Abstract
Bone tissue is comprised of collagen, non-collagenous proteins, and hydroxyapatite and the SIBLING (small integrin binding, N-linked glycoprotein) family of proteins is the primary group of non-collagenous proteins. By replicating the native interactions between collagen and the SIBLING family of proteins at the interface of an implant, it is believed that a bone scaffold will more easily integrate with the surrounding tissue. In this work bone sialoprotein (BSP), osteopontin (OPN), dentin sialoprotein (DSP), dentin phosphoprotein (DPP), C-terminal fragment of dentin matrix protein 1 (DMP1-C) and proteoglycan versions of DSP (DSP-PG) and DMP1 (DMP1-PG) were tested individually to determine their roles in collagen fibrillogenesis and the prevention of denaturation. It was shown that DSP and DPP slowed down fibrillogenesis, while other SIBLINGs had limited impact. In addition, the denaturation time was faster in the presence of DSP and OPN, indicating a negative impact. The role of calcium ions in these processes was also investigated. The presence of calcium ions sped up fibrillogenesis in all scenarios tested, but it had a negative impact by reducing the extent. Calcium also sped up the denaturation in most cases, with the exception of DMP1-C and DSP where the opposite was seen. Calcium had a similar effect on the proteoglycan variants in the fibrillogenesis process, but had no impact on the denaturation process in the presence of these two. It is believed that incorporating DMP1-C or DSP on the surface of a bone implant may improve the collagen interactions with the implant, thereby facilitating improved osteointegration.
Keywords: Collagen, SIBLING, fibrillogenesis, denaturation, calcium
1. Introduction
The extracellular matrix (ECM) of bone tissue is a highly hierarchical microenvironment based on hydroxyapatite (HAP) minerals and collagen fibrils. Depending on the exact location, dry bone tissue is approximately 70–90% by mass calcium phosphate mineral and 10–30% by mass protein. As much as 90% of the protein content is collagen, primarily collagen I, and it provides structural support to bone. The remaining protein content is believed to provide cues for bone physiological activity, including cell differentiation, maturation, growth, repair, and cellular adhesion [1].
Due to the naturally occurring foreign body response and the inability of bone tissue engineering scaffolds to replicate the native hierarchical bone structure, many critical size defect implants face challenges with rejection or incomplete closure [2, 3]. Therefore, there is interest in developing novel approaches for improving the integration of bone tissue engineering scaffolds with the native tissue through advanced design strategies [4]. One such approach is the incorporation of tissue specific bioactive signaling molecules on the surface of implant, with the goal of improving the host tissue response to that material upon implantation. In order for this approach to be successful, these signaling molecules should be identical to those naturally occurring in the bone ECM. However, the bioactive roles of the ECM constituents must be better understood before they can be incorporated into an advanced tissue engineering scaffold
The primary group of non-collagenous proteins in mineralized tissue is the small integrin-binding ligand, N-linked glycoprotein (SIBLING) family of proteins. This family of proteins consists of five members, some of which are naturally cleaved in bone tissue. The family consists of osteopontin (OPN), bone sialoprotein (BSP), matrix extracellular phosphoglycoprotein (MEPE), dentin matrix protein 1 (DMP1), and dentin sialophosphoprotein (DSPP). Typically, DMP1 is rapidly cleaved in the in vivo environment into a 37 kDa N-terminal fragment (DMP1-N) and a 57 kDa C-terminal fragment (DMP1-C) [5]. A third variant of DMP1 is also found in mineralized tissue consisting of a glycosaminoglycan-containing version of DMP1-N referred to as DMP1-PG, which consists of a single chondroitin sulfate chain [6]. Of these three variants, Dmp1-null mouse studies have suggested that DMP1-C is the most essential fragment [7]. Similarly, DSPP is also proteolytically cleaved into an N-terminal fragment known as dentin sialoprotein (DSP) and a C-terminal fragment known as dentin phosphoprotein (DPP). As with DMP1, there is also a proteoglycan form of the N-terminal fragment of DSPP referred to as DSP-PG [8, 9].
The SIBLING family of proteins has long been implicated as playing essential roles in bone development [10]. The SIBLING proteins all contain a collagen binding domain, a HAP binding domain, and a cell integrin binding arginine-glycine-aspartic acid (RGD) sequence [11, 12]. Many of the SIBLING family members have been immunolocalized at fracture sites, developing bone, and in mineralized tissues indicating their importance for bone formation and regeneration processes [13–15]. While many groups, including our own, have investigated the cell binding and mineralization induction capabilities of the SIBLING family of proteins, to the best of our knowledge no one has conducted extensive in vitro studies into the influence of these proteins on collagen fibrillogenesis [6, 11, 16–23]. Therefore, one motivation for this work is to screen the SIBLING family of proteins to determine which, if any, of the SIBLING family members play a role in promoting collagen fibrillogenesis from either a kinetics or extent perspective. Furthermore, this group of proteins will also be screened for their ability to reduce the occurrence or rate of collagen denaturation, albeit through thermal denaturation rather than enzymatic pathways.
Investigations into the mechanisms and kinetics of natural collagen fibrillogenesis have been completed by multiple teams dating back to at least the 1960s [24–28]. It is well understood that fibrillogenesis can be induced using defined substrates, solvents, and purified collagen molecules, resulting in fibrils that are very similar in morphology to native collagen fibrils [29]. The fibrillogenesis process has long been believed to occur in two steps, nucleation and growth, and these steps are easily distinguished using turbidity assays [26–28]. The nucleation step is often characterized with lag time measurements and the growth step can be determined by quantifying the extent of fibrillogenesis following equilibrium. At the same time, this system is highly sensitive to external conditions including temperature, pH, collagen concentration, collagen source, and additional bioactive species [29, 30].
In one recent example, Halász et al investigated the role of various forms of cartilage oligomeric matrix protein (COMP) on both the rate and extent of collagen fibrillogenesis [30]. The ability of external biomolecules like COMP to influence collagen fibrillogenesis was well demonstrated as pentameric COMP increased both the rate and extent of collagen fibrillogenesis, while monomeric COMP slowed the process, relative to collagen controls. Other extracellular matrix proteins including decorin and fibromodulin have also been studied to determine their influence on fibrillogenesis [31–33]. Furthermore, proteoglycan modifications to proteins have also been demonstrated to have an influence on collagen fibrillogenesis in a species dependent manner [34].
