Abstract
Despite aggressive multimodal treatment, survival for patients with glioblastoma (GBM) remains dismal. One obstacle to improving patient outcome is the difficulty in delivering adequate therapeutic to the CNS due to the presence of the blood brain barrier (BBB). While direct drug infusion by convection-enhanced delivery (CED) can bypass the BBB and facilitate delivery to intracranial tumors, determining the distribution of delivered therapeutic remains problematic. Image-guidance is a strategy that can optimize the accuracy of therapeutic delivery. Here, we performed an open-label clinical trial in ten pet dogs with spontaneous intracranial tumors to examine the target coverage accuracy of delivering polymeric magnetite nanoparticles (PMNPs) encapsulating temozolomide (TMZ). A modified small animal frame was applied to the head of each subject and PMNPs stereotactically delivered to the center of the tumor. An MRI was obtained immediately post-operatively to examine PMNP distribution and animals followed until death. Nine of ten dogs underwent PMNP infusion without complication. No infusate backflow was observed during any procedure. In 70% of the cases, the infusion accurately targeted the tumor mass as determined by the presence of PMNP signal in the tumor on immediate post-operative MRI. These data suggest that CED of PMNPs carrying TMZ is safe in dogs with intracranial tumors and can lead to nanoparticle distribution in the region of the target. Image-guidance is an important adjunct to CED as distribution is unpredictable with the potential for missed target delivery.
Keywords: Canine, Convection Enhanced Delivery, Glioma, Nanoparticles, Stereotactic, Temozolomide
Introduction
Glioblastoma (GBM) and other isocitrate dehydrogenase (IDH) wildtype gliomas have a very poor prognosis. Treatment of these tumors involves maximal surgical debulking followed by the combination of temozolomide (TMZ) chemotherapy and radiation therapy (RT). Despite the incremental success of this multimodal strategy 1, tumor recurrence is virtually universal. An important reason for recurrence is the survival of migrated tumor cells that lie outside of the resectable tumor mass and the standard RT field. Although chemotherapeutics like TMZ can kill these distant cells, the brain tissue into which tumor cells migrate retains an intact blood brain barrier (BBB) restricting the effectiveness of such systemically administered agents. One technique that can address these distant cells involves direct intracranial injection of therapeutic into the tumor and surrounding infiltrated tissue. Such an approach increases the local concentration of chemotherapeutic that can enhance tumor cell killing without increasing peripheral toxicity.
Multifunctional delivery vehicles such as polymeric nanoparticles are an important class of vector with several advantages for delivery of therapeutics to intracranial tumors. They can carry and protect a variety of payloads, can be functionalized to target specific cells and can incorporate contrast agents such as magnetite to facilitate real-time imaging 2. Our group and others have demonstrated the feasibility of using various polymeric or liposomal formulations for treating experimental glioma 3–7.
Convection-enhanced delivery (CED) is a direct injection technique that uses a hydrostatic pressure gradient to enhance the distribution of injected therapeutics via surgically inserted catheters 8–13. Although clinical trials have not yet shown benefit from the use of CED 14–18, the failures can at least in part be attributed to technical limitations associated with CED and tumor coverage 19. These trials underline the importance of verifying the exact distribution of each injected agent, as delivery can often be unpredictable and might miss the intended target (for a review of previous preclinical and clinical trials see ref 20). When used in conjunction with MRI-traceable vectors, real-time imaging can identify infusate distribution and provide valuable information for subsequent injections. While examination of CED in small animal xenograft models has been good for initial proof-of-principal studies, translation of these results into larger animal models that better represent human tumors is important for each individual vector system.
Spontaneously occurring canine gliomas represent an ideal model system for analysis of image-guided, CED-based therapy. Translational studies in dogs with spontaneous gliomas have the potential to lead to advances in both human and canine oncology care2,21,22. These spontaneous tumors are heterogeneous and relatively large making them ideal for pre-clinical exploration of novel therapeutics. Additionally, canine gliomas have many molecular similarities to their human counterparts 23–26. Finally, like humans, survival in dogs with gliomas is poor and minimally invasive techniques that can avoid extensive surgical resection represent a potential strategy that can improve outcome. Although studies in dogs are uncommon, CED of liposomal nanoparticles loaded with CPT-11 was previously shown to be safe and effective in canines with gliomas 4,27. In this pilot study, ten pet dogs with spontaneous intracerebral tumors deemed to be consistent with glioma on MRI were treated with stereotactically-guided CED of polymeric magnetite nanoparticles (PMNPs) encapsulating TMZ. Following infusion, the dogs underwent standard MRI to evaluate nanoparticle distribution in relation to the tumor.
