Supplemental Digital Content is available in the text
Keywords: early enteral nutrition, meta-analysis, severe acute pancreatitis
Abstract
Background:
Whether to conduct enteral nutrition in patients with severe acute pancreatitis (SAP) during the active phase of intestinal stress or to feed during remission remains controversial. This study was aimed to evaluate the efficacy and safety of enteral nutrition within 48 hours after admission in the patients with SAP or predicted severe acute pancreatitis (pSAP).
Methods:
We searched PubMed, EMBASE, Web of Science, and the Cochrane Library before December 2017. Randomized controlled trials of early enteral nutrition (starting within 48 hours after admission) versus late enteral nutrition or total parental nutrition in severe acute pancreatitis or predicted severe acute pancreatitis were selected.
Results:
Ten randomized controlled trials containing 1051 patients were included. Comparing early enteral nutrition to late enteral nutrition or total parental nutrition in SAP or pSAP, the pooled risk ratios were 0.53 (95% confidence interval [CI] 0.35–0.81, P = .003) for mortality, 0.58 (95% CI 0.43–0.77, P = .0002) for multiple organ failure (MOF), 0.50 (95% CI 0.33–0.75, P = .0008) for operative intervention, 0.75 (95% CI 0.61–0.93, P = .009) for systemic infection, 0.42 (95% CI 0.26–0.69, P = .0005) for local septic complications, 0.84 (95% CI 0.74–0.96, P = .01) for gastrointestinal symptoms. 0.87 (95% CI 0.74–1.02, P = .08) for systemic inflammatory response syndrome (SIRS), and 1.24 (95% CI 0.66–2.31, P = .50) for other local complications.
Conclusions:
Enteral nutrition within 48 hours after admission is efficient and safe for the patients with SAP or pSAP.
1. Introduction
Severe acute pancreatitis (SAP) is one of the devastating diseases leading to intensive care unit (ICU) admission.[1] The high mortality is due to the serious complications including systemic inflammatory response syndrome (SIRS) and multiple organ failure (MOF).[2] Animal and human studies[3,4] have demonstrated that the damage of intestinal barrier function accelerates the development of local and systemic infectious complications. In the early stage of SAP, the intestinal permeability has been significantly increased, which results in the translocation of inflammatory mediators and toxic products.[5] Furthermore, the gut microbiota gets the chance to the systemic circulation through the damaged intestinal epithelial cells. As a consequence, the sepsis or infected pancreatic necrosis occur in the early stage of SAP.[6] So, the maintenance of intestinal barrier function in the early stage is critical for the mortality and prognosis.
In SAP, the inflammatory response induced by necrosis or secondary infection leads to the increase of caloric requirement and loss of mass protein. This contributes to the nutritional deterioration and negative nitrogen balance, which further lead to the damage of function and structure of vital organs.[7] So, early nutritional management plays an important role in the patients with SAP. Parenteral nutrition (PN) has been regarded as the standard care for providing nutrients and can avoid pancreatic stimulation. But, PN may lead to intestinal atrophy and attenuate the intestinal barrier function.[8]
Enteral nutrition (EN) is found to be better at maintaining the function and structure of intestinal mucosa.[9] For avoiding the pancreatic stimulation, the route of EN is delivered beyond the Treitz’ ligament, which results in minimal or negligible stimulation.[10] Some reviews have suggested that early EN was associated with a reduction in mortality, MOF and infections compared with the late EN or PN in the patients with acute pancreatitis.[11–13] But the above studies were not strictly stratified on the basis of the severity of disease, which potentially led to selection bias and may misguide the therapy of SAP. Despite these data, whether to conduct EN in patients with SAP during the active phase of intestinal stress or to feed during remission remains controversial. Recent trials have yielded mixed results and the guidelines are vague and contradictory.[14–16] Therefore, we attempt to rigorously design and comprehensively reevaluate the efficacy and safety of early EN (starting within 48 hours after hospital admission) in the patients with SAP or predicted SAP.
2. Methods
2.1. Search strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[17] We independently searched PubMed, EMBASE, Web of Science, and the Cochrane Library before December 2017 for relevant studies. The detailed search strategy was in the appendix (Supplemental Digital Content, which illustrates the detailed search strategy). No restrictions were placed on language.
2.2. Selecting criteria
Studies included in this meta-analysis fulfilled the following criteria:
-
1)
patients diagnosed with SAP or predicted SAP.
-
2)
Intervention: EN initiated within 48 hours after admission, controlled by EN outside 48 hours or PN.
-
3)
randomized clinical trials (RCT); Studies were excluded if they were:
-
1)
not RCT;
-
2)
patients <18 years of age;
-
3)
the undefined timing of EN initiated within 48 hours after admission;
-
4)
not reporting detailed information on required clinical outcomes.
