Abstract
MutS and MutL homologs have been implicated in multiple genetic stabilization pathways. The activities participate in the correction of DNA biosynthetic errors, are involved in cellular responses to certain types of DNA damage, and serve to ensure the fidelity of genetic recombination. We show here that the rate of CAD (carbamyl-P synthetase/aspartate transcarbamylase/dihydroorotase) gene amplification is elevated 50- to 100-fold in human cell lines deficient in MLH1 or MSH6, as compared with mismatch repair-proficient control cells. Fluorescence in situ hybridization indicates that these amplification events are the probable consequence of unequal sister chromatid exchanges involving chromosome 2, as well as translocation events involving other chromosomes. These results implicate MutSα and MutLα in the suppression of gene amplification and suggest that defects in this genetic stabilization function may contribute to the cancer predisposition associated with mismatch repair deficiency.
Multiple genetic stabilization functions have been attributed to the MutS and MutL families of DNA mismatch repair proteins. In both prokaryotes and eukaryotes, MutS and MutL homologs play key roles in the correction of DNA biosynthetic errors and the transcription-coupled repair of DNA damage (1–4). In mammalian cells, MutSα (MSH2⋅MSH6 heterodimer), MutSβ (MSH2⋅MSH3 heterodimer), and MutLα (MLH1⋅PMS2 heterodimer) are also thought to function as lesion sensors for certain types of DNA damage that kill by triggering apoptosis (1, 4, 5). Inactivation of the mismatch repair system results in a large increase in the spontaneous occurrence of base substitution and frame-shift mutations, an effect that can be understood in terms of these genetic stabilization functions.
In addition to their roles in replication fidelity and DNA damage responses, MutS and MutL homologs act to ensure the fidelity of genetic recombination. In the bacterial system, where this function has been most thoroughly examined, MutS and MutL act as a barrier to recombination between diverged DNA sequences (homeologous recombination). This barrier not only serves to prevent intrachromosomal rearrangements, but also contributes to the stability of a bacterial species by blocking the production of recombinant chromosomes that would otherwise result from the interspecies exchange of related sequences (6). Although the mechanisms underlying these antirecombination activities of bacterial mismatch repair proteins are not fully understood, available evidence indicates that these effects are a consequence of MutS- and MutL-dependent editing of early strand-transfer intermediates when mismatched base pairs occur within the recombination heteroduplex (1, 4). Such structures presumably undergo subsequent disassembly, possibly by a DNA helicase II-dependent pathway (7).
Yeast MutS and MutL homologs also act to suppress homeologous recombination events in both mitotic and meiotic cells. As in the bacterial system, genetic studies suggest that Saccharomyces cerevisiae MutS and MutL homologs function in this manner by blocking expansion of a heteroduplex that contains mismatched base pairs (4). Furthermore, the efficiency of gene replacement (8, 9) and analysis of double-strand break repair (10) in murine embryonic stem cells suggest that the mammalian mismatch repair proteins MSH2 and MSH6 have a similar function. We show here that inactivation of the human MSH6 or MLH1 genes is associated with a large increase in the frequency of gene amplification. Because changes in DNA copy number and other chromosome rearrangements have been implicated in tumor development (11), this effect may contribute to the cancer predisposition associated with mismatch repair deficiency.
Experimental Procedures
Cell Lines and N-Phosphonacetyl-l-aspartate (PALA) Selection.
TK6 and MT1 cells (12, 13) were grown in RPMI medium 1640 supplemented with 10% (vol/vol) FBS (HyClone). HCT116 and HCT116.ch3 (14) were maintained in McCoy's 5A medium (HyClone) supplemented with 2 mM l-glutamine and 10% (vol/vol) FBS. For growth of HCT116.ch3 cells, the latter medium was supplemented with 400 μg/ml of geneticin disulfate.
PALA was a gift from the Drug Synthesis and Chemistry Branch (Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute). PALA sensitivity, defined as the concentration of PALA that inhibits cell growth by 50% (ID50), was determined as described by Perry et al. (15). Selection for resistance was performed at a PALA concentration of 3 times the ID50 concentration by using medium deficient in deoxyribonucleosides and ribonucleosides that was supplemented with 10% (vol/vol) dialyzed FBS (HyClone). In the case of attached HCT116 and HCT116.ch3 cell lines, each selection used ≈107 cells, plating 5 × 105 or 1 × 106 cells per 10-cm plate. Medium was changed every 5 days, and PALA-resistant colonies appeared after 2–3 weeks. Frequencies cited are corrected for plating efficiency. For TK6 and MT1 cells, which grow in suspension, 1 × 104 cells were seeded into each well of a set of 10 96-well dishes. PALA-resistant cells were observed after 3–4 weeks by nitro blue tetrazolium stain. Colonies containing 100–200 live cells were scored as PALA-resistant clones.