Methods
Animals
Ten pet dogs (two Boston terriers, two bulldogs, one maltese, one Doberman, one pitbull, one mastiff, one sheltie, and one mixed breed) presented to the Chicago Veterinary Specialty Group after onset of symptoms from the general pet population and were diagnosed with an intracranial mass after undergoing brain MRI. Dogs were enrolled into an open-label, single arm clinical trial examining CED of PMNPs encapsulating TMZ following formal written consent by their owners. Experimental protocols were performed in accordance with the National Institutes of Health laboratory animal guidelines and the Animal Welfare Act. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Nanoparticle Preparation
PMNPs were produced using a proprietary electrohydrodynamic (EHD) technology (LNK Chemsolutions LLC, USA), as described previously 3. Briefly, NPs were constructed using a mixture of functionalized polyethylene glycol (PEG), polylactide (PLA), and polycaprolactone (PCL) and have a diameter of < 100 nm and a slightly negative surface charge (zeta, ζ, potential = −7.75 mV). PMNPs contain 11% superparamagnetic iron oxide (magnetite), a concentration that was previously shown to offer the best balance between polymer content and magnetic susceptibility. The TMZ equivalent concentration within the PMNPs was 5mg/kg/dose and complete TMZ payload release is achieved approximately 20 hours after delivery under physiological conditions 3.
PMNPs were prepared under sterile procedures and were specifically manufactured upon subject enrollment.
Surgical protocol
Following verification of the tumor location by MRI, stereotactic guidance with a modified Knopf small animal frame was used for CED procedures. All surgeries were performed by a board certified veterinary neurosurgeon (M.P.) at the Chicago Veterinary Specialty Group. Briefly, animals were placed under general isoflorane anesthesia and the surgical site shaved and prepared in a standard manner for sterile surgery. Animals were secured in the frame (Fig. 1). Coordinates for infusion were determined based on the pre-operative MRI with the occipital protuberance serving as a fixed fiduciary point for rostral-caudal measurements. The target was set to the center of the tumor, and a visual triangulation planning method from the sagittal, axial, and coronal planes was used to determine the depth of penetration and angulation from the canine’s dorsal sagittal midline. Subsequently, the temporalis muscle was reflected, a small burr hole fashioned directly above the target site and the dura and pia mater punctured using a sterile needle. A sterile step-down catheter, previously described for CED 28, was used for all infusions. The catheter was secured to the stereotactic frame and the construct manually positioned using the Kopf frame instrumentation. Once over the infusion site, the catheter was slowly lowered to the desired depth. No repositioning of the catheter after insertion was performed. Infusion of PMNPs was performed using PEEK tubing and a standard infusion pump (Just Infusion Syringe Pump BS-300, Braintree Scientific). Total injection time was capped at 1 hour and a standard injection rate protocol was followed that involved starting at 0.1 μl/min, escalating to a maximum of 5.0 μl/min within 8 minutes, maintaining 5.0 μl/min for 45 minutes, and decelerating to 0.1μl/min over the final 8 minutes. Using this protocol, all animals received a total PMNP infusion volume of approximately 250 μl. Following completion of infusion, the cannula was slowly removed and the skin closed. An immediate post-operative MRI was performed to evaluate PMNP distribution. The animal was then extubated and recovered in the clinic overnight. In two cases, an additional follow-up MRI was performed.
Fig. 1.