-
1)
2.3. Types of outcome measures
The clinical outcomes are the following:
-
(1)
mortality;
-
(2)
MOF;
-
(3)
systemic inflammatory response syndrome (SIRS);
-
(4)
operative intervention;
-
(5)
systemic infection (septicemia, urinary tract infection, and pneumonia);
-
(6)
local septic complications (pancreatic abscess and infected pancreatic necrosis);
-
(7)
other local complications (fluid collection, pseudocyst, and fistula);
-
(8)
gastrointestinal symptoms (nausea, vomiting, and diarrhea).
2.4. Data extraction and management
Two independent reviewers used a standard form for data abstraction. The extracted data were cross-checked by the reviewers. The extracted information included: first author, publication year, country of origin, study design, patient demographics, sample size, type of intervention, and outcomes. Disagreements between reviewers were resolved by the third reviewer.
2.5. Assessment of risk of bias in included studies
The quality of the included randomized clinical trials (RCTs) was assessed according to the methodological criteria of the Cochrane Handbook for Systematic Reviews of Interventions. We assessed the risk of bias through seven domains, including allocation sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, management of incomplete outcome data, selective outcome reporting, and other potential sources of bias. The publication bias was evaluated by funnel plots if ten or more studies were included in an outcome. Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to create a summary of findings table and assess the quality of evidence.[18]
2.6. Statistical analyses
All statistical analyses were performed using RevMan Software (version 5.3) and STATA software (version 12.0). Binary variables were combined to estimate the pooled risk ratio (RR) with 95% confidence intervals (CIs). The I2 test was used to measure statistical heterogeneity among the included studies and P < .1 or I2 > 50% indicated significant heterogeneity. If significant heterogeneity was not observed, a fixed-effects model was used to make estimates, otherwise, a random-effects model was applied to statistical analysis. A P value < .05 was considered statistically significant.
3. Results
3.1. Search results
A total of 1424 articles were found from PubMed, EMBASE, Web of Science, and the Cochrane Library. The flow diagram for searching and screening of eligible studies is shown in Figure 1. Finally, 10 RCTs including enrolled 1051 patients were included in this meta-analysis.
Figure 1.
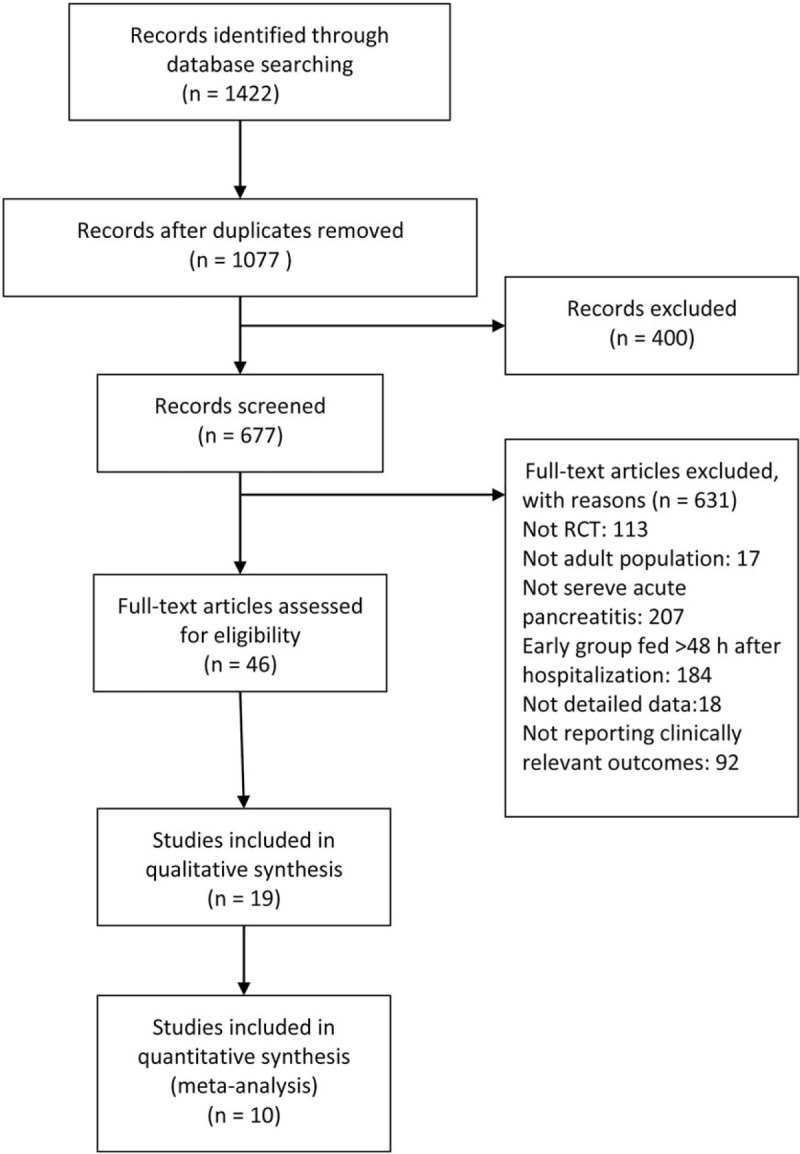
PRISMA flow diagram of study selection process. RCT = randomized controlled trials.