Fluctuation Analysis.
Wells of 24-well plates were inoculated with ≈103 cells. After growth without selection to 106 cells per well, cells were dispersed by trypsinization, cells from each well were plated onto 10-cm dishes, and treated immediately with 100 μM PALA. Medium was changed every 4–5 days until resistant colonies appeared (2–3 weeks). Plates were stained with methylene blue, and colonies containing 100–200 cells were scored as PALA-resistant. A single resistant clone was picked from each plate for further study. Controls for plating variation were derived from a single mass culture grown in a T175 flask. After dispersal by trypsinization as described previously, samples of 5 × 105 or 106 cells as indicated were distributed into 10-cm dishes and immediately treated with PALA as described previously.
Quantitative Southern Blot Analysis.
Genomic DNA was isolated from cultured cells by SDS lysis, proteinase K digestion, and phenol extraction (16). After digestion with PstI endonuclease, genomic DNA samples were ethanol-precipitated, dissolved in 0.01 M Tris⋅HCl (pH 7.6)/1 mM EDTA, and concentrations were determined by absorbance at 260 nm. Samples (10 μg) were subjected to electrophoresis through 0.8% agarose, and DNA was transferred to a nylon membrane (Amersham Pharmacia Biotech), which was hybridized with a mixture of 32P-labeled probes corresponding to CAD (carbamyl-P synthetase/aspartate transcarbamylase/dihydro-orotase) and p53 gene sequences. A 1.5-kb probe corresponding to nucleotides 2646–4146 of the CAD genomic sequence (17) was prepared by PCR in the presence of [α-32P]dCTP (3,000 Ci/mmol; NEN), using cosmid HU-CAD-69 (18) (generously provided by George Stark, Cleveland Clinic Foundation) as template. A 32P-labeled 1-kb probe corresponding to p53 cDNA nucleotides 569-1572 (19) was prepared in a similar manner from the cDNA clone pT7–7Hup53, which was kindly provided by David Lane (University of Dundee, Scotland). DNA hybridization was performed in a commercial buffer, using conditions recommended by the manufacture (Ambion, Austin, TX). Hybridized Southern blots were phosphorimaged by using a STORM imager system, and data was quantitated with imagequant software (Molecular Dynamics). Relative CAD gene dosage was estimated based on the ratio of 32P in the CAD band relative to that in the p53 band. The relative gene dosage for PALA-sensitive HCT116 and MT1 cells was assigned a value of 1.0.
Fluorescence in Situ Hybridization (FISH).
FISH experiments were performed as described (20, 21). Briefly, chromosomes of mitotic metaphase spreads were denatured in 70% formamide, 2× SSC (0.3 M NaCl/0.03 M sodium citrate, pH 7.0) at 75°C for 3 min, and then dehydrated in absolute ethanol. A fluorescent probe for human chromosome 2 alpha satellite DNA was purchased from Vysis (Downers Grove, IL). A fluorescent CAD DNA probe was prepared by nick translation of cosmid HU-CAD-69 (18) in the presence of fluorophore-labeled dUTP (Vysis). CAD and human chromosome 2 probes were denatured at 75°C for 5 min in hybridization buffer (Vysis) and hybridized at 37°C for 24 h to metaphase spreads according to recommendations of the manufacturer. Slides were then washed at 75°C with 0.1% Nonidet P-40/2× SSC for 5 min, with 0.3% Nonidet P-40/0.1× SSC at room temperature for 1 min, and air-dried at room temperature. Chromosome DNA was counterstained with DAPI (4′,6-diamidino-2-phenylindole, Vysis), and chromosomes were visualized by using a Zeiss Axioskop fluorescence microscope.
The PALA-resistant derivatives of HCT116 and MT1 cells that were examined by FISH were attributed to independent genetic events. In the case of HCT116, resistant derivatives were assumed to be independent if they occurred during different frequency-based selections (Table 1) or if they arose in different cultures during fluctuation analysis. For MT1 cells, resistant derivatives were assumed to be independent if they arose in different wells of 96-well plates used for their selection (see above).
Table 1.
MLH1 and MSH6 defects increase the frequency of PALA-resistant colonies
| Cell line | MMR status | PALA ID50, μM | PALAR colonies, frequency |
|---|---|---|---|
| HCT116 | MLH1− | 37 | 1.1 ± 0.1 × 10−5 |
| HCT116.ch3 | MLH+ | 34 | ≈1 × 10−7 |
| MT1 | MSH6− | 61 | 1.3 × 10−5 |
| TK6 | MSH6+ | 61 | <2 × 10−7 |
PALA resistance was scored as described in Experimental Procedures. Values shown for HCT116 and HCT116.ch3 are averages of three independent experiments. With HCT116.ch3, two experiments using 107 cells yielded no resistant clones, whereas a third experiment yielded three resistant colonies. The reason for this difference is not clear, although it could be due to occasional loss of the MLH1+ chromosome 3 in the third experiment.
p53 cDNA Sequences and Protein Levels.