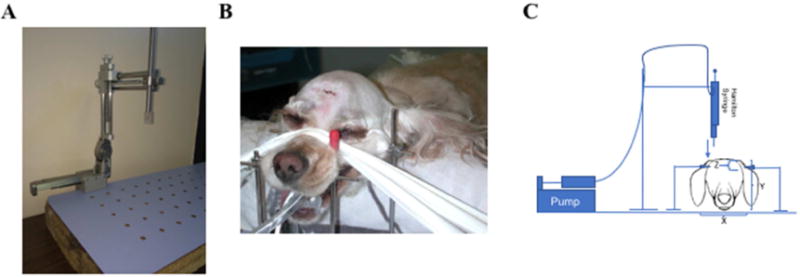
Canine nanoparticle CED System. A. Stereotactic infusion system. B. Photo of dog positioned for surgery for infusion. C. Schematic representation of stereotactic catheter delivery system showing infusion pump, Hamilton syringe, reservoir, frame, and canine subject.
MRI acquisition
Brain MRI scans were acquired using a standardized canine brain protocol. The initial diagnostic scan included T1 weighted, T2 weighted, Fluid-Attenuated Inversion Recovery (FLAIR), gradient echo, and T1 weighted post contrast (gadolinium 0.2 mg/kg IV) sequences. The post-CED scans included the same sequences and a T1 weighted 3D FFE sequence (TR 9.6 ms, TE 4.9 ms, FOV 150 mm × 150 mm, Slice Thickness 3 mm, Slice Gap 0 mm) to evaluate the volume of PMNP distribution.
Neuropathological Examination
For interested families, neuropathological consultation by the Department of Veterinary Biosciences at The Ohio State University was offered after the canine passed away. An excisional wedge procedure was performed on the brain in the region of gross pathology and serial 2 mm sections analyzed.
Results
The average age of the cohort was 8.1 years old with a range of 7 to 9 years (Table 1). While the most common presenting symptom was that of a new onset seizure, two animals had hemiparesis and one patient was stuporous and disoriented. Dogs were brought into the clinic by their owners and underwent an initial diagnostic MRI with and without gadolinium contrast. Once a supratentorial intracranial mass was identified, the animal was enrolled and a formal consent signed. All pre-operative scans were radiographically consistent with a glial neoplasm. Average tumor volume on FLAIR MR sequences was 7.3 cm3 ± 5.2 cm3.
Table 1.
Clinical characteristics, adjuvant therapy and overall outcome in each subject.
| Animal # | Age onset (yrs) | Gender | Primary sign | MRI Diagnosis | Path Diagnosis (if available) | Pre CED Tumor Volume (cm3) on FLAIR | Other Tumor Treatment | Euthanasia | Post-CED Survival (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | F | Seizures | Left temporal lobe glioma | Glioblastoma | 10.7 | None | Yes | 38 |
| 2 | 9 | M | Seizures | Right fronto-temporal lobe glioma | Anaplastic astrocytoma | 14.3 | TMZ | Yes | 82 |
| 3 | 9 | F | Seizures | Left parieto-occipital lobe glioma | None | 2.6 | None | Yes | 44 |
| 4 | 9 | M | Seizures | Right temporal lobe glioma | None | 2.2 | TMZ | Yes | 115 |
| 5 | 7 | M | Seizures | Right thalamic glioma | None | 1.1 | TMZ | No | Alive at 722 days |
| 6 | 7 | F | Seizures | Left fronto-parietal glioma | None | 4.3 | None | Yes | 62 |
| 7 | 8 | M | Behavior change and seizures | Right temporal lobe glioma | Cystic meningioma | 5.8 | None | Yes | 13 |
| 8 | 8 | M | Seizures | Right temporal lobe glioma | None | 4.9 | None | Yes | 263 |
| 9 | 9 | F | Right hemiparesis and circling | Right temporal glioma | None | 14.2 | RT | Yes | 59 |
| 10 | 8 | F | Seizures, left hemiparesis, stupor, and disorientation | Right parietal glioma | High grade astrocytoma | 12.6 | None | Died 1 day post-op | 1 |
Only one procedure was performed on each animal. For 7 canines, the initial catheter placement and trajectory resulted in MRI evidence of nanoparticle distribution within the tumor, giving a target delivery accuracy of 70%. Figure 2 shows three representative MRI slices from animals where the distribution of PMNPs following CED covers a significant portion of the tumor area. One of these animals had a very long clinical response. In 3 animals, PMNP delivery missed the target (Fig. 3). Interestingly, in one of these animals, evidence of tumor shrinkage, clinical improvement and prolonged survival was seen despite low tumor coverage by PMNPs on the post-operative MRI (Fig. 3B). Notably, this animal did not receive any other post-operative anti-glioma therapy. Only this dog and animal #5 underwent repeat MRI at a later time point. In both these animals an objective decrease in tumor volume was noted.