3.2. Characteristics of trials included
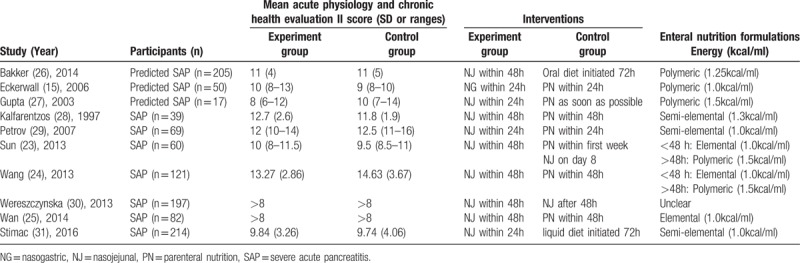
We included ten randomized controlled trials of published studies. The 5 of 10 RCTs were the patients with predicted SAP. Predicted SAP was defined as Acute Physiology and Chronic Health Evaluation (APACHE) II score ≥ 8 or C-reactive protein (CRP) levels ≥ 150 mg/L. Countries of publication were diversity, three from China,[19–21] one from the Netherlands,[22] Sweden,[23] the UK,[24] Greece,[25] Russia,[26] Poland,[27] Croatia,[28] and all were in English. All included studies were full-text papers. More details of the included studies were illustrated in Table 1.
Table 1.
Characteristics of included studies.
3.3. Risk of bias in included studies
Among 10 included RCTs, 6 studies provided complete data in allocation sequence generation. Four studies did not specifically describe the method of allocation sequence generation. Allocation concealment was adequate in 9 studies. Only 1 study did not provide enough information regarding the use of allocation concealment method. None of the studies provided enough information regarding the use of the blinding method (Figs. 2 and 3). No more than 10 studies were included in an outcome, so the publication bias was not evaluated by funnel plots. If the asymmetry were obvious in this minimum number of studies, the assessment of funnel plot asymmetry would have low power to differentiate real asymmetry.[29]
Figure 2.
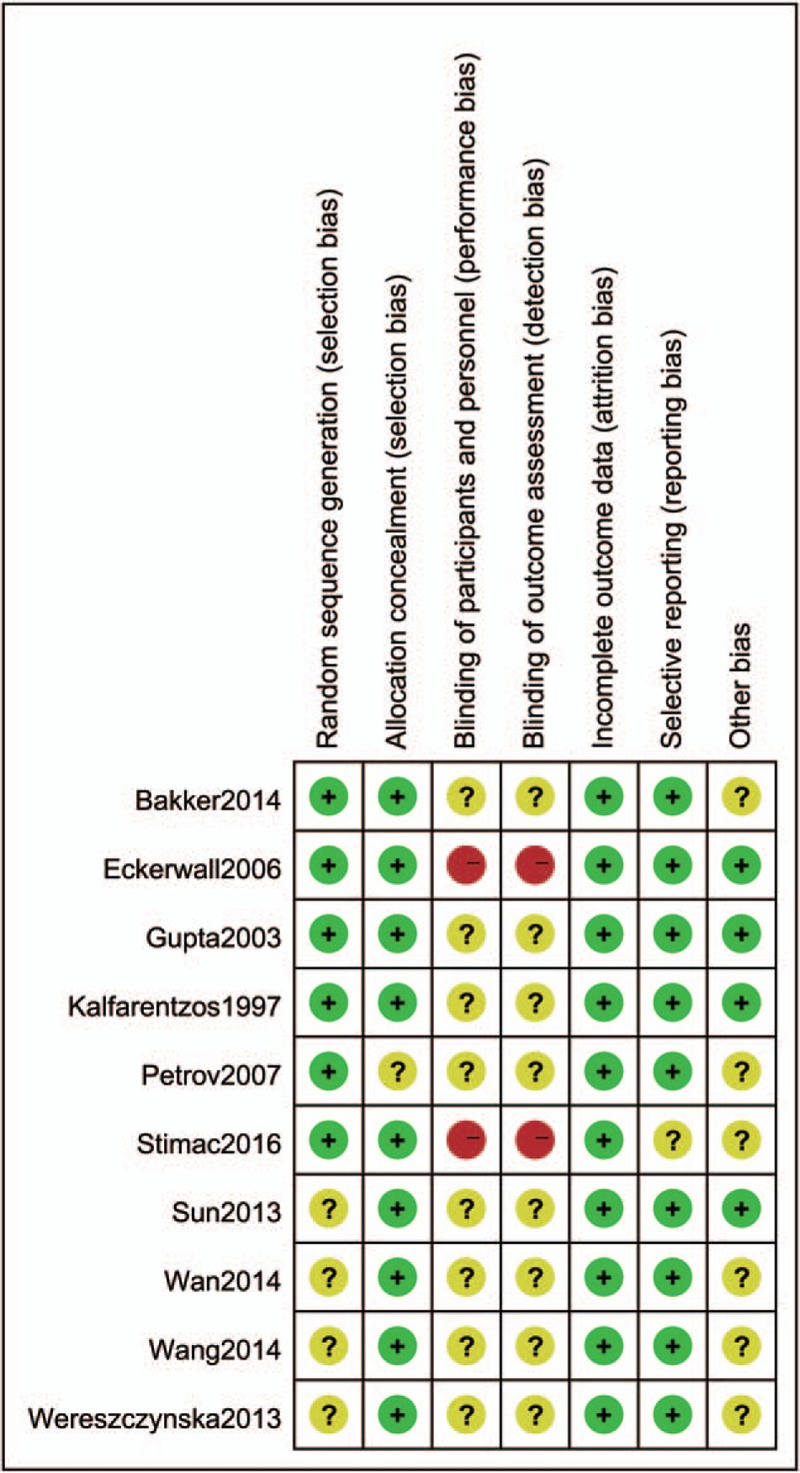
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study. “+”: low risk of bias; “?”: unclear risk of bias; or “−“: high risk of bias.