Total RNA was extracted from cells with Trizol reagent (GIBCO/BRL) as specified by the manufacturer, and cDNA was synthesized by using Superscript First-Strand Synthesis kit (GIBCO/BRL). A 1510-bp DNA fragment spanning the p53 coding region was amplified from the cDNA pool by using d(GGACACTTTGCGTTCGGGCTG) and d(AGTAAAAACCTTAAAATCTAAGCTGGTATG) as primers. Sequences of PCR products were determined by the Duke University DNA Analysis Facility. Cellular levels of p53 were determined by Western blot as described (22).
Results
The Frequency of PALA Resistance Is Highly Elevated in MLH1−/− and MSH6−/− Human Cell Lines.
Because selection for PALA resistance has proven useful for the study of gene amplification in mammalian cells (23, 24), we have exploited this system to assess the effects of mismatch repair deficiency on chromosome rearrangements. Two sets of human cell lines were used in these studies. Mismatch repair-deficient MT1 cells were derived from repair-proficient TK6 lymphoblastoid cells by single-step selection for high-level DNA methylator resistance (12). Methylator resistance and mismatch repair deficiency of MT1 cells are due to a hMutSα defect, a consequence of missense mutations in both alleles of MSH6 (13, 25). The HCT116 colorectal tumor cell line is defective in mismatch repair as a result of inactivation of both alleles of MLH1 and hMutLα deficiency (26). The control cell line in this case was HCT116.ch3, which was derived from HCT116 by introduction of a single copy of human chromosome 3 carrying a wild-type MLH1 gene (14). HCT116.ch3 cells are proficient in mismatch repair and are genetically stable.
As shown in Table 1, the sensitivity of these four human cell lines to the growth-inhibitory effects of PALA is independent of mismatch repair status, as judged by the concentration necessary to inhibit cell growth by 50% (ID50). However, the frequency of resistant colonies selected at a PALA concentration of 3 times the ID50 (15) was dramatically elevated in MSH6−/− and MLH1−/− cells as compared with the repair-proficient control cell lines TK6 and HCT116.ch3. The observed frequency of PALA-resistant colonies was 10−5 for both MLH1−/− HCT116 and MSH6−/− MT1cells, whereas HCT116.ch3 and TK6 cell lines yielded resistant clones with a frequency of 10−7 or less.
PALA-Resistant Colonies Arising in Mismatch Repair-Deficient Backgrounds Are Primarily Due to CAD Gene Amplification.
PALA blocks pyrimidine biosynthesis by inhibition of aspartate transcarbamylase, one activity of a trifunctional polypeptide encoded by the mammalian CAD gene (27). Although CAD gene amplification is a common mechanism of PALA resistance in rodent cell lines (28), CAD amplification is typically rare in cultured human cells (18, 29–31); however, exceptions to this generalization have been noted. Defective p53 function is sufficient to confer a permissive state for amplification of the human CAD locus, but other permissive pathways may also exist (32, 33). PALA resistance due to mechanisms other than CAD amplification have also been observed in human cells (18).
To determine whether CAD amplification contributes to development of PALA resistance in mismatch repair-deficient cells, the CAD gene copy number was estimated by quantitative Southern blot. Eight independent PALA-resistant derivatives of HCT116 and MT1 were examined in this manner by using a p53 sequence probe as internal standard (see Experimental Procedures). Eight of eight resistant isolates derived from MLH1−/− HCT116 cells displayed a 2- to 3-fold increase in CAD gene dosage as judged by this method, and seven of eight isolates derived from MSH6−/− MT1 cells behaved in a similar manner (Table 2). Because a 2-fold increase in CAD gene copy number is sufficient to confer a 5- to 10-fold increase in PALA resistance (18), these observations suggest that CAD gene amplification is the predominant mechanism responsible for development of PALA resistance in MLH1- and MSH6-deficient human cells.
Table 2.
PALA resistance is mainly due to amplification of the CAD gene
| PALAR clone | Relative gene dosage | PALAR clone | Relative gene dosage |
|---|---|---|---|
| HCT116 control | 1.0 | MT1 control | 1.0 |
| H1 | 2.5 | M1 | 1.1 |
| H2 | 2.0 | M2 | 3.1 |
| H3 | 1.8 | M3 | 2.8 |
| H4 | 1.8 | M4 | 2.1 |
| H5 | 2.9 | M5 | 2.3 |
| H6 | 2.5 | M6 | 2.2 |
| H7 | 2.1 | M7 | 2.0 |
| H8 | 1.8 | M8 | 1.7 |
| H1–H8 mean = 2.2 | M1–M8 mean = 2.2 | ||
PALA-sensitive HCT116 and MT1 and eight PALA-resistant derivatives of each cell line were analyzed for CAD gene dosage as described in Experimental Procedures. Gene dosages cited are relative to that of the p53 gene, which served as internal standard.