Despite extensive investigations both in vitro and in vivo into the bioactive roles of the SIBLING family of proteins, there has been little to no work to date into the influence that this family of proteins has on the collagen fibrillogenesis process. It is hypothesized that one or more of the SIBLING protein family is responsible for mediating the collagen fibrillogenesis process in developing bone, therefore, this work seeks to explore the roles of BSP, OPN, DPP, DSP, DSP-PG, DMP1-C, and DMP1-PG in the early stages of collagen fibrillogenesis. MEPE is not being included in this investigation because, while present in mineralized tissue, it is found only in very trace amounts making its isolation and purification not feasible at the present time. To better understand the roles of the SIBLING family of proteins in the early stages of collagen fibrillogenesis, the impact of each individual protein on the kinetics and extent of both fibrillogenesis and the denaturation of assembled fibrils was characterized using in vitro assays. The relative influence of the concentration of SIBLING proteins on the fibrillogenesis process was also investigated by varying the collagen concentration present. Elucidating the roles that these proteins play in collagen fibrillogenesis and denaturation would be useful in bone biology and could aid in guiding the design of bone tissue engineering scaffolds that improves implant osteointegration. This study is one of the first to explore the relationships between collagen and SIBLINGS under in vitro conditions
2. Materials and Methods
2.1. Materials
Collagen type I from rat tail tendon with a purity >90% was purchased from BD Biosciences (Bedford, MA). Ultrapure water (18.2 ΜΩ-cm) was obtained from a Millipore Synergy UV water purifier (Billerica, MA) and it was used for all experiments. NaCl, Na2HPO4, CaCl2 and Tris (hydroxymethyl) aminomethane (Tris-HCl) were purchased from Thermo Fisher Scientific (Waltham, MA). NaOH was purchased from Sigma-Aldrich (Saint Louis, MO). Fibrillogenesis buffer was made in ultrapure water with the following composition in grams per liter: NaCl, 15.75; Tris-HCl, 13.76; Na2HP04 7H20, 16.08; plus −1.4 ml of 10 N NaOH to obtain a measured pH of 7.45 to 7.50. The buffer was filtered sterilized and stored at 5 °C. The calcium supplemented fibrillogenesis buffer contained an additional 366.3 mg of CaCl2 [6].
2.2. SIBLING protein isolation procedures
All of the SIBLING proteins were isolated, purified, and confirmed using previously published procedures and detailed procedures can be found in the cited reference articles All proteins were isolated from either rat long bone or rat incisor dentin from wild-type animals euthanized for reasons unrelated to this study. BSP and OPN, were extracted from the femurs and tibiae of 10-week-old rats as described in detail previously [15, 35]. The total bone protein extracts were subjected to multiple chromatography isolation/purification steps using established protocols. The purity and identity of OPN and BSP were confirmed with polyacrylamide gel electrophoresis and Western immunoblots using antibodies specific to OPN and BSP.
DMP1 37 kDa and 57 kDa fragments were isolated from rat long bone extracellular matrix (ECM) [36]. Briefly, the rat bone protein extracts were first subjected to size-exclusion chromatography and then the high-molecular-mass protein was separated into several sub-fractions by anion-exchange chromatography. Finally, the fractions enriched with 37 kDa and 57 kDa fragments were passed over a mono-Q column (Bio-Rad, Hercules, California) connected to a fast protein liquid chromatography system resulting in highly purified samples.
DMP1-PG was isolated from the DMP1 sub-fractions by first eliminating the 37 kDa sub-fraction using Q-sepharose ion exchange chromatography [37]. Then the DMP1-PG-rich subfractions were passed over an affinity column composed of monoclonal anti-DMP1 37 kDa fragment antibody. After a series of careful analyses, including Stains-All staining, chondroitinase digestion, amino acid analysis, and tryptic peptide sequencing the identity and purity of DMP1-PG was confirmed.
DSP was isolated from rat incisor dentin [38]. Rat incisor pieces were first extracted with 0.5 M EDTA in solution containing protease inhibitors. Next, EDTA extracts were subjected to size-exclusion chromatography. Using this procedure, the high molecular weight protein fraction (ES1) was separated from osteocalcin into an included volume. ES1 was next chromatographed through an anion-exchange column and DSP eluted in a position referred as fraction B. Then fraction B was further purified by size-exclusion column, where a single major peak was isolated from the lower molecular weight proteins. The purity of the isolated DSP was assessed by SDS-PAGE and anti-DSP Western immunoblotting analyses.
DPP was extracted from the incisor dentin of 10-wk-old rats by standard procedures as described in detail elsewhere [39, 40]. Briefly, the total rat dentin protein extract was subjected to Sephacryl S200 size-exclusion chromatography, anion-exchange, and Bio-Gel A50m size-exclusion chromatography isolation/purification steps. Fractions containing DPP were combined, dialyzed, and lyophilized for use in this study. The purity of the collected DPP was confirmed by SDS-PAGE with Stains-All staining.
DSP-PG was extracted and purified from the incisor dentin of rats, as previously described [9, 40]. Briefly, the non-collagenous proteins extracted from rat incisors were first passed through an anion-exchange column to separate DSP-PG from DSP, and then, the DSP-PG rich and DSP-free fractions were passed over an affinity column made of monoclonal anti-DSP antibodies. The final eluted proteins from the anti-DSP affinity column were dialyzed extensively, lyophilized, reconstituted in water and analyzed. The purity of DSP-PG was confirmed by Stains-All staining and anti-DSP Western immunoblotting analyses. The DSP-PG isolated in this manner was highly pure and free of any contaminating proteins [9, 40].
Representative SDS-PAGE images for all of the purified protein fractions can be found in the Supplementary Information file.
2.3. Fibrillogenesis Assays
The fibrillogenesis assay was adapted from the procedures originally published by Williams [25]. The fibrillogenesis buffer, water, and well plate were preheated to 37 °C before beginning the assay. SIBLINGS were stored at 0 °C in water and the stock collagen solution was stored at 4 °C. Each individual SIBLING protein or proteoglycan-modified SIBLING protein was added to the well plate and then collagen was added to produce a molar ratio of 1:8, 1:16, or 1:40 SIBLING:collagen, with overall collagen concentrations of 0.05, 0.10, and 0.25 mg/mL, respectively. The amount of SIBLING protein in each well was held constant for all experiments. Following the addition of SIBLING proteins and collagen, 125 μL of fibrillogenesis buffer and enough ultrapure water to give a final volume of 250 μL in each well was added. The amount of water in each well was adjusted based on the volume of collagen and SIBLING protein solution to fix the total volume at 250 μL. All fibrillogenesis assays were performed in a PowerWave XS2 multi-well plate reader from BioTek (Winooski, VT) and absorbance was measured at 400 nm every 3 minutes over the entire assay. The plate reader was preheated to 37 °C prior to inserting the well plate and held at 37°C during each assay. Each trial consisted of six wells for each SIBLING and collagen combination (n = 6) and the assay was repeated at least three times for a minimum total of 18 replicates.