Figure 3.
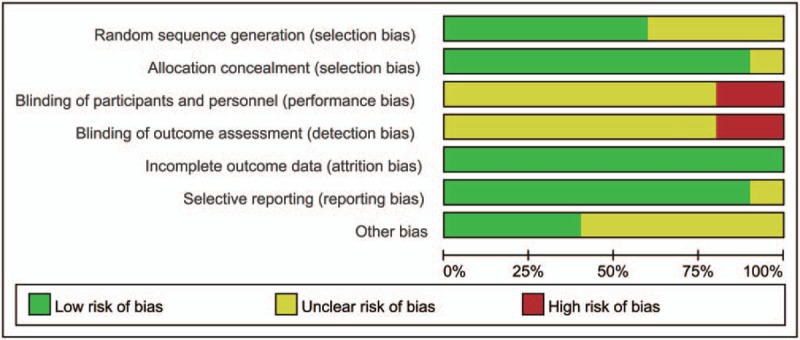
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
3.4. Effects of interventions
3.4.1. Mortality
This outcome was reported in nine studies with 969 patients. The results were homogenous and thus a fixed-effects model was used. After aggregating the data, a significant reduction was observed in the early EN group compared with the late EN or PN group (RR = 0.53, 95% CI 0.35–0.81, P = 0.003, I2 = 44%). (Fig. 4)
Figure 4.

Forest plot of the effect of early enteral nutrition on mortality, multiple organ failure and systemic inflammatory response syndrome in patients with severe acute pancreatitis or predicted severe acute pancreatitis.
3.4.2. Multiple organ failure (MOF)
Eight studies collected data for this outcome with 931 patients. Our results showed that early EN was associated with a significant reduction in the rate of MOF compared with the late EN or PN group (RR = 0.58, 95% CI 0.43–0.77, P = 0.0002, I2 = 0%) (Fig. 4).
3.4.3. Systemic inflammatory response syndrome (SIRS)
Four studies assessed this outcome including a total of 676 patients. This outcome showed that there was a tendency of decreased SIRS in early EN, but the difference was not significant (RR = 0.87 95% CI 0.74–1.02, P = 0.08, I2 = 27%) (Fig. 4).
3.4.4. Operative intervention
This outcome was reported in 7 studies with 643 patients. The results showed that early EN was significantly associated with lower risk of operative intervention than late EN or PN (RR = 0.50, 95% CI 0.33–0.75, P = 0.0008, I2 = 27%) (Fig. 5).
Figure 5.