Fig. 2.
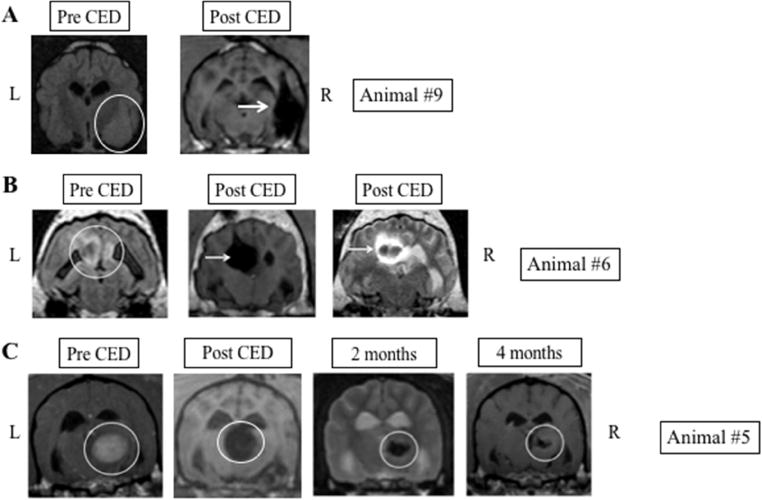
Representative images in canines with good PMNP coverage following CED. A. Large, poorly circumscribed temporal lobe tumor (white circle) with significant lesion coverage following CED of PMNPs (white arrows). Post-CED images show PMNP signal immediately post infusion. B. Parasagittal mass (white circle) with excellent tumor coverage following CED (white arrow). Some extension of signal within the ventricle is also seen. C. Thalamic tumor (white circle) with significant tumor coverage on immediate post-operative scan. Two and four month post CED treatment scans demonstrate durable decrease in tumor size. L: Left side of animal corresponds to left side of image; R: Right side of animal corresponds to right side of image.
Fig. 3.
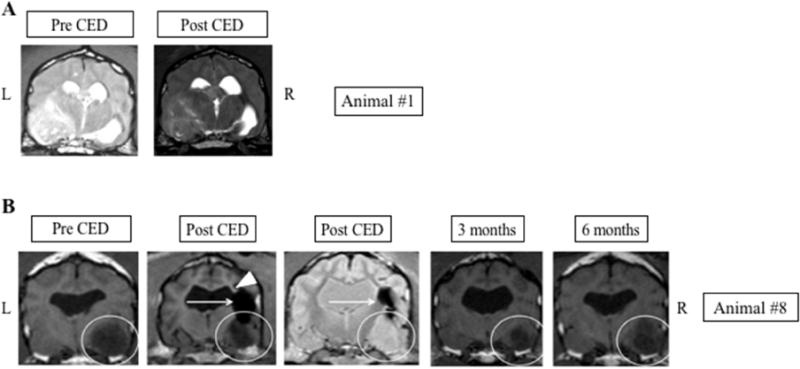
Tumors with poor CED coverage A. No evidence of PMNP signal within the tumor or surrounding brain is seen following CED. B. Temporal lobe tumor (white circle) demonstrates tumor shrinkage following CED treatment at 3 and 6 months. However, PMNP signal immediately following CED only covers a small region of the mass (horizontal arrows). Possible loss of PMNP into the left lateral ventricle is noted (arrowhead). L: Left side of animal corresponds to left side of image; R: Right side of animal corresponds to right side of image.
Nine out of ten canines recovered from the acute effects of the procedure to baseline function. One dog had a very large tumor causing significant pre-operative symptoms, including stupor, disorientation and left hemiparesis. This animal died on post-op day 1 from a likely herniation event. Pathology in this animal was consistent with a high grade astrocytoma. No other dogs had complications as a result of the procedure or drug delivery. During the course of care, seven of the families elected for euthanasia. One canine was still alive two years after PMNP administration. Excluding the perioperative mortality and the animal with a pathological diagnosis of meningioma (which is not typically treated with TMZ), the remaining 8 dogs had a median survival of 72 days (range: 13- 722 days) after surgery. The median time from onset of symptoms to death was 182 days (range: 54- 291 days) and median time from symptom onset to surgery was 45 days (range: 17- 205 days).
