 34, 140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conboy I.M., Yousef H., and Conboy M.J. Embryonic anti-aging niche. Aging (Albany NY) 3, 555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopinath S.D., and Rando T.A. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7, 590, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Silva H., and Conboy I.M. Aging and stem cell renewal. In: StemBook; Cambridge, MA, 2008 [PubMed] [Google Scholar]
- 33.Zhou Y., Lovell D., Bethea M., et al. Age-dependent changes cooperatively impact skeletal muscle regeneration after compartment syndrome injury. Am J Pathol 184, 2225, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Criswell T.L., Corona B.T., Ward C.L., et al. Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol 180, 787, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Villeda S.A., Luo J., Mosher K.I., et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson B.M., and Faulkner J.A. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol 256(6 Pt 1), C1262, 1989 [DOI] [PubMed] [Google Scholar]
- 37.Chakkalakal J.V., Jones K.M., Basson M.A., and Brack A.S. The aged niche disrupts muscle stem cell quiescence. Nature 490, 355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boldrin L., Neal A., Zammit P.S., Muntoni F., and Morgan J.E. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells 30, 1971, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delbono O. Neural control of aging skeletal muscle. Aging Cell 2, 21, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Taylor-Jones J.M., McGehee R.E., Rando T.A., Lecka-Czernik B., Lipschitz D.A., and Peterson C.A. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev 123, 649, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Conboy I.M., Conboy M.J., Smythe G.M., and Rando T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Tedesco F.S., and Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol 25, 597, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Gussoni E., Blau H.M., and Kunkel L.M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med 3, 970, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Tremblay J.P., Bouchard J.P., Malouin F., et al. Myoblast transplantation between monozygotic twin girl carriers of Duchenne muscular dystrophy. Neuromuscul Disord 3, 583, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Huard J., Bouchard J.P., Roy R., et al. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve 15, 550, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Huard J., Bouchard J.P., Roy R., et al. Myoblast transplantation produced dystrophin-positive muscle fibres in a 16-year-old patient with Duchenne muscular dystrophy. Clin Sci (Lond) 81, 287, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.