Analysis of PALA-Resistant Isolates by FISH.
To confirm the results of the Southern analyses and to clarify the nature of the CAD amplification events occurring in MLH1−/− and MSH6−/− backgrounds, PALA-sensitive HCT116 and MT1 cells and a set of independent PALA-resistant derivatives derived from each cell line were examined by FISH. These experiments demonstrated that both MT1 and HCT116 are nearly diploid (data not shown), confirming previous observations to this effect (34, 35). Both cell lines contained two copies of chromosome 2, with each containing a CAD gene on the short arm (Figs. 1 and 2). PALA-resistant derivatives of HCT116 and MT1 remained diploid with average chromosome numbers of 45.6 (SD = 0.75, n = 20) and 46.5 (SD = 0.53, n = 8), respectively.
Figure 1.
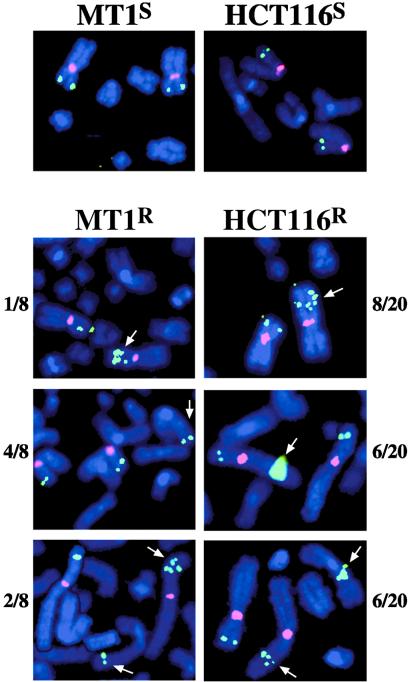
CAD gene amplification in PALA-resistant derivatives of MT1 and HCT116. FISH was performed as described in Experimental Procedures. The two micrographs (Left) illustrate results with PALA-sensitive MSH6−/− MT1 cells (Upper) and a PALA-resistant derivative (Lower). Similar results with MLH1−/− HCT116 cells are shown Right. The pink fluorophore corresponds to a probe for chromosome 2 alpha satellite DNA, while the green fluorophore is the CAD gene probe. Note that CAD amplification is also evident in interphase nuclei, which were present in the same field with the mitotic chromosome spreads in three of the cases shown.
Figure 2.
Three classes of CAD amplification events occur in mismatch repair-deficient cells. Eight independent PALA-resistant isolates derived from MT1 cells and 20 independently resistant derivatives of HCT116 were examined by FISH (see Experimental Procedures). Fluorophore labels are as in the legend to Fig. 1. The three classes of CAD amplification events (arrows) observed in MT1 and HCT116 PALA-resistant isolates are illustrated pictorially: gene amplification on the short arm of chromosome 2 (Top); translocation to another chromosome or to the other arm of chromosome 2 (Middle); or double events involving CAD amplification on the short arm of chromosome 2, as well as translocation to another chromosome (Bottom). The incidence of each type of event relative to the total number of resistant isolates examined by FISH is indicated to the side of each photograph. For this presentation, affected chromosomes in mitotic spreads were selected by using Adobe Systems (Mountain View, CA) photoshop and moved into proximity so all could be shown in a small field.
As expected from the results of quantitative Southern blots, 20 of 20 independent PALA-resistant HCT116 isolates and 7 of 8 independent MT1-resistant clones were found to contain additional copies of the CAD gene. These amplification events fell into three classes: amplification of CAD on the short arm of chromosome 2 producing a condensed array of gene copies similar to that described (23); amplification events leading to the presence of one or more CAD copies on other autosomes or on the long arm of chromosome 2; and double events resulting in nuclei containing both types of rearrangement (Fig. 2). Because nuclei with double events occur with a frequency similar to that of nuclei harboring a single type of rearrangement, it is clear that these two types of event are not independent. In fact, this observation suggests that the two types of event may involve a common intermediate that is capable of promoting several rearrangements. It is important to note that PALA-resistant derivatives of the mismatch repair-deficient cells we have examined do not contain additional copies of chromosome 2 or isochromosome 2p, as has been observed with several other human cell lines (18).
CAD Amplification Is Not Due to Inactivation of the p53 Gene.
Although CAD amplification is rare in normal human cells, p53 defects render cells permissive for gene amplification (32, 33). The p53 loci in the cell lines used here are wild type, and some p53-dependent signaling pathways are intact in these lines (22, 35–38). For example, the p53- and p21-dependent mitotic arrest that occurs on gamma irradiation is intact in HCT116 cells (38). Although HCT116 is defective in p14ARF, which is encoded by chromosome 9 (39), murine cells deficient in the p14ARF homolog respond normally to PALA (40).