2.4. Denaturation Assay
Based on the results obtained in the fibrillogenesis assay, the 1:8 SIBLING:collagen ratio was selected for use in the denaturation assay. To complete the denaturation process, the well plate was set up identically to that described in the fibrillogenesis assay above. Following five hours of fibrillogenesis, the temperature of the plate reader was increased to 45 °C to initiate denaturation. The denaturation process was tracked in the plate reader at 400 nm, with a data point taken every 3 minutes for an additional 10 hours. Each trial consisted of six wells for each SIBLING and collagen combination (n = 6) and the assay was repeated at least three times for a minimum total of 18 replicates.
2.5. Calcium Supplemented Fibrillogenesis and Denaturation
The calcium supplemented fibrillogenesis and denaturation assays were identical to those described above, with the exception that calcium supplemented buffer was used in place of the traditional fibrillogenesis buffer, and only the 1:8 SIBLING:collagen ratio was investigated. All other conditions were identical. As before, each trial consisted of six wells of each SIBLING and collagen combination (n = 6) and the assay was repeated at least three times for a minimum total of 18 replicates.
2.6. Lag Time and Denaturation Time
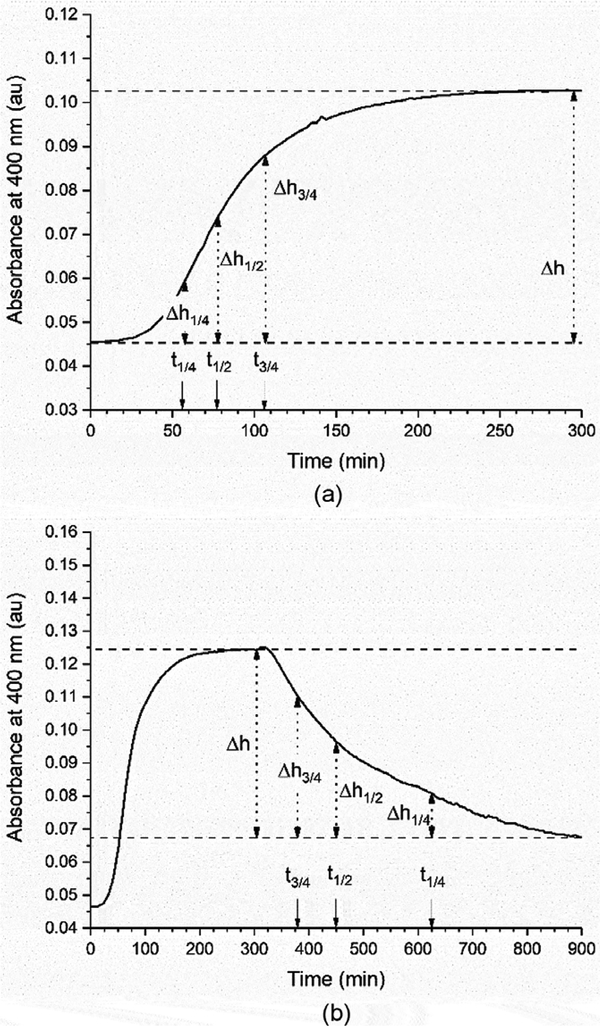
It is well known that the collagen fibrillogenesis process can be monitored using absorbance. The optical density of a collagen solution is proportional to the amount of sedimentable product (fibrils) and the final optical density is dependent upon the initial collagen concentration [26]. Therefore turbidity has been used to quantify fibrillogenesis and denaturation as far as back as 1979 [25]. It is possible to compare different fibrillogenesis conditions by quantifying the phases of fibrillogenesis which include the lag phase where there is no detectable change in turbidity, the growth phase during which turbidity increases rapidly, the plateau phase where turbidity remains constant, and the denaturation phase where turbidity decreases rapidly. The lag time associated with a given set of fibrillogenesis conditions is using Equation 1 [27].
| Equation 1 |
In this equation, t1/4, t1/2, and t3/4 represent the time to reach 1/4 Δh, and 3/4 Δh, where Δh is the total absorbance change that results from the fibrillogenesis or denaturation process. Figure 1a also shows a representative fibrillogenesis curve with each of these points identified. This approach was also adapted to determine the denaturation time in this study, using the same equation and replacing tlag with tdenature· Figure 1b shows a representative denaturation curve with each of these points identified
Figure 1:
Representative curves showing the (a) fibrillogenesis process and (b) denaturation process with the lag time and denaturation time points highlighted.
2.7. Statistical Analysis
All of the presented fibrillogenesis and denaturation curves are presented as the mean of all of the samples for a given data set. All of the lag time and denature time data are presented as the mean ± standard deviation of all of the samples for a given data set. The extent of fibrillogenesis was determined by averaging the absorbance measurement at 400 nm over the final three measurement time points (291–300 min) for all of the assays that were conducted.
Because so many proteins were studied, statistical analysis was completed by comparing the individual SIBLING protein results to the collagen results only from the same well plates. This was done because of the impact that subtle preparation conditions, such as temperature, have on the results of a given set of experiments. The results were identified as being statistically significant from each other at a 95% confidence interval (p < 0.05) using a one-way analysis of variance (ANOVA) test. The statistical analysis was conducted using OriginPro 9.1 software.