Dogs underwent routine post-operative care following infusion and the majority of dogs were discharged on post-operative day one. Treatment decisions regarding adjuvant chemotherapy, seizure prophylaxis and steroid management were made by the treating veterinarian in consultation with the family. All canines in this study received post-operative prednisone and seizure prophylaxis. Families who wished to pursue adjuvant therapies were offered oral TMZ, which was given to three out of ten animals (Table 1). One canine received RT. Tissue for histopathological analysis was available for four of the ten subjects. Three of these four specimens were consistent with a Grade 3 astrocytoma or GBM and one was a cystic meningioma. For animals that underwent post-mortem analysis, in the specimens where an inoculation tract was visible, areas of pannecrosis, hemorrhage and an extensive infiltrate of gitter cells was seen. Some of these cells were engaged in erythrophagocytosis, while others contained homogenous light brown material.
Discussion
In this open-label, single arm interventional proof-of-concept study, we enrolled 10 pet dogs presenting with an intracranial mass. The primary goal of this study was to examine the accuracy of targeting the intracranial mass with PMNPs encapsulating TMZ. Enrolled animals underwent stereotactic placement of a catheter into the center of the tumor via a burr hole overlying the lesion. Following infusion of PMNPs, animals underwent a post-surgical MRI to evaluate nanoparticle distribution using a standard 1.5T scanner. All ten animals underwent uneventful surgery and nine dogs recovered without acute treatment-associated complications. Delivery of PMNPs within the boundary of the tumor mass was noted in 70% of the animals. In three of the ten subjects the infusion missed the target. In one of these three animals the tumor was found to be a cystic meningioma. The firm nature of the capsule surrounding this mass may have prevented the catheter from penetrating into the tumor.
We previously demonstrated the efficacy of delivering PMNPs carrying a therapeutic payload in a rodent glioma model 3, and now examine this system in canine tumors that better recapitulate the biology of human glioma. The relatively high incidence of gliomas in dogs, the large number of dogs in the community, and the similarities in the genetic and morphological features of canine and human gliomas make these animals an ideal model for the evaluation of novel therapeutic agents 4,29. Here, we show that PMNPs can be injected into glioma tissue in dogs and distributed by CED to a localized region of the brain. Unfortunately, despite meticulous planning and tumor modeling, our results further confirm the observation that infusate distribution cannot always be accurately predicted following CED 30,31. PMNPs can be lost in the cerebrospinal fluid of the ventricles or subarachnoid space, or may be directed around the tumor due to the anisotropic nature of the surrounding tissue 32. Our results underline the importance of visualizing PMNP distribution following infusion. By identifying the distribution, subsequent adjustments or additional injections can be performed to maximize tumor coverage. While we failed to achieve adequate tumor coverage based on MRI in three dogs, in one of these animals clinical and imaging evidence of treatment effect was seen. It is possible that in some cases nanoparticle distribution does not reach the level of detection by a 1.5T MRI scanner. In such cases, more frequent imaging in real-time during the infusion may enable better understanding of PMNP distribution.
Initial studies examining CED in canines used liposomally encapsulated CPT-11 and gadoteridol in both healthy laboratory dogs and companion dogs with spontaneous gliomas 4,33. A subsequent study by a different group using CED of iron-oxide nanoparticles in healthy dogs confirmed the importance of delivery rate as faster infusion rate resulted in back-flow along the catheter track 27. Our work builds on these prior experiments to further demonstrate the potential of using nanoparticles in canines with gliomas. Notably, using a step-down catheter and an infusion rate not greater than 5 μl/min, no infusate back-flow was seen. Despite this observation and optimal catheter position, in three animals tumor coverage was poor highlighting the importance of imaging and the requirement of dynamic modulation of catheter position.