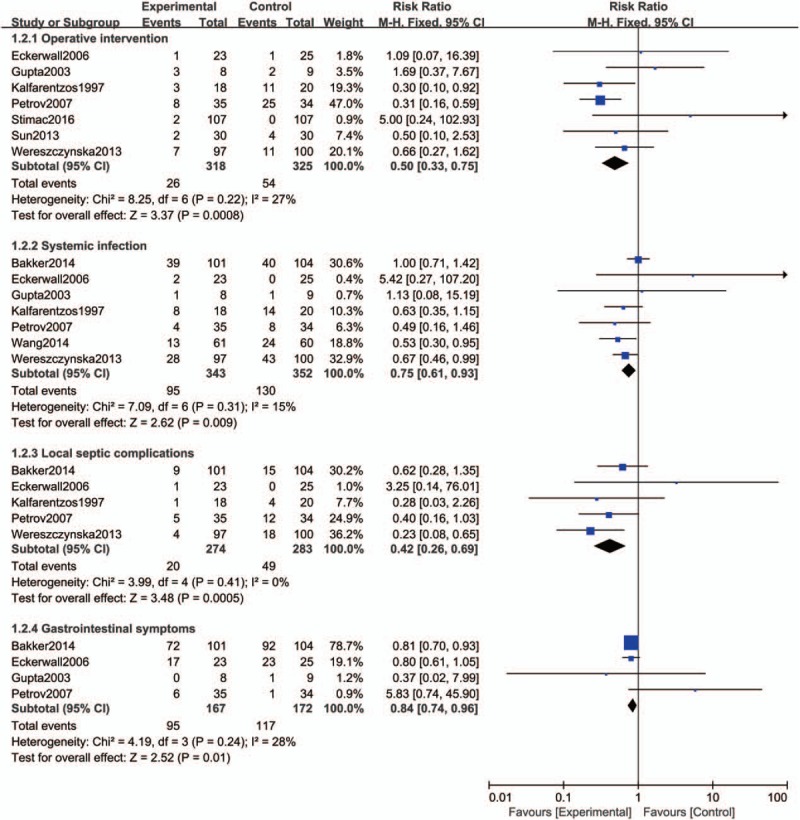
Forest plot of the effect of early enteral nutrition on operative intervention, systemic infection, local septic complications and gastrointestinal symptoms in patients with severe acute pancreatitis or predicted severe acute pancreatitis.
3.4.5. Systemic infection
This outcome was reported in 7 studies with a total of 695 patients. In this outcome, early EN displayed advantages over late EN or PN in reducing the rate of systemic infection (RR = 0.75, 95% CI 0.61–0.93, P = 0.009, I2 = 15%) (Fig. 5).
3.4.6. Local septic complications
Five studies assessed this outcome including a total of 557 patients. The outcome showed that early EN group was significantly associated with lower risk of local septic complications than late EN or PN group (RR = 0.42, 95% CI 0.26–0.69, P = 0.0005, I2 = 0%) (Fig. 5).
3.4.7. Gastrointestinal symptoms
Four studies reported the outcome of gastrointestinal symptoms with 339 patients. The result showed that early EN group was significantly associated with lower risk of other local complications than late EN or PN group (RR = 0.84, 95% CI 0.74–0.96, P = 0.01, I2 = 28%) (Fig. 5).
3.4.8. Other local complications
Four studies collected data for this outcome with 497 patients. The result showed that there was no significant reduction in the rate of other local complications when comparing the early EN group with the late EN or PN group (RR = 1.24, 95% CI 0.66–2.31, P = 0.50), and the significant heterogeneity was detected (I2 = 67%, P = 0.03), so a random-effects model was used (Fig. 6).
Figure 6.
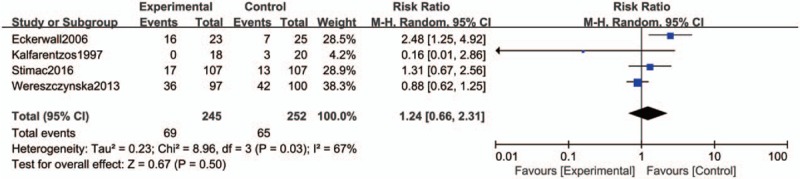
Forest plot of the effect of early enteral nutrition on other local complications in patients with severe acute pancreatitis or predicted severe acute pancreatitis.
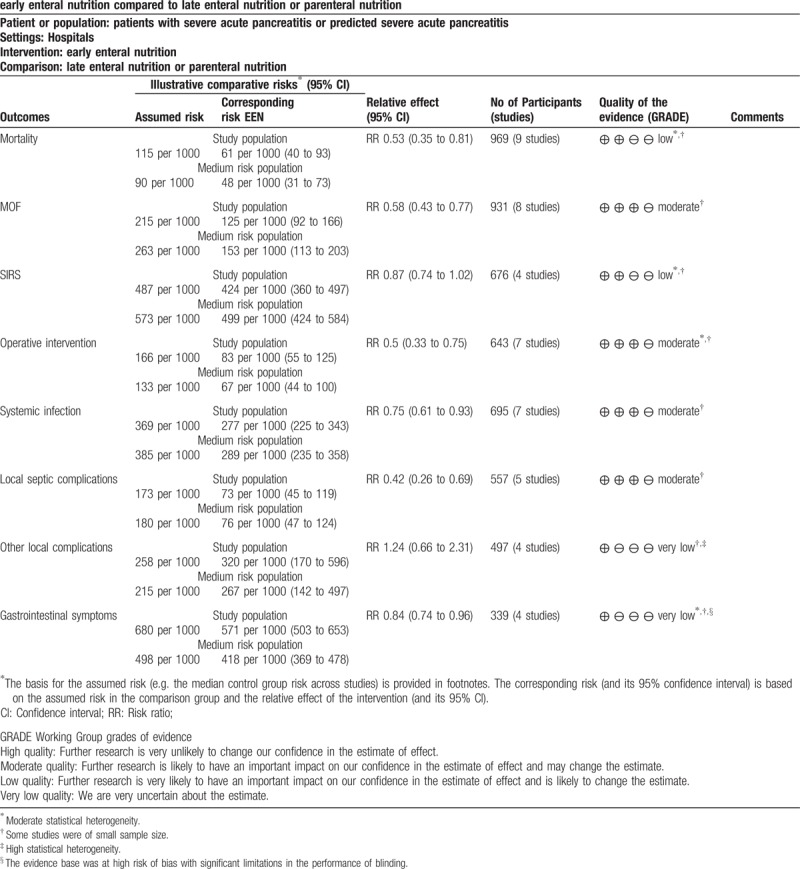
3.5. Summary of findings and quality of evidence
The summary of findings and the GRADE recommendations were illustrated in Table 2.