3. Results
3.1. Fibrillogenesis Assays
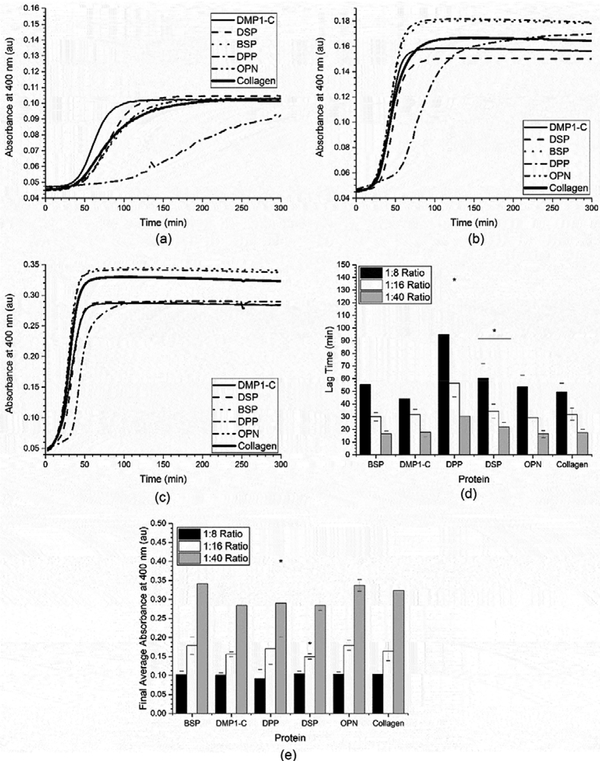
The first variable that was investigated was the influence of the SIBLING: collagen ratio on both the kinetics and extent of the fibrillogenesis process To investigate this, SIBLING: collagen molar ratios of 1:8, 1:16, and 1:40 were investigated by varying the concentration of collagen (0.05 mg/mL, 0.10 mg/mL, and 0.25 mg/mL, respectively), while holding the amount of SIBLING proteins constant. The results from these collagen fibrillogenesis assays are shown in Figures 2a-c. In Figure 2a, it can be seen that the collagen fibrillogenesis from 0.05 mg/mL collagen (1:8 SIBLING:collagen ratio) in the presence of either BSP or OPN was nearly identical to that of the collagen controls. When the lag time was determined for this data using Equation 1, this was confirmed, as the lag time in the presence of both BSP and OPN was not significantly different than the control, as shown in Figure 2d. In the presence of DMP1-C, it appears that the fibrillogenesis process occurs more quickly in Figure 2a. However, when the lag time was quantified it was determined that it was not significantly faster than the collagen control. All three of these systems also reached the same extent of fibrillogenesis as the collagen control, as shown in Figure 2e. The first statistically significant difference was seen when the fibrillogenesis process occurred in the presence of DSP. In this case, the fibrillogenesis process occurred a little more slowly as indicated by the larger lag time. Ultimately, though, the same extent of fibrillogenesis was achieved. Finally, it can clearly be seen that the largest impact on the fibrillogenesis process occurred in the presence of DPP, which significantly slowed the process as compared to both collagen and the other SIBLING proteins. The final extent of fibrillogenesis was not statistically different from the collagen controls based on a statistical analysis of the absorbance measurements after the process had proceeded for approximately 290 minutes as indicated in Figure 2e. However, the absorbance measurements in the presence of DPP were statistically lower (slower assembly) than those measured for collagen over the time range of 45 minutes to 291 minutes.
Figure 2:
Mean of the collagen fibrillogenesis assays performed with SIBLING proteins and a) 0.05 mg/mL of collagen (1:8 SIBLING to collagen ratio), b) 0.10 mg/mL of collagen (1:16 ratio), and c) 0.25 mg/mL of collagen (1:40 ratio). These curves were analyzed to obtain the mean ± standard deviation of the d) fibrillogenesis lag time and e) extent of fibrillogenesis for the SIBLING proteins at all three SIBLING: collagen ratios (n ≥ 18). A * represents a statistically significant difference between collagen and the indicated SIBLING protein at a 95% confidence interval (p < 0.05).
Similar results were found when the collagen concentration was increased to 0.10 mg/ml (1:16 SIBLING:collagen ratio) as seen in Figure 2b, 2d, and 2e, even though the fibrillogenesis process occurs more quickly in all cases as indicated by the lower fibrillogenesis lag times. There was no statistical difference in either the extent or kinetics of the process in the presence of BSP, OPN, or DMP1-C as compared to the collagen controls. Again, the fibrillogenesis process was statistically slower in the presence of both DSP and DPP, with DPP again having a greater impact on the process. However, in this situation the results for DSP indicated that the process did not achieve the same extent of fibrillogenesis because the final absorbance measurements for DSP (291–300 minutes) were statistically lower than those for the corresponding collagen controls, as shown in Figure 2e.
Figure 2c shows that the fibrillogenesis process occurs even more quickly in the presence of 0.25 mg/mL collagen (1:40 SIBLING:collagen ratio). When the results from the assay were analyzed it can be seen that the fibrillogenesis process was statically slower in the presence of DSP and DPP once again, as indicated by the larger values for the lag time indicated in Figure 2d. When the final absorbance values were compared to determine if any of the proteins had an impact on the extent of the fibrillogenesis process it was found that only DPP had a statistically lower value over the final absorbance measurements (291–300 minutes), as seen in Figure 2e. These results also confirmed that the 1:8 SIBLING:collagen ratio best highlighted the influence of the SIBLING proteins on the fibrillogenesis process.
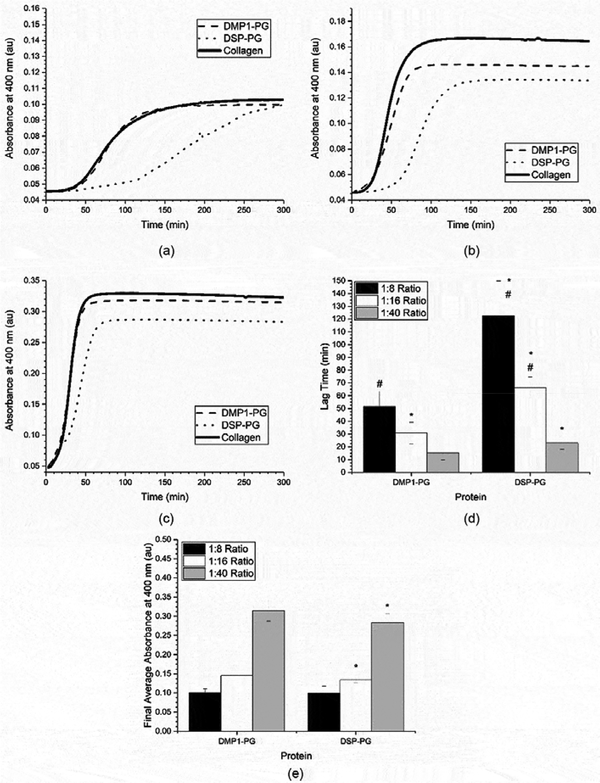
Next, the proteoglycan (PG) versions of DMP1 and DSP were tested across the same three ratios of protein to collagen. The fibrillogenesis curves for all three conditions can be seen in Figures 3 a-c, the corresponding lag times for the two PG species are summarized in Figure 3d, and the extent of fibrillogenesis is shown in Figure 3e. When the PG species were compared to their respective collagen controls, it can be seen that the fibrillogenesis process is statistically slower in the presence of DSP-PG, as indicated by its greater lag time values. This is similar to the result found with the native DSP. However, the fibrillogenesis process in the presence of DSP-PG is statistically slower than DSP at the 1:8 and 1:16 SIBLING:collagen ratios as well (0.05 mg/mL and 0.10 mg/mL collagen). In the presence of DMP1-PG, the fibrillogenesis process did not show as great of a difference from either the collagen or DMP1-C controls. In fact, the lag time was statistically different between the DMP1-PG and collagen control only at the 1:16 ratio of SIBLING:collagen (0.10 mg/mL collagen) and it was statistically different from the DMP1-C control only at the 1:8 ratio of SIBLING:collagen (0.05 mg/mL collagen). Because there is not a statistically significant difference across all of the SIBLING:collagen ratios in the presence of DMP1-PG these are likely due to experimental variability. When the extent of fibrillogenesis was evaluated based on the final absorbance measurements (291–300 minutes), it was seen that there was statistically lower levels of fibrillogenesis in the presence of DSP-PG at SIBLING:collagen ratios of 1:16 and 1:40 (0.10 mg/mL and 0.25 mg/mL collagen), as indicated in Figure 3e. There were no differences in the extent of fibrillogenesis in the presence of DMP1-PG.