Although the therapeutic strategy of encapsulating TMZ was safe in the current study and effective in a previous small animal model 3, it is notable that TMZ is efficacious when given orally 1. These observations suggest that encapsulating chemotherapeutics may not be the best use of an injected vector. Perhaps delivering a chemo- or radio-sensitizer that can improve the efficacy of systemic TMZ and RT is better. Regardless of the payload, CED of PMNPs represents a minimally invasive approach to directly target an intracranial tumor. Such a strategy is ideal either for deep, unresectable lesions, or to target the surrounding brain following resection of accessible tumors. One can envision a scenario where following imaging verification of an intrinsic tumor, a stereotactic biopsy is performed and one or several catheters left in place at the time of surgery. Subsequently, following molecular characterization of the tumor, personalized PMNPs can be fashioned that express a specific payload and are targeted to tumor antigens present on the tumor cells of that patient. Such personalized PMNPs can then be delivered by CED using the catheters placed during the initial surgery. In addition, CED parameters can be adjusted based on real-time imaging to optimize tumor coverage. In this manner, image-guided CED of PMNPs represents a model system for both spatial and molecular targeting of CNS malignancies.
This pilot study focused on the immediate distribution of PMNPs, so a shorter infusion time was employed rather than the multiple day infusion that would take place in the clinical setting. Given the limited scope of this study, the volume of infusate was kept constant. Of course, varying the volume of infusate is an important adjustment that would be used in the clinical setting to maximize tumor coverage 17. Another limitation of this study was the lack of serial follow-up MRIs. Such imaging could be used to monitor the disappearance rate of the PMNPs from the tumor. Also, the lack of pathology for the majority of tumors and the small number of canines limited our ability to investigate how tumor biology or location influenced the success of PMNP delivery and accuracy.
In conclusion, this study shows that convection-based drug delivery using PMNPs can be safely performed in a canine model of glioma. Moreover, given that even with optimal catheter placement only 70% target accuracy was seen, we confirm the importance of imaging to optimize the accuracy of nanoparticle delivery. Finally, although CED of PMNP was sufficient by itself to induce an observable decrease in tumor volume in one animal, additional studies are required to determine the efficacy of using this technique in dogs and ultimately in humans.
Highlights.
Spontaneous canine gliomas closely resemble human tumors
CED of polymeric nanoparticles is safe in dogs with supratentorial tumors
Distribution of nanoparticles is not predictable following CED
Following CED, imaging is essential to verify therapeutic agent distribution
Acknowledgments
We would like to thank Mike Oglesbee, The Ohio State University, for performing post-mortem analyses.
Funding: This work was supported by R44CA135906 (LN and BY) and by R01CA136937 (BY).
Abbreviations
- BBB
blood brain barrier
- CED
convection enhanced delivery
- CNS
central nervous system
- GBM
Glioblastoma
- MRI
magnetic resonance imaging
- PMNP
polymeric magnetite nanoparticles
- RT
radiation therapy
- TMZ
temozolomide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: LNK Chemsolutions, LLC have commercial interests in the nanoparticles described in this work.
Ethics Approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed
References
- 1.Stupp R, Mason W, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson PJ. Advances in Diagnostic and Treatment Modalities for Intracranial Tumors. J Vet Intern Med. 2014;28(4):1165–1185. doi: 10.1111/jvim.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal GM, LaRiviere MJ, Mansour N, et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine Nanotechnology, Biol Med. 2014;10(1):149–157. doi: 10.1016/j.nano.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: A translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12(9):928–940. doi: 10.1093/neuonc/noq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huo T, Barth RF, Yang W, et al. Preparation, Biodistribution and Neurotoxicity of Liposomal Cisplatin following Convection Enhanced Delivery in Normal and F98 Glioma Bearing Rats. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Lai X, Song S, Zhu X, Zhu J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2016;23(4):1369–1373. doi: 10.3109/10717544.2015.1038857. [DOI] [PubMed] [Google Scholar]