Table 2.
Summary of findings with GRADE recommendations.
4. Discussion
This systematic review evaluated ten RCTs and included 1424 patients with SAP or predicted SAP. The results showed that early EN (starting within 48 hours after admission) significantly reduced the mortality, MOF, operative intervention, systemic infections, local septic complications and gastrointestinal symptoms compared with late EN or PN. In addition, a decreasing trend is found in SIRS, but not significant. Meanwhile, no significant difference is also observed in the risk of other local complications. In GRADE recommendations, the quality of the evidence was moderate for MOF, operative intervention, systemic infections, and local septic complications, low for mortality and SIRS, and very low for other local complications and gastrointestinal symptoms.
The intestinal barrier function was described long ago as being involved in SAP, playing an active role in the progression of MOF and infections complications.[30] The early standard EN, even small amounts of EN, may improve the intestinal barrier function through affecting the intestinal permeability, bacterial translocation, and immunocompetent cells.[31–33] Three previous meta-analyses have addressed the issue of early EN (starting within 48 hours after hospital admission). Feng et al[11] included 6 trails with 1007 patients in acute pancreatitis (AP), of which 5 trails with 972 patients were in SAP or predicted SAP. They found that EN within 48 hours was related to a significant reduction in MOF compared to late EN or PN in AP. A decreasing trend is also found in mortality but not significant. We disagree with Feng et al[11] in the fact that they included 2 retrospective studies with 212 participants, which existed the high risk of bias, and they did not make a stratified analysis based on the severity of disease, so the conclusion could not truly reflect the validity of early EN in SAP or predicted SAP. Li et al[12] included eleven trails in AP, of which eight trails with 616 patients were in SAP or predicted SAP. They found a significant reduction in total infection complications, pancreatic infection complications and organ failure in EN within 48 hours compared to the late EN or PN group in SAP or predicted SAP. A significant reduction of mortality was also observed, but they did not make a stratified analysis in the indicator of mortality. They also included two retrospective studies with 327 patients in a subgroup of SAP or predicted SAP and these 2 studies were published in abstract form only, which existed great risk of bias. Vaughn et al[34] included eleven trails with 948 patients and indicated that EN within 48 hours did not seem to increase gastrointestinal symptoms, but they mainly focused on patients with mild to moderate AP and had limited data of SAP or predicted.
This meta-analysis had a number of limitations. First, some included RCTs were small in size and single center. The blinding was not addressed in all included RCTs, but we acknowledged that the blinding of different feeding routes was impossible, even to radiologists, for example, the nasojejunal tube could be found on radiological images. Second, the feeding routes of EN were different. In our study, the feeding route of one RCT was nasogastric feeding and the others are nasojejunal feeding. But, previous RCT studies indicated that between gastric and jejunal feeding was not a significant difference in SAP.[35–37] A meta-analysis also showed that no significant difference was detected in digestive complications.[38] Meanwhile, in our study, the EN formulations were different. Six RCTs were (semi) elemental formulation, three were polymeric formulation and one was unclear. But, a meta-analysis indicated that the risk of infectious complications and mortality did not significantly differ between (semi) elemental and polymeric formulation in acute pancreatitis.[39] Third, the predicted SAP in included RCTs was defined as APACHE II score ≥ 8 or CRP levels ≥ 150 mg/L, except two RCTs defined as APACHE II score ≥ 6.[24,28] In these two study, the mean of APACHE II score was in excess of 8, so this bias may not significantly influence the definition of predicted SAP. Fourth, the intervention of the control group was not consistent in all RCTs. The intervention of four RCTs was late EN in the control group and the others were PN. But, within 48 hours after admission, our study promised the “gut rest” in the control group, and the “gut rousing” in the experimental group.[40] Nevertheless, within the constraints in some included trials, this meta-analysis suggested that EN within 48 hours after admission may be beneficial to clinical outcomes in patients with SAP or predicted SAP.