Figure 3:
Mean of the collagen fibrillogenesis assays performed with proteoglycan proteins and a) 0.05 mg/ml of collagen (1:8 SIBLING to collagen ratio), b) 0.10 mg/mL of collagen (1:16 ratio), and c) 0.25 mg/mL of collagen (1:40 ratio). For reference, the collagen controls have been replicated from Figure 2. These curves were analyzed to obtain the mean ± standard deviation of the d) fibrillogenesis lag time and e) extent of fibrillogenesis for the proteoglycan modified proteins at all three SIBLING: collagen ratios (n ≥ 18). A * represents a statistically significant difference between collagen and the indicated SIBLING protein at a 95% confidence interval (p < 0.05). A # represents a statistically significant difference between the indicated proteoglycan modified protein and its native form at a 95% confidence interval (p < 0.05).
3.2. Denaturation Assay
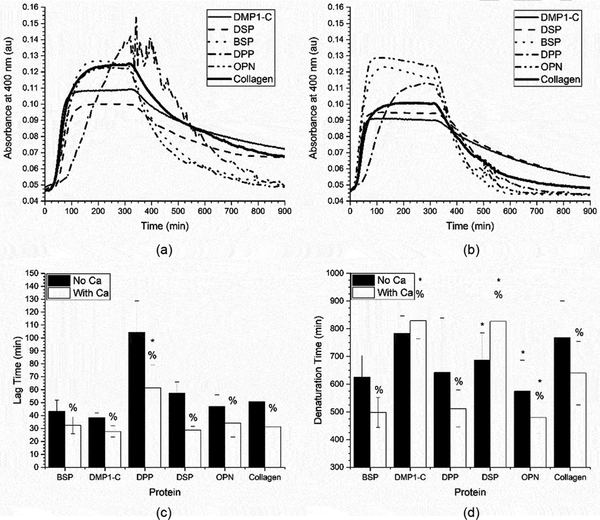
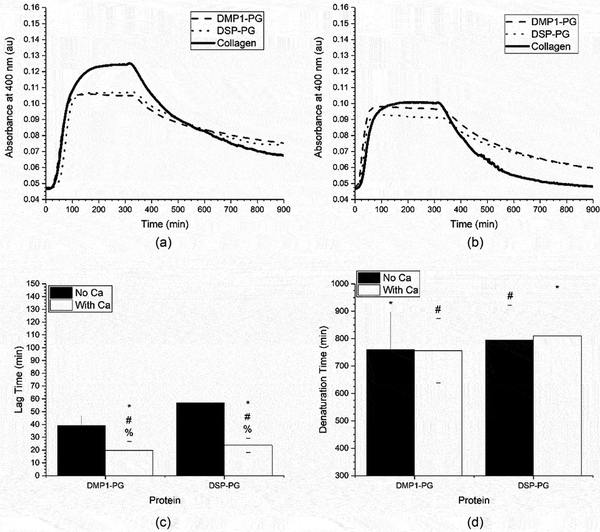
After identifying the 1:8 SIBLING: collagen ratio (0.05 mg/mL collagen) as the condition that best highlighted the impact of the SIBLING proteins on fibrillogenesis, the temperature induced collagen denaturation process was investigated. While thermal degradation is not a naturally occurring process, it does provide insight as a screening tool to identify candidate SIBLING family members for future in-depth studies. In addition to the heated denaturation process, the influence of calcium ions in the system was also investigated. The fibrillogenesis curves that were obtained for the SIBLING proteins are shown in Figures 4 a-b. Additionally, Figures 4 c-d show the results of the lag time and denaturation time analyses, both with and without calcium. This discussion will focus on the denaturation assay and the influence of calcium because the lag time was already discussed above.
Figure 4:
Mean of the collagen fibrillogenesis and denaturation assays performed with SIBLING proteins and 0.05 mg/ml of collagen (1:8 SIBLING to collagen ratio) in a) regular buffer and b) calcium supplemented buffer. These curves were analyzed to obtain the mean ± standard deviation of the c) fibrillogenesis lag time and d) denaturation lag time for the SIBLING proteins and 0.05 mg/ml of collagen (1:8 SIBLING to collagen ratio) in regular or calcium supplemented buffer (n ≥ 18). A * represents a statistically significant difference between collagen and the indicated SIBLING proteins and a % represents a statistically significant difference between the regular and calcium supplemented buffer for the indicated SIBLING protein, both at a 95% confidence interval (p < 0.05).
In Figure 4a, it can be seen that the collagen begins to denature immediately upon heating. However, after 10 hours of elevated temperatures the collagen control does not fully denature. The denaturation process in the presence of either DMP1-C or DSP appears to be similar to the collagen controls, but the denaturation time for DSP was statistically smaller than that for the collagen control indicating a faster denaturation process. Conversely, in the presence of both OPN and BSP the collagen appeared to fully denature, with the absorbance dropping to its initial value. While this was seen with both OPN and BSP, it was determined that only OPN has a statistically lesser denaturation time. Perhaps the most interesting response occurred when DPP was present. There was significant noise in the signal at the start of the heating process, as clearly seen in Figure 4a. The absorbance measurements in this system ultimately returned to their initial values, indicating full denaturation as well. However, the denaturation time in the presence of DPP was not statistically different than its corresponding collagen controls, likely due to the wide range of noise seen in this particular system.
Next, the influence of calcium on both the fibrillogenesis and denaturation processes was examined. In the presence of calcium, the collagen control assembled significantly faster during the fibrillogenesis process and degraded more quickly during the denaturation process as indicated by the lag time and denaturation times shown in Figures 4 c-d. The fibrillogenesis process also occurred more rapidly when calcium was added in system in the presence of all members of the SIBLING family investigated. The biggest impact on the measured lag time as compared to the process in the absence of calcium was seen in the DPP and DSP systems. However, in the presence of calcium, only the DPP system showed statistical differences as compared to the collagen controls. It is hypothesized that this is due to the strong calcium binding capacity of DPP, which therefore reduces the number of free calcium ions in solution available to influence the fibrillogenesis process. It is also clear that the presence of calcium plays a significant role in the fibrillogenesis process.