However, it could be argued that the PALA-resistant HCT116 and MT1 derivatives described above arose from p53-deficient subpopulations present in cultures of these mutator cell lines. Although this possibility seems unlikely, based on the frequencies with which resistant colonies were observed in this study as compared with those observed in p53-deficient cells (18, 32, 33), p53 cDNA sequences were determined for three independent PALA-resistant clones isolated from MLH1−/− HCT116 cells. In each case, the sequence was wild type with no evidence for a heterozygotic defect, and each isolate contained a normal level of p53 polypeptide as judged by Western blot (data not shown). Indeed, the nature of the MLH1-proficient HCT116.ch3 cell line (previous and ref. 14), used as control for studies with MLH1-deficient HCT116 cells, and the similarity of results obtained with HCT116 and MSH6−/− MT1 cells render unlikely the possibility that a p53-dependent signaling defect is responsible for the phenotype described here.
The Rate of CAD Amplification in Mismatch Repair-Deficient Cells.
The rate of production of CAD rearrangements in MLH1−/− HCT116 cells was estimated by fluctuation analysis (Table 3), using the method of Luria and Delbrück (41). This approach yielded a value of 4 to 5 × 10−6 per cell per generation, about an order of magnitude less than that observed with the pseudodiploid p53-deficient HT-1080 human cell line (18), but similar to values obtained with nontumorigenic rodent cells that are permissive for CAD amplification (29, 42).
Table 3.
Fluctuation analysis with HCT116 cells
| Replicate cultures (N) | 20 | 40 | 1 | 1 |
| Samplings per culture | 1 | 1 | 10 | 20 |
| Cells per culture (initial) | 103 | 103 | ||
| Cells per culture (final) | 1 × 106 | 1 × 106 | 1 × 107 | 1 × 107 |
| PALAR colonies per sample (N) | Replicates with N PALAR colonies | |||
| 0 | 0 | 0 | 0 | 0 |
| 1–5 | 0 | 0 | 0 | 12 |
| 5–10 | 6 | 10 | 5 | 7 |
| 11–20 | 7 | 13 | 5 | 1 |
| 21–30 | 5 | 6 | 0 | 0 |
| 31–40 | 0 | 4 | 0 | 0 |
| 41–50 | 1 | 2 | 0 | 0 |
| 51–60 | 0 | 3 | 0 | 0 |
| 61–70 | 0 | 1 | 0 | 0 |
| 71–80 | 0 | 0 | 0 | 0 |
| 81–90 | 0 | 1 | 0 | 0 |
| 100–150 | 1 | 0 | 0 | 0 |
| Mean | 22 | 23 | 11 | 5.0 |
| Variance | 510 | 320 | 11 | 7.2 |
| Variance/mean | 23 | 14 | 1.0 | 1.4 |
| Rate | 4.7 × 10−6 | 4.4 × 10−6 | ||
| SD/mean (Exp.) | 1.0 | 0.77 | ||
| SD/mean (calc.) | 0.98 | 1.2 | ||
Fluctuation analysis was performed as described in Experimental Procedures. Plating controls received 106 cells (fourth column) or 5 × 105 cells (fifth column) per 10-cm plate. Mutation rates and the calculated value for the ratio of the SD to the mean were determined by using equations 8 and 12, respectively, of Luria and Delbrück (41). Values for the expression CaNt were obtained from the numerical tables of Capizzi and Jameson (52).
As shown in Table 3, the ratio of variance to the mean for plating controls was near unity, as expected. By contrast, this value was much larger for both fluctuation experiments, ranging from 14 to 23, a consequence of the highly skewed nature of the experimental distributions. Furthermore, the value for the ratio of the experimental SD to the mean for these fluctuation experiments is similar to that predicted by the Luria–Delbrück theory (equation 12 of ref. 41). The simplest interpretation of these observations is that PALA selects for preexisting CAD amplification events that occur spontaneously in HCT116 cells, in agreement with previous conclusions concerning the origin of CAD amplification events which are obtained in fluctuation tests (29, 42). However, it has been shown that PALA exposure can induce CAD amplification in some cell lines during the selection step (43). This issue will be considered in Discussion.
Discussion
The experiments described here demonstrate that the probability of gene amplification is greatly enhanced in MT1 and HCT116 cell lines that are deficient in MSH6 or MLH1. It is important to note that high frequency CAD amplification in an MLH1-deficient cell line has been observed previously. Tlsty and colleagues (32) demonstrated that RKO cells, a pseudodiploid colorectal tumor cell line, are permissive for CAD amplification. Although unappreciated at the time, this cell line is now known to be MLH1-deficient as a result of epigenetic promoter silencing (44). Because illegitimate recombination is believed to contribute in a major way to amplification of chromosomal segments (23, 45), the simplest explanation for these observations that is consistent with known functions of mismatch repair is that this DNA repair system prevents this class of recombination events. MutSα and MutLα may act at several levels in this respect.