5. Conclusions
Early EN (starting within 48 hours after admission) significantly decreased the risk of MOF, operative intervention, systemic infections, and local septic complications based on a moderate quality of evidence, mortality with a low quality of evidence and gastrointestinal symptoms with the very low quality of evidence. In addition, early EN did not reduce the risk of SIRS based on a low quality of evidence and the other local complications with very low quality of evidence. Further, well-designed RCTs are required to explore this topic.
Author contributions
Conceptualization: Jianbo Song, Yilong Zhong.
Data curation: Jianbo Song, Yilong Zhong, Wenxiu Guo, Jie Liu, Yilun Yang.
Formal analysis: Wenxiu Guo, Jie Liu, Yilun Yang.
Project administration: Xiaoguang Lu.
Resources: Xiaoguang Lu.
Software: Xin Kang, Yi Wang, Liying Pei.
Supervision: Xiaoguang Lu.
Writing – original draft: Jianbo Song, Yilong Zhong.
Writing – review & editing: Xiaoguang Lu, Xin Kang, Yi Wang, Wenxiu Guo, Jie Liu, Yilun Yang, Liying Pei.
Supplementary Material
Footnotes
Abbreviations: MOF = multiple organ failure, pSAP = predicted severe acute pancreatitis, SAP = severe acute pancreatitis, SIRS = systemic inflammatory response syndrome.
JS and YZ contributed equally.
Funding/support: This work was supported by grants from the National Science Foundation of China (grant number: 81673801, 81473512)
Authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Warren M, McCarthy MS, Roberts PR. Practical application of the revised guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: a case study approach. Nut Clin Pract: Off Publ Am Soc Parent Enter Nutr 2016;31:334–41. [DOI] [PubMed] [Google Scholar]
- [2].Yang R, Tenhunen J, Tonnessen TI. HMGB1 and histones play a significant role in inducing systemic inflammation and multiple organ dysfunctions in severe acute pancreatitis. Int J Inflam 2017;2017: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li HC, Fan XJ, Chen YF, et al. Early prediction of intestinal mucosal barrier function impairment by elevated serum procalcitonin in rats with severe acute pancreatitis. Pancreatol: Off J Int Assoc Pancreatol 2016;16:211–7. [DOI] [PubMed] [Google Scholar]
- [4].Schietroma M, Pessia B, Carlei F, et al. Intestinal permeability and systemic endotoxemia in patients with acute pancreatitis. Ann Ital Chir 2016;87:138–44. [PubMed] [Google Scholar]
- [5].Qiao SF, Lu TJ, Sun JB, et al. Alterations of intestinal immune function and regulatory effects of L-arginine in experimental severe acute pancreatitis rats. World J Gastroenterol 2005;11:6216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schwarz M, Thomsen J, Meyer H, et al. Frequency and time course of pancreatic and extrapancreatic bacterial infection in experimental acute pancreatitis in rats. Surgery 2000;127:427–32. [DOI] [PubMed] [Google Scholar]
- [7].Luo XJ, Peng Y. Enteral nutrition in severe acute pancreatitis. World Chinese J Digest 2014;22:1658–62. [Google Scholar]
- [8].Ekelund M, Kristensson E, Ekelund M, et al. Total parenteral nutrition causes circumferential intestinal atrophy, remodeling of the intestinal wall, and redistribution of eosinophils in the rat gastrointestinal tract. Digest Dis Sci 2007;52:1833–9. [DOI] [PubMed] [Google Scholar]
- [9].Hua Z, Su Y, Huang X, et al. Analysis of risk factors related to gastrointestinal fistula in patients with severe acute pancreatitis: a retrospective study of 344 cases in a single Chinese center. BMC gastroenterology 2017;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Corcoy R, Sanchez JM, Domingo P, et al. Nutrition in the patient with severe acute-pancreatitis. Nutrition (Burbank, Los Angeles County, Calif) 1988;4:269–75. [Google Scholar]
- [11].Feng P, He C, Liao G, et al. Early enteral nutrition versus delayed enteral nutrition in acute pancreatitis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2017;96:e8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li JY, Yu T, Chen GC, et al. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PLoS One 2013;8:64926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qi D, Yu B, Huang J, et al. Meta-analysis of early enteral nutrition provided within 24 hours of admission on clinical outcomes in acute pancreatitis. J Parenter Enter Nutr 2018;1–9. [DOI] [PubMed] [Google Scholar]
- [14].Blaser AR, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017;43:380–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tenner S, Baillie J, DeWitt J, et al. Amican college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108:1400–15. 1416. [DOI] [PubMed] [Google Scholar]