The most interesting results were seen in the denaturation process when calcium was supplemented alongside the SIBLING proteins. As with the lag time analysis, all of the measured denaturation times were statistically different from the related cases where there was no calcium. The collagen denatured more quickly in both the control case and when it was denatured in the presence of BSP, DPP, and OPN in the calcium supplemented buffer. Conversely, the denaturation process occurred more slowly in the systems containing either DMP1-C or DSP. Therefore, it is possible that the binding interactions between calcium and these proteins facilitates more robust collagen assembly.
Finally, the proteoglycan versions of DMP1 and DSP were tested to determine their impact on the denaturation process both with and without a calcium supplement. The fibrillogenesis and denaturation curves can be seen in Figures 5 a-b and the lag time and denaturation time analysis can be seen in Figures 5 c-d. In the presence of calcium, both PG proteins had faster fibrillogenesis processes than their unmodified relatives. They were also faster than the collagen controls and the system without calcium. These trends were consistent with what was observed in the SIBLING protein systems. However, the presence of calcium did not have any effect on the denaturation time for either PG modified protein. These were the only two cases where calcium did not have an impact.
Figure 5:
Mean of the collagen fibrillogenesis and denaturation assays performed with proteoglycan proteins and 0.05 mg/ml of collagen (1:8 SIBLING to collagen ratio) in a) regular buffer and b) calcium supplemented buffer. For reference, the collagen control curves have been replicated from Figure 4. These curves have been analyzed to obtain the mean ± standard deviation of the c) fibrillogenesis lag time and d) denaturation lag time for proteoglycan proteins and 0.05 mg/ml of collagen (1:8 SIBLING to collagen ratio) in regular or calcium supplemented buffer (n=18). A * represents a statistically significant difference between collagen and the indicated SIBLING proteins, a # represents a statistically significant difference between the indicated proteoglycan protein and its native form, and a % represents a statistically significant difference between collagen and the regular and calcium supplemented buffer for the indicated SIBLING protein, all at a 95% confidence interval (p<0.05).
4. Discussion
The results presented above for the collagen fibrillogenesis process clearly demonstrated that as the amount of collagen was increased, the fibrillogenesis process occurred more quickly and to a greater extent. This was expected based upon the historical literature probing the fibrillogenesis process [25, 27]. Based on these results it was determined that the lowest collagen concentration was the best scenario for evaluating the impact of the SIBLING proteins on collagen fibrillogenesis and denaturation, because at the greater concentrations the process occurred so rapidly that the influence of the SIBLING proteins was often washed out. Furthermore, when calcium was supplemented into the system, it was also observed that both the fibrillogenesis and denaturation processes occurred more rapidly, as indicated by the reduced lag and denaturation times. It is well understood that the presence of various ions can influence the fibrillogenesis and denaturation process, as this naturally occurring assembly process is very sensitive to environmental cues including pH, temperature, and salt [29].
Three of the SIBLING proteins demonstrated no significant impact on either the fibrillogenesis or denaturation process in the absence of calcium, BSP, OPN, and DMP1-C. All three of these SIBLING proteins had similar kinetics and extent of fibrillogenesis relative to the collagen controls. However, when calcium was added to the system in the presence of DMP1-C there was a significant impact as the denaturation process occurred more slowly. Goldberg et al. have suggested that DMP1 is upregulated in fibromodulin deficient mice in dentin tissue where increased collagen fibrils have also been immunolocalized [31]. When this is combined with the results obtained here, it suggests that DMP1-C may play a role in binding calcium to collagen fibrils to improve the overall structural stability, while not playing a role in the assembly process. It should be noted the DMP1-C has a large number of the negatively charged phosphates that can attract the positively charged calcium ions [36].
Two of the SIBLING proteins also appear to have a negative impact on the native fibrillogenesis process, DSP and DPP. Both DSP and DPP reduced the extent of fibrillogenesis as compared to the collagen controls, although the results were only statistically significant under one SIBLING: collagen ratio for each. Furthermore, DPP had the most significant impacts on fibrillogenesis as indicated by its lag time results. Native DPP has a highly negatively charged state, due to the high number of aspartic acid and phosphorylated serine residues. DPP also had the most significant variation during the denaturation assay, suggesting that the stability of DPP structure is most readily impacted by small temperature increases. However, this variability was significantly stabilized by the presence of calcium ions, likely due to the strong binding capacity of DPP for calcium ions [11, 41, 42]. This stability is a result of the formation of additional β-sheet like secondary structure, not found in the native secondary structure, which are formed in the presence of calcium ions [43]. Furthermore, it may be possible to replicate this improved stability with additional di-cation species such as magnesium, which have been used as calcium analogues in DPP precipitation studies [18]. While the turbidimetric responses were smoothed due to the interaction of calcium and DPP, the impact of calcium on the fibrillogenesis and denaturation process was similar to that of the collagen controls with shorter lag and denaturation times. Conversely, in the presence of calcium DSP had a slower denaturation process, similar to that seen with DMP1-C. This is interesting because Verdelis et al. found that Dspp-null mice had greater collagen maturity, which would seem to contrast this finding that the collagen was less likely to denature in the presence of DSP and calcium [21]. However, it is possible that this bioactivity is replicated by another SIBLING protein like DMP1-C in the DSPP null mouse studies. For example, it has been suggested by Qin et al that subdomains of DMP1 and DSPP appear to have similar biological functions [37]. Furthermore, DSPP and both of its subdomains DSP and DPP have recently been immunolocalized in a number of soft tissues including salivary glands, kidney, and cartilage suggesting one or both of the subdomains play a biological role in soft tissues [44]. Another possibility that cannot be eliminated based on the results in this study is that the thermal denaturation process is mediated through different mechanisms than the naturally occurring enzymatic degradation pathways.
The final group of SIBLING proteins that were evaluated were DMP1-PG and DSP-PG. These are important variants to consider because previous work has shown that PG side chains can play a role in enhancing collagen fibrillogenesis [34]. However, it is important to point out that Kvis et al. concluded that only chondroitin-4, 6-sulfate showed an impact on the fibrillogenesis process, while these two SIBLING variants both contain chondroitin-4-sulfate side chains. The results obtained in this study suggest that the DSP-PG did play a role in slowing down the assembly of collagen fibrils. Additionally, DSP-PG reduced the extent of fibrillogenesis as compared to the collagen controls at the 1:16 and 1:40 SIBLING:collagen ratios (0.10 mg/mL and 0.25 mg/mL collagen concentrations, respectively). Furthermore, the PG modification had a significantly different reduction in the assembly kinetics as compared to the native DSP protein, suggesting that this PG side chain does influence the fibrillogenesis process directly. This is in contrast to the conclusions drawn by Kvist et al [34]. At the same time, the DMP1-PG did not have a significant impact on the fibrillogenesis process, and this may be due to the presence of only one chondroitin-4-sulfate side chain as compared to the two found in DSP-PG [6, 9, 37]. The final observation of interest was seen in the denaturation assays in the calcium supplemented buffers in the presence of both PG variants. Interestingly, these were the only two instances throughout the entire investigation where the presence of calcium ions did not influence the fibrillogenesis or denaturation process. One possible explanation is the sequestration of calcium in the chondroitin-4-sulfate side chains, thereby limiting its impact on the denaturation process. It is possible that this beneficial response can be replicated in a bone tissue engineering scaffold through either an in vivo or in vitro response. In the in vitro preparation of a tissue engineering scaffold, this beneficial response may be replicated by exposing bound DMP1-PG or DSP-PG to a calcium solution in advance of implantation. Alternatively, the sequestration of naturally occurring calcium ions typically found in the in vivo environment of developing bone by DMP1-PG or DSP-PG at the interface of an implant may also lead to this beneficial response. The best approach for incorporating this improvement in a bone tissue scaffold will become more evident with the completion of on-going mechanistic studies.