There is excellent evidence that microbial MutS and MutL homologs function to ensure the fidelity of genetic recombination (1, 4, 6), probably by responding to mismatched base pairs within the recombination heteroduplex, resulting in abortion of the recombination event. MSH2 and MSH6 have also been implicated in the fidelity of double-strand break repair in mouse embryonic stem cells (8–10), although there is little information concerning the underlying mechanism in this case.
Attempts to replicate damaged DNA can lead to replication fork demise and double-strand breaks, resulting in activation of recombinational repair systems (46). DNA breaks produced in this manner are thought to be early intermediates in the gene amplification process in those cells that are permissive for this process (43, 47). The mammalian mismatch repair system is known to respond to several types of chemical and photochemical genetic lesion, with lesion recognition and possibly processing by this system leading to activation of damage-response pathways (5). Because one model for MutSα- and MutLα-dependent damage-induced signaling posits that the activating lesions are produced after DNA biosynthetic bypass of template strand damage at the replication fork (13), inactivation of the repair system may result in a stalled replication fork that is prone to uncontrolled strand breakage. Thus, in addition to its role in recombination fidelity, the mismatch repair may stabilize the replication fork to damage that could otherwise lead to strand breakage and production of recombinogenic DNA termini.
The simplest interpretation of the fluctuation experiments described above is that PALA selects for preexisting CAD amplification events that occur spontaneously in MutSα- or MutLα-deficient cells. However, this interpretation may be simplistic because PALA exposure can promote CAD amplification in some cell lines, an effect attributed to DNA damage that may result as a consequence of PALA inhibition of pyrimidine biosynthesis (24, 47). Wahl and colleagues (43) have demonstrated directly that PALA exposure promotes gene amplification in p53-deficient HT1080 cells, a conclusion based on comparison of the CAD amplification frequency as scored by PALA selection with that for DHFR amplification using methotrexate selection. Although PALA selection revealed high-frequency CAD amplification, DHFR amplification was undetectable under these conditions. Because PALA exposure results in a p53-dependent arrest in G0/G1, whereas methotrexate leads to cell cycle arrest in early S phase by a p53-independent mechanism (48), these disparate observations were attributed to S-phase progression of p53-deficient cells in the presence of PALA, with the ensuing DNA damage promoting gene amplification events on selection plates (43).
We have used flow cytometry analysis of DNA content to assess the cell cycle response of MLH1-deficient HCT116 and MLH1-positive HCT116.ch3 cells to PALA exposure. Twenty-two to twenty-three percent of the cells in asynchronous populations of these two lines were found to be in S phase (these data are available in Table 4, which is published as supporting information on the PNAS web site, www.pnas.org). With both HCT116 and HCT116.ch3, this fraction increased to about 40% after 24 h of PALA exposure and then dropped to 6% or less by 48 h, with both cell lines arresting in G0/G1. Consequently, we regard as unlikely the possibility that PALA selection is inducing the amplification events that are observed in HCT116 cells. Although we have not used methotrexate selection, after this work was completed Lin et al. (49) reported high-frequency DHFR amplification in MLH1−/− HCT116 cells using methotrexate selection. The nature of these amplification events was not established, but this finding, coupled with the results described here, implies that the nature of the amplification-permissive state that exists in mismatch repair-deficient cells differs significantly from that in p53-deficient cells.
Analysis of karyotype variation and chromosomal rearrangements has revealed significant differences between RER+ tumor cells that display microsatellite instability (indicative of mismatch repair deficiency), as compared with mismatch repair-proficient RER− cancer cells. Whereas RER− tumor cells are usually aneuploid and harbor a large number of chromosomal rearrangements as judged by comparative genome hybridization (average value about 18 per aneuploid cell), RER+ tumor cells are usually diploid, or nearly so, and contain fewer chromosomal rearrangements (mean about 6 per cell; refs. 35, 50, 51). These observations indicate that similar biological endpoints can evolve by means of distinct genetic destabilization mechanisms.
The high-frequency occurrence of frame-shift and base substitution mutations has been extensively documented in mismatch repair-deficient mammalian cells, and the associated cancer predisposition has generally been interpreted in this context (1–4). However, mismatch repair-deficient cells are also prone to chromosomal rearrangements, which occur at a significant frequency in RER+ tumor cells. Because chromosome rearrangements have been implicated in tumor development, it seems likely that such events may also contribute to the cancer predisposition conferred by mismatch repair defects.