- [16].IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatol: Off J Int Assoc Pancreatol 2013;13(4 Suppl 2):e1–5. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res) 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sun JK, Mu XW, Li WQ, et al. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol 2013;19:917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang G, Wen J, Xu L, et al. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J Surg Res 2013;183:592–7. [DOI] [PubMed] [Google Scholar]
- [21].Wan B, Fu H, Yin J, et al. Efficacy of rhubarb combined with early enteral nutrition for the treatment of severe acute pancreatitis: a randomized controlled trial. Scand J Gastroenterol 2014;49:1375–84. [DOI] [PubMed] [Google Scholar]
- [22].Bakker OJ, Van BS, van Santvoort HC, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. New Eng J Med 2014;371:1983–93. [DOI] [PubMed] [Google Scholar]
- [23].Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg 2006;244:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gupta R, Patel K, Calder PC, et al. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II >6). Pancreatol: Off J Int Assoc Pancreatol 2003;3:406–13. [DOI] [PubMed] [Google Scholar]
- [25].Kalfarentzos DF, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial. Br J Surg 1997;84:1665–9. [PubMed] [Google Scholar]
- [26].Petrov MS, Kukosh MV, Emelyanov NV. A Randomized Controlled Trial of Enteral versus Parenteral Feeding in Patients with Predicted Severe Acute Pancreatitis Shows a Significant Reduction in Mortality and in Infected Pancreatic Complications with Total Enteral Nutrition. Dig Surg 2007;23:336–45. [DOI] [PubMed] [Google Scholar]
- [27].Wereszczynska-Siemiatkowska U, Swidnicka-Siergiejko A, Siemiatkowski A, et al. Early enteral nutrition is superior to delayed enteral nutrition for the prevention of infected necrosis and mortality in acute pancreatitis. Pancreas 2013;42:640–6. [DOI] [PubMed] [Google Scholar]
- [28].Stimac D, Poropat G, Hauser G, et al. Early nasojejunal tube feeding versus nil-by-mouth in acute pancreatitis: A randomized clinical trial. Pancreatol: Off J Int Assoc Pancreatol 2016;16:523–8. [DOI] [PubMed] [Google Scholar]
- [29].AL-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nrtrition for acute pancreatitis. Cochrane database of systematic reviews. 2010. Issue 1. Art. No.: CD002837; 10.1002/14651858.CD002837.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Landahl P, Ansari D, Andersson R. Severe acute pancreatitis: gut barrier failure, systemic inflammatory response, acute lung injury, and the role of the mesenteric lymph. Surg Infect 2015;16:651–6. [DOI] [PubMed] [Google Scholar]
- [31].Roberts KM, Nahikian-Nelms M, Ukleja A, et al. Nutritional aspects of acute pancreatitis. Gastroenterol Clin North Am 2018;47:77–94. [DOI] [PubMed] [Google Scholar]
- [32].Chen J, Wang XP, Liu P, et al. Effects of continuous early enteral nutrition on the gut barrier function in dogs with acute necrotizing pancreatitis. Zhonghua Yi Xue Za Zhi 2004;84:1726–31. [PubMed] [Google Scholar]
- [33].Foitzik T. Pancreatitis and nutrition. Significance of the gastrointestinal tract and nutrition for septic complications. Zentralblatt fur Chirurgie 2001;126:4–9. [DOI] [PubMed] [Google Scholar]
- [34].Vaughn VM, Shuster D, Rogers MAM, et al. Early versus delayed feeding in patients with acute pancreatitis: a systematic review. Ann Internal Med 2017;166:883–92. [DOI] [PubMed] [Google Scholar]
- [35].Singh N, Sharma B, Sharma M, et al. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: a noninferiority randomized controlled trial. Pancreas 2012;41:153–9. [DOI] [PubMed] [Google Scholar]
- [36].Kumar A, Singh N, Prakash S, et al. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. J Clin Gastroenterol 2006;40:431–4. [DOI] [PubMed] [Google Scholar]
- [37].Eatock FC, Chong P, Menezes N, et al. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol 2005;100:432–9. [DOI] [PubMed] [Google Scholar]
- [38].Nally DM, Kelly EG, Clarke M, et al. Nasogastric nutrition is efficacious in severe acute pancreatitis: a systematic review and meta-analysis. Br J Nutr 2014;112:1769–78. [DOI] [PubMed] [Google Scholar]
- [39].Petrov MS, Loveday BP, Pylypchuk RD, et al. Systematic review and meta-analysis of enteral nutrition formulations in acute pancreatitis. Br J Surg 2009;96:1243–52. [DOI] [PubMed] [Google Scholar]
- [40].Petrov MS, Windsor JA. Nutritional management of acute pancreatitis: the concept of ’gut rousing’. Curr Opin Clin Nutr Metabol Care 2013;16:557–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