5. Conclusions
The SIBLING family of proteins have long been investigated for their biological role in mineralized tissues, especially their role in the biomineralization process. Their role in collagen fibrillogenesis in developing bone has been less well understood and this investigation was the first to complete a comparison of the influence many of the SIBLING family members. Based on the results obtained, it was concluded that both DSP and DPP play a role in reducing the kinetics of the natural fibrillogenesis process even though the overall extent of fibrillogenesis was not significantly different. This suggests these two proteins may play a role in regulating the fibrillogenesis process during bone formation. The results also suggest that calcium typically acts as a catalyst, speeding up both the fibrillogenesis and denaturation processes. However, this effect is counterbalanced by both DMP1-C and DSP, which appear to play a role in stabilizing collagen fibrils in the presence of calcium as indicated by the longer denaturation time. This insight has been used to guide on-going, detailed mechanistic studies focused on these individual proteins. Furthermore, the results presented in this work demonstrate that individual SIBLING proteins may play a significant role in collagen assembly and stability in the in vivo environment, presenting an opportunity to better understand native bone development and design tissue engineering scaffolds that facilitate better host integration.
Supplementary Material
7. Acknowledgements
This research was supported in part by the University of Missouri College of Engineering (MTB) and by the National Institute of Health through grant R01DE022549 (CQ).
Footnotes
6. Supplementary Information
Images of SDS-PAGE gels for all purified and isolated proteins can be found in the Supplementary Information File. This information is available via the Internet at http://www.tandfonline.com.
8. References
- [1].Gokhale JA, Boskey AL, Robey PG, The Biochemistry of Bone, in: Marcus R, Felman D, Kelsey J (Eds.), Osteoporosis, Academic Press, San Diego, 2001, pp. 107–188. [Google Scholar]
- [2].Robling AG, Castillo AB, Turner CH, Biomechanical and Molecular Regulatio of Bone Remodeling, Annual Review in Biomedical Engineering 8(1) (2006) 455–498. [DOI] [PubMed] [Google Scholar]
- [3].Ratner BD, Bryant SJ, Biomaterials: Where We Have Been and Where We Are Going, Annual Review in Biomedical Engineering 6(1) (2004) 41–75. [DOI] [PubMed] [Google Scholar]
- [4].Kohn J, Langer R, Bioresorbable and Bioerodible Materials, in: Ratner BD, Hoffman F AS.Schoen J, Lemons JE (Eds.), Biomaterials Science: An Introduction to Materials in Medicine, Academic Press, San Diego, 1996, pp. 64–73. [Google Scholar]
- [5].Maciejewska I, Cowan C, Svoboda K, Butler WT, D’Souza R, Qin C, The NH2-terminal and COOH-terminal Fragments of Dentin Matrix Protein 1 (DMP1) Localize Differently in the Compartments of Dentin and Growth Plate of Bone, Journal of Histochemistry & Cytochemistry 57(2) (2008) 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL, Different Forms of DMP1 Play Distinct Roles in Mineralization, Journal of Dental Research 89(4) (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ, Studies of the DMP1 57-kDa Functional Domain both in vivo and in vitro, Cells Tissues Organs 189(1–4) (2009) 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT, Dentin Phosphoprotein and Dentin Sialoprotein Are Cleavage Products Expressed from a Single Transcript Coded by a Gene on Human Chromosome 4: DENTIN PHOSPHOPROTEIN DNA SEQUENCE DETERMINATION, Journal of Biological Chemistry 272(2) (1997) 835–842. [DOI] [PubMed] [Google Scholar]
- [9].Zhu Q, Sun Y, Prasad M, Wang X, Yamoah AK, Li Y, Feng J, Qin C, Glycosaminoglycan Chain of Dentin Sialoprotein Proteoglycan, Journal of Dental Research 89(8) (2010) 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].LeGeros RZ, Calcium Phosphate-Based Osteoinductive Materials, Chemical Reviews 108(11) (2008) 4742–4753. [DOI] [PubMed] [Google Scholar]
- [11].George A, Veis A, Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition, Chemical Reviews 108(11) (2008) 4670–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Qin C, Baba O, Butler WT, Post-Translational Modifications of SIBLING Proteins and Their Roles in Osteogenesis and Dentinogenesis, Critical Reviews in Oral Biology and Medicine 15(3) (2004) 126–136. [DOI] [PubMed] [Google Scholar]
- [13].Chen J, McCulloch CAG, Sodek J, Bone sialoprotein in developing porcine dental tissues: Cellular expression and comparison of tissue localization with osteopontin and osteonectin, Archives of Oral Biology 38(3) (1993) 241–249. [DOI] [PubMed] [Google Scholar]
- [14].Ganss B, Kim RH, Sodek J, Bone Sialoprotein, Critical Reviews in Oral Biology and Medicine 10(1) (1999) 79–98. [DOI] [PubMed] [Google Scholar]
- [15].Qin C, Brunn JC, Jones J, George A, Ramachadran A, Gorski JP, Butler WT, A Comparative Study of Sialic Acid-rich Proteins in Rat Bone and Dentin, European Journal of Oral Sciences 109(2) (2001) 133–141. [DOI] [PubMed] [Google Scholar]
- [16].Bernards MT, Qin C, Jiang S, MC3T3-E1 Cell Adhesion to Hydroxyapatite with Adsorbed Bone Sialoprotein, Bone Osteopontin, and Bovine Serum Albumin, Colloids and Surfaces B: Biointerfaces 64(2) (2008) 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bernards MT, Qin C, Ratner BD, Jiang S, Adhesion of MC3T3-E1 Cells to Bone Sialoprotein and Bone Osteopontin Specifically Bound to Collagen I, Journal of Biomedical Materials Research, Part A 86A(3) (2008) 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Butler WT, Ritchie H, The nature and functional significance of dentin extracellular matrix proteins, The International Journal of Developmental Biology 39(1) (1995) 169–179. [PubMed] [Google Scholar]