Supplementary Material
Acknowledgments
We thank Dr. Robert Schultz (Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute) for generously providing the PALA used in this work. We also thank Dr. George Stark (Cleveland Clinic Foundation) for much useful advice during the course of this study and Geoff Wahl (Salk Institute) for suggesting the cell cycle studies. We acknowledge Jennifer Powers and Elisabeth Penland for their gracious assistance with the FISH analysis and cell culture work. This work was supported in part by National Institutes of Health Grants GM45190 (to P.M.) and CA43722 (to S.H.B.). P.M. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- PALA
N-phosphonacetyl-l-aspartate
- FISH
fluorescence in situ hybridization
References
- 1.Modrich P, Lahue R. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 2.Kolodner R D, Marsischky G T. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 3.Buermeyer A B, Deschenes S M, Baker S M, Liskay R M. Annu Rev Genet. 1999;33:533–564. doi: 10.1146/annurev.genet.33.1.533. [DOI] [PubMed] [Google Scholar]
- 4.Harfe B D, Jinks-Robertson S. Annu Rev Genet. 2000;34:359–399. doi: 10.1146/annurev.genet.34.1.359. [DOI] [PubMed] [Google Scholar]
- 5.Li G M. Oncol Res. 1999;11:393–400. [PubMed] [Google Scholar]
- 6.Radman M, Matic I, Halliday J A, Taddei F. Philos Trans R Soc London B Biol Sci. 1995;347:97–103. doi: 10.1098/rstb.1995.0015. [DOI] [PubMed] [Google Scholar]
- 7.Stambuk S, Radman M. Genetics. 1998;150:533–542. doi: 10.1093/genetics/150.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wind N, Dekker M, Berns A, Radman M, te Riele H. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 9.de Wind N, Dekker M, Claij N, Jansen L, van Klink Y, Radman M, Riggins G, van der Valk M, van't Wout K, te Riele H. Nat Genet. 1999;23:359–362. doi: 10.1038/15544. [DOI] [PubMed] [Google Scholar]
- 10.Elliott B, Jasin M. Mol Cell Biol. 2001;21:2671–2682. doi: 10.1128/MCB.21.8.2671-2682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwab M. Semin Cancer Biol. 1999;9:319–325. doi: 10.1006/scbi.1999.0126. [DOI] [PubMed] [Google Scholar]
- 12.Goldmacher V S, Cuzick R A, Thilly W G. J Biol Chem. 1986;261:12462–12471. [PubMed] [Google Scholar]
- 13.Kat A, Thilly W G, Fang W H, Longley M J, Li G M, Modrich P. Proc Natl Acad Sci USA. 1993;90:6424–6428. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koi M, Umar A, Chauhan D P, Cherian S P, Carethers J M, Kunkel T A, Boland C R. Cancer Res. 1994;54:4308–4312. [PubMed] [Google Scholar]
- 15.Perry M E, Rolfe M, McIntyre P, Commane M, Stark G R. Mutat Res. 1992;276:189–197. doi: 10.1016/0165-1110(92)90008-w. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 17.Davidson J N, Rao G N, Niswander L, Andreano C, Tamer C, Chen K C. DNA Cell Biol. 1990;9:667–676. doi: 10.1089/dna.1990.9.667. [DOI] [PubMed] [Google Scholar]
- 18.Smith K A, Chernova O B, Groves R P, Stark M B, Martinez J L, Davidson J N, Trent J M, Patterson T E, Agarwal A, Duncan P, et al. Proc Natl Acad Sci USA. 1997;94:1816–1821. doi: 10.1073/pnas.94.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matlashewski G, Lamb P, Pim D, Peacock J, Crawford L, Benchimol S. EMBO J. 1984;3:3257–3262. doi: 10.1002/j.1460-2075.1984.tb02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagioli F, Cuneo A, Bardi A, Carli M G, Bigoni R, Balsamo R, Previati R, Pazzi I, Roberti G, Rigolin G M, et al. Cancer Genet Cytogenet. 1995;82:116–122. doi: 10.1016/0165-4608(94)00228-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Dodd L G, Powers J A, Bigner S H. Acta Cytol. 2000;44:368–374. doi: 10.1159/000328480. [DOI] [PubMed] [Google Scholar]
- 22.Duckett D R, Bronstein S M, Taya Y, Modrich P. Proc Natl Acad Sci USA. 1999;96:12384–12388. doi: 10.1073/pnas.96.22.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark G R. Adv Cancer Res. 1993;61:87–113. doi: 10.1016/s0065-230x(08)60956-2. [DOI] [PubMed] [Google Scholar]