- 7.Mangraviti A, Tzeng SY, Kozielski KL, et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano. 2015;9(2):1236–1249. doi: 10.1021/nn504905q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Huo T, Barth RF, et al. Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neurooncol. 2011;101(3):379–390. doi: 10.1007/s11060-010-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidros DS, Liu JK, Vogelbaum Ma. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 2010;6(1):117–125. doi: 10.2217/fon.09.135. [DOI] [PubMed] [Google Scholar]
- 10.Debinski W, Tatter S. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9(10):19831841. doi: 10.1586/ern.09.99.Convection-enhanced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allard E, Passirani C, Benoit J-P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30(12):2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SD, Foster K, Yamini B. Convection-enhanced delivery for treatment of brain tumors. Expert Rev Anticancer Ther. 2007;7(12 SUPPL) doi: 10.1586/14737140.7.12.S79. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Deliv. 2007;4(2):169–180. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 14.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf- CRM107) therapy of malignant gliomas. J Neurooncol. 2003;65(1):3–13. doi: 10.1023/A:1026246500788. [DOI] [PubMed] [Google Scholar]
- 18.Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL4- Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64(1):125–137. doi: 10.1227/00006123-200108000-00127. [DOI] [PubMed] [Google Scholar]
- 19.Vogelbaum MA, Iannotti CA. Convection-enhanced delivery of therapeutic agents into the brain. In: Vinken PJ, Bruyn GW, editors. Handb Clin Neurol. Vol. 104. 2012. pp. 355–362. [DOI] [PubMed] [Google Scholar]
- 20.Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK. Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg. 2016:1–10. doi: 10.3171/2016.1.JNS151591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentley RT, Ahmed AU, Yanke AB, Cohen-Gadol AA, Dey M. Dogs are man’s best friend: In sickness and in health. Neuro Oncol. 2017;19(3):312–322. doi: 10.1093/neuonc/now109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leblanc AK, Mazcko C, Brown DE, et al. Creation of an NCI comparative brain tumor consortium: Informing the translation of new knowledge from canine to human brain tumor patients. Neuro Oncol. 2016;18(9):1209–1218. doi: 10.1093/neuonc/now051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson PJ, Sturges BK, Higgins RJ, et al. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet Pathol. 2008;45(2):131–139. doi: 10.1354/vp.45-2-131. [DOI] [PubMed] [Google Scholar]
- 24.Higgins RJ, Dickinson PJ, Lecouteur RA, et al. Spontaneous canine gliomas: Overexpression of EGFR, PDGFRα and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neurooncol. 2010;98(1):49–55. doi: 10.1007/s11060-009-0072-5. [DOI] [PubMed] [Google Scholar]
- 25.Stoica G, Kim HT, Hall DG, Coates JR. Morphology, Immunohistochemistry, and Genetic Alterations in Dog Astrocytomas. Vet Pathol. 2004;41(1):10–19. doi: 10.1354/vp.41-1-10. [DOI] [PubMed] [Google Scholar]
- 26.Thomas R, Duke SE, Wang HJ, et al. “Putting our heads together”: Insights into genomic conservation between human and canine intracranial tumors. J Neurooncol. 2009;94(3):333–349. doi: 10.1007/s11060-009-9877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt S, Nduom E, Kent M, et al. Canine model of convection-enhanced delivery of cetuximab-conjugated iron-oxide nanoparticles monitored with magnetic resonance imaging. Clin Neurosurg. 2012;59:107–113. doi: 10.1227/NEU.0b013e31826989ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krauze MT, Saito R, Noble C, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103(5):923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicks J, Platt S, Kent M, Haley A. Canine brain tumours: a model for the human disease? Vet Comp Oncol. 2017;15(1):252–272. doi: 10.1111/vco.12152. [DOI] [PubMed] [Google Scholar]
- 30.Linninger AA, Somayaji MR, Mekarski M, Zhang L. Prediction of convection-enhanced drug delivery to the human brain. J Theor Biol. 2008;250(1):125–138. doi: 10.1016/j.jtbi.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Raghavan R, Brady ML, Rodríguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20(4):E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 32.Vavra M, Ali MJ, Kang EW-Y, et al. Comparative pharmacokinetics of 14C-sucrose in RG-2 rat gliomas after intravenous and convection-enhanced delivery. Neuro Oncol. 2004;6(2):104–112. doi: 10.1215/S1152.8517.03.00044.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickinson PJ, Lecouteur RA, Higgins RJ, et al. Canine model of convection- enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging. J Neurosurg. 2008;108(5):989–998. doi: 10.3171/JNS/2008/108/5/0989. [DOI] [PubMed] [Google Scholar]