- [19].Prasad M, Butler WT, Chunlin Q, Dentin sialophosphoprotein in biomineralization, Connective Tissue Research 51(5) (2010) 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sodek J, Ganss B, McKee MD, Osteopontin, Critical Reviews in Oral Biology and Medicine 11(3) (2000) 279–303. [DOI] [PubMed] [Google Scholar]
- [21].Verdelis K, Ling Y, Sreenath T, Haruyama N, MacDougall M, van der Meulen MCH, Lukashova L, Spevak L, Kulkarni AB, Boskey AL, DSPP effects on in vivo bone mineralization, Bone 43(6) (2008) 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zurick KM, Qin C, Bernards MT, Adhesion of MC3T3-E1 Cells Bound to Dentin Phosphoprotein Specifically Bound to Collagen Type I, Journal of Biomedical Materials Research Part A 100A(9) (2012) 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zurick KM, Qin C, Bernards MT, Mineralization Induction Effects of Osteopontin, Bone Sialoprotein, and Dentin Phosphoprotein on a Biomimetic Collagen Substrate, Journal of Biomedical Materials Research Part A 101A(6) (2013) 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evans HJ, Sullivan CE, Piez KA, The resolution of Ascaris cuticle collagen into three chain types, Biochemistry 15(7) (1976) 1435–1439. [DOI] [PubMed] [Google Scholar]
- [25].Gelman RA, Poppke DC, Piez KA, Collagen fibril formation in vitro. The role of the nonhelical terminal regions, Journal of Biological Chemistry 254(22) (1979) 11741–11745. [PubMed] [Google Scholar]
- [26].Wood GC, Keech MK, The formation of fibrils from collagen solutions 1. The effect of experimental conditions: kinetic and electron-microscope studies, Biochemical Journal 75(3) (1960) 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gelman RA, Williams BR, Piez KA, Collagen Fibril Formation Evidence for a Multistep Process, Journal of Biological Chemistry 254(1) (1979) 180–186. [PubMed] [Google Scholar]
- [28].Williams BR, Gelman RA, Poppke DC, Piez KA, Collagen Fibril Formation Optimal In Vitro Conditions and Preliminary Kinetic Results, Journal of Biological Chemistry 253(18) (1978) 6578–6585. [PubMed] [Google Scholar]
- [29].Jiang F, Hörber H, Howard J, Müller DJ, Assembly of collagen into microribbons: effects of pH and electrolytes, Journal of Structural Biology 148(3) (2004) 268–278. [DOI] [PubMed] [Google Scholar]
- [30].Halasz K, Kassner A, Morgelin M, Heinegard D, COMP Acts as a Catalyst in Collagen Fibrillogenesis, Journal of Biological Chemistry 282(43) (2007) 31166–31173. [DOI] [PubMed] [Google Scholar]
- [31].Goldberg M, Septier D, Oldberg Å, Young MF, Ameye LG, Fibromodulin-deficient Mice Display Impaired Collagen Fibrillogenesis in Predentin as Well as Altered Dentin Mineralization and Enamel Formation, Journal of Histochemistry & Cytochemistry 54(5) (2006) 525–537. [DOI] [PubMed] [Google Scholar]
- [32].Hansen U, Platz N, Becker A, Bruckner P, Paulsson M, Zaucke F, A secreted variant of cartilage oligomeric matrix protein carrying a chondrodysplasia-causing mutation (p.H587R) disrupts collagen fibrillogenesis, Arthritis & Rheumatism 63(1) (2011) 159–167. [DOI] [PubMed] [Google Scholar]
- [33].Mochida Y, Parisuthiman D, Pornprasertsuk-Damrongsri S, Atsawasuwan P, Sricholpech M, Boskey AL, Yamauchi M, Decorin modulates collagen matrix assembly and mineralization, Matrix Biology 28(1) (2009) 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszódi A, Fässler R, Sasaki T, Timpl R, Aspberg A, Chondroitin Sulfate Perlecan Enhances Collagen Fibril Formation: IMPLICATIONS FOR PERLECAN CHONDRODYSPLASIAS, Journal of Biological Chemistry 281(44) (2006) 33127–33139. [DOI] [PubMed] [Google Scholar]
- [35].Prince CW, Oosawa T, Butler WT, Tomana M, Bhown AS, Bhown M, Schrohenloher RE, Isolation, Characterization, and Biosynthesis of a Phosphorylated Glycoprotein from Rat Bone, Journal of Biological Chemistry 262(6) (1987) 2900–2907. [PubMed] [Google Scholar]
- [36].Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT, Evidence for the Proteolytic Processing of Dentin Matrix Protein 1: IDENTIFICATION AND CHARACTERIZATION OF PROCESSED FRAGMENTS AND CLEAVAGE SITES, Journal of Biological Chemistry 278(36) (2003) 34700–34708. [DOI] [PubMed] [Google Scholar]
- [37].Qin C, Huang B, Wygant JN, McIntyre BW, McDonald CH, Cook RG, Butler WT, A Chondroitin Sulfate Chain Attached to the Bone Dentin Matrix Protein 1 NH2-Terminal Fragment, Journal of Biological Chemistry 281(12) (2006) 8034–8040. [DOI] [PubMed] [Google Scholar]
- [38].Qin C, Cook RG, Orkiszewski RS, Butler WT, Identification and Characterization of the Carboxyl-terminal Region of Rat Dentin Sialoprotein, Journal of Biological Chemistry 276(2) (2001) 904–909. [DOI] [PubMed] [Google Scholar]
- [39].Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, Wygant J, Butler WT, Qin C, Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone, European Journal of Oral Sciences 116(2) (2008) 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT, Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein, European Journal of Oral Sciences 111(3) (2003) 235–242. [DOI] [PubMed] [Google Scholar]
- [41].Milan AM, Sugars RV, Embery G, Waddington RJ, Adsorption and interactions of dentine phosphoprotein with hydroxyapatite and collagen, European Journal of Oral Sciences 114(3) (2006) 223–231. [DOI] [PubMed] [Google Scholar]
- [42].Yamakoshi Y, Dentin Sialophosphoprotein (DSPP) and dentin, Journal of Oral Biosciences 50(1) (2008) 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Veis A, Mineral-Matrix Interactions in Bone and Dentin, Journal of Bone and Mineral Research 8(S2) (1993) S493–S497. [DOI] [PubMed] [Google Scholar]
- [44].Prasad M, Zhu Q, Sun Y, Wang X, Kulkarni A, Boskey A, Feng JQ, Qin C, Expression of Dentin Sialophosphoprotein in Non-mineralized Tissues, Journal of Histochemistry & Cytochemistry 59(11) (2011) 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.