- 24.Smith K A, Agarwal M L, Chernov M V, Chernova O B, Deguchi Y, Ishizaka Y, Patterson T E, Poupon M F, Stark G R. Philos Trans R Soc London B Biol Sci. 1995;347:49–56. doi: 10.1098/rstb.1995.0008. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos N, Nicolaides N C, Liu B, Parsons R, Lengauer C, Palombo F, D'Arrigo A, Markowitz S, Willson J K, Kinzler K W, et al. Science. 1995;268:1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulos N, Nicolaides N C, Wei Y-F, Ruben S M, Carter K C, Rosen C A, Haseltine W A, Fleischmann R D, Fraser C M, Adams M D, et al. Science. 1994;263:1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 27.Padgett R A, Wahl G M, Stark G R. Mol Cell Biol. 1982;2:293–301. doi: 10.1128/mcb.2.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl G M, Padgett R A, Stark G R. J Biol Chem. 1979;254:8679–8689. [PubMed] [Google Scholar]
- 29.Tlsty T D, Margolin B H, Lum K. Proc Natl Acad Sci USA. 1989;86:9441–9445. doi: 10.1073/pnas.86.23.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright J A, Smith H S, Watt F M, Hancock M C, Hudson D L, Stark G R. Proc Natl Acad Sci USA. 1990;87:1791–1795. doi: 10.1073/pnas.87.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tlsty T D. Proc Natl Acad Sci USA. 1990;87:3132–3136. doi: 10.1073/pnas.87.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livingstone L R, White A, Sprouse J, Livanos E, Jacks T, Tlsty T D. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- 33.Yin Y, Tainsky M A, Bischoff F Z, Strong L C, Wahl G M. Cell. 1992;70:937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- 34.Galloway S M, Greenwood S K, Hill R B, Bradt C I, Bean C L. Mutat Res. 1995;346:231–245. doi: 10.1016/0165-7992(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 35.Eshleman J R, Casey G, Kochera M E, Sedwick W D, Swinler S E, Veigl M L, Willson J K, Schwartz S, Markowitz S D. Oncogene. 1998;17:719–725. doi: 10.1038/sj.onc.1201986. [DOI] [PubMed] [Google Scholar]
- 36.Phillips E N, Xia F, Kelsey K T, Liber H L. Radiat Res. 1995;143:255–262. [PubMed] [Google Scholar]
- 37.D'Atri S, Tentori L, Lacal P M, Graziani G, Pagani E, Benincasa E, Zambruno G, Bonmassar E, Jiricny J. Mol Pharmacol. 1998;54:334–341. doi: 10.1124/mol.54.2.334. [DOI] [PubMed] [Google Scholar]
- 38.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 39.Burri N, Shaw P, Bouzourene H, Sordat I, Sordat B, Gillet M, Schorderet D, Bosman F T, Chaubert P. Lab Invest. 2001;81:217–229. doi: 10.1038/labinvest.3780230. [DOI] [PubMed] [Google Scholar]
- 40.Khan S H, Moritsugu J, Wahl G M. Proc Natl Acad Sci USA. 2000;97:3266–3271. doi: 10.1073/pnas.050560997. . (First Published March 14, 2000; 10.1073/pnas.050560997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luria S E, Delbrück M. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kempe T D, Swyryd E A, Bruist M, Stark G R. Cell. 1976;9:541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- 43.Paulson T G, Almasan A, Brody L L, Wahl G M. Mol Cell Biol. 1998;18:3089–3100. doi: 10.1128/mcb.18.5.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veigl M L, Kasturi L, Olechnowicz J, Ma A H, Lutterbaugh J D, Periyasamy S, Li G M, Drummond J, Modrich P L, Sedwick W D, Markowitz S D. Proc Natl Acad Sci USA. 1998;95:8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giulotto E, Saito I, Stark G R. EMBO J. 1986;5:2115–2121. doi: 10.1002/j.1460-2075.1986.tb04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox M M, Goodman M F, Kreuzer K N, Sherratt D J, Sandler S J, Marians K J. Nature (London) 2000;404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- 47.Poupon M F, Smith K A, Chernova O B, Gilbert C, Stark G R. Mol Biol Cell. 1996;7:345–354. doi: 10.1091/mbc.7.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linke S P, Clarkin K C, Di Leonardo A, Tsou A, Wahl G M. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- 49.Lin C T, Lyu Y L, Xiao H, Lin W H, Whang-Peng J. Nucleic Acids Res. 2001;29:3304–3310. doi: 10.1093/nar/29.16.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis L J, Georgiades I B, White S, Bird C C, Harrison D J, Wyllie A H. J Pathol. 2000;192:440–445. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH761>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Rahman W M, Katsura K, Rens W, Gorman P A, Sheer D, Bicknell D, Bodmer W F, Arends M J, Wyllie A H, Edwards P A. Proc Natl Acad Sci USA. 2001;98:2538–2543. doi: 10.1073/pnas.041603298. . (First Published February 20, 2001; 10.1073/pnas.041603298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capizzi R L, Jameson J W. Mutat Res. 1973;17:147–148. doi: 10.1016/0027-5107(73)90265-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.